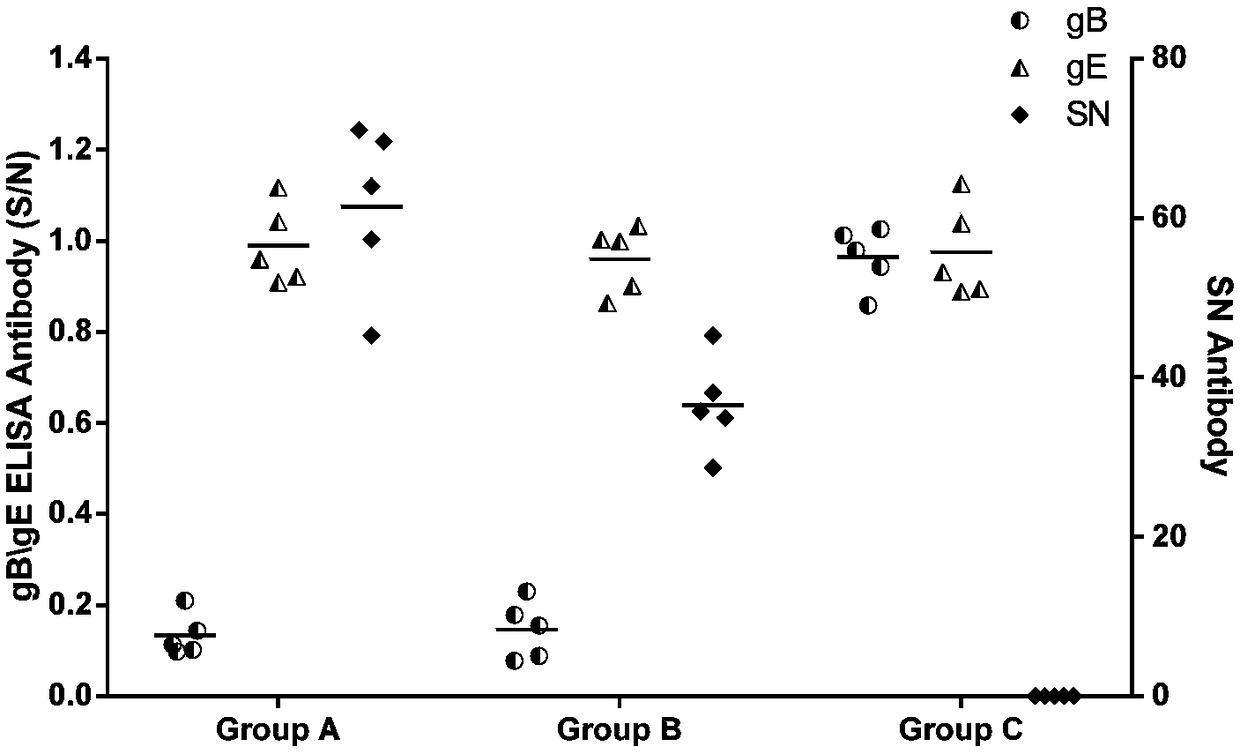
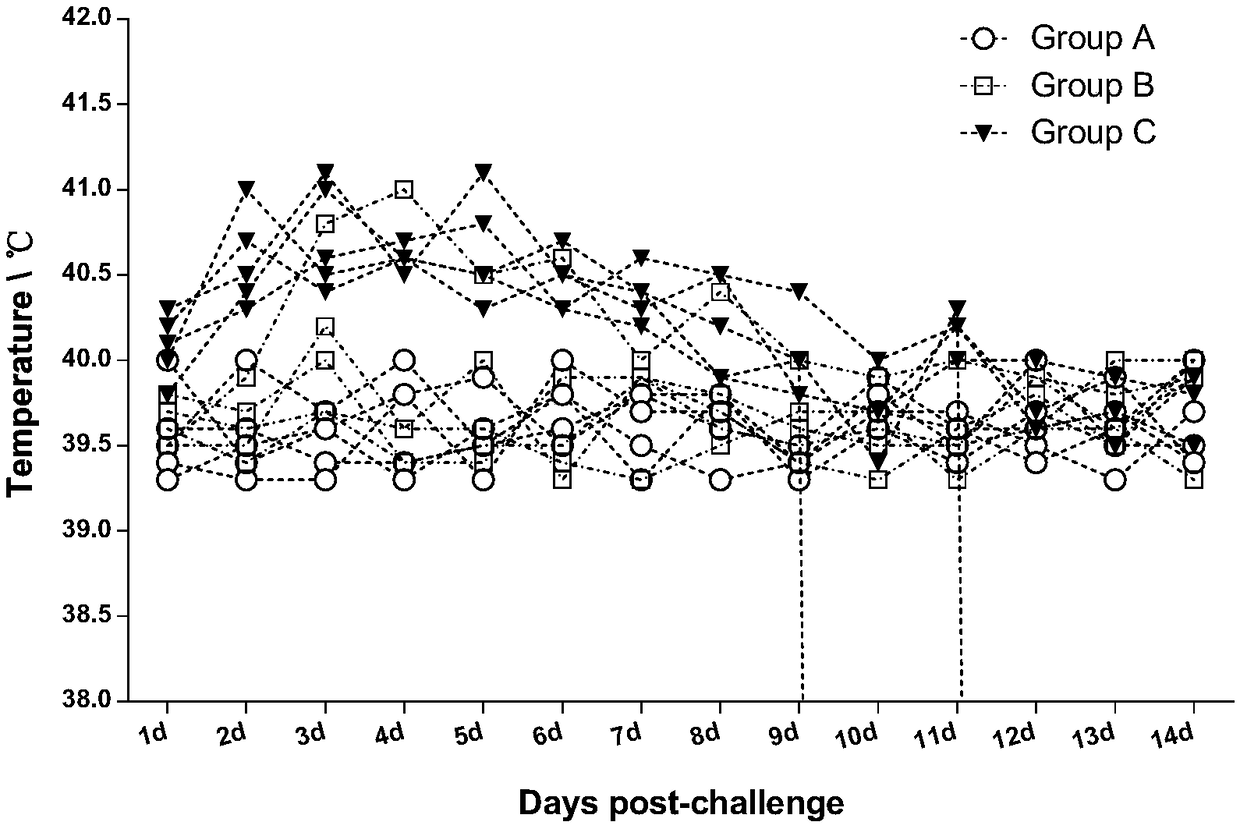
Patents
Literature
65results about How to "Preserve immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human Anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor
ActiveUS20110271358A1Reduced antigen binding affinityLess immunogenicAntibacterial agentsAntipyreticTransplant rejectionAutoimmune disease
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
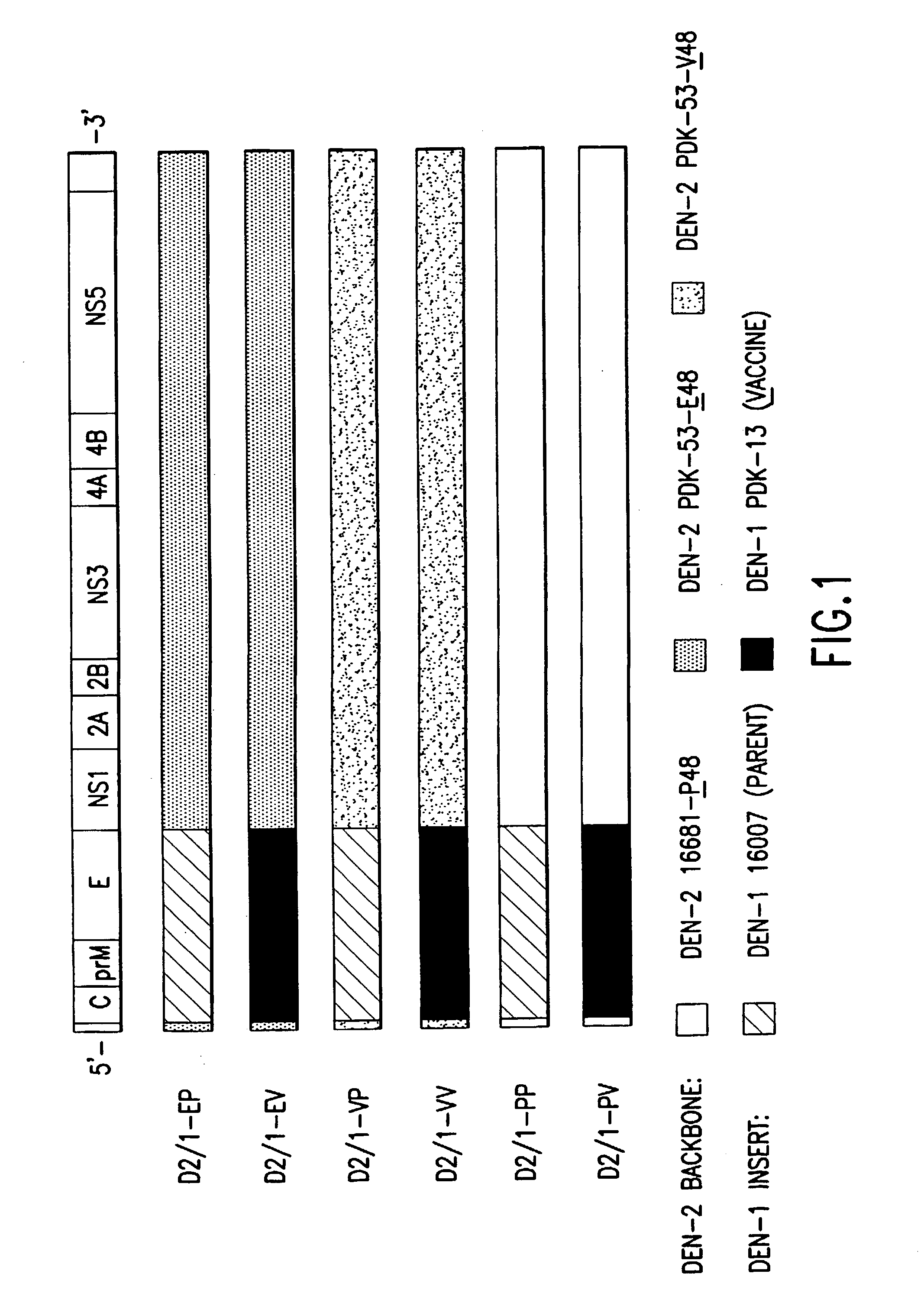
Avirulent, immunogenic flavivirus chimeras
InactiveUS7094411B2Minimize and inhibit infectionStable maintenanceOrganic active ingredientsVirusesViral diseaseAmino acid mutation
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Avirulent, immunogenic flavivirus chimeras
InactiveUS20060062803A1Inhibition effectPreserve immunogenicityBiocideOrganic active ingredientsViral diseaseFhit gene
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Pseudomonas aeruginosa vaccine and preparation method thereof
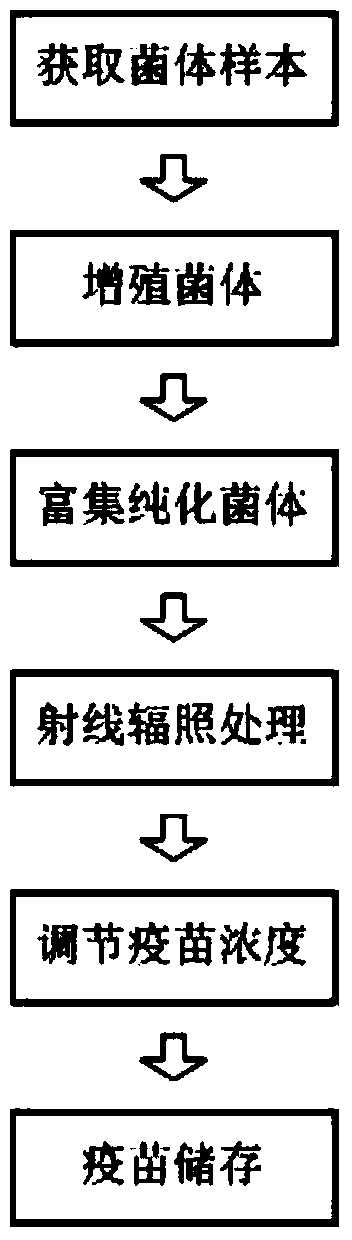
ActiveCN104189898ARetain metabolic activityPreserve immunogenicityAntibacterial agentsElectrical/wave energy microorganism treatmentBacteroidesDisease
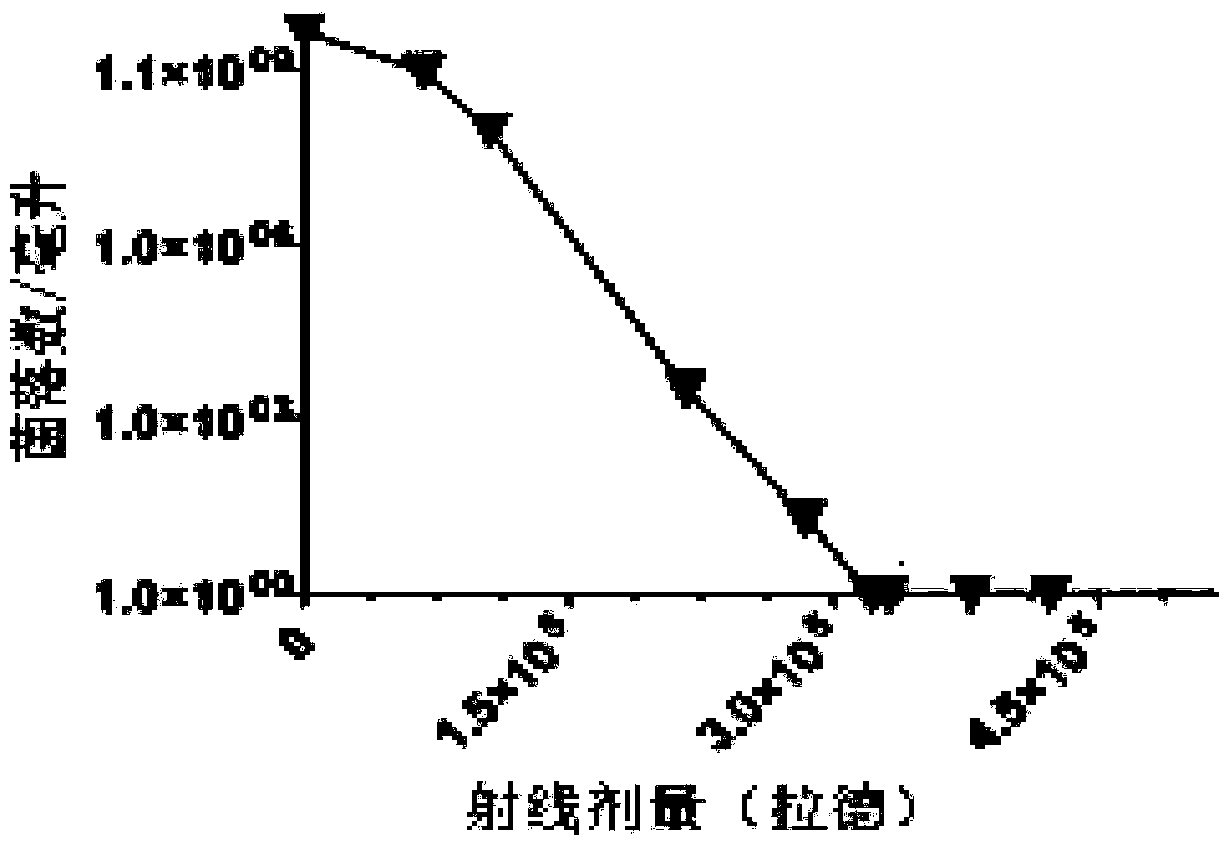
