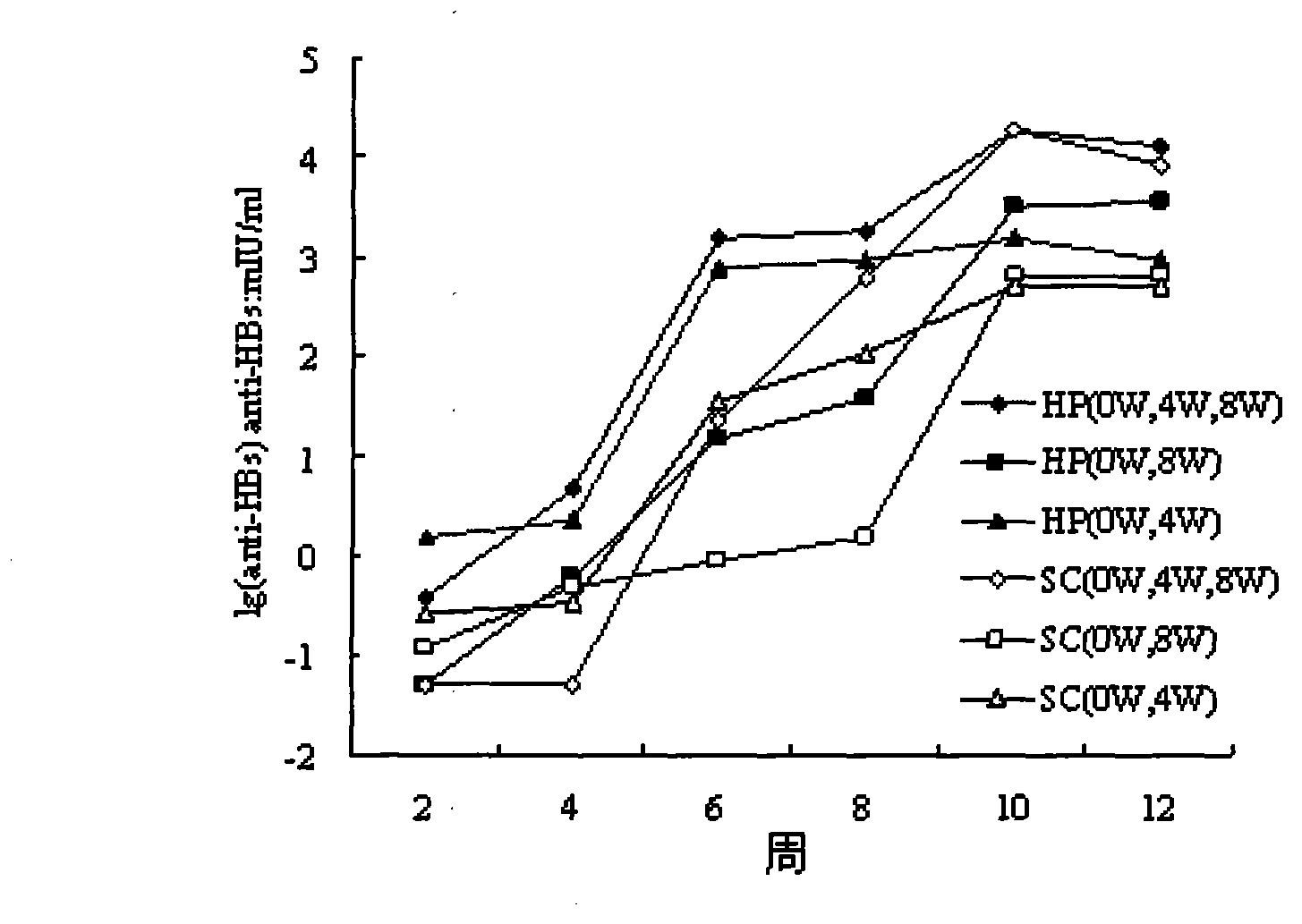
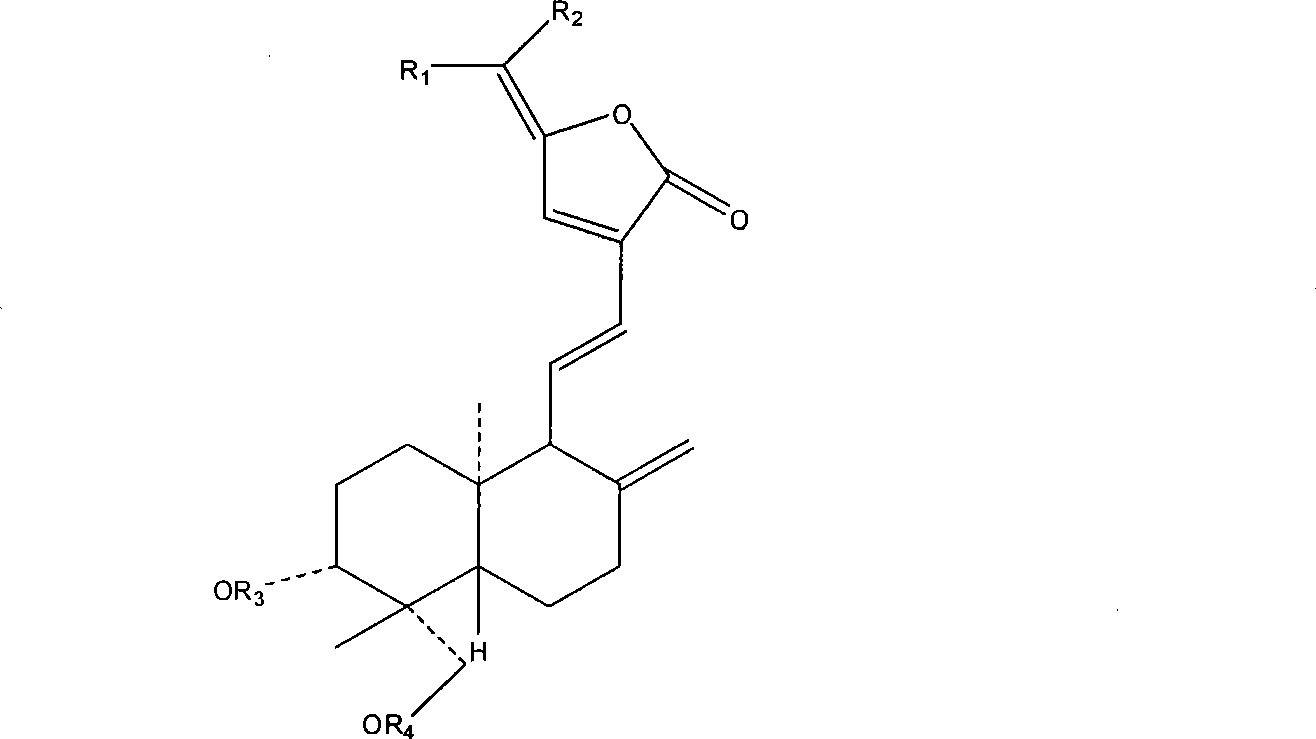
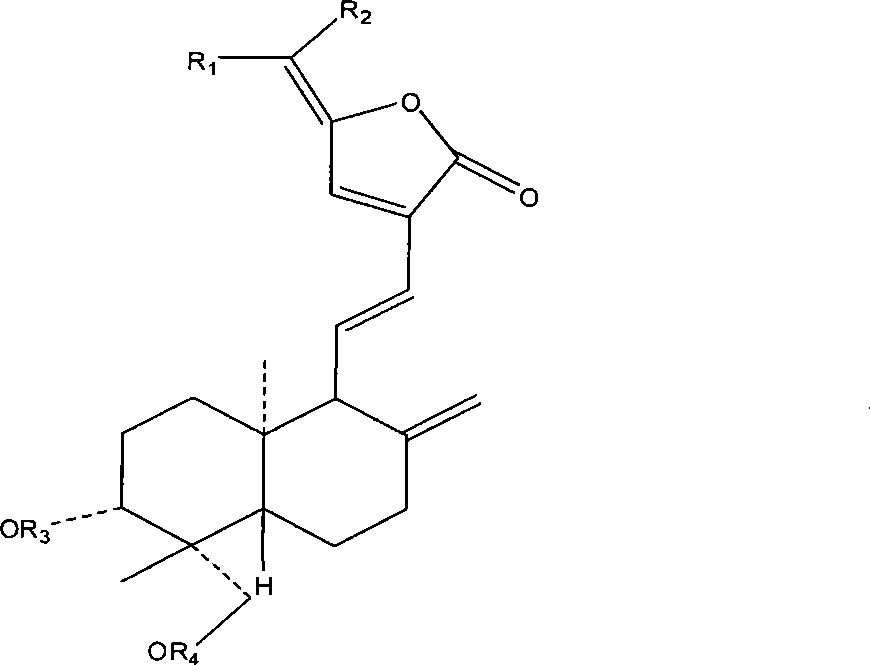
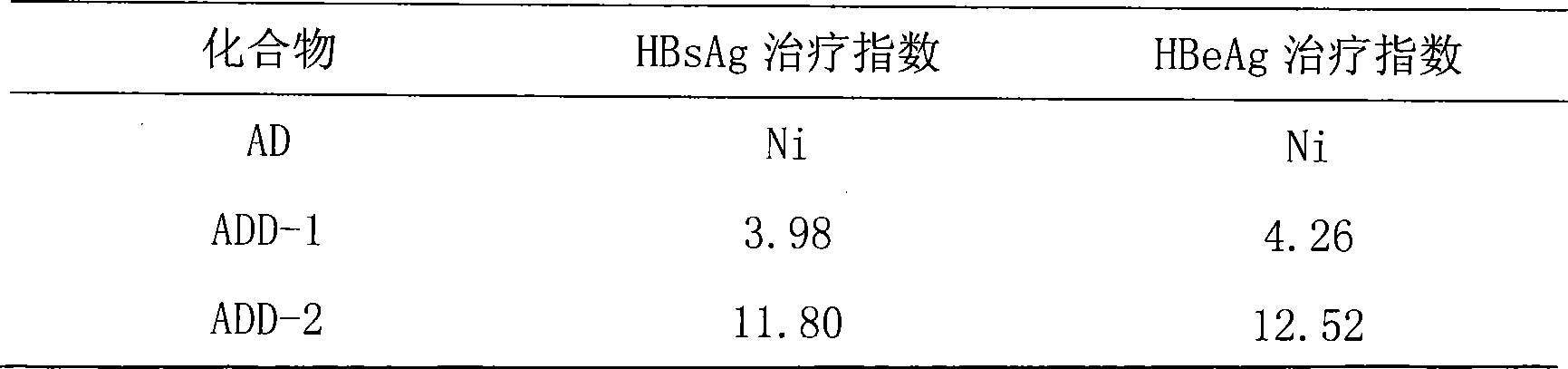
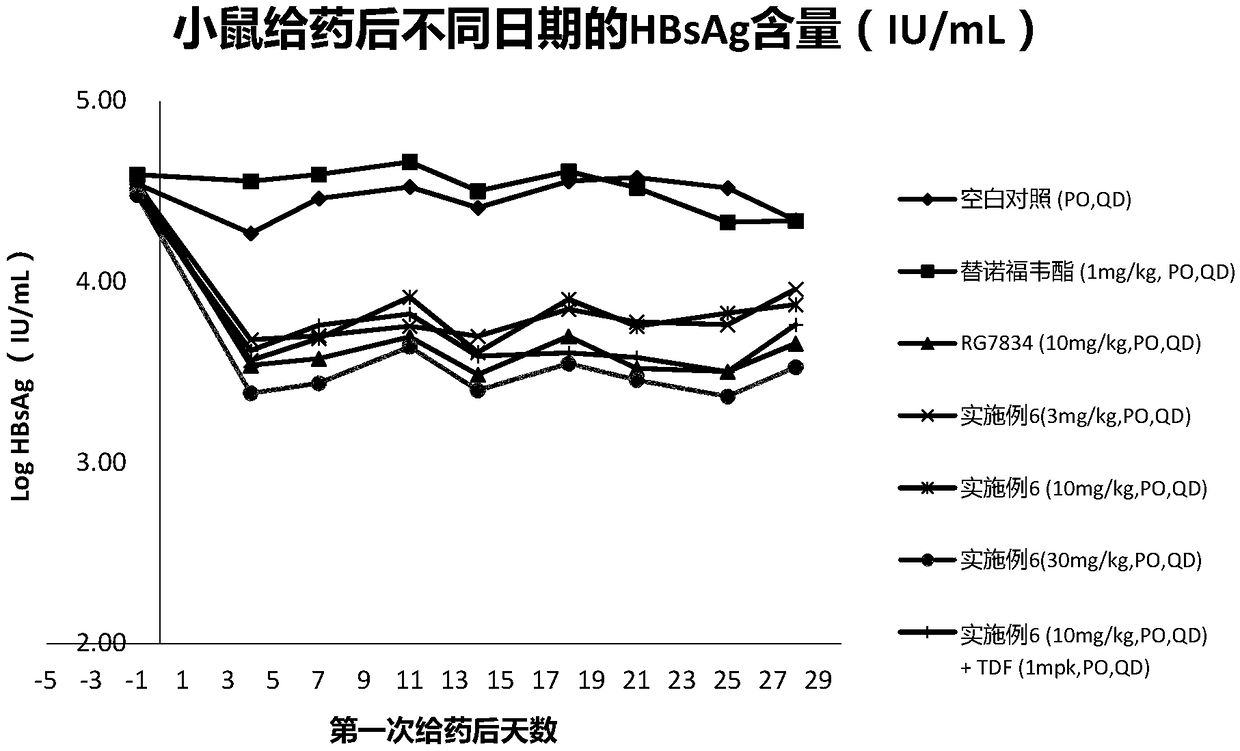
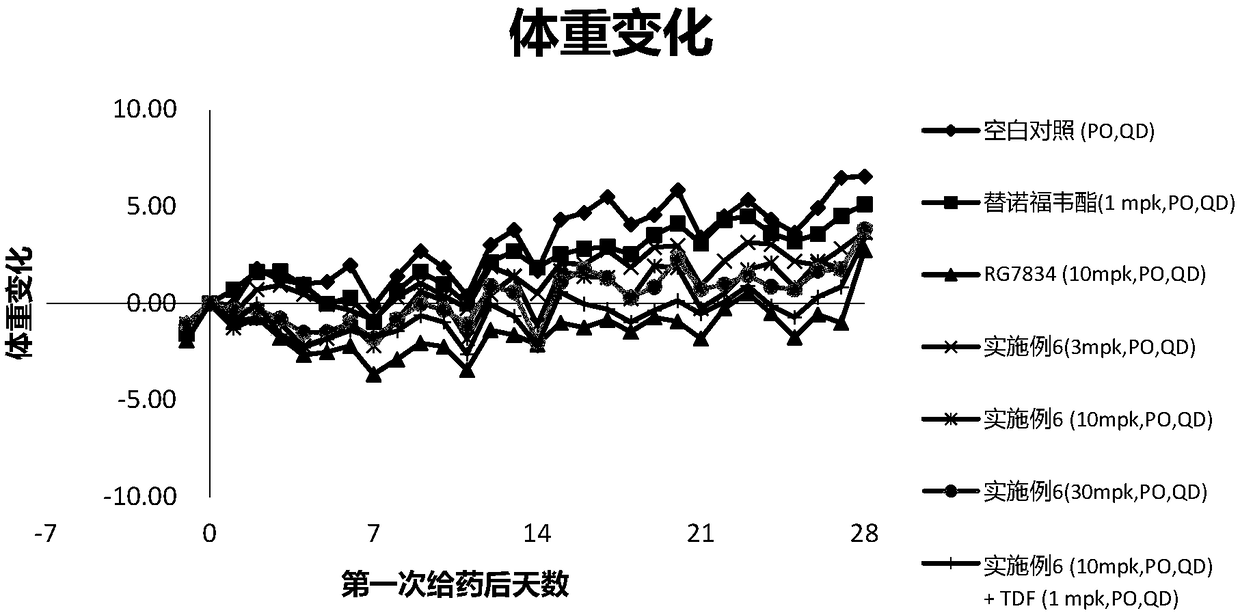
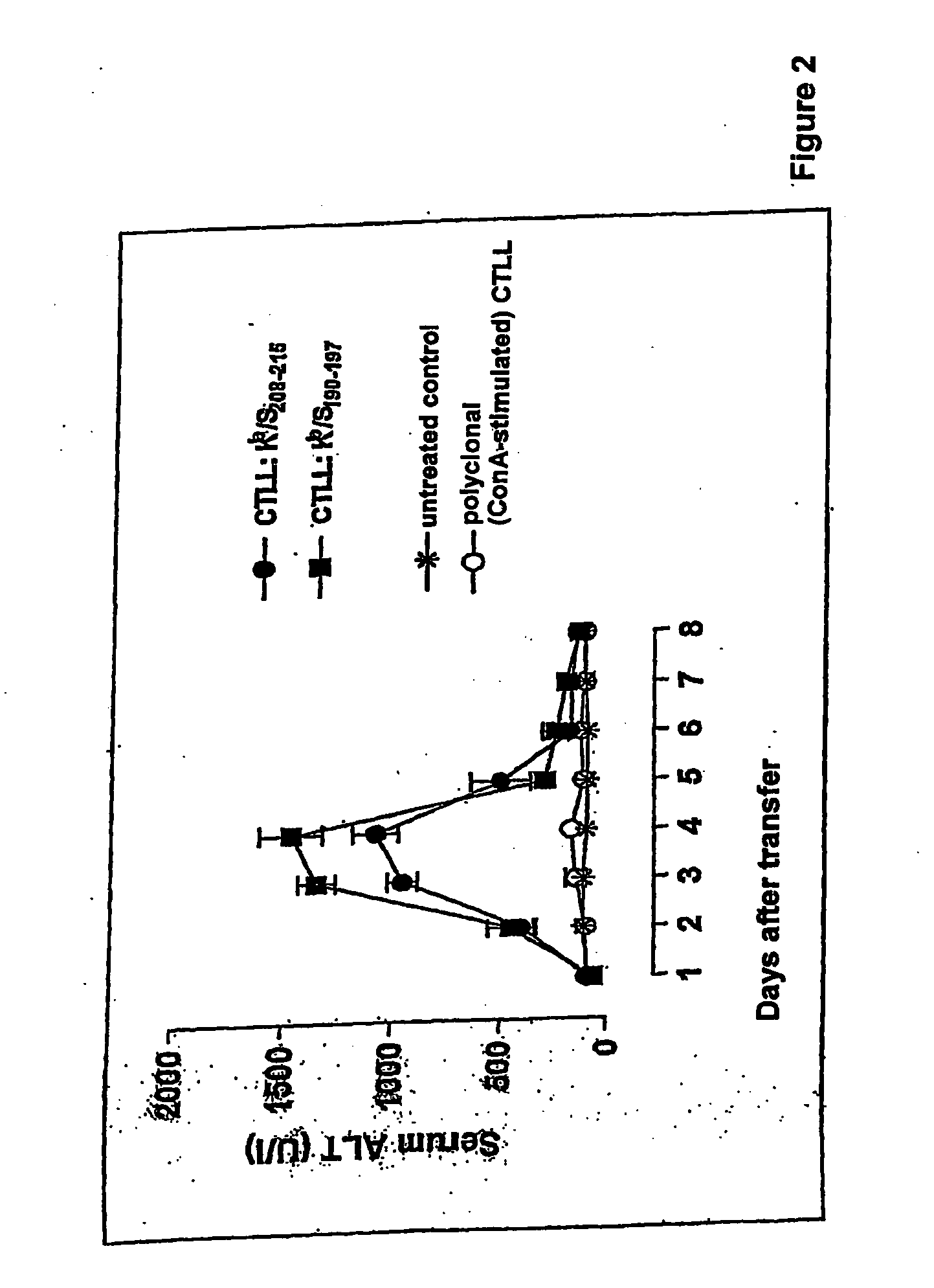
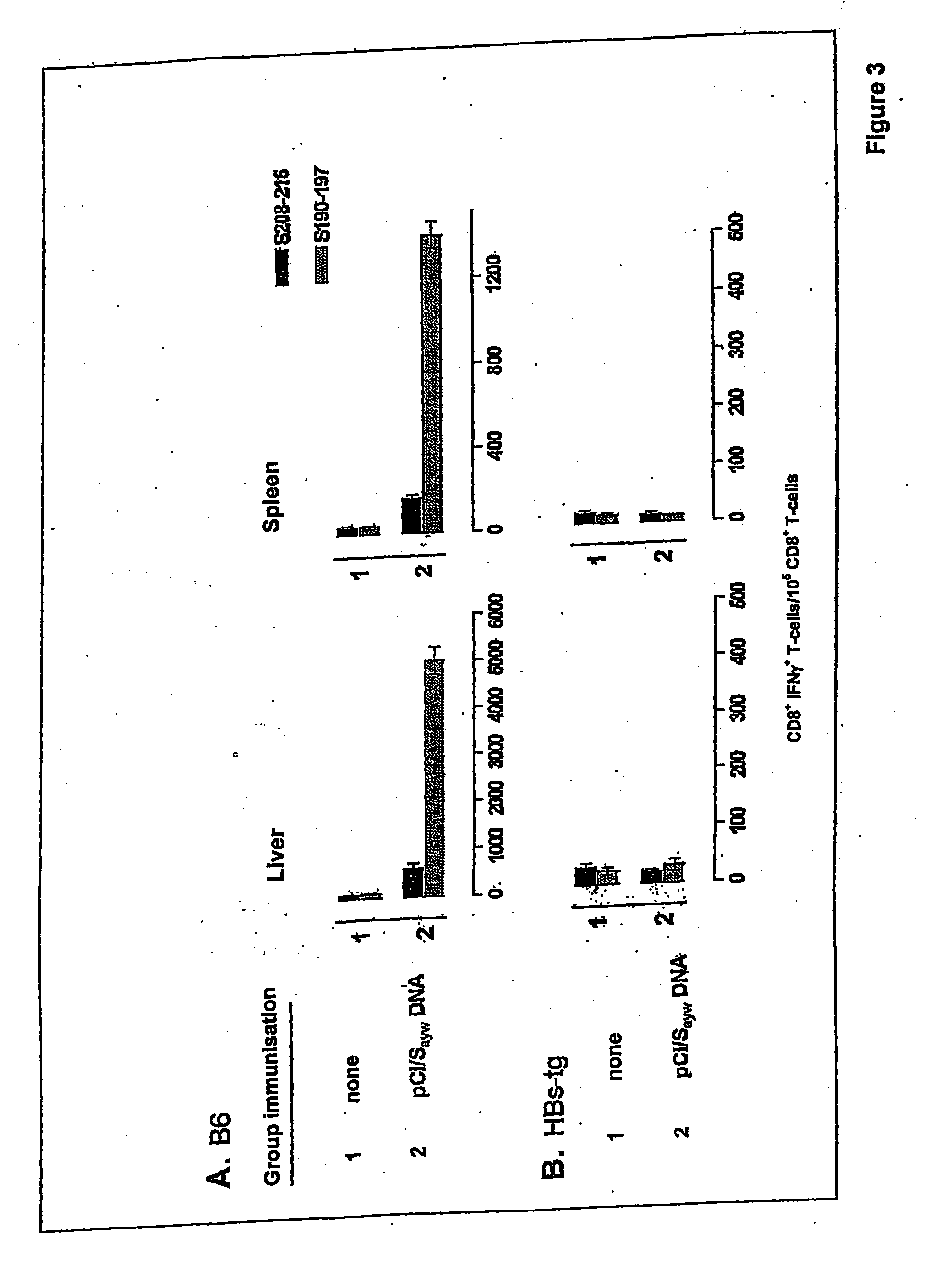
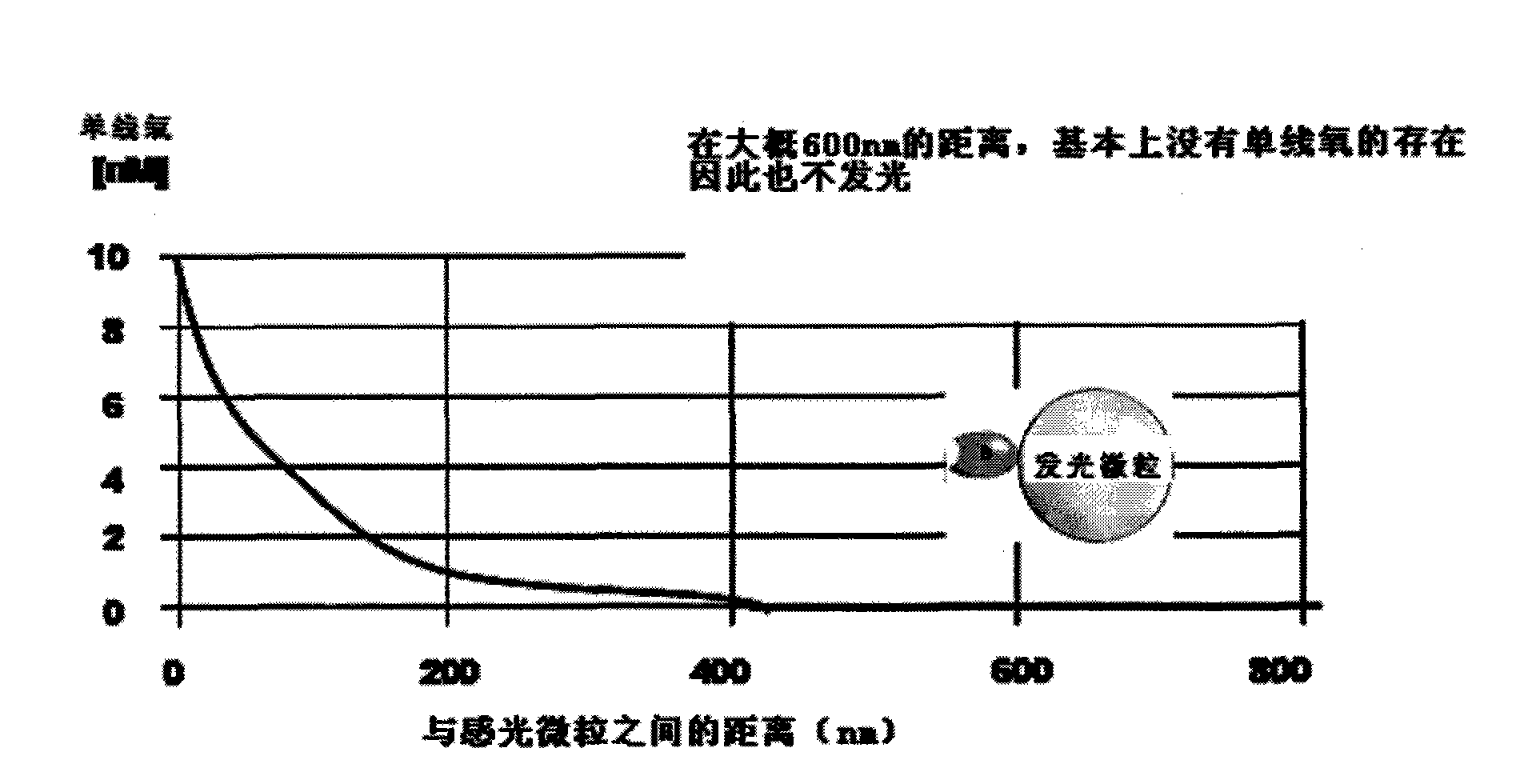
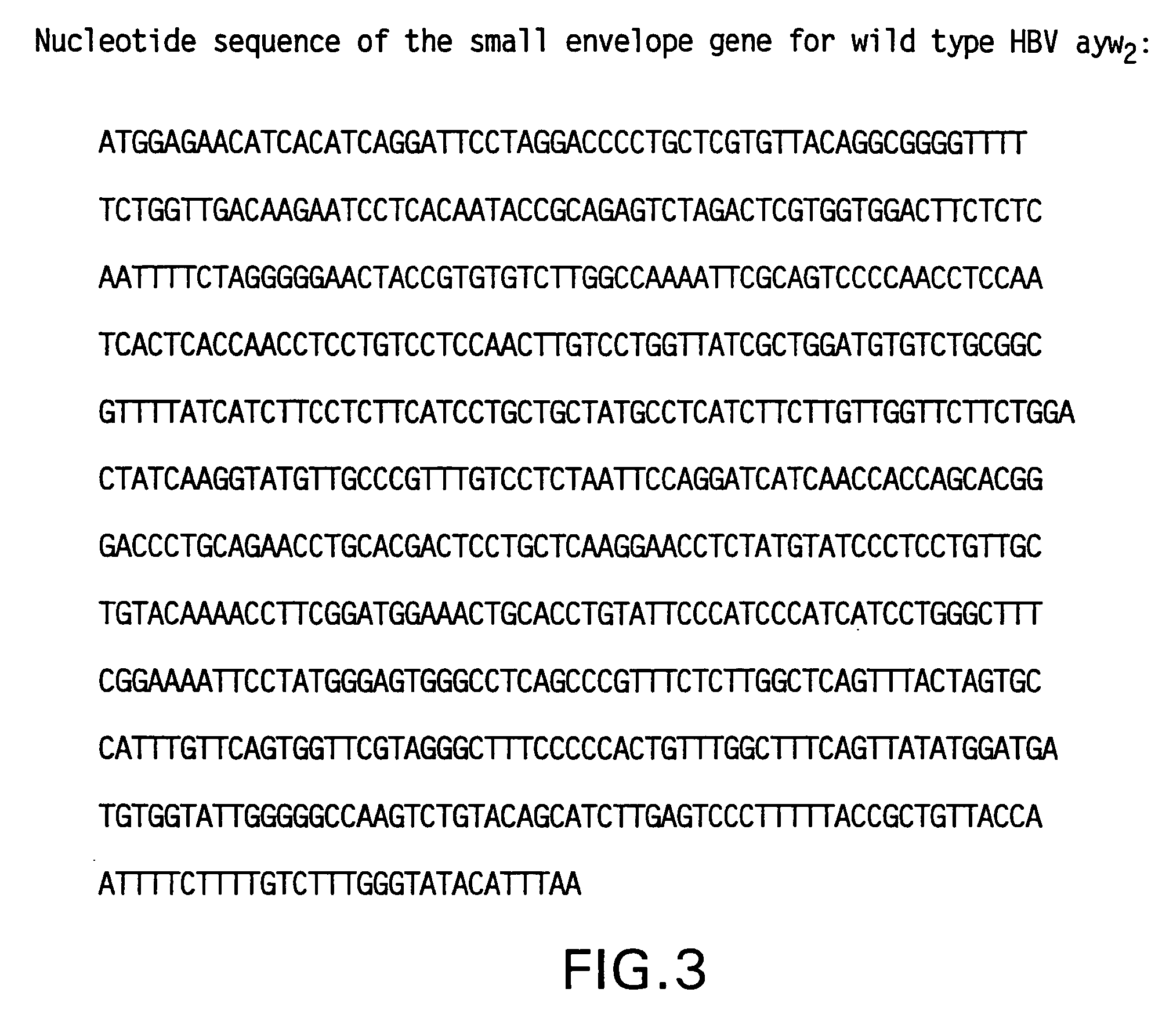
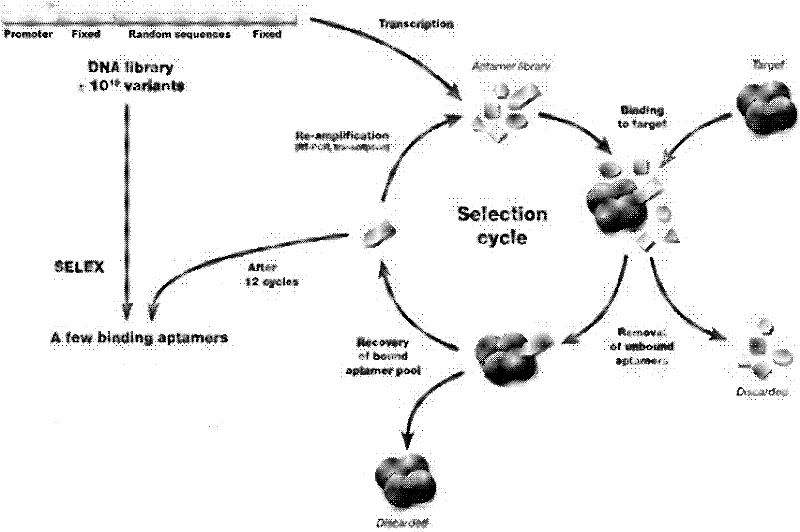
