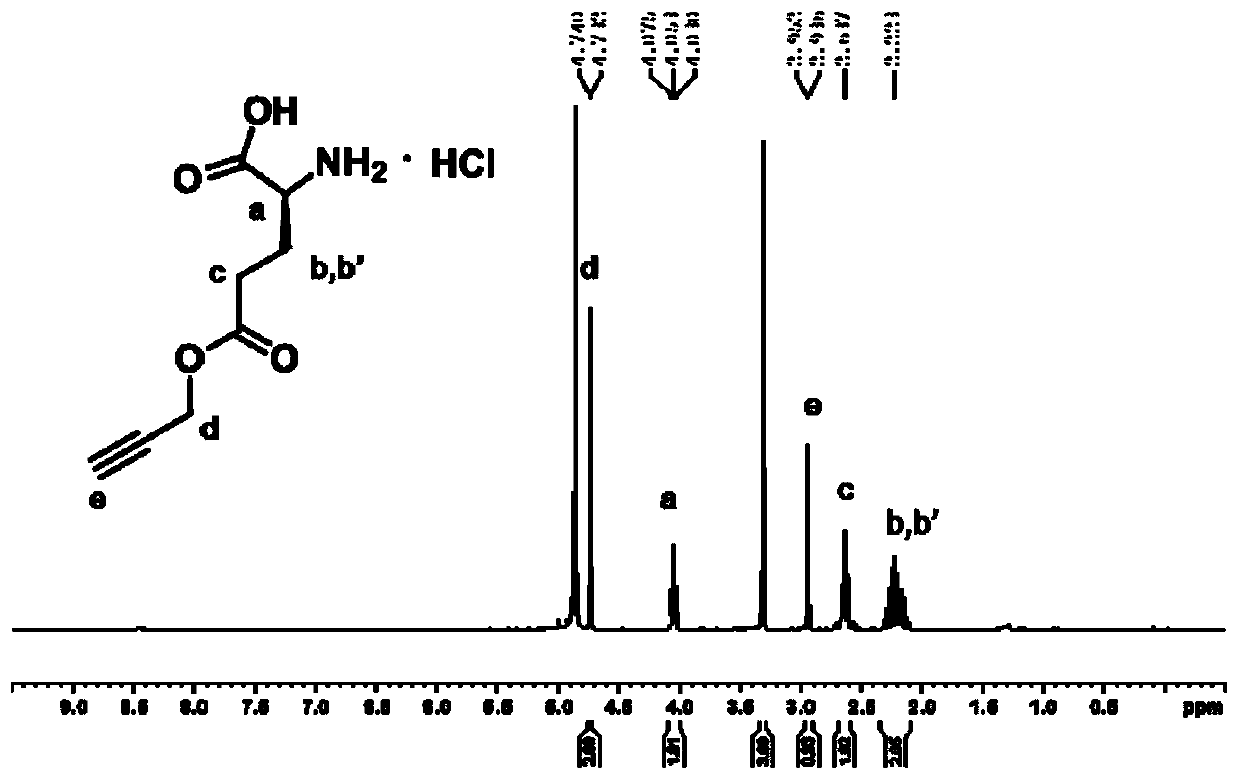
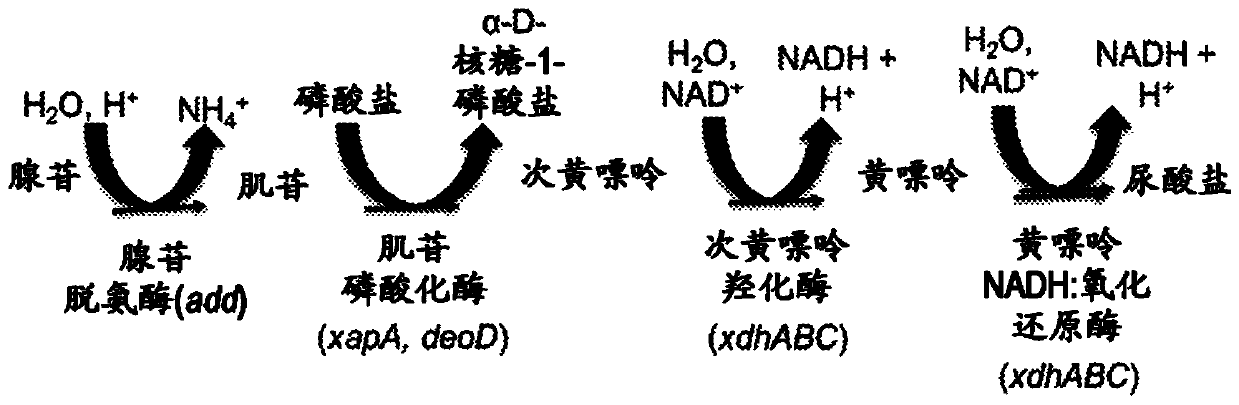
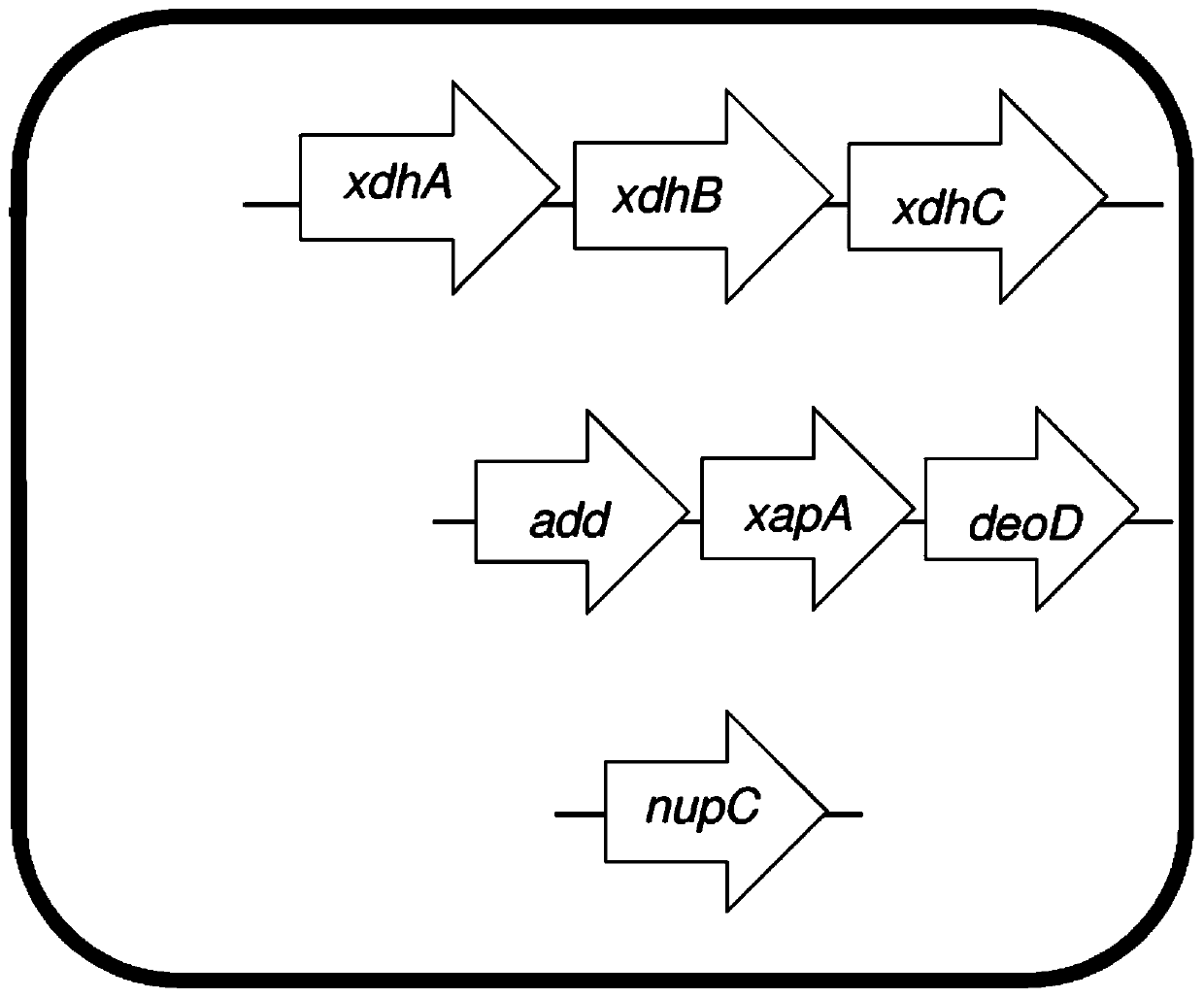
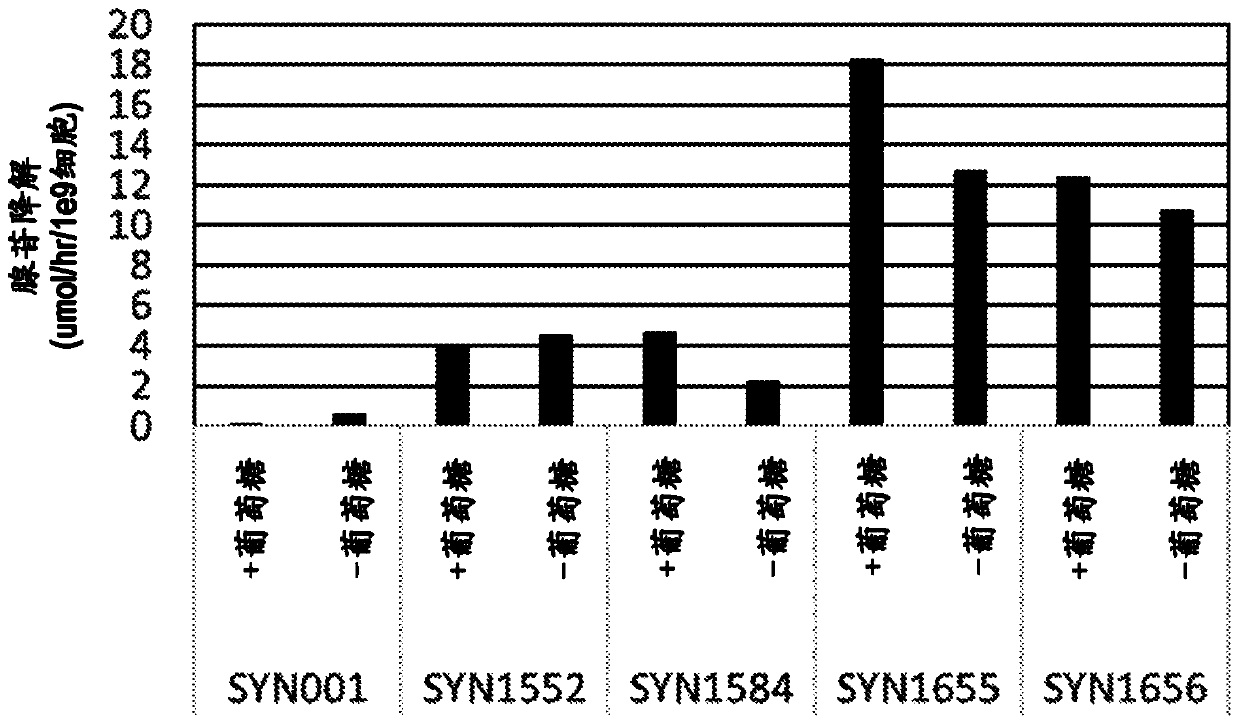
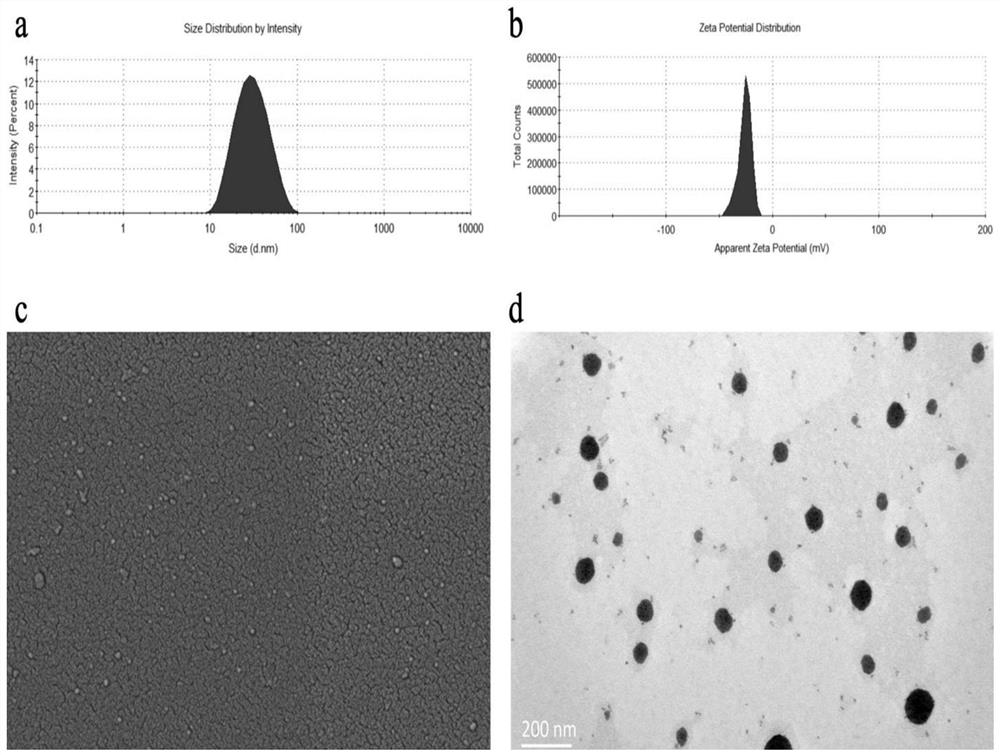
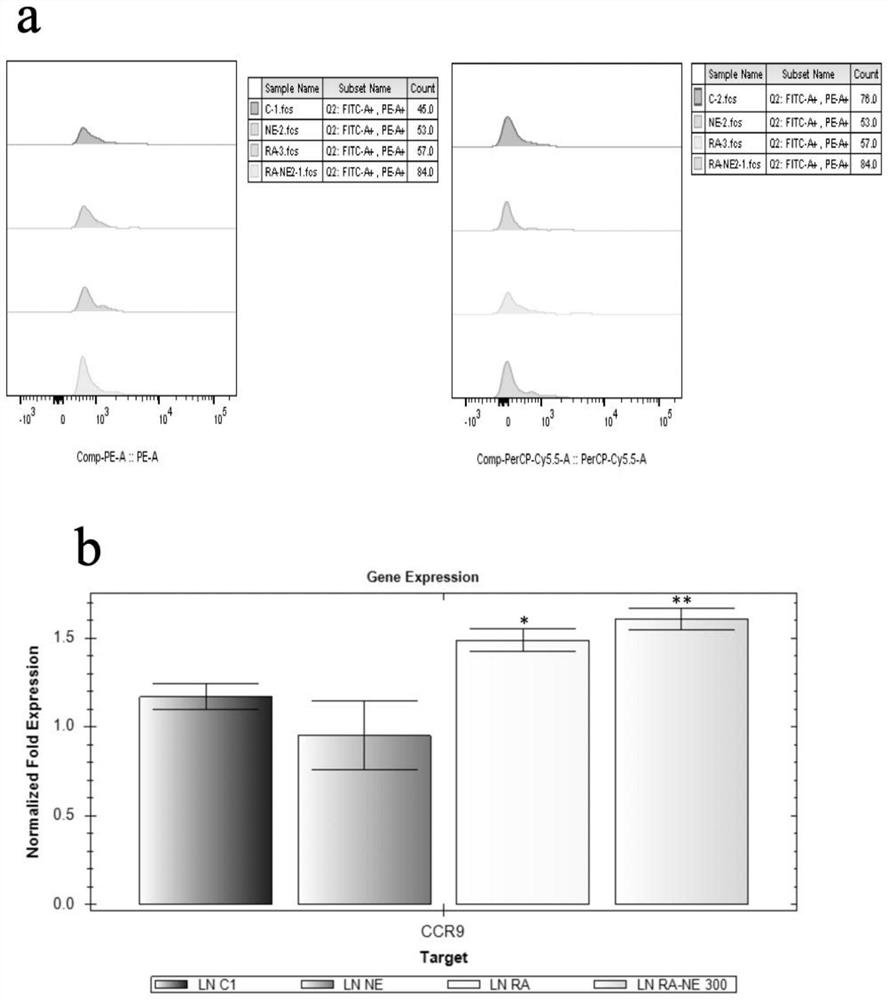
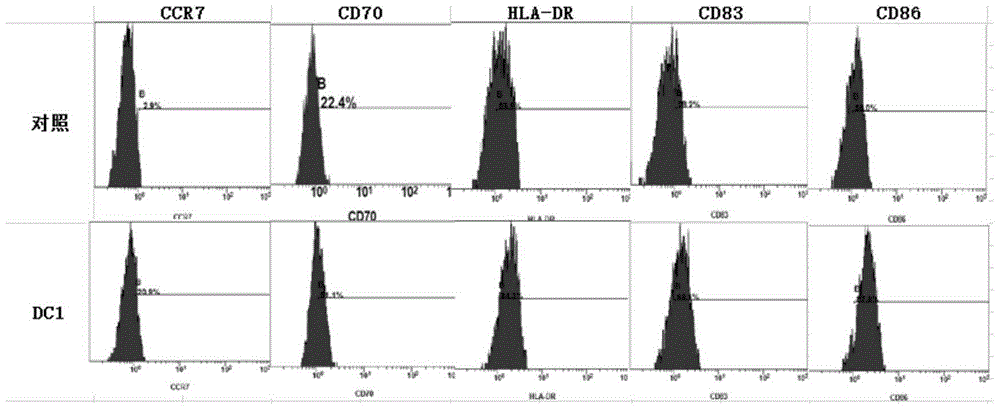
Patents
Literature
97results about How to "Activate immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
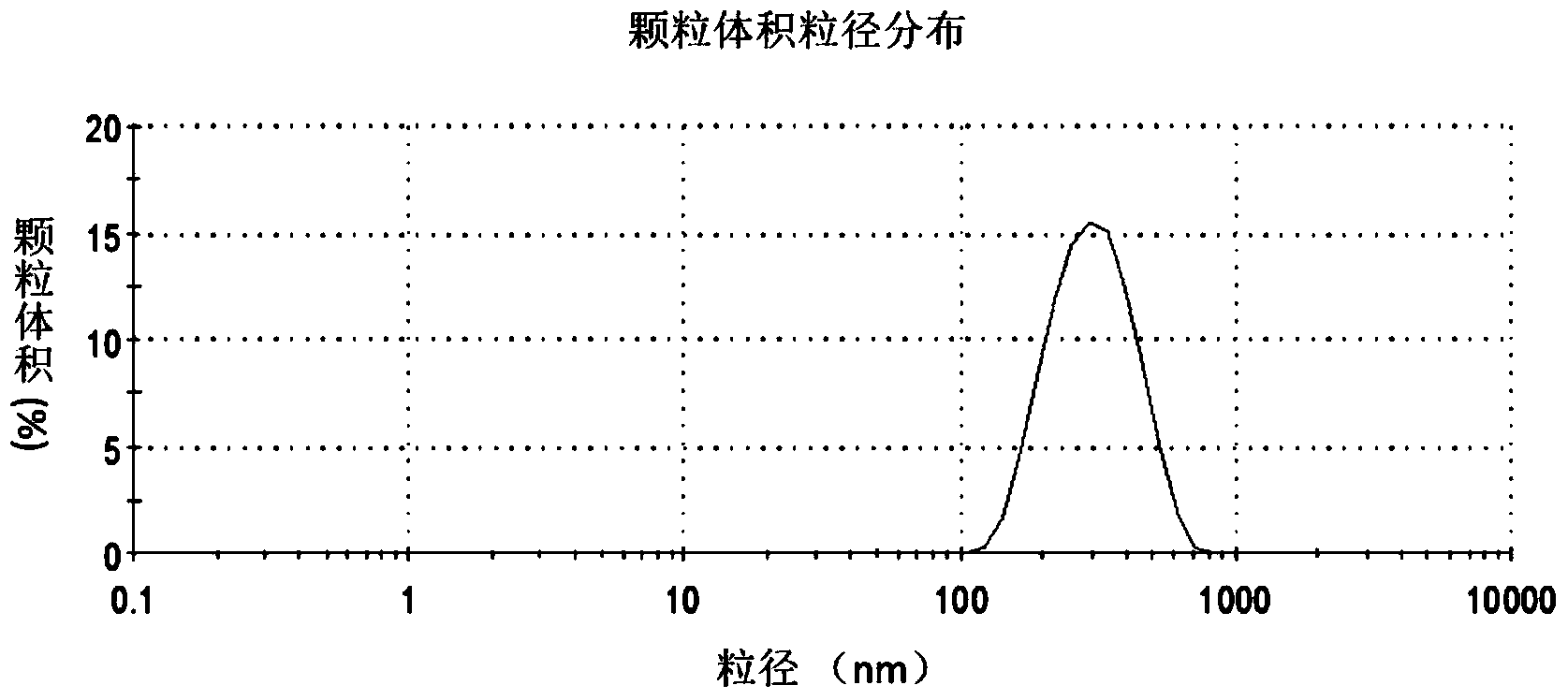
Oil-in-water emulsion free of surfactant and use thereof
ActiveCN104013955AThe nature is easy to controlGood biocompatibilitySsRNA viruses negative-sensePowder deliveryActive agentGlycerol
The invention discloses an oil-in-water emulsion free of surfactant. The emulsion comprises a metabolizable oil phase, a water phase and oil-water amphipathic solid particles dispersed in the water phase and having biocompatibility, wherein the oil phase comprises squalene or / and tocol; the water phase is any one or a combination of at least two of purified water, water for injection, aqueous liquid of glycerol, buffered saline liquid and clinically available infusion liquid; and the average grain diameter of the solid particles is at nanometer-to-micron grade. The emulsion can be used as a vaccine adjuvant or a medicine delivery or controlled release carrier; the properties of the emulsion can be controlled and regulated; the obtained emulsion is stable; the use of a surfactant is avoided; and harm to the human body and pollution on the environmental can be reduced.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Nano aluminum-encapsulating carrier and application thereof
InactiveCN104055736AImprove stabilityEnhance internal and external stabilityAntiinfectivesEmulsion deliveryBiocompatibility TestingALUMINUM PHOSPHATE
The invention discloses a nano aluminum-encapsulating carrier and an application of the nano aluminum-encapsulating carrier in constructing a vaccine adjuvant transfer system. The nano aluminum is nanoparticles of aluminum phosphate, aluminum sulfate and aluminum hydroxide or a mixture of aluminum phosphate, aluminum sulfate and aluminum hydroxide, wherein the grain size is below 1 mu m; the carrier is a lipidosome, a lipoid, an emulsion, a nano-capsule or a micro-capsule. Construction of the vaccine adjuvant transfer system is the main purpose of the nano aluminum-encapsulating carrier. The application of the nano aluminum-encapsulating carrier in constructing the vaccine adjuvant transfer system comprises the following step: encapsulating vaccine components in the nano aluminum-encapsulating carrier; or adsorbing the vaccine components on the surface of the carrier; or purely mixing with the carrier to exert the vaccine adjuvant and the transfer function. The nano aluminum-encapsulating carrier disclosed by the invention has the advantages that the nano aluminum-encapsulating carrier is wide in application range, so that the nano aluminum-encapsulating carrier is suitable for antigens with different pathogens; the nano aluminum-encapsulating carrier is high in stability and can encapsulate antigens so as to enhance in vivo and in vitro stability; the nano aluminum-encapsulating carrier is high in safety, and the use materials have good biocompatibility; the nano aluminum-encapsulating carrier is many in inoculation and wide in way, and the carrier can be inoculated through track mucosa or subcutaneous, intracutaneous and intramuscular injection; the carrier is strong in immunosuppression induction potency.
