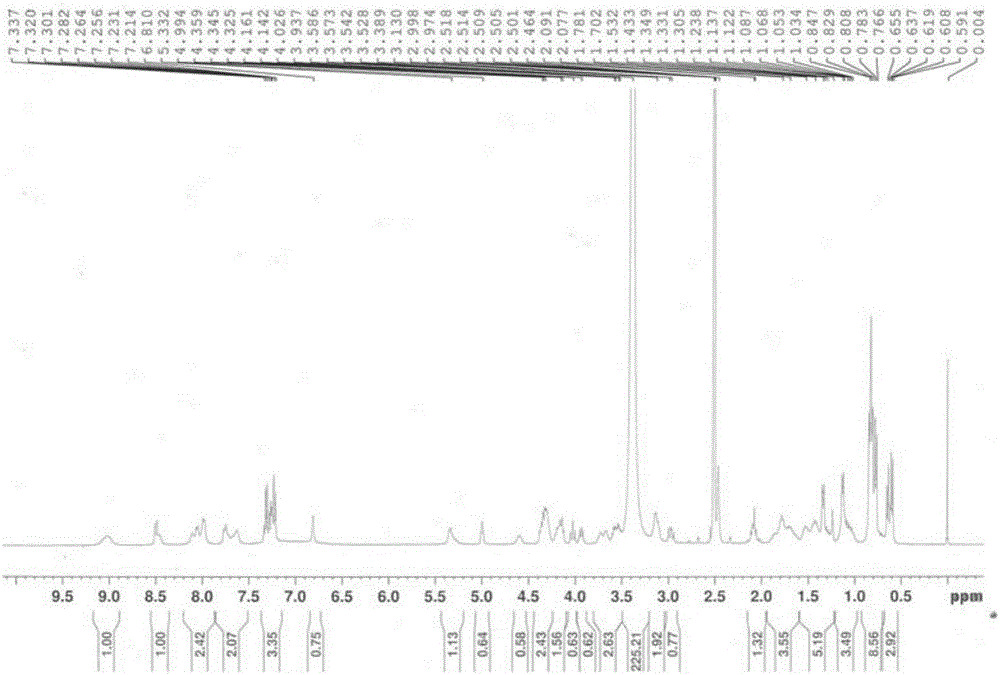
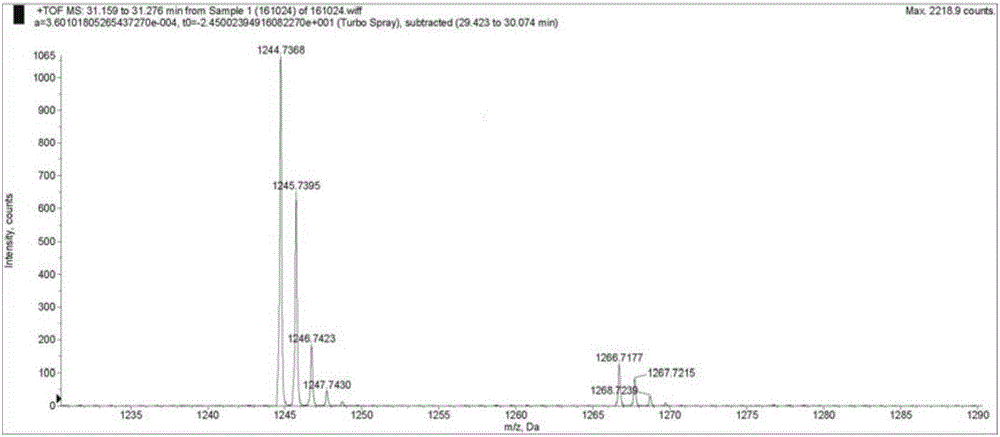
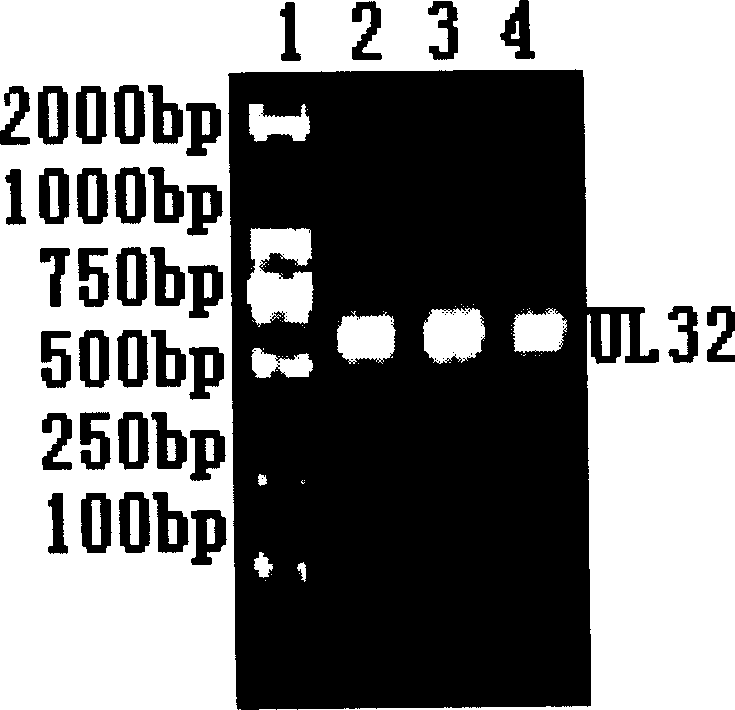
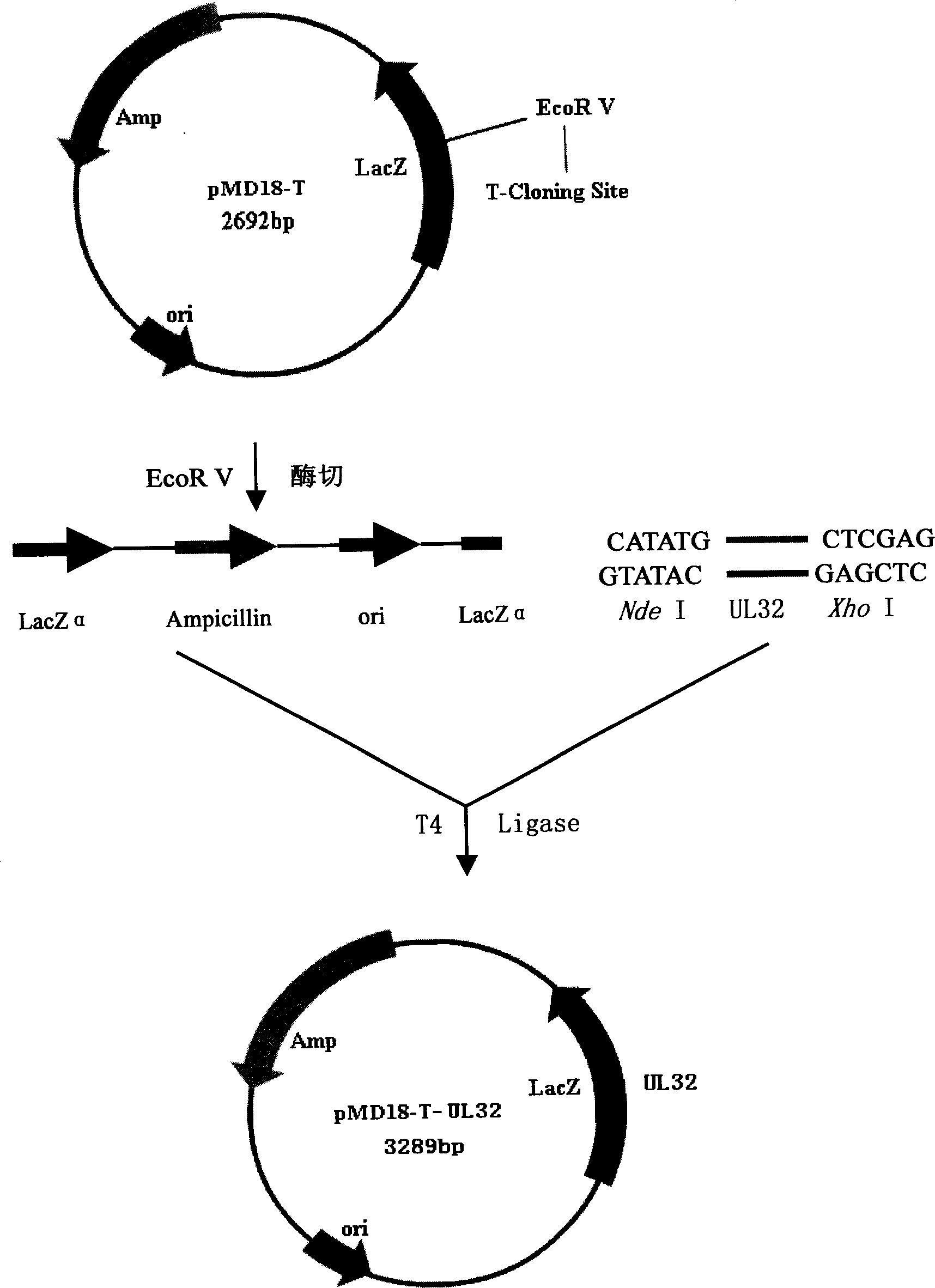
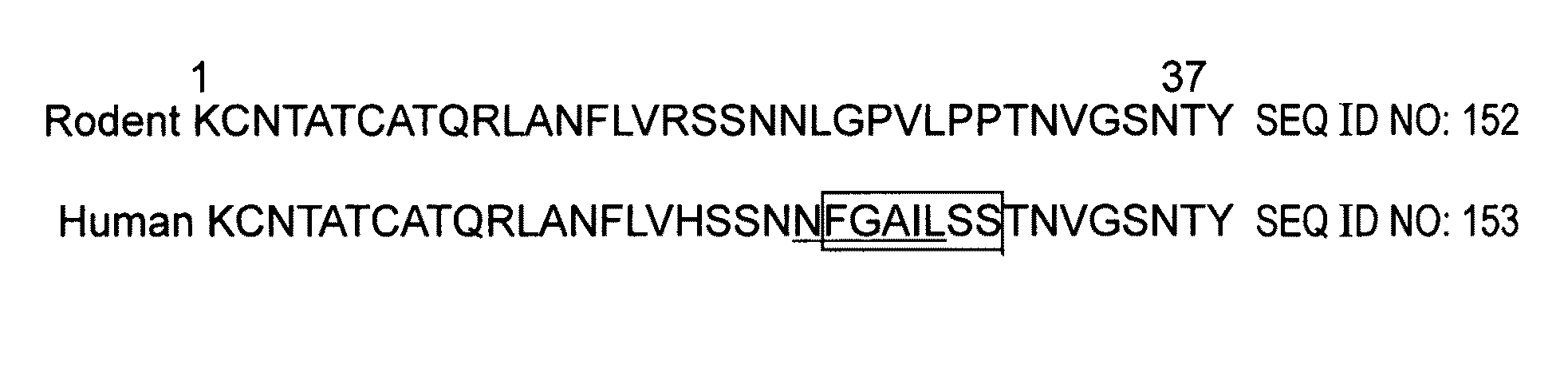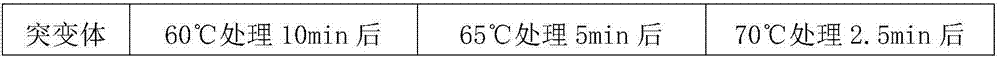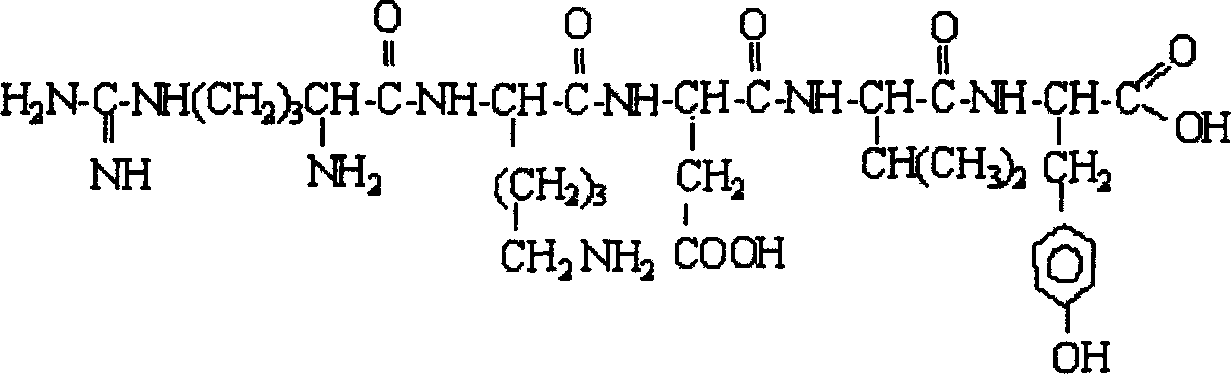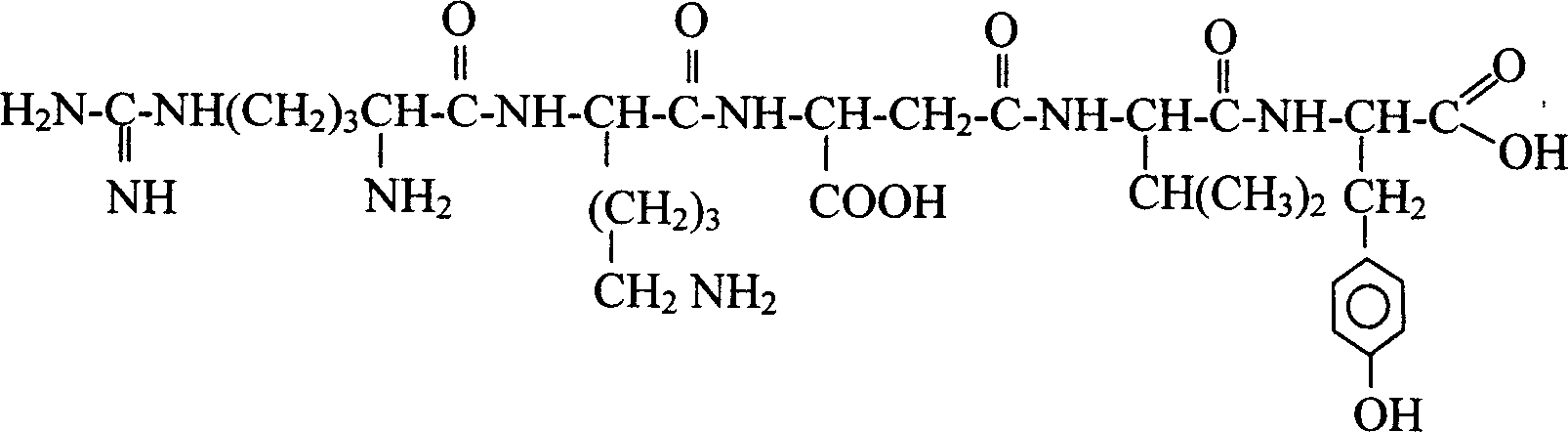Patents
Literature
32 results about "2-aminohistamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptides antibodies directed thereagainst and methods using same for diagnosing and treating amyloid-associated diseases
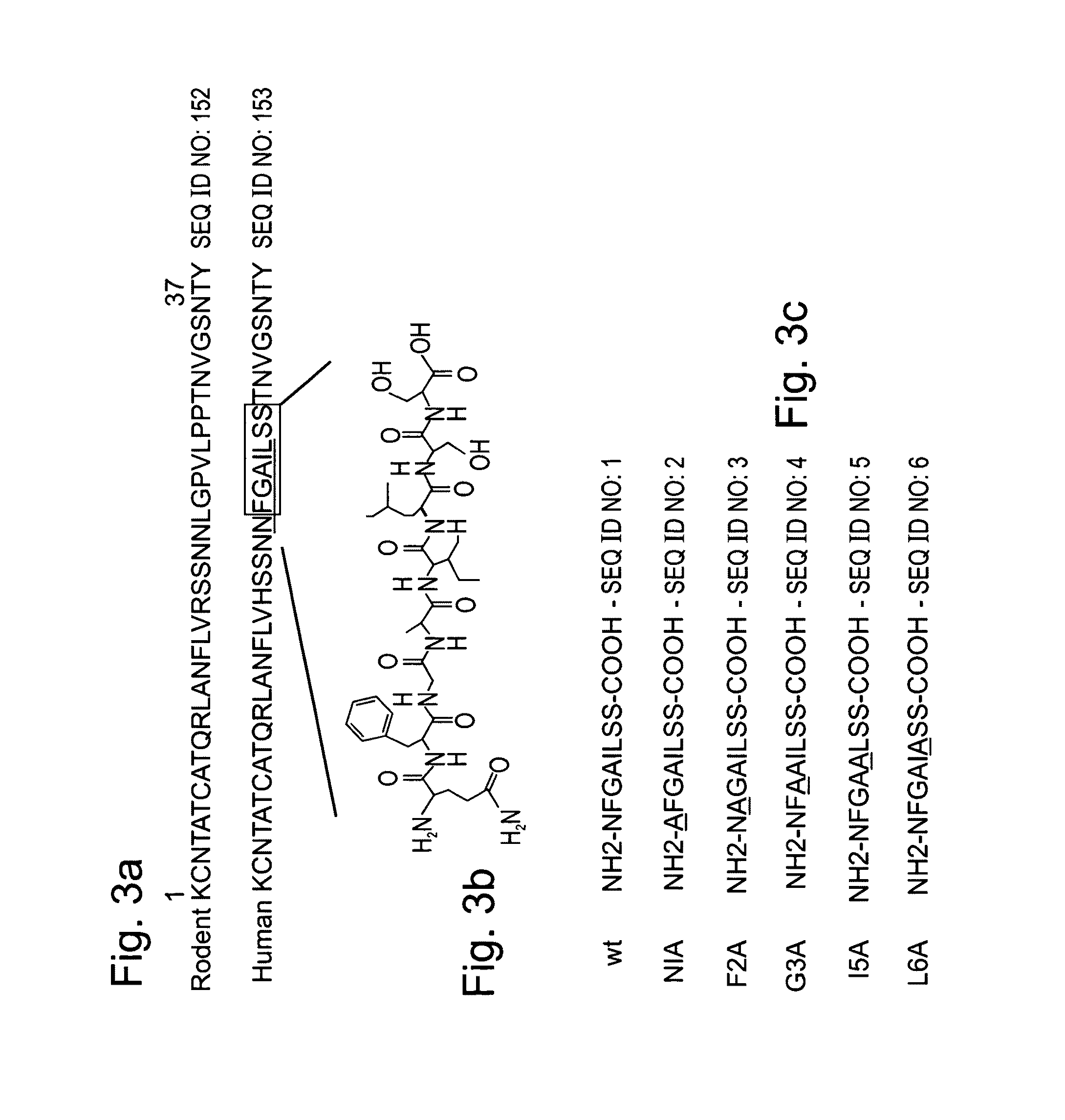
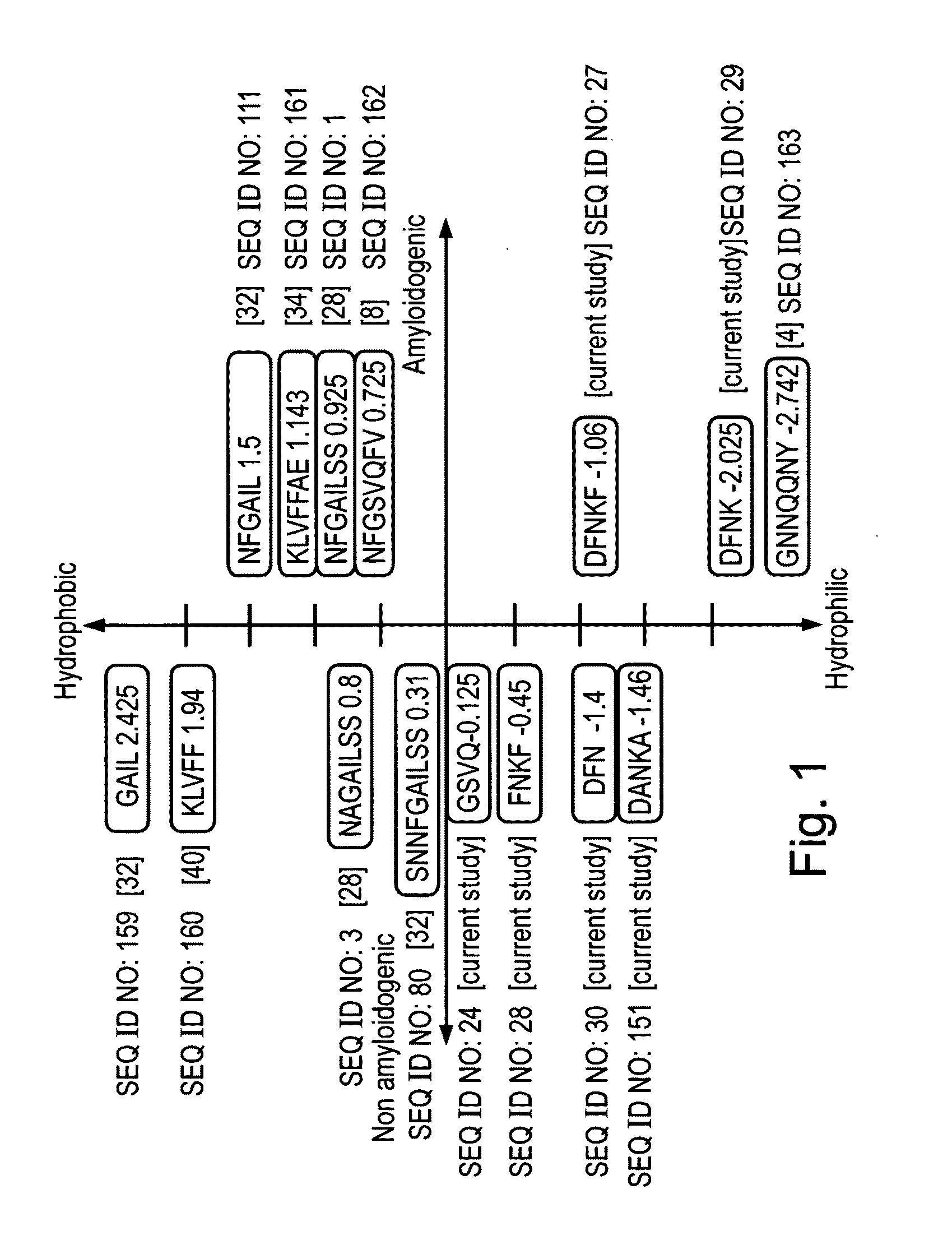
Peptides having at least 2 amino acids and no more than 15 amino acids are provided. The peptides comprise amino acid sequence X-Y or Y-X, wherein X is an aromatic amino acid and Y is any amino acid other than glycine. Also provided are pharmaceutical compositions and kits including such peptides as well as methods using same for diagnosing and treating amyloid associated diseases.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Peptides antibodies directed thereagainst and methods using same for diagnosing and treating amyloid-associated diseases