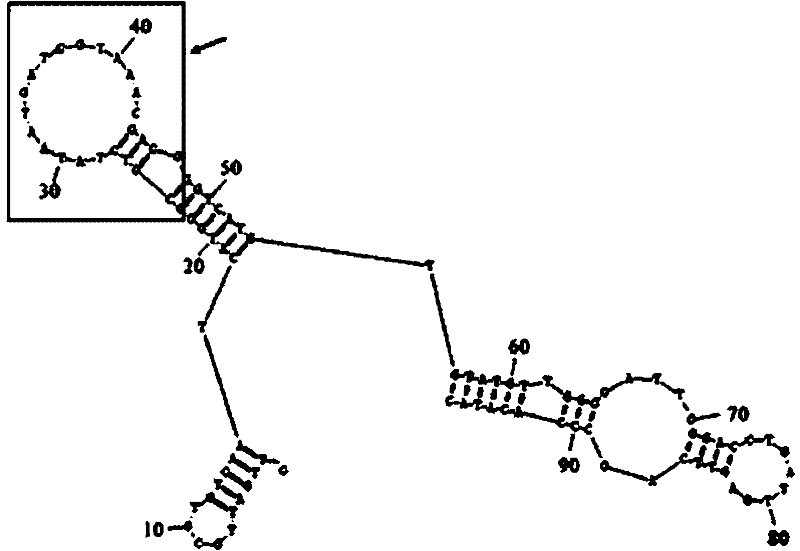
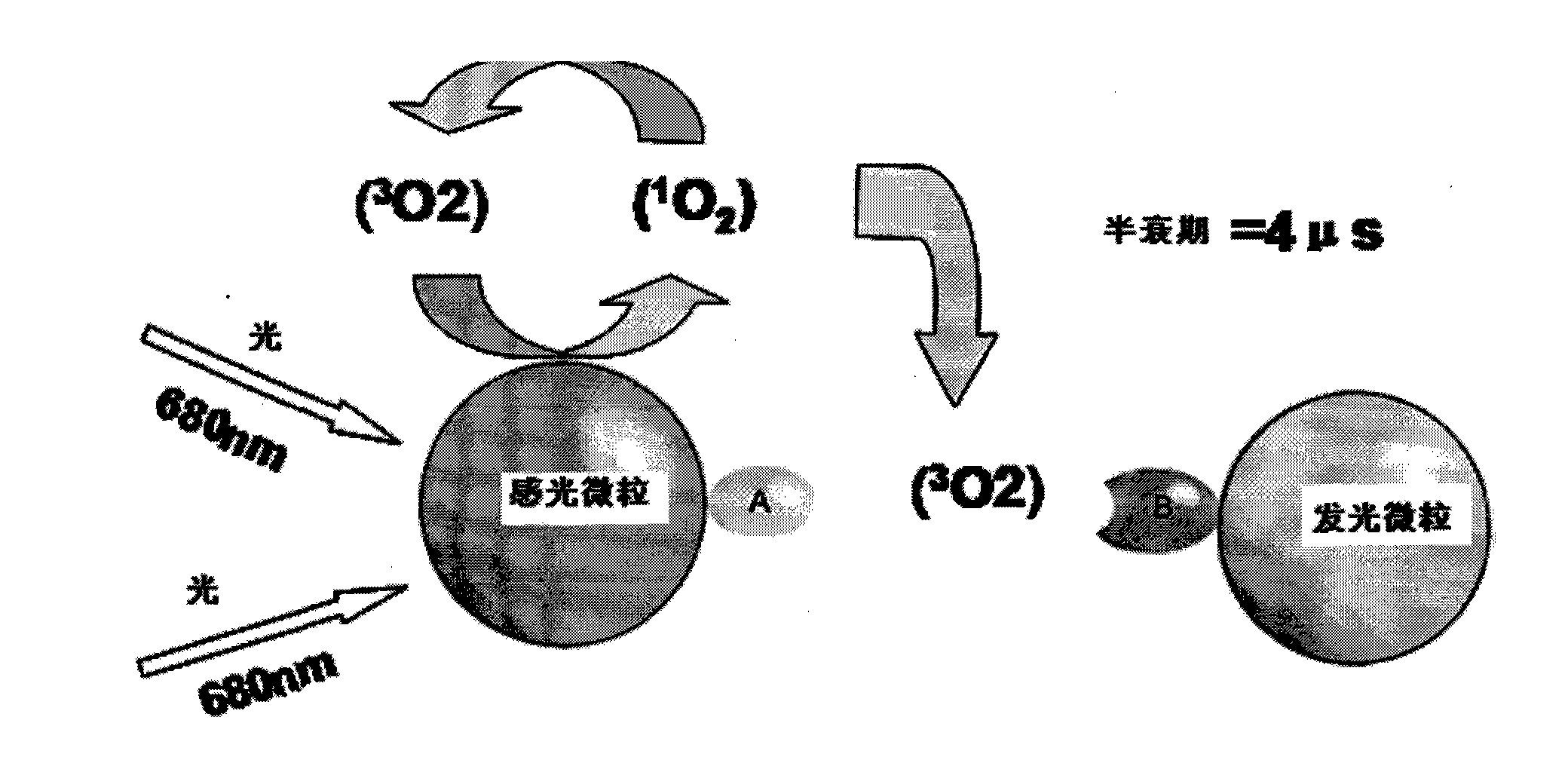Patents
Literature
170 results about "Hepatitis B virus surface Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis B Surface Antigen Blood Test. This test is used to screen for infection with the Hepatitis B (Hep B) virus. The Surface Antigen test looks for a protein which is present on the surface of the virus. This protein will be present in the blood with an acute or chronic Hep B infection.
