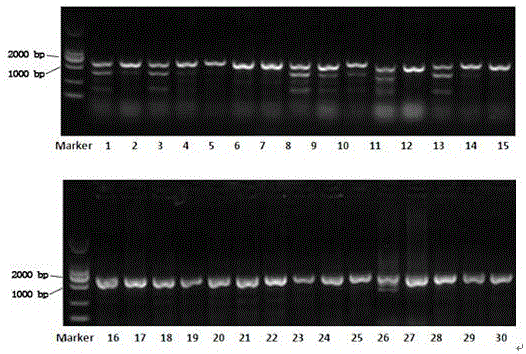
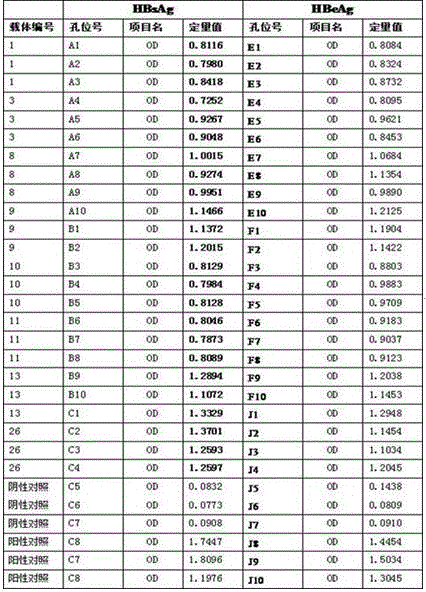
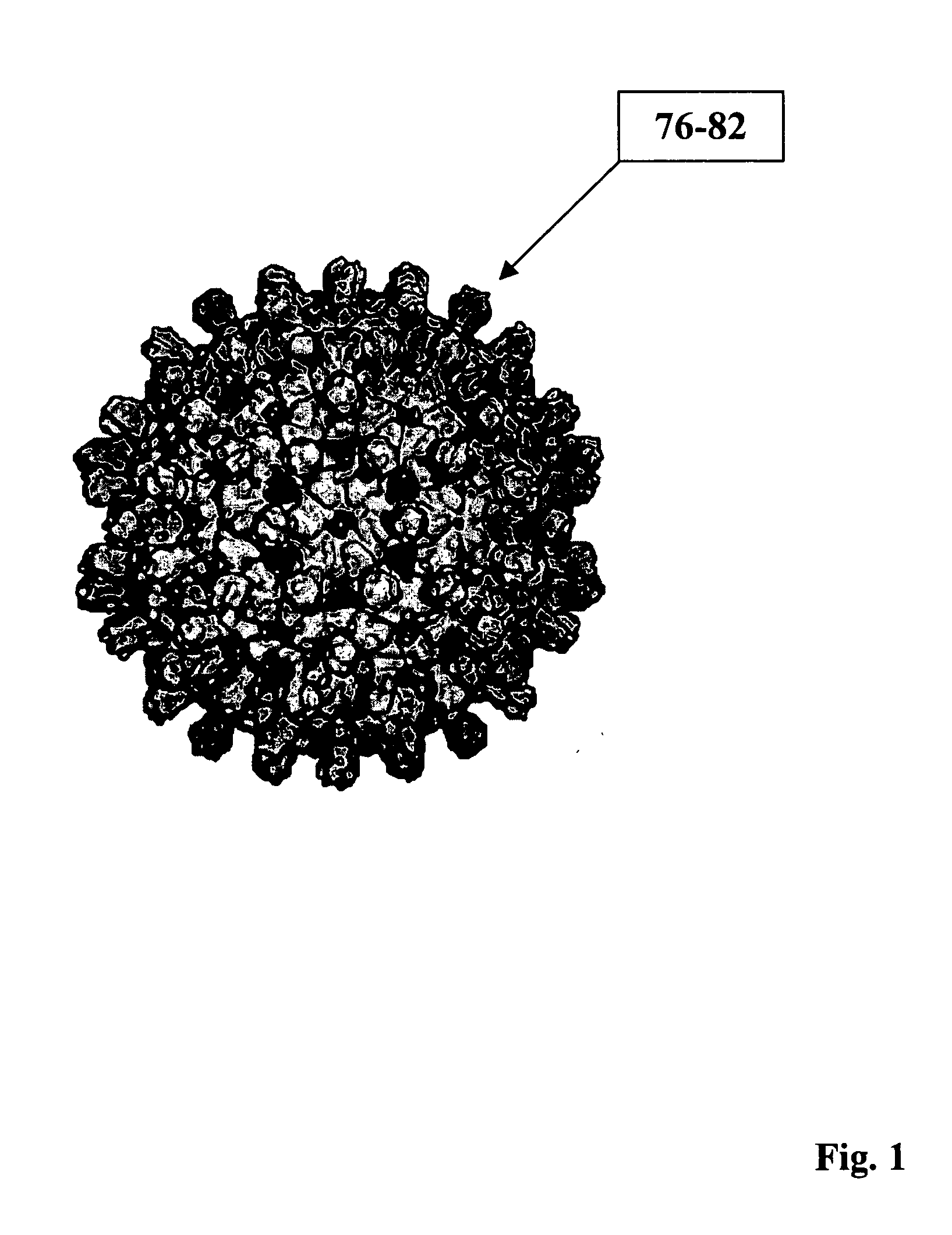
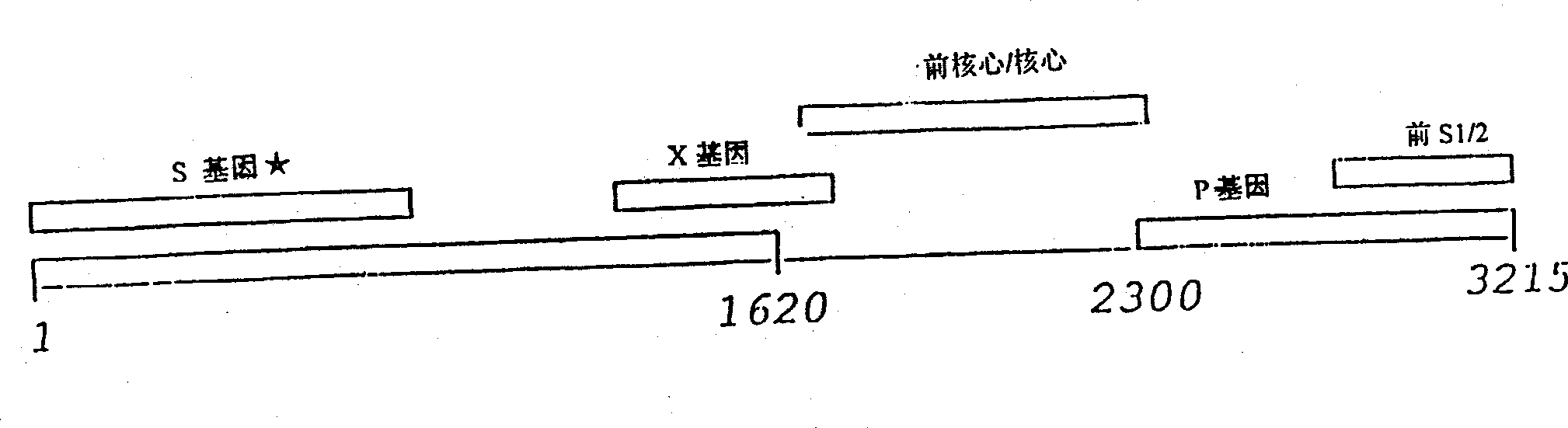
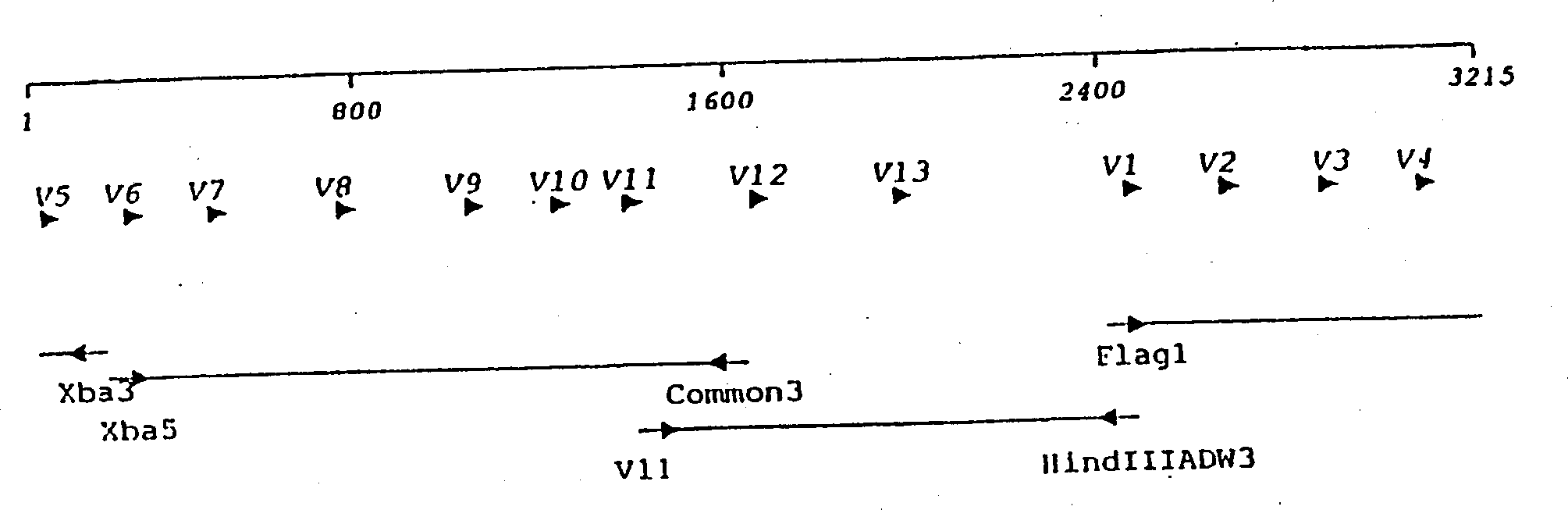
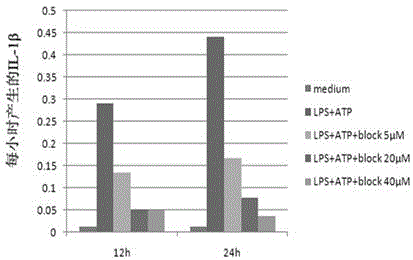
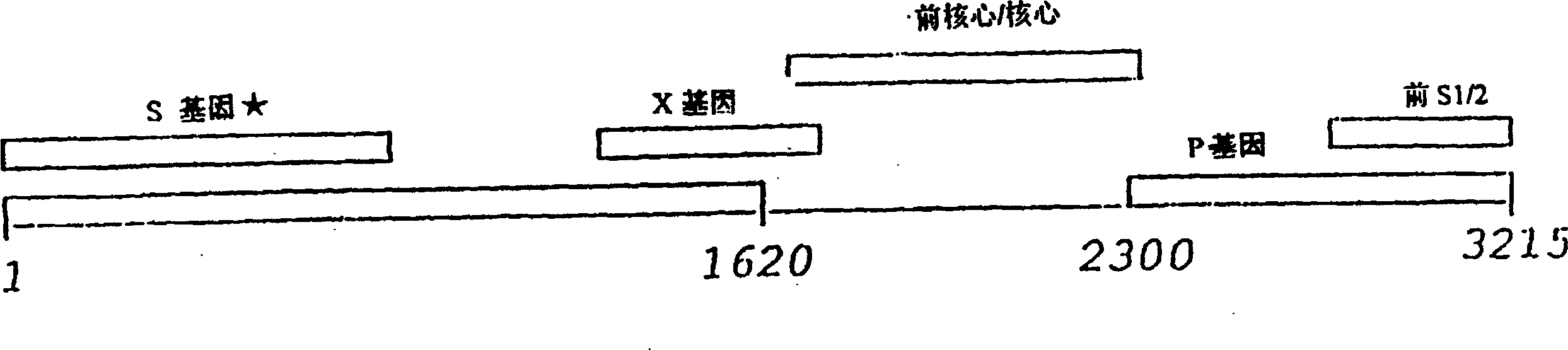
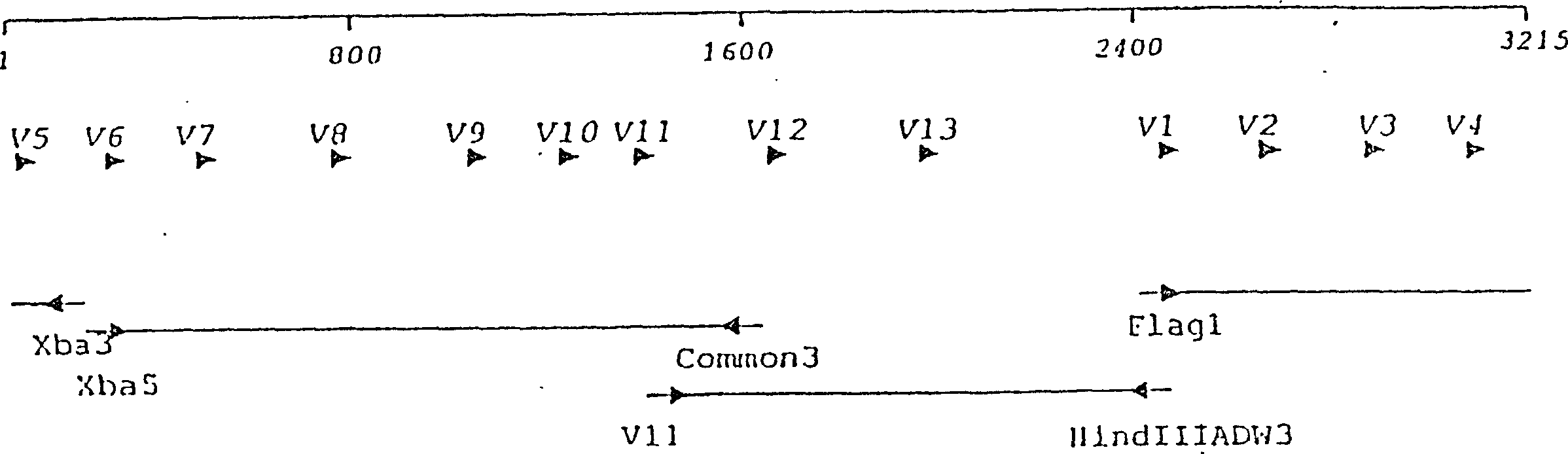
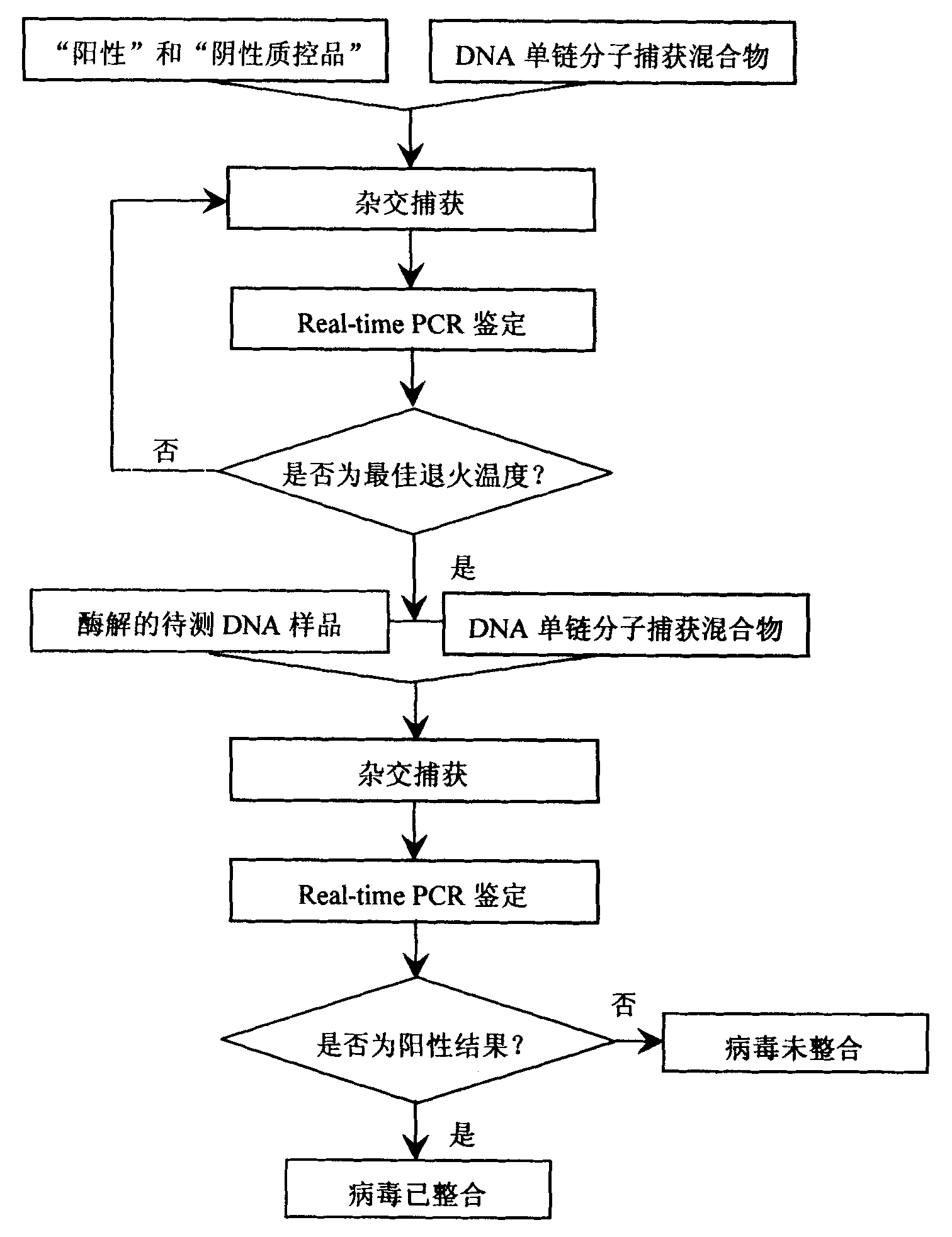
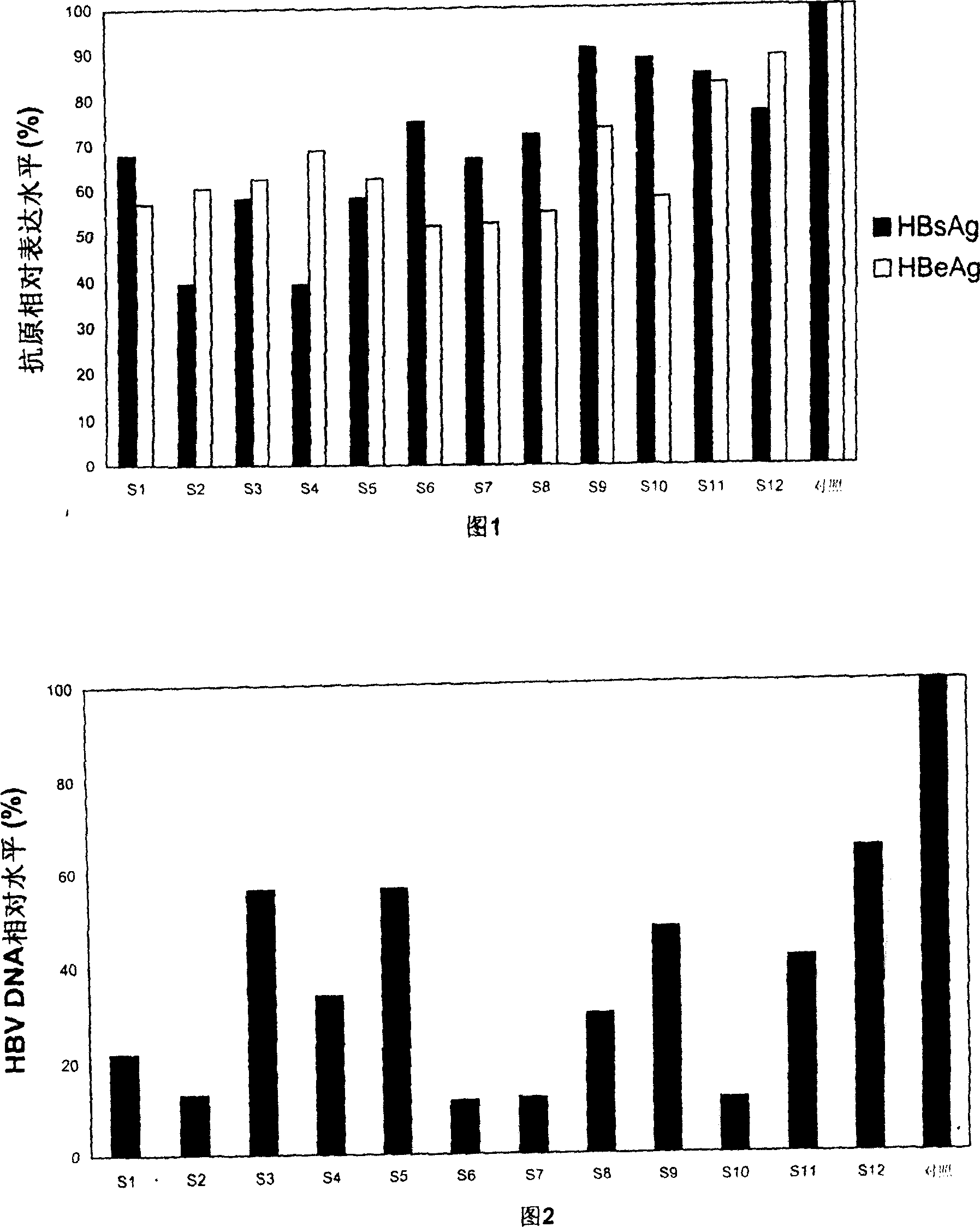
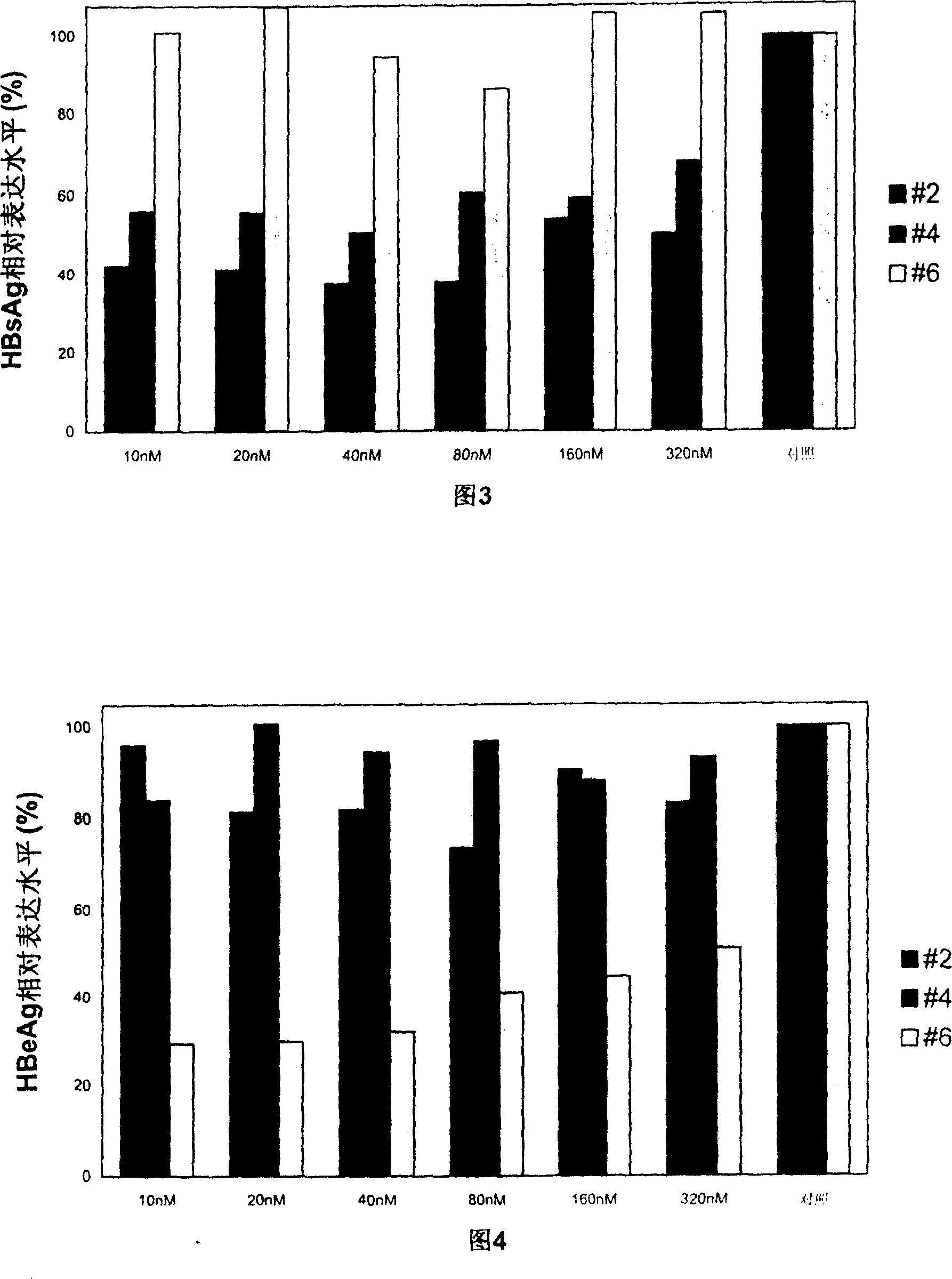
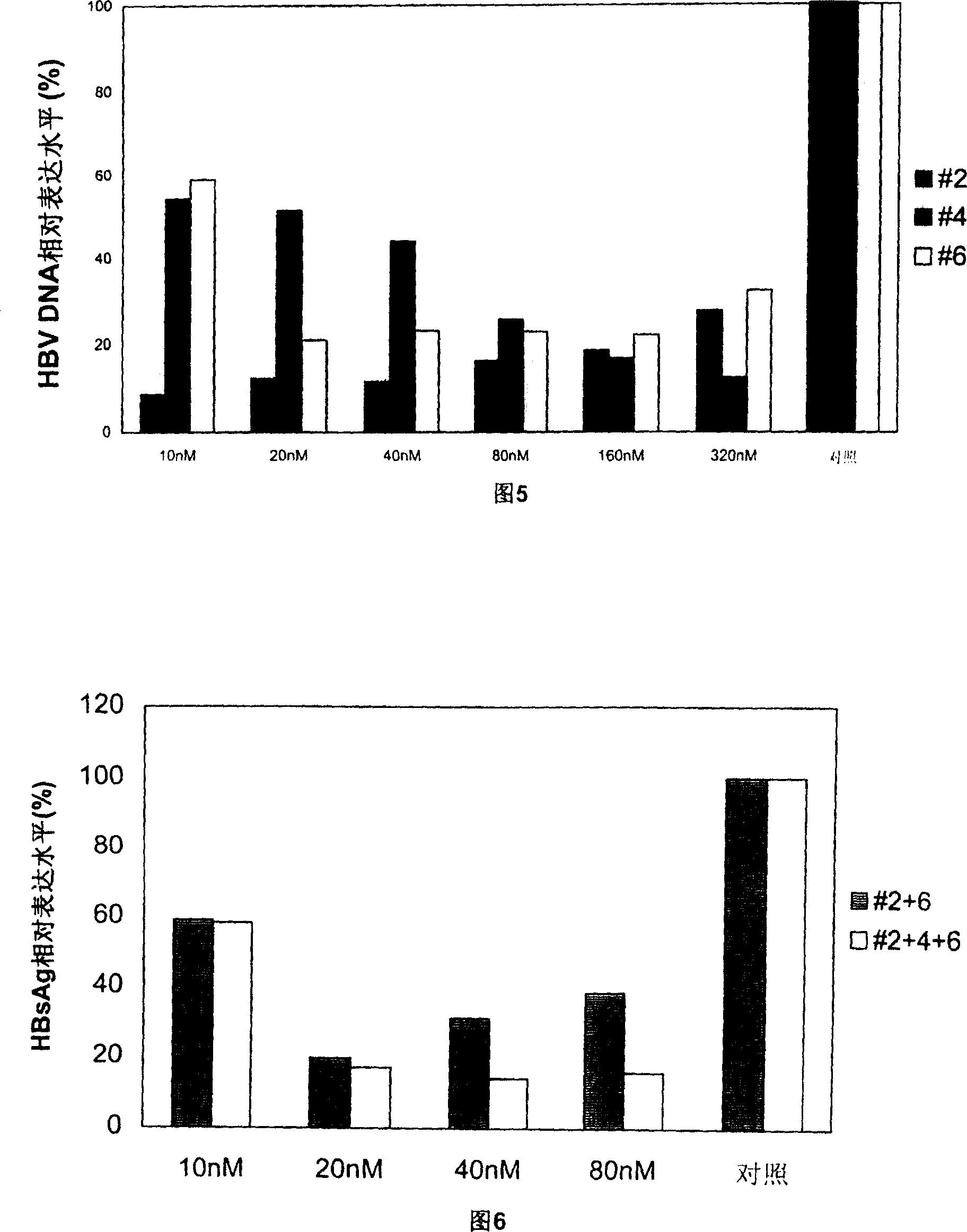