Peptides having at least 2 amino acid residues and no more than 15 amino acid residues are provided. The peptides comprise amino acid sequence X-Y or Y-X, wherein X is an aromatic amino acid and Y is any amino acid other than glycine. Also provided are pharmaceutical compositions and kits including such peptides as well as methods using same for diagnosing and treating amyloid associated diseases.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Peptides directed for diagnosis and treatment of amyloid-associated disease
Peptides having at least 2 amino acids and no more than 15 amino acids are provided. The peptides comprise amino acid sequence X-Y or Y-X, wherein X is an aromatic amino acid and Y is any amino acid other than glycine. Also provided are pharmaceutical compositions and kits including such peptides as well as methods using same for diagnosing and treating amyloid associated diseases.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Peptides directed for diagnosis and treatment of amyloid-associated diseases
Peptides having at least 2 amino acids and no more than 15 amino acids are provided. The peptides comprise amino acid sequence X—Y or Y—X, wherein X is an aromatic amino acid and Y is any amino acid other than glycine. Also provided are pharmaceutical compositions and kits including such peptides as well as methods using same for diagnosing and treating amyloid associated diseases.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Synthesis method of Bremelanotide
ActiveCN106589111AEasy to removeRaw materials are cheap and easy to getPeptide preparation methodsDepsipeptidesSynthesis methodsBremelanotide
The invention discloses a preparation method of polypeptide, particularly relates to a synthesis method of Bremelanotide and belongs to the field of drug synthesis. The synthesis method comprises the following steps: two fragments of Bremelanotide are synthesized firstly and comprise a fragment with two amino acids and a fragment with five amino acids, p-hydroxymethylbenzoic acid is used for protecting the right side of a Bremelanotide sequence, namely, a carboxyl functional group of lysine, and the final product Bremelanotide is obtained with a counter-clockwise ring-forming synthetic method. The synthesis method has the advantages of being short in synthesis route, high in yield and low in synthesis difficulty and cost, and adopting cheap and available raw materials, and is suitable for industrial production.