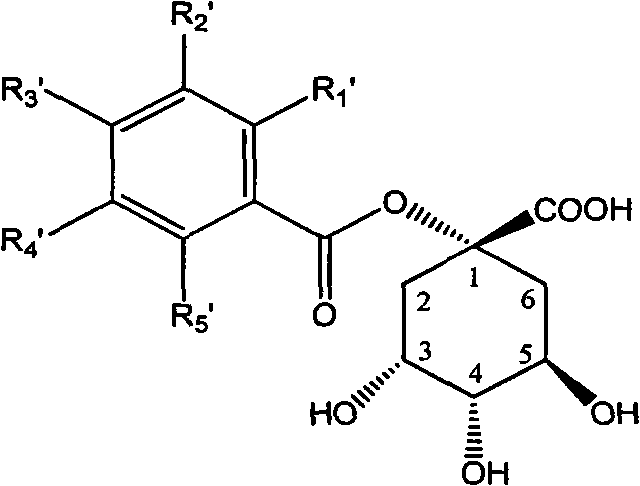
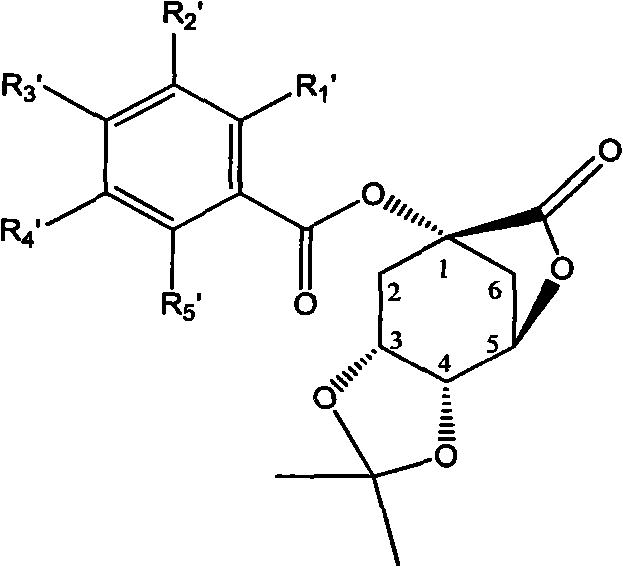
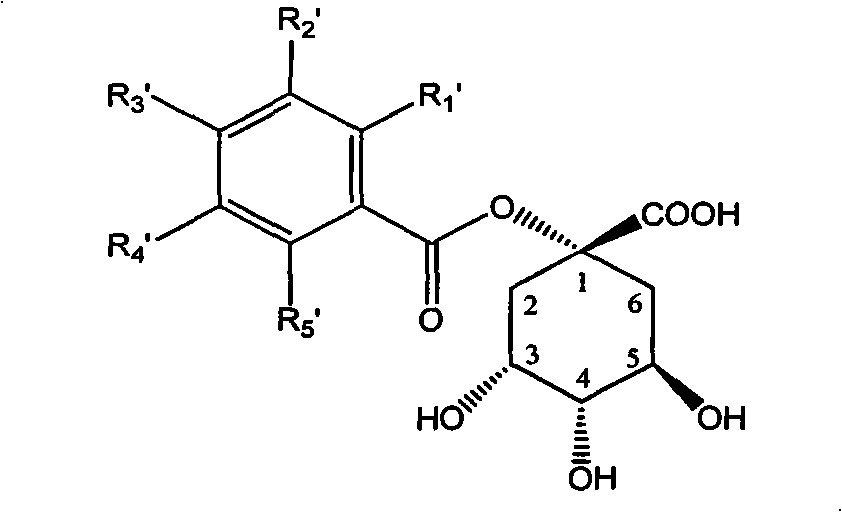
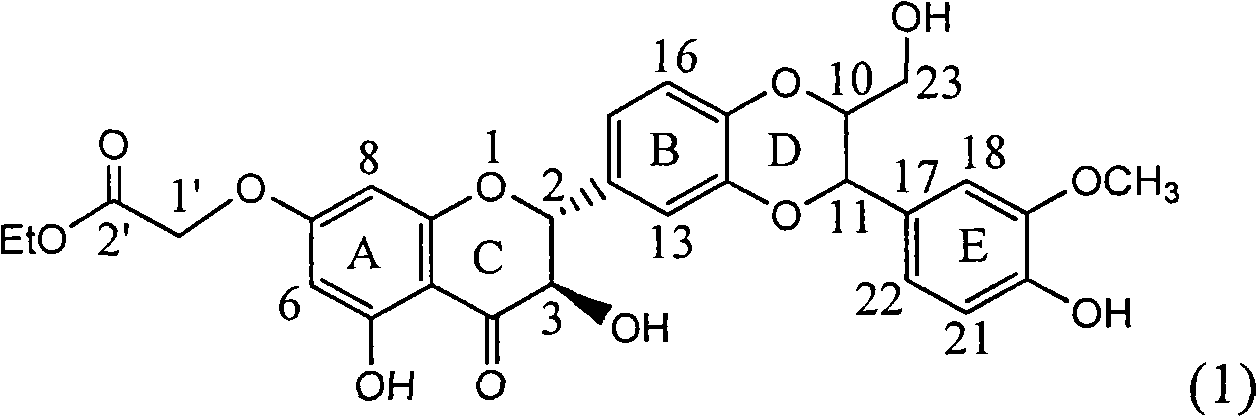
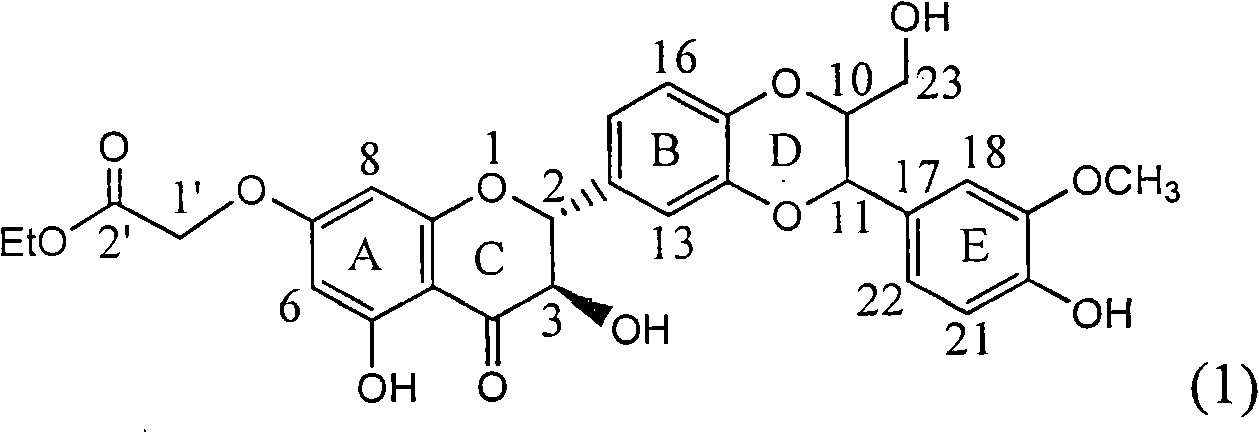
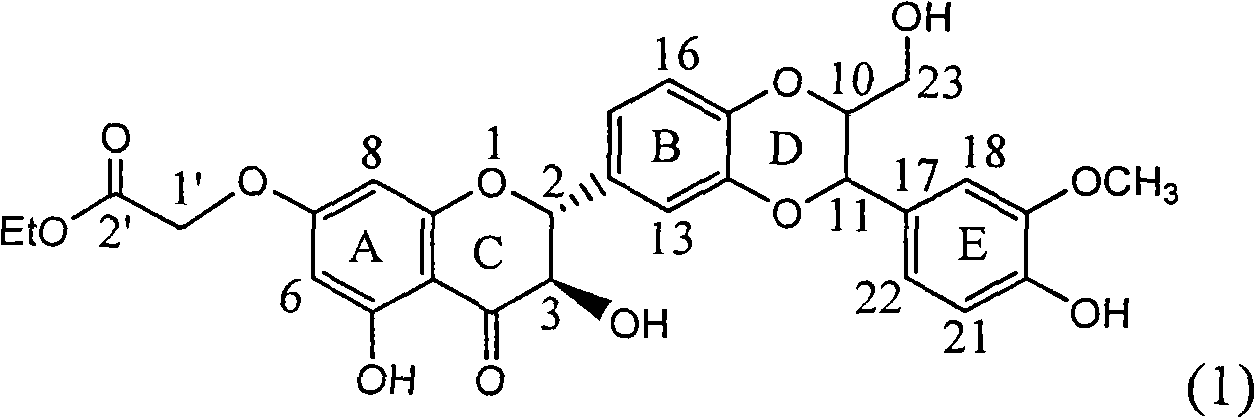
1-oxygen-substituted benzene formyl quinic acid pharmaceutical use of inhibiting hepatitis b virus
The invention relates to 1-o-substituted benzoyl quinic acid compounds having the formula of (I) and anti-hepatitis B virus activity and pharmaceutical salts thereof. The invention also relates to a preparation method of the quinic acid compounds and intermediate compounds of the quinic acid compounds. The invention also relates to a pharmaceutical application of the quinic acid compounds, and pharmaceutical compositions containing the same. The compounds (I) and intermediate compounds thereof, and pharmaceutical salts thereof inhibit hepatitis B virus DNA (HBVDNA) replication and reduces hepatits B virus surface antigen (HBsAg) expression. Thus, the 1-o-substituted benzoyl quinic acid compounds and intermediate compounds thereof have prospect pharmaceutical application in the preparation of a drug for treating correlated hepatitis B virus infectious disease.
Owner:WENZHOU MEDICAL UNIV
Enantiomorphous eremophilanic acid and its medical use for inhibiting hepatitis B surface antigen
The invention relates to the medicine technical field and concretely relates to a mixture formed by two enantiomorphous eremophilane acids separated from murrey ligularia and the officinal salt as well as the medicine combined material. The enantiomorphous eremophilane acid can be used for preparing the medicine curing hepatitis B virus infectious diseases due to the ability of suppressing the antigenic activity on the surface of hepatitis B virus.
Owner:WENZHOU MEDICAL UNIV
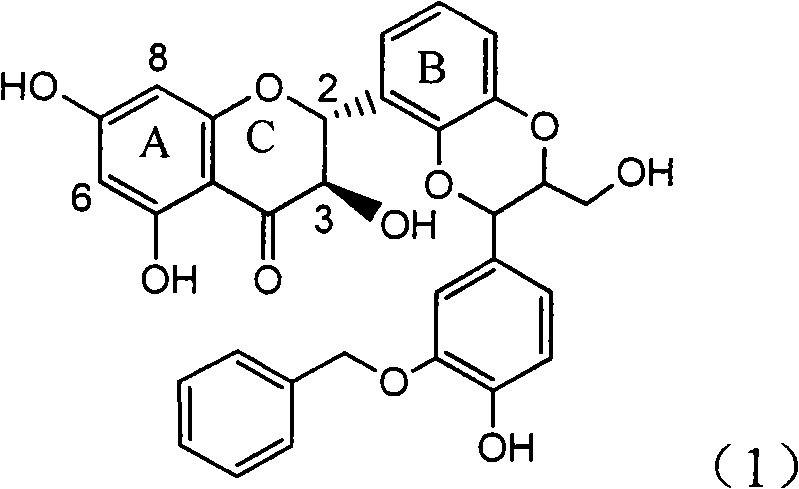
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
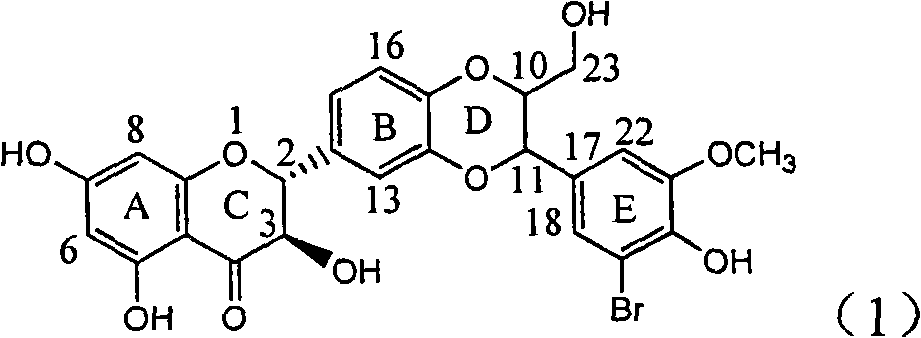
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
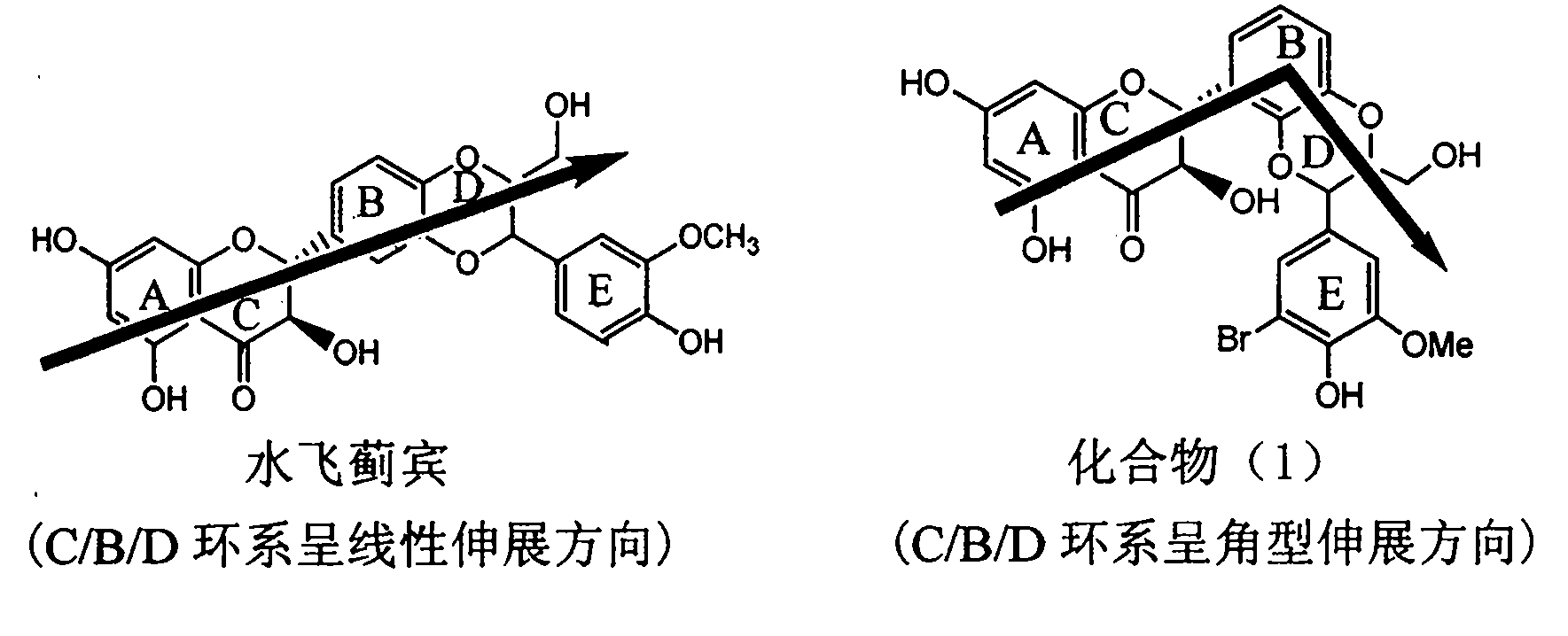
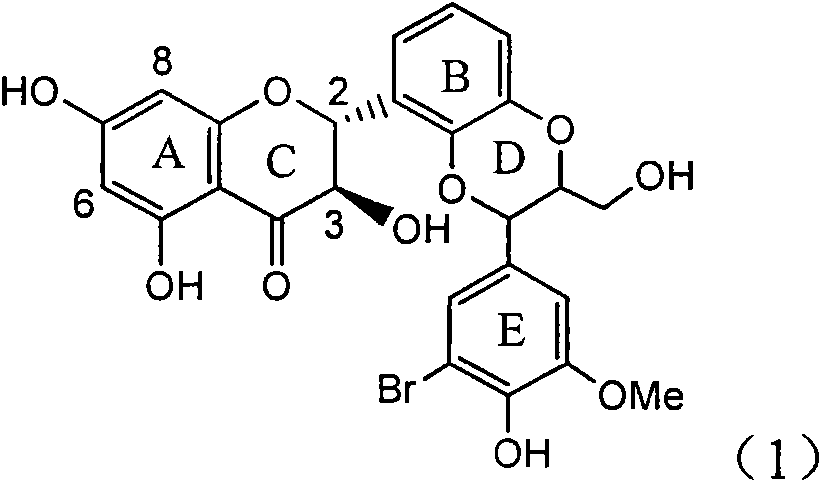
