Inactivated Japanese encephalitis vaccine freeze-drying preparation for injection and preparation method thereof
An inactivated vaccine, Japanese encephalitis technology, applied in the directions of biochemical equipment and methods, vaccines, freeze-dried delivery, etc., can solve problems such as poor freezing type, achieve the effect of speeding up the reconstitution speed and solving the collapse of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Screening experiment of process parameters for preparation of freeze-dried preparation of Japanese encephalitis inactivated vaccine.
[0025] 1. Screening experiment of carbohydrates in lyoprotectant.
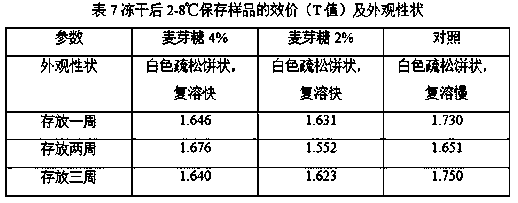
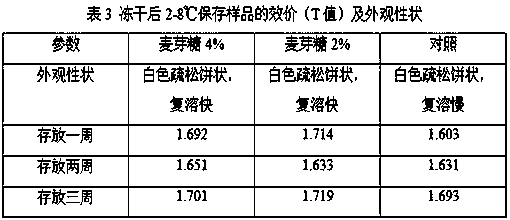
[0026] (1) Preparation method: Weigh each component: 30g of human serum albumin, optionally 45g of trehalose, lactose, and maltose, and add PBS buffer to 500ml. The liquid prepared above is sterilized and filtered with a filter with a pore size of 0.22 μm to obtain the lyoprotectant of the present invention.
[0027] (2) The Japanese encephalitis inactivated vaccine strains were cultured in microcarrier suspension at 37°C for 20 days, then the virus liquid was harvested, and then the harvested liquid was concentrated and inactivated, and finally purified by molecular sieves. Mix the obtained purified solution with the prepared lyoprotectant at a ratio of 1:3, mix well, and immediately freeze-dry after dispensing according to a predetermined volume.
[0028] (...
Embodiment 2
[0055] Example 2 Formula and preparation process parameters of the optimized freeze-dried preparation of Japanese encephalitis inactivated vaccine.
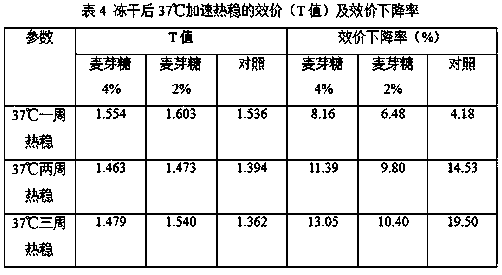
[0056] 1. The optimized formula of the lyoprotectant: it is characterized by maltose 2-4g / 100ml and human serum albumin 2g / 100ml.
[0057] 2. Optimized freeze-drying process: pre-freeze at -45°C, maintain at this temperature for 4-6 hours, then anneal at -15°C for 20 minutes. After annealing, lower the temperature to -45°C, evacuate to 0.1mbar, then raise the temperature to -30°C, then raise the temperature to -20°C after 8~10 hours, then raise the temperature to 27°C after 4~6 hours and maintain it for 6~8 hours Get it later.
[0058] 3. Preparation method.
[0059] (1) Preparation method: Weigh each component: human serum albumin 30g, maltose 30g, add PBS buffer to 500ml. The liquid prepared above is sterilized and filtered with a filter with a pore size of 0.22 μm to obtain the lyoprotectant of the present invention.
[00...
Embodiment 3
[0062] Example 3 Comparison of optimized freeze-dried formulations of Japanese encephalitis inactivated vaccines and original freeze-dried formulations.
[0063] Table 9 compares the reconstitution time and thermal stability of the lyophilized preparation prepared in Example 2 and the lyophilized preparation prepared by the original process.
[0064]
[0065] It can be drawn from the results in Table 9.
[0066] (1) After the introduction of annealing process, the problem of collapse of freeze-dried products can be solved, and the frozen shape is ideal.
[0067] (2) After replacing the original dextran with maltose, the reconstitution time of Japanese encephalitis inactivated vaccine was shortened by half, from 20-30 seconds to 8-10 seconds. The drug dissolves quickly and fully, and is convenient to use.
[0068] (3) After replacing the original dextran with maltose, the protection against Japanese encephalitis inactivated vaccine was significantly improved, especially in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com