Owner:江苏中泰药业有限公司
Recombinant Toxoplasma fusion protein antigen and preparation process and use thereof
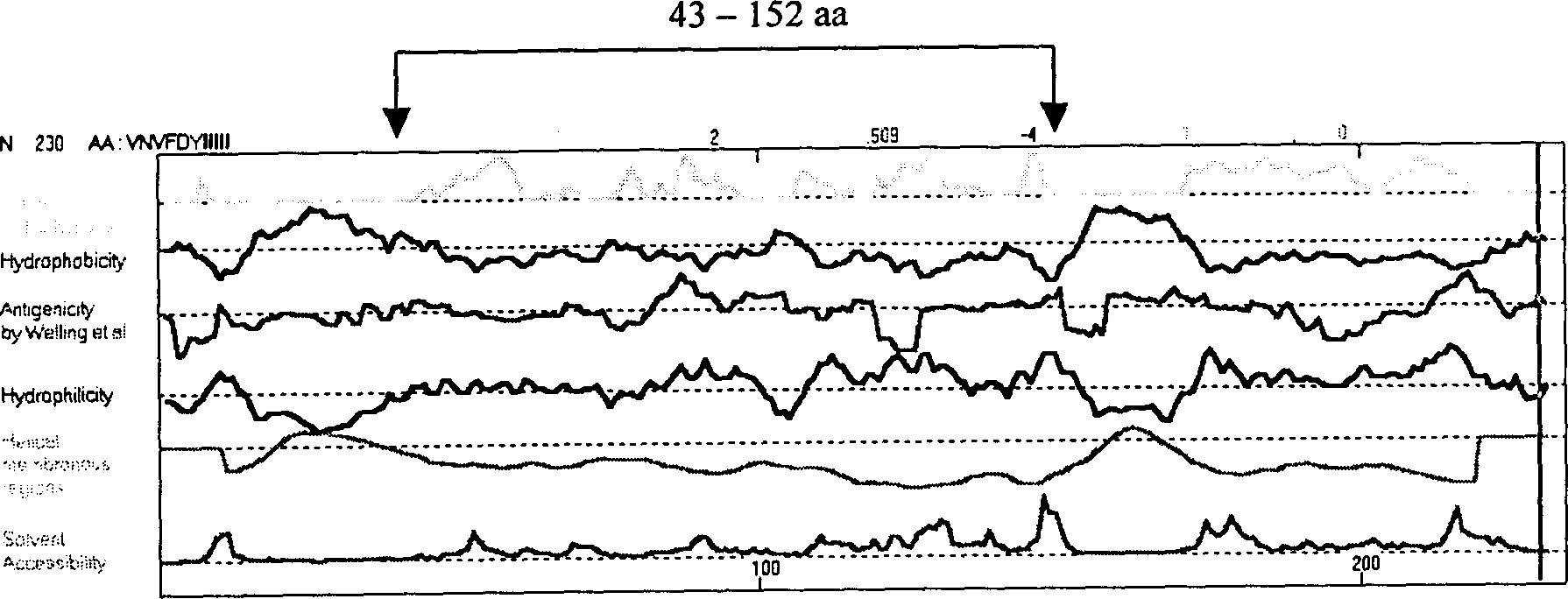
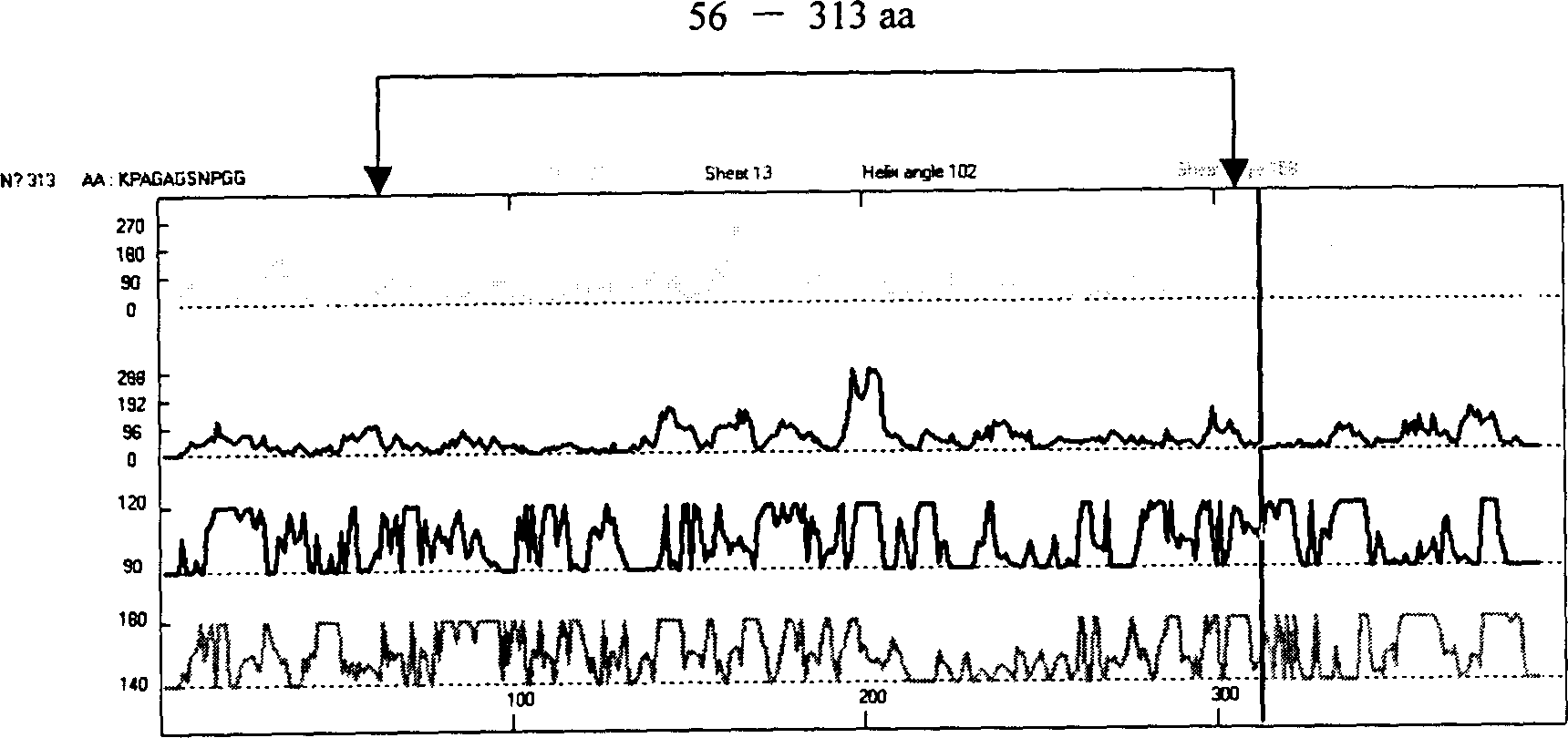
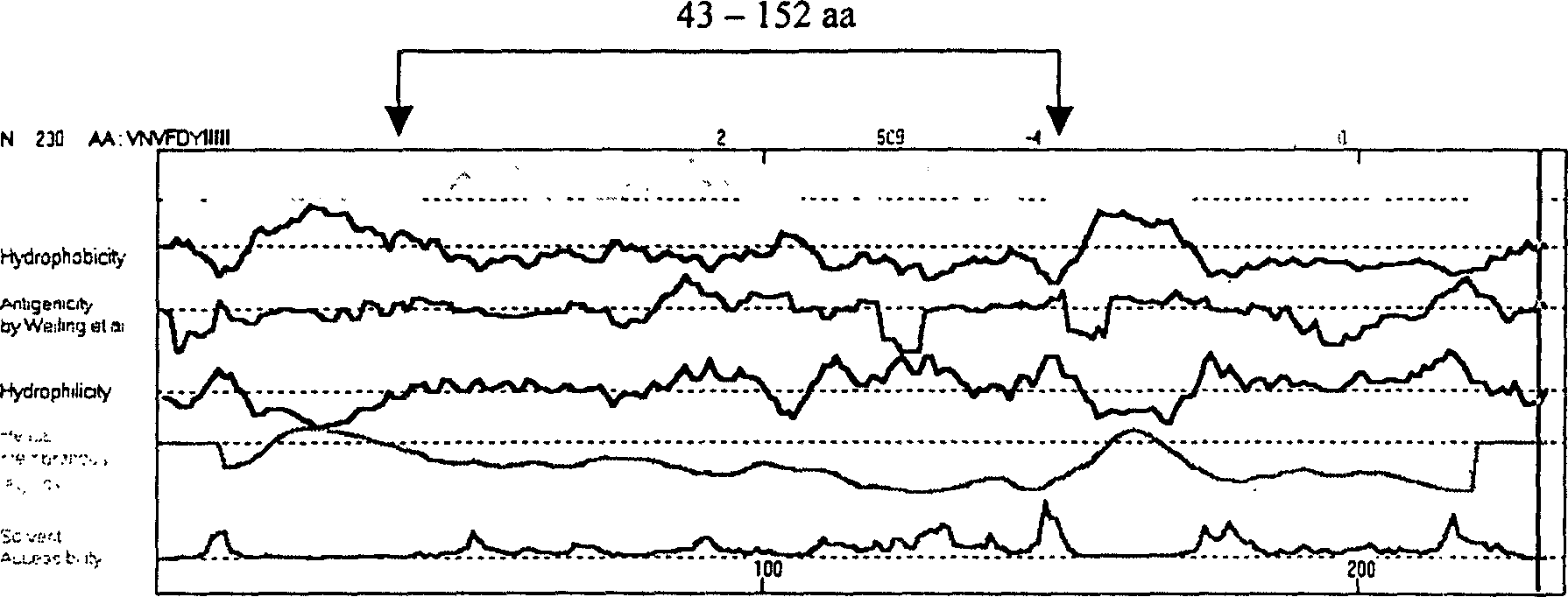
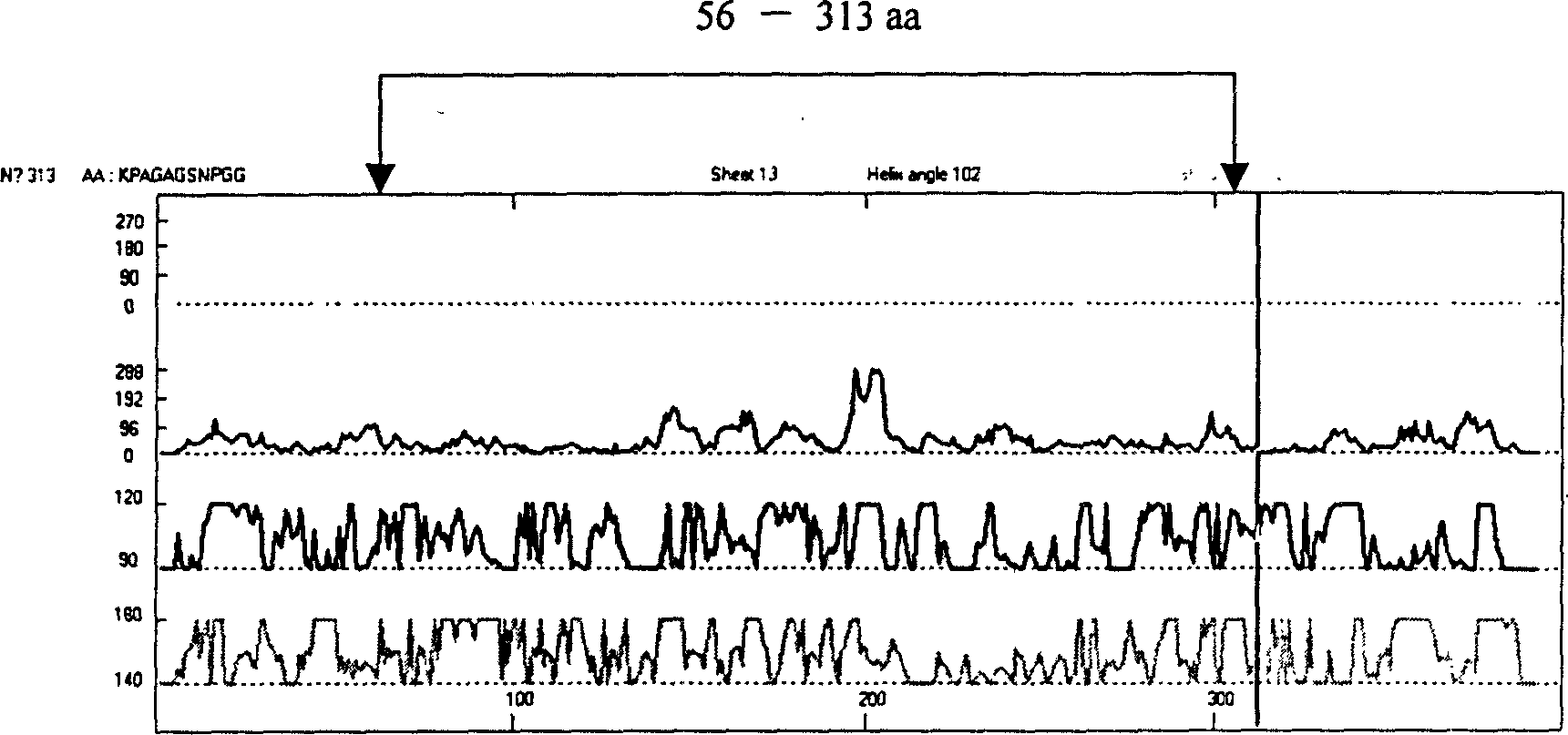
The present invention relates to recombinant toxoplasma fusion protein, its preparation method and application. It utilizes gene engineering technology to prepare a new-type recombinant toxoplasma protein, i.e. the fusion protein formed from 110 amino acids from 43-th amino acid to 152-th amino acid of toxoplasma GRA6 protein N end and 258 amino acids from 56-th amino acid to 313-th amino acid ofP30 protein which are series-connected. The above-mentioned 110 amino acids of GRA6 protein N end is positioned at fusion protein N end, and the 258 amino acids of P30 protein is positioned at C end of the fusion protein, and between them 2 amino acids of Gly and Ser are connected, and one methionine (Met) is added at N end of fusion protein, its total length has 371 amino acids.