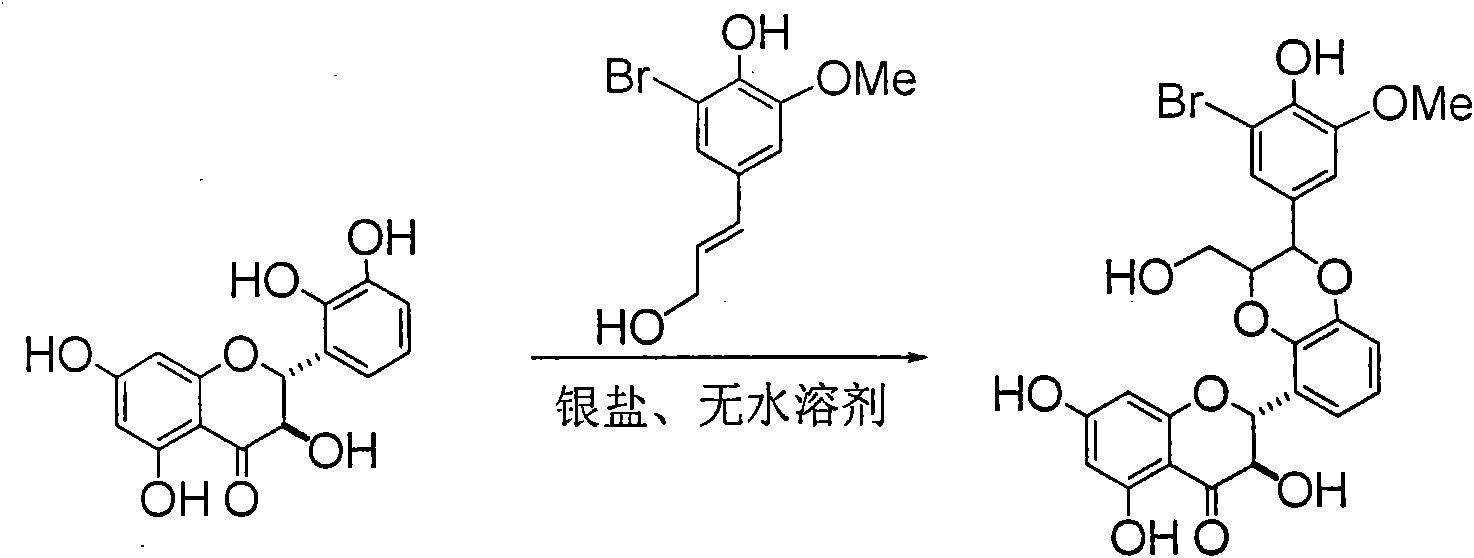
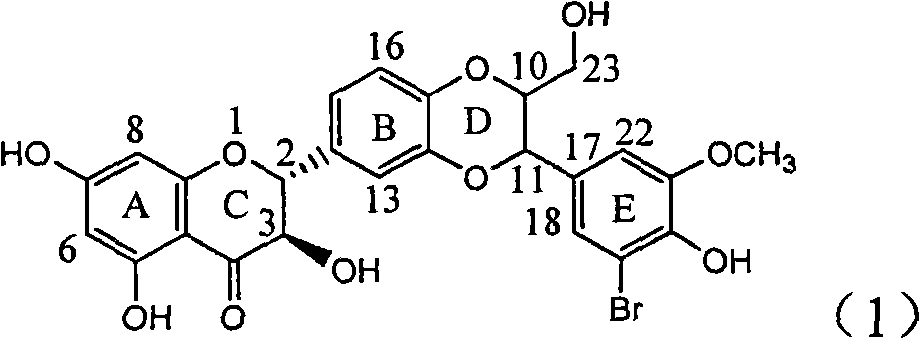
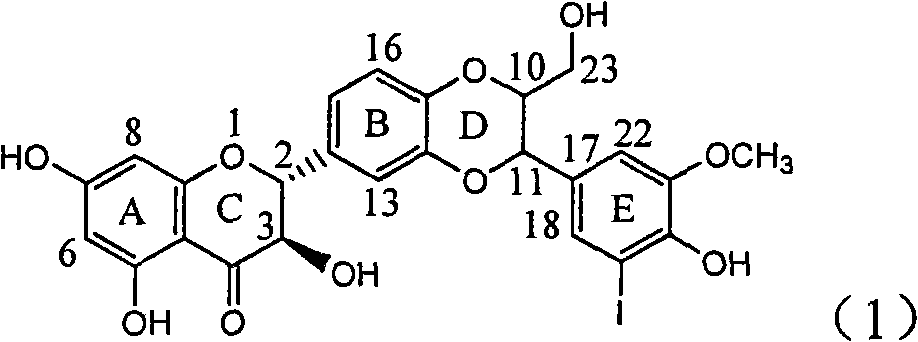
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
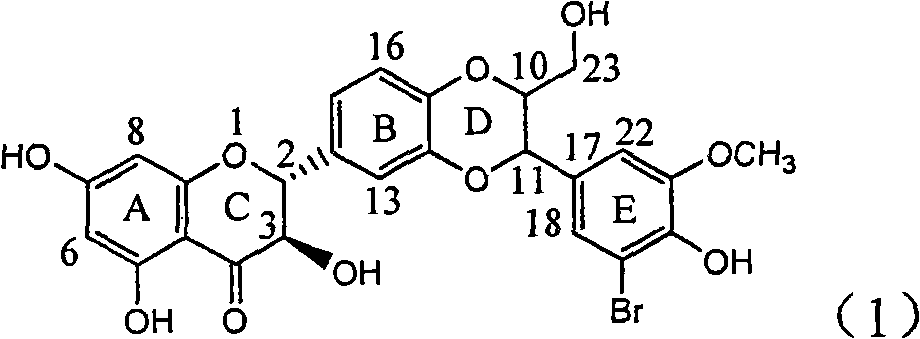
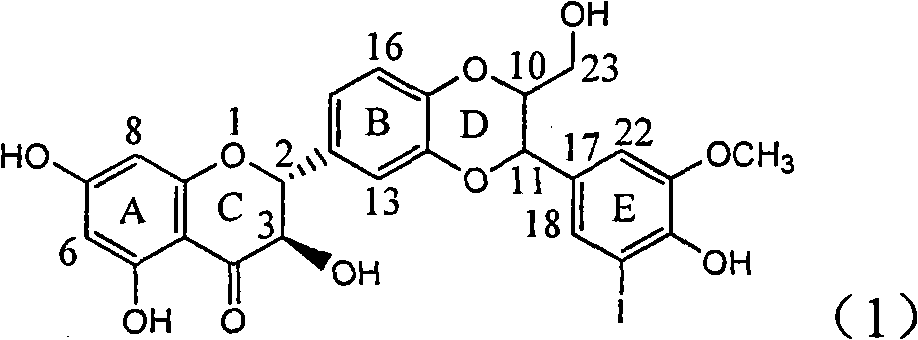
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
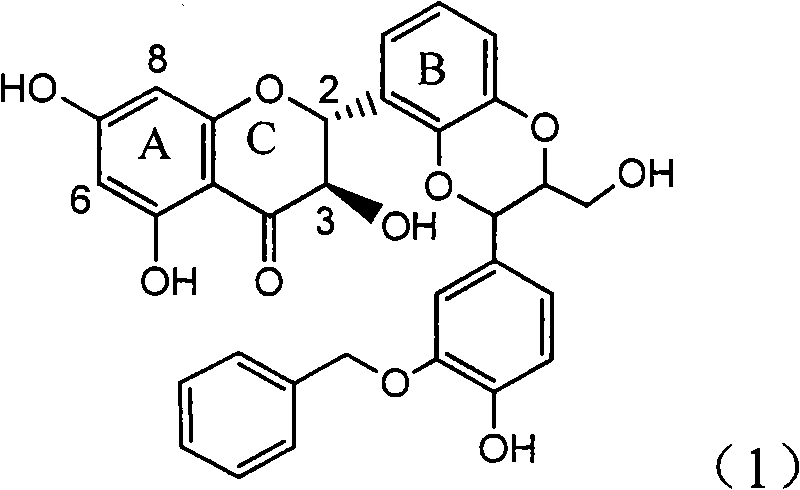
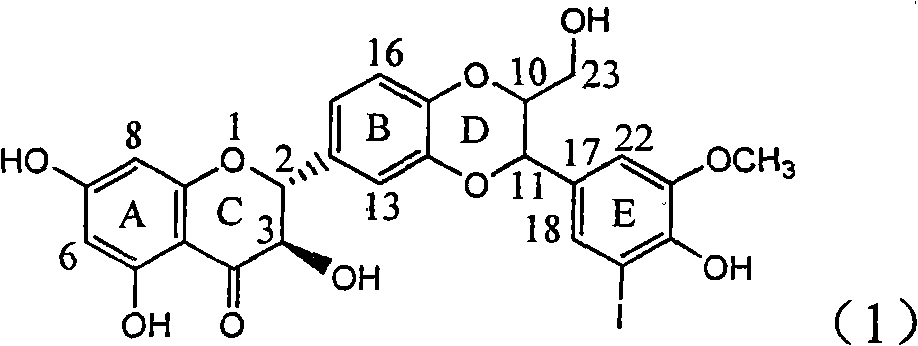
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
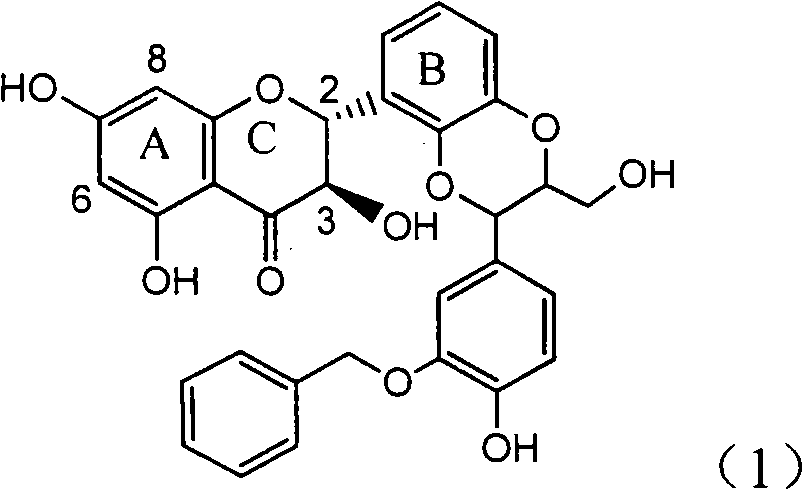
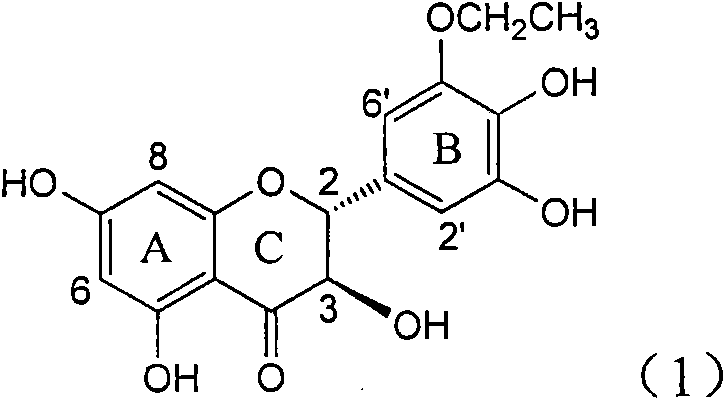
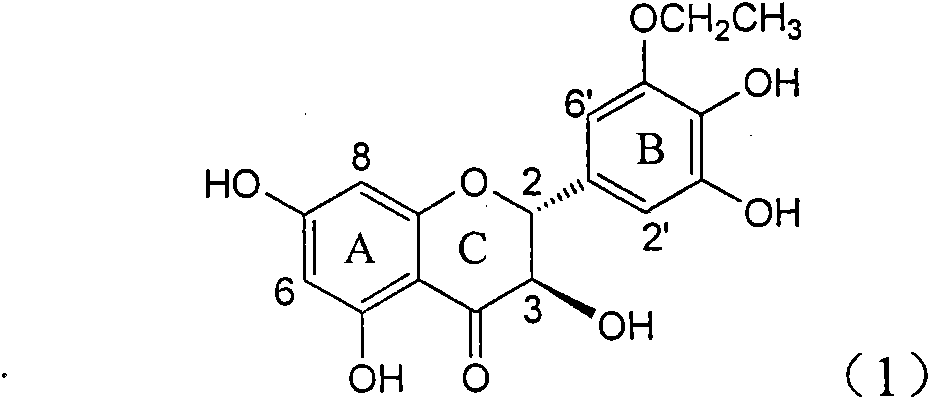
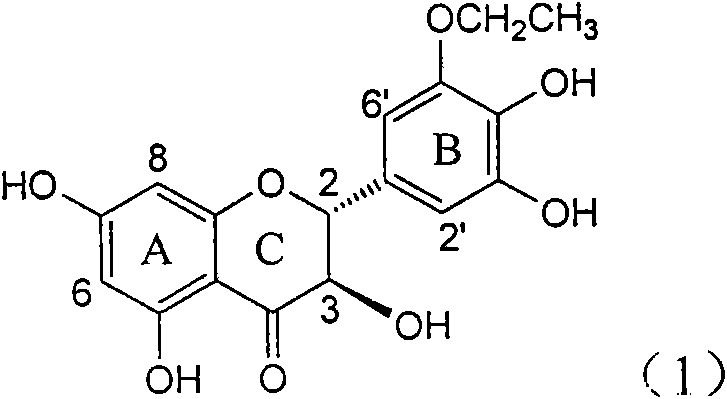
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
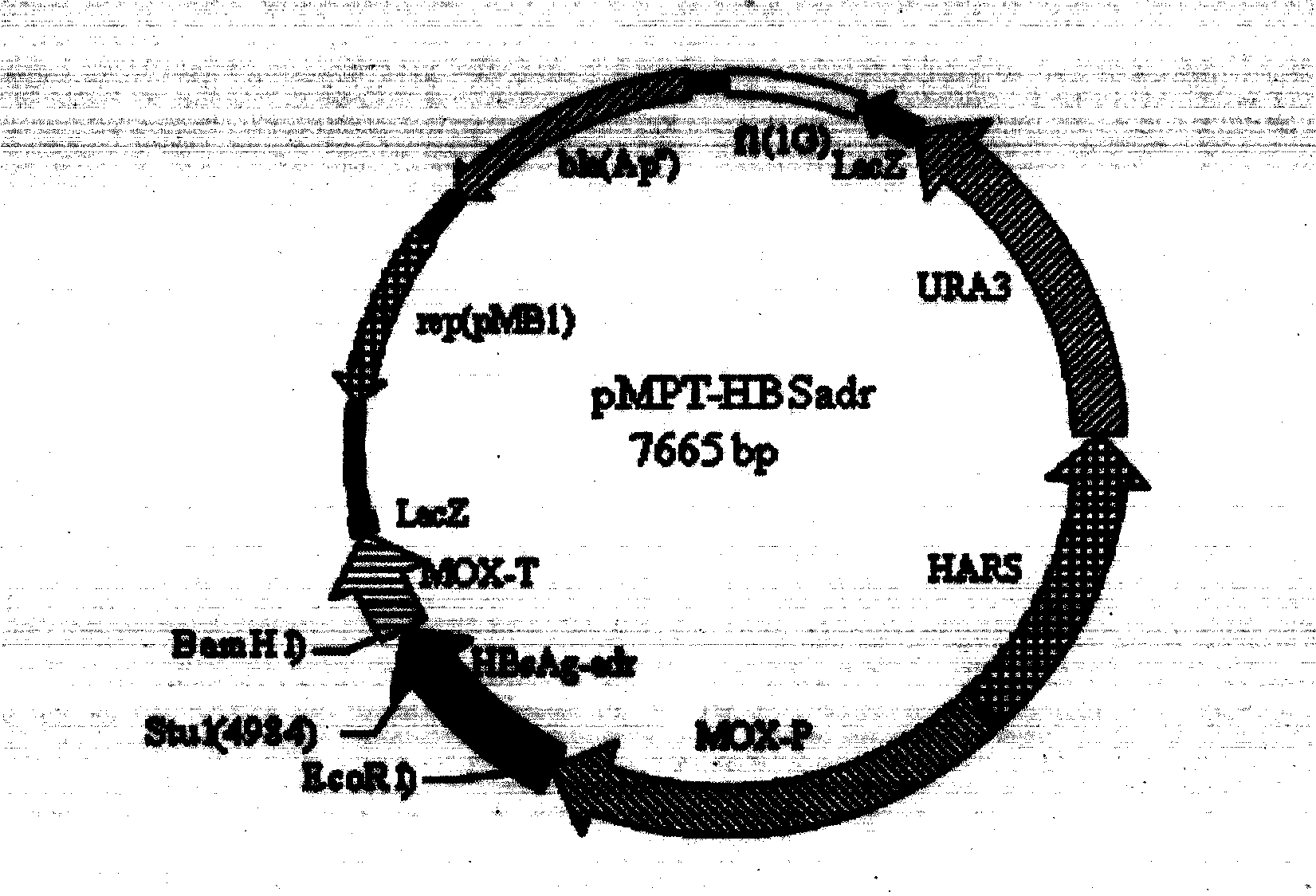
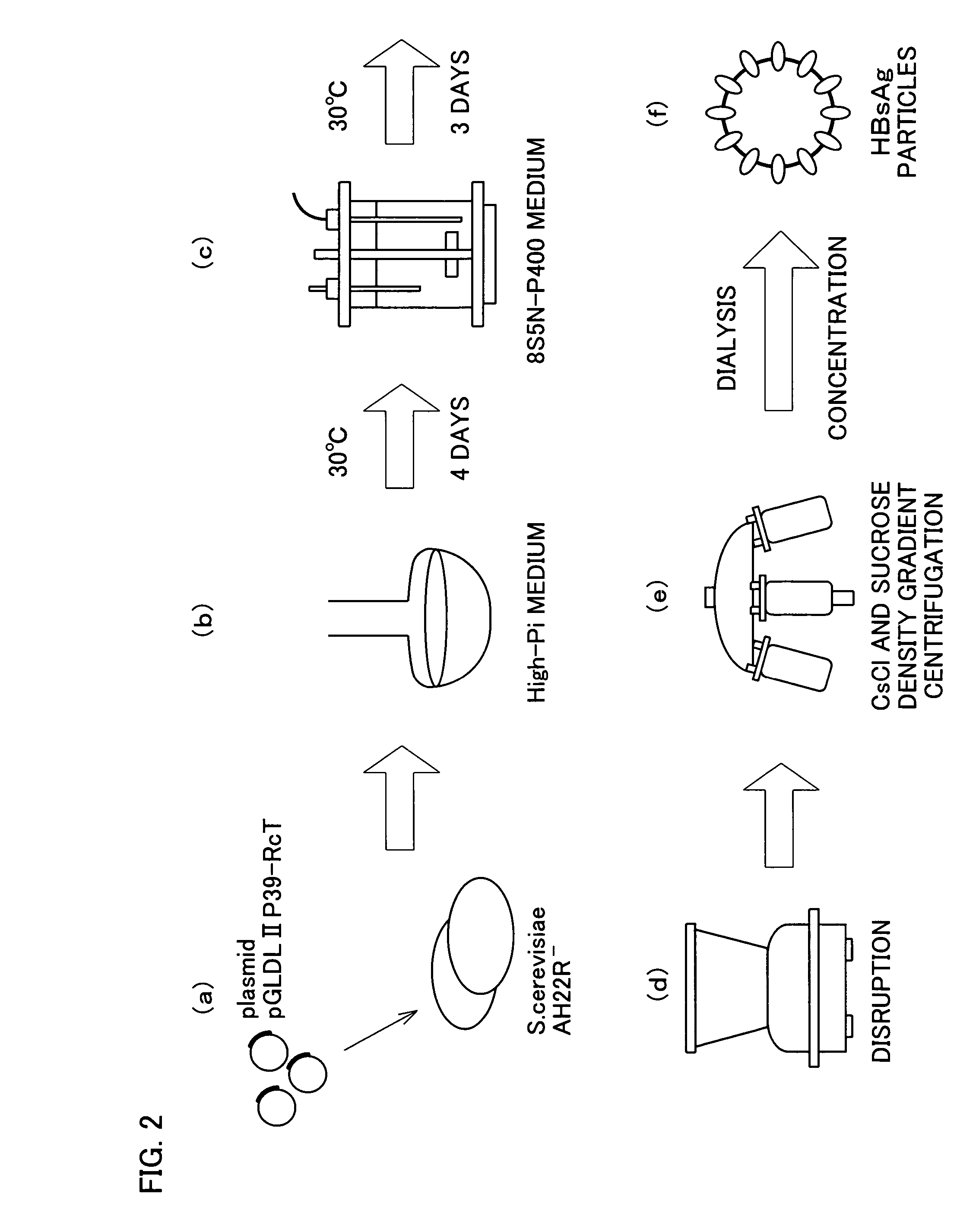
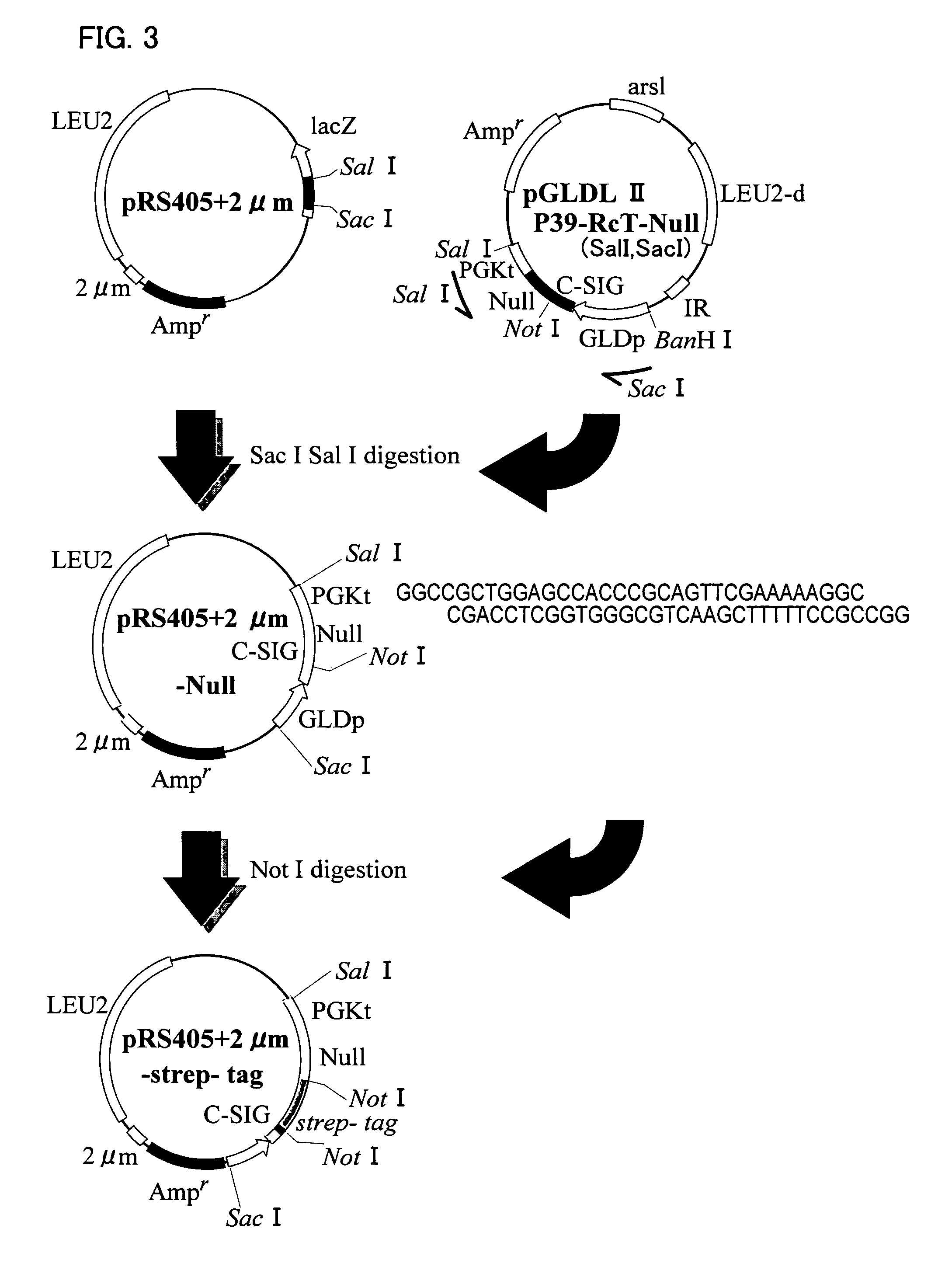
Recombinant DNA sequence, hansenula polymorpha, preparation method for hepatitis B surface antigen, and hepatitis B vaccine
InactiveCN104232661AImprove expression levelSplit genetic stability is highFungiMicroorganism based processesChemical synthesisHigh cell
The invention provides a recombinant DNA sequence, hansenula polymorpha, a preparation method for hepatitis B surface antigen, and a hepatitis B vaccine. The recombinant DNA sequence is obtained by codon optimization of coding genes of the hepatitis B virus surface antigen according to codon usage frequency of the hansenula polymorpha. The invention also provides the hansenula polymorpha comprising the recombinant DNA sequence, a method for preparing adr sub-type hepatitis B surface antigen by using the recombinant DNA sequence, and the hepatitis B vaccine. The adr sub-type hepatitis B surface antigen has high expression level of the recombinant DNA sequence. The recombinant hansenula polymorpha is fast in growth speed, has high HBsAg yield, can be fermented in high cell density by using a cheap chemically synthetic medium, has low fermentation contamination rate and is beneficial to large-scale production; and HBsAg adr vaccine provided by the invention has high trend of Th1 and Th2 type cellullar immunologic response.
