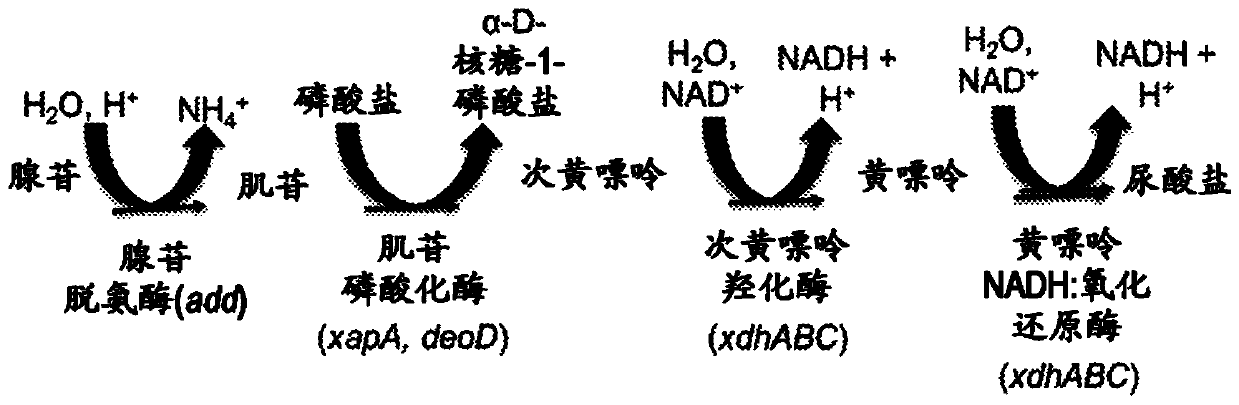
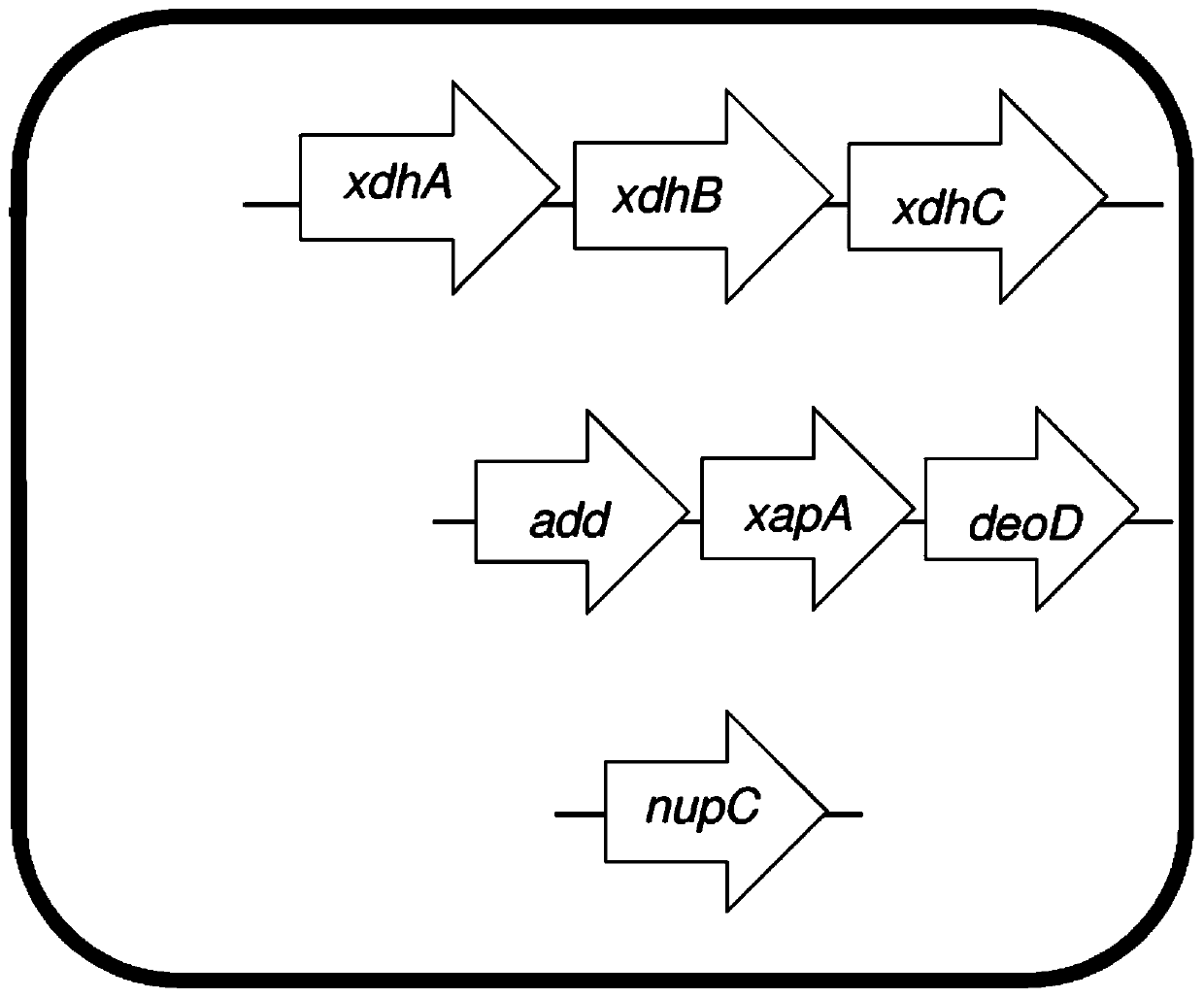
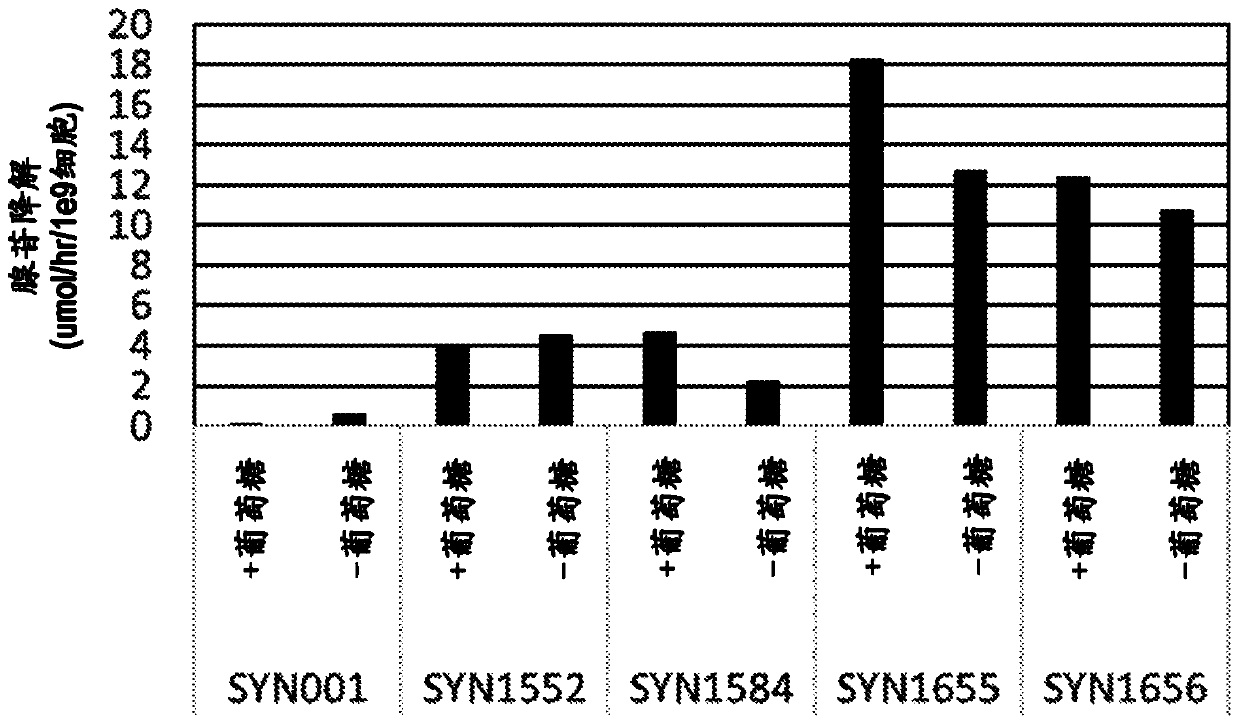
Microorganisms programmed to produce immune modulators and Anti-cancer therapeutics in tumor cells
A technology of microorganisms and cells, applied in the direction of microorganisms, tumor necrosis factor, cytokines/lymphokines/interferons, etc., to achieve the effect of increasing the immune response in tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1543] Example 1 Anti-cancer molecules
[1544] The exemplary nucleic acid sequence used in the construction of the single-chain anti-CTLA-4 antibody is described in the international patent application PCT / US2017 / 013072 filed on January 11, 2017 and published as WO2017 / 123675, the entire content of which is incorporated herein by reference, For example, in Example 1. In some embodiments, the genetically engineered bacteria comprise a nucleic acid sequence that is at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least about 99% homologous or comprising a DNA sequence The DNA sequence, the DNA sequence encoding and SEQ ID NO: 765, SEQ ID NO: 766, SEQ ID NO: 767, SEQ ID NO: 768, SEQ ID NO: 769, SEQ ID NO: 770, SEQ ID NO : 771, SEQ ID NO772, SEQ ID NO: 773, SEQ ID NO: 774, SEQ ID NO: 775 and / or SEQ ID NO: 776 at least about 80%, at least about 85%, at least about 90%, at least about 95%, or at least About 99% homologous polypeptide. In another...
