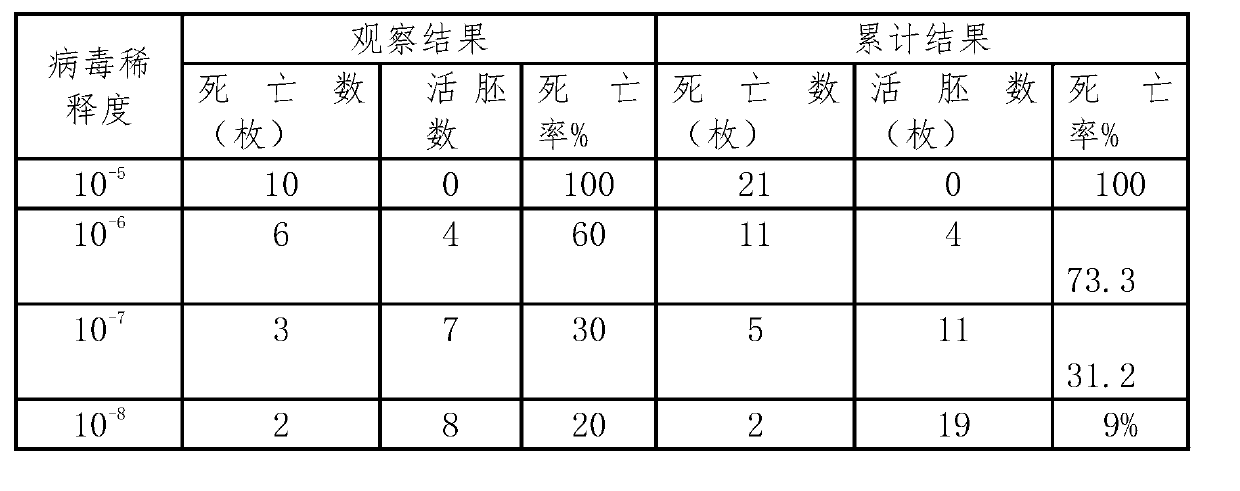
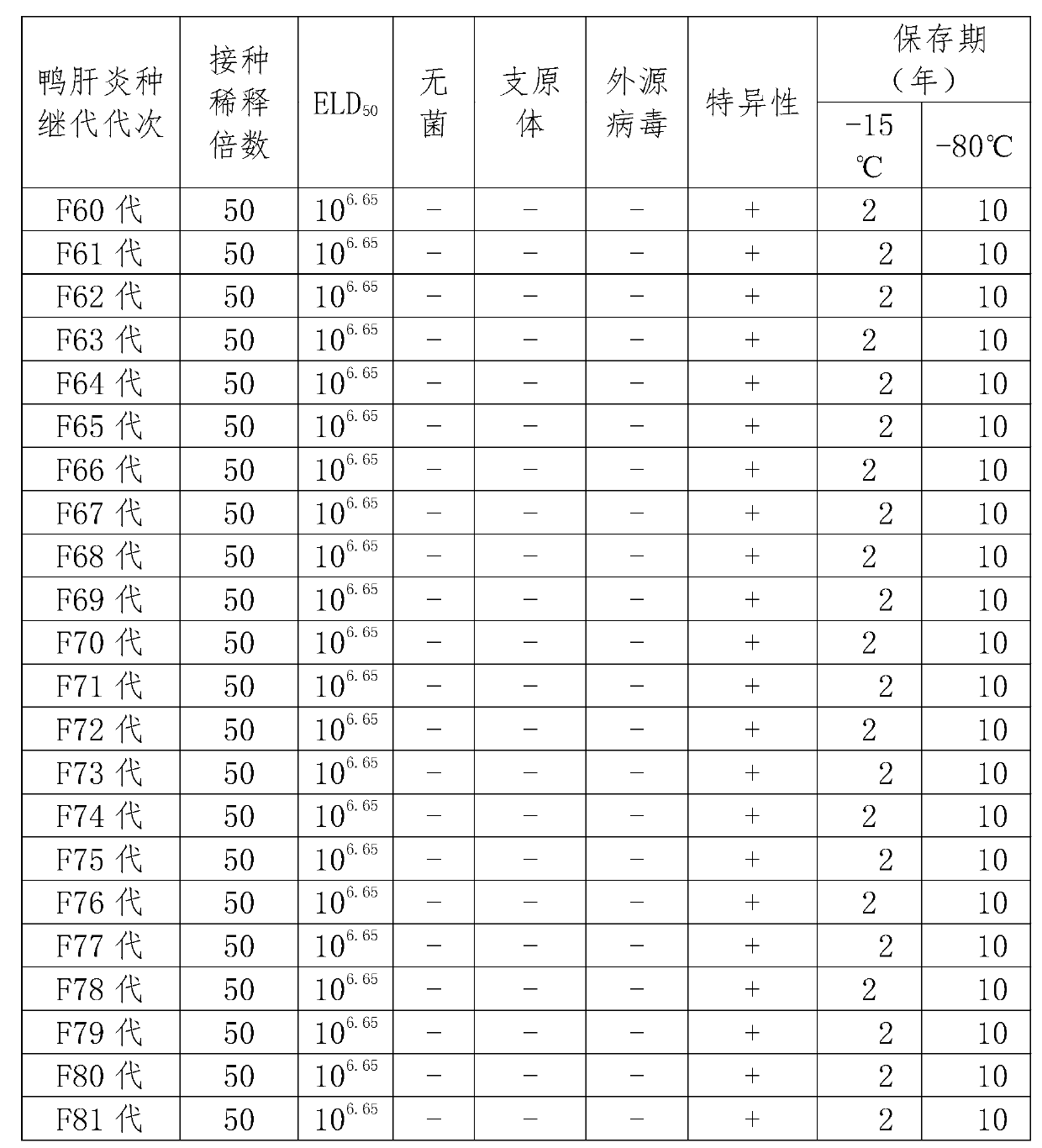
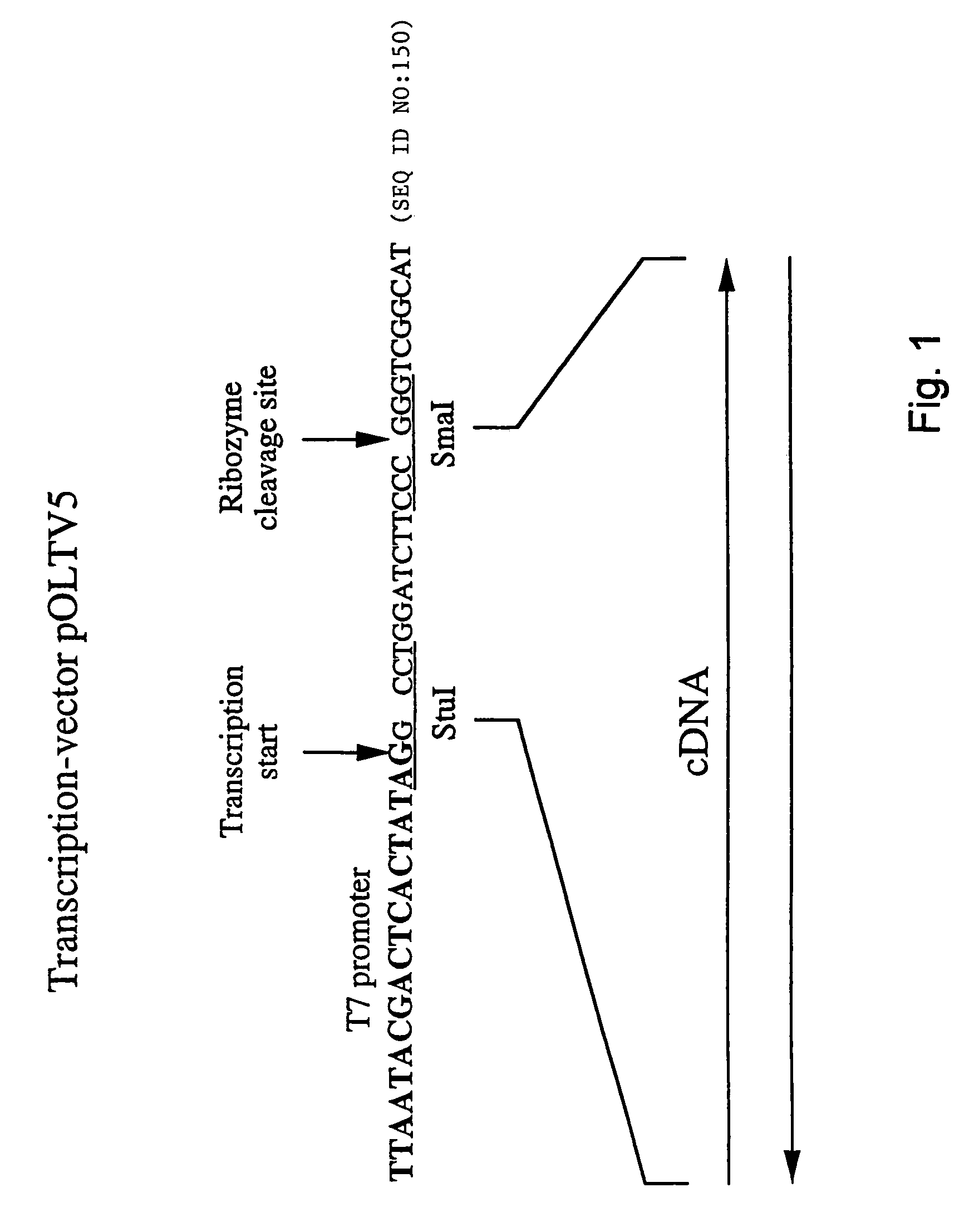
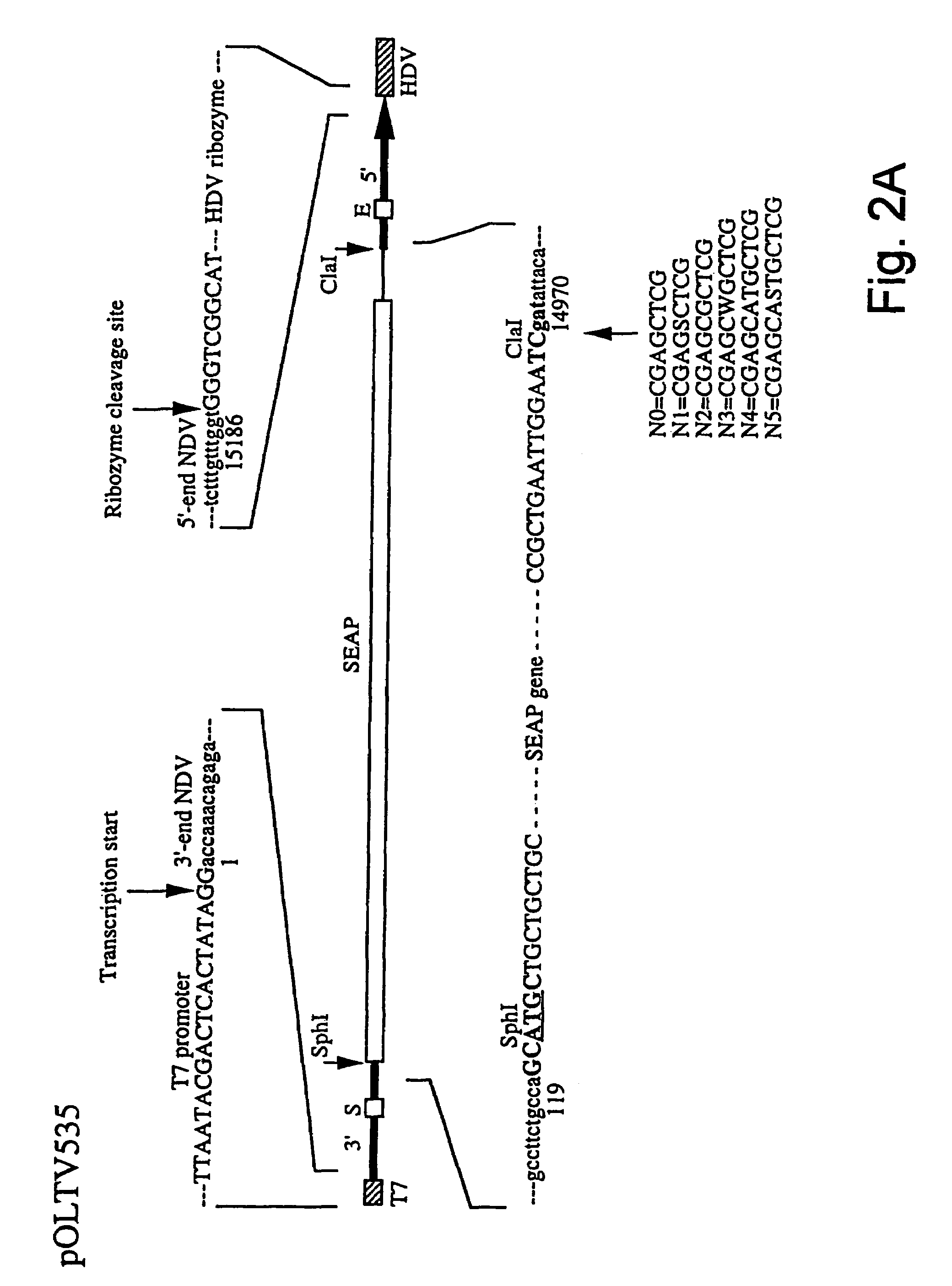
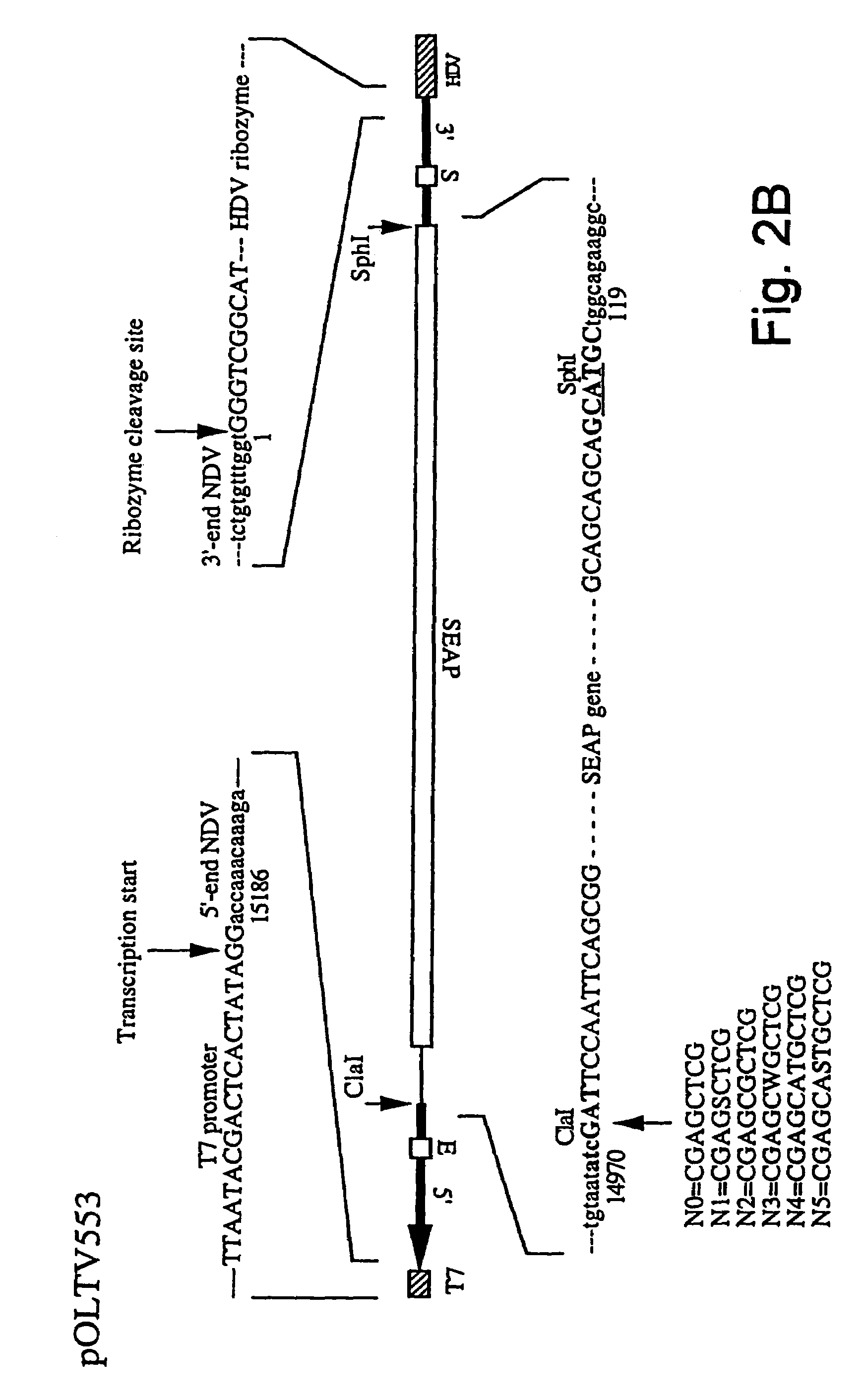
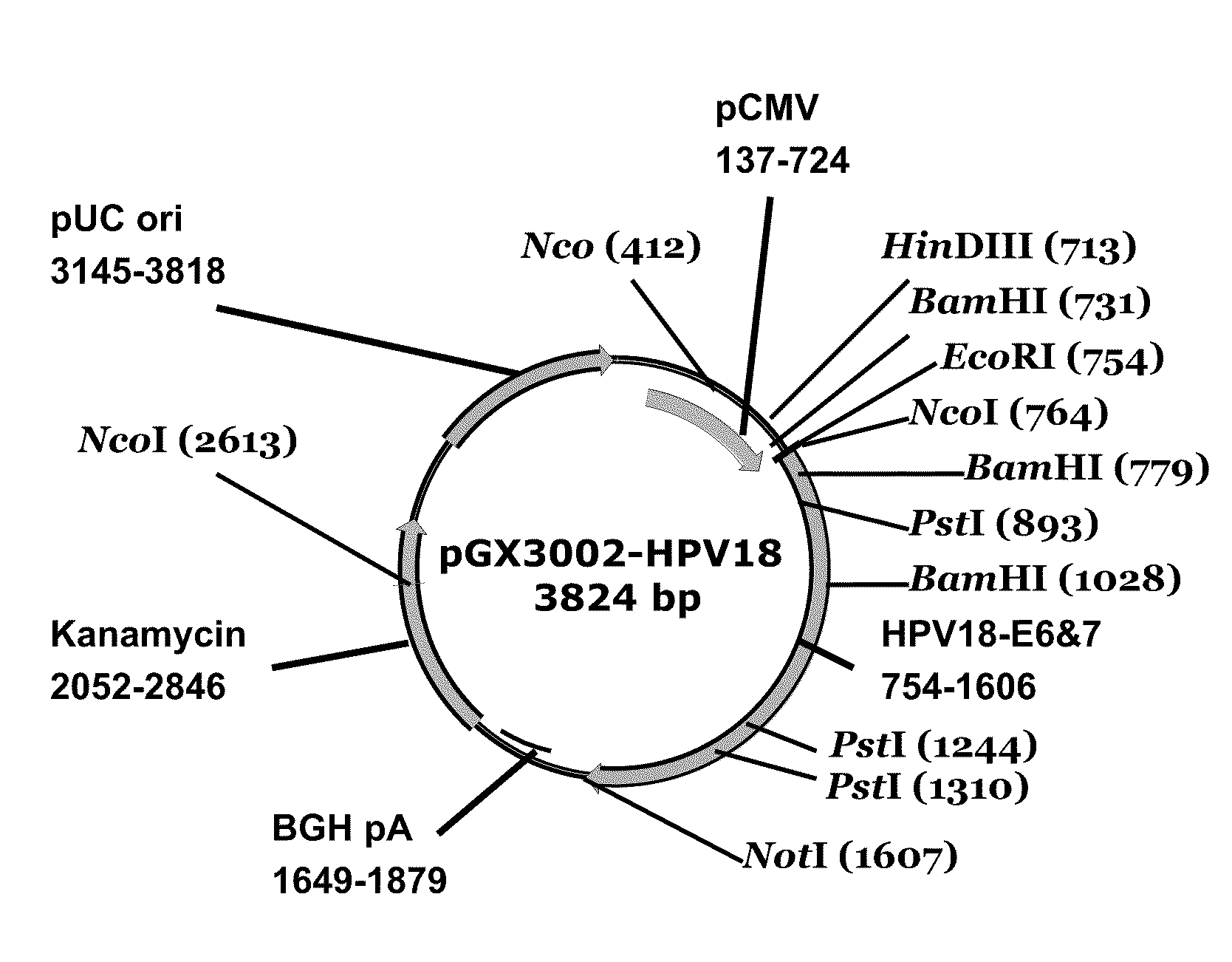
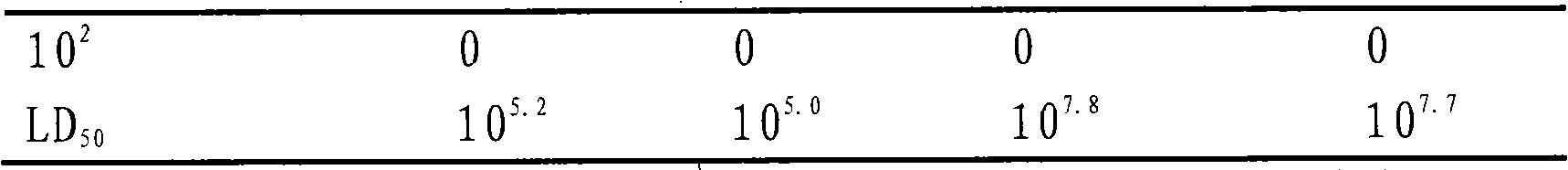Patents
Literature
354 results about "Attenuated Live Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel methods for therapeutic vaccination
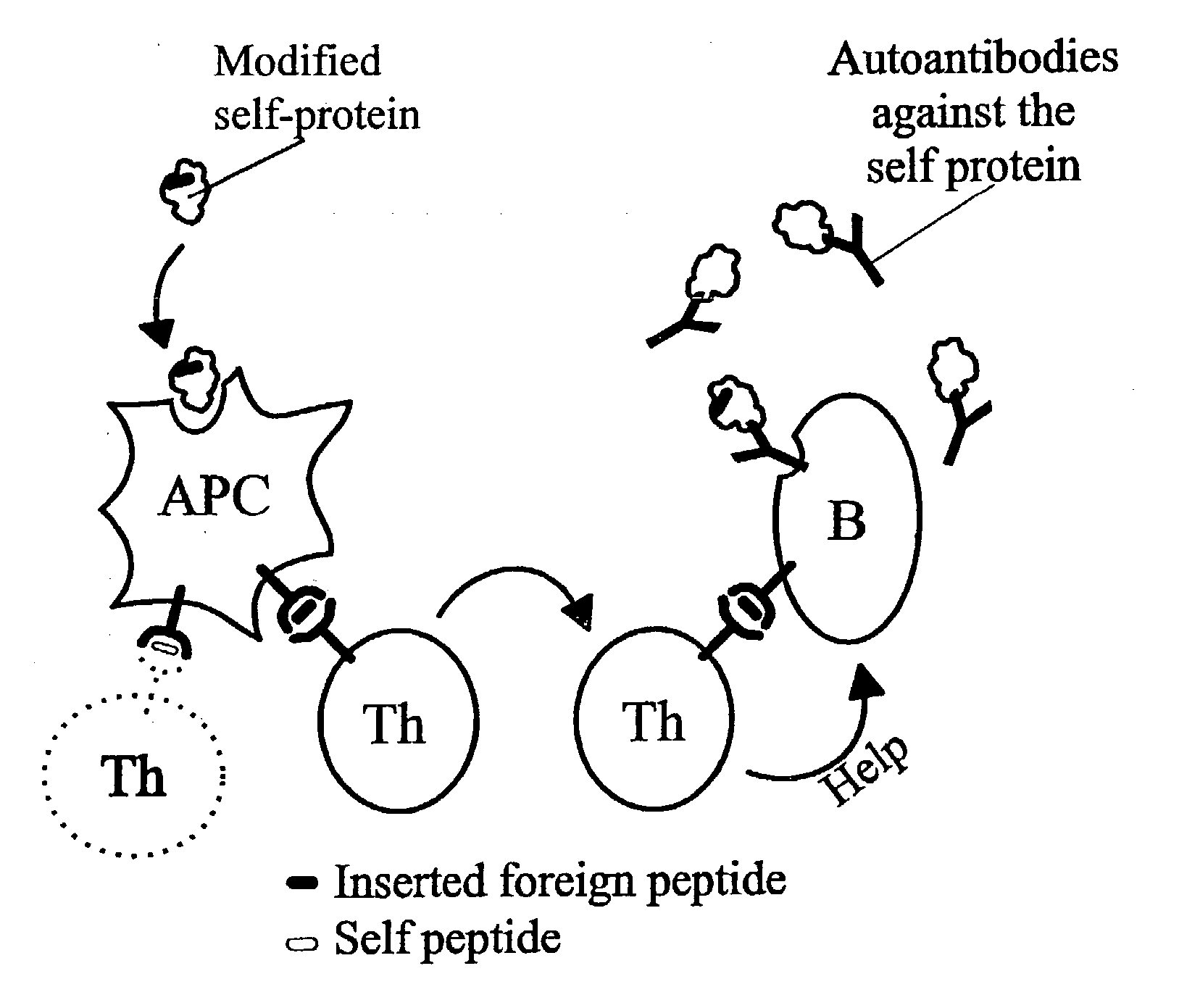
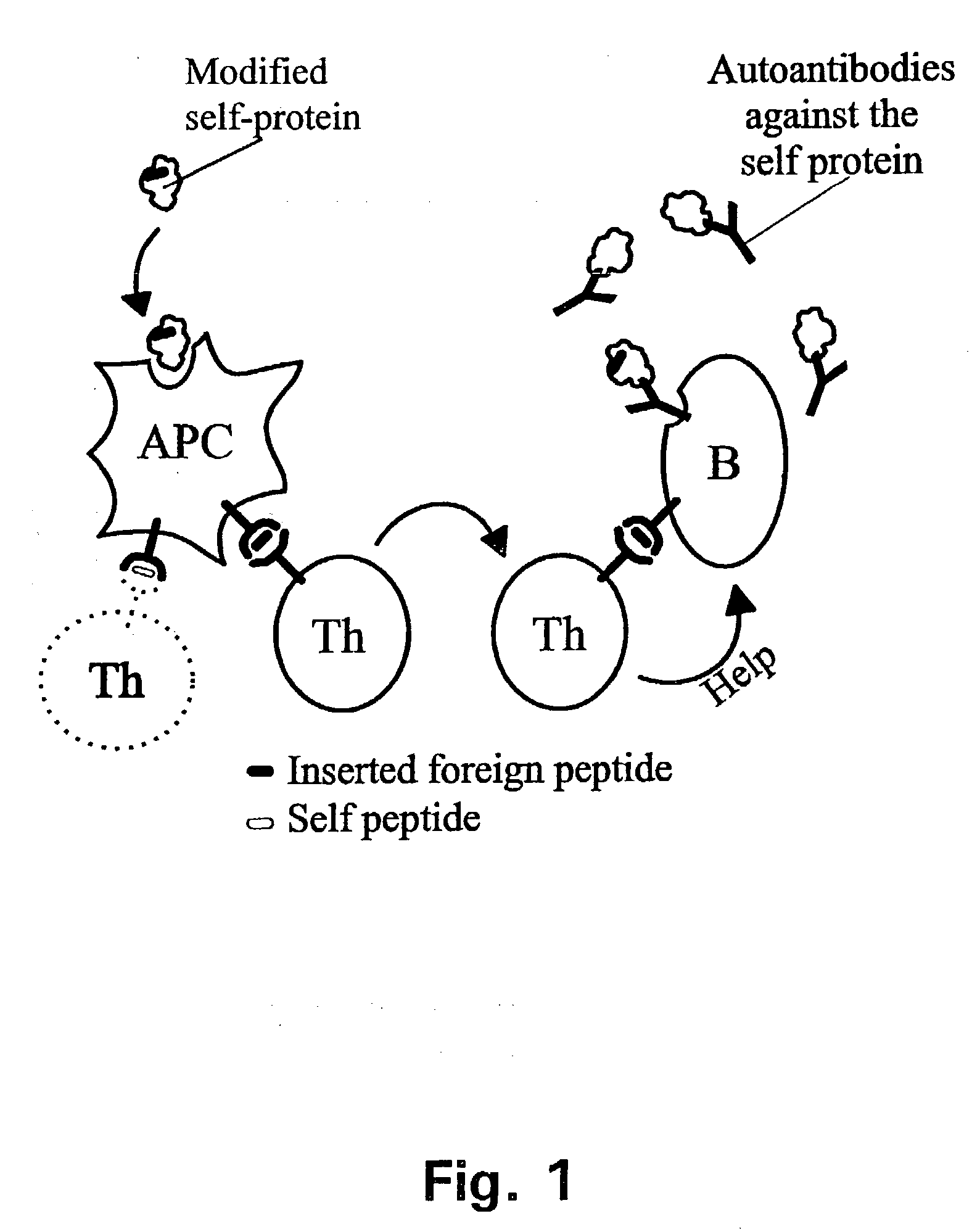
