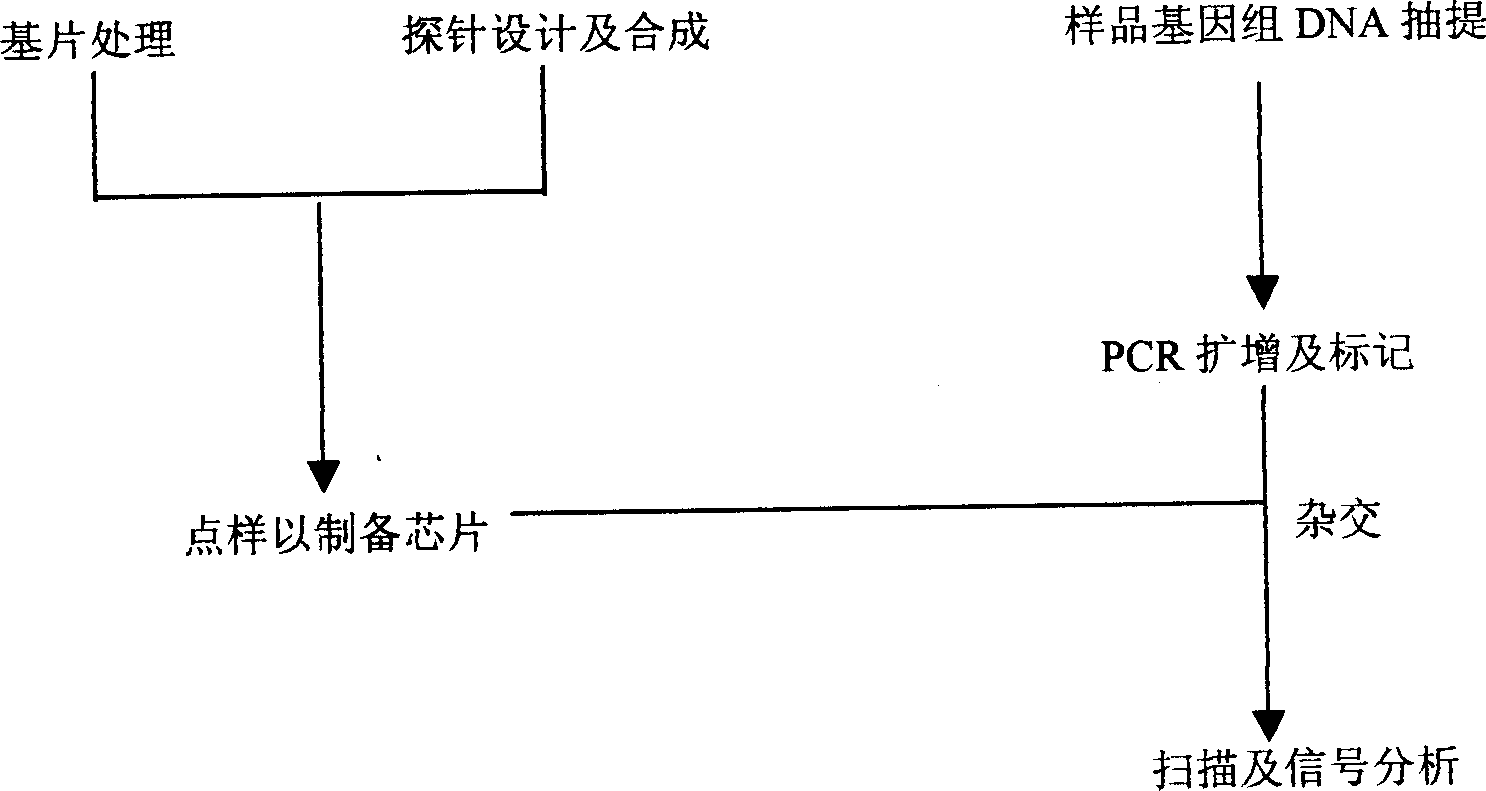
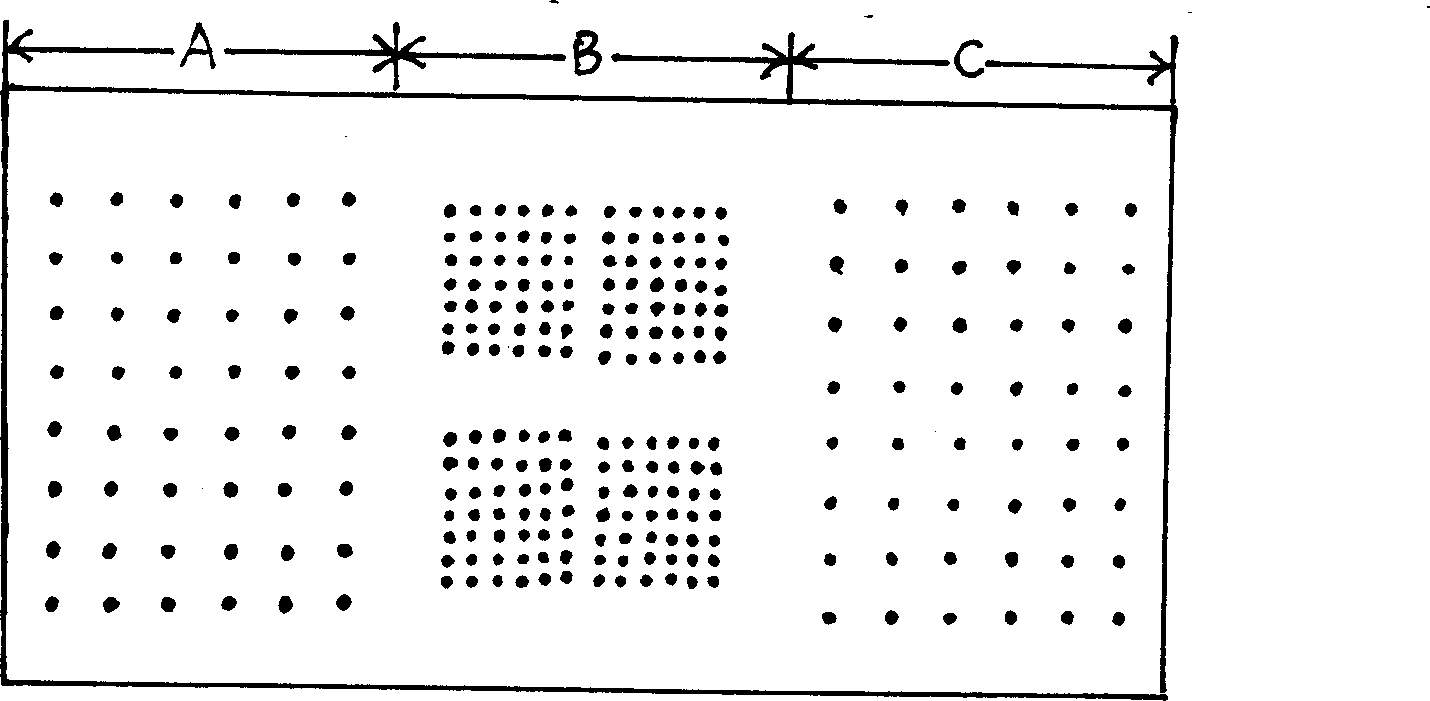
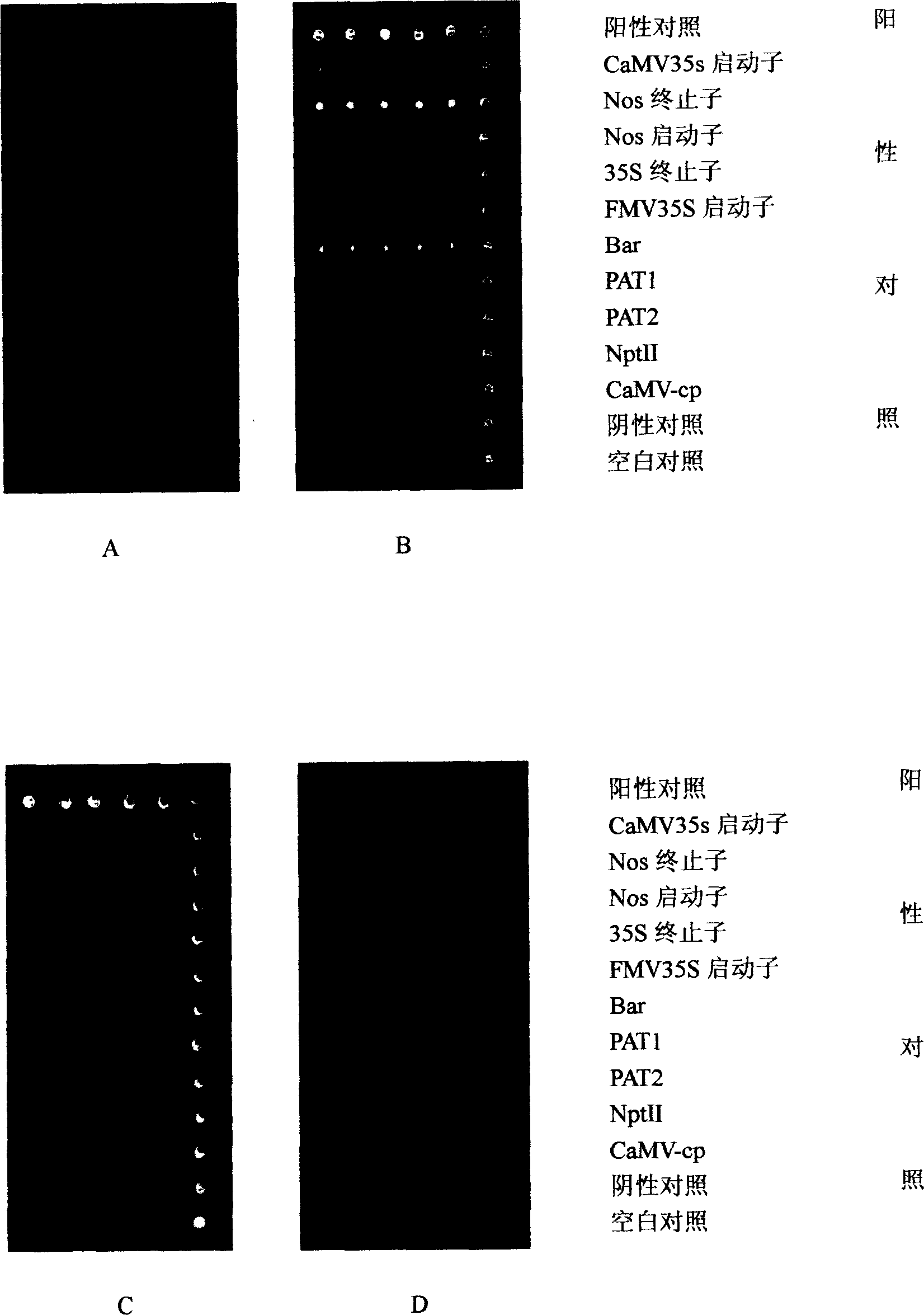
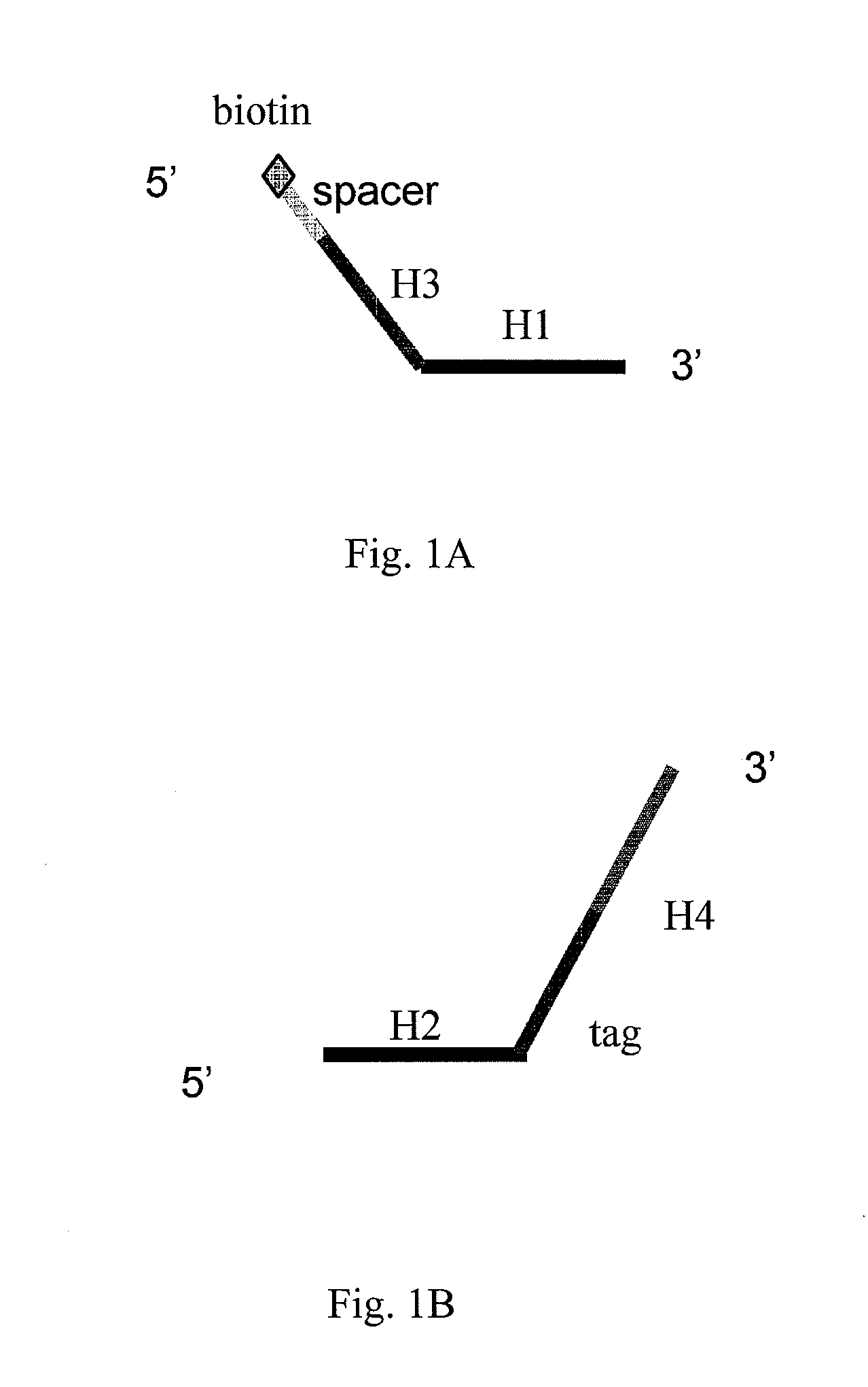
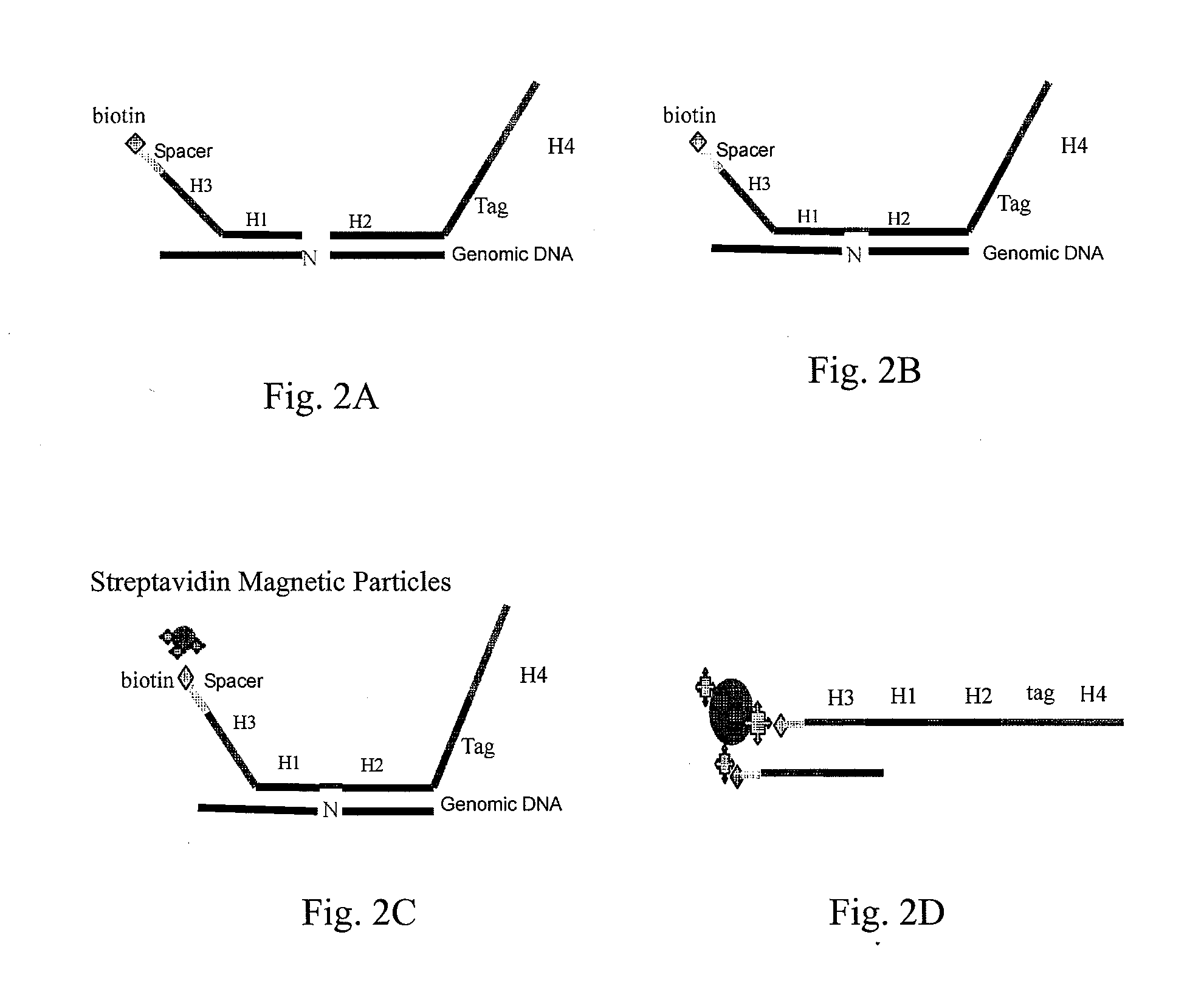
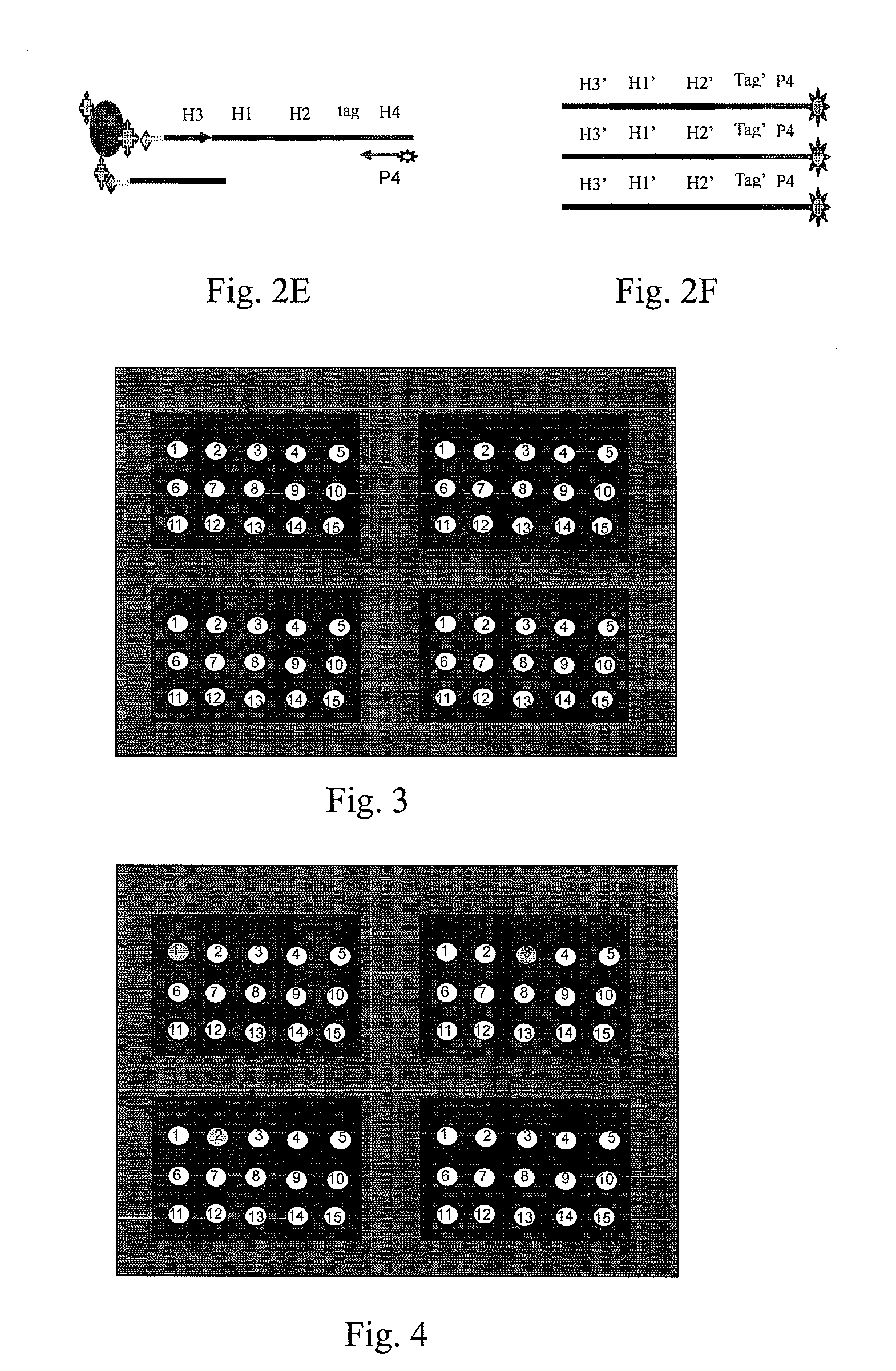
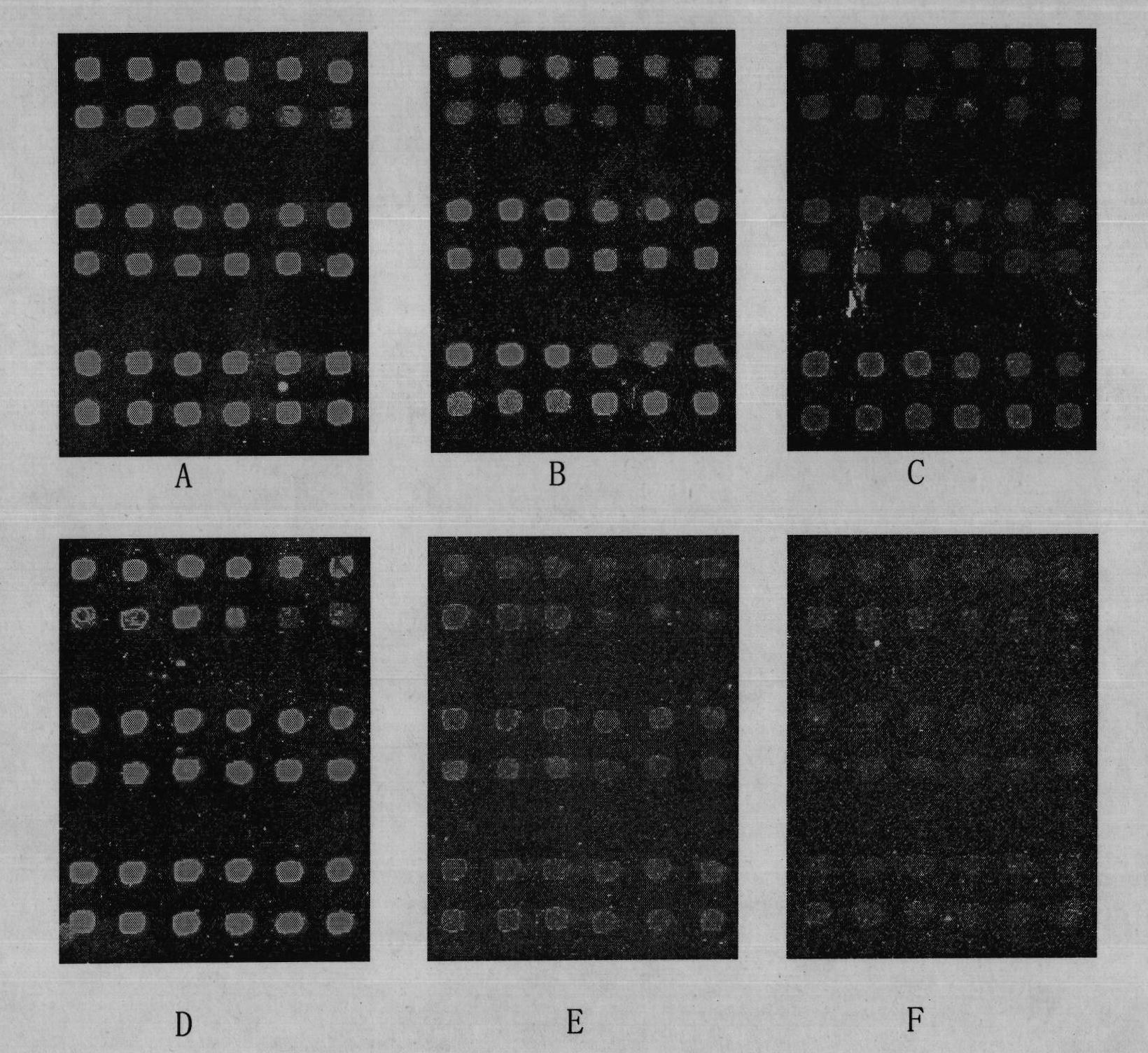
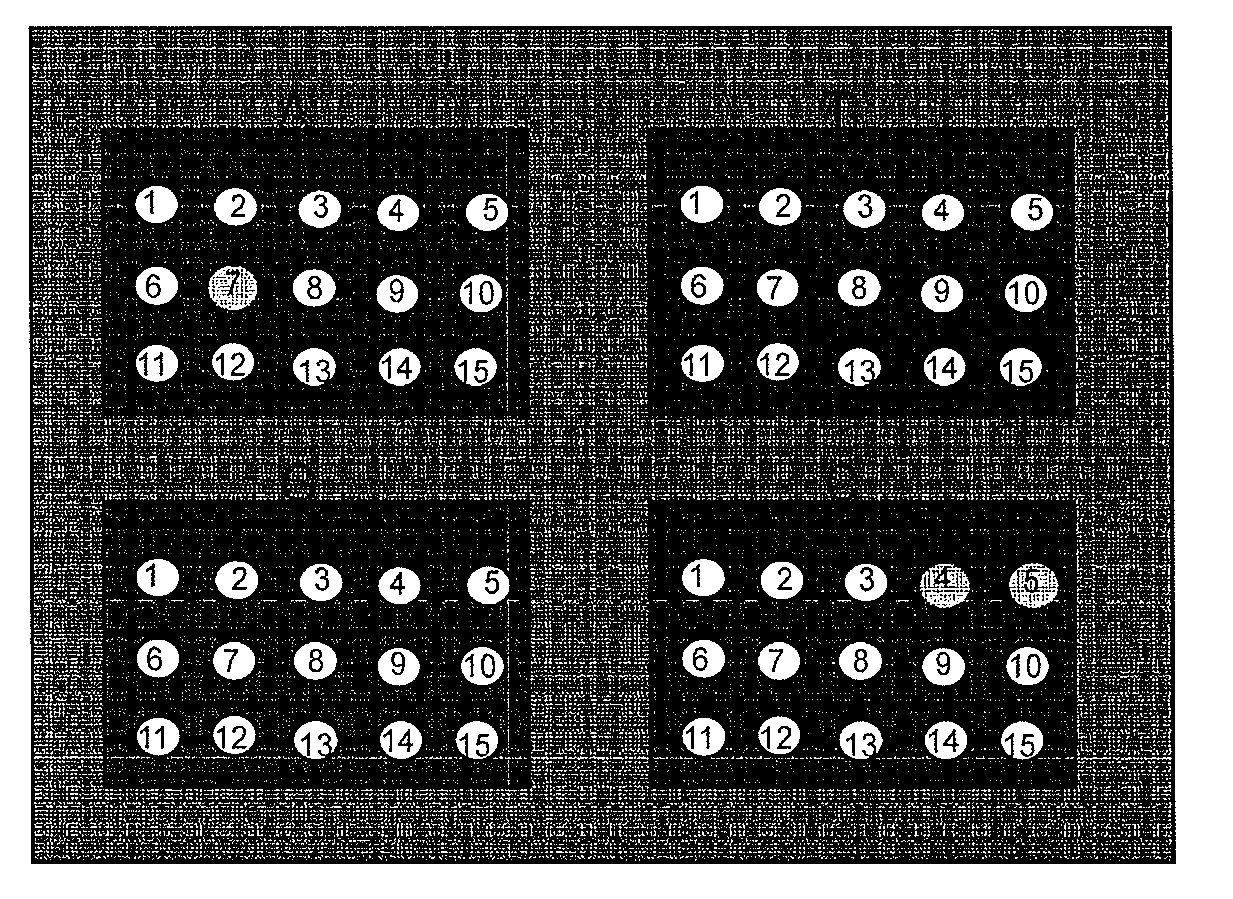
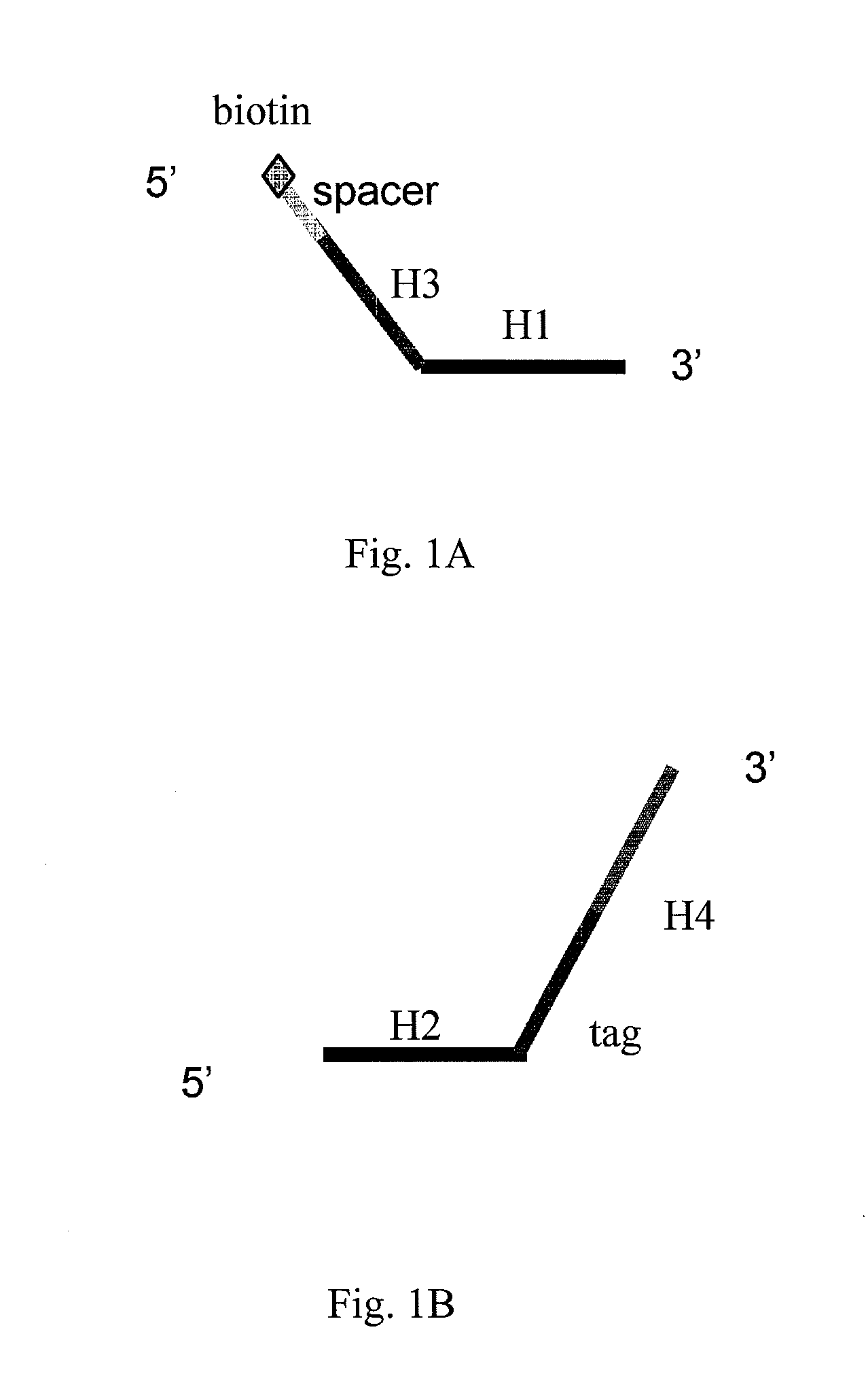
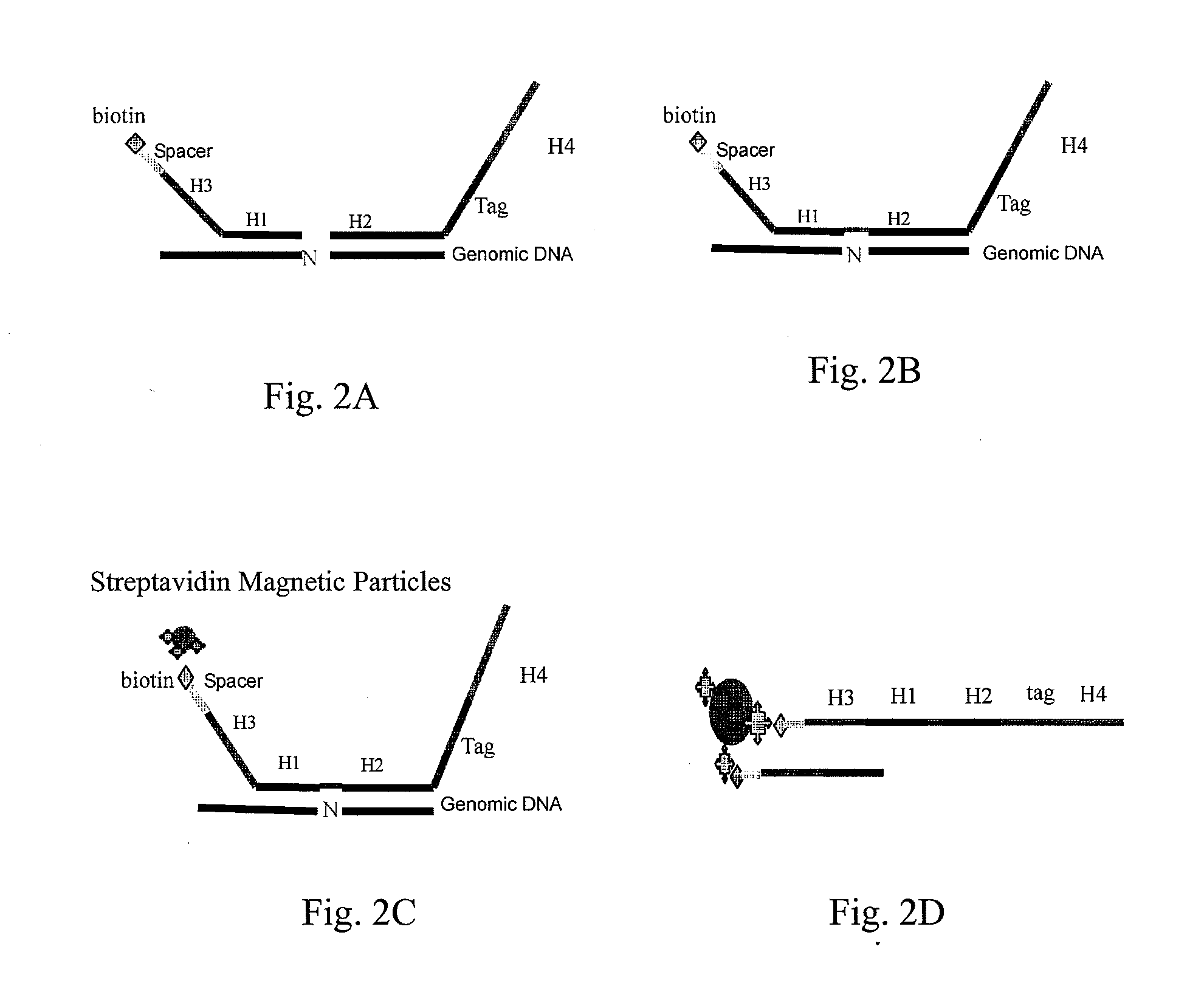
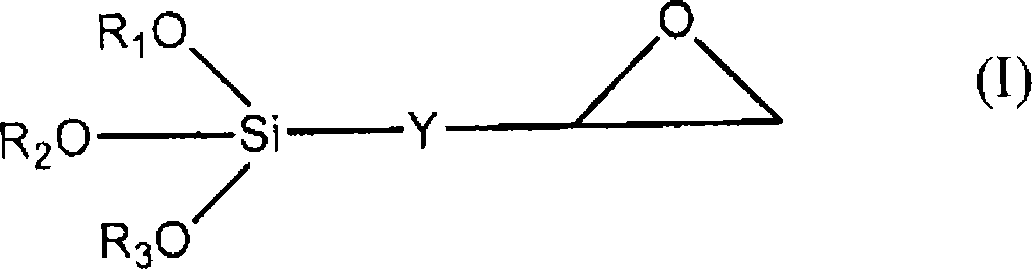Patents
Literature
51 results about "Oligonucleotide chip" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparing method for tr-gene products detecting oligonucleotides chip and use thereof
InactiveCN1584049AGuaranteed specificityTo achieve the purpose of mutual verificationMicrobiological testing/measurementHeterologousOligonucleotide chip
A method for preparing oligonucleotide chip for transgene product detection and use are disclosed. It includes: designing specific oligonucleotide probe and related primer by heterogeneric inserting gene, species internal standard gene, specific boundary sequence in transgene product gene set, fixing probe on glass slide to form transgene product detecting chip, amplifying DNA of plant to be tested by related primmer, marking by fluorescence, and hybridizing with chip. It can be use to acquire variable information.
Owner:国家质量监督检验检疫总局动植物检疫实验所 +2
Test probes, common oligonucleotide chips, nucleic acid detection method, and their uses
ActiveUS8501459B2Strong specificityHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsRe sequencingPathogenic microorganism
High-throughput detection for the interesting base or the mutation site in the nucleic acid sample can be achieved by means of the linear test probe pairs P1 and P2. The test probe pairs P1 and P2 respectively comprise either of the flanking complementary sequences which are adjacent to the interesting base or the mutation site in the nucleic acid sample. The invention can be applied to the re-sequencing the target nucleic acid sequence, the detection and analysis for the mutation, insertion, or deletion sites of a known nucleic acid sequence, and the genotyping of the pathogenic microorganism.
Owner:SHANXI LIFEGEN
High-flux fast detecting method for virus pathogen
InactiveCN101210270AImprove throughputQuick Troubleshooting and DetectionMicrobiological testing/measurementFluorescenceOligonucleotide chip