Owner:北京天坛生物制品股份有限公司
Use of 15-methano-substituted-andrographolide derivative in preparing anti-hepatitis B medicine
ActiveCN101416958AExpand the range of optionsTo clarify the anti-HBV activity in vitroOrganic active ingredientsDigestive systemHepatitis B virus core AntigenMedicine
The invention discloses the medical application of a 15-methylene replaced andrographolide derivant as shown in general formula 1, more particularly relates to the application thereof in preparing anti-hepatitis B virus drugs, pertaining to the pharmaceutical chemistry field. HepG2.2.15 cells are used for detecting the secretory volumes of HBsAg and HBeAg and the HBV DNA level related to viral particles in the supernatant liquid of a nutrient solution, and the result shows that the 15-methylene replaced andrographolide derivant has good in-vitro anti-HBV effect. The 15-methylene replaced andrographolide derivant has better development and application prospect by being applied in preparing drugs used for treating and preventing Hepatitis B.
Owner:ZHENGZHOU UNIV
Kit for detecting hepatitis B surface antigen and detection method and application of kit
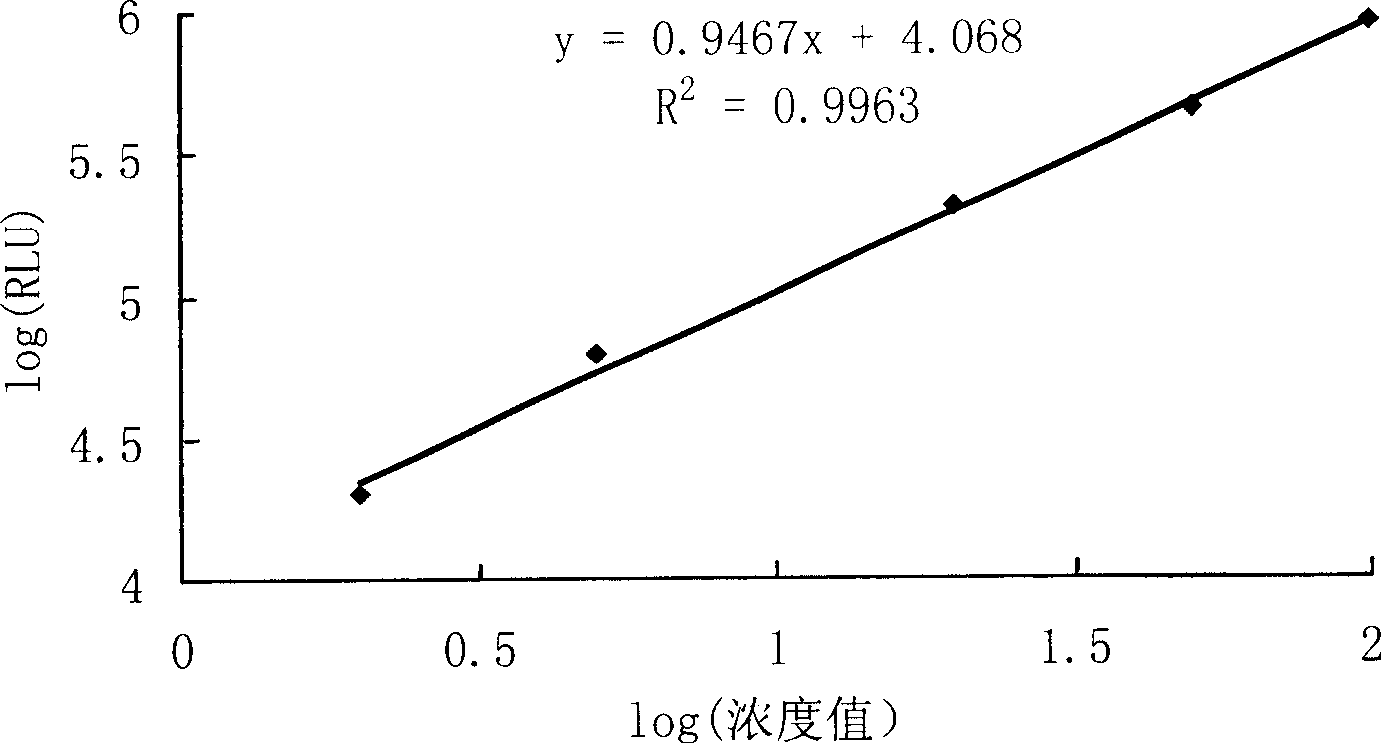
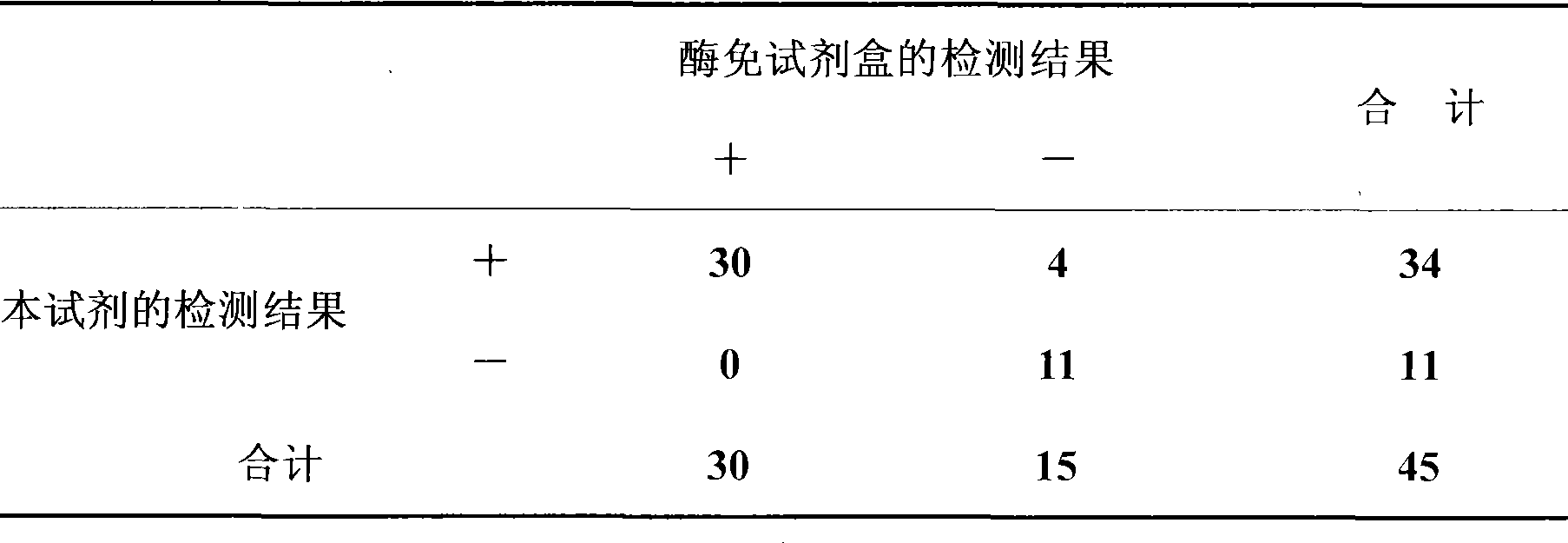
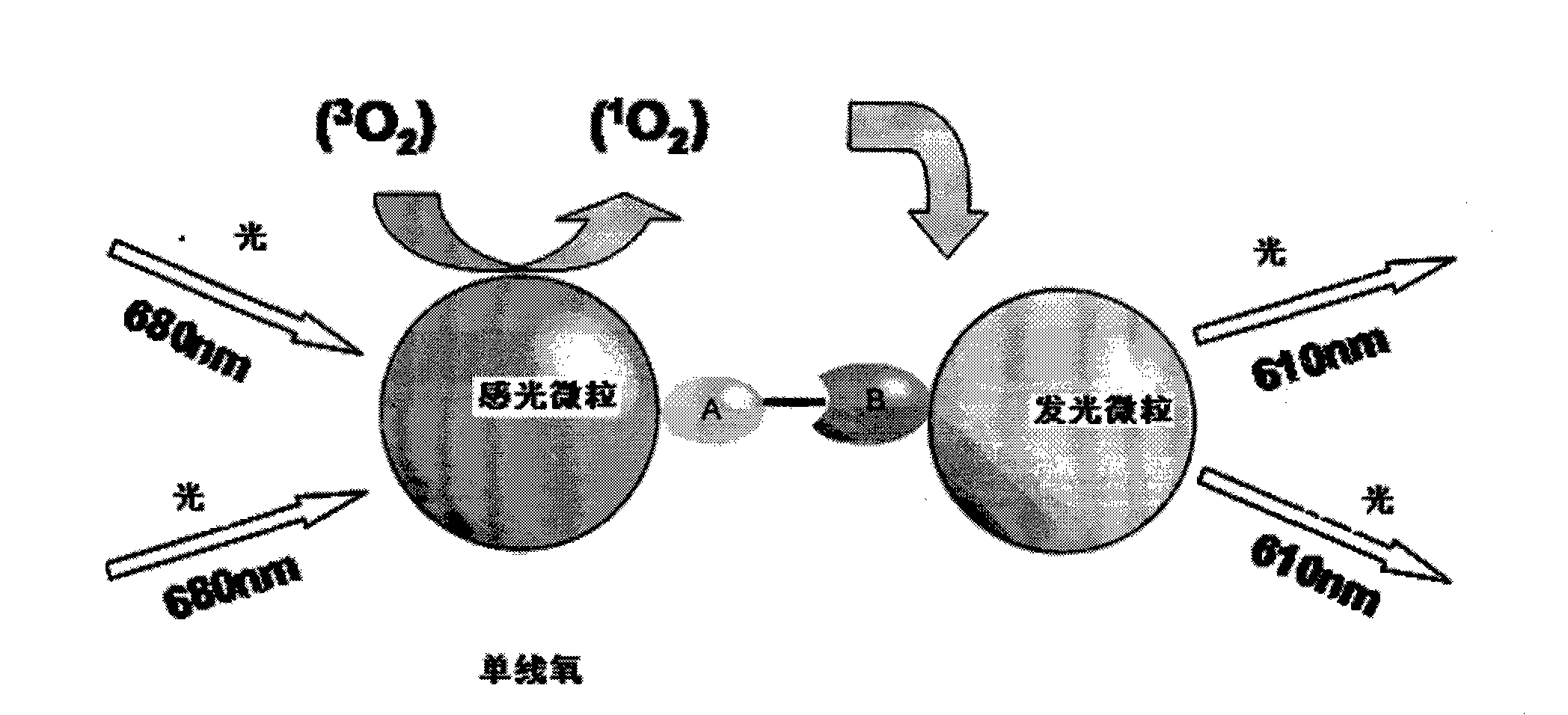
ActiveCN104698172AHigh sensitivityAvoid the problem of losing antigenBiological material analysisAnalysis by material excitationMicrosphereHook effect
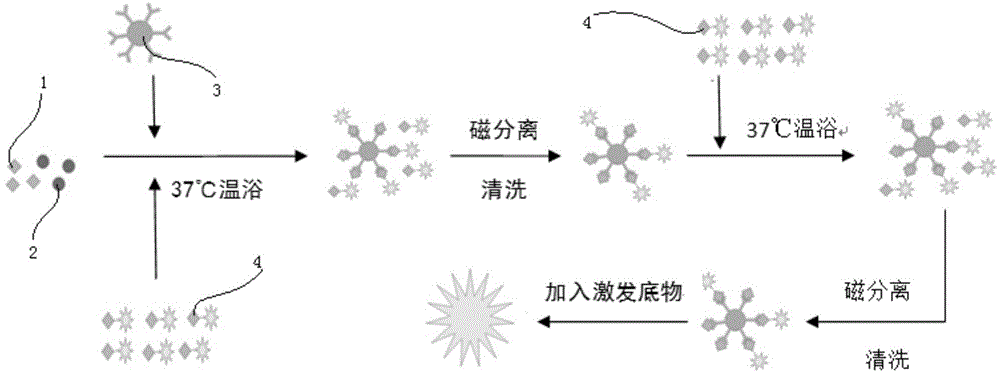
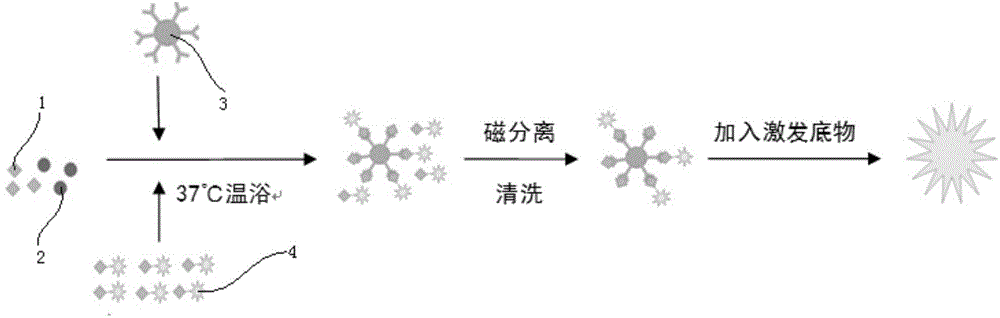
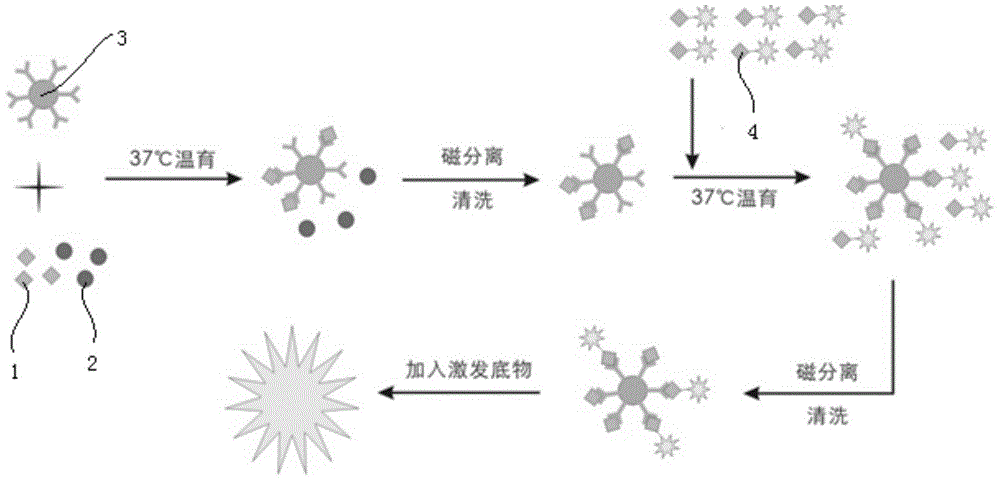
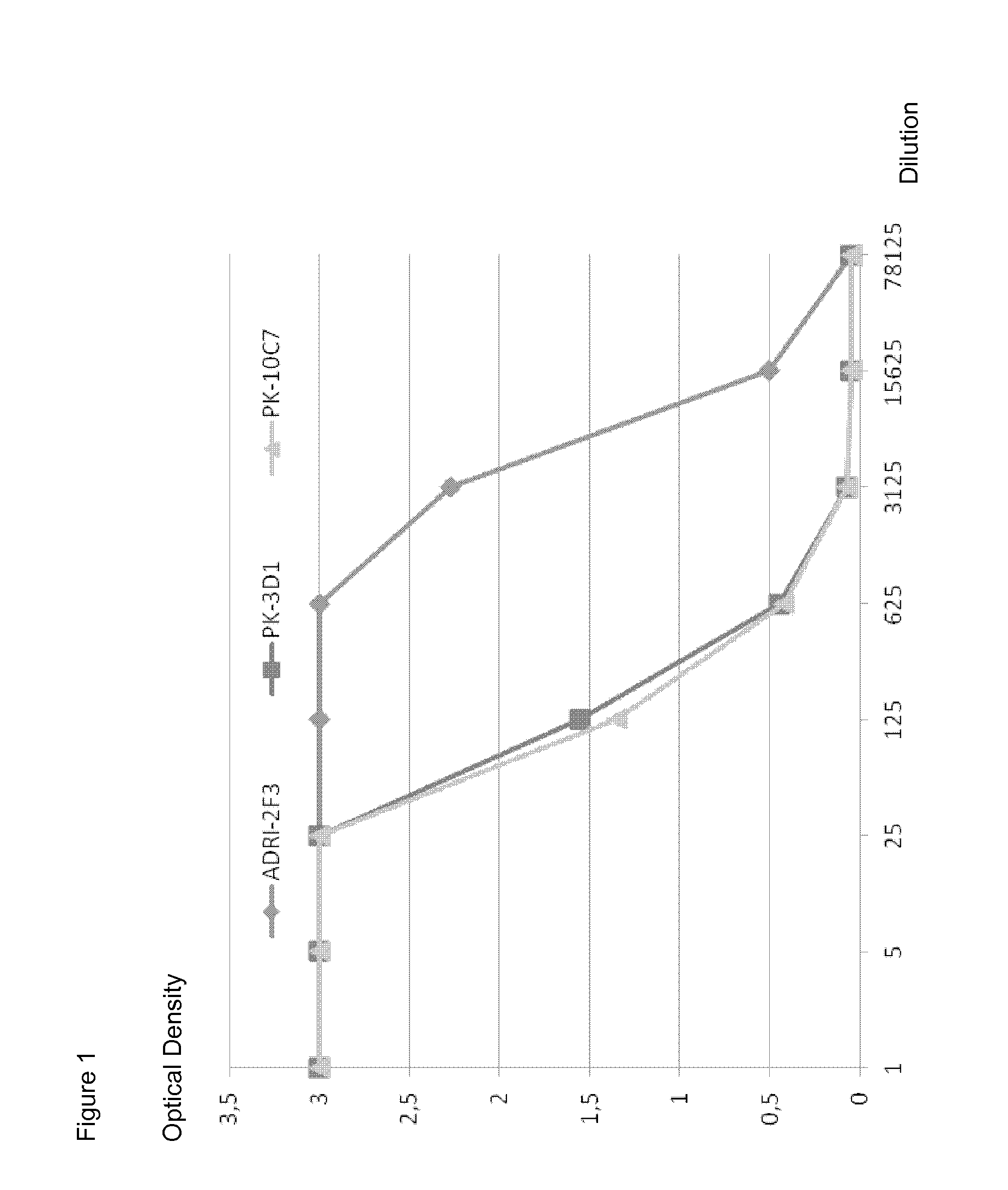
The invention discloses a kit for detecting a hepatitis B surface antigen and a detection method and application of the kit, belonging to the technical field of in vitro diagnosis and detection. The kit comprises the following components: (1) a magnetic micro-spherical system which comprises magnetic microspheres directly connected or indirectly connected with an antiBsAg antibody 1, (2) a first marker system which comprises an anti-HBsAg antibody 2 directly or indirectly connected with a first tracer or a second tracer, and (3) a second marker system which comprises an anti-HBsAg antibody 2 directly or indirectly connected with the first tracer or the second tracer or an anti-HBsAg antibody 3 directly or indirectly connected with the first tracer or the second tracer. The kit and the detection method can be used for detecting the hepatitis B surface antigen and have the advantages of high sensitivity and no HOOK effect.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Neutralizing human monoclonal antibodies against hepatitis b virus surface antigen
ActiveUS20160326233A1Efficient and effectiveImmunoglobulins against virusesAntiviralsHeavy chainHepatitis B Surface Antigens
The invention is in the field of medical treatment and prevention. The invention provides an antibody or a part thereof capable of specifically binding to the Hepatitis B surface antigen (HBsAg) and having Hepatitis B Virus (HBV) neutralizing activity, wherein said antibody or fragment thereof comprises a light chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 5, a light chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 6, a light chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 7, a heavy chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 8, a heavy chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 9 and a heavy chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 10.