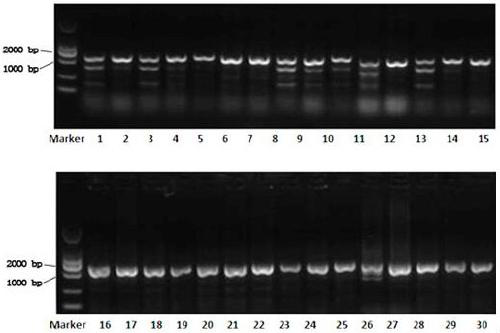
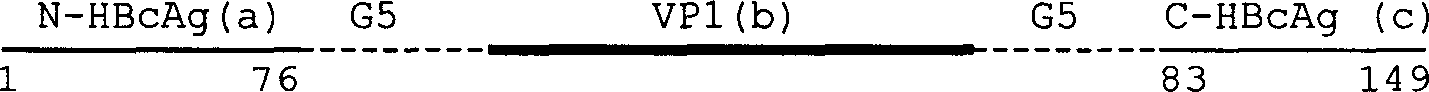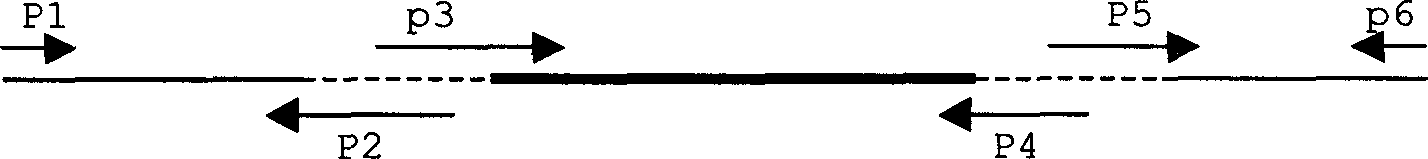Patents
Literature
36 results about "Human hepatitis B virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
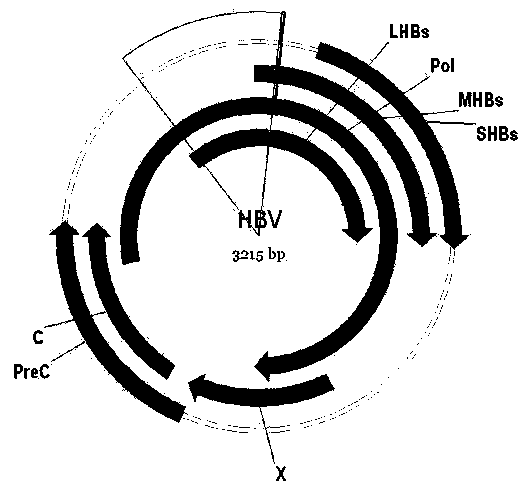
Hepatitis B virus (HBV) is a human pathogen of major significance, as chronic infection can lead to cirrhosis and primary liver cancer, resulting in over a million deaths worldwide each year.
