Patents
Literature
59 results about "Hepatitis B virus DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hepatitis B virus is a DNA virus, meaning that its genetic material is made up of deoxyribonucleic acids. It belongs to a family of viruses known as Hepadnaviridae. The virus is primarily found in the liver but is also present in the blood and certain body fluids.
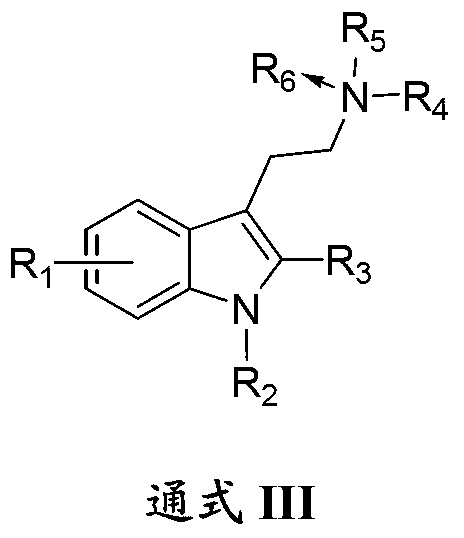
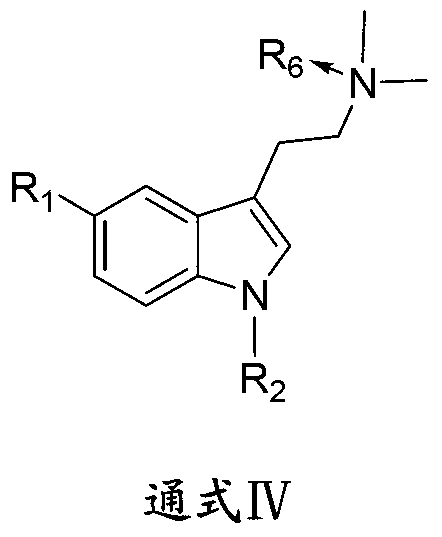
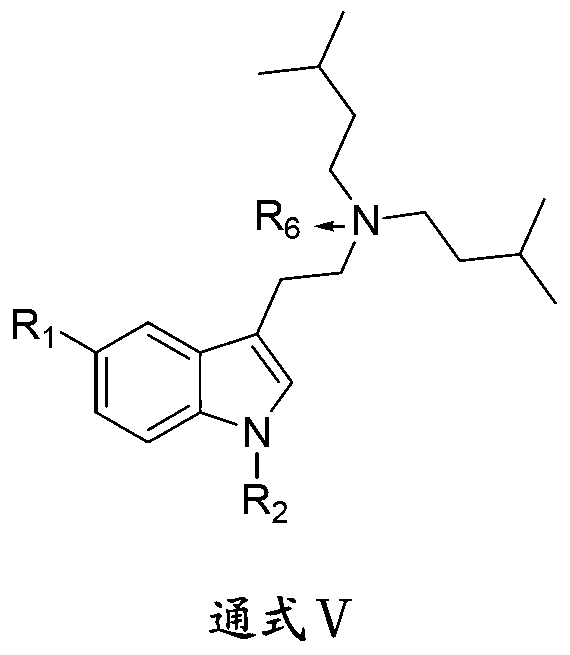
1-oxygen-substituted benzene formyl quinic acid pharmaceutical use of inhibiting hepatitis b virus
The invention relates to 1-o-substituted benzoyl quinic acid compounds having the formula of (I) and anti-hepatitis B virus activity and pharmaceutical salts thereof. The invention also relates to a preparation method of the quinic acid compounds and intermediate compounds of the quinic acid compounds. The invention also relates to a pharmaceutical application of the quinic acid compounds, and pharmaceutical compositions containing the same. The compounds (I) and intermediate compounds thereof, and pharmaceutical salts thereof inhibit hepatitis B virus DNA (HBVDNA) replication and reduces hepatits B virus surface antigen (HBsAg) expression. Thus, the 1-o-substituted benzoyl quinic acid compounds and intermediate compounds thereof have prospect pharmaceutical application in the preparation of a drug for treating correlated hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
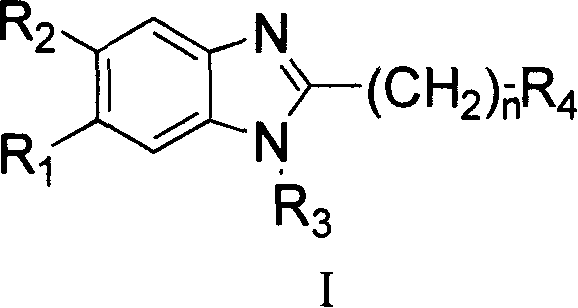
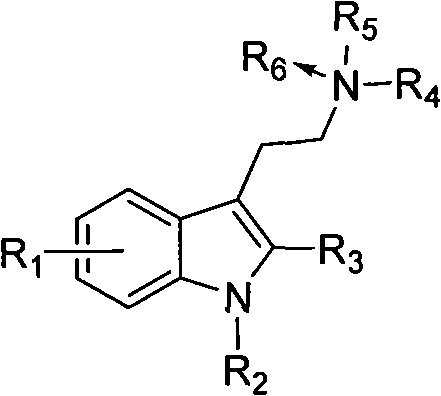
Benzimidazole compounds, its prepn. and uses
InactiveCN1834090AMild reaction conditionsAbundant and easy to get raw materialsOrganic chemistryDigestive systemHepatitis B virus DNASolvent
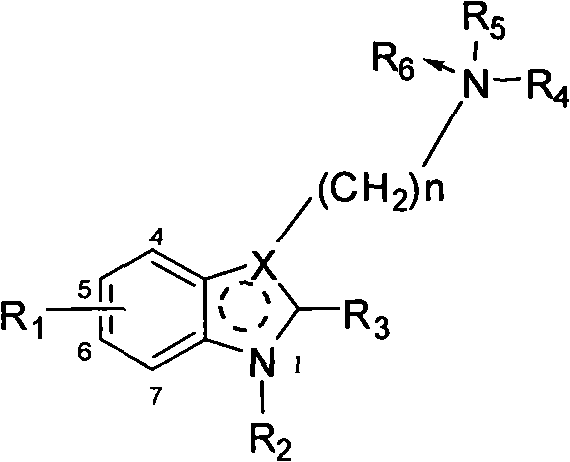
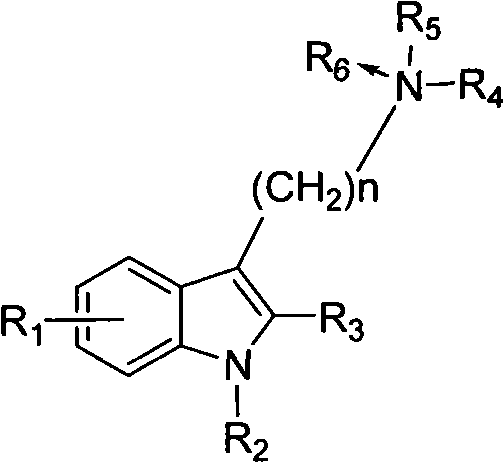
This invention provides substituted benzimidazole compounds shown in common formula (I) and their pharmacologically permitted salts. The definitions of R1, R2, R3, R4 and n in common formula (I) are mentioned in the instruction. Pharmacological experiments show that, this kind of compounds and their pharmacologically permitted salts or their solvates or hydrates perform excellent anti-hepatitis B virus (anti-HBV) activities and are especially inhibitory to DNA replication of HBV. They are thus promising as anti-HBV drugs. This invention also provides a method to prepare this kind of compounds.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
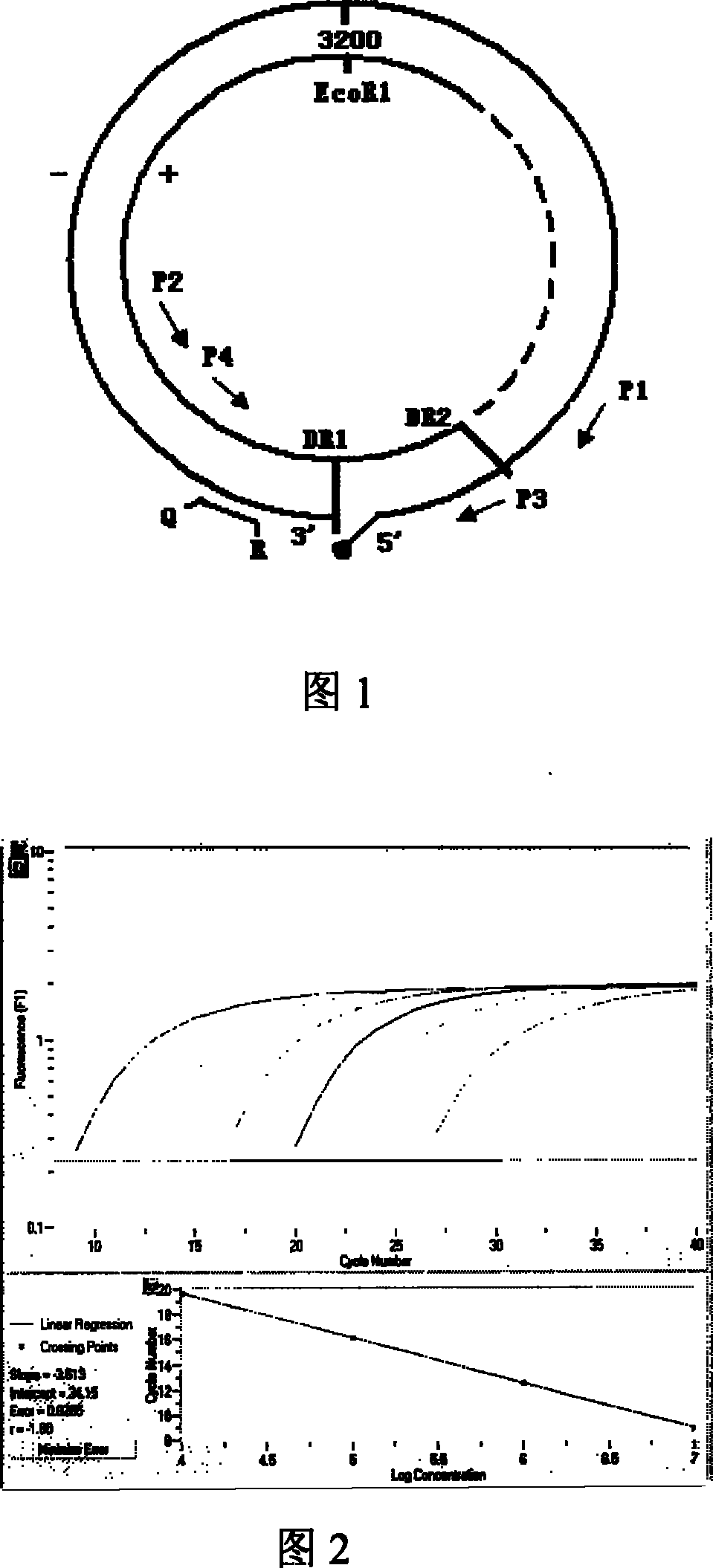
Nest type-real time quantitative PCR method for detecting hepatitis B virus cccDNA
InactiveCN101104867AImprove featuresHigh sensitivityMicrobiological testing/measurementNegative strandFluorescence
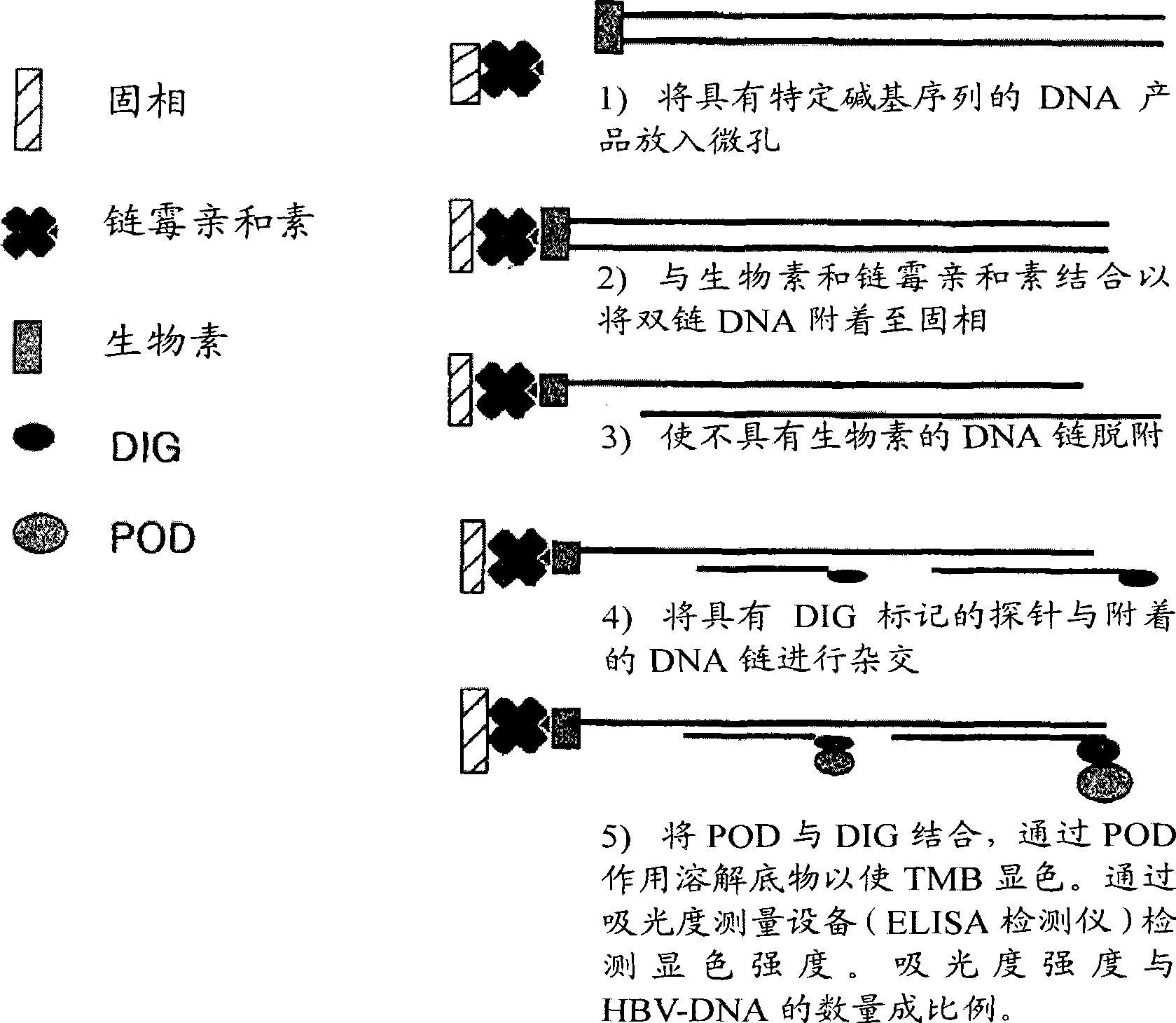
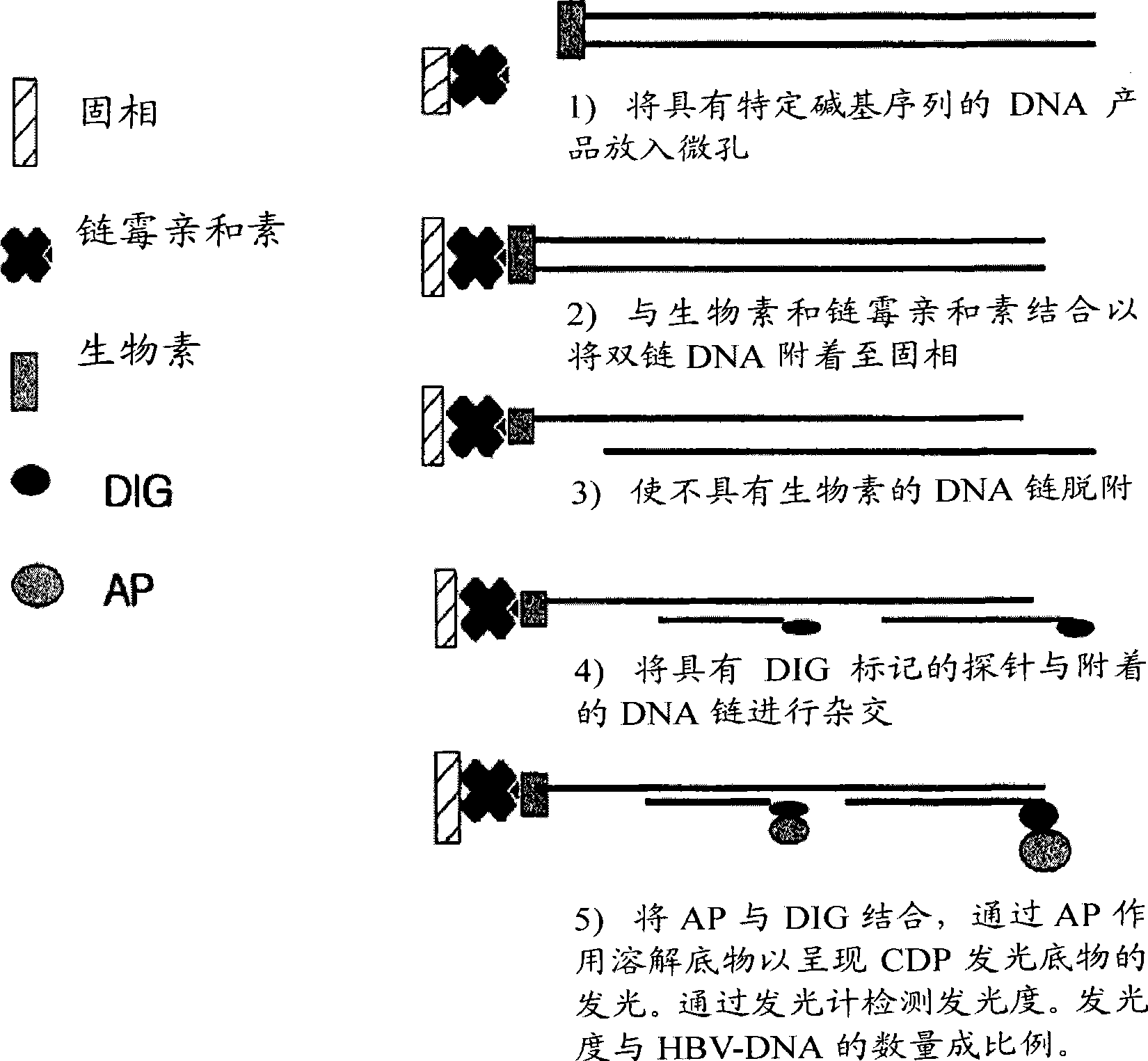
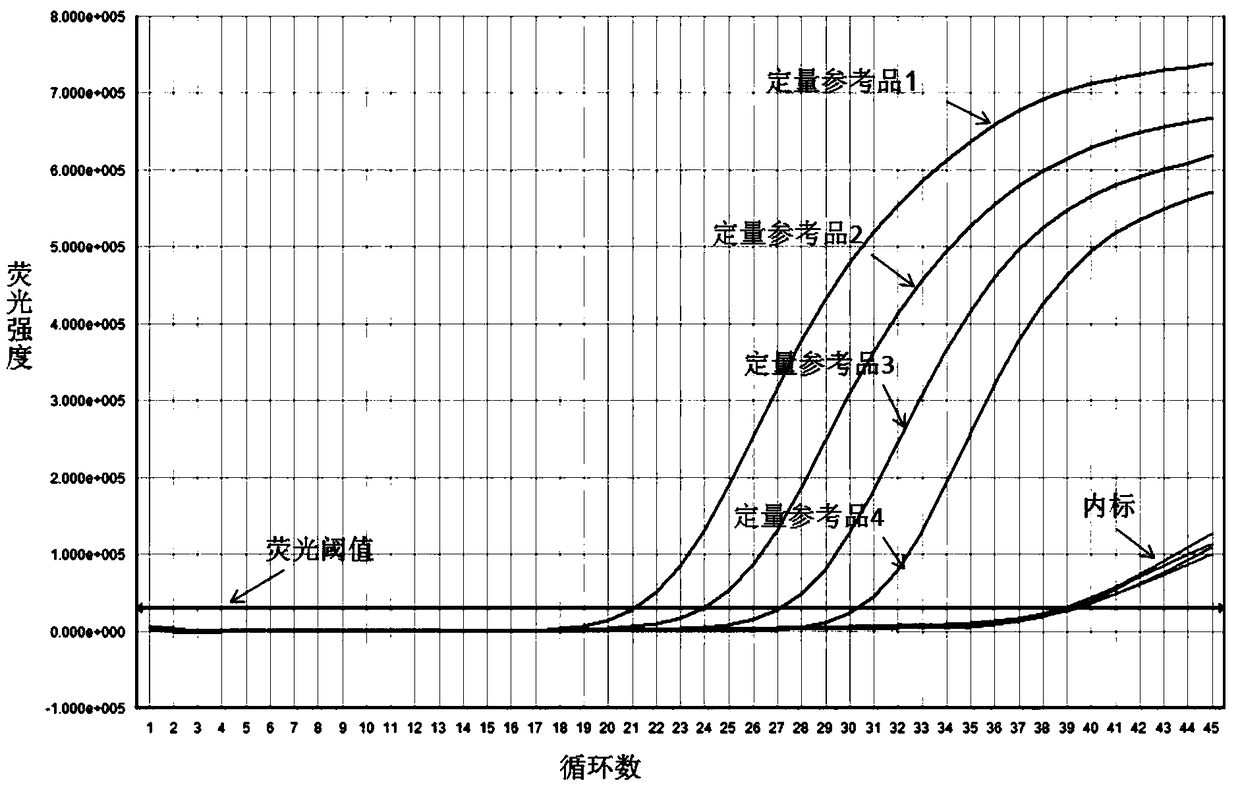
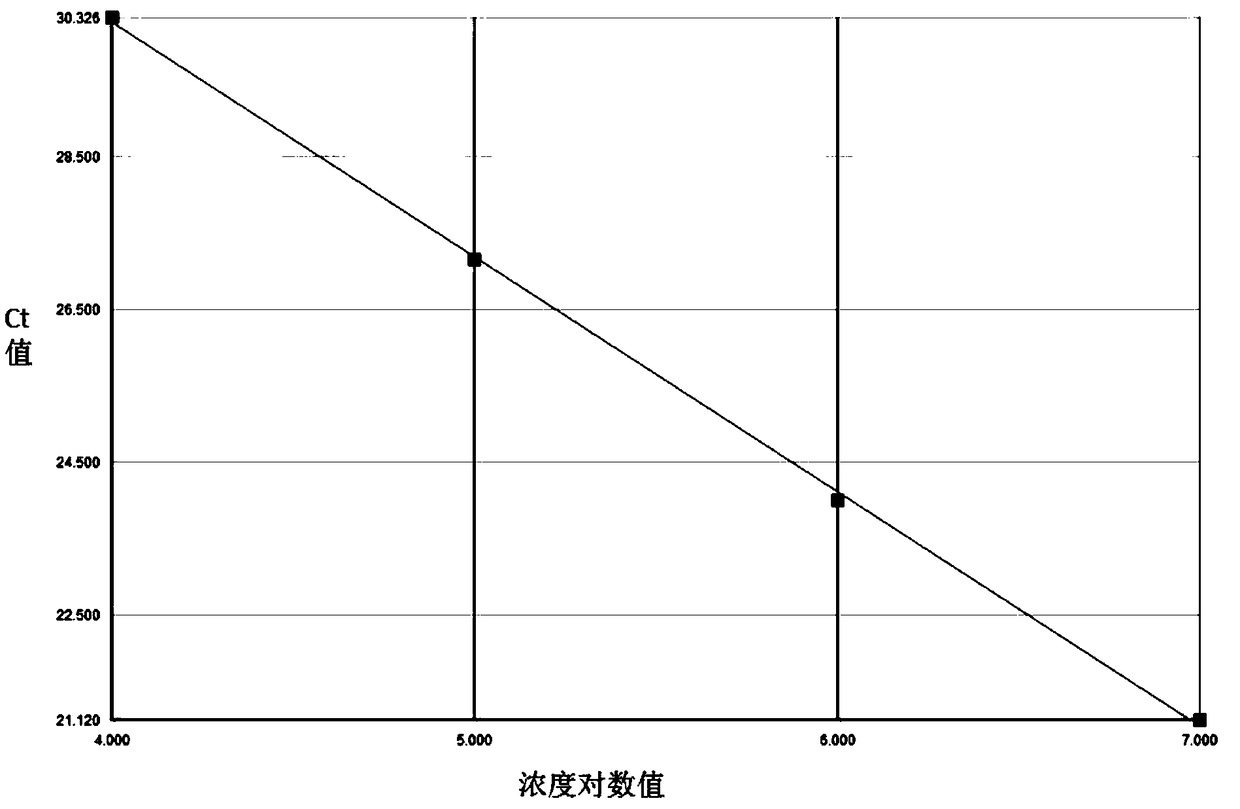
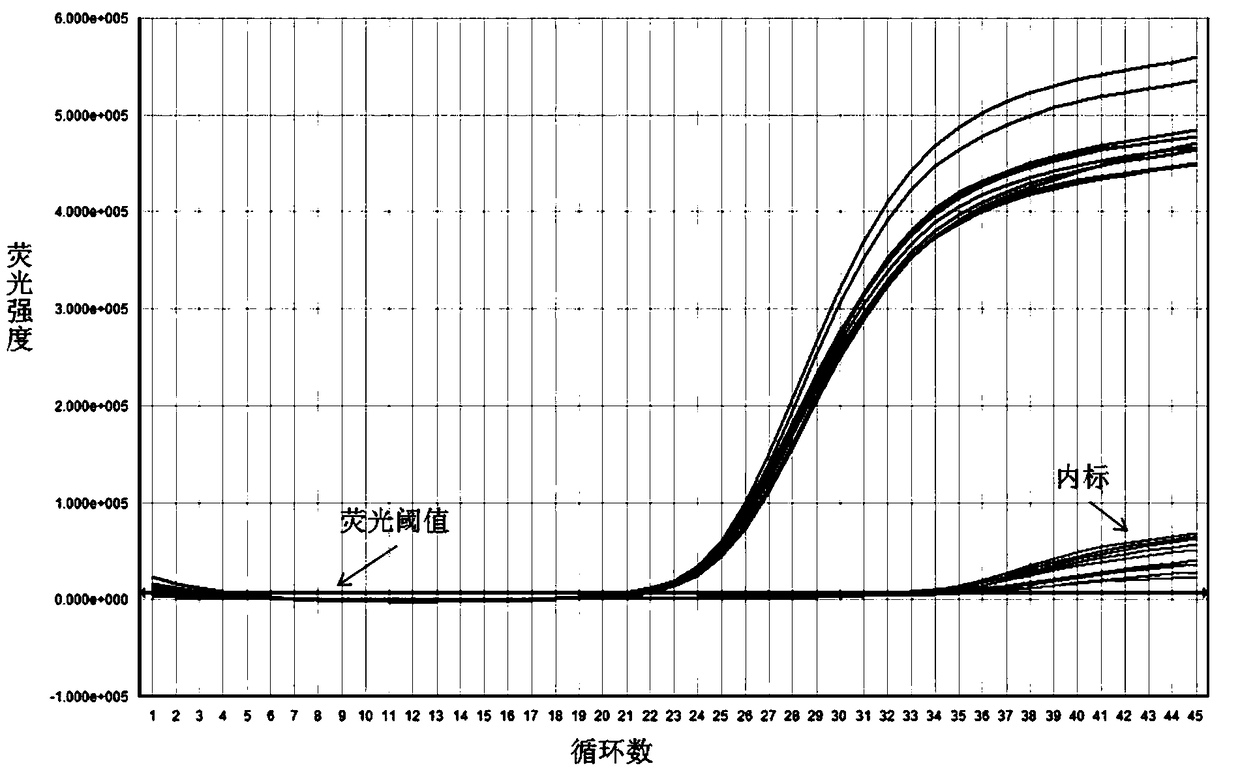
