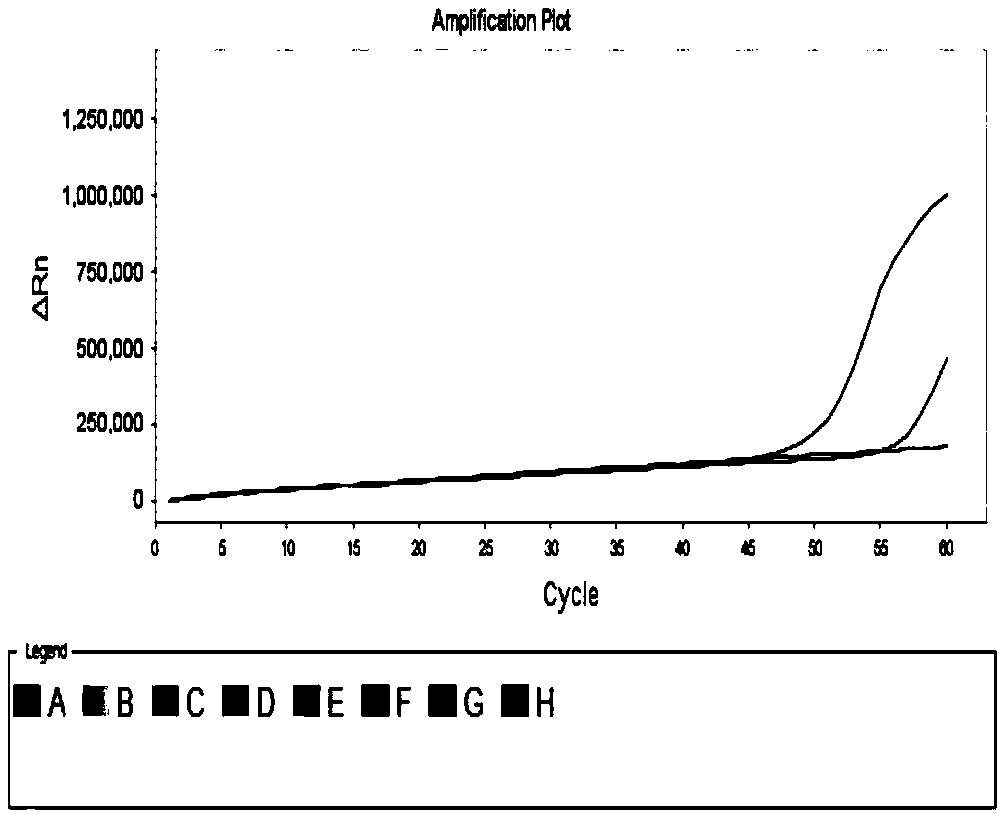
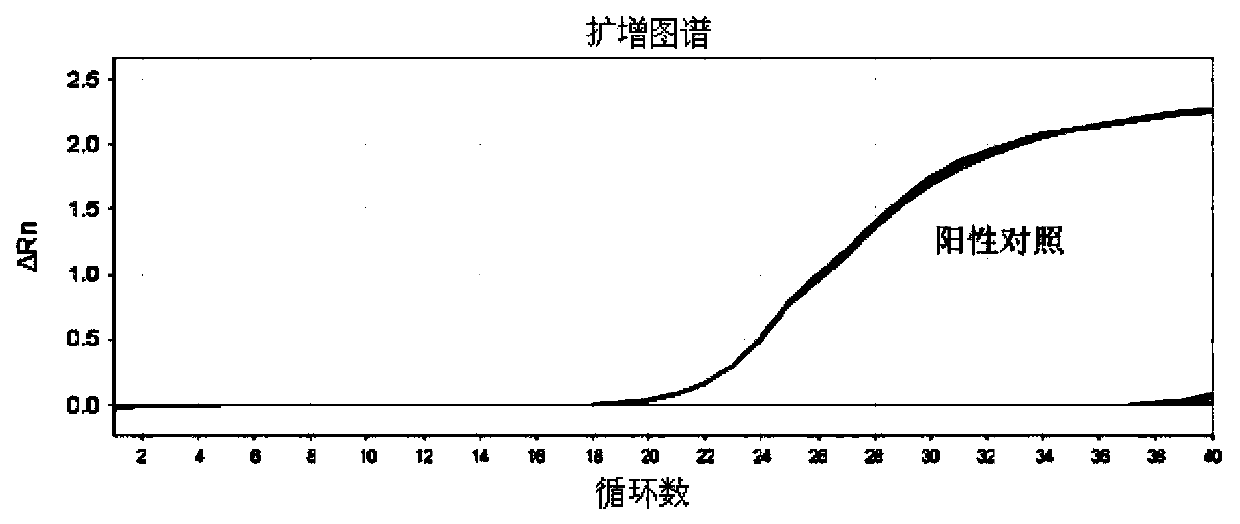
Patents
Literature
43 results about "Swine influenzavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine strain of HIN1 swine influenza virus and application thereof
ActiveCN102899294AImprove protectionLong durationMicroorganism based processesAntiviralsEngineeringImmunogenicity
The invention relates to a vaccine strain of HIN1 swine influenza virus, wherein a classification naming is Swine influenza virus A / Swine / Nanjing / 50 / 2011 (H1N1), and a microbial accession number is CCTCCNO: V201218. According to the invention, isolation, identification and whole genome sequencing of the HIN1 swine influenza virus strain are carried out, and an inactivated vaccine against the swine flu is prepared by using the HIN1 swine influenza virus strain. The inactivated vaccine against the H1NI swine flu prepared from the virus strain can provide good protection for pigs attacked by homologous virulent strains, has good immunogenicity, and can effectively prevent H1NI swine flu with single vaccine or combined vaccines.
Owner:JIANGSU ACAD OF AGRI SCI
New swine influenza vaccine
ActiveUS20180080044A1Low costSimilar efficiencySsRNA viruses negative-senseViral antigen ingredientsNucleotideHerpes simplex virus DNA
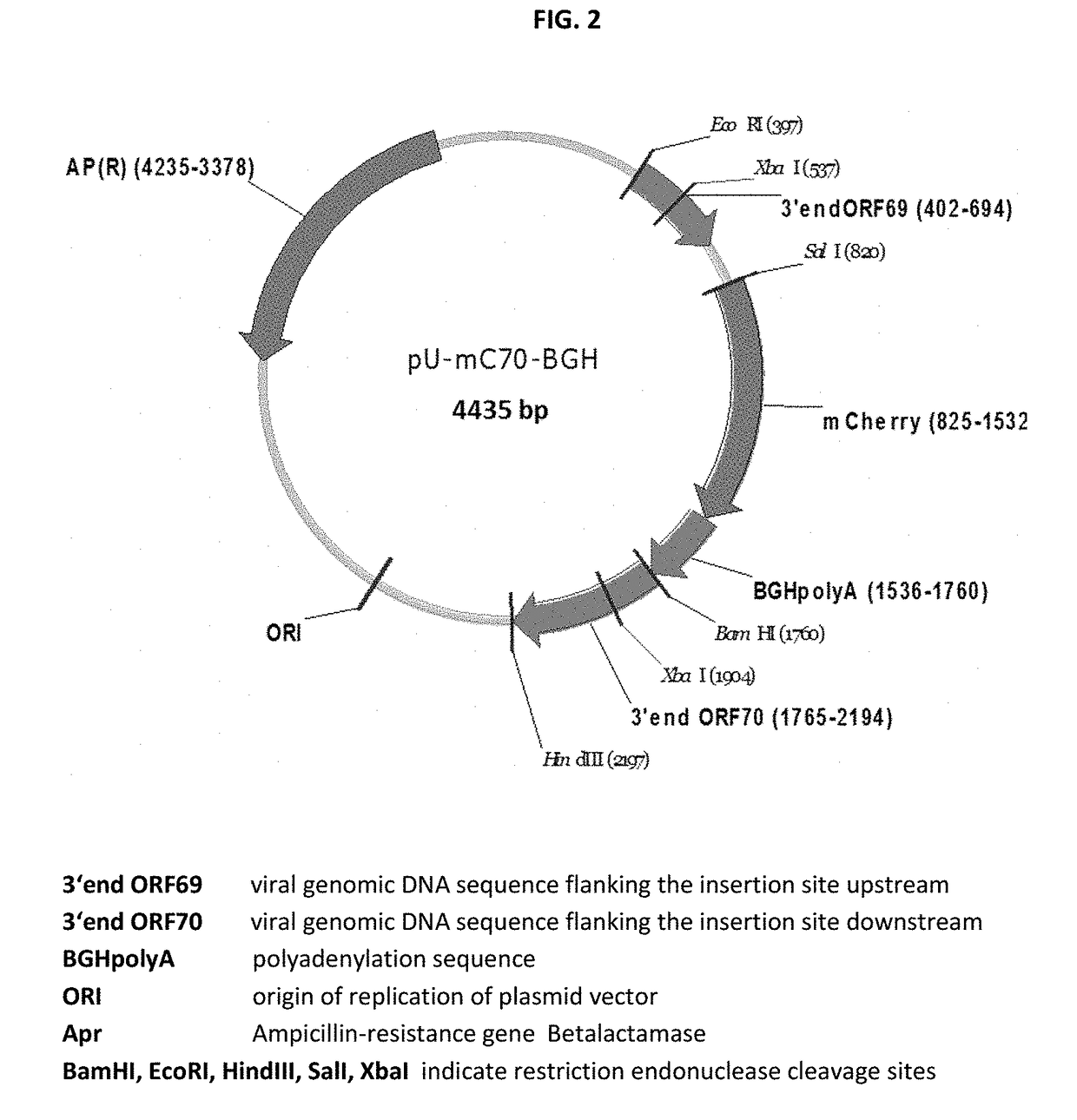
The present invention relates to Equine Herpes Virus (EHV) vectors comprising at least one exogenous antigen encoding sequence relating to a pathogen infecting food producing animals, wherein said exogenous antigen encoding sequence is inserted into an insertion site, preferably ORF70, and said exogenous antigen encoding sequence is operably linked to a promoter sequence, preferably the promoter sequence comprising 4pgG600 (SEQ ID NO:1) or 4pMCP600 (SEQ ID NO:2) or the complementary nucleotide sequences thereof or a functional fragment or a functional derivative thereof or the complementary nucleotide sequences thereof. Furthermore, the present invention relates to methods for immunizing a food producing animal comprising administering to such food producing animal an immunogenic composition comprising embodiments of the present invention. Moreover, the present invention relates to methods for the treatment or prophylaxis of clinical signs caused by swine influenza virus in a food producing animal.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Nucleic-acid sequence-based amplification (NASBA) method for detecting swine influenza virus (SIV)
InactiveCN102534052AMicrobiological testing/measurementMicroorganism based processesQuarantineSwine influenzavirus
The invention discloses a nucleic-acid sequence-based amplification (NASBA) method for detecting a swine influenza virus (SIV). The NASBA method mainly comprises the following steps of: extracting the ribonucleic acid (RNA) from the SIV, preparing an NASBA system, preparing an NASBA program, carrying out identification on an NASBA product and carrying out PCR (Polymerase Chain Reaction) amplification. According to the NASBA method, the NASBA rapid detection method is established by taking the NP gene of the SIV as a target gene and is used for the diagnosis of the SIV. The method has higher specificity and sensitivity so as to detect a virus solution with the dilution factor of 10<-5>, so that the method can be used for the rapid detection of clinically-suspected SIV samples, meanwhile, the technical level of China in the diagnosis, epidemic surveillance, inspection and quarantine of swine influenza can be improved so as to ensure the healthful and rapid development of swine industry.
