Patents
Literature
61 results about "Senecavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Senecavirus is a genus of viruses in the order Picornavirales, in the family Picornaviridae. Pig and maybe also cow serve as natural hosts. There is currently only one species in this genus: the type species Senecavirus A.' Senecavirus is a replication-competent oncolytic picornavirus. It has selective tropism for cancers with neuroendocrine features including small cell lung cancer (SCLC) and several pediatric solid tumors including retinoblastoma, neuroblastoma, and medulloblastoma. A Phase I clinical trial of Senecavirus in adults with neuroendocrine tumors showed that senecavirus is apparently safe to administer at doses up to 1E11 vp/kg. It has potential antineoplastic activity.
Novel genetic engineering vaccine of porcine Seneca virus as well as preparation method and application of novel genetic engineering vaccine
ActiveCN110279855AEasy to assembleGood antigenicitySsRNA viruses positive-senseVirus peptidesVaccine ProductionImmunogenicity
The invention discloses an immunological composition which comprises porcine Seneca virus structural protein VP3 and VP1 proteins, as well as porcine Seneca virus structural protein VP2 and / or VP4 protein. Further, the immunological composition can further comprise a porcine Seneca virus structural protein VP0. The immunological composition can be used for preparing a novel genetic engineering subunit vaccine of porcine Seneca virus, the antigenicity, immunogenicity and function of the vaccine are similar to those of natural proteins, the expression level is relatively high, the immunogenicity is strong, and no pathogenicity is caused to animals; the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, thereby greatly reducing the cost of vaccine production.
Owner:苏州世诺生物技术有限公司
Solid phase competition ELISA kit for detecting Seneca valley virus antibody, and applications thereof,
InactiveCN107894508AAvoid cross reactionShorten detection timeBiological testingElisa kitSenecavirus
The invention discloses a solid phase competition ELISA kit for detecting Seneca valley virus antibody, and applications thereof, wherein the kit comprises Seneca valley virus inactivated antigen-coated enzyme label plate and HRP-labeled Seneca valley virus rabbit anti-IgG. According to the present invention, the primary antibody and the secondary antibody in the traditional ELISA are replaced with the HRP-labeled rabbit anti-IgG so as to simplify the operation step; by changing the coating stabilization process, the surface of the solid phase carrier is coated with the SVV inactivated antigen, such that the enzyme-labeled antibody diluent preparation process is changed, the enzyme-labeled antibody working liquid can be stably stored without the change of the activity and the titer, and the solid phase competition ELISA kit for the specific detection of the SVV antibody, and the detection method thereof are established; and with the kit, the blank in the SVV ELISA antibody detection ismade up, the problems of low repeatability, low sensitivity and cumbersome operation procedures in the SVV antibody detection in the existing VNT detection are overcome, and the effective technical means is provided for the SVV antibody detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof
InactiveCN107741495AAvoid cross reactionShorten detection timeBiological material analysisElisa kitSenecavirus
The invention discloses a double antibody sandwich ELISA kit used for Seneca viral antigen detection and application thereof. The kit comprises a Seneca viral guinea pig anti-IgG enveloped elisa plateand an HRP marked Seneca viral rabbit anti-IgG. The HRP marked rabbit anti-IgG is used for replacing primary antibodies and secondary antibodies in traditional ELISA, so that the operating steps aresimplified. Simultaneously, the SVV guinea pig anti-IgG is enveloped to the surface of a solid-phase carrier by changing an enveloping stabilization technology, the preparation technology of an enzyme-labeled antibody diluent is changed, an enzyme-labeled antibody working solution can be stored stably without changing the activity and valence, and a double antibody sandwich ELISA kit for SVV antigen specific detection and a detection method thereof are established. The vacancy of making up SVV ELISA antigen detection is proposed, the problem that existing virus separation and detection for SVVantigens are low in repeatability and sensitivity and complex in operational program is overcome, and effective technical means is provided for SVV antigen detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Senecavirus ELISA antibody detection kit and preparation method and application
InactiveCN108761074AQuick checkIncreased sensitivityBiological material analysisEscherichia coliSerum ige