The invention relates to the technical field of biology and specifically relates to a pseudomonas aeruginosa vaccine and a preparation method thereof. The pseudomonas aeruginosa vaccine is used for solving the technical problem that the existing pseudomonas aeruginosa vaccine at the development and clinical test stages are low in protective efficacy, high in toxicity to organisms and incapable of meeting the requirements of clinical immunology due to failure in curing infection. The technical scheme adopted to solve the technical problem is as follows: a preparation method of a novel vaccine is provided. The preparation method comprises the steps of firstly obtaining pseudomonas aeruginosa bacteria and then irradiating the bacteria by use of rays to obtain the pseudomonas aeruginosa vaccine. The pseudomonas aeruginosa vaccine is used for either preventing diseases or treating diseases, and has good application prospect.
Owner:SICHUAN UNIV
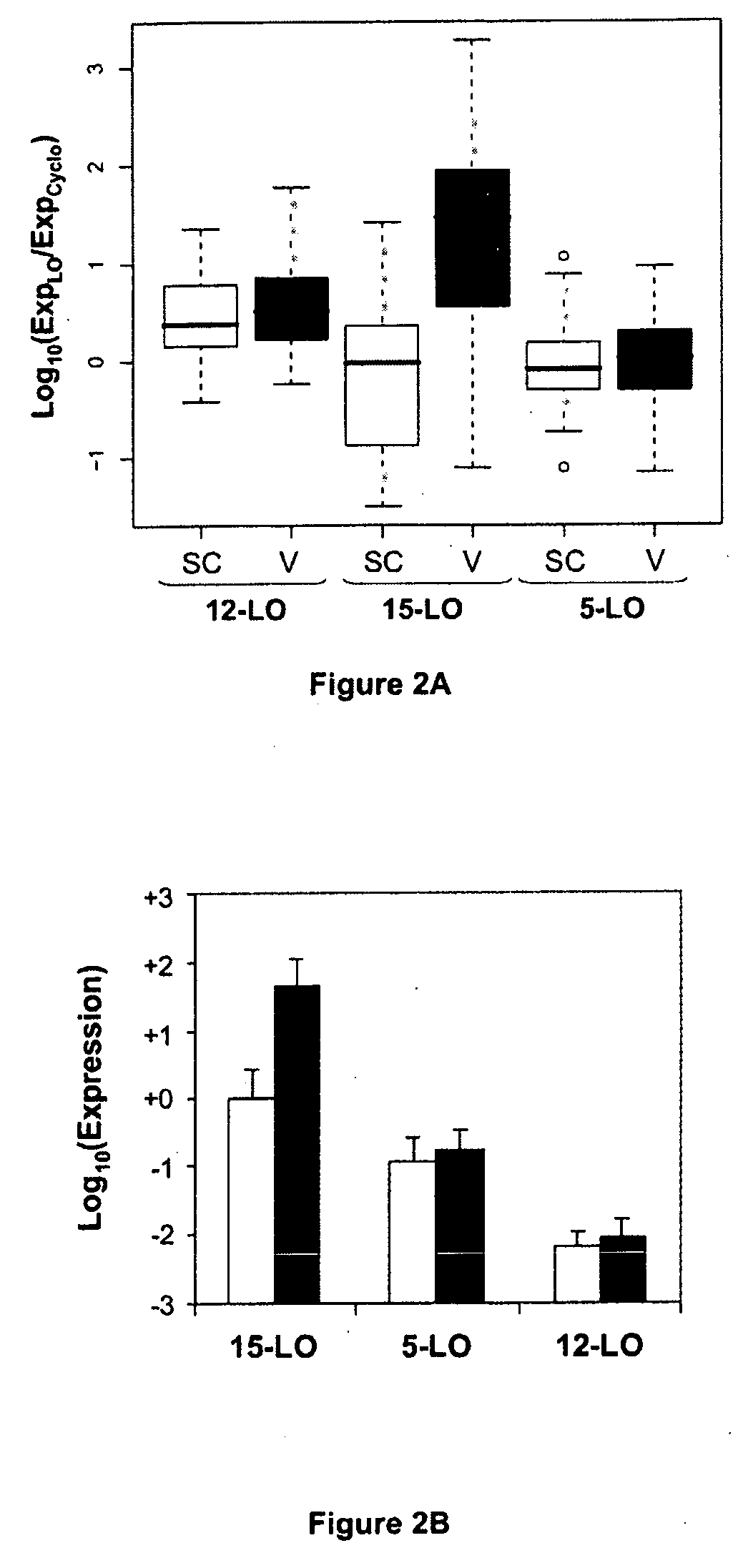
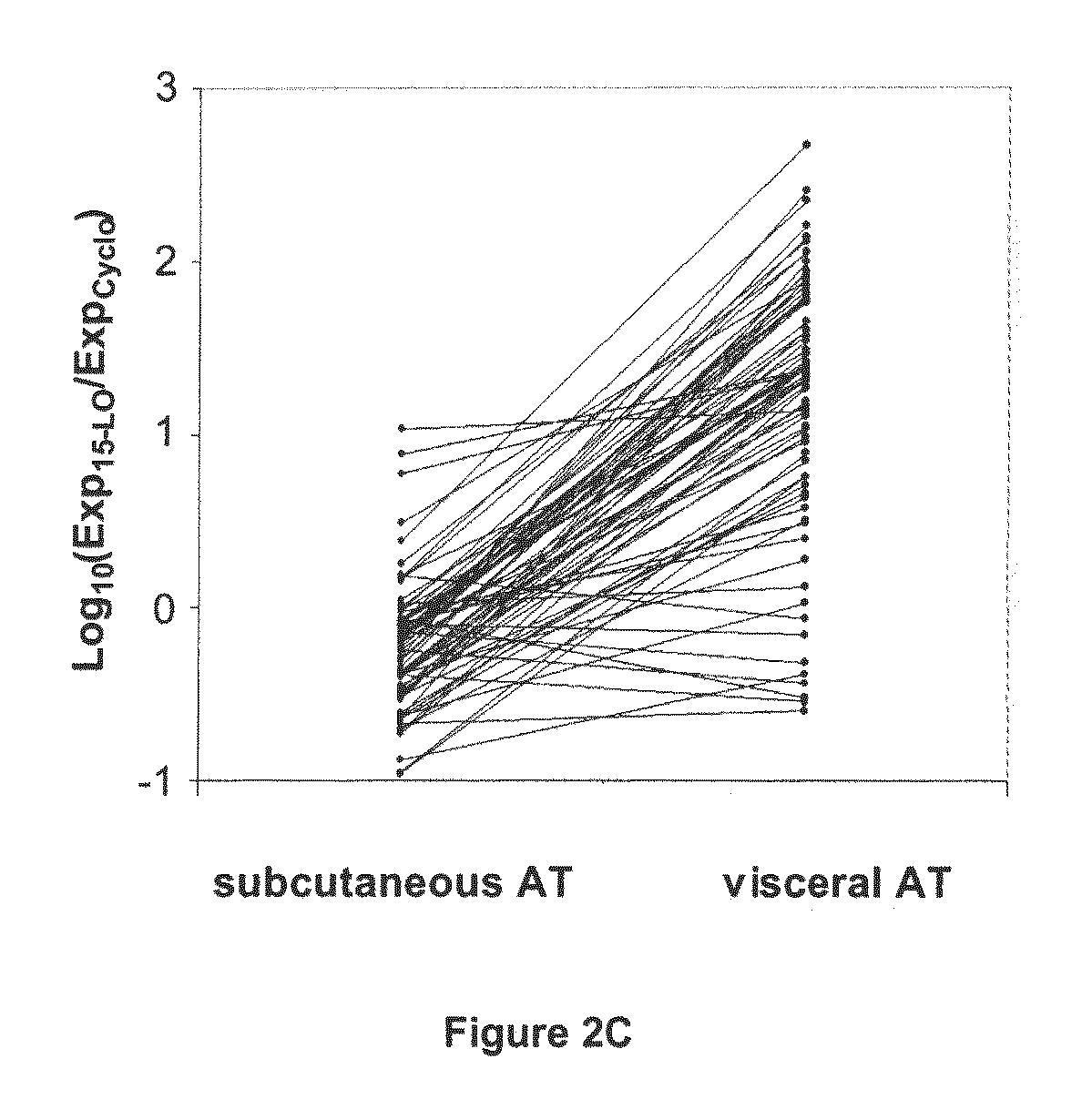
Use of 15-Lipoxygenase Inhibitors for Treating Obesity
ActiveUS20090060905A1Preserve immunogenicityImprove the situationHalogenated hydrocarbon active ingredientsBiocideObesityVisceral Obesity
The invention concerns the treatment of obesity, in particular abdominal visceral obesity. More specifically, the invention concerns the use of selective 15-lipoxygenase (LO) inhibitors for preparing medicines useful in the treatment of obesity, or at least abdominal visceral obesity, and / or its consequences.
