Porcine circovirus type 2d and type 3 cap protein dual subunit vaccine and its preparation method and application
A subunit vaccine, porcine circovirus technology, applied in the biological field to achieve the effect of activating immune response, retaining immunogenicity and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
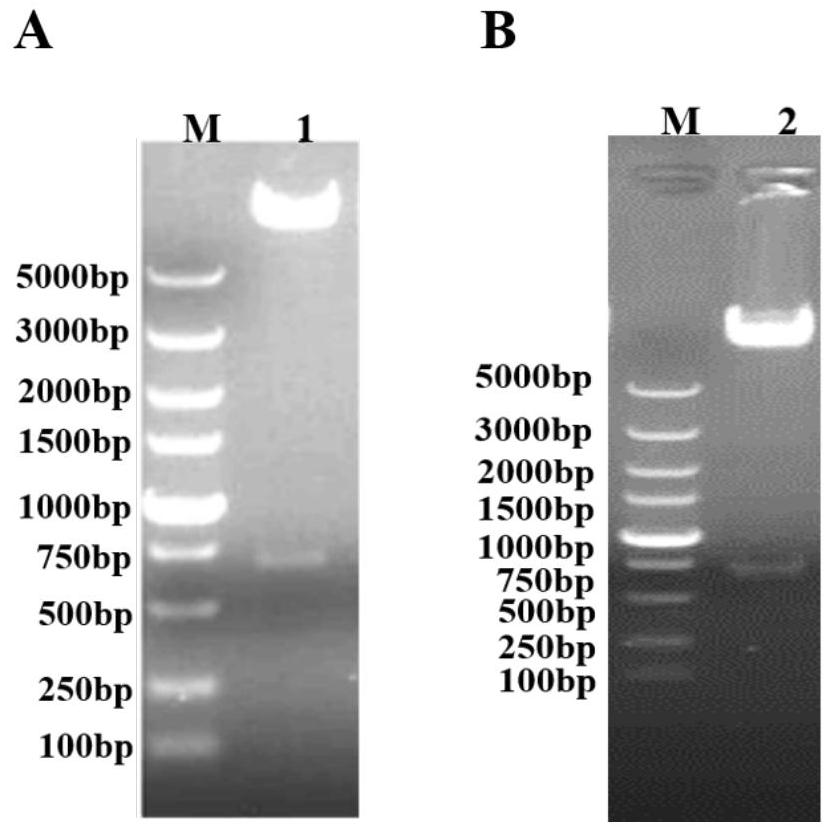
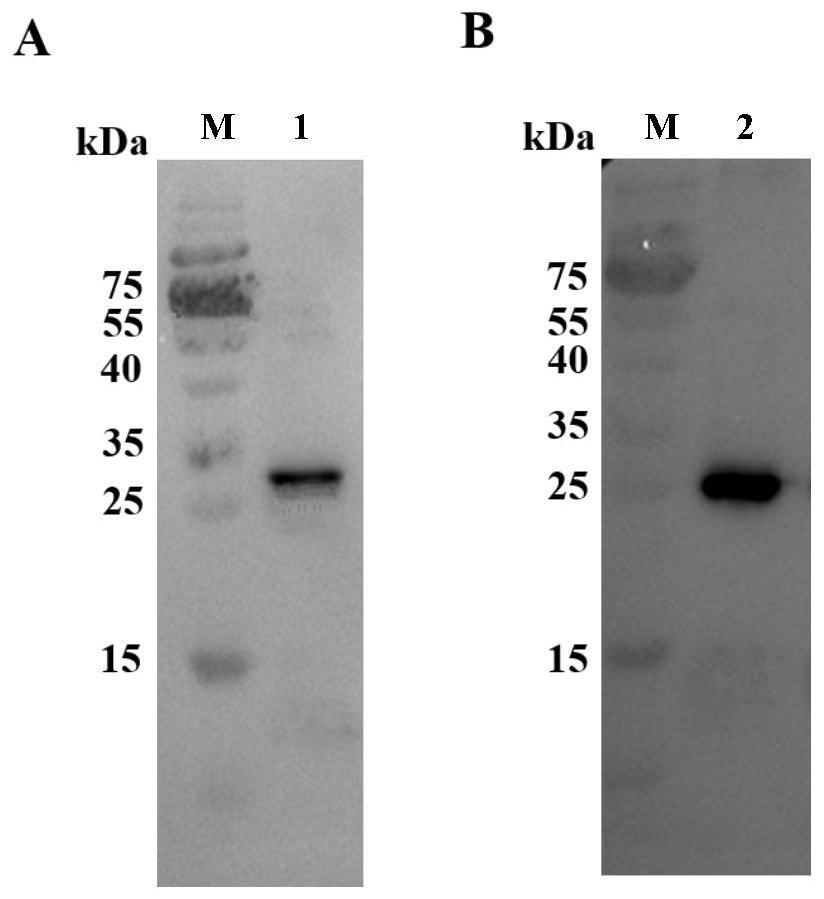
[0045] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. Unless otherwise specified, the examples are all in accordance with conventional experimental conditions, such as Sambrook et al. Molecular Cloning Experiment Manual (Sambrook J & Russell DW, Molecular Cloning: a Laboratory Manual, 2001), or in accordance with the conditions suggested by the manufacturer's instructions. Example 1 Expression and purification of porcine circovirus type 2d and type 3 Cap proteins
[0046] The present embodiment provides two kinds of recombinant baculoviruses, respectively named as Ac-PCV2d Cap and Ac-PCV3 Cap, Ac-PCV2d Cap comprises the Cap protein gene sequence (SEQ ID NO: 1) of porcine circovirus type 2d, and Ac- PCV3Cap contains the Cap protein gene sequence of porcine circovirus type 3 (SEQ ID NO: 2). The gene sequences are all derived from artificial synthesis. After codon optimization, the optimized C...
Embodiment 2
[0071] Example 2 Preparation of porcine circovirus type 2d and type 3 Cap protein dual subunit vaccine
[0072] The purified PCV2d Cap and PCV3 Cap proteins were filtered after their concentrations were measured, and the two were mixed in an equal mass ratio, and then emulsified with sterile 201 adjuvant in a mass ratio of 3:1 to prepare a dual subunit vaccine. The