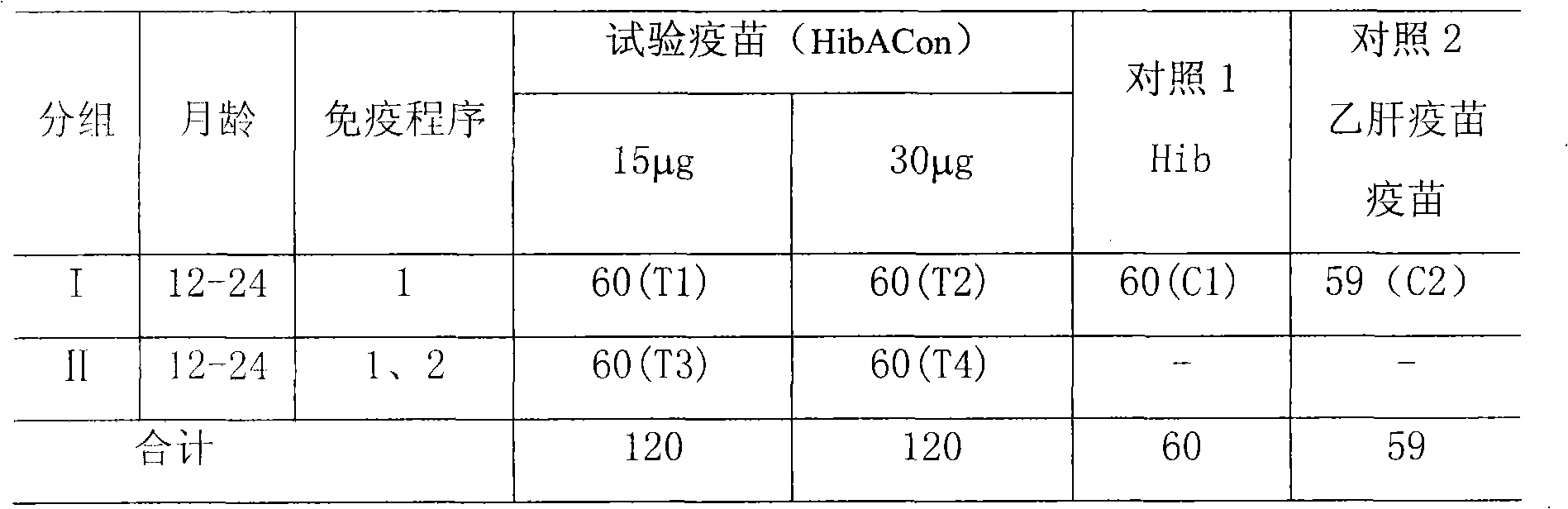
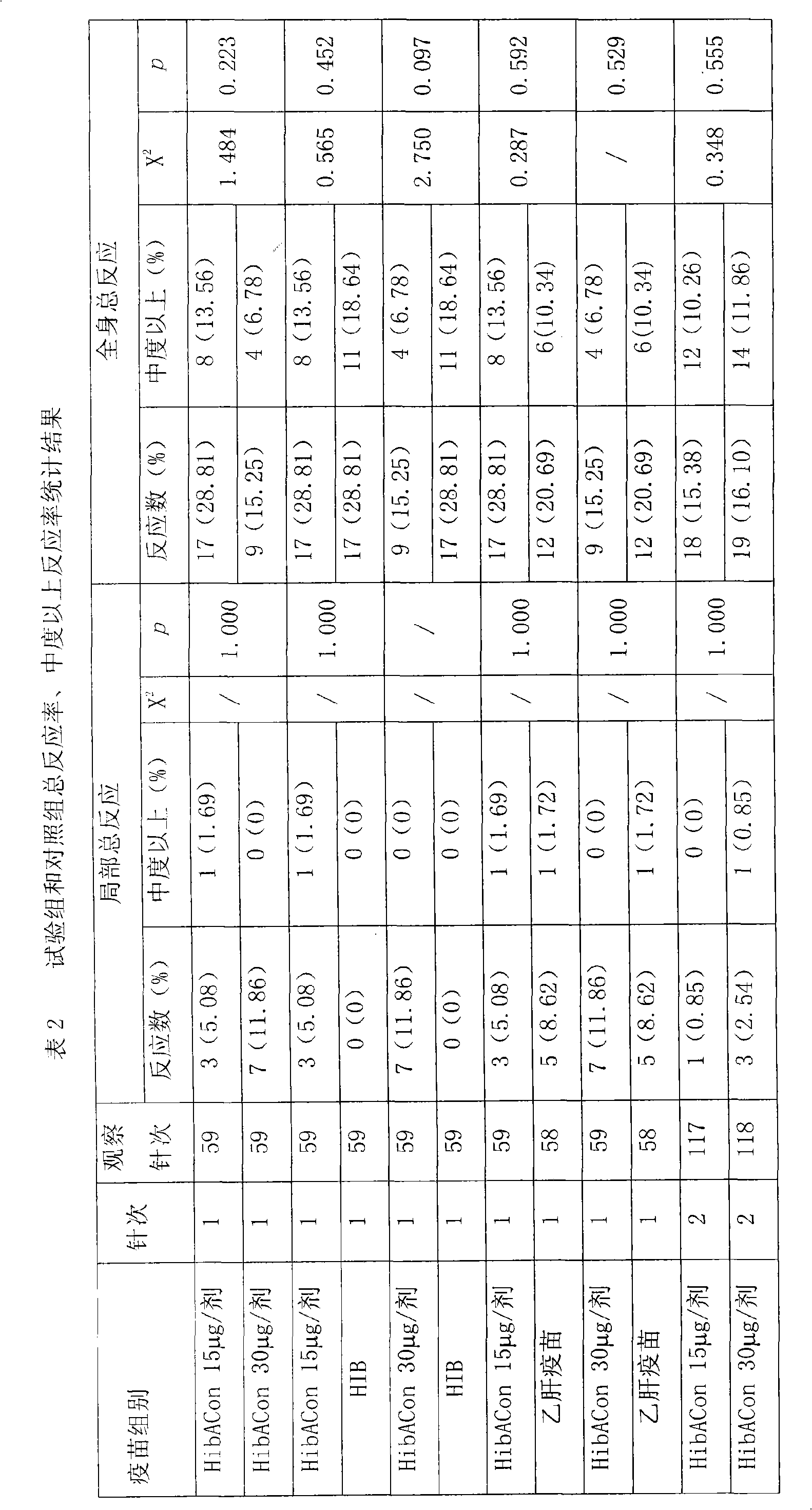
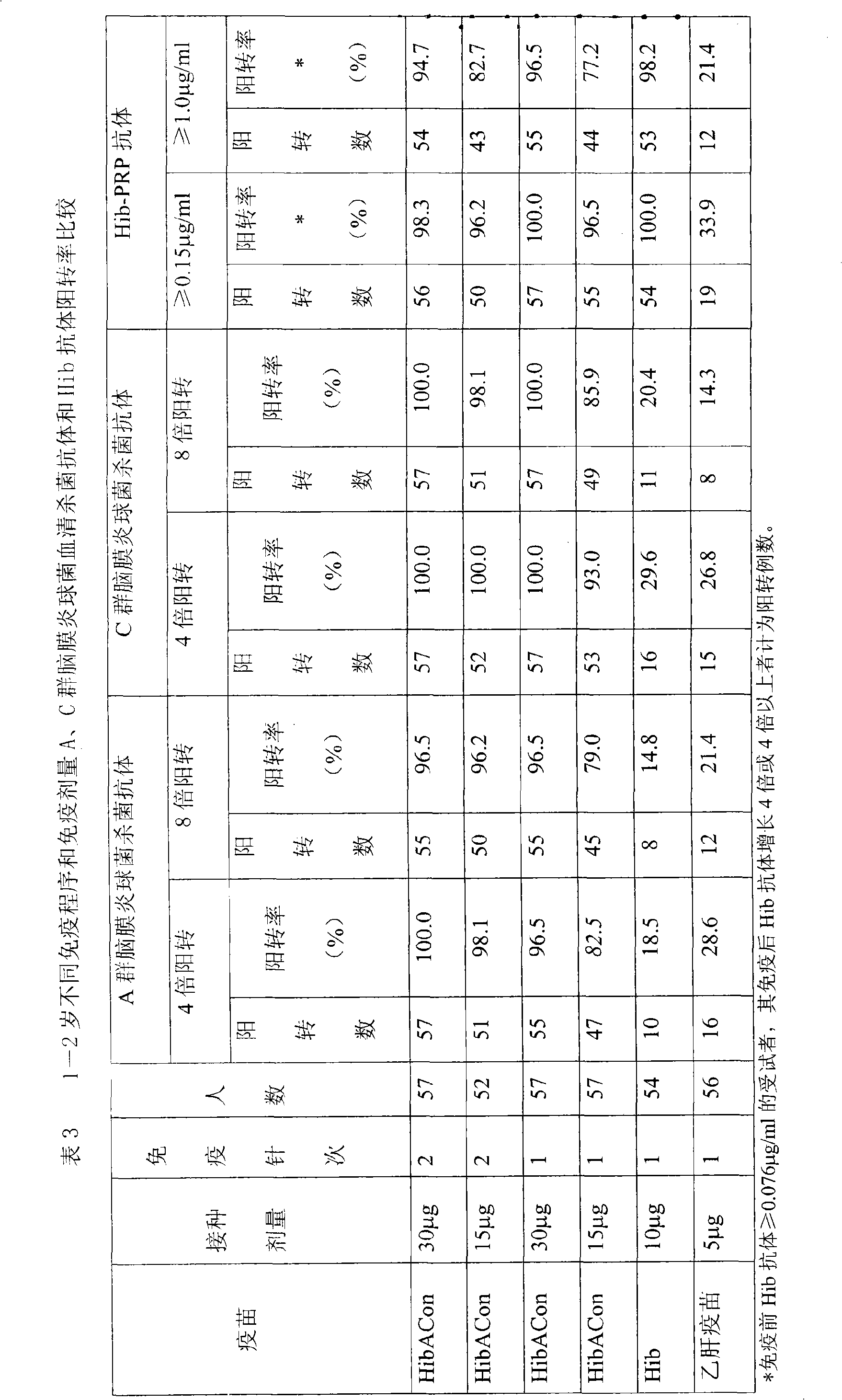
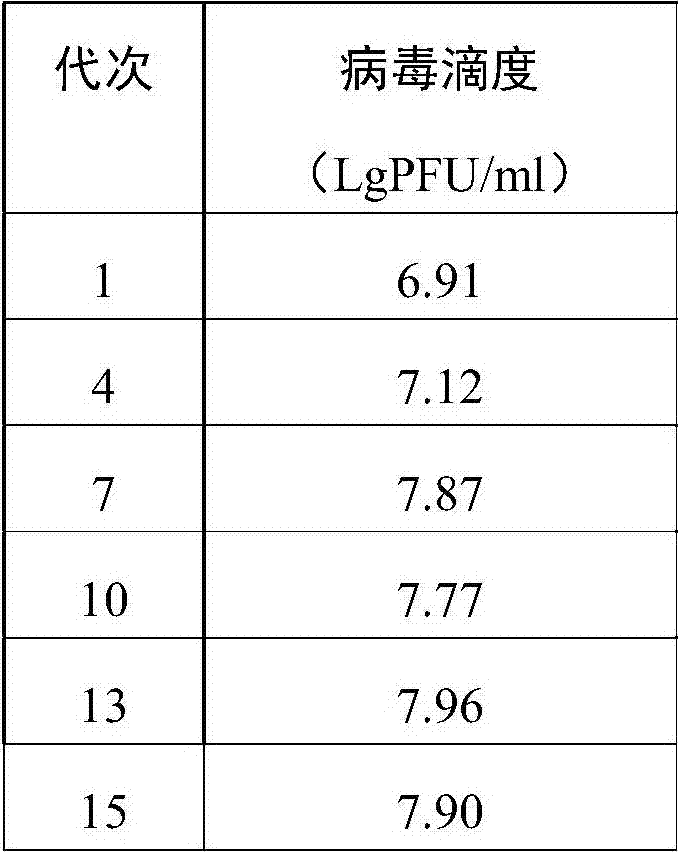
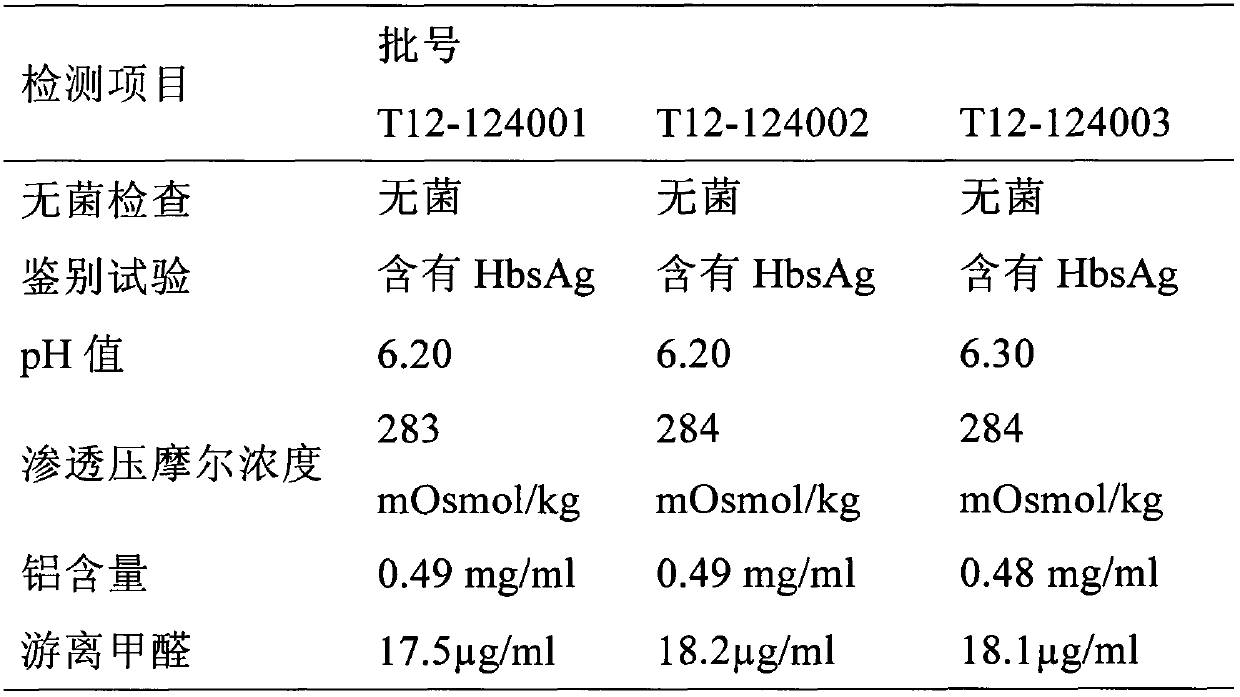
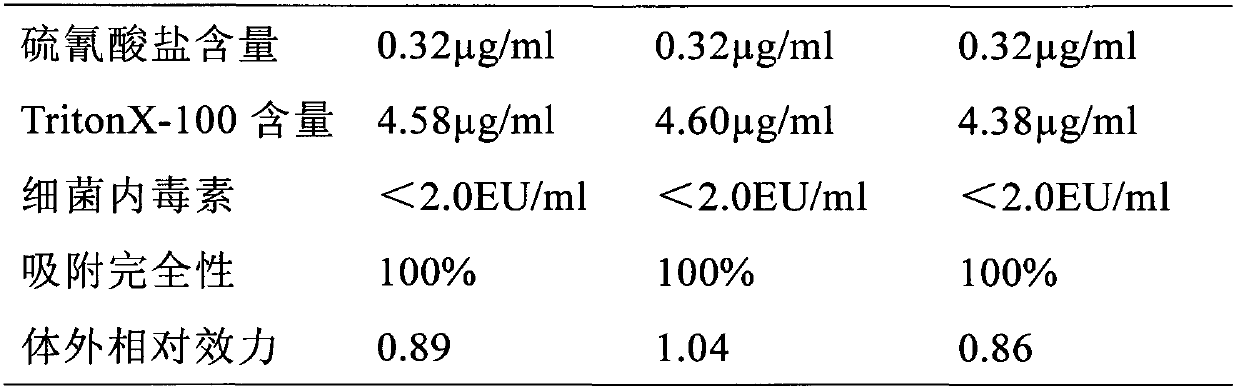
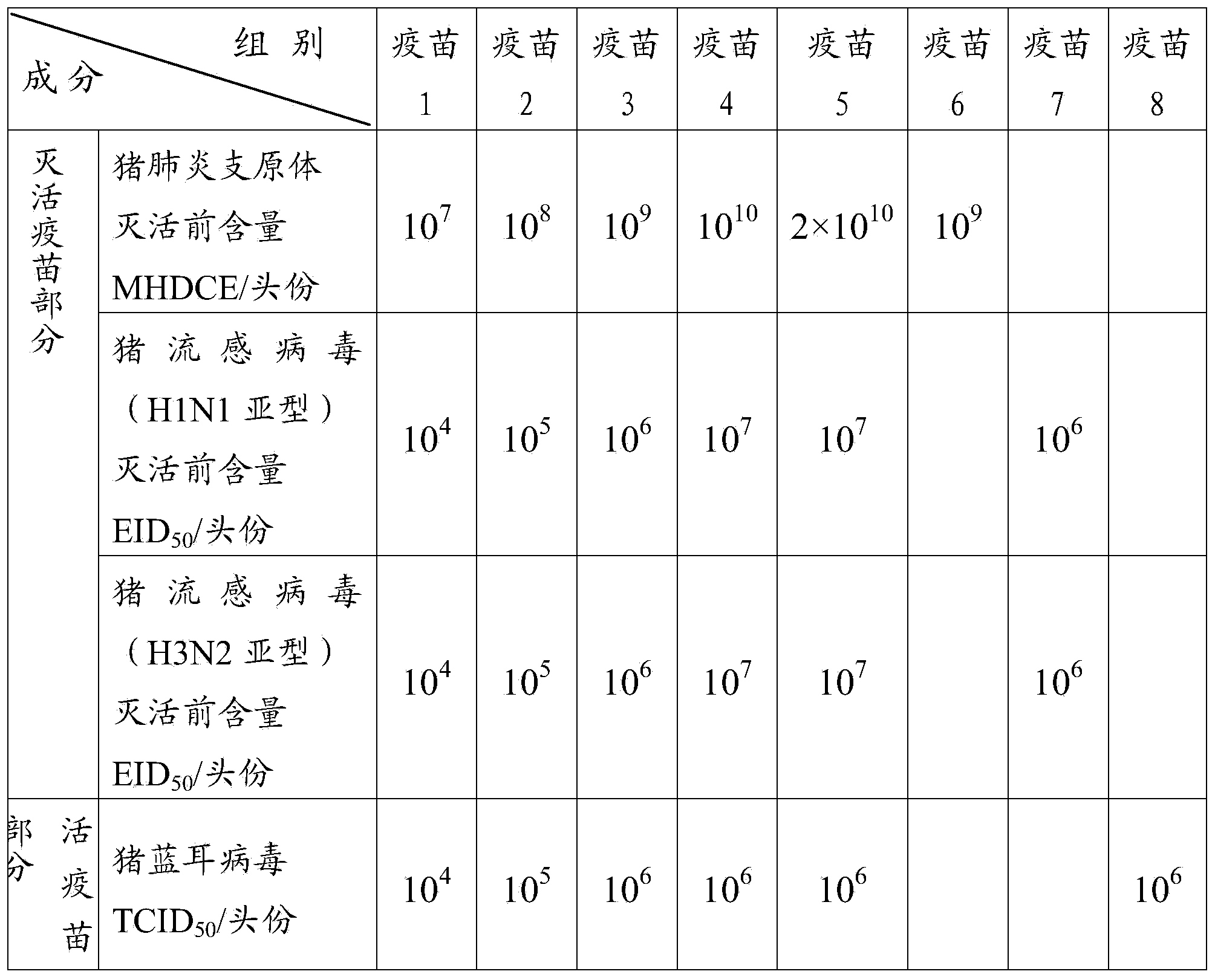
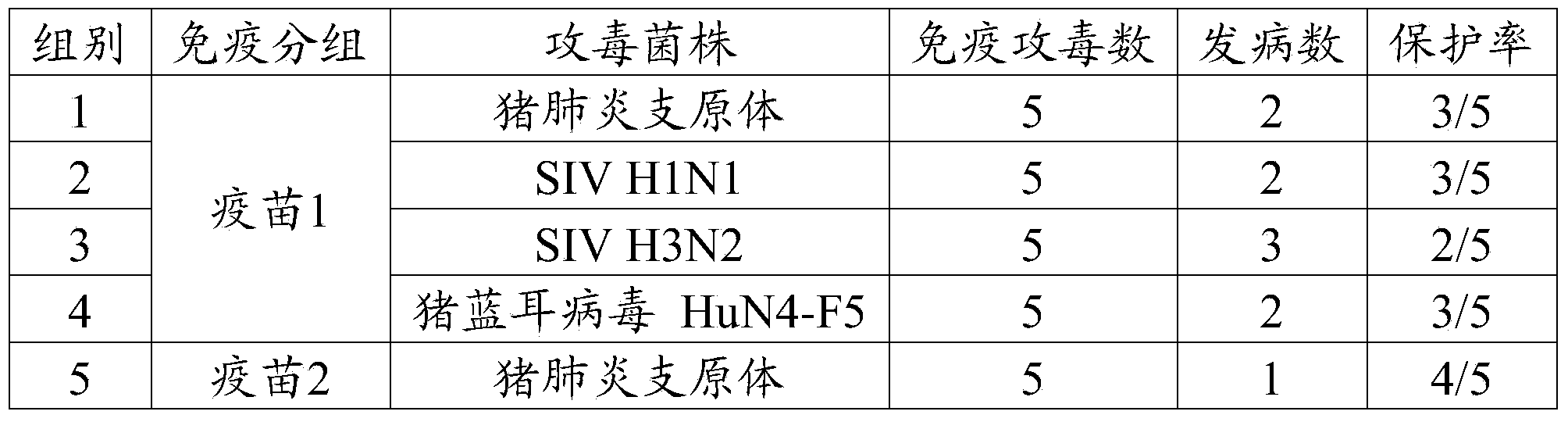
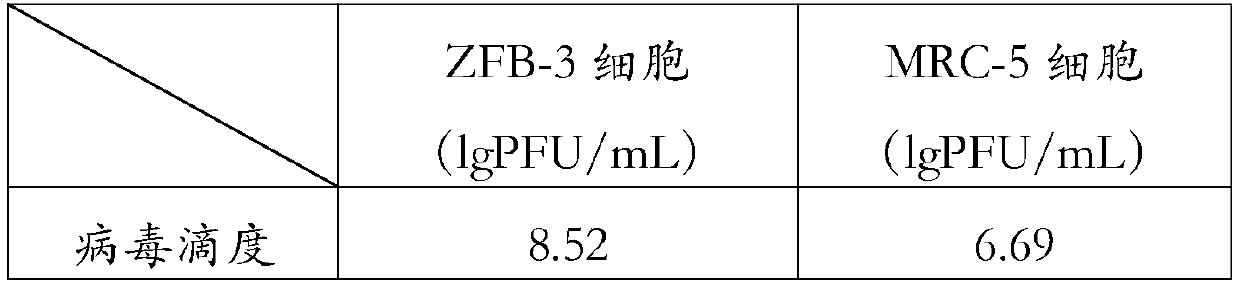
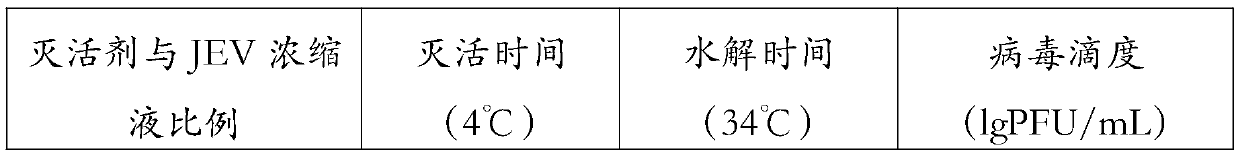
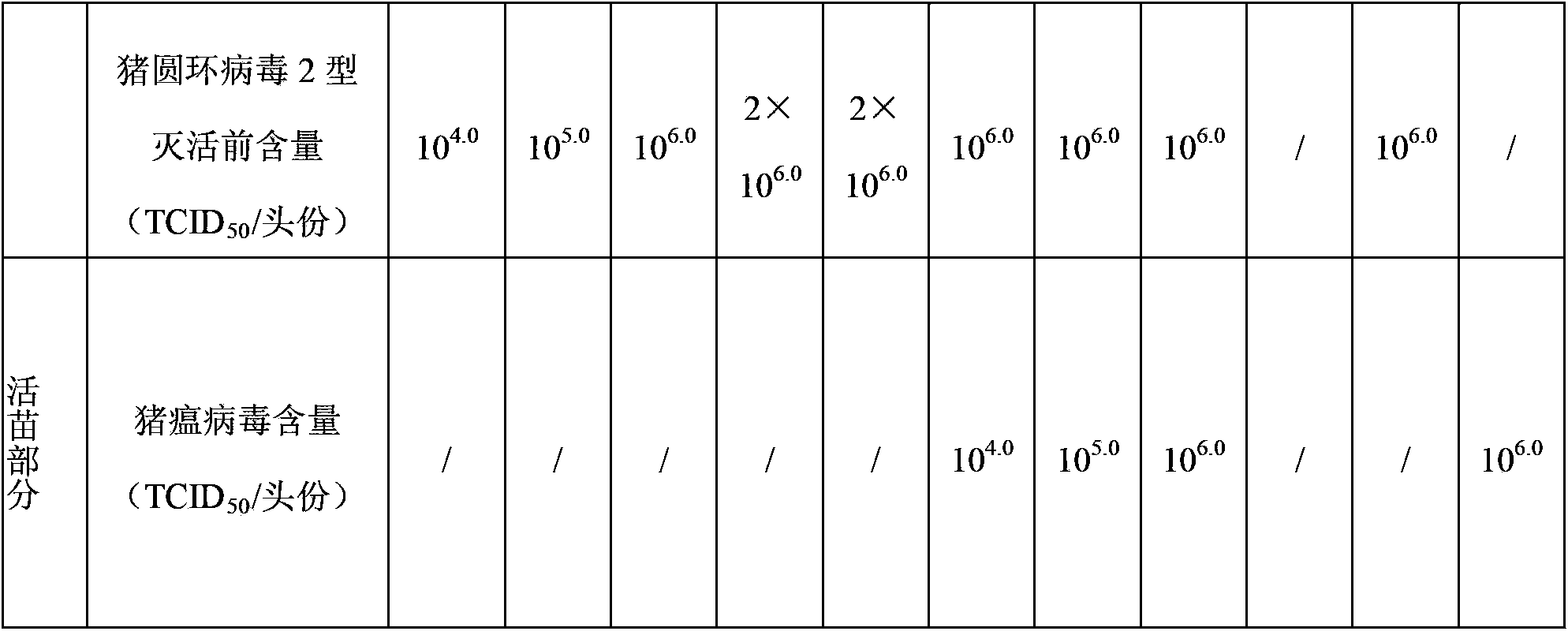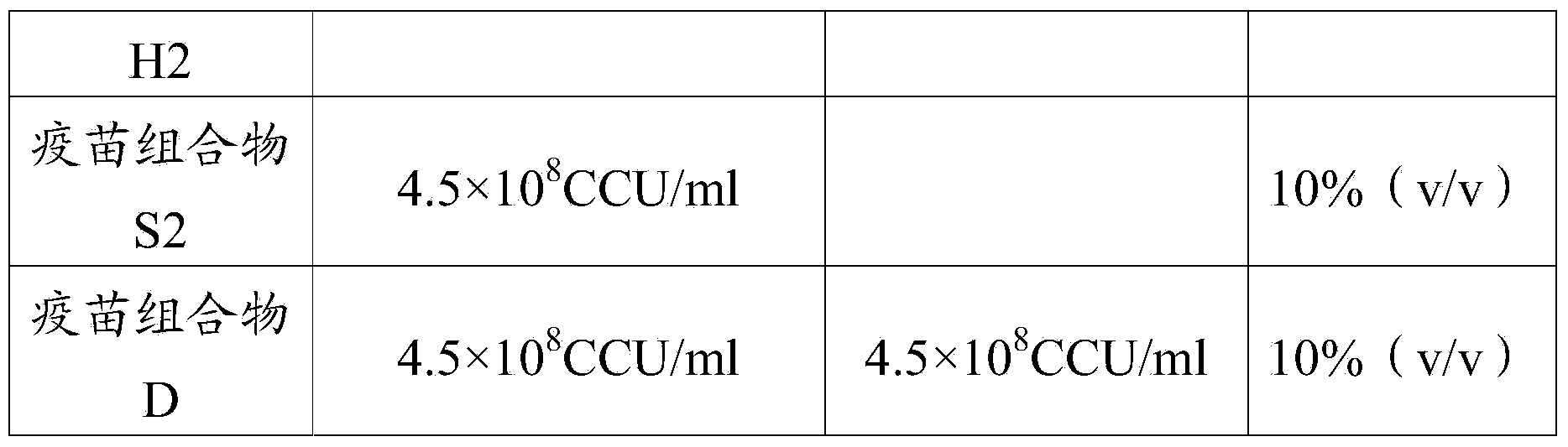Patents
Literature
57results about How to "Reduce the number of vaccinations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for culturing orchid special strain thereof
InactiveCN101338290ALoose textureImprove breathabilityBacteriaMicroorganism based processesMicrobiologySeedling
The invention discloses a method for culturing an orchid and a special bacterium thereof. The name of the bacterium of the invention is GDB181 the preservation number of which is CGMCCNo.2574. The method of the invention includes the steps for together culturing the bacterium of the invention and the orchid. The bacterium of the invention is applicable for the root culturing of an orchid bacteria and builds an epiphyte bacterium with excellent root which effectively coexists with the orchid. The method of the invention for culturing the orchid can better control the inoculation amount and can fix the inoculation position, thus fully ensuring the tissue culture seedling of the orchid to build the coexistence relation with the epiphyte bacterium with excellent root, simultaneously simplifying the inoculation steps and reducing the inoculation times. Permanent effect can be achieved by being inoculated for once, thus avoiding the dead seedling phenomenon caused by a plurality of manual inoculations, thereby improving the survival rate of transplanting and obtaining the root orchid seedling which grows strongly. Therefore, the method for culturing bacterium, the culture medium of the bacterium and the culture medium of the orchid of the invention has important economic values and is applicable for popularization and application.