A high-throughput method for rapid detection of virus pathogens comprises the following steps of: (1) designing probes and spotting on a chip to obtain an oligonucleotide chip; (2) designing and synthesizing primers to obtain primers corresponding to a detected pathogen; (3) performing synchronous asymmetric PCR amplification to a detected sample with the primers corresponding to the detected pathogen to obtain a single-stranded DNA product specifically complementary with the oligonucleotide; (4) labeling a fluorescent substance on the single-stranded DNA product to obtain a fluorescent-labeled single-stranded DNA product; (5) hybridizing with the oligonucleotide chip, and cleaning the chip; and (6) scanning with a fluorescent scanner to obtain virus detection and analysis results. The invention solves the time-consuming, labor-consuming, low sensibility and specificity and inaccurate detection result problems in the detection method of virus causing porcine epidemic diseases in the prior art. The invention can be used for rapid, accurate and efficient detection of multiple genes.
Owner:SHANXI LIFEGEN
Preparation and application of adenovirus parting gene chip
InactiveCN105671212AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipEpidemiologic survey
The invention relates to preparation and application of a gene chip capable of detecting adenoviruses in a parting mode.A preparation method comprises the steps of preparing specific primers and probes for different types of adenoviruses, preparing oligonucleotides chips, establishing a plurality of PCR systems, and establishing a hybridization system.The gene chip prepared through the method can screen different types of adenoviruses including a 3-type adenovirus, a 7-type adenovirus, a 14-type adenovirus, an 11-type adenovirus and a 55-type adenovirus.The gene chip has the advantages of quickness, accuracy, high throughput and high specificity.A new detection means is provided for clinical diagnoses of different types of adenovirus infections and epidemiological surveys.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Preparation and application of rickettsia discrimination detection gene chip
InactiveCN104651498AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipAnaplasma phagocytophilum DNA
