Double adjuvant-neoantigen tumor nanometer vaccine, and preparation method and application thereof
A nano-vaccine and antigen technology, which is applied in the field of preparation of tumor nano-vaccine drugs, can solve the problems of water solubility and pharmacokinetic limitation of joint use, and achieve the effect of inhibiting tumor cell growth, clear and simple synthesis steps, and activating immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
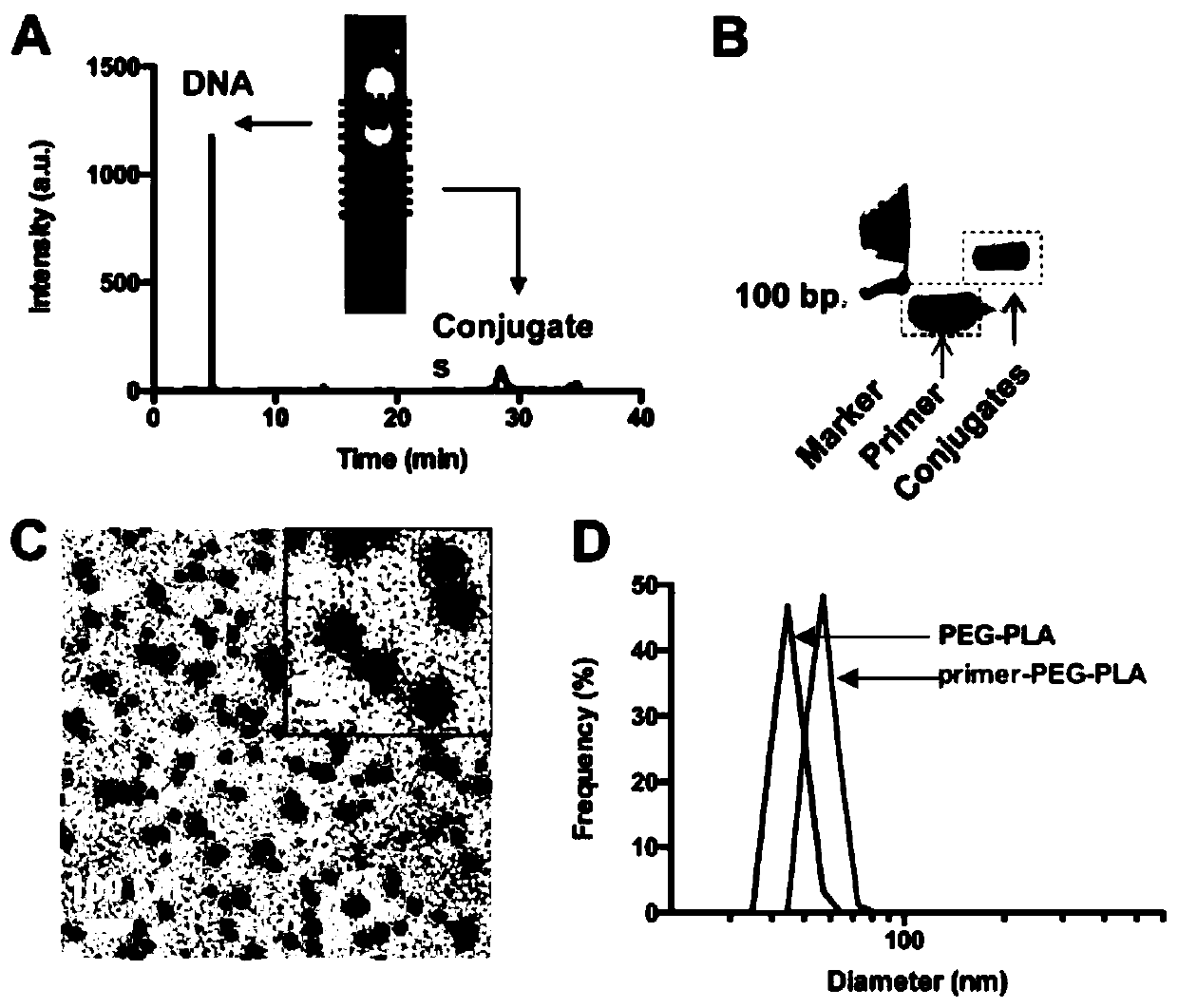
[0065] Example 1: Preparation and related characterization of DNA primer-PEG-PLA nanoparticles
[0066] Take 50 μl of DNA primer template with a concentration of 120 μM (the 5’ end is modified with a disulfide bond), add 10 μl of dithiothreitol (DTT) at a concentration of 1 M, add 10 μL of 10× phosphate buffered saline solution and 30 μL of DEPC Water was used to prepare a 100 μL reaction system. Stir at 37°C for 1 hour. Prepare one NAP 5 purification column, wash it with ascorbate 3 times, after the above stirring, mix 100 μL of the stirred product and 400 μL phosphate-buffered saline into the NAP 5 purification column, wait until the 500 μL solution is exhausted, and then add Add 1 mL of phosphate-buffered saline to the purification column, and collect 1 mL of the filtered liquid to obtain DNA primers modified with sulfhydryl groups at the 5' ends. Subsequently, the concentration of polyethylene glycol-polylactic acid (MAL-PEG3000-PLA2000) with terminally modified maleamid...