Owner:金辉
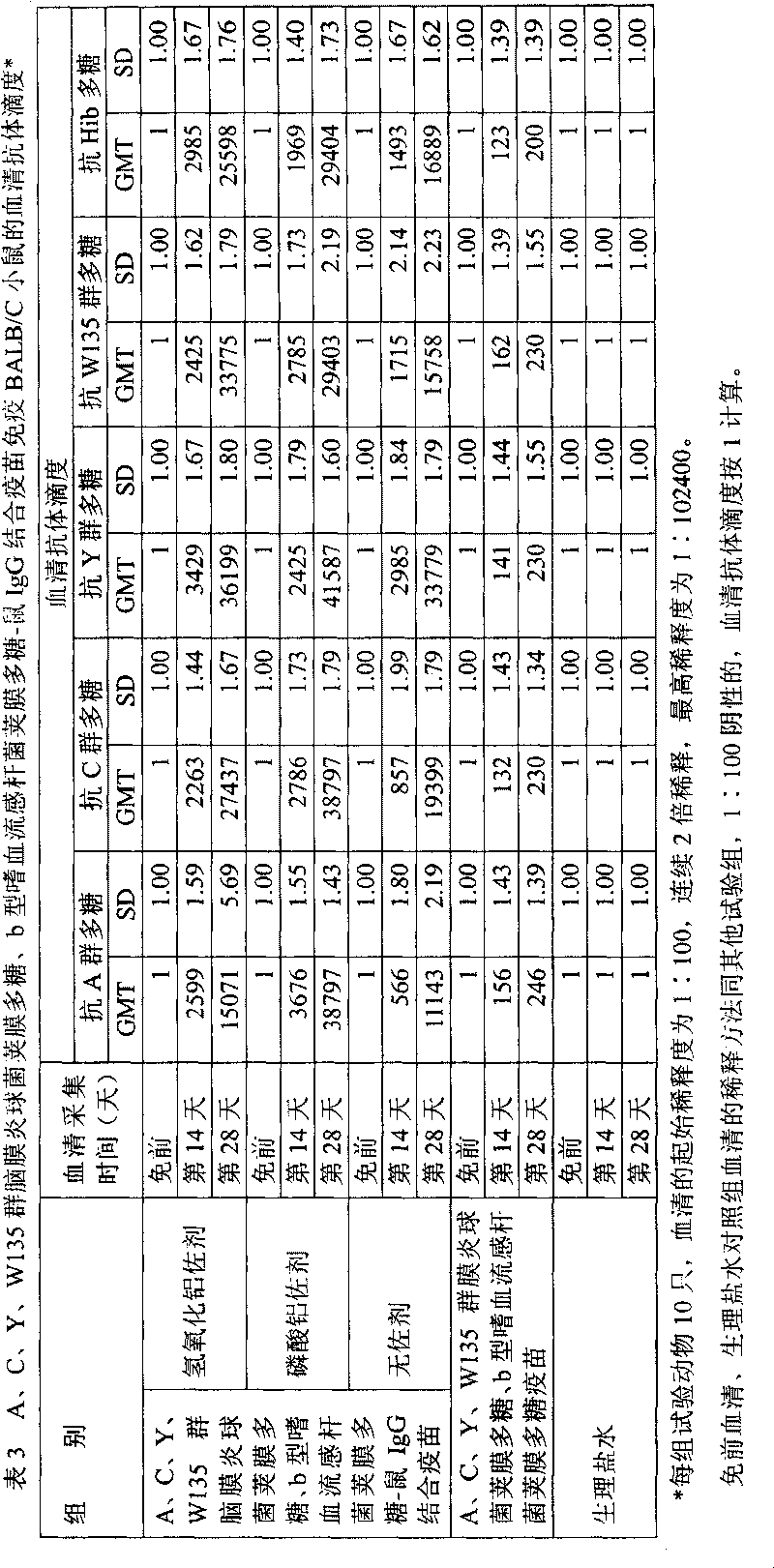
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
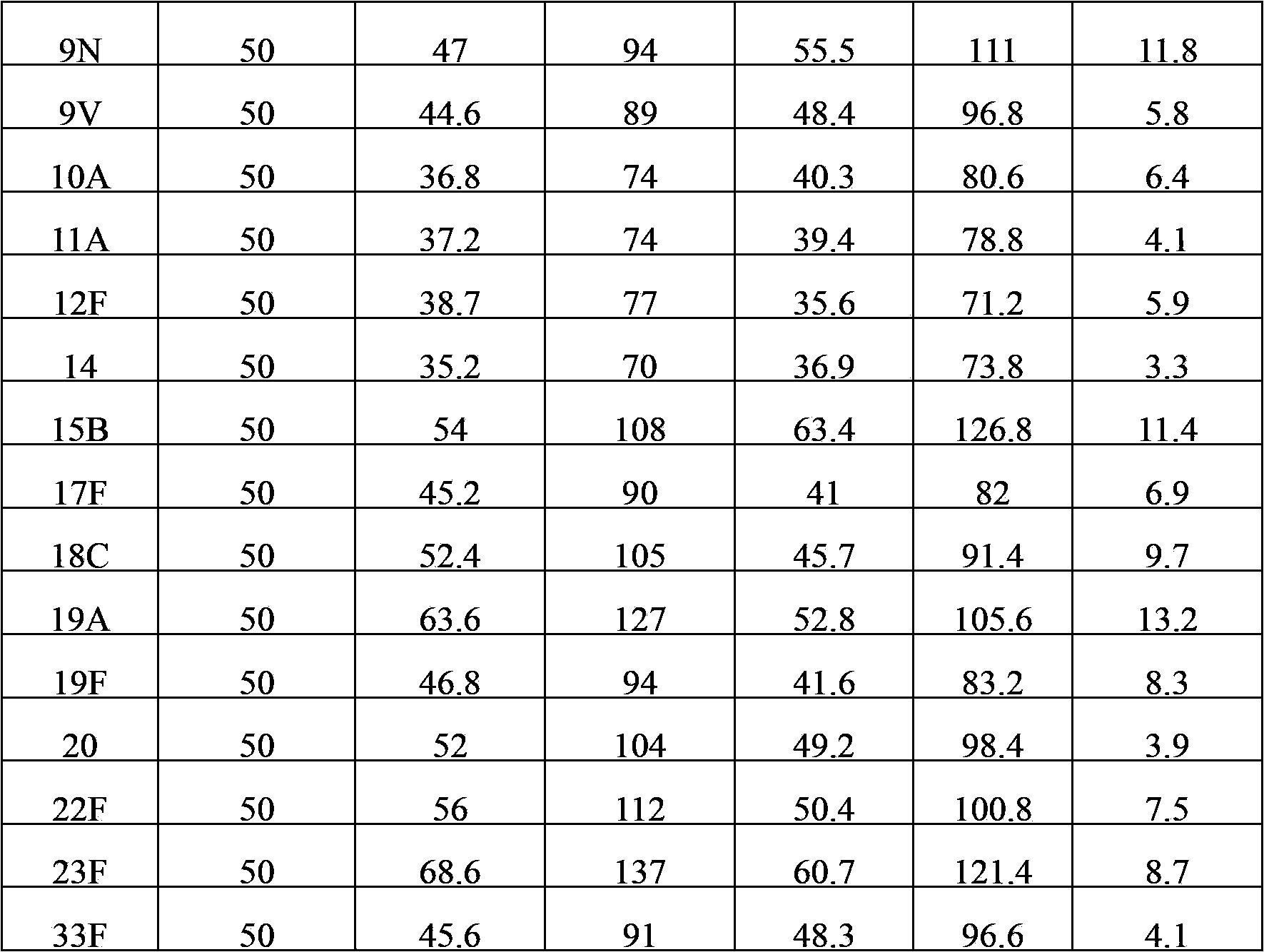
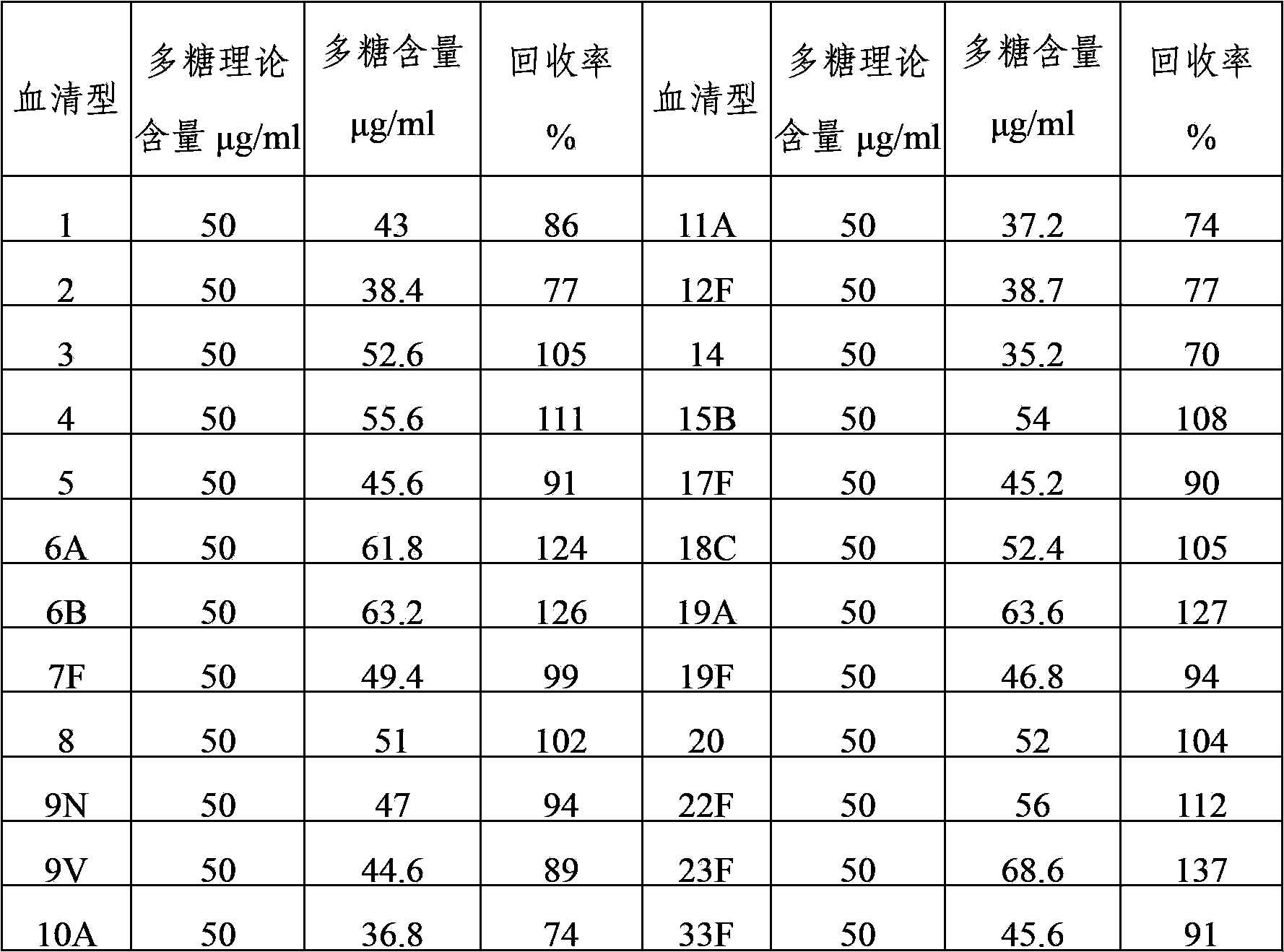
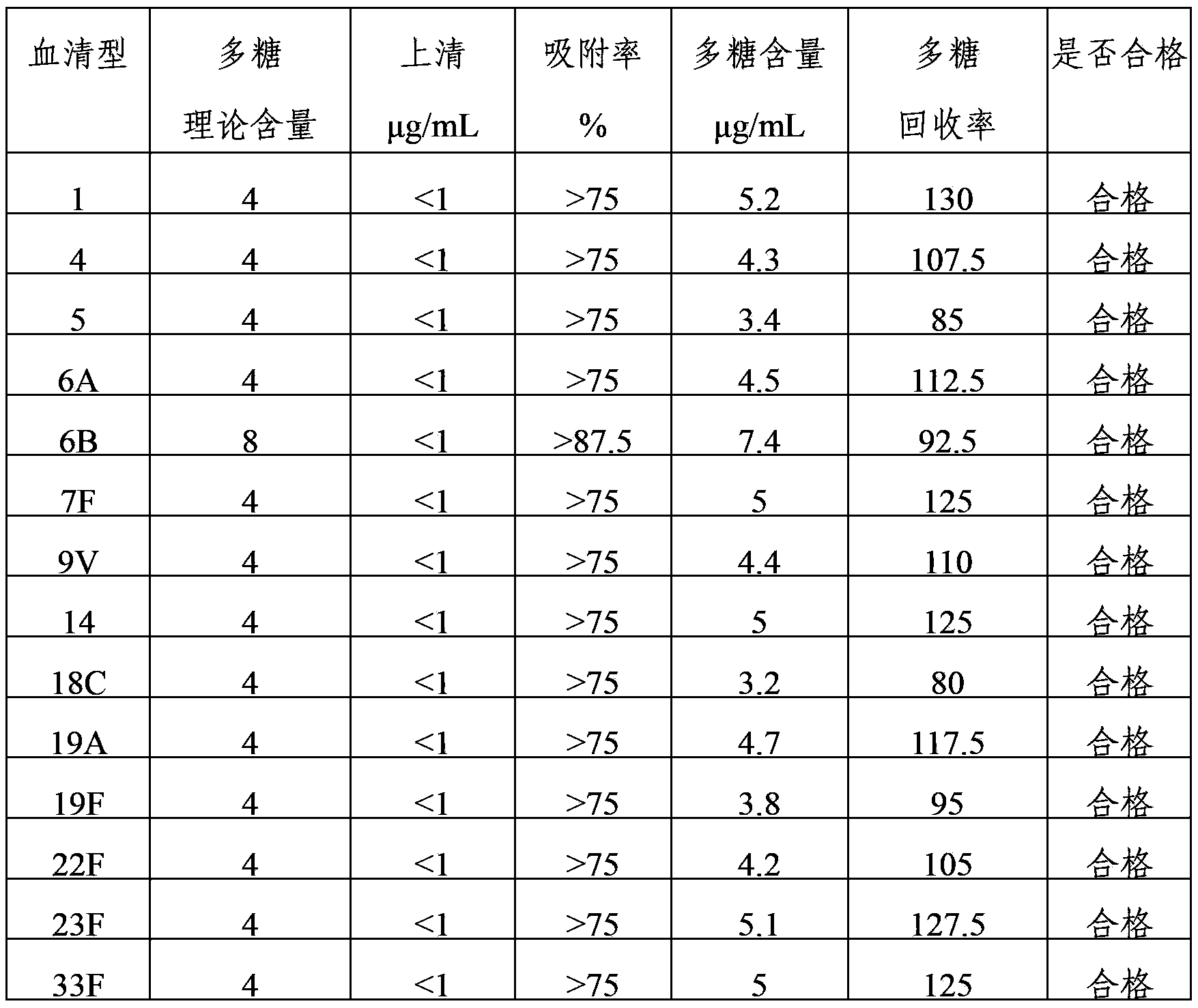
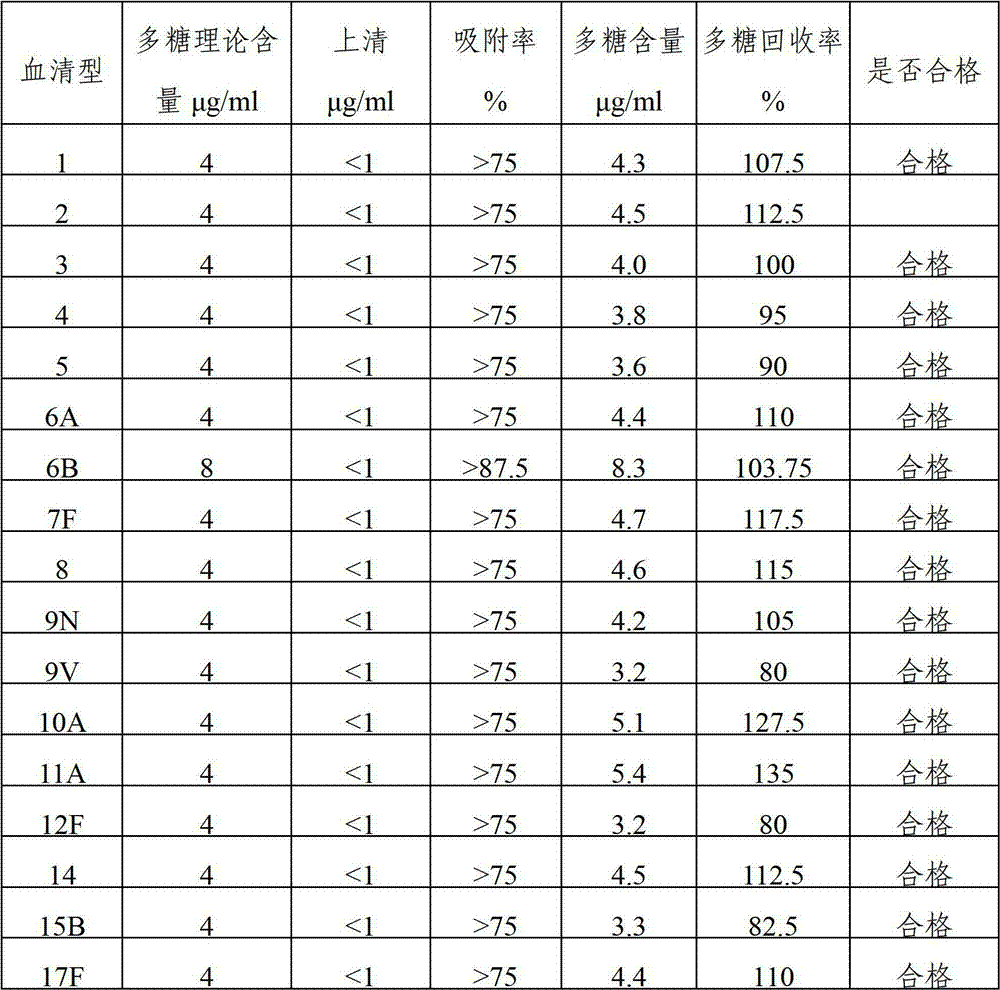
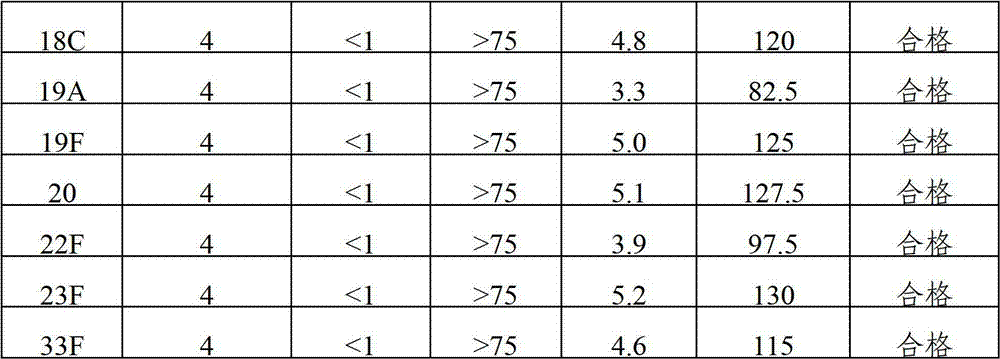
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN104069488AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
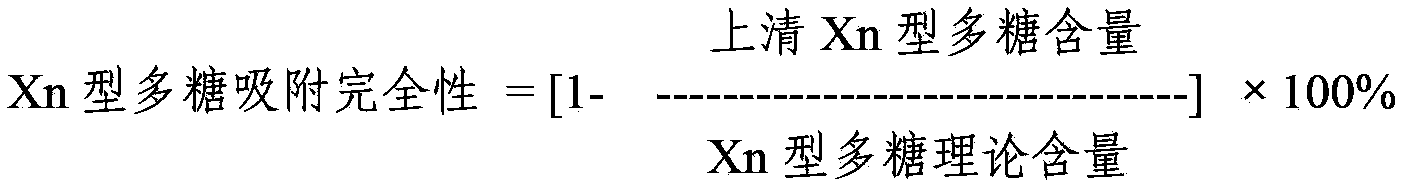
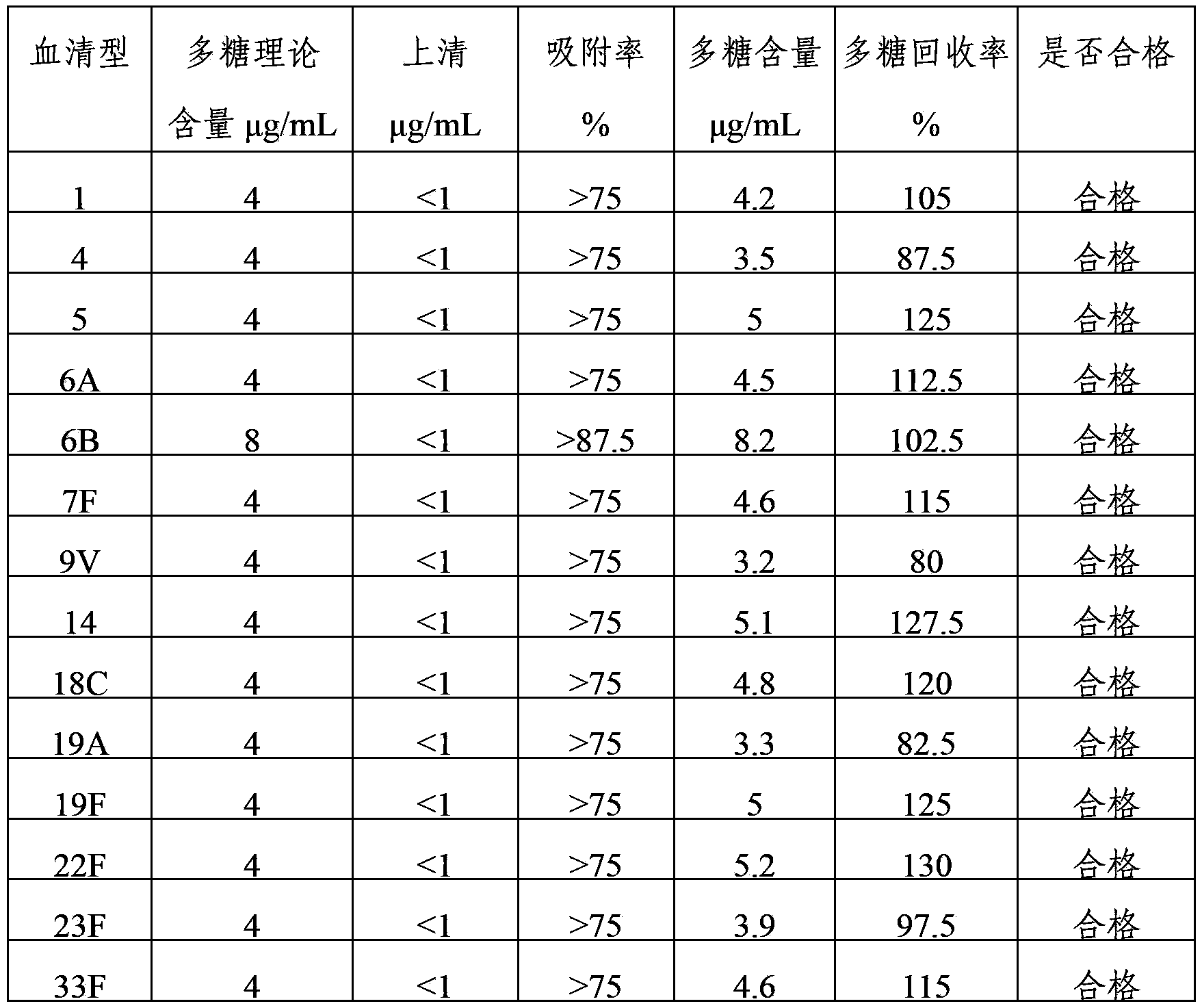
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed by covalent linkage of multivalent pneumococcus capsular polysaccharides of 14 different serotypes and carrier protein, wherein the 14 serotypes include 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. The conjugated composition has good adsorption effect and good stability, has multiple immunogenicity and protective performance against invasion of the pneumococcus of 14 serotypes, is superior to on-sale low-valent pneumonia compositions, and the immune response of the conjugated composition disclosed by the invention is higher than that of an uncombined composition. The inoculating injection frequency can be reduced by using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the immune process can be simplified, and diseases of human and animals caused by the 14 serotypes of pneumococcal bacteria can be effectively prevented. The conjugated composition has wider coverage and better immune effect.