The invention relates to a nested quantitative PCR detection method for virus covalently closed circular DNA (ccc DNA) of hepatitis b virus. The particular detection steps are: 1) extraction DNA of hepatitis b virus; 2) design and synthesis primers and probe: design an internal and an external primer at conservative region of both sides of negative chain gap of HVB DNA, with Taqman fluorescence probe arranged at the lower part of the negative chain gap; 3) Plasmid-SafeTM ATP-Dependent DNase restriction enzyme purification; 4) nested real time quantitative PCR amplification, comprising: common PCR-template with external primer amplification purification and real time quantitative PCR-fluorescence quantitative amplification to common PCR production with internal primer and fluorescence probe. The invention can improve the specificity and sensitivity of detection reactive to make the operation process and result analysis much more accurate, simple and time saving.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
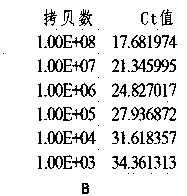
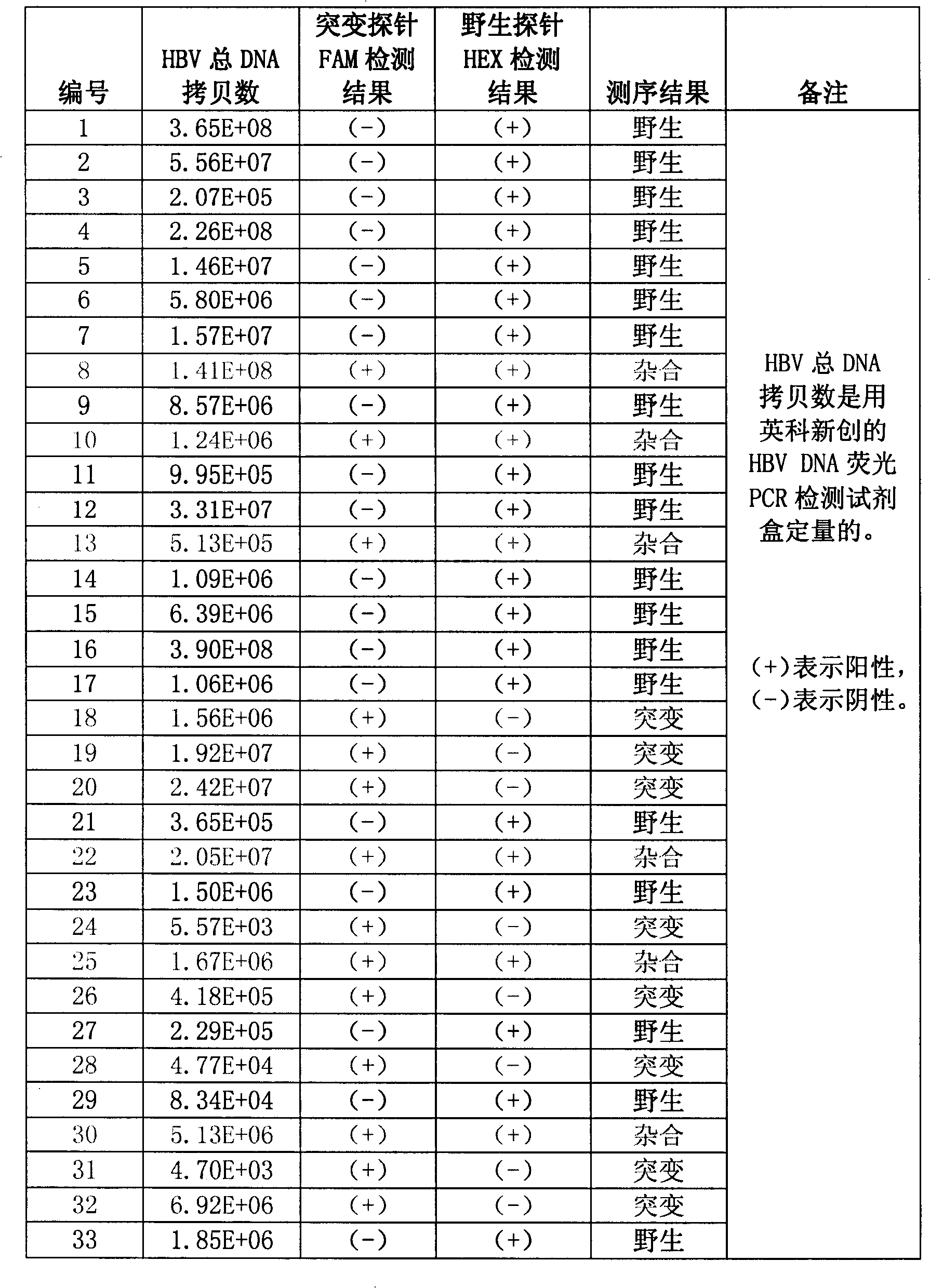
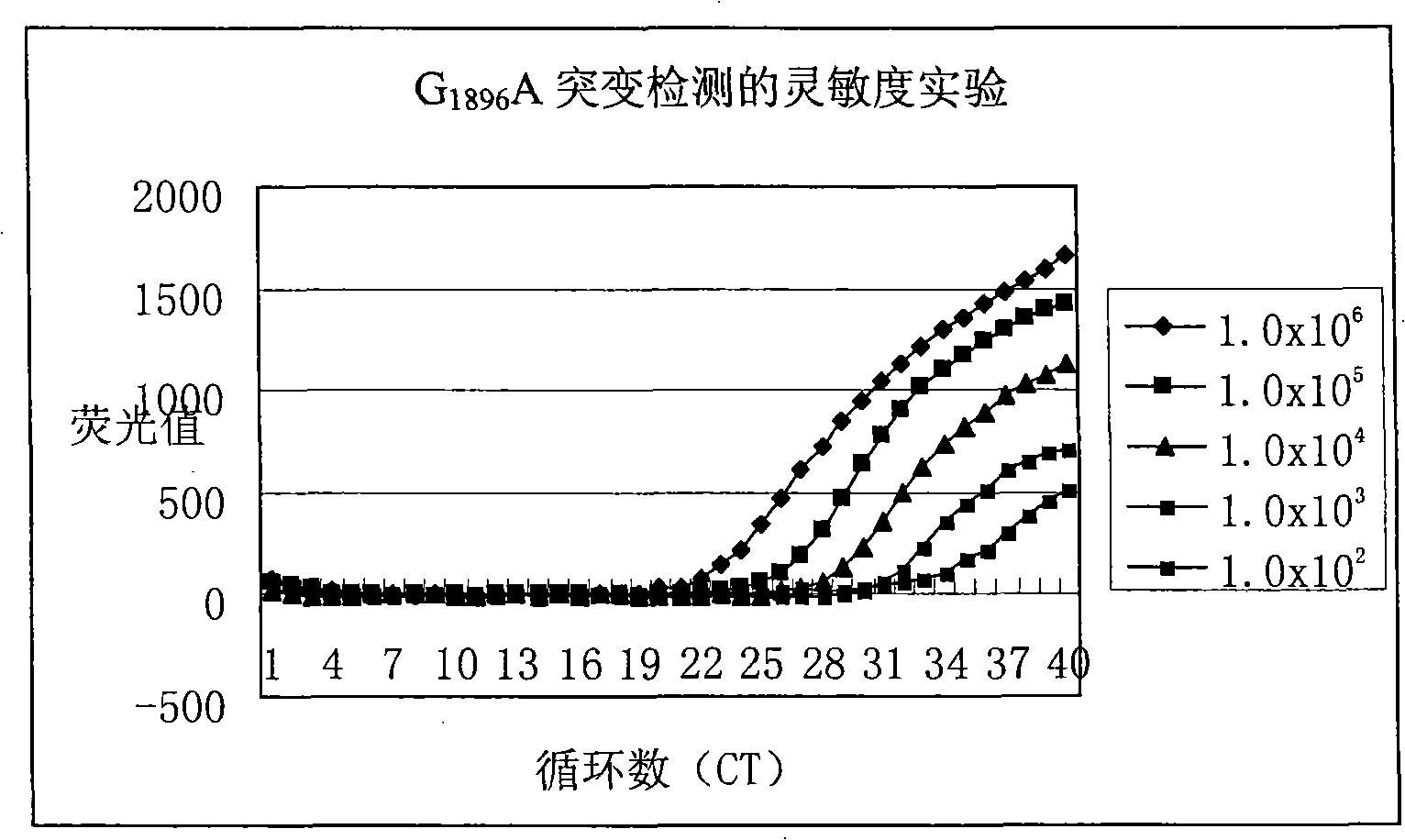
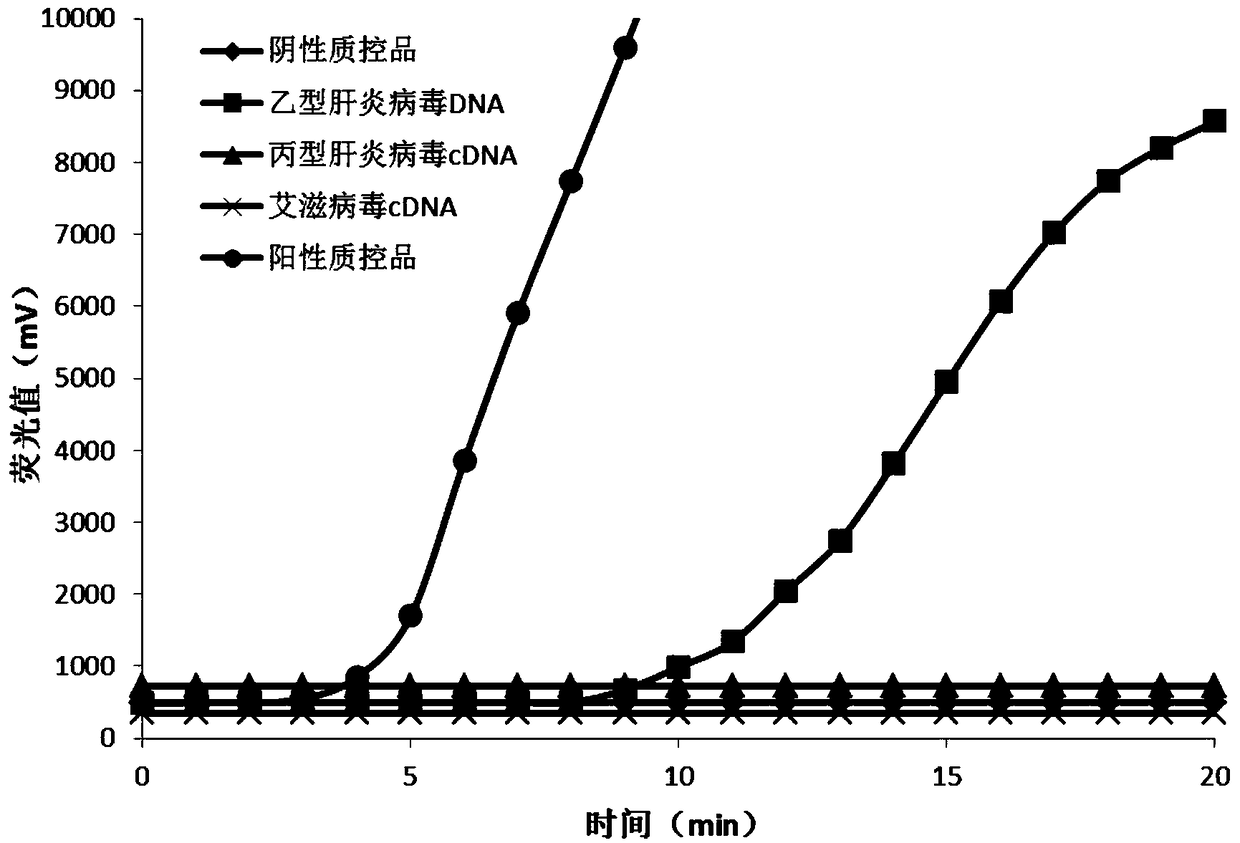
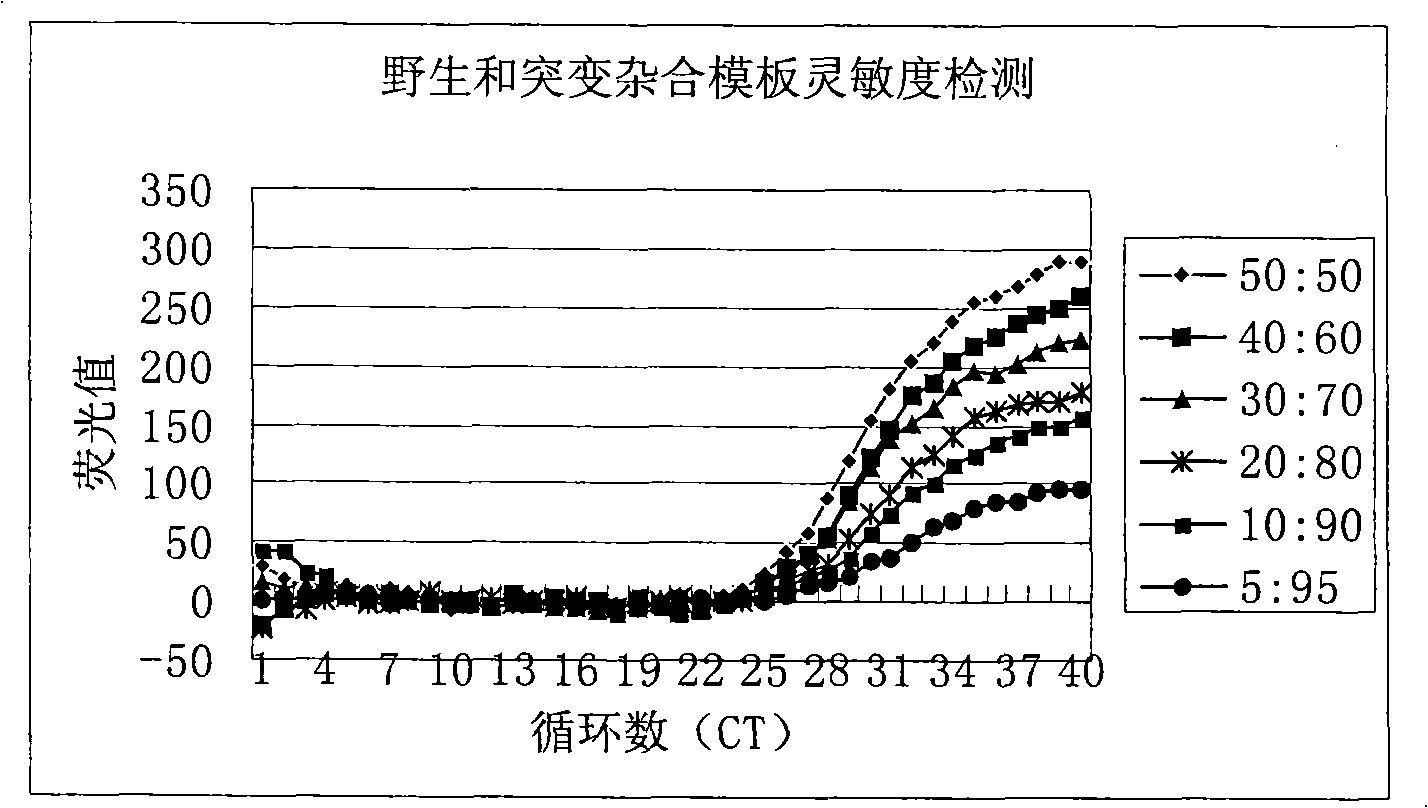
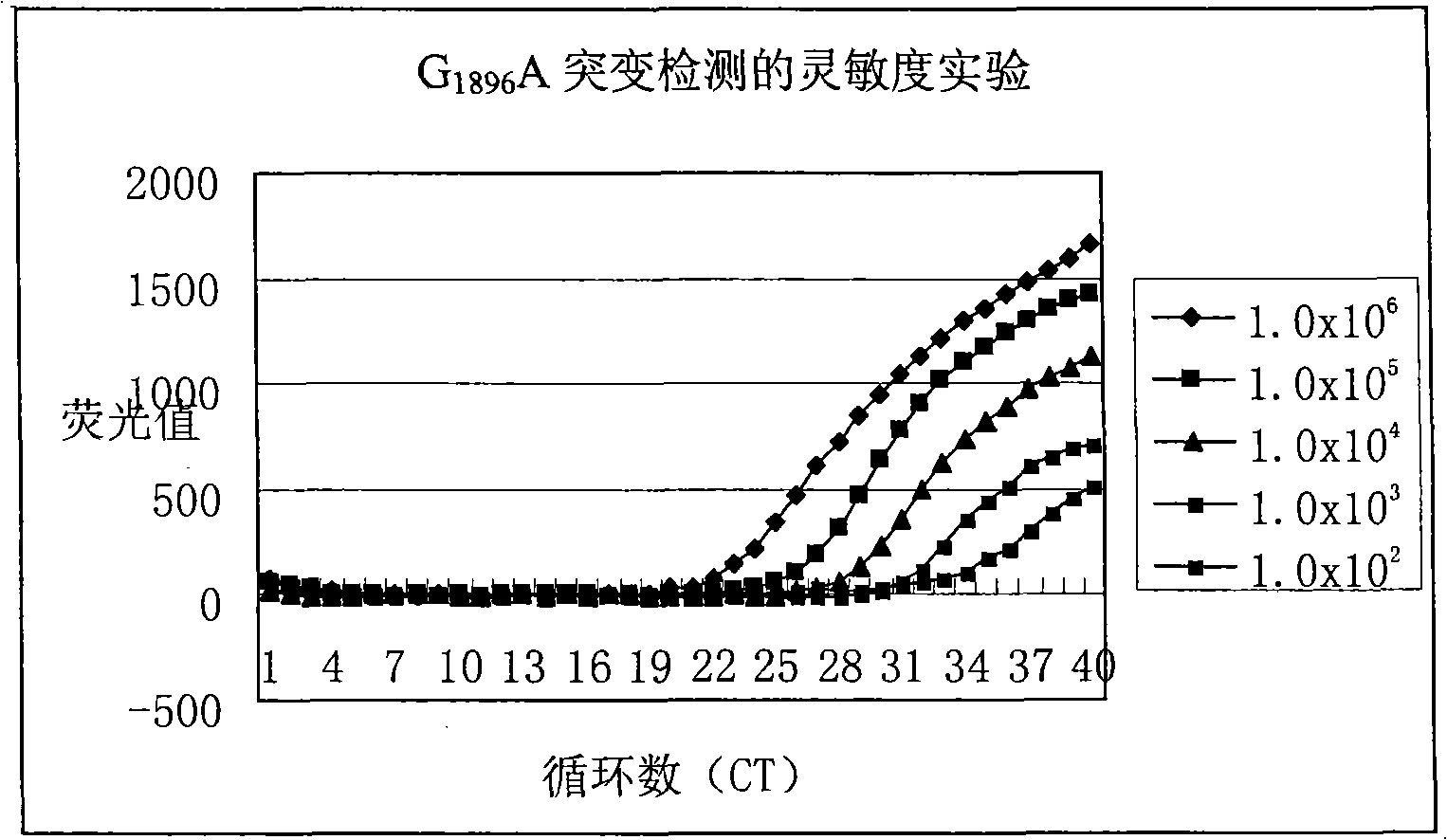
Method for detecting hepatitis B virus DNA and G1896A mutation thereof and kit
ActiveCN101338343ABeautiful and stable amplification curveMeet clinical needsMicrobiological testing/measurementHepatitis B virus DNAWild type
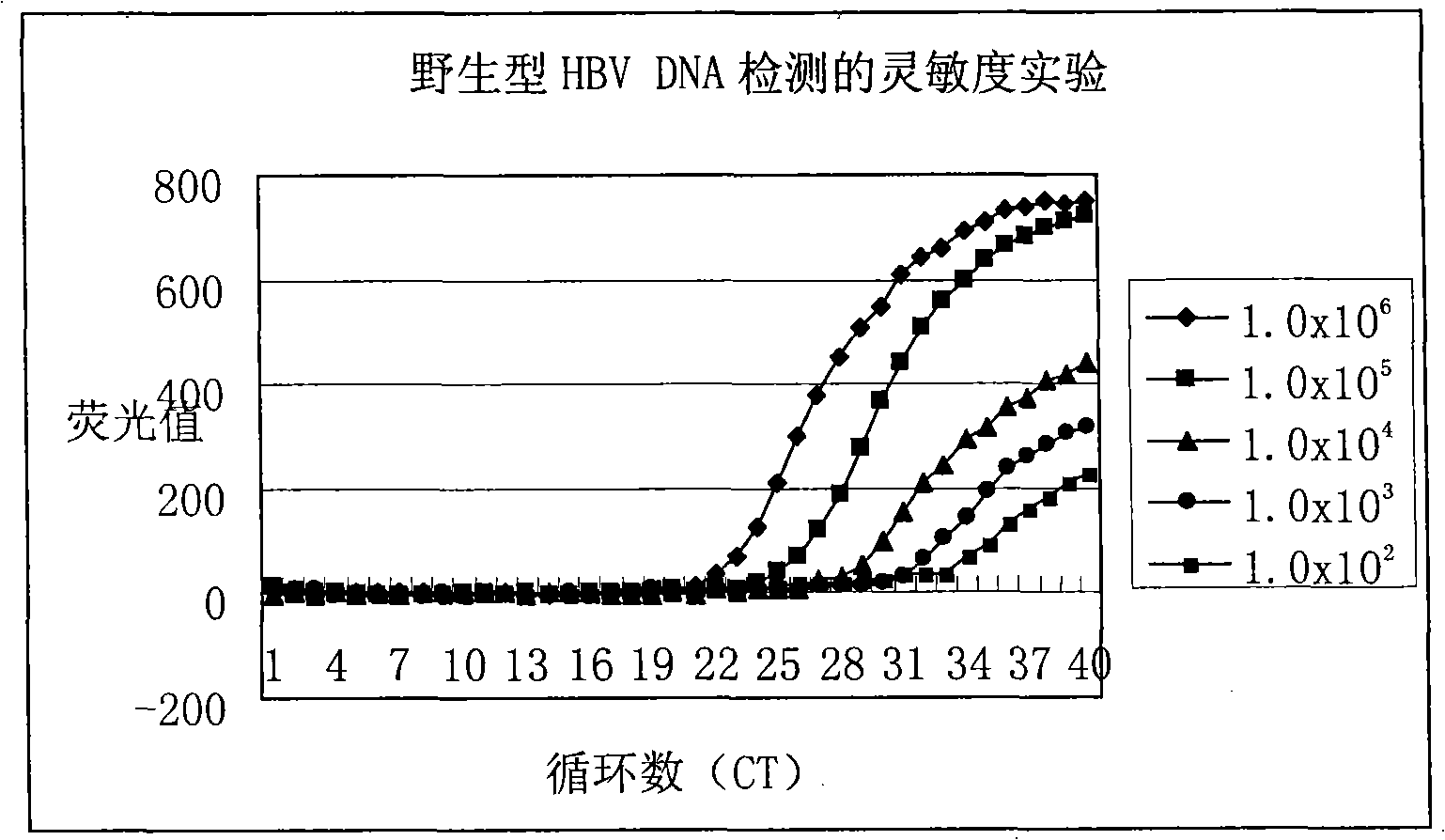
The invention relates to a detecting method and a reagent box for the DNA of the hepatitis B viruses of wild type and G1896A mutant, in particular to a method for detecting the DNA of the hepatitis B viruses of wild type and G1896A mutant by using the technology of combining the Locked Nucleic Acids with a molecular beacon probe as well as a real time PCR method and a reagent box used for finishing the detection. The method of the invention can specially and sensitively detect the DNA of the hepatitis B viruses of wild type and G1896A mutant in a clinic blood sample.
Owner:INTEC PROD INC
Benzohetercyclic compound as well as preparation method and applications thereof
ActiveCN102219725AInhibition of replicationSimple structureOrganic active ingredientsOrganic chemistryHepatitis B virus DNAChemical compound
The invention provides a non-nucleoside antiviral inhibitor, especially a benzohetercyclic compound shown by a general formula I or pharmaceutically acceptable salt or hydrate. The invention further provides a method for preparing the compound or the pharmaceutically acceptable salt or hydrate. Pharmacology tests show that the compound or salt or hydrate accepted on the pharmacy can effectively inhibit replication of HBV (hepatitis B virus) DNA (deoxyribonucleic acid) and HCV (hepatitis C virus) RNA (Ribonucleic acid), therefore, the invention further provides applications on preparing medicaments in preventing and / or treating viral infection, especially the HBV and the HCV. The general formula I is as follows.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
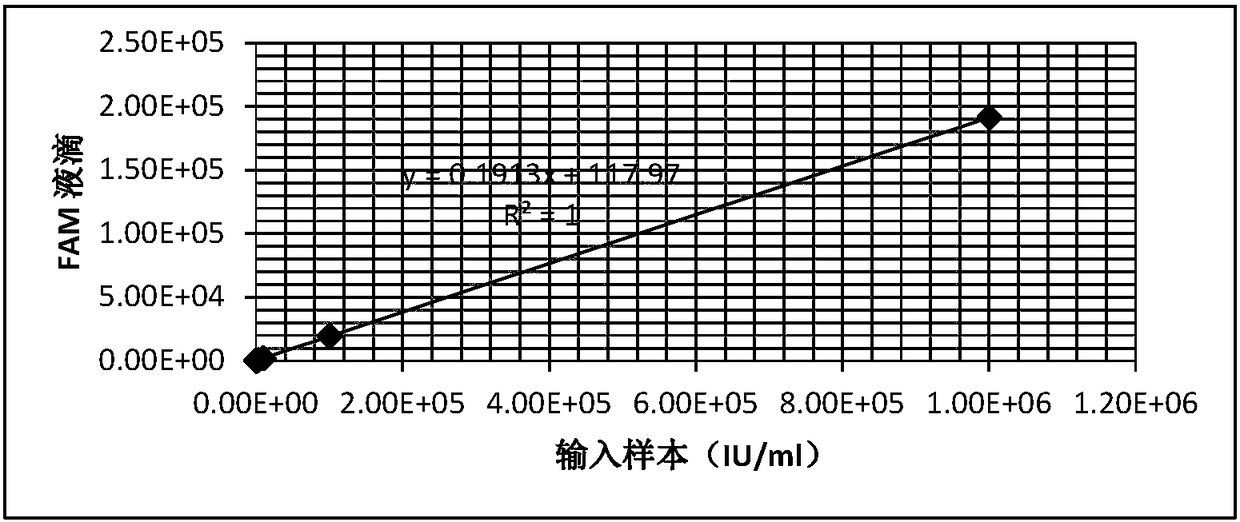
HBV nucleic acid quantitative detection system based on micro-droplet digital PCR technology
InactiveCN108342507AAccurate Absolute QuantificationMicrobiological testing/measurementHepatitis B virus DNAPcr ctpp
The invention provides a HBV nucleic acid quantitative detection system based on a micro-droplet digital PCR technology, the HBV nucleic acid quantitative detection system comprises an upstream primerand a downstream primer for amplifying hepatitis B virus DNA, a specific probe for detecting the hepatitis B virus DNA; a non-infected linearizing double-stranded plasmid quality control standard product and a probe for detecting the quality control standard product; and a buffer system comprising deoxyribonucleoside triphosphates dATP, dCTP, dGTP, and dUTP, and UNG. The HBV nucleic acid quantitative detection system is based on the micro-droplet digital PCR technology to realize the accurate absolute quantification of HBV DNA, faces the clinical actual demand, and has an important application prospect.
