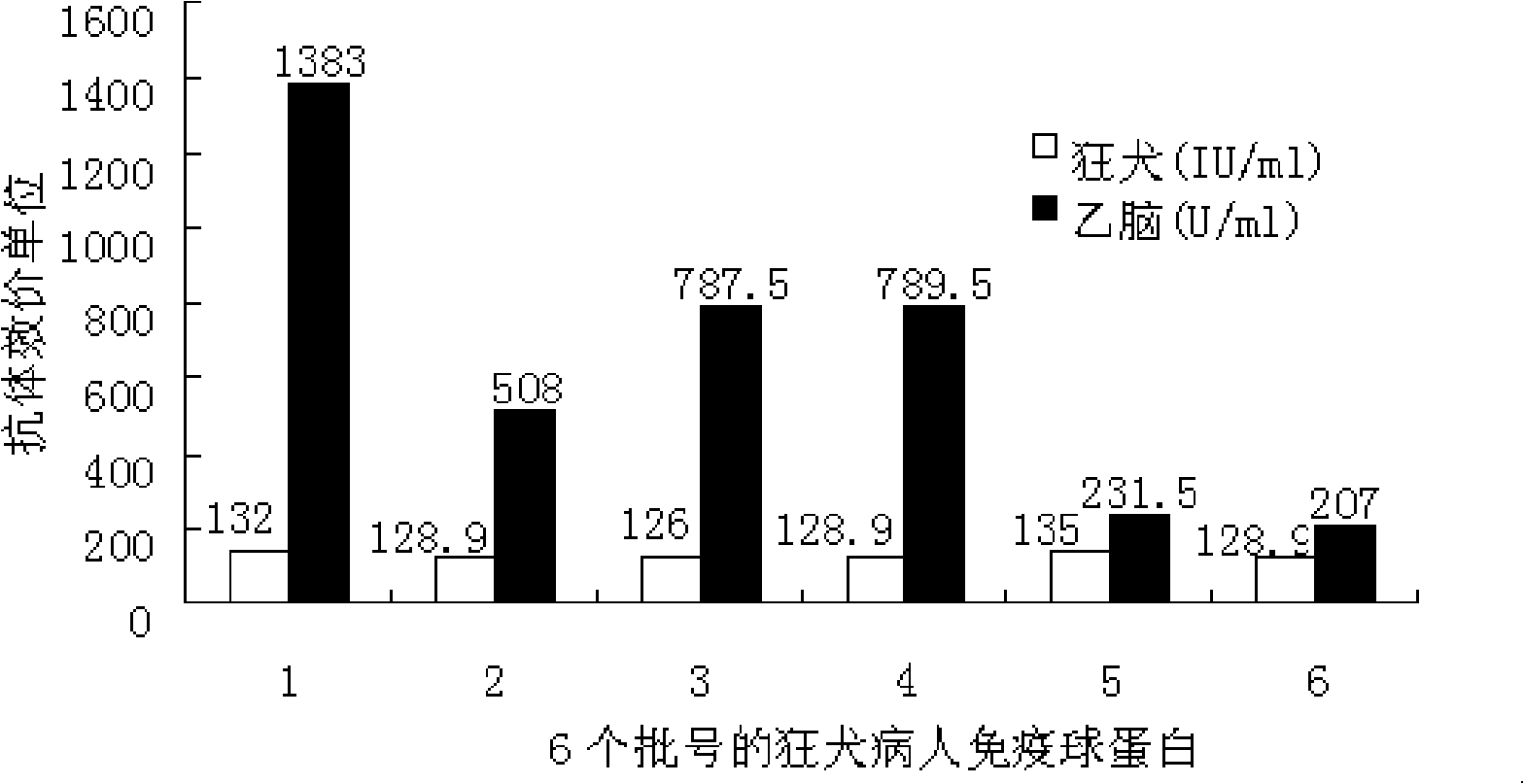
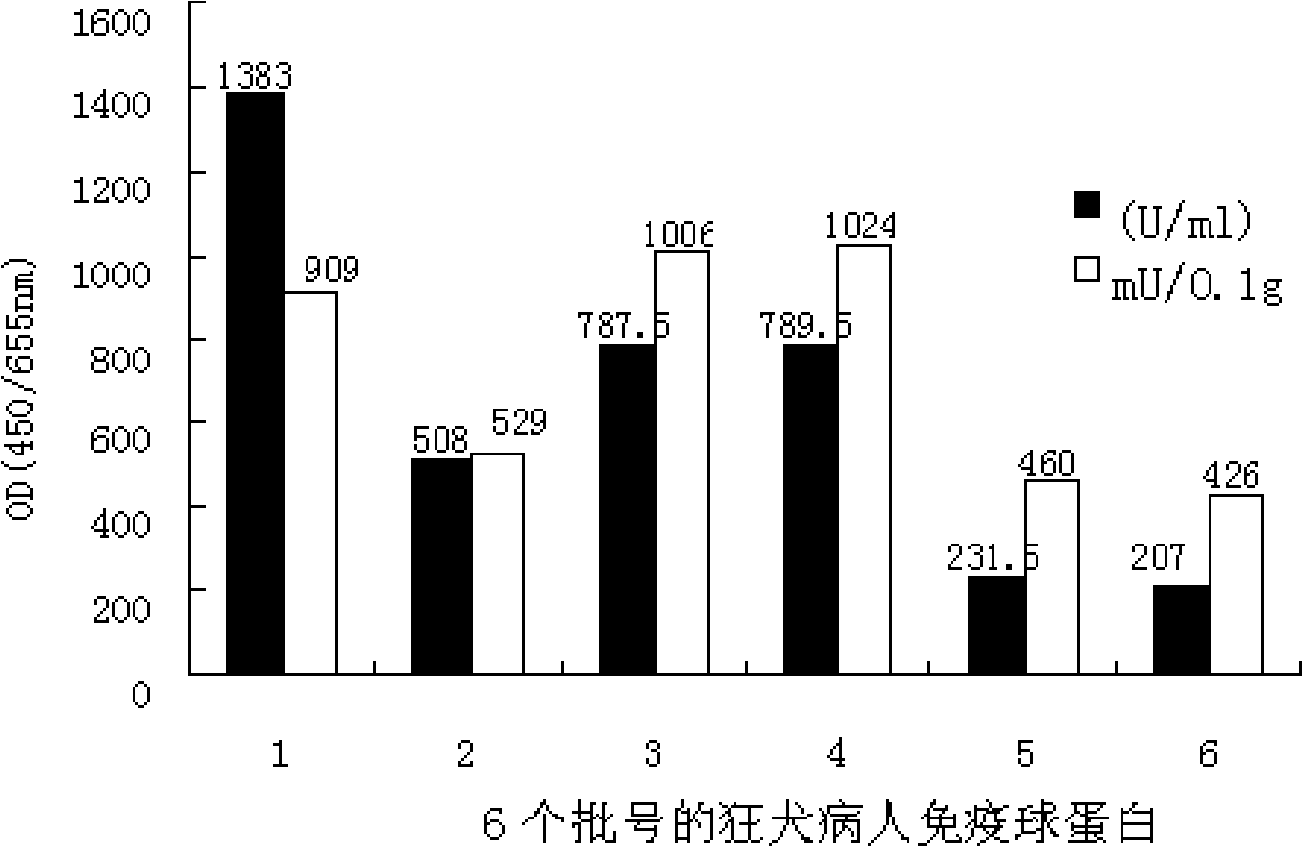
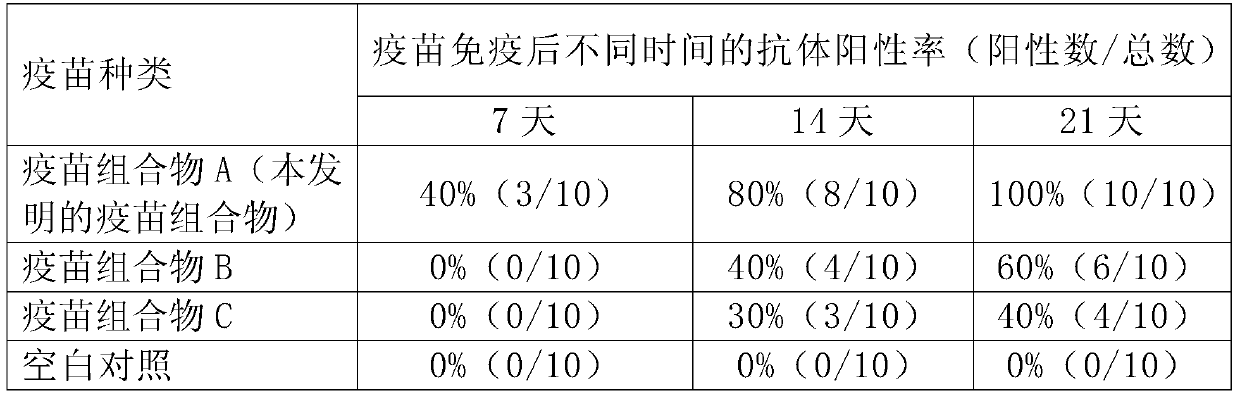
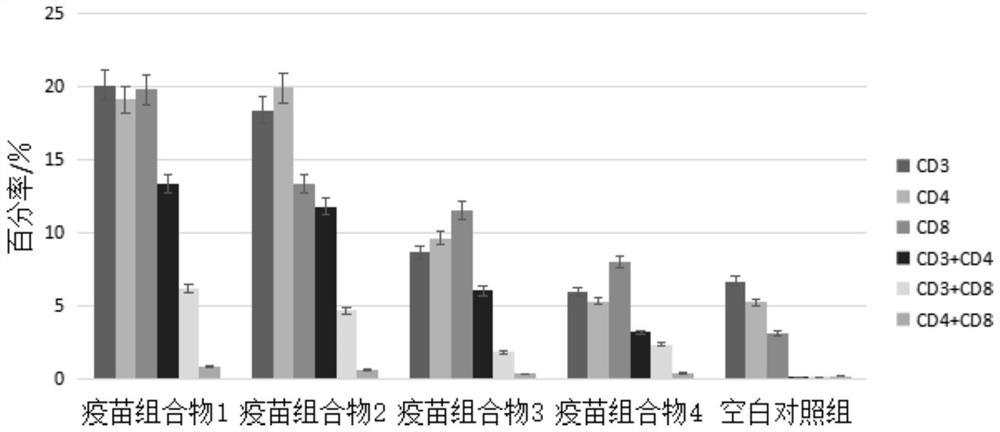
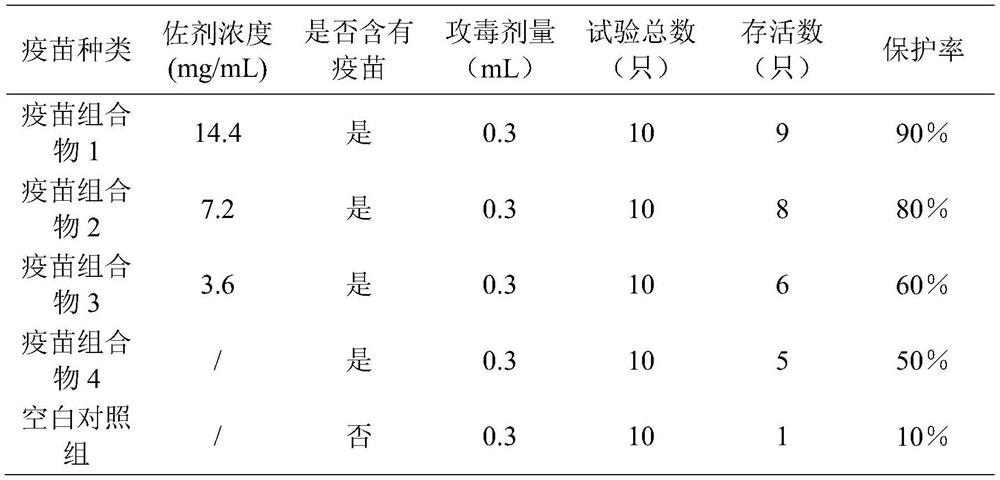
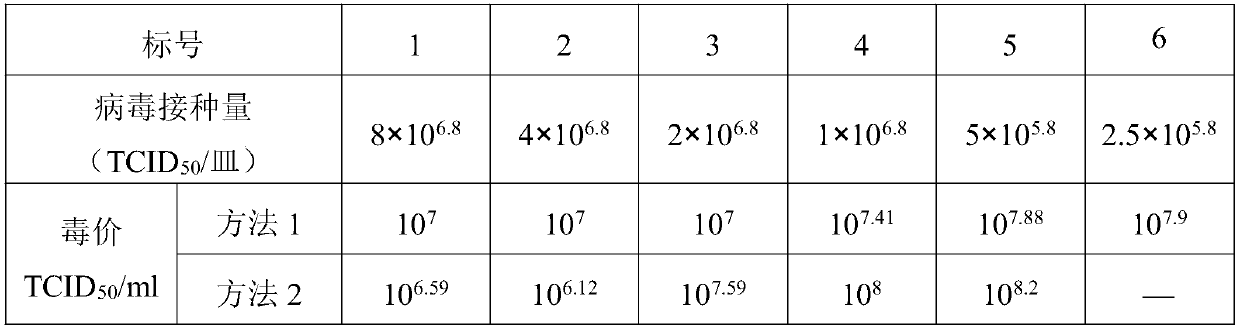
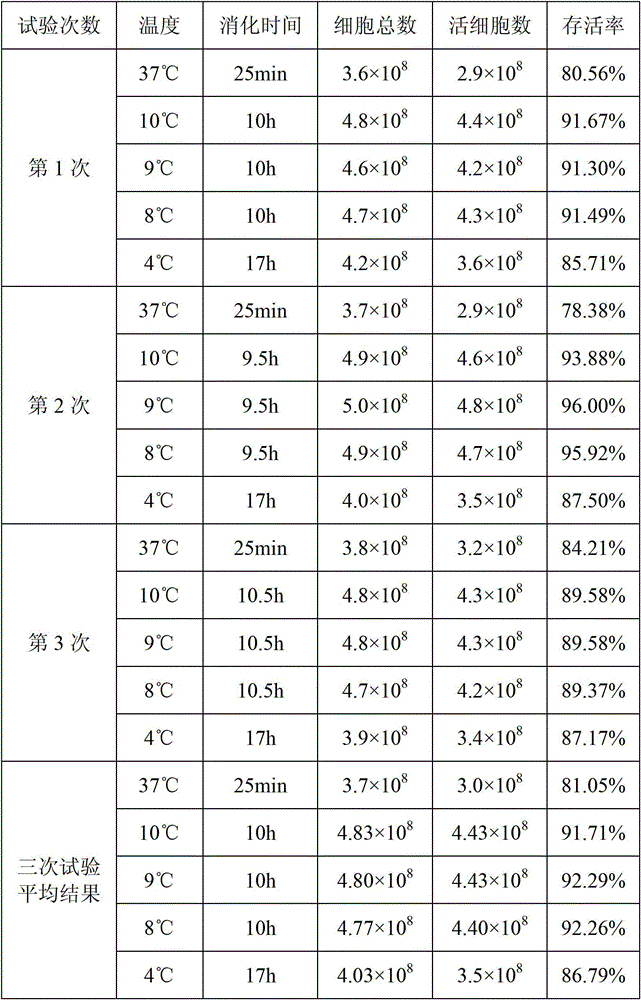
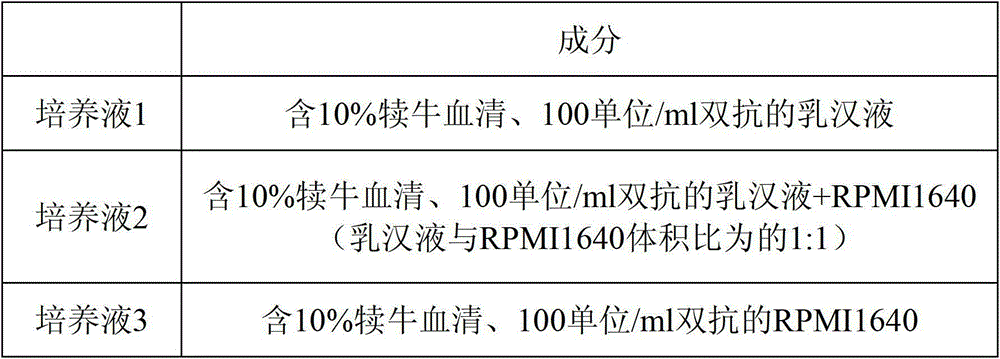
Patents
Literature
34 results about "Japanese encephalitis vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Japanese encephalitis vaccine is a vaccine that protects against Japanese encephalitis. The vaccines are more than 90% effective. The duration of protection with the vaccine is not clear but its effectiveness appears to decrease over time. Doses are given either by injection into a muscle or just under the skin.