Owner:ANHUI MEDICAL UNIV
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
InactiveCN101451145AActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsIntramuscular injectionTreating tuberculosis
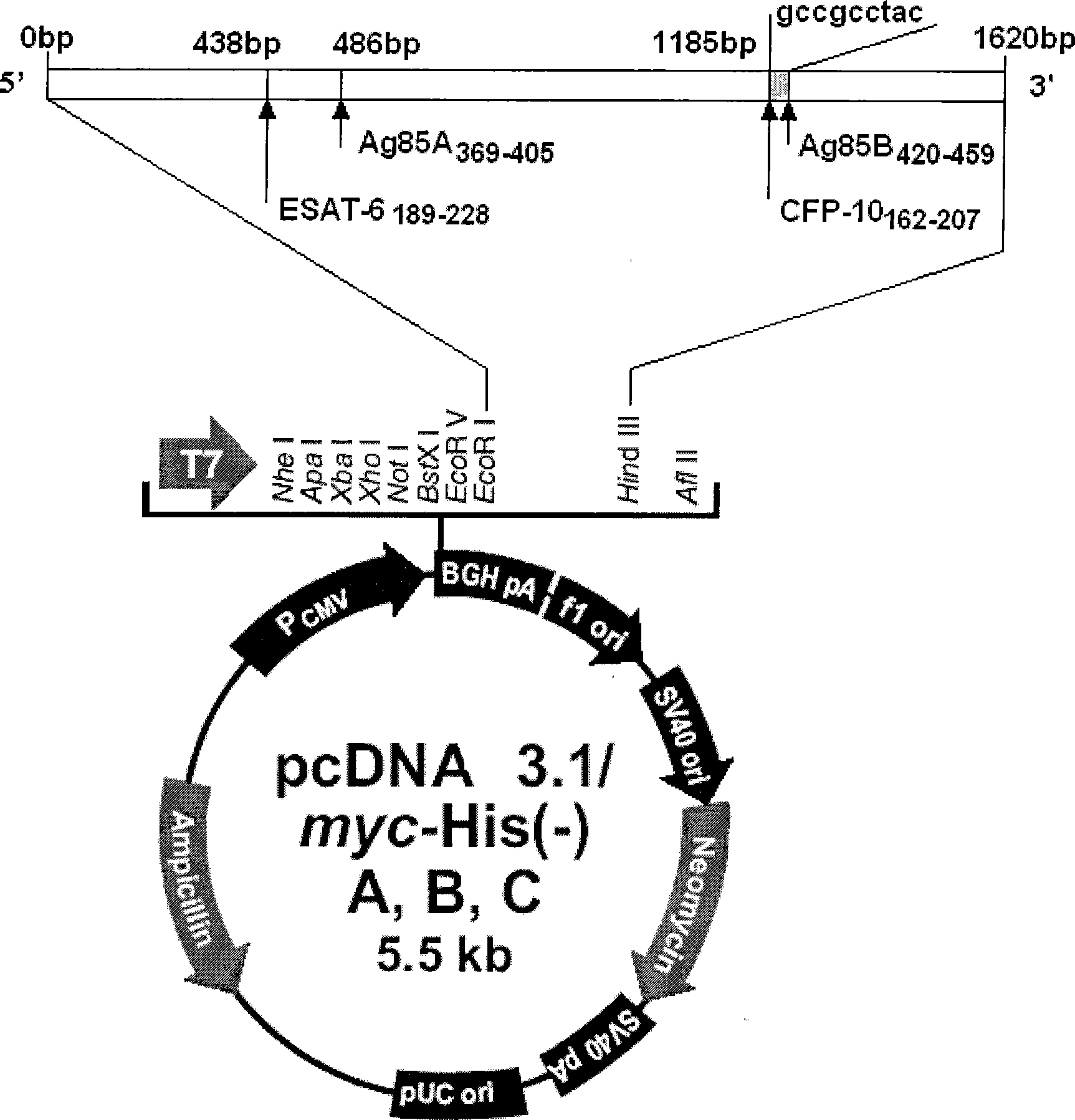
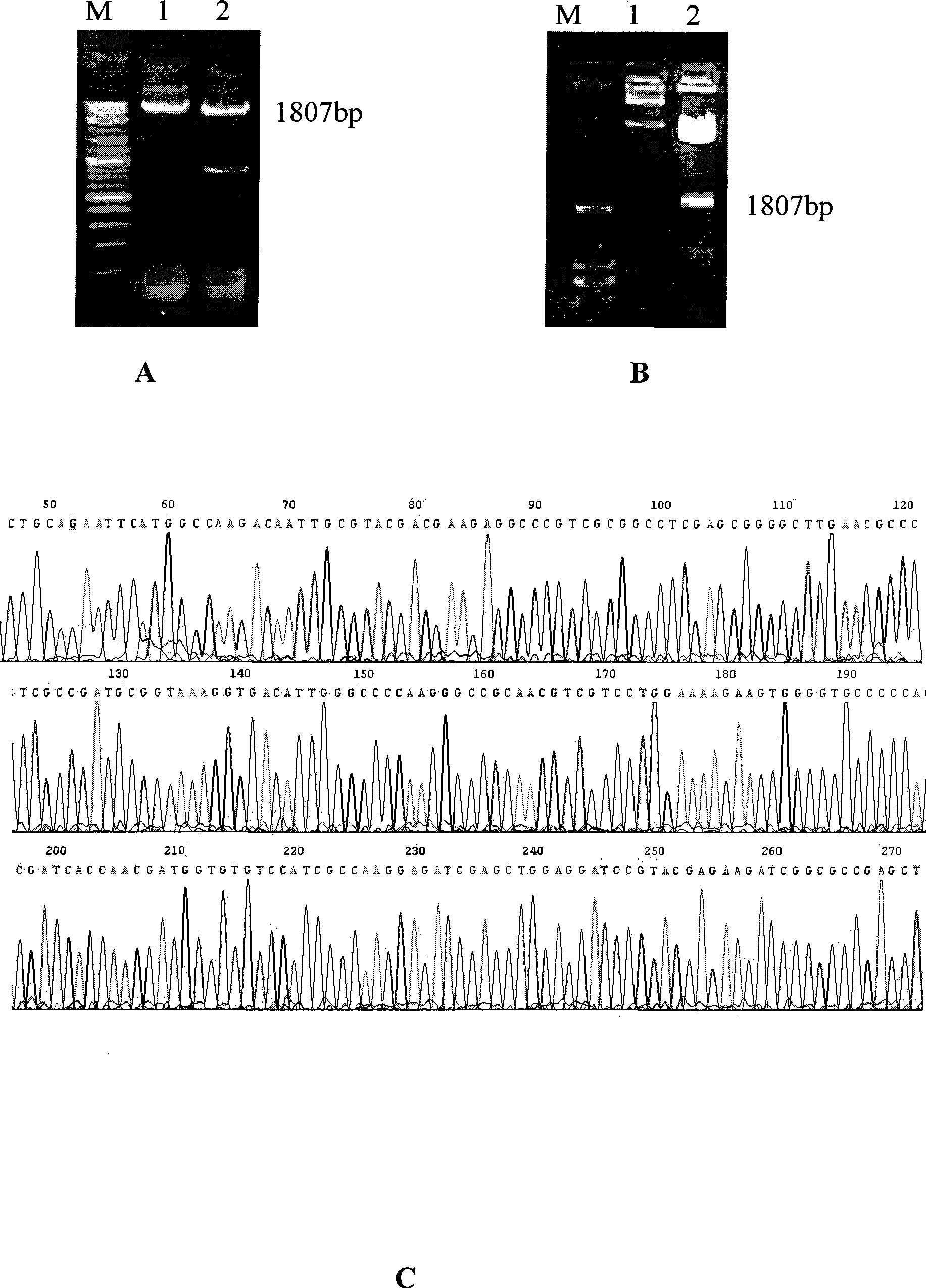
The invention discloses a tuberculosis gene vaccine based on T cell epitopes, wherein a full-length gene, embedded with four T cell epitope polypeptide genes which come from mycobacterium tuberculosis antigen, of mycobacterium tuberculosis heat shock protein is inserted into a vector. The invention also discloses a method for preparing the vaccine, which comprises the following steps: four T cell epitope genes, namely EAST-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 which come from the mycobacterium tuberculosis antigen are inserted into an HSP65 full-length gene. The invention also discloses application of an ECANS tuberculosis gene vaccine. Through the intramuscular injection of the gene vaccine into an immune mouse, the experiment proves that the vaccine can induce a specific antibody which aims at a plurality of tuberculosis antigens to response, can induce stronger tuberculosis specific killing response, can induce Th1 immune response at the same time, secrete high-level IFN gamma, and is a good vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
TGF-beta specific siRNA containing free triphosphoric acid group and application thereof
InactiveCN101974529AImprove recognitionPromote tumor cell apoptosisGenetic material ingredientsDigestive systemSense strandGuanine nucleoside
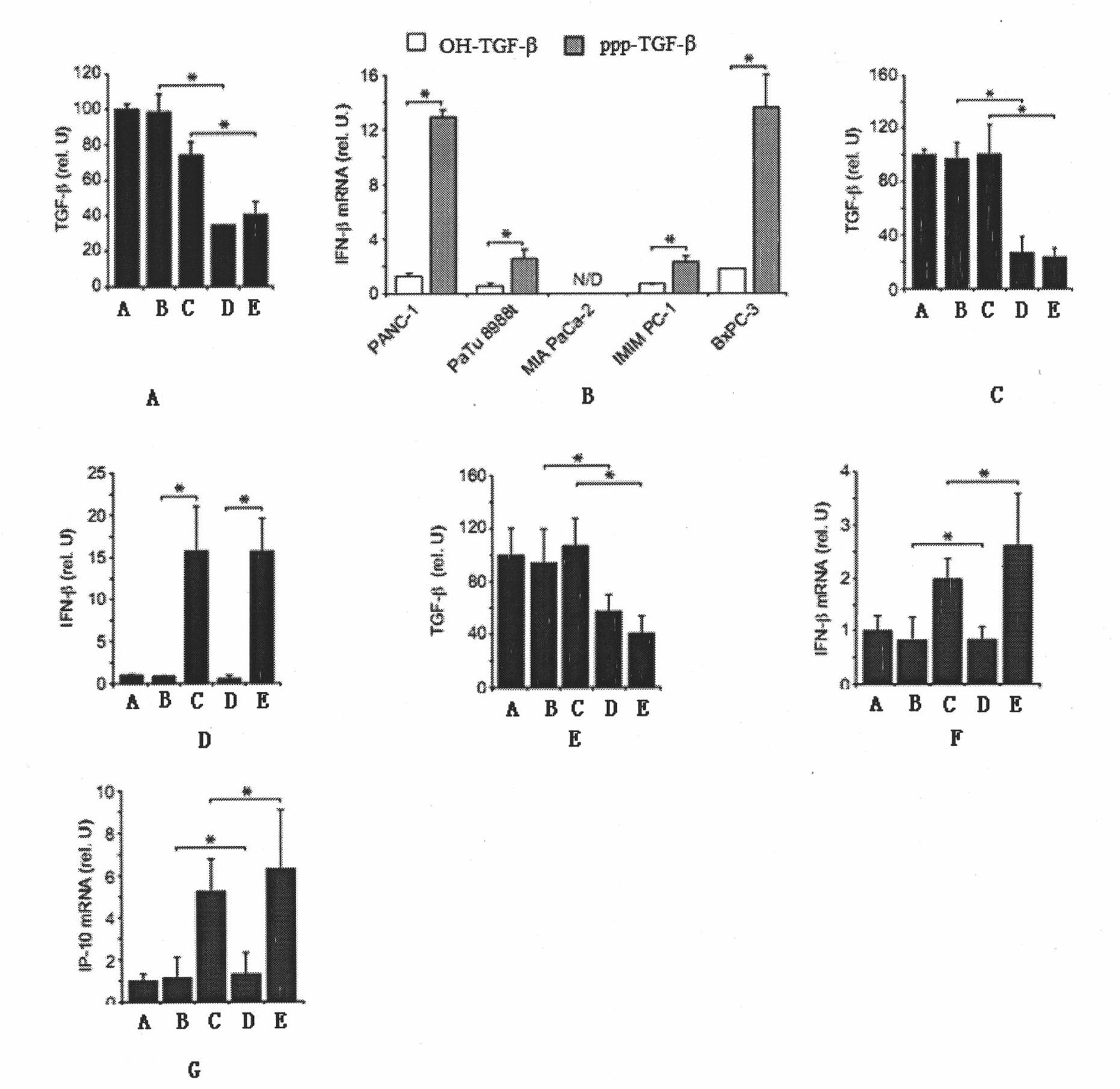
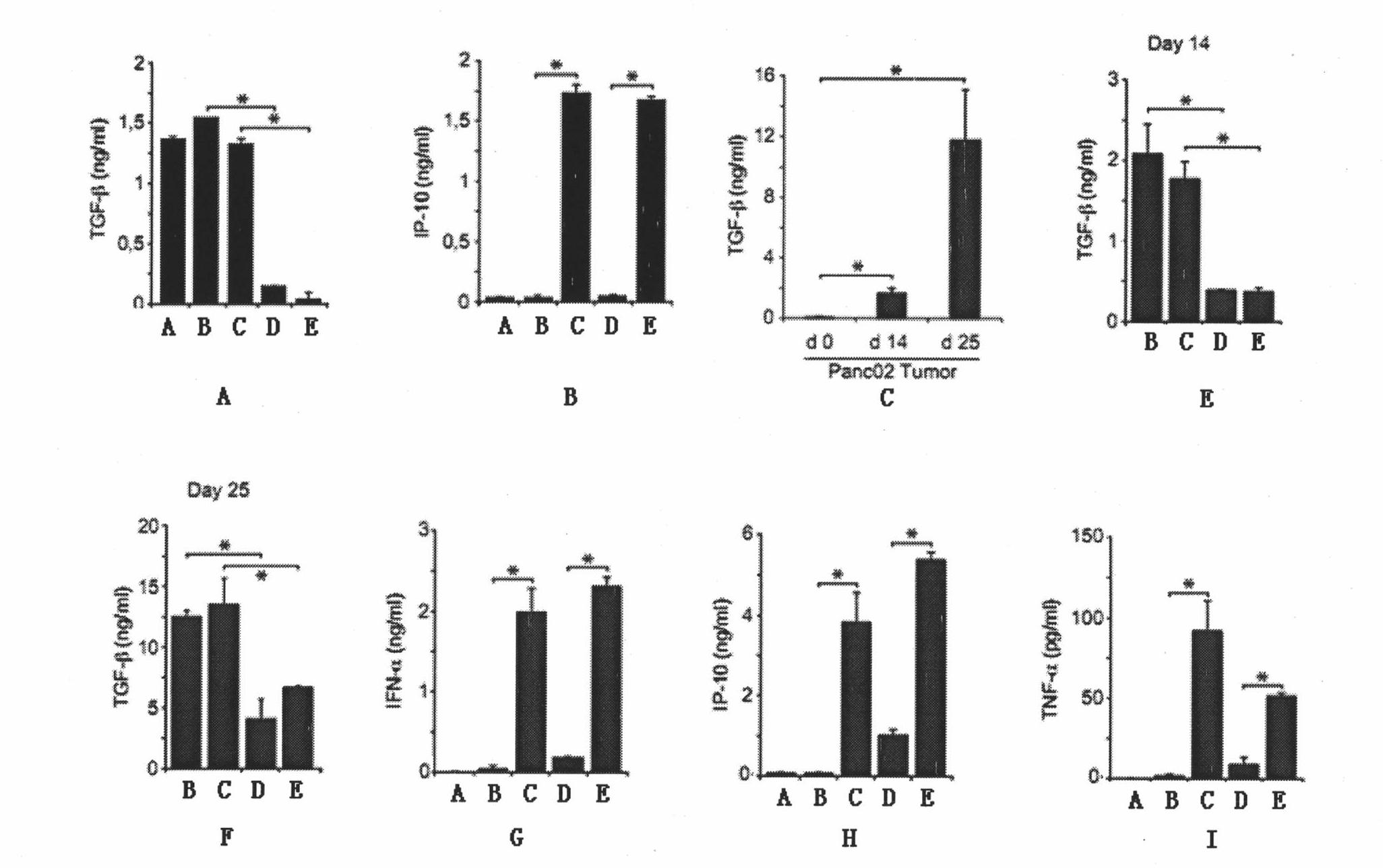
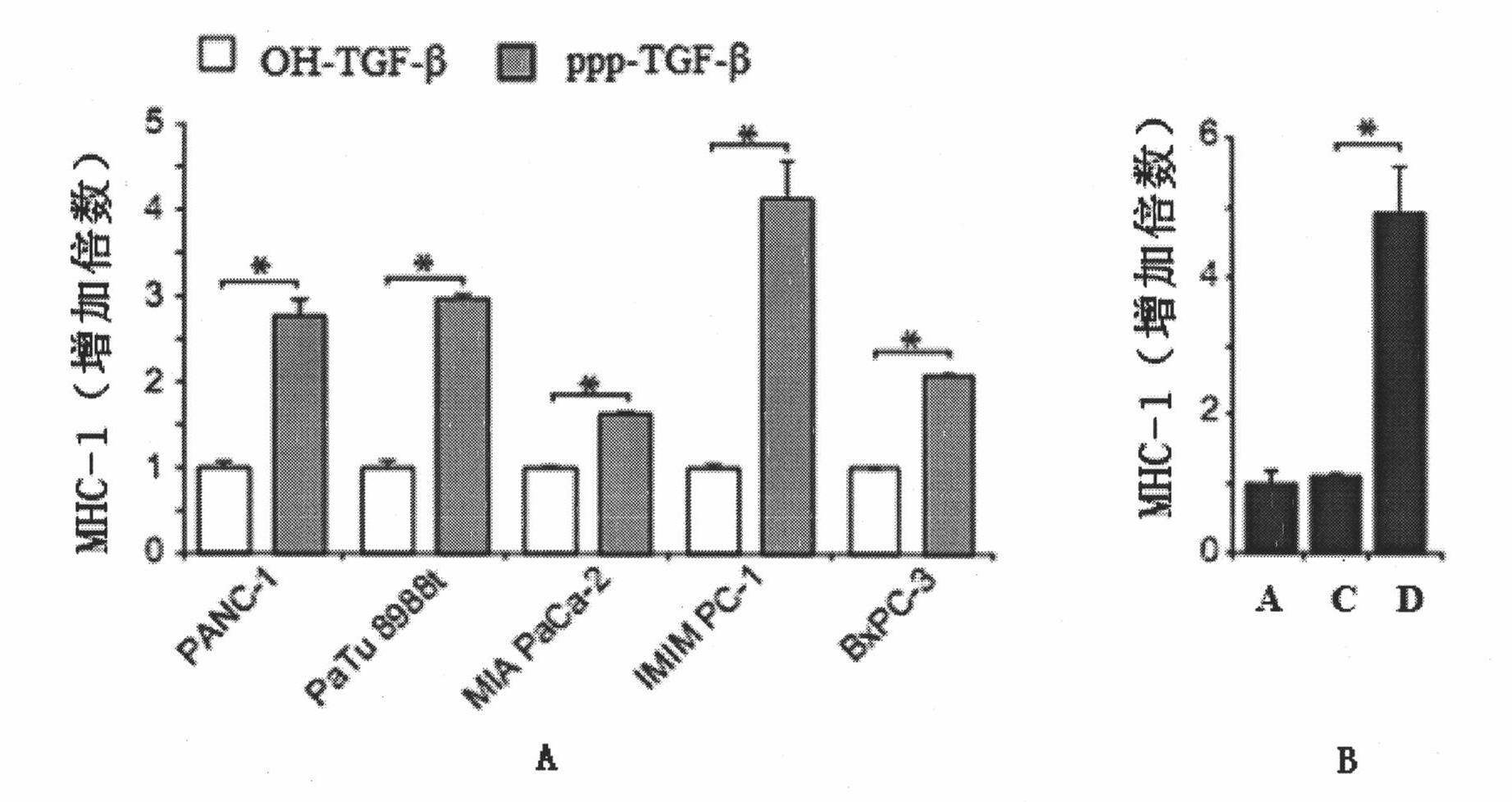
The invention belongs to the technical field of RNA interference, and discloses TGF-beta specific siRNA containing a free triphosphoric acid group and application thereof. The 5' ends of antisense strand and sense strand sequences of the TGF-beta specific siRNA containing the free triphosphoric acid group are guanosine, and the free triphosphoric acid group is modified on the pentose 3' site of the guanosine. By combining the TGF-beta specific gene silencing mechanism and the antiviral inherent immune mechanism of an induced eukaryotic cell, the siRNA can inhibit important molecule TGF-beta of mediated tumor immunologic escape, effectively activate the anti-tumor immunity of the body and remarkably improve the effect of treating tumor. The siRNA effectively solves the problem that the traditional siRNA (OH-RNA) only has single gene silencing function but poor tumor treatment effect. The ppp-TGF-beta can be applied to preparing a medicament for treating the tumor, in particular pancreatic cancer.
Owner:NANJING UNIVERSTIY SUZHOU HIGH TECH INST
PD-L1 targeting polypeptide and application thereof
ActiveCN108840923APromote degradationReduce interactionPeptide/protein ingredientsGenetic engineeringProtein targetPD-L1
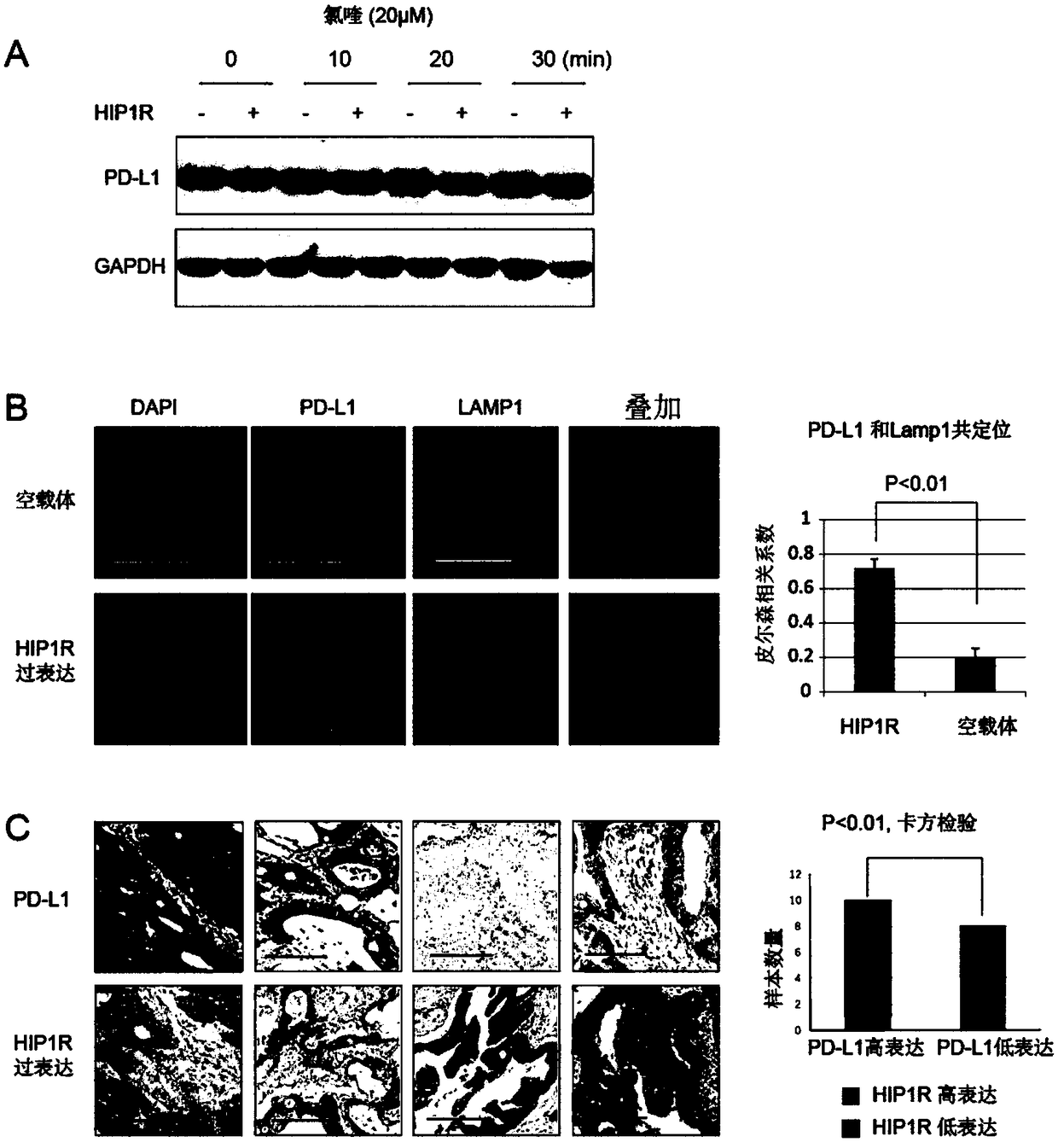
The invention provides PD-L1 protein targeting polypeptide, isolated nucleic acid, a nucleic acid construct, a recombinant expression vector and an antitumor medicine, and further provides applicationof the PD-L1 protein targeting polypeptide to preparation of the antitumor medicine. The polypeptide has the function of regulating PD-L1 protein, and can reduce the expression quantity of the PD-L1protein in cells, reduce the PD-1 protein bonding ability of tumor cells, increase the tumor cell killing ability of T cells and inhibit the in-vivo growth of the tumor cells. Different from the current commonly-used antibody blocking method, a technical scheme provided by the invention is beneficial to activation of T cell immune response and improvement on tumor immunity, is a new breakthrough as a tumor immunotherapeutic method, and has a wide meaning in biomedical and pharmaceutical research in allusion to PD-L1 regulation and control.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Pseudosciaena crocea interferon (IFN) gene and cloning method thereof