Owner:TARGETINGONE CORP +1
Hepatitis B virus detection method based on DNA (deoxyribonucleic acid) zyme probe
ActiveCN103952497AAvoid pollutionGood heartbeatMicrobiological testing/measurementMicroorganism based processesHepatitis B virus DNAHepatitis B virus
The invention belongs to the technical field of molecular biology, and discloses a method for qualitatively and quantificationally detecting the DNA (deoxyribonucleic acid) of hepatitis B virus by using a DNAzyme probe. The method is characterized in that specific to the limitations of the prior art, the single-step qualitative and quantificational detection of the hepatitis B virus is realized by using PCR (Polymerase Chain Reaction) amplification as a basic detection platform and introducing the 3'-amino modified DNAzyme probe. The method has the advantages of high sensitivity, strong specificity, wide detection linearity range, low cost, high accuracy, convenient operation and the like. A simple, rapid, accurate, high-efficiency, economic and practical detection method is supplied for the hepatitis B virus.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Oligonucleotide chip and its application of detecting muatatonal site of hepatitis B virus
InactiveCN1431317AShorten detection timeHigh strengthMicrobiological testing/measurementFluorescenceHepatitis B virus DNA
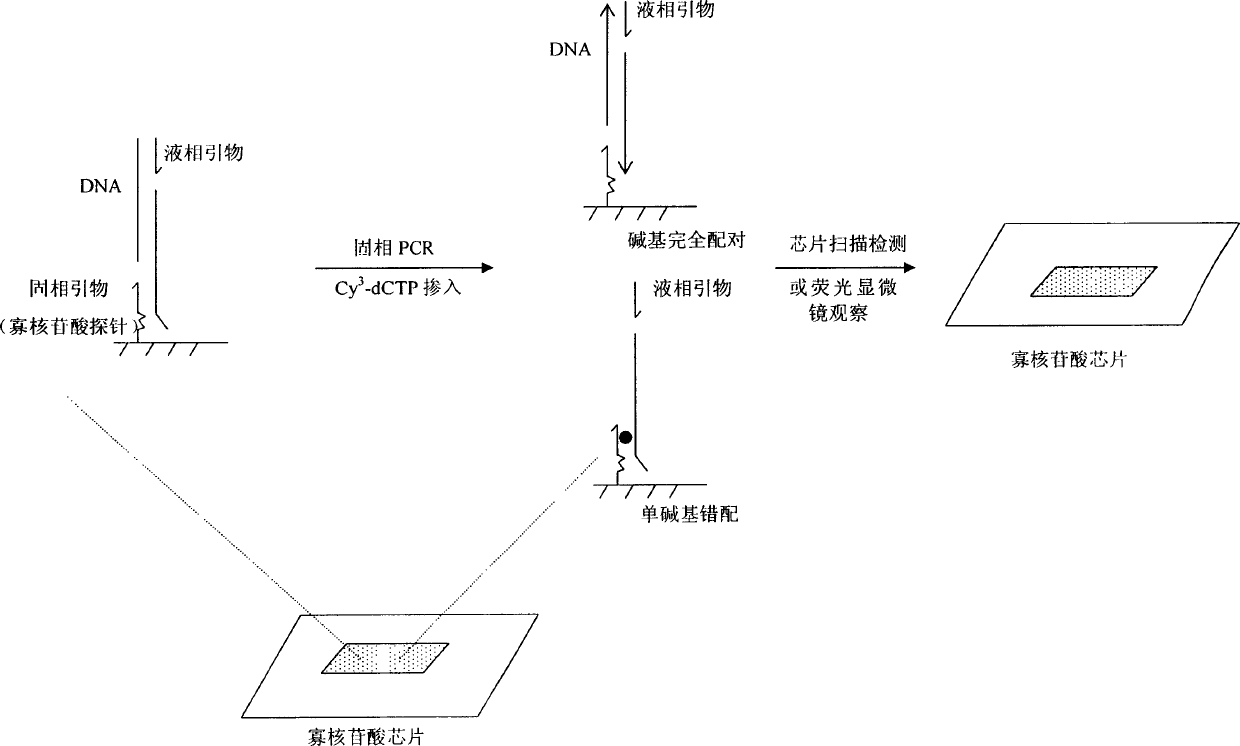
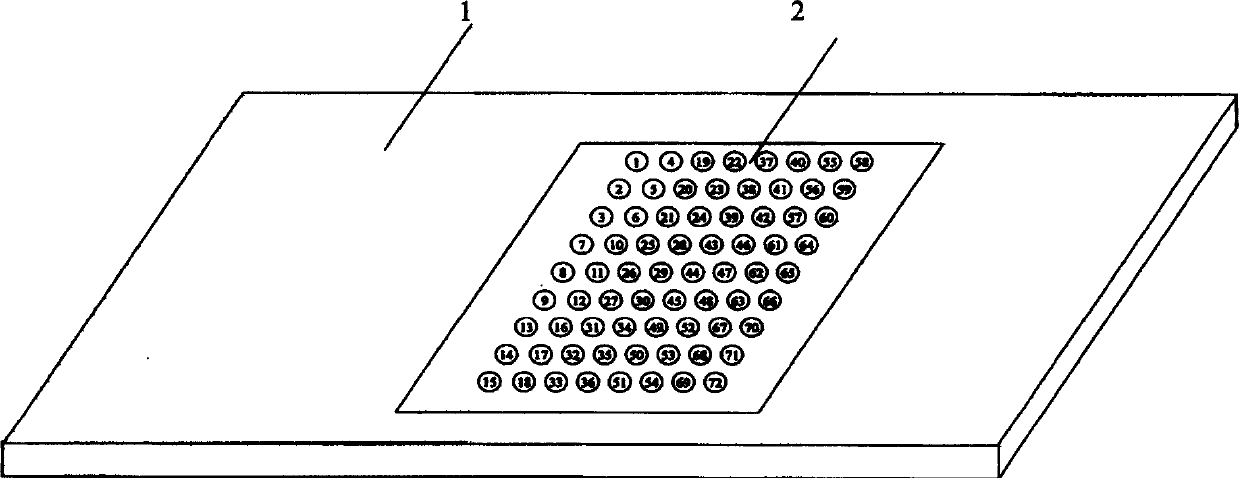
An oligonucleotide chip for detecting the mutation sites of hepatitis B virus is composed of glass substrate, oligonucleotide probes array and reference point-type coated laeyr. The said probes arrayhas 72 probes, including 12 known mutation sites and relative wild sites. The process for detcting mutation sites of hepatitis B virus includes such steps as mixing the probes as solid primer, hepatitis B virus DNA and template, and three pairs of liquid primers, PCR amplification via fluorescence labeled Cy3-dCTP, and analyzing mutation sites according to intensity of fluorescent signal. Its advantages are high speed, efficiency and correctness.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Extraction reagent and extraction method of hepatitis B virus DNA
The invention discloses an extraction method of hepatitis B virus deoxyribonucleic acid (DNA) and belongs to the field of nucleic acid extraction in molecular biology. An alkaline lysis technology is adopted according to particular characteristics of blood and viruses; impurities such as proteins, polysaccharides, lipids and the like are not required to be pre-removed; and the virus DNA is directly extracted through lysis buffer 1 and lysis buffer 2. The method has the characteristics of high efficiency, high speed, simplicity and no pollution, and the whole operation process only lasts for 50 minutes. The obtained hepatitis B virus DNA can be applied to molecular biology experiments such as the common polymerase chain reaction (PCR), fluorescence quantitative PCR and the like.
