Primer sets and probes for detection of human hepatitis B virus
A hepatitis B virus and primer set technology, which is applied in biochemical equipment and methods, recombinant DNA technology, microbial measurement/testing, etc., can solve the problem of low sensitivity, unfavorable evaluation of hepatitis B treatment effect, and inability to determine the virus in the body capacity issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Material
[0097] 1. Kit
[0098] 1.1 The kit of the present invention comprises the following components: HBVHSPCR solution, HotstarTaqplus polymerase (5U / μl) and UNG (1U / μl)).
[0099] HBVHSPCR solution contains the following components: ultrapure water, MultiplexVirusPCR buffer 20x, Q-solution5x, 25mmol / LMgCl 2 , 100mmol / LdATP, 100mmol / LdCTP, 100mmol / LdGTP, 100mmol / LdUTP, primer set 1 (forward primer 1 and reverse primer 1), probe 1 (probe 1), primer set 2 (forward primer 2 and reverse primer 2) and probe 2 (probe 2).
[0100] 1.2 Control Kit
[0101] The control kit is "Hepatitis B virus (HBV) nucleic acid quantitative detection kit (PCR-fluorescent probe method)" produced by Kaijie Bioengineering (Shenzhen) Co., Ltd. (trade name: careHBVPCRASSAY; approval number: China Food and Drug Supervisory Equipment (quasi) word 2009 No. 3401037).
[0102]1.3 Third-party kits
[0103] The third-party kit is COBASAmpliPrep / COBASTaqManHBVTest, version2.0 produced by Roch...
Embodiment 2
[0162] Embodiment 2—the lowest limit of detection (LOD) of the kit of the present invention
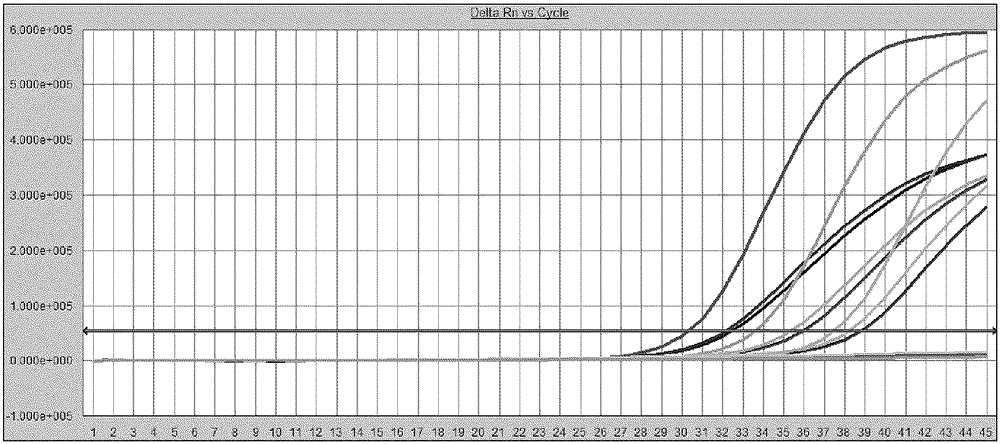
[0163] Using the WHO quantitative standard described in Example 1, dilute to 200, 100, 30, 20, 10 IU / ml with negative plasma (IU / ml represents the amount of virus contained per milliliter). Standard HBV DNA was prepared by the method described in Example 1. Using the kit of the present invention, each concentration gradient was repeatedly detected 25 times by fluorescent quantitative PCR reaction. Determine the detection rate of the kit of the present invention at each standard concentration, thereby determining the minimum detection limit. The result is as figure 1 As shown, the detection rate of 20IU / ml samples can reach 96%.