Owner:SINOVAC RES & DEV
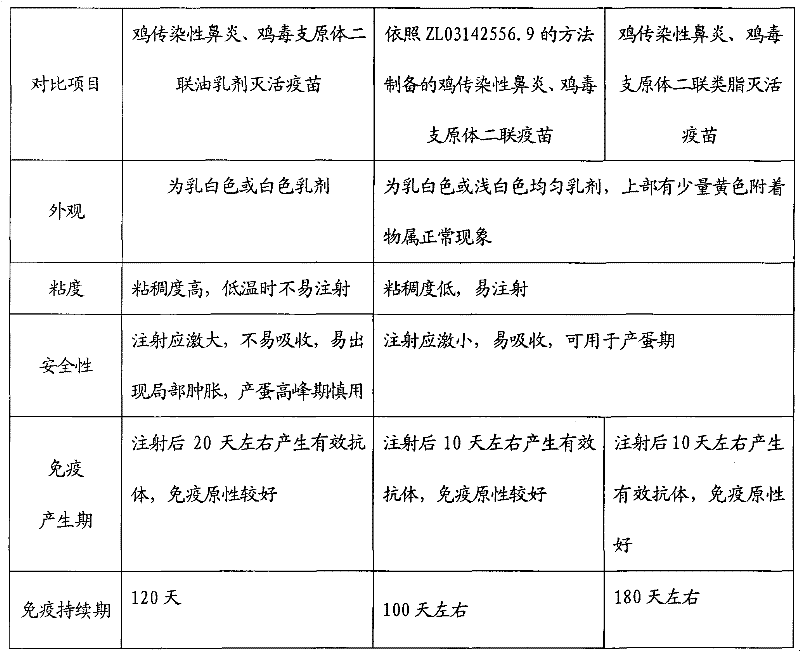
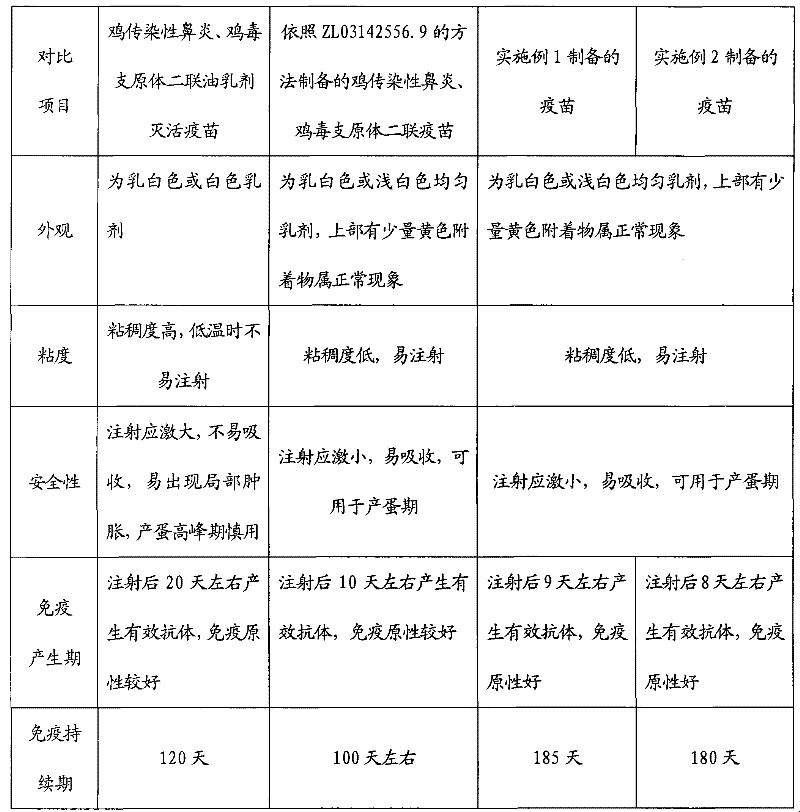
Preparation method of chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine
InactiveCN102406925AReduce manufacturing costIncrease production costAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma gallisepticum antigen
The invention provides a preparation method of a chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine, belonging to the technical field of biological products for animals. The preparation method is implemented by the following steps of: preparing a type A haemophilus paragallinarum inactivated antigen bacterial liquid, a type C haemophilus paragallinarum inactivated antigen bacterial liquid and a mycoplasma gallisepticum antigen liquid; uniformly mixing the type A haemophilus paragallinarum inactivated antigen bacterial liquid with the type C haemophilus paragallinarum inactivated antigen bacterial liquid in the volume ratio of 1:1 to obtain a mixed haemophilus paragallinarum inactivated antigen bacterial liquid; uniformly mixing the mixed haemophilus paragallinarum inactivated antigen bacterial liquid with the mycoplasma gallisepticum antigen liquid in the ratio of 1:1-1:1.5, pouring into an emulsifying tank and stirring; slowly adding poplar bark lipid till the final volume percentage concentration of the poplar bark lipid in the mixture is 2.0-3.8 percent; and continually stirring and emulsifying for 30-60 minutes. The vaccine has the advantages of easiness for absorbing, no toxic or side effect, short immunogenic time, long immune duration, good immune effect and capability of effectively preventing chicken infectious rhinitis and mycoplasma gallisepticum.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
Vaccine composition, preparation method and application thereof
ActiveCN103908665AReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsDiluentWater soluble
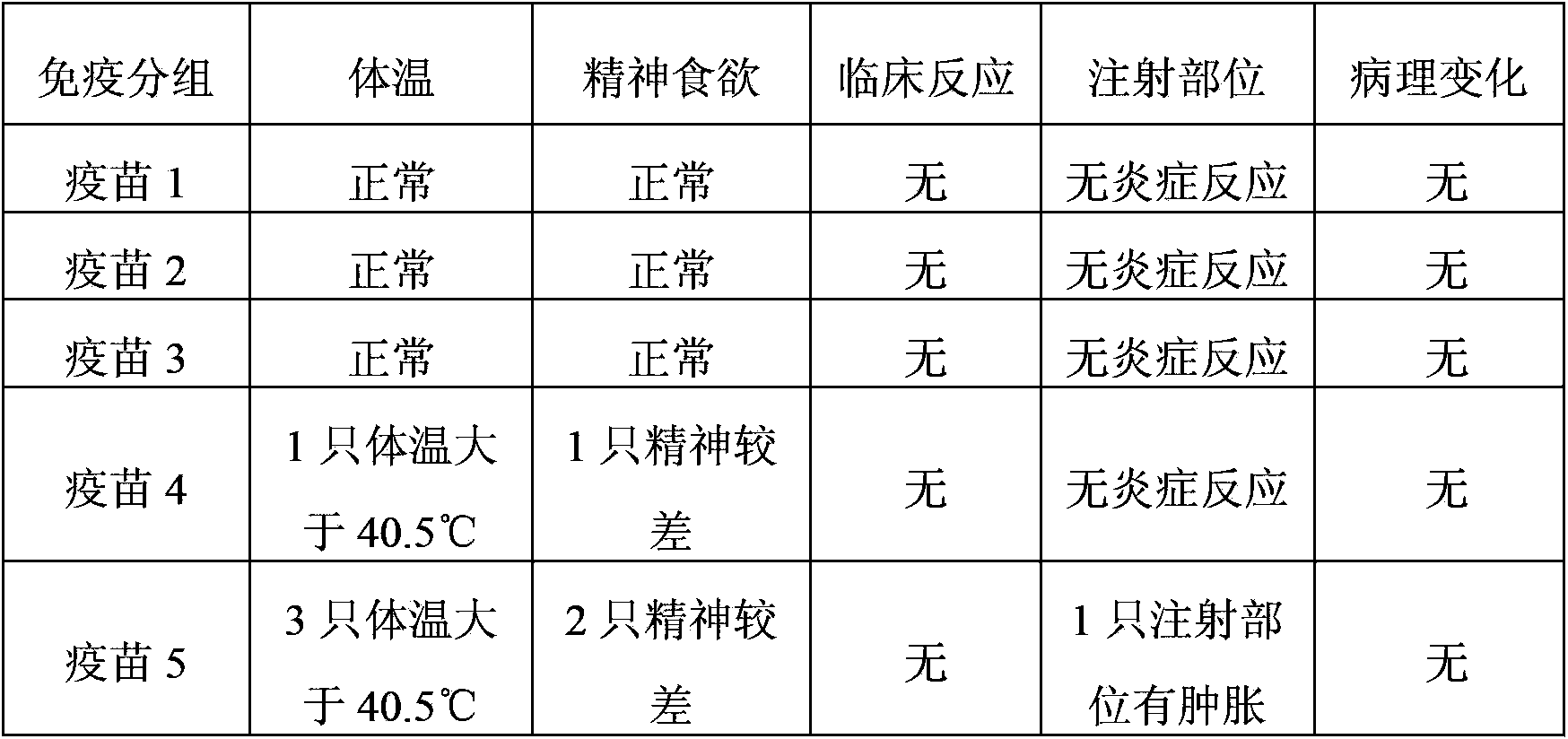
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
Bacterial polysaccharide protein conjugate vaccine using hepatitis B surface antigen as carrier protein and preparation method of bacterial polysaccharide protein conjugate vaccine
InactiveCN104383532AAddressing Immunization IssuesPlay a role in preventionAntibacterial agentsAntiviralsAntigenConjugate vaccine
The invention discloses a bacterial polysaccharide protein conjugate vaccine using a hepatitis B surface antigen as carrier protein and a preparation method of the bacterial polysaccharide protein conjugate vaccine. According to the vaccine, protein is the hepatitis B surface antigen, and a bacterial polysaccharide is selected from any one or more of a haemophilus influenza type b polysaccharide, group A, group C, group Y and group W135 meningococcal polysaccharides, a salmonella typhi type Vi polysaccharide, a group B streptococcus type Ia polysaccharide and the like, pneumococcus serotype type 1, 2 and the like, and salmonella paratyphi type A or salmonella paratyphi type B. Animal experiments show that the antibody positive conversion rates of the bacterial polysaccharide and the hepatitis B surface antigen in the vaccine are both more than 85%, so that the vaccine is relatively high in antibody positive conversion rate; carrier protein plays a role in transforming the bacterial polysaccharide from a T-cell-independent antigen into a T-cell-dependent antigen, and also can be used for preventing diseases caused by hepatitis B virus; and by adopting the bacterial polysaccharide protein conjugate vaccine disclosed by the invention, the problem of performing immunization inoculation on infants and young children under 2 years old can be solved, the function of one injection with multiple immune effects also can be achieved, and the use crowd and coverage rate of the vaccine can be expanded.
Owner:云南沃森生物技术股份有限公司
Antigen composition for preventing and treating secondary infected respiratory system diseases of pigs, preparation method and application thereof
ActiveCN103784951AChange cognitive biasImprove immunityAntibacterial agentsViral antigen ingredientsDiseaseAntigen
The invention provides an antigen composition for preventing and treating secondary infected respiratory system diseases of pigs. The antigen composition comprises at least one porcine reproductive and respiratory syndrome antigen with immune amount, and at leas one haemophilus parasuis antigen with immune amount and an adjuvant, wherein the porcine reproductive and respiratory syndrome antigen and the haemophilus parasuis antigen respectively and independently comprise attenuated live holoantigen, inactivated holoantigen, subunit antigen, recombinant live carrier antigen and DNA (deoxyribonucleic acid) carrier antigen. The antigen composition cannot cause mutual immune interference or influence of two antigen components, and enables the immune effects of porcine reproductive and respiratory syndrome antigen and the haemophilus parasuis antigen to be strengthened, and is convenient in vaccination process and good in safety.