Owner:上海裕隆医学检验所股份有限公司
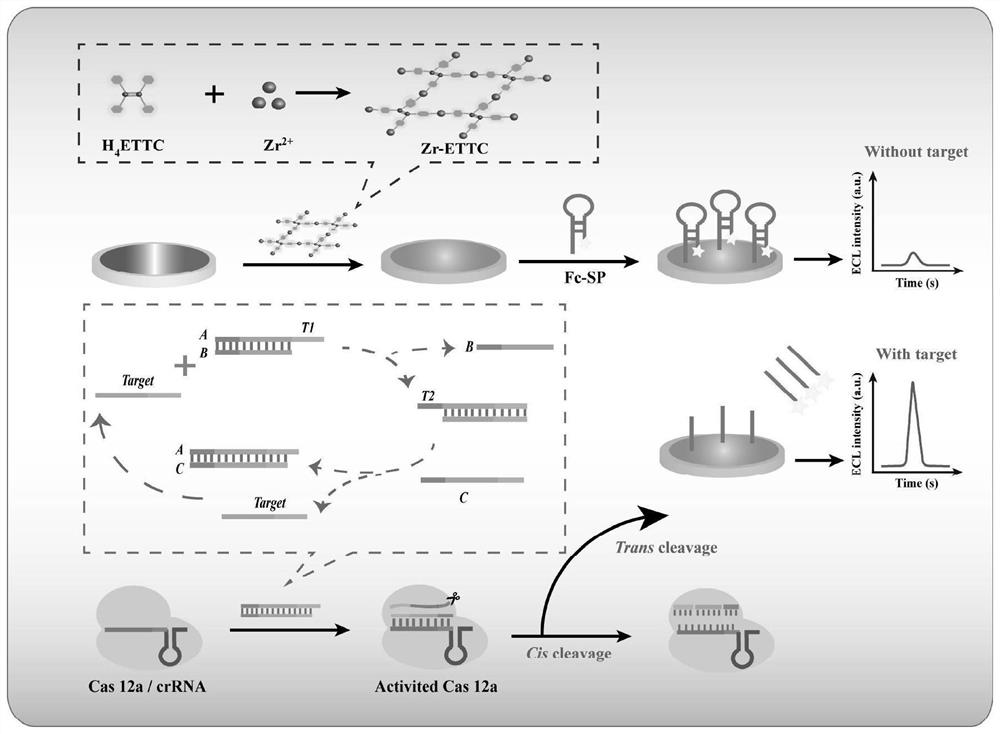
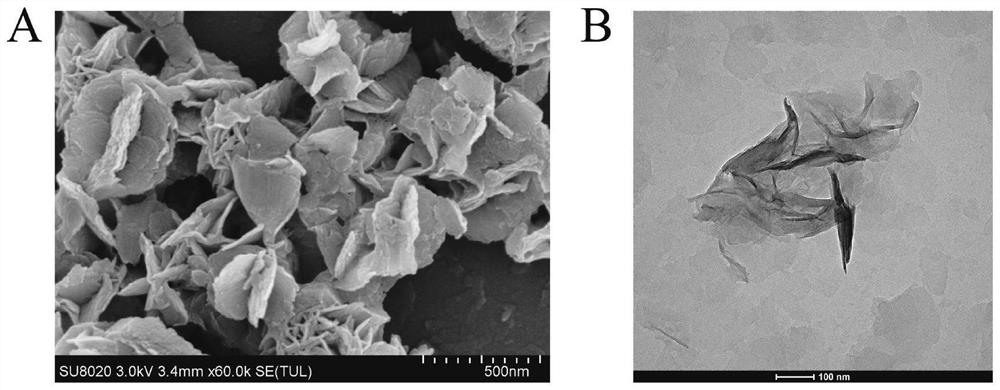
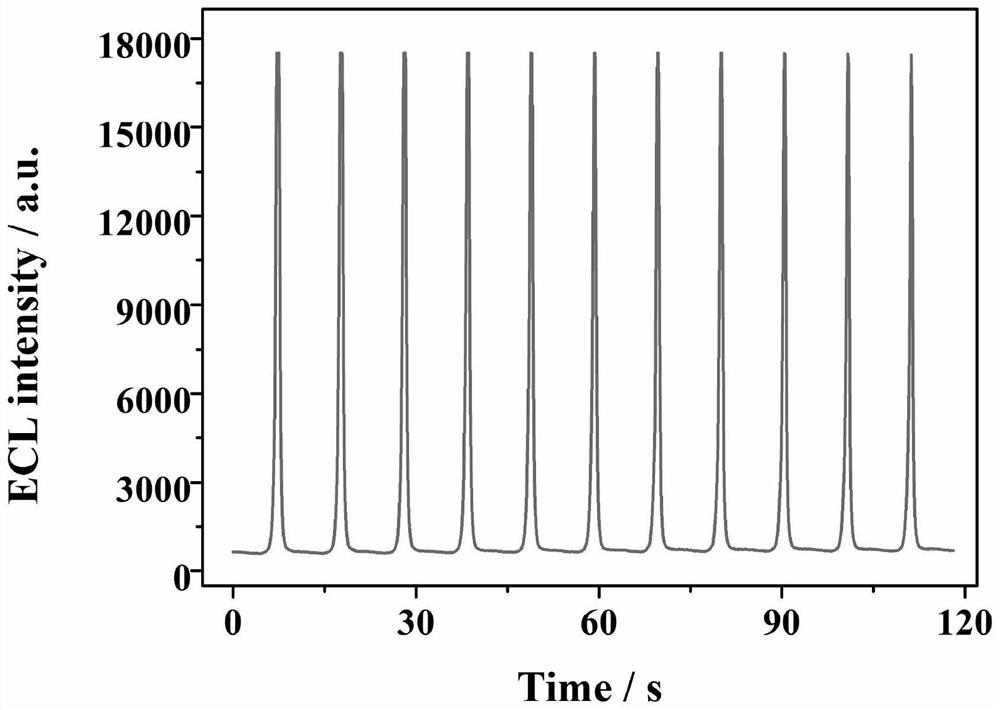
Electrochemiluminescence sensor for detecting hepatitis B virus DNA and preparation and application thereof
ActiveCN113237928AQuick checkHigh sensitivity detectionMicrobiological testing/measurementChemiluminescene/bioluminescenceHepatitis B virus DNAElectrochemiluminescence
The invention discloses an electrochemiluminescence sensor for detecting hepatitis B virus DNA and preparation and application thereof. The electrochemiluminescence sensor is constructed based on an aggregation-induced electrochemiluminescence body, a toehold-mediated strand displacement reaction (TSDR) and a CRISPR / Cas12a system. A Zr-ETTC@ Fc-SP compound is prepared and fixed to the surface of an electrode, and an ECL signal of Zr-ETTC can be quenched by Fc; a TSDR system is constructed by using a target substance T chain, an A-B compound and a single chain C, and a displacement reaction is carried out to obtain a large amount of Cas12a activator; and the Cas12a-crRNA compound can recognize the Cas12a activator, activate the cleavage activity of the Cas12a activator and perform trans-cleavage on SP, so that Fc is separated from the surface of the electrode, the ECL signal is recovered, and HBV DNA detection is realized. The HBV DNA detection method disclosed by the invention has the advantages of high sensitivity, strong stability, good reproducibility, high reaction speed and the like.
Owner:CHONGQING MEDICAL UNIVERSITY
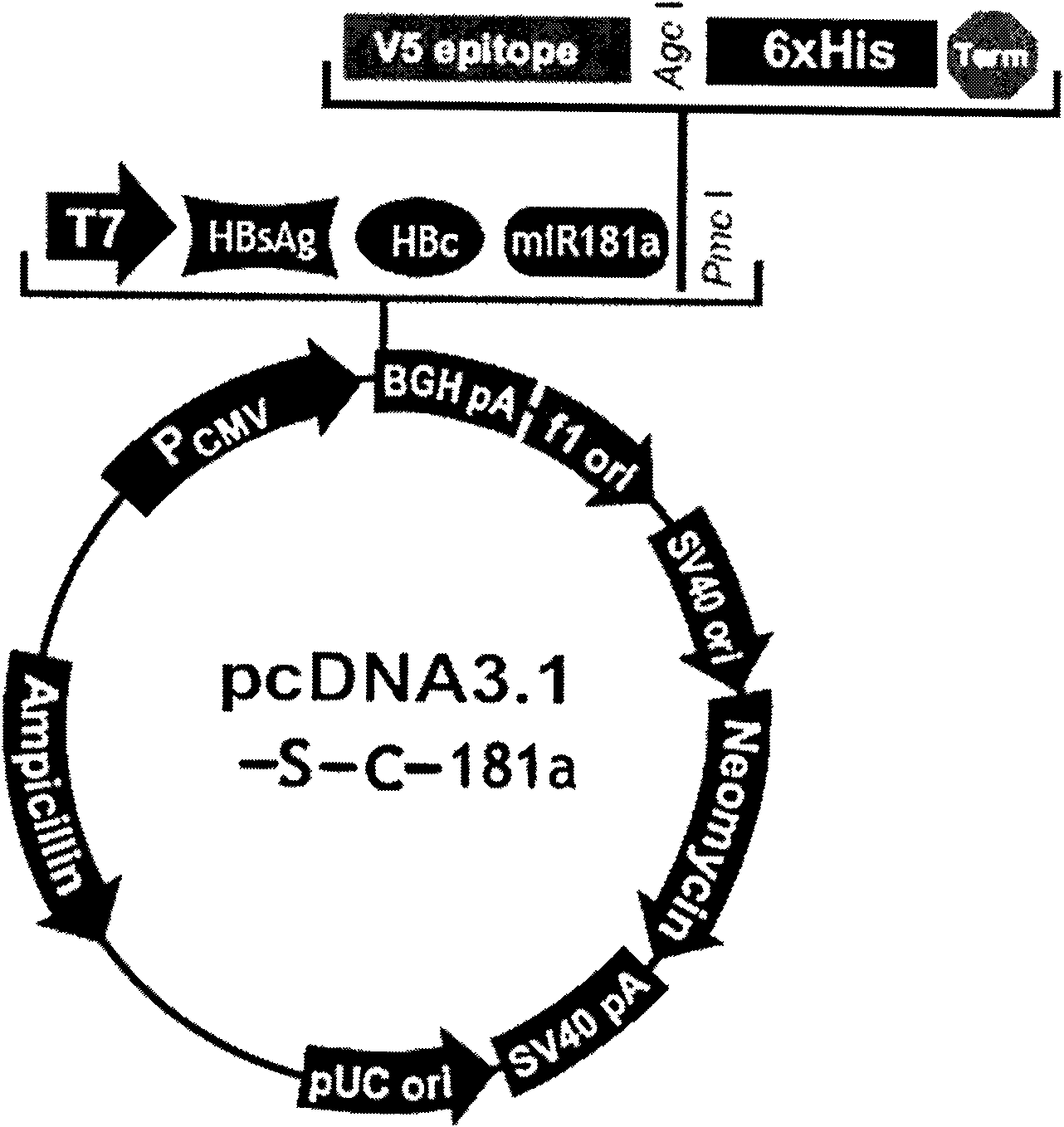
Hepatitis B nucleic acid vaccine and construction method thereof
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
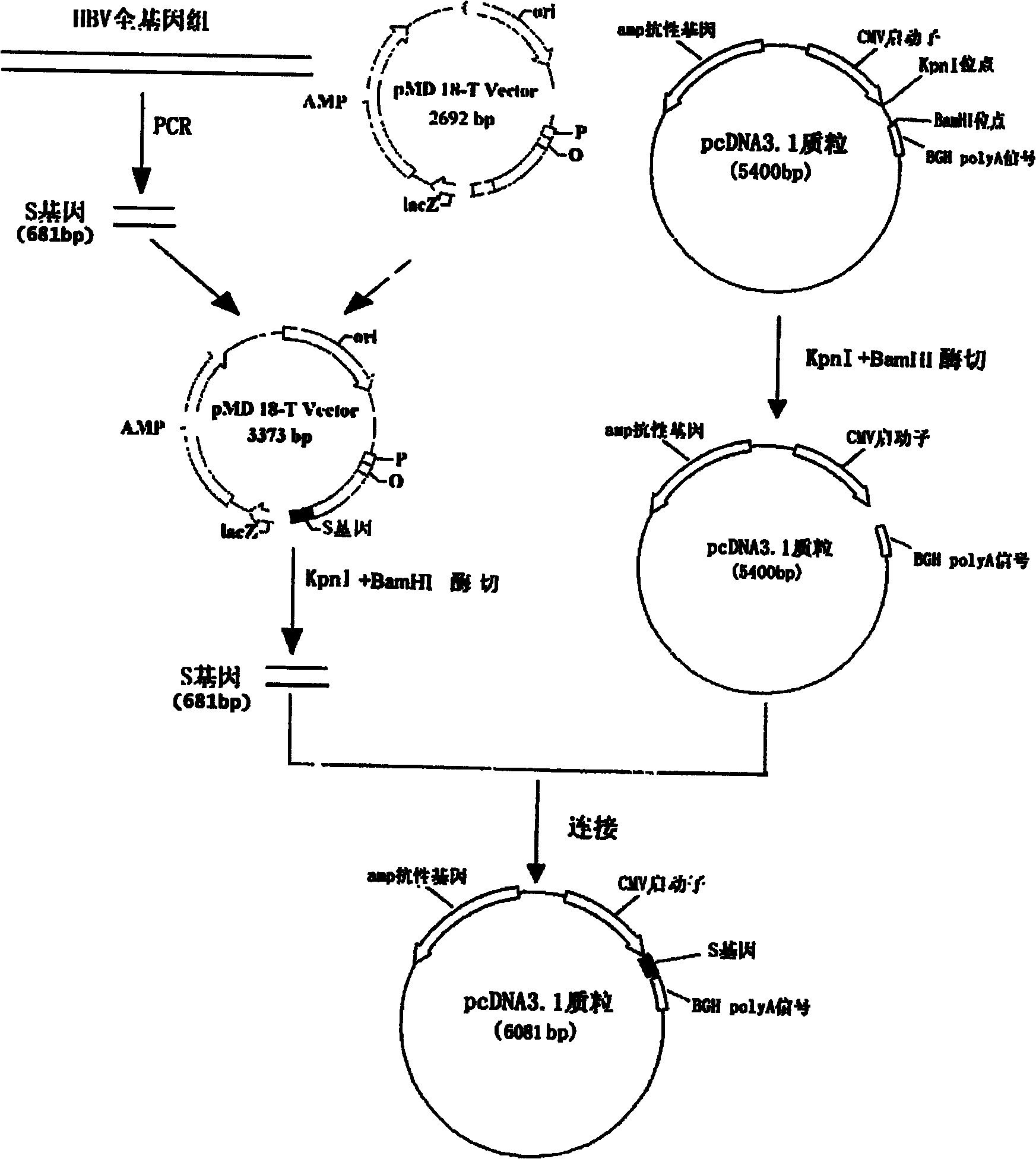
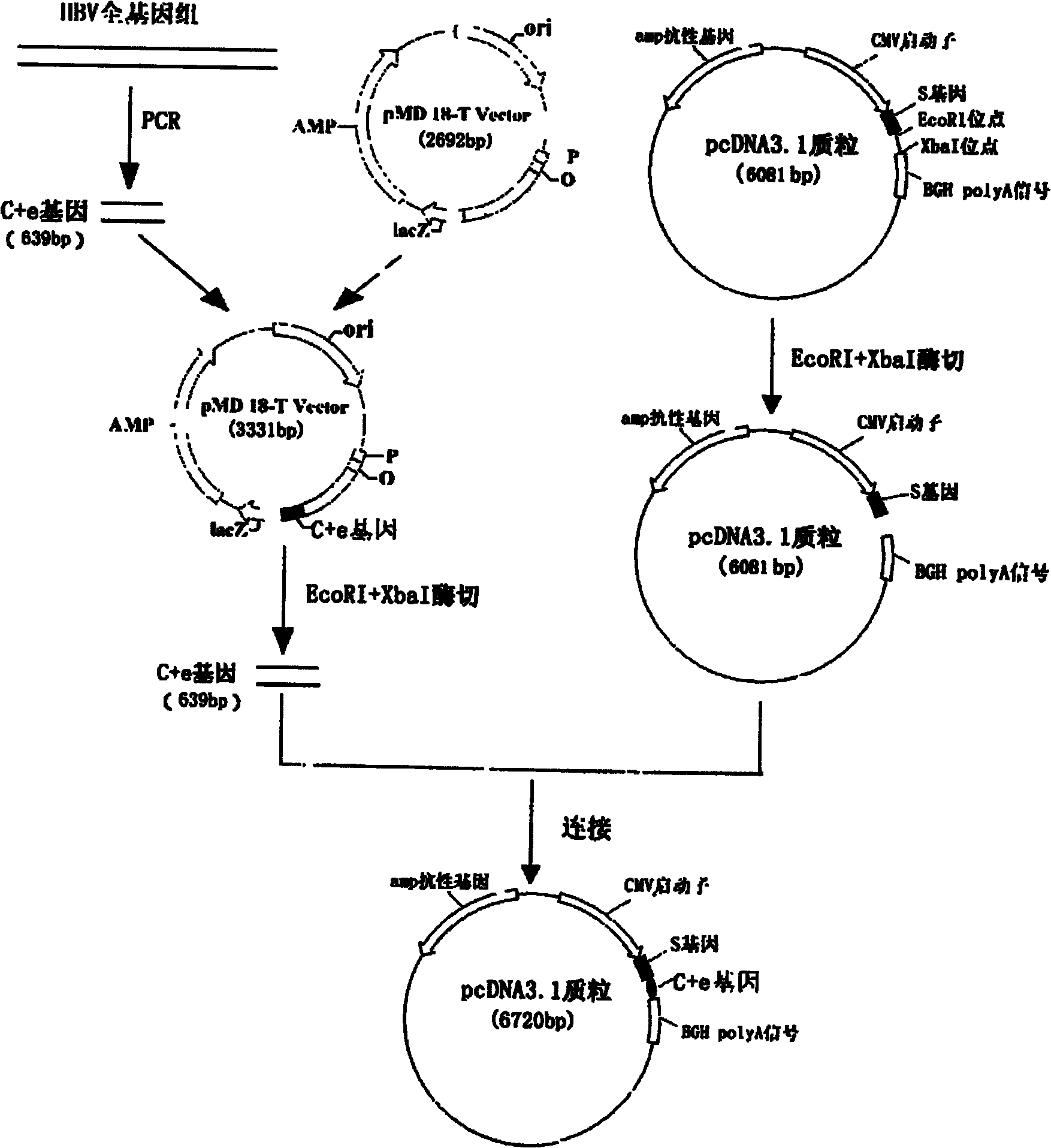
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for separating and purifying hepatitis B virus particles
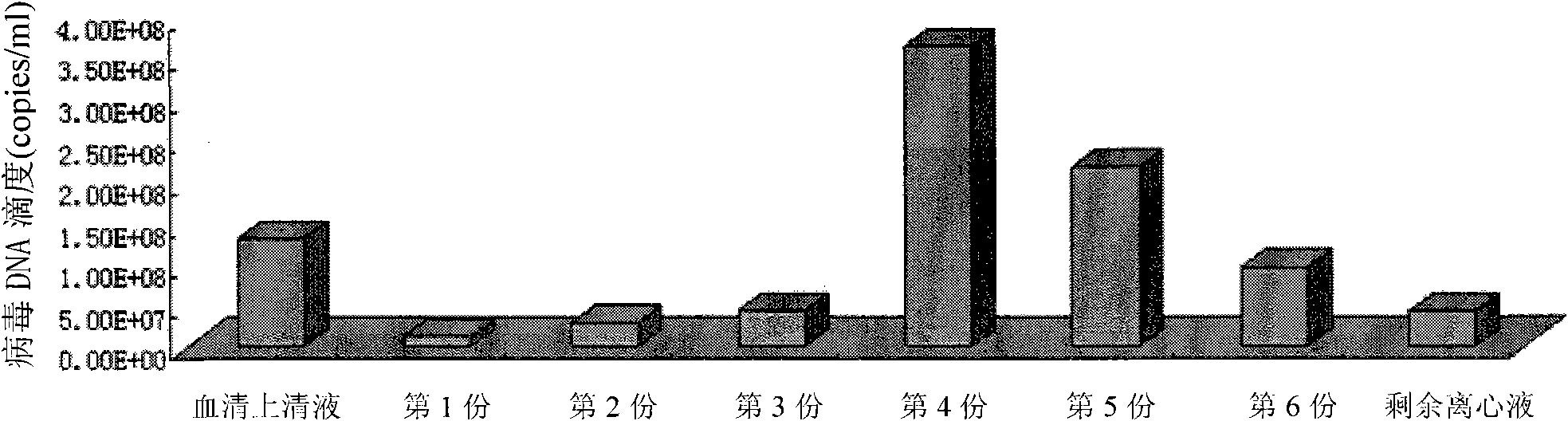
ActiveCN101875921AHigh yieldTypical HBV characteristicsRecovery/purificationSucroseHepatitis B virus DNA
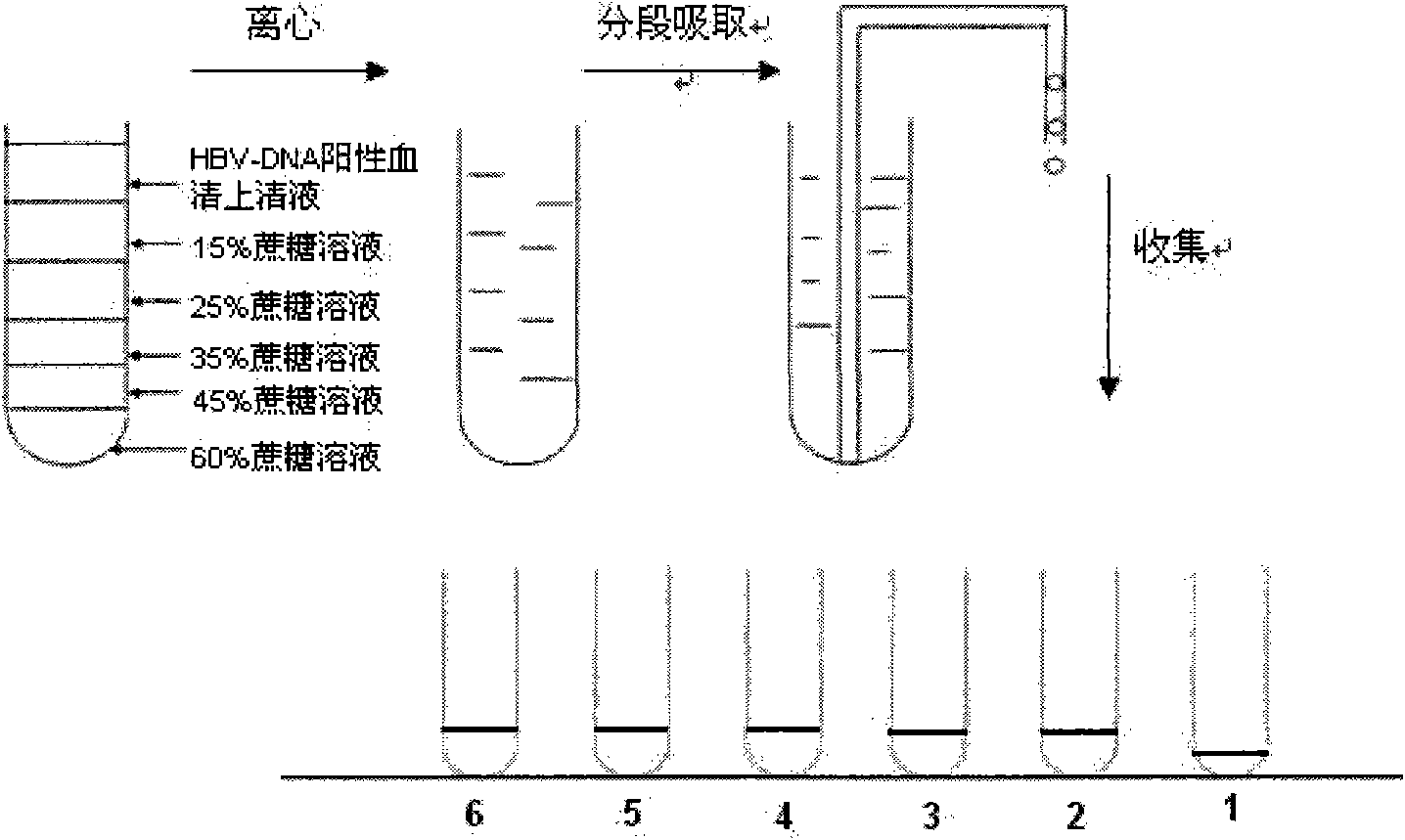
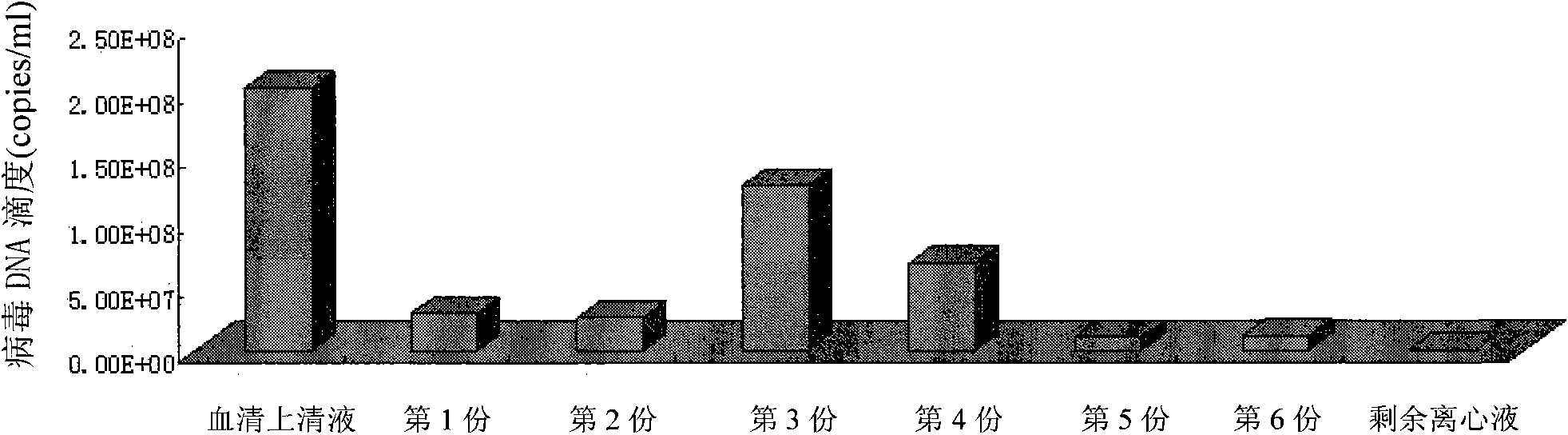
The invention discloses a method for separating and purifying hepatitis B virus particles, which comprises the following steps of: centrifuging 70,000 grams of hepatitis B virus DNA positive serum for 5 hours with discontinuous sucrose density gradients (60 percent, 45 percent, 35 percent, 25 percent and 15 percent), absorbing centrifugal liquid from the bottom of a centrifugal tube section by section, collecting the centrifugal liquid into different containers respectively, merging the colorless transparent collected liquid, and removing sucrose by ultra-filtration and centrifugation to obtain the hepatitis B virus particles. The method can efficiently separate and purify the hepatitis B virus particles in short time and has high virus particle yield; and the obtained virus particles have typical hepatitis B virus characteristics and good biological infection activity, and can effectively infect primary liver cells of tree shrews.
