Replication-defective recombinant influenza virus for simultaneously expressing HA and HEF
A technology for replication-deficient influenza virus, applied in the field of preparation of recombinant influenza virus, which can solve the problems of virulence reversal mutation, poor antigenicity, and long development cycle of cold-adapted virus strains, so as to maintain immunogenicity and strong mucosal immunity Response and cellular immune response levels, effects of strong and long-lasting immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Reverse transcribe the RNA fragments of PB2, PB1, PA, HA, NP, M, and NS of type A influenza virus subtype H1N1 in vitro to make cDNA, use the cDNA as a template to amplify the gene fragments of influenza virus, and clone them into Expression vector plasmid pHW2000. Seven plasmids were obtained, namely pHW-PB2, pHW-PB1, pHW-PA, pHW-HA, pHW-NP, pHW-M, and pHW-NS.
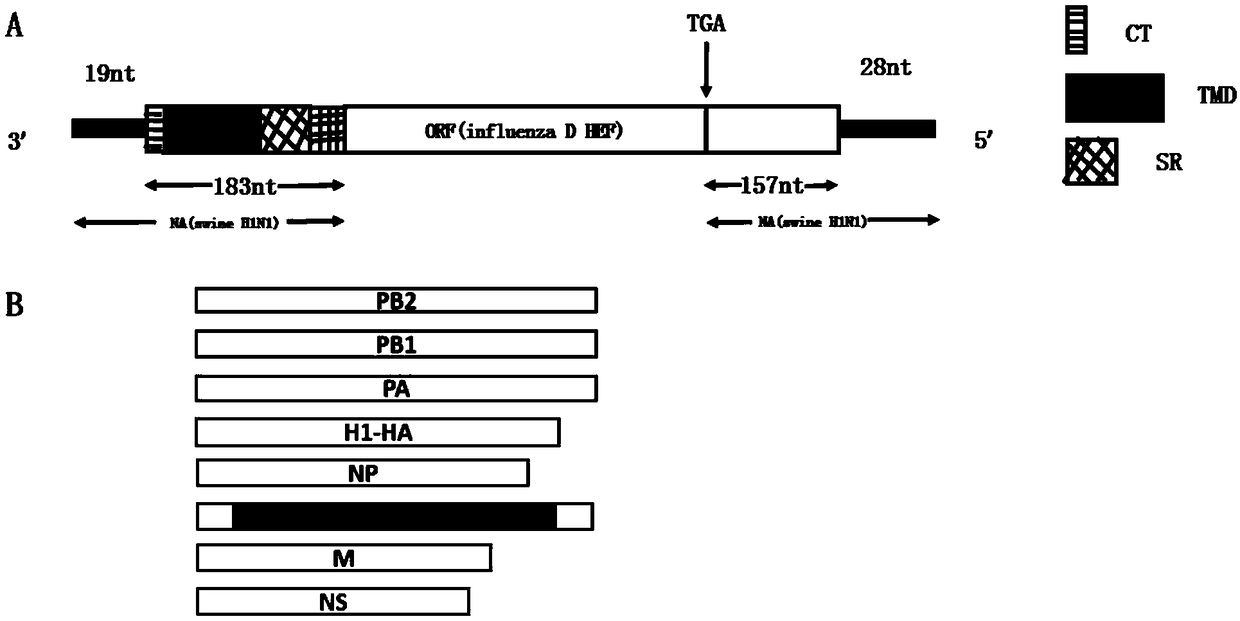
[0028] 2. Construction of plasmid pHW-NA-HEF
[0029] The newly constructed plasmid pHW-NA-HEF ( figure 1 ): at first the DNA sequence NA-HEF (Genscript Company) was synthesized, which retained 202 nucleotides of the 3' non-coding region of the H1N1NA fragment of type A influenza virus and 185 nucleotides of the 5' non-coding region Wrap signal. Utilize PCR technology to carry out the amplification of target fragment NA (202nt)-ORF (HEF)-NA (185nt) (such as figure 2 ).
[0030] Upstream primer sequence: TATTGGTCTCAGGGAGCAAAAGCAGGAGT,
[0031] Downstream primer sequence: ATATGGTCTCGTATTAGTAGAAACAAGGAGT...
Embodiment 2
[0034] Embodiment 2 rescues recombinant virus
[0035] Construction of cell lines: construction of MDCK cell lines that can stably express A / Califronia / 2009 / 07 (H1N1) influenza virus surface glycoprotein neuraminidase NA.
[0036] Specific steps are as follows:
[0037] G418 selection of stable expression cell lines:
[0038] Before screening, determine the optimal concentration of G418 for screening MDCK cells is 500 μg / mL.
[0039] Preparation of G418: Dissolve 1g of G418 in 1mL of 1M HEPES solution, add ultrapure water to 10mL, filter, and store at 4°C for later use.
[0040] (1) The RNA fragment of the surface glycoprotein neuraminidase NA of type A influenza virus subtype H1N1 was reverse-transcribed in vitro to make cDNA, and the NA gene fragment of influenza virus was amplified with cDNA as a template, and cloned into the vector plasmid pD2EGFP- on N1. The plasmid pD2EGFP-NA was obtained.
[0041] (2) Spread MDCK cells on a 6-well plate and culture them in MEM medi...
Embodiment 3
[0053] Embodiment 3 tests the growth curve of recombinant virus
[0054] MDCK cells stably expressing NA were cultured in MEM medium containing 10% fetal bovine serum (Fetal Bovine Serum, FBS) and 1% double antibody (Penicillin-Streptomycin Solution, PS) in a 37°C incubator. (Media and serum were purchased from Biological Industries Company.) The transformed MDCK cells were plated in 6-well cell culture plates, 3×10 per well 5 cells, the virus will be amplified when the cell growth density reaches 50%-60%. Before virus amplification, wash the transformed MDCK cells with phosphate buffer saline (Phosphate Buffer Saline, PBS) twice, infect the cells with the recombinant virus with MoI=0.001, and replace it with 2 mL containing 0.2% bovine serum albumin after 1 hour of adsorption. Protein (Bovine SerumActin, BSA) and 1 μg / mL TPCK in MEM culture solution, and then the supernatant was collected at 24, 48 and 72 hours after infection and stored at -80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com