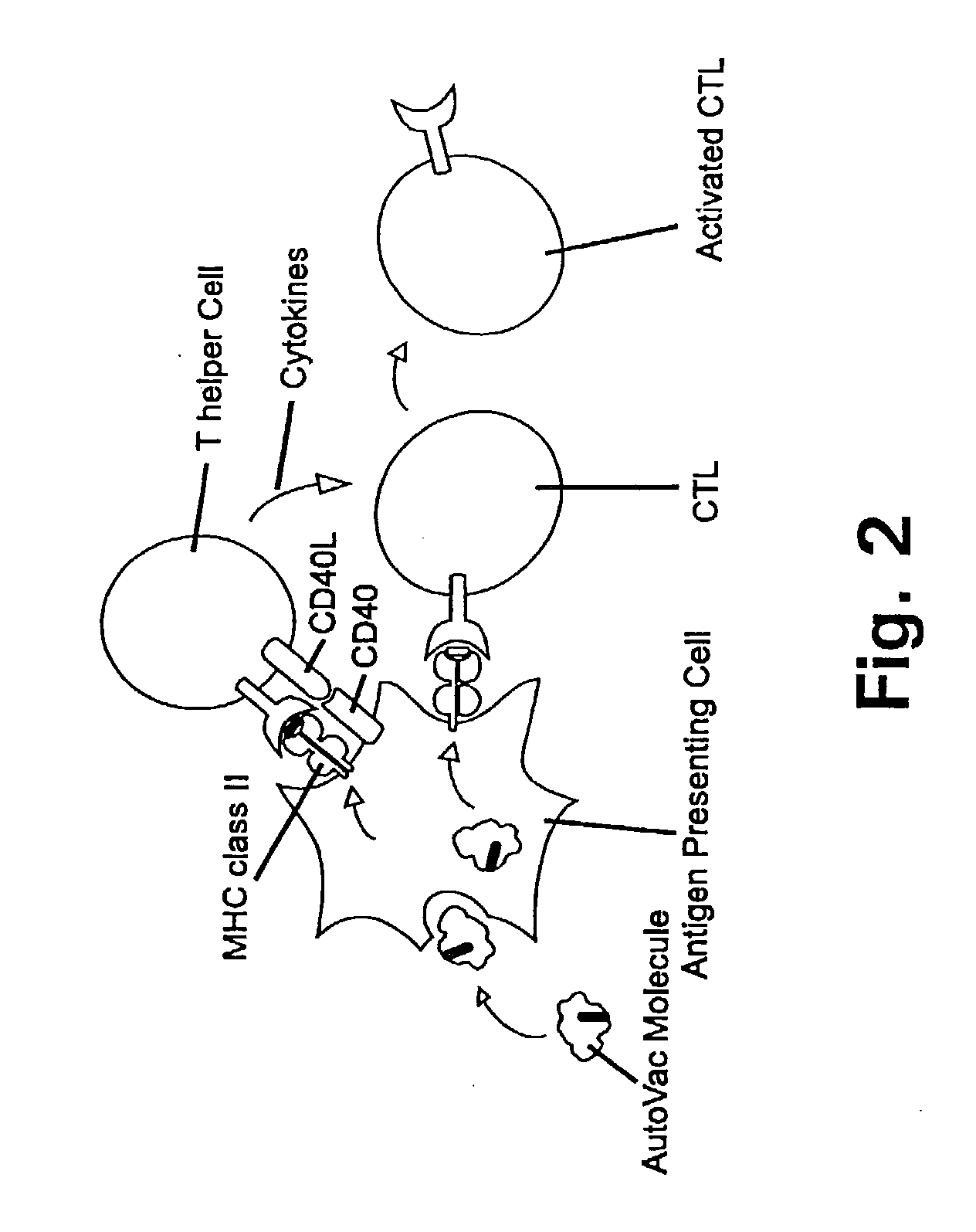
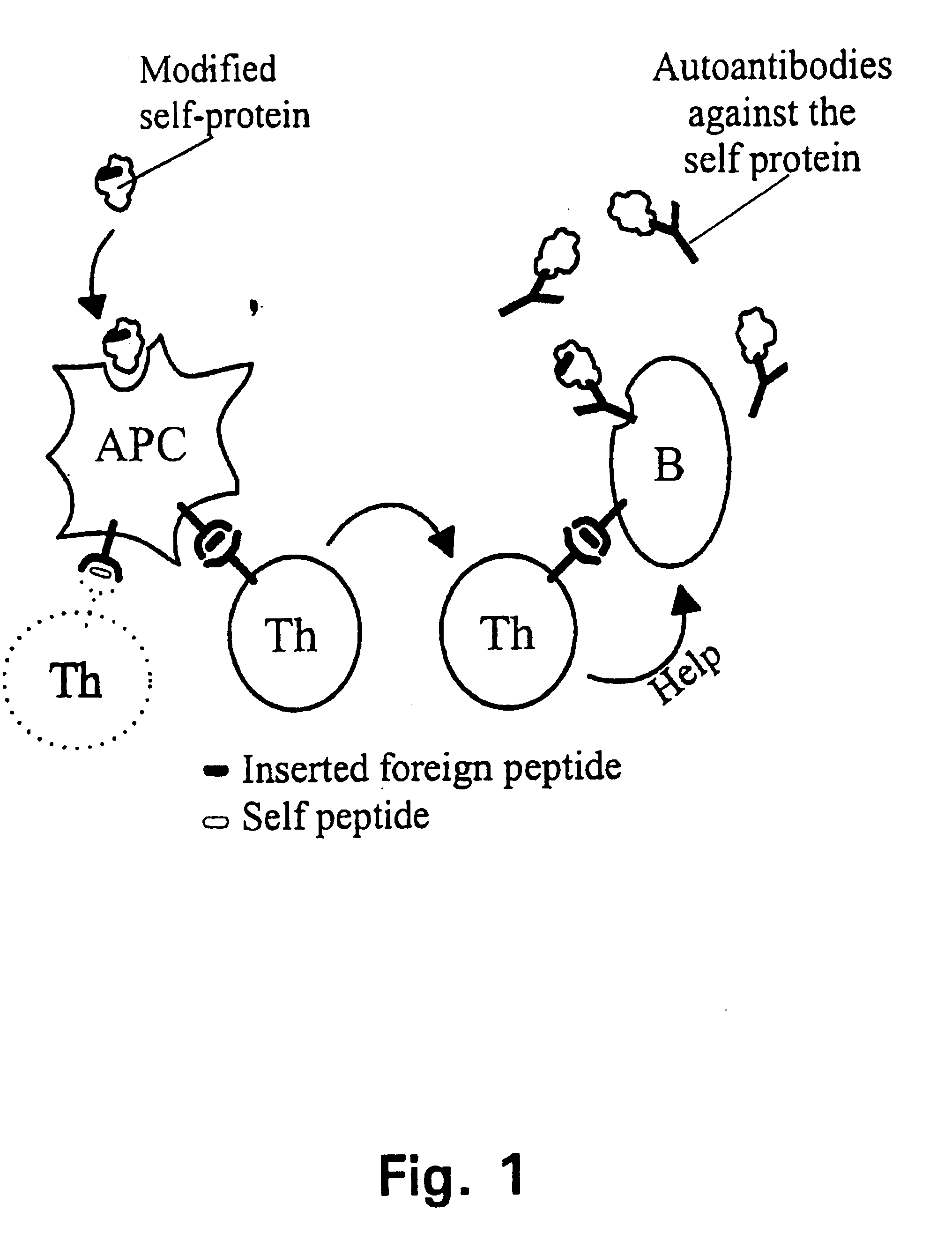
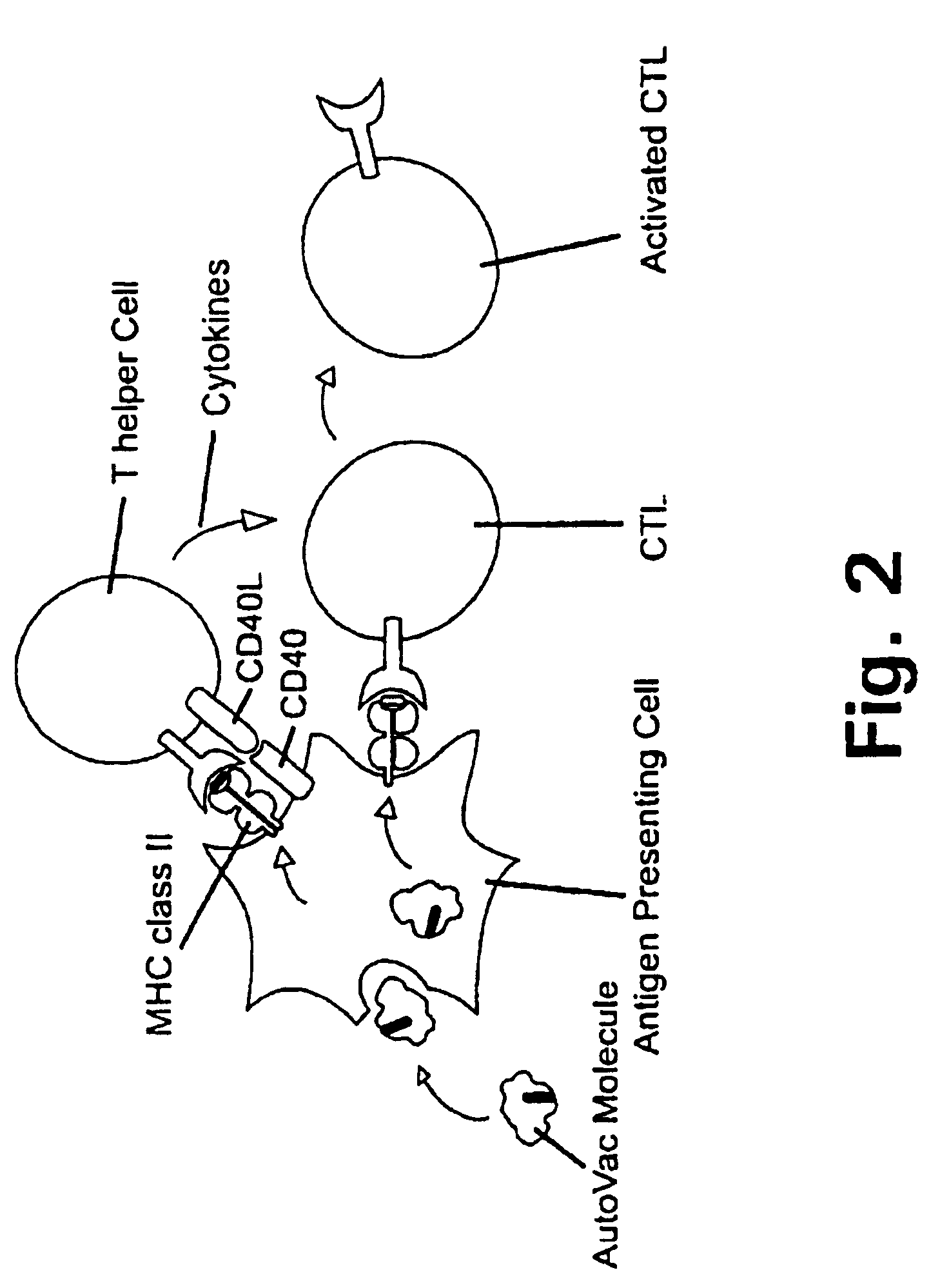
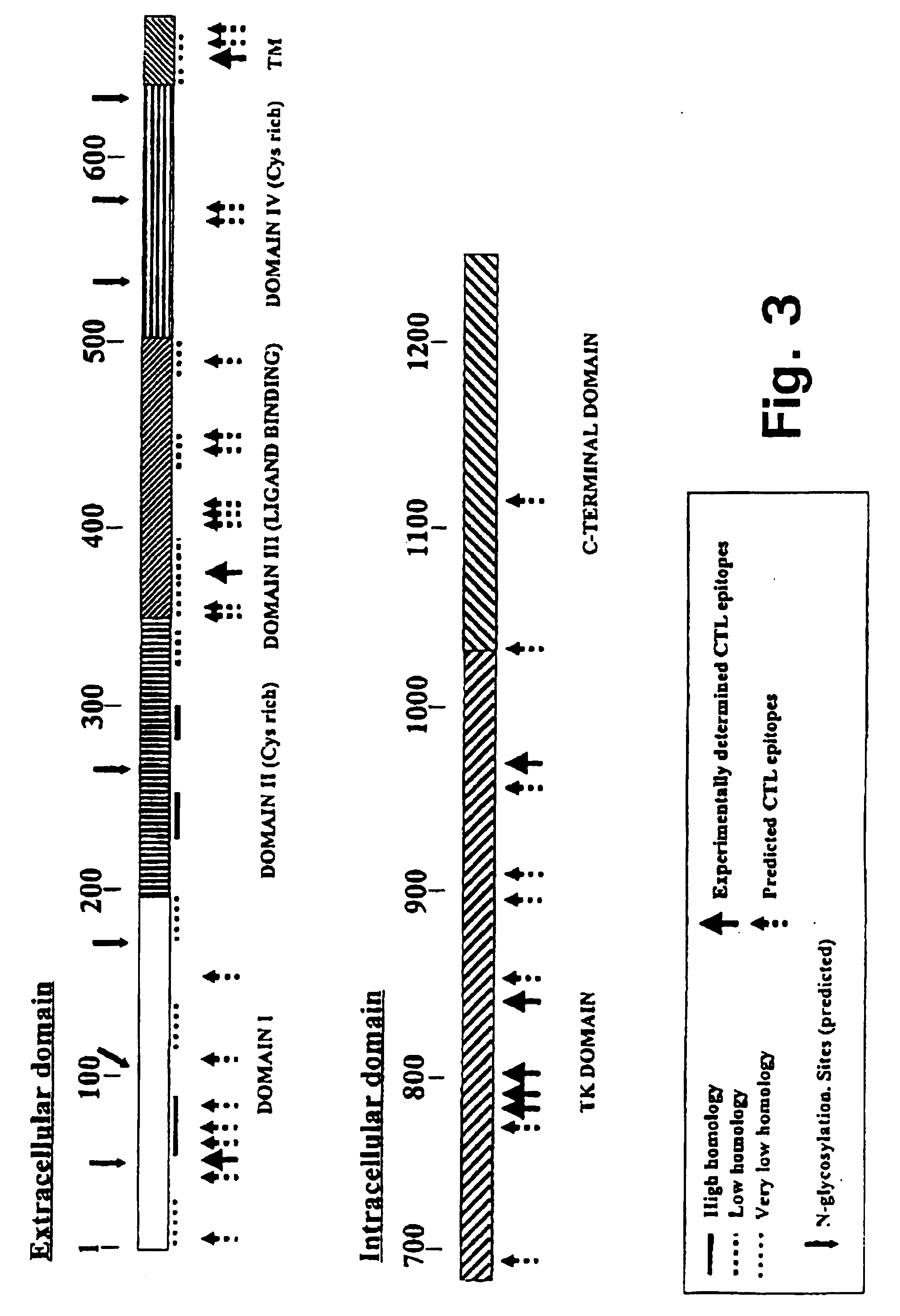
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
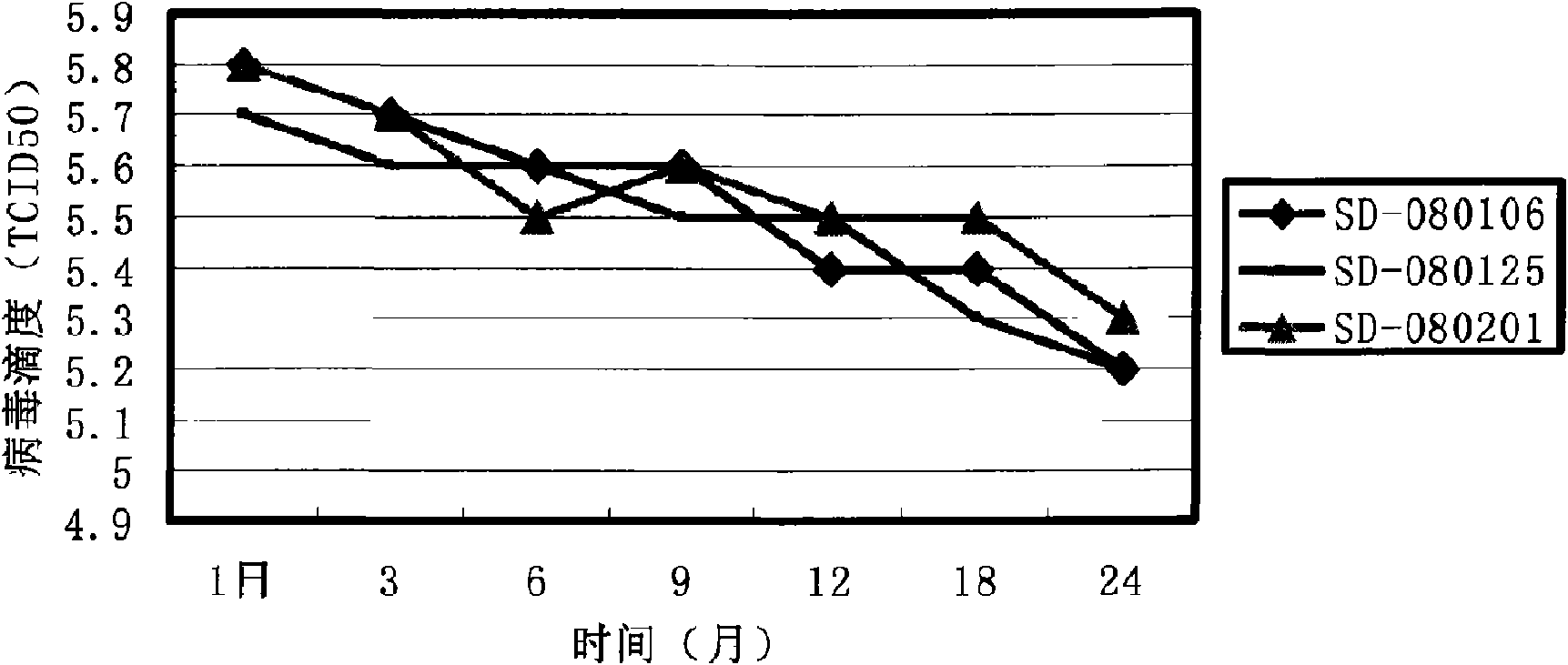
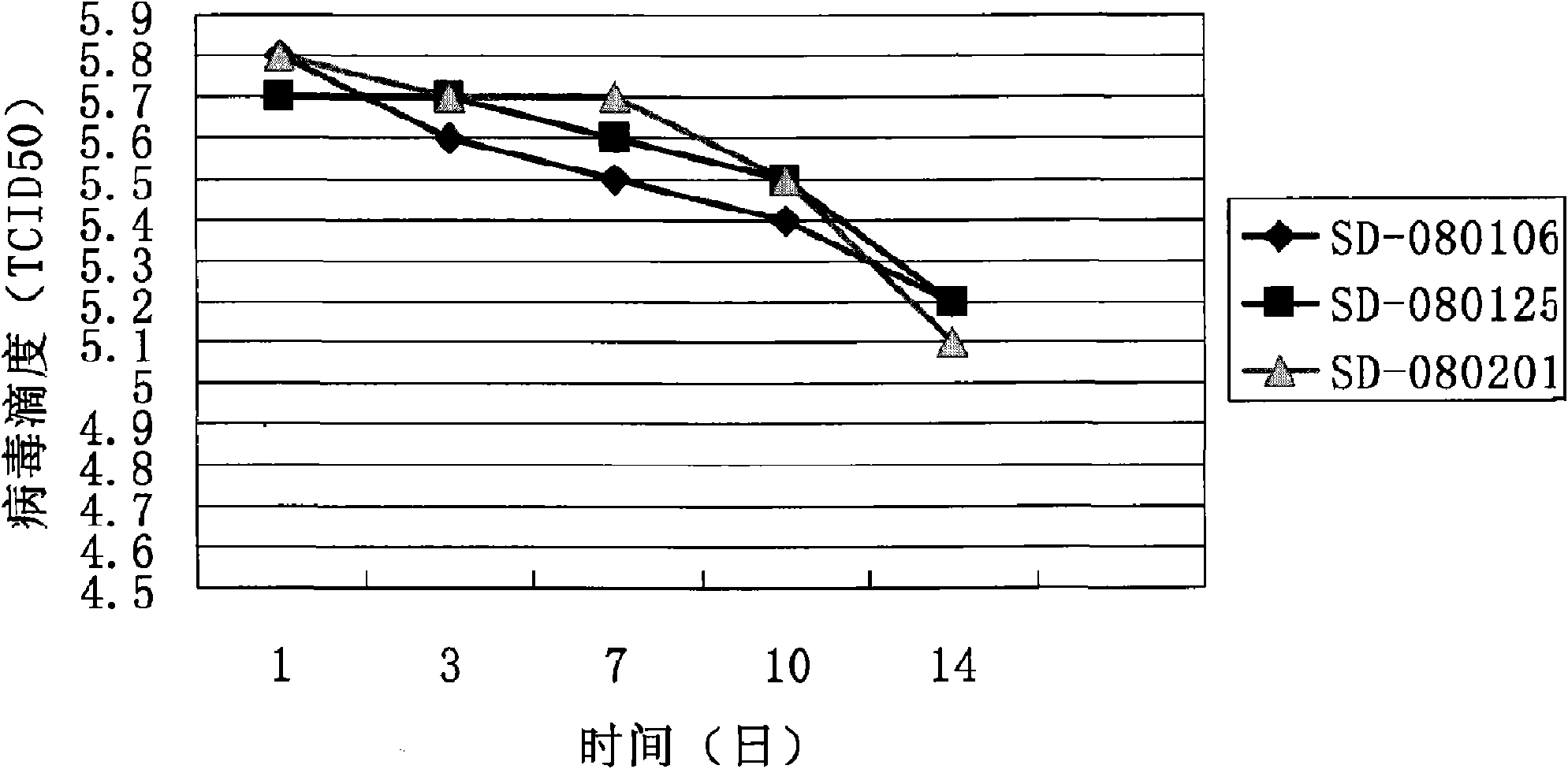
Attenuated live vaccine strain for preventing pig-pig infection breeding and respiratory syndrome
ActiveCN101633909AAvoid breedingPrevention of Respiratory SyndromeViral antigen ingredientsMicrobiological testing/measurementBiologyAttenuated Live Vaccine
The invention provides an attenuated live vaccine strain for preventing pig-pig infection breeding and respiratory syndrome, a formulation thereof and a preparation method of the attenuated live vaccine strain and the formulation thereof. The attenuated live vaccine strain has obvious immunity protection effect on the pig-pig infection breeding and respiratory syndrome. The vaccine formulation also has long protection period and stable storage.
