Method for removing residual dna in human Japanese encephalitis vaccine products by using hollow fiber membrane
A Japanese encephalitis and fiber membrane technology is applied to the field of removing residual DNA in human Japanese encephalitis vaccine products by using hollow fiber membranes, which can solve problems such as adverse reactions and cancer occurrence, and achieve easy operation and management, low cost, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
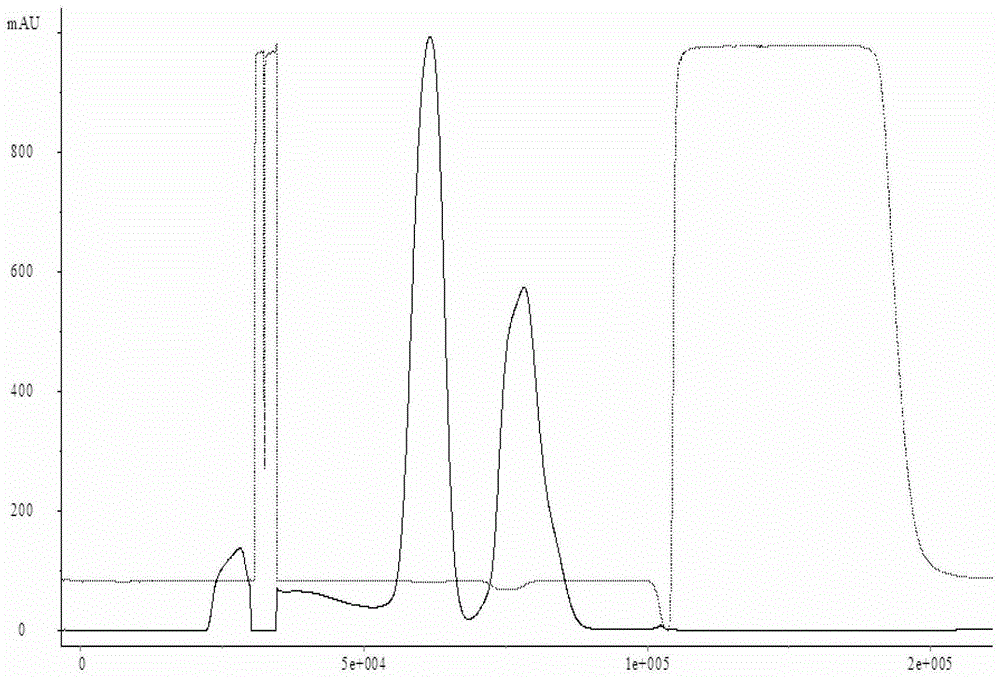
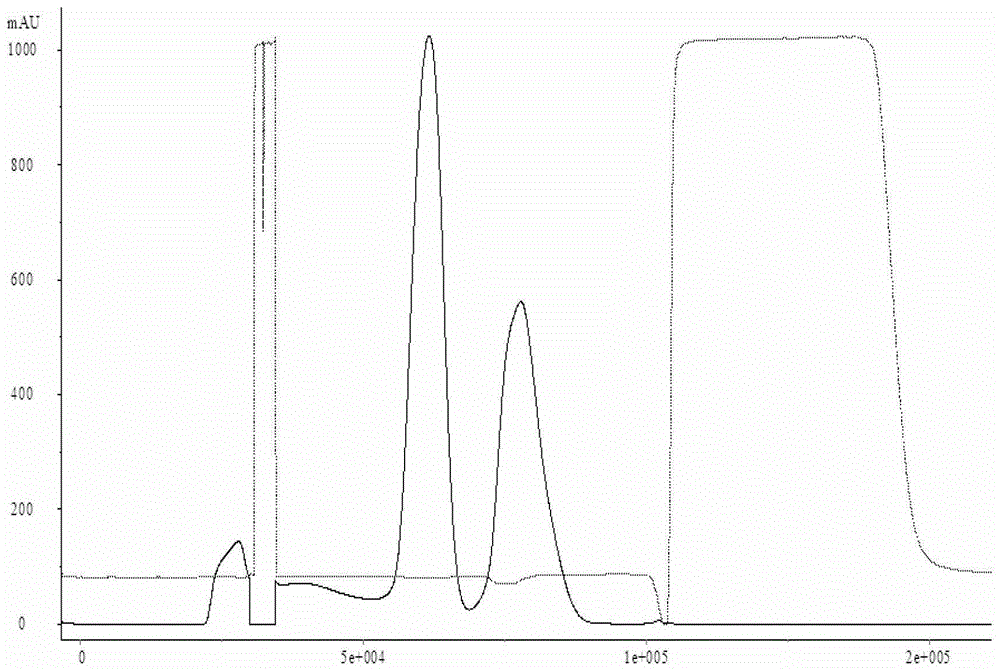
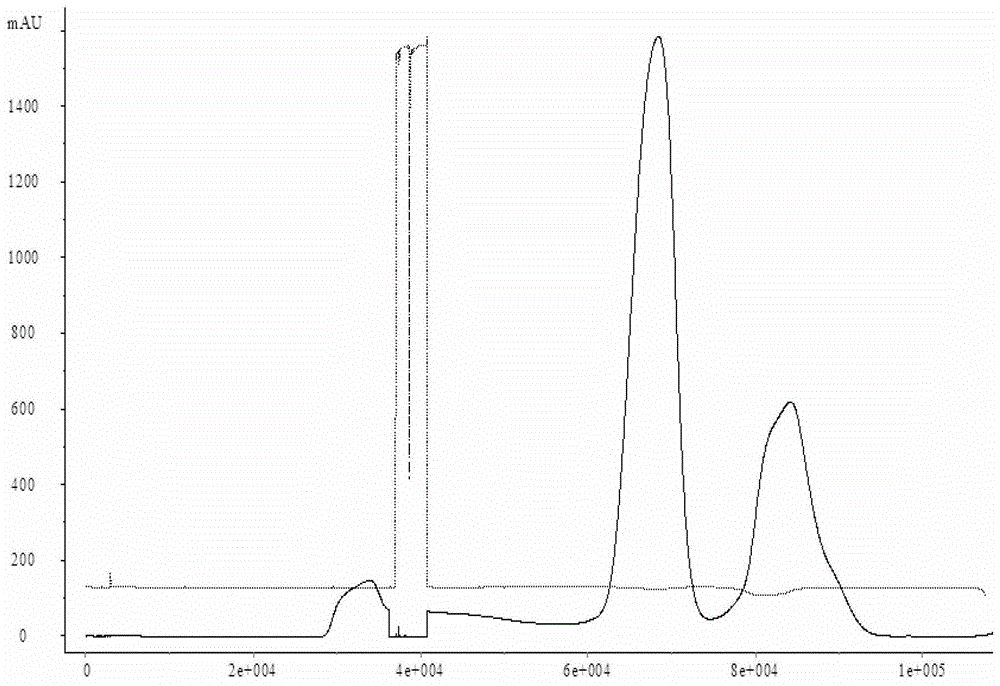
[0022] Select a batch of Japanese encephalitis vaccine virus harvest liquid, and use a sleeve filter to clarify and filter it. The type of the sleeve filter system is TP012TV25TCTFELB, the pore size is 0.65 μm, and the membrane area is about 4.0m 2 , the inlet pressure of the feed liquid is less than 10PSI, the outlet pressure of the feed liquid is about 0PSI, the total volume of the processed human Japanese encephalitis vaccine harvest liquid is 230L-240L, and the total working time is 1.5h-2.0h. The whole clarification and filtration process The temperature of the feed liquid is guaranteed to be between 18°C and 26°C. The liquid obtained after filtration enters the concentration process, and is made into a concentrated liquid of Japanese encephalitis vaccine for human use through an ultrafiltration concentration system. The batch number after inactivation is SA- 01, and then purified by a molecular sieve chromatography system, the batch number of the purified Japanese encep...
Embodiment 2