The invention discloses a Senecavirus ELISA antibody detection kit and a preparation method and application. The Senecavirus ELISA antibody detection kit comprises a Senecavirus VP1 protein-precoatedELISA plate, a blocking solution, a diluted sample solution, an enzyme conjugate, a concentrated washing solution, an enzyme substrate solution and a stopping solution. Senecavirus VP1 protein is usedas a coating antigen for the first time, a kit capable of detecting Senecavirus is established, and the antibody level of the Senecavirus in serum can be rapidly detected; the detection kit is high in sensitivity, good in specificity, good in repeatability and stable in result; the detection kit can be applied to monitoring of the antibody level of the Senecavirus so as to understand the status of the Senecavirus antibody in a whole pig herd; in addition, the detection kit uses an Escherichia coli expression system, and has the advantages of economy and cheapness.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
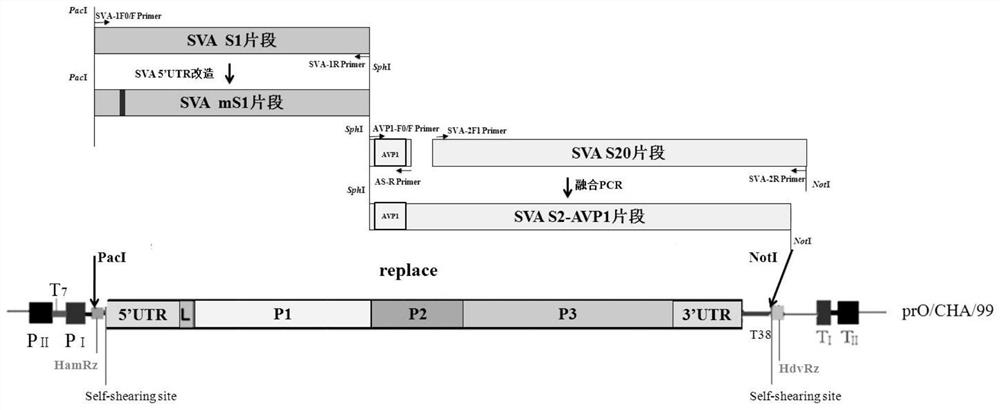
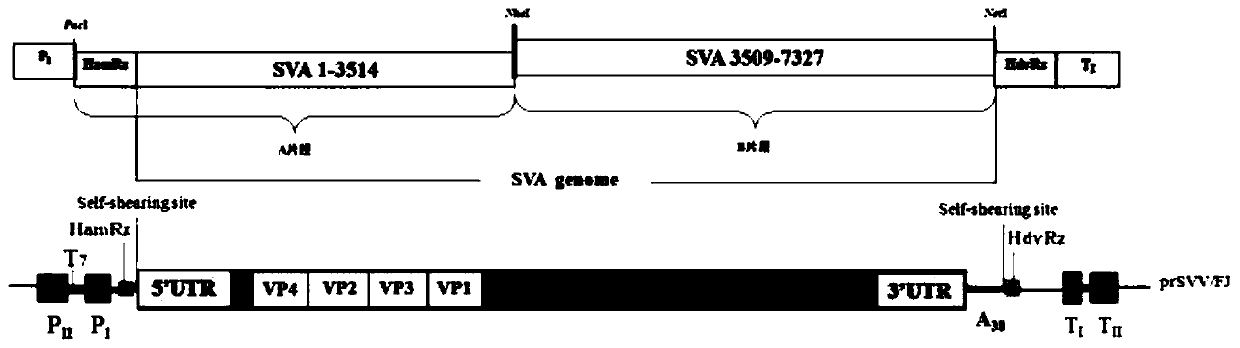
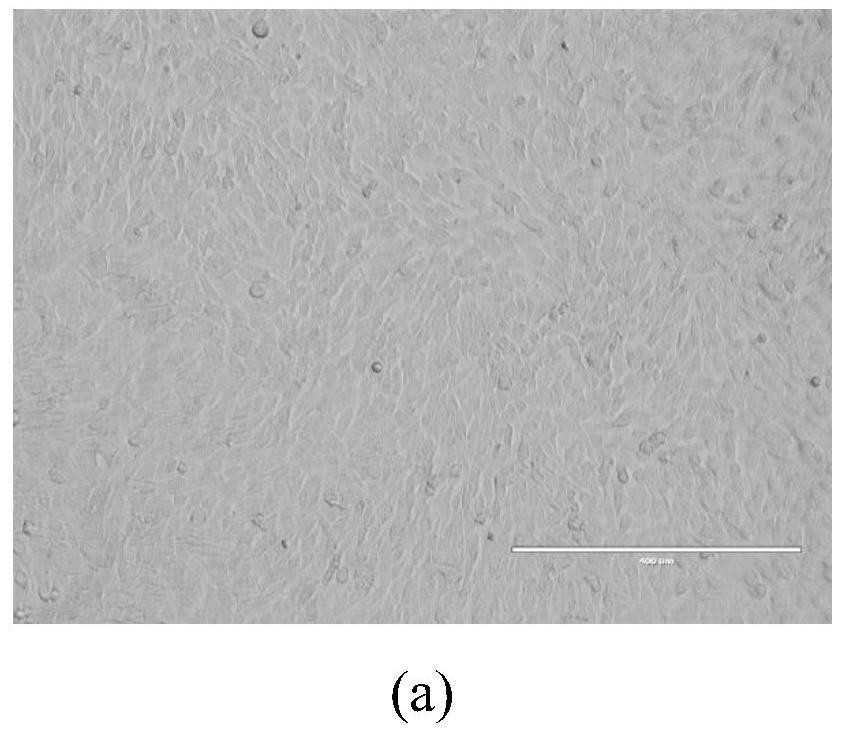
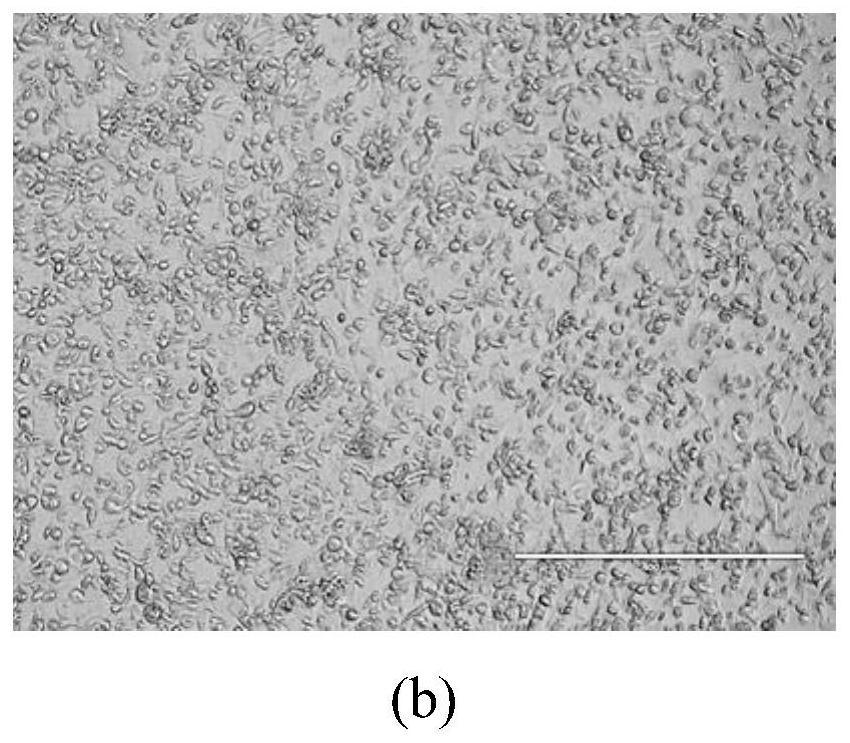
Preparation method and application of full-length infectious clone of porcine SVV (Seneca Valley Virus)
ActiveCN109810976ANo gene deletions were foundMicrobiological testing/measurementViruses/bacteriophagesSenecavirusViral infection
The invention relates to a preparation method and an application of a full-length infectious clone of a porcine SVV (Seneca Valley Virus), comprising the following steps: (1) amplification of a full sequence of an SVV / GD05 strain genome; (2) construction of infectious clone plasmid of a pSVV-GD05 virus; (3) construction of pSVV-GD05-iLOV recombinant plasmid; and (4) rescue of a parental virus (SVV-GD05) and a recombinant virus (SVV-GD05-iLOV). The method provided by the invention utilizes a strain of the SVV isolated from a Chinese pig herd to construct a viral infectious clone pSVV-GD05 withbacterial plasmid as a skeleton, and can successfully rescue the virus. At the same time, a reporter gene iLOV is inserted into an SVV infectious clone virus genome, and the recombinant SVV virus capable of expressing the reporter gene is successfully rescued. The preparation method and application of the invention provide an effective platform for deep and basic research and application of SVV, and have important scientific application value.
Owner:YANGZHOU UNIV
Method for preparing Seneca virus by suspension cell line
ActiveCN110564698AIncrease production capacityIncrease virus titerSsRNA viruses positive-senseArtificial cell constructsSenecavirusVirus vaccine
The invention provides a method for preparing Seneca virus by a suspension cell line. The method comprises the steps as follows: (1) an adherent ST cell line is domesticated into a suspension ST-S cell line; and (2) Seneca virus is cultured and prepared by the suspension ST-S cell line obtained in the step (1). The Seneca virus is cultured by the domesticated suspension ST-S cell line, the capacity of the obtained virus is effectively improved, and a foundation is laid for large-scale mass production of Seneca virus vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca virus blocking ELISA (enzyme-linked immuno sorbent assay) antibody detection kit
The invention provides a blocking ELISA (enzyme-linked immuno sorbent assay) kit for detecting Seneca virus serum antibodies, a preparation method of the kit and a use method of the kit. The Seneca virus blocking ELISA antibody detection kit is excellent in sensitivity and specificity, the adopted blocking ELISA technology belongs to open operations, technical requirements are less strict, operation steps are simple, convenient and rapid, and the kit can be widely popularized and applied.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Seneca virus isolation strain, Seneca virus disease inactivated vaccine and preparation method of Seneca virus disease inactivated vaccine
PendingCN110923211AProtectSsRNA viruses positive-senseViral antigen ingredientsPig farmsOil emulsion
The invention discloses a Seneca virus isolation strain, Seneca virus disease inactivated vaccine and a preparation method of the Seneca virus disease inactivated vaccine. A swine Seneca virus CH-HLJstrain is separated from a pig farm with symptoms of suspected Seneca virus infections in the Heilongjiang Province, the complete genome sequence of the strain is shown in SEQ ID No.1 in the description, and the microbial preservation number is CGMCC NO.18851. The invention further provides a method for preparing the Seneca virus disease inactivated vaccine. The method comprises the following steps: (1) performing viral multiplication and inactivation; (2) heating a vaccine adjuvant so as to obtain an oil phase; (3) heating a virus liquid so as to obtain a water phase; and (5) mixing and emulsifying the oil phase with the water phase into a double-phase oil emulsion. Swine immunological experiment results show that the Seneca virus disease inactivated vaccine prepared by the invention is capable of stimulating a body to generate an antibody of a high level. Immunoprotection efficacity evaluation results of the inactivated vaccine show that the inactivated vaccine prepared by the invention is capable of providing good protection on attacking of swine Seneca viruses.