Owner:天津市宁河原种猪场有限责任公司
Breeding method for transgenic pigs expressing sIFITM3 genes
InactiveCN102391990ASimple methodSimple and fast operationAnimal reproductionMicrobiological testing/measurementAnimal virusEmbryo transfer
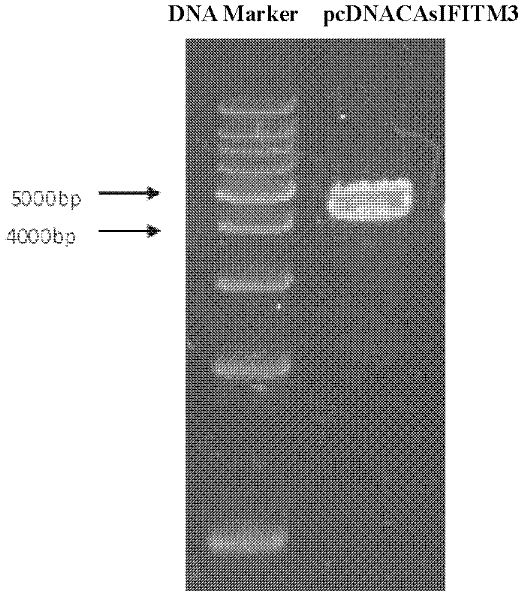
The invention discloses a breeding method for transgenic pigs expressing sIFITM3 (swine interferon induced transmembrane 3) genes. The breeding method comprises the steps as follows: firstly, the construction of swine fibroblast cell lines stably expressing the sIFITM3 genes comprises the following steps: the step a refers to the construction and the linearization of pcDNACAsIITM3 vectors, wherein, primers are designed, lymph node tissue RNA (Ribose Nucleic Acid) of pigs is extracted, the sIFITM3 genes are obtained through RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification, ethanol precipitation is carried out, and sterile water is used for dissolving; the step b refers to liposome transfection, wherein, swine fibroblasts expressing sIFITM3 are constructed; the step c refers to screening and proliferation of nuclear donor cells transfected with the sIFITM3 genes; secondly, recombination blastula acquisition based on a manual nucleus transplantation method comprises the following steps: the step a refers to the preparation of recipient cells; the step b refers to enucleation and injection of the nuclear donor cells; and thirdly, the embryo transplantation and the acquisition of the transgenic pigs comprise the following steps: the step a refers to embryo transplantation, wherein, bred blastulas are transferred to a fallopian tube of a surrogate sow, and then detection is carried out; and the step b refers to transgenic individual identification. The breeding method is feasible, and is simple and convenient to operate; in addition, the pigs are the transgenic pigs expressing sIFITM3, and have the potential capability to resist animal virus such as foot-and-mouth disease virus, Japanese encephalitis virus, swine influenza virus and the like.
Owner:HUAZHONG AGRI UNIV
RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection primers for specifically detecting SIV (swine influenza virus), application of primers, detection reagent and method
InactiveCN109468416AAccurate detectionHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiologySample handling
The invention relates to the biotechnology field, in particular to RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection primers for specifically detecting SIV (swine influenza virus), an application of the primers, a detection reagent and a method. The RT-LAMP detection primers are selected from one or several of the primer groups: (1) a primer group SIV1; (2) a primergroup SIV2; (3) a primer group SIV3. The RT-LAMP primer group can accurately detect the SIV and has no cross reaction with HP-PRRSV, PRRSV, PPV, PCV2, CSFV, PRV and the like. The three screened primers cover more than 300 SIV sequences in an NCBI database and have high detection sensitivity and specificity, the established detection method has the advantages of high accuracy, short detection timeand allowance of real-time observation of results, only 1 h is taken from sample treatment to result report, operation is simple, and pollution is avoided as midway opening is not required.
Owner:CAPITALBIO CORP
Porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion vaccine as well as preparation method and application thereof
ActiveCN107375920AEnsure safetySafe and effectiveSsRNA viruses negative-senseViral antigen ingredientsAntigenRespiratory complex II
The invention discloses a porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion vaccine as well as a preparation method and an application thereof. The vaccine consists of porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion antigen and a stable adjuvant compound; a porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion is formed through direct adsorption or covalent binding of porcine reproductive and respiratory syndrome virus to the surface of a swine influenza virus reconstructed virion, wherein the swine influenza virus reconstructed virion is a small vesicle containing a phospholipid bilayer; and haemagglutinin and neuraminidase are bound to the surface of the phospholipid bilayer. The vaccine prepared by the invention has the characteristics of being safe, efficient, convenient to use, long in efficacy time and the like. Purposes of reducing and / or removing respiratory syndrome virus and / or swine influenza virus can be achieved through intranasal immunization.
Owner:CHINA AGRI VET BIO SCI & TECH
Mixed virus-like particles of swine influenza virus and foot and mouth disease virus, preparation method and application thereof
ActiveCN102370976AStrong immunityLong durationMicroorganism based processesAntiviralsAdjuvantFoot-and-mouth disease virus
The invention provides a mixed virus-like particles, containing stromatin M1 of influenza virus, surface antigen erythrohemagglutinin HA of influenza virus and a nexus protein containing main antigenic epitope contained capsid protein of foot and mouth disease virus and a transmembrane zone and an inner membrane zone of the erythrohemagglutinin HA of influenza virus. The main antigenic epitope contained capsid protein of foot and mouth disease virus substitutes a basically same length outer membrane zone of a 5' terminal of the erythrohemagglutinin HA of influenza virus. Surface antigen erythrohemagglutinin HA of influenza virus and the main antigenic epitope contained capsid protein of foot and mouth disease virus are expressed simultaneously on surfaces of the mixed virus-like particles. The invention also provides a divalent vaccine of swine influenza and foot and mouth disease; and the vaccine contains the mixed virus-like particles and adjuvants. The invention also provides a method for preparing the mixed virus-like particles.