The invention relates to preparation and application of a rickettsia discrimination detection gene chip. The preparation method comprises the following steps: preparing universal and specific primers, preparing seven rickettsia nucleic acid typing probes, preparing an oligonucleotide chip, establishing a multiplex PCR (polymerase chain reaction) system, and establishing a hybridization system. The gene chip can simultaneously discriminate seven important rickettsiae, including rickettsia prowazeki, rickettsia mooseri, spotted fever group rickettsia, Coxiella burnetii, Orientia tsutsugamushi, Ehrlichia chaffeensis and Anaplasma phagocytophilum. The gene chip has the advantages of high speed, high accuracy, high flux and high specificity, and can provide a novel detection means for clinical diagnosis and epidemiological investigation of rickettsia infection.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Oligonucleotide chip capable of detecting five enteroviruses simultaneously and application thereof
InactiveCN101654713AStrong specificityHigh compliance rateNucleotide librariesMicrobiological testing/measurementFluorescenceOligonucleotide chip
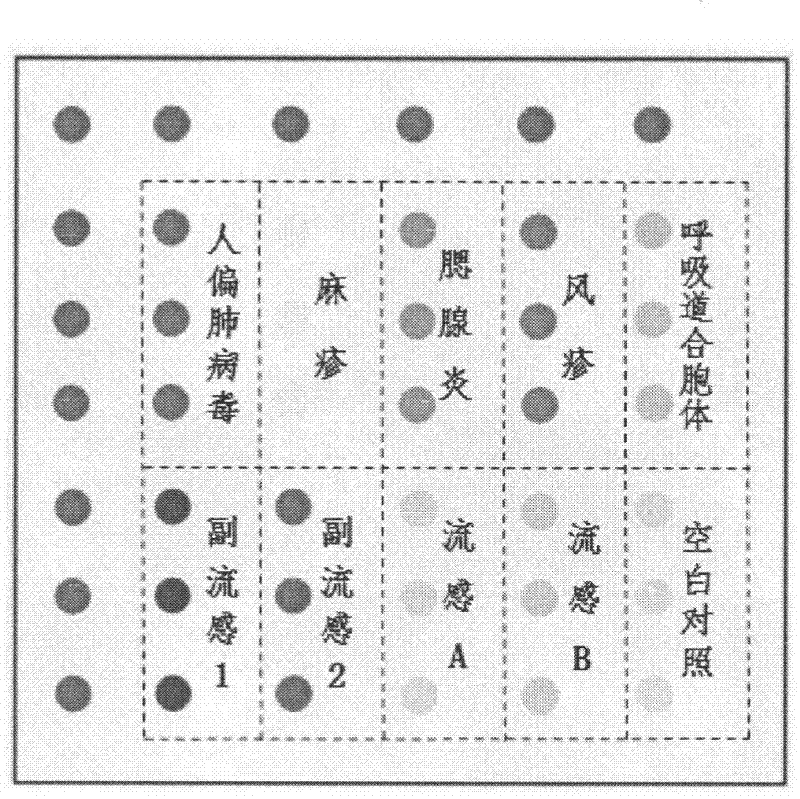
The invention discloses an oligonucleotide chip capable of detecting five enteroviruses simultaneously and an application thereof. The chip comprises a substrate, probes of pathogens of five enteroviruses coated on the substrate, negative contrast, positive contrast and blank contrast, wherein the probes of pathogens are five 54-70mer of HAV-P, ROV-P, NOV-P, ASV-P and ADV-PV1 probes. The chip of the invention adopts multiple PCR to amplify a plurality of target sequences of pathogens simultaneously and uses the downstream primer Tamara labeling method to perform fluorescence labeling to PCR product, the labeled PCR product is hybridized with the chip to realize the accurate detection of pathogens, the detection sensitivity is equal to that of PCR and the specificity is high. The detectionefficiency and accuracy are obviously shortened. The technology system of the invention is applicable to fields such as sea water and marine life specimens monitoring, food hygiene surveillance, customs quarantine, related clinical detection and the like.