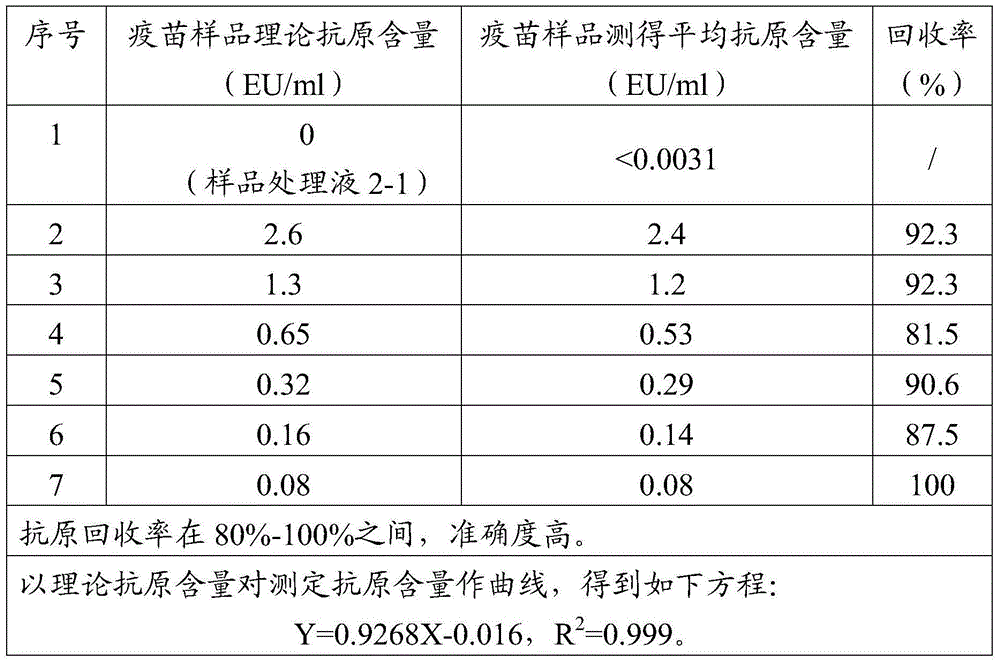
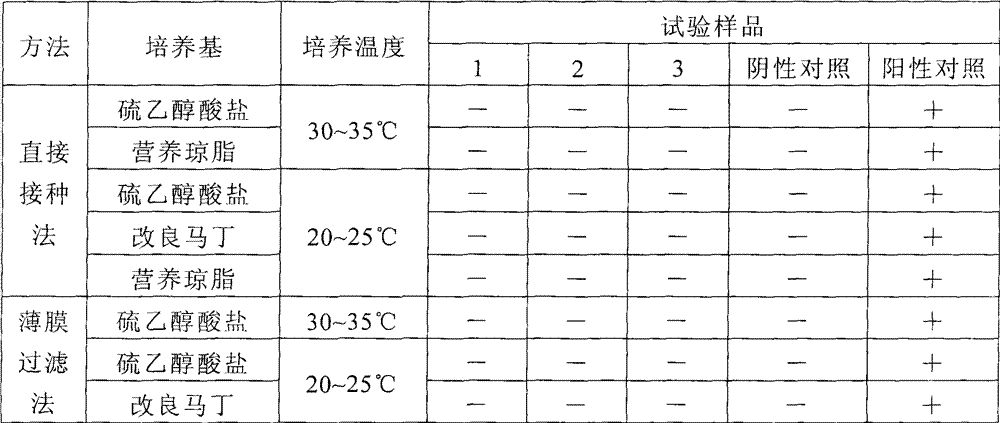
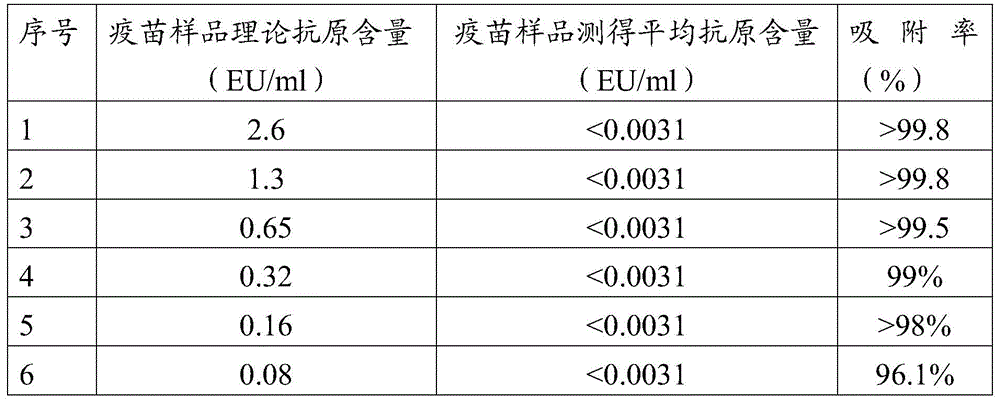
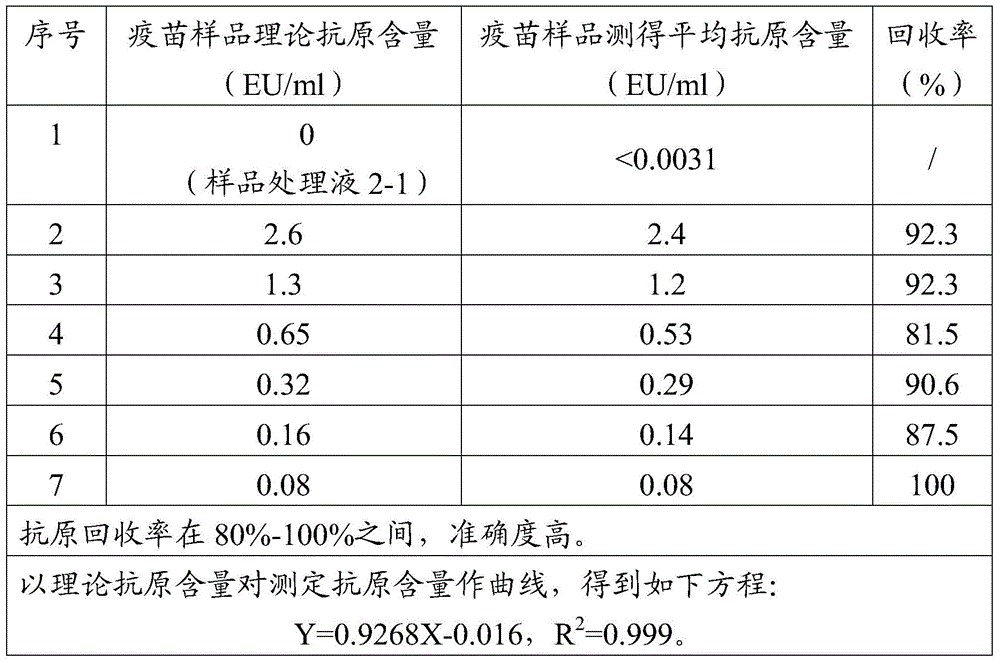
Treatment liquid and method using same to measure antigen content of aluminum salt adsorption type vaccines
ActiveCN104634959AAccurate detectionEfficient detectionMaterial analysisJapanese encephalitis vaccineAntigen
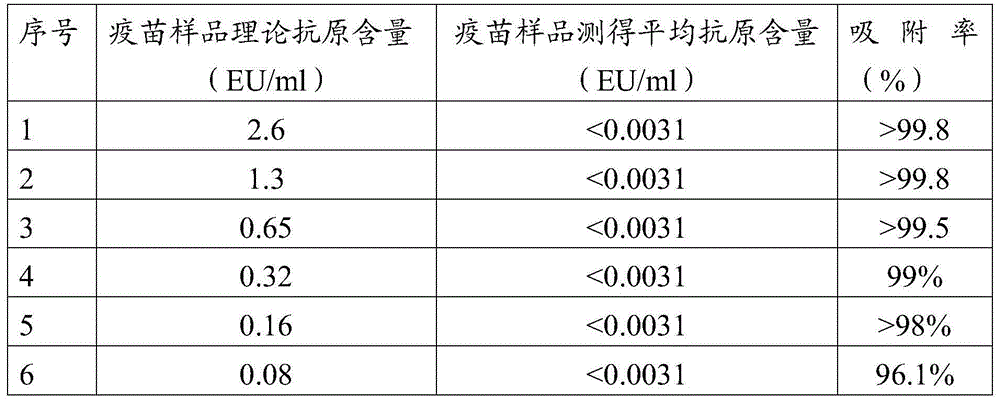
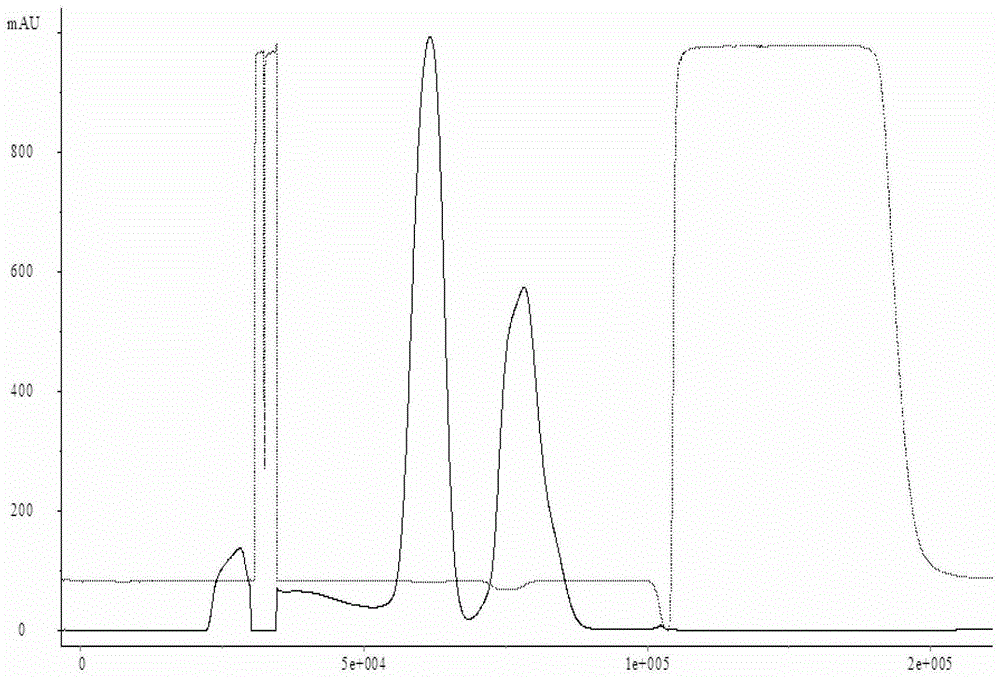
The invention discloses a treatment liquid for desorbing antigens in an aluminum salt adsorption type vaccine. The treatment liquid is a phosphate buffer solution or a citrate buffer solution, wherein the buffer solution contains proteins and at least one acid and / or salts of the acids. The invention further discloses a method using the provided treatment liquid to measure the antigen content of an aluminum salt adsorption type vaccine. The provided method reduces the interference brought by the aluminum adjuvant in Japanese encephalitis vaccine, is capable of rapidly and precisely measuring the antigen content in an adsorption type Japanese encephalitis inactivated vaccine, has the characteristics of good durability, high accuracy, and high precision, and can provide references for quality control of aluminum adjuvant adsorption type Japanese encephalitis inactivated vaccines.