Owner:华威特(江苏)生物制药有限公司
Vaccines, immunotherapeutics and methods for using the same
InactiveUS8119395B1Enhance and modulate immune responseUseful for immunotherapyAntibacterial agentsVirusesDiseaseAntigen
Improved vaccines which include a nucleotide sequence that encodes immunomodulating protein operably linked to regulatory elements are disclosed. The improved vaccines include DNA vaccines, recombinant vaccines for delivering foreign antigen and live attenuated vaccines. Methods of immunizing individuals are disclosed. Compositions for and methods of treating individuals with autoimmune diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Attenuated Pasteurella piscicida vaccine for fish
Live-attenuated vaccines against Edwardsiella ictaluri or against Pasteurella piscicida are disclosed. Both vaccines are incapable of reversion to virulence, because both are made by deletion mutations in the aroA gene, the purA gene, or both. These vaccines may be used not only to vaccinate fish against Edwardsiella ictaluri or Pasteurela piscicida, but also to serve as vectors to present antigens from other pathogens to the fish, thereby serving as vaccines against other pathogens as well, with no risk of infection by reversion to the virulent form of the pathogen in which the antigen occurs naturally.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Site-directed mutated influenza virus, and live vaccine, preparation method and application thereof
InactiveCN106929482ASsRNA viruses negative-senseViral antigen ingredientsGenomeAttenuated Live Vaccine
The invention relates to an influenza virus subjected to site-directed mutation or an influenza virus with simultaneous mutation of multiple sites. The influenza virus can be derived from human and other animals. The invention further relates to a method of the site-directed mutated influenza virus. The method includes introducing UAG into an influenza virus genome according to a reverse inheritance technology and introducing unnatural amino acid into an influenza virus gene in a site-directed way according to a gene codon extension technology. The invention further relates to application of the site-directed mutated influenza virus or the influenza virus with multiple mutation site combinations, such as serving as attenuated live vaccines and copying controllable safe influenza virus models.
Owner:PEKING UNIV
Avian influenza virus live attenuated vaccine and uses thereof
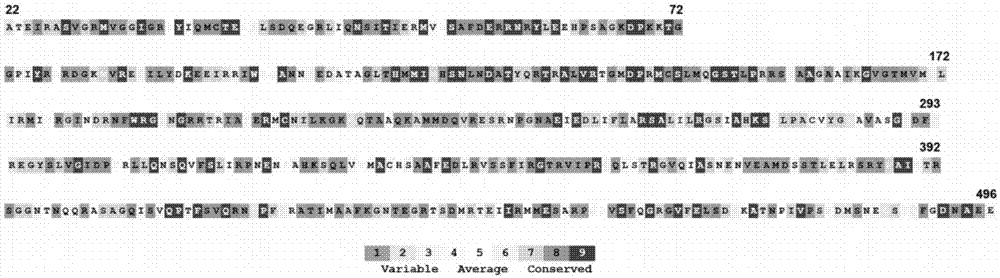
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
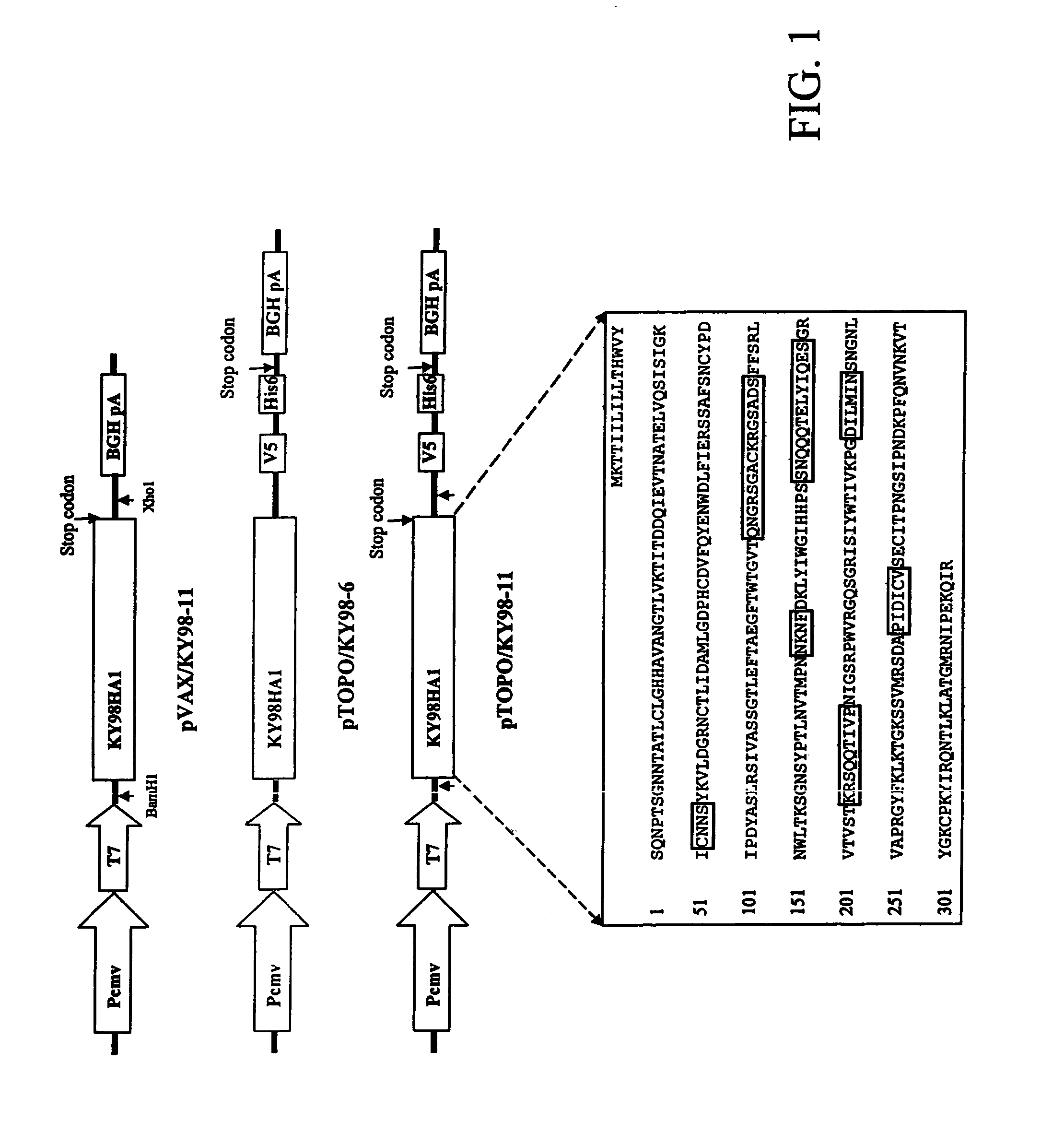
DNA vaccine expressing HA1 of equine-2 influenza virus
InactiveUS7244435B2Reduce riskReduce dosageSsRNA viruses negative-senseViral antigen ingredientsHemagglutininA-DNA
The invention is for a DNA vaccine expressing the hemagglutinin (HA1) gene of equine-2 influenza virus. By engineering a stop codon within HA1, expression of HA1 is ensured. By encapsulation of the DNA vaccine in liposome and by intranasal inoculation, it is sufficient to elicit protective immunity at a significantly lower dosage compared to a DNA vaccine expressing the full length HA gene. Lower dosage reduces the risk of induction of anti-DNA antibodies. Intranasal inoculation directly to the respiratory epithelial cells reduces the risk of DNA integration. The inventive vaccine is advantageous over current inactivated or live attenuated vaccines, as updating of the vaccine requires only the replacement of the encoding sequence with the new virus.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Attenuated Live Vaccine for Prevention of Porcine Reproductive and Respiratory Syndrome
ActiveUS20120189655A1Organic active ingredientsSsRNA viruses positive-senseBiologyAttenuated Live Vaccine
The present disclosure provides an attenuated live vaccine strain and the formulations thereof, for preventing pigs from infection of porcine reproductive and respiratory syndrome (PRRS). The preparation methods for the vaccines and the formulations are also provided. The attenuated live vaccine strain provided herein offers significant immunological protection to pigs against PRRS. The vaccine formulations of the present disclosure also have advantages in long shelf lives as well as good stability during storage.
Owner:WU HUA
Use of flavivirus for the expression of protein epitopes and development of new live attenuated vaccine virus to immunize against flavivirus and other infectious agents
InactiveUS20060159704A1Stable expressionSafe and effectiveSsRNA viruses positive-senseVirus peptidesEpitopeSpecific immunity
The present invention relates to a vaccine against infections caused by flavivirus. More particularly to the use of the YF vaccine virus (17D) to express at the level of its envelope, protein epitopes from other pathogens which will elicit a specific immune response to the parental pathogen.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
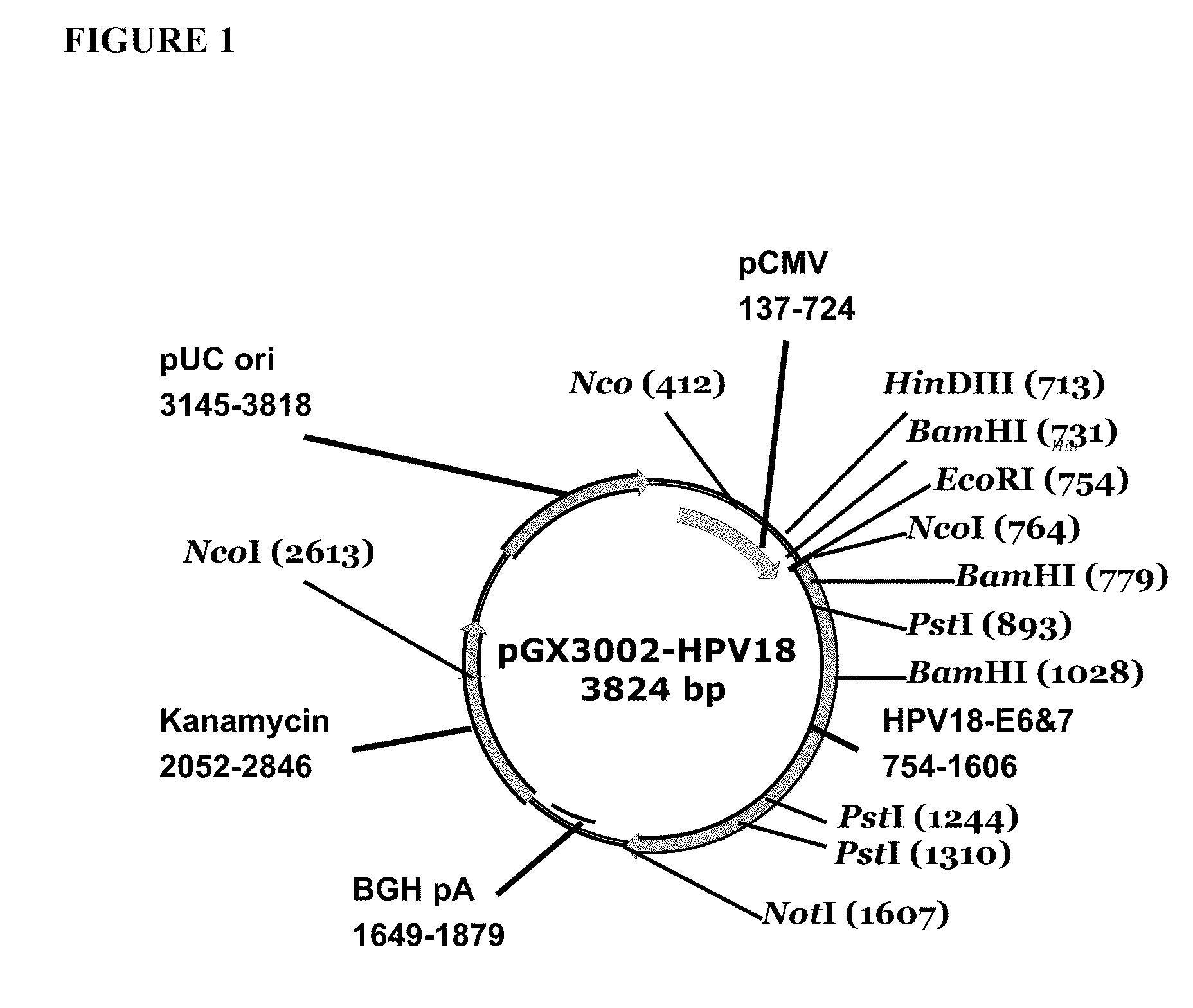
Vaccines for human papilloma virus and methods for using the same
ActiveUS20100189730A1Improved immunogenic targetImproved immunogenic targetsSsRNA viruses negative-senseOrganic active ingredientsRecombinant vaccinesAttenuated vaccine
Improved anti-HPV immunogens and nucleic acid molecules that encode them are disclosed. Immunogens disclosed include those having consensus HPV 18 E6 and E7. Pharmaceutical composition, recombinant vaccines comprising and live attenuated vaccines are disclosed as well methods of inducing an immune response in an individual against HPV are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Hcv vaccines and methods for using the same
ActiveUS20120034256A1Improved immunogenic targetOrganic active ingredientsSsRNA viruses positive-senseRecombinant vaccinesAttenuated vaccine
Owner:INOVIO PHARMA +1
Attenuated live vaccine for prevention of porcine reproductive and respiratory syndrome
ActiveUS8728487B2Organic active ingredientsSsRNA viruses positive-senseBiologyAttenuated Live Vaccine
The present disclosure provides an attenuated live vaccine strain and the formulations thereof, for preventing pigs from infection of porcine reproductive and respiratory syndrome (PRRS). The preparation methods for the vaccines and the formulations are also provided. The attenuated live vaccine strain provided herein offers significant immunological protection to pigs against PRRS. The vaccine formulations of the present disclosure also have advantages in long shelf lives as well as good stability during storage.
Owner:WU HUA
Freeze-dried hepatitis A attenuated live vaccine and its stabilizer
InactiveUS6884422B1Improve abilitiesIncreased thermo-resistance and storage stabilitySsRNA viruses negative-sensePowder deliveryMedicineHepatitis A vaccine
The present invention relates to hepatitis A vaccine, especially to a lyophilized attenuated hepatitis A vaccine which can be stored at ambient temperature for extended periods of time, and to a method for producing the same. The present invention further relates to a stabilizer for lyophilized live vaccine and its use in improving thermostability of lyophilized live vaccine during lyophilization processing and storage period after lyophilization.
Owner:CHANGCHUN INST OF BIOLOGICAL PRODS
Lentogenic CH60 strain of duck virual hepatitis virus and attenuated live vaccine thereof
ActiveCN103103163AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsDuck viral hepatitisAttenuated vaccine
The invention discloses a lentogenic CH60 strain of duck virual hepatitis virus. The lentogenic CH60 strain of the duck virual hepatitis virus is preserved in the China Center for Type Culture Collection of Wuhan University in China; and the preservation number is CCTCC NO: V201248. As the strain of duck virus hepatitis attenuated vaccine, the lentogenic CH60 strain of duck virual hepatitis virus provided by the invention is good in security and good in immunogenicity.