Owner:SUN YAT SEN UNIV
Eurasian avian like H1N1 swine influenza A virus cold-adapted attenuated virus strain and application thereof
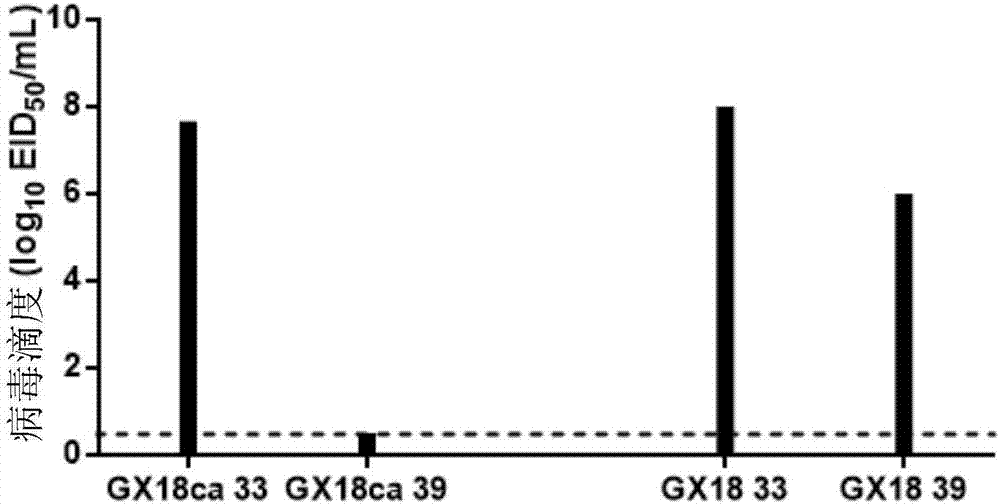
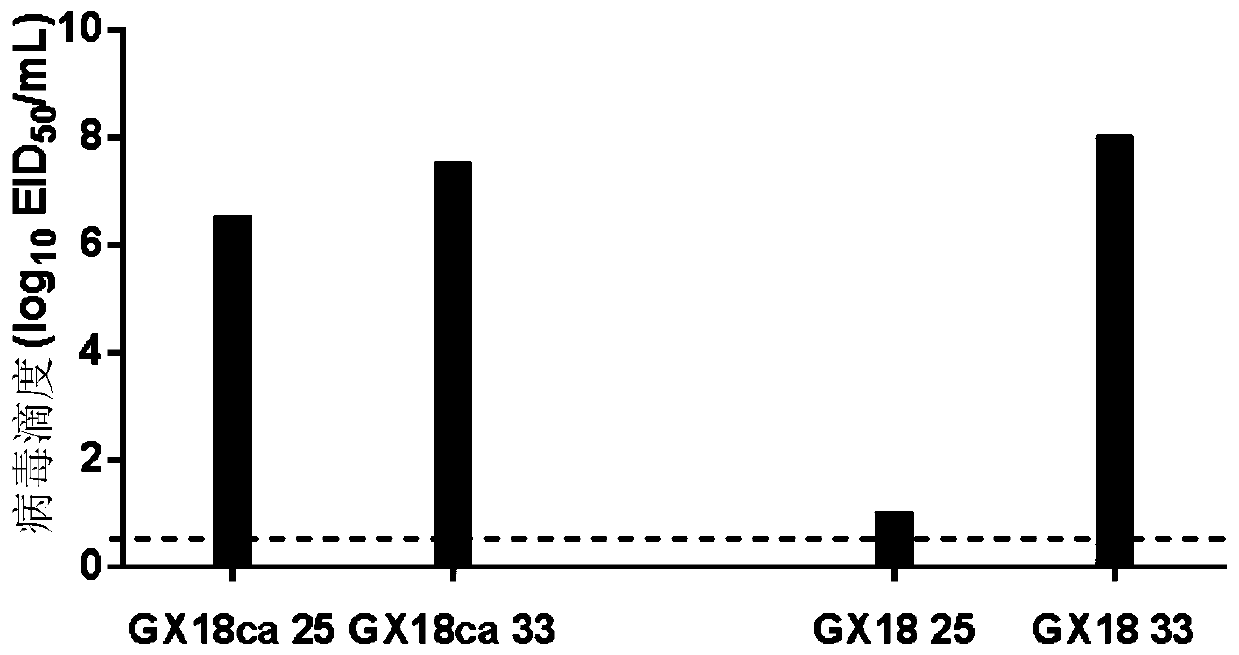
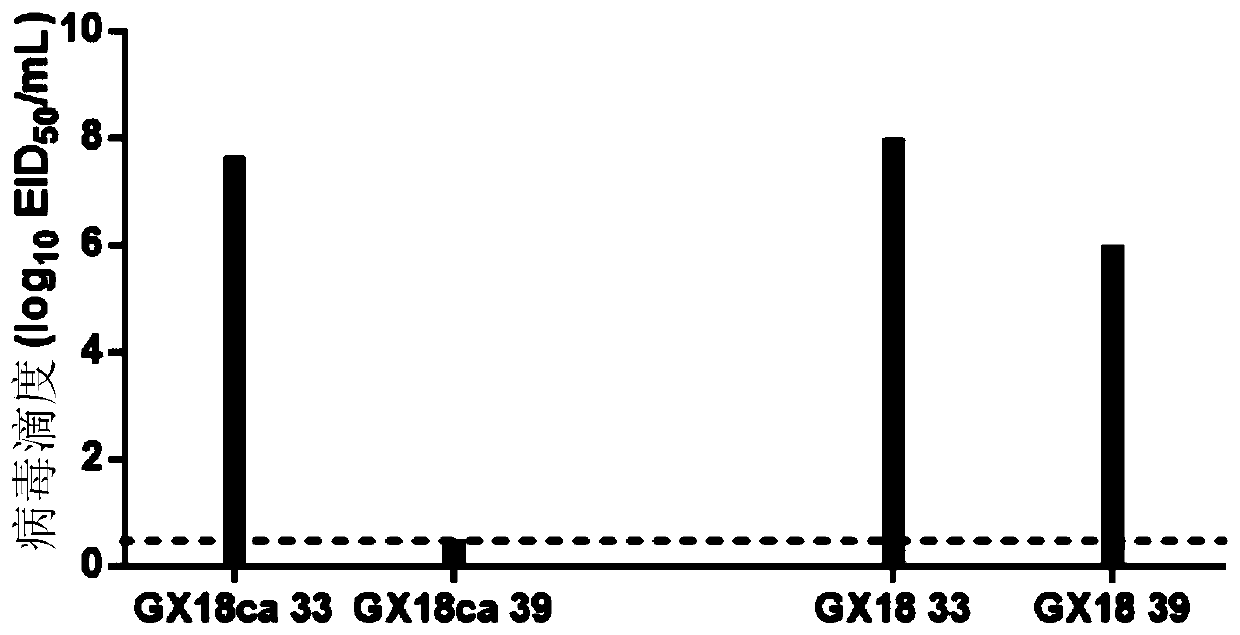
The invention discloses a Eurasian avian like H1N1 swine influenza A virus cold-adapted attenuated virus strain and an application thereof. Eurasian avian like H1N1 swine influenza A viruses A / swine / Guangxi / 18 / 2011 (H1N1) are subjected to serial sub-culturing at the temperature of 33-25 DEG C to obtain the Eurasian avian like H1N1 swine influenza A virus cold-adapted attenuated virus strain named as a swine influenza cold-adapted attenuated virus strain A / swine / Guangxi / 18 / 2011ca (H1N1), the strain is collected in a China Center for Type Culture Collection, and the collection number of the strain is CCTCC NO. V201720. Researches show that an attenuated live vaccine prepared from the cold-adapted attenuated virus strain can resist attack of swine influenza A virus wild strains mainly epidemic in Chinese swine herds, and is of great significance for controlling prevalence and human infection of Eurasian avian like H1N1 and H1N2 swine influenza A viruses.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Inactivated virus compositions and methods of preparing such compositions
ActiveUS9956281B2Easy to store and transportEnhance disinfectant and antimicrobial propertySsRNA viruses negative-senseViral antigen ingredientsAntigenMedicine
The present invention is directed at a composition comprising a live swine flu virus having an infectious component and a plurality of surface antigens in contact with a formaldehyde donor agent having a molecular weight that is less than about 400 g / mol. Preferably, the formaldehyde donor agent is selected from a non-crosslinking chemical fixative that contains urea.
Owner:STRECK LLC
Quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, porcine influenza virus and haemophilus parasuis
InactiveCN112386685AProlonged occurrenceExtension of timeSsRNA viruses negative-senseAntibacterial agentsAntigenAdjuvant
The invention belongs to the field of veterinary vaccines, particularly relates to a quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, a porcine influenza virus and haemophilus parasuis and further discloses a preparation method for the quad inactivated vaccine and application of the quad inactivated vaccine. The quad inactivated vaccine disclosed by the inventioncontains inactivated protein Cap expressed by the PCV2-type baculovirus, inactivated swine mycoplasma hyopneumoniae, an inactivated porcine influenza virus H1N1subtype virus, inactivated haemophilus parasuis types 4, 5 and 13 and an adjuvant of the vaccine; four kinds of antigens are free of interference to one another, four kinds of protection can be achieved by one injection, and four kinds of epidemic diseases can be prevented through one-time immunization; and meanwhile, in view of toxic substance counteracting protection and serum antibody level, the immunization effect reaches or exceedsthat of each single commodity vaccine, the immunization duration is long, the efficacy is durable, and the quad inactivated vaccine has the advantages that the safety is good, the preparation methodis simple, the immunization is convenient, the immunization cost is reduced, and the like.
Owner:BEIJING KEMUFENG BIOLOGICAL PHARMA +3
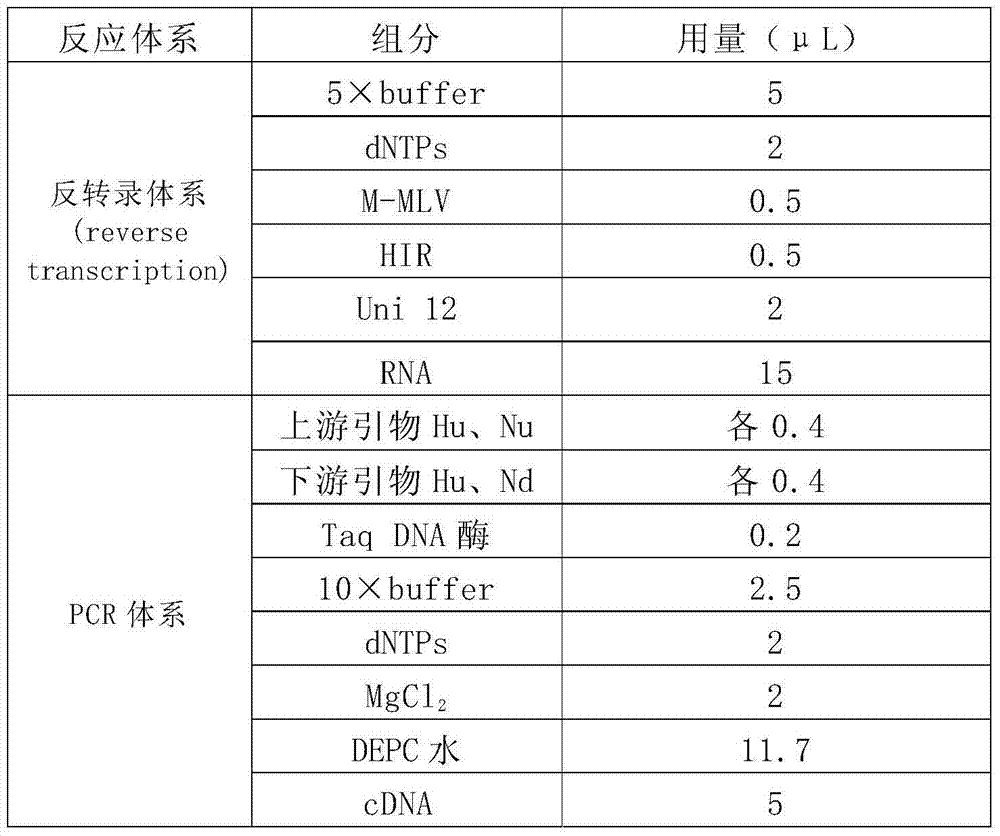
RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer for detecting poultry-like type H1N1 swine influenza virus and kit
InactiveCN103571974AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention discloses an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer for detecting poultry-like type H1N1 swine influenza virus and a kit. The RT-PCR primer comprises two pairs: primers for H1 gene and primers for N1 genes, wherein the nucleotide sequence of an upstream primer Hu on the primers for the H1 gene is 5'-GACTCTAGATTTCCATGACCTT-3', and the nucleotide sequence of a downstream Hd is 5'-ACCCATTAGAACACATCCAGAA-3'; the nucleotide sequence of an upstream primer Nu on the primers for the N1 gene is 5'-TCCAAGGGGGATGTGTTTG-3', and the nucleotide sequence of a downstream primer Nd is 5'-GACCAAGCGACTGACTCAA-3'; the kit comprises the primer. The RT-PCR primer and the kit are adopted for detecting the poultry-like type H1N1 swine influenza virus and have the advantages of high sensitivity, strong specificity, fastness and convenience and the like.