Owner:PU LIKE BIO ENG
Influenza-pandemic influenza bivalent combined vaccine and preparation method thereof
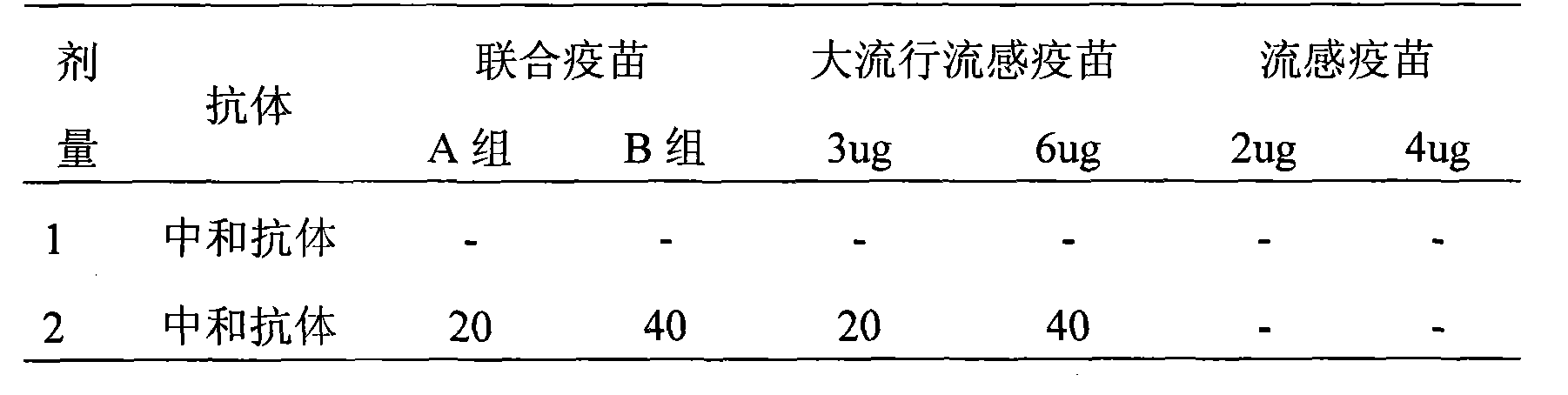
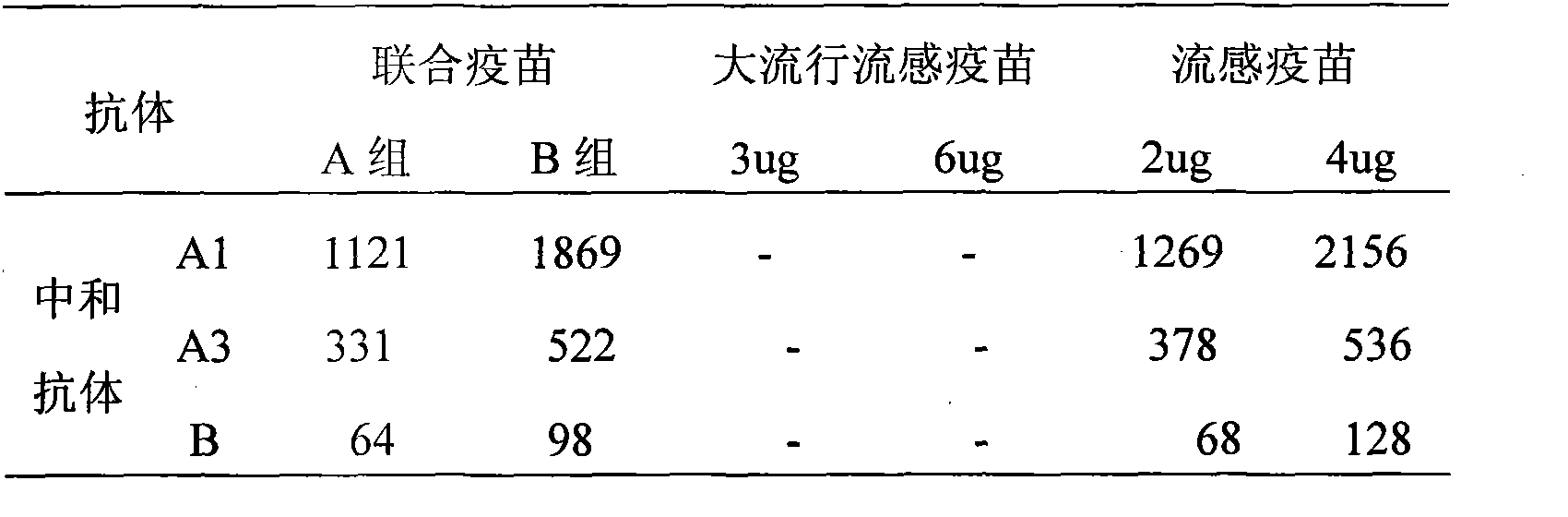
InactiveCN101524538AFlu preventionReduce the number of vaccinationsAntiviralsAntibody medical ingredientsAntigenBird flu
The invention provides a high-efficiency low-cost influenza-pandemic influenza bivalent combined vaccine which can be produced in large amount and a preparation method thereof, relating to a novel influenza-pandemic influenza vaccine preparation used for injection and a preparation process thereof and comprising preparation of virus seed banks and production and preparation of vaccine stock solution. The preparation method refers to that of whole-virus inactivated vaccines, split vaccines and subunit vaccines of the influenza and split vaccines of the pandemic influenza. The influenza-pandemic influenza bivalent combined vaccine preparation provided by the invention comprises two antigens for influenza and bird flu and can conveniently prevent viral epidemics and outbreak of the influenza and bird flu.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
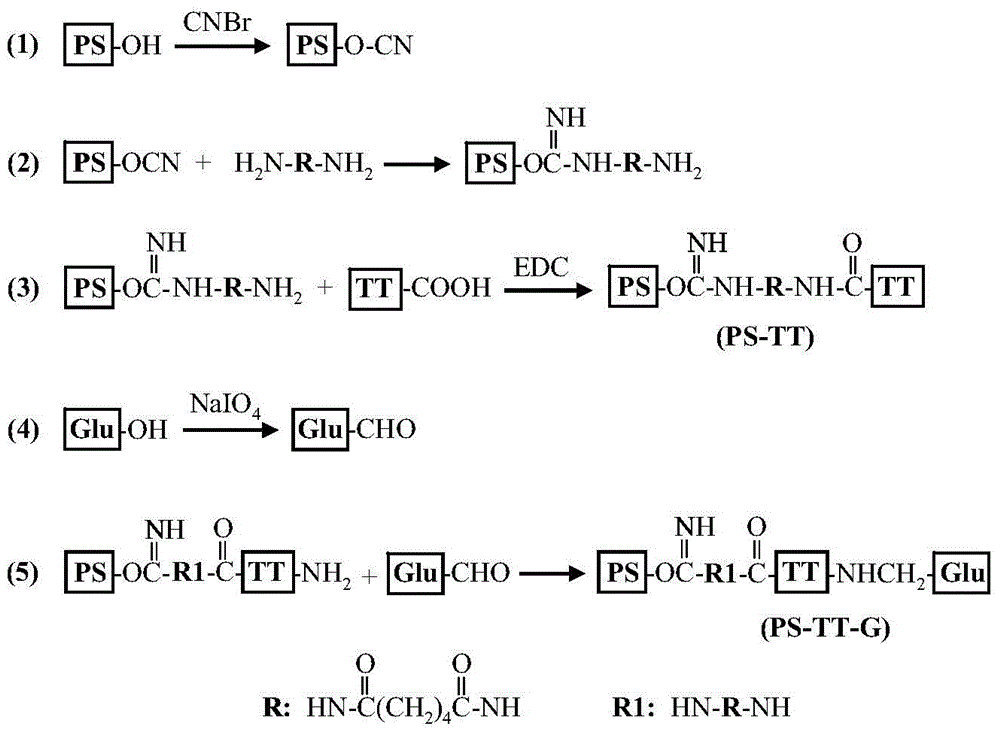
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
InactiveCN102178949ASimplified and expanded immunization programsReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseTetanus toxoids
The invention provides combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin (DTaP-sIPV) inactivated poliovirus and preparation thereof. The DTaP-sIPV is characterized in that each 100ml of the combined vaccine comprises the following components: 400-1800ug (PN) of acellular pertussis (AP) stock solution, 300-700Lf of tetanus toxoid (TT), 1000-2500Lf of diphtheria toxoid (DT), 126-154mg of Al(OH)<3>, 3000-6000DU of sIPV I, 5700-7100DU of sIPV II, 4500-9000DU of sIPV III, 765-935mg of NaCl, 0-600mg of 2-phenoxyethanol and the balance of H<2>O. Compared with the existing products, the DTaP-sIPV has the advantages of higher biological safety, better side reaction and the like; and the DTaP-sIPV has the beneficial effects of preventing a plurality of target diseases, reducing inoculating needles, simplifying immunization programs, improving inoculation rate, reducing opportunity of cross infection, being popular with a majority of parents and children, saving various expenses and facilitating smooth promotion of immunization plan.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
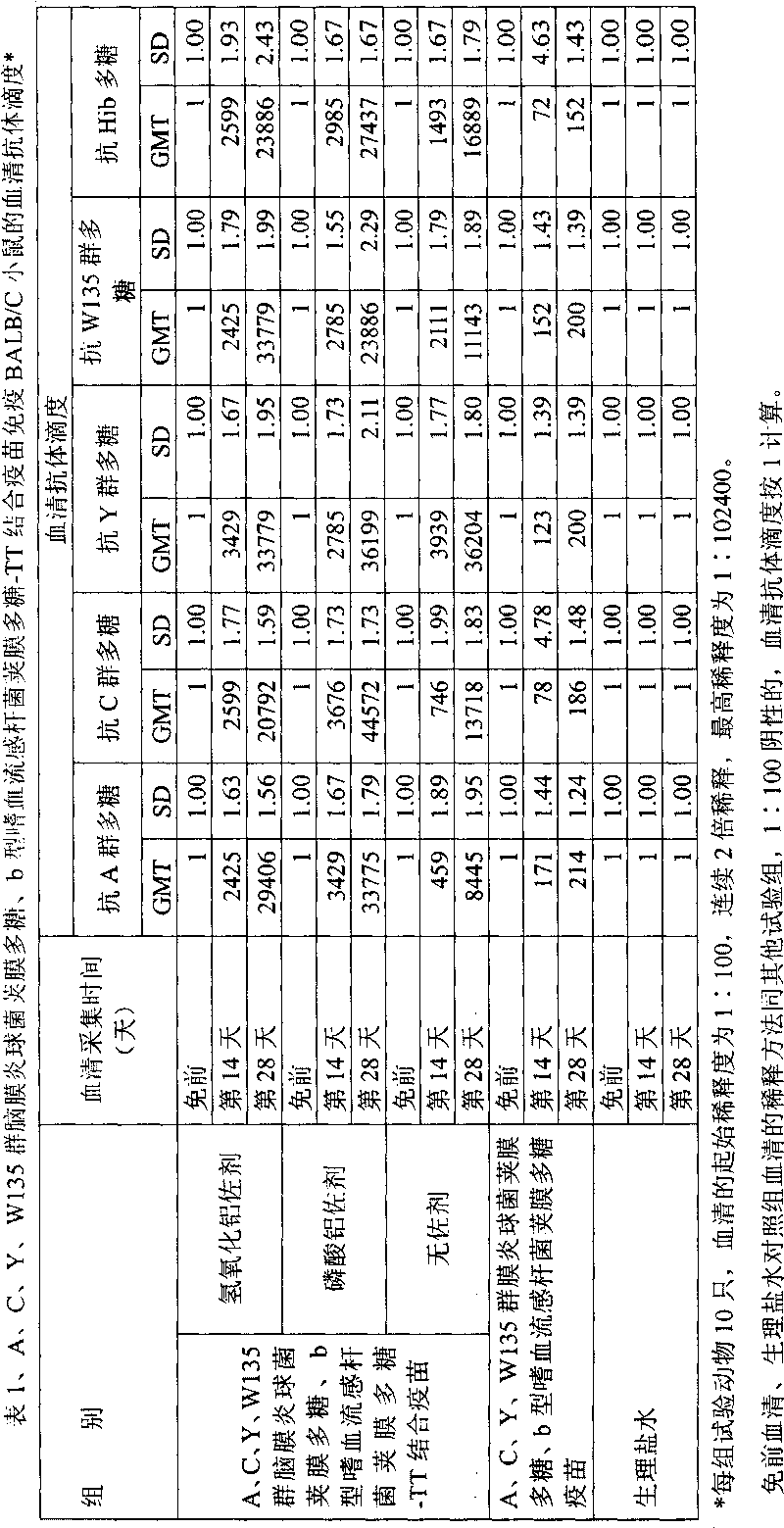
Combined vaccine bonding bacterial polysaccharides and protein for human
InactiveCN101559222AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsBacterial polysaccharideHaemophilus influenzae
The invention discloses a bacterial polysaccharides and protein binding combined vaccine for human, wherein Group A and Group B epidemic cerebrospinal meningitis Neisser's coccus capsular polysaccharides and Type B haemophilus influenzae capsular polysaccharide are bonded on an effective protein carrier with covalent bonds by a chemical method to prepare a preventive combined vaccine bonding polysaccharides and protein for human. Three vaccinations are conducted on children with the age of three to eight months and each for one month, and one vaccination is conducted on children older than one year. More than 96.5 percent of vaccine inoculators can be immunized from the Group A and Group C epidemic cerebrospinal meningitis Neisser's coccus and 91.2 percent of the vaccine inoculators can obtain long-term immunity from the Type B haemophilus influenzae one month after immunization. The vaccine is applied to the prevention of the infection caused by the Group A and Group C epidemic cerebrospinal meningitis Neisser's coccus and the Type B haemophilus influenzae.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +1
Zika-virus-and-yellow-fever-virus combined inactivated vaccine
ActiveCN107537029AStable physical and chemical propertiesReduce the number of vaccinationsViral antigen ingredientsInactivation/attenuationDiseaseZika virus
The invention provides a Zika-virus-and-yellow-fever-virus combined inactivated vaccine, and belongs to the technical field of biological product preparing. 0.5 microgram / ml-10 microgram / ml of yellowfever viruses and 0.5 microgram / ml-10 microgram / ml of Zika viruses are contained in the combined inactivated vaccine. The invention also provides a preparing method for the Zika-virus-and-yellow-fever-virus combined inactivated vaccine; the preparing method includes the steps of Zika-virus and yellow-fever-virus inoculating, purifying and inactivating, wherein yellow-fever-virus inoculated MOI is0.01 PFU / ml to 1 PFU / ml, and Zika-virus inoculated MOI is 0.001 CCID<50> / ml to 0.1 CCID<50> / ml. By means of the Zika-virus-and-yellow-fever-virus combined inactivated vaccine, the Zika viruses and theyellow fever viruses can be immunized at the same time, infection and anaphylaxis which are caused by an attenuated vaccine are avoided, and the Zika-virus-and-yellow-fever-virus combined inactivatedvaccine has the good capacity for controlling diseases caused by the Zika viruses and the yellow fever viruses.
Owner:SINOVAC BIOTECH
Recombinant adenovirus and construction method thereof
InactiveCN102154225ASolve the problem of not being immune at the same timeReduce the number of vaccinationsViruses/bacteriophagesGenetic engineeringVaccinationSwine Fever Virus
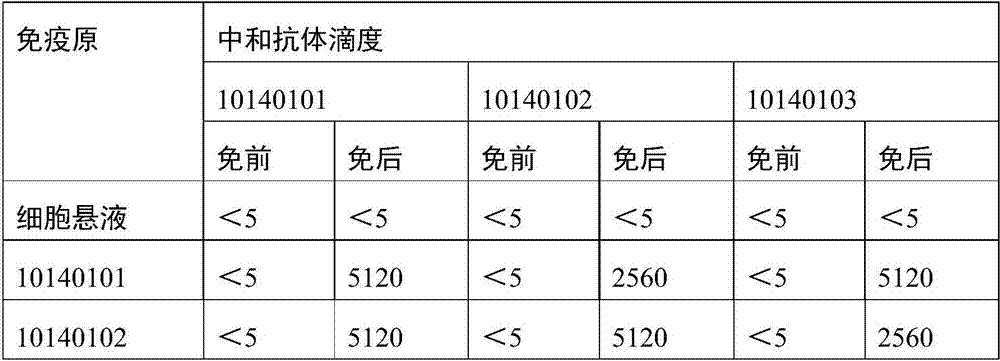
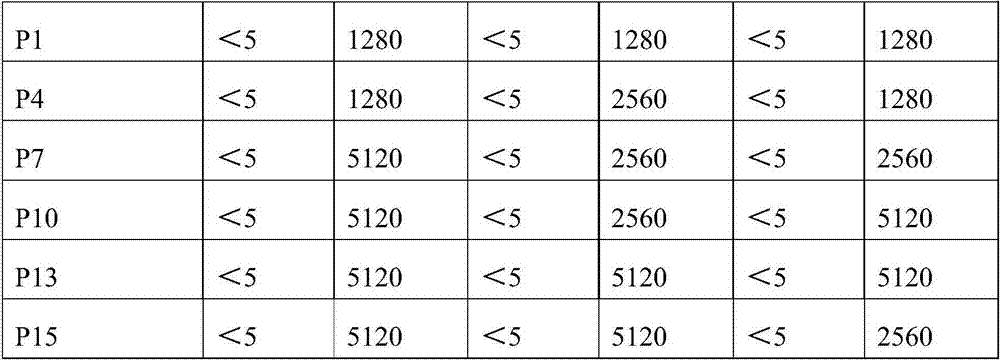
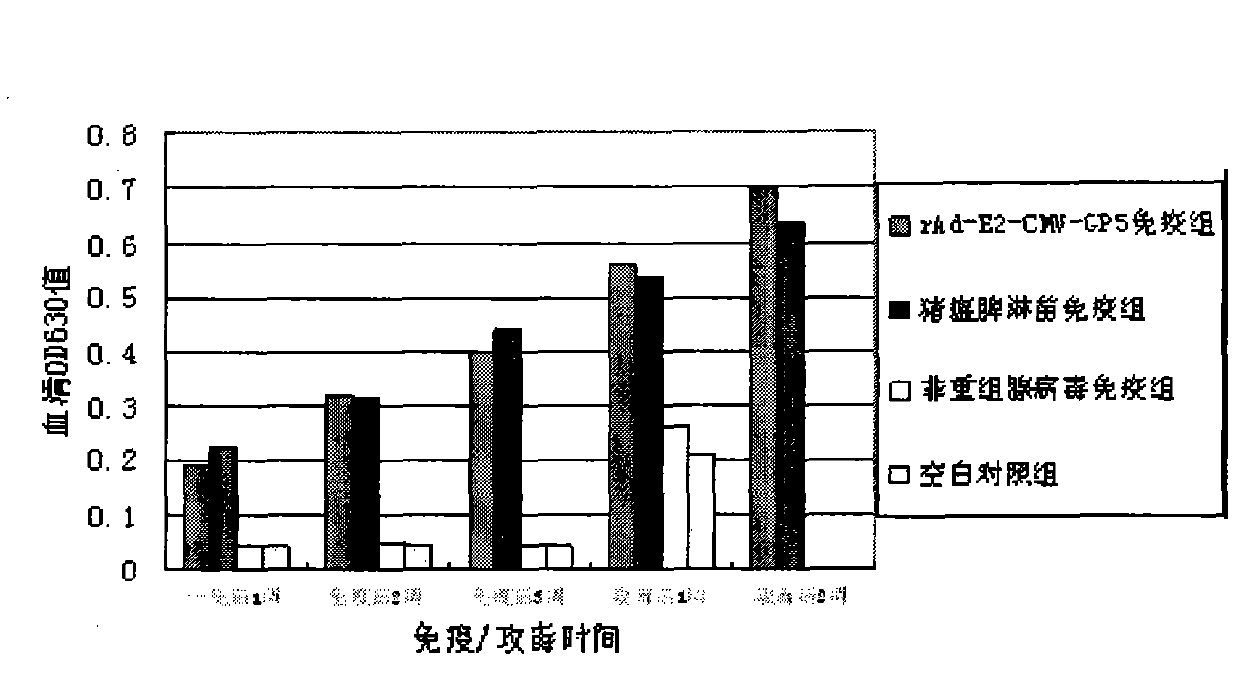
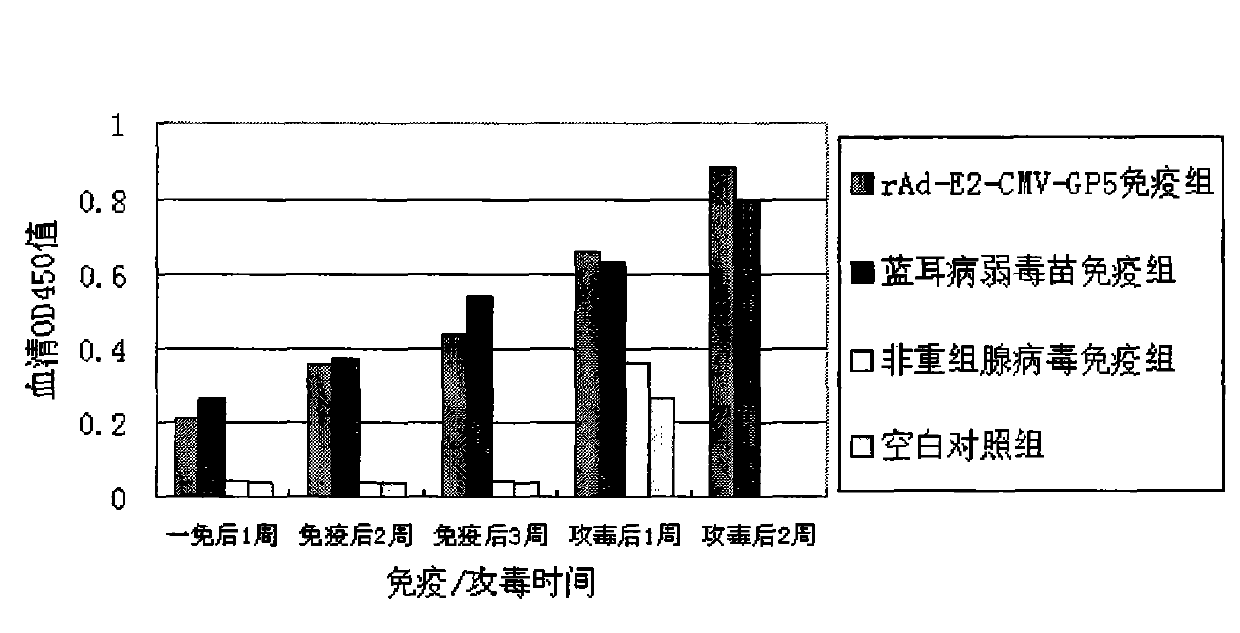
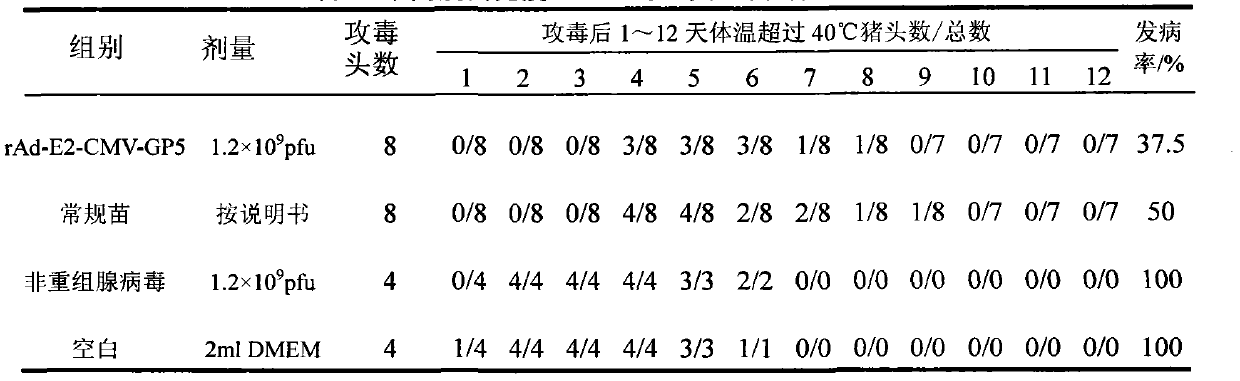
The invention provides a recombinant adenovirus and a construction method thereof. The construction method comprises the following steps of: inserting swine fever virus E2 genes into a multiple cloning site Bg1 II and a Sa1 I enzyme cutting site on the downstream of a CMV (cytomegalovirus) promotor of the adenovirus; and inserting porcine reproductive and respiratory syndrome virus GP5 genes into enzyme cutting sites Kpn I and Not I on the downstream of the CMV promotor of the adenovirus. The problems of low expression yield and weak protection effect when the E2 and GP5 genes are fused and connected in series are solved. Only by once immune vaccination, a pig can obtain antibodies for a swine fever virus and a porcine reproductive and respiratory syndrome virus. The recombinant adenovirus is a nonreplicative virus and is not reproduced in a pig body. The phenomena of virus atavism and toxicity strengthening caused by long-term vaccination of an attenuated vaccine are avoided. Moreover, the recombinant adenovirus only contains main protection genes of the swine fever virus and the porcine reproductive and respiratory syndrome virus and does not contain other genes of viruses, so that recombination cannot occur between the attenuated vaccine and a wild strain. A vaccine prepared from the recombinant adenovirus has a protection effect of 87.5 percent on coalition attack of the swine fever virus and the porcine reproductive and respiratory syndrome virus after used for immunizing an animal. The problem that the swine fever and porcine reproductive and respiratory syndrome vaccines cannot be immunized simultaneously is solved. The problems of strengthened toxicity and homologous recombination after the immunization of the attenuated vaccine are also solved.