Owner:GENFIT SA
Recombinant poxvirus vector comprising tetanus toxin fragment c
ActiveUS20140322265A1Negligible pathogenicityReduce capacityBacterial antigen ingredientsBacteriaBacilliViral vector
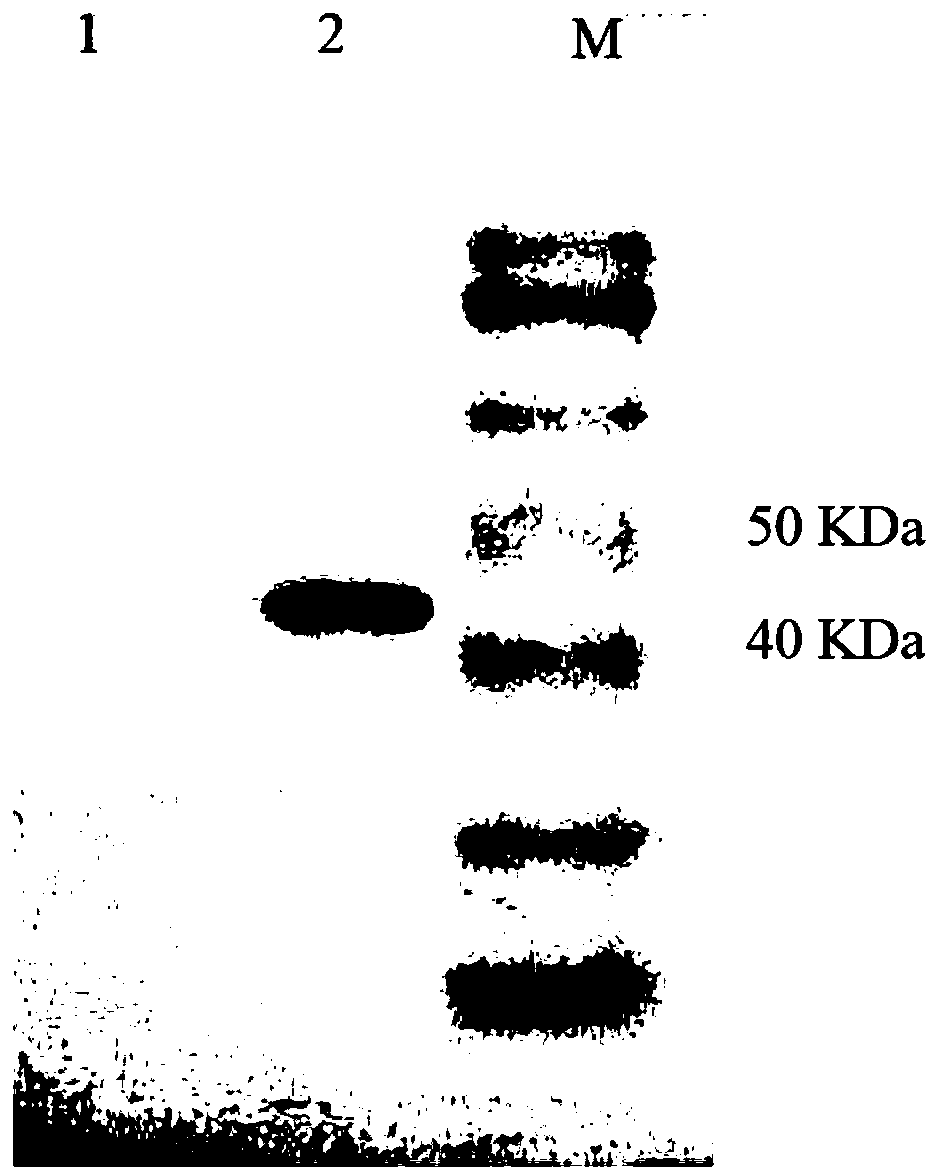
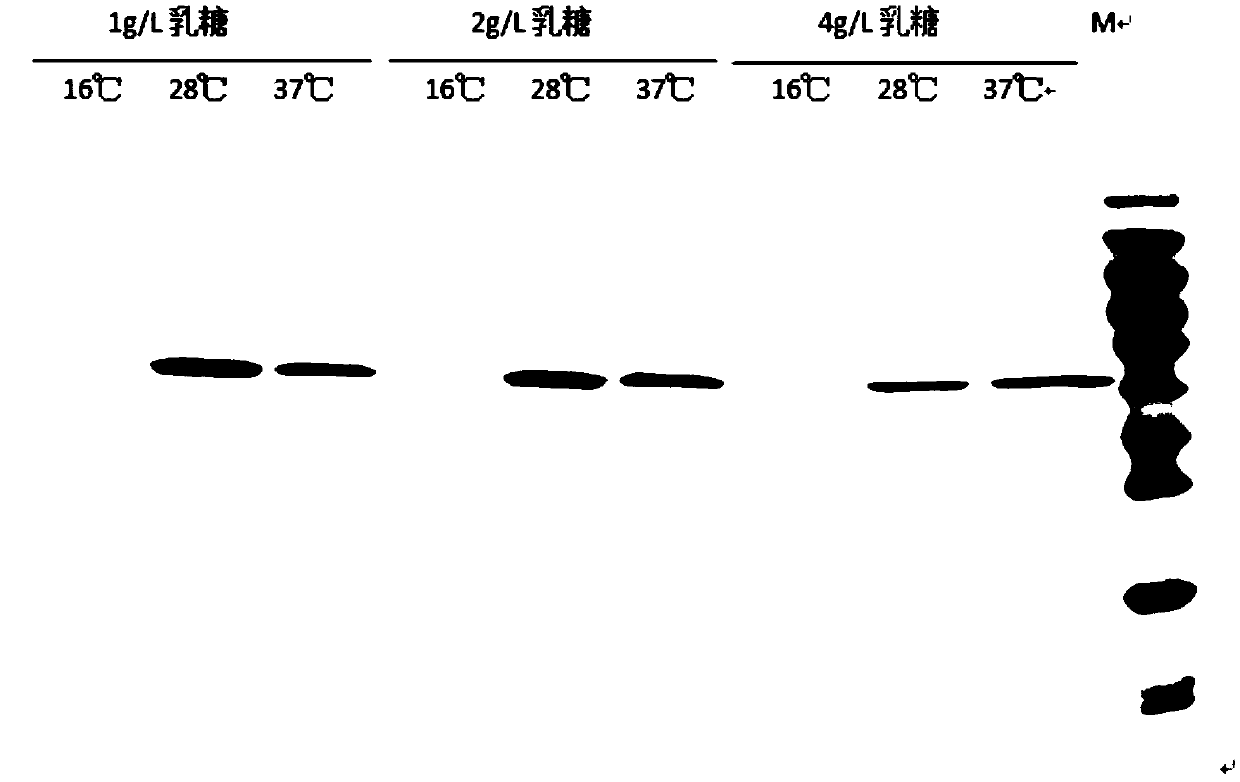
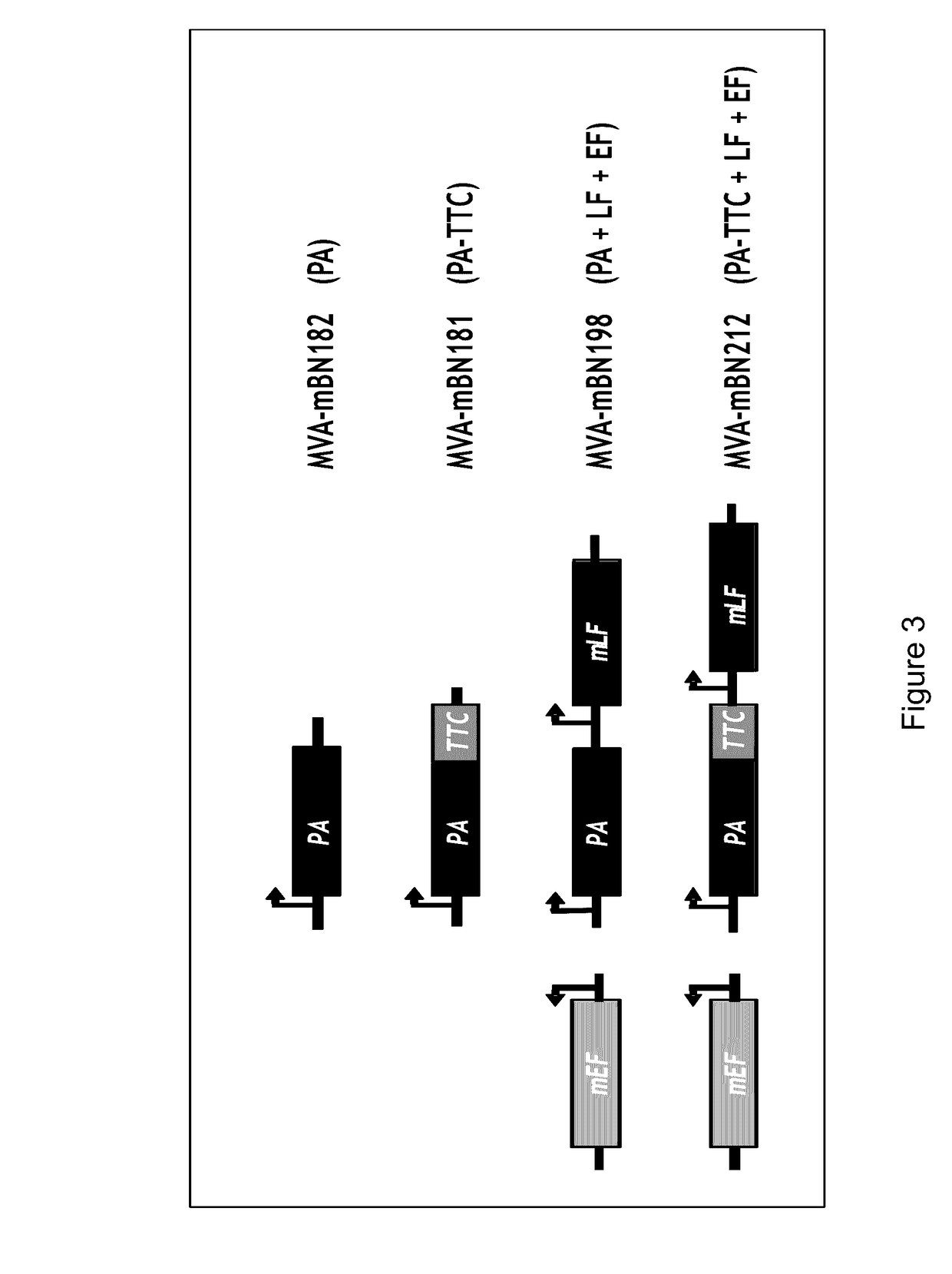
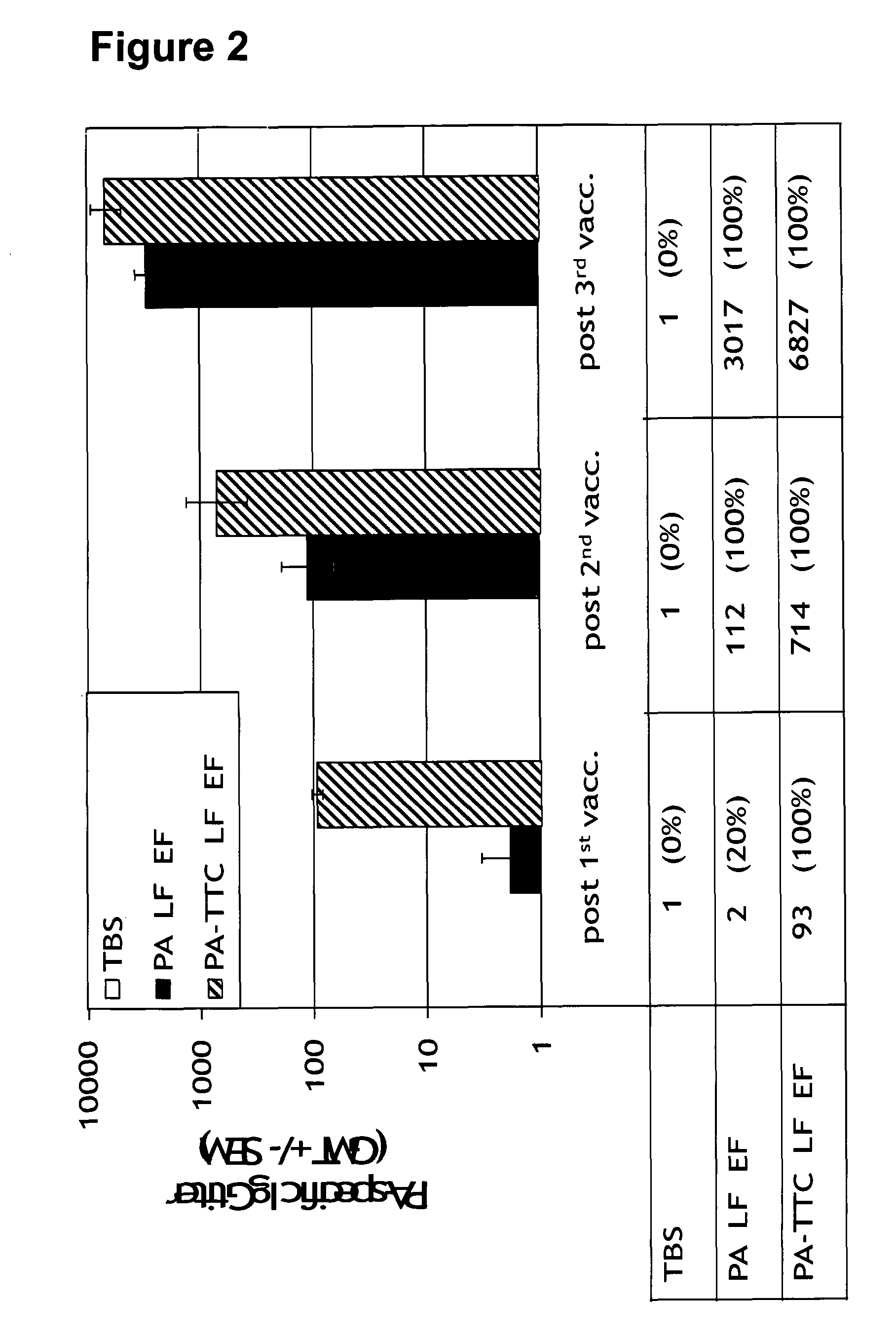
The present invention relates to a recombinant poxvirus comprising tetanus toxin fragment C for improved immunogenicity of an antigen and related methods and uses. Specifically, the present invention generally relates to genetically engineered (recombinant) poxvirus vectors comprising a tetanus toxin fragment C (TTC) coding sequence operably linked to a bacterial antigenic determinant as well as to uses thereof, e.g., to affect an immune response in a subject.
Owner:BAVARIAN NORDIC BAVARIAN NORDIC
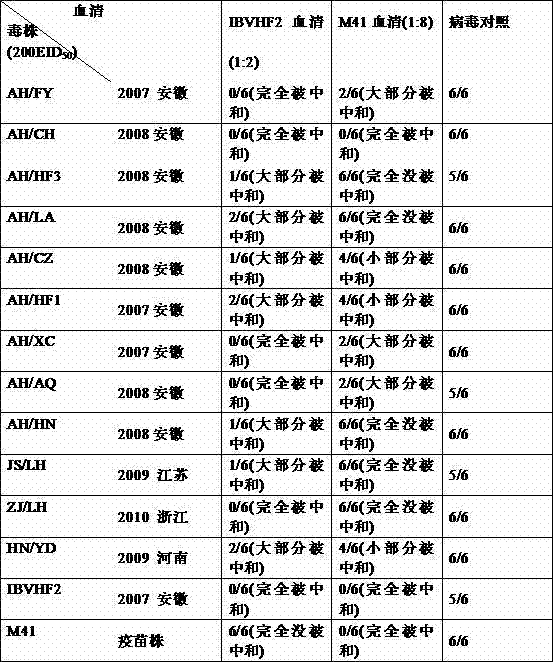
Avian infectious bronchitis virus vaccine strain (HF2 strain) and application thereof
ActiveCN103497934ASolved and still sickSolve the current situation of the diseaseMicroorganism based processesAntiviralsCombined VaccinesVirus strain
The invention belongs to the field of biological medicines of veterinarians and relates to an avian infectious bronchitis virus vaccine strain (HF2 strain) as well as application and an application effect of the vaccine strain in prevention and control of avian infectious bronchitis. The microbial preservation number of the vaccine strain is 7708. The vaccine strain disclosed by the invention is good in security and has a good protection effect on the avian infectious bronchitis. The vaccine strain can be used for preparing an inactivating single vaccine or combined vaccine for preventing and controlling the avian infectious bronchitis; a virus strain can be used for preparing an attenuated vaccine or combined vaccine for preventing and controlling the avian infectious bronchitis after being attenuated.