Owner:SHANDONG MEDICAL BIO TECH RES CENT +1
Oligonucleotide chip and application thereof
InactiveCN101586149ASuitable for clinical testing applicationsStrong specificityMicrobiological testing/measurementPositive controlOligonucleotide chip
The invention discloses an oligonucleotide chip labeling a random primer PCR amplification based on a biotin-avidin-cy3 reaction system, which is made by a substrate, and CMV, HSVI, RV, EBV or TB pathogen probes, a negative control, a positive control and a blank control that are coated on the substrate in an array way, wherein the substrate is a surface aldehyde glass slide; the pathogen probes are 10 of CMV-1, CMV-2, HSVI-1, HSVI-2, RV1, RV2, EBV1, EBV2, TB1 and TB2 probes with 70mer; the positive control is a synthesized marker probe; and the blank control is double distilled water. The invention performs the biotin-11-dUTP amplified labeling to various pathogen target sequences by using the random primer and detects the pathogen by adopting a method of increasing the labeling efficiency through using a biotin-avidin-cy3 signal amplification system, has high detection sensitivity and obviously shortens the detection cost and the detection time. The technical system can be suitable to the fields of clinical detection, health supervision, customs quarantine control, scientific research, and the like.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Preparation method and application of gene chip for detecting important respiratory pathogenic viruses
InactiveCN102586475AStrong specificityImprove the consistency rateMicrobiological testing/measurementOligonucleotide chipMeasles virus IgG
The invention relates to a gene chip for detecting important respiratory pathogenic viruses. A preparation method of the gene chip comprises the following steps of: preparing a specific primer; preparing a virus specific probe; preparing an oligonucleotide chip; establishing an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; establishing a hybrid system; preparing a visual detection reagent; and establishing a developing method. The gene chip prepared by the invention can be used for simultaneously discriminating nine general respiratory pathogenic viruses comprising A and B type influenza viruses, parainfluenza viruses type 1 and 2, human metapneumovirus, respiratory syncytial virus, measles virus, rubella virus and mumps virus, provides a new solution for quickly detecting the general respiratory pathogenic viruses at high throughout and can provide guidance for monitoring, clinically diagnosing and treating the respiratory pathogenic viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
Preparation and application of DNA (deoxyribonucleic acid) chip for carbapenem antibiotic drug-resistant gene detection
InactiveCN104774941AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipA-DNA
The invention relates to preparation and application of a DNA (deoxyribonucleic acid) chip for carbapenem antibiotic drug-resistant gene detection. The preparation method comprises the following steps: preparing nine carbapenem drug-resistant gene specific primers, preparing nine carbapenem antibiotic drug-resistant gene specific probes, preparing an oligonucleotide chip, establishing a multiplex PCR (polymerase chain reaction) system, and establishing a hybrid system. The gene chip can simultaneously detect nine common carbapenem drug-resistant genes, including KPC, NDM-1, OXA-23, OXA-48, OXA-51, IMP, VIM, SIM and DIM. The DNA chip has the advantages of high speed, high accuracy, high flux and high specificity, and can provide a new detection means for drug-resistant gene confirmation and molecular epidemiological survey of carbapenem antibiotic drug-resistant phenotypes.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Space transcriptome database building and sequencing method and device adopted for same
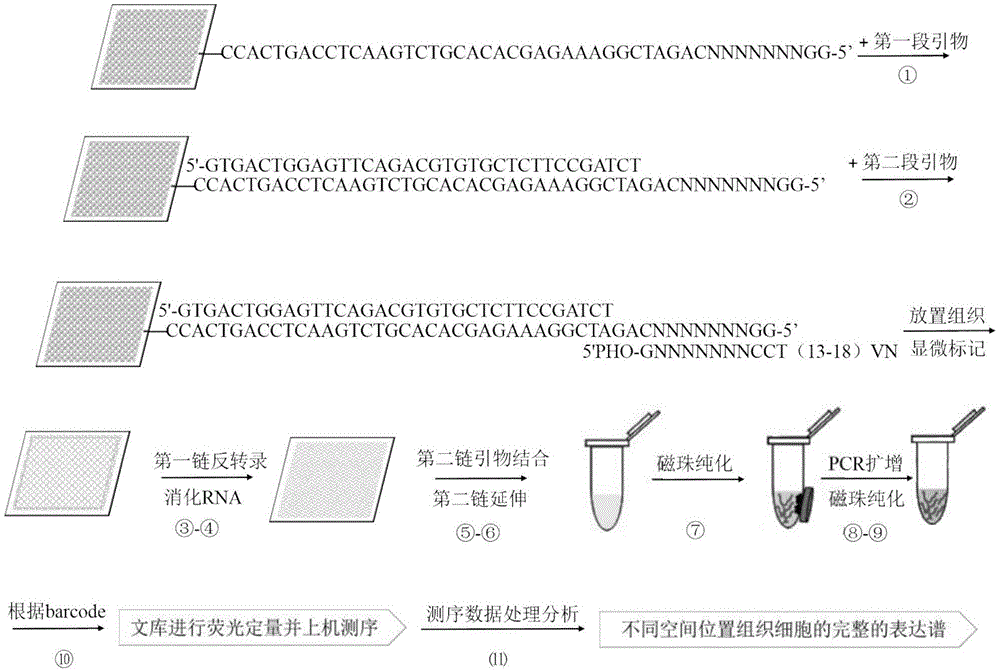
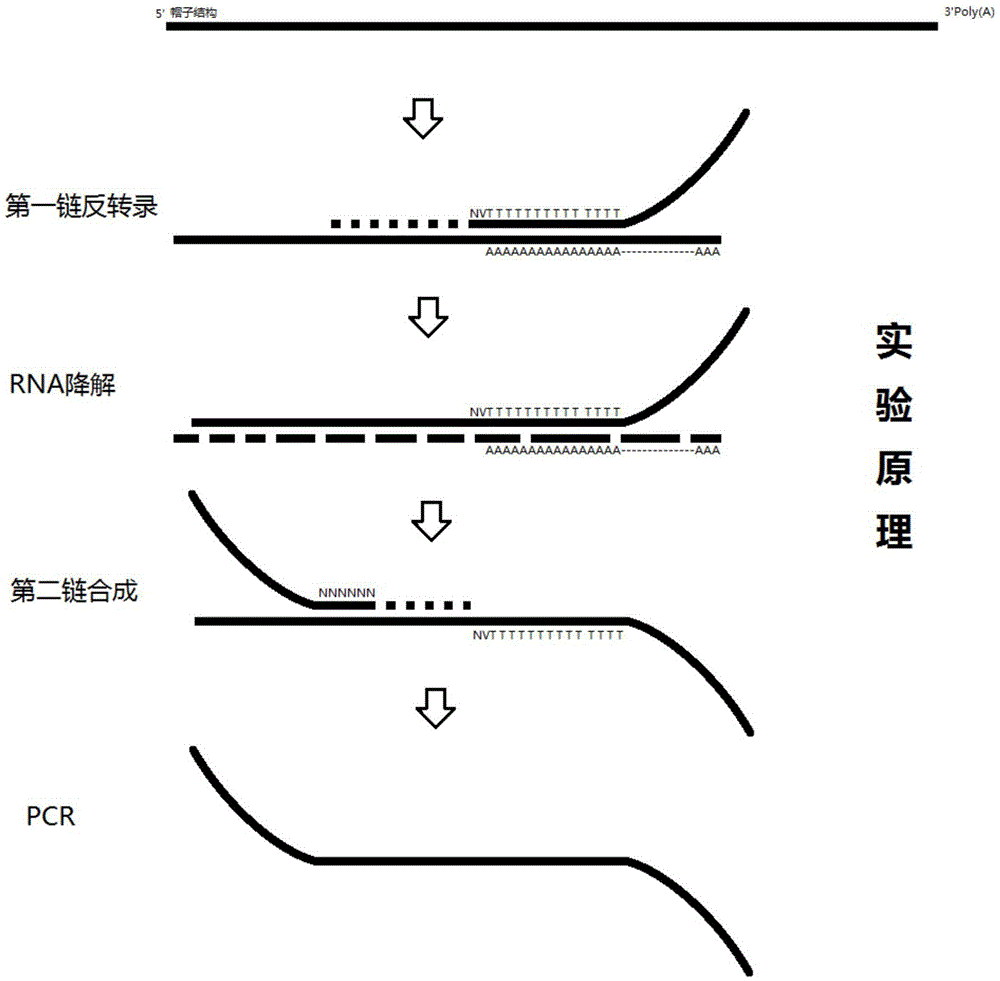
InactiveCN105505755AAchieve positioningImprove throughputBioreactor/fermenter combinationsBiological substance pretreatmentsOligonucleotide chipTranscriptome Sequencing
The invention discloses a space transcriptome sequencing technology device based on tissue and chip space position information. The device comprises a cover slip and a silicon-based oligonucleotide chip, wherein an anchoring probe is arranged on the silicon-based oligonucleotide chip; a gap between the cover slip and the silicon-based oligonucleotide chip is used for accommodating a tissue freezing section with the thickness of 10-50 um. Meanwhile, the invention further provides a space transcriptome sequencing technology method adopting the device. According to the device and the method, sufficient RNA (ribonucleic acid) samples can be effectively acquired from a small quantity of cells, uniform or overall characterization of a sequencing result is avoided, further, difference of different phenotypes of cell transcript information in tissue can be distinguished according to cell heterogeneity, and transcript information with low abundance is mined, so that that more accurate transcript information with high specificity is provided for fields of biology, medicine and agriculture.
Owner:HANGZHOU GUHE INFORMATION TECH CO LTD
Sol-gel process for the functionalisation of a surface of a solid substrate
InactiveCN101010133AReduce consumptionFast and uniform functionalizationMaterial nanotechnologyPeptide librariesOligonucleotide chipSolid substrate
The invention relates to a sol-gel process for the functionalisation of a surface of a solid substrate in order to provide said surface with chemical functions that can react with chemical or biological molecules, i.e. chemical functions that can immobilise, attach, graft biological or chemical molecules. The invention can be used, for example, in the preparation of chemical and biological sensors, such as for the production of chips comprising DNA, oligonucleotides, sugars, peptides and small organic molecules.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
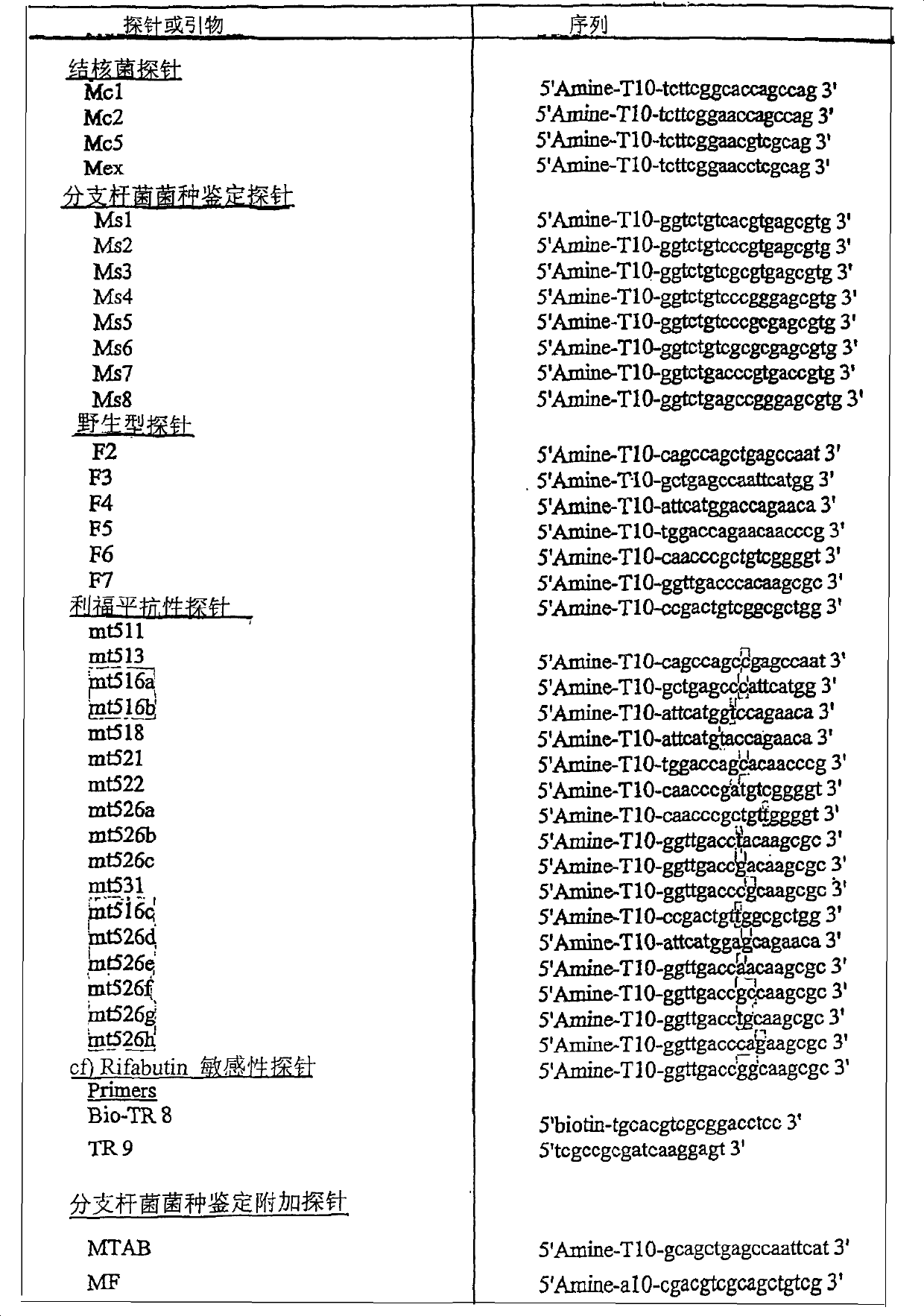
Diagnosis kit for mycobacterium species identification and drug-resistance detection and mfg. method thereof
InactiveCN1444662AEfficient amplificationBioreactor/fermenter combinationsBiological substance pretreatmentsHybridization probeOligonucleotide chip
The present invention relates to diagnosis kit for Mycobacterium species identification and drug-resistance detection and manufacturing method thereof, which can discriminate a Mycobacterium Tuberculosis rpoB gene point mutation relating to the Mycobacterium species identification and drug-resistance swiftly, exactly and in large quantities using an oligonucleotide chip. The diagnosis kit for Mycobacterium species identification and drug-resistance detection in accordance with the present invention consists of an oligonucleotide chip including a Mycobacterium tuberculosis complex probe, a Mycobacterium species identification probe and a drug-resistance detection probe of a Mycobacterium tuberculosis rpoB gene, and a fluorescent material containing a biotin-binding protein so as to detect hybridization of amplified products of a specimen marked as biotine and the corresponding probe.
Owner:BIOMEDLAB CORP
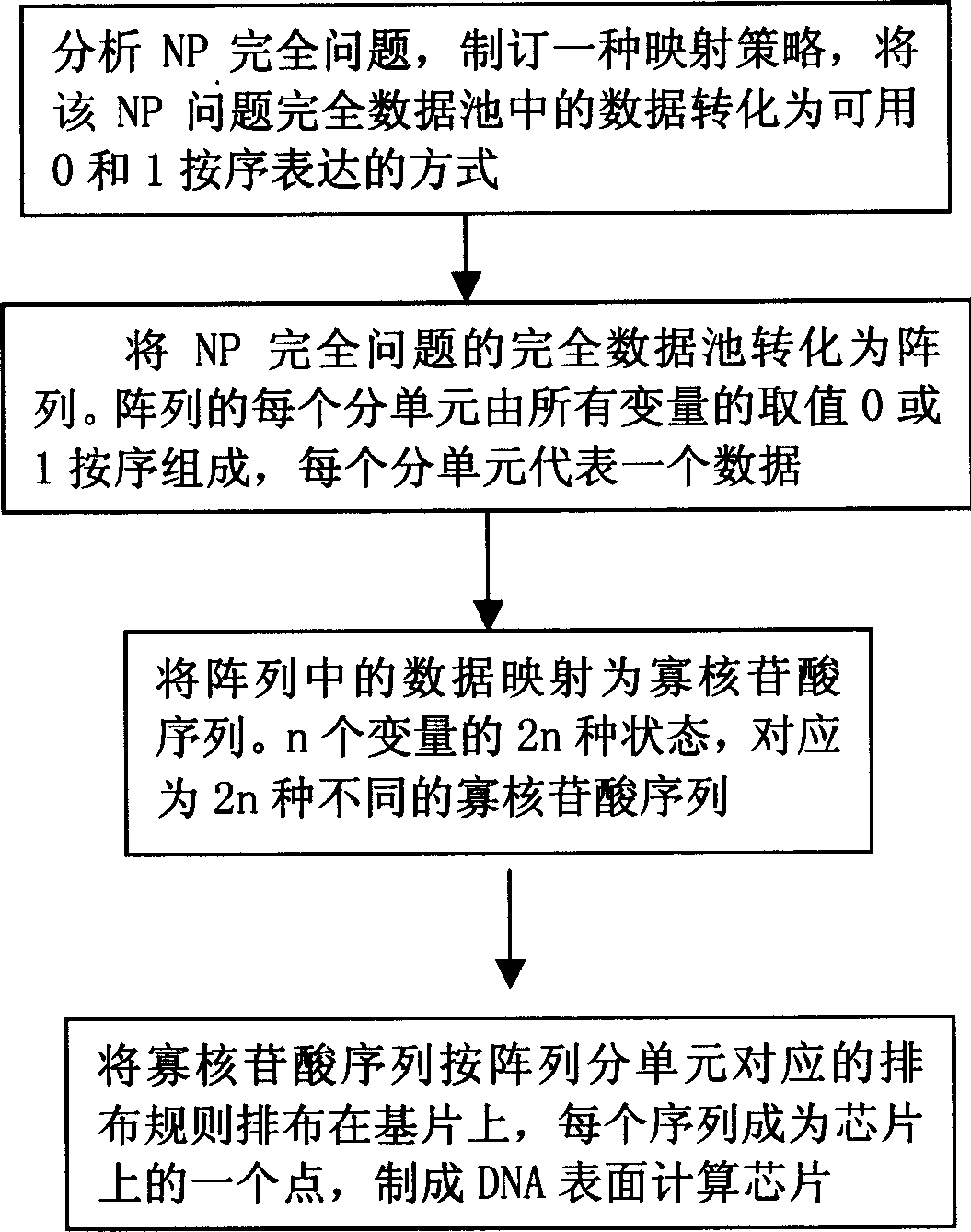
Method for preparing oligonucleotide chip in use for DNA surface computer in heterozygosis with computer
InactiveCN1661101AThe operation process is simple and convenientReduce false positive rateDigital data processing detailsMicrobiological testing/measurementOligonucleotide chipComputer science
A process for preparing oligonucleotide chip used in DNA surface calculation to solve the NP question is disclosed, which features that the coding and arranging of the oligonucleotide sequenes on DNA surface calculation chip are in such manner that the data in data pool is converted to the binary form, the data pool is converted to an array, and each data in the array is mapped to an oligonucleotide sequence, that is, a point on the chip.