Owner:MONDELLI MARIO UMBERTO FRANCESCO
Hepatitis b virus surface antigen as a mucosal immunostimulator and the resulting formulations
InactiveUS20050025780A1Enhance immune responseViral antigen ingredientsReverse transcribing DNA virusesHeterologous AntigensAdjuvant
The invention relates to a mucosal surface antigen which is used to promote and increase in the immune response against co-administered antigens in the formulations out line in the invention. Said novel formulations are obtained from the dual use of the surface antigen as an immunostimulatory agent and, at the same time, as a vaccine antigen. In this way it is possible to obtain multiple formulations of the hepatitis B surface antigen and heterologous antigens, with immunogenicity levels similar to those obtained following parenteral administration and with a reduction in components that can dispense with the use of nasal adjuvants, thereby converting same antigens into elements that can promote an increase in the response to the other co-administered antigens. Said novel use of the hepatitis B virus surface antigen and the resulting antigen formulations can be used in the pharmaceutical industry as therapeutic and preventive vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Application of andrographolide C15 substituted series derivatives to preparation of medicine for resisting hepatitis B
ActiveCN102302487AClear anti-HBV activityExpand the range of optionsOrganic active ingredientsOrganic chemistryBULK ACTIVE INGREDIENTViral hepatitis b
The invention belongs to the technical field of medicinal chemistry, and discloses application of andrographolide C15 substituted series derivatives to the preparation of a medicine for resisting hepatitis B. HepG2.2.15 cells are used for detecting the secretion amount of hepatitis B surface antigen (HBsAg) in culture solution supernatant, a large number of andrographolide derivative compounds are screened, and compounds which have high hepatitis B virus (HBV) resistance are optimally selected and have a structure shown in a general formula 1; and the compounds have high HBV-resistant activity, high efficiency and low toxicity and are taken as active ingredients to be used for preparing a medicine for resisting viral hepatitis B, and a new medicine is provided for treating hepatitis, so that the selectable range of clinical medicines is expanded.
Owner:ZHENGZHOU UNIV
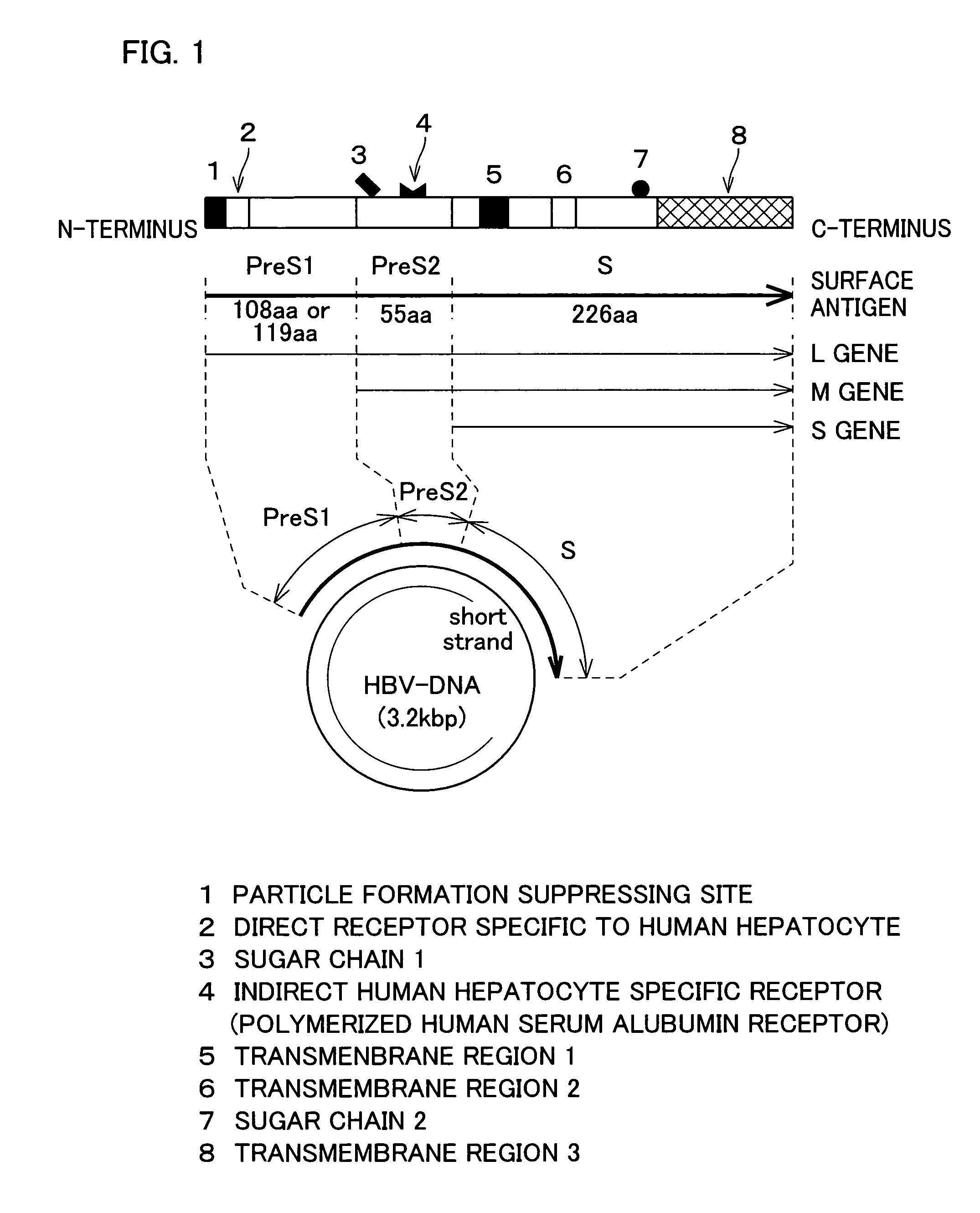
Hollow nanoparticle of NBsAg large protein for drug delivery
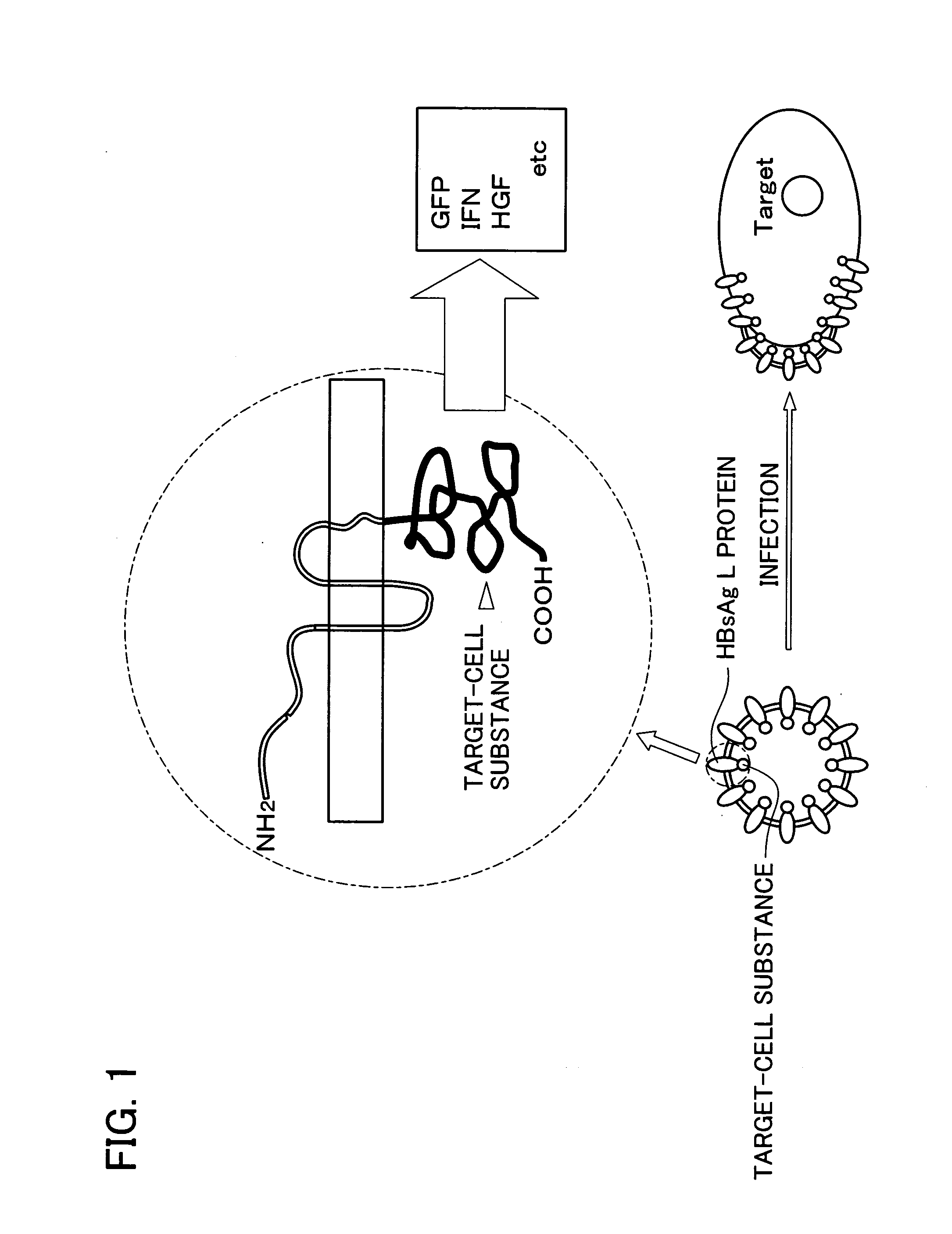
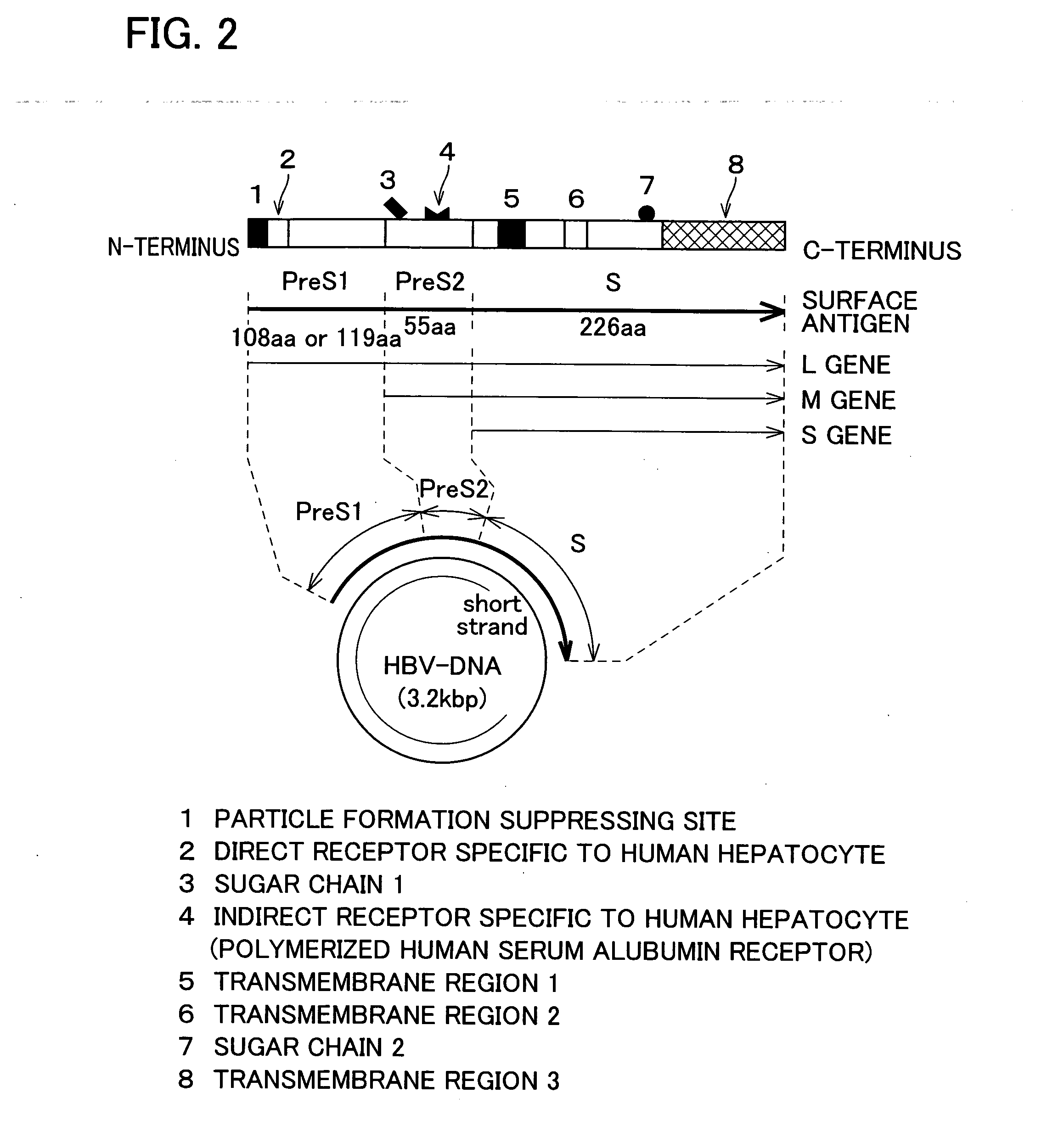
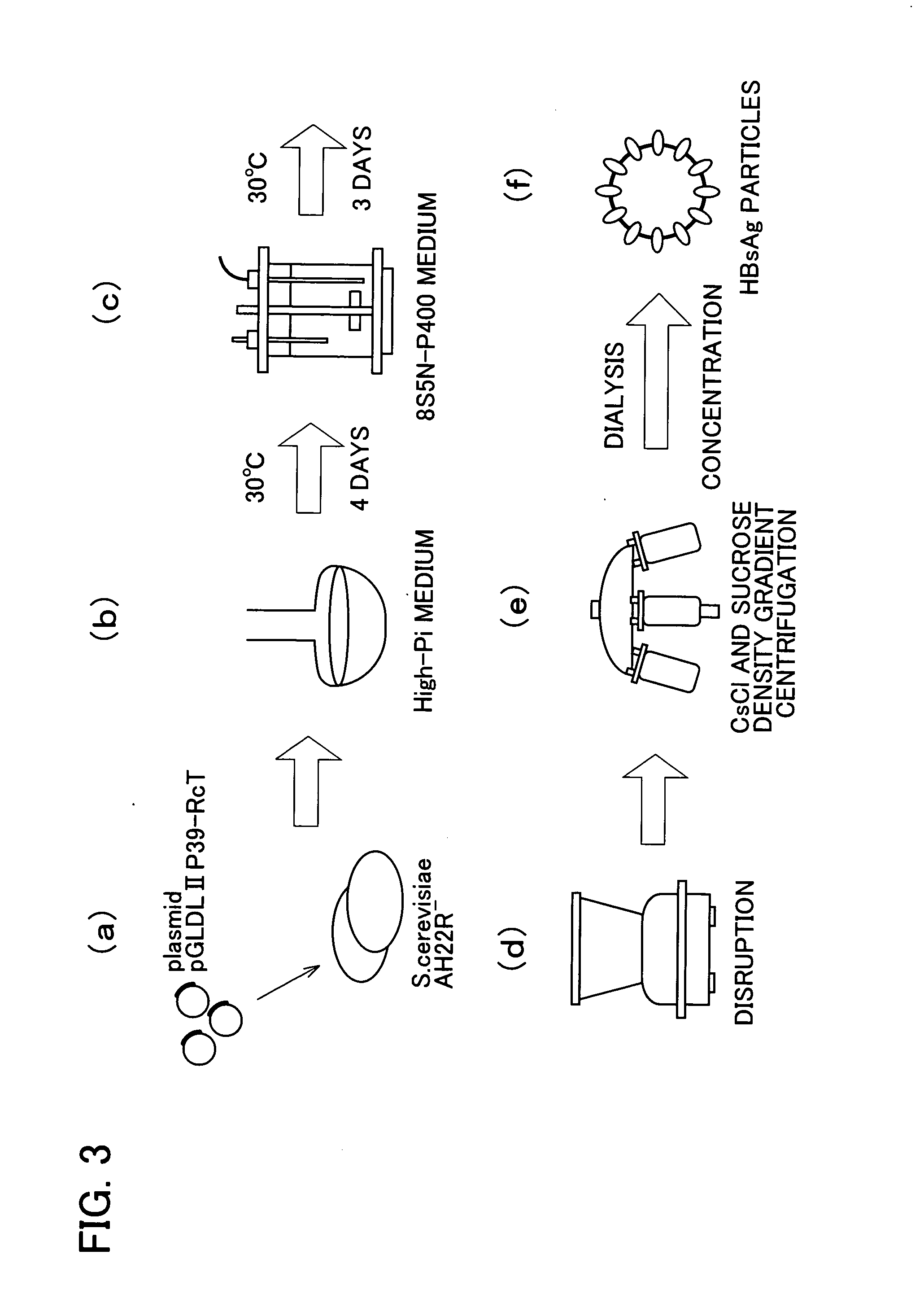
The invention provides a therapeutic drug that uses hollow protein nanoparticles displaying an antibody against a specific cell or specific tissue. The effectiveness of the drug has been proved by animal testing. The invention also provides a therapeutic method using such a drug. In a drug according to the present invention, a substance to be transferred into a cell for treating a disease (for example, a cancer treating gene such as a thymidine kinase gene derived from simple herpes virus) is encapsulated in hollow nanoparticles of a particle-forming protein (for example, hepatitis B virus surface-antigen protein that has been modified to lack its infectivity to hepatocytes and display an antibody). The particle surface of the drug displays an antibody, such as a cancer specific antibody, that recognizes an antigen molecule displayed on the surface of a specific cancer cell.