Owner:ARMY MEDICAL UNIV
Anti-hepatitis b virus X protein polypeptide drug
ActiveCN104744564AImprove stabilitySignificant pharmacological effectPeptide/protein ingredientsDigestive systemAntigenHepatitis B virus DNA
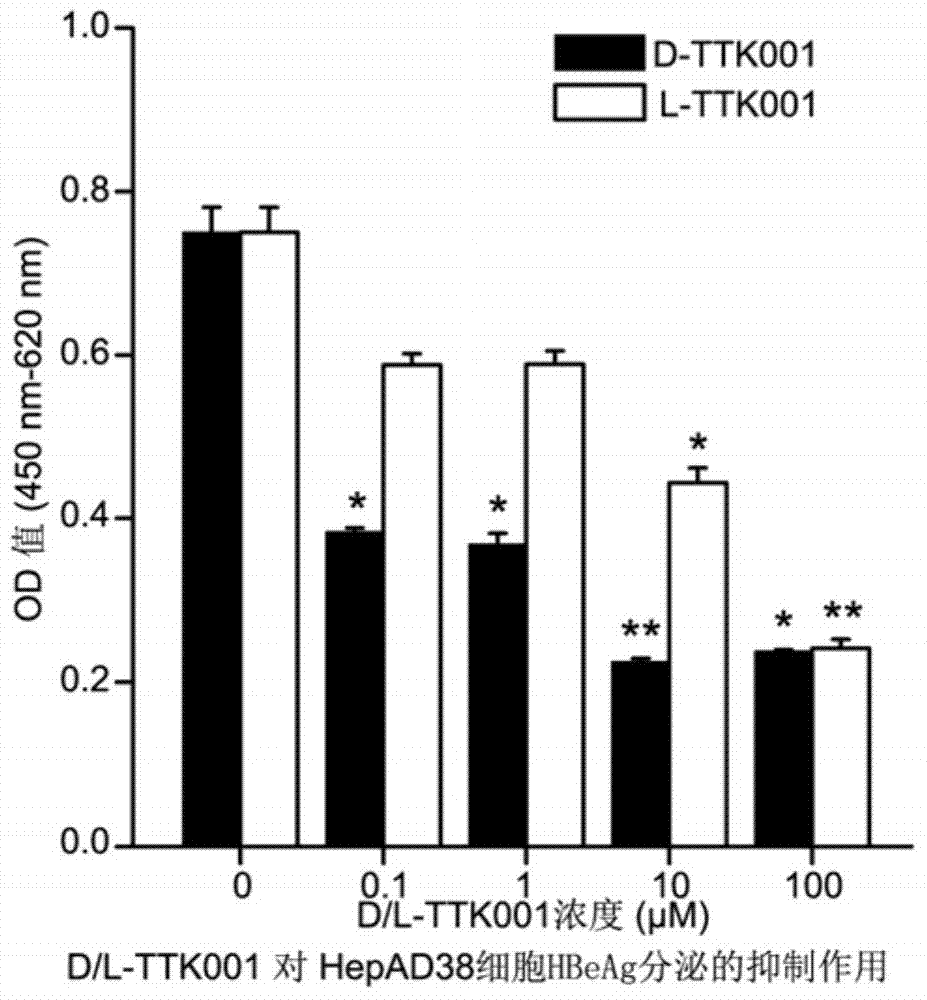
The invention relates to the field of polypeptide drugs and relates to a polypeptide of an anti-hepatitis b virus X protein and applications thereof. Particularly, the invention relates to a polypeptide containing a D-amino acid; the polypeptide has a function of inhibiting a hepatitis B virus X protein (HBx), has an effect of inhibiting the activity of the HBx at molecular level, cellular level and animal level and can inhibit the replication of hepatitis B virus DNA and the expression of associated antigens (such as HBeAg) and further inhibit hepatitis and cirrhosis caused by hepatitis B virus infection and hepatic carcinoma based on cirrhosis. The polypeptide can be widely applied to the prevention and treatment of liver diseases including hepatitis, cirrhosis and hepatic carcinoma caused by hepatitis B virus infection.
Owner:TIANJIN TOPTECH BIO SCI & TECH
5-oxygen-substituted benzene alkene propionyl quinic acid compounds and medicine uses thereof
The invention relates to 5-o-substitued phenyl propionyl quinic acid compounds and pharmaceutical application thereof, specifically 5-o-[3-substitued phenyl propionyl] quinic acid compounds having the formula of (I) and anti-hepatitis B virus activity and pharmaceutical salts thereof. The invention also relates to a preparation method of the quinic acid compounds, pharmaceutical application thereof, and drugs and pharmaceutical compositions containing the quinic acid compounds. The compounds having the formula of (I) and pharmaceutical salts thereof inhibit hepatitis B virus DNA (HBVDNA) replication and reduces hepatits B virus surface antigen (HBsAg) expression thereby to have prospect pharmaceutical application in the preparation of a drug for treating correlated hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
Peptide nucleic acid chip for detecting muatatonal site of hepatitis B virus and its preparing method
InactiveCN1431318AIncrease the Tm valueTm value decreasedMicrobiological testing/measurementSingle-Stranded RNAFluorescence
A peptide nucleic acid chip for detecting the mutation sites of hepatitis B virus is composed of glass substrate or nylon film, peptide nucleic acid probes array and reference point-type coated layer. Its preparing process includes PCR amplification of hepatitis B virus DNA speciment, fluorescence labelling, and hybridization. The mutation sites can be determined according to the intensity of hybrid signals. Its advantages are high speed, high effect, and high correctness.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
A quantitative method for hbv-dna, a primer and probe for detecting hbv-dna, and a detecting kit comprising the same
InactiveCN101400802AHigh sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationHepatitis B virus DNAVirology
The present invention relates to a method for quantifying hepatitis B virus DNA (HBA-DNA) by means of the PCR hybridization process, and to primers and probes used for such method. The present invention also relates to a kit for detecting HBV-DNA comprising said primers and probes. Specifically, the present invention is characterized in that said HBV is either an adr sub-type or an adw sub-type.
Owner:凯奇拜基因有限公司 +1
Kit for detecting hepatitis B virus
InactiveCN101824493AHigh sensitivityEasy to operateMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementHepatitis B virus DNANanoparticle
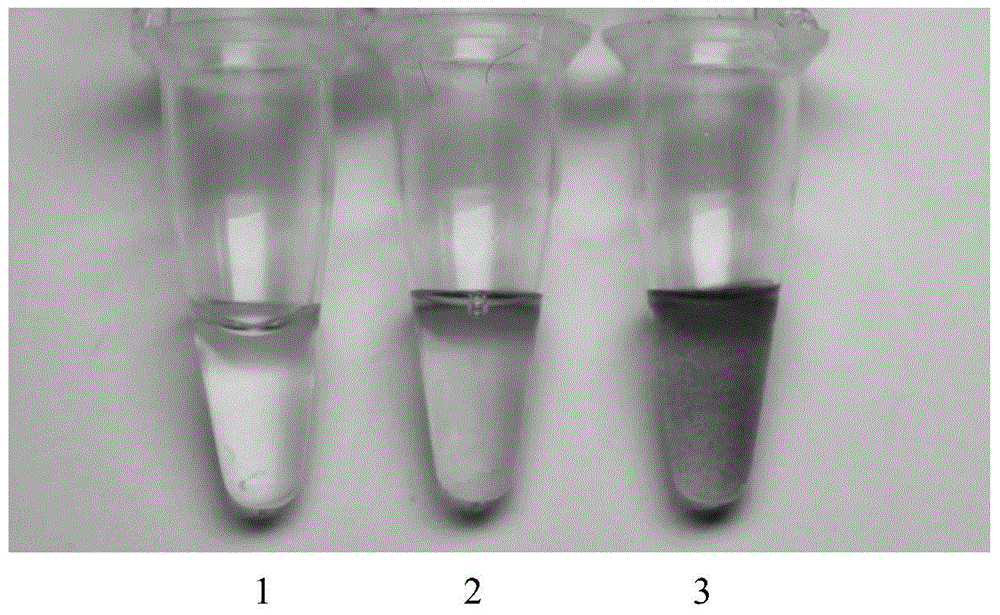
The invention relates to a kit for detecting product DNA of hepatitis B virus DNA amplified through a loop-mediated isothermal nucleic acid amplification technology by using gold nanoparticles, belonging to the technical field of biological detection. In combination with the loop-mediated isothermal nucleic acid amplification technology and a biological nanotechnology, the invention has the advantages that the sensitivity of biological molecular detection is improved, the operation of the method is simple, the detection is rapid, the accuracy is high, special instruments and devices are not required, and the kit can be widely used for high-sensitivity hepatitis B virus detection in fields such as family diagnosis, clinical diagnosis, infectious disease control, environmental monitoring, inspection and quarantine, biotechnology and the like. The kit comprises: (1) gold nanoparticles labeled with molecular probes capable of identifying specific sequences of hepatitis B viruses; and (2) a loop-mediated isothermal nucleic acid amplification system capable of amplifying hepatitis B virus DNA in a sample to be detected or capable of amplifying hepatitis B virus RNA in the sample to be detected after reverse transcription.
Owner:天津朝海科技有限公司
Method and kit for assaying hepatitis B virus DNA (deoxyribonucleic acid) sequence
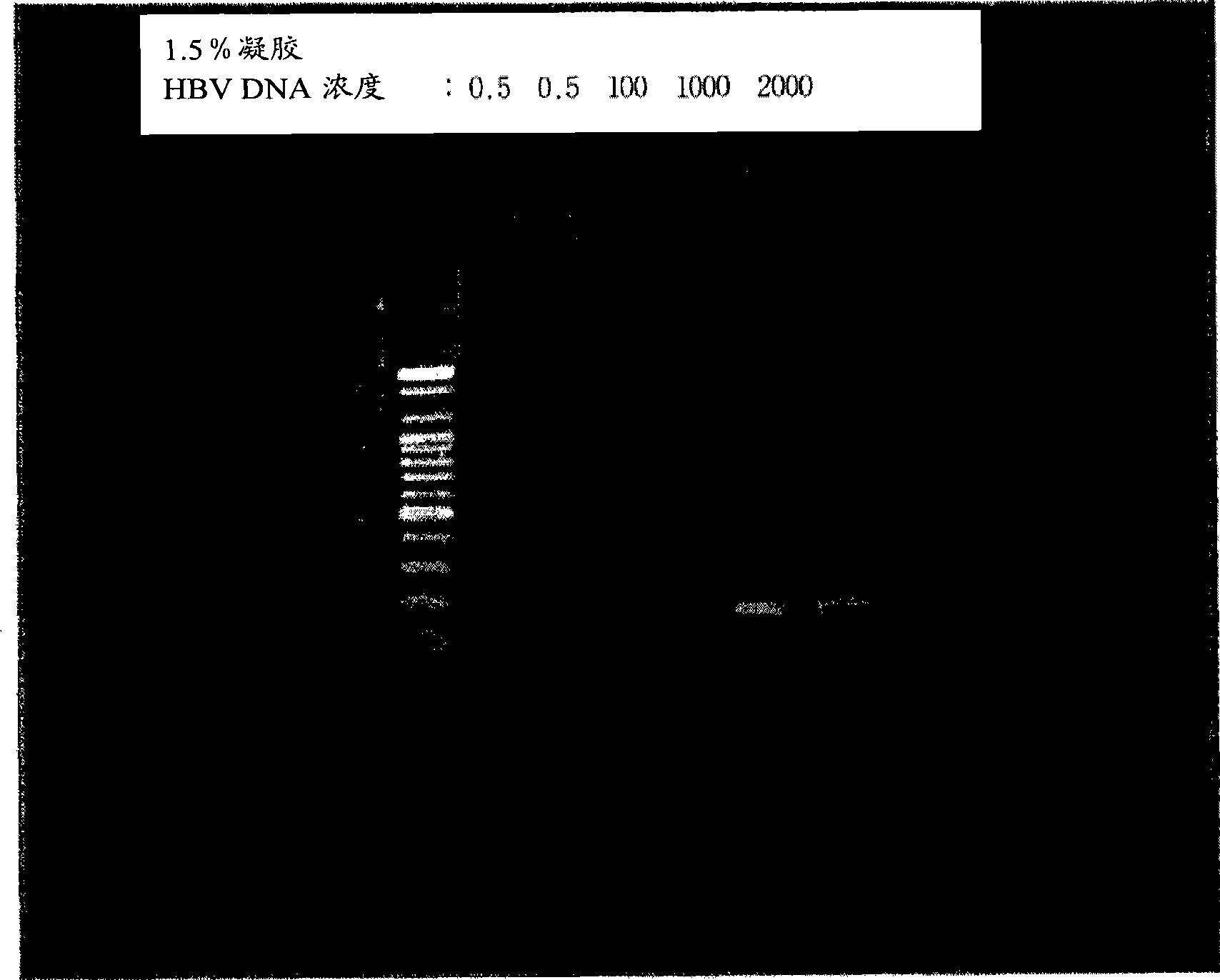
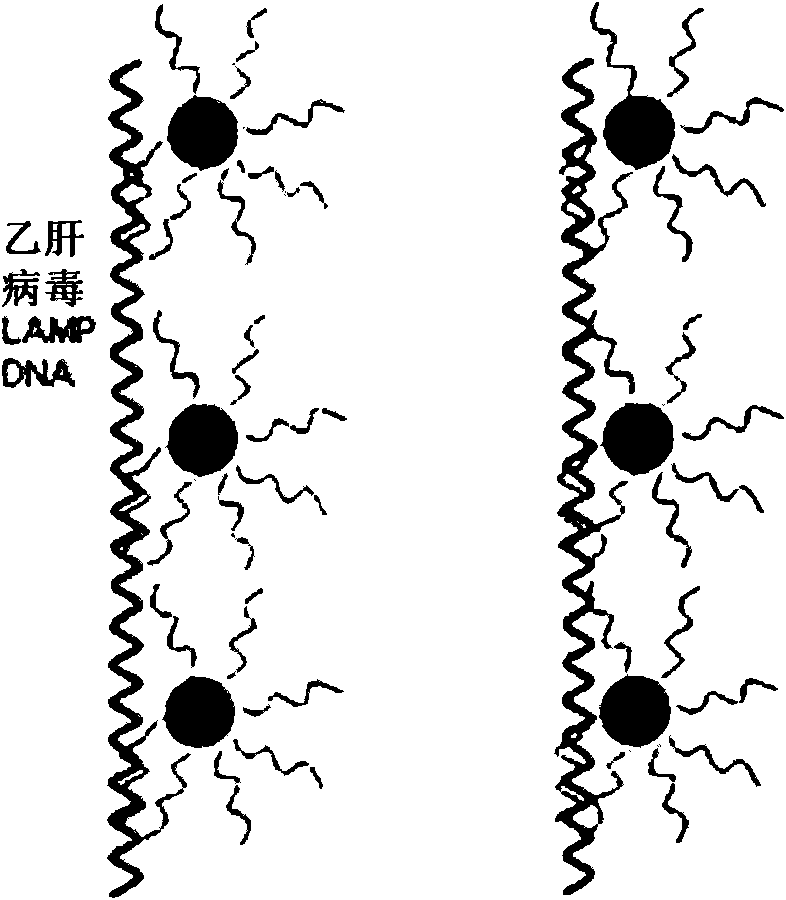
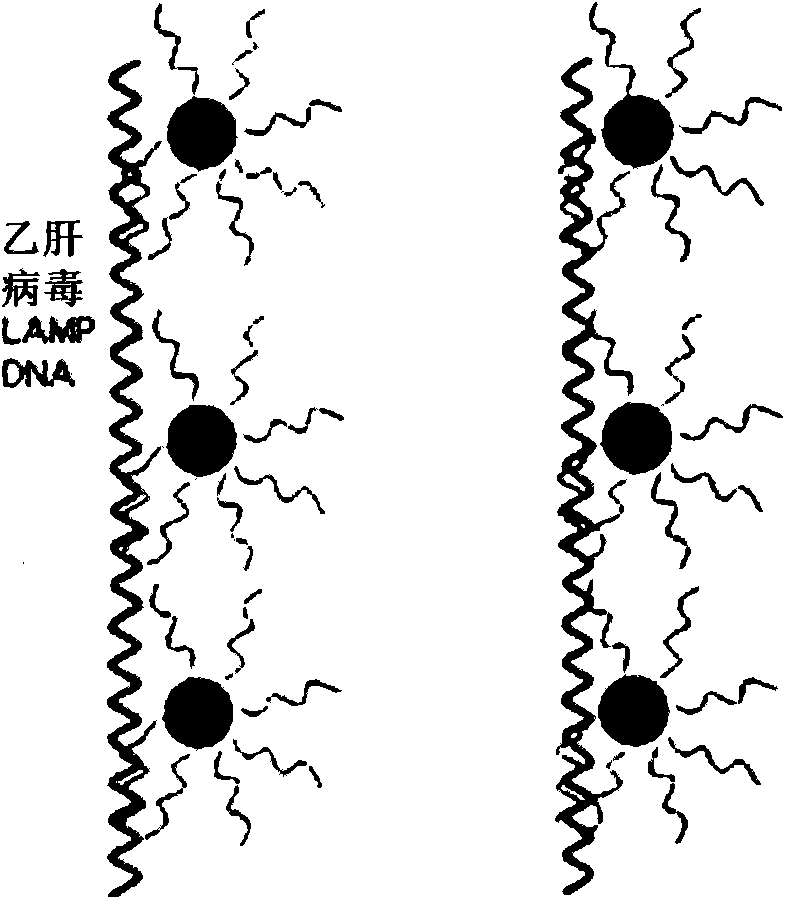
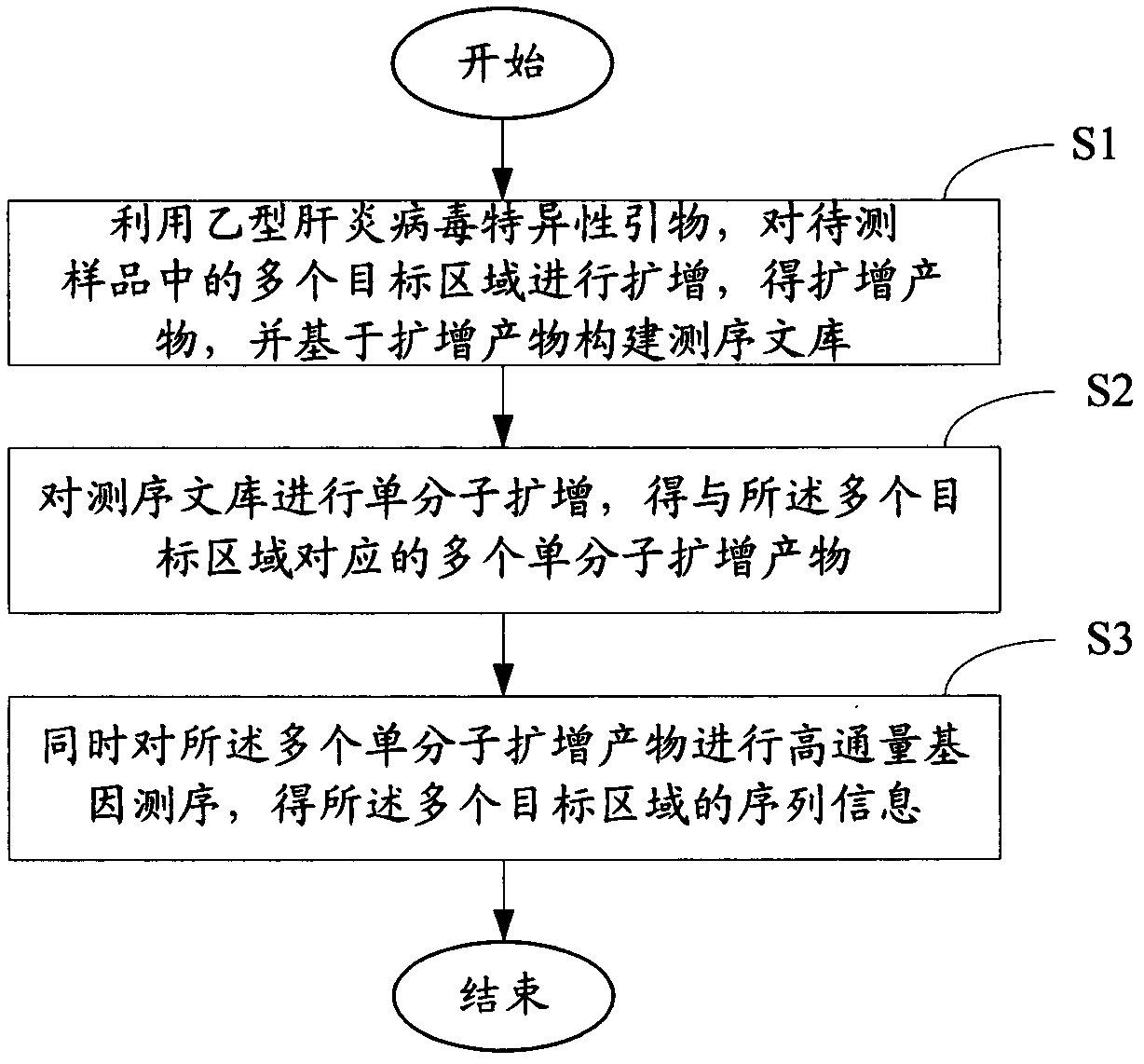
ActiveCN102586472AImprove detection efficiencyImprove accuracyMicrobiological testing/measurementMicroorganism based processesHepatitis B virus DNAHepatitis B virus
The invention relates to the field of genetic engineering, and provides a method and a kit for assaying the hepatitis B virus DNA (deoxyribonucleic acid) sequence. The method includes the following steps: (A) hepatitis B virus specific primers are utilized to amplify a plurality of target regions in a sample to be assayed, and a sequencing library is constructed on the basis of amplification products; (B) the single-molecule amplification of the sequencing library is carried out, so that a plurality of single-molecule amplification products corresponding to the target regions are obtained; (C) the high-throughput gene sequencing of the single-molecule amplification products is carried out simultaneously, so that the sequence information of the target regions is obtained. The kit comprises the hepatitis B virus specific primers and a connector element. The method and the kit can carry out deep region sequencing on a plurality of target regions of hepatitis B virus at the same time, consequently, the efficiency of assay is increased, meanwhile, the accuracy and sensitivity of assay can be increased as well, and furthermore, multi-region sequencing can be carried out on a large quantity of samples at the same time.