Owner:LIVZON GROUP VACCINE ENG
Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and preparation method thereof
ActiveCN101524536AFully identifiedFully standardizedViral antigen ingredientsAntiviralsImmune effectsJapanese encephalitis vaccine
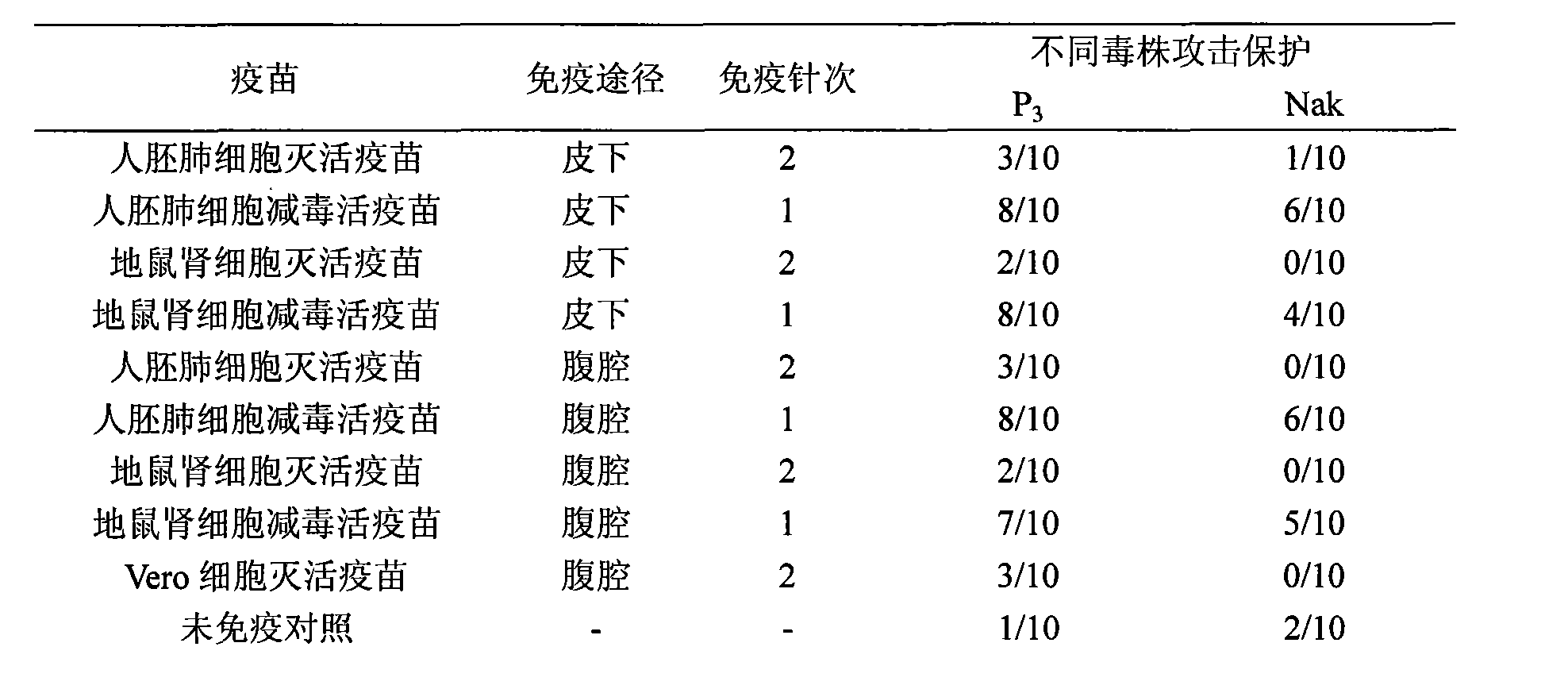
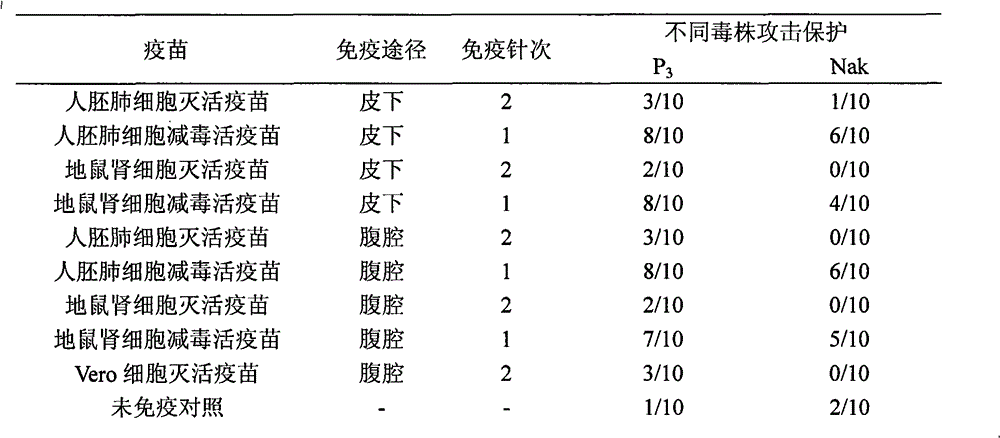
The invention discloses a Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and a preparation method thereof, comprising culture and expansion of the human embryonic lung fibroblasts. The method comprises the following steps: Japanese encephalitis virus strain P3, strain SA14-14-2 or strain Nakayama is naturalized and inoculated to fit the human embryonic lung fibroblasts, and the seeds of Japanese encephalitis viruses are prepared on the human embryonic lung fibroblasts; wherein, an inactivated Japanese encephalitis vaccine also comprises the steps of harvesting viruses, inactivating viruses, concentrating, purifying and the like; an attenuated live vaccine also comprises the steps of harvesting viruses, concentrating, purifying and the like. Due to being prepared by healthy human embryonic lung fibroblasts, the two kinds of Japanese encephalitis vaccines do not contain any adventitious pollution agent and tumorigenicity, has high purity after being purified and has the advantages of good immune effect and high security. The preparation method of the invention is suitable for large-scale industrial production and can meet the preparation processes of Japanese encephalitis vaccines required by domestic and abroad markets.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Diploid somatic cell encephalitis B vaccine and method for preparing purified encephalitis B vaccine
ActiveCN101352569ATotal protein content decreasedImprove immunityViral antigen ingredientsAntiviralsJapanese encephalitis vaccineSerum ige
The invention relates to a preparation method for diploid cell Japanese encephalitis vaccine and purified encephalitis vaccine. The method uses a serum-free medium to culture human diploid cell Japanese encephalitis purified vaccine, thus greatly reducing anaphylactic reaction and being beneficial to purification.
Owner:崔栋
Lyophilized inactivated Japanese encephalitis vaccine
ActiveCN102631672AMeet quality requirementsQuality improvementPowder deliveryViral antigen ingredientsJapanese encephalitis vaccineLactose
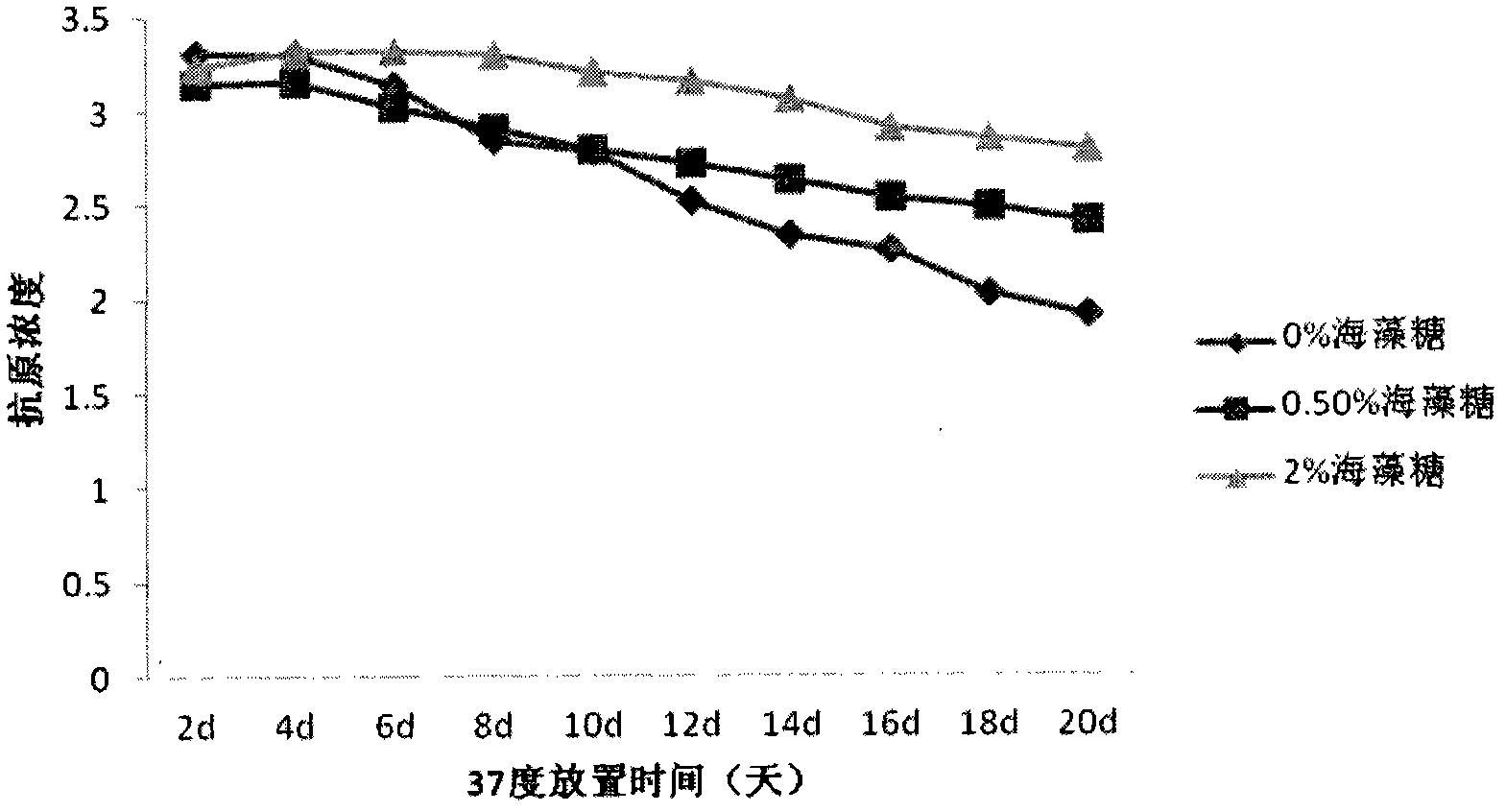
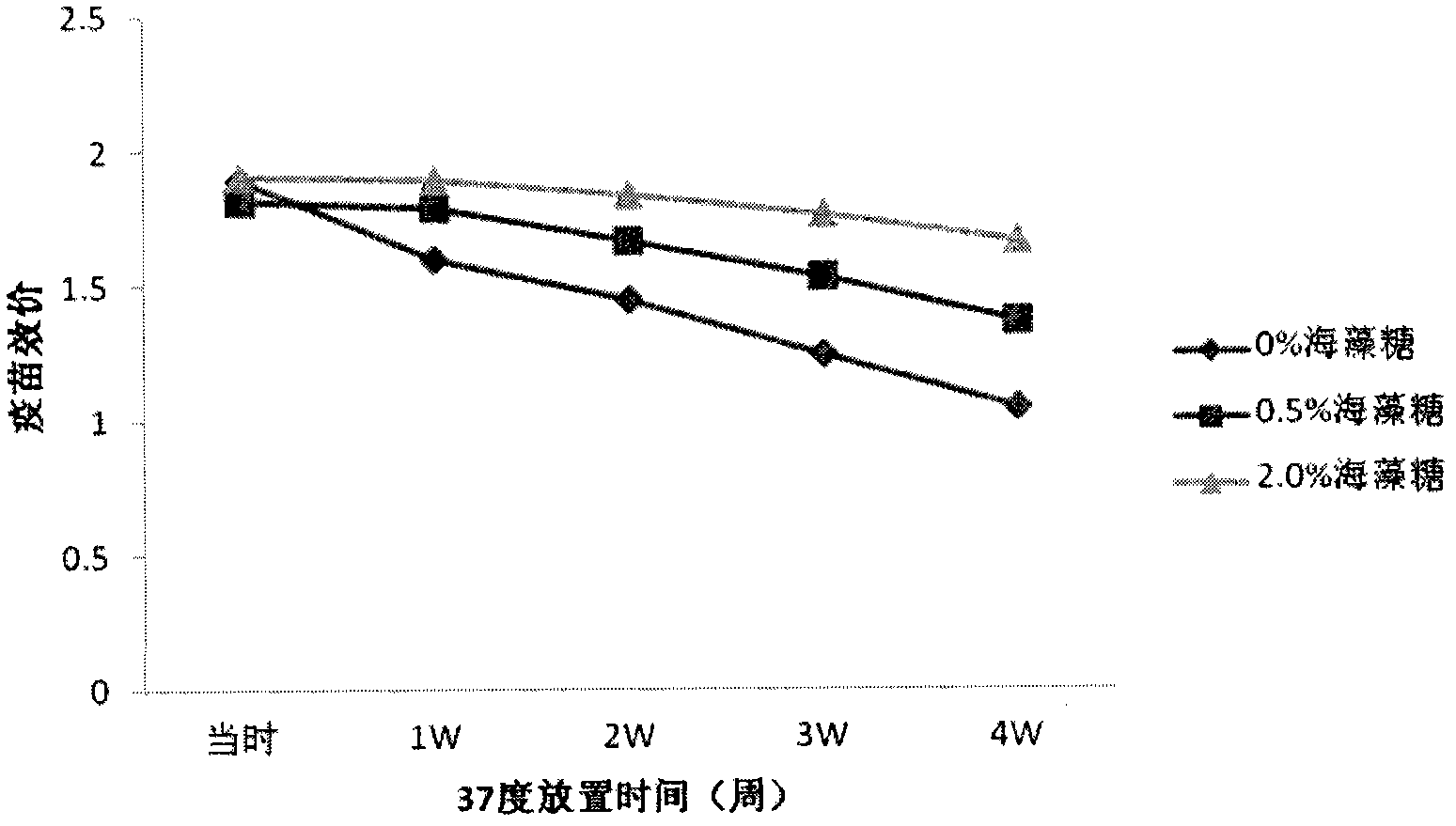
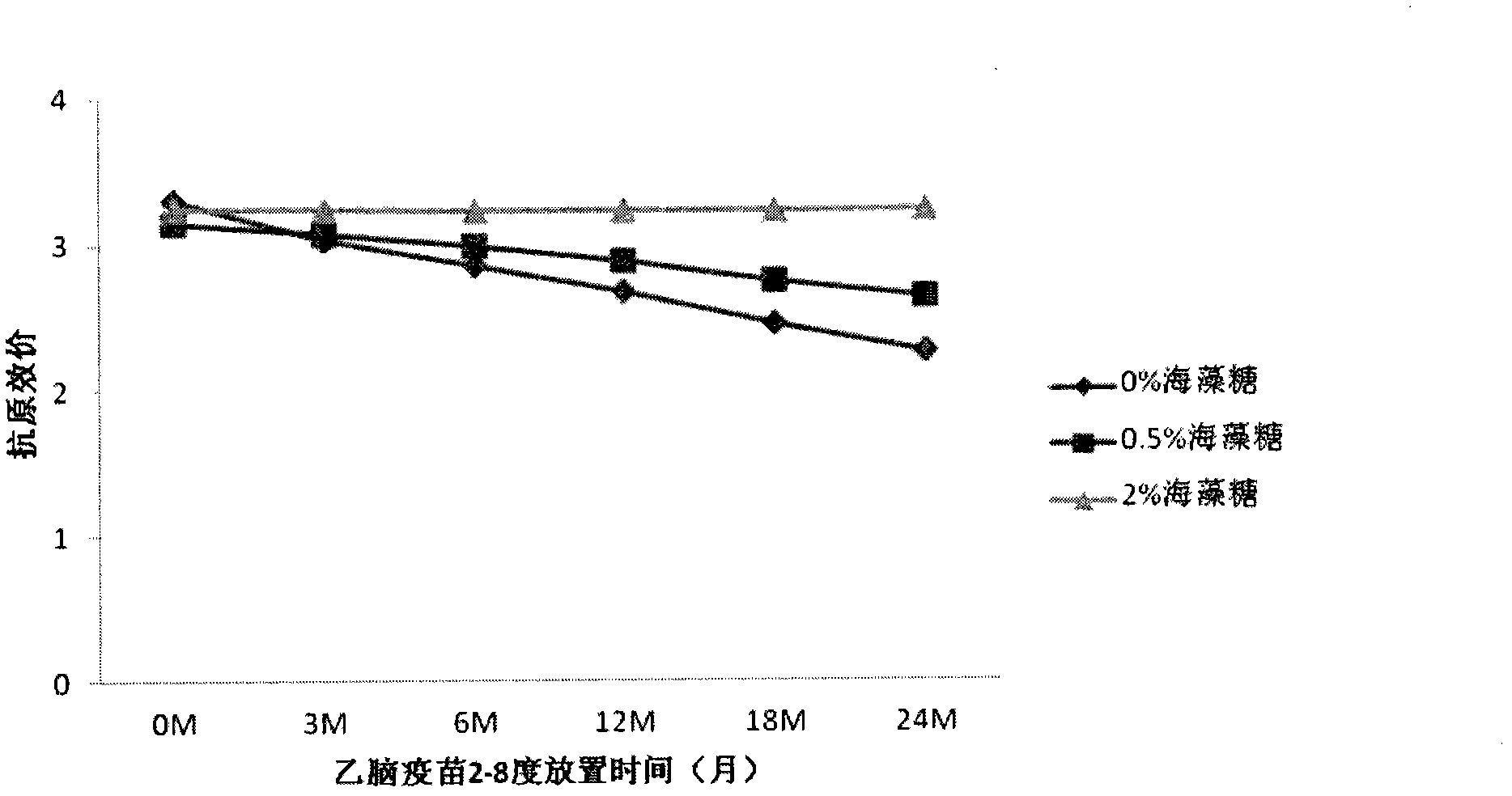
The invention provides a lyophilized inactivated Japanese encephalitis vaccine, comprising: (a) inactivated Japanese encephalitis totivirus; (b) stabilizer, which comprises maltose, lactose, and optionally mycose, mannite, sorbitol and / or amino acid; and optionally (c) buffer, surfactant, isotonic regulator and / or chelating agent.