Owner:哈药集团生物疫苗有限公司
Reagent and method for identifying FMDV and SVA, and application
InactiveCN108034761AEfficient identificationRapid prevention and controlMicrobiological testing/measurementMicroorganism based processesSenecavirusMicrobiology
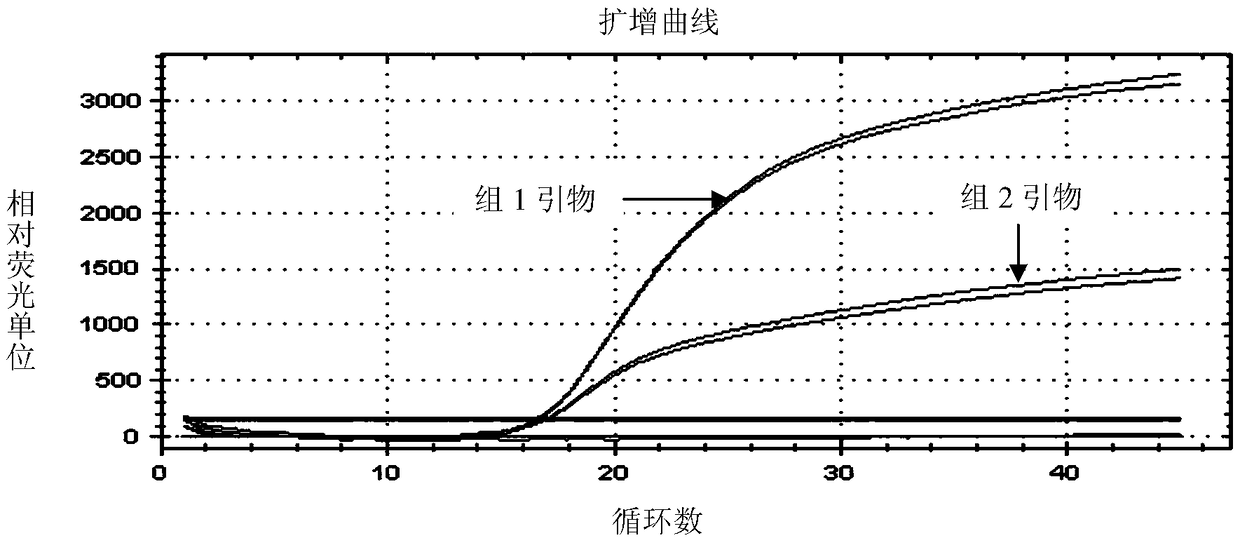
The invention discloses a reagent and method for identifying FMDV and SVA and application. The reagent comprises an FMDV primer probe group and an SVA primer probe group; an upstream primer and a downstream primer of the FMDV primer probe group are respectively sequences shown by Seq ID No. 1 and Seq ID No. 2, and the probe is a sequence shown by Seq ID No. 3 or a reverse complementary sequence; and an upstream primer and a downstream primer of the SVA primer probe are respectively sequences shown by Seq ID No. 4 and Seq ID No. 5, and the probe is the Seq ID No. 6 or the reverse complementarysequence. By adopting the reagent of the invention, the FMDV and the SVA can be simultaneously detected and identified, the specificity is high, the sensitivity is high, and a high-efficient technicalmeans is provided for identifying and detecting foot and mouth disease viruses and swine type-A Seneca viruses. The reagent of the invention is suitable for rapidly identifying and detecting two diseases.
Owner:SHENZHEN AUDAQUE DATA TECH
Duplex real-time fluorescent quantitative PCR detection kit for Seneca virus A and foot and mouth disease virus
InactiveCN109097495AEnsure safetyEnsure reasonablenessMicrobiological testing/measurementDNA/RNA fragmentationSenecavirusVaccine Production
The invention provides a duplex real-time fluorescent quantitative PCR detection kit for Seneca virus A (SVA) and foot and mouth disease virus (FMDV) and special primers and TaqMan probes of the duplex real-time fluorescent quantitative PCR detection kit. The kit contains two pairs of primers and two probes; the primers and the probes are respectively designed for an SVA conservative gene region and an FMDV conservative gene region. The kit and the detection method provided by the invention have the advantages of simplicity and convenience in operation, high specificity, high sensitivity, highrepeatability and broad application prospect; accurate quantitation of the SVA and the FMDV can be realized; and the duplex real-time fluorescent quantitative PCR detection kit and the detection method play an important role in detection and vaccine production of the SVA and the FMDV, such as quality monitoring and reasonable seedling distribution in the production process of vaccines, and have wide application prospects.
Owner:JINYUBAOLING BIO PHARMA CO LTD
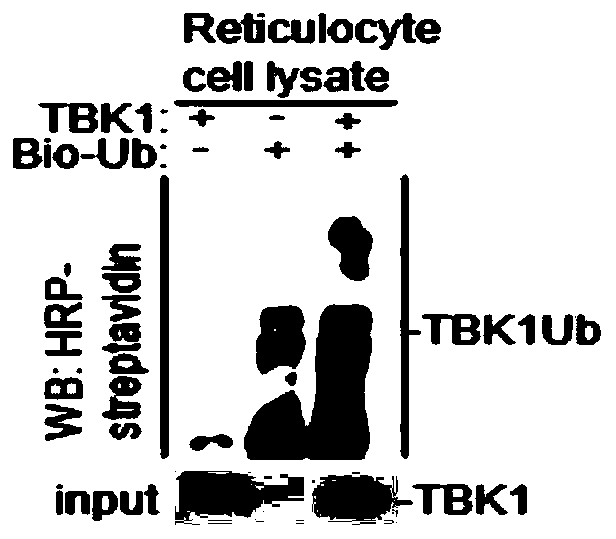
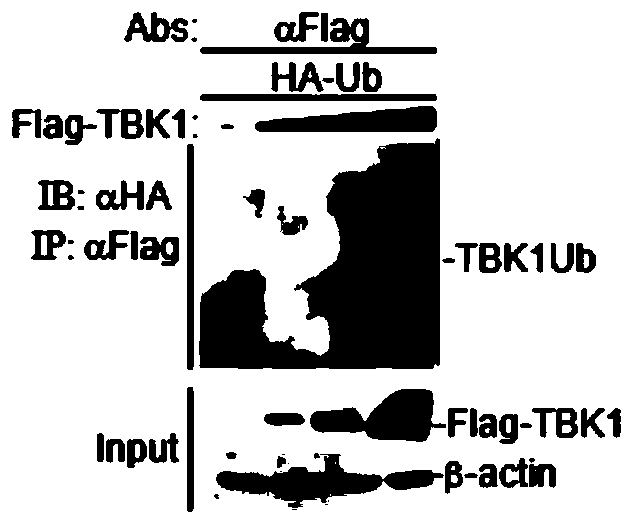
Application of TBK1 as E3 ubiquitin ligase
ActiveCN110129288AHas ubiquitination functionSpecificPeptide/protein ingredientsAntiviralsTANK-binding kinase 1Senecavirus
The invention belongs to the field of bio-medicine, in particular to a new application of TBK1 as E3 ubiquitin ligase. The TBK1 (TANK binding kinase 1) is found to have a ubiquitination function, cannot only undergo ubiquitination in vitro, but also undergo ubiquitination in vivo, and is a new E3 ubiquitin ligase; The invention further finds that the TBK1 can degrade structural protein VP3 of picornaviridae viruses including foot-and-mouth disease virus (FMDV), enterovirus (EV71), encephalomyocarditis virus (EMCV) and seneca virus (SVV), in particular can specifically degrade the structural protein VP3 of the FMDV, and can be used for preparing drugs related to degradation of the foot-and-mouth disease virus protein, preventing the foot-and-mouth disease virus protein from assembling related drugs, and preventing or treating foot-and-mouth disease virus infection related drugs.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Reagent and detection for detecting porcine senecavirus A and application of reagent
InactiveCN107974514AQuick checkRapid prevention and controlMicrobiological testing/measurementMicroorganism based processesSenecavirusFluorophore
The invention discloses a reagent and a detection method for detecting porcine senecavirus A and an application of the reagent. The reagent for detecting the porcine senecavirus A provided by the invention comprises a primer pair and a probe, wherein in the primer pair, the sequence an upstream primer is shown as SEQ ID No.1 and the sequence a downstream primer is shown as SEQ ID No.2; the sequence of the probe is shown as SEQ ID No.3 or a reverse complementary sequence of the sequence; in the probe sequence shown as SEQ ID No.3, a 6-fluorophore-dT is modified by the 31st base group, the 32ndbase group is substituted into a base analogue; a fluorescent quencher-dT is modified by the 33rd base group; and C3 Spacer is modified by 3' terminal. The reagent provided by the invention, through recombinase polymerase amplification, can conduct sensitive, specific and efficient detection on the SVA (the senecavirus A). In comparison with existing detection methods, the reagent and the method provided by the invention are short in time and easy to operate, and a conclusion can be reached rapidly; the reagent and the method are applicable to on-site detection; and the reagent and the methodare significant for the rapid prevention and control of the SVA.