[0025] Select a batch of human Japanese encephalitis vaccine virus harvest liquid, and use the hollow fiber membrane to micro-filter it. The model of the hollow fiber membrane system is CFP-6-D-8A, the pore size is 0.65 μm, and the membrane area is about 4.0m 2 , the pressure at the inlet end of the feed liquid is less than 10PSI, the pressure at the outlet end of the feed liquid is about 0PSI, and the pressure at the return end of the feed liquid is less than 3PSI. 1.5h-2.0h, the temperature of the feed liquid is guaranteed to be between 18°C and 26°C during the entire microfiltration process. The liquid obtained after filtration enters the concentration process, and is made into Japanese encephalitis vaccine concentrate through an ultrafiltration concentration system. After inactivation, the batch number is SA-03, and then purified through a molecular sieve chromatography system. The collected human The batch number of Japanese encephalitis vaccine purified liquid is SA-0...
Embodiment 3
[0028] Select a batch of human Japanese encephalitis vaccine virus harvest liquid, and use the hollow fiber membrane to micro-filter it. The model of the hollow fiber membrane system is CFP-6-D-8A, the pore size is 0.65 μm, and the membrane area is about 4.0m 2 , the pressure at the inlet end of the feed liquid is less than 10PSI, the pressure at the outlet end of the feed liquid is about 0PSI, and the pressure at the return end of the feed liquid is less than 3PSI. 0.8h-1.0h, the temperature of the feed liquid is guaranteed to be between 18°C and 26°C throughout the microfiltration process. The liquid obtained after filtration enters the concentration process, and is made into Japanese encephalitis vaccine concentrate through an ultrafiltration concentration system. After inactivation, the batch number is SA-05, and then purified through a molecular sieve chromatography system. The collected human The batch number of the Japanese encephalitis vaccine purified liquid is SA-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com