Owner:天津嘉诚顺隆商贸有限公司
Method of Producing Japanese Encephalitis Vaccine Stably Storable Over Long Time and Use of the Vaccine
ActiveUS20110020393A1Good storage stabilityProcess stabilityAntibacterial agentsSsRNA viruses positive-senseJapanese encephalitis vaccineViral Vaccine
The present inventors improved methods for inactivating Japanese encephalitis virus vaccines, and assessed the safety of vaccines produced by combining multiple vaccines. The present inventors successfully produced safer Japanese encephalitis vaccines by cell culture, which can be stored more stably over a long period than conventional Japanese encephalitis vaccines. Furthermore, it is also expected that the production methods can be used to produce other viral vaccines with excellent storage stability.
Owner:DAIICHI SANKYO CO LTD
Method for preparing positive serum of antibody against porcine Japanese encephalitis virus
ActiveCN105548536ASafe and reliableImproving immunogenicityChemiluminescene/bioluminescenceSerum igeJapanese encephalitis vaccine
The invention provides a method for preparing the positive serum of an antibody against the porcine Japanese encephalitis virus. The method comprises the following steps: vaccine preparation; negative pig screening; immunization; and harvesting and identification of the positive serum. According to the method, a negative pig is basically immunized with a live porcine Japanese encephalitis vaccine (strain SA14-14-2); and then a high-titer inactivated porcine Japanese encephalitis vaccine (strain SA14-14-2) prepared by using concentration and purification technology is used for supplementary immunization so as to prepare the positive serum of the antibody against the porcine Japanese encephalitis virus. The positive serum of the antibody against the porcine Japanese encephalitis virus prepared in the invention avoids the risk of usage of virulent vaccines and has the advantages of high safety compared with preparation of positive serum from virulent Japanese encephalitis vaccines; and compared with serum prepared from mice, the positive serum prepared from the pig has the advantages of a larger harvesting amount of serum, lower cost and prominent large-scale application prospects.
Owner:TIANJIN RINGPU BIO TECH
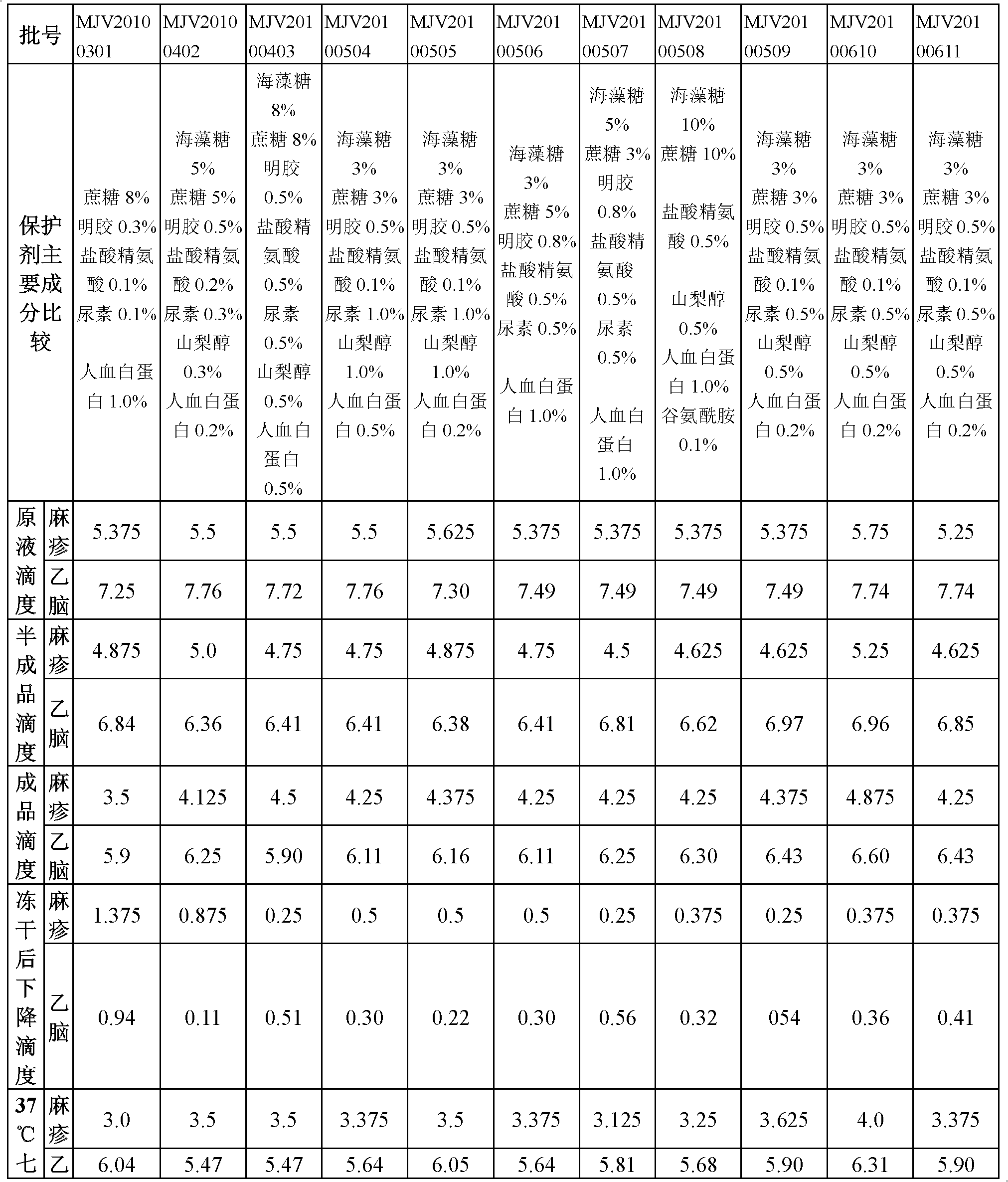
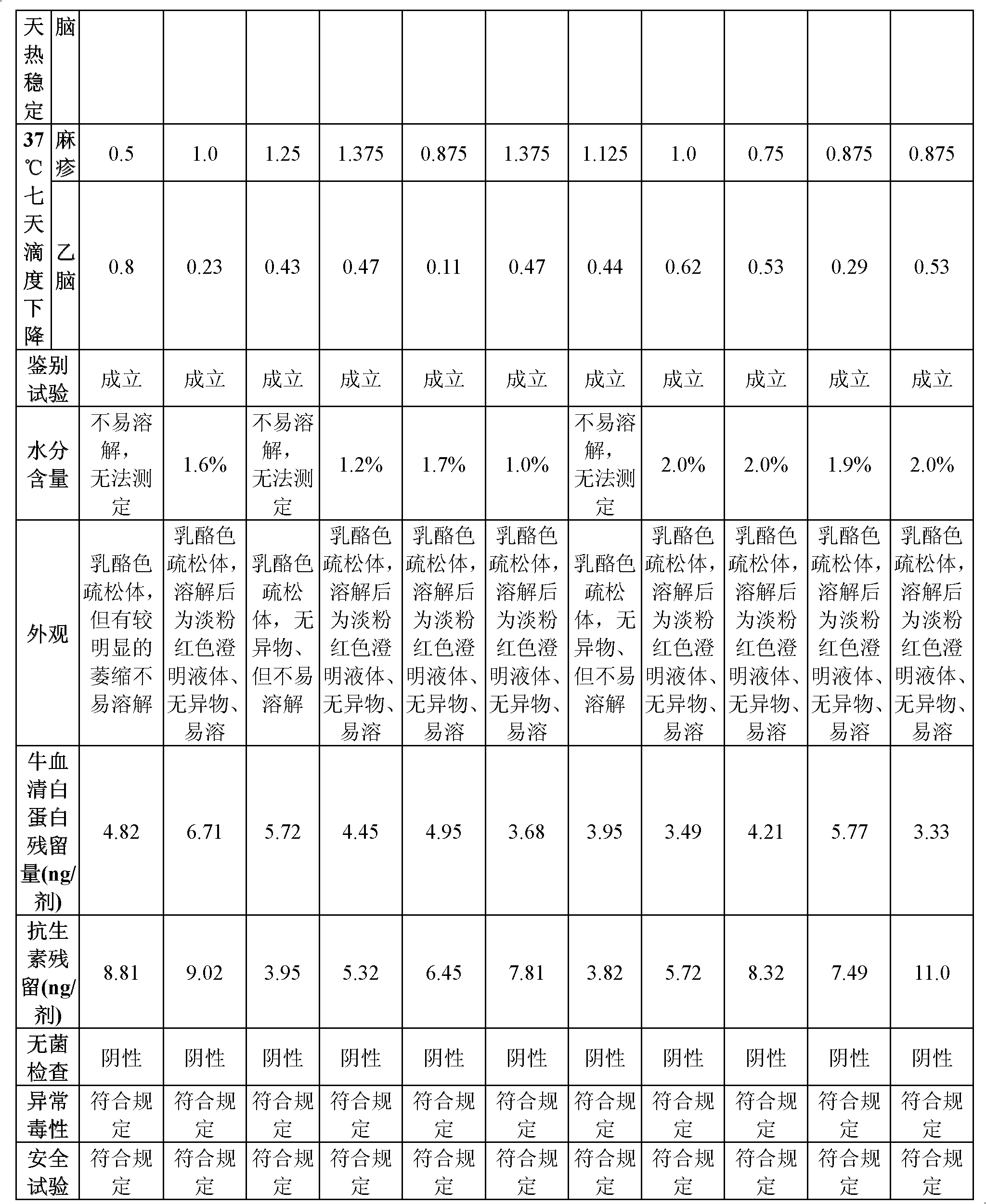
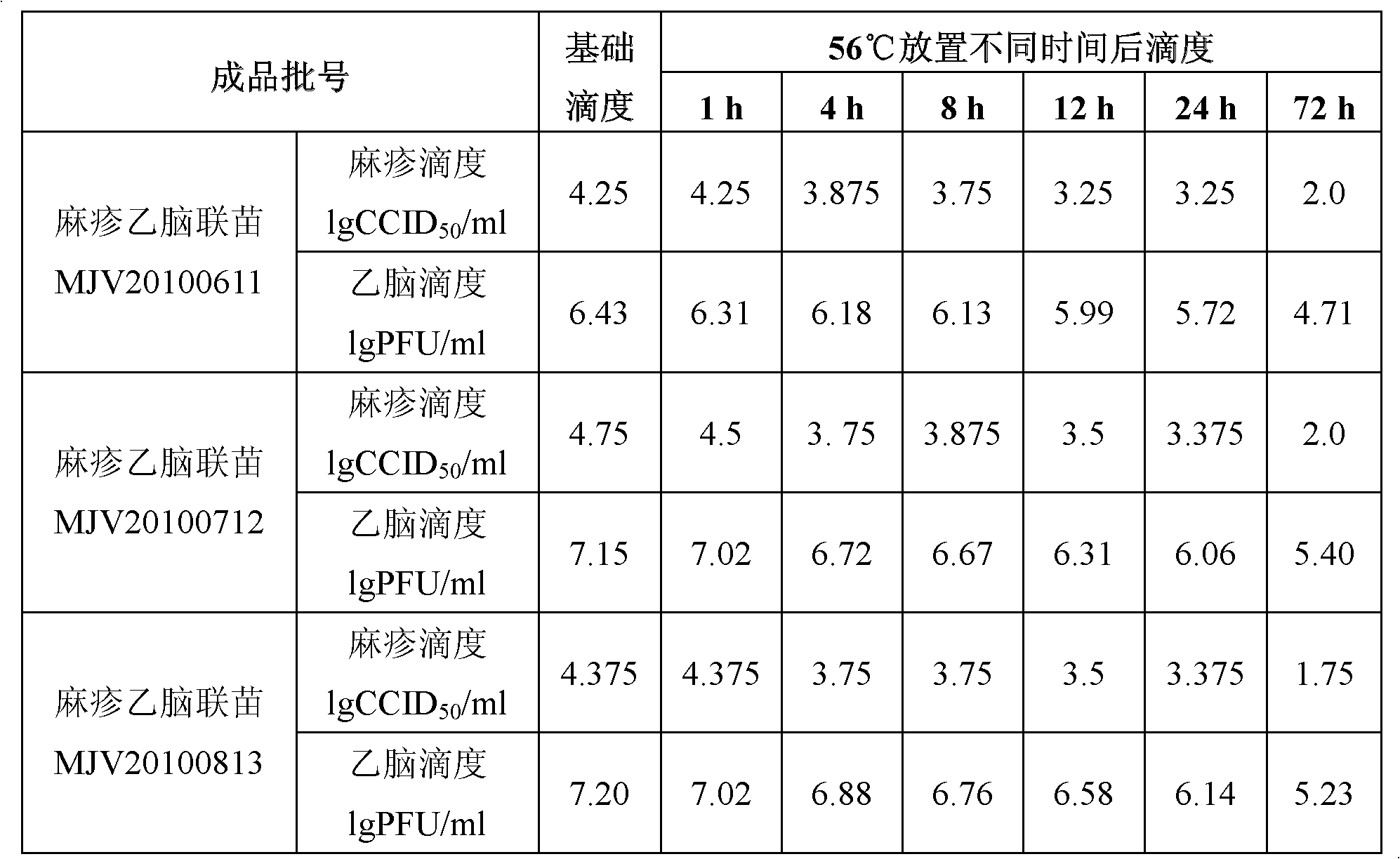
Vaccine protection agent, and combined measles and Japanese encephalitis vaccine and preparation method thereof
ActiveCN102512685AImprove stabilityIncreased sensitizationViral antigen ingredientsAntiviralsJapanese encephalitis vaccineEncephalitis
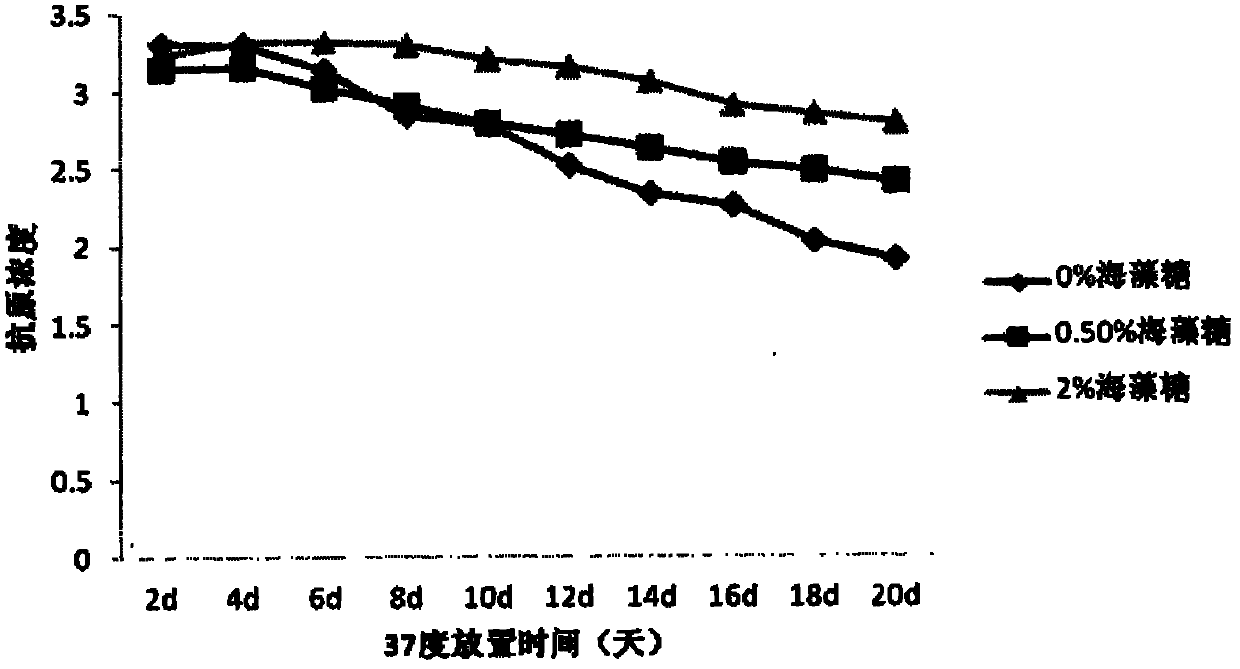
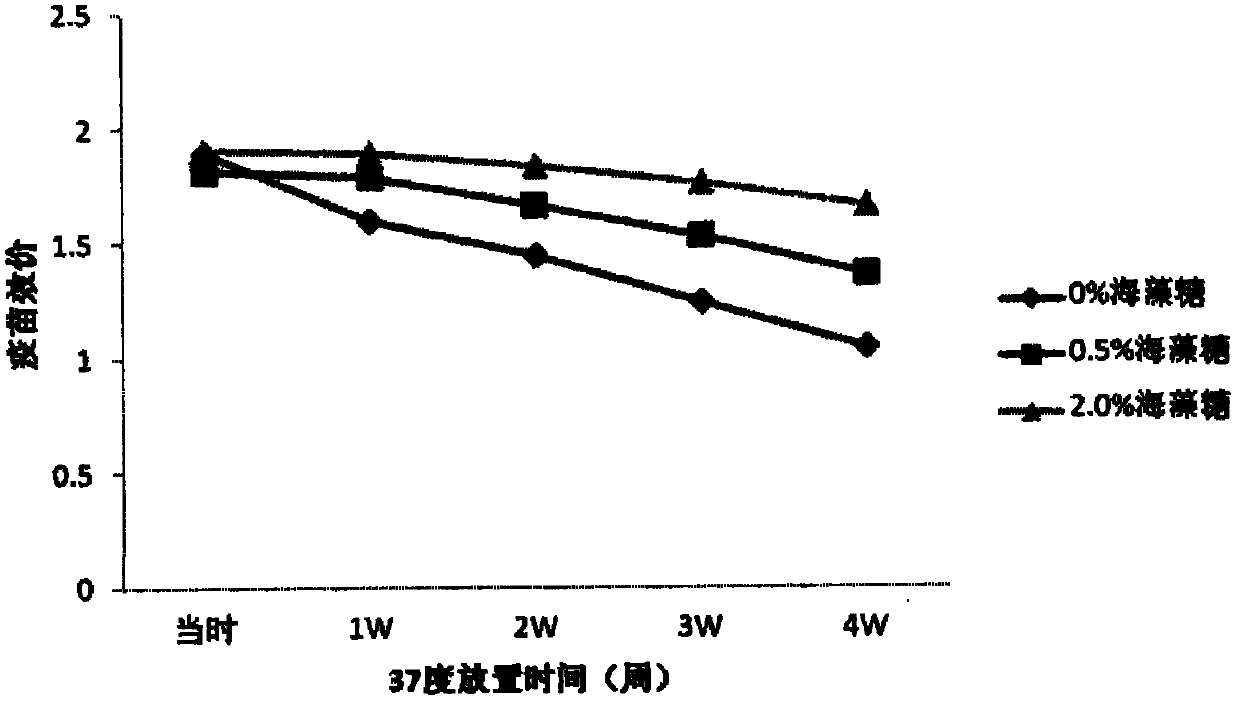
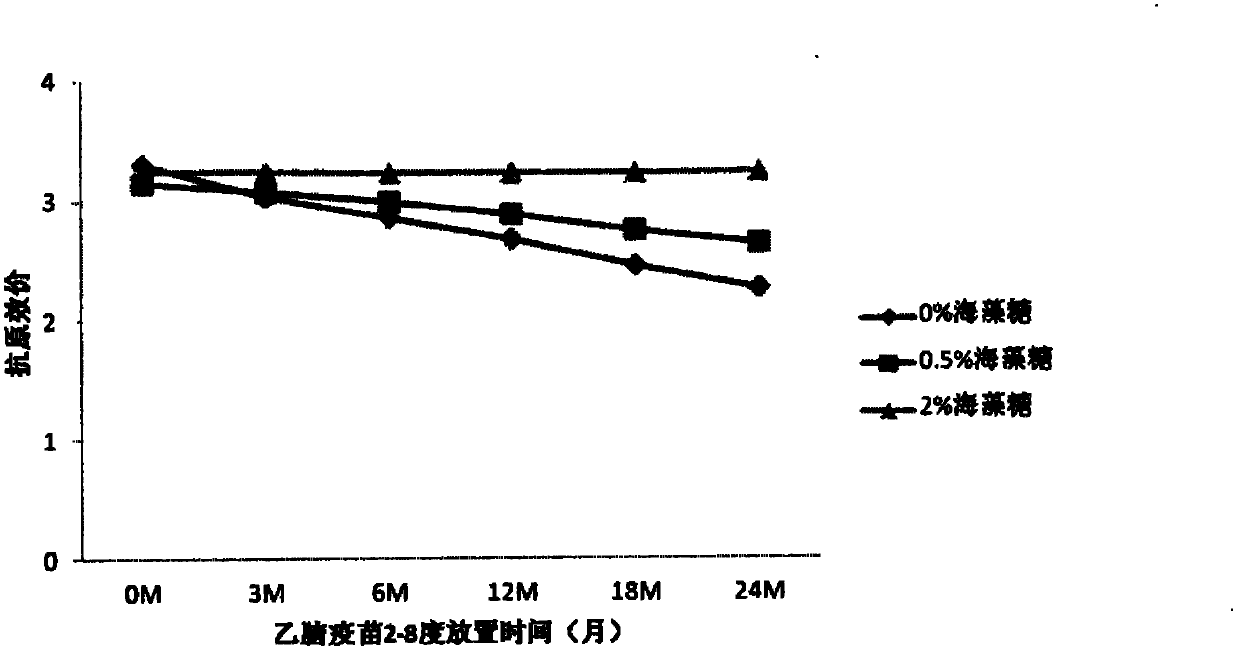
