Short peptide for inhibiting human hepatitis B virus infection and its application
A hepatitis B virus, short peptide technology, applied in the direction of viral peptides, applications, antiviral agents, etc., can solve the problems of drug cross-resistance, single target, etc., to achieve good specificity, high inhibition efficiency, good application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
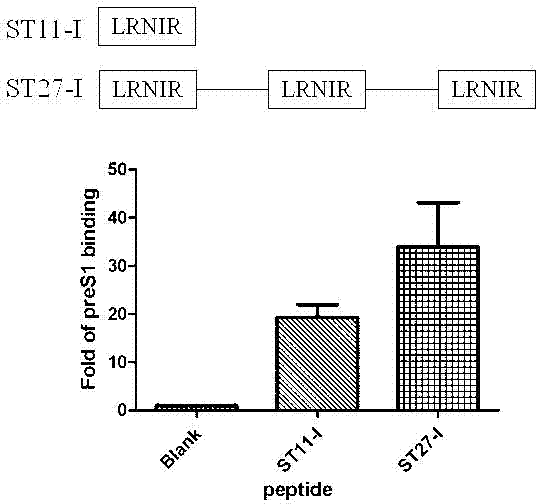
[0042] Example 1: Short peptides are able to bind preS1 13-59 .
[0043] Biotin-coupled short peptides were bound to Streptavidin-coated 96-well plates, 50ug Biotin-coupled short peptides were added to each well, and combined in 100ul PBS at 4°C for 4 hours. Gently tap off the PBS, rinse with PBS again. Re-add 100ul PBS and add 50ug myristoylated preS1 13-59 , 4°C for 8-12 hours. Gently tap off the PBS, rinse with PBS again. Add 200ul of PBS containing 0.1% BSA, combine at 37°C for 1 hour, wash once with PBST, 5 seconds each time. Then add 100ul anti-preS1 monoclonal antibody (1:1000, diluted in PBS containing 0.1% BSA), bind at 4°C for 4 hours, and then wash three times with PBST, 5 seconds each time. Add 100ul rabbit anti-mouse secondary antibody (1:1000, diluted in PBS containing 0.1% BSA), combine for 2 hours at 4 degrees, and then wash three times with PBST, 5 seconds each time. Finally, 100ul of TMB (premixed hydrogen peroxide) was added to each well, and the color...
Embodiment 2
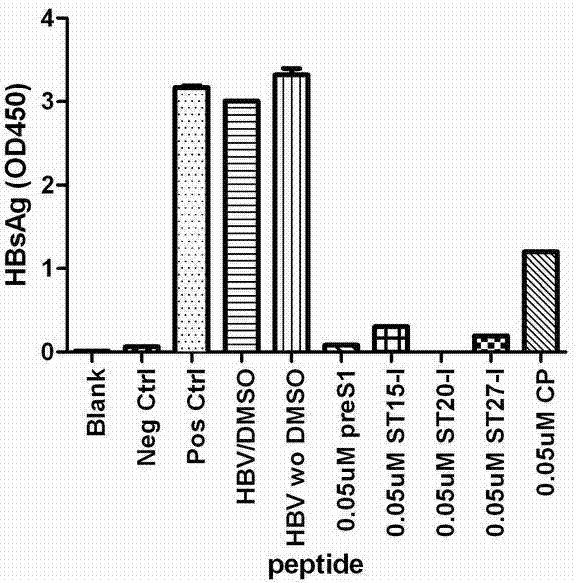
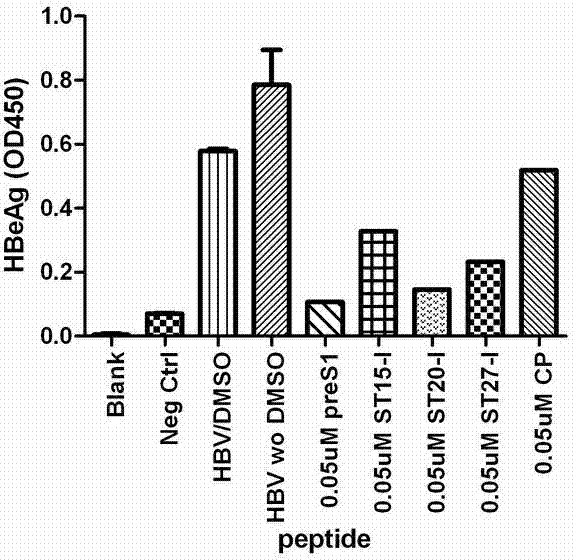
[0046] Example 2: Short peptides can inhibit the infection of hepatitis B virus in tree shrew primary liver cell model
[0047] 2.1 Culture of tree shrew primary hepatocytes
[0048] Tree shrews were purchased from the Experimental Animal Center of Kunming Institute of Zoology, Chinese Academy of Sciences. Tree shrew primary hepatocytes were isolated by a two-step perfusion method. Select adult tree shrews with a weight of 160-200 grams. After feeding for 5-7 days, fast for 12 hours before operation. Anesthesia: inject ketamine hydrochloride 5mg / 100g body weight into each muscle, add xylazine 1mg / 100g body weight. Perfusion: Fix the tree shrew on the support, open the abdominal cavity, expose the portal vein, insert the catheter, and start perfusing Hanks solution preheated at 42°C (5mM EGTA, 1% glucose, 50 u / ml ampicillin, 100 μg / ml streptomycin, 100mM L-glutamine, 15mM HEPES, pH7.4), 20 ml / min, perfuse for 5 minutes. Hanks solution (Hanks solution Add 0.5 mg / ml t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com