CRISPR/Cas9 targeted knockout human hepatitis B virus P gene and specific gRNA thereof
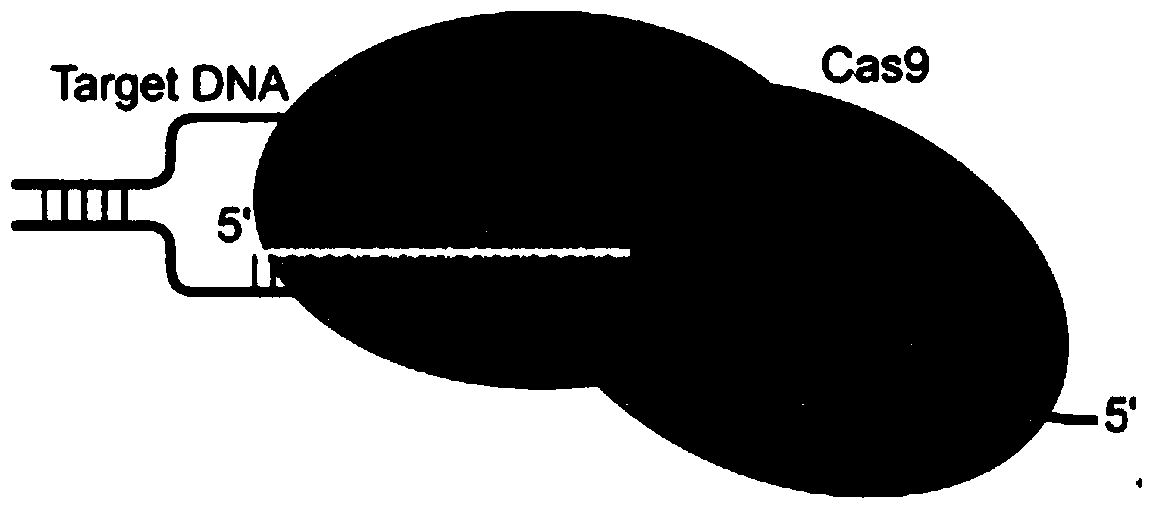
The invention relates to the technical field of genetic engineering, and particularly relates to a guide RNA (gRNA) sequence based on a CRISPR / Cas9 system and combinations thereof, as well as gRNA for a specific targeted knockout hepatitis B virus cccDNA P gene. In the invention, 30 gRNAs are designed according to design principles of CRISPR / Cas9, have a sequence table as shown in SEQ ID NO.1-30, and are constructed on a PX458 vector, to screen out four more efficient gRNAs. The CRISPR / Cas9 system guided by the four gRNAs and combinations thereof is utilized in a human hepatocellular carcinoma cell line (HepG2.2.15) to effectively knock out the human hepatitis B virus cccDNA P gene. The gRNA of the specific targeted hepatitis B virus cccDNA prepared in the invention can accurately target the hepatitis B virus cccDNA and realize gene knockout. The preparation method is simple in operation; the gRNA has a good targeting property; the CRISPR / Cas9 system is high in knockout efficiency.
Owner:重庆高圣生物医药有限责任公司
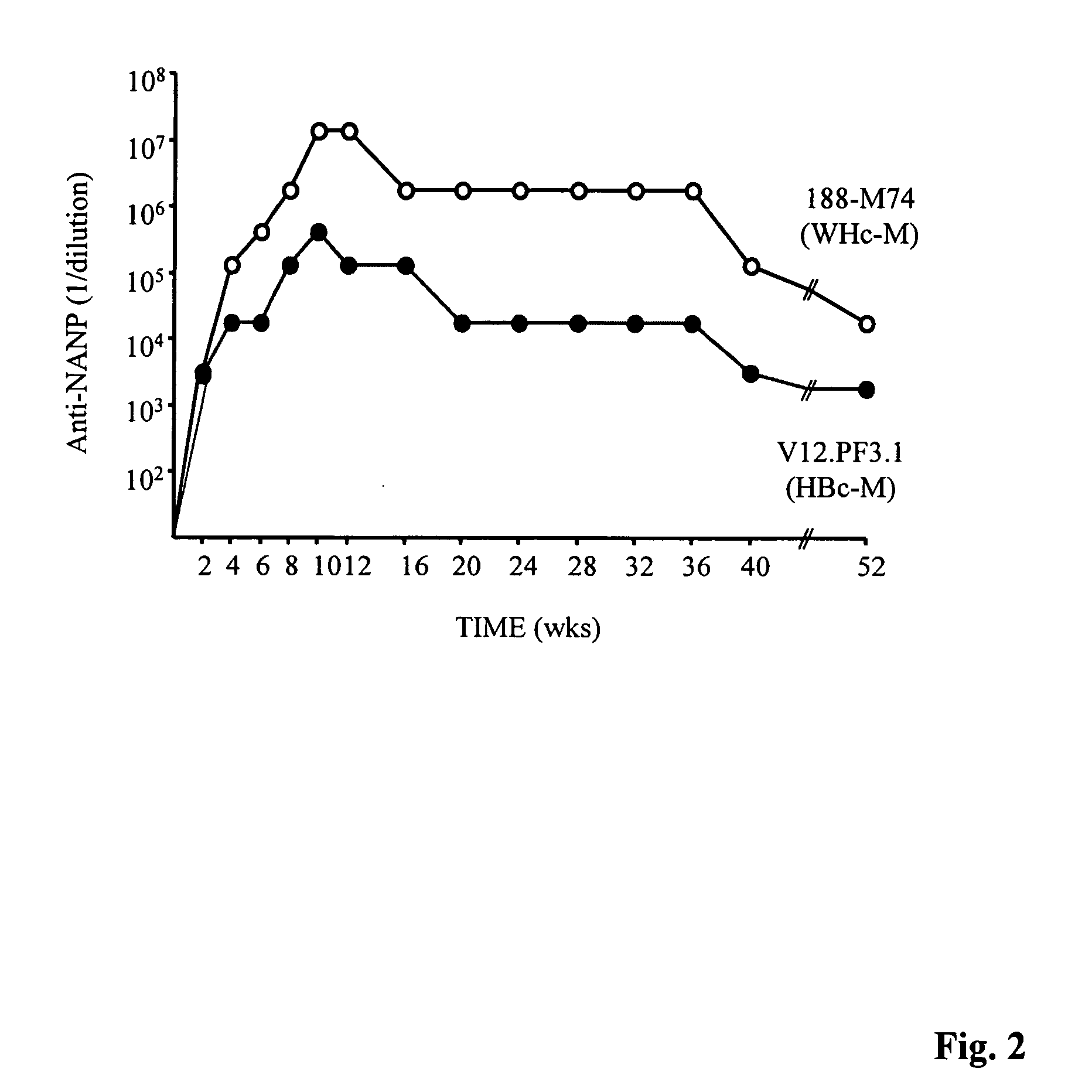
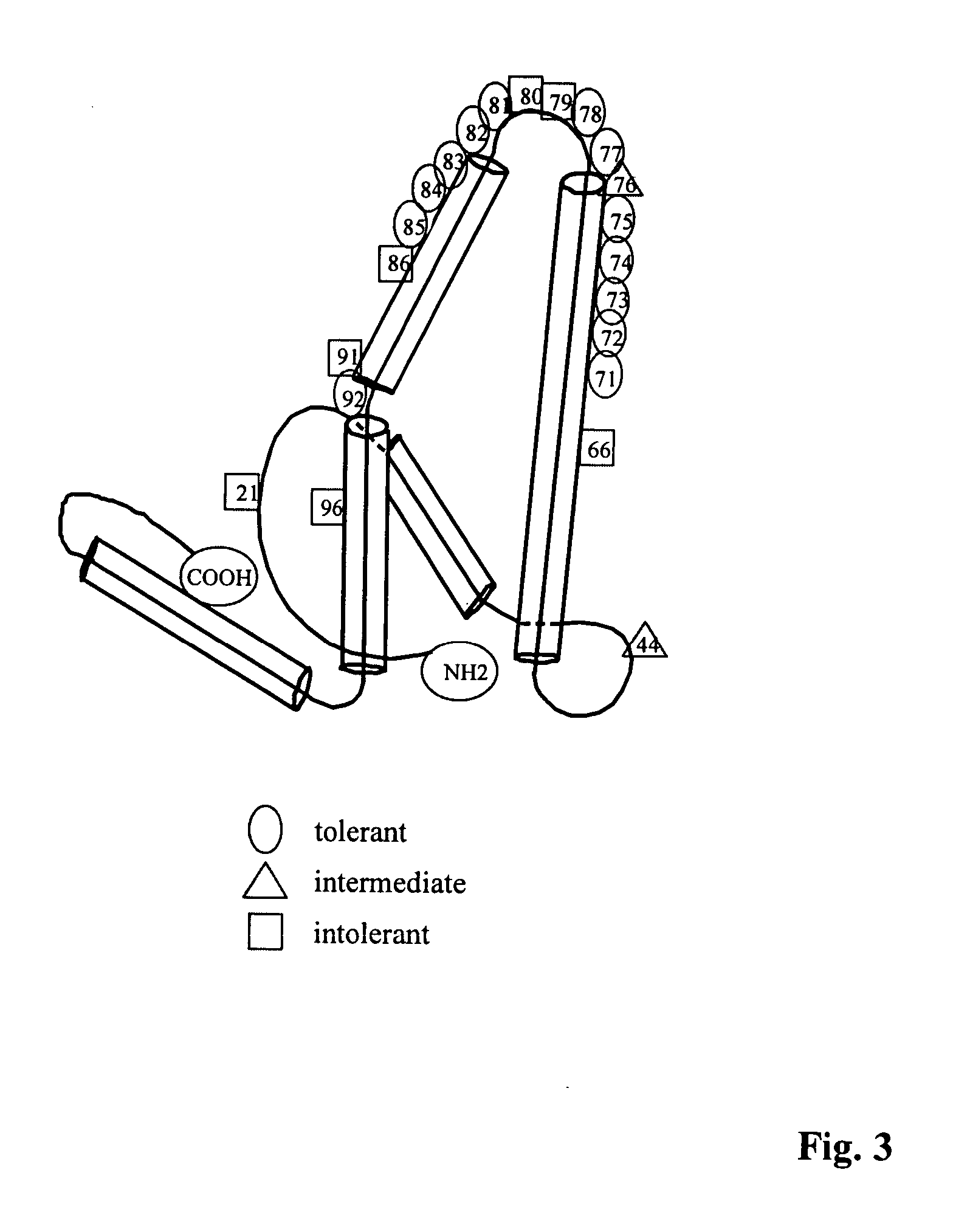
Human hepatitis B virus core proteins as vaccine platforms and methods of use thereof
ActiveUS20050025782A1Uptake of nucleic acids by cells is enhancedPromote absorptionAntibody mimetics/scaffoldsViral antigen ingredientsNucleic acidHepatitis virus
Owner:VLP BIOTECH
Small interference RNA molecule SiRNA capable of attacking human hepatitis B virus and application thereof
InactiveCN1566131ABlocking reproductionBlock replicationOrganic active ingredientsSugar derivativesDiseaseNucleotide
The invention provides a small interference RNA molecule SiRNA capable of attacking human hepatitis B virus, which is double chain RNA molecule whose sequence has at least 70% of consanguinity degree with the sequence (I), wherein the antisense chain of the sequence (I) and one nucleic acid mutant has no consanguinity with the known human gene and gene expression segment. The SiRNA can be used for preparing medicament or preparation for prevention or treating hepatitis B and any diseases relating to hepatitis B viral infection.
