Application of andrographolide C15 substituted series derivatives to preparation of medicine for resisting hepatitis B
A technology of andrographolide and preparation of drugs, which is applied in the direction of drug combination, digestive system, antiviral agents, etc., can solve the problems of easy drug resistance, adverse reactions, rebound, etc., to expand the range of options and clarify the anti-HBV active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
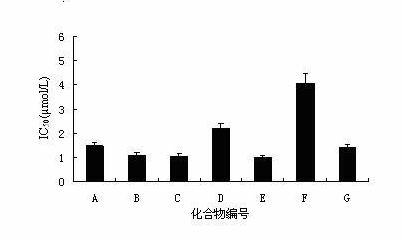
[0035] Example 1 Anti-HBV Activity Experiment of Andrographolide Derivatives in Vitro
[0036] 1. Cell culture and drug treatment
[0037] Human liver cancer HepG2.2.15 cells transfected with human hepatitis B virus gene are used to detect the effect of the drug of the present invention on the secretion of HBV surface antigen (HBsAg) in the culture supernatant of HepG2.2.15 cells. The HepG 2.2.15 cell suspension was seeded in a 48-well plate with a cell number of 1.25×10 4 To each well, add 0.5 mL of RPMI1640 culture medium, which contains 10% fetal bovine serum, 380 μg / mL G418, 100 μg / mL streptomycin, 100 IU / mL penicillin, and 5% CO 2 Place them in a carbon dioxide incubator at 37 °C for culture, and change the drug-containing culture medium every 24 h, and set 5 gradients of drug concentration. Lamivudine (3TC) was used as positive drug control. The culture medium was changed once at 3d and 6d of culture respectively, and the supernatant in the culture well was taken out ...
Embodiment 2
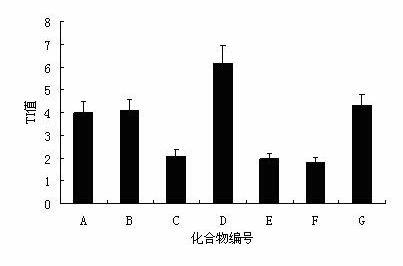
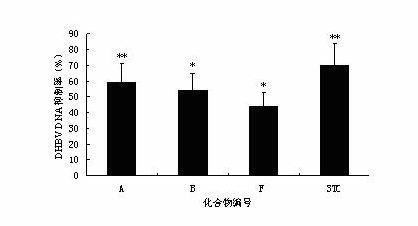
[0046] Example 2 In vivo anti-DHBV activity experiment of andrographolide derivatives
[0047] 1. Experimental animals and materials: Cherry Valley duck, male, purchased from a duck factory; DHBV positive serum was collected and preserved by our laboratory.
[0048] 2. Instruments, drugs and their preparation: the model LightCycler? 1.5 fluorescent quantitative PCR system produced by Swiss Roche Company; the Milli-Q-B.S ultrapure water instrument produced by Millipore Co., Ltd. of the United States; centrifuge. Compounds A-G of the present invention were synthesized by the applicant; lamivudine is commercially available. The above-mentioned experimental drugs were formulated into 0.5% CMC-Na solution, and dissolved with Tween-80 (final concentration 0.1%). SYBR Green I is a product of Bao Biological Engineering (Dalian) Co., Ltd. The upstream and downstream primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0049] 3. Experimental method:
[0050] Abo...
Embodiment 3
[0055] Example 3 Limit Toxicity Test of Compounds A and B
[0056] Animals: clean-grade Kunming mice, weighing 20±2 g, half male and half male, provided by the Experimental Animal Center of Henan Province. Certificate number: 0009898.
[0057] Drugs: Compounds A and B synthesized by the applicant.
[0058] Experimental method: Animals were randomly divided into groups, and after fasting for 12 hours (drinking water was not restricted), compound A and B at a dose of 5.00 g / kg were administered orally once. Observe and record the state of the animals, whether there is any poisoning performance. The results are shown in Table 1.
[0059] Experimental results: The mice did not show obvious signs of poisoning, and did not die, indicating that the acute toxicity of this type of compound is minimal. As preparation of anti-HBV medicine, it has good practical and development value.
[0060]
[0061] Table 1 Limited Toxicity Test
[0062] compound Dose (g / kg) n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com