Owner:SHENZHEN AUDAQUE DATA TECH
Method for preparing Seneca valley virus by utilizing suspension cell line
ActiveCN110628698AIncrease production capacityIncrease virus titerSsRNA viruses positive-senseArtificial cell constructsSenecavirusVirology
The invention provides a method for preparing Seneca valley virus by utilizing a suspension cell line. The method for preparing the Seneca valley virus by utilizing the suspension cell line comprisesthe following steps: (1) domesticating a non-suspension BHK-21 cell line into a suspension BHK-21-S cell line; and (2) culturing the Seneca valley virus by using the suspension BHK-21-S cell line obtained in the step (1). By culturing the Seneca valley virus by using the suspension BHK-21-S cell line obtained by performing domestication, yield of the Seneca valley virus can be effectively increased; and thus, foundation is laid for large-scale production of vaccines to the Seneca valley virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant nucleic acid of seneca virus, recombinant vaccine strain as well as preparation method and application thereof
ActiveCN111394367AReduce pathogenicityPathogenicity NoneSsRNA viruses positive-senseViral antigen ingredientsHighly pathogenicRecombinant vaccines
The invention provides a recombinant nucleic acid of a seneca virus, a recombinant vaccine strain as well as a preparation method and application thereof and relates to the technical field of gene engineering. The invention provides a recombinant nucleic acid of a seneca virus, a seneca recombinant virus containing the recombinant nucleic acid, a seneca recombinant virus coded by the recombinant nucleic acid, a seneca recombinant vaccine strain containing the seneca recombinant virus as well as a preparation method and application of the seneca recombinant vaccine strain. According to the invention, the vaccine strain having the characteristics of high antigen productivity, remarkably reduced pathogenicity and even no pathogenicity for pigs, strong antibody response, high immune protectionrate and the like is prepared, and the vaccine strain remarkably improves the biosafety and may be used for preventing and controlling the seneca virus in China and surrounding countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Polypeptide for detecting Seneca virus antibody and application of polypeptide
ActiveCN112225780AStrong specificityIncreased sensitivitySsRNA viruses positive-senseAntibody mimetics/scaffoldsSenecavirusViral infection
The invention discloses a polypeptide for detecting a Seneca virus antibody and application of the polypeptide. The polypeptide is composed of an eVP1-1 and an eVP2-1. The eVP1-1 is a polypeptide of P11 or P12: P11, wherein the P11 is a polypeptide of which the amino acid sequence is SEQ ID No. 1, and the P12 is a polypeptide of which the amino acid sequence is the 2-16th site of SEQ ID No. 1; theeVP2-1 is a polypeptide of P21 or P22, wherein the P21 is a polypeptide of which the amino acid sequence is SEQ ID No.2, and the P22 is a polypeptide of which the amino acid sequence is the 2-15th site of SEQ ID No.2. The antibody detection kit prepared from the polypeptide is high in sensitivity, high in accuracy, simple and rapid to operate, and suitable for rapid and large-scale screening anddetection of Seneca virus infected serum antibodies by veterinary departments and entry and exit inspection and epidemic prevention bureaus at all levels of grassroots.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Purification and concentration method of Seneca virus
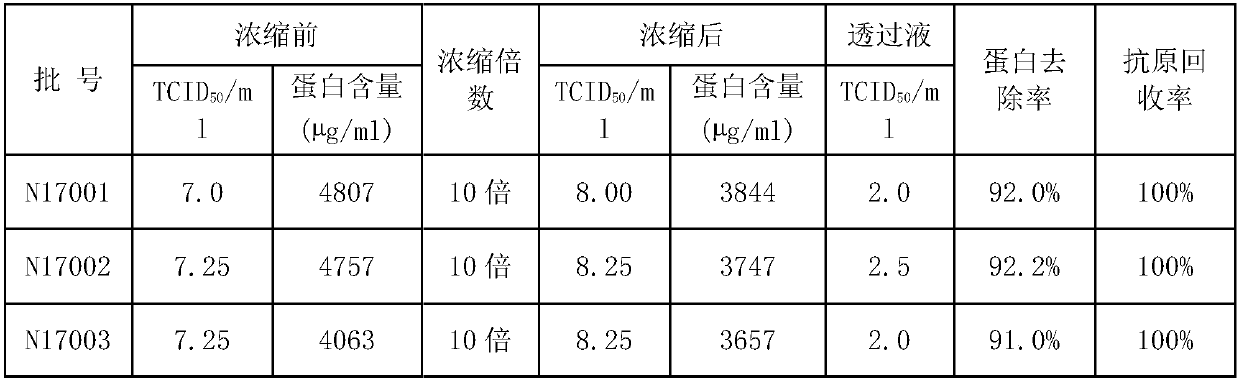
InactiveCN111172121AEasy to operateEasy to zoom inSsRNA viruses positive-senseMicroorganism based processesSenecavirusAntigen retrieval
The invention discloses a concentration method of Seneca virus. The method comprises pretreatment of a Seneca virus liquid, and concentration after the pretreatment, wherein the concentration sequentially comprises to filtration concentration, filter wash and filtration concentration. The invention relates to an SVV antigen concentration and purification method suitable for large-scale industrialproduction. The method subjects an SVV antigen to concentration and purification by combining tangential flow filtration and filter wash, and has the characteristics of convenient operation, easy amplification, mild and stable process, high antigen recovery rate and the like. The method can be used for concentration and purification of live viruses and inactivated viruses, has an antigen recoveryrate of greater than 80% and a protein removal rate of greater than 90%, and can prepare a purified SVV antigen or provide raw materials for subsequent further fine purification of the antigen.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Seneca recombinant virus of recombinant A-type foot-and-mouth disease virus VP1 gene, recombinant vaccine strain and preparation method and application of recombinant vaccine strain