Embodiment 2
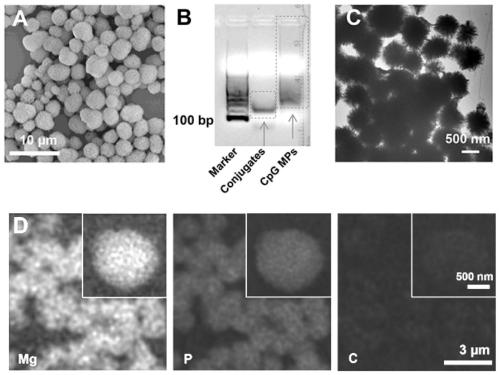
[0067] Example 2: Preparation and Characterization of CpG Microparticles and Small Molecule R848 Loading
[0068]Take 20 μL of the final product of Example 1 (1.2 μM DNA primer concentration), add 1 μL (1 μM) of CpG or GpC single-stranded template, and add 4 μL 10×T4 ligase buffer solution and 15 μL aqueous solution to configure a 40 μL reaction system. The 40 μL reaction solution was heated at 95° C. for 5 minutes and gradually cooled to 25° C. within 1.5 hours to complete the complementary hybridization of the DNA primer fragment and the CpG (GpC) template fragment. The hybridized product was reacted with 1 μL of T4 ligase (20000 U / μL) at room temperature for 2 hours to seal the gap of the CpG (GpC) ring-mounted template. Then the circular DNA product was mixed with DNA polymerase (12μL, 2U / μL), dNTP (20μL, 2mM) and BSA (0.8μL, 100×), and left to react at 30°C for 2 days (Note: when synthesized To label CpG microparticles of fluorescent molecules, add 3 μL of Cy5-UTP to the...
Embodiment 3
[0070] Embodiment 3: Polypeptide-g-polyethylene glycol (PPT-g-PEG) synthesis
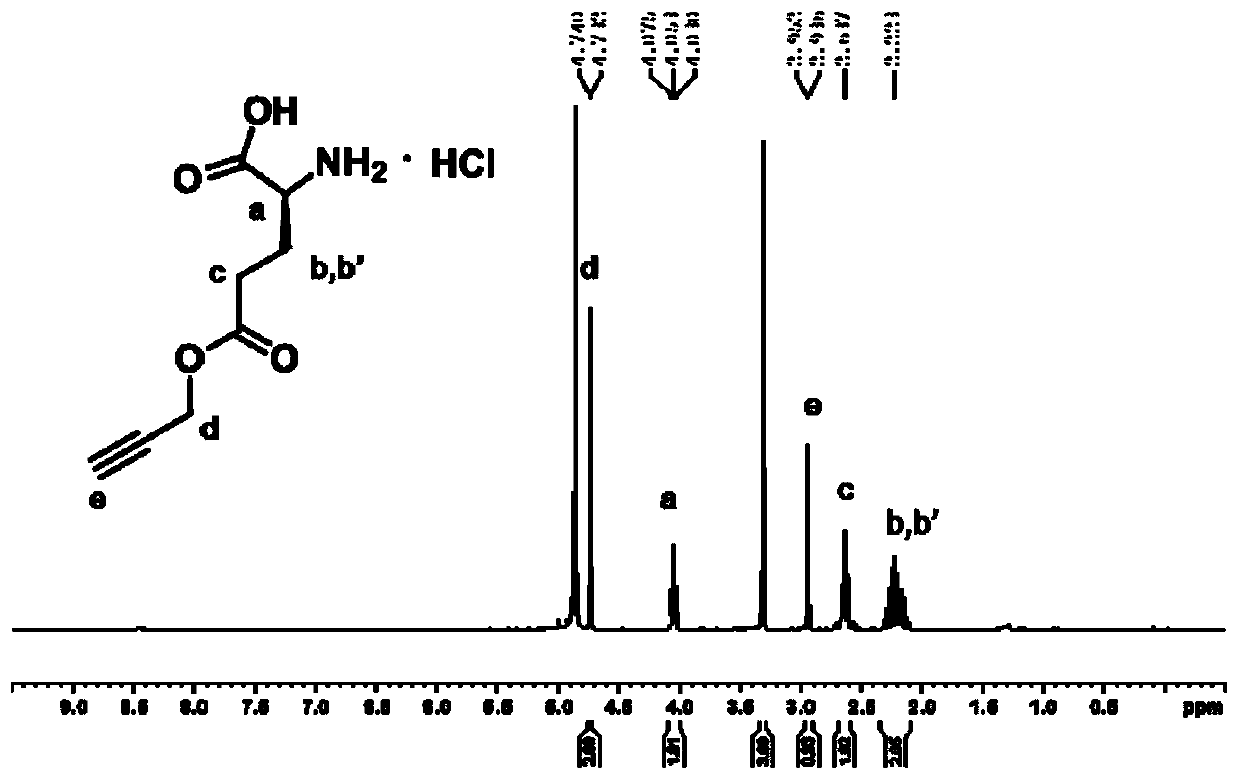
[0071] Synthesis of γ-propargyl-L-glutamic acid hydrochloride
[0072] Take a 500 mL round bottom flask, and disperse L-glutamic acid (6.0 g, 40.8 mmol) in 200 mL propargyl alcohol. Under the protection of nitrogen at room temperature, chlorotrimethylsilane (14.3 mL, 113 mmol) was added dropwise into the round bottom flask within 2 hours, and the stirring was continued for two days. After the reaction, the above reaction product was precipitated with diethyl ether (1.5 L) to obtain a black solid. The solid was first washed with methanol (50 mL), and then precipitated with diethyl ether (0.5 mL). This was repeated three times to purify the product. The product was filtered and dried under vacuum to obtain γ-propargyl-L-glutamic acid hydrochloride (4.71 g, yield: 52%) as a white solid. For its structural characterization see image 3 . Synthesis of γ-propargyl-L-glutamic acid N-carboxyanhydride (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com