InactiveCN102559695AActivate immune responseNo pollution in the processFermentationPlant genotype modificationBiotechnologyDisease
The present invention discloses a Pseudosciaena crocea interferon gene, which is characteristics in that: the cDNA (complementary DNA) sequence of the coding region of the interferon protein has the base sequence shown in SEQ ID NO.1 and the amino acid sequence shows in SEQ ID NO.2 in the sequence list; as well as a cloning method thereof. The Pseudosciaena crocea interferon gene disclosed by the invention has a good application prospect in prevention and control of Pseudosciaena crocea disease and further development of biological fish drugs, and lays the foundation for Pseudosciaena crocea molecular marker-auxiliary disease resistance breeding and detection of health status of fishes.
Owner:JIMEI UNIV
Nano vaccine and preparation method thereof
ActiveCN109998998AImprove endocytic efficiencyEfficient activationInorganic non-active ingredientsAntibody ingredientsPolymer modifiedDendritic cell
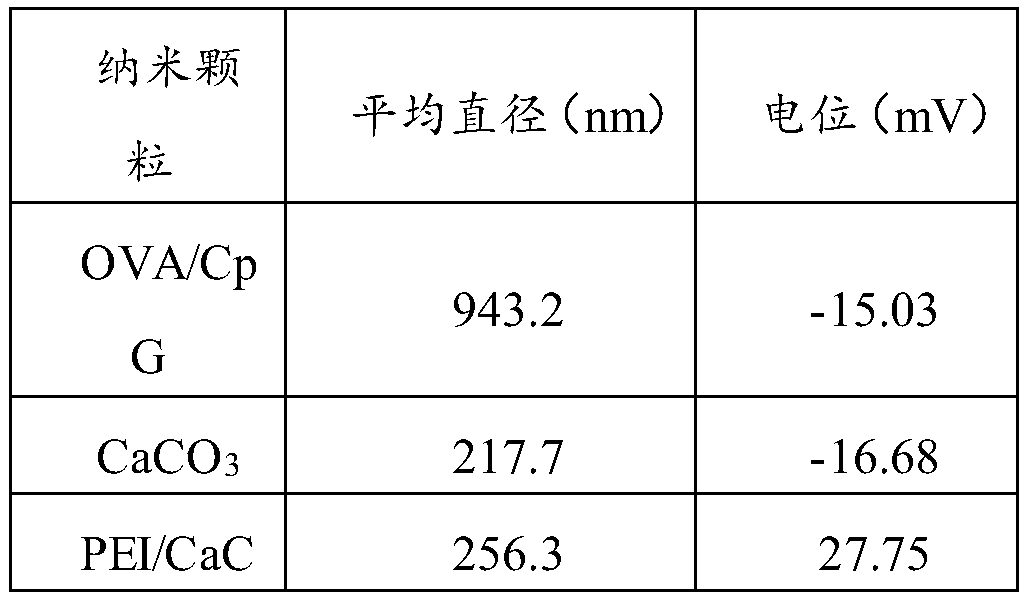
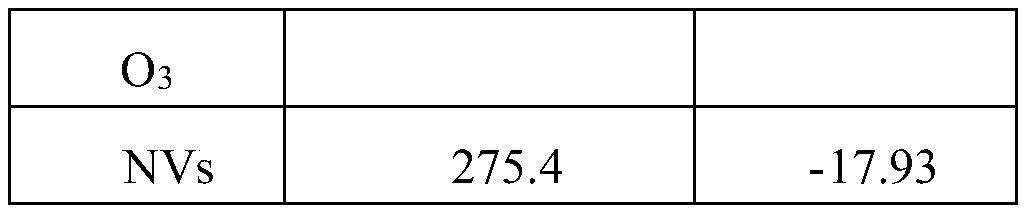
The invention provides a nano vaccine and a preparation method thereof. The nano vaccine comprises a positively charged carrier particle and a negatively charged wrapper loaded on the surface of the carrier particle; the carrier particle is a nano-calcium carbonate particle with the surface adsorbed with a cationic polymer; the wrapper is an antigen and an adjuvant. Accordingly, the cationic polymer modified nano-calcium carbonate particle serves as an electropositive vaccine carrier to load the negative antigen and the adjuvant, and the endocytosis efficiency of the antigen and the adjuvant can be effectively improved; meanwhile, dendritic cells can be effectively activated, and the in vivo immune response is activated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Anti-PD-1-anti-VEGFA bispecific antibodies, pharmaceutical compositions and uses thereof
PendingCN112830972AReduced activityEliminate binding activityHybrid immunoglobulinsImmunoglobulins against growth factorsMolecular ImmunologyIntravenous gammaglobulin
The invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-VEGFA-anti-PD-1 bispecific antibody and an application thereof. Specifically, the anti-VEGFA-anti-PD-1 bispecific antibody comprises a PD-1-targeting first protein functional region and a VEGFA-targeting second protein functional region, according to an EU numbering system, a heavy chain constant region of immune globulin contained in the bispecific antibody mutates at two sites of 234 and 235, and after mutation, the affinity constant of the bispecific antibody is reduced compared to the affinity constant of Fc [gamma] RI, Fc [gamma] RIIa, Fc [gamma] RIIIa and / or C1q before mutation. The bispecific antibody disclosed by the invention can be specifically combined with VEGFA and PD-1, specifically relieve immunosuppression of VEGFA and PD-1 on an organism and inhibit angiogenesis caused by tumors at the same time, and has a good application prospect.