Owner:广西壮族自治区动物疫病预防控制中心
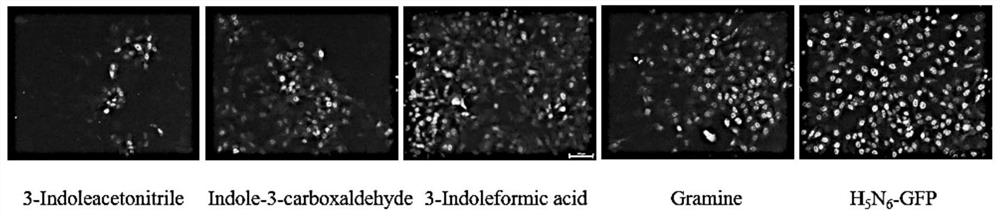
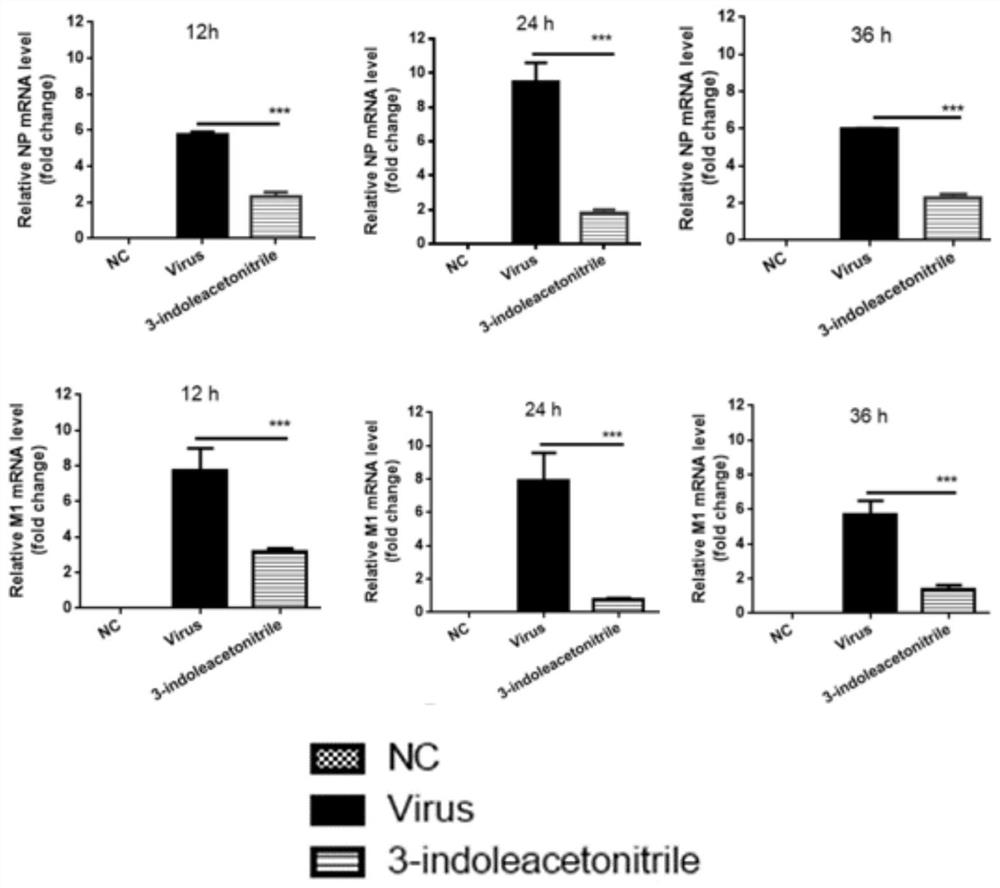
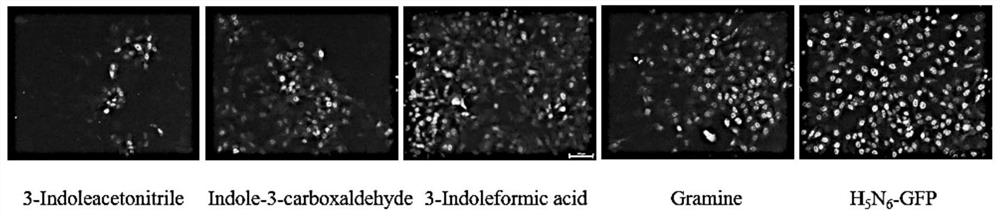
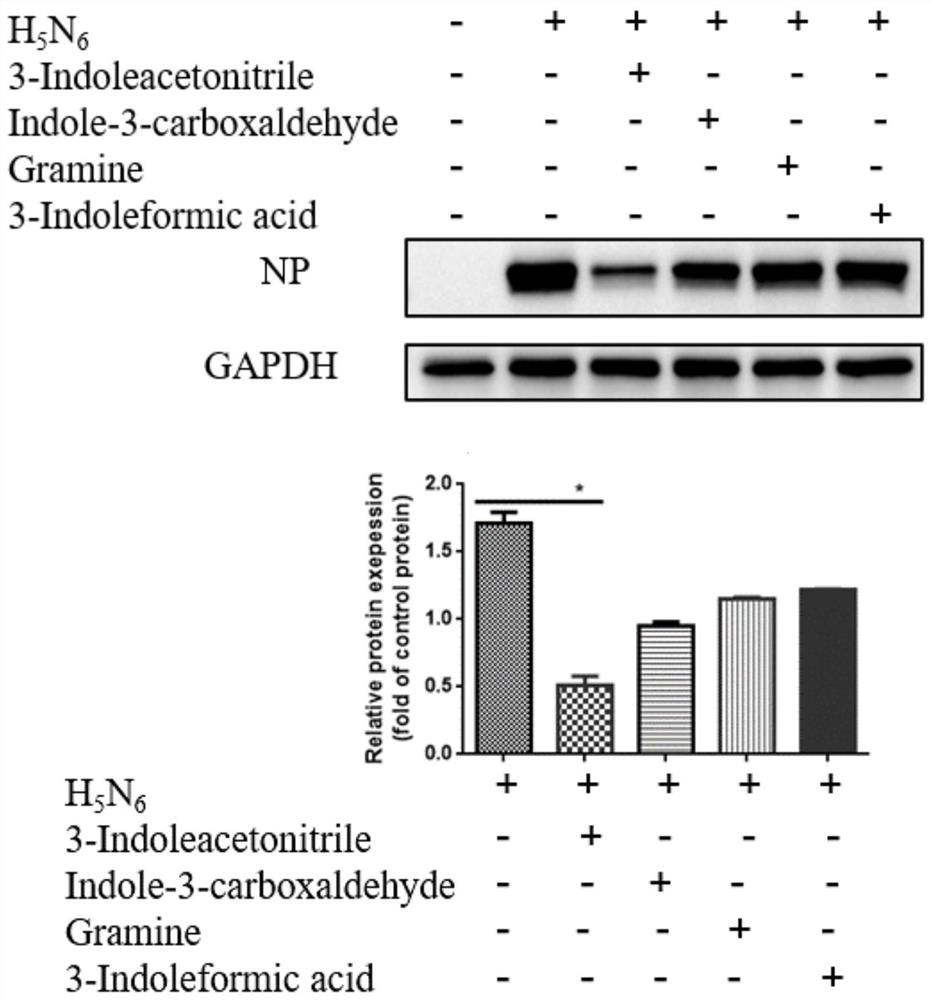
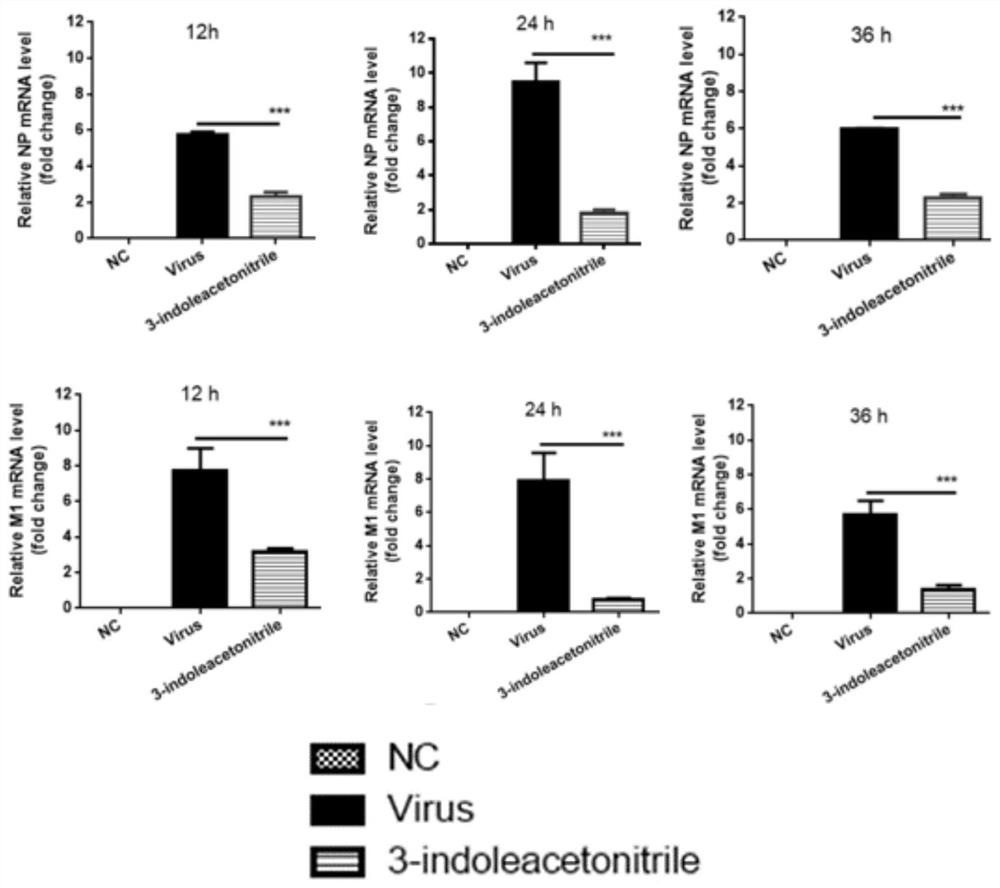
Application of indole-3-acetonitrile in preparation of medicine for treating or preventing influenza virus infection
ActiveCN112353797APrevent proliferationSuppressed expression levelOrganic active ingredientsAntiviralsSwine influenzavirusInfluenza a
The invention belongs to the field of biological medicines, and particularly discloses an application of indole-3-acetonitrile in preparation of a medicine for treating or preventing influenza virus infection. The applicant finds that indole-3-acetonitrile can improve weight loss caused by infection of influenza viruses, remarkably inhibit proliferation of the influenza viruses in a mouse body andimprove lung tissue lesion caused by infection of the influenza viruses, and has an obvious anti-influenza virus infection effect. Meanwhile, indole-3-acetonitrile can inhibit proliferation of various influenza A viruses including human influenza virus PR8, swine influenza virus G4 and avian influenza virus H5N6, has broad spectrum, is simple in synthesis process, economical and rapid, is easy toproduce on a large scale, and can be developed into anti-influenza virus drugs.
Owner:HUAZHONG AGRI UNIV
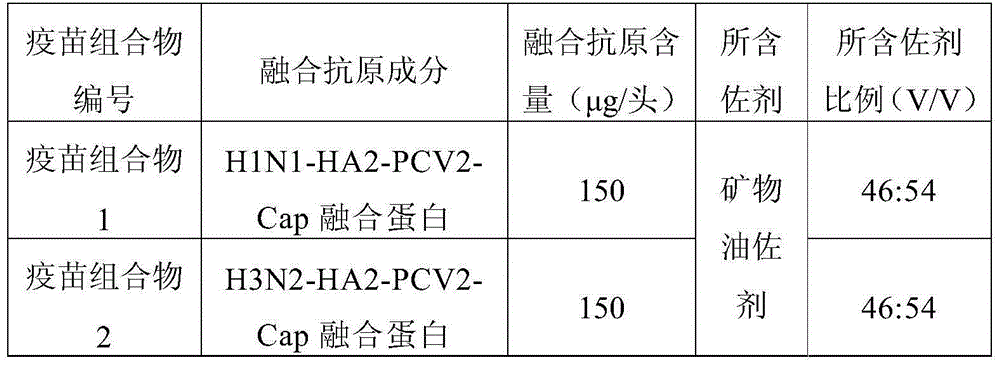
Vaccine composition for porcine circovirus and swine influenza and its preparation method and use
ActiveCN105709220AImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The invention relates to a vaccine composition for preventing and / or treating porcine circovirus diseases and swine influenza and its preparation method and use. The vaccine composition comprises i, a porcine