Owner:GUANGZHOU KANGXINRUI GENE HEALTH TECH CO LTD
Nucleic acid releasing agent and hepatitis B virus (HBV) nucleic acid releasing method
The invention discloses a nucleic acid releasing agent and a hepatitis B virus nucleic acid releasing method. The nucleic acid releasing agent comprises 0.02 to 0.2 vol.% of Gemini quaternary ammoniumsalt surfactant, 50 to 300 mM of potassium hydroxide, isopropanol (0.2-2 mL / 100 mL), and the balance being sterile water. The nucleic acid releasing agent with a trace amount can rapidly destroy theprotein structure of the shell of pathogen, encloses and precipitates substances that can influence the PCR amplification such as serum proteins, and makes HBV DNA carry out complexing so as to completely precipitate out HBV DNA. The releasing agent is safe to use, the operation is simple and convenient, and the manufacturing cost is low. The releasing agent is used to release HBV DNA of a serum,blood plasma, or whole blood sample without heating or centrifugation; only through one-step sample loading, DNA can be released and extracted. According to the method, HBV nucleic acid extraction andamplification-fluorescence PCR quantitative detection can be carried out in one step, and the problems of loss and contamination of nucleic acid in a multi-step nucleic acid extraction method are solved.
Owner:PULUOMAIGE BIOLOGICAL PRODS SHANGHAI
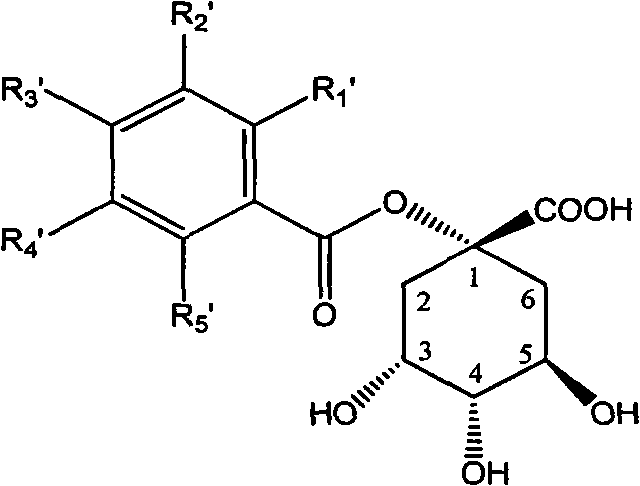
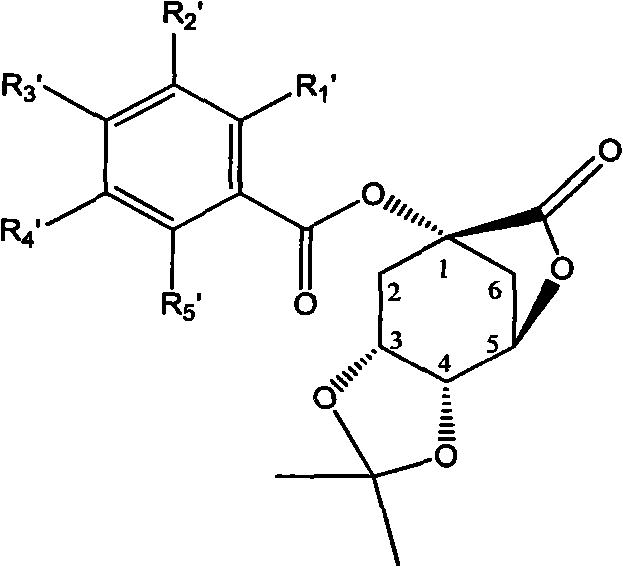
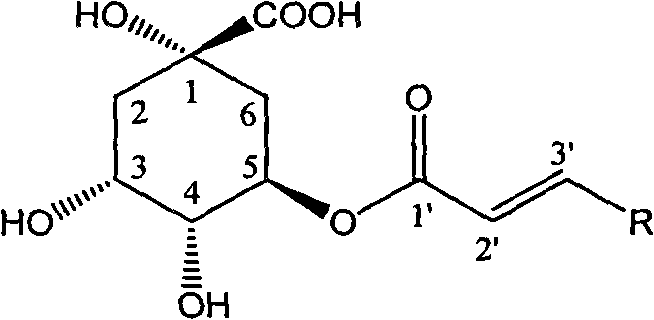
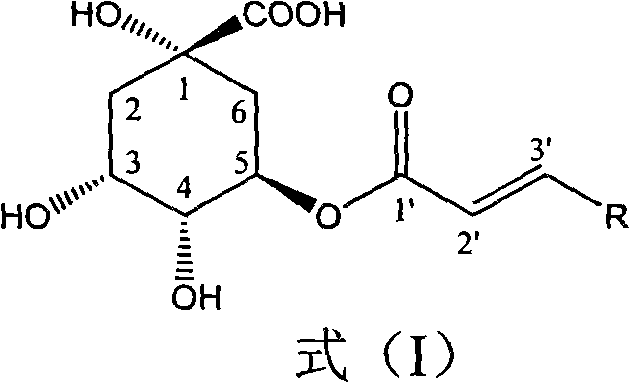
1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses
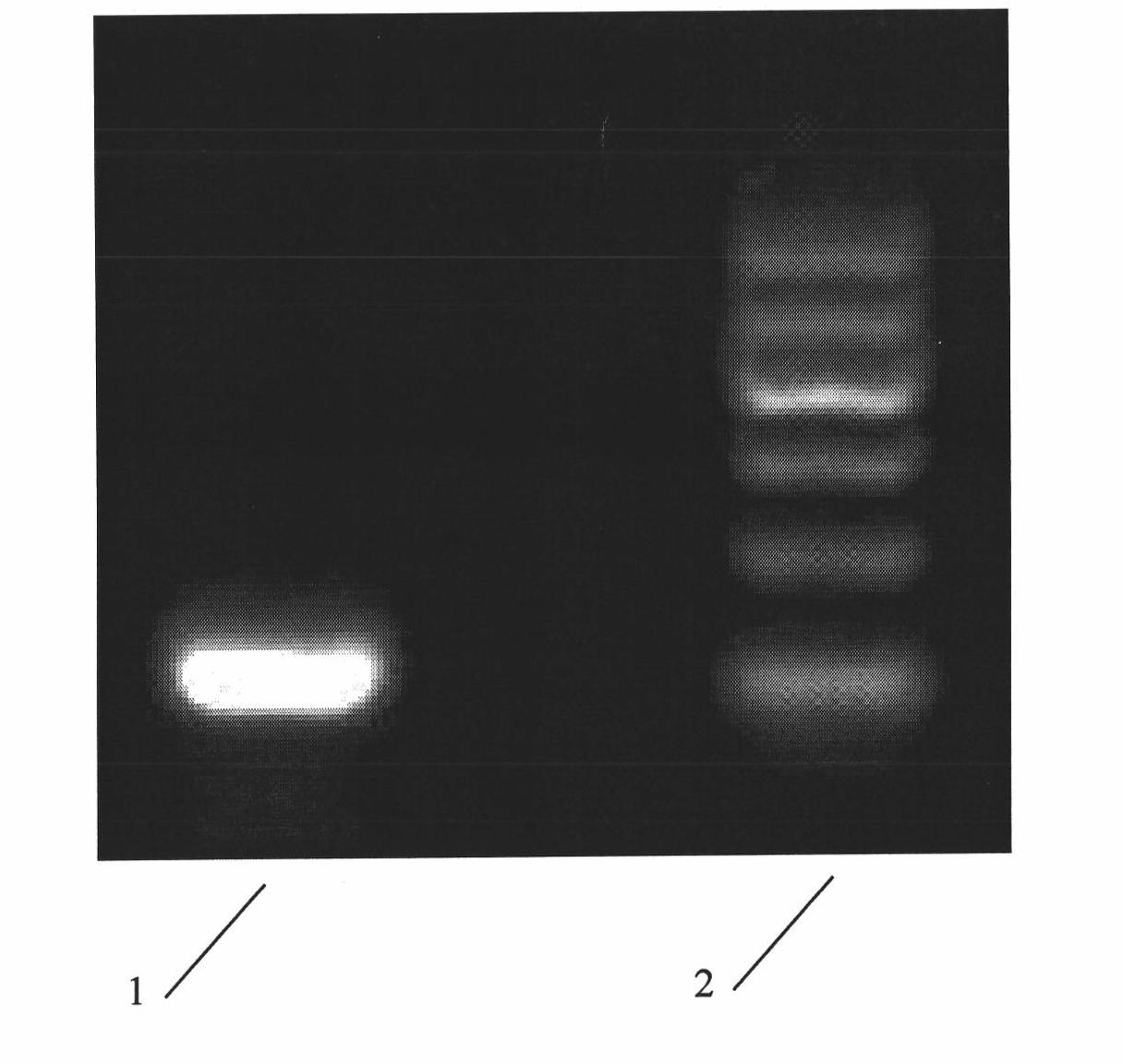
InactiveCN101293830AOrganic active ingredientsOrganic chemistryHepatitis B virus DNAHepatitis B virus
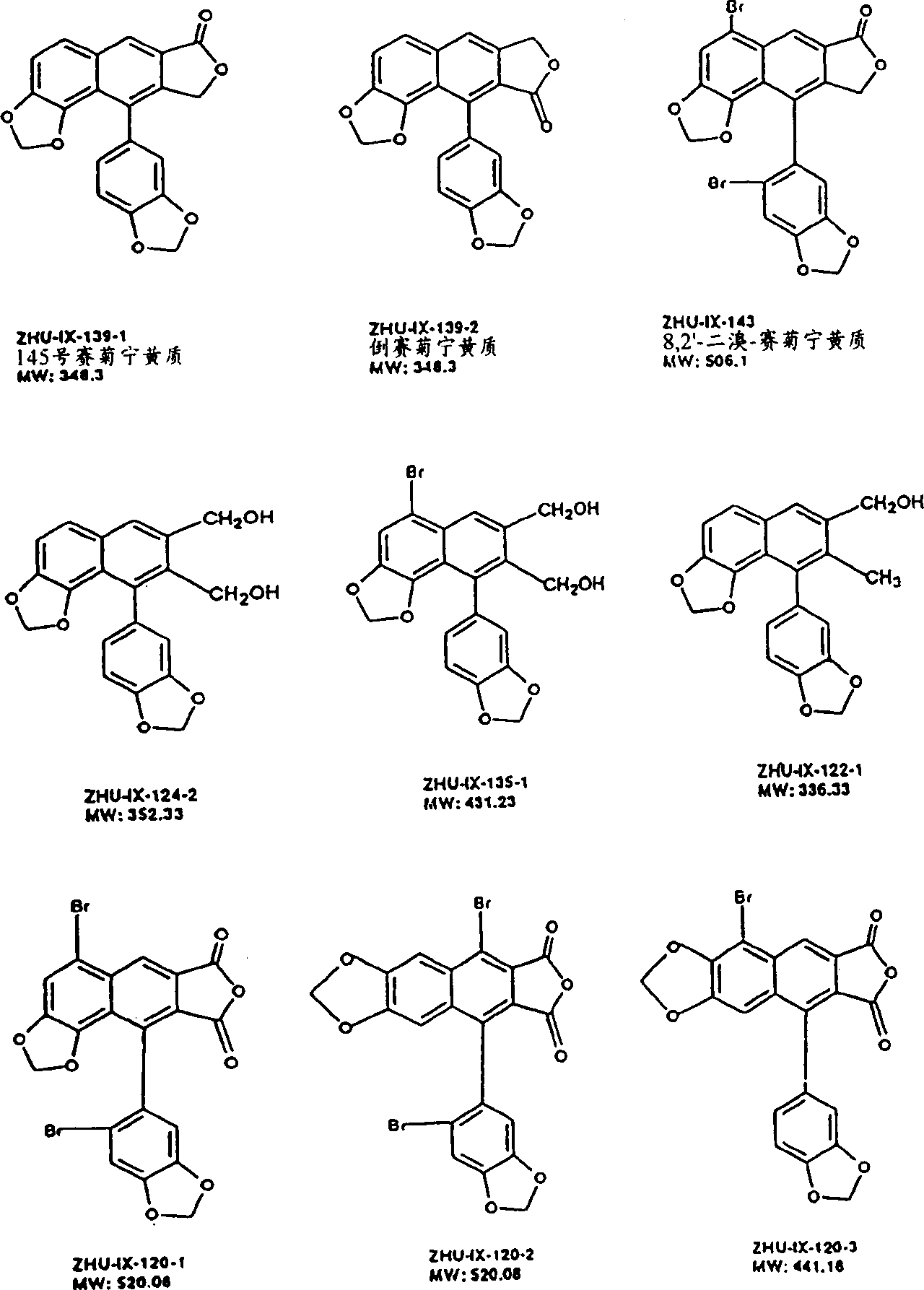
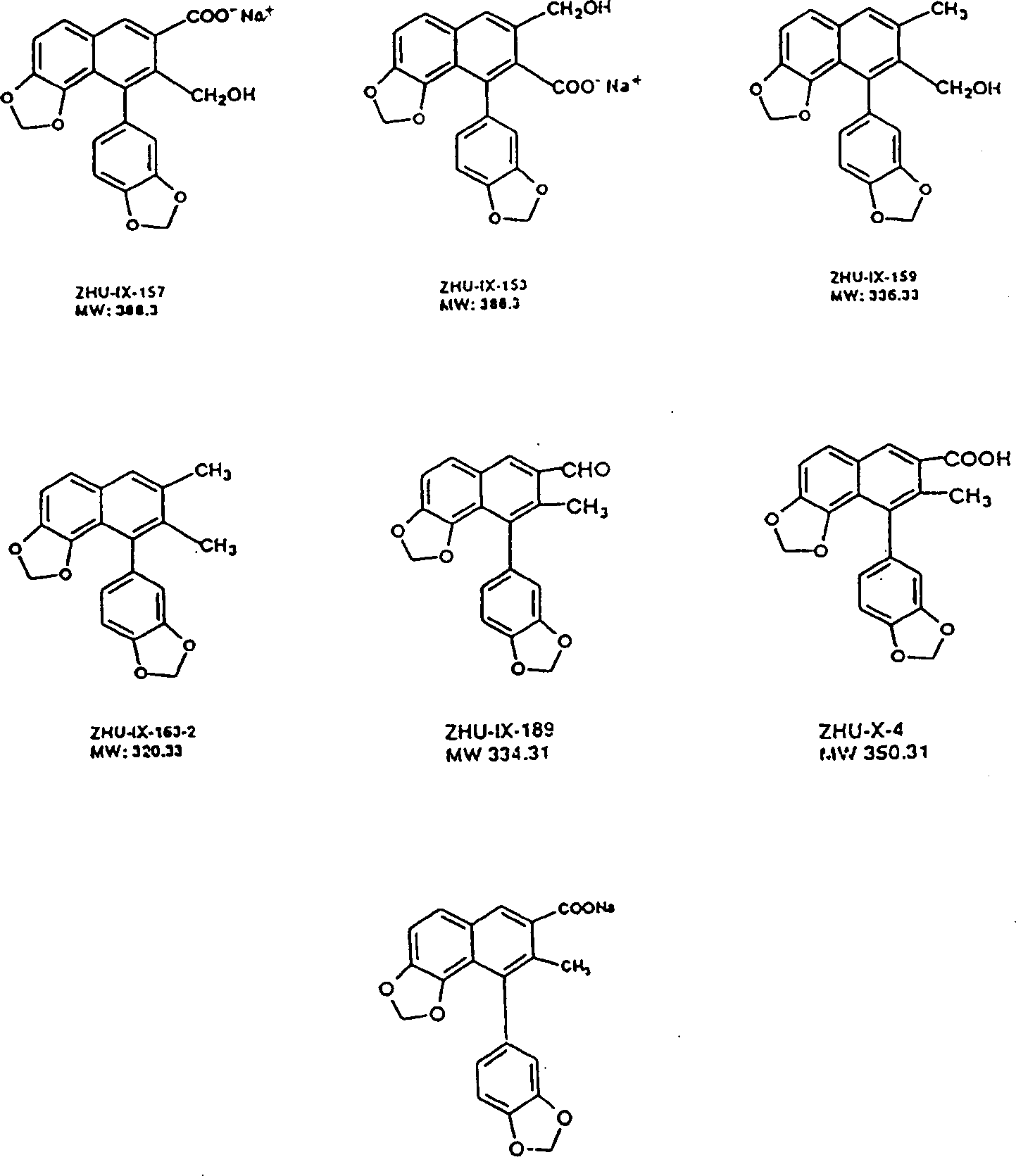
The invention relates to 1-o-[3-aryl substituted-acrylyl]quinic acid compounds having the formula of (I), and an application thereof. The invention also relates to a preparation method of the quinic acid compounds, and intermediate compounds (II) of the quinic acid compound, i.e. 1-o-[3-aryl substituted-acrylyl]-3,4-o-isopropylidene quinic acid-1.5-lactone. The invention relates to pharmaceutical application of the quinic acid compounds, and pharmaceutical compositions containing the same. The compounds having the formula (I) and the compounds having the formula of (II), and pharmaceutical salts thereof have the effects of inhibiting hepatitis B virus DNA replication and reducing hepatitis B virus surface antigen expression. Thus, the compounds having the formula of (I) and the compounds having the formula of (II) have pharmaceutical prospect application in the preparation of a drug for preventing and treating hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
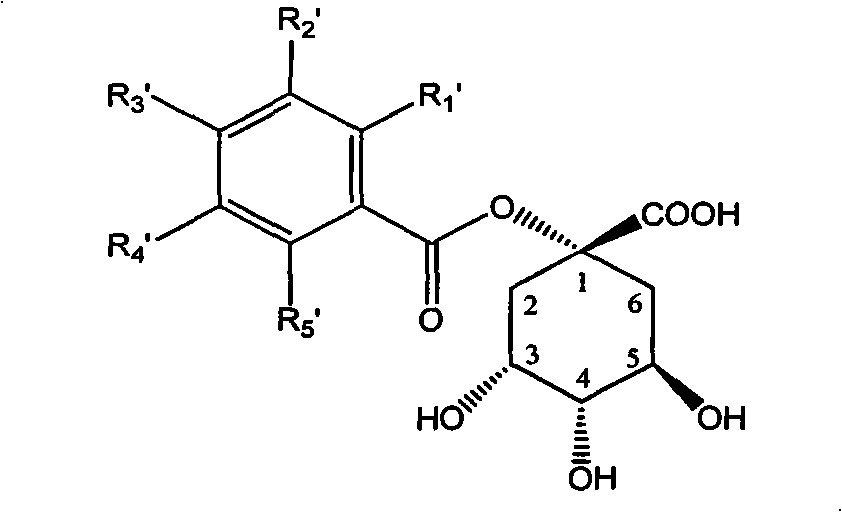
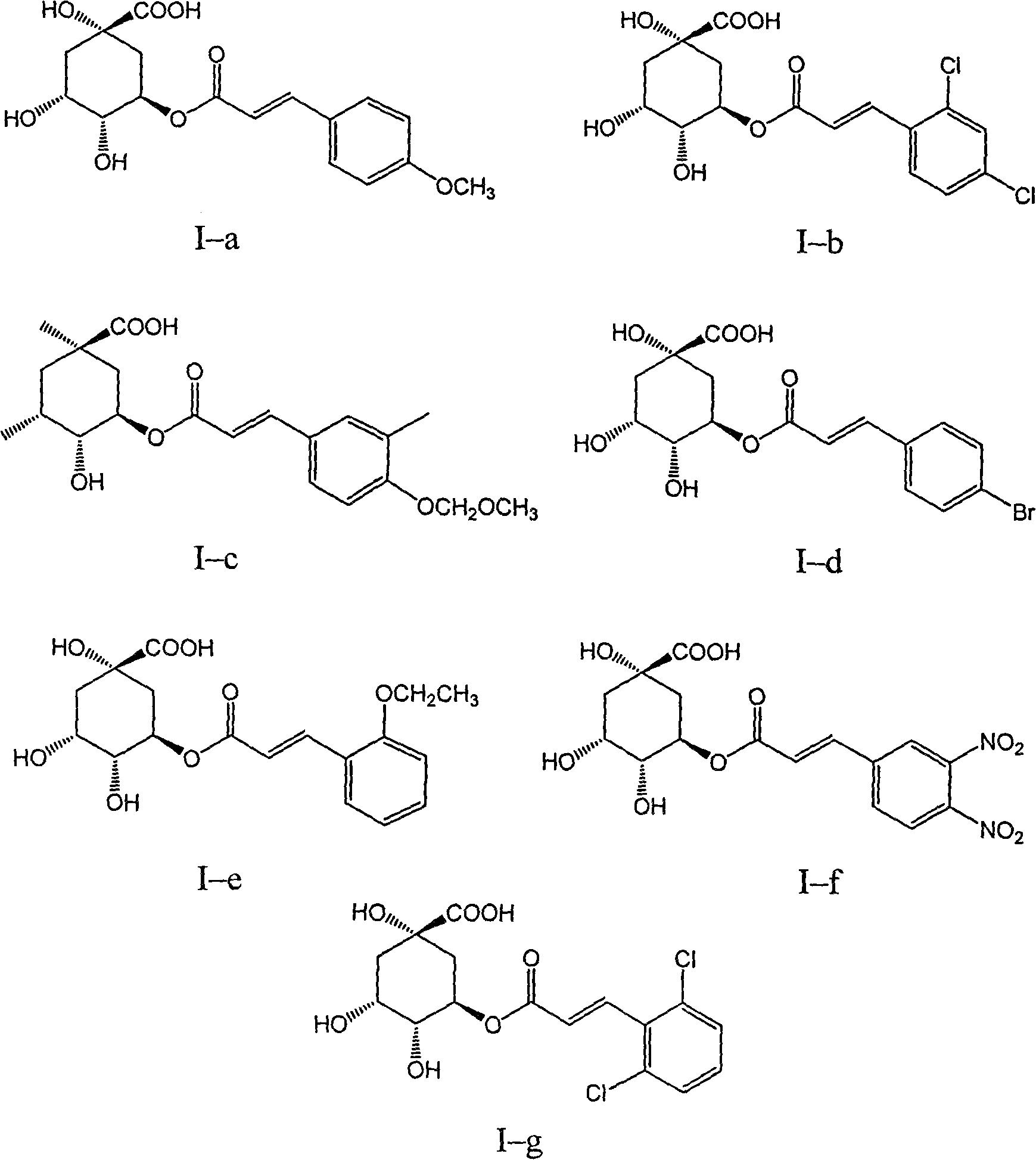
3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof
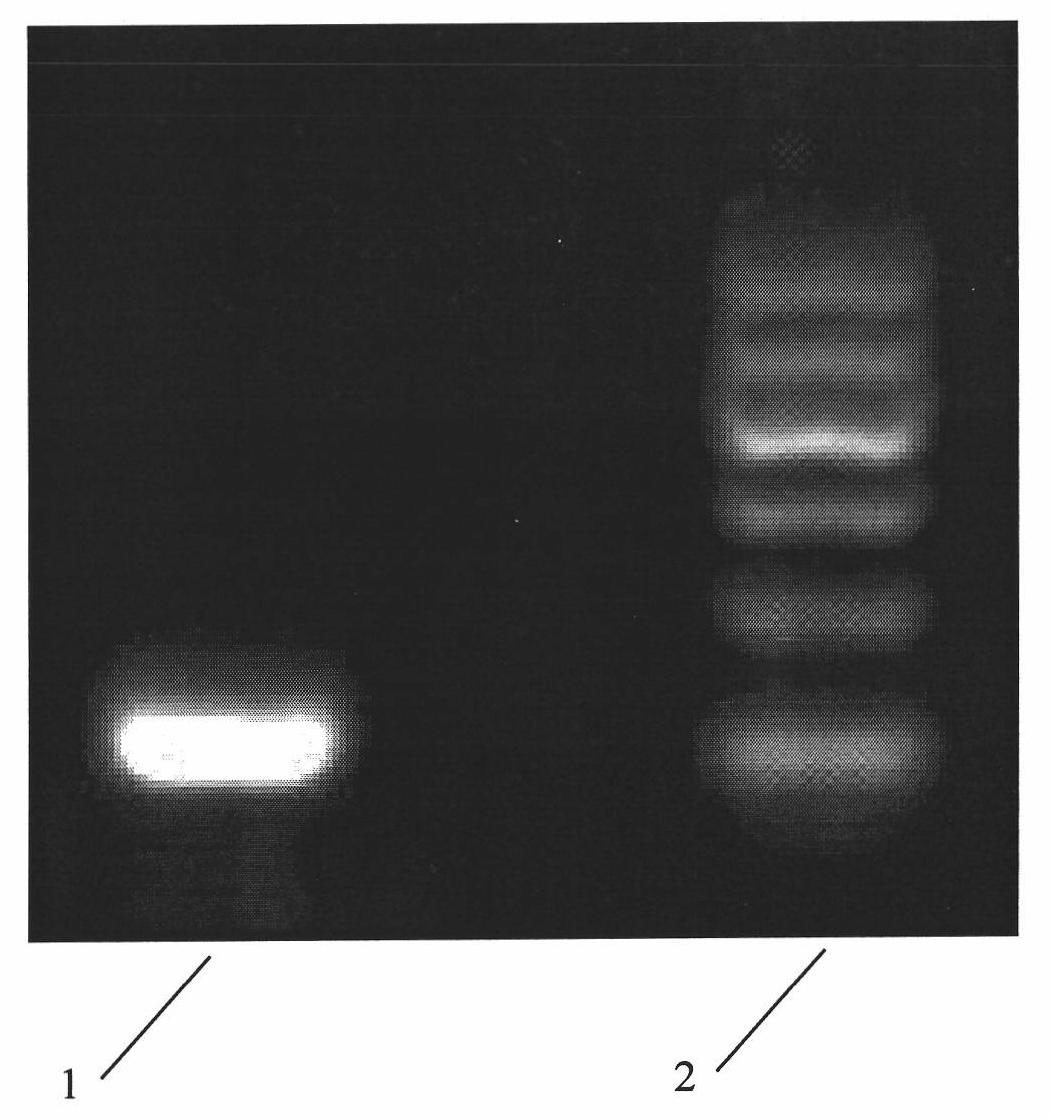
InactiveCN101293834AOrganic active ingredientsOrganic compound preparationAntigenHepatitis B virus DNA
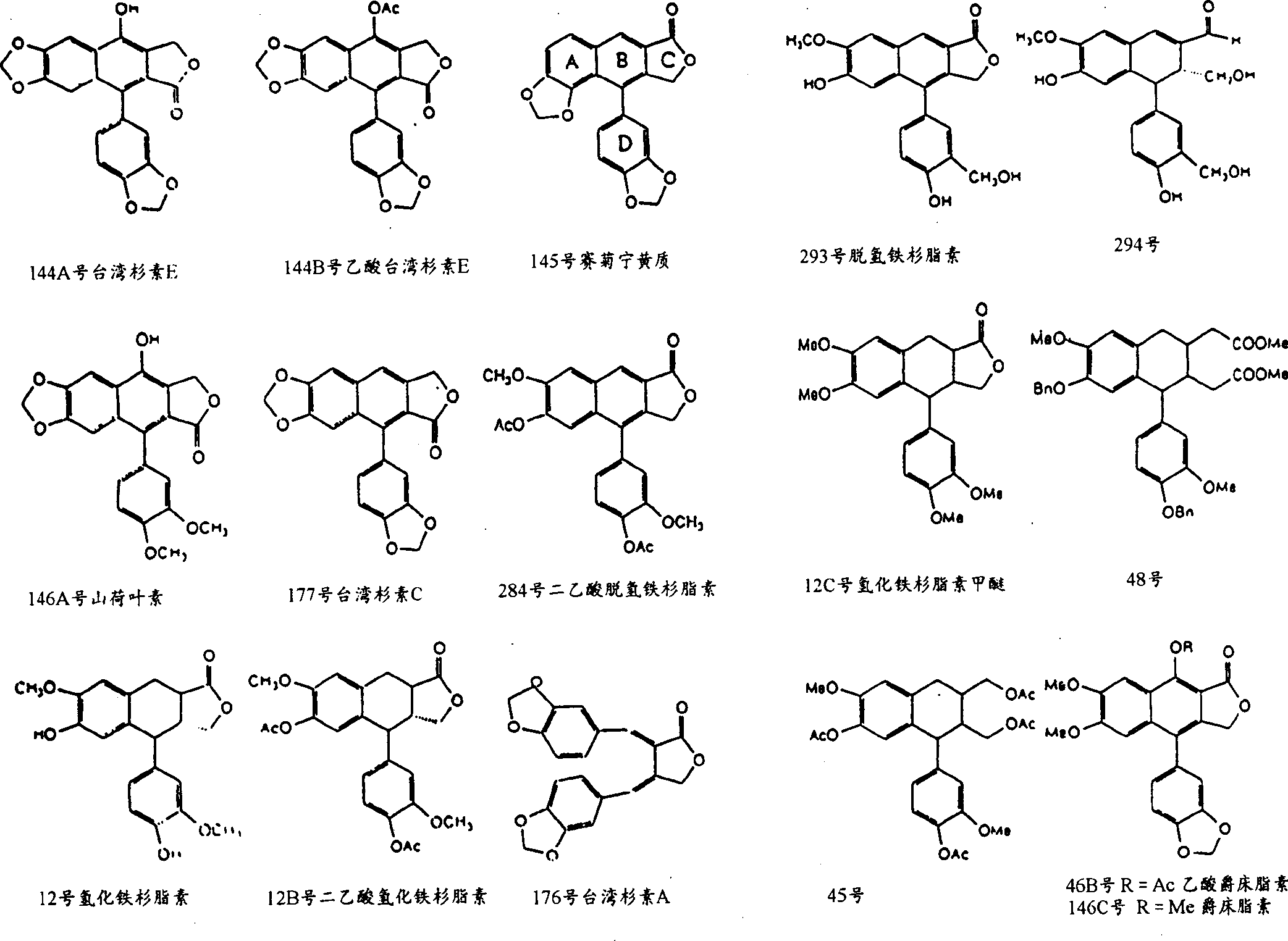
The invention relates to 3,4-di-o-[3-substitued phenyl propionyl] quinic acid compounds having the formula of (I) and anti-hepatitis B virus activity and a pharmaceutical application thereof. The invention also relates to key intermediate compounds (II) for preparing the quinic acid compounds having the formula of (I), pharmaceutical application of the compounds having the formula of (I) and the compounds having the formula of (II), and drugs and pharmaceutical compositions containing the compounds (I) and (II). The compounds (I) and (II) inhibit hepatitis B virus DNA (HBVDNA) replication and reduces hepatits B virus surface antigen (HBsAg) expression. Thus, the compounds (I) and (II) and pharmaceutical application thereof have prospect pharmaceutical application in the preparation of a drug for treating hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
Benzohetercyclic compound as well as preparation method and applications thereof
ActiveCN102219725BInhibition of replicationSimple structureOrganic active ingredientsOrganic chemistryHepatitis c rnaHepatitis B virus DNA
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Traditional Chinese medicine drug for eliminating hepatitis B virus
InactiveCN105664064AFree from destructionEasy to useAntiviralsUnknown materialsHepatitis B virus DNAFlos chrysanthemi