Owner:AKESO BIOPHARMA
Double adjuvant-neoantigen tumor nanometer vaccine, and preparation method and application thereof
InactiveCN111068047AThe synthesis steps are clear and simpleDetermine the topographyPowder deliveryPharmaceutical non-active ingredientsAdjuvantPolyethylene glycol
The invention provides a nanometer vaccine. The nanometer vaccine is a nanoparticle simultaneously loaded with tumor neoantigen and two adjuvants thereof. The invention also provides a composition used for preparing the nanometer vaccine. The composition comprises a micromolecular adjuvant R848, an amphiphilic diblock polymer containing a polyethylene glycol chain segment and with terminal modified with a maleamide bond, a nucleic acid adjuvant CpG, polyethylene glycol modified polypeptide and a tumor neoantigen. The nanometer vaccine disclosed by the invention has a significant immune activation effect and a good antigen-specific tumor inhibition effect in vivo and in vitro. The invention also provides a method for preparing the nanometer vaccine and an application of the nanometer vaccine in preparation of tumor vaccine drugs.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
Microorganisms programmed to produce immune modulators and Anti-cancer therapeutics in tumor cells
PendingCN110913875AActivate immune responseActivation inherentOrganic active ingredientsPeptide/protein ingredientsMicroorganismImmune modulator
Owner:SYNLOGIC OPERATING CO INC
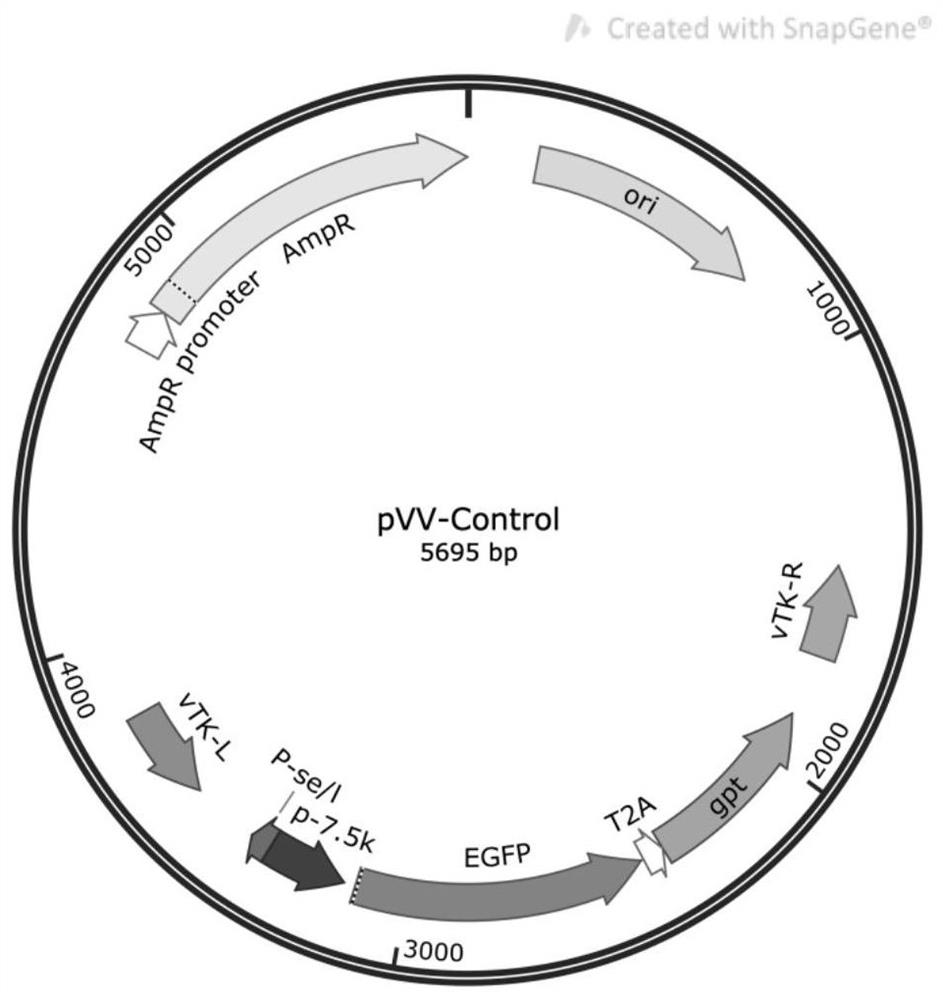
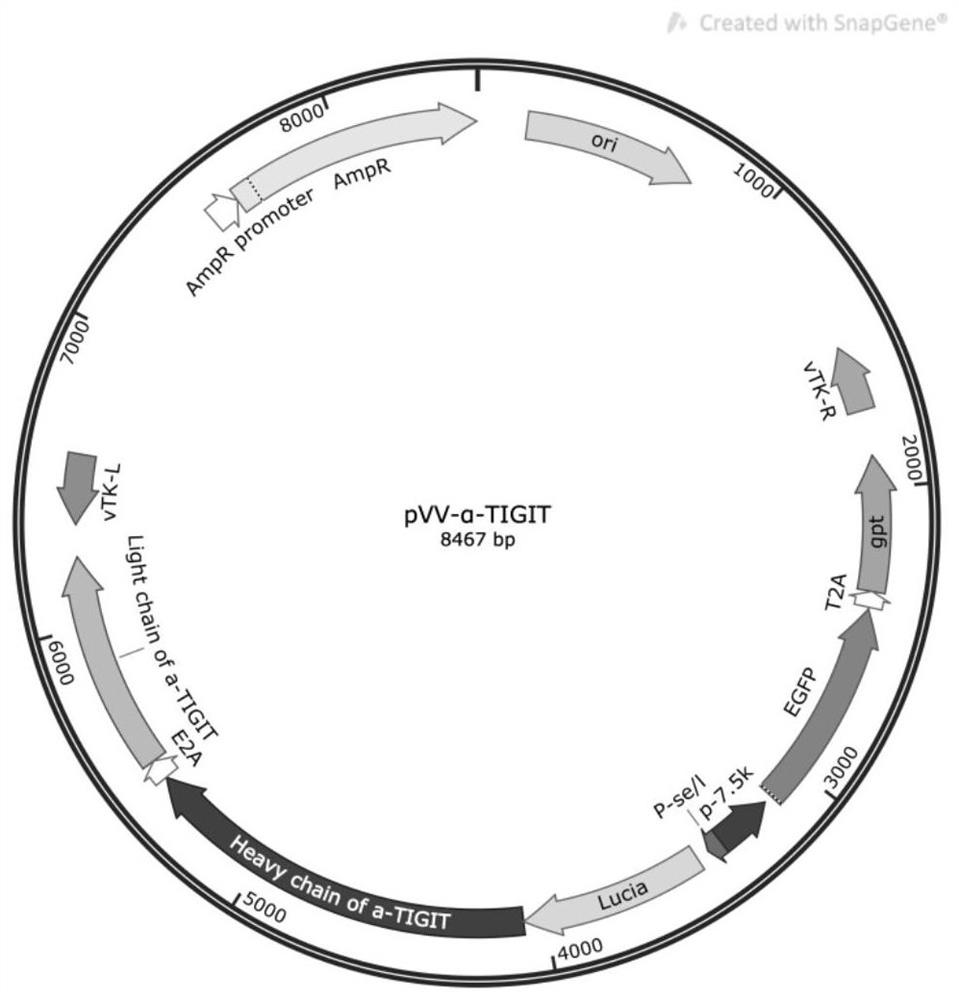
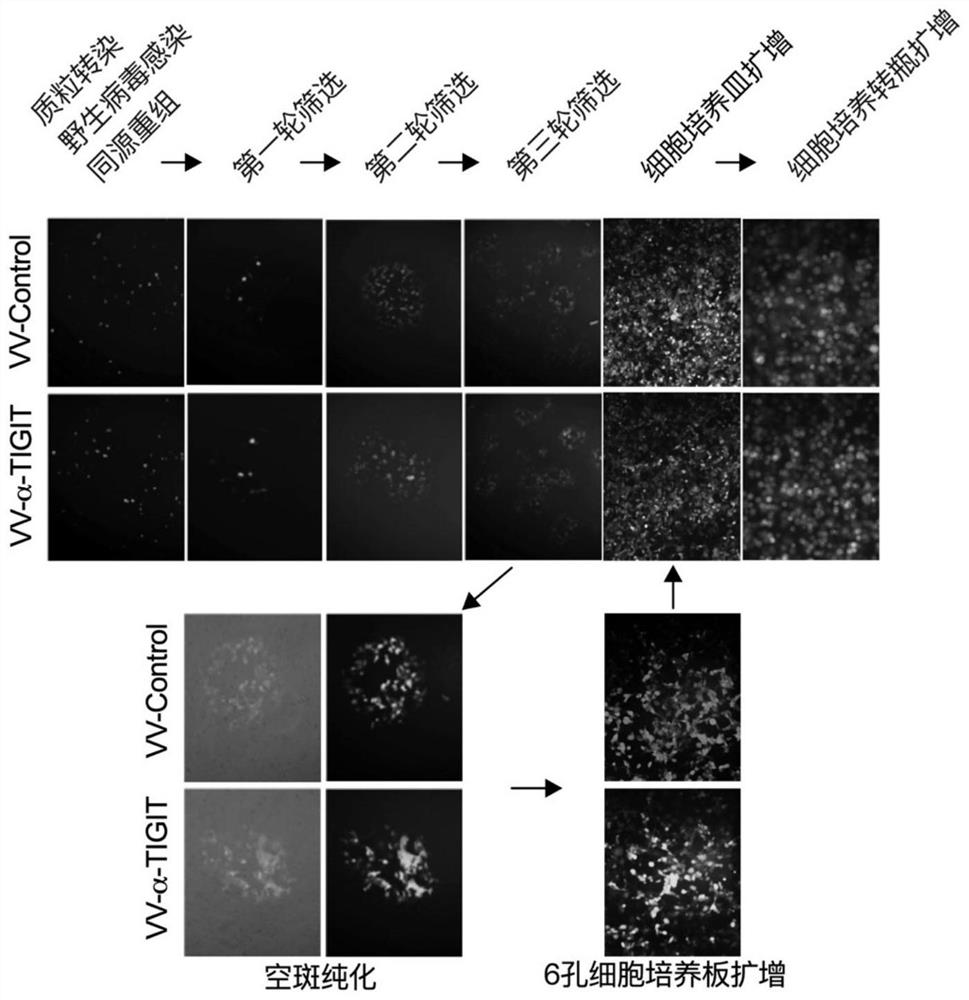
Recombinant oncolytic vaccinia virus, preparation method and application thereof
PendingCN111763660AEffective infiltrationImprove securityStable introduction of DNAUnknown materialsNucleotideTIGIT
The invention discloses a recombinant oncolytic vaccinia virus, a preparation method and application thereof. A thymidine kinase TK region of a genome of a recombinant oncolytic vaccinia virus comprises an anti-mouse / human TIGIT antibody genetic sequence, and the thymidine kinase TK region of the genome of the recombinant oncolytic vaccinia virus can express an anti-mouse / human TIGIT antibody. Theanti-mouse / human TIGIT antibody genetic sequence is formed by connecting an antibody heavy chain gene, 2A peptide and an antibody light chain gene in series, and the nucleotide sequence of the anti-mouse / human TIGIT antibody gene is shown as SEQ ID NO. 1. The recombinant oncolytic vaccinia virus oncolytic virus kills tumors through direct oncolysis and activation of a human immune system. The oncolytic vaccinia virus can effectively activate the immune response of T cells to tumor cells so as to exert multiple anti-tumor effects.
Owner:NANJING VIROTHER BIOPHARMACEUTICAL CO LTD
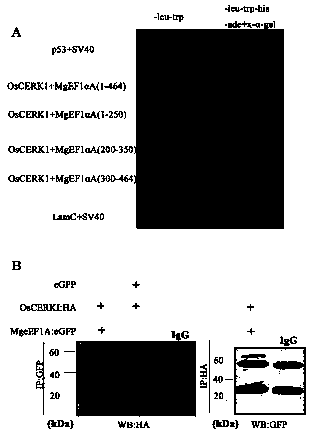
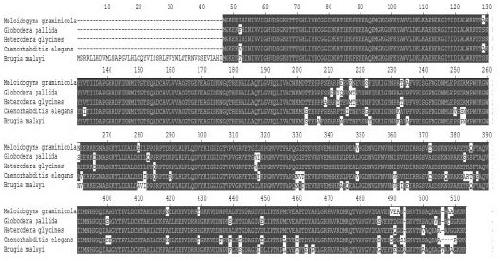
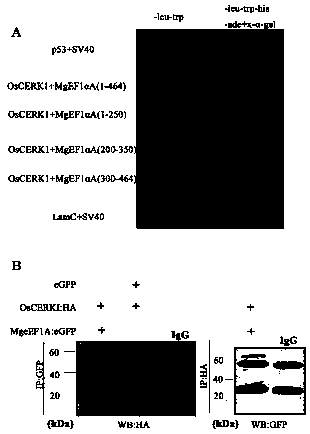
Meloidogyne graminicola translation elongation factor Mg-eFF1A and application thereof to control of plant diseases
ActiveCN108611352AActivate the immune systemActivate immune responseBiocideFungicidesDiseaseNematode
The invention discloses a meloidogyne graminicola translation elongation factor Mg-eFF1A and the application thereof to the control of plant diseases. A genomic sequence of the Mg-eFF1A is as shown inSEQ ID NO.1, and an amino acid sequence is as shown in SEQ ID NO.2. The Mg-eFF1A is firstly discovered, the fact that the Mg-eFF1A can be secreted to the exterior of body of meloidogyne graminicola,and the Mg-eFF1A can activate an immune system of a host during invasion of plant-parasitic nematodes, induce the fundamental immune response of paddies and disease resistance to pathogen, activate PTI immune response, such as pathogenesis-related gene expression, callose accumulation and MAP kinase phosphorylation, of the paddies, and enhance the resistance of the paddies to meloidogyne graminicola and magnaporthe grisea. In addition, researches find that the Mg-eFF1A can induce plant OsCERK1 expression during invasion of the meloidogyne graminicola, and the researches open a direction and strategy with huge potential for the prevention and control of the plant-parasitic nematodes.
Owner:SOUTH CHINA AGRI UNIV
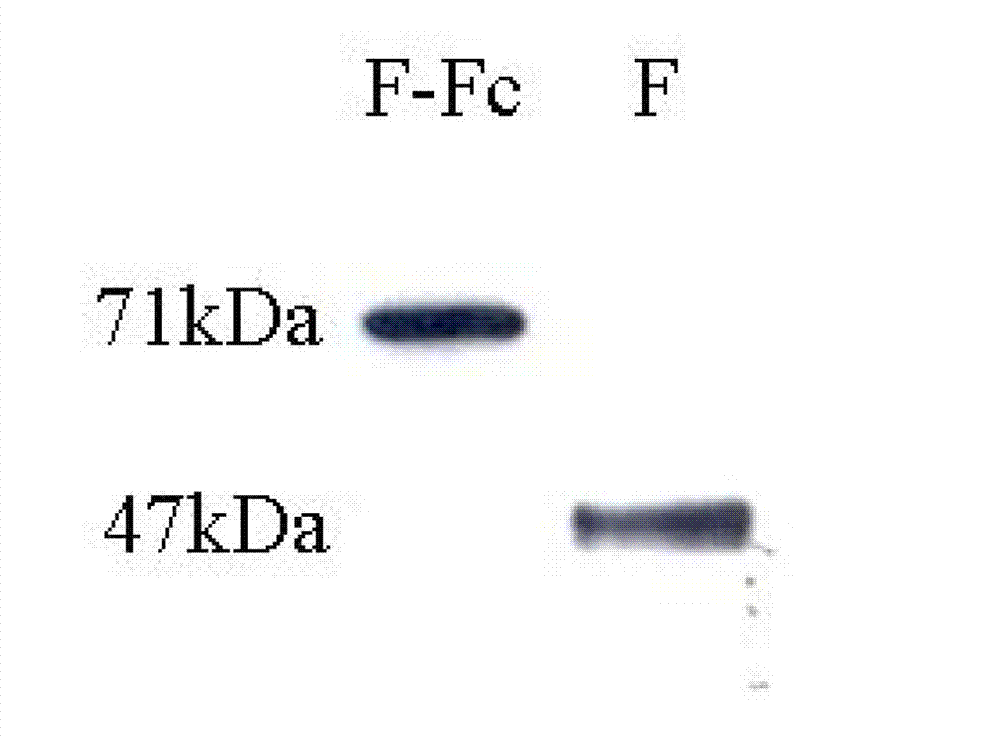
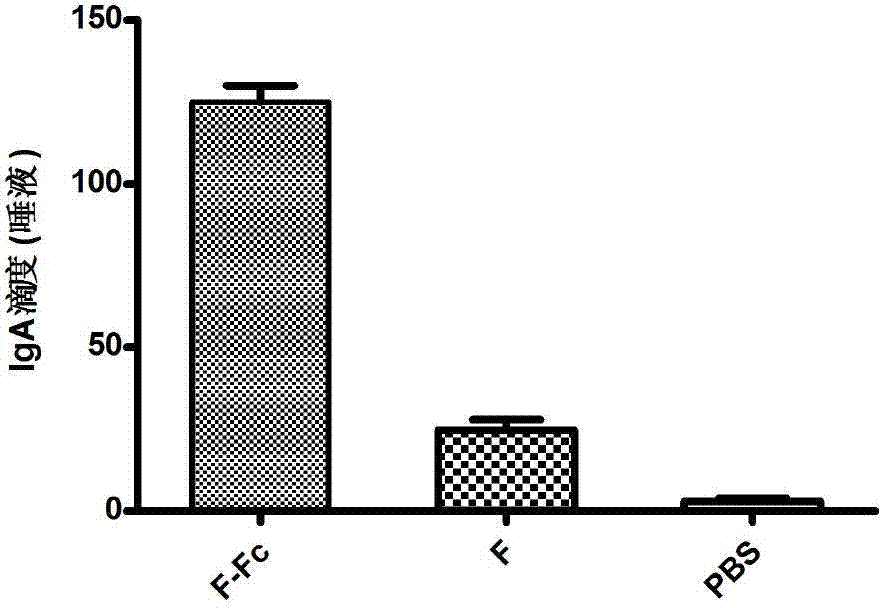
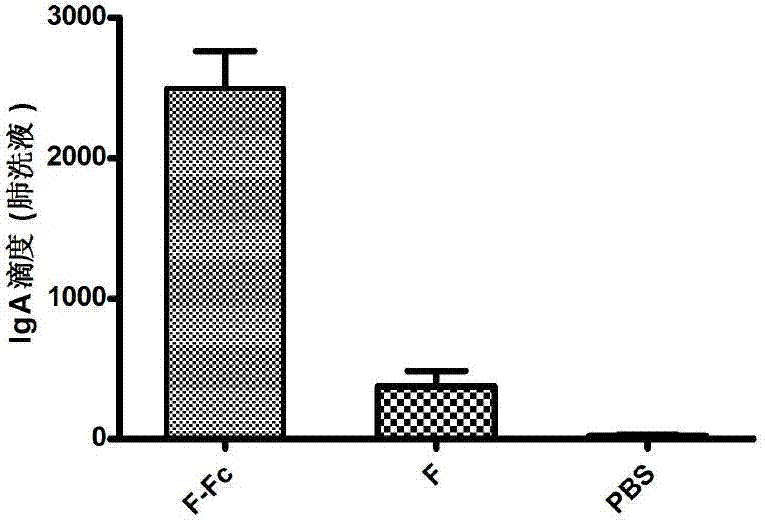
Fusion protein of RSV (respiratory syncytial virus) protein F and Fc, and application thereof
ActiveCN103204943AEnhanced viral infectionActivate immune responseAntiviralsPharmaceutical non-active ingredientsViral antigensHamster
The invention relates to the technical field of biomedical engineering and discloses a production method of fusion protein (F-Fc) of RSV (respiratory syncytial virus) antigen protein F and antibody constant region Fc, and application of the fusion protein as RSV subunit vaccine. The method includes: establishing a eukaryotic expression vector of the fusion protein containing RSV protein F and human IgG2Fc by molecular cloning, transfecting CHO (Chinese hamster ovary) cells, and expressing the fusion protein; performing protein A affinity chromatography to obtain high-purity fusion protein; performing fusion protein nasal dripping to immunize BABL / c mice to trigger specific mucosal immunity against the RSV, humoral immunity and 'Th1 / Th2 balanced' cellular immunity partial to Th1 type, and thereby effectively inhibiting RSV infections.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
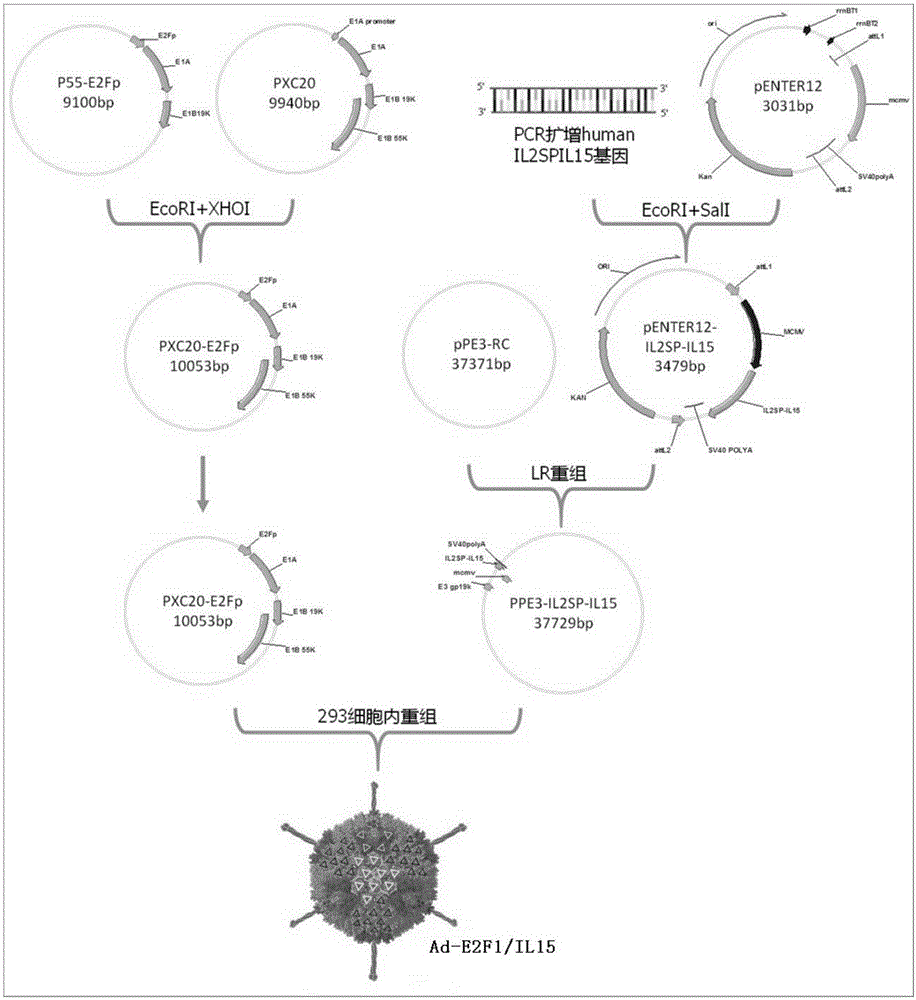
Recombinant oncolytic adenovirus expressing human interleukin 15 and construction method thereof
ActiveCN105177045AImprove anti-tumor effectHigh activityPeptide/protein ingredientsGenetic material ingredientsHigh concentrationOncolytic adenovirus
The invention provides a novel recombinant oncolytic adenovirus expressing human interleukin 15. Specifically, the gene promoter of a type 5 adenovirus E1 region is replaced with a transcription factor E2F-1 gene, and an hIL-15 gene is inserted to an E3 region to construct the recombinant oncolytic adenovirus. According to the invention, E2F-1 is used as the promoter to realize replication of virus specificity in tumor cells, and at the same time, loading of human IL-15 gene in a virogene E3 region can further enhance the anti-tumor effect of the virus. As the pRb / E2F pathway defects exist extensively in solid tumors, through the tumor resolving effect of the virus, a variety of tumor antigens from the individual itself can be acquired, and are not limited by antigen subcellular localization, thus being conducive to producing anti-tumor immune response with self tumor specificity, and having individualized and general treatment significance. In addition, the virus replication process drives the IL-15 gene expression, high concentration IL-15 can be obtained from a part of the virus-infected tumor cell, thereby being in favor of stimulating the activity of immune cells, activating the general immune response and strengthening the antitumor effect.