The invention provides a vaccine protection agent which is characterized by comprising the following components by weight: 60 to 200 parts of disaccharides, 3 to 10 parts of gelatin, 0.5 to 10 parts of amino acid, 3 to 10 parts of urea, 3 to 10 parts of sorbitol and 2 to 10 parts of human albumin. The invention also provides a combined measles and Japanese encephalitis vaccine and a preparation method thereof. The vaccine protection agent provided in the invention can effectively enhance stability of the combined measles and Japanese encephalitis vaccine and has a high application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD
New application of rabies human immunoglobulin in preparing medicine for preventing and controlling Japanese encephalitis and combined vaccine for rabies and Japanese encephalitis
InactiveCN101524539AReduce the number of vaccinationsVaccination promotion is easyViral antigen ingredientsAntiviralsJapanese encephalitis vaccineHuman immunoglobulins
The invention belongs to the field of biological products, in particular to a new application of the existing product-rabies human immunoglobulin. The technical proposal of the invention is the new application of rabies human immunoglobulin in preparing medicine for preventing and controlling Japanese encephalitis. The invention also provides a combined vaccine for rabies and Japanese encephalitis. The vaccine of the invention has better effect than conventional Japanese encephalitis vaccine in preventing and controlling Japanese encephalitis and fundamental immunity for resisting the rabies virus is obtained. The combined vaccine features scientific immunization schedule, few times of vaccine inoculation, easy promotion of inoculation and good effect in boostering immunization in case of emergency in preventing and controlling the rabies. The antibody and the vaccine jointly solve the technical and application problems of preventing and controlling the Japanese encephalitis and the rabies, thus enjoying great social and economic significance.
Owner:魏宪义
High-titer genotype I Japanese encephalitis virus strain and application thereof
InactiveCN105543179AHigh growth titerGood antigenicitySsRNA viruses positive-senseViral antigen ingredientsJapanese encephalitis vaccineGenotype
The invention relates to a high-titer genotype I Japanese encephalitis virus strain. The high-titer genotype I Japanese encephalitis virus strain is characterized in that the high-titer genotype I Japanese encephalitis virus strain is preserved in the China Center for Type Culture Collection with the preservation number of CCTCC NO: V201526. The invention further discloses a genotype I Japanese encephalitis vaccine. The genotype I Japanese encephalitis virus strain is high in growth titer and antigenicity, the valence of an antibody generated by the vaccine prepared with the virus strain is high, protective efficacy is high, safety performance is high, and clinical application prospects are broad.
