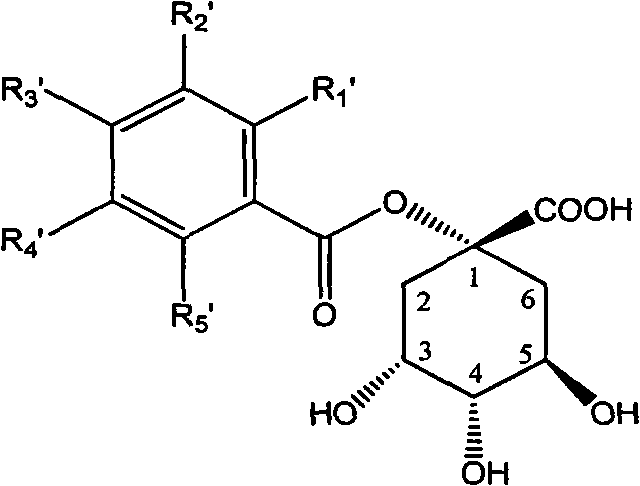
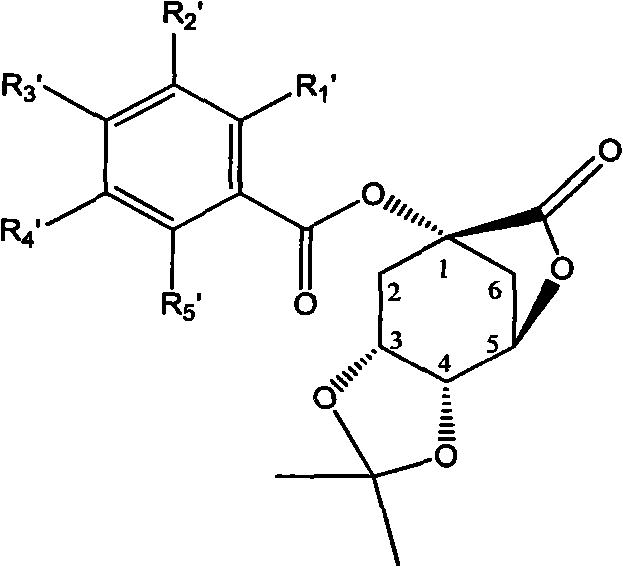
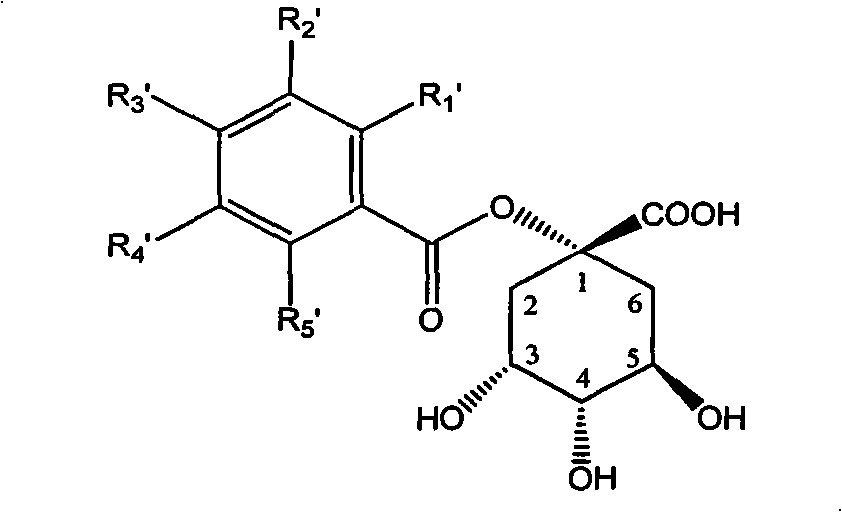
1-oxygen-substituted benzene formyl quinic acid pharmaceutical use of inhibiting hepatitis b virus
A technology of benzoylquinic acid and quinic acid, which can be used in antiviral agents, drug combinations, pharmaceutical formulations, etc., can solve the problems of drug resistance, high price, and adverse effects of nucleoside drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 : compound 3, the preparation of 4-acetonylidene quinic acid lactone
[0039] 3,4-Acetonylidene quinic acid lactone
[0040] In the reaction flask, add quinic acid (500 mg, 2.6 mmol), anhydrous sodium sulfate (2.5 g, 17.6 mmol), 15 milliliters of anhydrous acetone, stir for several minutes, then add 3 microliters of Concentrated sulfuric acid was heated to reflux for 5 hours. Cool to room temperature, add sodium bicarbonate to adjust the pH to about 7, remove insoluble matter by suction filtration, and concentrate the filtrate. The solid obtained by precipitation was dispersed in 3 ml of chloroform and 3 ml of distilled water, and the aqueous layer was extracted 3 times with chloroform (5 ml × 3), and all organic phases were combined, washed with water, washed several times with saturated brine, and dried over anhydrous sodium sulfate . The solvent was distilled off under reduced pressure to obtain a white solid, which was recrystallized in ethyl acetate...
Embodiment 2
[0042] Example 2 : Preparation of Compound II-a[1-oxo-(4-methoxybenzoyl)-3,4-acetonylidene quinic acid lactone]
[0043]
[0044] Add p-methoxybenzoic acid (90 mg, 0.58 mmol) to the reaction flask, carbonyldiimidazole (190 mg, 1.17 mmol), 8 ml of anhydrous tetrahydrofuran, reflux for 2 hours, then add 3,4- Acetonide (100 mg, 0.47 mmol), 1,8-diazabicyclo[5,4,0]11alk-7-ene (DBU) (90 mg, 0.58 mmol), whole The solution was refluxed for 8 hours. Precipitation gave a pale yellow viscous solid, which was separated by HPLC to obtain 50 mg of a white solid with a yield of 31.8%.
[0045] Melting point: 63~64℃; R f (chloroform / methanol / formic acid: 50 / 2 / 1): 0.75; H NMR spectrum ( 1 H NMR, 400MHz, deuterated methanol): δ1.33 (3H, singlet), 1.43 (3H, singlet), 2.46~2.76 (4H, multiplet), 3.91 (3H, singlet), 4.09 (1H, Double doublet), 4.51 (1H, multiplet), 4.74 (1H, double doublet), 7.07 (2H, singlet), 8.03 (2H, singlet).
[0046] According to the same method of embodiment 1 and 2...
Embodiment 7
[0056] Example 7 : Preparation of Compound I-a[1-oxo-(4-methoxybenzoyl)-quinic acid]
[0057]
[0058] Add compound 1-oxygen-(4-methoxybenzoyl)-3,4-acetonylidene quinic acid lactone II-a (50 mg, 0.144 mmol) in a two-necked flask, add 5 ml of tetrahydrofuran, Add 5 ml of 1N hydrochloric acid dropwise, react at room temperature for 72 hours, add saturated salt to the reaction system, extract with chloroform, wash the combined chloroform layer with a large amount of water, wash with saturated brine, and dry over anhydrous sodium sulfate. After separation by HPLC, 29 mg of white solid was obtained with a yield of 62.5%.
[0059] Melting point: 84~87℃, R f (chloroform / methanol / formic acid: 50 / 2 / 1): 0.25; H NMR spectrum ( 1 H NMR, 400MHz, deuterated methanol): δ2.46~2.76 (4H, multiplet), 3.91 (3H, singlet), 4.09 (1H, double doublet), 4.51 (1H, multiplet), 4.74 (1H , double doublet), 7.07 (2H, singlet), 8.03 (2H, singlet).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com