Owner:SHANGHAI JIAO TONG UNIV
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
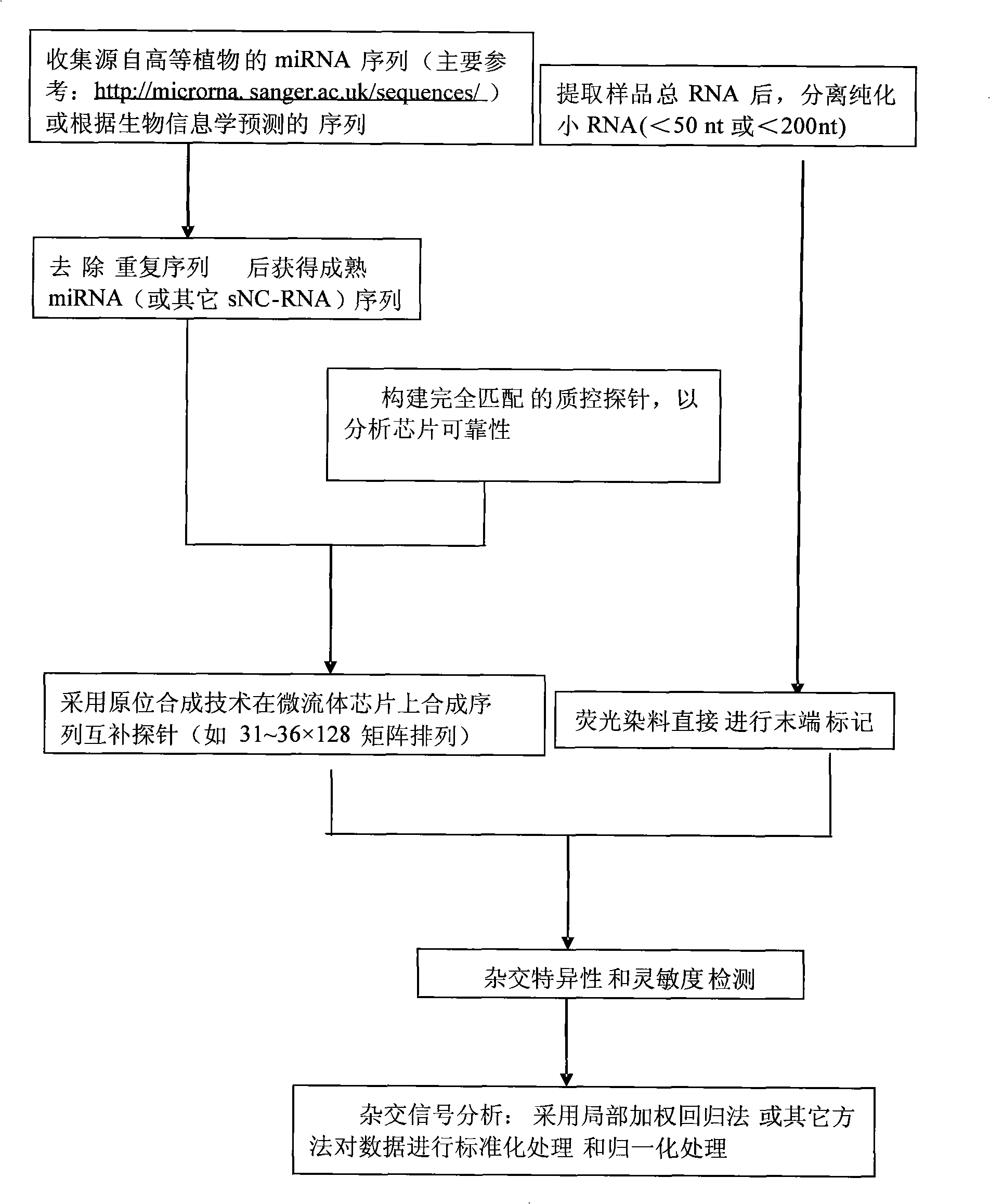
Detection process for synthesizing microfluid chip by in-situ method
InactiveCN101285100AHigh sensitivityImprove throughputMicrobiological testing/measurementOligonucleotide chipSense strand
The invention discloses a detection method synthesizing microfluidic chips in situ, which comprises the following steps that: 1) a sequence of non-coding small RNAs reported on the prior model plant is utilized, sequence information of miRNA is preferably selected, and complementary oligonucleotide hybridization probes are designed according to the sequence of a sense strand or an antisense strand of miRNA; 2) an oligonucleotide chip is prepared by an in-site synthetic method; 3) small RNAs smaller than a 50 oligonucleotide group are extracted from a plant to be detected, then are hybridized with the probes in the chip after making end labeling, and the result of chip hybridization is obtained to perform qualitative and quantitative analysis. The detection method can be used for the validation of miRNA (and other sNC-RNAs) obtained through the forecast by a bioinformatics method, can also be used for the detection and identification of miRNA (and other sNC-RNAs) in new plant species, and can also be used for the determination of miRNA (and other sNC-RNAs) variation relation under different treatment conditions (eg. under the condition of virus infection).
Owner:ZHEJIANG SCI-TECH UNIV
Oligonucleotide chip and its application of detecting muatatonal site of hepatitis B virus
InactiveCN1431317AShorten detection timeHigh strengthMicrobiological testing/measurementFluorescenceHepatitis B virus DNA
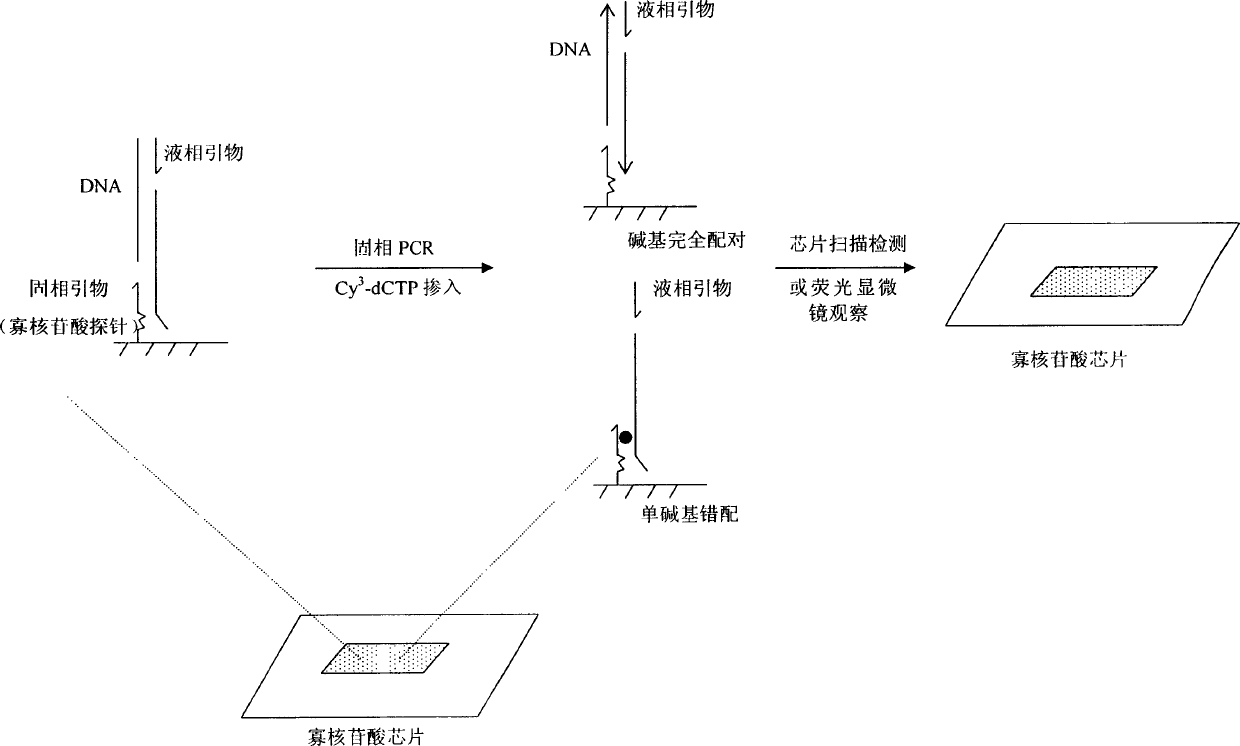
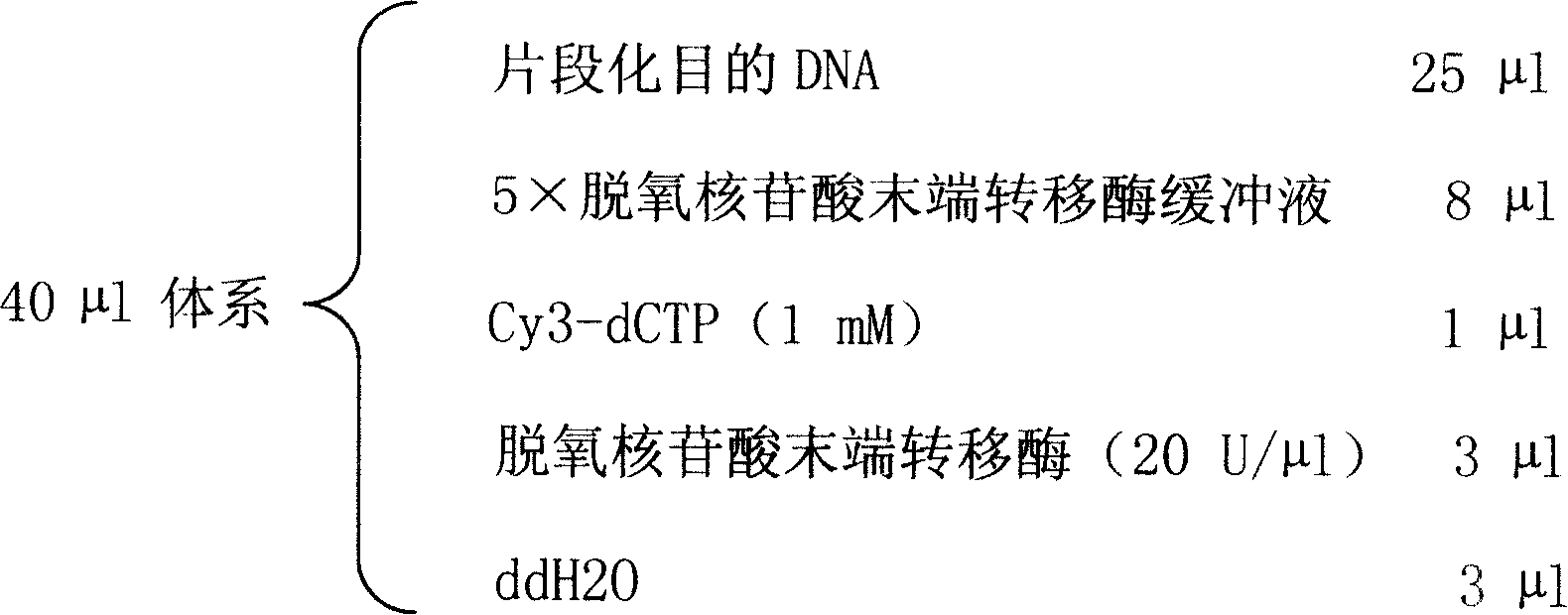
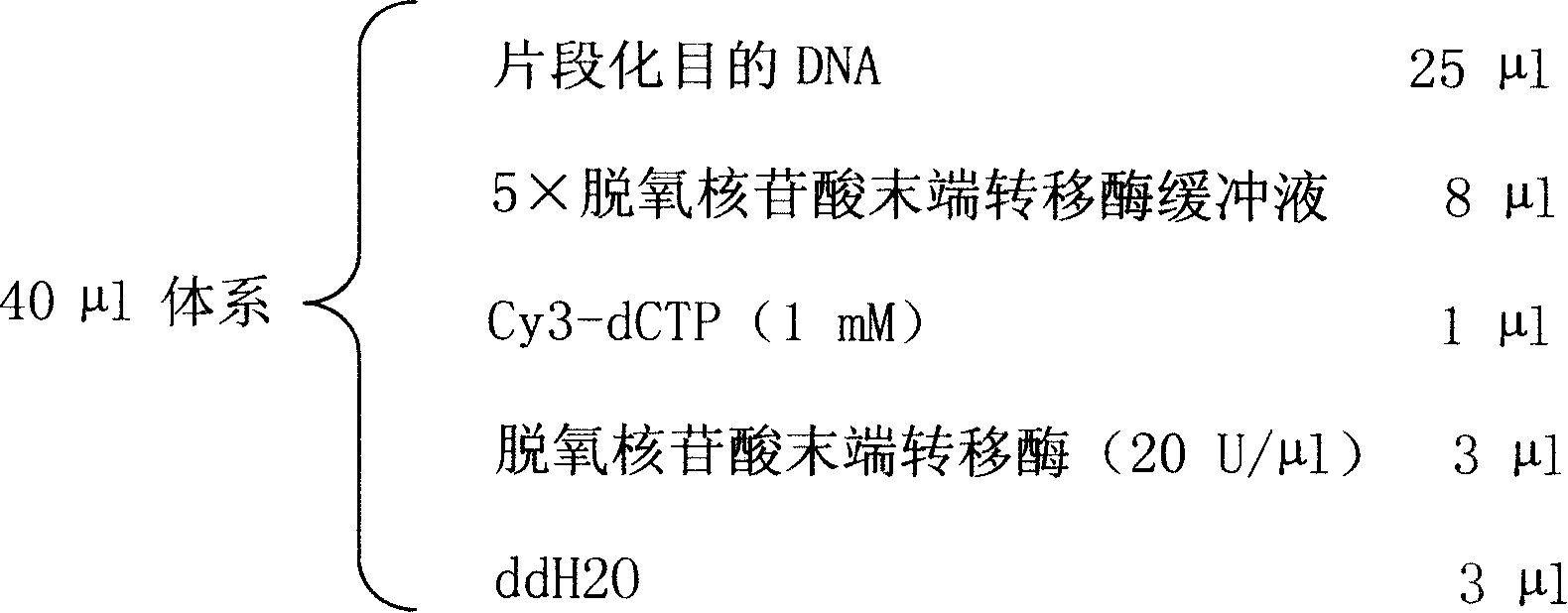
An oligonucleotide chip for detecting the mutation sites of hepatitis B virus is composed of glass substrate, oligonucleotide probes array and reference point-type coated laeyr. The said probes arrayhas 72 probes, including 12 known mutation sites and relative wild sites. The process for detcting mutation sites of hepatitis B virus includes such steps as mixing the probes as solid primer, hepatitis B virus DNA and template, and three pairs of liquid primers, PCR amplification via fluorescence labeled Cy3-dCTP, and analyzing mutation sites according to intensity of fluorescent signal. Its advantages are high speed, efficiency and correctness.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Preparation method and application of VRE/MRSA/KPC/NDM-1 detection gene chip
InactiveCN107130016AImprove throughputStrong specificityMicrobiological testing/measurementOligonucleotide chipAntibiotic Y
The invention relates to a preparation method and application of a VRE / MRSA / KPC / NDM-1 detection gene chip. The preparation method includes: preparing specific primers, preparing specific probes, preparing an oligonucleotide chip, and building a multiple PCR system and a hybrid system. By the prepared gene chip, 5 drug-resistant genes and 3 related pathogenic bacteria can be detected at the same time, and the 5 drug-resistant genes and 3 related pathogenic bacteria include Vancomycin-resistant Enterococcus VRE drug-resistant genes VanA and VanB, an encoding New Delhi metallo-beta-lactamase-1 NDM-1 drug-resistant gene, a methicillin-resistant staphylococcus aureus MRSA drug-resistant gene mecA, an encoding carbapenemase KPC gene, an enterococcus faecalis specific gene Fddl, an enterococcus faecium specific gene Sddl and a staphylococcus aureus specific gene nuc. The chip which provides a new detection manner for the confirmation of the drug-resistant genes of common antibiotics resistance phenotypes has advantages of quickness, accuracy, high flux and the like.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA +1
Beta-catenin oligonucleotide microchip and method for detecting beta-catenin mutations employing same
InactiveUS20050176016A1Fast and reliableBioreactor/fermenter combinationsBiological substance pretreatmentsBeta-cateninΒ catenin mutation
Owner:NAT CANCER CENT
Oligonucleotides chip composition for analyzing HCV gene type and its detecting method
InactiveCN1535319AMicrobiological testing/measurementRecombinant DNA-technologyHCV GenotypingOligonucleotide chip
The invention discloses a method for analyzing HCV genotype by extracting RNA from plasma and serum, carrying out RT-PCR of hepatitis C virus (HCV) and reacting it on an oligonucleotide chip. The present invention also provides a simple and accurate method for assaying 4 human HCV genotypes on one slide.
