Recombinant porcine pseudorabies virus and application thereof and recombinant porcine live pseudorabies vaccine
A porcine pseudorabies virus, pseudorabies virus technology, applied in the direction of veterinary vaccines, vaccines, viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Artificially Modified Pseudorabies Virus
[0040] 1. Virus strain
[0041] The PRV-FJ strain was isolated from the Sichuan Provincial Key Laboratory of Livestock and Poultry Infectious Diseases from the pig disease materials of Fujian pig pseudorabies in 2015; the diseased pigs mainly showed symptoms such as elevated body temperature, poor appetite, and sneezing. PRV-FJ strain with 10 7 TCID 50 The dose per head infected 28-day-old weaned piglets without neurological symptoms, and all died 7 days after the challenge.
[0042] PEDV-CV777 strain and SA014 strain were provided by Sichuan Provincial Key Laboratory of Livestock and Poultry Infectious Diseases.
[0043] ST cells and 293T cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco).
[0044] 2. Construction of recombinant vector
[0045] Artificially synthesized SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, and then directional cloned SEQ ID NO.1 into the Ase I restriction site of th...
Embodiment 2
[0059] Pathogenicity of rPRV-TIE18(RBD)
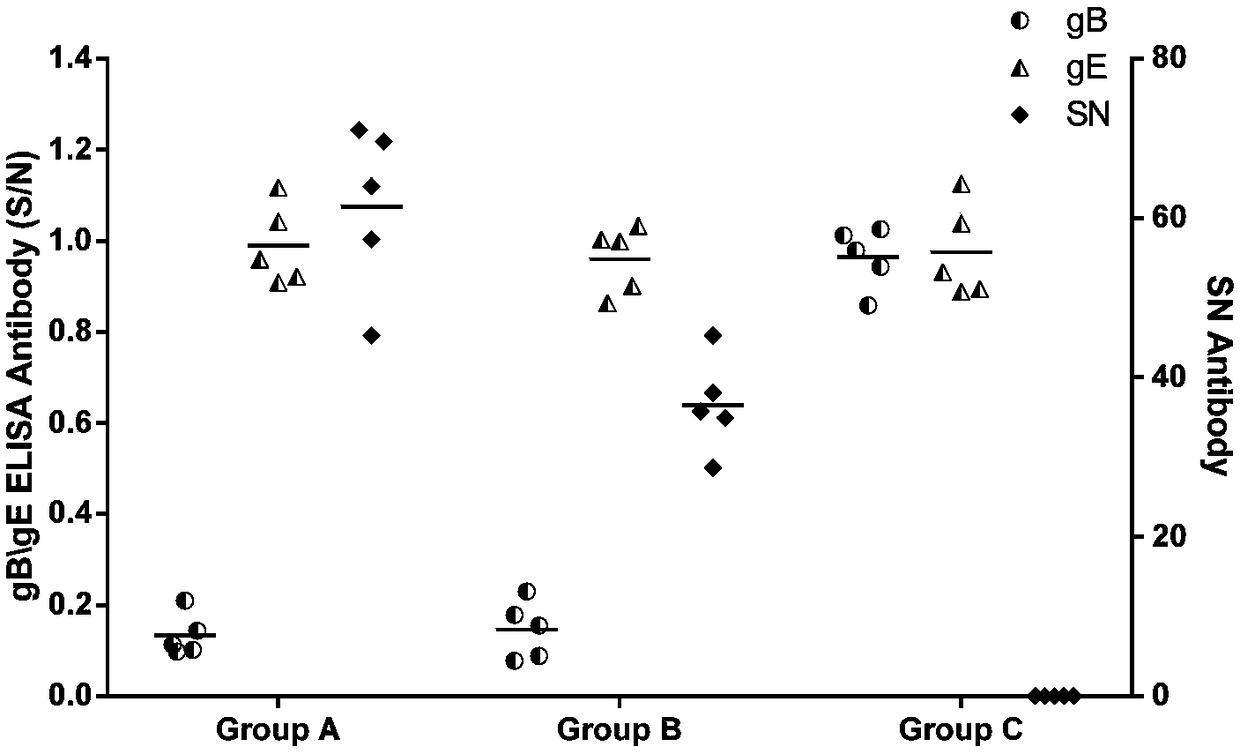
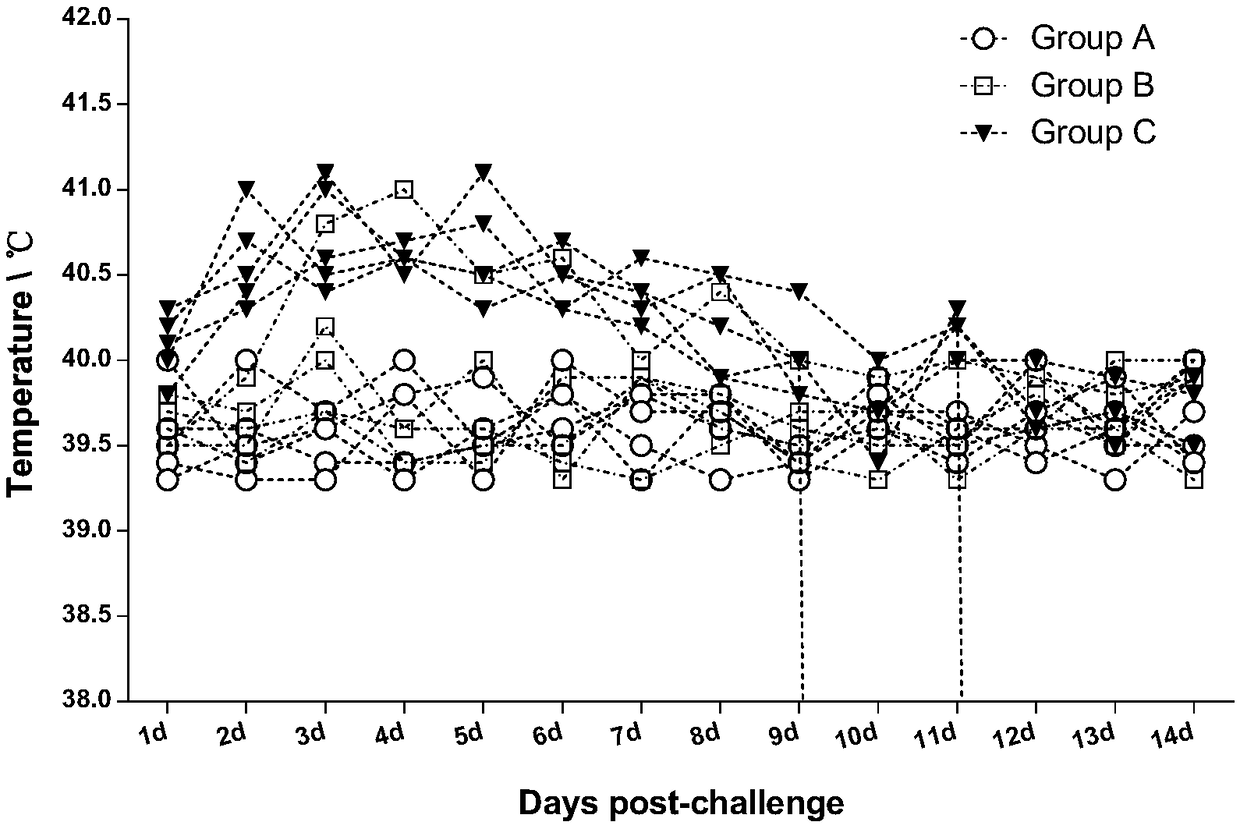
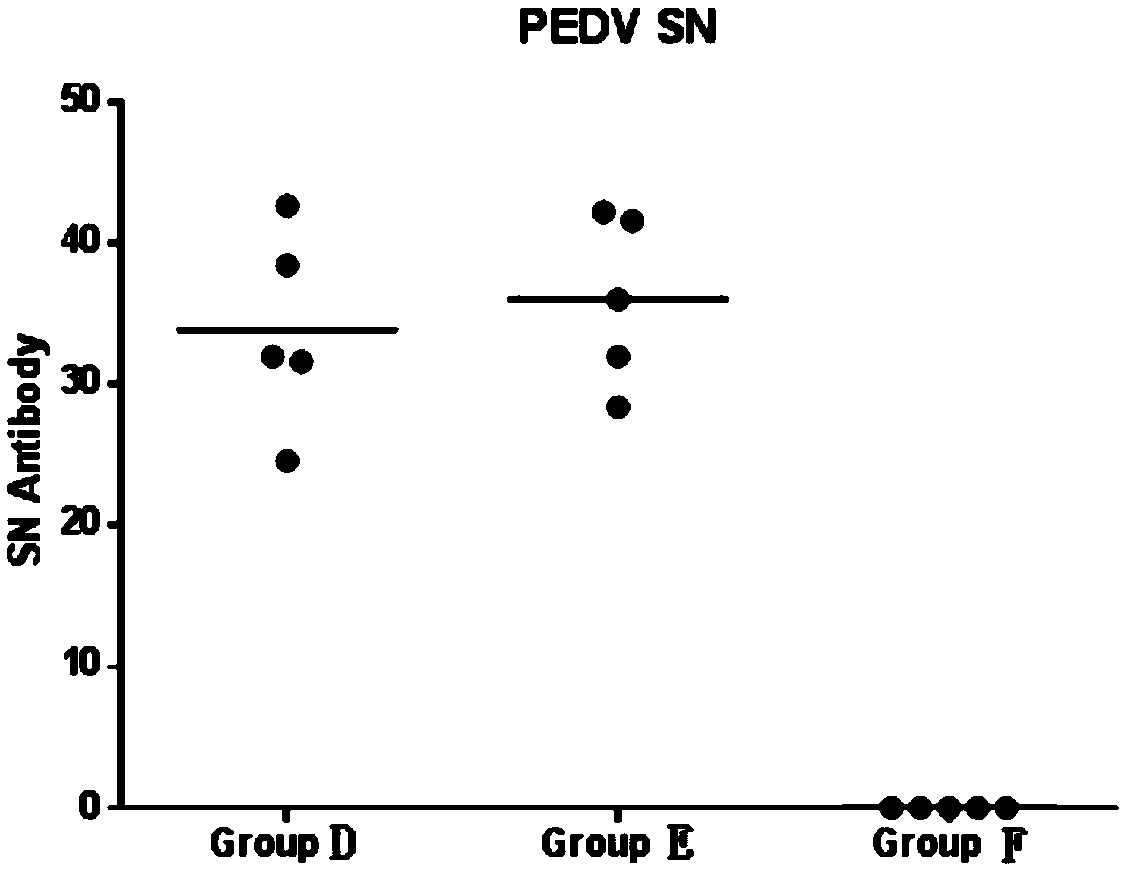
[0060] Take 20 PRV antibody-negative piglets aged 1-3 days, and randomly divide them into 4 groups, marked as A, B, C, and D groups, and conduct the challenge test according to the table below. Continuous observation for 14 days after the challenge.
[0061] Table 2 Pathogenicity test of rPRV-TIE18(RBD) and PRV-FJ on newborn piglets
[0062] group
Challenge strain
Vaccination method
Remark
A
rPRV-TIE18(RBD)
10 8 TICD 50
Nasal drip
B
rPRV-TIE18(RBD)
10 7 TICD 50
Nasal drip
C
DMEM medium
2.0ml
Nasal drip
blank control
D
PRV-FJ
10 7 TICD 50
Nasal drip
[0063] After the challenge, the temperature of the piglets was observed and measured every day to observe whether there were clinical symptoms and death of pseudorabies. The results are shown in Table 3 below. After rPRV-TIE18 (RBD) was instil...
Embodiment 3
[0067] Preparation and Test of Porcine Pseudorabies Live Vaccine rPRV-TIE18(RBD)
[0068] Inoculate BHK cells with rPRV-TIE18(RBD), and harvest the virus liquid when more than 90% of the cells are damaged. After 2-fold dilution of the harvested virus liquid, it was mixed with gelatin sucrose protectant (containing 2% gelatin and 10% sucrose) in equal volumes, and then divided into 2ml / bottles for freeze-vacuum drying. According to the appendix of "Chinese Veterinary Drug Store", there is no bacterial and mycoplasma pollution, and no exogenous virus pollution. The titer of cultured virus solution is 10 8.0 TCID 50 / ml, the freeze-dried vaccine was restored to the original volume and the virus content was 10 7.25 TCID 50 / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Potency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com