ActiveCN111996201AAchieving antigen matchingAchieve immune responsivenessSsRNA viruses positive-senseViral antigen ingredientsAntigenDisease
The invention provides a Seneca recombinant virus of a recombinant A-type foot-and-mouth disease virus VP1 gene, a recombinant vaccine strain and a preparation method and application of the recombinant vaccine strain, and relates to the technical field of gene engineering. The invention provides a Seneca recombinant nucleic acid, the Seneca recombinant virus containing the recombinant nucleic acid, the Seneca recombinant vaccine strain containing the Seneca recombinant virus, and a preparation method and application of the vaccine strain. An SVV / FJ / 001 strain is subjected to gene deletion mutation transformation, the VP1 gene of the A-type FMDV is fused into cDNA of the SVV / FJ / 001 strain to obtain the Seneca recombinant virus, the recombinant virus can express the fused gene, and an expression product has good reactogenicity; and the obtained vaccine strain is high in antigen productivity, the pathogenicity is remarkably reduced, even no pathogenicity is caused to pigs, the immune response of SVA can be stimulated after animals are immunized by the inactivated vaccine, the immunocompetence aiming at fusion genes can be generated, and the vaccine strain can be used for preventing and controlling Seneca virus and one or more non-Seneca virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant baculovirus expressing senecavirus VP2 gene and preparation method and application thereof
ActiveCN110358741APreserve immunogenicitySimplify the extraction and purification processSsRNA viruses positive-senseViral antigen ingredientsSenecavirusVp2 gene
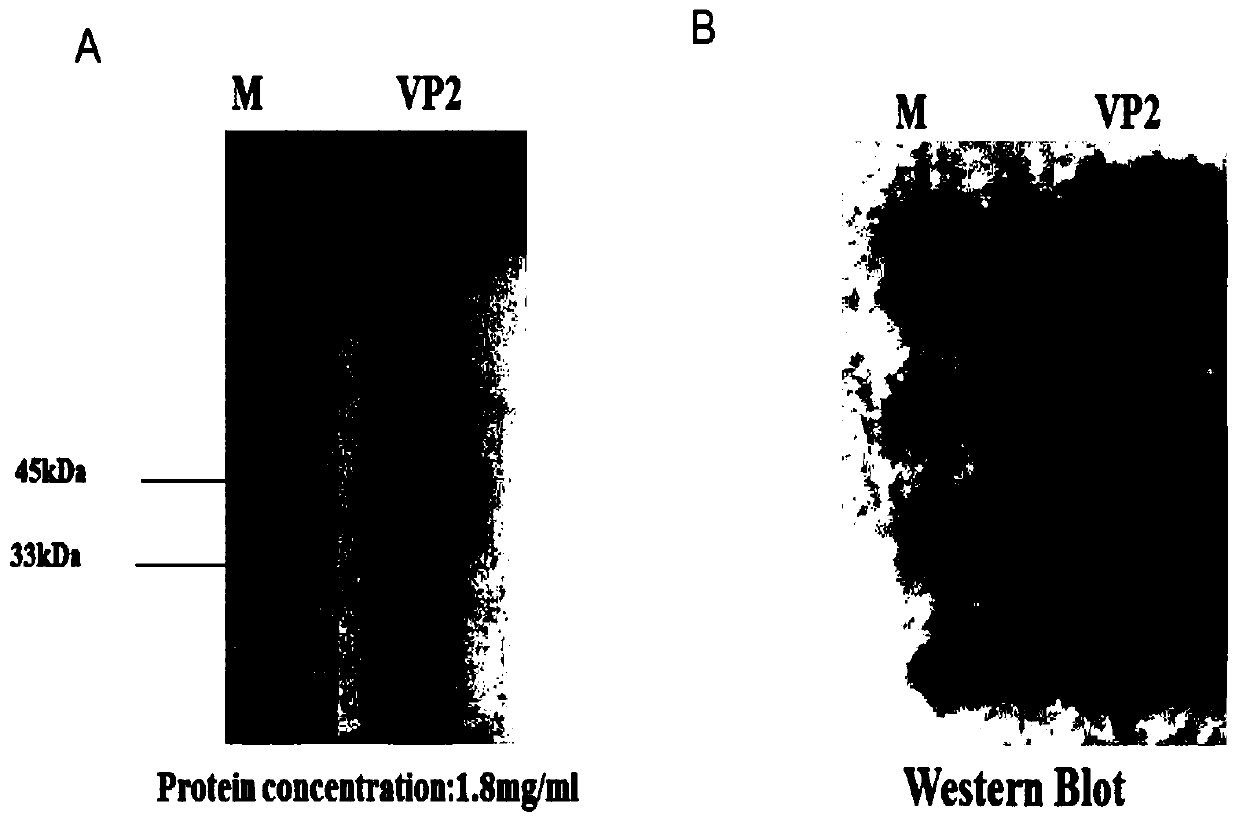
The invention relates to the field of biotechnology, in particular to recombinant baculovirus expressing senecavirus VP2 gene and a preparation method and application thereof. The recombinant baculovirus comprises one or more copies of senecavirus VP2 protein encoding gene. The invention further provides a senecavirus subunit vaccine which comprises the recombinant baculovirus expressed senecavirus VP2 protein. Accordingly, by means of artificial codon optimization, high-level and high-purity expression of senecavirus VP2 protein is achieved, and the immunogenicity of the VP2 protein is retained to the maximum extend. The senecavirus subunit vaccine has the excellent immunogenicity and high safety, and an ideal immune protection effect is achieved for infection of senecavirus.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
One-step triple real-time fluorescent quantitative PCR detection primer and probe for SVA, type O FMDV and type A FMDV
PendingCN110305986AReduce workloadLow costMicrobiological testing/measurementDNA/RNA fragmentationDiseaseSenecavirus
The invention provides a one-step triple real-time fluorescent quantitative PCR detection primer and probe capable of simultaneously detecting and identifying Seneca virus, type O foot and mouth disease virus, and type A foot and mouth disease virus, and belongs to the technical field of biological detection. A kit is developed based on the provided primer and probe and used for simultaneous and rapid detection of the Seneca virus, the type O foot and mouth disease virus, and type A foot and mouth disease virus. According to the kit and the detection method of the one-step triple real-time fluorescent quantitative PCR detection primer and probe capable of simultaneously detecting and identifying the Seneca virus, the type O foot and mouth disease virus, and the type A foot and mouth disease virus, one-time sample loading and one-time quantification analysis can be realized, and the purpose of simultaneously and rapidly detecting and identifying and accurately quantifying the Seneca virus, the type O foot and mouth disease virus, and the type A foot and mouth disease virus can be achieved, the workload and cost of detection are reduced, and the detection can be completed in the shortest time. The one-step triple real-time fluorescent quantitative PCR detection primer and probe capable of simultaneously detecting and identifying the Seneca virus, the type O foot and mouth diseasevirus, and the type A foot and mouth disease virus play an important role in the detection and vaccine production of the Seneca virus, the type O foot and mouth disease virus, and the type A foot andmouth disease virus and have broad application prospects.