Owner:株式会社生物核心
Preparation and application of guiding gene chip for HCV infection individual treatment
InactiveCN104357584AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementHybrid systemOligonucleotide chip
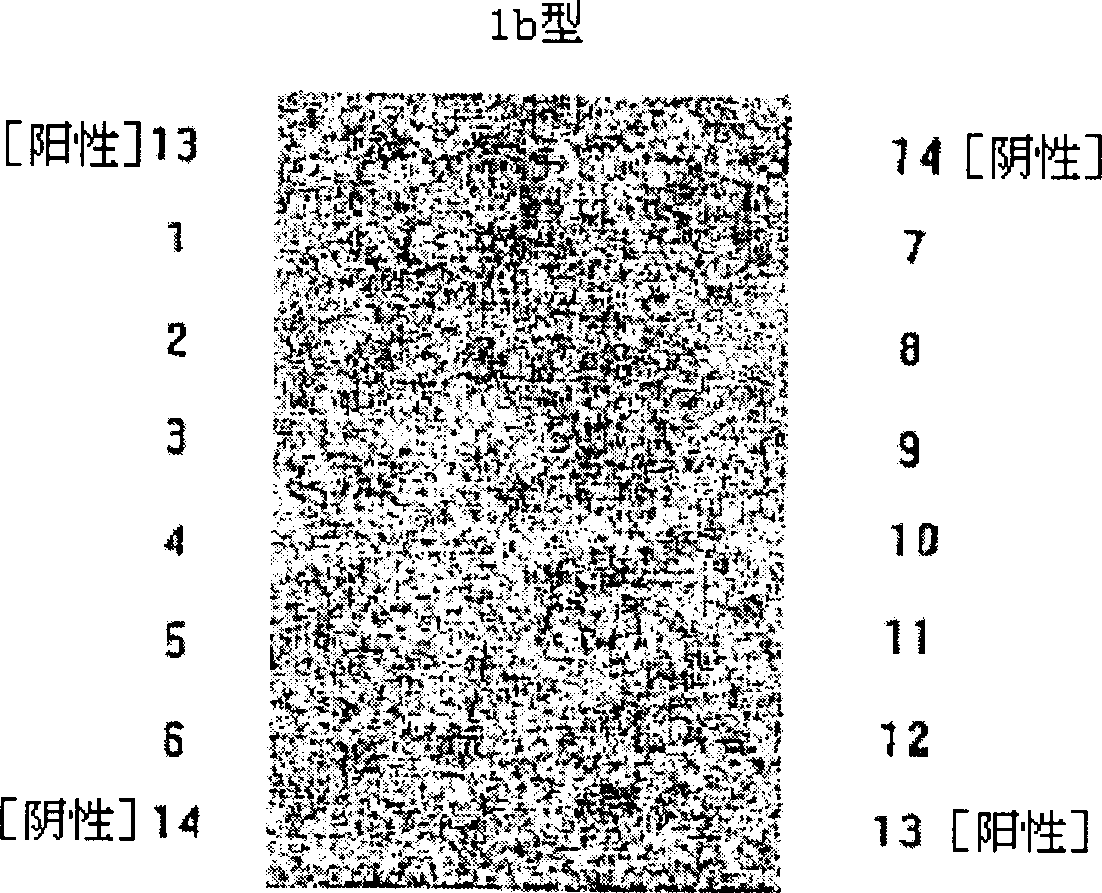
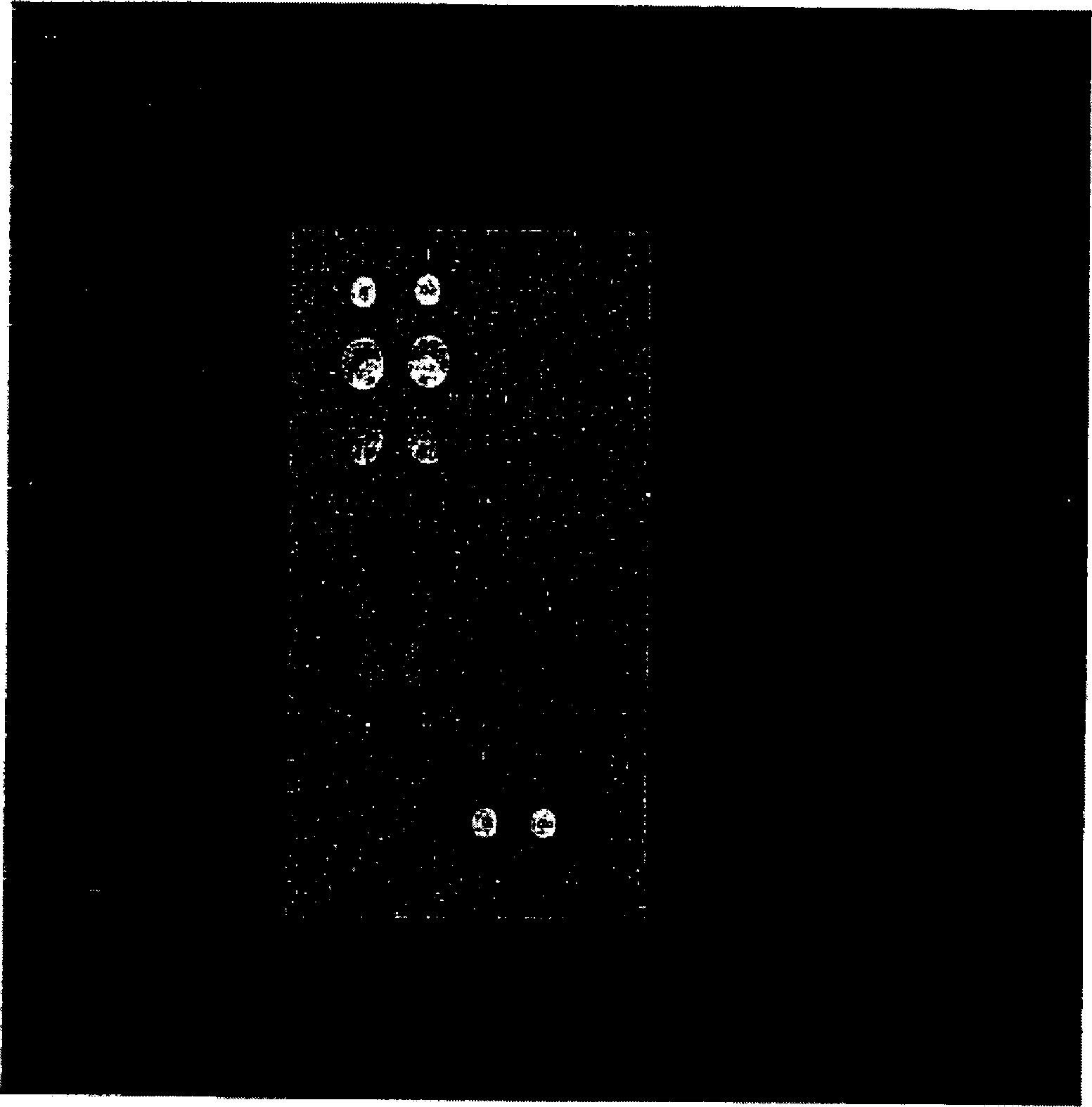
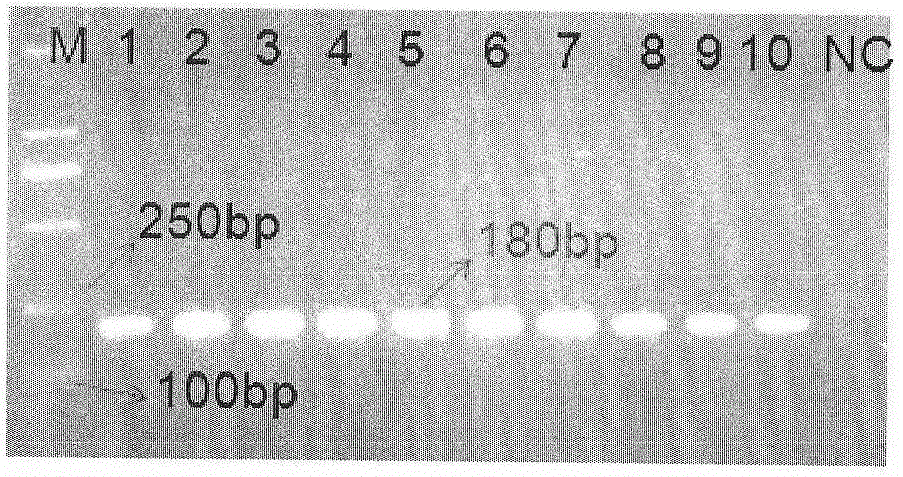
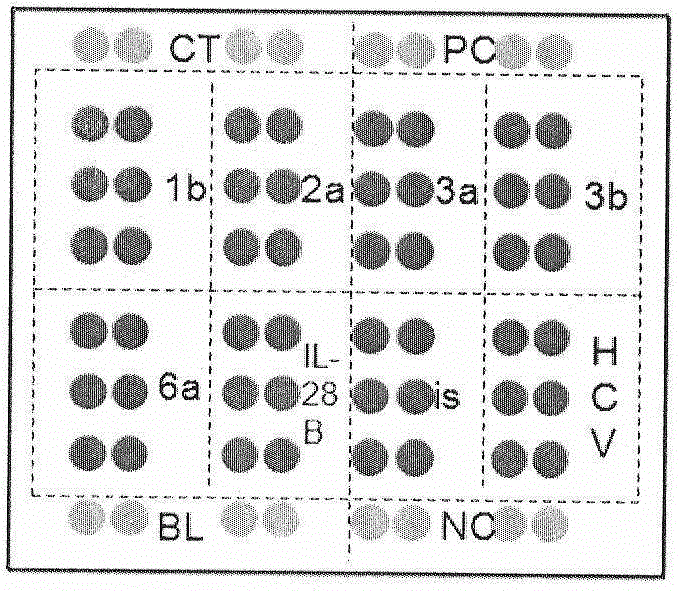
The invention relates to a guiding gene chip for HCV infection individual treatment. The preparation method of the guiding gene chip comprises the following steps: preparing a universal primer; preparing an HCV hypotype nucleic acid parting probe and a human IL-28B rs12979860 investigation of polymorphism probe; preparing an oligonucleotide chip; building a polyfunctional RT-PCR system; building a hybrid system. The gene chip prepared by the method can discriminate five hypotypes of 1b, 2a, 3a, 3b and 6a of HCV at the same time, can detect polymorphism of CC, TT and CT three genes of human rs12979860, has the advantages that the gene chip is quick, accurate, and high in flux and specificity, and can provide guiding for HCV clinical diagnosis and individual treatment.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Oligonucleotide chip for detecting complete genome CpG island and uses thereof
InactiveCN101205559AImprove throughputMicrobiological testing/measurementFluorescence/phosphorescenceNucleotideOligonucleotide chip
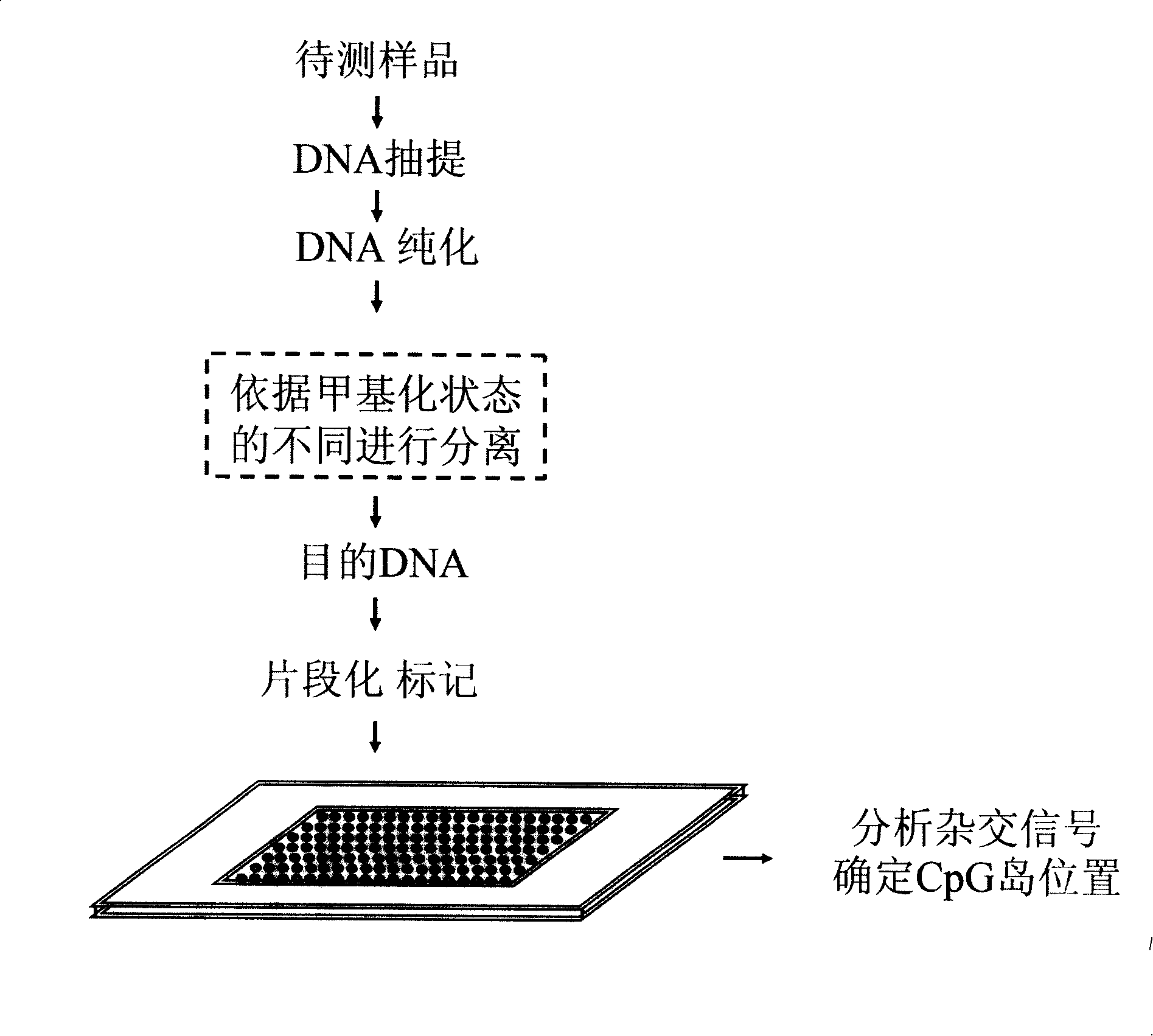
The invention relates to a gene chip, and discloses an oligonucleotide chip for detecting a whole genome CpG island. The invention consists of solid-phase carriers and probes, wherein, the probes are hybridized with nucleotide sequences and / or complementary sequences thereof of the CpG island in the whole genome and 2kb areas of the upstream and downstream of the CpG island. The invention also discloses a method of detecting the position of the whole genome CpG island with the oligonucleotide chip. The oligonucleotide chip of the invention can carry out the CpG island detection of methylate and / or methylate DNA rapidly and accurately with high flux, and provides a powerful tool for correlation studies on the whole genome CpG island.
Owner:SHANGHAI BIOCHIP
Method for designing oligonucleotide chip probe and HIV oligonucleotide probe and quality control method thereof
InactiveCN101979537AIncreased sensitivityImprove efficiencyMicrobiological testing/measurementDNA preparationFluorescenceOligonucleotide chip
The invention belongs to the technical field of biology, and relates to a detection method for nucleic acid, in particular to a method for designing an oligonucleotide probe. The method for designing the oligonucleotide probe comprises the following steps of: (1) designing a target gene oligonucleotide probe with length of 50mer aiming at target genes; (2) designing general sequences, the homologies of which are not more than 40 percent compared with the target genes and the lengths of which are 10mer; and (3) connecting the general sequences at two ends of the target gene oligonucleotide probe to construct the oligonucleotide probe respectively. The sequence of the human immunodeficiency virus oligonucleotide probe constructed by adopting the design method of the invention is SEQ ID No: 1. Fluorescence labeled complementary sequences are obtained by fluorescence labeling of the complementary sequences of the obtained general sequences. The fluorescence labeled complementary sequences can be used for detecting the quality of an oligonucleotide chip, have the advantage of high sensitivity, and are particularly suitable for detection chip design of clinical pathogen genes.
Owner:SOUTHERN MEDICAL UNIVERSITY
Test probes, common oligonucleotide chips, nucleic acid detection method, and their uses
ActiveUS20110218115A1Strong specificityHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsOligonucleotide chipDna polymerasen
High-throughput detection for the interesting base or the mutation site in the nucleic acid sample can be achieved by the linear test probe pairs P1 and P2. The test probe pairs P1 and P2 respectively comprise either of the flanking complementary sequences which are adjacent to the interesting base or the mutation site in the nucleic acid sample. When the test probe pairs P1, P2 are annealed and hybridized to the nucleic acid sample, a gap will be generated at the interesting base or the mutation site position between the probe pairs and the sample. Divide the annealed hybrid sample into four equal reaction systems to which add dATP, dTTP, dCTP, dGTP, respectively. The test probe pairs P1 and P2 will be ligated into one single probe when adding the complementary nucleotide system under the DNA polymerase or ligase. After purified and amplified, the generated single probes are hybridized to the corresponding area in a common oligonucleotide microarray. The generated single probe will give a signal in the hybrid area, and therefore detect and analyze the hybrid signal to determine the base type or the mutation genotype at the detection position. The invention can be applied to the re-sequencing the target nucleic acid sequence, the detection and analysis for the mutation, insertion, or deletion sites of a known nucleic acid sequence, and the genotyping of the pathogenic microorganism.