Owner:丽水瑞德佳生物医药有限公司
Human hepatitis B virus core proteins as vaccine platforms and methods of use thereof
Owner:VLP BIOTECH
B hepatitis virus X protein transduction system expression vector and its construction
InactiveCN1546668ARealize "quantitative" importAchieving Quantitative ImportMicrobiological testing/measurementFermentationCell membraneHepatitis B Virus-X
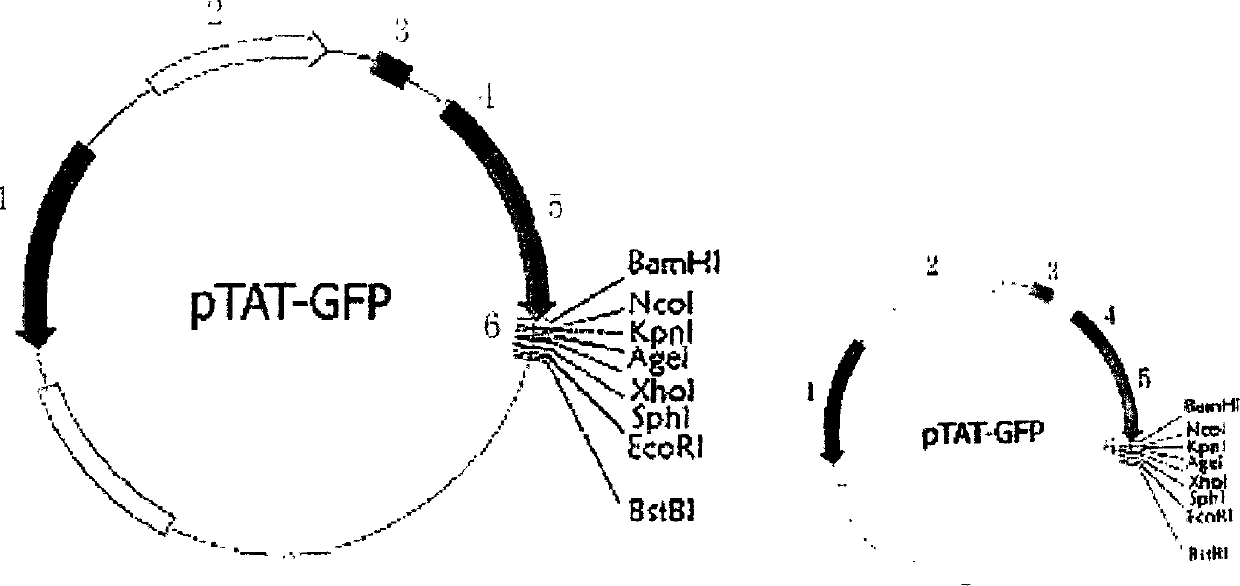
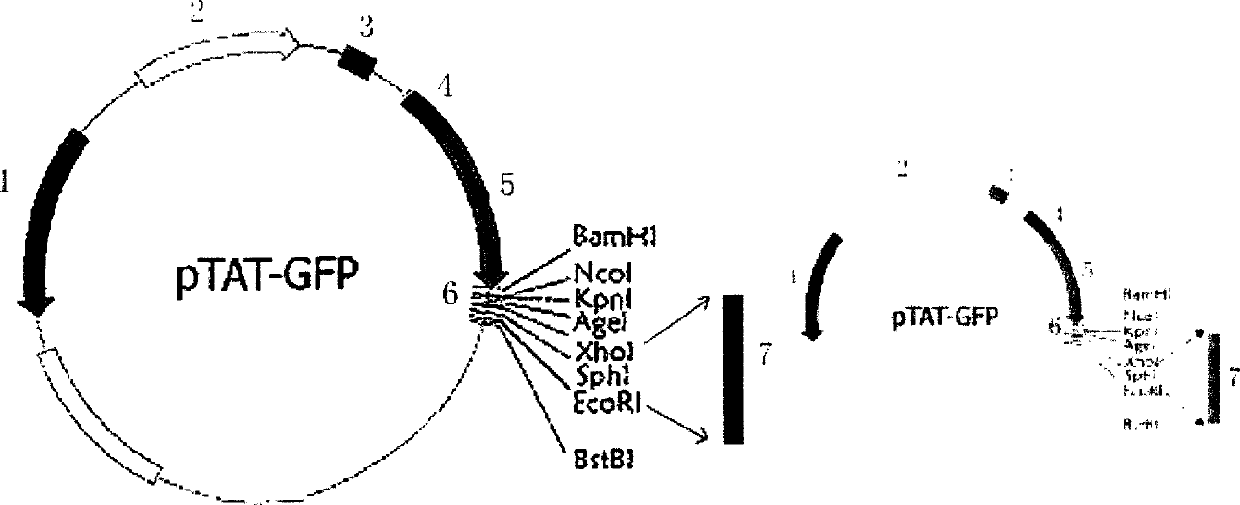
The invention discloses a type B hepatitis virus X protein transduction system expression vector and its construction, by constructing restructured plasmid pTAT-GFP-X through the X gene of the human hepatitis B virus and pTAT-GFP vector, wherein the constructed pTAT-GFP-X restructured plasmid can express the fusion protein containing X protein of hepatitis B viruse, and a vast amount of expression fusion proteins can be evoked from isopropylthiog-alactoside (IPTG), purification can be carried out through 6 histidines (His6), the TAT polypeptide has the structure capable of traversing cell membrane.
Owner:NANKAI UNIV
Recombinant human hepatitis B virus core protein fused protein
The invention discloses a recombinant human hepatitis B virus core protein fused protein. The fused protein comprises a protein (X), a linker peptide (L) and a hepatitis B virus core protein (HBc) from the end N to the end C in sequence; the linker peptide (L) has the amino acid sequence of Gly-Ser-(Gly-Gly-Gly-Gly-Ser)n, and n is an integer between 2 and 20 and is 9 or 18, particularly; the end C of the linker peptide (L) is connected with the end N of the hepatitis B virus core protein (HBc); the end C of the protein (X) is connected with the end N of the linker peptide (L); and the protein (X) is a red fluorescent protein or vesicular stomatitis virus G glycoprotein. The hepatitis B virus core protein (HBc) is connected with the functional protein, and the functions of the proteins on two ends of the linker peptide (L) can be both guranteed. The problem in the prior art that the functions of the hepatitis B virus core protein (HBc) and the functions of the functional protein can not be both guaranteed after the hepatitis B virus core protein (HBc) is fused with the functional protein is solved. The fused protein is of great importance to the research on the hepatitis B virus (HBV).
Owner:CHONGQING MEDICAL UNIVERSITY
An i(in vitro) activity assay for human hepatitis B virus (HBV) DNA polymerase, and its use for screening for inhibitors of HBV DNA polymerase
InactiveCN1320167AAssayable priming activityMicrobiological testing/measurementTransferasesSerum samplesHbv dna polymerase
The present invention provides an in vitro activity assay for human hepatitis B virus (HBV) DNA polymerase, which comprises using, as the 5' oligonucleotide in PCR amplification of HBV DNA polymerase from a sample, an oligonucleotide into which has been incorporated the SP6 viral polymerase promoter, directly transcribing and translating the PCR products in the presence of a radio-labelled agent and measuring the priming of the HBV DNA polymerase. The present invention also provides the use of such an assay to assay activity of various serum samples, to screen for inhibitors of the HBV DNA polymerase and to test and / or screen potential anti-HBV drugs for their ability to inhibit DNA priming activity of human HBV DNA polymerase.
Owner:GOVERNMENT OF THE REPUBLIC OF SINGAPORE
Assay for detecting hepatitis b virus (HBV)
The disclosure is directed to methods, kits, and compositions, for amplifying and detecting a human hepatitis B virus (HBV) in a sample, which comprises a variety of combinations of forward oligonucleotide primers, reverse oligonucleotide primers, and oligonucleotide probes.
Owner:ABBOTT MOLECULAR INC
Vaccine-induced hepatitis B viral strain and uses thereof
This invention provides an isolated strain of Hepatitis B virus designated Human Hepatitis B Virus Surface Antigen-'S'-145 Singapore Strain (Glycine to Arginine) which constituent viral genome is deposited under Accession Nos. P97121504, P97121505 and P97121506 with the European Collection of Cell Culture on 15th Dec. 1997. This invention also provides an isolated nucleic acid encoding a polypeptide which is a mutant major surface antigen of a strain of hepatitis B virus, such polypeptide having an amino acid sequence which differs from the amino acid sequence of a major surface antigen of a wild type hepatitis B virus in that the amino acid at position number 145 of such polypeptide is an arginine rather than a glycine, and the purified peptide This invention further provides an isolated nucleic acid which encodes a peptide, wherein the peptide is encoded by a nucleic acid molecule comprising nucleotides 527 through 595 of SEQ. I.D. No. 1, and the purified peptide. This invention also provides various methods using the disclosed isolated nucleic acids and polypeptides.
Owner:GOVERNMENT OF THE REPUBLIC OF SINGAPORE
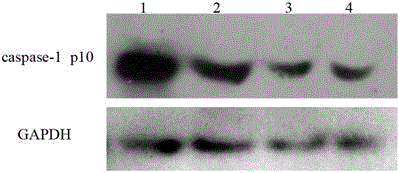
Caspase-1 inhibitor targeting liver and application thereof
ActiveCN102977215AInhibition of activationActivation facilitates terminationPeptide/protein ingredientsAntipyreticAmino acid compositionPolymer
The invention discloses a caspase-1 inhibitor targeting liver. The caspase-1 inhibitor is formed by connection of human hepatitis B virus Pre-S1 peptide and caspase-1 inhibitor through a flexible connector, wherein the Pre-S1 peptide consists of amino acids from first to fiftieth of the Pre-S1 protein; and the caspase-1 inhibitor is formed by connection of trimolecular polymer of YVAD tetrapeptide and trimolecular polymer of VAD tripeptide. According to the inhibitor, the caspase-1 inhibitor is specifically targeted to a liver cell by utilizing the carrier Pre-S1 peptide which specifically targets liver, so that intrahepatic persistent chronic inflammation caused by endogenous danger signals released by injured liver cells can be blocked by terminating caspase-1 activation in the liver cell, sequential spinal cord injury of the liver cell can be blocked, body adverse response which is possibly caused by spread inhibition of caspase-1 can be reduced, and the liver cell can be specifically and effectively protected. The caspase-1 inhibitor can be used for preparing a medicament for treating intrahepatic chronic inflammation caused by endogenous danger signals released by injured liver cells.
Owner:ARMY MEDICAL UNIV
Vaccine-induced hepatitis B viral strain and uses thereof
The present invention provides a hepatitis B virus isolate named as the surface antigen of human hepatitis B virus-'S'-145 Singapore strain (change glycine to arginine), and the virus genome composed of it has been obtained in December, 1997 It was preserved in the European Cell Culture Collection on the 15th, with the registration numbers P97121504, P97121505 and P97121506. The present invention also provides isolated nucleic acids and purified peptides encoding a polypeptide, wherein said polypeptide is a mutant major surface antigen of a hepatitis B virus strain, said polypeptide having an amino acid sequence similar to that of wild-type hepatitis B virus The amino acid sequence of the major surface antigen of said polypeptide differs in that the amino acid at position 145 of said polypeptide is arginine instead of glycine. The present invention further provides an isolated nucleic acid encoding a peptide encoded by a nucleic acid molecule comprising nucleotides 527 to 595 of SEQ ID NO:1, and purified peptides thereof. The invention also provides various methods of using the disclosed isolated nucleic acids and polypeptides. The present invention also provides various applications of said virus strain and its protein.
Owner:GOVERNMENT OF THE REPUBLIC OF SINGAPORE
Kit for detecting integrated viruses of genome in hybrid capture
InactiveCN101974652AMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingBiomedicine
The invention belongs to the field of biomedicine, in particular to a kit for detecting integrated viruses of a genome in hybrid capture. The kit comprises the following main components: (1) a solid phase medium fixed with a capture mixture of human genome DNA (Deoxyribonucleic Acid) single-chain molecules, (2) positive and negative quality control materials for identifying the hybrid capture efficiency and specificity, and (3) a primer pair and a molecular probe for carrying out real-time PCR (Polymerase Chain Reaction) identification to the captured mixture of the target DNA molecules, obtained by capture. By utilizing all or partial components in the invention, a complete integrated genome virus detection kit or an integrated genome virus detection kit with the type specificity can be assembled. The obtained virus detection kit can be used for detecting and identifying the integrated state of the genome in a certain common integrated and oncogenic viruses, such as human hepatitis B viruses, human papilloma viruses, Epstein-Barr viruses and human herpes simplex viruses, in tissue or cytology specimens.