Owner:云南生物制药有限公司 +2
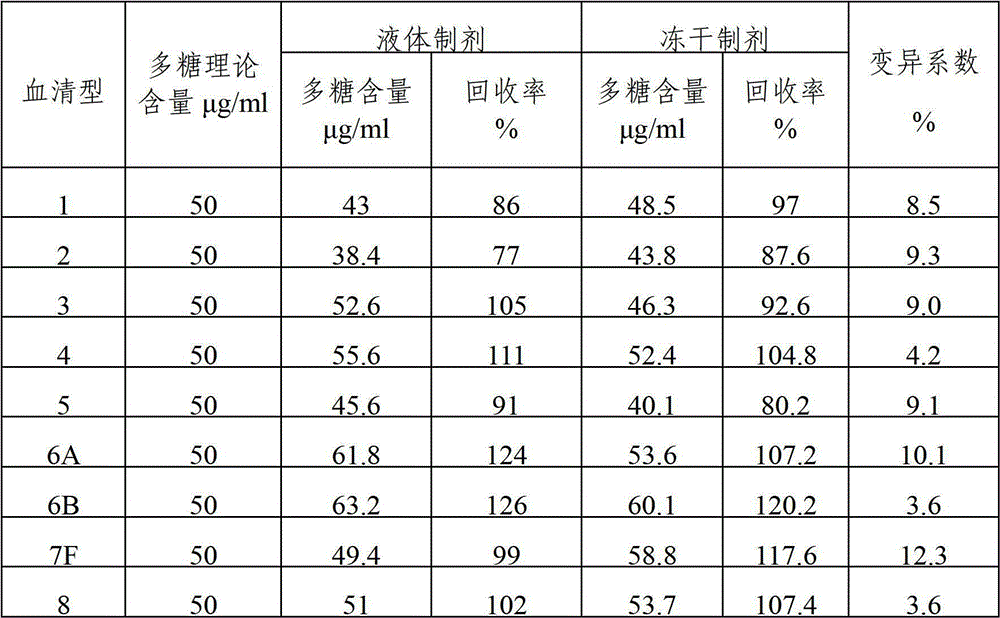
Polyvalent pneumococcal capsular polysaccharide-protein conjugate composition and preparation method thereof
ActiveCN103656631BImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
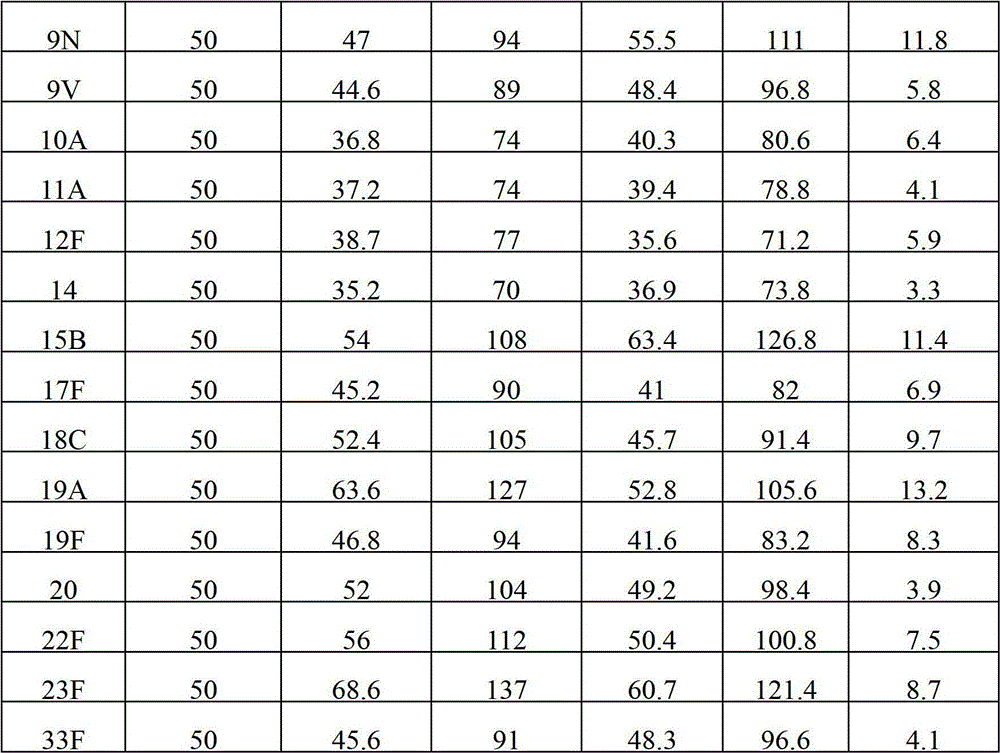
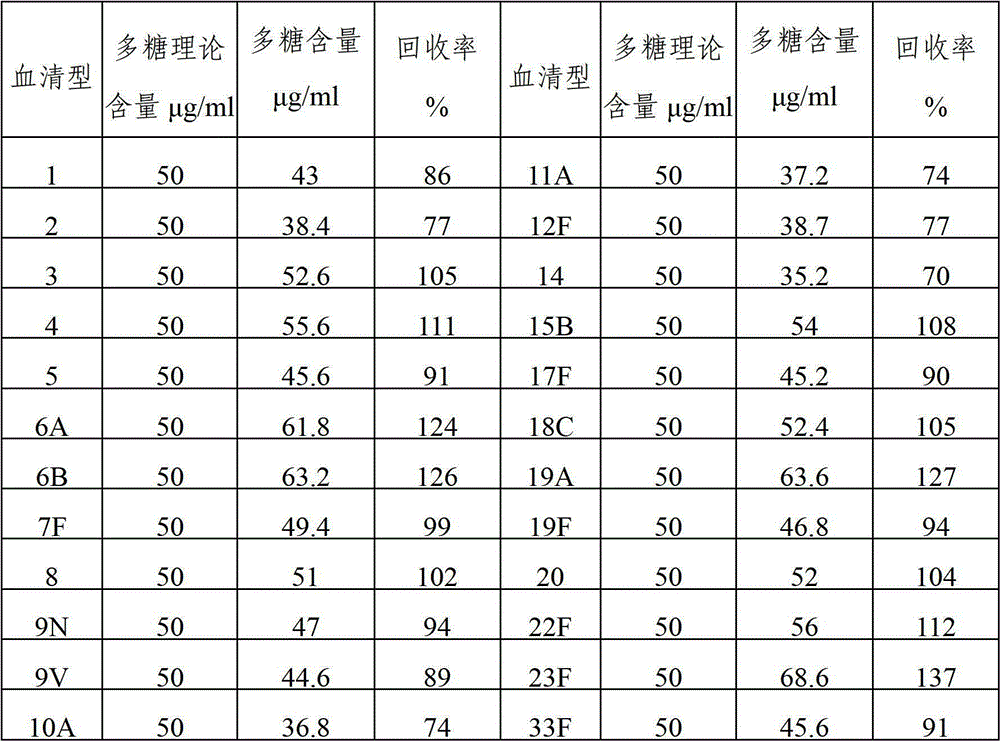
The invention provides a multivalence pneumococcus capsular polysaccharide-protein conjugate composition and a preparation method thereof. The conjugate composition is prepared from capsular polysaccharides of pneumococcus of 24 different serotypes and a carrier protein in a covalence connection manner, wherein the 24 different serotypes are 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The conjugate composition is good in adsorption effect and stability, has multiple immunogenicity and protection properties aiming at invasion of pneumococcus of the 24 serotypes, and is superior to the low-valence pneumonia composition in the market, and the immune response is higher than that of uncombined compositions. By inoculating a multivalence pneumococcus capsular polysaccharide conjugate vaccine prepared from the conjugate composition, the inoculation injection times can be reduced, the immunization procedure can be simplified, and meanwhile human beings and animals can be effectively prevented from diseases resulted from the pneumococcus of the 24 serotypes, and the conjugate composition is wide in coverage range and good in immune effect.
Owner:SINOVAC RES & DEV
Hepatitis A-hepatitis E combined vaccine and preparation method thereof
InactiveCN1883704AImproving immunogenicityLow production costDigestive systemAntiviralsAttenuated Live VaccineHepatitis E
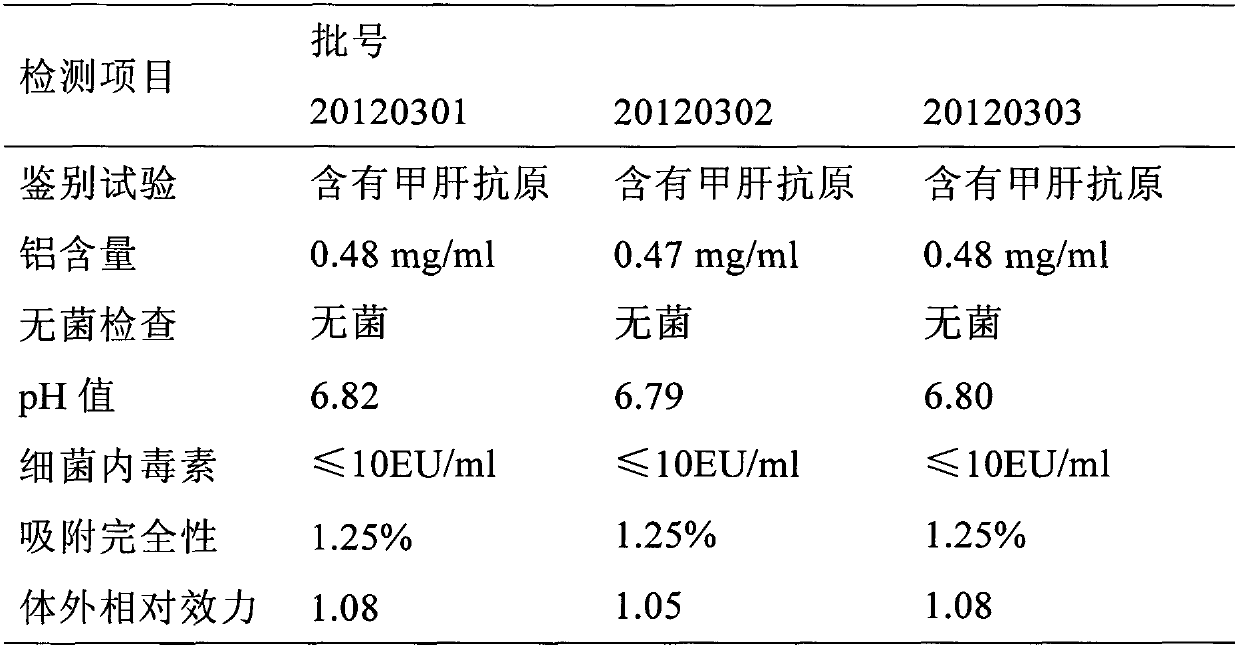
Disclosed are a combined vaccine for hepatitis A and hepatitis E, and preparation method thereof. Said vaccine comprises hepatitis E virus recombined peoteins, hepatitis A attenuated live vaccine and hepatitis A inactivated vaccine. Said method comprises before the paretion of the final combined vaccine, absorbing hepatitis A attenuated live vaccine viruses and / or hepatitis A inactivated vaccine viruses on aluminum hydroxide gel, absorbing hepatitis E virus recombined peoteins on aluminum hydroxide gel, adjusting the pH value of the two compositons, and mixing the two compositons together. The inventiion is provided with convenience, multiple-effect, low cost, and effective prevention of hepatitis A and hepatitis E for human and animals.
Owner:SOUTHEAST UNIV
Combined hepatitis A and B vaccine and preparation method thereof
InactiveCN102988975AReduce the number of vaccinationsReduce the chance of missed seedsAntiviralsViruses/bacteriophagesViral antigensAntigenic protein
The invention discloses a combined hepatitis A and B vaccine comprising a hepatitis A viral antigen and a hepatitis B virus surface antigen protein, wherein the hepatitis A viral antigen is obtained by carrying out human embryo lung diploid cell culture on hepatitis A viruses, collecting and purifying, and the content of the hepatitis A viral antigen is 630-660EU / ml; and the hepatitis B virus surface antigen protein is obtained by fermenting recombinant liquor-making yeasts, expressing and purifying, and the content of the hepatitis B virus surface antigen protein is 15-30 microgram per milliliter. According to the invention, frequent inoculation times and the opportunity of the leakage of inoculation are reduced, the success rate of inoculation is improved and the cost of inoculation is reduced, and a more effective way is provided to control the infection and prevalence of hepatitis A and B. The combined hepatitis A and B vaccine is good in security, immunogenicity and stability.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Vaccine composition, preparation method and application thereof
ActiveCN104248759AImprove immunityReduce stress responseAntibacterial agentsBacterial antigen ingredientsImmune effectsAdditive ingredient
The invention provides a vaccine composition, which includes an immune amount of a mycoplasma hyopneumoniae antigen, an immune amount of a swine influenza virus antigen and an immune amount of a porcine reproductive and respiratory syndrome virus antigen. All antigens of the vaccine composition not only do not generate mutual interference or influence of antigen components, but have the effect of mutually enhancing the immune effect instead. One immunization can achieve the immune effect and the antibody continuous level of single antigen twice immunization. Also, the vaccine composition has the advantages of good security, simple preparation method, convenient and fast immunization, and reduction of the immunization cost, etc.