Owner:SICHUAN AGRI UNIV
Marker-free gene deletion attenuated mutant strain of Edwardsiella tarda wild strain as well as relevant preparations and application thereof
ActiveCN101974472AGood control effectImprove immunityAntibacterial agentsBacterial antigen ingredientsChorismic acidAttenuated Live Vaccine
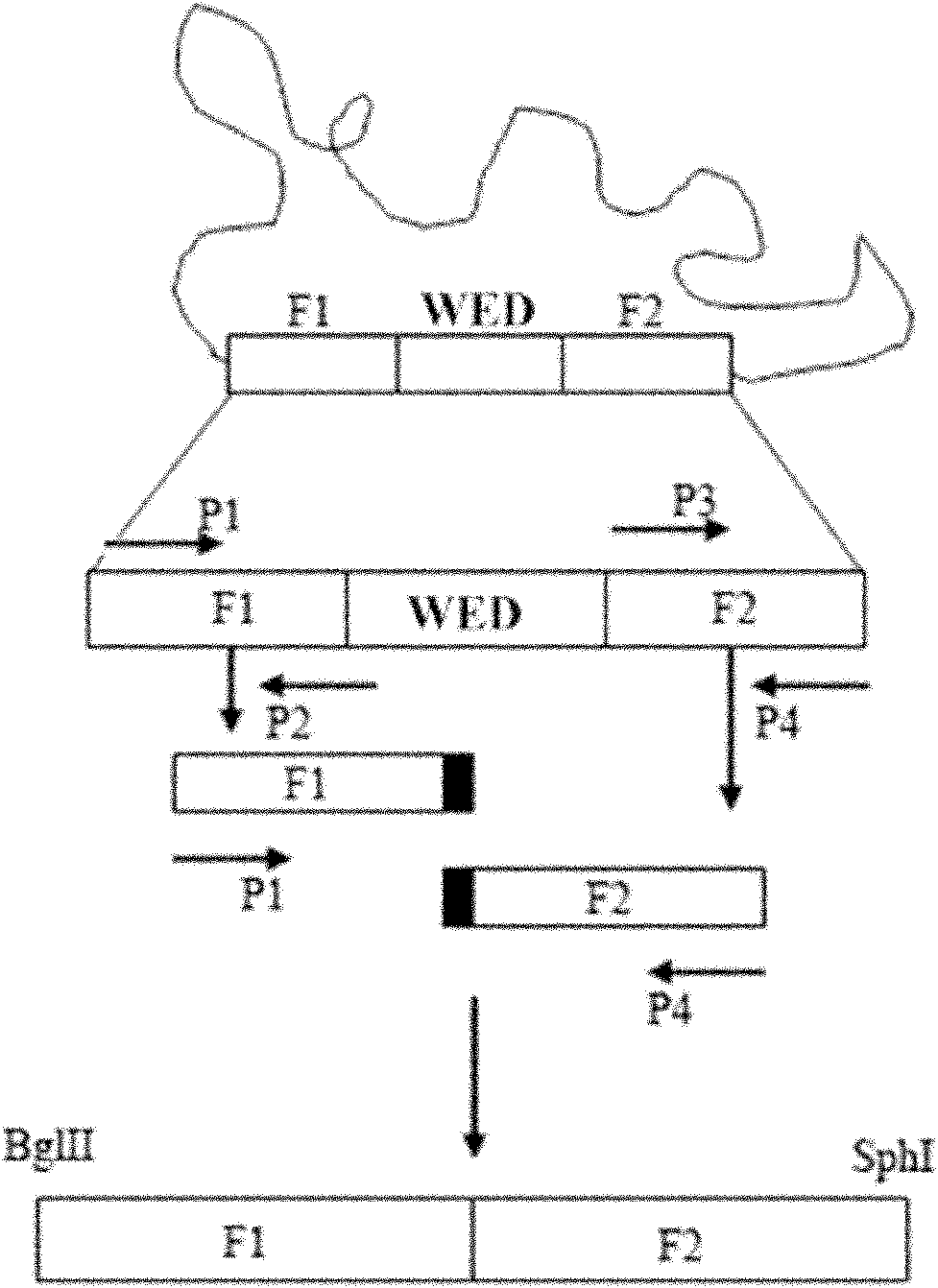
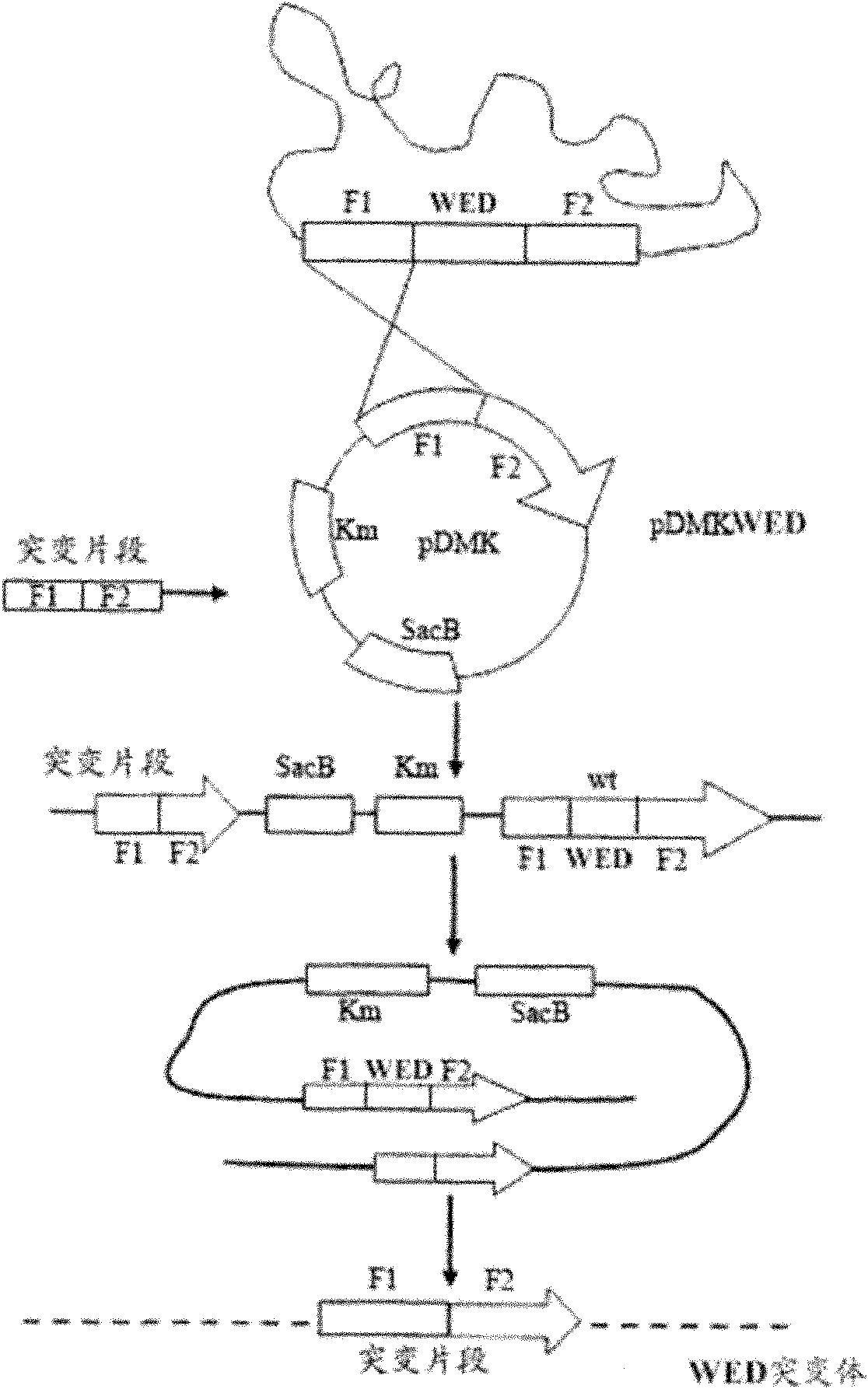
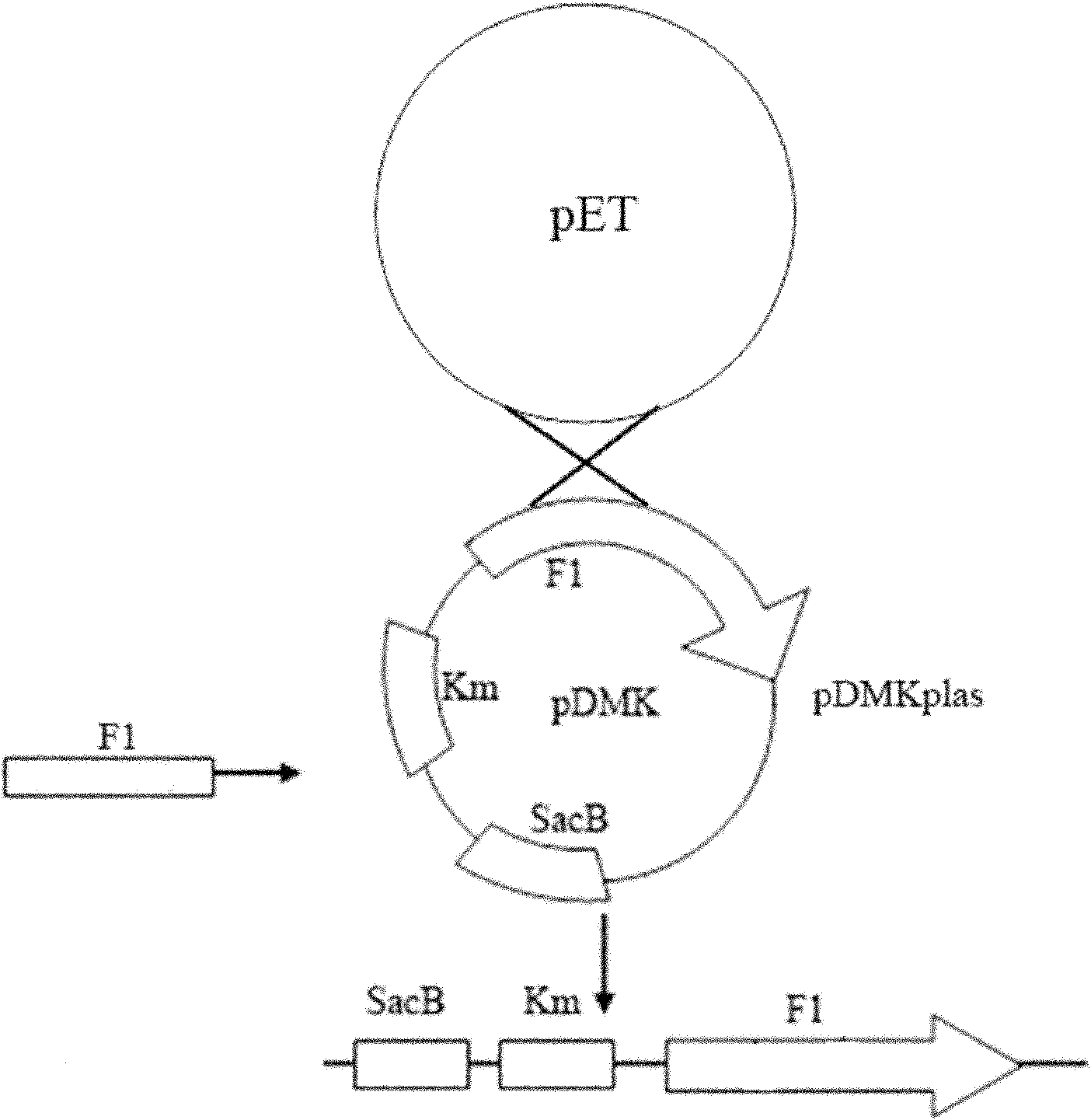
The invention relates to a marker-free gene deletion attenuated mutant strain of an Edwardsiella tarda wild strain. The marker-free gene deletion attenuated mutant strain is an attenuated live vaccine of an Edwardsiella tarda virulent strain, which deletes the chorismic acid synthase gene aroC of the Edwardsiella tarda virulent strain, three types of secretion system response element genes of eseB, escA, eseC and eseD and an endogenous plasmid, preferably, the Edwardsiella tarda virulent strain is an Edwardsiella tarda virulent strain EIB202 with the preservation number of CCTCC No:M208068; the endogenous plasmid is a plasmid of pEIB202; and the marker-free gene deletion attenuated mutant strain of the Edwardsiella tarda virulent strain is an attenuated strain WED with the preservation number of CCTCC No:M2010278. The invention also provides relevant preparations and application of the marker-free gene deletion attenuated mutant strain. The attenuated mutant strain or relevant preparations eliminate the potential environment and safety risk of products existing in the traditional attenuated live vaccines generally and is a safe, effective and economic vaccine aiming at Edwardsiella tarda diseases of cultured fishes.
Owner:EAST CHINA UNIV OF SCI & TECH
Avian influenza virus live attenuated vaccine and uses thereof
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Freeze-dried vaccine protective agent, freeze-dried varicella attenuated live vaccine and preparation methods of freeze-dried vaccine protective agent and freeze-dried varicella attenuated live vaccine
ActiveCN104258404ALess prone to allergic reactionsExtensive and neat lesionsAntiviralsPharmaceutical non-active ingredientsArginineVaccine Production
The invention discloses a freeze-dried vaccine protective agent, a freeze-dried varicella attenuated live vaccine and preparation methods of the freeze-dried vaccine protective agent and the freeze-dried varicella attenuated live vaccine, belongs to the field of vaccine production and preparation processes, and solves the problem that an existing freeze-dried vaccine protective agent of a varicella vaccine contains gelatin, or contains dextranum and still contains human serum albumin even if the gelatin is removed, so that a potential hazard still exists in the safety of medication of the vaccine. The freeze-dried vaccine protective agent contains mycose, sodium glutamate, urea, L-arginine and a 199 culture medium, and does not contain macromolecular allergen ingredients such as gelatin, dextranum and human serum albumin; the bacterial endotoxin content is low; the varicella vaccine prepared by using the protective agent is low in endotoxin content and residual content of bovine serum albumin and antibiotics, good in stability, safe and effective; the main point is direct infection after passage; the cells are cleaned in the next day after infection, and are replaced with a serum-free maintenance fluid; the operation is simple; the cost is low; regular and uniform formation of cytopathy is facilitated; and the residual content of the bovine serum albumin can be greatly lowered.
Owner:陈安明
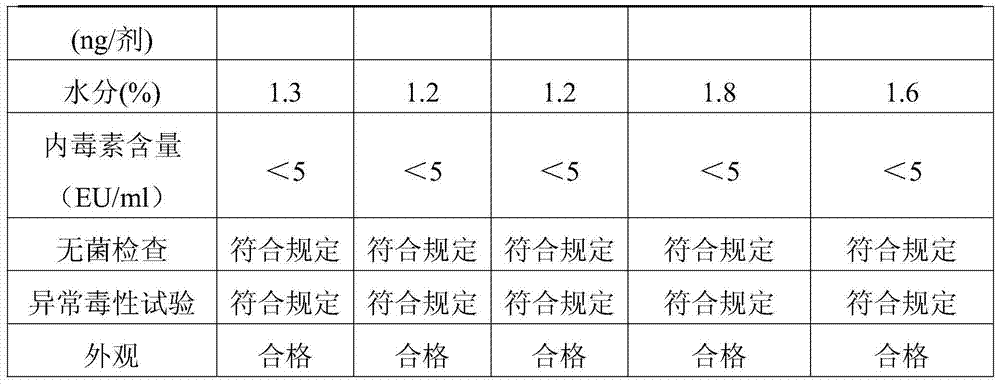
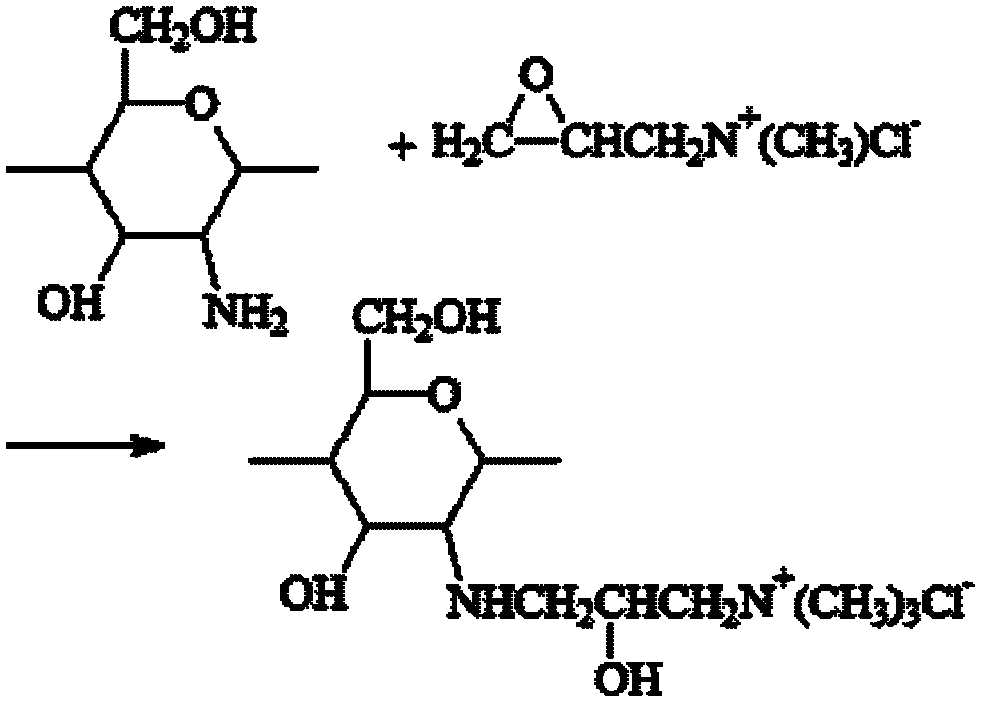
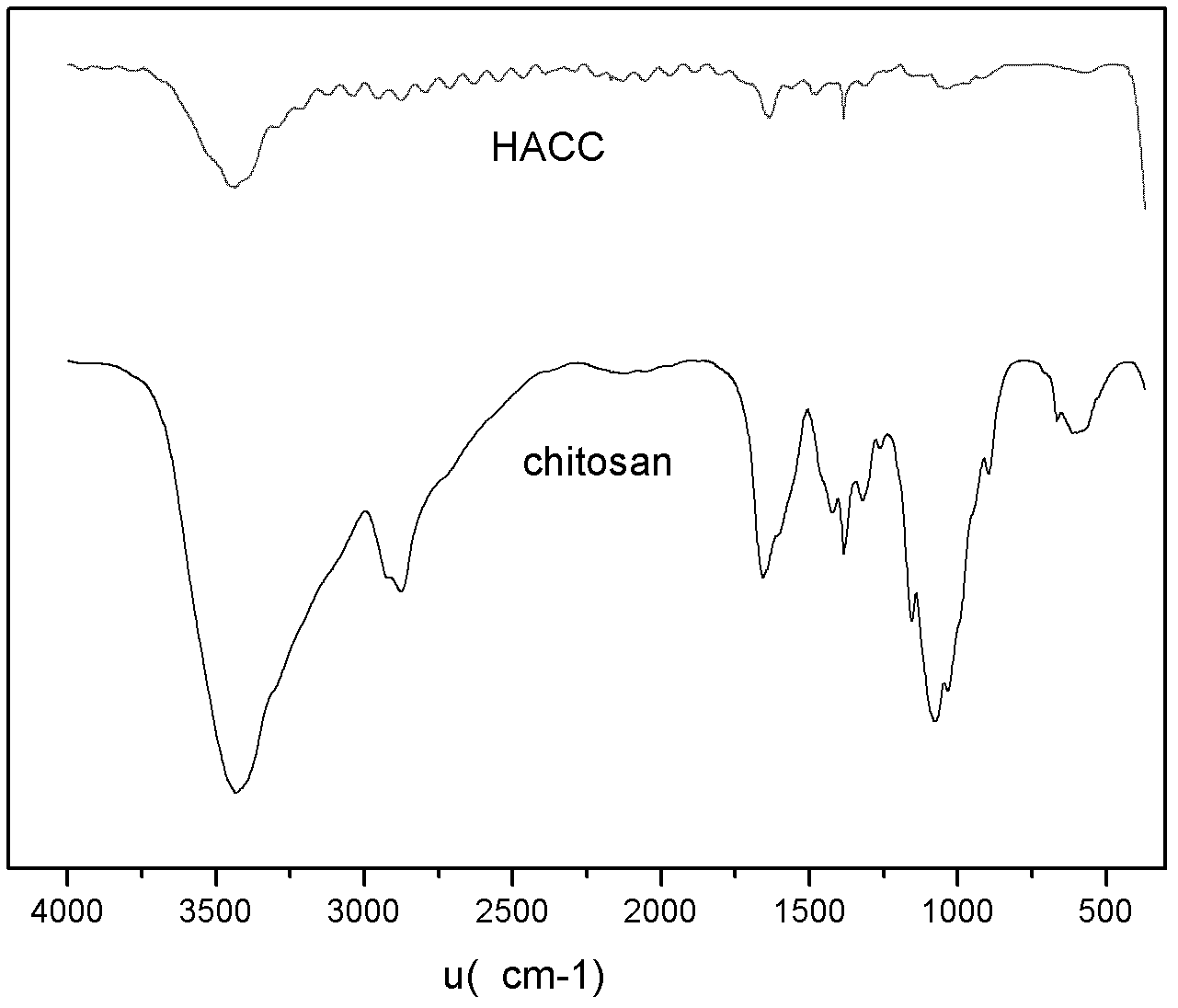
Synthetic method of N-2-hydroxypropyl trimethyl ammonium chloride chitosan and preparation method of Newcastle disease attenuated live vaccine-loaded nanoparticles of N-2-hydroxypropyl trimethyl ammonium chloride chitosan
ActiveCN102432695ARaw materials are easy to getEasy to operatePowder deliveryViral antigen ingredientsSide effectFreeze-drying
The invention provides a synthetic method of N-2-hydroxypropyl trimethyl ammonium chloride chitosan and a preparation method of Newcastle disease attenuated live vaccine-loaded nanoparticles of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan, relating to a synthetic method of chitosan and a preparation method of vaccine-loaded nanoparticles of the chitosan. The synthetic method comprises the following steps of: deacelation of the chitosan; dip-treatment of the chitosan; crude preparation of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan; and refined preparation of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan. The preparation method comprises the following steps of: adding a Newcastle disease virus solution into an N-2-hydroxypropyl trimethyl ammonium chloride chitosan solution to obtain a solution A; adding sodium tripolyphosphate, PBS (phosphate buffer solution) and span-80 into the solution A to obtain a solution B; and centrifuging the solution B to obtain a deposit, adding PBS for suspension, adding mycose skimmed milk, and performing freeze drying to finish the preparation. The nanoparticles prepared by using the method has the advantages of easiness in control of particle size, small particle size of drug-loaded nanoparticles, high entrapment efficiency, large drug-loading quantity, mild preparation conditions, low drug toxic or side effect, long slow release time, simple preparation process, lower production cost and easiness in large-scale production.