Owner:何以丰
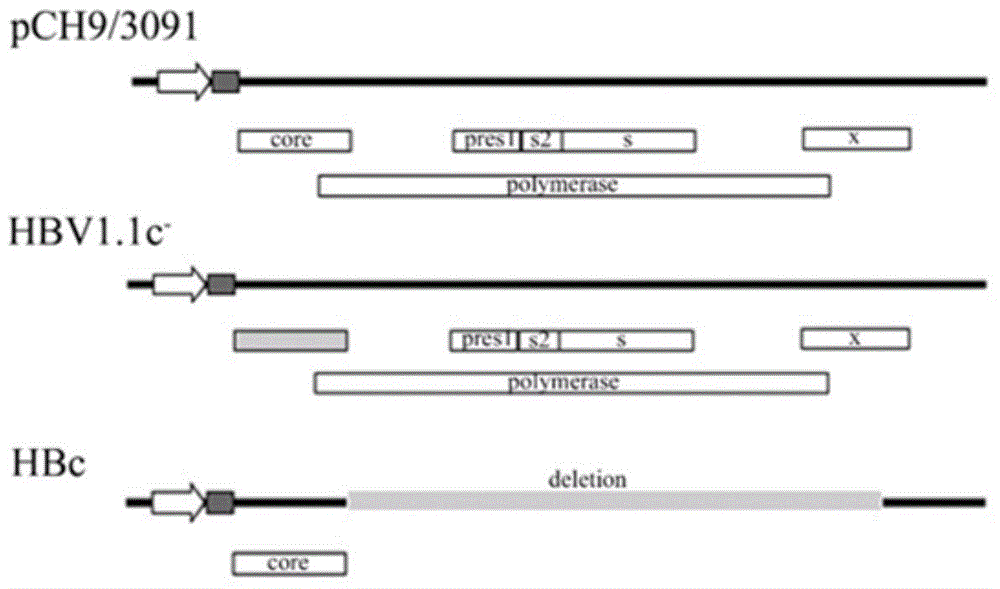
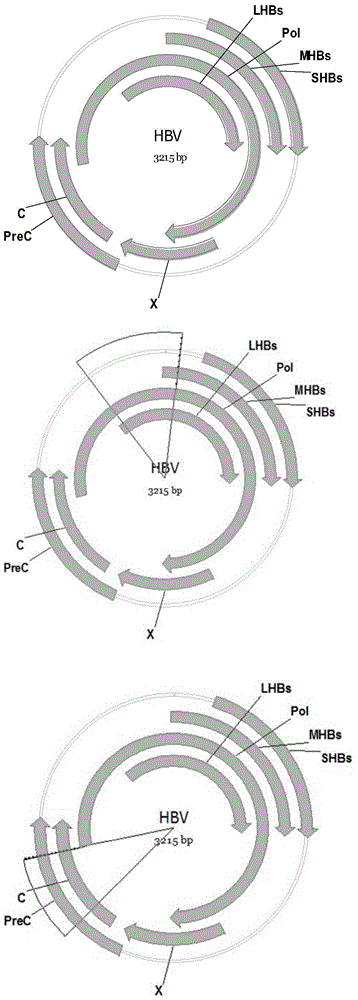
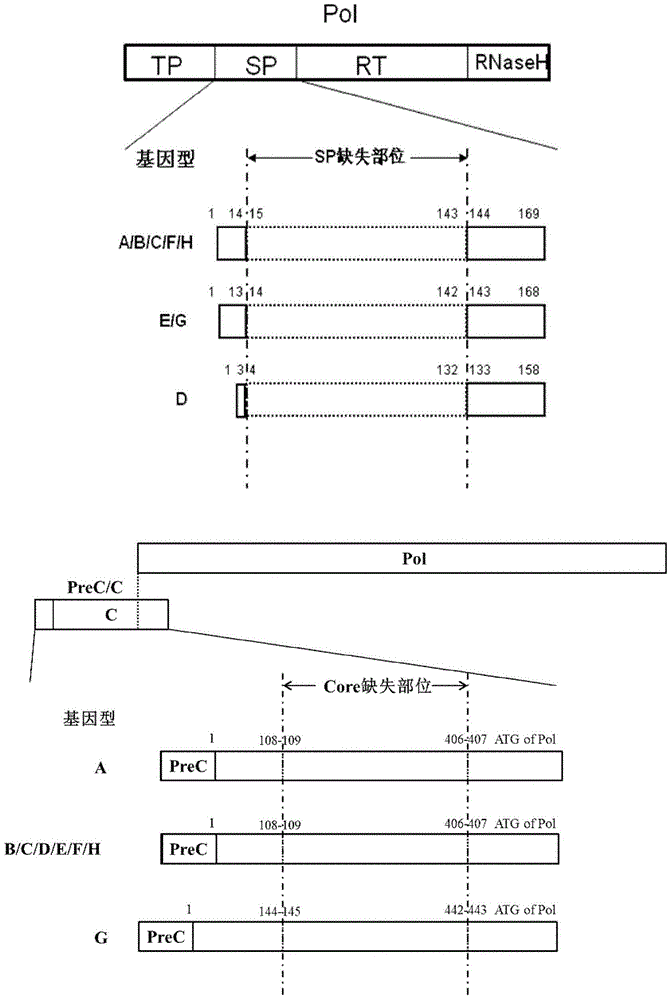
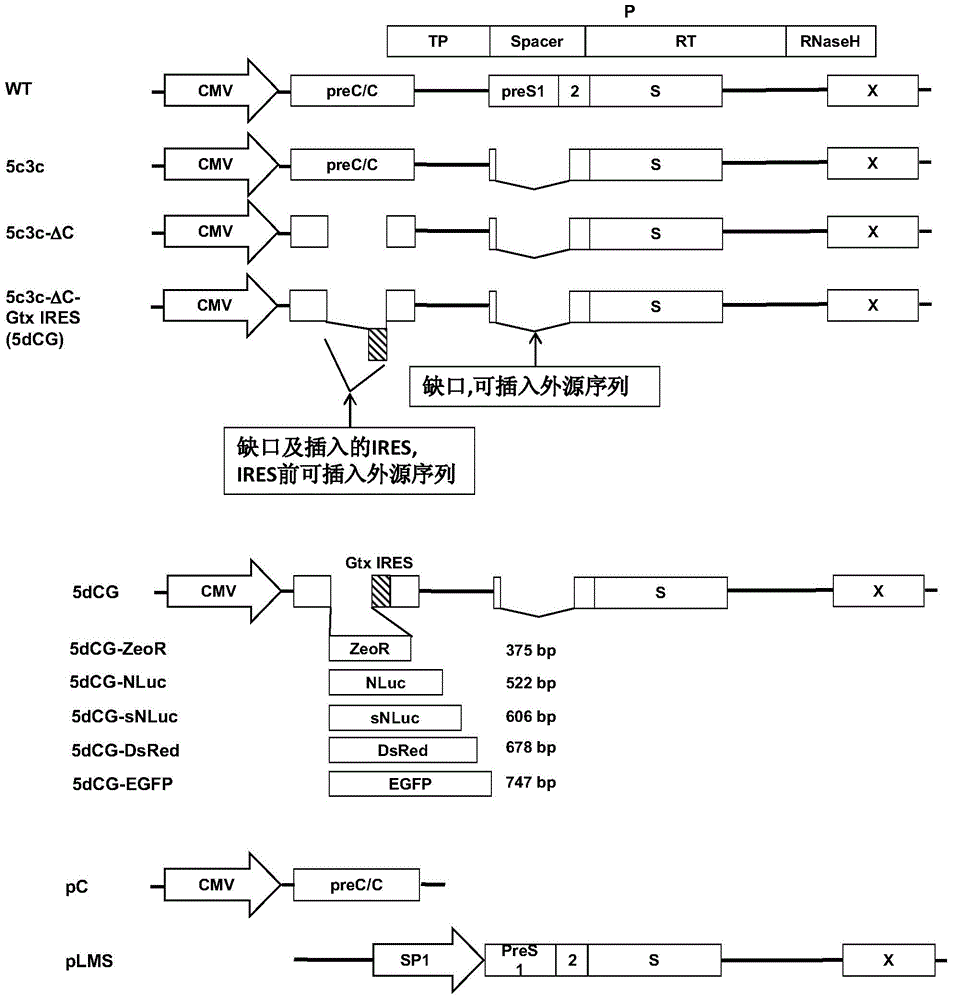
Recombinant vector for human hepatitis B virus and application thereof
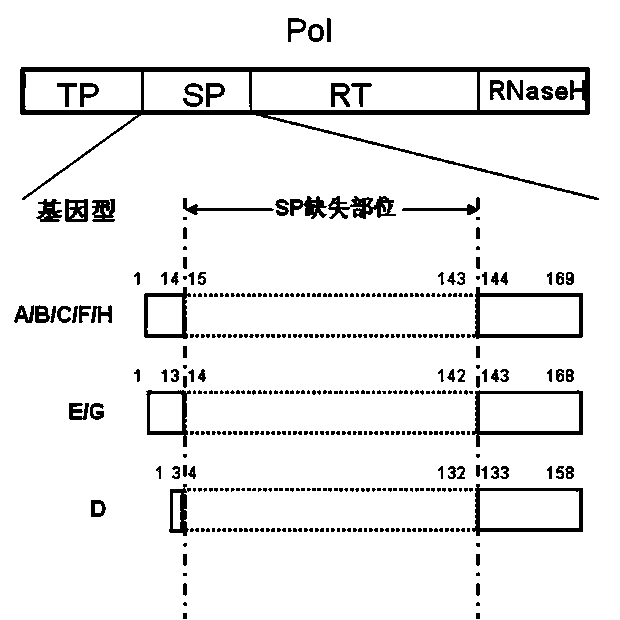
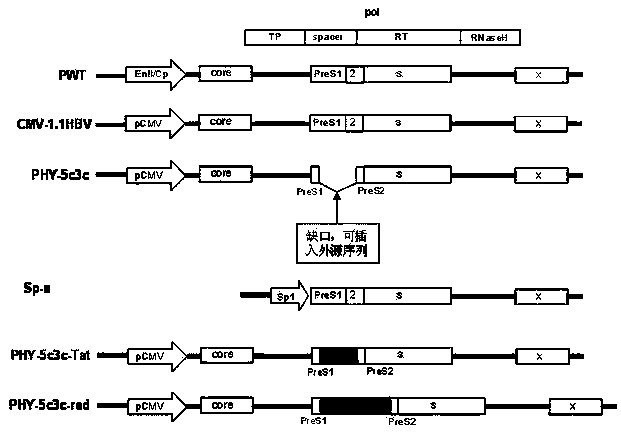
The invention relates to a recombinant vector for the human hepatitis B virus, belonging to the fields of biological medicine and genetic engineering. Compared with a wild human hepatitis B virus, viral genome involved in the invention lacks a part of a spacer region or the whole spacer region of hepatitis B virus polymerase and a part of a core protein and has a ribosome entry site inserted before the initiation codon of the polymerase. The invention also provides a preparation method for the recombinant vector and application of the recombinant vector to gene therapy targeting to the liver or hepatocytes. The recombinant vector for the human hepatitis B virus provided by the invention supports insertion of a plurality of exogenous sequences and gene expression. A recombinant hepatitis B virus does not have genome replication capability, but recombinant hepatitis B virus genome can realize high-efficiency replication under the condition of supply of the core protein, and packages and secretes infectious intact recombinant hepatitis B virus particles under the condition of simultaneous supply of the core protein and surface protein; so it is proved that the recombinant vector for the human hepatitis B virus provided by the invention has good application prospects as a liver-specific gene introduction vector.
Owner:FUDAN UNIV
Vaccine preparation for fused virus-like granule, its production method and process of using
InactiveCN1481898AElicit a complete immune responseTrigger immune responseViral antigen ingredientsImmunological disordersHepatitis B virus core AntigenAdjuvant
The fusion virus-like particle contains protein capable of forming virus-like particle as carrier and fused protective target virus antigen. Specifically, human hepatitis B virus core antigen forming virus-like particle is used as the carrier, and protective target virus antigen gene segment is fused in to form fused virus-like particle, which is further mixed with adjuvant, if any, to form vaccine. The target antigen may display its surface to produce relatively high B cell activity and stimulate T cell to produce protective immunity. During use, the vaccine may be made to enter body via injection, spraying, oral taking, dropping to nose or eye, penetration, absorption or physical and chemical mediation.
Owner:CHINA AGRI UNIV
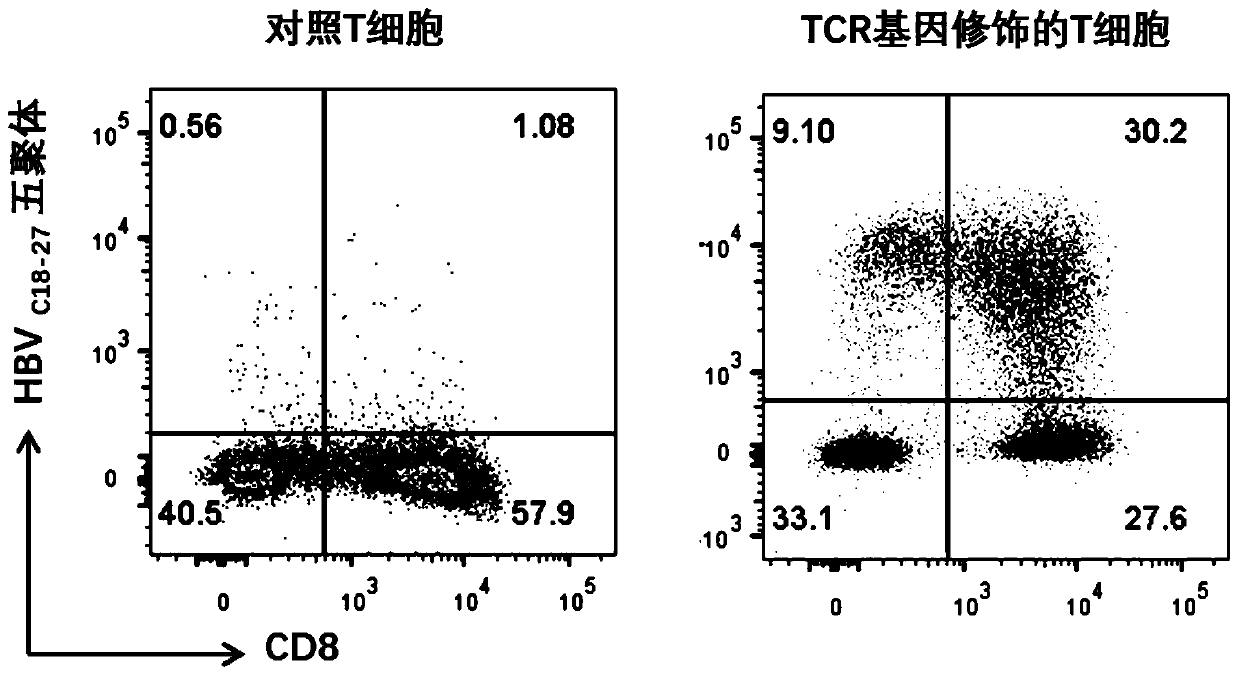
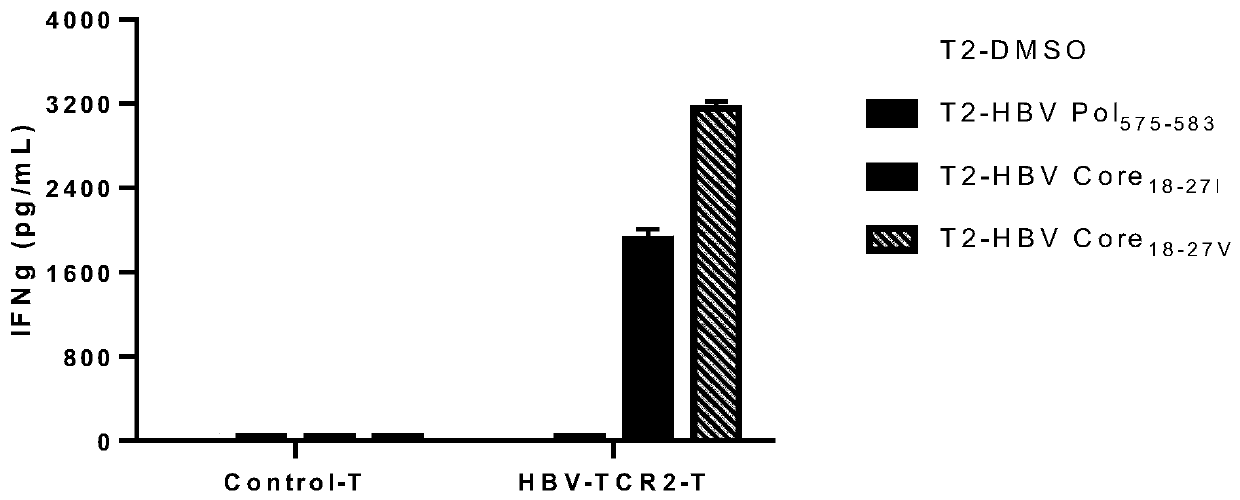
T cell receptors for recognizing human hepatitis B virus core antigens
ActiveCN111592590AHigh affinityMammal material medical ingredientsAntiviralsHepatitis B immunizationNucleotide
The invention discloses a T cell receptor (TCR) for recognizing a human hepatitis B virus core antigen, and relates to the technical field of cellular immunotherapy. The TCR has the characteristic ofcombination with an HLA-A2-HBV-C18-27 antigen complex, and the TCR comprises a TCR alpha-chain variable region and / or a TCR beta-chain variable region. The invention also provides nucleic acid molecules which comprise nucleotide sequences for encoding the TCR or complementary sequence of the nucleotide sequences, and the invention provides a carrier which comprises the nucleic acid molecules, cells for transducing the nucleic acid molecules or the carrier, a pharmaceutical composition which contains the TCR, the nucleic acid molecules, the carrier or the cells as active ingredients and application of the TCR, the nucleic acid molecules, the carrier, the cells and the pharmaceutical composition. The TCR for recognizing the human hepatitis B virus core antigen has the properties of specificrecognition of the human hepatitis B virus core antigen and good affinity.