Owner:晏阳
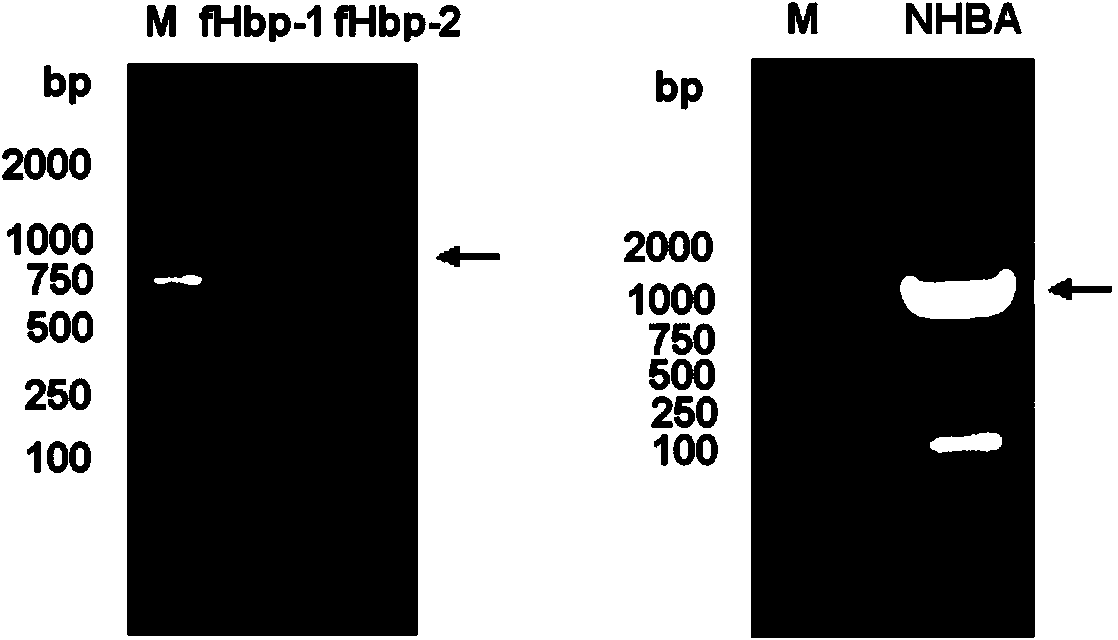
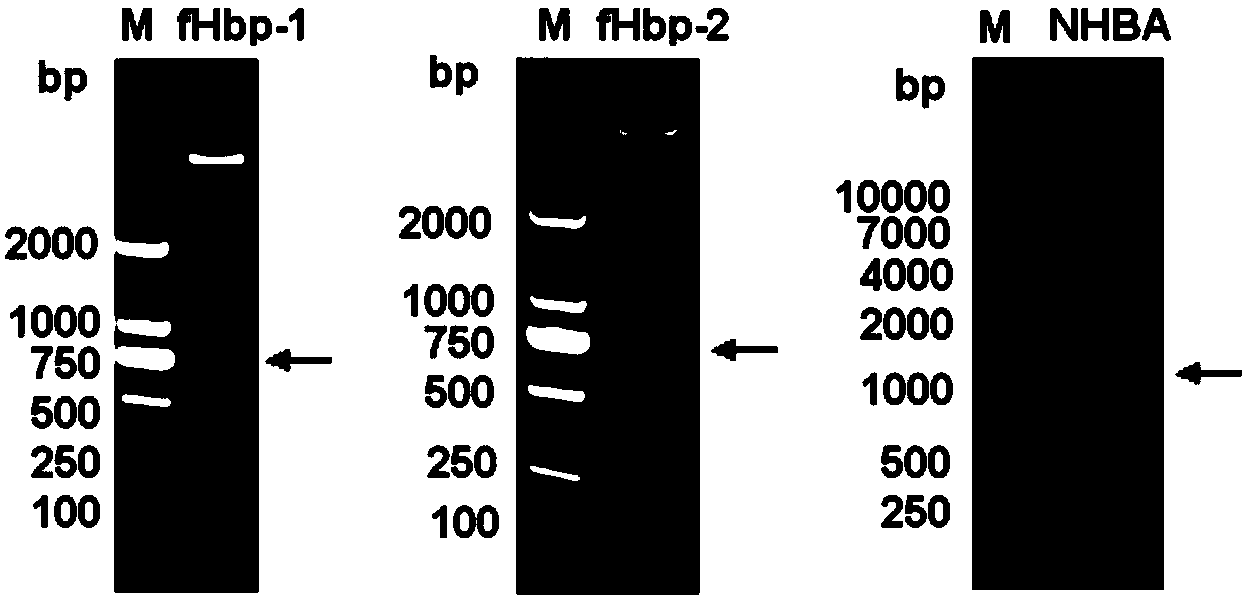
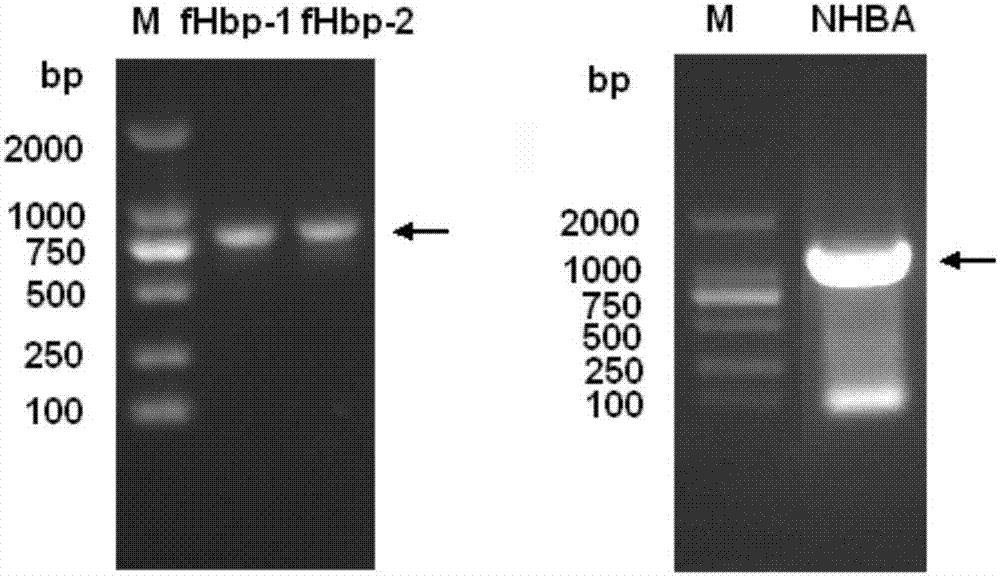
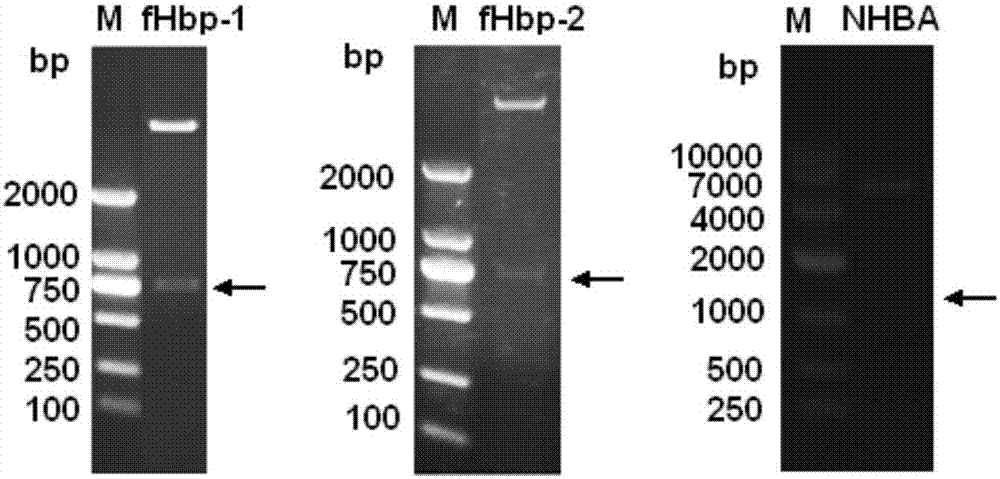
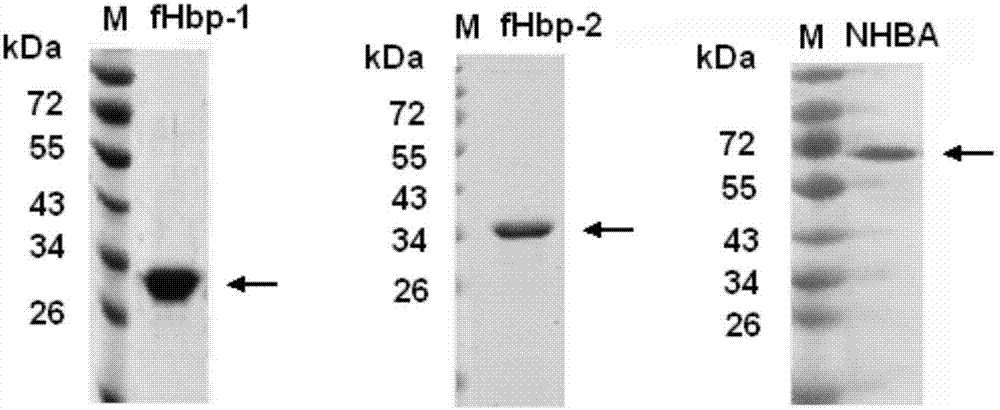
Meningococcus antigen combination and application thereof
ActiveCN104072590AEffective immunityActivate immune responseAntibacterial agentsBacteriaAntigenMeningococcal carriage
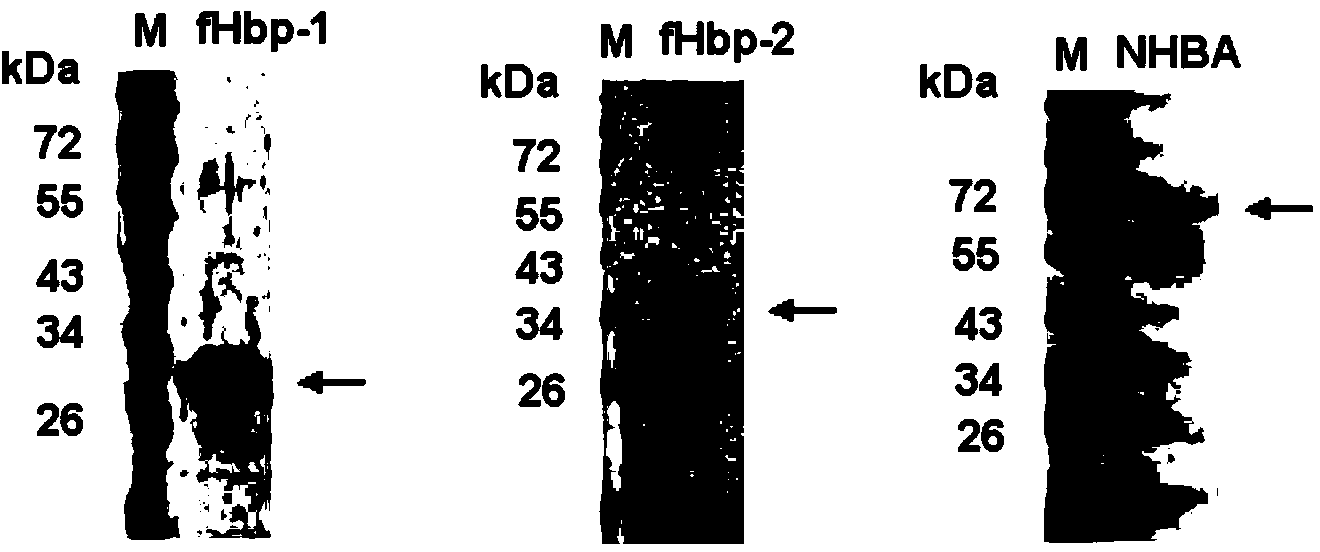
The invention provides a novel antigen combination of meningococcus and an application thereof. The antigen combination comprises fHbp V1 variant proteins, fHbp V2 variant proteins and NHBA proteins. The experiment shows that vaccines prepared from the antigen combination have an obvious synergistic effect and have high broad-spectrum meningococcus resistant capability.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
AHFGDS (aeromonas hydrophila five-gene deletion strain) attenuated bacteria without antibiotic markers and application
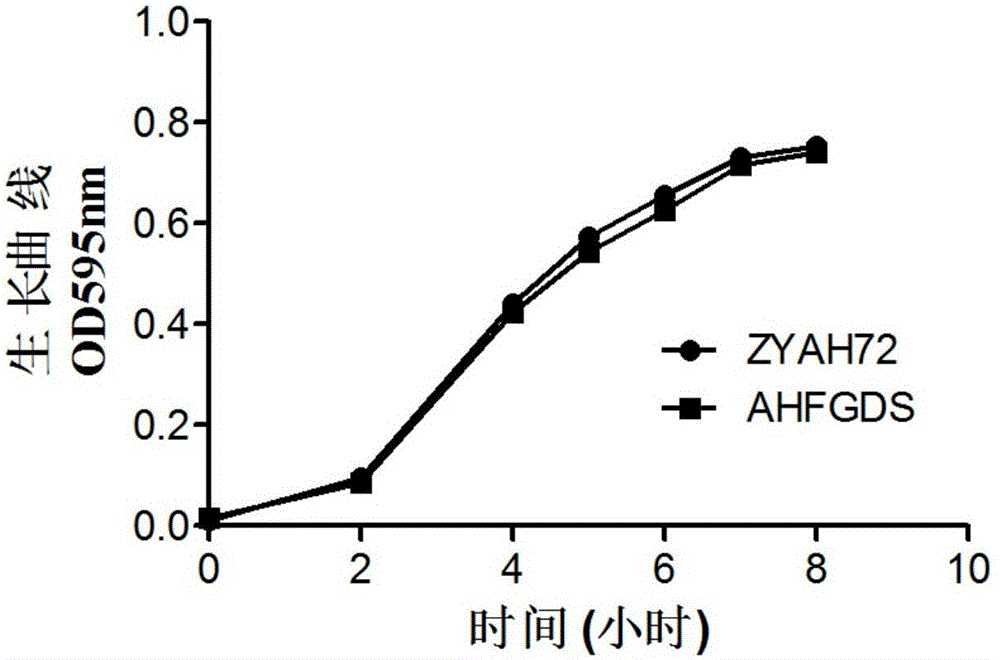
ActiveCN105886429ANo potential hazardGood genetic stabilityAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsAquatic animal
The invention discloses AHFGDS (aeromonas hydrophila five-gene deletion strain) attenuated bacteria without antibiotic markers and an application. Based on high-virulent strain ZYAH72, five virulence factors including aerA, alt, ahp, ast and hly are deleted with a genetic engineering method and a marker-free screening strategy, an AHFGDS with five genes deleted is obtained, and the preservation number of the strain is CCTCC (China Center for Type Culture Collection) No:M2015798. Compared with wild bacteria, the strain can still be subjected to a strong reaction with positive serum even if the pathogenicity is remarkably weakened. Experiments prove that the strain serving as an attenuated live vaccine can effectively protect susceptible aquatic animals against infection of wild AHFGDSs.