Owner:HEILONGJIANG UNIV
Newcastle disease virus infectious clones, vaccines and new diagnostic assays
InactiveUS7332169B2Immune responseImprove propertiesSsRNA viruses negative-senseGenetic material ingredientsAntigenVirulent characteristics
The invention relates to a process for generating infectious Newcastle disease virus (NDV) entirely from cloned full-length cDNA and to the use of vaccines and diagnostic assays generated with and derived from the process. The process offers the possibility to modify the NDV genome by means of genetic modification and allows for the introduction of mutations, deletions and / or insertions. The process can be used to modify the virulence of NDV, thus generating new attenuated live vaccines with enhanced properties. The process can be used to modify the antigenic make-up of NDV, to allow the generation of live NDV marker vaccines that can be serologically distinguished from NDV field strains.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Vaccines for human papilloma virus and methods for using the same
ActiveUS8389706B2Improved immunogenic targetsSsRNA viruses negative-senseOrganic active ingredientsRecombinant vaccinesAttenuated vaccine
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Live attenuated vaccines
ActiveUS20160235834A1Reliable analysisAntibacterial agentsImmunoglobulins against bacteriaBacteroidesMicrobiology
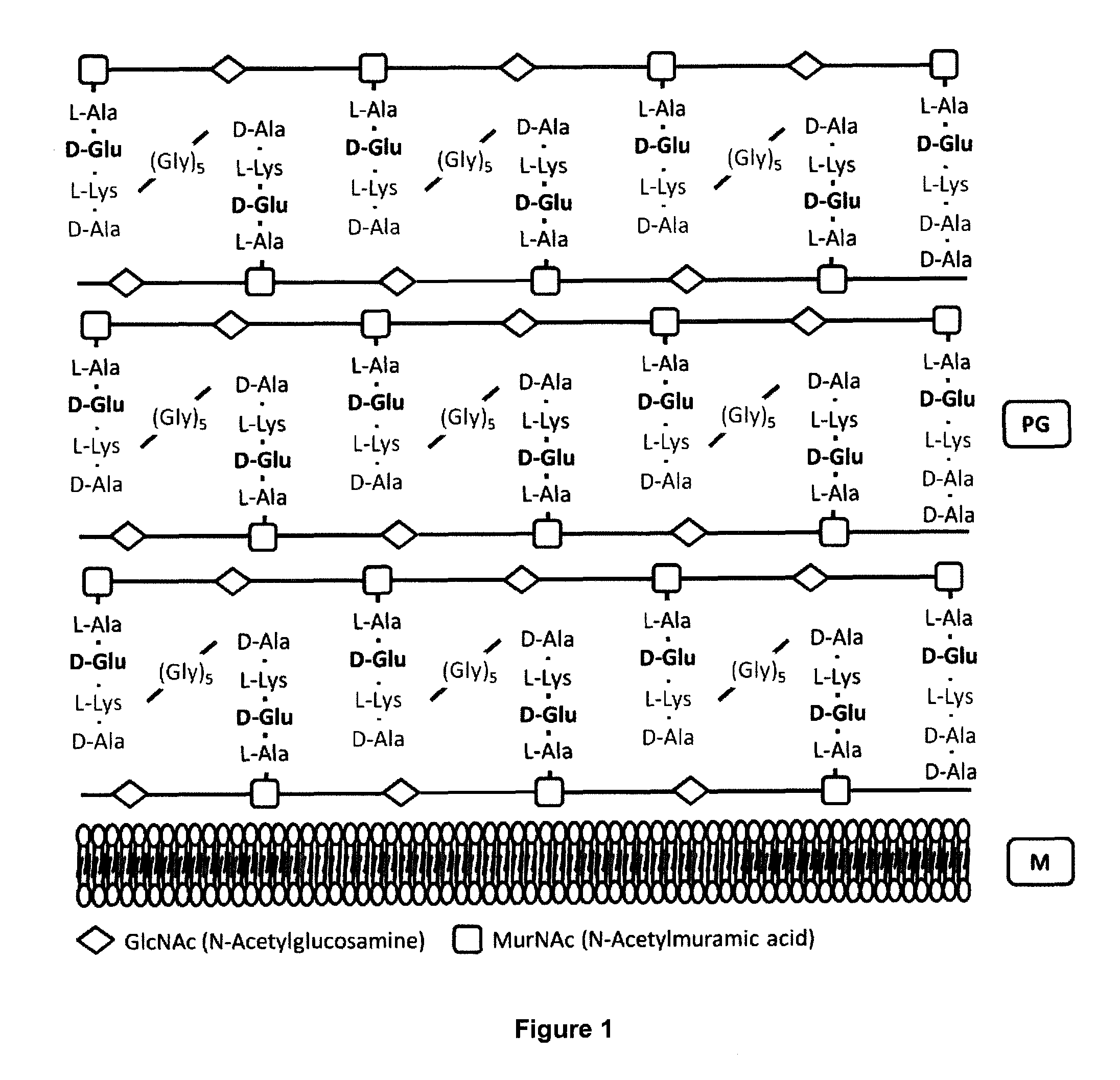
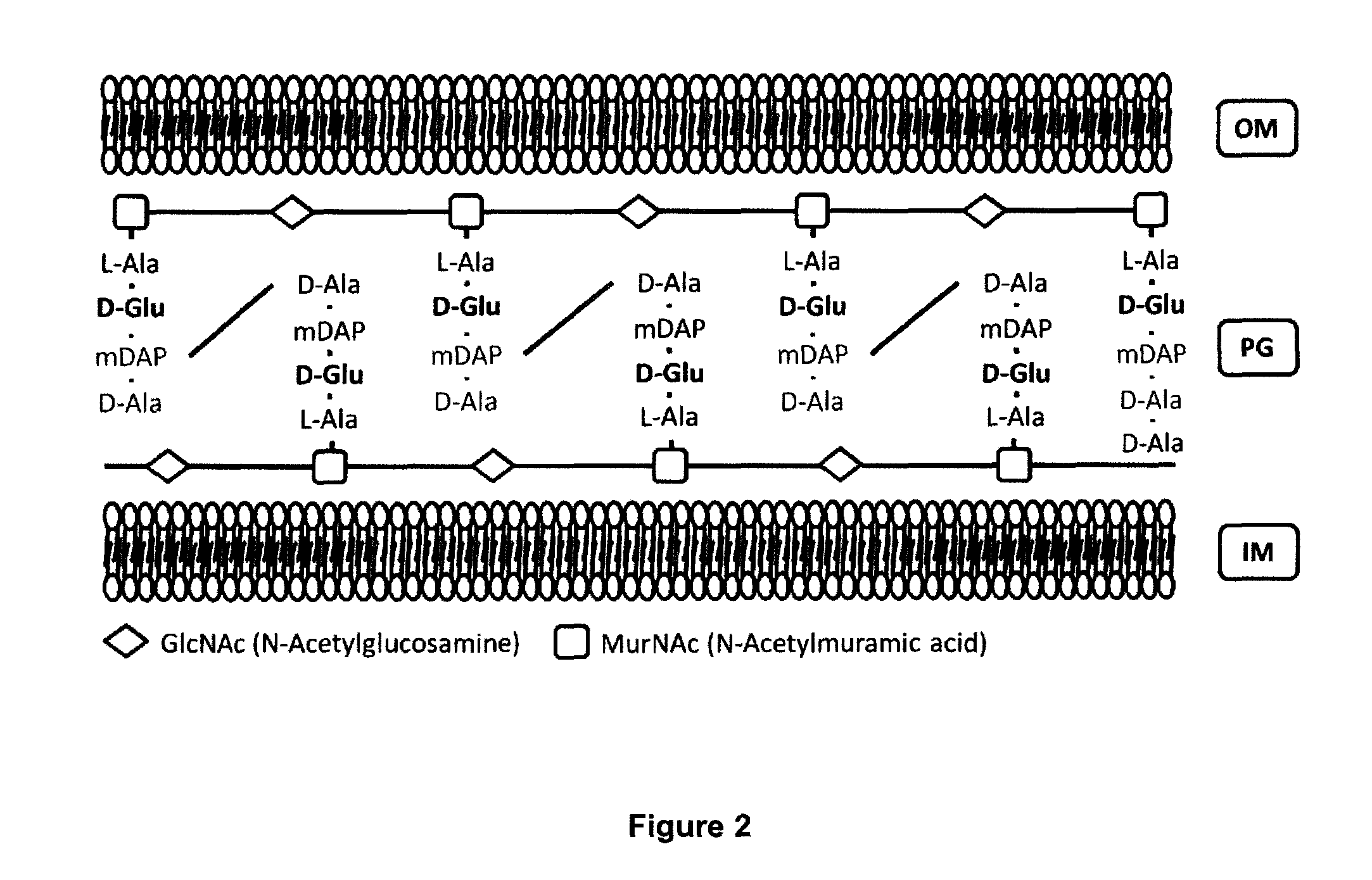
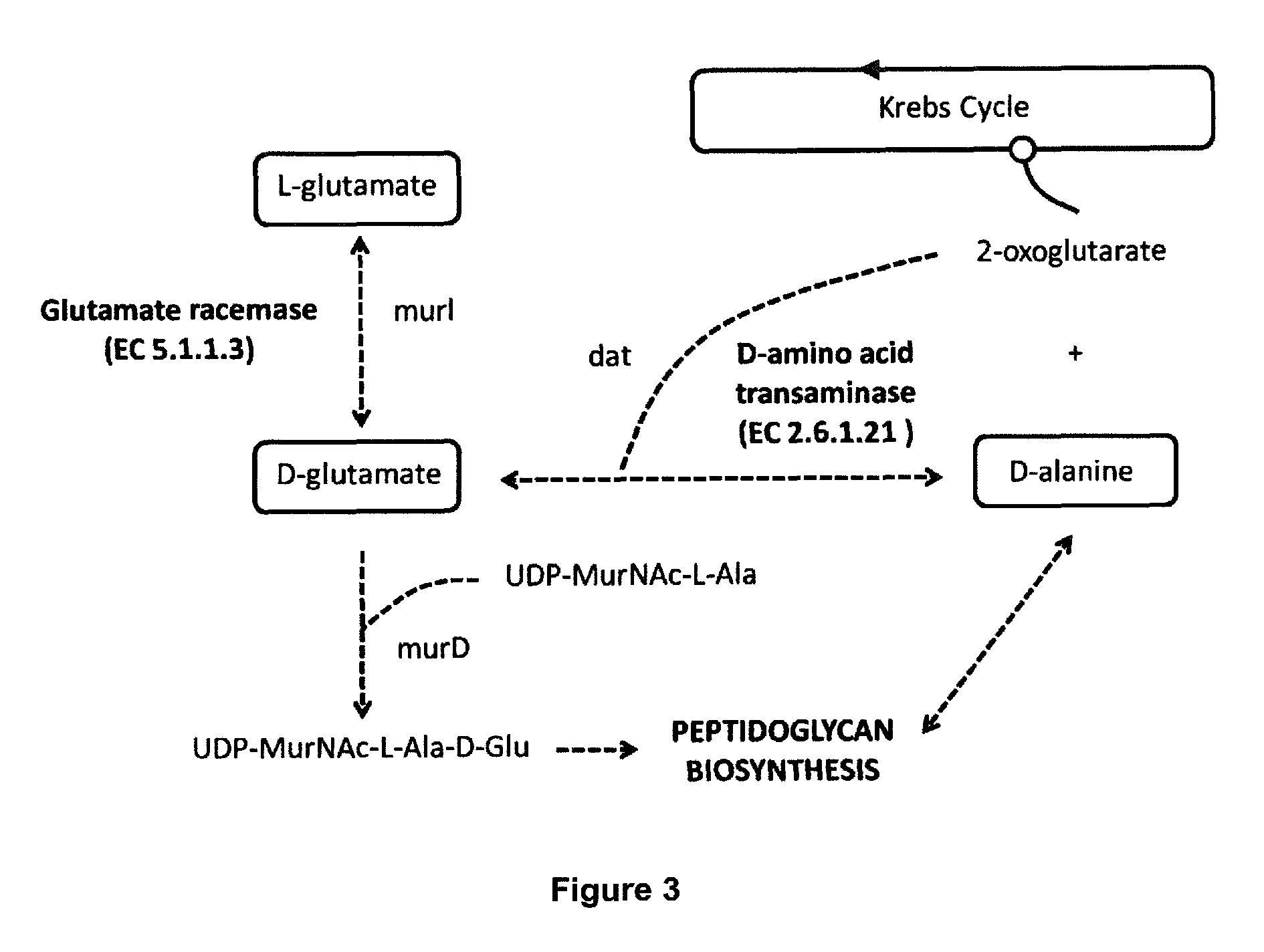
The present invention refers to a method for the production of live attenuated bacterial strains, suitable as vaccine candidates, comprising the steps of: A. providing a bacterial strain capable of expressing glutamate racemase and possibly D-amino acid transaminase and comprising a peptidoglycan cell wail, and B. inactivating the gene or genes encoding for the glutamate racemase enzyme and, if needed, the gene or genes encoding for the enzyme D-amino acid transaminase in such way that the bacterial strain is no longer capable of expressing a functional glutamate racemase and / or a functional D-amino acid transaminase; wherein the inactivation of said genes causes said bacterial strain to be auxotrophic for D-glutamate.