Owner:深圳市因诺转化医学研究院 +1
In vitro activity assay for human hepatitis B virus (HBV) DNA polymerase, and its use for screening for inhibitors of HBV DNA polymerase
InactiveUS7037682B2Simple processSugar derivativesMicrobiological testing/measurementSerum igeHbv dna polymerase
The present invention provides an in vitro activity assay for human hepatitis B virus (HBV) DNA polymerase, which comprises using, as the 5′ oligonucleotide in PCR amplification of HBV DNA polymerase from a sample, an oligonucleotide into which has been incorporated the SP6 viral polymerase promoter, directly transcribing and translating the PCR products in the presence of a radio-labelled agent and measuring the priming of the HBV DNA polymerase. The present invention also provides the use of such an assay to assay activity of various serum samples, to screen for inhibitors of the HBV DNA polymerase and to test and / or screen potential anti-HBV drugs for their ability to inhibit DNA priming activity of human HBV DNA polymerase.
Owner:GOVERNMENT OF THE REPUBLIC OF SINGAPORE
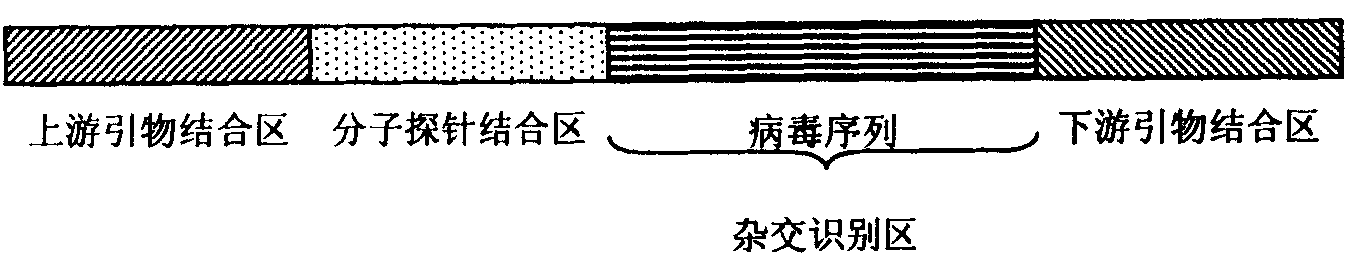
Primer group and probe for detecting human hepatitis B virus
ActiveCN103849690AHigh precisionReduce false negative rateMicrobiological testing/measurementDNA/RNA fragmentationHepatitis B immunizationGene
The invention relates to a primer group and / or a probe for specific detection of human hepatitis B virus (HBV). The invention aslo relates to a kit and a microarray which comprise the primer groups and / or the probes and are used for specific detection of the human hepatitis B virus (HBV). The invention aslo relates to applications of the primer group and / or the probe in preparation of the kit and the microarray for specific detection of the hepatitis B virus in a sample. The invention aslo relates to a method of for detecting HBV genes in the sample by using the primer group and / or the probe.
Owner:QIAGEN SHENZHEN CO LTD
Method for detection and evaluation of biological functions of molecule and medicine and use thereof
InactiveCN103977425AInfection controlAbility to control diseaseAntibody ingredientsAntiinfectivesImmune toleranceDrug biological activity
The present invention relates to antibody engineering and anti-infection drug field, provides a method for detection and evaluation of biological functions of a molecule and a medicine and use thereof, and particularly relates to a method for detection of biological functions of (human) anti-human hepatitis B virus antibody. The method can effectively detect the level of the biological functions of the molecule, the medicine and the (human) anti-human hepatitis B virus antibody. According to the method, biological activity of a forerunner molecule and drug can be defined by detection of the ability of the to-be-detected forerunner molecule and drug to neutralize pathogene key molecules in animal body and the ability of the to-be-detected forerunner molecule and drug to break immune tolerance and reconstruct immune protection in the animal body. The method can be used for rapid detection of virus clearing ability and virus infection and pathogenesis control ability of antibodies and other anti-infection drugs. The method has the advantages of simple operation process and good the repeatability, and is a supplement to methods in the prior art for detection and evaluation of functionality of antiviral drugs, the method is established for providing a new choice for research and development of drugs of cell and animal infection model virus.
Owner:FUDAN UNIV
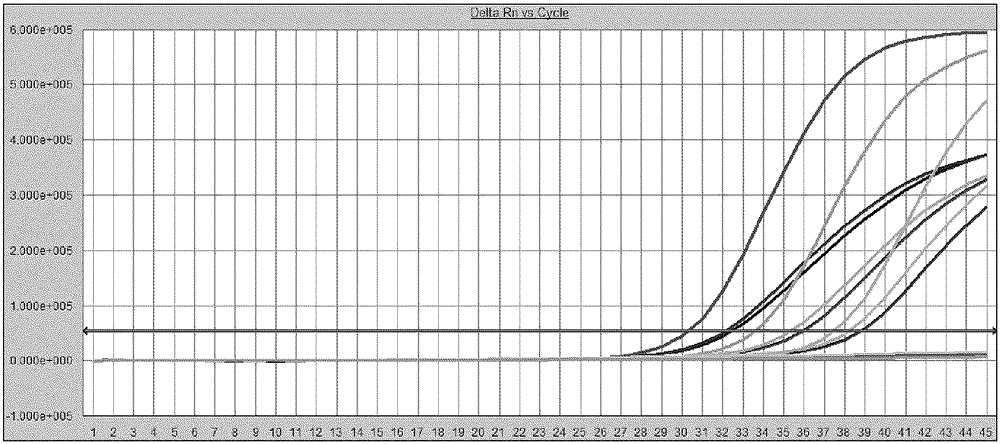
High-sensitivity HBV DNA digital PCR quantitative detection kit and application thereof
InactiveCN110592289AImprove toleranceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationForward primerPositive control
The invention relates to a high-sensitivity HBV DNA digital PCR quantitative detection kit and application thereof. The high-sensitivity HBV DNA digital PCR quantitative detection kit comprises a forward-primer container, a reverse-primer container, a probe container, a digital PCR buffer solution container, a positive control container and a negative control container. According to the high-sensitivity HBV DNA digital PCR quantitative detection kit, a method of using digital PCR for absolutely quantifying the content of target nucleic acid in samples is utilized for accurately measuring the quantity of HBV DNA, and therefore, the high-sensitivity HBV DNA digital PCR quantitative detection kit can be applied to the fields of biology, agriculture, medicine and the like and has good development prospects especially in the aspect of human hepatitis B virus detection.
Owner:SUZHOU CHIEN SHIUNG INST OF TECH
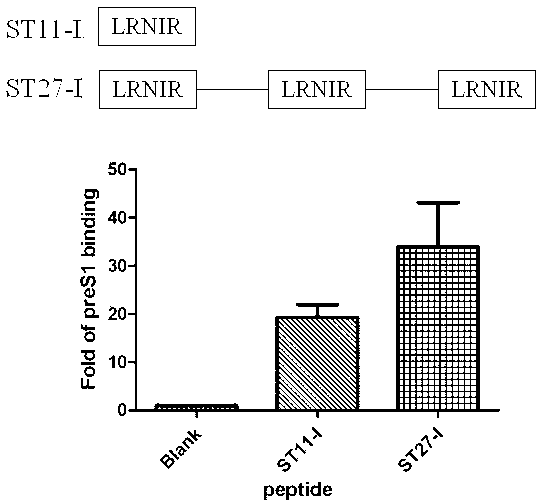
Short peptide for inhibiting human hepatitis B virus infection and its application
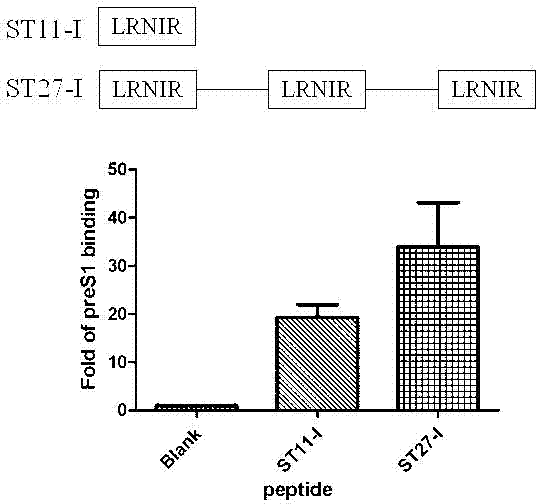
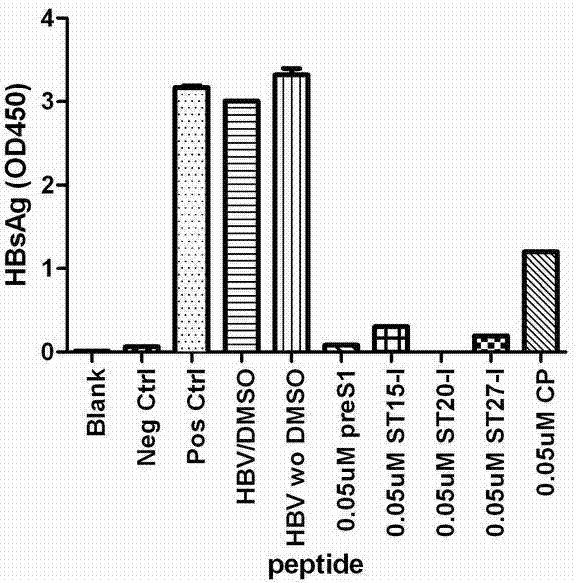
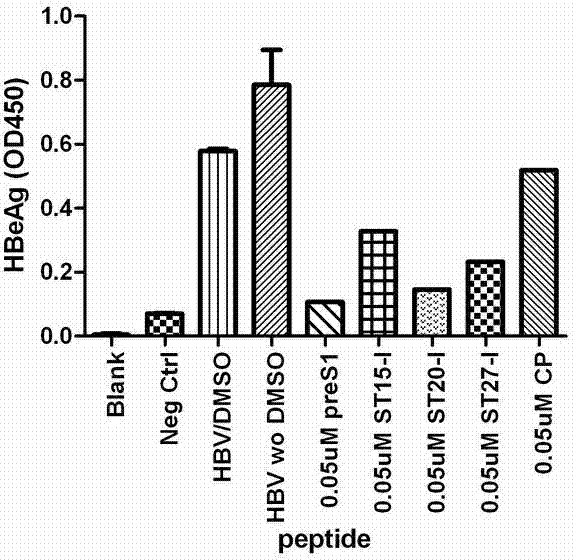
ActiveCN102775470AHigh suppression efficiencyStrong specificityBacteriaDigestive systemArginineBiomedicine
The invention belongs to the biomedicine field, and relates to a short peptide for inhibiting human hepatitis B virus infection. The short peptide comprises anyone of the following amino acid sequences: 1, N-leucine-arginine-asparagine-isoleucine-arginine-C; 2, an amino acid sequence obtained through deleting one or two amino acids from the above amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 3, an amino acid sequence obtained through substituting one or two amino acids in the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 4, adding one or more amino acids to the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; and 5, an amino acid sequence obtained through repeated splicing of the amino acid sequences 1-4 and capable of inhibiting the human hepatitis B virus infection. The invention also provides a construct and a cell of an amino acid sequence comprising the short peptide or of a nucleic acid coding sequence of the short peptide, and an application of the short peptide.
Owner:FUDAN UNIV
Small interference RNA molecule SiRNA capable of attacking human hepatitis B virus and application thereof
The invention provides a small interference RNA molecule SiRNA capable of attacking human hepatitis B virus, which is double chain RNA molecule whose sequence has at least 70% of consanguinity degree with the sequence (I), wherein the antisense chain of the sequence (I) and one nucleic acid mutant has no consanguinity with the known human gene and gene expression segment. The SiRNA can be used for preparing medicament or preparation for prevention or treating hepatitis B and any diseases relating to hepatitis B viral infection.
Owner:丽水瑞德佳生物医药有限公司
CRISPR/Cas9 targeted knockout of human hepatitis B virus p gene and its specific gRNA
The invention relates to the technical field of genetic engineering, and particularly relates to a guide RNA (gRNA) sequence based on a CRISPR / Cas9 system and combinations thereof, as well as gRNA for a specific targeted knockout hepatitis B virus cccDNA P gene. In the invention, 30 gRNAs are designed according to design principles of CRISPR / Cas9, have a sequence table as shown in SEQ ID NO.1-30, and are constructed on a PX458 vector, to screen out four more efficient gRNAs. The CRISPR / Cas9 system guided by the four gRNAs and combinations thereof is utilized in a human hepatocellular carcinoma cell line (HepG2.2.15) to effectively knock out the human hepatitis B virus cccDNA P gene. The gRNA of the specific targeted hepatitis B virus cccDNA prepared in the invention can accurately target the hepatitis B virus cccDNA and realize gene knockout. The preparation method is simple in operation; the gRNA has a good targeting property; the CRISPR / Cas9 system is high in knockout efficiency.