circovirus subunit antigen or a nucleic acid which is used for coding the porcine circovirus subunit antigen and can be expressed in a pig, and ii, a swine influenza virus subunit antigen or a nucleic acid which is used for coding the swine influenza virus subunit antigen and can be expressed in a pig. The invention also relates to a nucleic acid molecule, a recombinant plasmid and a host cell for treating and / or preventing two types of pig diseases.
Owner:PU LIKE BIO ENG
Swine influenza virus H1N1 subtype hemagglutinin (HA)-1 protein recombinant suipoxvirus and preparation method thereof
InactiveCN102154365AObvious blue spotEasy to filterMicroorganism based processesAntiviralsHemagglutininRecombinant virus vaccine
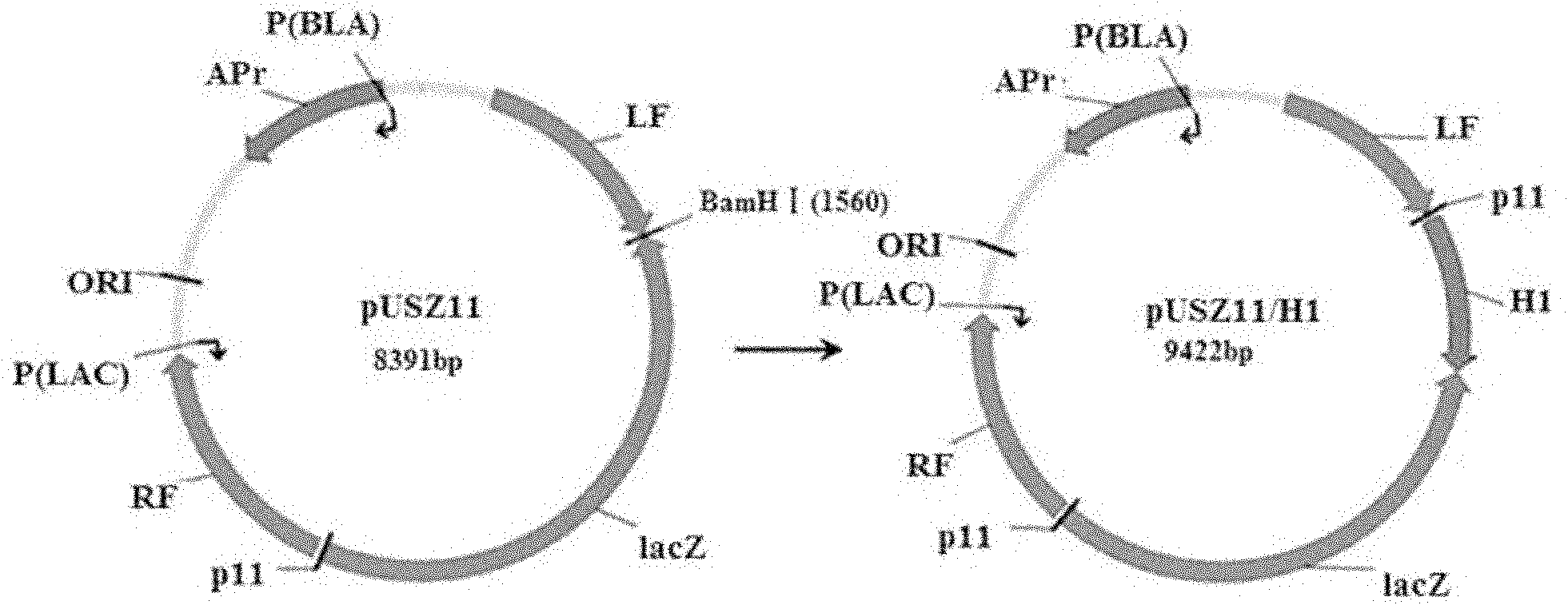
The invention belongs to the technical field of biology and discloses swine influenza virus H1N1 subtype HA-1 protein recombinant suipoxvirus and a preparation method thereof. An HA1 gene is designed and synthesized according to the gene sequence of HA1 of an A / swine / Shanghai / 1 / 2005(H1N 1) strain of the swine influenza virus, and the gene is cloned in a suipoxvirus vector pUSZ11 to obtain a transfer vector pUSZ11 / H1. The recombinant virus is a positive clone obtained by infecting and transfecting PK15 cells with the suipoxvirus and the transfer vector pUSZ11 / H1 and performing homologous recombination. The recombinant suipoxvirus can express HA1 protein stably, and after infection with the virus, the cytopathy becomes regular, the toxic effect of the breeding virus is stabilized at 107TCID50 / mL, the breeding virus can effectively stimulate the immune protect reaction of organisms and can be used in the preparation of medicines for preventing and / or treating infection with swine influenza virus H1N1 subtype.