Owner:FUNDACION PROFESOR NOVOA SANTOS +1
Anti-swine SC protein monoclonal antibody and application of monoclonal antibody in preparing mycoplasma hyopneumoniae SIgA antibody ELISA detection kit
ActiveCN104877027AAvoid cross interferenceAvoid interferenceTissue cultureImmunoglobulinsImmune effectsProtein.monoclonal
The invention provides an anti-swine SC protein monoclonal antibody and an application of the monoclonal antibody in preparing a mycoplasma hyopneumoniae SIgA antibody ELISA detection kit, and relates to the technical field of animal virological and epizootiological detection. The anti-swine SC protein monoclonal antibody is secreted by a hybridoma cell strain 4H11 and the collection number of the anti-swine SC protein monoclonal antibody is CCTCC NO: C201526. The invention also discloses the monoclonal antibody and the application of the monoclonal antibody in preparing the mycoplasma hyopneumoniae SIgA antibody ELISA detection kit. The kit has high specificity, high stability and high sensitivity; a detection sample can be sampled conveniently; and the kit is capable of distinguishing porcine mycoplasma pneumonia inactivated vaccine immunization and natural infection, and can be applied to the early diagnosis of pneumonic porcine mycoplasma infection and the evaluation of the immune effect after attenuated live vaccine immunization.
Owner:JIANGSU ACAD OF AGRI SCI
Method for preparing thermal-stability vaccine
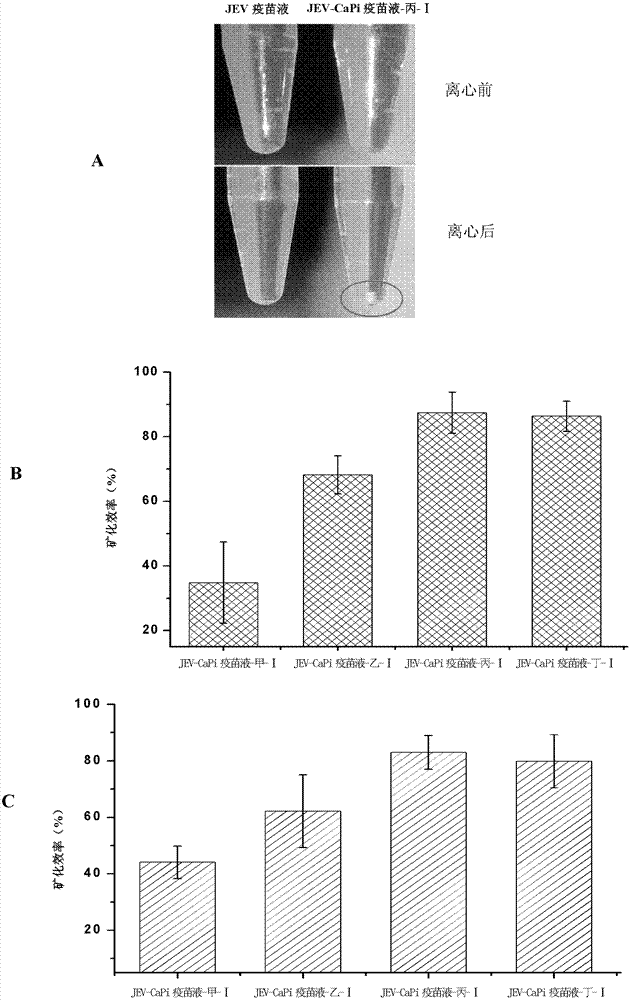
ActiveCN102784390AImprove securityImprove effectivenessViral antigen ingredientsInorganic non-active ingredientsCalcium biphosphateVaccine Production
The invention discloses a method for preparing thermal-stability vaccine and particularly relates to a method for preparing thermal-stability vaccine based on calcium phosphate mineralization. The invention provides a method for preparing calcium phosphate coated viruses. The method comprises the following steps that (1) compounds with Ca<2+> ions are added into virus liquid, an initial system is obtained, and PO4<3-> ions are contained in the virus liquid; (3) the initial system is subjected to incubation; and (3) precipitation is centrifugally collected, and the calcium phosphate coated viruses are obtained. The method has the advantages that through virus surface mineralization modification, the problem of heat inactivation phenomenon in the vaccine transportation and storage process can be effectively solved, and the financial expenditure of the attenuated live vaccine is obviously reduced. The method can be widely applied to thermal-stability vaccine production and preparation.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Transdermal immune influenza multivalent vaccine and preparation method thereof
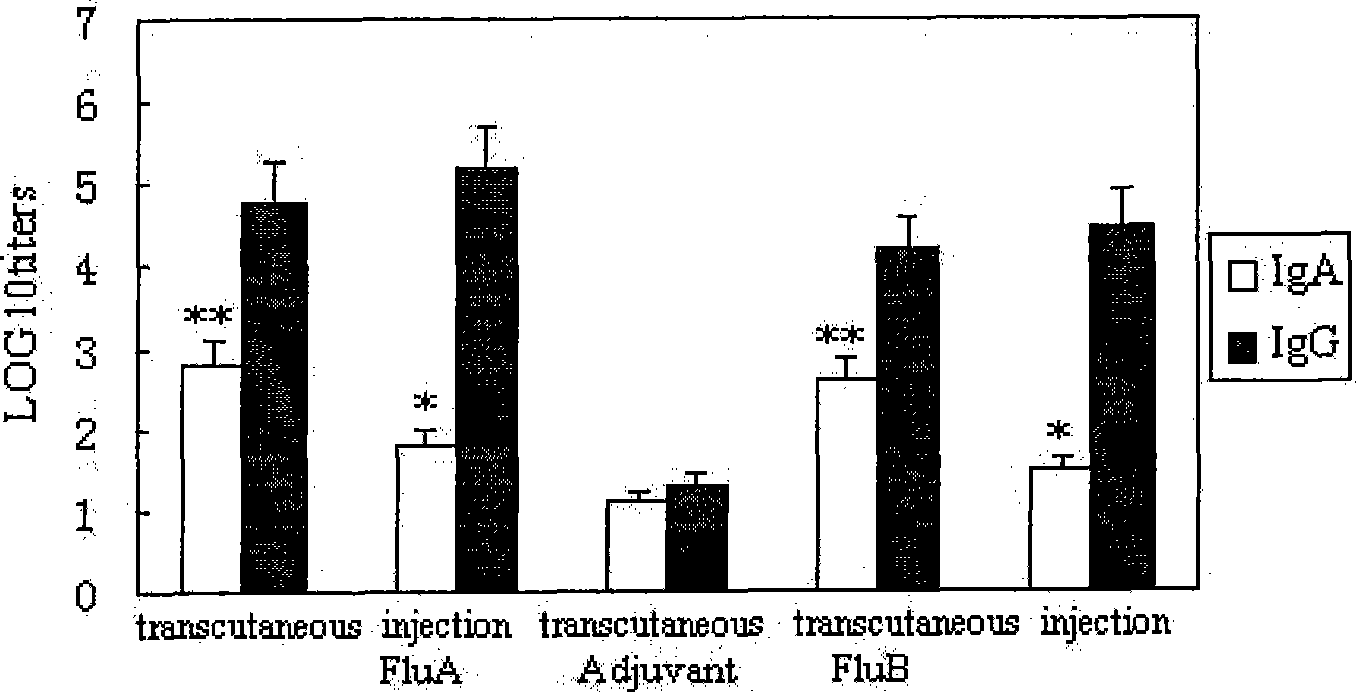
InactiveCN101450209AEnhanced antigen presentation functionFacilitated DiffusionPowder deliveryAntiviralsBALB/cAdjuvant
The invention provides a transdermal immunity flu polyvalent vaccine and a preparing method. The transdermal immunity flu polyvalent vaccine comprises transdermal immunity adjuvant, flu polyvalent vaccine antigen, permeation agent and medical dressing. The flu polyvalent inactivation or attenuated live vaccine is differ from prior vaccine immunity approach and adjuvant. A result through permeation purpose immunity to Balb / c mouse, ferret, monkey and human body improves that the transdermal immunity flu polyvalent vaccine can generate IgA and IgG antibody with high valency, namely, can induce immune system and mucosal immune simultaneously, also can be used for immunoprophylaxis to popular flu and highly pathogenic avian influenza.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and preparation method thereof
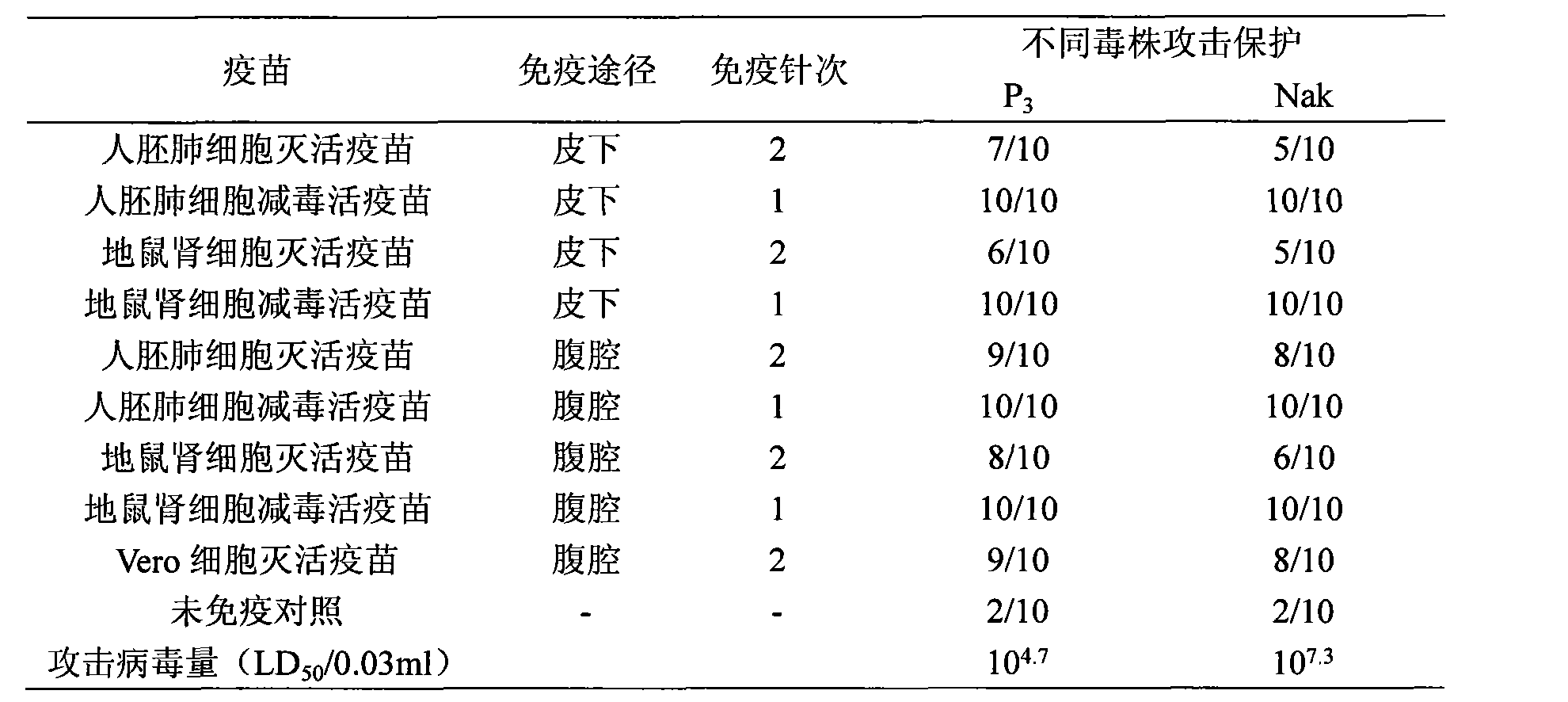
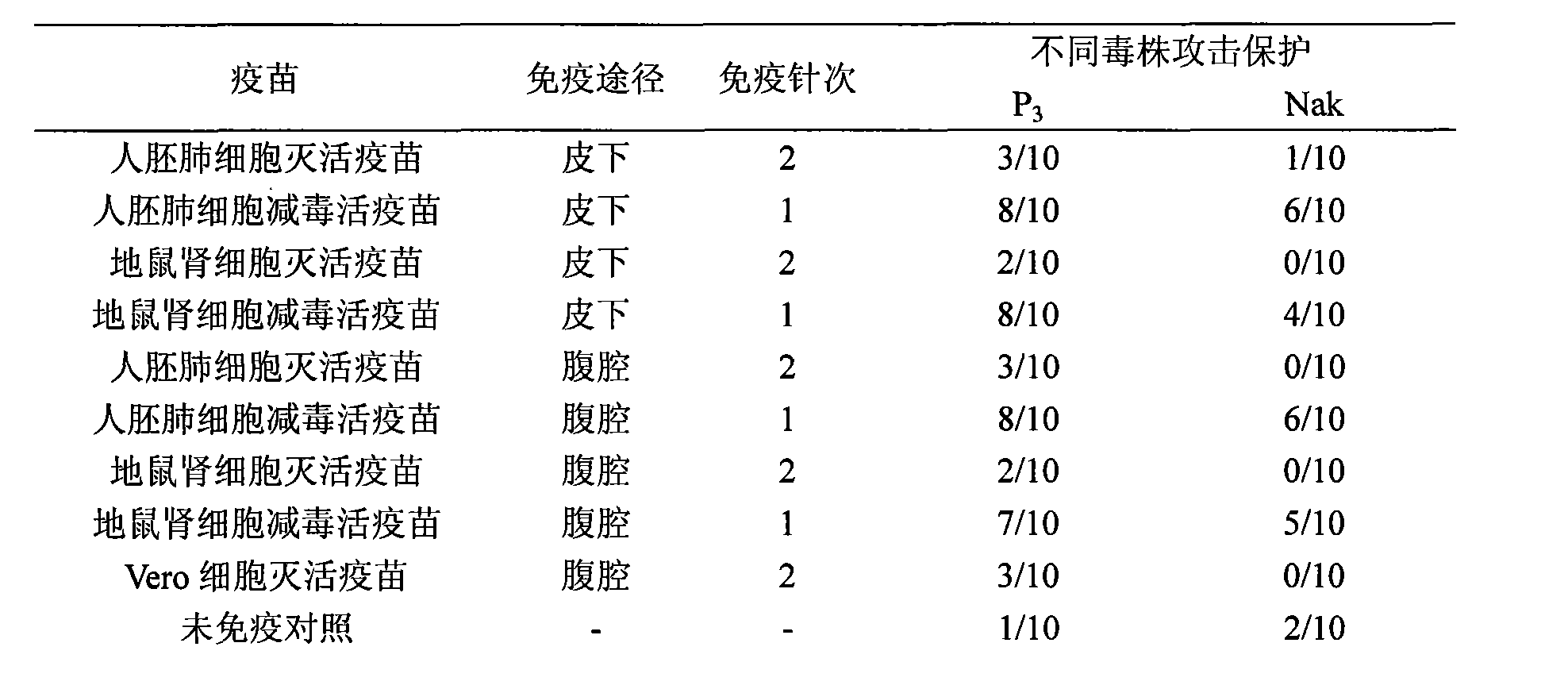
ActiveCN101524536AFully identifiedFully standardizedViral antigen ingredientsAntiviralsImmune effectsJapanese encephalitis vaccine
The invention discloses a Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and a preparation method thereof, comprising culture and expansion of the human embryonic lung fibroblasts. The method comprises the following steps: Japanese encephalitis virus strain P3, strain SA14-14-2 or strain Nakayama is naturalized and inoculated to fit the human embryonic lung fibroblasts, and the seeds of Japanese encephalitis viruses are prepared on the human embryonic lung fibroblasts; wherein, an inactivated Japanese encephalitis vaccine also comprises the steps of harvesting viruses, inactivating viruses, concentrating, purifying and the like; an attenuated live vaccine also comprises the steps of harvesting viruses, concentrating, purifying and the like. Due to being prepared by healthy human embryonic lung fibroblasts, the two kinds of Japanese encephalitis vaccines do not contain any adventitious pollution agent and tumorigenicity, has high purity after being purified and has the advantages of good immune effect and high security. The preparation method of the invention is suitable for large-scale industrial production and can meet the preparation processes of Japanese encephalitis vaccines required by domestic and abroad markets.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
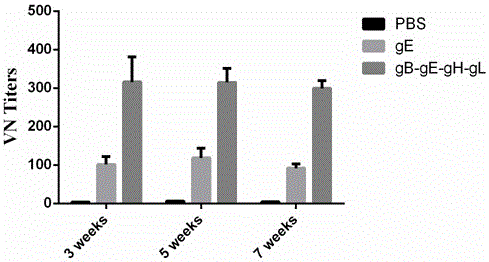
Varicella-zoster virus gB-gE-gH-gL fusion protein, genetic engineering subunit vaccine and preparation methods
ActiveCN105906721AImprove immunityStrong immune memoryAntibody mimetics/scaffoldsViral antigen ingredientsEscherichia coliAdjuvant
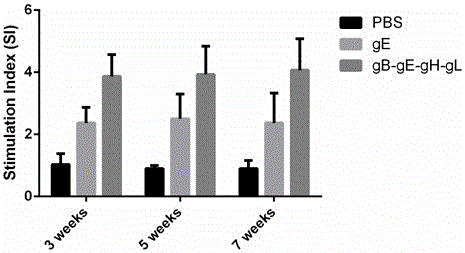
The invention relates to the technical field of biomedicine, and particularly provides varicella-zoster virus gB-gE-gH-gL fusion protein, a genetic engineering subunit vaccine and preparation methods. The fusion protein comprises a VZV gB extracellular region, a gE extracellular region, a gH truncated fragment and a gL truncated fragment. The amino acid sequence of encoded protein of the fusion protein is SEQ ID NO:1, and one amino acid sequence is SEQ ID NO:2. A prokaryotic expression vector is utilized for constructing escherichia coli BL21 (DE3) host bacteria capable of expressing the VZV gB-gE-gH-gL fusion protein. The fusion protein is purified and mixed with a medicinal adjuvant to be prepared into the genetic engineering subunit vaccine. Compared with a currently-used VZV attenuated live vaccine, the vaccine can induce immune mice to generate higher specific humoral immunity and cell-mediated immunity and can prevent dormant infection that VZV is spread to dorsal root ganglions and intestinal ganglions through blood flow, the safety of the VZV vaccine is effectively improved, and therefore the genetic engineering subunit vaccine is an alternative vaccine with potential clinical application value.