Owner:兆丰华生物科技(南京)有限公司
Bacterial polysaccharide protein conjugate vaccine using hepatitis B surface antigen as carrier protein and preparation method of bacterial polysaccharide protein conjugate vaccine
InactiveCN104383532AAddressing Immunization IssuesPlay a role in preventionAntibacterial agentsAntiviralsAntigenConjugate vaccine
The invention discloses a bacterial polysaccharide protein conjugate vaccine using a hepatitis B surface antigen as carrier protein and a preparation method of the bacterial polysaccharide protein conjugate vaccine. According to the vaccine, protein is the hepatitis B surface antigen, and a bacterial polysaccharide is selected from any one or more of a haemophilus influenza type b polysaccharide, group A, group C, group Y and group W135 meningococcal polysaccharides, a salmonella typhi type Vi polysaccharide, a group B streptococcus type Ia polysaccharide and the like, pneumococcus serotype type 1, 2 and the like, and salmonella paratyphi type A or salmonella paratyphi type B. Animal experiments show that the antibody positive conversion rates of the bacterial polysaccharide and the hepatitis B surface antigen in the vaccine are both more than 85%, so that the vaccine is relatively high in antibody positive conversion rate; carrier protein plays a role in transforming the bacterial polysaccharide from a T-cell-independent antigen into a T-cell-dependent antigen, and also can be used for preventing diseases caused by hepatitis B virus; and by adopting the bacterial polysaccharide protein conjugate vaccine disclosed by the invention, the problem of performing immunization inoculation on infants and young children under 2 years old can be solved, the function of one injection with multiple immune effects also can be achieved, and the use crowd and coverage rate of the vaccine can be expanded.