Owner:重庆高圣生物医药有限责任公司
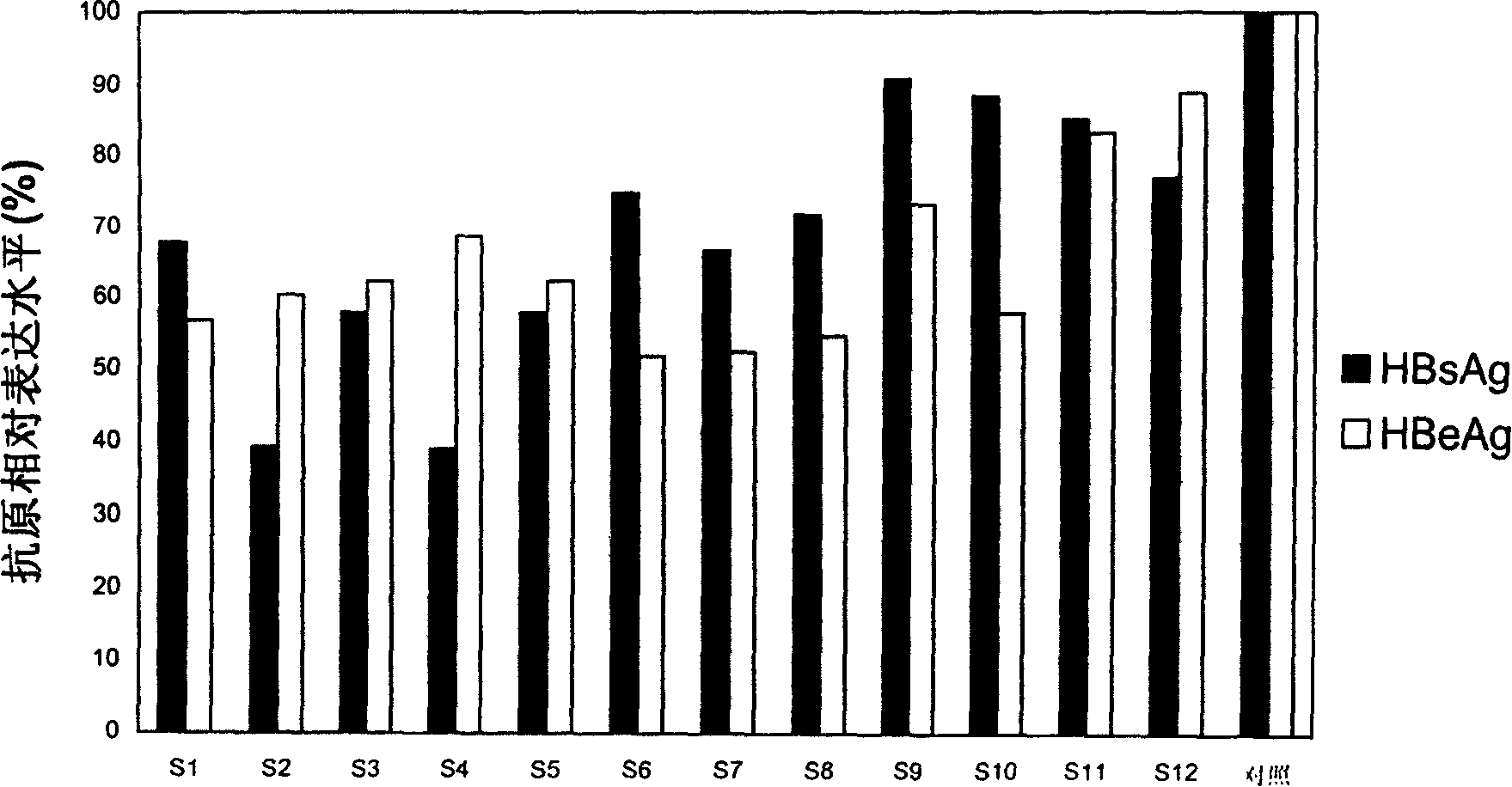
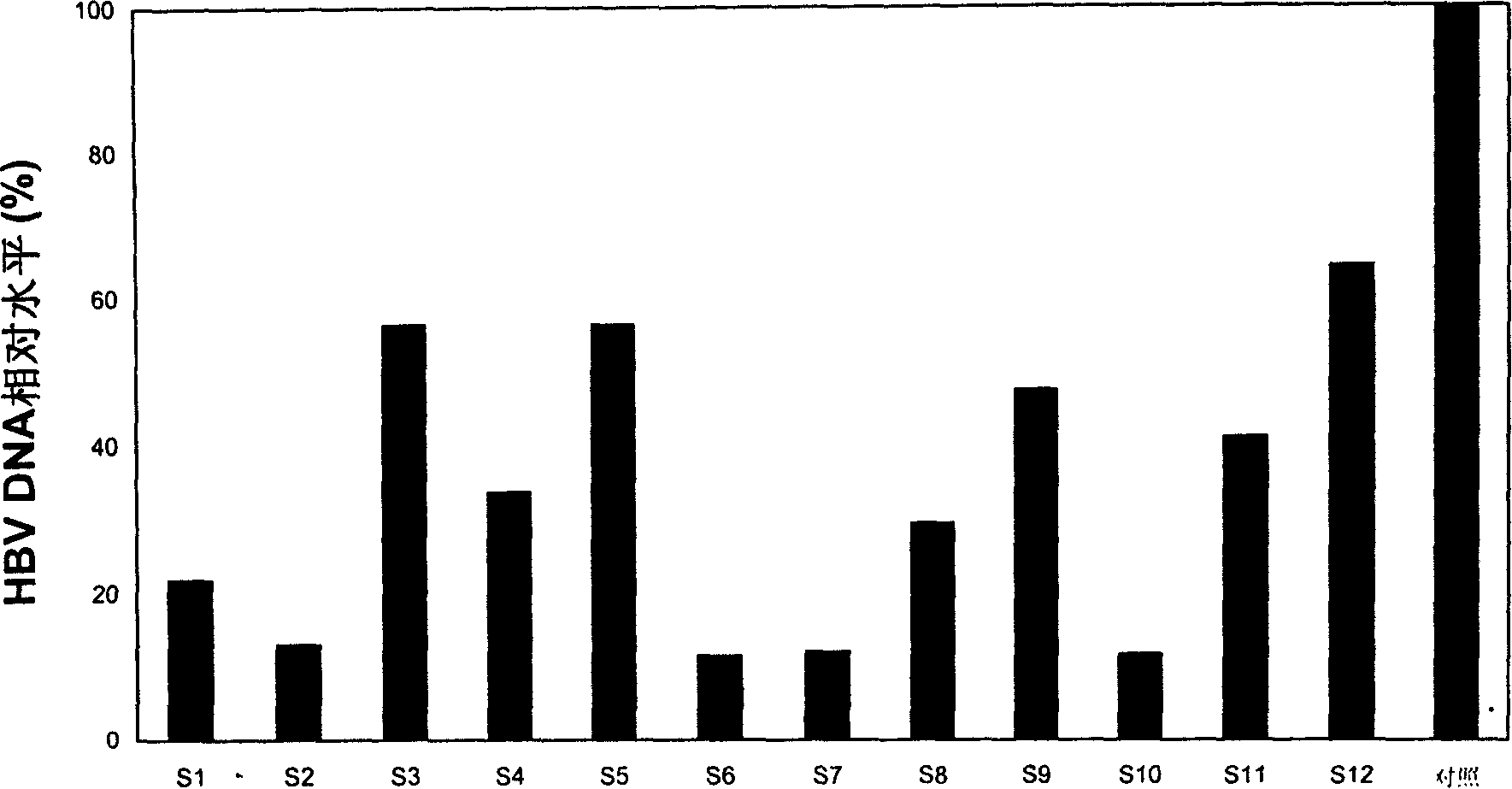
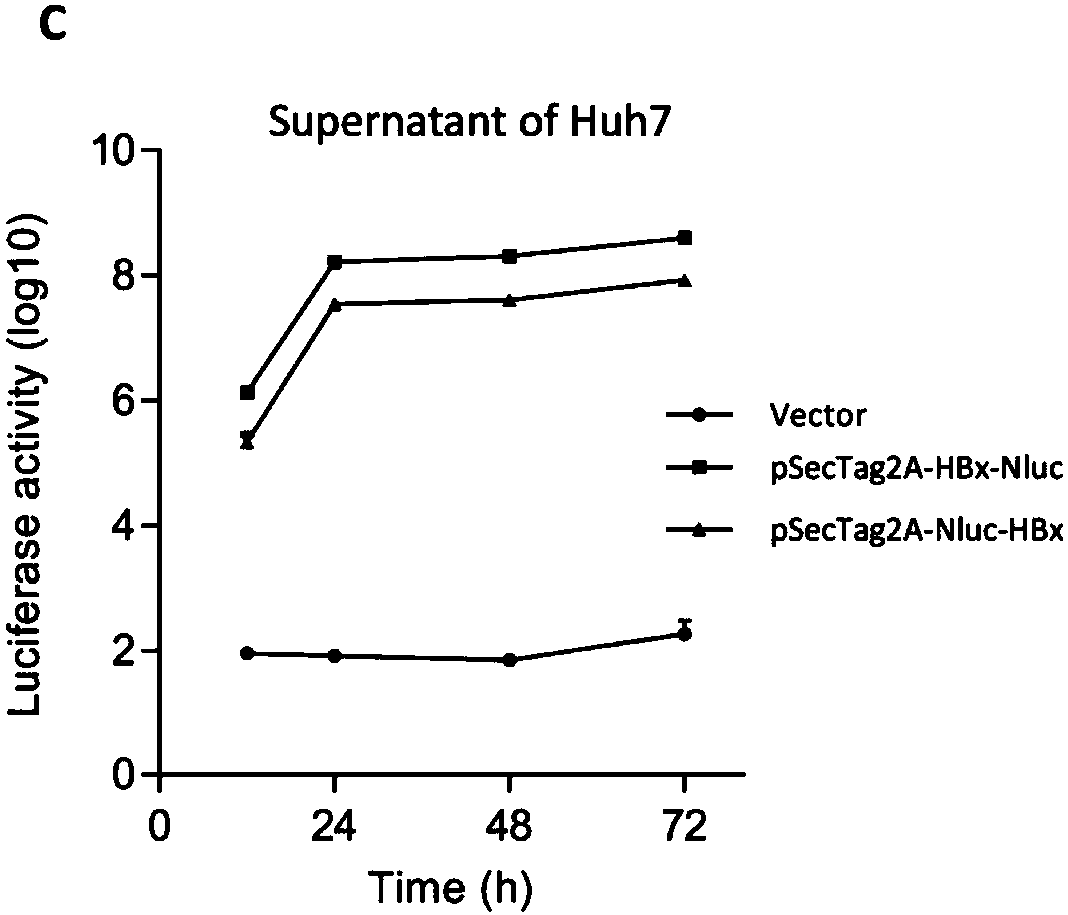
Method for evaluating X protein binding molecule of human hepatitis C virus
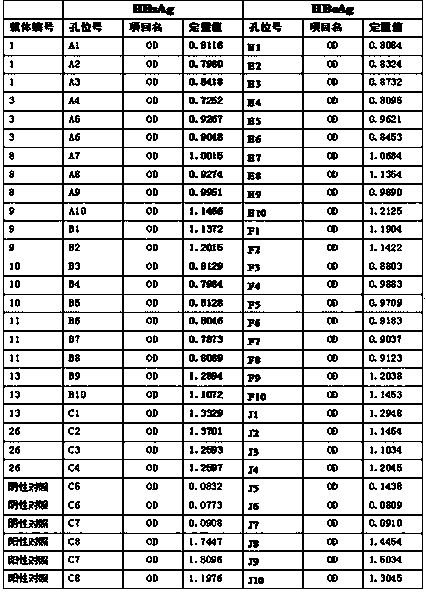
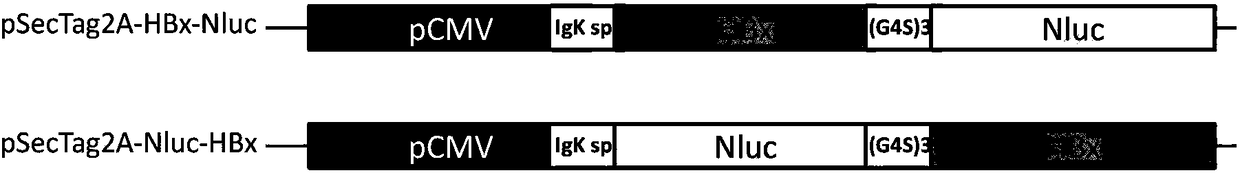
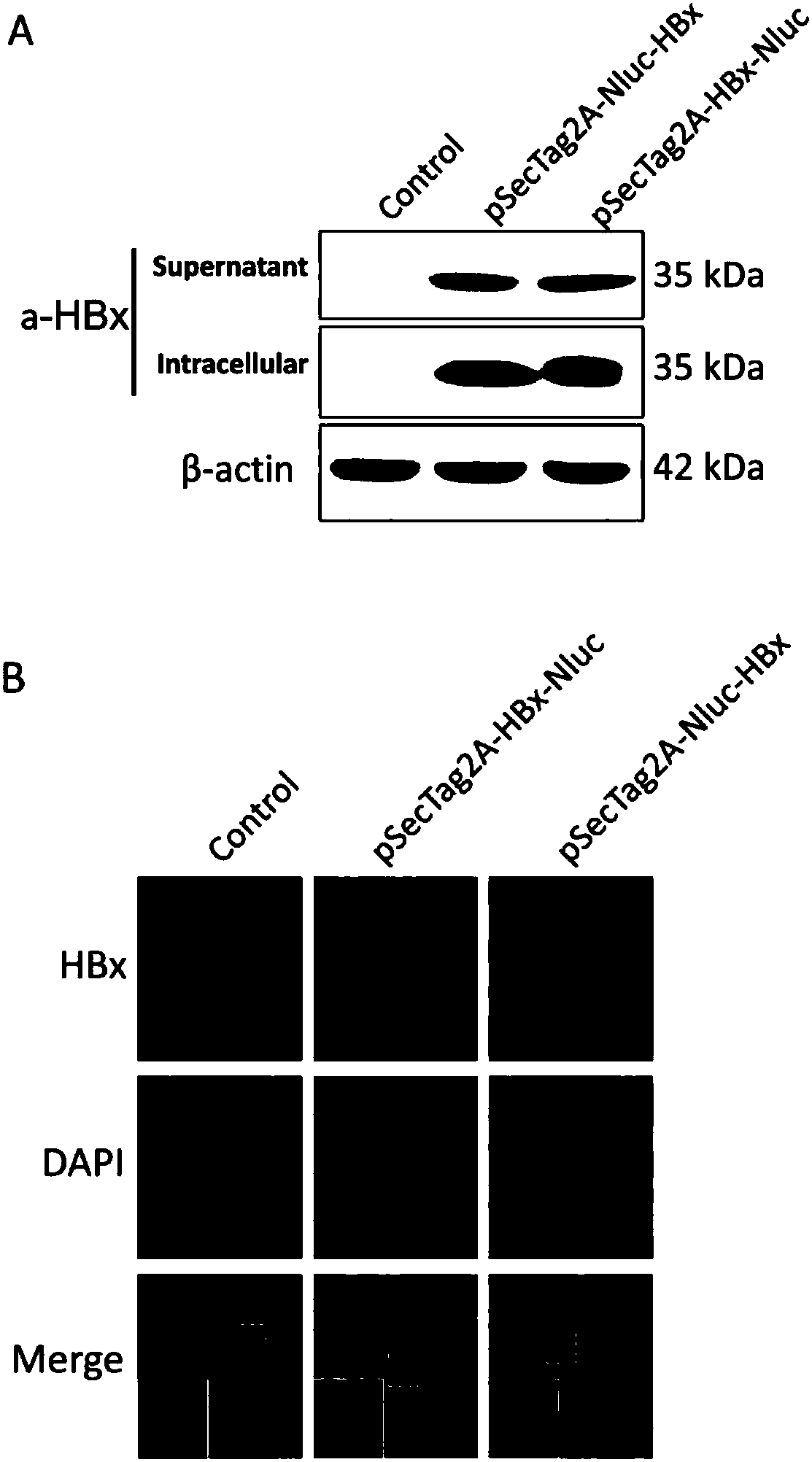
ActiveCN108239657ASimple methodSignal sensitiveVirus peptidesNucleic acid vectorHepatitis B immunizationProtein target
The invention belongs to the field of biotechnology and genetic engineering, and relates to a method for evaluating an X protein (HBx protein) binding molecule of human hepatitis C virus. The method provided by the invention comprises the following steps: expressing a secreting type fusion protein containing the HBx protein and luciferase NanoLuciferase (Nluc) in a cell; and quantifying the levelof the secreted HBx protein by detecting the activity of luciferase of the fusion protein. The method can be used for screening and identifying the HBx protein binding molecule, provides a support forresearching an HBx protein targeted drug, and has good application prospect.