Owner:JINYUBAOLING BIO PHARMA CO LTD
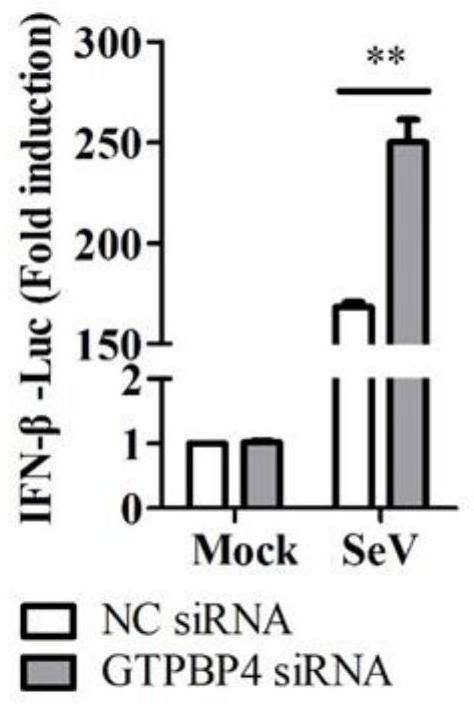
Application of GTPBP4 protein as immunosuppressant and construction of knockdown or overexpression GTPBP4 cell line
ActiveCN112353939AIncrease production capacityHigh titerOrganic active ingredientsHydrolasesIMMUNE SUPPRESSANTSVaccine Production
The invention belongs to the technical field of biology, and particularly relates to an application of GTPBP4 protein as an immunosuppressant and construction of a knockdown or overexpression GTPBP4 cell line. Firstly, it is accidentally found that GTPBP4 inhibits activation of an IFN-beta promoter induced by SeV (Sendai virus) and mRNA expression of IFN beta and downstream genes of IFN beta, andinhibits IFN-beta protein expression induced by SeV; and targeted IRF3 (interferon reaction factor 3) inhibits innate immunity; secondly, overexpression of the GTPBP4 protein in the cell line significantly promotes expression of the Senecavirus VP1 protein, improves the Senecavirus titer, and can be used for construction of Senecavirus and vaccine production cell lines; and finally, the GTPBP4 protein is knocked down in the cell line, expression of the Senecavirus VP1 protein is obviously inhibited, the titer of the Senecavirus is reduced, and the cell line can be used for breeding animals resisting Senecavirus infection. The GTPBP4 protein inhibitor can be used for preparing medicines, pharmaceutical compositions or vaccine compositions for preventing or treating virus infection of the small ribonucleic acid viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca virus infectious clone based on single plasmid rescue system, construction method and application
PendingCN111394389ASolve the technical problem of rapid preparation of vaccine inoculumSimple and fast operationSsRNA viruses positive-senseViral antigen ingredientsRecombinant vaccinesGenetic engineering
The invention provides a single plasmid rescue system aiming at a positive strand RNA virus, a Seneca virus infectious clone based on the single plasmid rescue system, a construction method and an application, and relates to the technical field of gene engineering. The invention provides the single plasmid rescue system aiming at the positive strand RNA virus and an application of the single plasmid rescue system in directional design of an SVA reverse recombinant vaccine and construction of an SVA directional mutation strain in researches of virus gene functions, virus variation, pathogenesisand the like. According to the invention, a single plasmid Seneca virus rescue system is constructed by using elements like promoters, terminators and ribozyme of polymerase I and polymerase II, so editing of a virus genome on a plasmid level is realized, and a technical platform is provided for directional design, construction and rescue of viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Bivalent inactivated vaccine for pigs, preparation method and application thereof
PendingCN110974949AEffective controlSsRNA viruses positive-senseViral antigen ingredientsDiseaseSenecavirus
The invention discloses a bivalent inactivated vaccine for pigs, a preparation method and application thereof. The bivalent inactivated vaccine for pigs disclosed by the invention comprises: a recombinant baculovirus for expressing an O-type FMDV VP1 gene, a porcine senecavirus and an immunoadjuvant. The invention further discloses a preparation method of the bivalent inactivated vaccine for pigs,and the method includes: (1) amplifying the recombinant baculovirus expressing the O-type FMDVVP1 gene, and then performing inactivation, amplifying the porcine senecavirus, and then performing inactivation; (2) mixing the inactivated recombinant baculovirus liquid expressing the O-type FMDV VP1 gene with the inactivated porcine senecavirus liquid uniformly to obtain a water phase; (3) heating the immunoadjuvant to obtain an oil phase; and (4) adding the oil phase into the water phase, and performing mixing and emulsification. Immune protection efficacy detection results prove that the bivalent inactivated vaccine for pigs prepared according to the invention can effectively control O-type foot-and-mouth disease virus and porcine senecavirus at the same time.
Owner:哈药集团生物疫苗有限公司
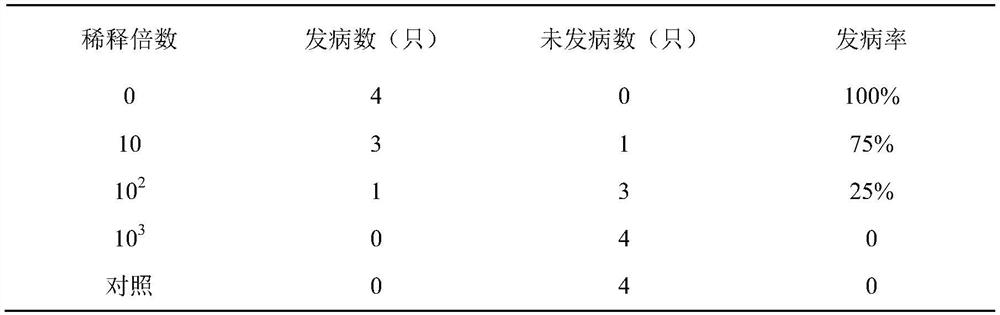
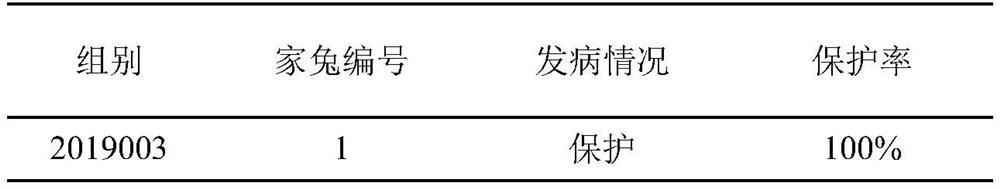
Method for testing efficacy of porcine Seneca virus disease inactivated vaccine by using rabbits
ActiveCN111729091AEliminate the effects ofReduce feeding costsCompounds screening/testingSsRNA viruses positive-senseVaccine PotencySenecavirus
