Application of timosaponin AIII in anemarrhena to preparation of antitumor drugs
A timosaponin and anti-tumor technology, which is applied in the field of pharmacy, can solve the problems of anti-tumor and anti-cancer, and achieve the effect of expanding the range of options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
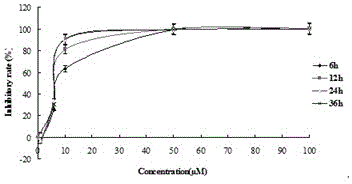
[0030] Example 1. MTT method was used to determine the growth inhibitory effect of timosaponin AⅢ on HeLa, HepG2, MCF-7, HCT116, HT1080, T475-S2, K562, HL60, U937 and A549 cells.
[0031] Experimental Materials:
[0032] 1. Cells
[0033] HeLa cells (cultured in RPMI-1640 medium), HepG2 cells (cultured in RPMI-1640 medium), MCF-7 cells (cultured in RPMI-1640 medium), HCT116 cells (cultured in RPMI-1640 medium), HT1080 cells (cultured in RPMI -1640 medium), A375-S2 cells (MEM medium), K562 cells (RPMI-1640 medium), HL60 cells (RPMI-1640 medium), U937 cells (RPMI-1640 medium) , A549 cells (cultured in DMEM medium) were purchased from American Type Culture Collection (ATCC, Manasas, MD, USA). Inoculate in RPMI-1640 medium or MEM medium or DMEM medium containing 10% fetal bovine serum at 37°C in 5% CO 2 cultured in an incubator.
[0034] 2. Drugs and reagents
[0035] Timosaponin AⅢ was dissolved in dimethyl sulfoxide. Under sterile conditions, after dissolving DMSO, dilute ...
Embodiment 2
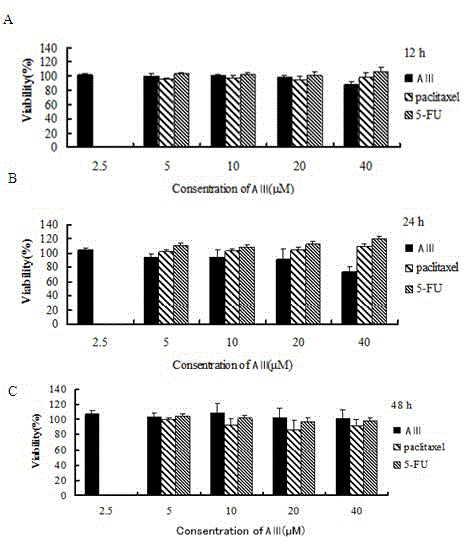
[0053] Example 2, MTT method to measure the growth inhibitory effect of timosaponin AⅢ, 5-fluorouracil and paclitaxel on hPBMC
[0054] Experimental Materials:
[0055] 1. Cells
[0056] Human peripheral blood mononuclear cells (hPBMC) were collected from three healthy adult volunteers by the Shenyang Military Region Army General Hospital entrusted by the Sino-Japanese Medical Research Institute of Shenyang Pharmaceutical University.
[0057] 2. Drugs and reagents
[0058] Timosaponin AⅢ was dissolved in dimethyl sulfoxide. Under sterile conditions, after dissolving DMSO, dilute with RPMI-1640 culture solution or MEM culture solution or DMEM culture solution to the required concentration (DMSO≤2.5‰).
[0059] 5-Fluorouracil and paclitaxel were purchased from China Institute for the Control of Pharmaceutical and Biological Products and dissolved in dimethyl sulfoxide. Under sterile conditions, after dissolving DMSO, dilute with RPMI-1640 culture solution or MEM culture solu...
Embodiment 3
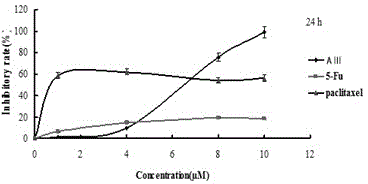
[0075] Example 3. Determination of growth inhibitory effects of timosaponin AⅢ, 5-fluorouracil and paclitaxel on T475-S2 cells by MTT method (repeated screening)
[0076] Experimental Materials:
[0077] 1. Cells
[0078] A375-S2 cells (cultured in MEM medium) were purchased from American Type Culture Collection (ATCC, Manasas, MD, USA). Inoculated in MEM medium at 37°C, 5% CO 2 cultured in an incubator.
[0079] 2. Drugs and reagents
[0080] Timosaponin AⅢ was dissolved in dimethyl sulfoxide. Under sterile conditions, after dissolving DMSO, dilute with RPMI-1640 culture solution or MEM culture solution or DMEM culture solution to the required concentration (DMSO≤2.5‰).
[0081] 5-Fluorouracil and paclitaxel were purchased from China Institute for the Control of Pharmaceutical and Biological Products and dissolved in dimethyl sulfoxide. Under sterile conditions, after dissolving DMSO, dilute with RPMI-1640 culture solution or MEM culture solution or DMEM culture solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com