Owner:NANJING AGRICULTURAL UNIVERSITY
H9N2 swine influenza virus and its kit
InactiveCN103320392ASignificant technological progressMicrobiological testing/measurementMicroorganism based processesGenotypeBiology
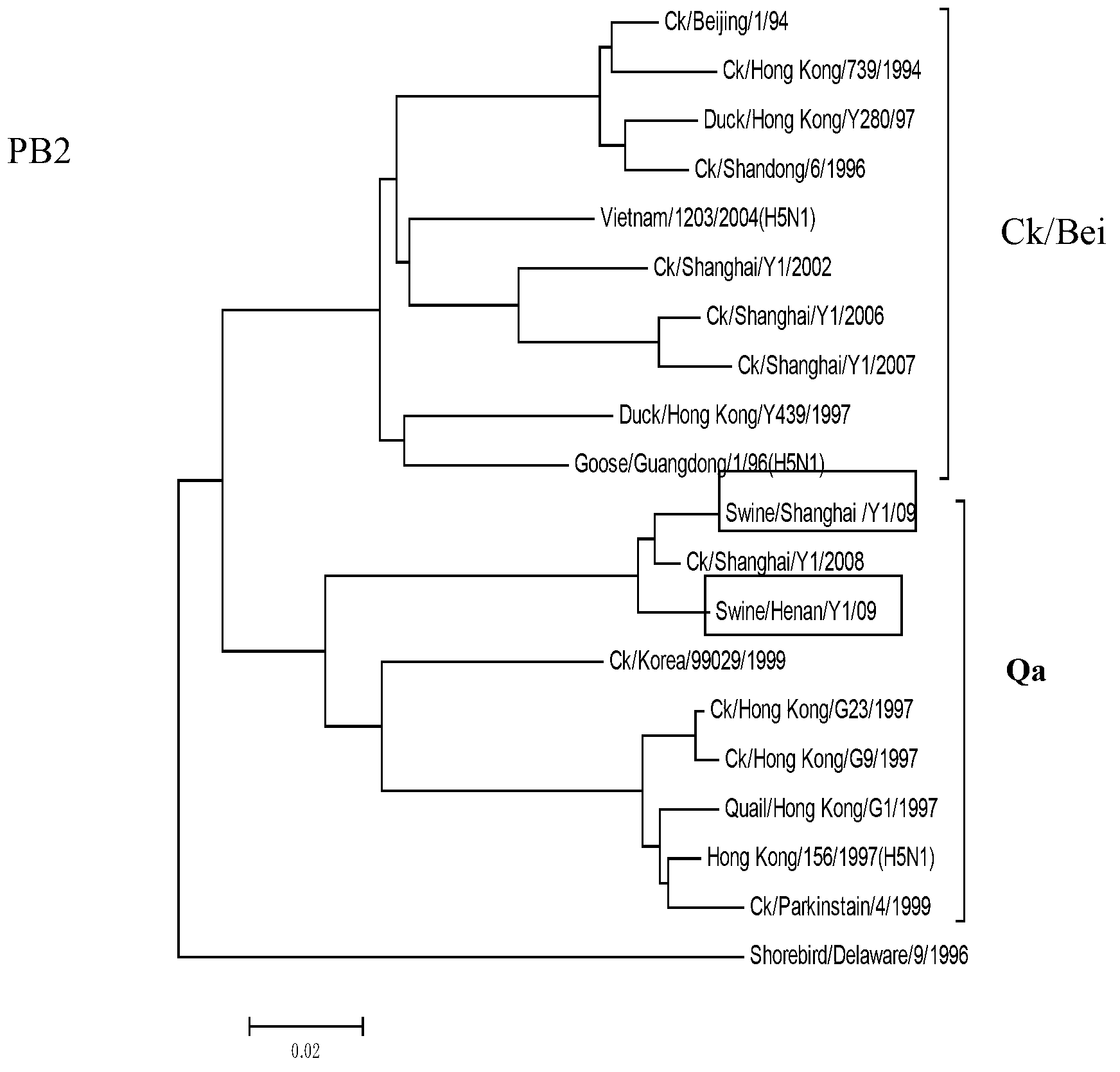
The invention belongs to the field of biotechnology and relates to a novel separated strain swine influenza virus. The invention discloses two separated H9N2 swine influenza viruses. According to the invention, it firstly proves that the two strains both belong to Ck / Bei series. The invention also discloses a kit for detecting the H9N2 swine influenza viruses. Through the kit provided by the invention, whether swine flu is caused by the genotype of H9N2 swine influenza viruses can be rapidly detected or eliminated so as to control the swine flu epidemic situation as soon as possible. In addition, by the use of the kit, bioinformation for a swine influenza virus monitoring system can be provided.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Triple inactivated vaccine for pigs and preparation method and application thereof
PendingCN111000995ASsRNA viruses negative-senseSsRNA viruses positive-senseDiseaseSwine influenzavirus
The invention discloses a triple inactivated vaccine for pigs and a preparation method and application of the triple inactivated vaccine. The triple inactivated vaccine for pigs comprises recombinantbaculovirus for expressing an O-type FMDV VP1 gene, swine senecavirus, H3N2 subtype swine influenza virus and an immunologic adjuvant. The invention also discloses a method for preparing the triple inactivated vaccine for pigs. The method comprises the following steps: (1) amplifying and inactivating the recombinant baculovirus for expressing the O-type FMDV VP1 gene; (2) mixing the inactivated recombinant baculovirus fluid for expressing the O-type FMDV VP1 gene, the inactivated swine senecavirus fluid and the H3N2 subtype swine influenza virus to obtain a water phase; (3) heating the immunologic adjuvant to obtain an oil phase; and (4) adding the oil phase into the water phase, mixing and emulsifying. Immune protective efficacy detection results prove that the prepared triple inactivatedvaccine for pigs can effectively prevent and treat O-type foot-and-mouth disease virus, swine senecavirus and H3N2 subtype swine influenza virus simultaneously.
Owner:哈药集团生物疫苗有限公司
A porcine reproductive and respiratory syndrome virus-swine influenza virus reconstituted virion vaccine and its preparation method and application
ActiveCN107375920BAvoid infectionReduce or clear viremiaSsRNA viruses negative-senseViral antigen ingredientsAntigenAdjuvant
The invention discloses a porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion vaccine as well as a preparation method and an application thereof. The vaccine consists of porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion antigen and a stable adjuvant compound; a porcine reproductive and respiratory syndrome virus-swine influenza virus reconstructed virion is formed through direct adsorption or covalent binding of porcine reproductive and respiratory syndrome virus to the surface of a swine influenza virus reconstructed virion, wherein the swine influenza virus reconstructed virion is a small vesicle containing a phospholipid bilayer; and haemagglutinin and neuraminidase are bound to the surface of the phospholipid bilayer. The vaccine prepared by the invention has the characteristics of being safe, efficient, convenient to use, long in efficacy time and the like. Purposes of reducing and / or removing respiratory syndrome virus and / or swine influenza virus can be achieved through intranasal immunization.
Owner:CHINA AGRI VET BIO SCI & TECH
Hybridoma cell strain, avian-like H1N1 swine influenza virus HA protein MAb, antigen epitope and application thereof
ActiveCN112359020AStrong specificityHigh potencyImmunoglobulins against virusesTissue cultureLinear epitopeBinding peptide
The invention provides a hybridoma cell strain, and belongs to the technical field of monoclonal antibody (MAb) preparation. The hybridoma cell strain disclosed by the invention can secrete avian-likeH1N1 swine influenza virus HA protein MAb, and the obtained MAb has the characteristics of good specificity, high titer and the like. By utilizing the MAb and combining a peptide scanning technology,the linear epitope 216VSVGSS221 located on the HA protein is obtained through screening, and the epitope is highly conservative in the avian-like H1N1 virus. The MAb and the epitope can be obtained to be used for establishing immunological diagnosis methods of the avian-like H1N1 SIV or the antibody thereof, such as ELISA, colloidal gold and the like. The HA protein epitope information of the H1N1 SIV can be perfected by obtaining the epitope information, the structure and the function of the HA protein antigen can be better understood, and theoretical and technical supports are provided forpredicting the dynamic state of virus antigenic variation and better preventing and controlling epidemic diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method and application of gene chip for determination and drug resistance detection of influenza A virus
InactiveCN102433391APracticalImprove throughputMicrobiological testing/measurementOligonucleotide chipReverse transcriptase
The invention relates to a gene chip for determination and drug resistance detection of influenza A virus. The preparation method of the gene chip comprises the steps of preparing a universal primer, preparing a subtype nucleic acid parting probe and a drug resistance probe, preparing an oligonucleotide chip, establishing an RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) system and establishing a hybrid system. The gene chip prepared by the method disclosed by the invention can be used for simultaneously determining five kinds of subtype influenza A viruses, including influenza A H1N1 influenza virus, seasonal H1N1 influenza virus, seasonal H3N2 influenza virus, poultry H5N1 influenza virus and swine H1N1 influenza virus. In addition, the gene chip can be used for prompting the condition that the influenza A virus is non-drug resistant, has the advantages of quickness, accuracy, high throughput and high specificity and can be used for providing guidance for monitoring, clinical diagnosis and treatment of the influenza viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
Detection of modified live swine influenza virus vaccines
The present invention relates i.a. to diagnostic kits and methods for detecting an animal vaccinated with a modified live Swine Influenza virus specific vaccine and diagnostic kits and methods for differentiating animals vaccinated with a modified live Swine Influenza virus specific vaccine from animals infected with Swine Influenza virus, respectively.