Owner:李越希
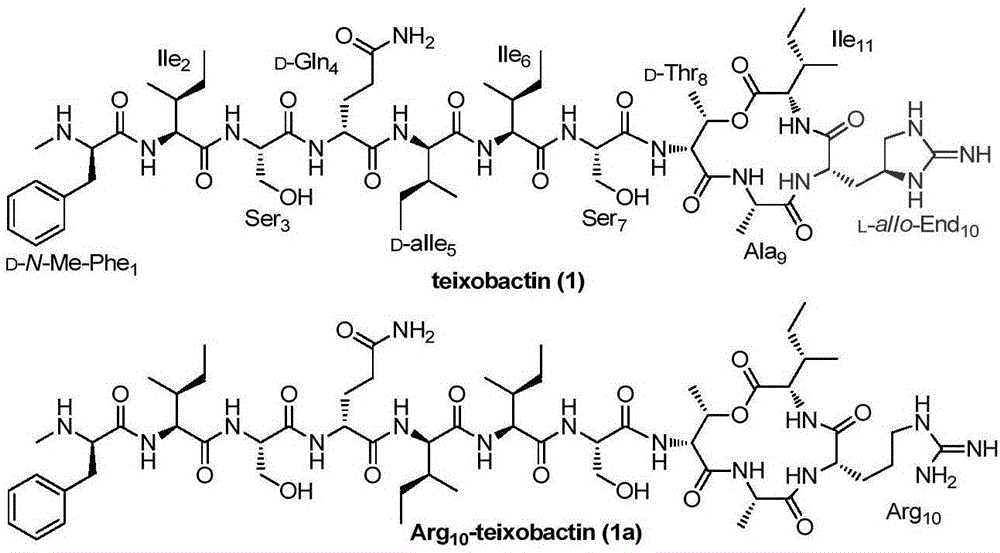
Teixobactin analogs, and preparation method and application thereof
The invention discloses Teixobactin analogs, and a preparation method and application thereof. The preparation method comprises the following steps: 1) sequentially coupling 6-12 amino acids onto a solid-phase synthetic resin with amino groups or diazanyl groups to obtain a straight-chain peptide AA1-AA2-...-AAn-1-AAn-solid-phase synthetic resin, wherein AAn-1 is selected from amino acids with hydroxyl on the side chain; 2) coupling 2 amino acids onto the AAn-1 side chain by condensation to obtain a precursor AA1-AA2-...-AAn-1(OCOAAi-AAii)-AAn-solid-phase synthetic resin; and 3) cracking the solid-phase synthetic resin, and carrying out condensation coupling on AAii and AAn to obtain the cyclization products. The preparation method can be used for preparing the compounds from the simple and cheap raw material amino acids in a simple and fast way.
Owner:SHENZHEN INST OF ADVANCED TECH
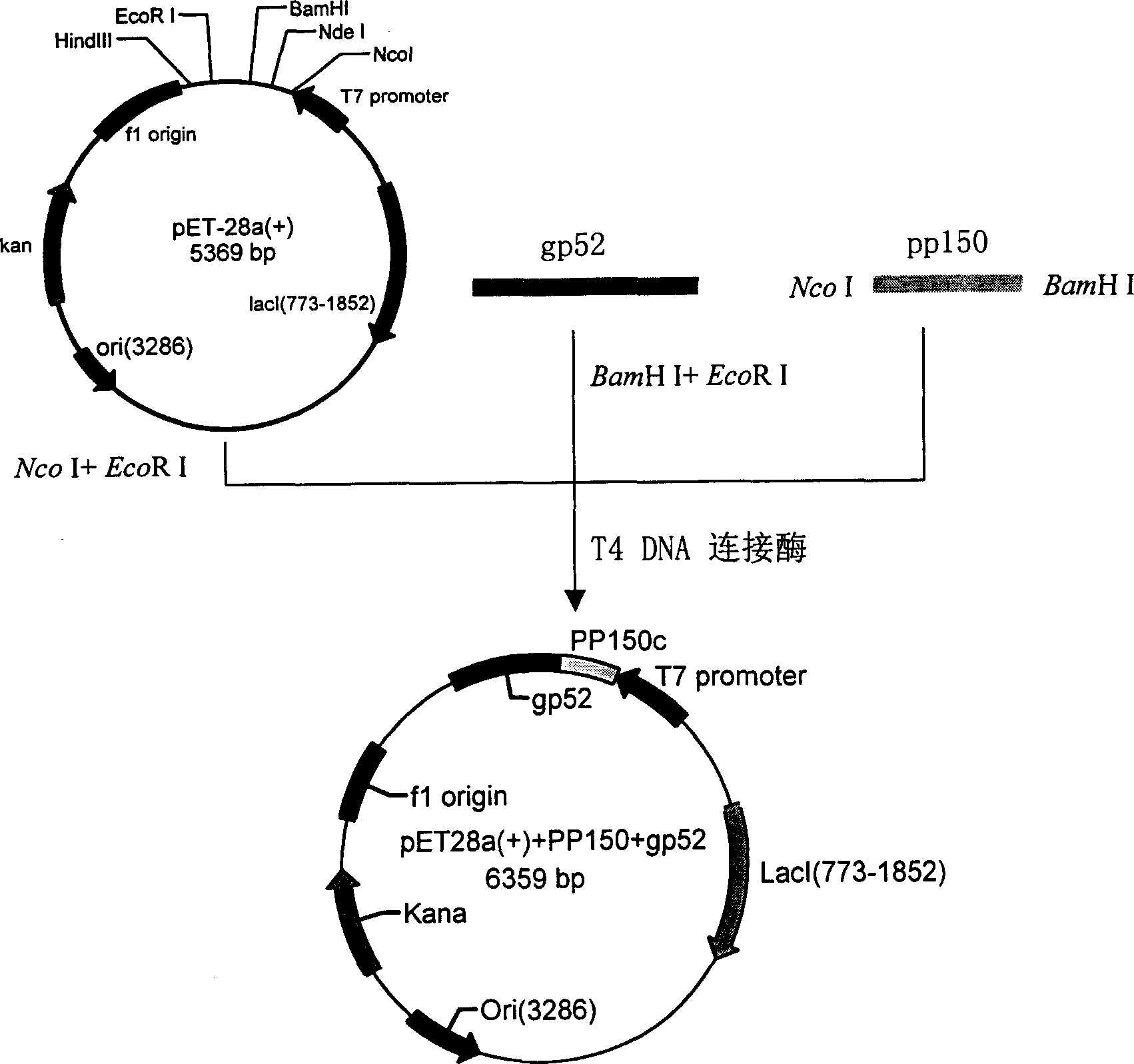
Gene recombinant human cytomegalovirus fusion protein pp150/MDBP, preparation process and application thereof
InactiveCN1763203AHigh expressionHigh yieldPeptide/protein ingredientsBiological testingAntigenC-terminus
The present invention discloses one kind of gene recombinant human cytomegalovirus fusion protein pp150 / MDBP and its preparation process and application, and relates to gene engineering technology, preventing vaccine, diagnosis reagent and other technology. The recombinant fusion protein pp150 / MDBP is fusion protein formed through connecting serially the 197th amino acid in the 495-691 amino acid segment of human cytomegalovirus pp150 and the 64th amino acid in the 538-601 amino acid segment of MDBP protein. The 197th amino acid pp150 in the N-terminal of the fusion protein and the 64th amino acid of MDBP protein in the C-terminal of the fusion protein are connected through two amino acids including one Leu and one Glu, and the fusion protein has one increased Met and has whole length of 264 amino acids. The fusion protein is used in detecting human cytomegalovirus antibody and antigen, preparing antibody and protein chip, vaccine, ELISA detecting kit and other products.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Compounds comprising lpa
InactiveCN1863820AAvoid deathConnective tissue peptidesCell receptors/surface-antigens/surface-determinantsDiseasePathology diagnosis
The present invention relates to new peptide compounds capable of binding to fibroblast growth factor receptor (FGFR), said compounds comprising two individual amino acid sequences, wherein at least one of the two amino acid sequences is capable of binding to FGFR. The invention discloses the amino acid sequences of the compounds and features pharmaceutical compositions comprising thereof. Invention also relates to uses of the compounds and pharmaceutical compositions comprising thereof for the treatment or prevention of different pathological conditions, wherein FGFR plays a role in pathology and / or recovery from the disease. New peptide compounds of the invention are obtainable by the ligand presenting assembly (LPA) method.