Owner:云南沃森生物技术股份有限公司
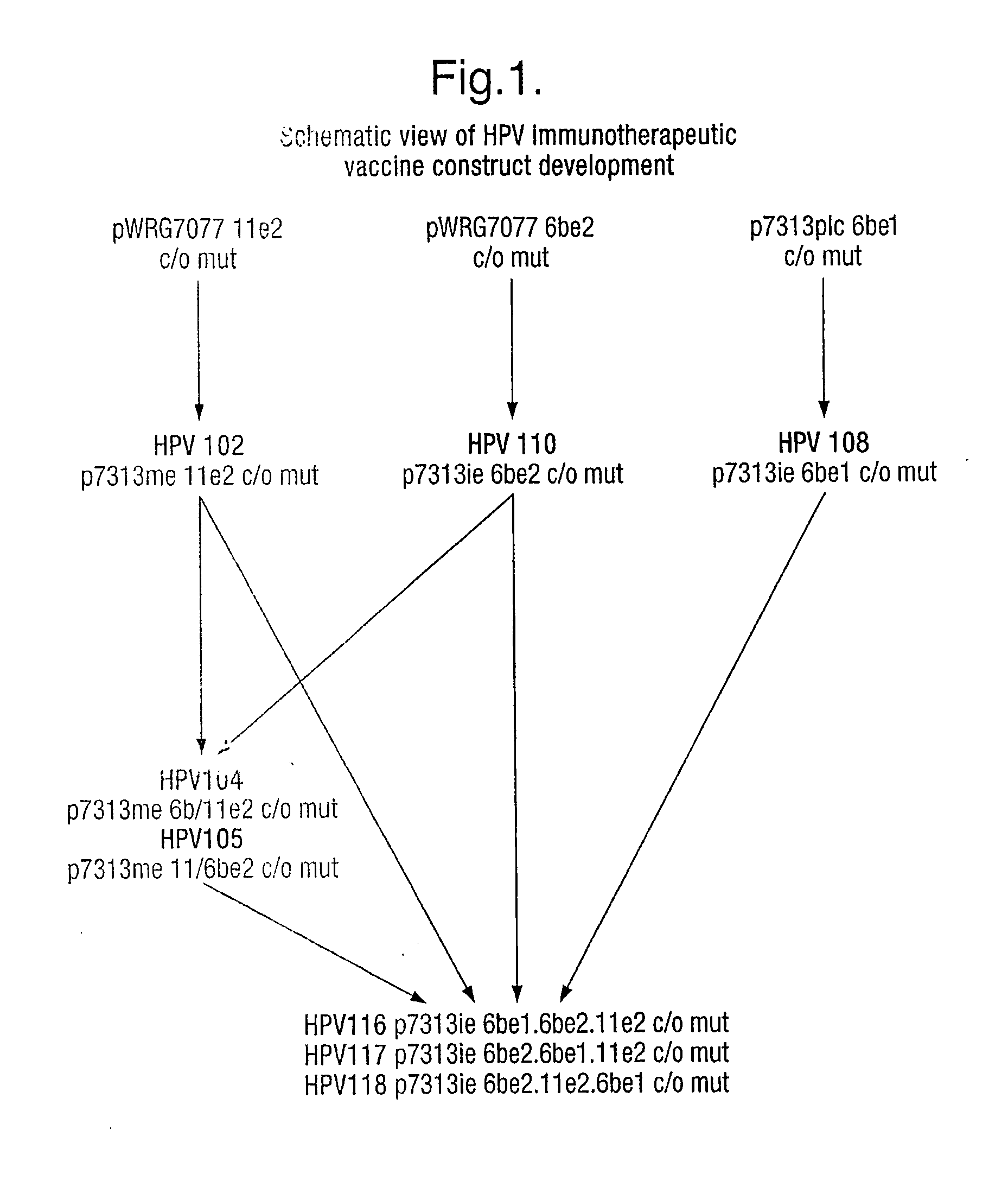
Vaccine
InactiveUS20060165713A1Easy to manufactureSimple and cheap to manufactureGenetic material ingredientsVirus peptidesVaccine deliveryHuman papilloma virus infection
The present invention relates to methods and compositions useful in the treatment and prevention of human papilloma virus infections. In particular the invention relates to nucleic acid molecules encoding E1 and / or E2 and vectors suitable for DNA vaccine delivery, and pharmaceutical compositions containing them. Methods for manufacturing said molecules, vectors and composition are also contemplated.
Owner:GLAXO GRP LTD
Porcine circovirus-mycoplasma pneumonia duplex subunit vaccine and preparation method thereof
ActiveCN109055412AFor proper foldingSmall steric hindranceAntibacterial agentsBacterial antigen ingredientsVaccine ProductionPorcine circovirus
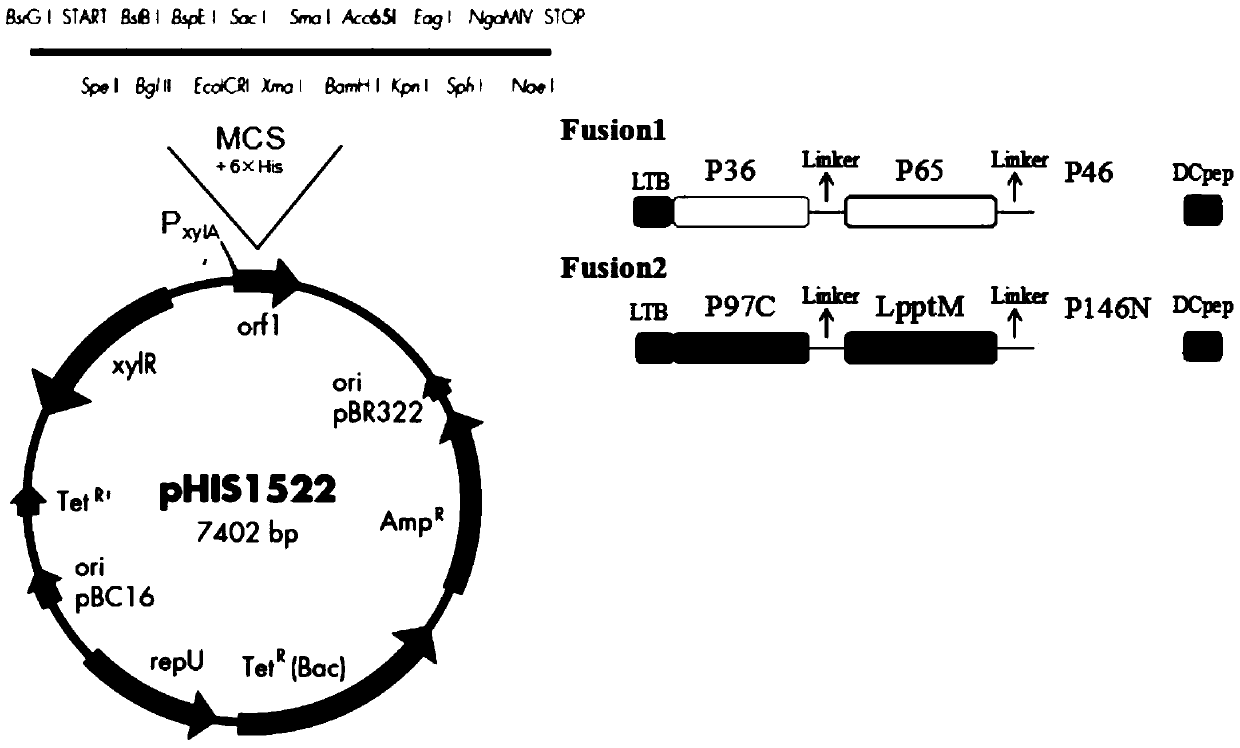
The invention belongs to the technical field of vaccine production, and specifically discloses a porcine circovirus-mycoplasma pneumonia duplex subunit vaccine and a preparation method thereof. The preparation method comprises the following steps: (1) dividing six coding genes of coded mycoplasma hyopneumoniae membrane proteins P36, P46, P65, P97, Lppt and P146 into two groups, constructing two mosaic genes which separately contain two groups of coding genes, and separately constructing the two mosaic genes in an expression vector; (2) constructing a coding gene of the porcine circovirus Cap protein into an expression vector; (3) constructing the expression vector comprising different genes into engineering bacteria, and expressing two mosaic genes to obtain two fused proteins and expressthe porcine circovirus Cap protein; and (4) utilizing the expressed protein to prepare the porcine circovirus-mycoplasma pneumonia duplex subunit vaccine. Multiple protens of the mycoplasma hyopneumoniae are fused, so that the growth period is greatly shortened, and production cost is greatly reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
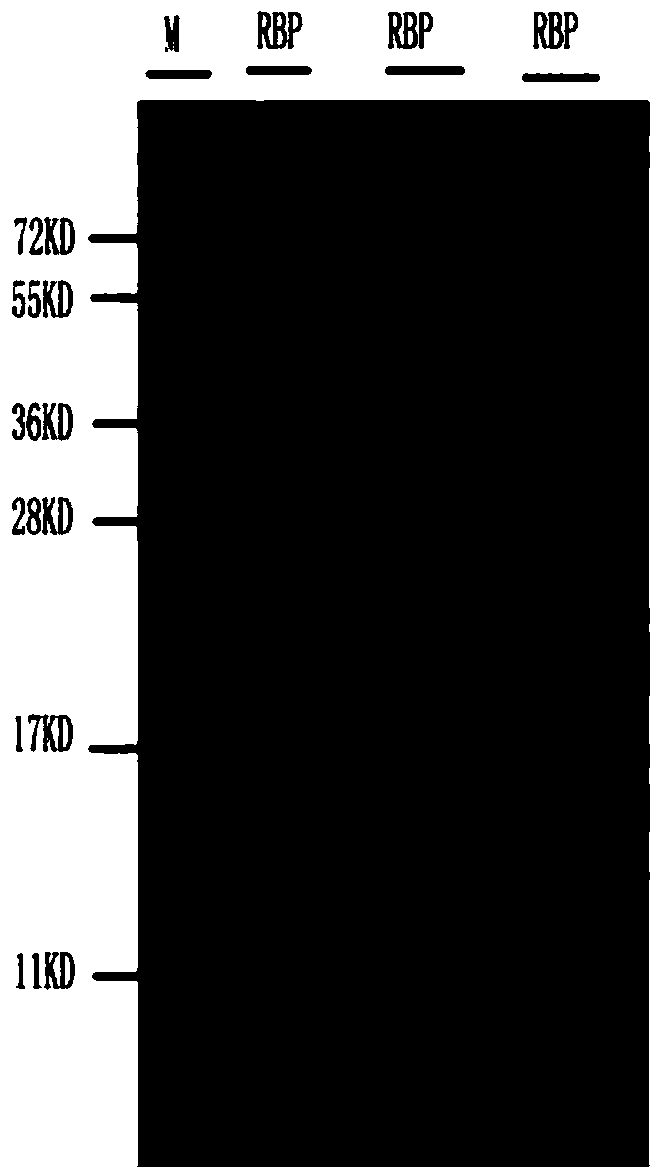
Process for purifying human retinol binding protein and preparation process of polyclonal antibody thereof
ActiveCN104193817APreserve immunogenicitySerum immunoglobulinsImmunoglobulins against animals/humansSeparation technologyDEAE-Sepharose
The invention discloses a process for purifying a human retinol binding protein and a preparation process of a polyclonal antibody thereof. According to the invention, by using a separation technology by multiple chromatographic columns such as a DEAE-Sepharose Fast Flow (DEAE-S) ion exchange column, a molecular sieve (Superdex 75, Sephacryls-200) and a hydrophobic column Phenyl SepharoseTM High Performance (PHSP), the human retinol binding protein is successfully purified from urine of a patient suffering from renal injury, and the immunogenicity of the RBP protein is remained to the greatest degree. The obtained human RBP antigen is used for immunizing animals, thus obtaining a polyclonal antibody of the human retinol binding protein. The obtained polyclonal antibody of the human retinol binding protein can be applied to an immunonephelometry kit.