Owner:SHANXI LIFEGEN
Diagnosis kit for mycobacterium species identification and drug-resistance detection and mfg. method thereof
InactiveCN1193103CBioreactor/fermenter combinationsBiological substance pretreatmentsOligonucleotide chipRpoB
The present invention relates to diagnosis kit for Mycobacterium species identification and drug-resistance detection and manufacturing method thereof, which can discriminate a Mycobacterium Tuberculosis rpoB gene point mutation relating to the Mycobacterium species identification and drug-resistance swiftly, exactly and in large quantities using an oligonucleotide chip. The diagnosis kit for Mycobacterium species identification and drug-resistance detection in accordance with the present invention consists of an olignucleotide chip including a Mycobacterium tuberculosis Mycobacterium species identification probe and a drug-resistance detection probe of a complex probe, a Mycobacterium tuberculosis rpoB gene, and a fluorescent material containing a biotin-binding protein so as to detect hybridization of amplified products of a speciment marked as biotine and the corresponding probe.
Owner:BIOMEDLAB CORP
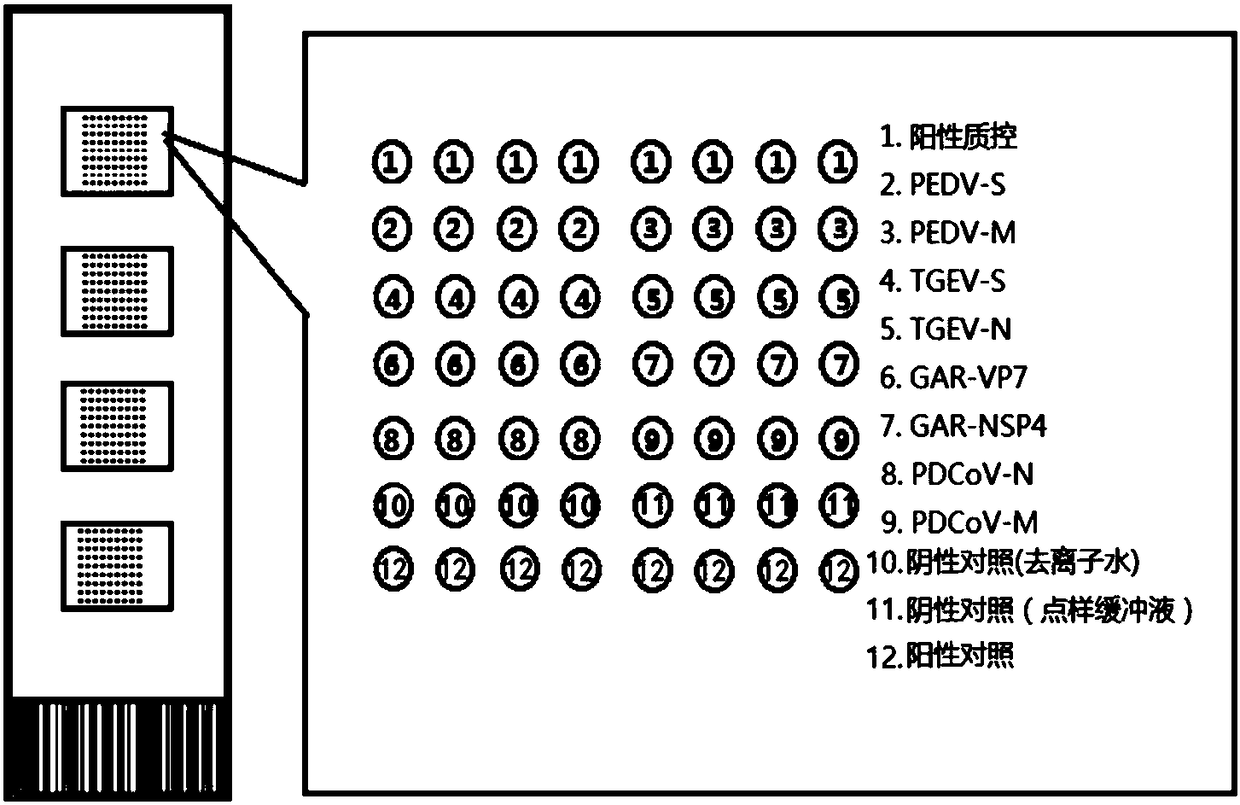
Oligonucleotide chip for synchronous detection of four porcine diarrhea viruses, and application of oligonucleotide chip
ActiveCN108531648ANucleotide librariesMicrobiological testing/measurementRotavirus RNAOligonucleotide chip
The invention provides an oligonucleotide chip for synchronous detection of four porcine diarrhea viruses. The oligonucleotide chip comprises a solid phase carrier and oligonucleotide probes fixed onthe solid phase carrier, wherein the oligonucleotide probes comprise a probe shown in SEQ ID No. 1-2, a probe shown in SEQ ID No. 3-4, a probe shown in SEQ ID No. 5-6, and a probe shown in SEQ ID No.7-8; the probes are respectively used for detecting porcine epidemic diarrhea virus (PEDV), porcine transmissible gastroenteritis virus (TGEV), porcine group A rotavirus (GAR) and porcine delta coronavirus (PDCoV). The invention also provides a kit and a method for the synchronous detection of the four porcine diarrhea viruses. The chip and the method can realize the high flux co-detection of thefour porcine diarrhea viruses, i.e., PEDV, TGEV, GAR and PDCoV, and can be applied to mass detection of clinical diseases, thus having a wide application prospect.
Owner:SICHUAN AGRI UNIV
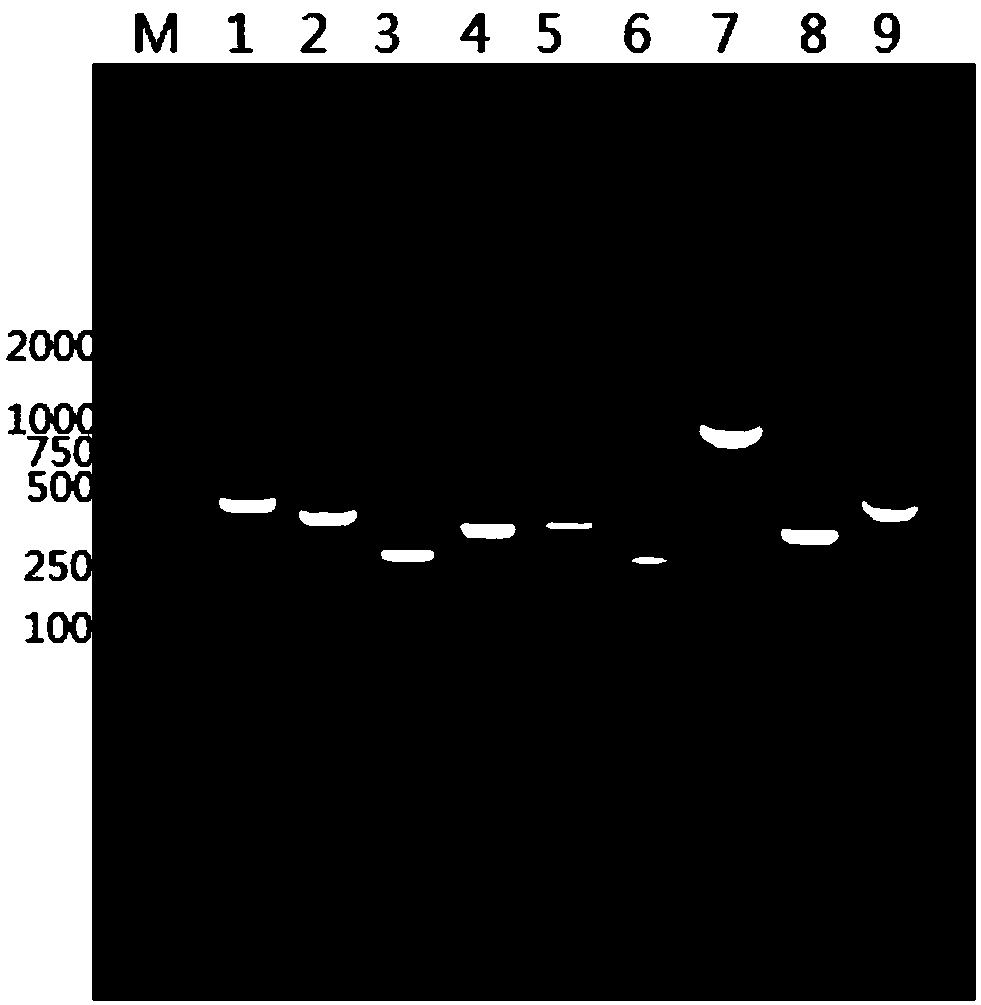
Method for detecting transgenic tomato by using in-situ synthetic microfluidic chip
InactiveCN103290128AMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotide chipFluorescence
The invention discloses a method for detecting a transgenic tomato by using an in-situ synthetic microfluidic chip. The method comprises the following steps of: 1) designing a chip probe for detecting an exogenous gene of the transgenic tomato; 2) preparing an oligonucleotide chip; 3) extracting genome deoxyribonucleic acid (DNA) in a tomato sample to be detected; 4) marking a specific segment of the genome or the entire genome by a fluorescent agent; 5) chip hybridization; 6) judging whether the sample belongs to the transgenic tomato variety. By adopting the method, an oligonucleotide probe is designed according to the sequence information of the known tomato exogenous gene which has been publically reported; the microfluidic gene chip is prepared by utilizing an in-situ synthesis method, so as to achieve simultaneous detection of existence of the exogenous genes related to insect resistance, antiviral action, herbicide resistance, delayed fruit, softening resistance, storage resistance and the like on the same chip. Thus, an effective method is provided for rapid detection and identification of the transgenic ingredients of the tomato and highly processed products; a foundation is provided for building and perfecting a transgenosis detection system and a supervisory system.
Owner:ZHEJIANG SCI-TECH UNIV
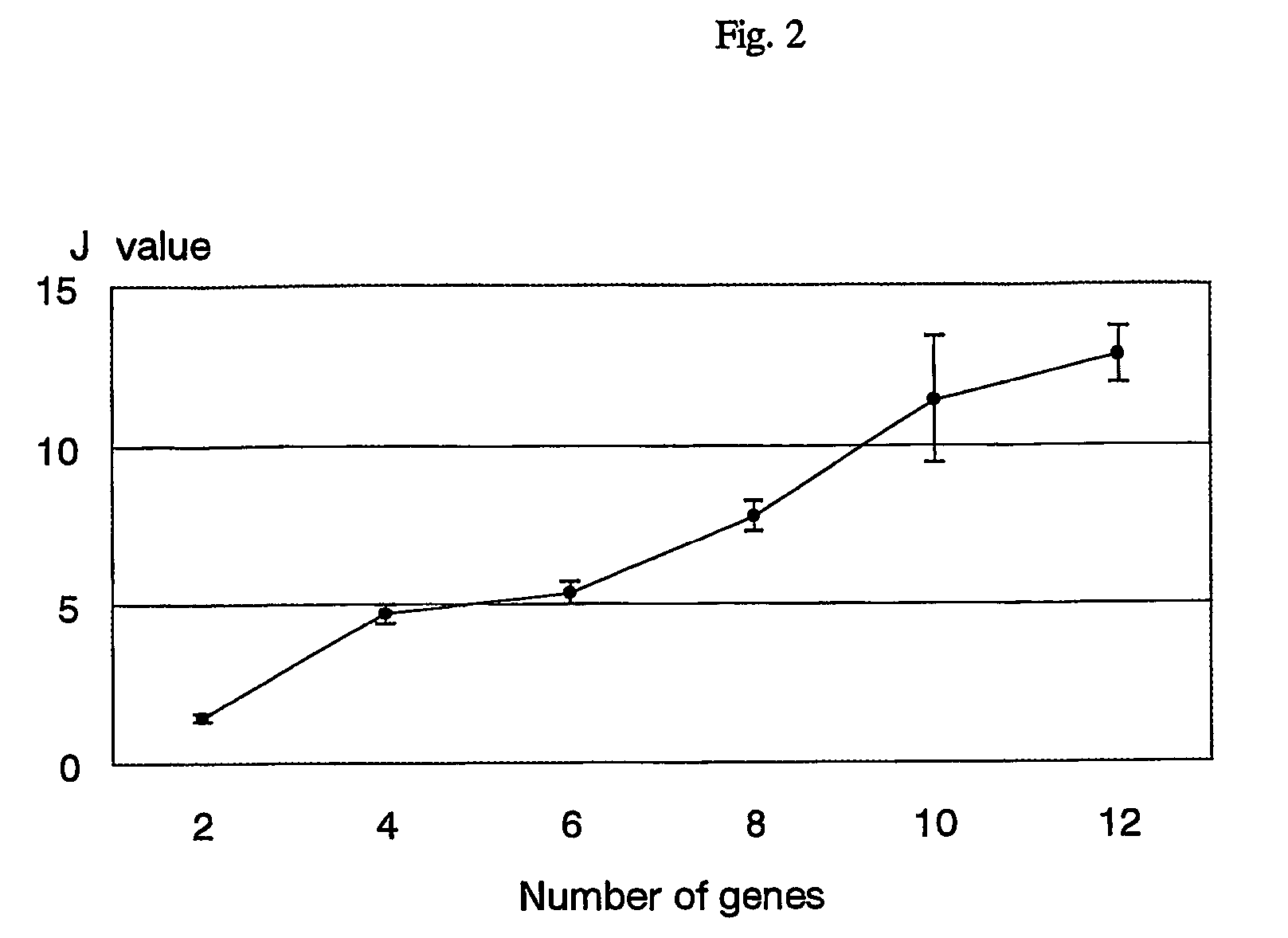
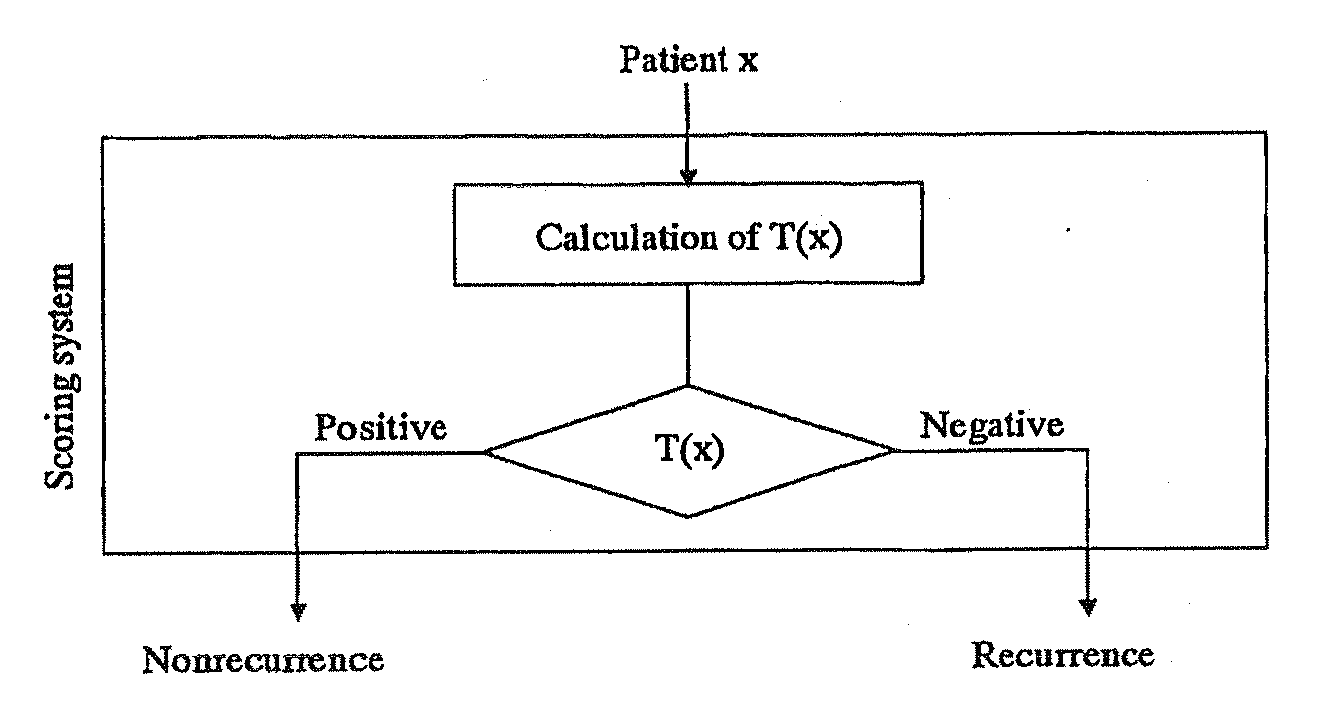
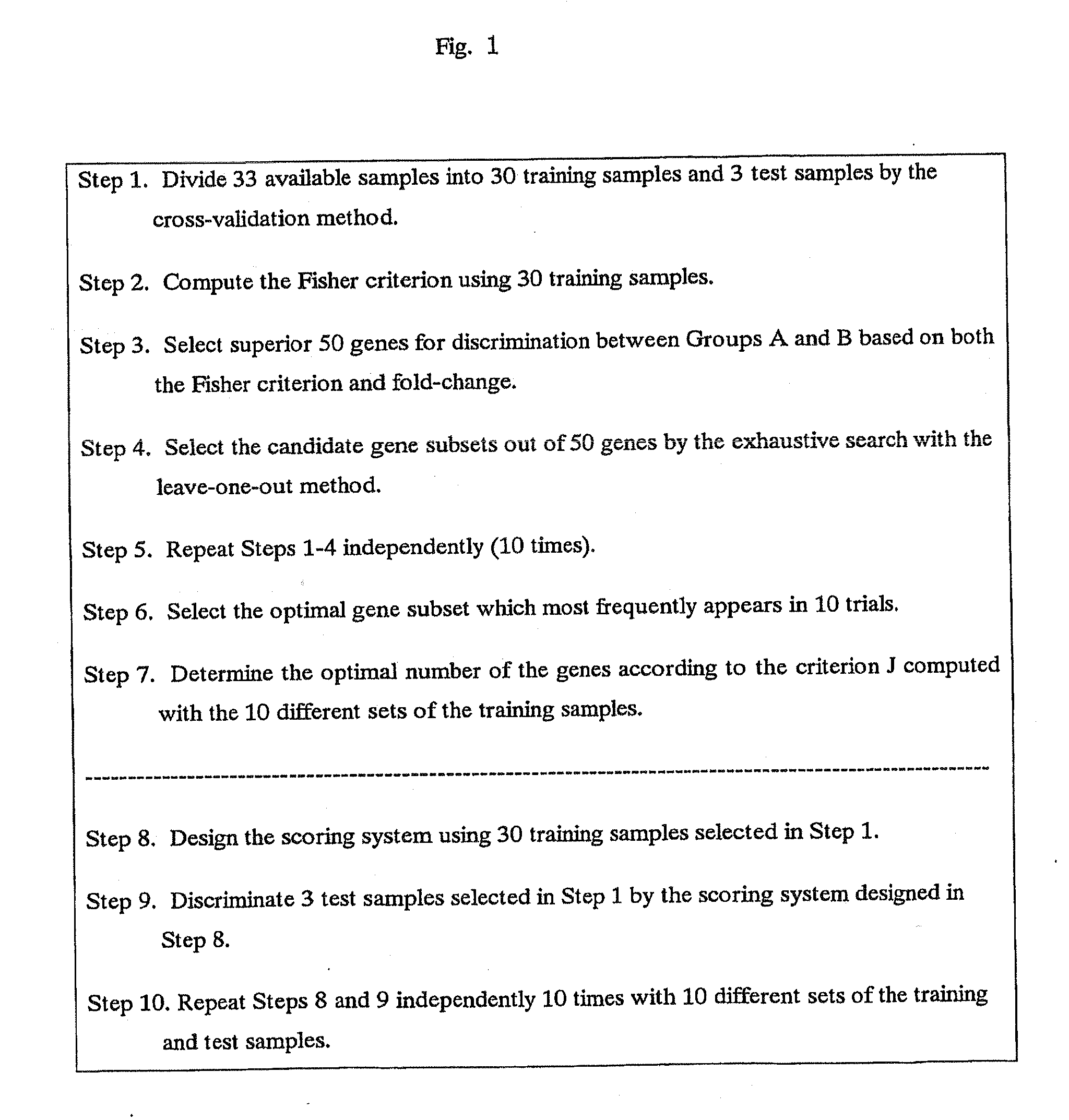
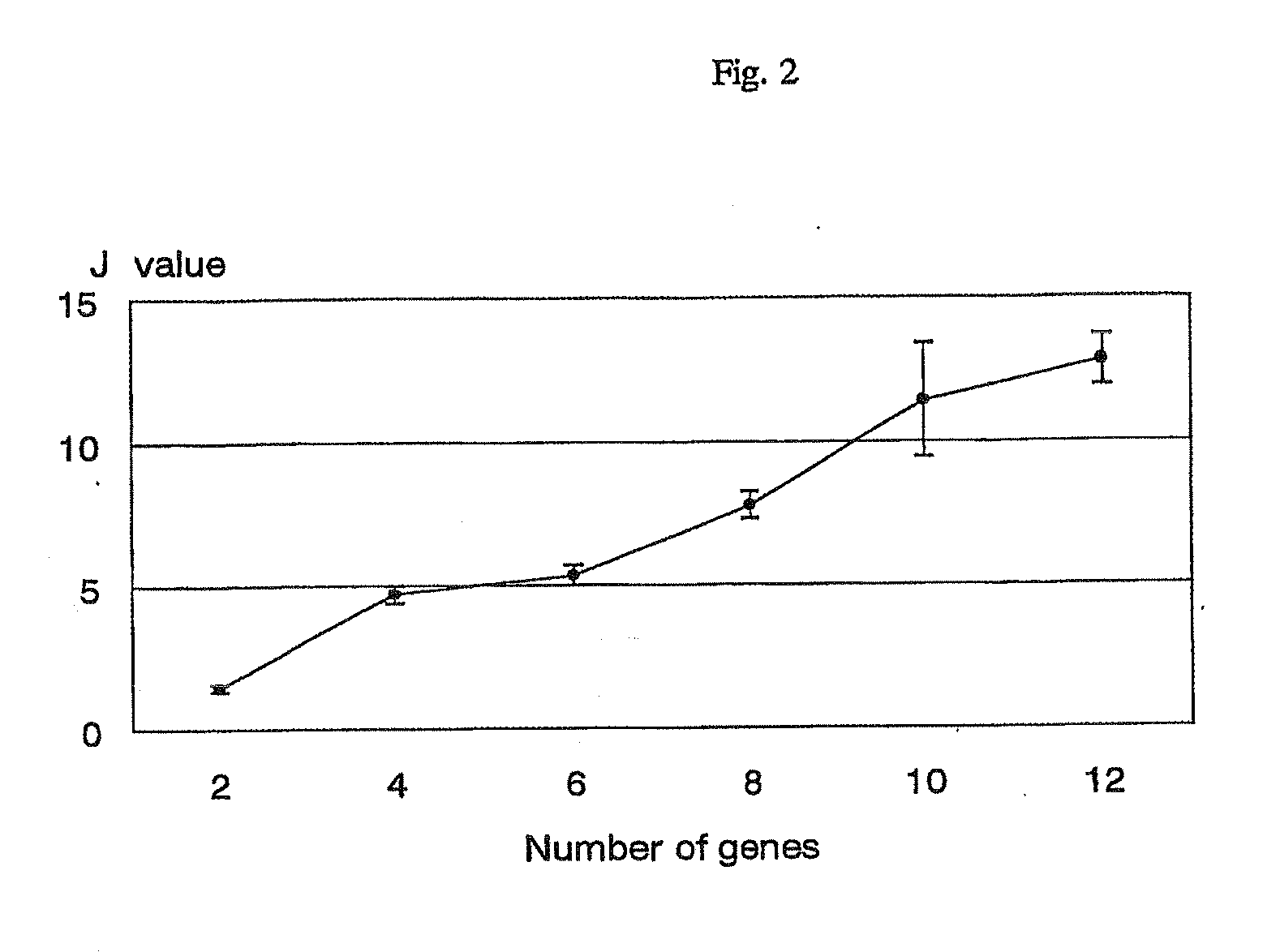
Scoring system for the prediction of cancer recurrence
ActiveUS7747389B2Digital data processing detailsMicrobiological testing/measurementTumour tissueHuman tumor