Owner:PU LIKE BIO ENG
Combined vaccine, and preparation method and application thereof
ActiveCN110743007AReduce the number of vaccinationsSsRNA viruses positive-senseViral antigen ingredientsChickenpoxEncephalitis Viruses
The invention relates to a combined vaccine, and a preparation method and application thereof. The combined vaccine comprises an inactivated japanese encephalitis virus and an inactivated varicella zoster virus; the ratio of the total protein content of the inactivated japanese encephalitis virus in the combined vaccine to the total protein content of the inactivated varicella zoster virus in thecombined vaccine is (0.25-4):1; the preparation method of the combined vaccine comprises the steps that an inactivated japanese encephalitis virus purification solution and an inactivated varicella zoster virus purification solution are mixed to obtain a mixed virus purification solution; and the inactivated japanese encephalitis virus and the inactivated varicella zoster virus are prepared by adopting the same human diploid cells as a virus culture medium to realize mixture of the inactivated japanese encephalitis virus and the inactivated varicella zoster virus to obtain the combined vaccine. The prepared combined vaccine is safe and effective, and the number of vaccinations is effectively reduced.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
Mink hemorrhagic pneumonia and botulism combined inactivate vaccine and preparing method thereof
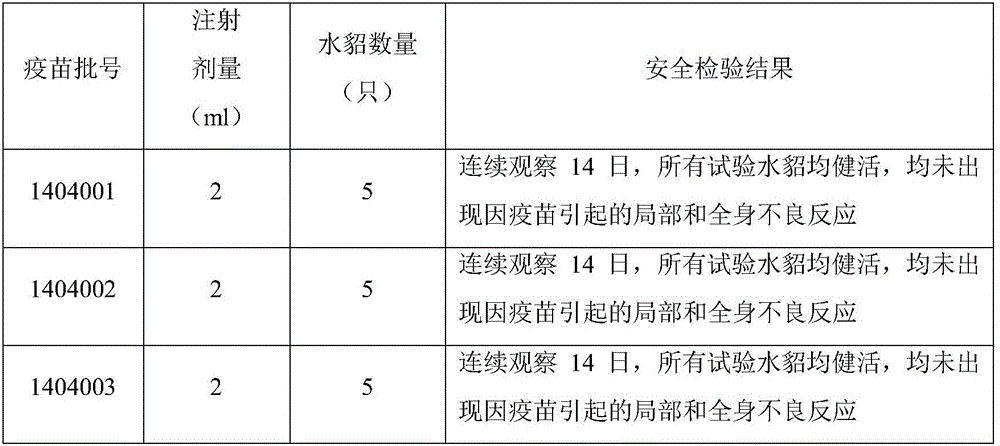
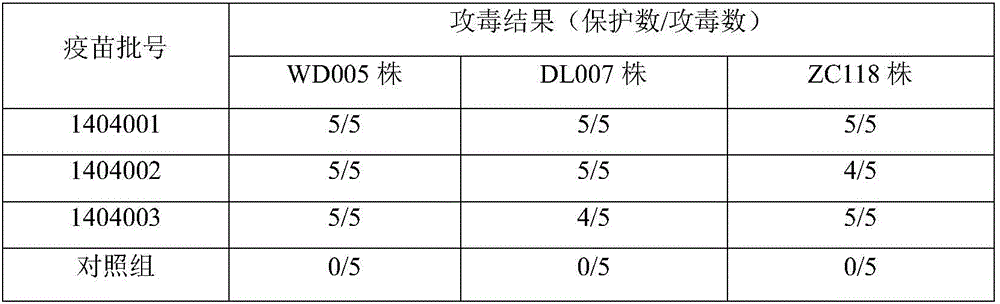
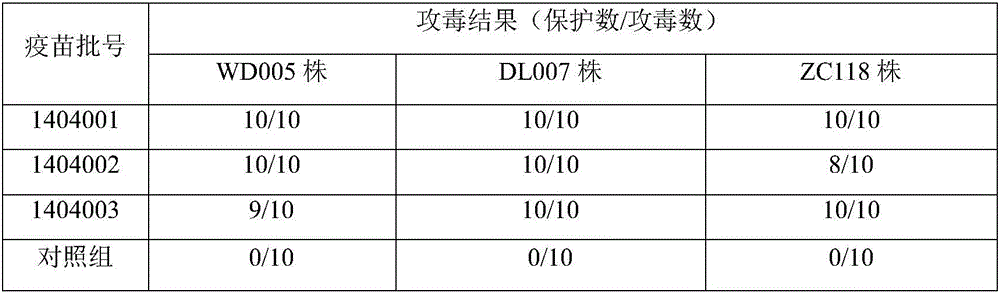
ActiveCN105749266AStrong targetingImprove protectionAntibacterial agentsBacterial antigen ingredientsVaccine manufacturingMink
The invention relates to a mink hemorrhagic pneumonia and botulism combined inactivate vaccine and a preparing method thereof.Strain WD005, strain DL007 and strain ZC118 of pseudomonas aeruginosa for vaccine manufacturing and detecting are obtained through clinical isolation, and the strains are higher in pertinence and more comprehensive in protection for current mink hemorrhagic pneumonia epidemic serotype; by means of virulence tests and immunogenicity tests, the immunogenicity is good; a strain C-type clostridium botulinum C62-4 has the advantage of being superior in immunogenicity.The combined inactivate vaccine can prevent attack of G-type pseudomonas aeruginosa, B-type pseudomonas aeruginosa, C-type pseudomonas aeruginosa and C-type clostridium botulinum to minks at the same time, and has the advantages that the number of inoculation times is reduced, and using is convenient.The labor intensity of immunization is relieved, the immune cost is reduced, the stress reaction of animals is reduced, and the vaccine is more economical and reliable.
Owner:QILU ANIMAL HEALTH PROD
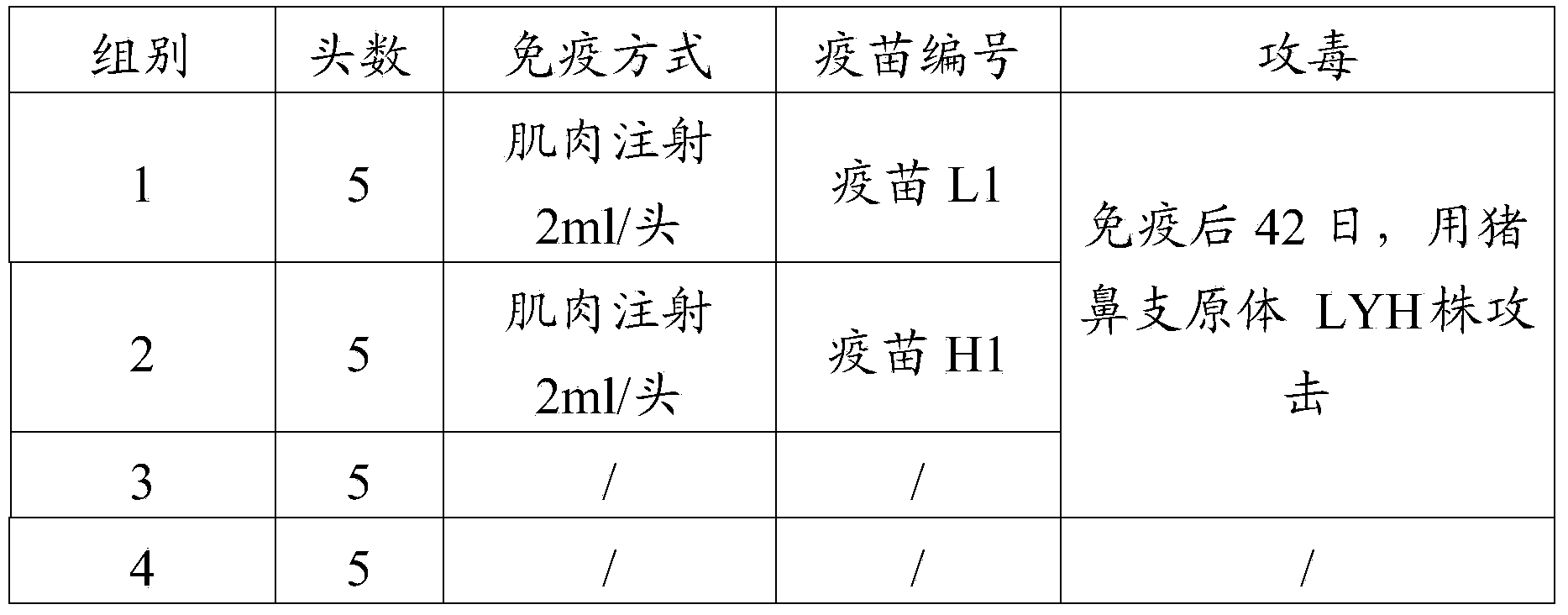
Mycoplasma hyorhinis strain, vaccine composition, preparation method and application thereof
ActiveCN104250623AImprove the effect of prevention and controlImmunity overAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
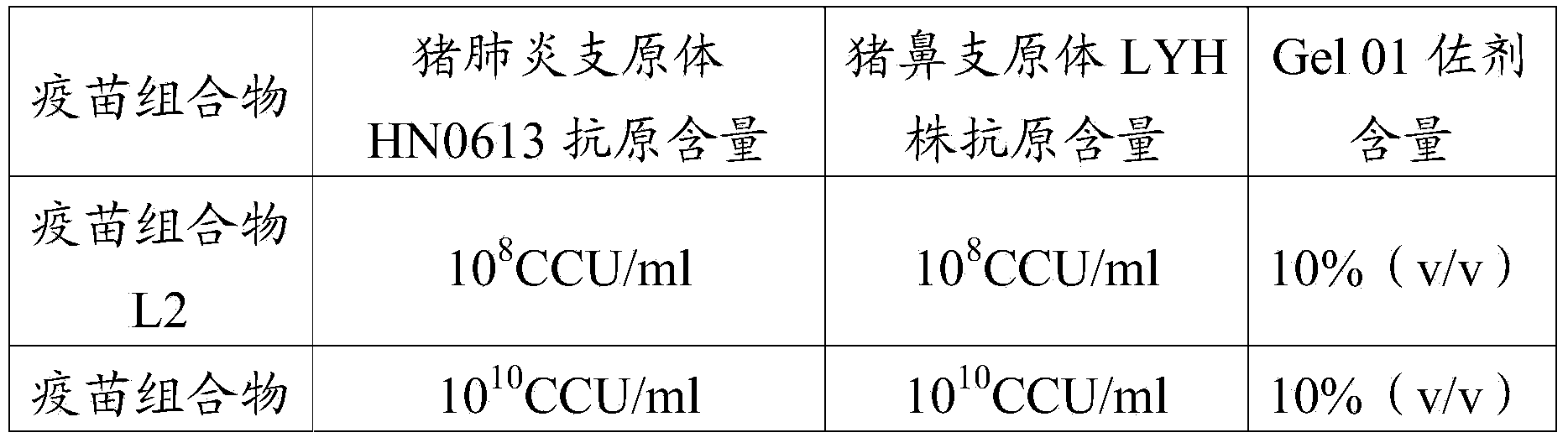
The invention provides a Mycoplasma hyorhinis strain LYH, and a vaccine composition prepared from the Mycoplasma hyorhinis strain LYH, in particular to a vaccine composition comprising the Mycoplasma hyorhinis and Mycoplasma hyopneumoniae. The vaccine composition can be effective in prevention and treatment of swine enzootic pneumonia caused by Mycoplasma hyorhinis, Mycoplasma hyopneumoniae single infection or mixed infection. Especially in the circumstance of mixed infection, immune effect of the vaccine composition significantly exceeds that of each single vaccine.
Owner:PU LIKE BIO ENG
Vaccine composition, and preparation method and application thereof
ActiveCN104248761ASolve the problem of immune failureLow costAntibacterial agentsBacterial antigen ingredientsDiseasePestivirus
The invention relates to the field of biological products, and specifically relates to a Pestivirus suis and streptococcus suis vaccine composition and a preparation method thereof. The composition includes Pestivirus suis and streptococcus suis antigens. The invention also relates to application of the vaccine composition to preparation of a composition for the prevention and / or treatment of diseases associated with Pestivirus suis and streptococcus suis, and infections caused by Pestivirus suis and streptococcus suis.