Owner:桂林英美特生物技术有限公司
O-type foot and mouth disease virus strain with improved replication titer as well as construction method and application of O-type foot and mouth disease virus strain
ActiveCN114854698AReplication titer increasedGood genetic stabilitySsRNA viruses positive-senseViral antigen ingredientsInfected cellDisease
The invention provides an O-type foot and mouth disease virus strain with improved replication titer as well as a construction method and application thereof, and belongs to the technical field of biological products. Through a reverse genetic manipulation technology of the foot and mouth disease virus, a G-H ring gene of the foot and mouth disease virus O / NXYCh / CHA / 2018 strain is further used for replacing a corresponding gene on a recombinant virus skeleton of a main immune gene of the chimeric foot and mouth disease virus O / XJ / CHA / 2017 strain, the time of causing 100% infected cell lesion by the constructed recombinant virus rHN / XJ / NXGH is remarkably shortened to 12 h, and the time of causing 100% infected cell lesion by the recombinant virus rHN / XJ / NXGH is remarkably shortened to 12 h. And the replication titer of infected cells for 12 hours is improved by more than 5 times. The recombinant virus is continuous in passage and good in hereditary stability, and replacement of the G-H ring does not affect immunogenicity of the vaccine. Therefore, the recombinant foot-and-mouth disease virus strain rHN / XJ / NXGH provided by the invention has the potential to be used as a vaccine candidate strain for effectively preventing and controlling the O-type foot-and-mouth disease in China.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
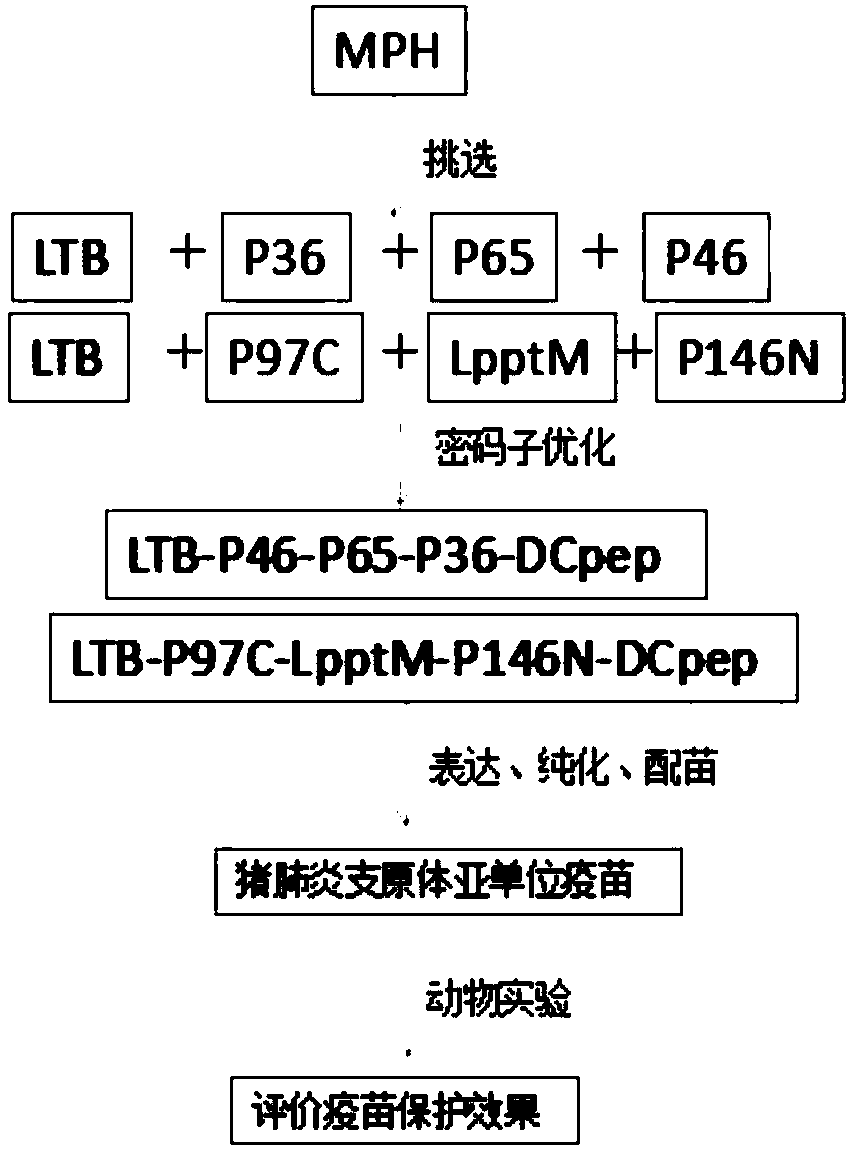
Mycoplasma hyopneumoniae subunit vaccine and preparation method and application thereof
ActiveCN109678968AFor proper foldingPreserve immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliDendritic cell
The invention provides a mycoplasma hyopneumoniae subunit vaccine and a preparation method and application thereof. A plurality of antigenic proteins of mycoplasma are connected by a flexible Linker and expressed as a chimeric protein, and the proteins are mutually unaffected, and can retain respective immunogenicity. The mucosal immune protection effect of the vaccine is greatly enhanced by adding the escherichia coli heat-labile enterotoxin B subunit and the dendritic cell-inducing peptide to the chimeric protein. The invention also relates to a preparation method of a mycoplasma hyopneumoniae chimeric antigen and application of the mycoplasma hyopneumoniae chimeric antigen to the subunit vaccine for preventing mycoplasma hyopneumoniae infection. The mycoplasma chimeric antigen is expressed in bacillus megaterium and can be used for industrial preparation of safe mycoplasma hyopneumoniae subunit vaccines.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Clostridium perfringen alpha toxin genetic engineering vaccine and preparation method thereof
ActiveCN109602898APreserve immunogenicityReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsAlpha-toxinWild type
The invention provides a clostridium perfringen alpha toxin genetic engineering vaccine and a preparation method thereof. Compared with wild type alpha toxins, clostridium perfringen alpha toxin recombination protein is characterized in that the 176th site histidine of an amino acid sequence is mutated to obtain asparagine. The clostridium perfringen alpha toxin genetic engineering vaccine is prepared from detoxifcation clostridium perfringen alpha toxin recombination protein of self-induction secretory expression. The vaccine has the advantages of being safer, better in immune efficacy, simpler in technology, lower in cost and the like, and can effectively solve the problems that in the prior art, the production technology of the vaccine is complexer, and the cost is higher.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Recombinant poxvirus vector comprising tetanus toxin fragment c
InactiveUS20170081642A1Negligible pathogenicityReduce capacityBacterial antigen ingredientsDepsipeptidesBacteroidesViral vector
The present invention relates to a recombinant poxvirus comprising tetanus toxin fragment C for improved immunogenicity of an antigen and related methods and uses. Specifically, the present invention generally relates to genetically engineered (recombinant) poxvirus vectors comprising a tetanus toxin fragment C (TTC) coding sequence operably linked to a bacterial antigenic determinant as well as to uses thereof, e.g., to affect an immune response in a subject.
Owner:BAVARIAN NORDIC AS
Low-allergenicity fish allergen parvalbumin, and preparation method and application thereof
ActiveCN105384804APromote the formation of flavor substancesPreserve immunogenicityPeptide/protein ingredientsPeptide preparation methodsDrugLysine residue
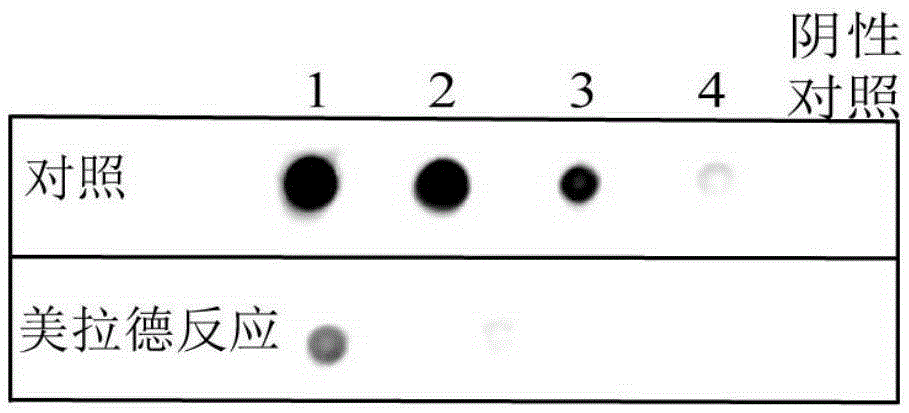
The invention discloses low-allergenicity fish allergen parvalbumin, and a preparation method and application thereof. The low-allergenicity fish allergen parvalbumin has galactosylated modification of three amino acid residues, i.e., the 88th lysine residue, the 97th lysine residue and the 108th lysine residue. Moreover, the low-allergenicity fish allergen parvalbumin has galactosylated modification of the 46th lysine residue, the 55th lysine residue and the 84th lysine residue. The low-allergenicity fish allergen parvalbumin has the characteristic of low allergenicity and maintains immunogenicity. The low-allergenicity fish allergen parvalbumin is prepared by uniformly mixing parvalbumin and a sugar solution and then successively subjecting the obtained mixture to freeze drying, a Maillard reaction, redissolving and sugar removal, and can be used for preparing a drug for preventing allergen sensitization, especially fish allergen sensitization and a low-allergenicity foodstuff, especially a low-allergenicity fish-related foodstuff.