contents of PCV2dCap and PCV3 Cap antigens are both 40 μg, and stored at 4°C until use. At the same time, after mixing PCV2d Cap and PCV3 Cap proteins in an equal mass ratio, the vaccine was prepared according to the mass ratio of antigen and Gel01 water adjuvant 4:1, and stored at 4°C for later use.
[0073] 1. Comparative test of vaccine stability prepared with different adjuvants
[0074] The two prepared porcine circovirus type 2d and type 3 Cap protein subunit vaccines were placed at 37°C, 25°C and 4°C for observation respectively. The stability of the vaccine was regularly sampled and observed, and the test ...
Embodiment 3
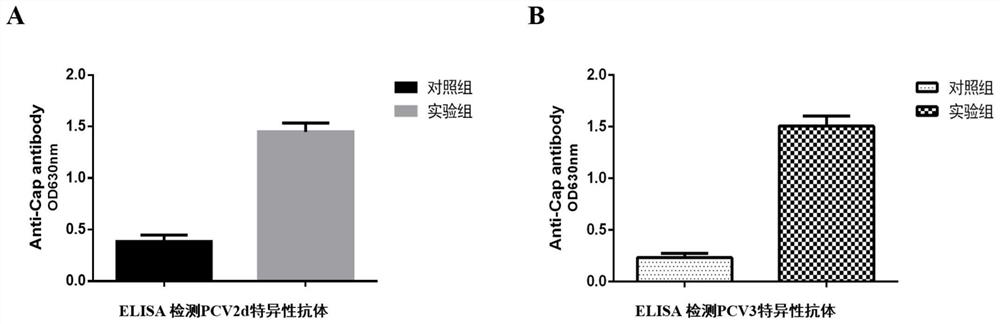
[0089] Example 3 Evaluation of immune protection effect and safety evaluation of porcine circovirus type 2d and type 3 Cap protein dual subunit vaccine
[0090] Evaluation of porcine circovirus type 2d and type 3 Cap protein dual subunit vaccines in mice:
[0091] 1. Safety evaluation of porcine circovirus type 2d and type 3 Cap protein dual subunit vaccine in mice
[0092] Purchase 10 6-8-week-old female Balb / C mice and divide them into two groups, A and B, with 5 in each group. Each mouse in group A was subcutaneously injected with 0.3ml of PCV type 2d and type 3 dual vaccine (201 adjuvant) prepared in Example 2; each mouse in group B was injected with 0.3ml of dialysis buffer (20mM Tris, 200mM NaCl); After 14 days of continuous observation, the mice in the two groups were in the same state and had no abnormal reaction, which indicated that the vaccine was safe for the mice.
[0093] 2. Immunogenicity evaluation of porcine circovirus type 2d and type 3 Cap protein dual sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com