Owner:ENKAM PHARMA
High enzyme activity aspartokinase mutant, engineering bacterial strain and preparation method of mutant
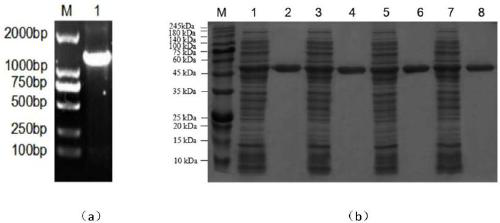
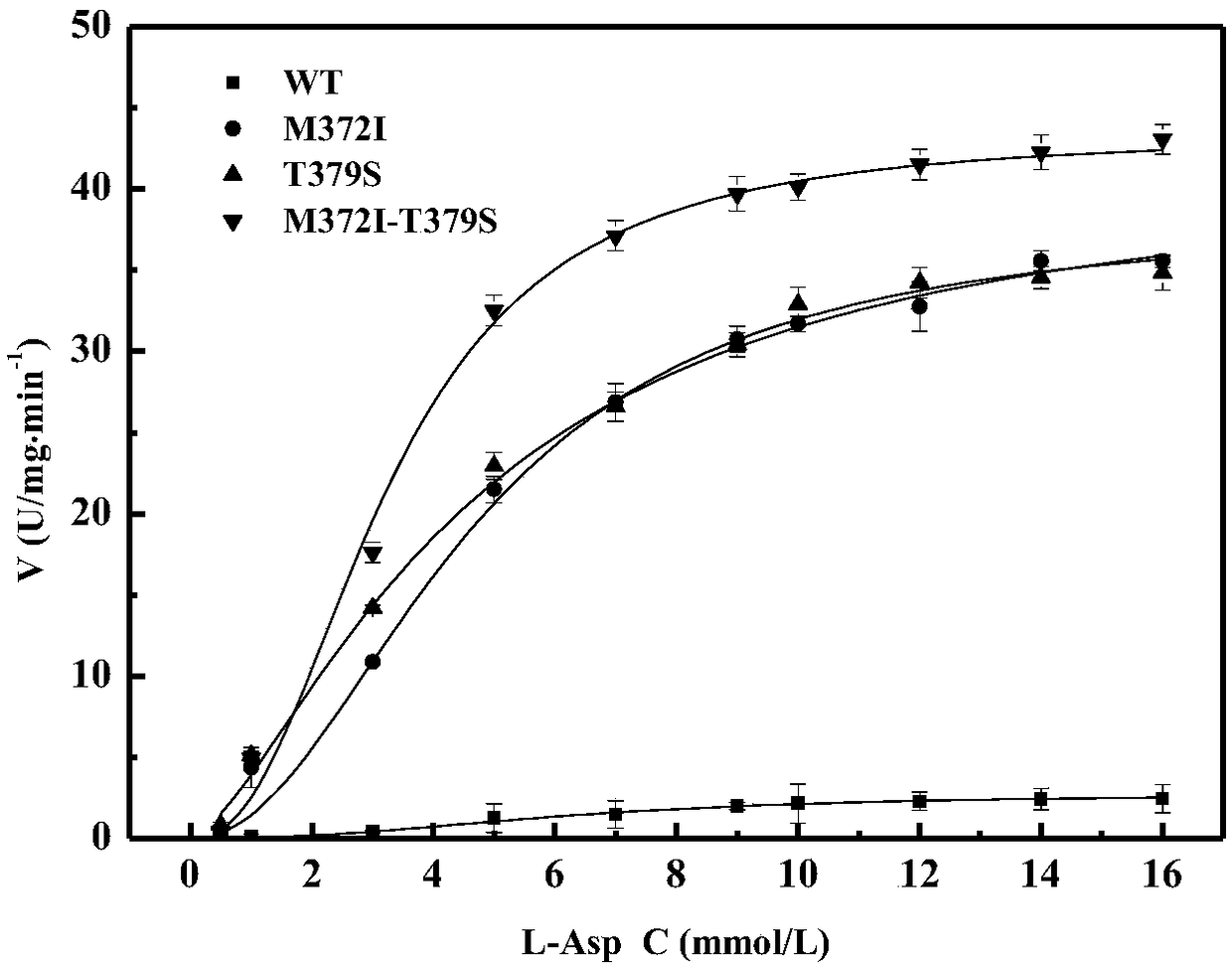
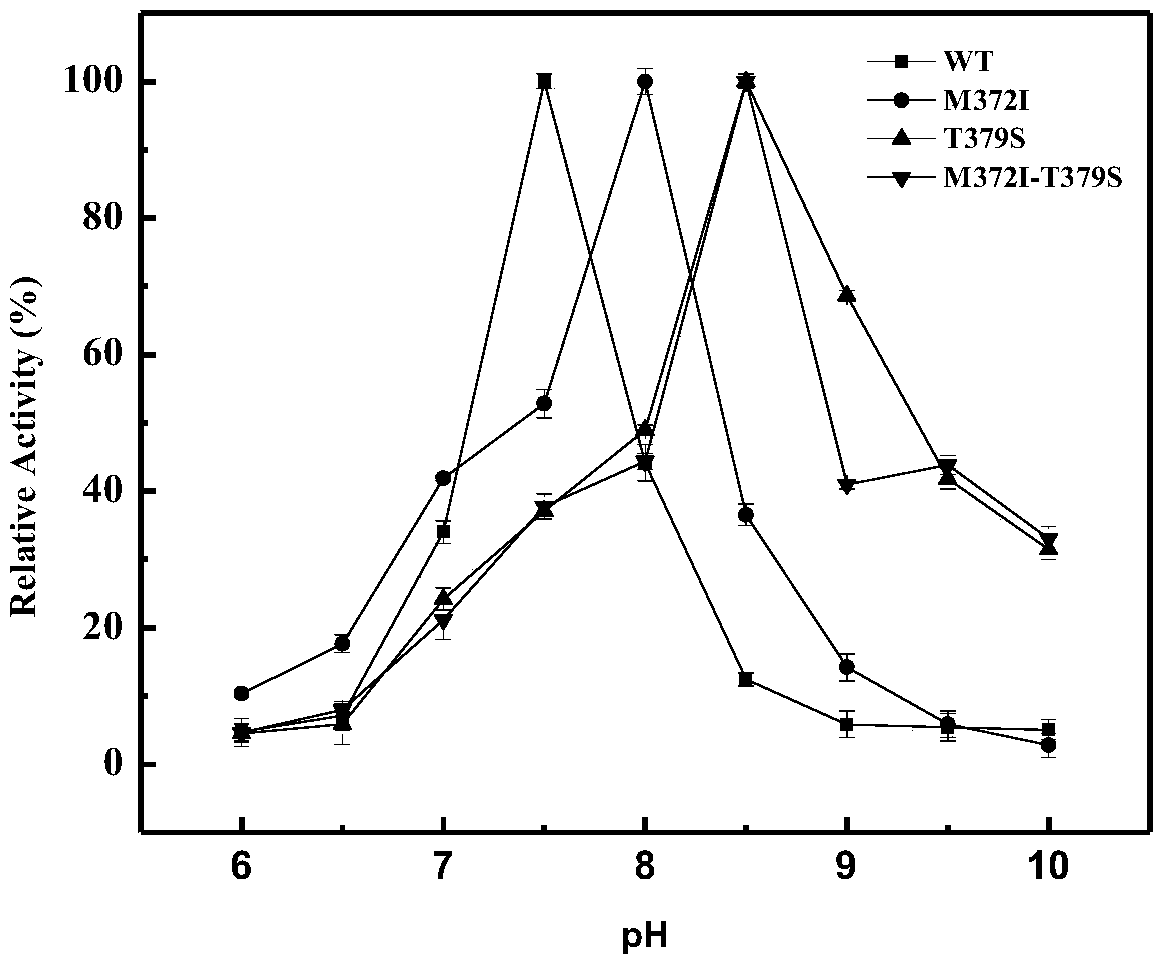
Belonging to the technical field of bioengineering, the invention relates to an aspartokinase mutant, an engineering bacterial strain and a preparation method of the mutant. The high enzyme activity aspartokinase mutant has an amino acid sequence formed by substitution, deletion or addition of 2-10, further 2-6, and even further 2-3 amino acid residues to an exogenous aspartokinase with an amino acid sequence shown as SEQ ID NO:2, and has the functions of aspartokinase. Preferably, the sequence is formed by substitution, deletion or addition of 2 amino acid residues. In particular, preferably,site-specific saturation mutation is carried out to the 372nd and 379th amino acids in the amino acid sequence, and a high throughput screening method is utilized to select the high enzyme activity aspartokinase mutant. The invention further utilizes the electrotransformation method to transfer a pEC-AK recombinant shuttle expression vector of the aspartokinase mutant that has high enzyme activity and can relieve or weaken Lys feedback inhibition effect into Corynebacterium pekinense, finally the recombinant Corynebacterium pekinense M372I-T379S engineering bacterial strain can be obtained, and fermentation product analysis is conducted on the engineering bacterial strain.