Owner:BOEHRINGER INGELHEIM VETMEDICA GMBH
Sulfated yeast beta glucan application in preparation of anti-animal-virus drugs
The invention belongs to the technical field of veterinary drug application, relates to a sulfated yeast beta glucan application in preparation of anti-animal-virus drugs and particularly relates to the application in preparation of anti-swine-flu viruses, porcine reproductive and respiratory syndrome viruses and porcine pseudorabies virus drugs. The sulfated yeast beta glucan application in preparation of the anti-animal-virus drugs has the advantages of being wide in antiviral spectrum, high in effective rate, safe and toxic-and-side-effect-free and residue-free, reducing or replacing utilization of antibiotics, belonging to a novel antiviral drug and having good application and development prospects.
Owner:天津市农业科学院
Primer set for real-time fluorescent quantitative PCR detection of swine influenza virus
ActiveCN103014176BReduce testing costsRapid typing testMicrobiological testing/measurementMicroorganism based processesHypotypeSwine influenzavirus
The invention discloses a set of primers for swine influenza virus SYBGREEN I Real-time PCR detection method, including swine influenza virus universal M gene, H1 subtype, N1 subtype, H3 subtype and N2 subtype primers. The present invention provides a group of Real-time PCR primers for swine influenza virus detection and typing, the primers are suitable for SYBR Green I dye method, to reduce the detection cost of SIV, and realize the rapid typing detection of SIV, for Provide technical support for clinical monitoring of SIV epidemic dynamics and epidemic trends.
Owner:兆丰华生物科技(南京)有限公司 +3
Vaccine composition for porcine circovirus and swine influenza, preparation method and application
ActiveCN105709220BImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The present invention relates to a vaccine composition for preventing and / or treating porcine circovirus disease and swine influenza and its preparation method and application. Circular virus subunit antigen and nucleic acid capable of being expressed in pigs, and ii) swine influenza virus subunit antigen or nucleic acid encoding swine influenza virus subunit antigen and capable of being expressed in pigs. The present invention also relates to nucleic acid molecules, recombinant plasmids and host cells for simultaneous treatment and / or prevention of two porcine diseases.
Owner:PU LIKE BIO ENG
Eurasian Avian H1N1 Subtype Swine Influenza Cold Adapted Attenuated Strain and Its Application
The invention discloses a Eurasian avian like H1N1 swine influenza A virus cold-adapted attenuated virus strain and an application thereof. Eurasian avian like H1N1 swine influenza A viruses A / swine / Guangxi / 18 / 2011 (H1N1) are subjected to serial sub-culturing at the temperature of 33-25 DEG C to obtain the Eurasian avian like H1N1 swine influenza A virus cold-adapted attenuated virus strain named as a swine influenza cold-adapted attenuated virus strain A / swine / Guangxi / 18 / 2011ca (H1N1), the strain is collected in a China Center for Type Culture Collection, and the collection number of the strain is CCTCC NO. V201720. Researches show that an attenuated live vaccine prepared from the cold-adapted attenuated virus strain can resist attack of swine influenza A virus wild strains mainly epidemic in Chinese swine herds, and is of great significance for controlling prevalence and human infection of Eurasian avian like H1N1 and H1N2 swine influenza A viruses.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
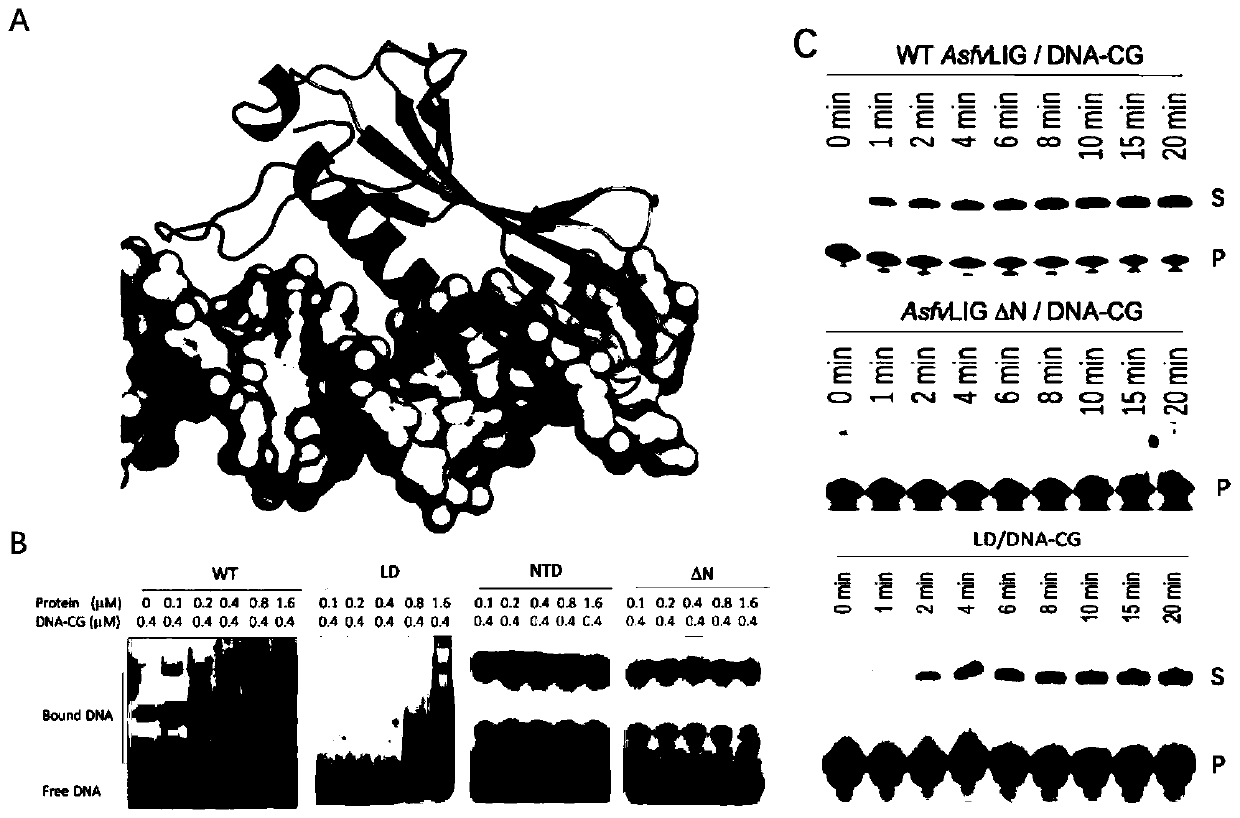
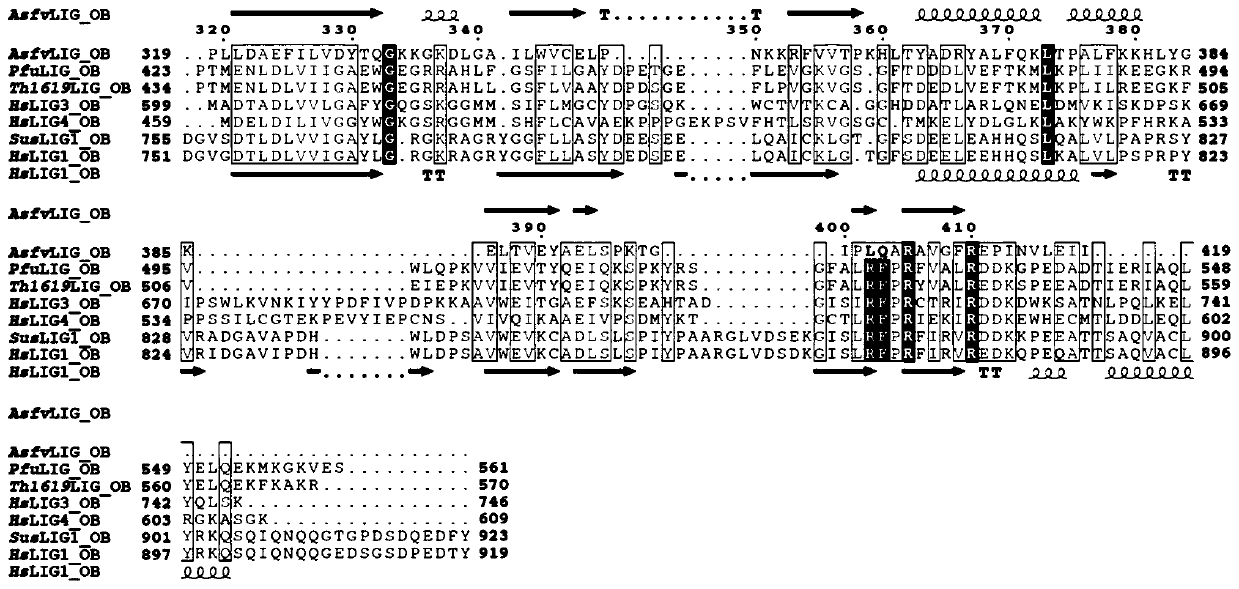
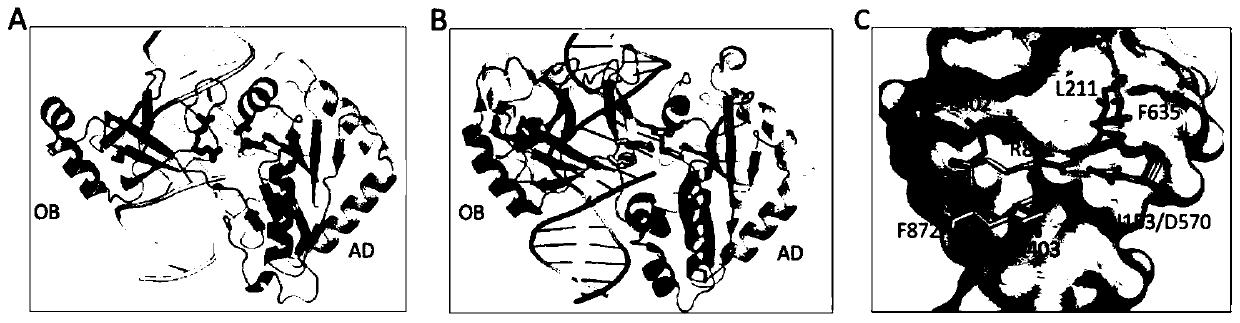
Crystal of African swine fever virus (ASFV) DNA ligase, and preparation method and application of crystal
The invention belongs to the technical field of biology, and particularly relates to a crystal of African swine fever virus (ASFV) DNA ligase, and a preparation method and application of the crystal.The DNA ligase AsfvLIG contains 419 amino acids, has the molecular weight of about 45 kDa, and has highly soluble expression in an expression strain Escherichia coli BL21 (DE3). A three-dimensional structure of the crystal is obtained by X-ray diffraction, with the resolution reaching 2.5 angstroms. A crystallographic asymmetric unit has a protein molecule consisting of 9 alpha helixes and 17 betapleated sheets, and is combined with DNA of a catalytic reaction substrate. The 153th asparagine, the 211th leucine, the 402th leucine and the 403th glutamine in the structure have different conformations from human DNA ligase. The crystal structure of the DNA ligase AsfvLIG is widely used in drug design or drug screening for African swine fever viruses (ASFV).