Owner:SICHUAN AGRI UNIV
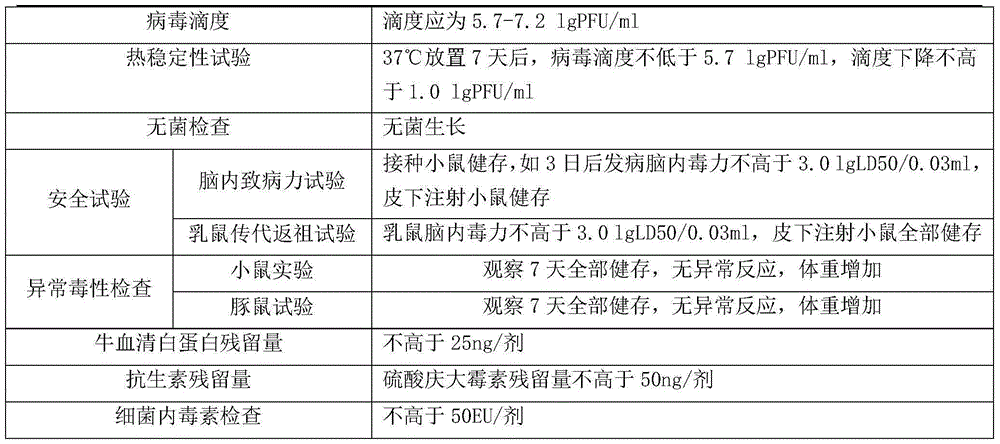
Method for preparing inactivated Japanese encephalitis vaccine by bioreactor
InactiveCN102949715AQuality improvementImproved oxygen deliveryViral antigen ingredientsMicroorganism based processesAdjuvantCytopathic effect
The invention provides a method for preparing inactivated Japanese encephalitis vaccine by a bioreactor. The method includes by the aid of the bioreactor and a Cytodex-1 microcarrier system, subjecting high-density Vero cells adaptive to Japanese encephalitis virus P3-strain to virus infection by utilizing the P3-strain Japanese encephalitis virus with good antigenicity; according to multiplication conditions of the Japanese encephalitis virus P3-strain on the Vero cells, harvesting virus liquid after the cytopathic effect reaches a certain degree so as to increase virus titer from 105-6PFU / ml to 107-8PUF / ml at least and increase virus content by 10 times at least; and inactivating harvest virus liquid, adding additives, and then packing to obtain high-quality vaccine products. The prepared vaccine is safe and effective, production process is stable, and production quality is controllable.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
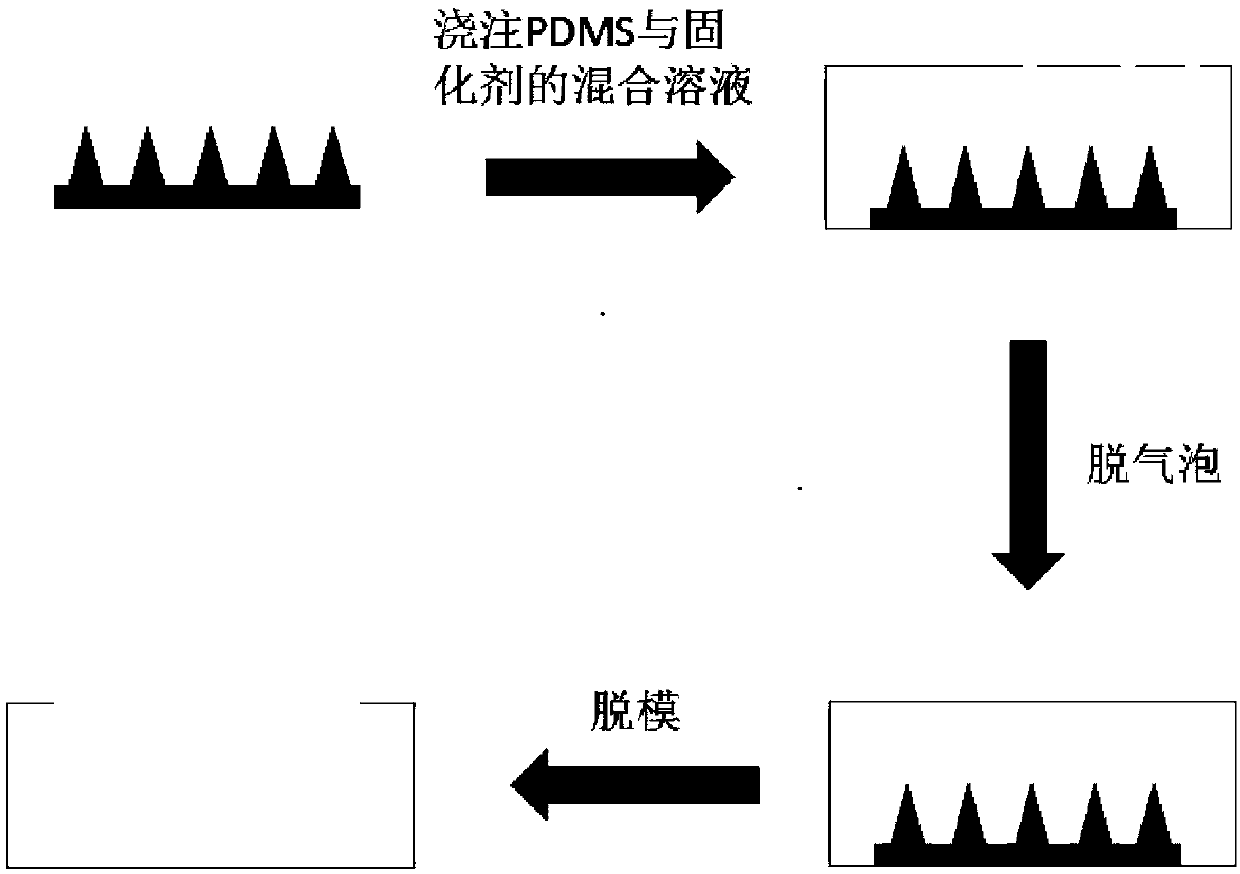
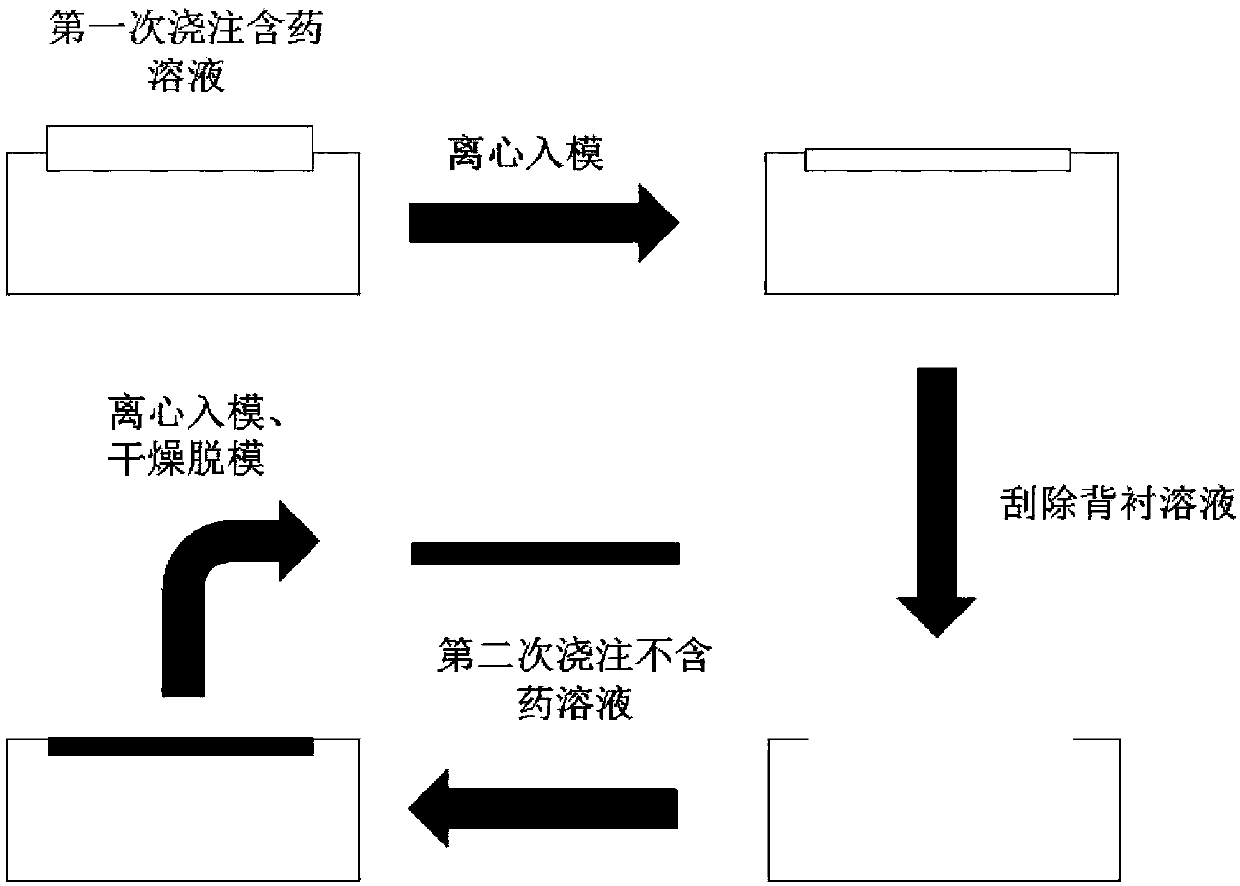
JE vaccine soluble microneedle patch and preparation method thereof
PendingCN110680911AGood immune boosting effectModerate mechanical propertiesSsRNA viruses positive-senseViral antigen ingredientsJapanese encephalitis vaccineImmune effects
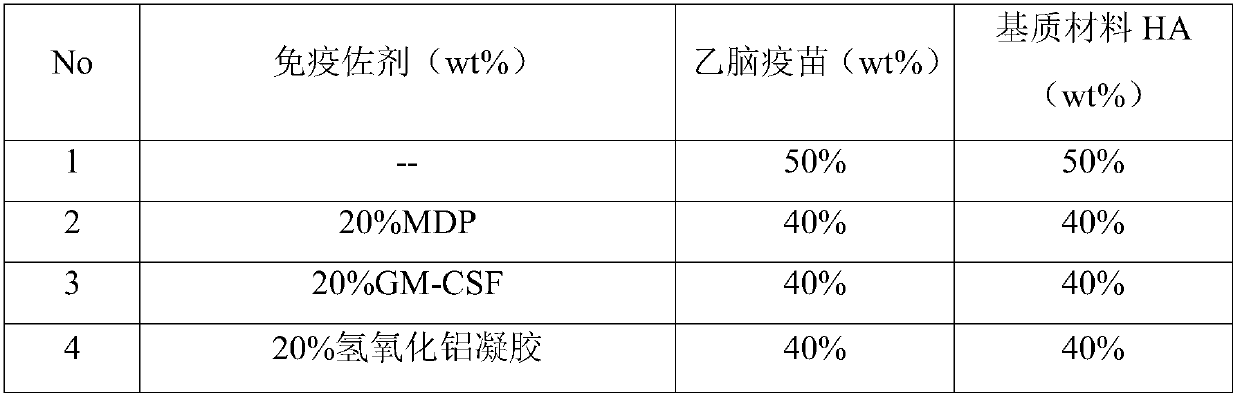
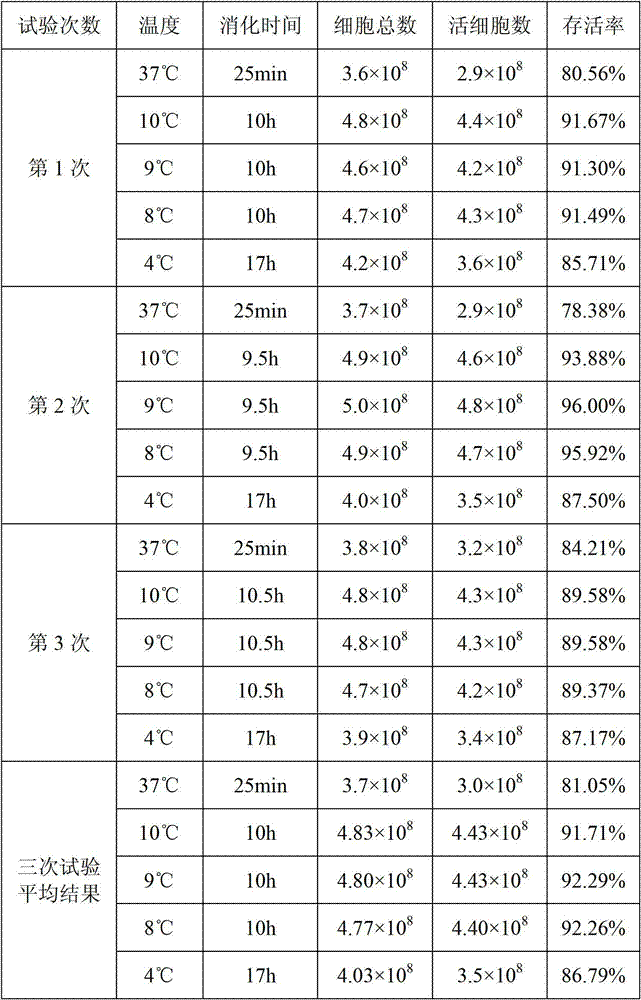
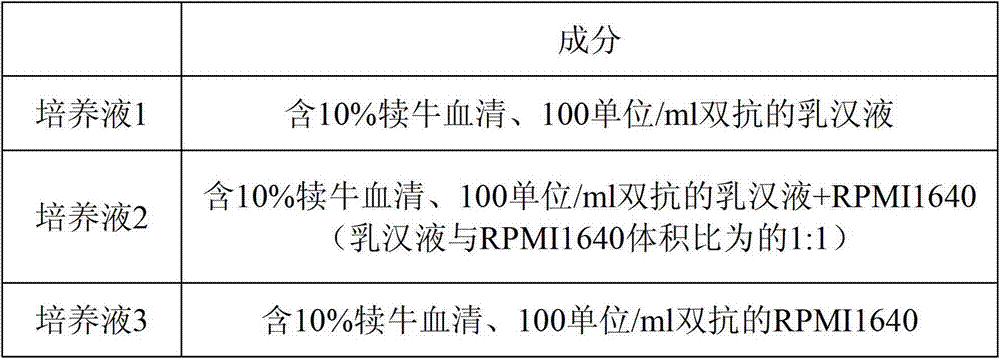
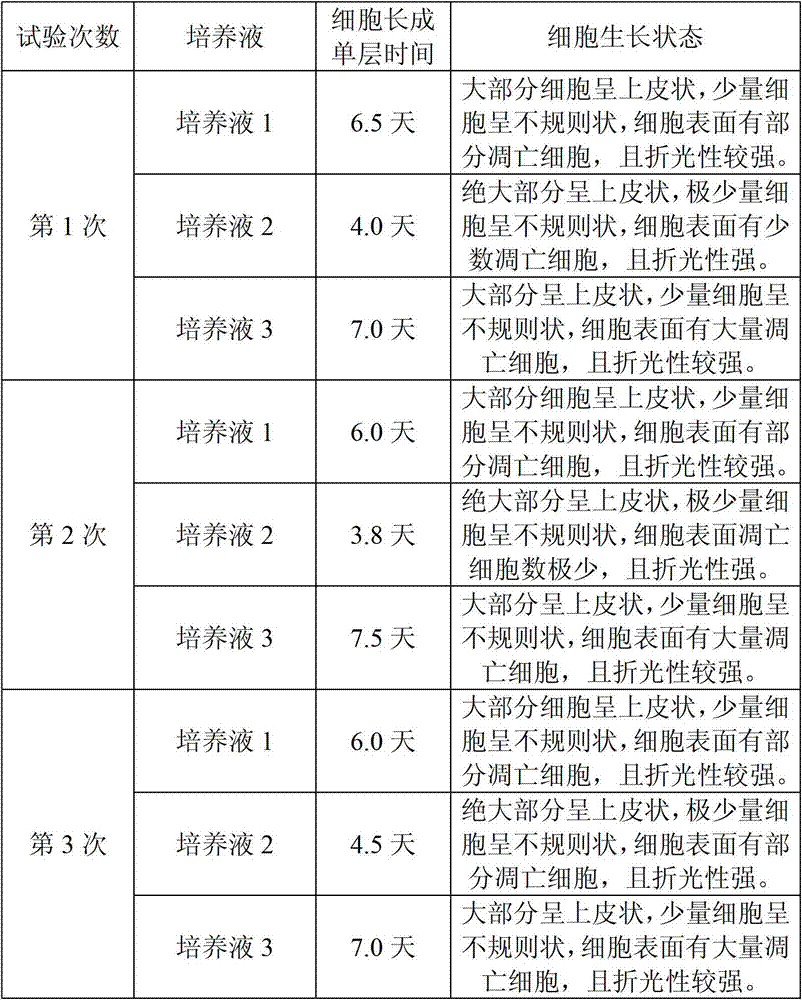
The invention provides a JE (Japanese encephalitis) vaccine soluble microneedle patch, which comprises a needle body and a backing. The needle body is composed of a JE vaccine, a matrix material and an adjuvant, wherein the adjuvant is a mixture of MDP and GM-CSF; and the content ratio of the adjuvant to the Japanese encephalitis vaccine is (1: 1) to (1: 3). The JE vaccine soluble microneedle prepared in the invention has good immune effect and high mechanical strength of the needle body, and is expected to replace a JE vaccine injection and realize painless minimally invasive administration.
Owner:LIAONING CHENGDA BIOTECH
Method for improving Japanese encephalitis virus titer
ActiveCN103160477AHigh potencyImproving immunogenicityViral antigen ingredientsAntiviralsJapanese encephalitis vaccineCytopathic effect
The invention provides a method for proliferation of a Japanese encephalitis virus. The method for proliferation of the Japanese encephalitis virus comprises the steps of culturing cells, inoculating Japanese encephalitis viruses to the cells which are provided with mono-layers in an overgrowing mode, and directly obtaining a virus solution until a rate of a cytopathic effect reaches to 75%-95% after inoculation. The invention further provides a preparation method of Japanese encephalitis vaccines. The method for proliferation of the Japanese encephalitis virus and the preparation method of the Japanese encephalitis vaccines can remarkably improve a Japanese encephalitis virus titer, and meanwhile shorten production periods, and can prepare the Japanese encephalitis vaccines which are better, stable, and good in immunogenicity.