The invention discloses a traditional Chinese medicine drug for eliminating hepatitis B virus. The traditional Chinese medicine drug comprises the following raw materials in parts by weight: 4-7 parts of Chinese angelica, 3-6 parts of radix curcumae, 3-6 parts of bear gall powder, 3-6 parts of liquorice, 7-10 parts of radix isatidis, 9-12 parts of radix paeoniae alba, 7-10 parts of lucid ganoderma, 3-6 parts of bitter gourd, 2-5 parts of flos chrysanthemi, 3-6 parts of schisandra chinensis, 4-7 parts of orange peel, 9-11 parts of rhizoma dioscoreae, 3-6 parts of poria cocos, 3-5 parts of ligusticum wallichii, 3-6 parts of lycium barbarum, 3-6 parts of gorgon euryale seed, 9-12 parts of Chinese date, 2-5 parts of stevia rebaudian and 3-6 parts of radix bupleuri. The traditional Chinese medicine drug is a natural pure traditional Chinese medicine and has important functions of soothing the liver and promoting bile secretion, relieving liver qi depression and liver depression transforming into fire, clearing heat and removing toxin and restoring liver functions, avoids damage to the liver and liver cells and is safe and healthy to use. The DNA replication of hepatitis B virus is reduced to zero; and the traditional Chinese medicine drug is safe and healthy, can completely overcome the shortcomings of western medicine treatment of hepatitis B virus and deserves large-area application and popularization.
Owner:徐世棋
SYBR GreenI fluorogenic quantitative PCR detection method for hepatitis B virus
PendingCN109251997AMicrobiological testing/measurementMicroorganism based processesConserved sequenceHepatitis B virus DNA
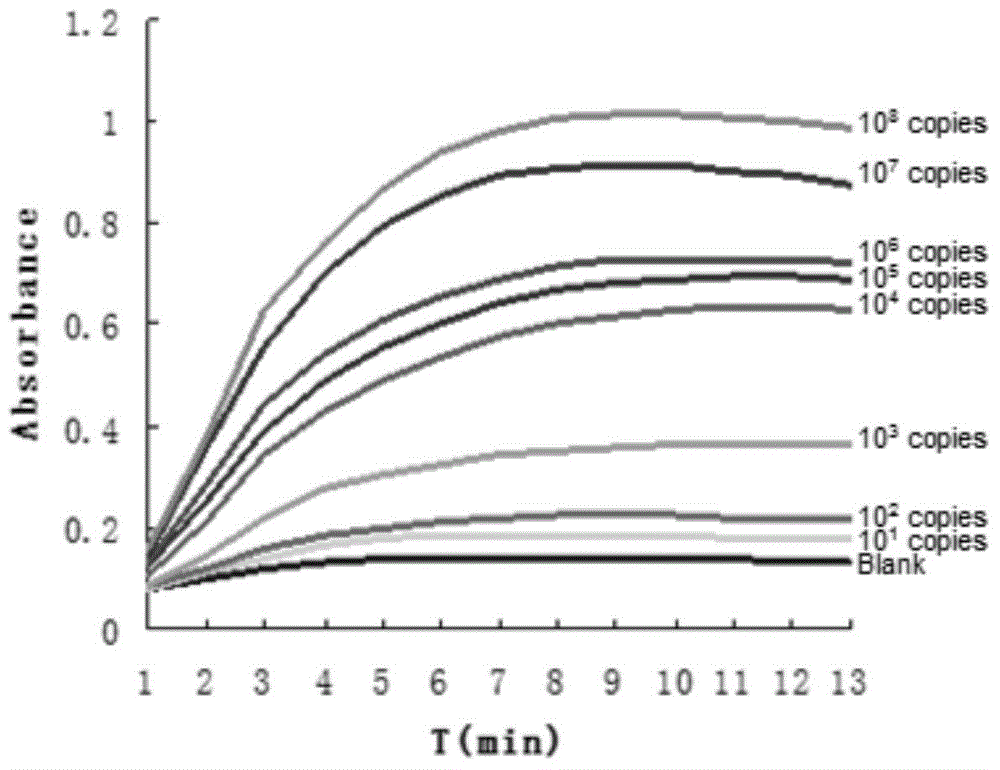
The invention establishes a rapid SYBR GreenI fluorogenic quantitative PCR detection method for hepatitis B virus. A primer is designed based on the conserved sequence of the C gene region of hepatitis B virus DNA published by GenBank, and the method for detecting hepatitis B virus and conducting negative / positive result determination through SYBR GreenI fluorogenic quantitative PCR detection is established. The method of the invention has the advantages of high sensitivity, good repeatability, high specificity, low cost, short time consumption and accurate detection.
Owner:重庆高圣生物医药有限责任公司
Method for detecting hepatitis B virus DNA and G1896A mutation thereof and kit
ActiveCN101338343BImprove accuracyNo missed detectionMicrobiological testing/measurementHepatitis B virus DNAWild type
The invention relates to a detecting method and a reagent box for the DNA of the hepatitis B viruses of wild type and G1896A mutant, in particular to a method for detecting the DNA of the hepatitis B viruses of wild type and G1896A mutant by using the technology of combining the Locked Nucleic Acids with a molecular beacon probe as well as a real time PCR method and a reagent box used for finishing the detection. The method of the invention can specially and sensitively detect the DNA of the hepatitis B viruses of wild type and G1896A mutant in a clinic blood sample.
Owner:INTEC PROD INC
Inhibition and treatment of hepatitis B virus and flavivirus by helioxanthin and its analogs
This invention relates to anti-viral drugs such as Helioxanthin and its analogs. The present compounds may be used alone or in combination with other drugs for the treatment of Hepatitis B virus (HBV), Hepatitis C virus (HCV), Yellow Fever, Dengue Virus, Japanese Encephalitis, West Nile virus and related flaviviruses. In addition, compounds according to the present invention can be used to prevent hepatoma secondary to virus infection as well as other infections or disease states which are secondary to the virus infection.
Owner:YALE UNIV +3
Primer probe and kit for detecting hepatitis B virus based on RAA fluorescence method
InactiveCN109055614AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesHepatitis B virus DNAFluorescence
The invention provides a primer probe group for detecting hepatitis B virus based on RAA fluorescence method, and belongs to the technical field of molecular biological detection. The primer probe group comprises an upstream primer, a downstream primer and a probe, wherein the nucleotide sequence of the upstream primer is shown by SEQ ID NO.1, the nucleotide sequence of the downstream primer is shown by SEQ ID NO.2, and the nucleotide sequence of the probe is shown by SEQ ID NO.3; the probe is a modified by a fluorescence reporter group and a fluorescence quenching group. By adopting the primer probe group provided by the invention, detection of the hepatitis B virus DNA can be finished within 15min, high temperature is not required for DNA unwinding, and the detection can be finished simply by performing isothermal amplification at 30-42 DEG C; moreover, the detection is fast and sensitive and has high specificity while avoiding false positive.