Owner:JIMEI UNIV
Attenuated virus for prevention of infectious bursal disease and use
ActiveCN108929864APreserve immunogenicityDevelopmental impactViral antigen ingredientsInactivation/attenuationInfectious bursal disease virus IBDVInfectious bursitis
The invention discloses an attenuated infectious bursal disease virus, which has a preservation name of: infectious bursal disease virus IBDV / 1F, and is preserved in China Center for Type Culture Collection at Wuhan University in Wuhan, China. The preservation number is CCTCC NO: V201834, and the preservation date: July 3, 2018. The invention also provides the use of the attenuated infectious bursal disease virus in preparation of vaccines. The viral strain does not cause bursal damage in chickens and can induce high titer antibodies directed at infectious bursal disease virus. The vaccine canresist the attack of virulent strains and has no influence on the development of chickens.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Controlled-release hepatoma cell vaccine depending on granulocyte-macrophage colony-stimulating factor (GM-CSF) wrapped by nanoparticles
InactiveCN102743745AStable secretionProlonged secretion timePharmaceutical non-active ingredientsAntibody medical ingredientsControlled releaseChitosan nanoparticles
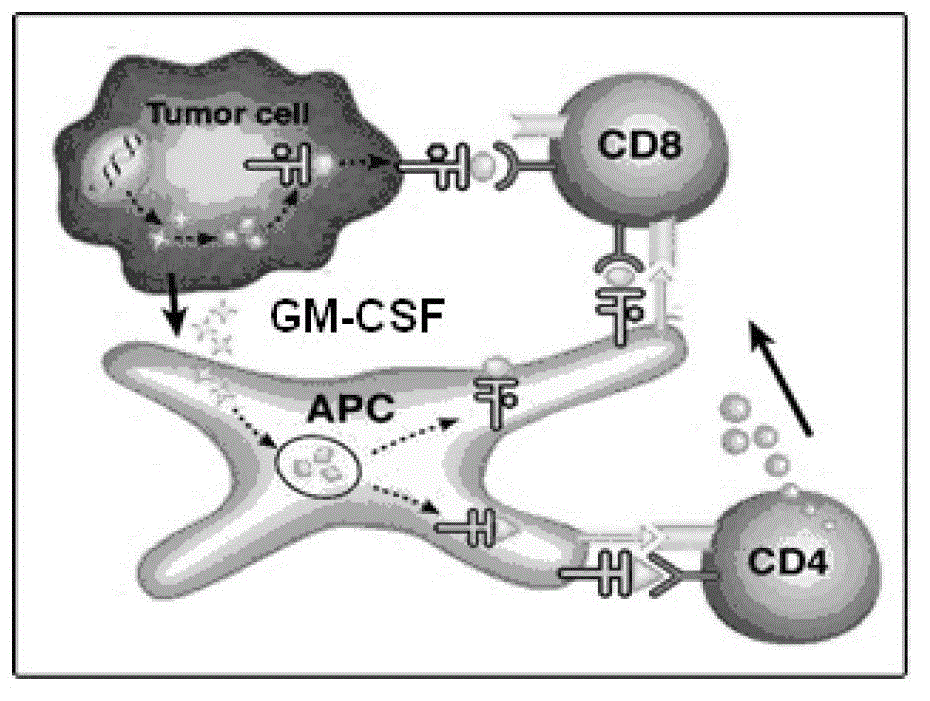
A controlled-release hepatoma cell vaccine depending on granulocyte-macrophage colony-stimulating factor (GM-CSF) wrapped by nanoparticles is prepared from chitosan nanoparticles carrying GM-CSF and inactivated hepatoma cell. The method for preparing the vaccine comprises the steps of: stirring the mixed solution which consists of GM-CSF, sodium tripolyphosphate and chitosan solution for 2-3 hours, then collecting the reaction liquid, centrifuging utilizing glycerol at 8000-12000rpm for 10-15 minutes, carrying out 15-20KHz ultrasound resuspension using 0.9% sodium chloride for 30-45 seconds after finishing the centrifuging to obtain the uniform chitosan nanoparticles carrying GM-CSF, wherein the wrapping rate is 87.08+ / -2.32% and the particle size is 100+ / -23.68 nanometers; and mixing and suspending the chitosan nanoparticles carrying GM-CSF and the inactivated hepatoma cell to prepare the controlled-release hepatoma cell vaccine depending on GM-CSF wrapped by nanoparticles. The vaccine is simple to prepare, and has strong safety and high effectiveness.
Owner:NANJING XINSAIERSI BIOLOGICAL TECH
Porcine rotavirus recombinant protein, recombinant adenovirus expressing same protein and application
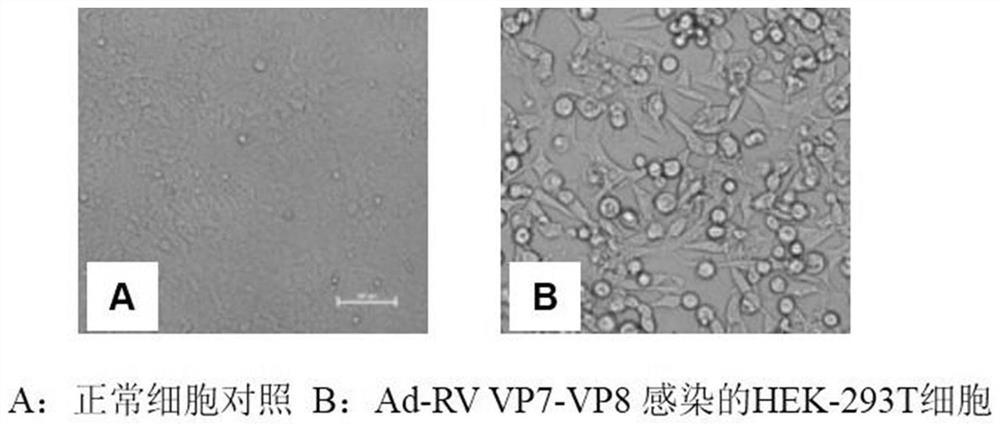
ActiveCN112625095AEasy to identifyQuick identificationViral antigen ingredientsVirus peptidesPorcine rotavirusImmunogenicity
The invention relates to the technical field of veterinary biology, and particularly relates to a porcine rotavirus recombinant protein, a recombinant adenovirus expressing same protein and application. The recombinant protein is formed by connecting a porcine rotavirus type 5 VP7 protein and a porcine rotavirus type 9 VP8 protein, and specifically comprises an amino acid sequence shown as SEQ ID NO: 1. Gene sequences of coding genes of the porcine rotavirus type 5 VP7 protein and the porcine rotavirus type 9 VP8 protein are constructed into an adenovirus expression vector, codon optimization is carried out, and the recombinant adenovirus for the porcine rotavirus is prepared. The structure and function of the target protein expressed by the recombinant adenovirus are completely consistent with those of the natural state, and the recombinant adenovirus has high safety and immunogenicity.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
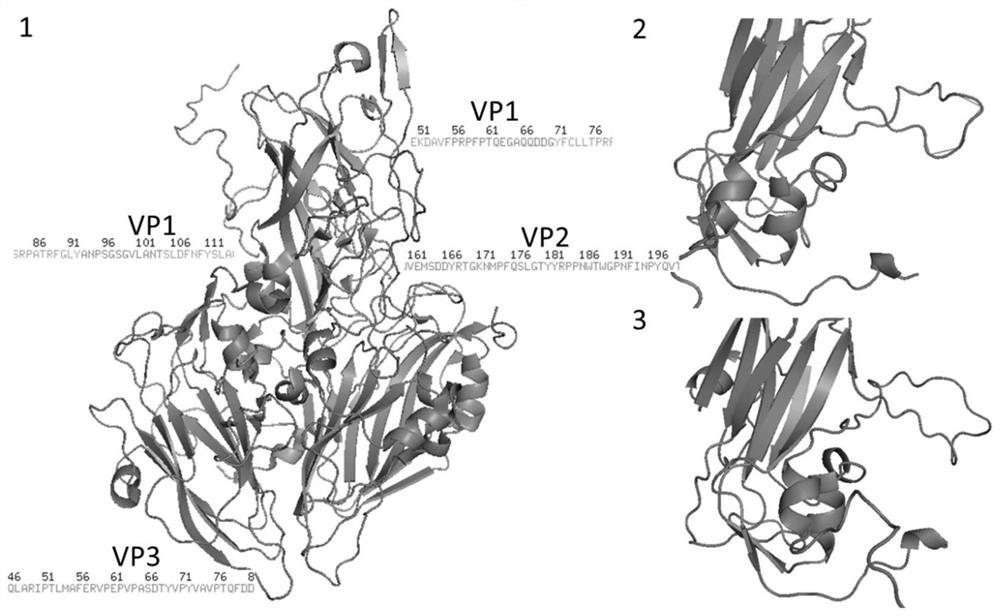
Chimeric virus-like particle vaccine and preparation method therefor and application of chimeric virus-like particle vaccine
ActiveCN111647087AIncrease productionHigh puritySsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseEpitope
The invention relates to a chimeric virus-like particle vaccine and a preparation method therefor and application of the chimeric virus-like particle vaccine, in particular to a porcine Seneca valleyvirus and porcine circovirus-2 chimeric virus-like particle vaccine. According to the chimeric virus-like particle vaccine, sequences of VP0, VP3 and VP1 are connected in tandem for expression, wherein part of the VP3 sequences are replaced with porcine circovirus-2 ORF2 gene C-terminal epitope sequences to form a recombination sequence; the recombination sequence transfects sf9 cells after beingconstructed on recombinant bacmid for further expression to obtain porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particles. It is the first time for the invention to developthe porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine by utilizing the chimeric virus-like particles, and the porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine can exert a relatively good immune protection effect on the porcine Seneca valley virus and the porcine circovirus-2; and the vaccine of the invention is economical and practical, can effectively reduce the epidemic prevention cost of diseases, and provides a new method of preventing two diseases at the same time for breeding enterprises in China.