Owner:JILIN AGRICULTURAL UNIV
Configuration of cell facter IL-24 eucargon expression carrier and application
InactiveCN1618978AObvious inhibitoryOvert apoptosisGenetic material ingredientsFermentationMelanomaSmall intestine cancer
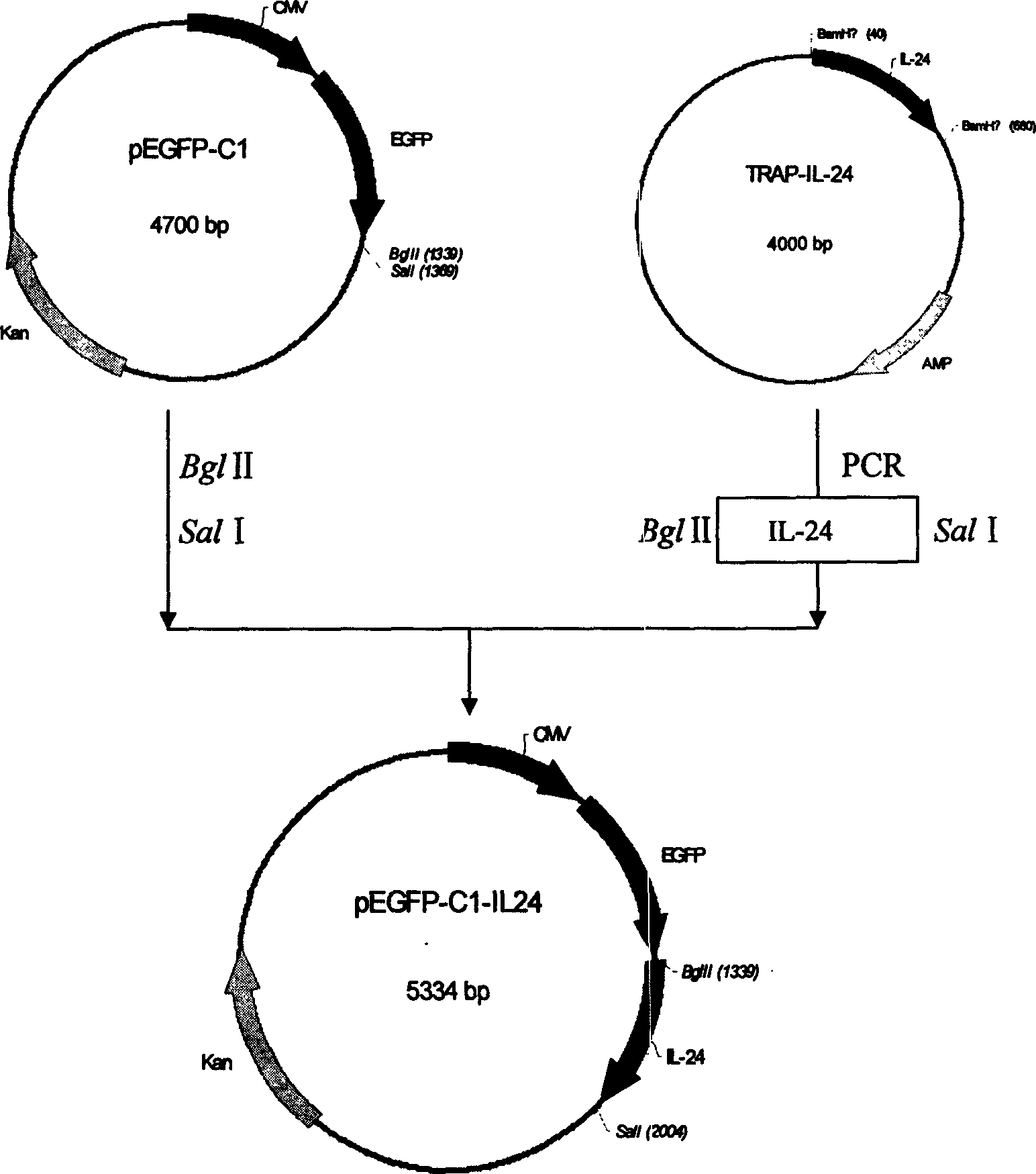
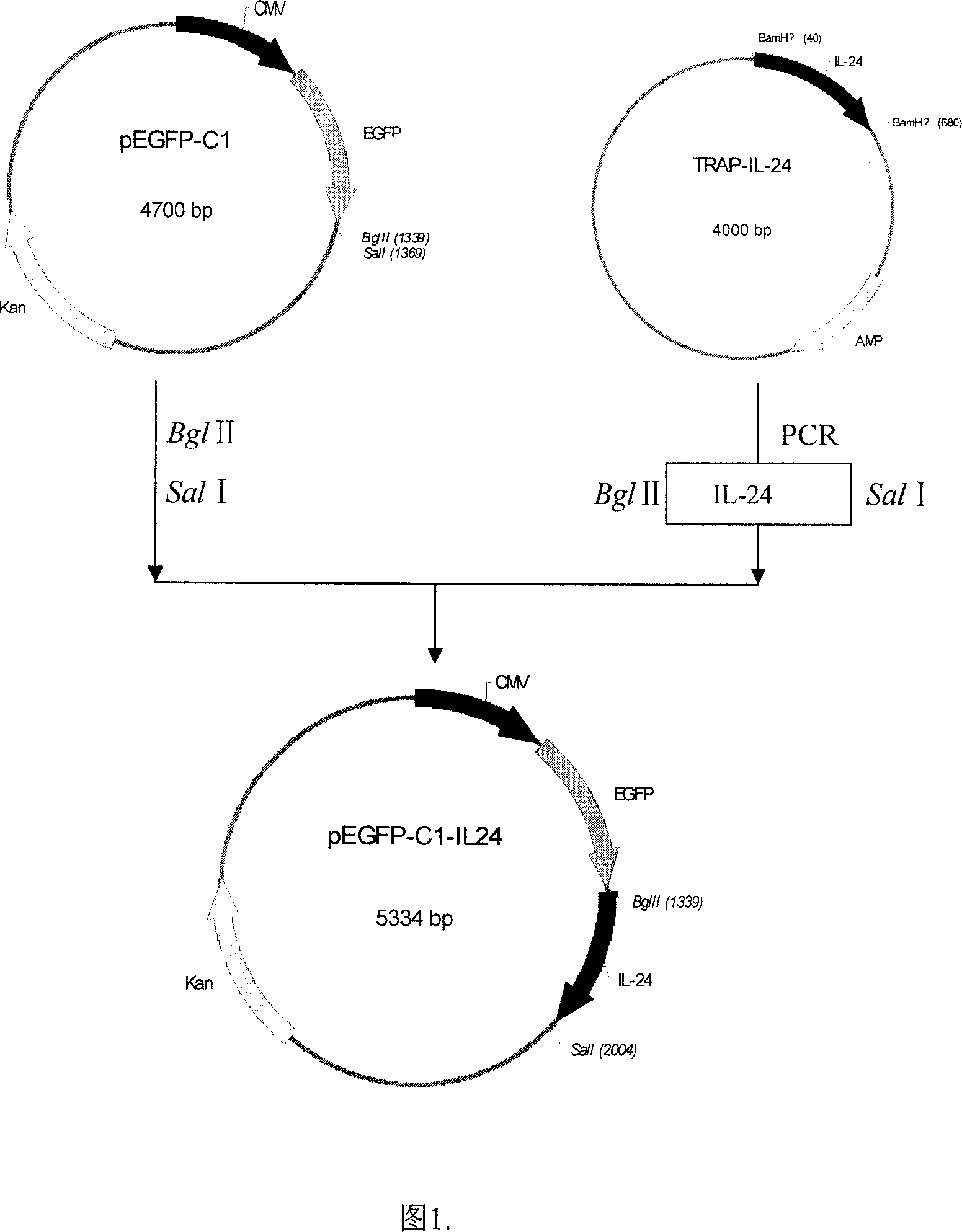
A method for configuring the eucaryotic expression carrier of cell factor IL-24 includes designing the ascending and descending primers, PCR amplifying begining from the second amino acid in the code at human IL-24 N terminal by using TRAPIL-24 plasmid as template, cutting off target band, purifying it with reagent kit, configuring and determining recombinant plasmid pEGFP-CI-IL-24, respectively enzyme-severing the PCR product and carrier pEGFP by Bgl II and Sal I, purifying them by reagent kit, linking fragment, culturing, cloning, determining and sequencing. The recombinant cell factor Il-24 can be used to treat liver cancer, small intestine cancer and melanoma.