Owner:HUNAN SINOLAND BIOLOGICAL PHARMA
Inactivated Japanese encephalitis vaccine freeze-drying preparation for injection and preparation method thereof
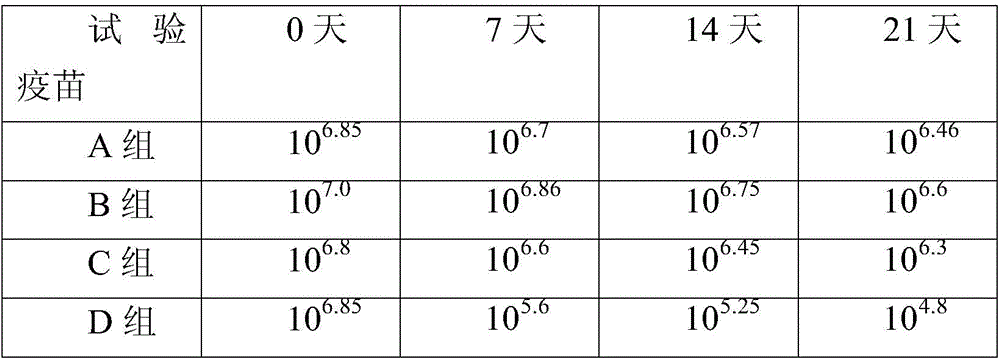
PendingCN109395074AImprove thermal stabilityLong validity periodPowder deliverySsRNA viruses positive-senseJapanese encephalitis vaccineVaccine Production
The invention belongs to the technical field of vaccine production processes and particularly relates to an inactivated Japanese encephalitis vaccine freeze-drying preparation for injection and a preparation method thereof. The inactivated Japanese encephalitis vaccine freeze-drying preparation for injection is prepared from an inactivated Japanese encephalitis vaccine and a freeze-drying protection agent, wherein the freeze-drying protection agent is one of human serum albumin, trehalose, lactose and maltose, and a surfactant is Tween 20 or 80. The freeze-drying protection agent in an inactivated Japanese encephalitis vaccine freeze-drying product can shorten the vaccine re-dissolving time, further improve the heat stability of the inactivated Japanese encephalitis vaccine and accordinglyprolong the useful life of the inactivated Japanese encephalitis vaccine.
Owner:LIAONING CHENGDA BIOTECH
A kind of freeze-dried preparation of Japanese encephalitis vaccine
ActiveCN108175854BSimple compositionControl activity loss ratePowder deliverySsRNA viruses positive-senseBiotechnologyJapanese encephalitis vaccine
The invention discloses a freeze-dried preparation of JE vaccine. The preparation contains JE vaccine stock solution and a vaccine stabilizer, and the vaccine stabilizer is composed of human serum albumin and mannitol. The beneficial effects of the present invention are: (1) The ingredients in the auxiliary materials are simple in composition, without ingredients derived from animal sources, and the risk is lower. (2) The vaccine activity loss rate in the freeze-drying process is effectively controlled. (3) After the preparation is made into a finished product, the product shows good stability through stability research.
Owner:GUANGZHOU GALAXY SUN BIOLOGICAL PROD CO LTD
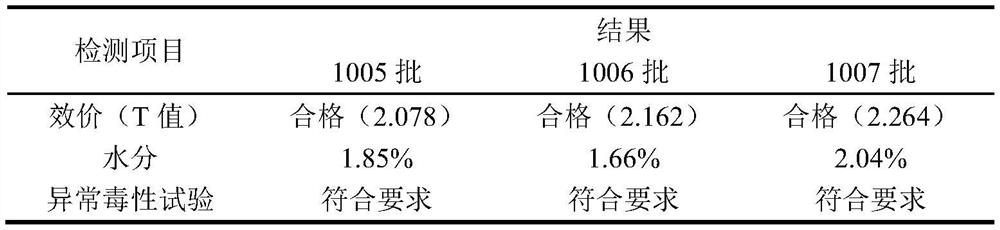
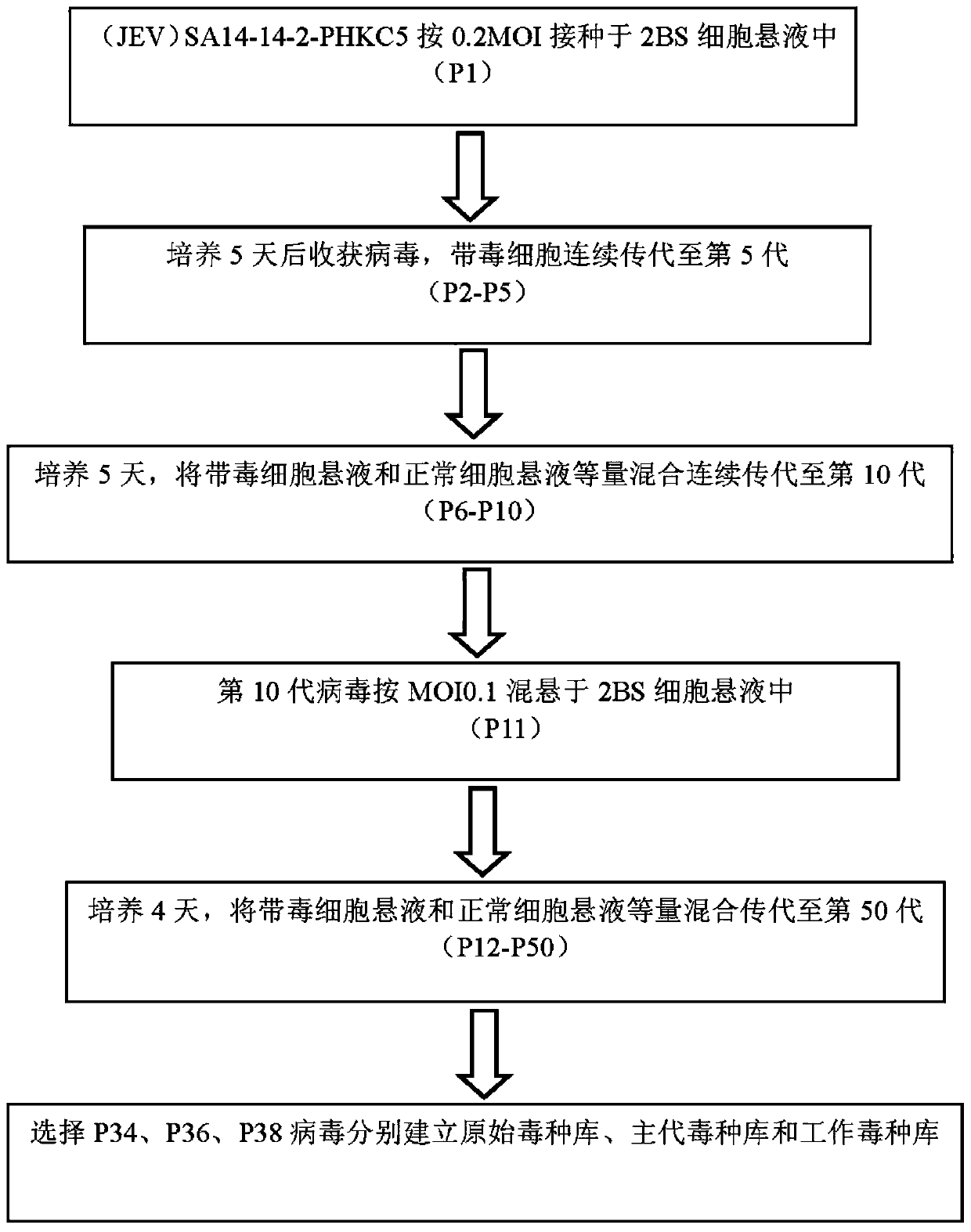
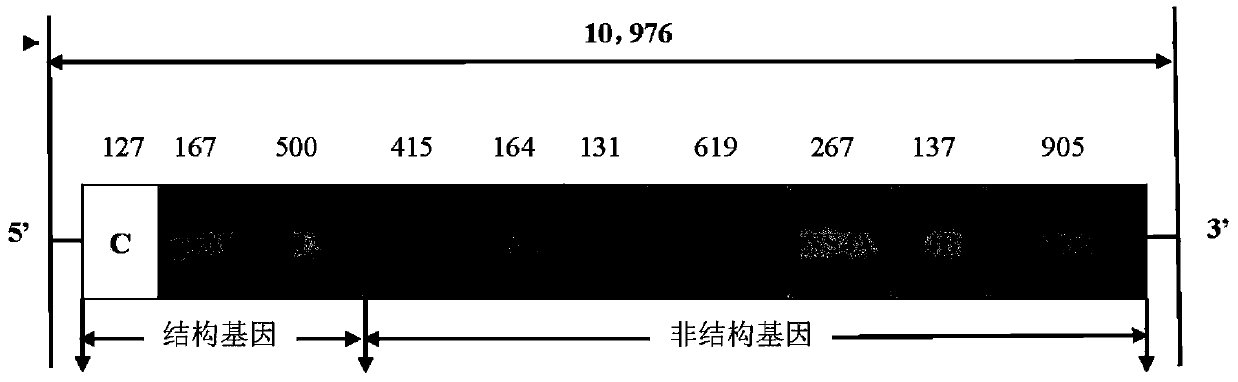
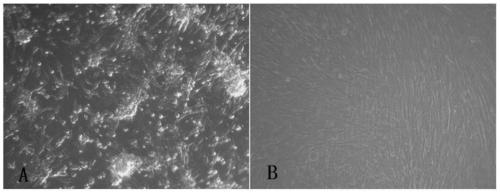
Live attenuated Japanese encephalitis vaccine strain sa14-14-2 adapted to human diploid cell 2bs and its vaccine
ActiveCN105695424BEasy to adaptHigh titerSsRNA viruses positive-senseViral antigen ingredientsThree levelJapanese encephalitis vaccine
Owner:NAT INST FOR FOOD & DRUG CONTROL
Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and preparation method thereof
ActiveCN101524536BProve safety and effectivenessRealize the cell seed bank systemViral antigen ingredientsAntiviralsJapanese encephalitis vaccineImmune effects
The invention discloses a Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and a preparation method thereof, comprising culture and expansion of the human embryonic lung fibroblasts. The method comprises the following steps: Japanese encephalitis virus strain P3, strain SA14-14-2 or strain Nakayama is naturalized and inoculated to fit the human embryonic lung fibroblasts, and the seeds of Japanese encephalitis viruses are prepared on the human embryonic lung fibroblasts; wherein, an inactivated Japanese encephalitis vaccine also comprises the steps of harvesting viruses, inactivating viruses, concentrating, purifying and the like; an attenuated live vaccine also comprises the steps of harvesting viruses, concentrating, purifying and the like. Due to being prepared by healthy human embryonic lung fibroblasts, the two kinds of Japanese encephalitis vaccines do not contain any adventitious pollution agent and tumorigenicity, has high purity after being purified and has the advantages of good immune effect and high security. The preparation method of the invention is suitable for large-scale industrial production and can meet the preparation processes of Japanese encephalitis vaccines required by domestic and abroad markets.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
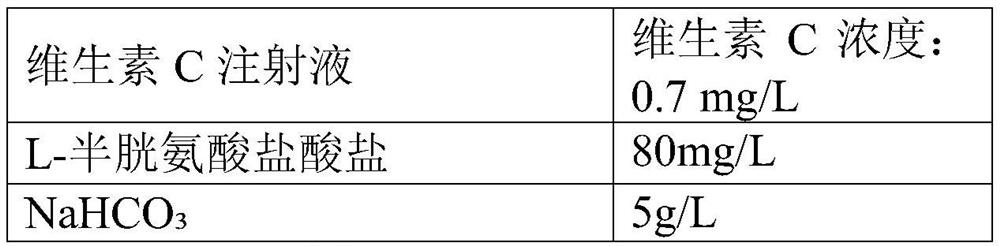
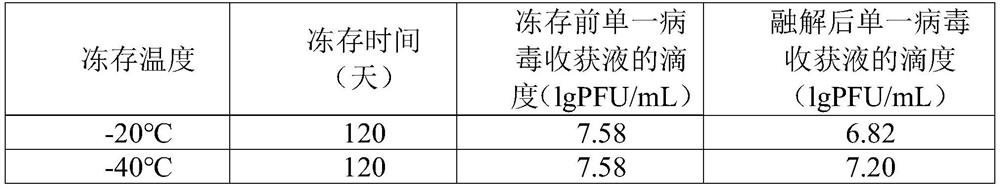
Freeze-thaw method for single virus harvest
ActiveCN113943713BReduce the risk of obsolescenceExtended storage timeSsRNA viruses positive-senseMicroorganism based processesJapanese encephalitis vaccineFreeze and thaw
The invention discloses a method for freezing and thawing a single virus harvest liquid, which belongs to the field of vaccine preparation technology. The freezing and thawing method includes the following steps: (1) cryopreservation: freezing a single virus harvest solution; (2) thawing: thawing the single virus harvest solution after freezing in step (1). Freezing and thawing the single virus harvest solution of the live attenuated Japanese encephalitis vaccine under the freeze-thaw method of the present invention can enable the single virus harvest solution to be stored for a long time under the condition of ≤-60°C, and then put into the next step after all the verification items are completed. Use, for the preparation of Japanese encephalitis live attenuated vaccine. By combining the single virus harvest solution of the Japanese encephalitis live attenuated vaccine of the present invention with the freezing-thawing method of the present invention, the storage time can be significantly prolonged, and the loss of virus titer can also be significantly reduced, and there is no need to carry out the risk release of intermediate products. The arrangement is more flexible and controllable, and the risk of a single virus harvest liquid being discarded due to changes in production plans is greatly reduced.