Owner:PU LIKE BIO ENG
Porcine pseudorabies virus and porcine circovirus type II bivalent vaccine, and applications thereof
ActiveCN106511993AImprove securityImprove protection efficiencyViral antigen ingredientsAntiviralsAntigenDisease
The invention belongs to the technical field of vaccine, and more specifically relates to a porcine pseudorabies virus and porcine circovirus type II bivalent vaccine, and applications thereof. The porcine pseudorabies virus and porcine circovirus type II bivalent vaccine comprises inactivated porcine pseudorabies virus XF-1 strain, and porcine circovirus type II-WH strain. It is confirmed by the porcine pseudorabies virus and porcine circovirus type II bivalent inactivated vaccine that XF-1 strain can be prepared into combined vaccines used for preventing two or more than two diseases with other well-known antigens in the field of vaccine, a cognition error that concurrent infection of porcine circovirus type II and porcine pseudorabies virus is caused is eliminated, combination application of porcine circovirus type II and porcine pseudorabies virus at an appropriate rate is capable of achieving excellent effect, and vaccine development prospect is promising.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Preparation method of type B haemophilus influenzae capsular polysaccharide and combined vaccine
InactiveCN105646726AHigh purityLess impuritiesAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus toxoids
The invention discloses a preparation method of a type B haemophilus influenzae capsular polysaccharide. The preparation method comprises the steps of fermentation culture, sterilization and precipitation, polysaccharide extraction and purification, polysaccharide derivatization and polysaccharide protein conjugate preparation. The invention further discloses a multivalent combined vaccine which is prepared through the steps that an acellular pertussis stock solution, refined diphtheria toxoid and refined tetanus toxoid are added into aluminum hydroxide to be adsorbed and then added into a type B haemophilus influenzae capsular polysaccharide stock solution, and the mixed solution is diluted with normal saline. According to the preparation method, the prior art is optimized, and the high-purity low-impurity-content type B haemophilus influenzae capsular polysaccharide can be obtained; meanwhile, the type B haemophilus influenzae capsular polysaccharide is combined with multiple component vaccines to form the multivalent vaccine, the multivalent vaccine can be immune to multiple diseases simultaneously, the inoculating times are decreased, the medical risk is reduced, and the better immune effect can be obtained through one-time inoculating.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
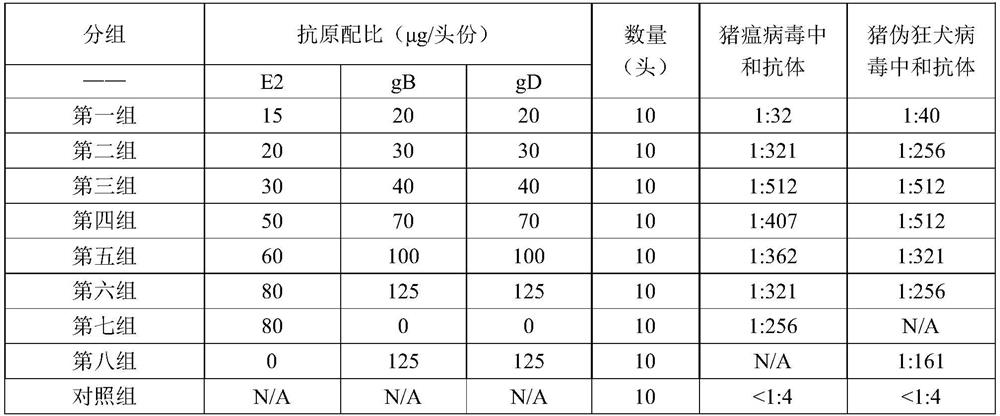
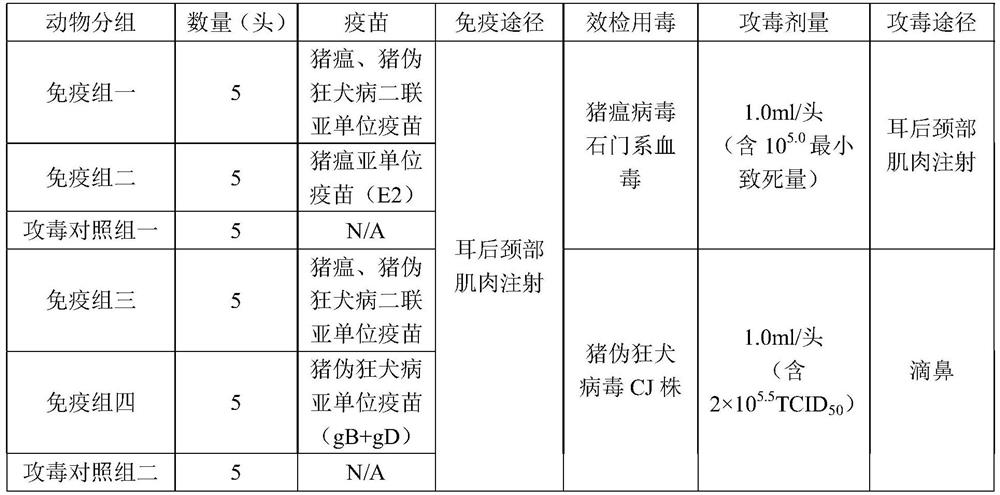
Swine fever and porcine pseudorabies virus bivalent subunit vaccine and preparation method thereof
ActiveCN114163505AEasy to prepareAntigen immunity is strongSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVAntigen
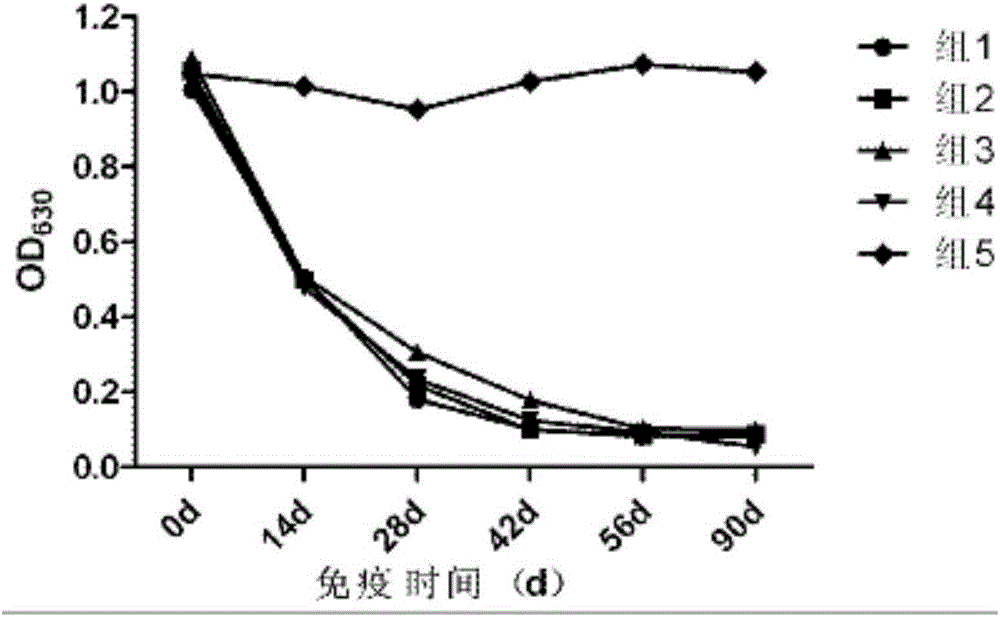
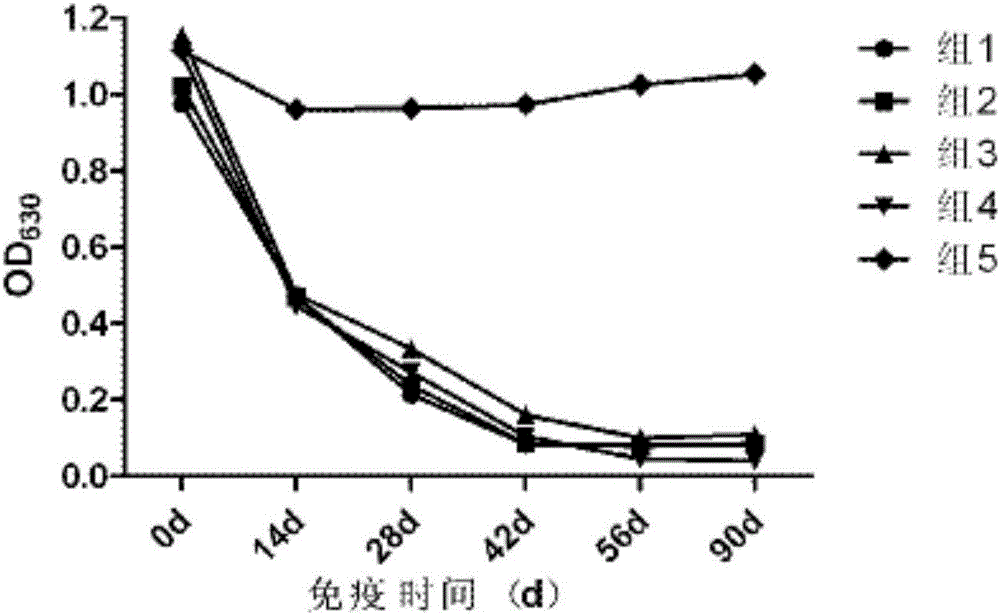
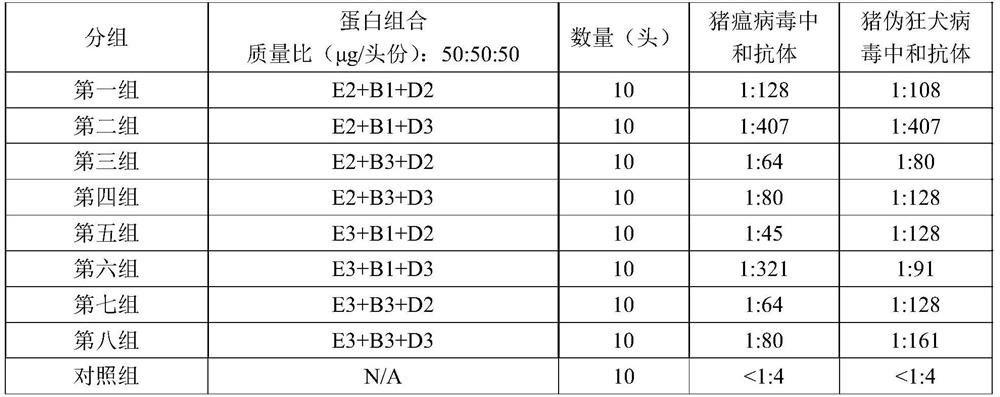
The invention provides a swine fever and porcine pseudorabies virus bivalent subunit vaccine and a preparation method thereof. The effective components of the bivalent subunit vaccine are classical swine fever virus E2 protein, porcine pseudorabies virus gB protein and porcine pseudorabies virus gD protein. The preparation method of the bivalent subunit vaccine is simple, the swine fever virus E2 protein, the porcine pseudorabies virus gB protein and the porcine pseudorabies virus gD protein are high in yield, high in purity and strong in antigen immunity, the immune response of a body can be effectively activated, and the bivalent subunit vaccine has an ideal immune protection effect on swine fever and porcine pseudorabies. Compared with a monovalent vaccine, the artificial cost can be reduced, the number of vaccination times (double prevention by one injection) is reduced, the stress reaction of pigs caused by vaccine immunization is reduced, the protection effect is equivalent to that of the monovalent vaccine, and the antibody neutralizing result is superior to that of the monovalent vaccine.
Owner:天康制药股份有限公司
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505BImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsBacteroidesHaemophilus
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Method for continuously culturing tribonema
PendingCN111500463AEfficient continuous productionEliminate protozoan contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringPure culture
A method for continuously culturing tribonema comprises the following steps: 1, building of a continuous culture reactor; 2, expanding culture of algae species; 3, expanding culture of algae species in the reactor; and 4, algae species harvesting and re-culturing. The method for continuously culturing tribonema realizes stable and efficient continuous production of the tribonema, and can finally realize the continuous industrial production of the tribonema through pure culture of algae species, secondary expanding culture of the algae species and industrial culture of the tribonema in the closed circulating reactor, the inoculation frequency of the tribonema in industrial expanding culture can be reduced, and the continuous circulating industrial production of the tribonema is realized through a fed-batch cultivation mode.
Owner:QINGDAO XUNON BIOLOGICAL ENG
Polyvalent pneumococcal capsular polysaccharide composition, its preparation method and application
ActiveCN103656632BStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Bivalent subunit vaccine of porcine circovirus type 2b and type 2d and preparation method thereof
ActiveCN110358742BImprove the level ofHigh purityViral antigen ingredientsVirus peptidesEngineeringImmunogenicity
The invention relates to the field of biotechnology, in particular to bivalent subunit vaccines of type 2b and 2d porcine circoviruses and preparation methods thereof. The invention provides recombinant baculoviruses comprising one or more copies of a Cap protein encoding gene of type 2b or 2d porcine circovirus. The invention also provides the bivalent subunit vaccines of the type 2b and 2d porcine circoviruses which comprise Cap proteins of the type 2b and 2d porcine circoviruses expressed by the recombinant baculoviruses respectively. Through artificial codon optimization, high-level and high-purity expression of the Cap proteins of the type 2b and 2d porcine circovirus is achieved, and the natural structure and immunogenicity of the Cap proteins is ensured. The bivalent subunit vaccines of the type 2b and 2d porcine circoviruses have high immunogenicity and safety, and exert an excellent immunoprotective effect on the porcine circoviruses.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
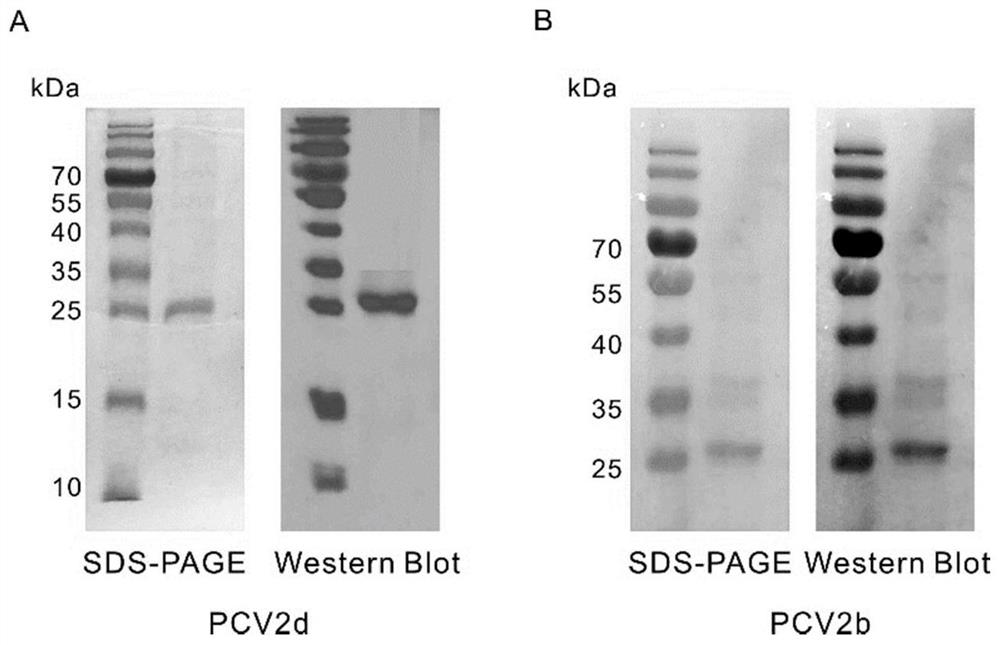
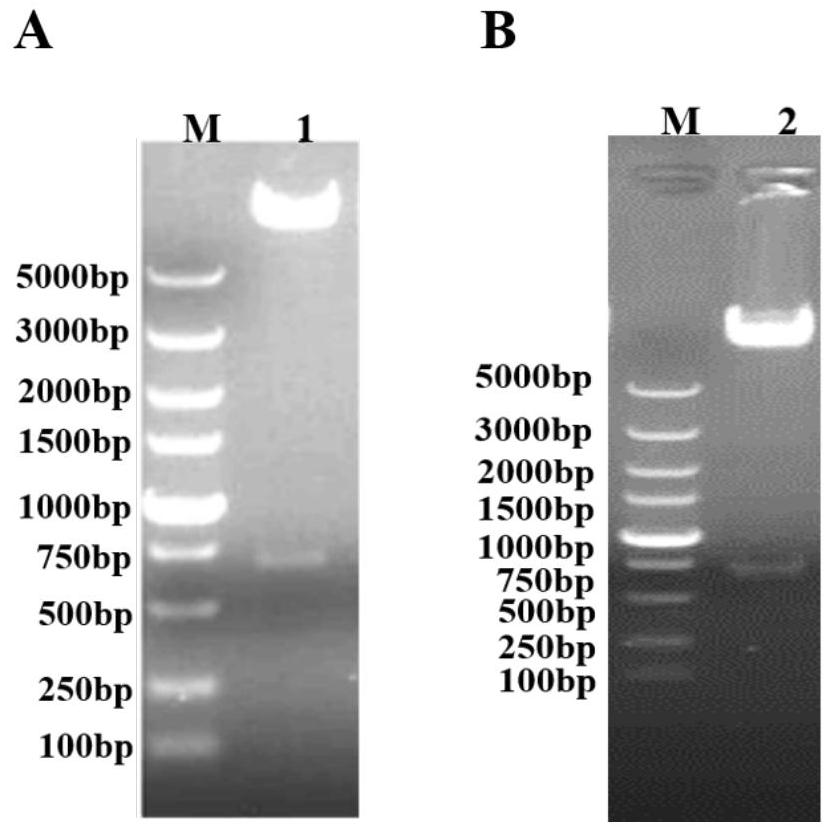
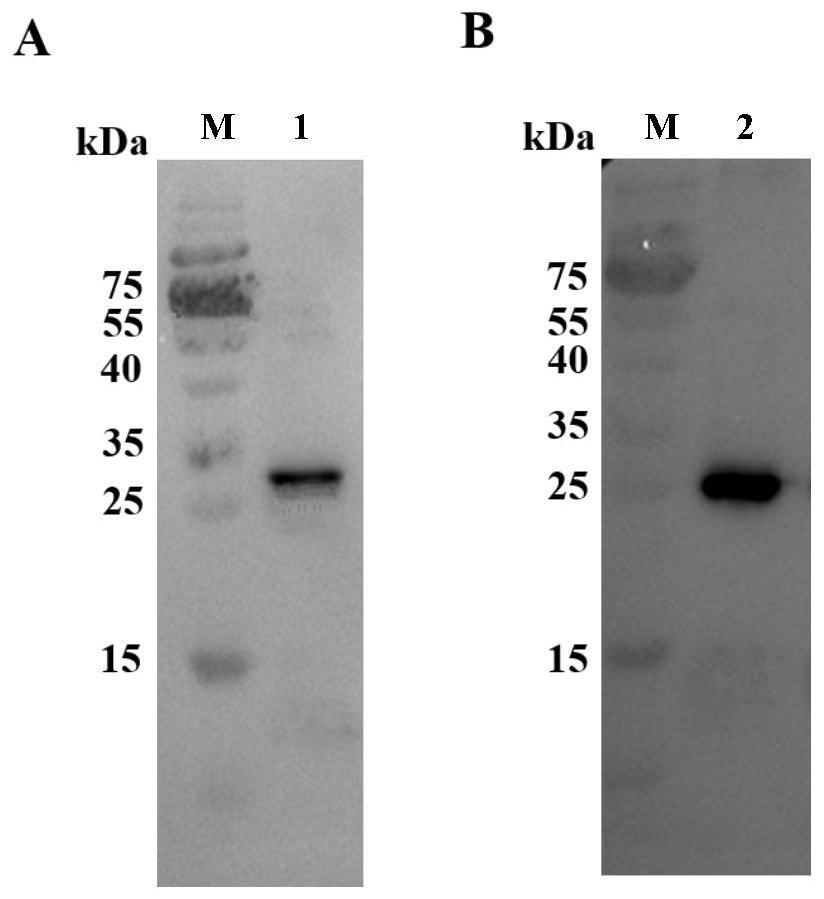
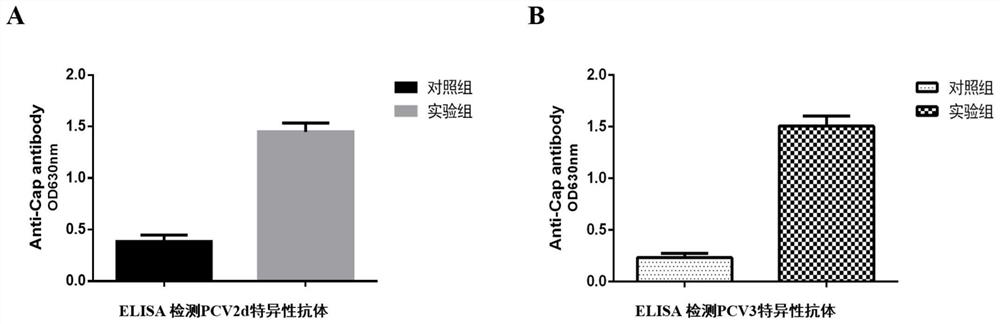
Porcine circovirus type 2d and type 3 cap protein dual subunit vaccine and its preparation method and application
ActiveCN110606873BEasy to operatePreserve immunogenicityVirus peptidesAntiviralsBaculovirus expression vector systemEpitope
The invention provides a porcine circovirus type 2d and type 3 Cap protein dual subunit vaccine as well as a preparation method and application thereof. By cloning porcine circovirus type 2d and type 3 Cap protein epitope genes, the highly purified type 2d and type 3 Cap proteins were successfully expressed using the insect-baculovirus expression vector system. Utilizing the two expressed proteins, the porcine circovirus type 2d and type 3 Cap protein dual subunit vaccine was developed for the first time. The prepared dual vaccine has strong immunity and high safety and is effective against porcine circovirus type 2d and type 3 Viruses can play an ideal role in immune protection, providing effective means for the prevention and control of porcine circovirus.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Vaccine composition containing swine mycoplasmal pneumonia antigen and swine streptococcosis antigen, and preparation method and application thereof
ActiveCN103861095APreserve immune efficiencyLow costAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com