The invention discloses a method for testing the efficacy of a porcine Seneca virus disease inactivated vaccine by using rabbits, and belongs to the field of veterinary biological products. The methodcomprises the following steps: performing immunization on a plurality of healthy susceptible rabbits by using the porcine Seneca virus disease inactivated vaccine; after 28 days of immunization, performing virus attack on the immunized rabbits by using a Seneca virus solution; and after virus attack, continuously observing for 15 days, and when at least 4 / 5 of the rabbits do not show clinical symptoms after virus attack, judging that the protection efficacy of the porcine Seneca virus disease inactivated vaccine is qualified. According to the method, the rabbits can be effectively used for replacing experimental pigs to establish a replacement method for testing the efficacy of the Seneca virus disease inactivated vaccine, so that the cost is effectively reduced; and the method is simpleand feasible to operate, safe and convenient.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Method for efficiently expressing structural proteins of Senecavirus A
ActiveCN113248574AImprove expression efficiencyIncrease the soluble ratioSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliProtein target
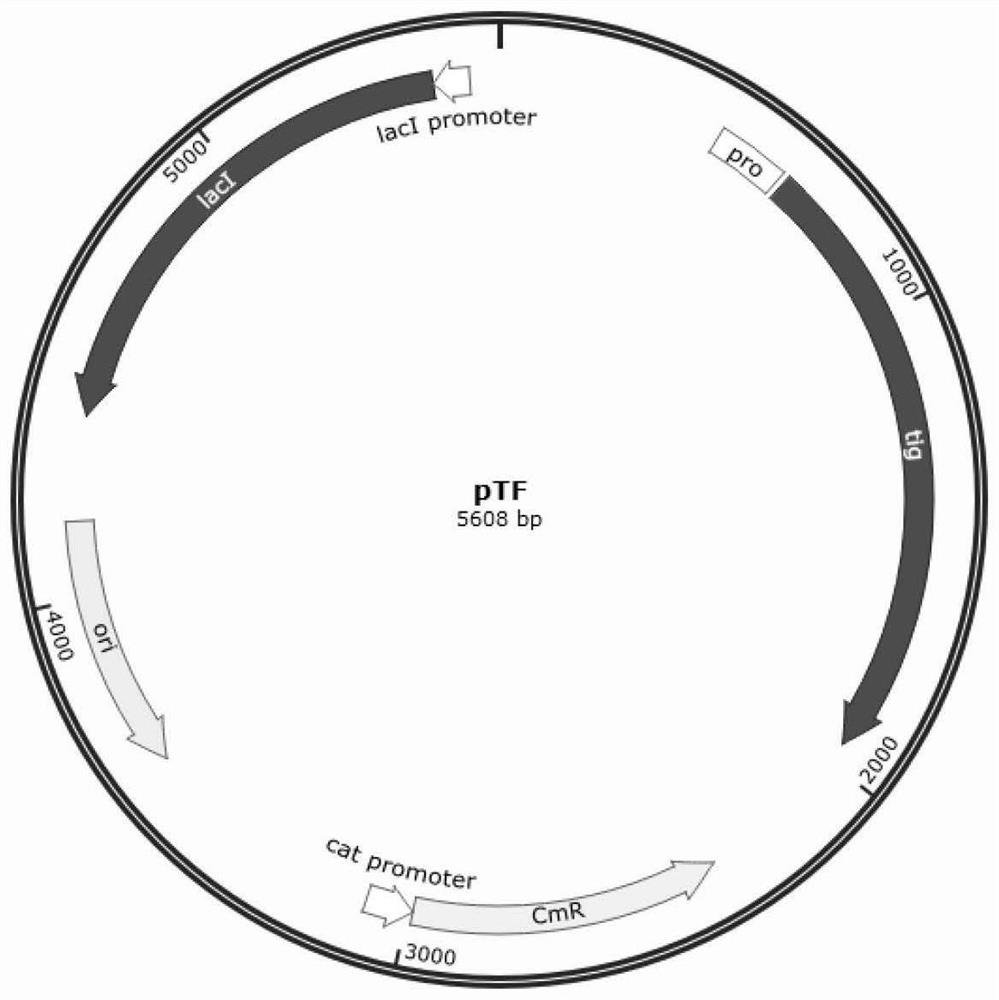
The invention relates to the technical field of molecular biology, in particular to a method for efficiently expressing structural proteins of Senecavirus A. According to the method, firstly, prokaryotic expression codon optimization is carried out on genes for coding three structural proteins VP0, VP3 and VP1 of the Senecavirus A, and a single plasmid for simultaneously carrying out soluble expression of the three structural proteins in escherichia coli is obtained by means of a small ubiquitin-like fusion protein; secondly, molecular chaperone plasmids and plasmids of the structural proteins of the Senecavirus A are transferred into the escherichia coli, and thus, the soluble expression of the structural proteins of the Senecavirus A is further improved, and the problem of non-uniform expression of target proteins is solved, and the obtained target protein accounts for about 25% or more of the total protein of bacteria. A Senecavirus subunit vaccine prepared from the three structural proteins obtained by expression can stimulate a pig body to generate a high-level neutralizing antibody, and has a good protection effect on a Senecavirus epidemic strain.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca virus type A genetic engineering composite epitope protein, vaccine and application of Senecavirus type A genetic engineering composite epitope protein and vaccine
ActiveCN113527516AGood immune protectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsAdjuvantGenetic engineering
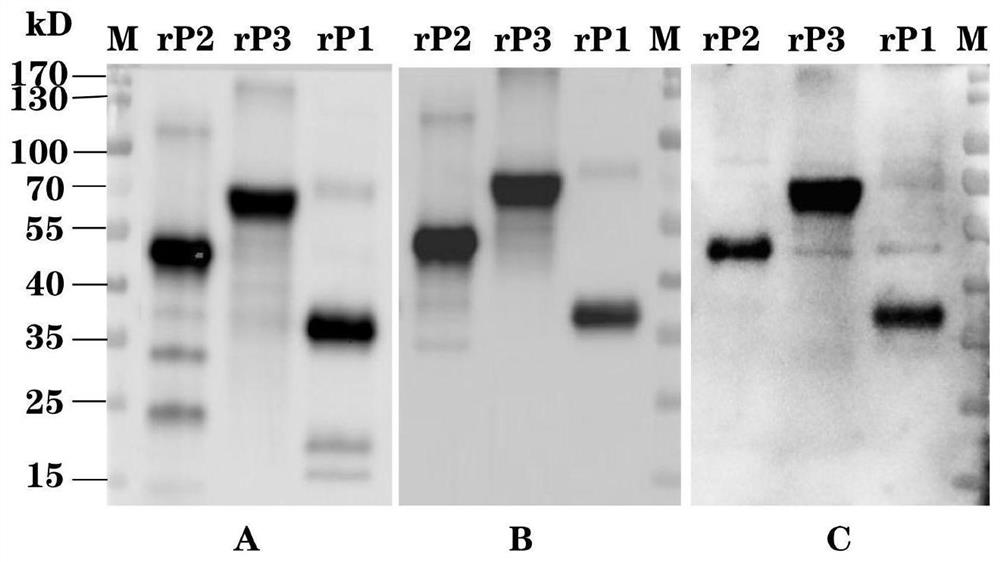
The invention provides a Seneca virus type A genetic engineering composite epitope protein, a vaccine and application of the Seneca virus type A genetic engineering composite epitope protein and the vaccine, and belongs to the technical field of veterinary biological products. The invention provides an amino acid sequence of a Seneca virus A genetic engineering composite epitope protein as shown in SEQ ID NO: 14. After the recombinant composite epitope protein is obtained through in-vitro recombinant expression, the vaccine prepared by mixing the recombinant composite epitope protein with an adjuvant is used for immunizing healthy susceptible piglets, the generation of a specific antibody and a neutralizing antibody can be detected in 28 days, and the composite epitope protein rP2 protected by the application has a poison attack protection rate of 80% or more, and has a higher poison attack protection effect compared with a control composite epitope protein. The prepared novel Seneca virus A gene engineering composite epitope protein vaccine has a wide application prospect in the aspect of preventing and controlling the Seneca virus A.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Triple inactivated vaccine for pigs and preparation method and application thereof
PendingCN111000995ASsRNA viruses negative-senseSsRNA viruses positive-senseDiseaseSwine influenzavirus
The invention discloses a triple inactivated vaccine for pigs and a preparation method and application of the triple inactivated vaccine. The triple inactivated vaccine for pigs comprises recombinantbaculovirus for expressing an O-type FMDV VP1 gene, swine senecavirus, H3N2 subtype swine influenza virus and an immunologic adjuvant. The invention also discloses a method for preparing the triple inactivated vaccine for pigs. The method comprises the following steps: (1) amplifying and inactivating the recombinant baculovirus for expressing the O-type FMDV VP1 gene; (2) mixing the inactivated recombinant baculovirus fluid for expressing the O-type FMDV VP1 gene, the inactivated swine senecavirus fluid and the H3N2 subtype swine influenza virus to obtain a water phase; (3) heating the immunologic adjuvant to obtain an oil phase; and (4) adding the oil phase into the water phase, mixing and emulsifying. Immune protective efficacy detection results prove that the prepared triple inactivatedvaccine for pigs can effectively prevent and treat O-type foot-and-mouth disease virus, swine senecavirus and H3N2 subtype swine influenza virus simultaneously.