Owner:ANHUI MEDICAL UNIV
Edwardsiella tarda attenuated live vaccine
InactiveCN101342367AGood low toxicityImprove securityAntibacterial agentsBacterial antigen ingredientsBacteroidesDisease
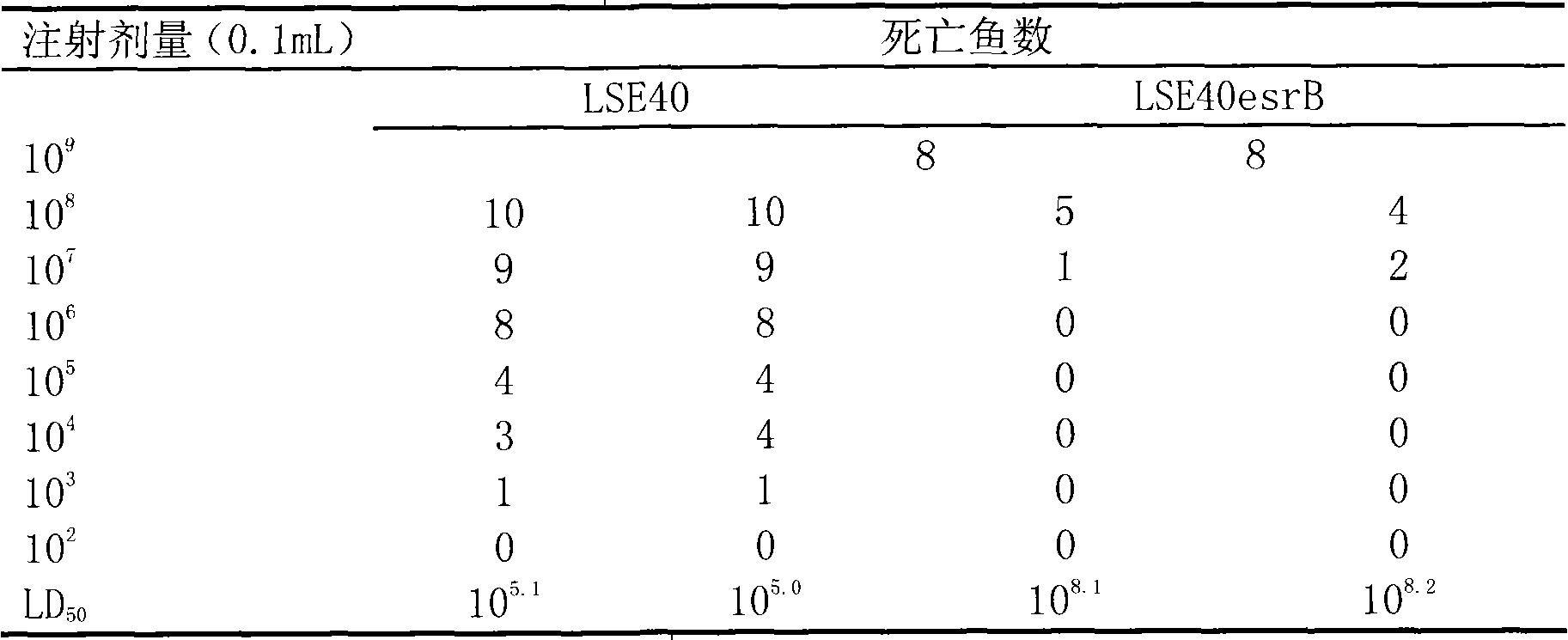
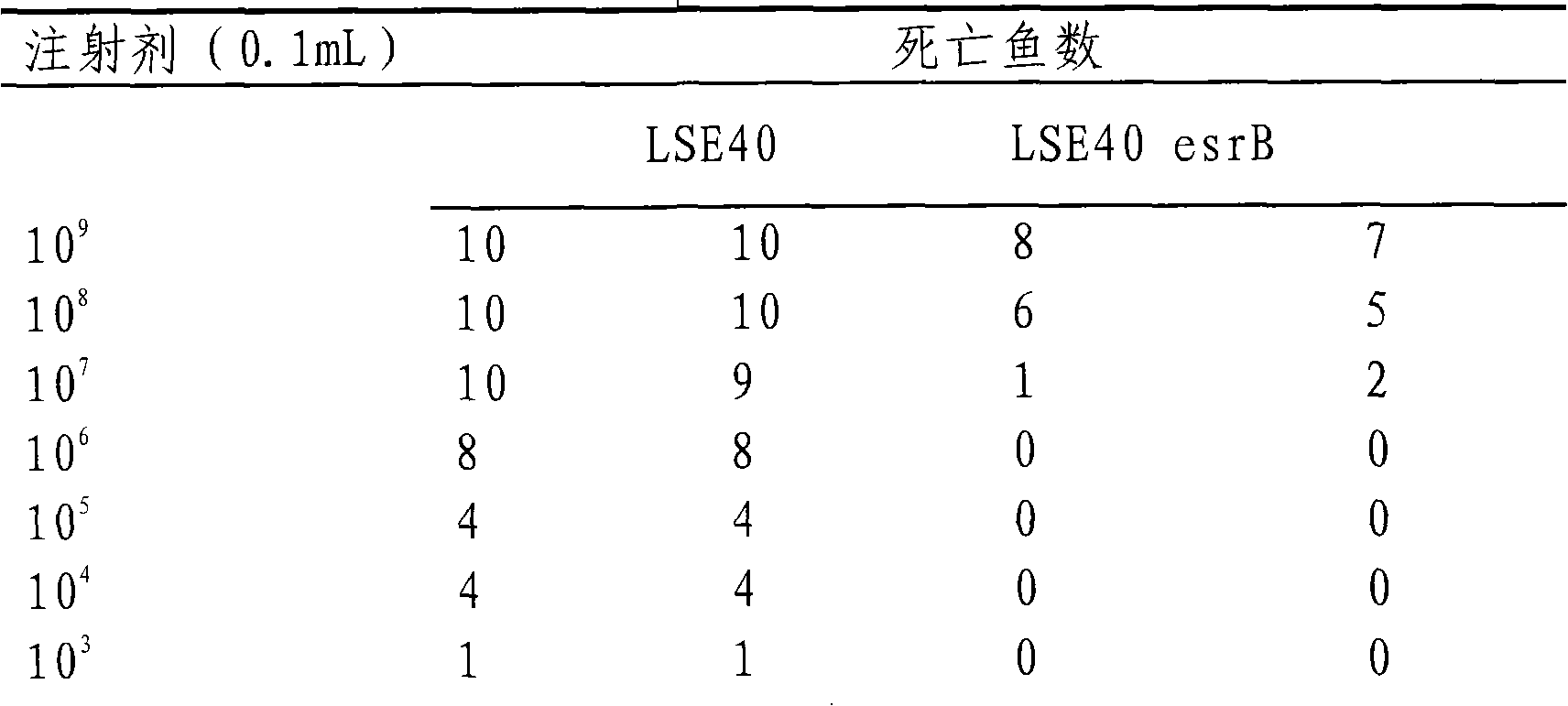
The present invention discloses a technique for preventing and curing the diseases of aquacultural animals, which relates to an attenuated vaccine for aquacultural bacterial diseases, in particular to an attenuated live vaccine for Edwardsiella tarda. The esrB gene of Edwardsiella tarda strain LSE40 loses an attenuated mutant strain, the Edwardsiella tarda mutant strain is MZLSE40esrB, which is preserved in China General Microbiological Culture Collection Center (CGMCC) and the accession number of which is CGMCC No.2087, and the mutant strain MZLSE40esrB does not contain exogenous antibiotic selectable markers and exogenous gene fragments. In respect to the wide type Edwardsiella tarda, the attenuated live vaccine has obviously low toxicity and immune protective rate and does not contain any exogenous antibiotic resistance marker and exogenous gene fragment. Experiments show that the attenuated live vaccine can effectively protect susceptible fishes from being infected with the pathogenic Edwardsiella tarda.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Attenuated Salmonella pullorum and application thereof
InactiveCN102154184ALow toxicityImproving immunogenicityAntibacterial agentsBacteriaHorizontal transmissionMicroorganism
The invention discloses an attenuated Salmonella pullorum and application thereof. By using gene knockout technology, the in vivo colonization gene and the main virulence gene of Salmonella pullorum are deleted, thus obtaining an dual-gene-deleted attenuated Salmonella pullorum SM091-DED strain; and the microbiological preservation number of the strain is CGMCC NO.4604. The virulence of the attenuated Salmonella pullorum disclosed by the invention is obviously reduced, and the in vivo colonization time is short after the attenuated Salmonella pullorum is inoculated into a host; the herd infection test shows that the attenuated Salmonella pullorum has no horizontal transmission capability; the attenuated Salmonella pullorum provides full cross protection for homotype and allotype high-virulence Salmonella pullorum; and after SPF (Specific Pathogen Free) chicks are immunized, the infection of the high-virulence strain can be effectively eliminated, and the herd infection can not be caused. Thus, the invention provides a safe, fine-immunogenicity and low-virulence Salmonellosis avium attenuated live vaccine for preventing Salmonellosis avium.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Herpes simplex virus I-type gene recombinant attenuated live vaccine and preparation method thereof
InactiveCN102657861ARelapse controlControl spreadInactivation/attenuationMicroorganism based processesShuttle vectorWild type
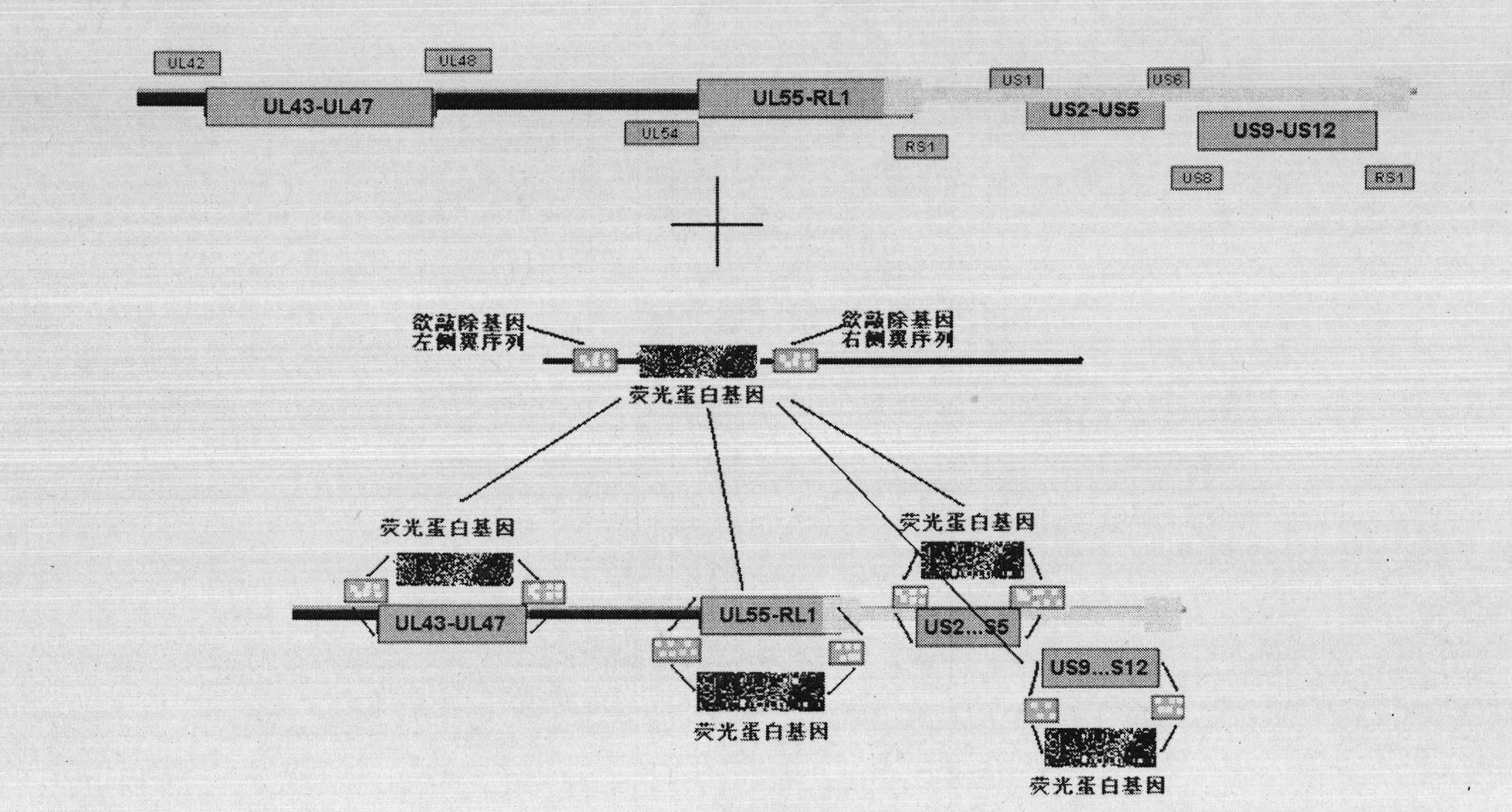
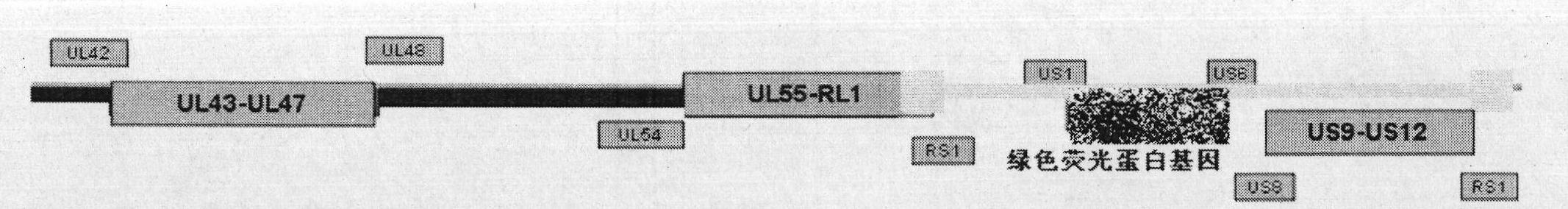
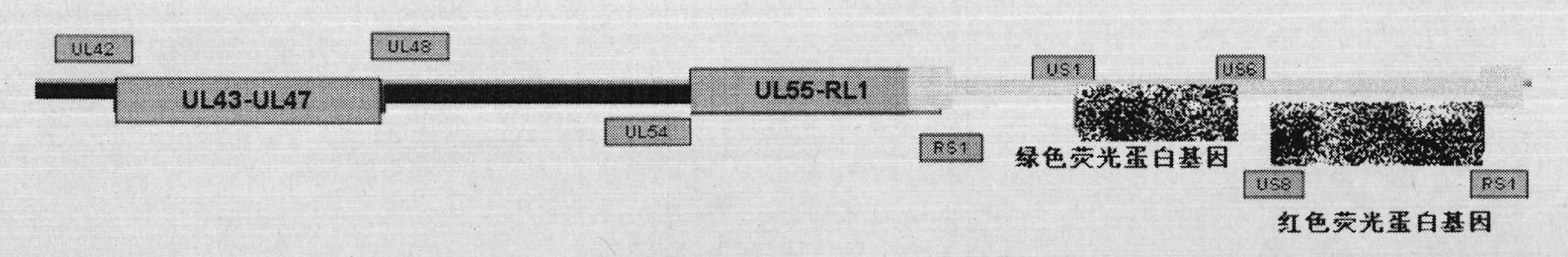
The invention discloses a herpes simplex virus I-type gene recombinant attenuated live vaccine and a preparation method thereof. The attenuated live vaccine is characterized in that: US2, US3, US4 and US5 genes in a genome and other duplicated or infected nonessential gene fragments are jointly knocked out, wherein other duplicated or infected nonessential gene fragments are US9-US12 genes. The method comprises the following steps: separating and identifying wild HSV-1 (herpes simplex virus-1) from blister fluid of a patient with remarkable herpes labialis and herpes progenitalis; propagating and extracting a complete genome of virus; co-transfecting a Vero cell with a shuttle vector containing homologous flanking sequences of 700bp to 2000bp on the left and right sides of a fluorescent expression gene and the HSV gene to be knocked off; and selecting the recombinant virus by using one or combination of multiple fluorescent protein genes serving as a marker under fluorescent microscope, and acquiring the required recombinant attenuated live vaccine after plaque purification.
Owner:郑州金森生物科技工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com