Embodiment 3
[0164] The quantitative detection limit (LOQ) of embodiment 3-kit of the present invention
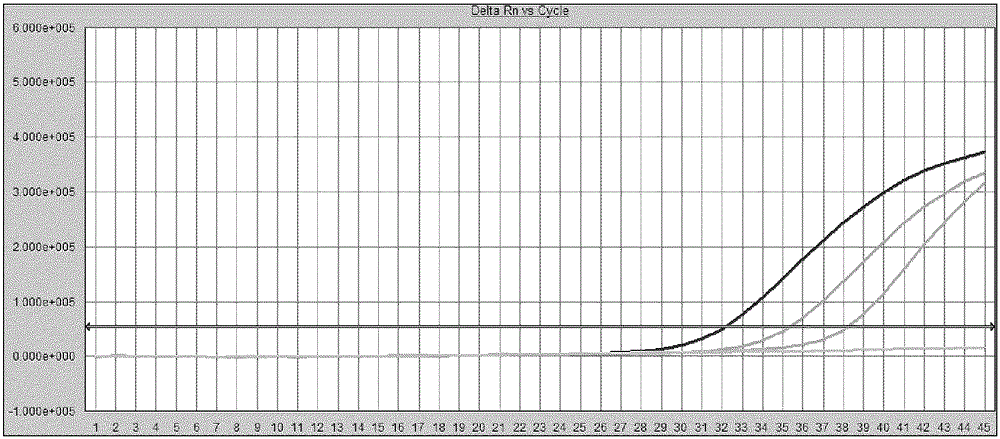
[0165] Using the WHO quantitative standard, L0 standard and B / C / D genotype standard described in Example 1, they were diluted to 40 and 20 IU / ml with negative plasma, respectively. The HBV DNA of the standard was prepared by the method described in Example 1. Using 3 batches of the kit of the present invention, each concentration gradient was repeatedly detected 25 times by fluorescent quantitative PCR reaction.
[0166] Determine the quantitative detection limit (LOQ) of the kit of the present invention according to the following criteria: calculate the logarithmic value of the quantitative result, count the ratio (quantitative accuracy) of the logarithmic value of the quantitative result under each sample concentration within the range of ± 0.5log10 of the theoretical value, The ratio should be greater than or equal to 22 / 25.
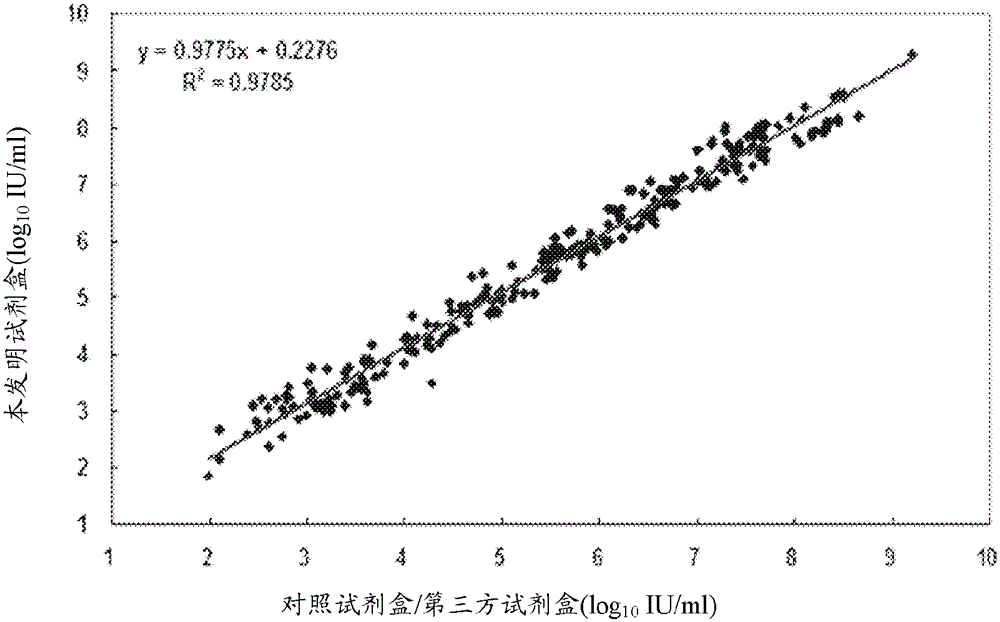
[0167] Experimental results such as figure 2As sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com