Owner:JIANGSU QITIAN GENE BIOTECHNOLOGY CO LTD +1
Method for detecting A1762T-G11764A double-mutation of hepatitis B virus DNA and kit
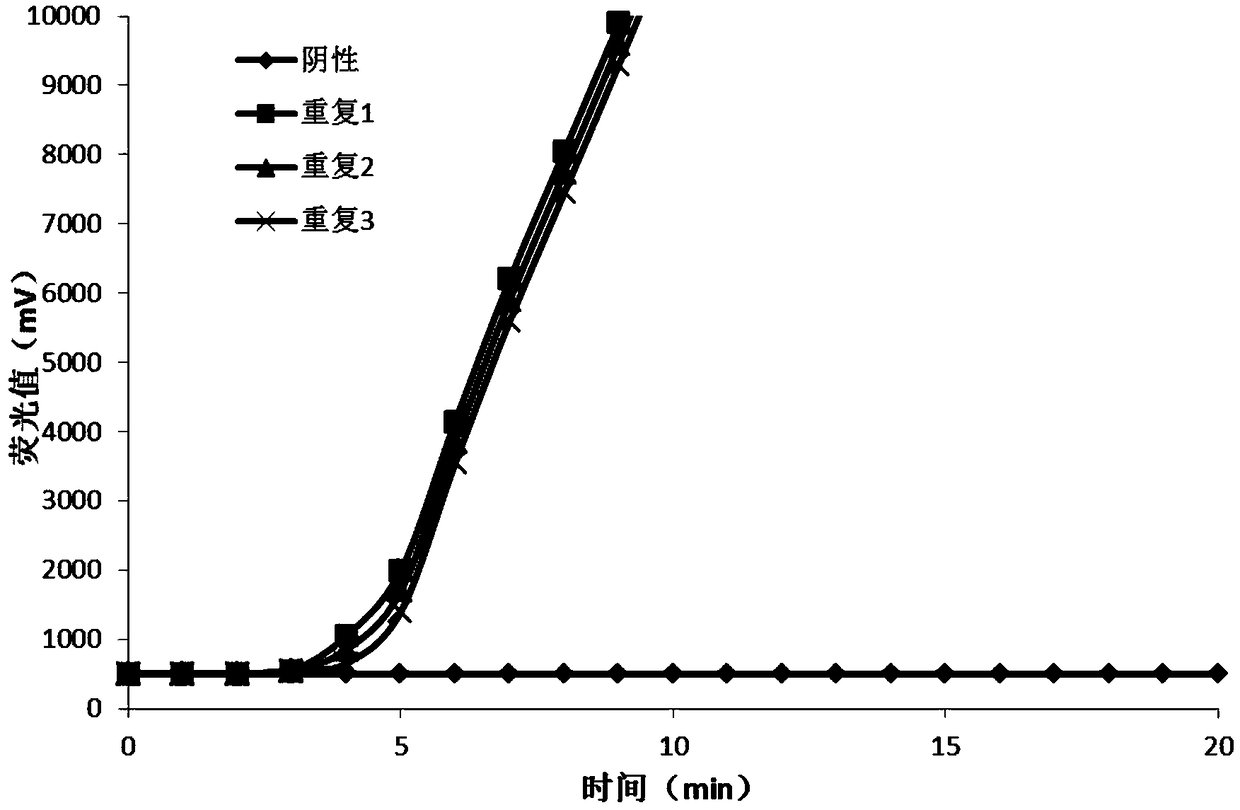
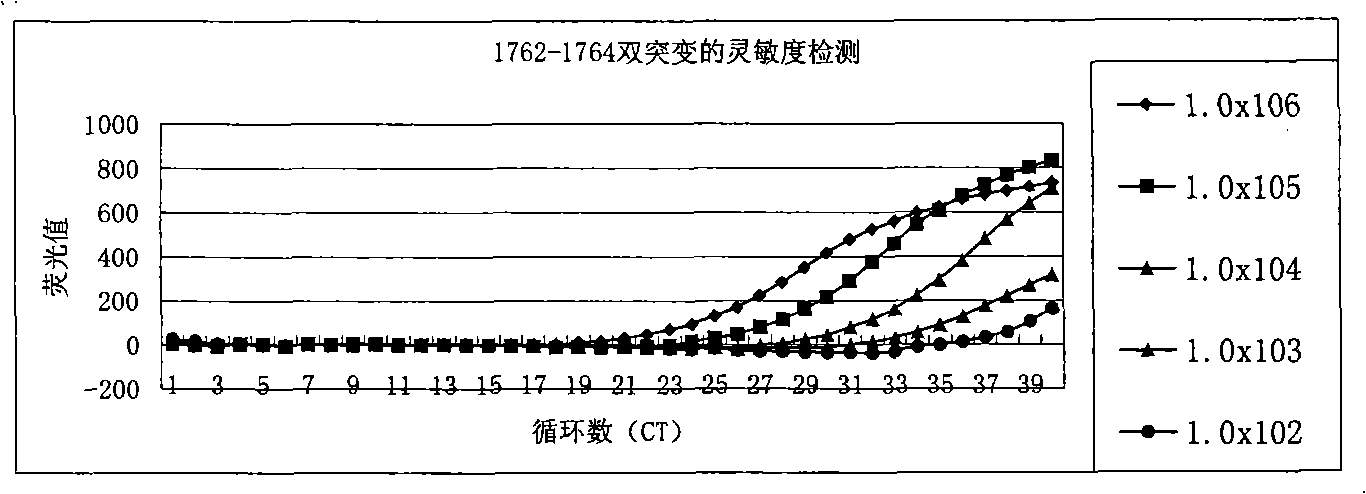
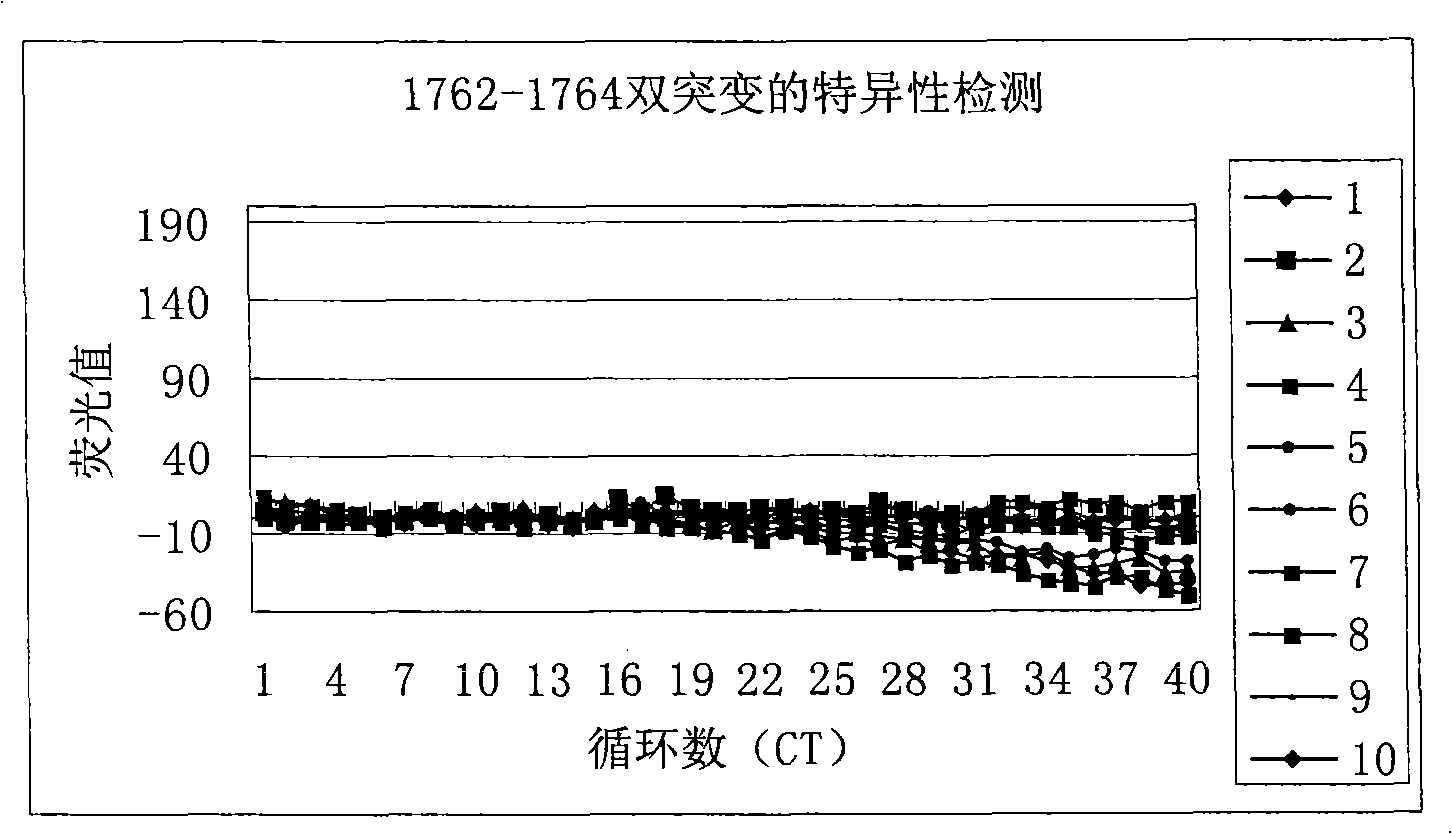
ActiveCN101338344AThe test results are beautiful and stableReliable test resultsMicrobiological testing/measurementDouble mutationHepatitis B virus DNA
The invention relates to a double mutation detection method and a reagent box for a hepatitis B virus DNA A1762T-G1764A, in particular to a double mutation detection method for detecting the A1762T-G1764A, in the DNA sequence of the hepatitis B virus by utilizing a molecular beacon probe technology and a real time PCR method as well as a reagent box for finishing the detection. The method of the invention can specially and sensitively detect the double mutation hepatitis B virus DNA of the A1762T-G1764A in a clinic blood sample.
Owner:INTEC PROD INC
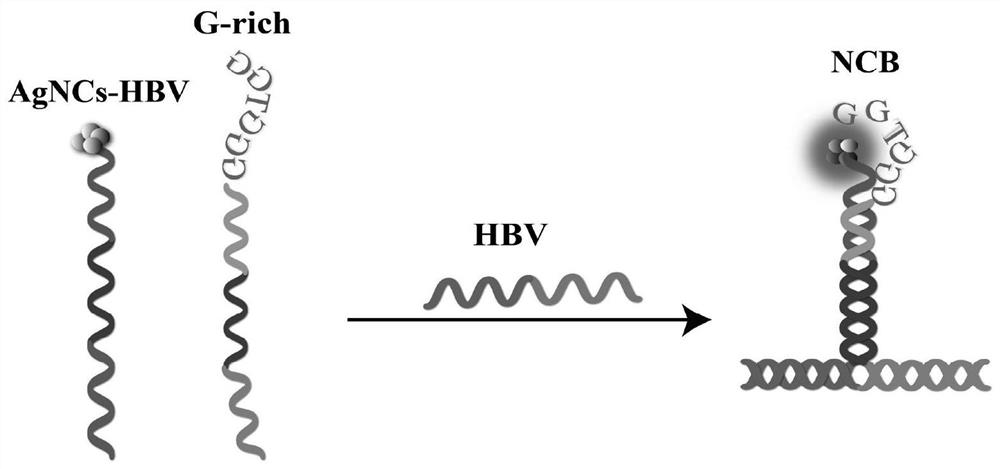
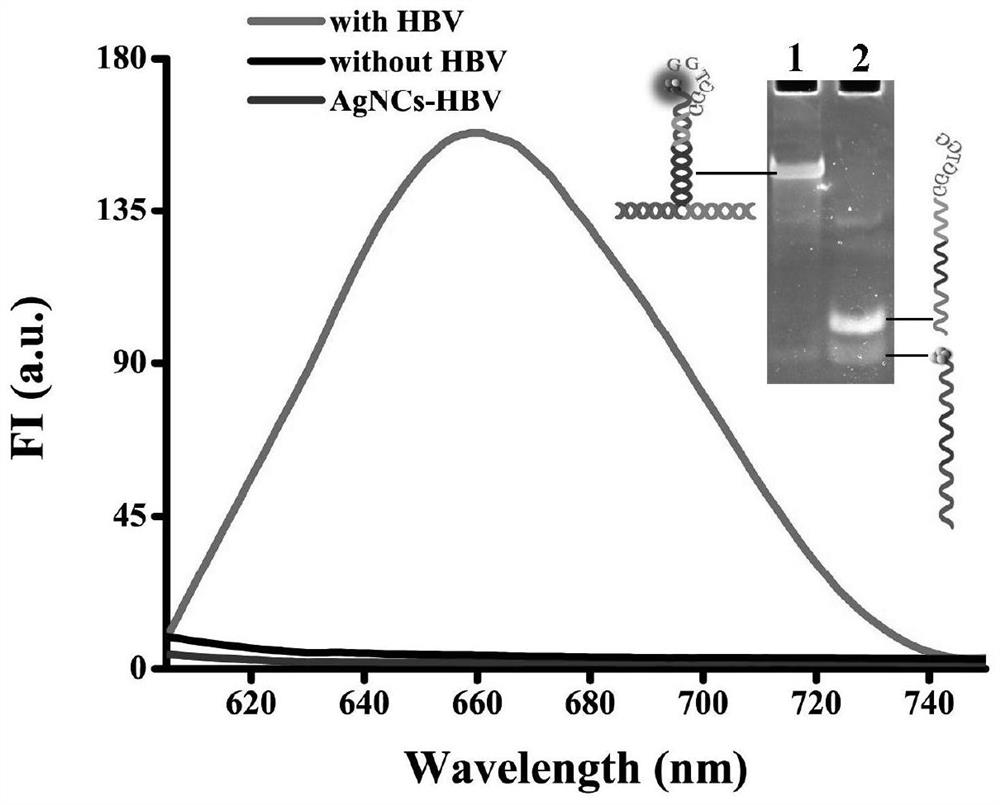
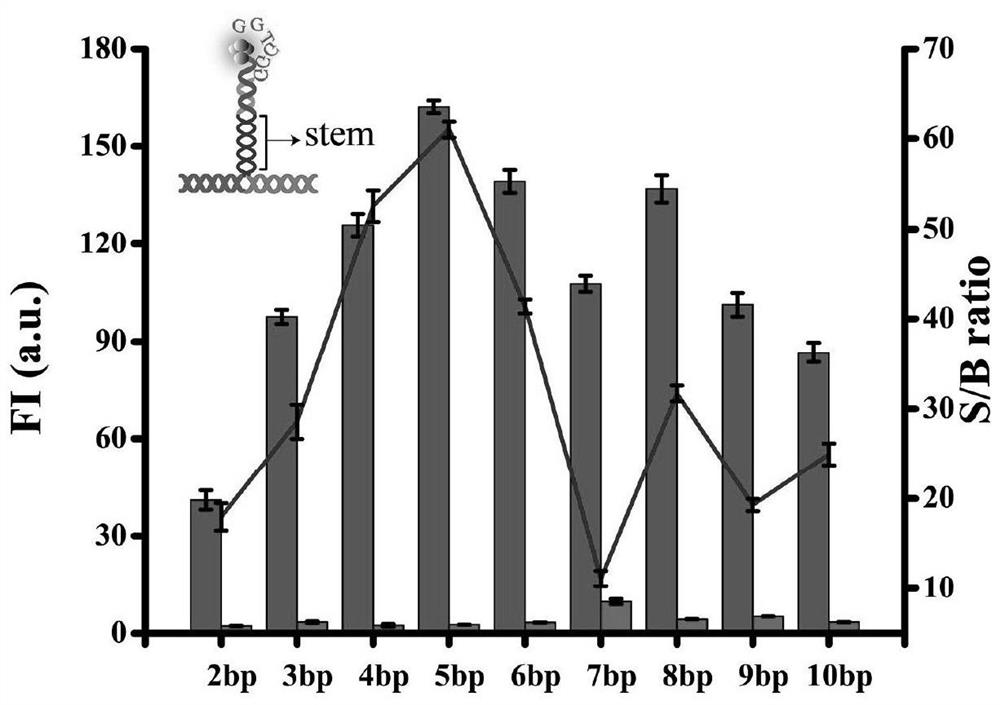
Probe and fluorescence sensor for quantitatively detecting hepatitis B virus DNA and method
PendingCN113249520ARestricted Site RequirementsLimit personnel requirementsMicrobiological testing/measurementFluorescence/phosphorescenceHepatitis B virus DNAPcr method
The invention discloses a probe and a fluorescence sensor for quantitatively detecting hepatitis B virus DNA and a method. The fluorescence sensor comprises a first probe enhanced sequence G-rich and a second probe DNA silver nanocluster sequence AgNCs-HBV, the G-rich and the AgNCs-HBV dissolve in a buffer solution, a target substance to be detected is added, mixed incubation is performed, a fluorescence reaction system is constructed, fluorescence detection is performed, and a fluorescence signal can be obtained. The fluorescence sensor provided by the invention realizes high-sensitivity detection of HBV based on a silver nano-cluster beacon, can improve the detection speed by tuning DNA silver nano-cluster sequence AgNCs-HBV fluorescence through the proximity enhancement sequence G-rich, is low in synthesis cost, can overcome the limitation of the traditional PCR method for detecting HBV on site and personnel requirements, is good in universality, stability and reproducibility, and is suitable for large-scale screening or quantitative detection of the hepatitis B HBV in communities or hospitals.
Owner:CHONGQING MEDICAL UNIVERSITY
Kit for evaluating lamivudine dosage
The invention discloses a kit for detecting the sensitivity of an individual patient to Lamivudine drugs. The kit comprises a specific primer pair and DNA sequencing primer for detecting the YMDD region gene type of the DNA of hepatitis B in vivo of a patient, a PCR reaction assembly, a PCR product purification assembly, a DNA sequencing reaction assembly and the like. The kit is used for analyzing and evaluating the sensitivity of the patient to the Lamivudine drugs.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
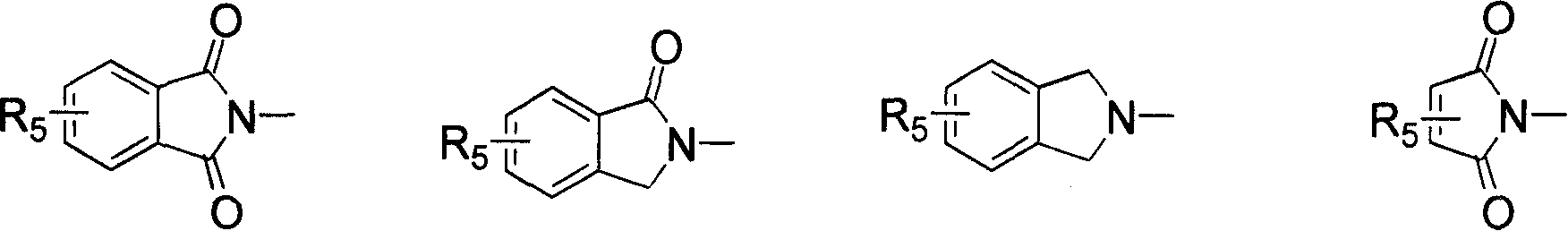
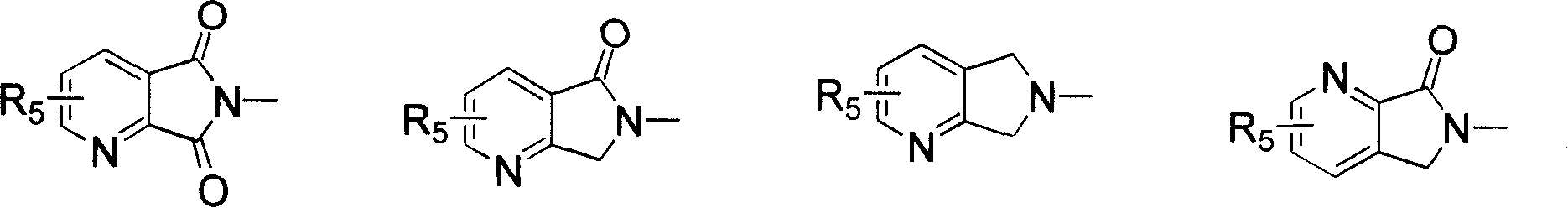

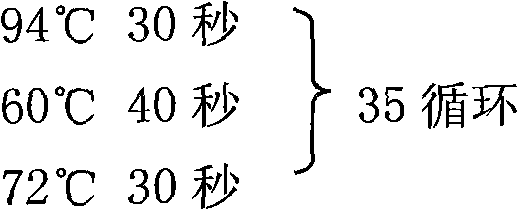

![1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses 1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses](https://images-eureka.patsnap.com/patent_img/c28639d1-88a2-4ab3-8692-aa3efa56c0c6/a2008100625470002c1.PNG)
![1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses 1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses](https://images-eureka.patsnap.com/patent_img/c28639d1-88a2-4ab3-8692-aa3efa56c0c6/a2008100625470002c2.PNG)
![1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses 1-oxygen-[3-aryl substituted-alkene propionyl]quinic acid compounds and uses](https://images-eureka.patsnap.com/patent_img/c28639d1-88a2-4ab3-8692-aa3efa56c0c6/a20081006254700061.PNG)
![3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof 3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof](https://images-eureka.patsnap.com/patent_img/c4b4e213-e59f-445e-b924-f79274457abf/a20081006255100061.PNG)
![3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof 3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof](https://images-eureka.patsnap.com/patent_img/c4b4e213-e59f-445e-b924-f79274457abf/a20081006255100062.PNG)
![3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof 3,4-di-oxygen-[3-substituted alkene propionyl]quinic acid compounds and medicine uses thereof](https://images-eureka.patsnap.com/patent_img/c4b4e213-e59f-445e-b924-f79274457abf/a20081006255100071.PNG)