Owner:CHINA ANIMAL HUSBANDRY IND
Recombinant baculovirus expressing senecavirus VP2 gene and preparation method and application thereof
ActiveCN110358741APreserve immunogenicitySimplify the extraction and purification processSsRNA viruses positive-senseViral antigen ingredientsSenecavirusVp2 gene
The invention relates to the field of biotechnology, in particular to recombinant baculovirus expressing senecavirus VP2 gene and a preparation method and application thereof. The recombinant baculovirus comprises one or more copies of senecavirus VP2 protein encoding gene. The invention further provides a senecavirus subunit vaccine which comprises the recombinant baculovirus expressed senecavirus VP2 protein. Accordingly, by means of artificial codon optimization, high-level and high-purity expression of senecavirus VP2 protein is achieved, and the immunogenicity of the VP2 protein is retained to the maximum extend. The senecavirus subunit vaccine has the excellent immunogenicity and high safety, and an ideal immune protection effect is achieved for infection of senecavirus.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
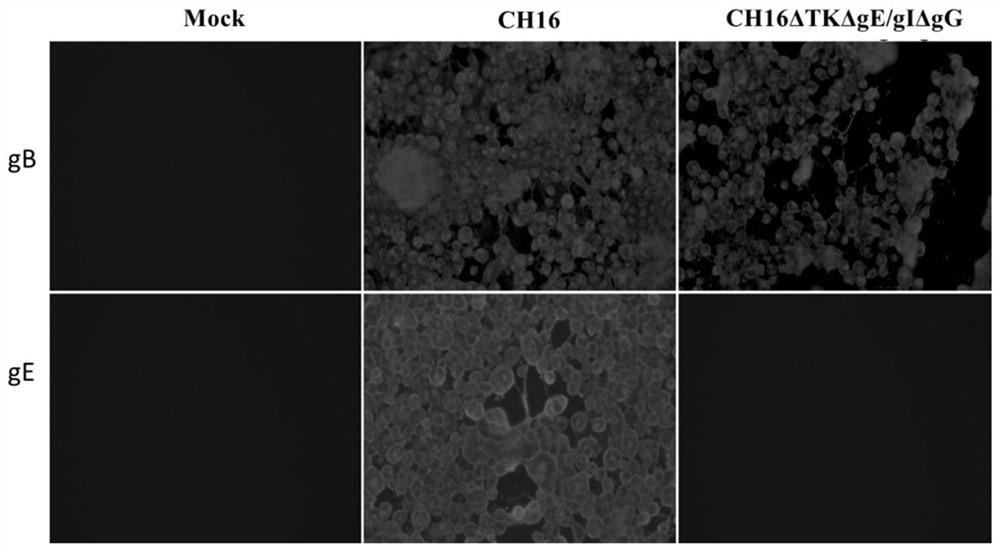
Pseudorabies virus TK, gE, gI and gG gene deletion strain as well as preparation method and application thereof
ActiveCN112280753ALow toxicityImprove efficiencyViral antigen ingredientsVirus peptidesPseudorabiesImmunosuppression
The invention relates to a pseudorabies virus TK, gE, gI and gG gene deletion strain as well as a preparation method and an application thereof. A CRISPR / Cas9 mediated homologous recombination technology specifically comprises the steps that CRISPR / Cas9 serves as a medium to break DNA double strands, then a homologous recombination method is used, homologous sequences transferred into cells are supplemented to notches, DNA double strand repairing is conducted, genome editing is completed, accurate fixed-point editing can be conducted according to the will of a researcher, the gene editing efficiency is greatly improved, and the research and development period of vaccine can be shortened. According to the present invention, the partial sequences of the virulence-related genes TK, gE and gIand the immunosuppression-related gene gG of the pseudorabies virus epidemic strain are deleted by using the technology to obtain the TK, gE, gI and gG gene deleted strain, and the vaccine of the genedeleted strain has advantages of high safety, good hereditary stability, good immunoprotection effect and the like.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Use of 15-lipoxygenase inhibitors for treating obesity
ActiveUS8048900B2Preserve immunogenicityImprove the situationBiocideHalogenated hydrocarbon active ingredientsObesityVisceral Obesity
The invention concerns the treatment of obesity, in particular abdominal visceral obesity. More specifically, the invention concerns the use of selective 15-lipoxygenase (LO) inhibitors for preparing medicines useful in the treatment of obesity, or at least abdominal visceral obesity, and / or its consequences.
Owner:GENFIT SA
Porcine pseudorabies virus with six-gene deletion, porcine pseudorabies vaccine and preparation method
ActiveCN109609468AGood immune effectPreserve immunogenicityViral antigen ingredientsMicroorganism based processesPseudorabiesImmunogenicity
The invention discloses a porcine pseudorabies virus with six-gene deletion, a porcine pseudorabies vaccine and a preparation method, and relates to the field of animal biological products. The accession number of the porcine pseudorabies virus with six-gene deletion provided by present invention is CGMCC No:16290. The porcine pseudorabies virus with six-gene deletion lacks six genes, the pathogenicity is removed, and the immunogenicity is effectively retained. When in use, the vaccine prepared by the porcine pseudorabies virus can induce a higher dose of interferon in the body, and has a better immune effect in emergency immunization.
Owner:SICHUAN HUASHEN ANIMAL BIOLOGICAL PRODS
Avirulent, immunogenic flavivirus chimeras
InactiveUS20090010961A1Minimize and inhibit and immunize individualStable maintenanceOrganic active ingredientsSsRNA viruses positive-senseViral diseaseAmino acid mutation
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:UNITED STATES OF AMERICA +1
Recombinant poxvirus vector comprising tetanus toxin fragment C
ActiveUS9463238B2Negligible pathogenicityReduce capacityBacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsBacteroidesViral vector
The present invention relates to a recombinant poxvirus comprising tetanus toxin fragment C for improved immunogenicity of an antigen and related methods and uses. Specifically, the present invention generally relates to genetically engineered (recombinant) poxvirus vectors comprising a tetanus toxin fragment C (TTC) coding sequence operably linked to a bacterial antigenic determinant as well as to uses thereof, e.g., to affect an immune response in a subject.
Owner:BAVARIAN NORDIC BAVARIAN NORDIC
Bivalent subunit vaccine of porcine circovirus type 2b and type 2d and preparation method thereof
ActiveCN110358742BImprove the level ofHigh purityViral antigen ingredientsVirus peptidesEngineeringImmunogenicity
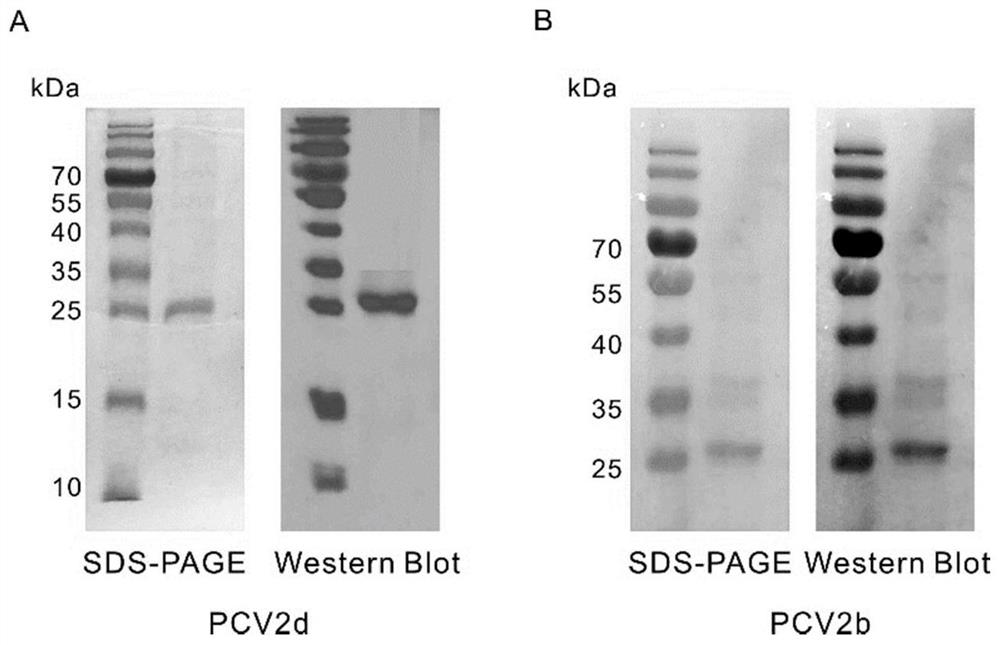
The invention relates to the field of biotechnology, in particular to bivalent subunit vaccines of type 2b and 2d porcine circoviruses and preparation methods thereof. The invention provides recombinant baculoviruses comprising one or more copies of a Cap protein encoding gene of type 2b or 2d porcine circovirus. The invention also provides the bivalent subunit vaccines of the type 2b and 2d porcine circoviruses which comprise Cap proteins of the type 2b and 2d porcine circoviruses expressed by the recombinant baculoviruses respectively. Through artificial codon optimization, high-level and high-purity expression of the Cap proteins of the type 2b and 2d porcine circovirus is achieved, and the natural structure and immunogenicity of the Cap proteins is ensured. The bivalent subunit vaccines of the type 2b and 2d porcine circoviruses have high immunogenicity and safety, and exert an excellent immunoprotective effect on the porcine circoviruses.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Marked Pestivirus suis C strain expressing enhanced green fluorescent protein and construction method and application thereof
InactiveCN105861453ARetain biological propertiesPreserve immunogenicitySsRNA viruses positive-senseVirus peptidesMicroorganismBiological property
The present invention discloses a marked Pestivirus suis C strain expressing enhanced green fluorescent protein and construction method and application thereof, and belongs to construction and application of marked Pestivirus suis C strain. A C strain infectious clone is used as a skeleton, the Pestivirus suis Shimen strain Npro protein is used to substitute a C strain Npro protein, then an EGFP gene is introduced between No. 13 and 14 amino acids of the Npro protein to obtain an marked Pestivirus suis C strain expressing enhanced green fluorescent protein, wherein the strain has microbial preservation number: CGMCC No. 12039. The marked Pestivirus suis C strain can stably express EGFP gene, is successfully observed with green fluorescence, retains biological properties and immunogenicity of the parental viruses, and can be applied to the preparation of marked vaccines for prevention of Pestivirus suis. The present invention confirms that the C strain Npro protein can be modified to obtain marked Pestivirus suis, so as to lay foundation for the development of marked C strain vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant porcine pseudorabies virus and application thereof and recombinant porcine live pseudorabies vaccine
ActiveCN109337874ANot pathogenicDefense against invasionViral antigen ingredientsAntiviralsPathogenicityTruncated protein
The invention discloses a recombinant porcine pseudorabies virus and application thereof and a recombinant porcine live pseudorabies vaccine, and relates to the field of biological products for animals. The recombinant porcine pseudorabies virus provided by the invention has the collection number of CGMCC No: 16289. The recombinant porcine pseudorabies virus is obtained after artificial transformation, the pathogenicity thereof is removed, pseudorabies virus TK, gI, gE, 11K and 28K genes are deleted, and fusion protein obtained through fusion of porcine epidemic diarrhea virus S truncated protein and porcine CD40L protein can be expressed. After a pig is immunized by the recombinant porcine live pseudorabies vaccine prepared by using the recombinant porcine pseudorabies virus, the pig canacquire the immunity against the porcine epidemic diarrhea virus challenge and the porcine pseudorabies virus challenge. The recombinant porcine live pseudorabies vaccine has a relatively good immuneeffect and has a broad application prospect.
Owner:SICHUAN HUASHEN ANIMAL BIOLOGICAL PRODS
Method for inactivating bacterium exotoxin antigen
InactiveCN105695539ACompletely detoxifiedPreserve immunogenicityMicroorganism based processesPeptide preparation methodsImmunogenicityToxoid
The invention relates to a method for inactivating bacterium exotoxin antigen. The method is characterized by including the steps of strain passage and amplification, fermentation tank culture, exotoxin separation and purification, exotoxin inactivation and toxoid purification. Formalin, lysine and hydrogen peroxide are all used as detoxification agents, exotoxin detoxification can be completed within a shorter period of time, detoxification efficiency is improved, the immunogenicity of toxoid is kept to a larger extent, and the method has the advantages of being reliable in detoxification effect, little in influence on exotoxin structure, and capable of being widely used for detoxifying bacterium exotoxin and preparing toxoid antigen.
Owner:LIAONING CHENGDA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com