Owner:哈药集团生物疫苗有限公司
Porcine senecavirus nucleic acid standard substance and application thereof
InactiveCN111286491AAccurate target value settingTraceableSsRNA viruses positive-senseMicrobiological testing/measurementPathogenic microorganismNucleic acid detection
The invention relates to a porcine senecavirus nucleic acid standard substance and application thereof, and belongs to the technical field of virus standard substance preparation. A senecavirus strainwith the preservation number of CCTCC NO: V201767 is subjected to continuous passage and domestication to obtain an SVA strain with high virus content, the SVA strain is named as SVA YRQ / 2016, and the preservation number of the SVA strain is CCTCC NO: V202013. The virus content of each milliliter of the SVA strain is more than or equal to 10<12.5>TCID<50>. A preparation method comprises steps: preparing a porcine senecavirus solution from the SVA strain; inactivating, and adding a stabilizer and a protective agent; and carrying out uniformity evaluation, stability evaluation and valuation toobtain the porcine senecavirus nucleic acid standard substance which is accurate in valuation, has good traceability, stability and uniformity, can be used for SVA pathogenic microorganism nucleic acid detection and test mechanism evaluation and control test methods, and has huge quality control, quality arbitration and application values.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Method for extracting, concentrating and purifying porcine senecavirus particles by two aqueous phases
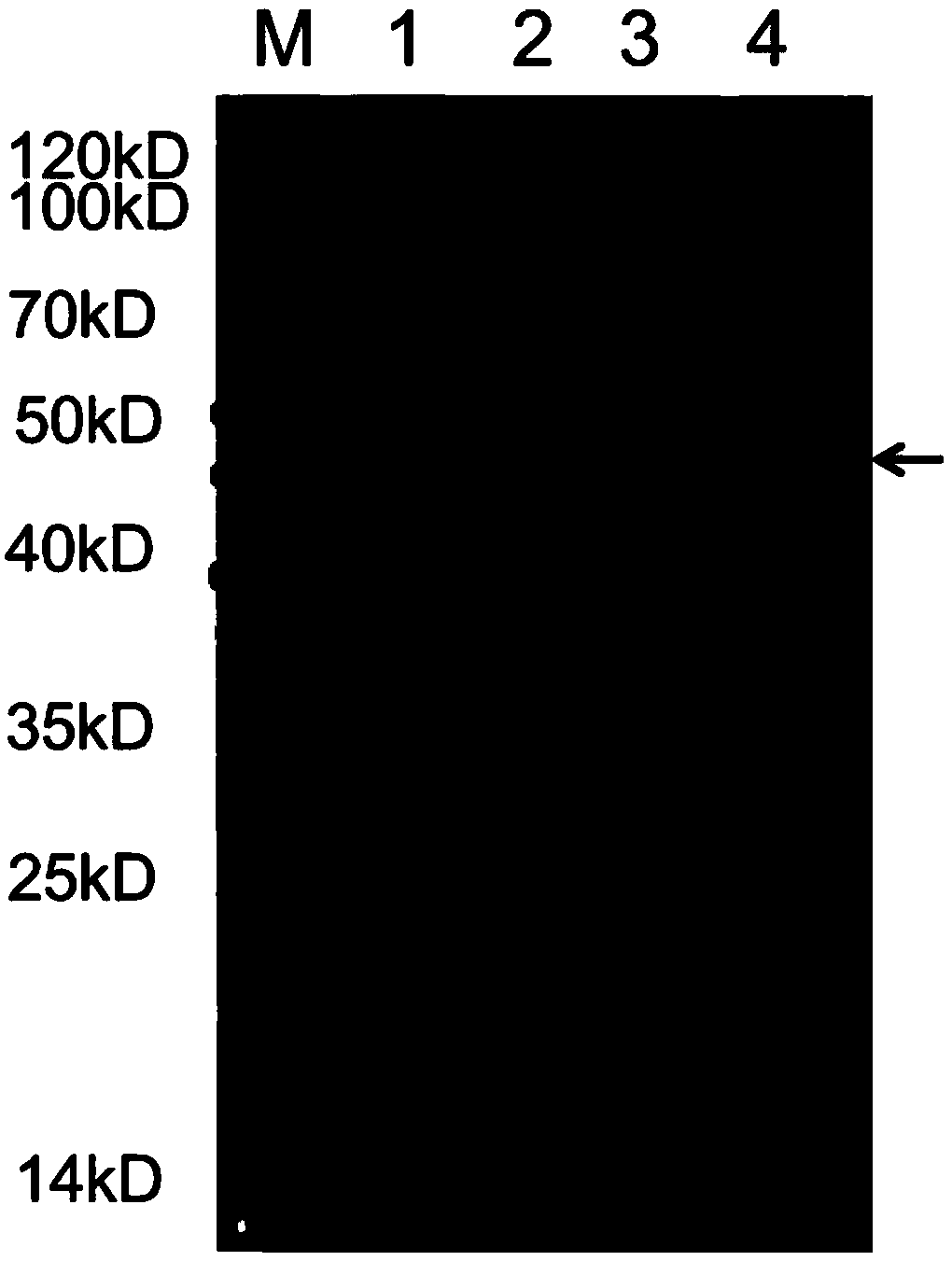
ActiveCN112725293AEasy to separateHigh recovery rateSsRNA viruses positive-senseMaterial analysis by optical meansPolyethylene glycolBlot
The invention belongs to the technical field of two-aqueous-phase extraction of viruses, and discloses a method for concentrating and purifying porcine SenecaviusA (SVA) particles through two-aqueous-phase extraction. The method comprises the following steps: performing SVA reproduction; carrying out indirect immunofluorescence identification on the SVA; establishing a three-step aqueous two-phase extraction method; determining the content of SVA; carrying out a western blot test; and carrying out electron microscope identification on SVA. After low-concentration polyethylene glycol and inorganic salt are added into an SVA culture solution, cell debris is removed, and then polyethylene glycol is added to form an aqueous two-phase system, so that concentrated and purified porcine SVA virus particles can be quickly obtained; rapid separation of virus particles can be realized through an aqueous two-phase extraction technology, the recovery rate and the purity of the virus particles are relatively high, the process is simple, and the cost is low; through a three-step aqueous two-phase extraction method, the purpose of concentrating and purifying the porcine SVA virus particles from the cell culture fluid is achieved, and the method can be applied to laboratory and large-scale production at the same time.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Seneca recombinant virus of recombinant O-type foot-and-mouth disease virus epitope genes, recombinant vaccine as well as preparation method and application of recombinant vaccine
ActiveCN111996203AHigh titerReduce pathogenicitySsRNA viruses positive-senseViral antigen ingredientsGenetic engineeringSenecavirus
The invention provides a Seneca recombinant virus of recombinant O-type foot-and-mouth disease virus epitope genes, a recombinant vaccine and a preparation method and application of the recombinant vaccine, and relates to the technical field of genetic engineering. According to the invention, the full-length cDNA of SVV / FJ / 001 strain is obtained, deletion and mutation transformation are carried out on the 5'UTR, meanwhile, the tandem O-type FMDV recombinant epitope genes are fused into SVA cDNA, and the Seneca recombinant virus of recombinant foot-and-mouth disease antigen epitope is constructed. The recombinant virus can express the foot-and-mouth disease B cell epitope and T cell epitope fused into the SVA cDNA, and an expression product has good reactogenicity. The pathogenicity of therecombinant virus is remarkably reduced, even no pathogenicity is caused to pigs, and the biological safety of the virus strain is remarkably improved; and the prepared inactivated vaccine has good immunogenicity, can generate a specific antibody for FMDV while effectively stimulating an SVA neutralizing antibody, and can be used for preventing and controlling Seneca virus and one or more non-Seneca viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Kit for rapidly detecting Seneca virus and detection method of kit
ActiveCN108796123AIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesSenecavirusPhosphate
The invention discloses a kit for rapidly detecting Seneca viruses and a detection method of the kit. The kit comprises an RPA (Recombinase Polymerase Amplificatio) primer probe mixed liquid, a PBST (Phosphate Buffered Solution), a flow measurement chromatography test paper strip, RPA lyophozyme and an RPA reaction premixed liquid, wherein the primer in the RPA primer probe mixed liquid has gene sequences as follows: an upstream primer: 5'-AATTCCTGTTTGACCGATCTCGGCTACTGA-3'; a downstream primer: 5'-biotin-CCAGTAAAGTGGTAGGGTACGTGCAGTTGGTC3'; the RPA primer probe has a gene sequence as follows: 5'-FAM-GGCCTGTACGTCAACCCGTCTGACAGTGGCG-THF-TCTCGCTAACACTTC-Spacer C3-3'. The kit for rapidly detecting Seneca viruses, which is disclosed by the invention, is good in specificity and accurate in diagnosis, has sensitivity better than that of a conventional PCR (Polymerase Chain Reaction), the Seneca virus detection method disclosed by the invention is very simple, convenient and rapid to operate, and is not only applicable to clinical diagnosis, but also applicable to multiple fields such as food security detection, on-site detection in breeding fields and environment assessment.
Owner:CHINA AGRI VET BIO SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com