Owner:BEACLE +1
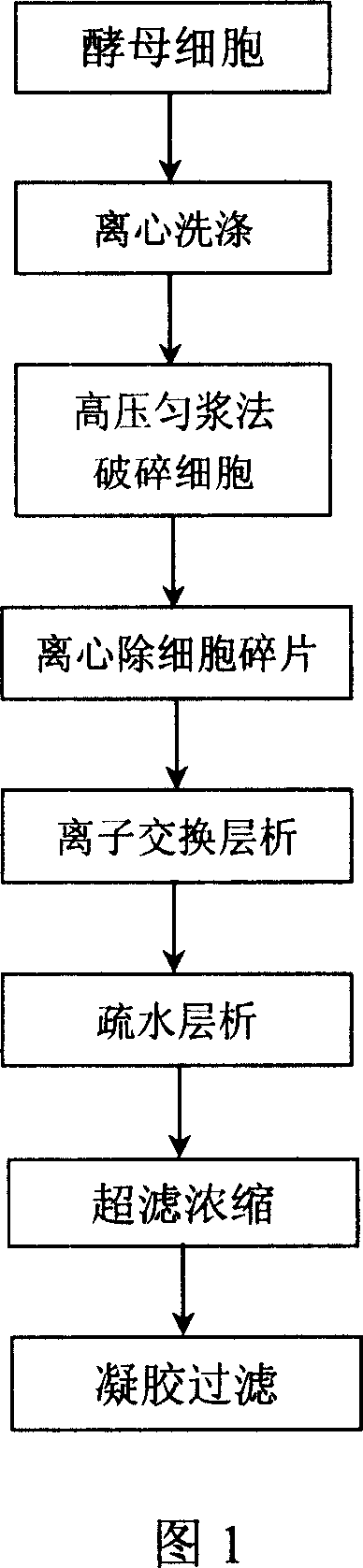
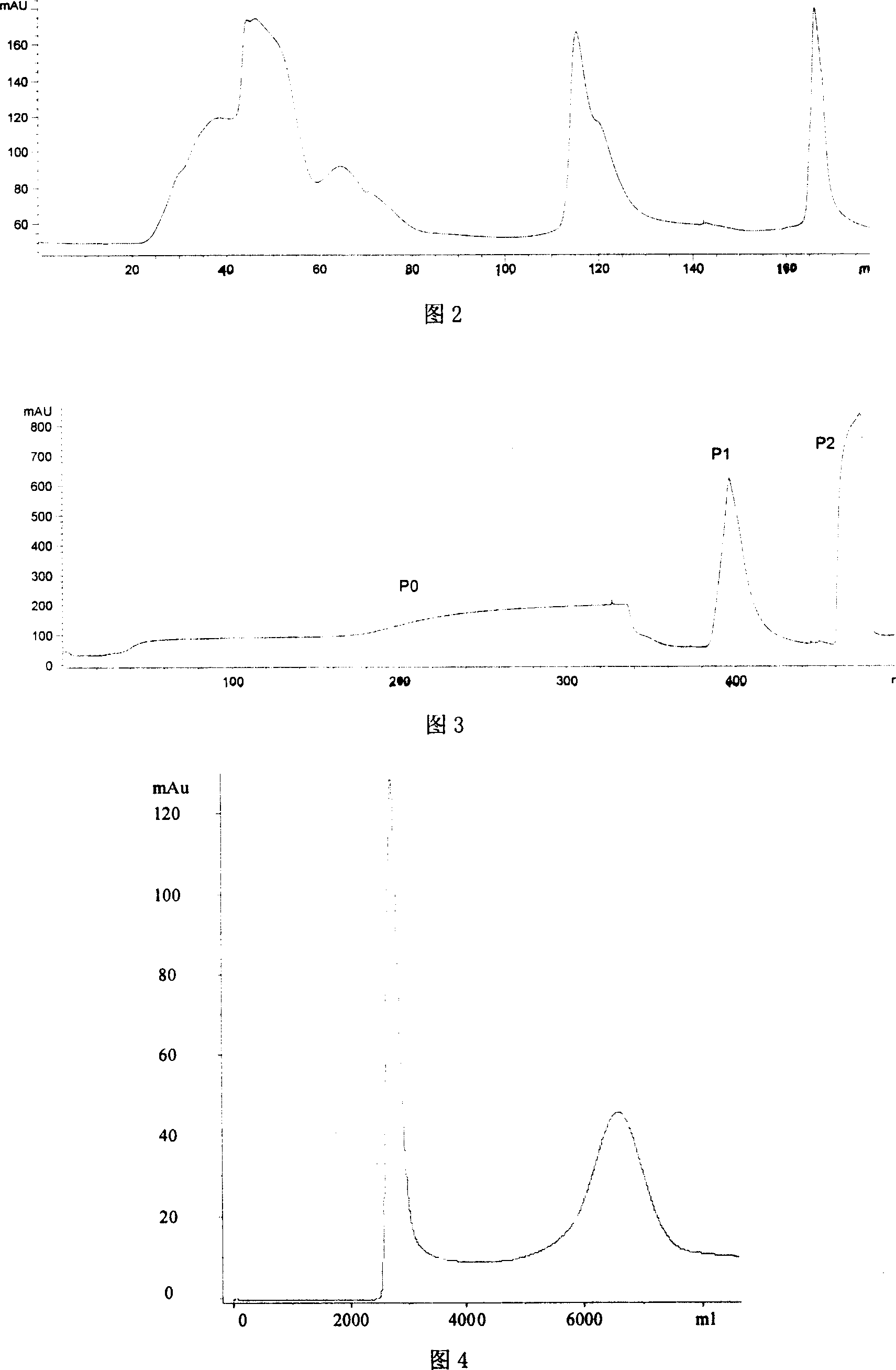
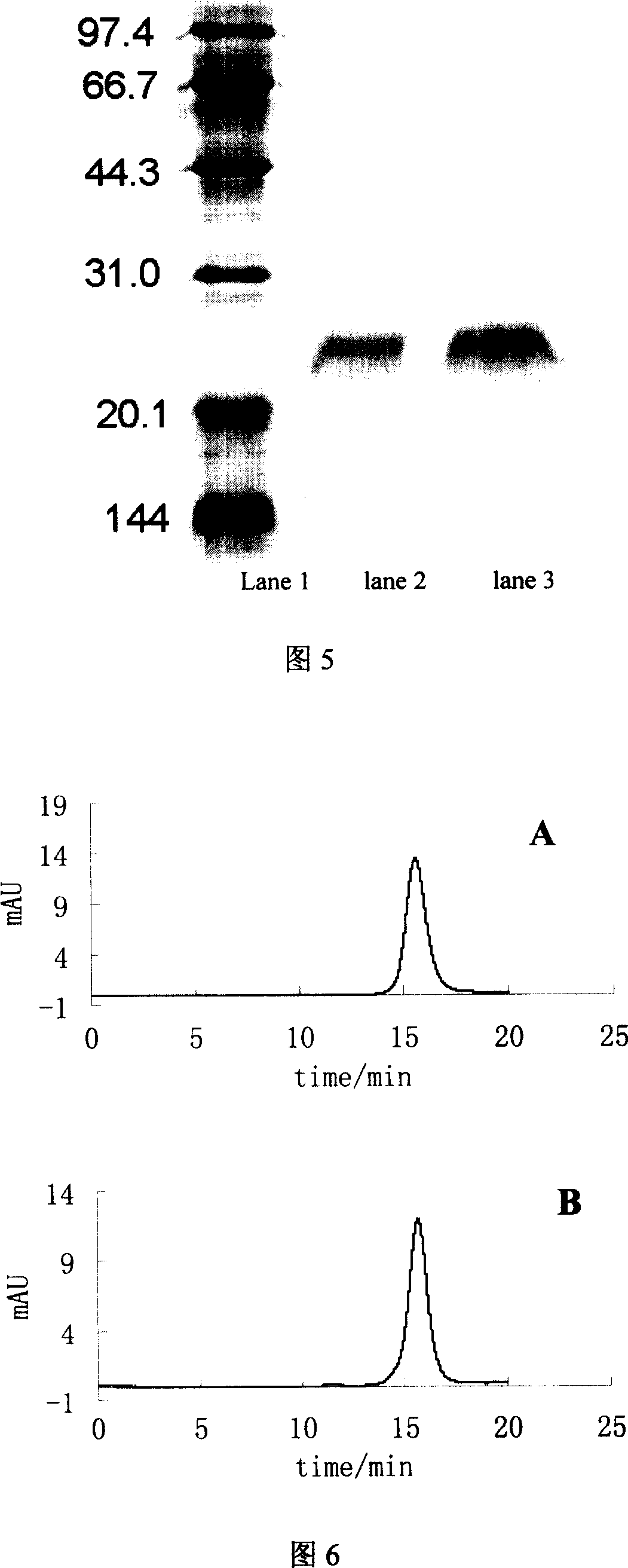
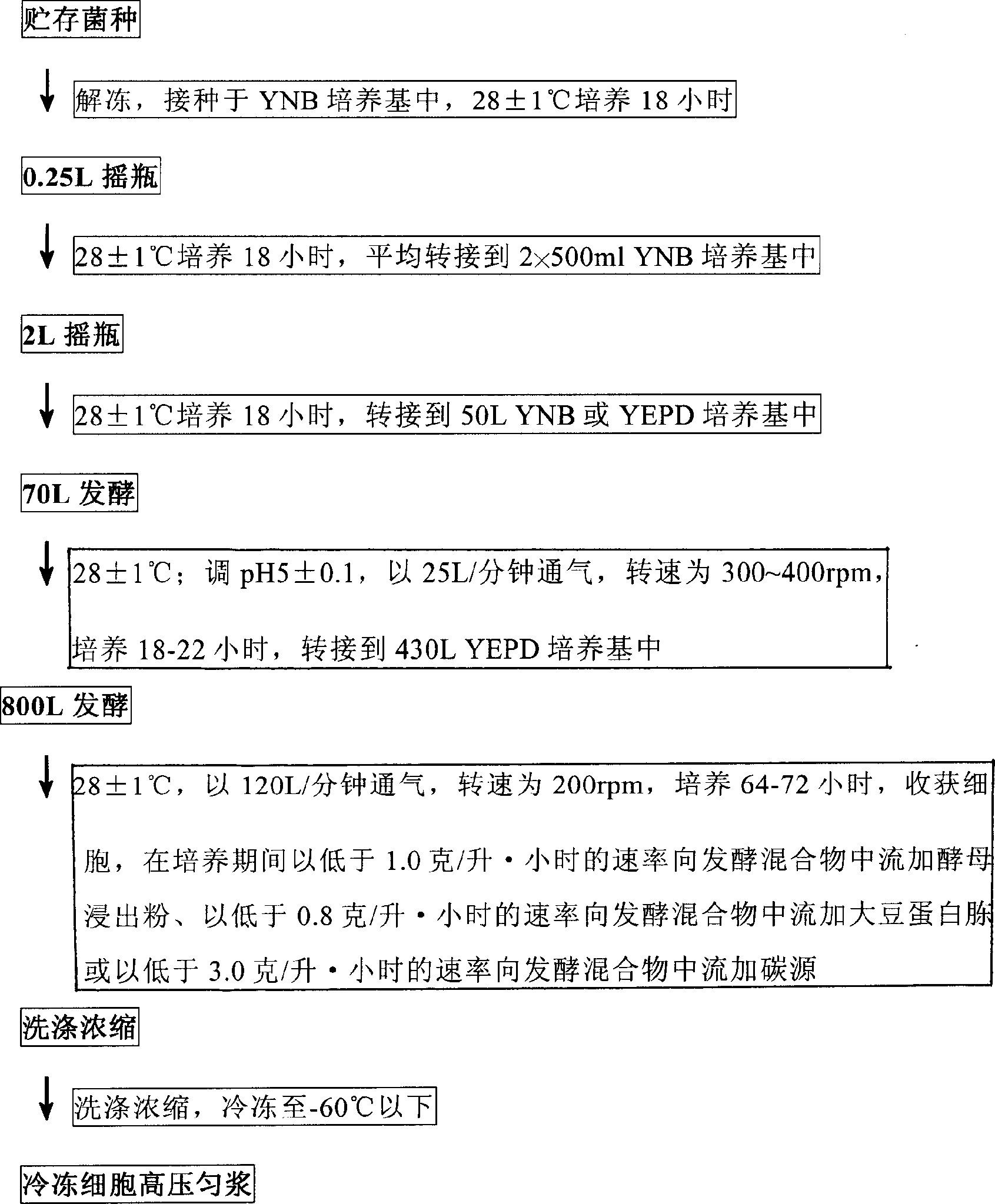
Method for separating and purifying recombined hepatitis b surface antigen expressed by Hansenula yeast
InactiveCN101003564AHigh recovery of purification activityReduce stepsVirus peptidesPeptide preparation methodsAntigenFiltration
This invention relates to a method for separating and purifying recombinant hepatitis B virus surface antigen expressed in Hansenula polymorpha. The method comprises: (1) crushing culture solution of recombinant B virus surface antigen expressed in Hansenula polymorpha till 50-90% cells are crushed; (2) centrifuging to remove cell debris, adjusting pH value and electrical conductivity, performing anion exchange chromatography, and collecting the eluates with activity higher than 20%; (3) incorporating the eluates, adjusting the pH value and electrical conductivity, and performing hydrophobic chromatography; (4) concentrating the eluate with ultrafiltration membrane; (5) separating by a gel filtration column to obtain recombinant B virus surface antigen expressed in Hansenula polymorpha with purity higher than 99%. The method has such advantages as high recovery rate, high product purity, few steps, and short time.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Kit and method for detecting hepatitis B virus surface antigen (HBsAg)
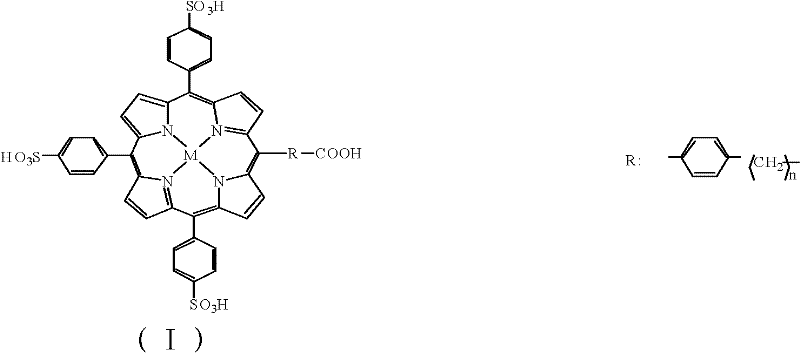
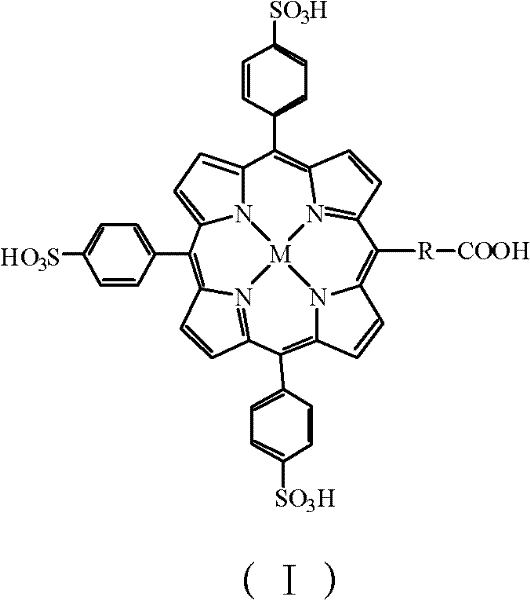
InactiveCN102183647AHigh purityReduce system errorOrganic chemistryMaterial analysisHepatitis B Virus Surface AntibodyPorphyrin
The invention discloses a kit and a method for detecting hepatitis B virus surface antigen (HBsAg). The detection kit is used for marking hepatitis B virus surface antibody (HBsAb) by using metalloporphyrin obtained by using water-soluble A3B type metalloporphyrin to mark the HBsAb. The detection kit and the detection method have the advantages that: (1) free metalloporphyrin molecules are separated from a coupled substance easily after an A3B type metalloporphyrin complex is coupled with the HBsAb to obtain a high-purity labelled antibody, so that non-specific adsorption background signals are reduced when the HBsAg is detected by chemical luminescence immunodetection, and detection sensitivity is greatly improved; (2) the method is simple, convenient and quick; automation is realized easily; and specificity of immunoreaction, high sensitivity of chemical luminescence reaction and stability of a chemical marker are achieved; and (3) a new method is provided for detecting the HBsAg quickly and accurately, and preventing and treating the hepatitis B virus.
Owner:HANGZHOU NORMAL UNIVERSITY
Drugs comprising protein forming hollow nanoparticles and therapeutic substance to be transferred into cells fused therewith
InactiveUS20050181064A1Easy to manufactureEfficient executionPeptide/protein ingredientsGenetic material ingredientsHepatitis B virusHepatitis B virus surface Antigen
The subject invention provides a disease-treating drug that uses hollow protein nanoparticles to specifically act on a target cell or tissue. The present invention allows a protein drug to be effectively capsulated in the particles. The invention also provides a therapeutic method using such a drug. The drug according to the present invention is capable of recognizing a specific cell, such as hepatocytes, and manufactured by fusing a disease-treating substance for a target cell (for example, interferon, hepatocyte growth factor etc.) with hollow nanoparticles of a particle-forming protein (for example, hepatitis B virus surface-antigen protein).
Owner:JAPAN SCI & TECH CORP
Hepatitis B virus surface antigen inhibitor
The invention discloses a novel 11-oxo-7, 11-dihydride-6H-benzo [f] pyrido [1, 2-d] [1, 4] azepine-10-carboxylic acid derivative as a hepatitis B virus surface antigen inhibitor, and concretely discloses compounds represented by formula (V) or pharmaceutically acceptable salts thereof, and applications of the compounds represented by formula (V) or pharmaceutically acceptable salt and pharmaceutically acceptable compositions in the treatment of viral hepatitis B.