Owner:HUAZHONG AGRI UNIV
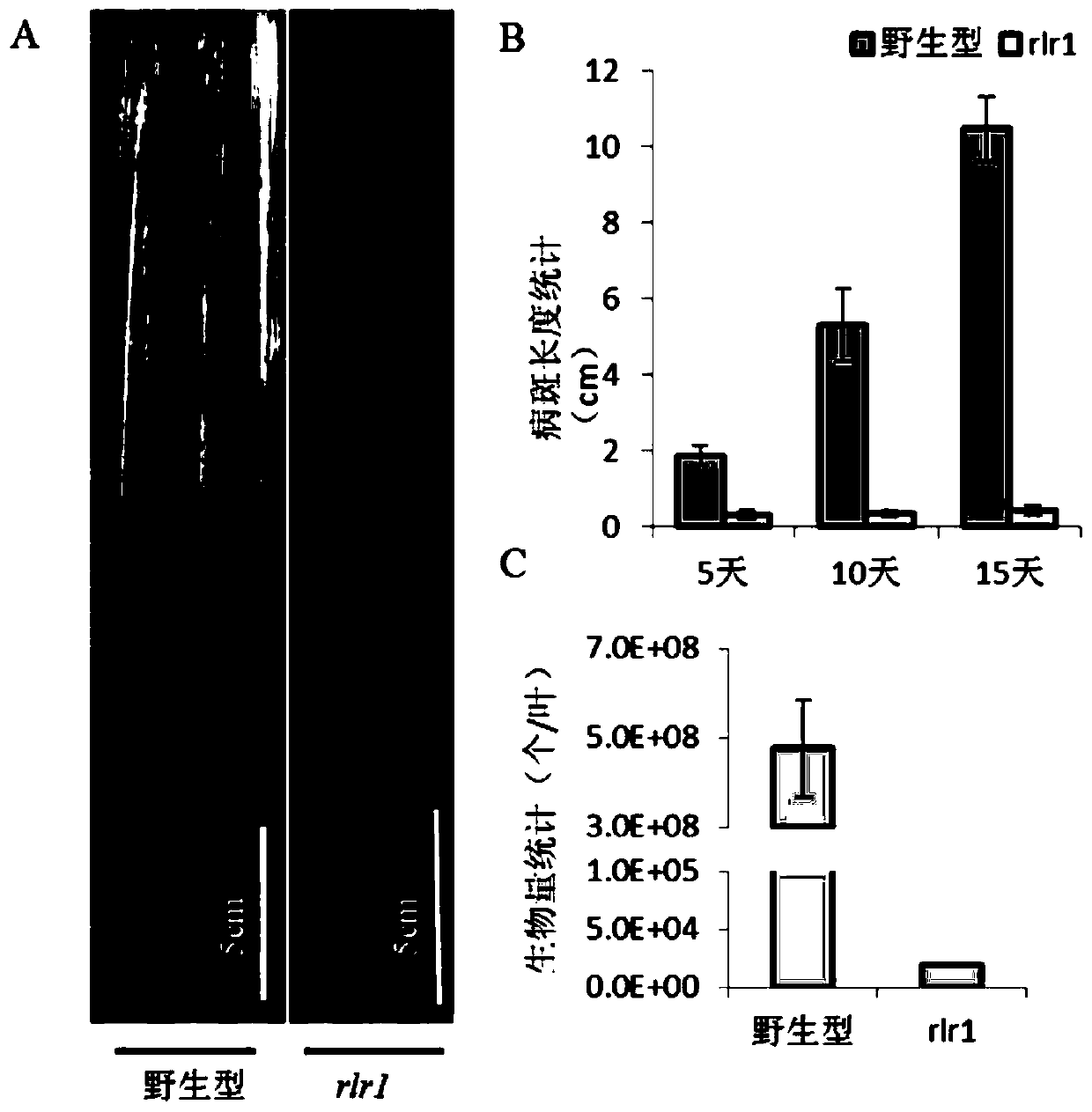
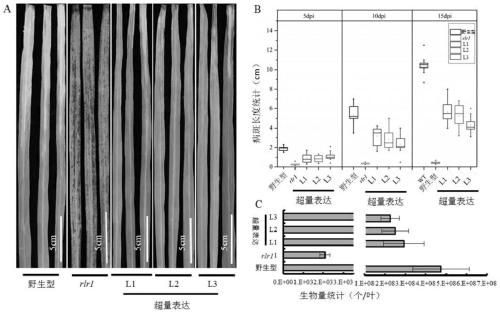
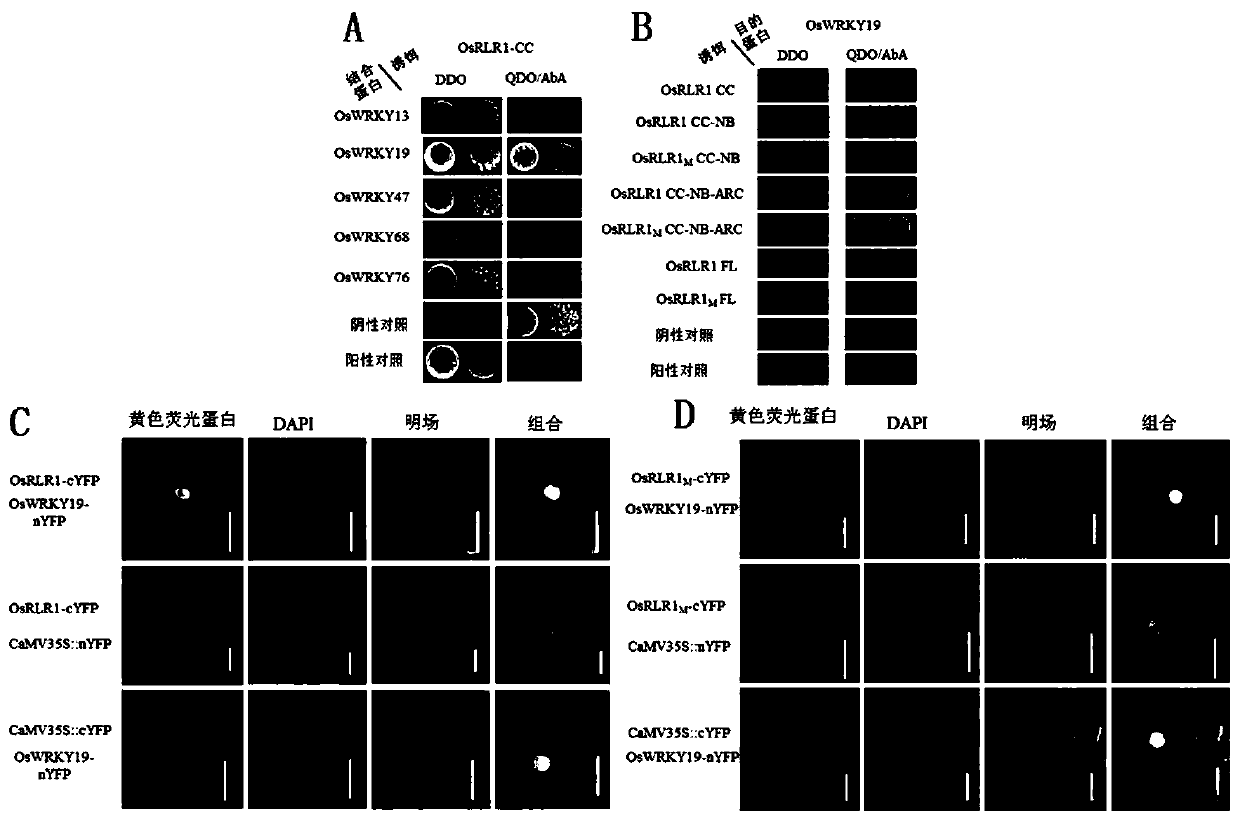
Anti-disease gene OsRLR1, transcription factor OsWRKY19 and application of anti-disease gene OsRLR1 and transcription factor OsWRKY19 in bacterial leaf blight resistant breeding of rice
ActiveCN111187779AIncrease resistanceEnhanced immune responsePlant peptidesFermentationBiotechnologyDisease resistant
The invention relates to an anti-disease gene OsRLR1, a transcription factor OsWRKY19 and an application of the anti-disease gene OsRLR1 and the transcription factor OsWRKY19 in bacterial leaf blightresistant breeding of rice. Molecular biology and a biochemical technique show that overexpression of OsRLR1 enhances the resistance to bacterial leaf blight. The anti-disease gene OsRLR1 and the transcription factor OsWRKY19 have mutual effect on protein and can enhance the resistant reaction to the bacterial leaf blight. Mutation of the rice anti-disease gene OsRLR1 causes brownness of disease like spots of leaves, expression of related genes of disease course of rice is enhanced, immunoreaction in a body is activated, and the resistance to the bacterial leaf blight is enhanced. A new way isdeveloped for enhancing the bacterial leaf blight resistance of rice and improving the yield of the rice.
Owner:SOUTHWEST UNIVERSITY
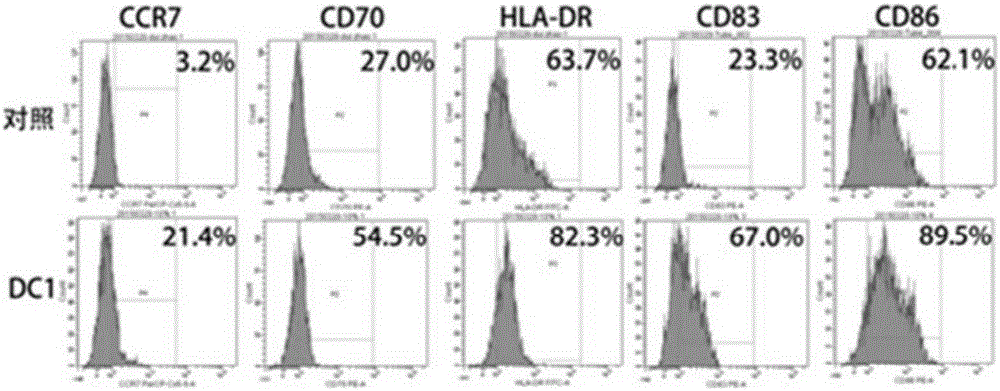
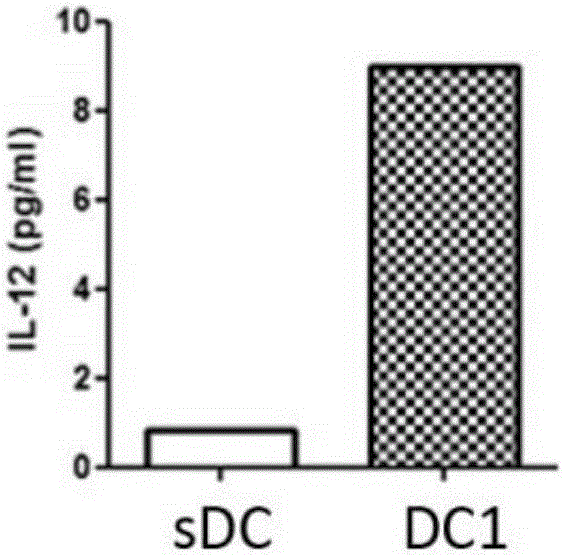
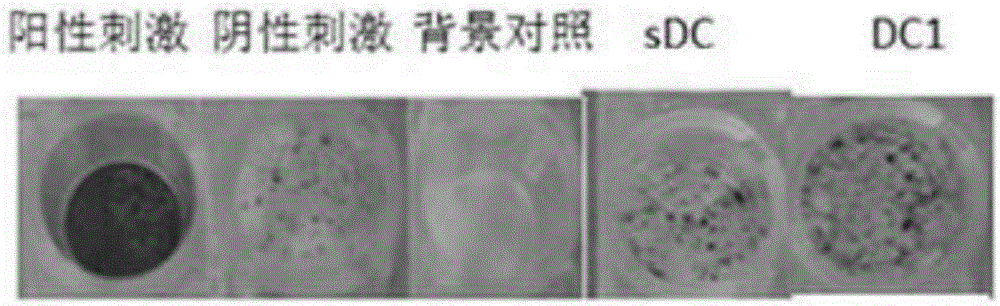
Type 1 polarized dendritic cells and inducing method and application thereof
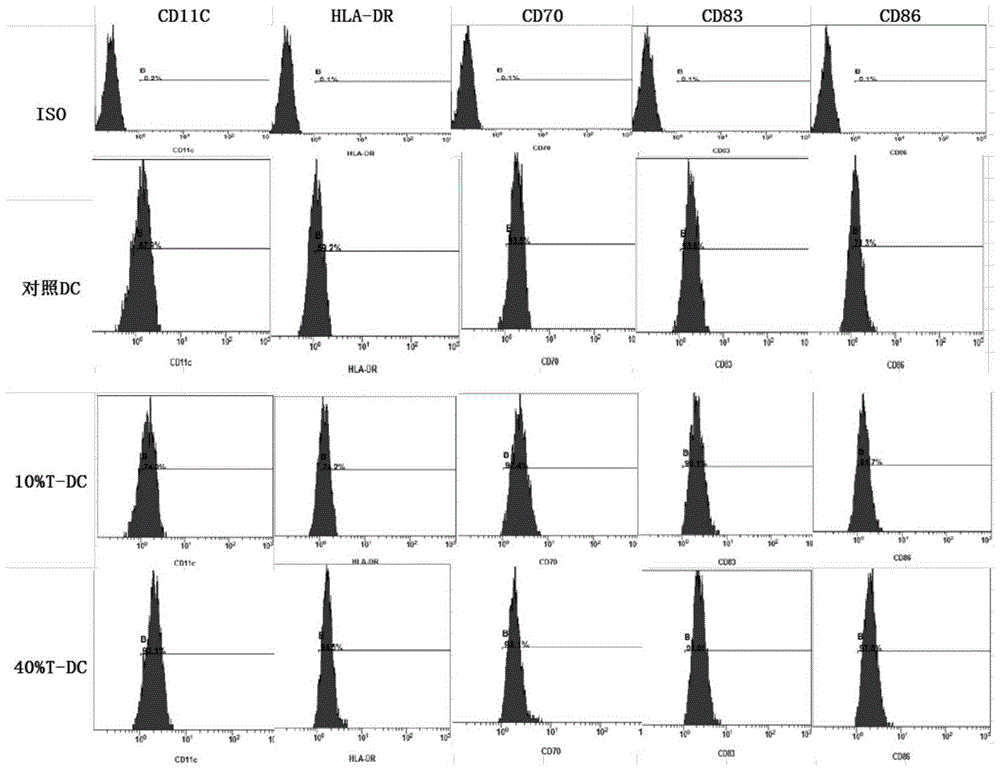
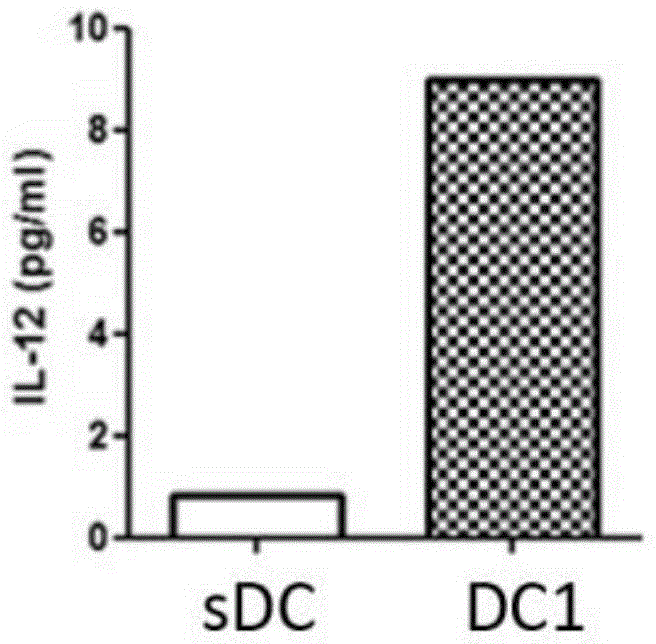
InactiveCN105219717AReduce usageReduce manufacturing costMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthDendritic cell
The invention provides type 1 polarized dendritic cells and an inducing method and application thereof. A novel DC1 preparation method is built through the assistant function of Natural Killer cells of umbilical cord blood in the adaptive immunity activating process of DCs, and in the preparation process, usage of cell factors is reduced, the cost is lowered, and quality control is improved. The invention further provides a method for applying the prepared DC1s to preparation of CTLs with the tumor antigen specificity; after the DC1s are loaded with tumor specific antigens, part of the cells are transfused to a patient body, the other part of the cells are used for in-vitro inductive cytotoxic T lymphocytes (CTL) with the tumor antigen specificity, and the immunoreaction of the tumor specificity is activated through the mode of combining active induction with passive induction in vivo and vitro.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Artificial breeding method for spring water fish
InactiveCN109757412AFast growthSoft and delicious meatFood processingClimate change adaptationBroodstockZygote formation
The invention discloses an artificial breeding method for spring water fishes. The method comprises the following steps: (1) screening mature and active spring water fishes, and putting into a fish pond; (2) launching fish fries, namely launching fish fries in November every year, cleaning and sterilizing the fish pond, putting spring water into the fish pond, launching the fish fries at an appropriate temperature, feeding water from one end of the fish pond, discharging water from the other end of the fish pond to construct a living water environment, and breeding the fish fries for more thanone year to obtain adult fishes; (3) managing adult fishes, namely when 60% of the spring water fish fires grow into adult fishes of 20g, putting a traditional Chinese medicine granule feed which accounts for 3-5% of the mass of a feed into the feed used every day, and fishing up the spring water fishes which are 1500-3000g on average, and delivering to the market. Due to adoption of mature and efficient spring water fish parental breeding and artificial production acceleration techniques, zygotes are increased, the hatchability can be improved, and the survival rate of offspring seeds can beincreased.