Owner:CHENGDU INST OF BIOLOGICAL PROD
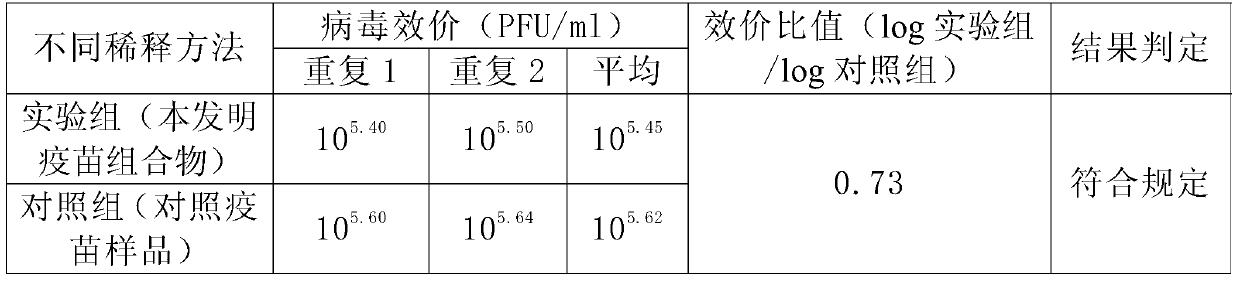
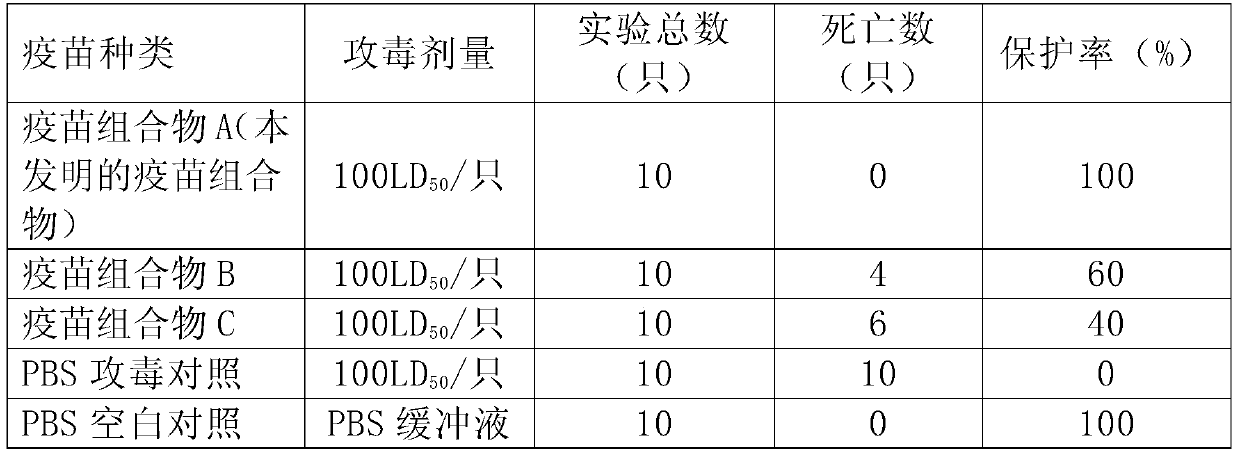
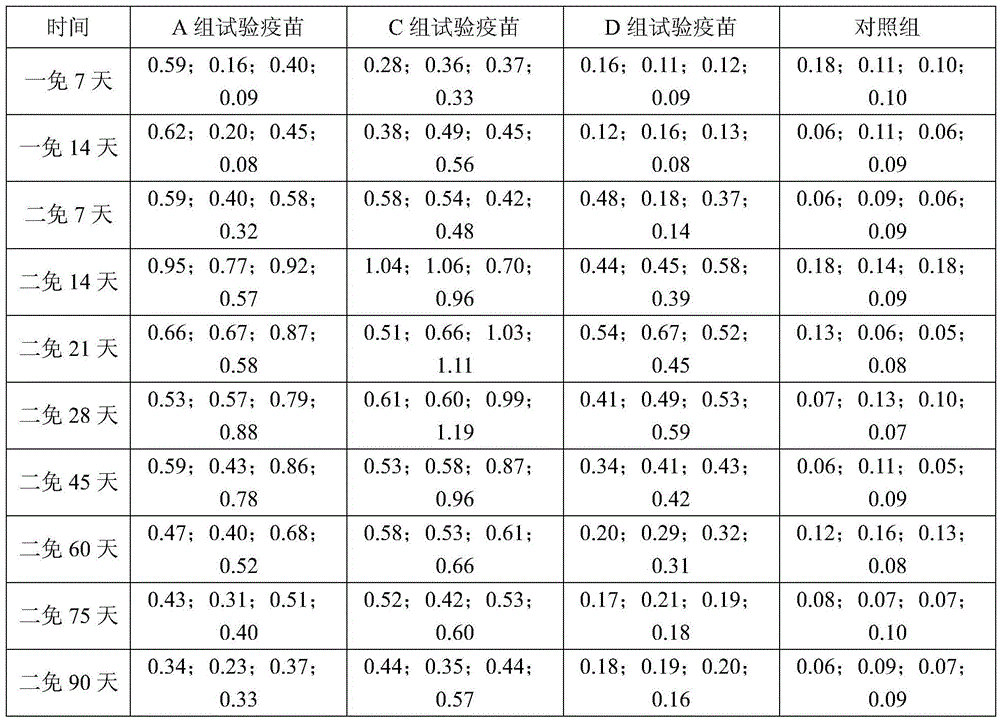
A Japanese encephalitis vaccine composition and preparation method thereof
InactiveCN105688202BImproving immunogenicityImprove protectionSsRNA viruses positive-senseViral antigen ingredientsJapanese encephalitis vaccineImmune effects
The invention provides Japanese encephalitis (JE) vaccine composition. The JE vaccine composition comprises a JE virus antigen of immunization effective quantity and water based vaccine adjuvant. The invention further provides a preparing method and application of the JE vaccine composition. The vaccine composition has good immunogenicity and immunizing protection. The immune effect of the JE vaccine composition is remarkably superior to that of single pig JE live vaccine and other live vaccine diluted by immunologic adjuvant. The clinical application prospects are good.
Owner:SICHUAN AGRI UNIV
Preparation and application of a porcine Japanese encephalitis vaccine composition
ActiveCN108969759BImprove immunityImprove the level ofViral antigen ingredientsAntiviralsDiseaseJapanese encephalitis vaccine
The invention provides a vaccine composition, which comprises polymer Carbomer 934P and Japanese encephalitis virus strain SCYA201201 (cell passage attenuated strain) cell culture fluid. Compared with live vaccines, the vaccine composition of the invention can greatly improve the immunogenicity, enhance the body's resistance to porcine Japanese encephalitis virus on the original basis, and provide new technical support for the prevention and control of the disease.
Owner:SICHUAN AGRI UNIV
Large-scale culture method of porcine Japanese encephalitis vaccine antigens
ActiveCN111394319AIncrease productionSuitable for large-scale productionSsRNA viruses positive-senseViral antigen ingredientsAntigenJapanese encephalitis vaccine
The invention discloses a large-scale culture method of porcine Japanese encephalitis vaccine antigens. According to the large-scale culture process of the porcine Japanese encephalitis vaccine antigens, porcine kidney cells PK-15 are used for culturing porcine Japanese encephalitis virus, multiple batches of virus can be continuously harvested through one-time virus inoculation, and the titer ofthe virus is equivalent to that of BHK-21 and VERO cells or higher than an existing culture process, the yield of the virus is greatly increased, the production cost is reduced, and the method is a porcine Japanese encephalitis vaccine antigen culture process suitable for large-scale production.
Owner:SICHUAN ANIMAL SCI ACAD +1
A kind of vaccine protection agent, measles and Japanese encephalitis combined vaccine and preparation method thereof
ActiveCN102512685BImprove stabilityIncreased sensitizationViral antigen ingredientsAntiviralsJapanese encephalitis vaccineEncephalitis
The invention provides a vaccine protection agent which is characterized by comprising the following components by weight: 60 to 200 parts of disaccharides, 3 to 10 parts of gelatin, 0.5 to 10 parts of amino acid, 3 to 10 parts of urea, 3 to 10 parts of sorbitol and 2 to 10 parts of human albumin. The invention also provides a combined measles and Japanese encephalitis vaccine and a preparation method thereof. The vaccine protection agent provided in the invention can effectively enhance stability of the combined measles and Japanese encephalitis vaccine and has a high application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Mercury-free Japanese encephalitis inactivated vaccine composition and application thereof
InactiveCN101732707BAvoid adverse reactionsKeep healthyViral antigen ingredientsAntiviralsJapanese encephalitis vaccineThiomersalate
The invention discloses a mercury-free Japanese encephalitis inactivated vaccine composition, which consists of inactivated Japanese encephalitis virus, phenol red free 199 culture solution and virus protective agent. Through comparison with fungus inspection of a mercury-containing preparation, the composition can achieve the quality standard required by Chinese Pharmacopoeia on sterile products. In the preparation of a Japanese encephalitis vaccine, thiomersalate is not used so as to avoid the adverse effect caused by mercury pollution on users, and ensures body health of the users.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Combined vaccine composed of ABCYW135 group meningococcal vaccine and Japanese encephalitis vaccine
InactiveCN111281972AAntibacterial agentsSsRNA viruses positive-senseTGE VACCINEMeningococcus vaccine
The invention provides an ABCYW135 group meningococcus / Japanese encephalitis combined vaccine, which is prepared by the following steps: respectively extracting capsular polysaccharides from A group meningococcus, C group meningococcus, W135 group meningococcus and Y group meningococcus, purifying, and combining the activated capsular polysaccharides with diphtheria CRM197 protein; using PorA protein on outer membrane vesicles of group B meningococcus; using Vero cells for culturing encephalitis B viruses, after inactivation and purification, mixing the components according to a certain proportion, and the mixture is used for preventing infection caused by ABCYW135 group meningococci and Japanese encephalitis viruses.
Owner:北京成大天和生物科技有限公司 +1
A treatment solution and a method for determining antigen content in aluminum salt-adsorbed vaccines using it
ActiveCN104634959BAccurate detectionEfficient detectionMaterial analysisJapanese encephalitis vaccineAdjuvant
Owner:LIVZON GROUP VACCINE ENG
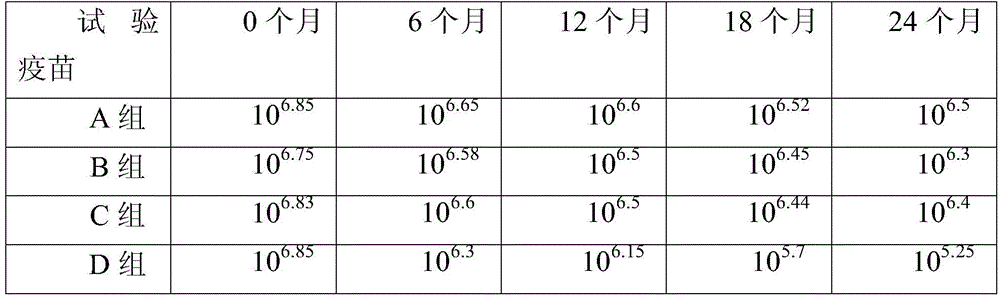
Heat-resistant freeze-drying protectant for live vaccine and its preparation method and application
ActiveCN104083769BRequirements for reduced temperature storage conditionsEasy to storeViral antigen ingredientsAntiviralsArgininePyrrolidinones
Owner:HUNAN SINOLAND BIOLOGICAL PHARMA
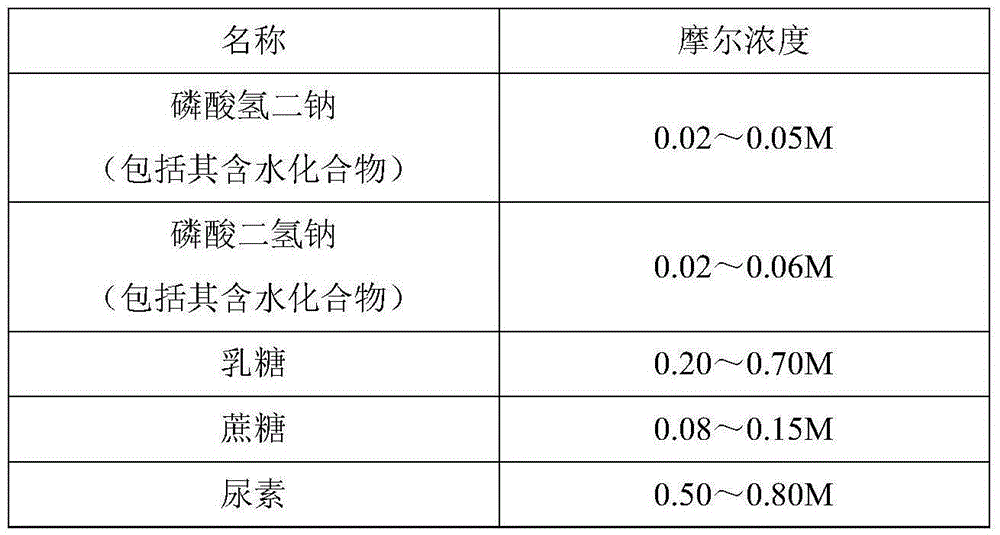
Gelatin-free freeze-drying stabilizer used for human Japanese encephalitis vaccine
InactiveCN105106964ALess irritatingSafe to usePowder deliveryViral antigen ingredientsSucroseSaccharum
The invention relates to a gelatin-free freeze-drying stabilizer used for a human Japanese encephalitis vaccine. The freeze-drying stabilizer is free of gelatin; and according to test and detection results, the prepared vaccine has high stability and security and small irritation and is free of known allergic population. A formula for the freeze-drying stabilizer comprises 0.02 to 0.05 M of disodium hydrogen phosphate (including aquo-compounds thereof), 0.08 to 0.15 M of cane sugar, 0.02 to 0.06 M of sodium dihydrogen phosphate (including aquo-compounds thereof), 0.50 to 0.80 M of urea and 0.20 to 0.70 M of lactose, and the pH value of the freeze-drying stabilizer is 6.5 to 7.8.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Lyophilized inactivated Japanese encephalitis vaccine
ActiveCN102631672BMeet quality requirementsQuality improvementPowder deliveryViral antigen ingredientsJapanese encephalitis vaccineLactose
The invention provides a lyophilized inactivated Japanese encephalitis vaccine, comprising: (a) inactivated Japanese encephalitis totivirus; (b) stabilizer, which comprises maltose, lactose, and optionally mycose, mannite, sorbitol and / or amino acid; and optionally (c) buffer, surfactant, isotonic regulator and / or chelating agent.
Owner:天津嘉诚顺隆商贸有限公司
Method for removing residual dna in human Japanese encephalitis vaccine products by using hollow fiber membrane
ActiveCN103768589BGood physicochemical toleranceSolution to short lifeViral antigen ingredientsAgainst vector-borne diseasesJapanese encephalitis vaccineMolecular sieve
Owner:LIAONING CHENGDA BIOTECH
Method of producing japanese encephalitis vaccine stably storable over long time and use of the vaccine
ActiveUS9453055B2Stable storageGood storage stabilityAntibacterial agentsSsRNA viruses positive-senseJapanese encephalitis vaccineJapanese encephalitis virus vaccine
The present inventors improved methods for inactivating Japanese encephalitis virus vaccines, and assessed the safety of vaccines produced by combining multiple vaccines. The present inventors successfully produced safer Japanese encephalitis vaccines by cell culture, which can be stored more stably over a long period than conventional Japanese encephalitis vaccines. Furthermore, it is also expected that the production methods can be used to produce other viral vaccines with excellent storage stability.
Owner:DAIICHI SANKYO CO LTD
Method for improving Japanese encephalitis virus titer
ActiveCN103160477BHigh potencyImproving immunogenicityViral antigen ingredientsAntiviralsJapanese encephalitis vaccineCytopathic effect
The invention provides a method for proliferation of a Japanese encephalitis virus. The method for proliferation of the Japanese encephalitis virus comprises the steps of culturing cells, inoculating Japanese encephalitis viruses to the cells which are provided with mono-layers in an overgrowing mode, and directly obtaining a virus solution until a rate of a cytopathic effect reaches to 75%-95% after inoculation. The invention further provides a preparation method of Japanese encephalitis vaccines. The method for proliferation of the Japanese encephalitis virus and the preparation method of the Japanese encephalitis vaccines can remarkably improve a Japanese encephalitis virus titer, and meanwhile shorten production periods, and can prepare the Japanese encephalitis vaccines which are better, stable, and good in immunogenicity.
Owner:HUNAN SINOLAND BIOLOGICAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com