Embodiment 2
[1546] Example 2. Tumor pharmacokinetics of E. coli Nissle
[1547] The tumor pharmacokinetics was measured and determined as described in the international patent application PCT / US2017 / 013072 published as WO2017 / 123675 filed on January 11, 2017, the entire contents of which are incorporated herein by reference, for example, Examples 58-61. The CT26 tumor model was used to determine the tumor pharmacokinetics of Nissle (1e7 and 1e8 cells / dose) within 7 days. The counts of bacteria in tumor tissues were similar at both doses. No bacteria were detected in the blood at any point in time.
[1548] The tumor pharmacokinetics of streptomycin-resistant Nissle and Nissle DOM mutant (Nissle deltaPAL::CmR) were compared in the CT26 tumor model. The counts of bacteria in tumor tissues were similar in both strains. No bacteria were detected in the blood. These results indicate that both wild-type and DOM mutant Nissle can survive in the tumor environment.
[1549] The CT26 tumor model was...
Embodiment 3A
[1551] Example 3A. LC-MS / MS quantification of metabolites
[1552] As described in the international patent application PCT / US2017 / 013072 published as WO2017 / 123675 filed on January 11, 2017, the bacterial supernatant and the adenosine, kynurenine, The quantification of tryptophan and arginine, the entire contents of which are incorporated herein by reference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com