Owner:程自衡
A translation elongation factor mg-eef1a of root-knot nematode graminaceae and its application in the control of plant diseases
ActiveCN108611352BActivate the immune systemActivate immune responseBiocideFungicidesBiotechnologyCallose
The invention discloses a meloidogyne graminicola translation elongation factor Mg-eFF1A and the application thereof to the control of plant diseases. A genomic sequence of the Mg-eFF1A is as shown inSEQ ID NO.1, and an amino acid sequence is as shown in SEQ ID NO.2. The Mg-eFF1A is firstly discovered, the fact that the Mg-eFF1A can be secreted to the exterior of body of meloidogyne graminicola,and the Mg-eFF1A can activate an immune system of a host during invasion of plant-parasitic nematodes, induce the fundamental immune response of paddies and disease resistance to pathogen, activate PTI immune response, such as pathogenesis-related gene expression, callose accumulation and MAP kinase phosphorylation, of the paddies, and enhance the resistance of the paddies to meloidogyne graminicola and magnaporthe grisea. In addition, researches find that the Mg-eFF1A can induce plant OsCERK1 expression during invasion of the meloidogyne graminicola, and the researches open a direction and strategy with huge potential for the prevention and control of the plant-parasitic nematodes.
Owner:SOUTH CHINA AGRI UNIV
Lipid cochleate carrier based on aluminum ions
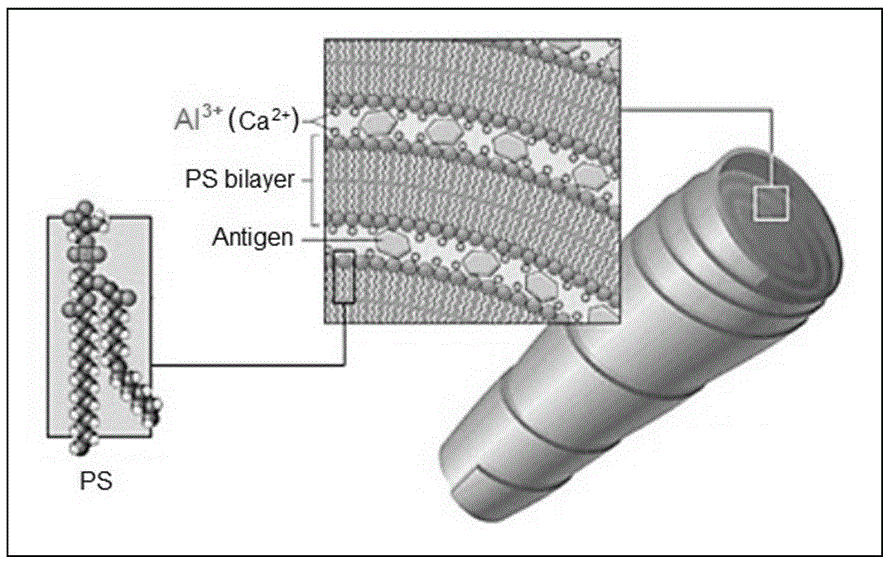
InactiveCN105944098AOvercome adverse reactions such as local irritationStrong adjuvant functionPowder deliveryPharmaceutical non-active ingredientsAluminum IonBiocompatibility Testing
The invention discloses a lipid cochleate carrier based on aluminum ions. ACO is mainly used as a vaccine carrier and has the strong adjuvant function, so that a vaccine adjuvant / transfer system is formed, and immunity induction potency is improved. Being used as the vaccine carrier, the lipid cochleate carrier has the obvious advantages, the ACO carrier is wide in application range, applicable to carrying different vaccines such as subunit vaccine antigen, inactivated / attenuated vaccines and DNA vaccines, ACO stability is high, meanwhile, vaccine interior and exterior stability can be improved by packaging the vaccine, safety is high, materials adopted for ACO have good biocompatibility, dosage amount is large, multiple dosage ways are provided, dosage can be achieved through sinus tract mucosa comprising oral administration and inoculation and can also be achieved through subcutaneous, intracutaneous and muscle injection, immune induction validity is strong, and ACO has the adjuvant function, and can increase cell uptake and improve immune induction validity when carrying vaccines.
Owner:ANHUI MEDICAL UNIV
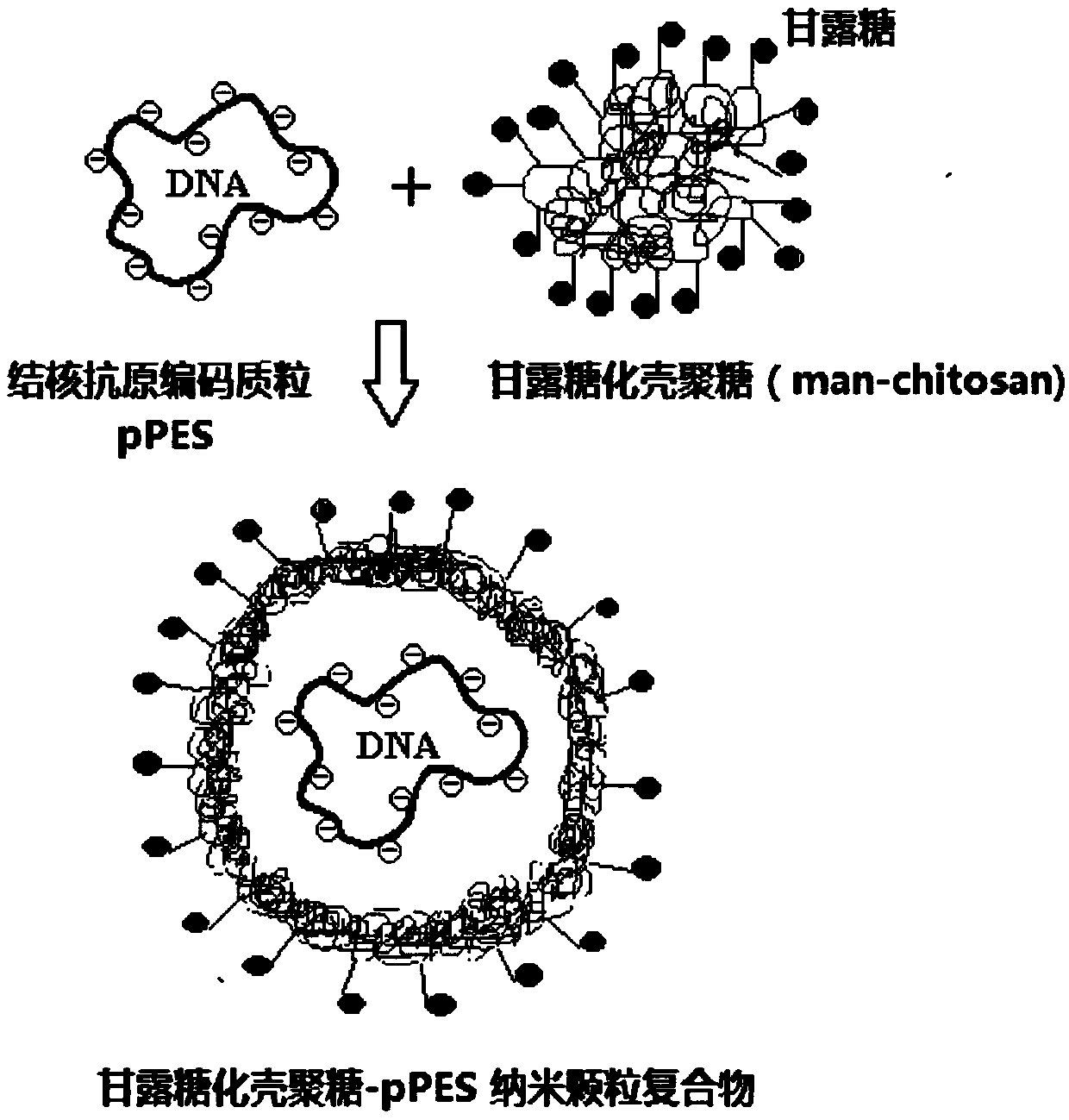
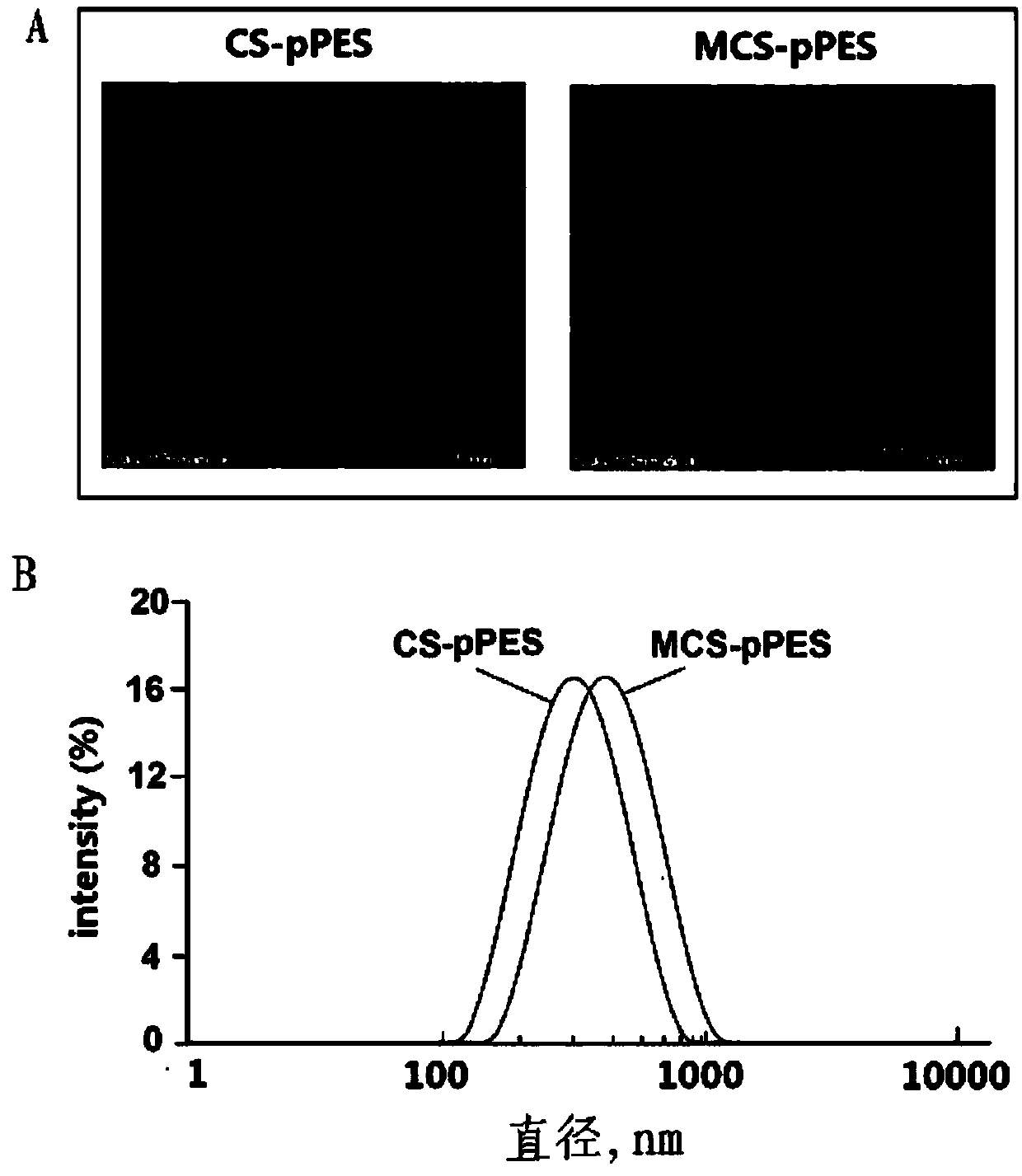
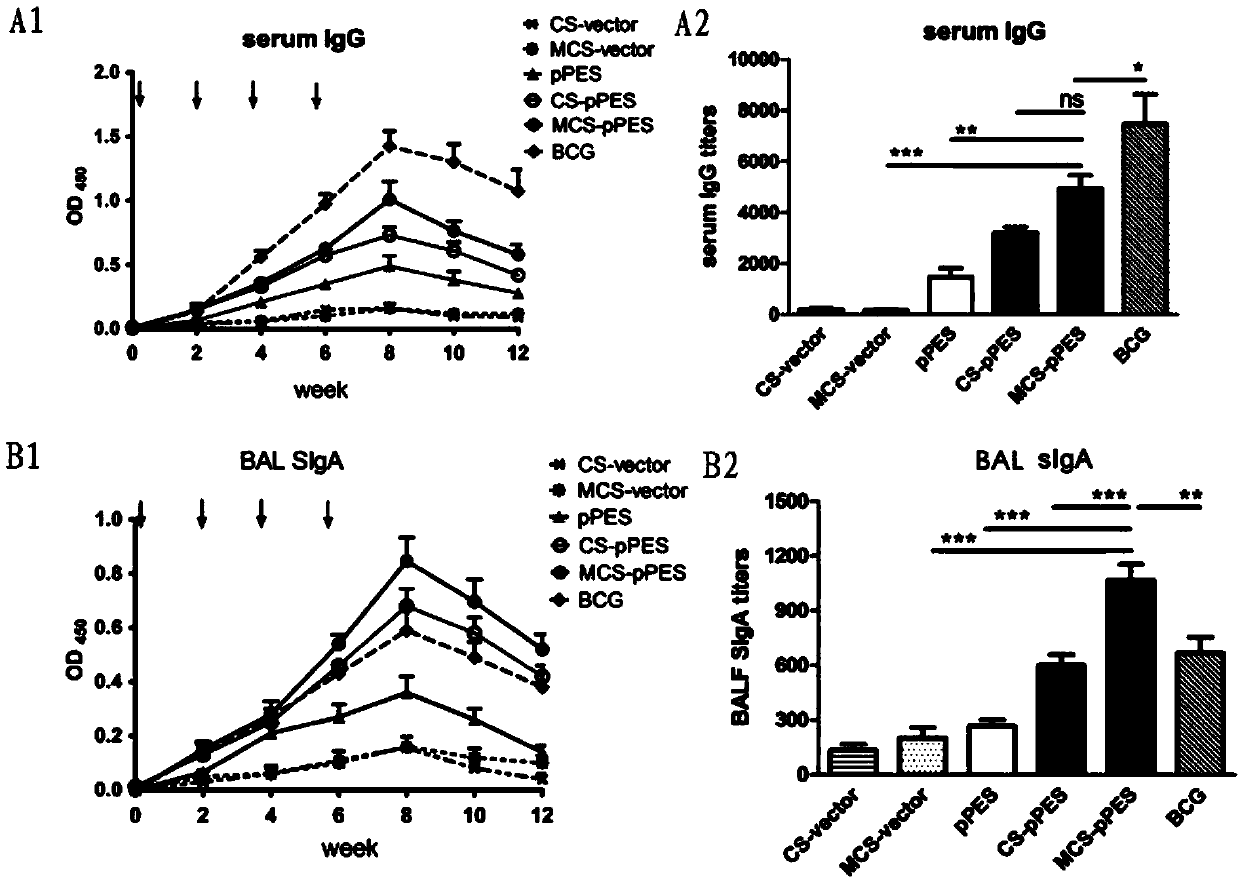
Mannosylated chitosan delivery system assembled tuberculosis mucosa gene vaccine
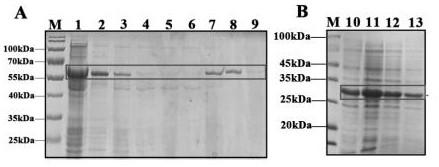
ActiveCN107583045ASafe and non-toxicReduce releaseAntibacterial agentsBacterial antigen ingredientsNanoparticle ComplexGenetic vaccine
The invention relates to a mannosylated chitosan delivery system assembled tuberculosis mucosa gene vaccine. The mannosylated chitosan delivery system assembled tuberculosis mucosa gene vaccine is a nanoparticle complex compounded through copolymerization and crosslinking of mannosylated chitosan and a tuberculosis antigen encoding plasmid pPES; the tuberculosis antigen encoding plasmid pPES is amulti-T cell epitope tuberculosis gene vaccine with heat shock protein 65 as an epitope scaffold, which is disclosed in the invention with the application number of 201310682146.4. The invention further discloses a preparation method of the mannosylated chitosan delivery system assembled tuberculosis mucosa gene vaccine. The preparation method comprises the following steps: preparing a mannosylated chitosan solution with the concentration of 0.015-0.025% and the pH of 5.2-5.5; dissolving the tuberculosis antigen encoding plasmid pPES into a buffer solution to obtain a tuberculosis antigen encoding plasmid solution with the concentration of 800 [mu]g / ml-1 mg / ml; and mixing the mannosylated chitosan solution and the tuberculosis antigen encoding plasmid solution at 54-57 DEG C at a high speed of 15000-17000 rpm, and performing a copolymerization and chemical crosslinking reaction. The invention further discloses application of the mannosylated chitosan delivery system assembled tuberculosis mucosa gene vaccine to preparation of medicines for preventing or treating tuberculosis. The tuberculosis mucosa gene vaccine has the potential as a novel preventive and therapeutic tuberculosis vaccine.
Owner:SUZHOU UNIV
Bovine akabane disease virus vaccine
PendingCN114507272AHigh purityAntigen immunity is strongSsRNA viruses negative-senseViral antigen ingredientsNucleotideNucleotide sequencing
The invention relates to the technical field of biology, and particularly provides a cattle akabane disease virus vaccine. The protein for preparing the bovine akabane disease virus vaccine comprises G1 protein of bovine akabane disease virus and G2 protein of bovine akabane disease virus, a nucleotide sequence for coding the G1 protein is shown as SEQ ID NO.1, and a nucleotide sequence for coding the G2 protein is shown as SEQ ID NO.2. The invention further provides a preparation method of the bovine akabane disease virus vaccine. The protein is suitable for a eukaryotic cell expression system, the process for preparing active ingredients of bovine akabane disease virus vaccines is changed, and meanwhile the cost is reduced. In addition, the protein has broad-spectrum antigenicity, and a good cross protection effect can be achieved.
Owner:天康制药股份有限公司
Crude sargassum pallidum polysaccharide, and preparation method, separation and purification method and application thereof
PendingCN113621088AHigh yieldAvoid destructionOrganic active ingredientsImmunological disordersCelluloseMonosaccharide composition
The invention discloses a crude sargassum pallidum polysaccharide and a preparation method thereof. The crude sargassum pallidum polysaccharide is extracted by taking sargassum pallidum frond as a raw material, and monosaccharide comprises galactose, fucose and mannose, and structurally has typical S=O symmetrical stretching vibration peaks and alpha-configuration glucosidic bonds. The preparation method of thecrude sargassum pallidum polysaccharide comprises the following steps: performing hot water extraction, concentration, dialysis, alcohol precipitation and freeze drying on sargassum pallidum frond to obtain the crude sargassum pallidum polysaccharide. The invention also discloses a separation and purification method of the crude polysaccharide. The separation and purification method comprises the following steps: deproteinizing the crude polysaccharide through a Sevag reagent, eluting and separating through a DEAE-cellulose anion exchange column, and then concentrating, dialyzing, carrying out alcohol precipitation and drying to obtain powder of each component of the sargassum pallidum polysaccharide. The sargassum pallidum polysaccharide obtained by the invention has the effects of improving the immunity of the organism and inhibiting the growth of tumor cells, and can be applied to healthy foods and / or pharmaceutical preparations for adjuvant therapy of tumors or improvement of the immune regulation ability of the organism.