Owner:푸지엔에이키링크바이오테크놀로지컴퍼니리미티드
Single clone antibody of antimutagen hepatitis B virus surface antigen
InactiveCN1680581AAvoid losing toQuality assuranceImmunoglobulins against virusesFused cellsAntigenVariant virus
A process of preparing single-colon antibody resisting surface antigen of hepatitis B virus mutant from CGMCCHBSP2. The single-colon antibody can detect 14 types hepatitis B virus containing mutant and wild strain and replace HBsAb in fluorogence diagnostic kit. It can avoid mistake induced by normal agent and be used widely.
Owner:徐钧
Composition for the prophylaxis and treatment of HBV infections and HBV-mediated diseases
InactiveUS20060233832A1Easy to handleSimple preparation processAntipyreticGenetic material ingredientsAntigenGenotype
The present invention is a composition that comprises at least two hepatitis B virus surface antigens (HBsAgs), fragments thereof and / or nucleic acids encoding them, the HBsAgs differing in HBV genotype in the S region and / or pre-S1 region and the composition containing no HBV core antigen (HBcAg) or nucleic acid encoding that antigen. The present invention also includes pharmaceutical compositions, especially vaccines, comprising these compositions for the prevention and / or treatment of an HBV infection or an HBV-mediated disease. The present invention further includes a method of preparing a patient-specific medicament for the therapeutic treatment of hepatitis B.
Owner:RAJN BIOTEKH GEZELLSHAFT FJUR NOJE BIOTEKHNOLOGISHE PROTSESSE & PROD MBKH
Large protein pre-S surface antigen for hepatitis B virus chemiluminescence immune assay kit and method for making same
InactiveCN101382553AHigh sensitivityNo cross reactionChemiluminescene/bioluminescenceBiological testingAntigenImmune profiling
The invention relates to the immunoassay medical field, concretely, the invention provides a determination kit of the front S area of the large protein (HBV-LP) of hepatitis B virus surface antigen, and a preparation method thereof. The kit based on the invention comprises: 1) a working calibrator of the front S area of the HBV-LP; 2) a streptavidin carrier; 3) a biotinylation monoclonal antibody of the front S area of the HBV-LP; 4) an alkaline phosphatase-marked monoclonal antibody of the front S area of the HBV-LP; and 5) a chemical luminescent substrate. Further, the preparation method of the kit based on the invention comprises the steps of: 1) preparing the working calibrator by the sterling product of the front S area of the HBV-LP; 2) enveloping the carrier by the streptavidin; 3) carrying out biotinylation to the monoclonal antibody of the front S area of the HBV-LP; 4) marking the monoclonal antibody of the front S area of the HBV-LP by the alkaline phosphatase; 5) preparing the chemical luminescent substrate; 6) subpackaging the working calibrator, an enzyme marker and the chemical luminescent substrate; and 7) assembling and installing for forming finished goods. The kit has the advantages of being simple and convenient, fast, sensitive, stable and the like.
Owner:北京科美东雅生物技术有限公司
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Hepatitis B virus surface antigen mutant and methods of detection thereof
The subject invention relates to a novel hepatitis B surface antigen mutant and methods of detecting this mutant, and / or antibodies thereto, in patient samples. In particular, the mutant contains a substitution of amino acid threonine for the amino acid alanine at position 123 in the amino acid sequence of the hepatitis B surface antigen (HBsAg) protein.
Owner:ABBOTT LAB INC
Medicine for curing hepatitis B
The medicine for treating hepatitis B is prepared with ten kinds of Chinese medicinal materials including human placenta, fresh-water turtle shell, capillary artemisia, etc. and pig bile. It has the functions of strengthening body's resistance, clearing away toxic matter, dispersing the stagnated liver energy, regulating vital energy, promoting diuresis, regulating and activating body's immunity system, eliminating hepatitis virus, promoting liver cell restoration, etc. It has obvious curative effect on hepatitis B.
Owner:罗明才
Nucleic acid aptamer combining hepatitis B virus surface antigen and sequence thereof
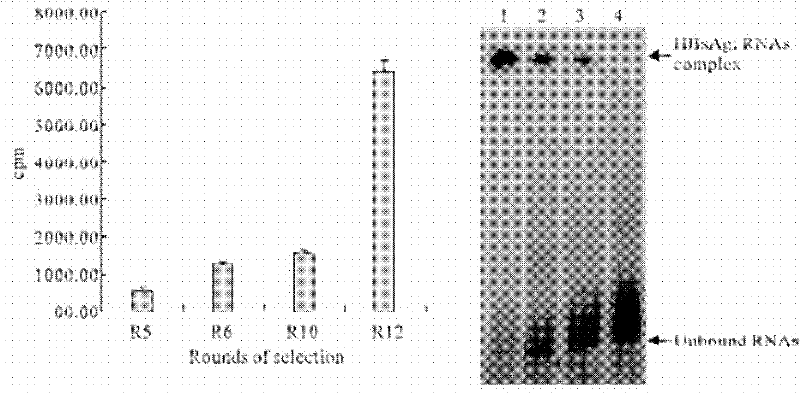
The invention discloses a nucleic acid aptamer of a targeted human infectious hepatitis B virus cell and a sequence thereof and belongs to the fields of gene engineering and biological medicine. According to the invention, a novel combining chemical technology SELEX is utilized to screen out an RNA aptamer capable of specifically binding to a hepatitis B virus surface antigen from single chain RNA random library by using the hepatitis B virus surface antigen as a target protein. The RNA aptamer has a sequence of 5'-GUUGAUUGCGUGUCAAUCAUGGCCGUCUAUAAUGAUCGUAAACGACGGGUCAUGUGUAUGUUGGGGAUUGGGACCUGAUUGAGUUCAGCCCACAUAC-3', can form a special loop structure in a random sequence area thereof and combine with the hepatitis B virus surface antigen singularly and with high affinity or combine to hepatocyte infected by the hepatitis B virus by target bonding. The RNA aptamer provides a specific efficient mark molecule for hepatitis B diagnosis and treatment and also provides a new option for exploiting diagnostic reagent and treatment medicament for chronic hepatitis B.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
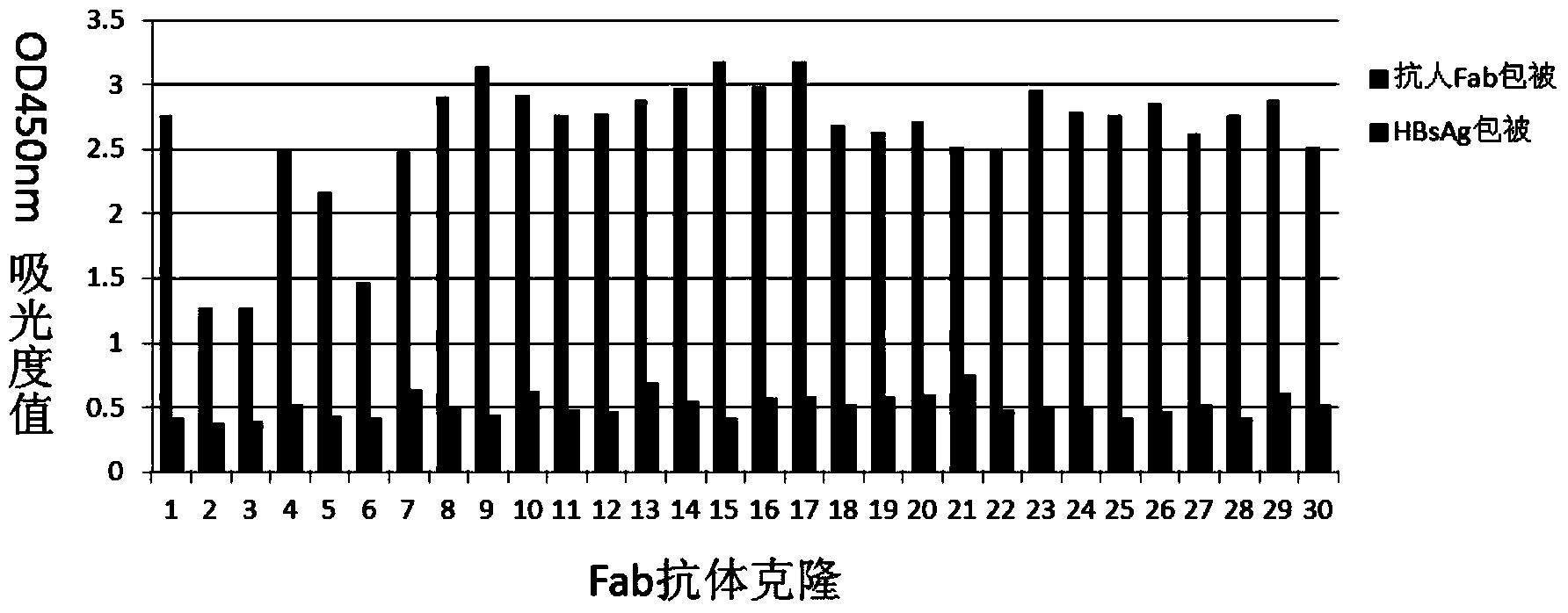
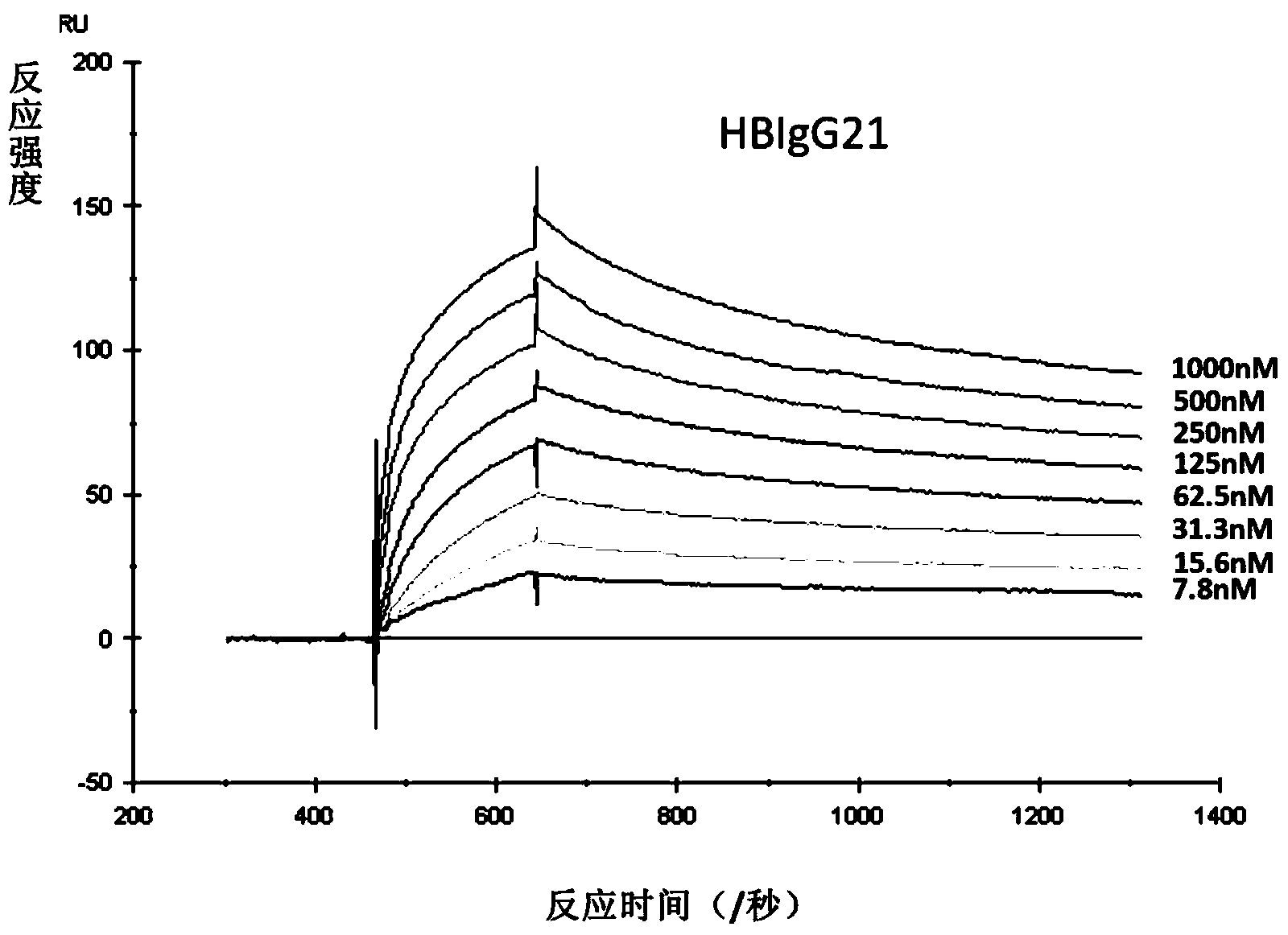
Human anti-HBV surface antigen genetic engineering antibody, and preparation method and application thereof
The invention discloses a human anti-HBV surface antigen genetic engineering antibody, and a preparation method and application thereof. According to the invention, the phage surface display technology is adopted, peripheral blood lymphocyte of person having high titer surface-antibody after immunization of hepatitis B vaccine is collected is obtained, a human anti-HBV surface antigen genetic engineering antibody library is established through a genetic engineering means, and a Fab section of the specific anti-HBV surface antigen genetic engineering antibody is obtained through screening. The obtained Fab section of the antibody is named to be HBFab21. The amino acid sequence of a light chain variable region of the HBFab21 is shown in SEQ ID No.1, and the amino acid sequence of a heavy chain variable region of the HBFab21 is shown in SEQ ID No.2. According to the invention, a foundation for hepatitis B virus infection prevention and researches on treatment to hepatitis B virus is laid; through the foundation, an antibody product having an effect of neutralizing hepatitis B virus infection is obtained, and can be applied to preparation of a clinical drug or diagnostic reagent for liver diseases relating to hepatitis B virus.
Owner:中国疾病预防控制中心病毒病预防控制所
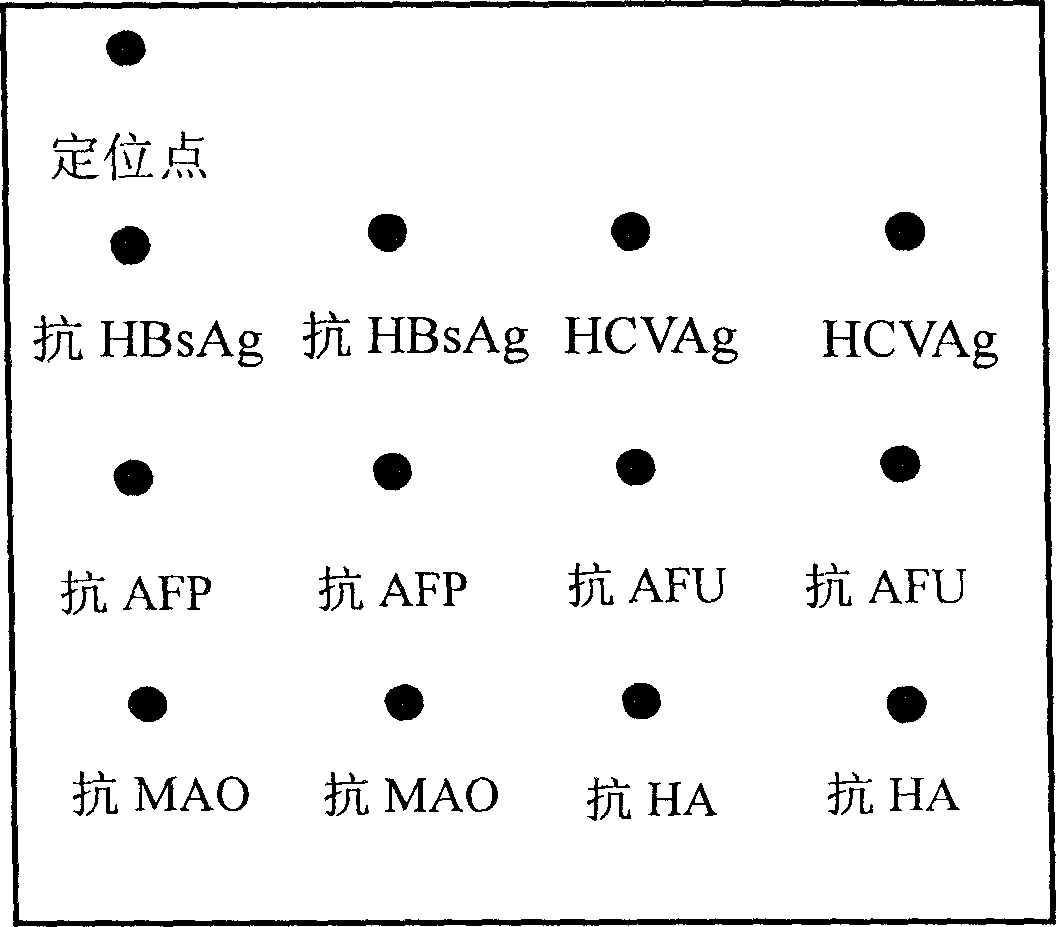
Integral detection reaction plate and protein chip kit of hapetitis and cirrhosis
The invention relates to an integrated detection reaction orifice plate and protein chip reagent box for detecting six indexes of diagnosis, forecast and prognosis of hepatitis, hepatocirrhosis and liver cancer, including hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibody (HCVAb), alpha-fetoprotein (AFP), alpha-L-Fucosidase (AFU), mono amine oxidase (MAO), hyaluronic acid (HA). And the orifice plate comprises a substrate and reaction orifices on the substrate, where the reaction orifices comprise 2-384 sample orifices and 2-8 standard product orifices, and at the bottom of each reaction orifice is solid carrier coated with micro lattice of HBsAg, HCVAg, AFP, AFU,MAO,and HA antigens / antibodies or more. And the reaction plate and reagent can simply and conveniently, quickly and accurately implement simultaneous detection of the six indexes of diagnosis, forecast and prognosis of hepatitis, hepatocirrhosis and liver cancer for many persons.
Owner:穆海东
Brewer's yeast recombined hepatitis B virus surface antigen stream plus addition femrentation
ActiveCN1552857AImprove toleranceIncreased weight-to-weight productivityFungiImmunoglobulins against cell receptors/antigens/surface-determinantsHepatitis B Surface AntigensAdenosine diphosphate
A hepatitis B surface antigen is produced with beer yeast engineering bacteria. The process is carried out by: 1) fermenting seeds of the beer yeast engineering bacteria, 2) fermenting by the said seeds, and 3) separating and purifying fermented mixture to obtain hepatitis B surface antigen, specially, flow adding adenine, adenosine, adenosine monophosphate, adenosine diphosphate or adenosine triphosphate at the rate of 14mg / L.h by weight into its culture medium in the said step 2. It achieves in higher expression level of recombined HBsAg.
Owner:BEIJING BIOLOGICAL PROD INST CO LTD +1
B-type hepatitis vaccine
InactiveCN1404875ABright future for treatmentImprove humoral immunity/cellular immunityDigestive systemAntiviralsAdjuvantChronic hepatitis
The present invention relates to a hepatitis B vaccine, the main composition of said vaccine comprises gene engineering hepatitis B virus surface antigen, muramyl dipeptide (MDP) and aluminium adjuvant. Said hepatitis B vaccine can be used for immunity of adult, renal transplanted patient and patient with renal dialysis therapy and for preventing infection of hepatitis B virus, and said hepatitis B vaccine also can be used for immunotherapy of chronic hepatitis B patient, and the immunogenicity of said vaccine is superior to that of existent aluminium adjuvant hepatitis B vaccine.
Owner:BEIJING LUZHU BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com