The present invention relates to a scoring system for the prediction of cancer recurrence. More particularly, the present invention concerns with the selection of genes and / or proteins, and generation of formulae with the selected genes and / or proteins for the prediction of cancer recurrence by measuring the expression of genes and / or proteins of human tumor tissues, and comparing their patterns with those of the gene and / or protein expression of human primary tumors from patients who have cancer recurrence and those who do not have cancer recurrence. The present invention also relates to a kit for performing the method of the present invention comprising DNA chip, oligonucleotide chip, protein chip, peptides, antibodies, probes and primers that are necessary for effecting DNA microarrays, oligonucleotide microarrays, protein arrays, northern blotting, in situ hybridization, RNase protection assays, western blotting, ELISA assays, reverse transcription polymerase-chain reaction (hereinafter referred to as RT-PCR) to examine the expression of at least 2 or more of genes and / or proteins, preferably 4 or more of genes and / or proteins, more preferably 6 or more of genes and / or proteins, and most preferably 12 or more of genes and / or proteins, that are indicative of cancer recurrence.
Owner:F HOFFMANN LA ROCHE & CO AG
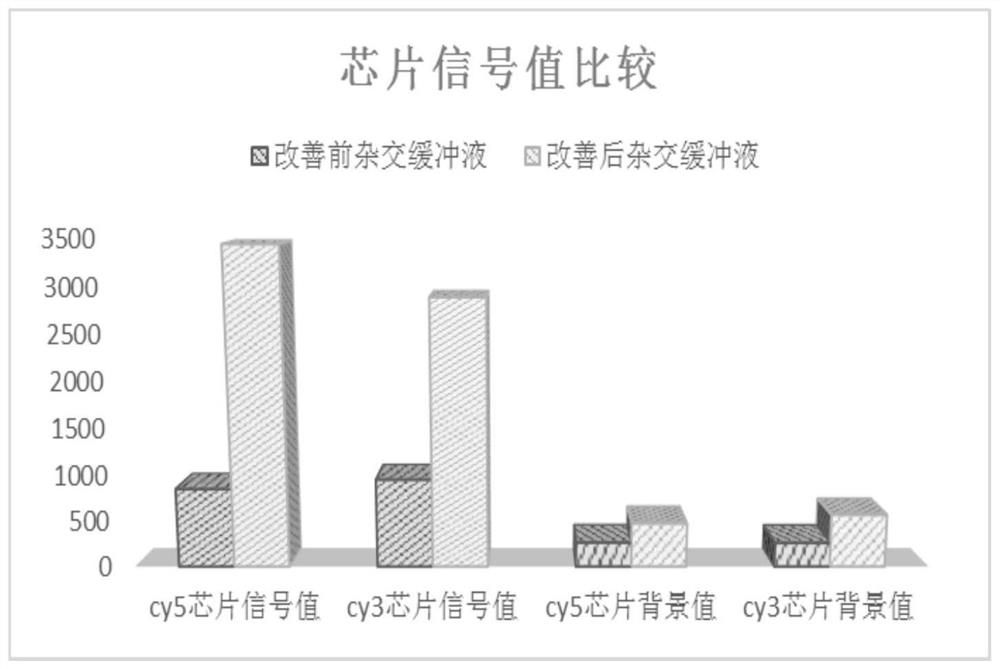
A hybridization buffer, a kit for oligonucleotide acgh chips, and a method for detecting variation in chromosome copy number
ActiveCN107893102BImprove detection accuracyMicrobiological testing/measurementOligonucleotide chipGenome
The invention belongs to the field of molecular biology and provides a hybridization buffer solution, a reagent kit of an oligonucleotide comparison genome hybridization chip and a method for detecting variation in chromosome copy number. The hybridization buffer of the present invention is composed of buffer Mix, formamide, lithium dodecyl sulfate and Cot-1DNA, which can significantly improve the problems of hybridization leakage, weak hybridization signal, uneven hybridization, and poor specificity, and improve the known The detection accuracy of variant CNV.
Owner:CAPITALBIO CORP +1
Information storage method based on oligonucleotide chip hybridization
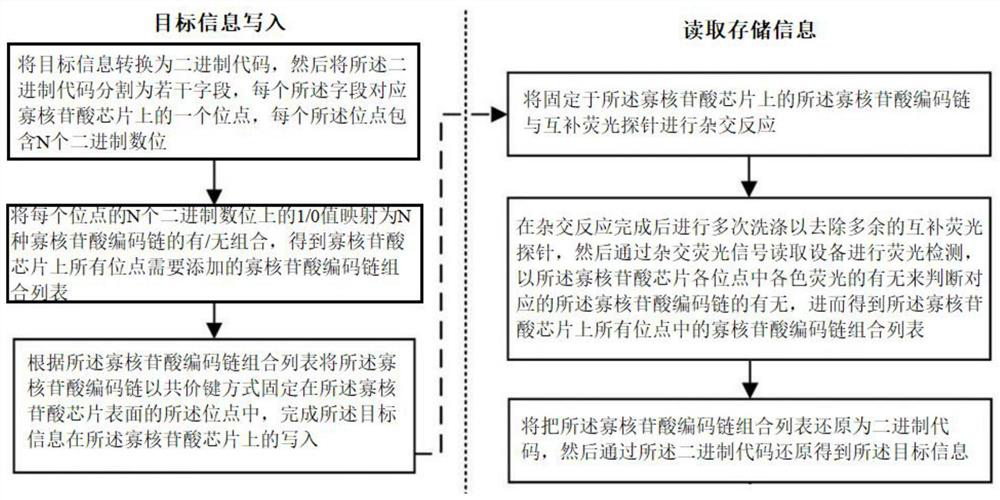
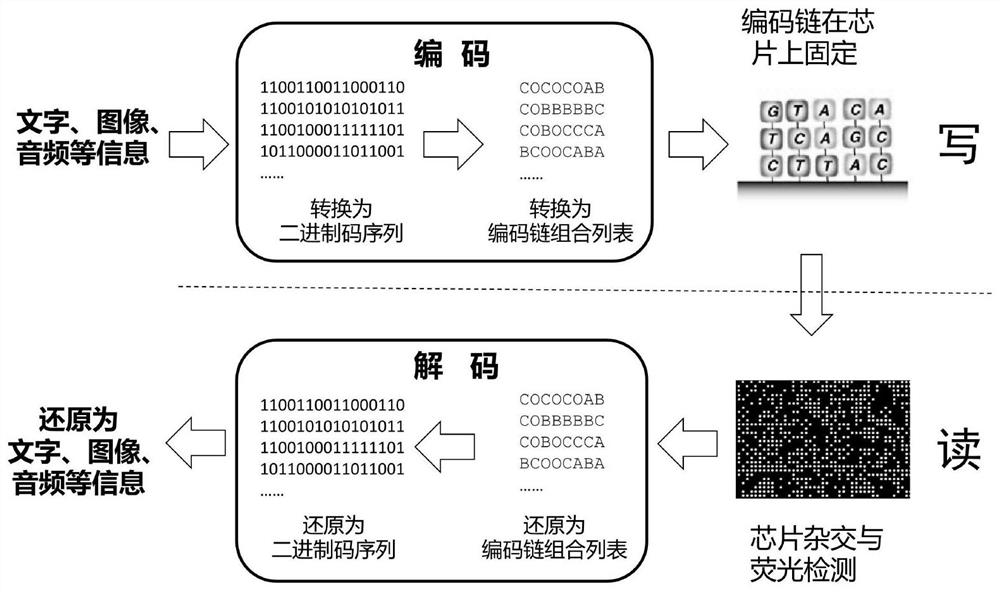
PendingCN114093401AImprove writing efficiencyImprove reading efficiencyDigital storageDNA computersOligonucleotide chipEngineering
The invention discloses an information storage method based on oligonucleotide chip hybridization, which comprises the following target information writing steps: (1) converting target information into a binary code, then segmenting the binary code into a plurality of fields, each field corresponding to a site on an oligonucleotide chip, and each site comprising N binary digits; (2) mapping the 1 / 0 value on the N binary digits of each site into a presence / absence combination of N oligonucleotide coding chains to obtain a combination list of oligonucleotide coding chains needing to be added to all the sites on the oligonucleotide chip; and (3) fixing the oligonucleotide coding chains in the sites on the surface of the oligonucleotide chip in a covalent bond manner according to the oligonucleotide coding chain combination list, thereby completing the writing of the target information on the oligonucleotide chip. According to the invention, the access of various complex file formats is realized, the parallelism is good, the signal-to-noise ratio is high, the access speed is high, and the accuracy is good.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI +1
Scoring system for the prediction of cancer recurrence
The present invention relates to a scoring system for the prediction of cancer recurrence by selecting genes and / or proteins whose expression patterns associated with recurrence of cancer, and generating formulae with the selected genes and / or proteins for the prediction of cancer recurrence. The present invention relates to a kit for determining the likelihood of recurrence of cancer, comprising DNA chip, oligonucleotide chip, protein chip, peptides, antibodies, probes and primers that are necessary for effecting DNA microarrays, oligonucleotide microarrays, protein arrays, northern blotting, in situ hybridization, RNase protection assays, western blotting, ELISA assays, reverse transcription polymerase-chain reaction to examine the expression of at least 2, 4, 6, 10, 12 or more genes and / or proteins, that are indicative of cancer recurrence.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com