Owner:QINGDAO AGRI UNIV
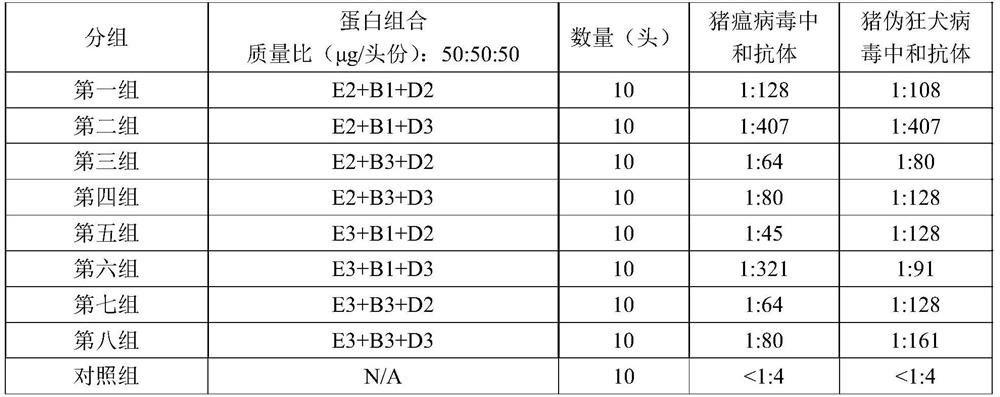
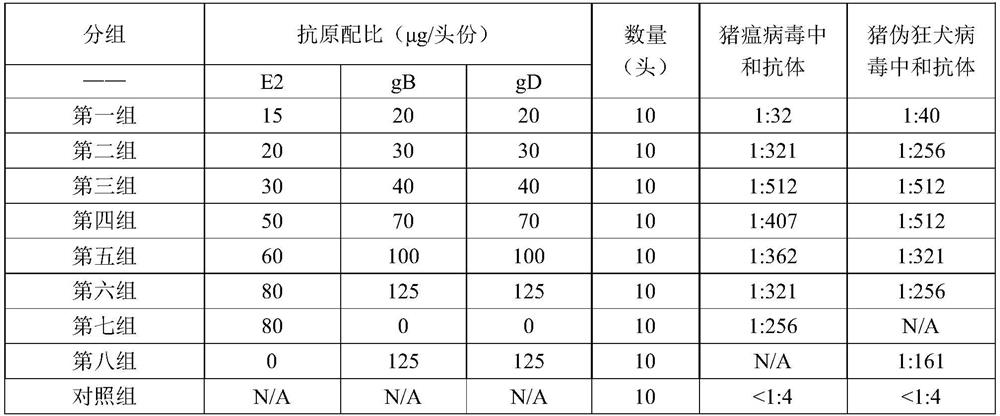
Swine fever and porcine pseudorabies virus bivalent subunit vaccine and preparation method thereof
ActiveCN114163505AEasy to prepareAntigen immunity is strongSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVAntigen
The invention provides a swine fever and porcine pseudorabies virus bivalent subunit vaccine and a preparation method thereof. The effective components of the bivalent subunit vaccine are classical swine fever virus E2 protein, porcine pseudorabies virus gB protein and porcine pseudorabies virus gD protein. The preparation method of the bivalent subunit vaccine is simple, the swine fever virus E2 protein, the porcine pseudorabies virus gB protein and the porcine pseudorabies virus gD protein are high in yield, high in purity and strong in antigen immunity, the immune response of a body can be effectively activated, and the bivalent subunit vaccine has an ideal immune protection effect on swine fever and porcine pseudorabies. Compared with a monovalent vaccine, the artificial cost can be reduced, the number of vaccination times (double prevention by one injection) is reduced, the stress reaction of pigs caused by vaccine immunization is reduced, the protection effect is equivalent to that of the monovalent vaccine, and the antibody neutralizing result is superior to that of the monovalent vaccine.
Owner:天康制药股份有限公司
Meningococcus antigen composition and applications thereof
ActiveCN107349423AEffective immunityActivate immune responseAntibacterial agentsPeptidesAntigenMutated protein
The invention provides a novel meningococcus antigen composition and applications thereof. Specifically, the antigen composition comprises fHbp V1 mutant protein, fHbp V2 mutant protein, and NHBA protein. The experiment results show that a vaccine prepared from the antigen composition has a prominent synergistic effect and an excellent performance on resisting meningitis in a broad spectrum.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Recombinant baculovirus expressing senecavirus VP2 gene and preparation method and application thereof
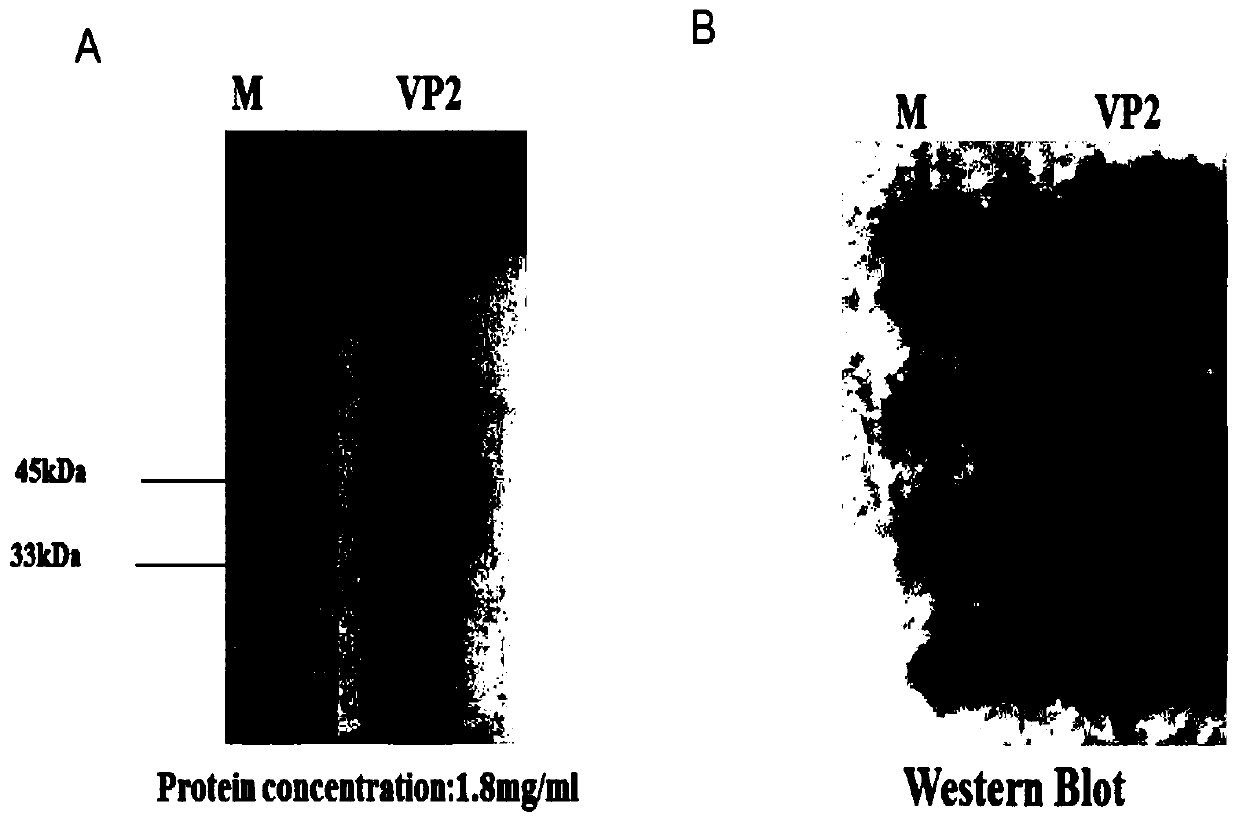
ActiveCN110358741APreserve immunogenicitySimplify the extraction and purification processSsRNA viruses positive-senseViral antigen ingredientsSenecavirusVp2 gene
The invention relates to the field of biotechnology, in particular to recombinant baculovirus expressing senecavirus VP2 gene and a preparation method and application thereof. The recombinant baculovirus comprises one or more copies of senecavirus VP2 protein encoding gene. The invention further provides a senecavirus subunit vaccine which comprises the recombinant baculovirus expressed senecavirus VP2 protein. Accordingly, by means of artificial codon optimization, high-level and high-purity expression of senecavirus VP2 protein is achieved, and the immunogenicity of the VP2 protein is retained to the maximum extend. The senecavirus subunit vaccine has the excellent immunogenicity and high safety, and an ideal immune protection effect is achieved for infection of senecavirus.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
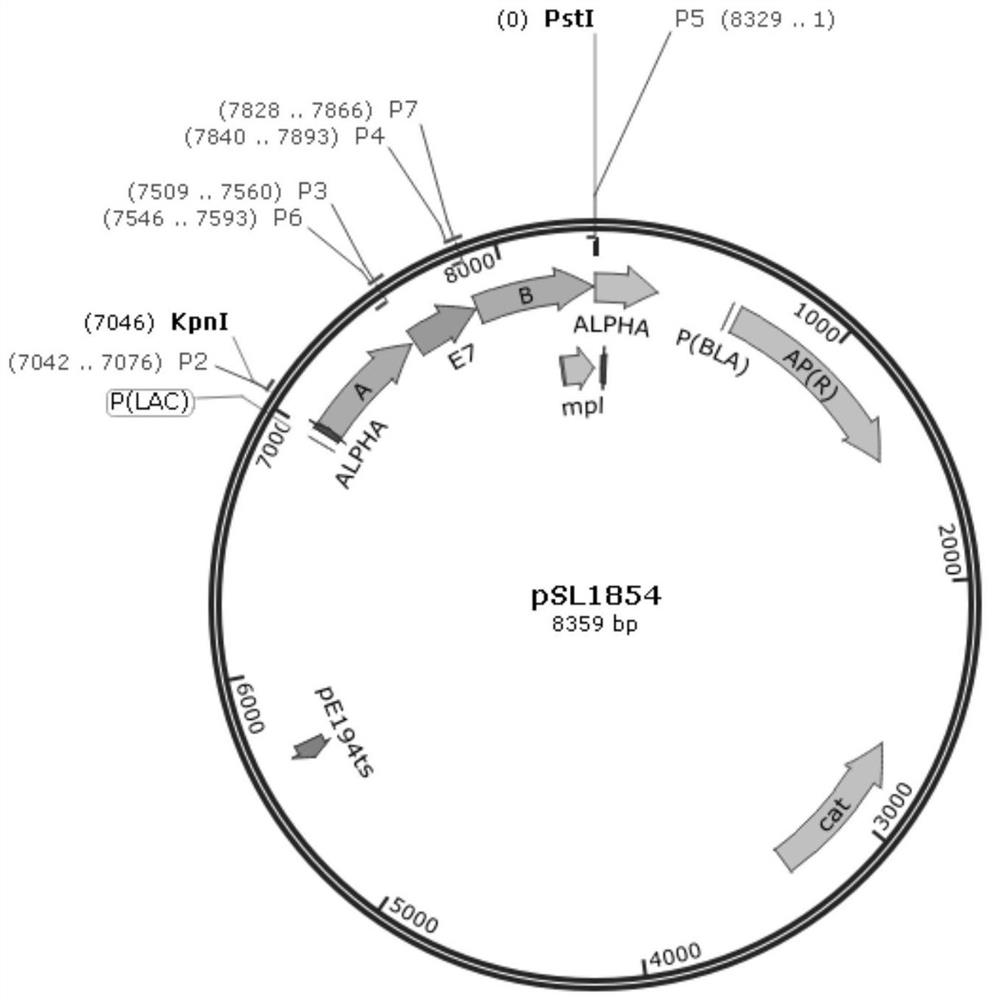
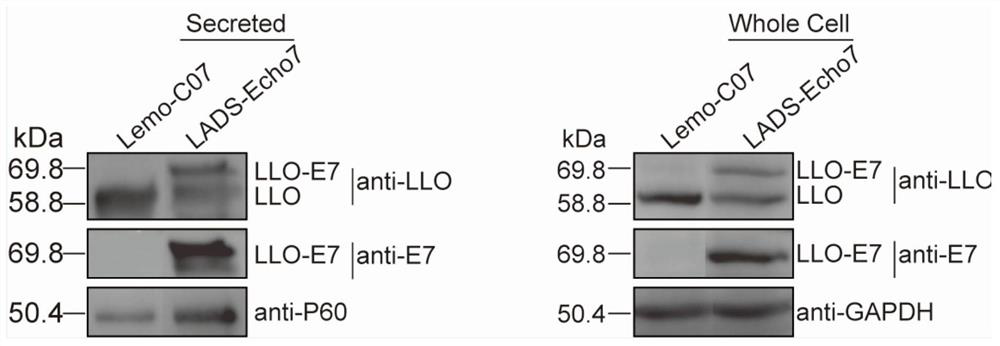
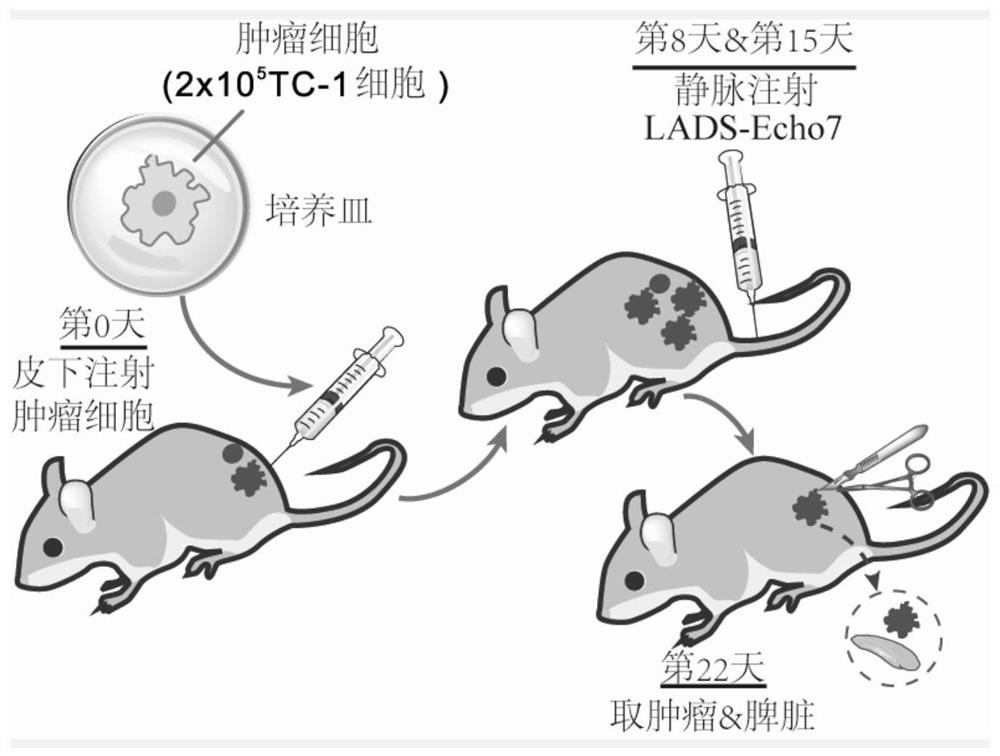
Cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes and preparation method of cervical cancer therapeutic vaccine
PendingCN112538452AStable expressionNot easy to loseBacteriaViral antigen ingredientsTumor therapyCervical ca
The invention provides a cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes and a preparation method of the cervical cancer therapeutic vaccine, and relates tothe field of genetic engineering. The attenuated Listeria monocytogenes Lemo-C07 are taken as a background, and a virulence factor LLO protein of the strain carries two key mutant amino acid sites, sothat the virulence of the strain is greatly reduced, but part of the proliferation ability in the cells is still retained. According to the vaccine, on the basis of the attenuated Listeria monocytogenes, a molecular biological technique is utilized to integrate a full-length gene of a specific antigen E7 of the cervical cancer to the downstream part of an LLO gene of the Listeria monocytogenes, thereby constructing and obtaining the recombinant attenuated Listeria monocytogenes containing the tumor specific antigen E7. The vaccine has the capabilities of presenting the specific antigen and activating the anti-tumor cellular immunity of the body, and has the remarkable tumor treatment effect.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Novel retinoic acid nanoemulsion adjuvant for efficiently enhancing humoral immune response and mucosal immune response and preparation method and application of adjuvant
PendingCN113713093APromote aggregationEnhance immune responseImmunological disordersAntibody medical ingredientsMucosal Immune ResponsesAdjuvant
The invention discloses a novel retinoic acid nanoemulsion adjuvant for efficiently enhancing humoral immune response and mucosal immune response and a preparation method and application of the adjuvant. The retinoic acid nanoemulsion adjuvant is prepared from retinoic acid, an oil phase, a surfactant, a cosurfactant and a water phase, wherein the retinoic acid is wrapped by a formed oil-in-water type nanoemulsion adjuvant system. The retinoic acid nanoemulsion adjuvant can be delivered together with multiple antigens (a model antigen OVA, a staphylococcus aureus recombinant protein antigen and a neocoronavirus recombinant protein antigen) respectively, the immune response level of a vaccine antigen can be enhanced after injection immunization, the adjuvant has a good immune protection effect, can induce humoral immune response of a system and efficiently activate immune response of mucosal parts (intestinal mucosa, vaginal mucosa, lung mucosa, gastric mucosa and nasal mucosa) and has great market application value and broad application prospect.
Owner:ARMY MEDICAL UNIV
Kit for activating colorectal cancer specific immunity response
InactiveCN105219727AActivate adaptive immune responseIncrease contentBlood/immune system cellsAntibody medical ingredientsAntigenSpecific immunity
The invention provides a kit for activating colorectal cancer specific immunity response. The kit comprises an RPMI-1640 culture medium, a cell culture supernatant, a GM-CSF, an IL-4, an INF gamma and a colorectal cancer specific antigenic peptide combination. The colorectal cancer specific antigenic peptide combination includes MAGE-A3-A2 antigenic peptides, MAGE-A4-A2 / 3 antigenic peptides, SSX-2-A2 antigenic peptides, PLAC1-A2 antigenic peptides, hTERT-A2 antigenic peptides, hTERT-A3 antigenic peptides, HER2-A2 antigenic peptides, HER2-A3 antigenic peptides, Survivin-A2 antigenic peptides, Survivin-A3 antigenic peptides, COX-2-A2 / 3 antigenic peptides and MTA1-A2 antigenic peptides. The kit can efficiently activate colorectal cancer specific immunity response in an active inducing / passive inducing combined mode.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com