Owner:FUDAN UNIV
Assay for detecting hepatitis B virus (HBV)
Owner:ABBOTT MOLECULAR INC
Short peptide for inhibiting human hepatitis B virus infection and its application
ActiveCN102775470BHigh suppression efficiencyStrong specificityBacteriaDigestive systemArginineTert-leucine
The invention belongs to the biomedicine field, and relates to a short peptide for inhibiting human hepatitis B virus infection. The short peptide comprises anyone of the following amino acid sequences: 1, N-leucine-arginine-asparagine-isoleucine-arginine-C; 2, an amino acid sequence obtained through deleting one or two amino acids from the above amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 3, an amino acid sequence obtained through substituting one or two amino acids in the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 4, adding one or more amino acids to the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; and 5, an amino acid sequence obtained through repeated splicing of the amino acid sequences 1-4 and capable of inhibiting the human hepatitis B virus infection. The invention also provides a construct and a cell of an amino acid sequence comprising the short peptide or of a nucleic acid coding sequence of the short peptide, and an application of the short peptide.
Owner:FUDAN UNIV
Primer sets and probes for detection of human hepatitis B virus
ActiveCN103849690BHigh precisionReduce false negative rateMicrobiological testing/measurementDNA/RNA fragmentationHepatitis B immunizationGene
The present invention relates to primer sets and / or probes for the specific detection of human hepatitis B virus (HBV). The present invention also relates to kits and microarrays for specific detection of human hepatitis B virus comprising primer sets and / or probes. The present invention also relates to the use of primer sets and / or probes in the preparation of kits and microarrays for specific detection of hepatitis B virus in samples. The present invention also relates to a method for detecting HBV genes in samples using primer sets and / or probes.
Owner:QIAGEN SHENZHEN CO LTD
In vitro activity assay for human hepatitis B virus (HBV) DNA polymerase, and its use for screening inhibitors of HBV DNA polymerase
InactiveCN1197978CAssayable priming activityMicrobiological testing/measurementTransferasesSerum samplesHbv dna polymerase
The present invention provides an in vitro activity test of human hepatitis B virus (HBV) DNA polymerase, which comprises utilizing an oligonucleotide incorporated into the SP6 viral promoter as a 5' oligonucleotide to amplify HBV from a sample by PCR DNA polymerase, direct transcription and translation of the PCR product in the presence of a radiolabeled reagent, and determination of HBV DNA polymerase priming. The present invention also provides the application of such a test in the activity test of various serum samples, in the screening of HBV DNA polymerase inhibitors, and in the detection and / or screening of potential anti-HBV drugs on human HBV DNA polymerization Applications in the inhibitory capacity of DNA-priming activity of enzymes.
Owner:GOVERNMENT OF THE REPUBLIC OF SINGAPORE
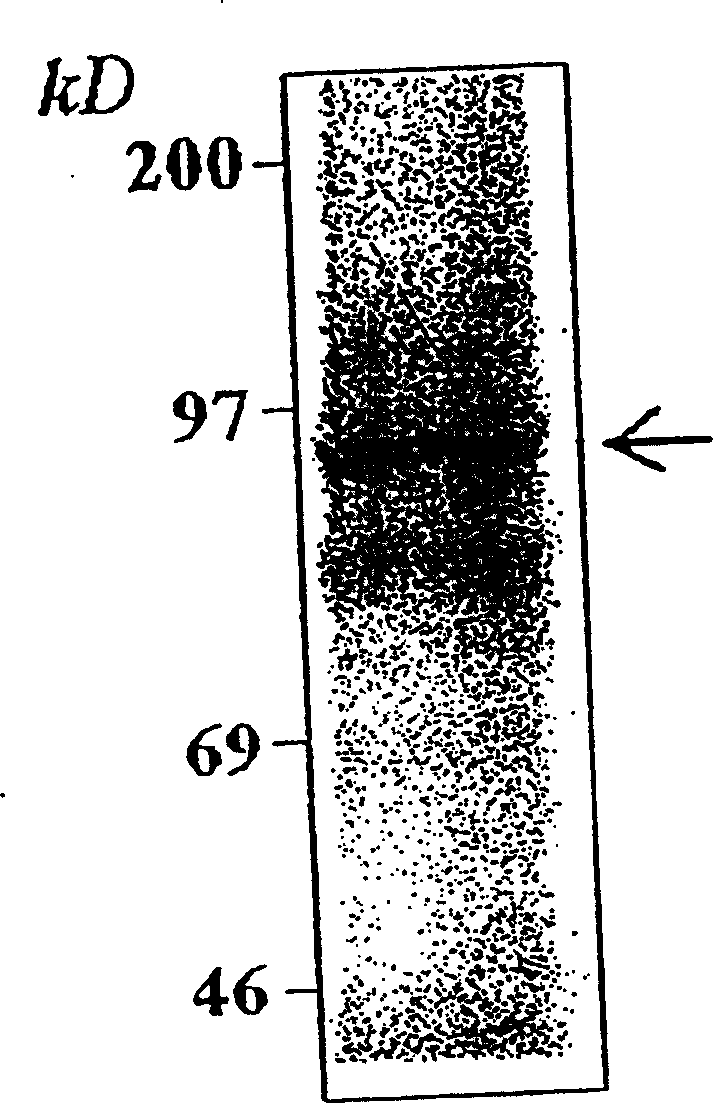
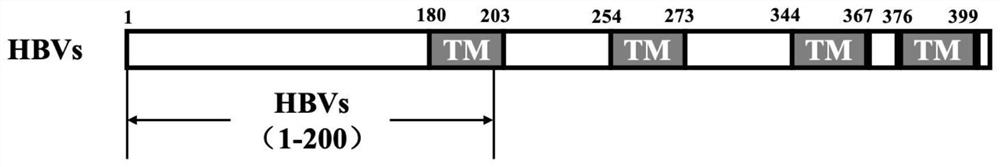
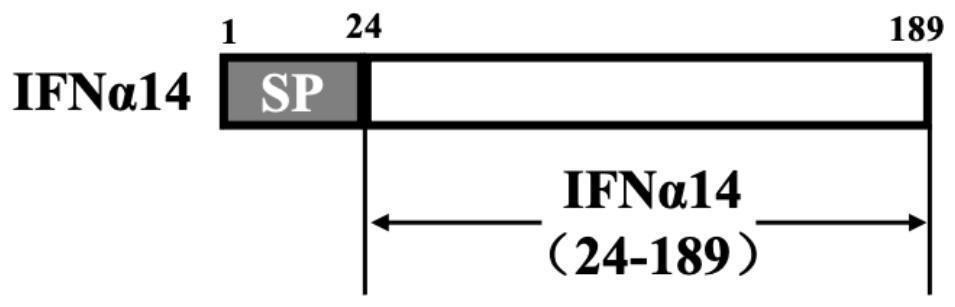
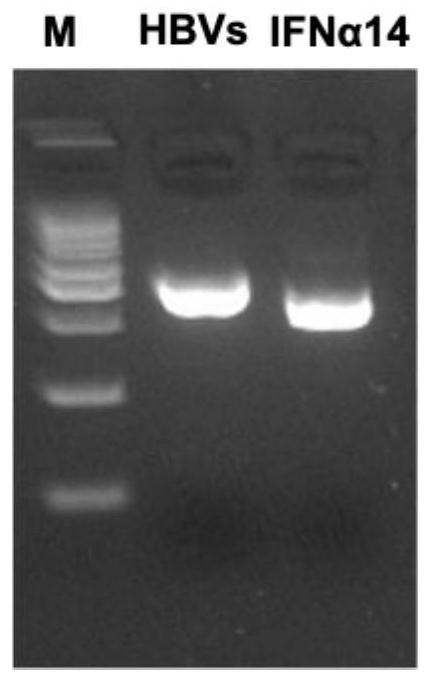
Recombinant saccharomyces cerevisiae strain capable of simultaneously expressing IFNa14 protein and human hepatitis B virus S protein as well as preparation method and application
ActiveCN113817621AAchieve immune effectAchieve therapeutic effectFungiPeptide/protein ingredientsHepatitis B immunizationSecretory protein
The invention discloses a recombinant saccharomyces cerevisiae strain capable of simultaneously expressing IFNa14 protein and human hepatitis B virus S protein as well as a preparation method and application. The recombinant saccharomyces cerevisiae strain contains 1-200 amino acids of HBVs protein and 24-190 amino acids of IFN alpha 14. Two phenotypes are integrated into one yeast strain, so that the recombinant yeast strain JDY52-HBVs-IFN alpha 14 capable of simultaneously displaying the HBVs protein and secreting the IFN alpha 14 protein on the surface is obtained. The recombinant saccharomyces cerevisiae strain is used for preparing a hepatitis B virus oral vaccine, the protective immune response of an organism is stimulated through an oral administration way, and more effective immune protection is exerted by virtue of the regulating effect of yeast cell wall polysaccharide on the congenital immune system of the organism. Compared with the existing vaccine, the oral recombinant yeast preparation is low in cost, can realize large-scale enlarged production, is safe and reliable, has good application and development prospects, and provides a choice for immunization and treatment of hepatitis B virus.
Owner:TIANJIN UNIV
Efficiently-replicated human hepatitis B virus recombinant vector and application thereof
ActiveCN102643858BEfficient replicationEfficient expression of foreign genesGenetic material ingredientsAntineoplastic agentsWild typeGene engineering
The invention belongs to the field of medicine and gene engineering, and relates to a recombinant vector based on human hepatitis B viruses; the virus genome has partially or all deleted hepatitis B virus polymerase spacers relative to wild-type hepatitis B virus vectors. The invention also provides a preparation method of the recombinant vector and an application in gene therapy of targeting livers or hepatocytes. The human hepatitis B virus recombinant vector of the invention can enable the expression of inserted exogenous genes and the efficient replication of recombinant hepatitis B viruses, and can form complete recombinant hepatitis B virus particles with the supply of surface protein, which shows the application prospects that the human hepatitis B virus recombinant vector of the invention can be used as a liver-specific gene introduction vector.
Owner:FUDAN UNIV
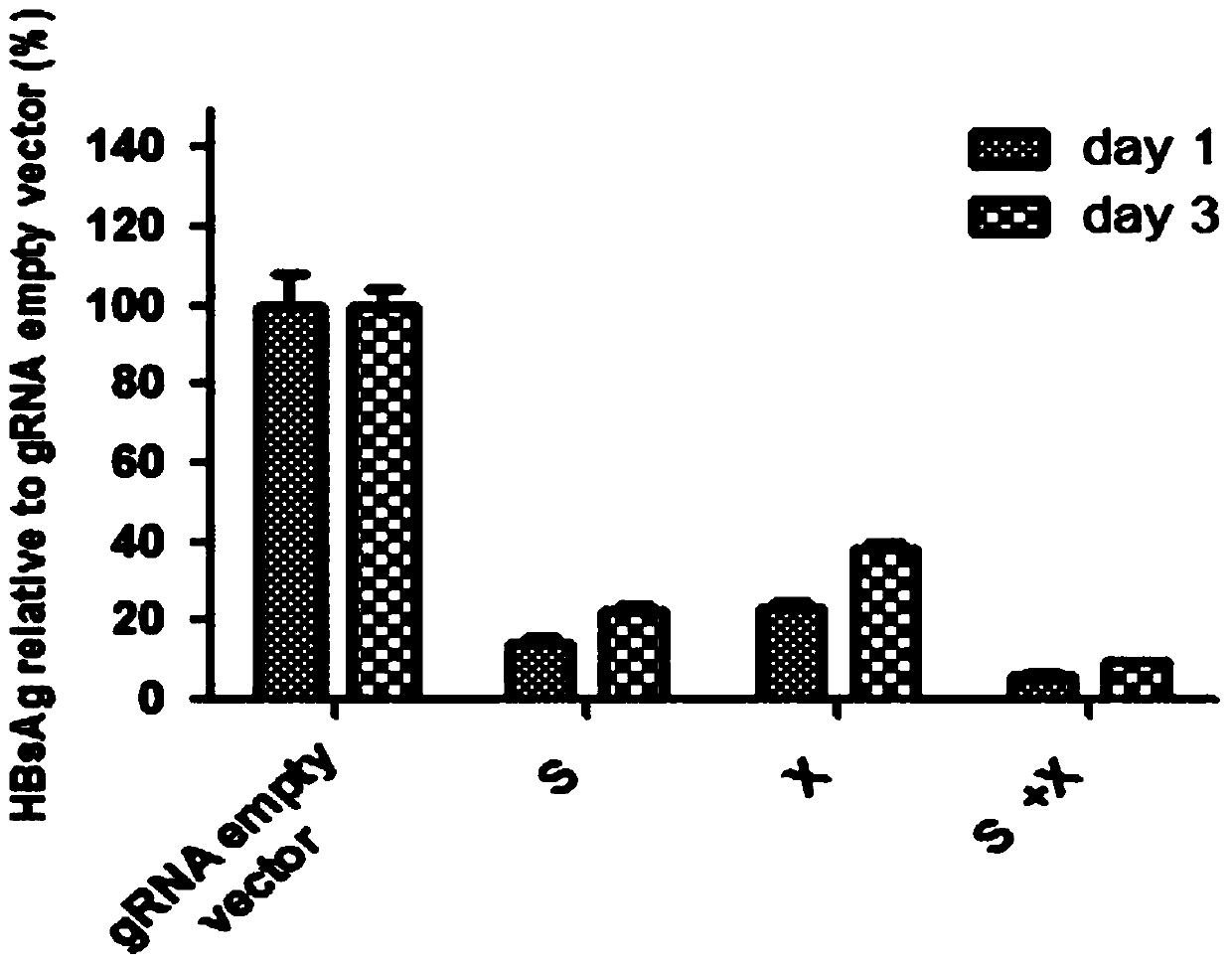
Specific sgRNA, expression vector and application of combined immune gene inhibition of HBV replication
ActiveCN105821039BImprove targetingHigh knockout efficiencyOrganic active ingredientsGenetic material ingredientsAntigenHepatitis B Virus Antigen
The invention provides a specific sgRNA combined with an immunogene to inhibit HBV replication, an expression vector thereof, and an application of the specific sgRNA and the expression vector. The sgRNA sequences of a human hepatitis B virogene suitable for CRISPR-Cas9 targeting editing and a PD-1 gene are designed, the plasmid vector of the sgRNA inhibiting HBV and PD-1 genes is constructed, and the plasmid vector and a nuclease gene expression vector are transferred to HBV transgenic mice, and can obviously inhibit HBV DNA replication. The gene expression vector prepared in the invention has the advantages of simple method steps, good sgRNA targeting property, and high CRISPR-Cas9 knockout efficiency. The sgRNAs specifically targeting the HBV and PD-1 genes can accurately splice the HBV and PD-1 genes, inhibit in vivo hepatitis B virus replication, and reduce the hepatitis B virus antigen expression.
Owner:李旭
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com