Owner:FUDAN UNIV
Swine flu virus identification primer and application thereof
InactiveCN102399906BMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseNucleotide
The invention discloses a swine flu virus identification primer and an application thereof. The swine flu virus identification primer provided by the invention consists of primer groups A to E, wherein the primer group A consists of a primer 1 and a primer 2, the primer group B consists of a primer 3 and a primer 4, the primer group C consists of a primer 5 and a primer 6, the primer group D consists of a primer 7 and a primer 8, the primer group E consists of a primer 9 and a primer 10, nucleotide sequences of the primer 1 to the primer 10 are respectively the sequence 1 to the sequence 10 in a sequence table. Experiments prove that the primer groups provided by the invention are characterized in that the subtype and the lineage of the swine flu virus is identified in one step through extracting sample ribonucleic acid (RNA), synthesizing complementary deoxyribonucleic acid (cDNA) via reverse transcriptase and simultaneously amplifying the genes with the possibly existence of H1, H3, H9 and H5 in one step. Compared with the typical virus separation identification and serology grouping tests, the primer has the advantage that the speed and the accuracy are high.
Owner:山东省动物疫病预防与控制中心
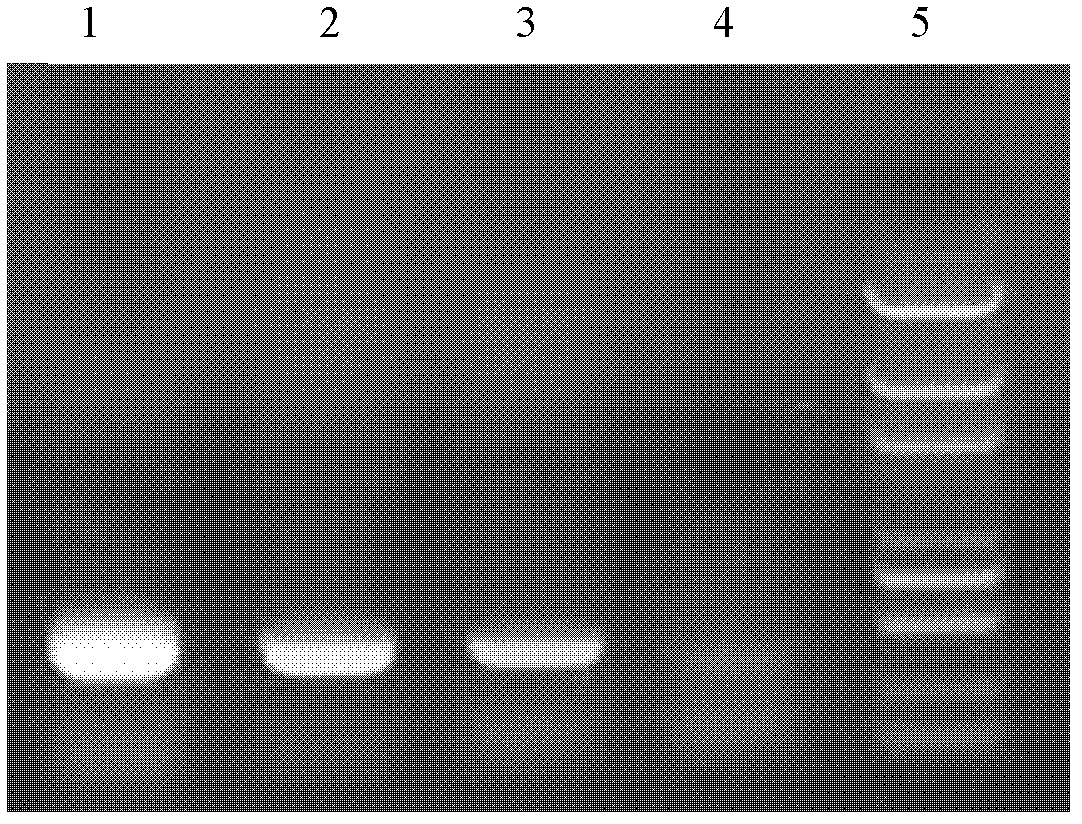
Application of porcine β-defensin 2 gene in anti-swine influenza
ActiveCN108866069BReduce the severity of the diseaseLower titerFermentationCationic antimicrobial peptidesInfectious DisorderFeed additive
The invention belongs to the technical fields of zoonosis and animal transgenes, and particularly relates application of a PBD (porcine beta-defensins 2) gene in resisting of swine influenza. The application has the advantages that the segments of the PBD-2 gene are excessively expressed in a swine body, so as to obviously improve the resistance ability of the transgenic swine on the swine influenza, namely that the excessively expressed PBD-2 can obviously reduce the virus titer of swine influenza virus in lung tissues of the infected swine and the lesion degree of swine lung due to infectionby the swine influenza virus; by synthesizing or recombining the PBD-2 gene, the duplication of the swine influenza virus can be effectively inhibited; the polypeptide medicines or feed additives canbe prepared by in-vitro expression or synthesizing of the PBD-2 gene.
Owner:HUAZHONG AGRI UNIV
A kind of hybridoma cell line, poultry type h1n1 swine influenza virus ha protein mab, antigenic epitope and application
ActiveCN112359020BStrong specificityHigh potencyImmunoglobulins against virusesTissue cultureEngineeringPepscan
The invention provides a hybridoma cell strain and belongs to the technical field of monoclonal antibody (MAb) preparation. The hybridoma cell strain of the invention can secrete the HA protein MAb of poultry-like H1N1 swine influenza virus, and the obtained MAb has the characteristics of good specificity, high potency and the like. The present invention uses the above-mentioned MAb, combined with peptide scanning technology, to screen and obtain a linear epitope located on the HA protein 216 VSVGSS 221 , this epitope is highly conserved among avian H1N1 viruses. The acquisition of MAb and antigenic epitope can be used to establish immunological diagnostic methods for avian-like H1N1 SIV or its antibody, such as ELISA, colloidal gold, etc. The acquisition of epitope information is helpful to improve the epitope information of H1N1 SIV HA protein, to better understand the structure and function of HA protein antigen, to predict the dynamics of virus antigenic variation, and to better prevent and control the epidemic. Theoretical and technical support.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of indole-3-acetonitrile in preparation of medicine for treating or preventing influenza virus infection
ActiveCN112353797BPrevent proliferationSuppressed expression levelOrganic active ingredientsAntiviralsBiomedicineSwine influenzavirus
The invention belongs to the field of biomedicine, and specifically discloses the application of indole-3-acetonitrile in the preparation of medicines for treating or preventing influenza virus infection. The applicant found that indole-3-acetonitrile can improve the weight loss caused by influenza virus infection, significantly inhibit the proliferation of influenza virus in mice, can improve the lung tissue lesions caused by influenza virus infection, and has obvious anti-influenza virus infection role. At the same time, indole-3-acetonitrile can inhibit the proliferation of a variety of influenza A viruses, including human influenza virus PR8, swine influenza virus G4, avian influenza virus H 5 N 6 , has a broad spectrum, and the synthesis process of indole-3-acetonitrile is simple, economical and fast, easy for large-scale production, and can be developed into an anti-influenza virus drug.
Owner:HUAZHONG AGRI UNIV
Mixed virus-like particles of swine influenza virus and foot and mouth disease virus, preparation method and application thereof
ActiveCN102370976BImprove immunityLong durationMicroorganism based processesAntiviralsAdjuvantFoot mouth disease virus
The invention provides a mixed virus-like particles, containing stromatin M1 of influenza virus, surface antigen erythrohemagglutinin HA of influenza virus and a nexus protein containing main antigenic epitope contained capsid protein of foot and mouth disease virus and a transmembrane zone and an inner membrane zone of the erythrohemagglutinin HA of influenza virus. The main antigenic epitope contained capsid protein of foot and mouth disease virus substitutes a basically same length outer membrane zone of a 5' terminal of the erythrohemagglutinin HA of influenza virus. Surface antigen erythrohemagglutinin HA of influenza virus and the main antigenic epitope contained capsid protein of foot and mouth disease virus are expressed simultaneously on surfaces of the mixed virus-like particles. The invention also provides a divalent vaccine of swine influenza and foot and mouth disease; and the vaccine contains the mixed virus-like particles and adjuvants. The invention also provides a method for preparing the mixed virus-like particles.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com