Owner:WUHAN UNIV
Peptides and methods using same for diagnosis and treatment of amyloid-associated disease
Peptides having at least 2 amino acids and no more than 15 amino acids are provided. The peptides comprise amino acid sequence X-Y or Y-X, wherein X is an aromatic amino acid and Y is any amino acid other than glycine. Also provided are pharmaceutical compositions and kits including such peptides as well as methods using same for diagnosing and treating amyloid associated diseases.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Recombination human cytomegalovirus fusion protein and its preparing method, application
InactiveCN1431223AStrong specificityImprove the detection rateRecombinant DNA-technologyBiological testingEngineered geneticC-terminus
A novel human cytomegalovirus fusion protein used for detecting human cytomegalovirus antibody or antigen and preparing monoclonal or polyclonal antibody is prepared by recombining human cytomegalvirus PP150 protein with gp52 protein in such manner that the 25 amino acids at c terminal of pp150 and the 164 amino acids at C terminal of gp52 are serially linked via 2 amino acids Gly and Ser.
Owner:李越希 +1
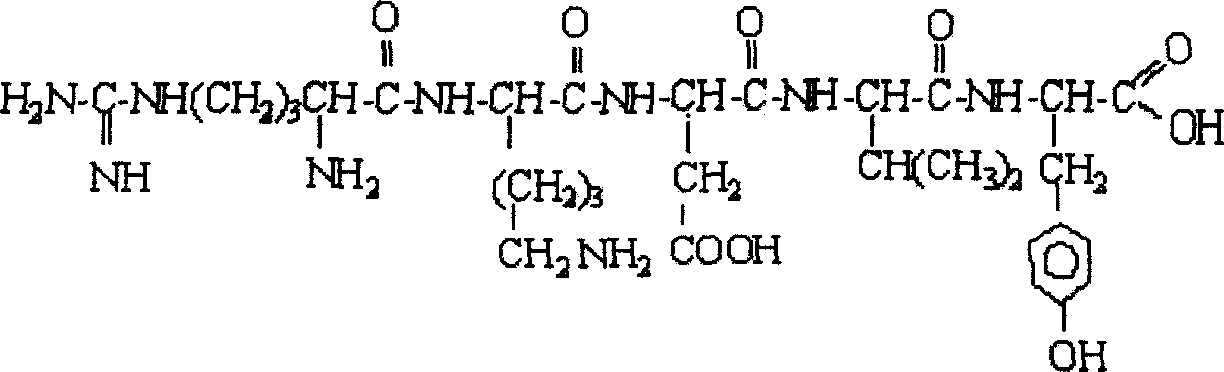
Thymopentapeptide active isomer and application thereof in pharmaceutical preparation
InactiveCN1927879AImprove stabilityProlong half-life in vivoPeptide/protein ingredientsPill deliveryHalf-lifeCurative effect
The present invention is active thymic peptide 5 (TP-5) isomer and its pharmaceutical application, and belongs to the fields of organic chemistry and chemical medicine. Structurally, the active TP-5 isomer has TP-5 amino sequence of Arg-Lys-Asp-Val-Tyr with one or two amino acids substituted by corresponding beta-amino acids or gamma-amino acids. It is prepares through a conventional Fmoc solid peptide synthesizing process. Phamacodynamical experiment shows that the active TP-5 isomer possesses obvious immunological enhancement effect, antitumor effect and antioxidant effect. Extracorporeal activity and stability measurement shows that the active TP-5 isomer has activity not lower than that of TP-5, raised stability, longer half life and thus enhanced curative effect. The present invention provides theoretic base for the research on the activity mechanism of thymic peptide and the development of new medicine.
Owner:JILIN UNIV
Recombinant Toxoplasma fusion protein antigen and preparation process and use thereof
InactiveCN1194991CAntigen specificity is not strongProne to false positivesBiological testingHybrid peptidesAntigenGlycine
The present invention relates to recombinant toxoplasma fusion protein, its preparation method and application. It utilizes gene engineering technology to prepare a new-type recombinant toxoplasma protein, i.e. the fusion protein formed from 110 amino acids from 43-th amino acid to 152-th amino acid of toxoplasma GRA6 protein N end and 258 amino acids from 56-th amino acid to 313-th amino acid of P30 protein which are series-connected. The above-mentioned 110 amino acids of GRA6 protein N end is positioned at fusion protein N end, and the 258 amino acids of P30 protein is positioned at C end of the fusion protein, and between them 2 amino acids of Gly and Ser are connected, and one methionine (Met) is added at N end of fusion protein, its total length has 371 amino acids.
Owner:李越希
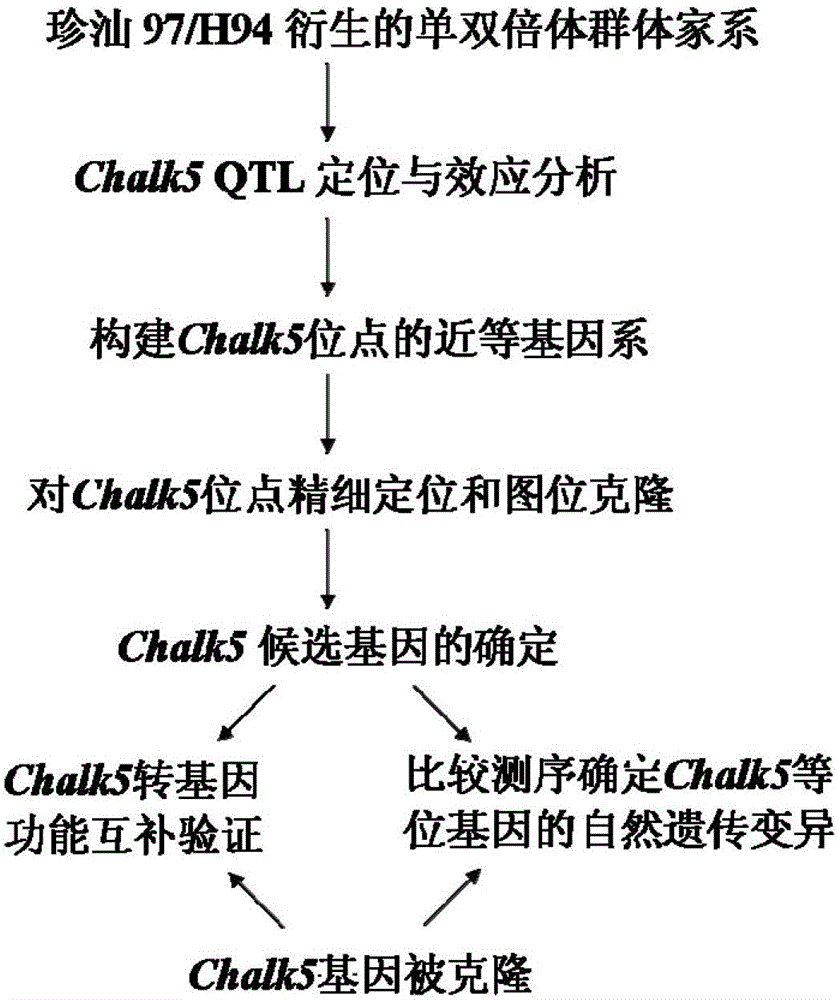
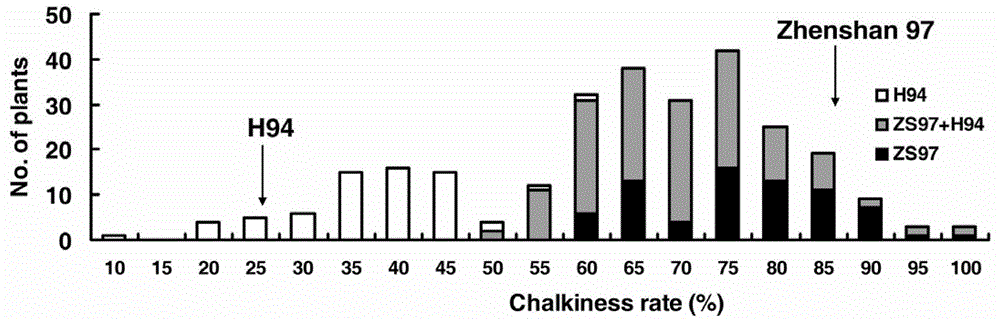
Cloning and application of major gene Chalk5 for chalkiness rate of paddy rice
The invention belongs to the technical field of plant genetic engineering. The invention discloses the DNA sequences of a separated and cloned major gene Chalk5 for regulating and controlling chalkiness characters of paddy rice and an allele (Chalk5-H94) of the major gene Chalk5, wherein the nucleotide sequence of the major gene is represented by SEQ ID No. 1, and the nucleotide sequence of the allele of the major gene is represented by SEQ ID No.3 in a sequence table. A high-chalkiness variety and two low-chalkiness varieties of paddy rice are utilized for comparison and sequencing, and it is discovered that the high-chalkiness variety and the low-chalkiness varieties have 39 common base differences in a whole-genome range, wherein 10 mutations are located in a promoter region, and 5 mutations are located in a coding region, resulting in changes of two amino acids. According to the invention, transgenic technology is employed to obtain paddy rice plants with a trans-Chalk5 gene, and the chalkiness rate of the transgenic plants is substantially increased compared with that of control groups. Character changes of the transgenic plants are identical to those of two genotypes of a nearly isogenic line of Chalk5 of Zhenshan 97.
Owner:HUAZHONG AGRI UNIV
Self-assembled polypeptide nanorod and preparation method thereof
ActiveCN106699841AUniform sizeSpecial structurePeptide preparation methodsBulk chemical productionSide chainStructural formula
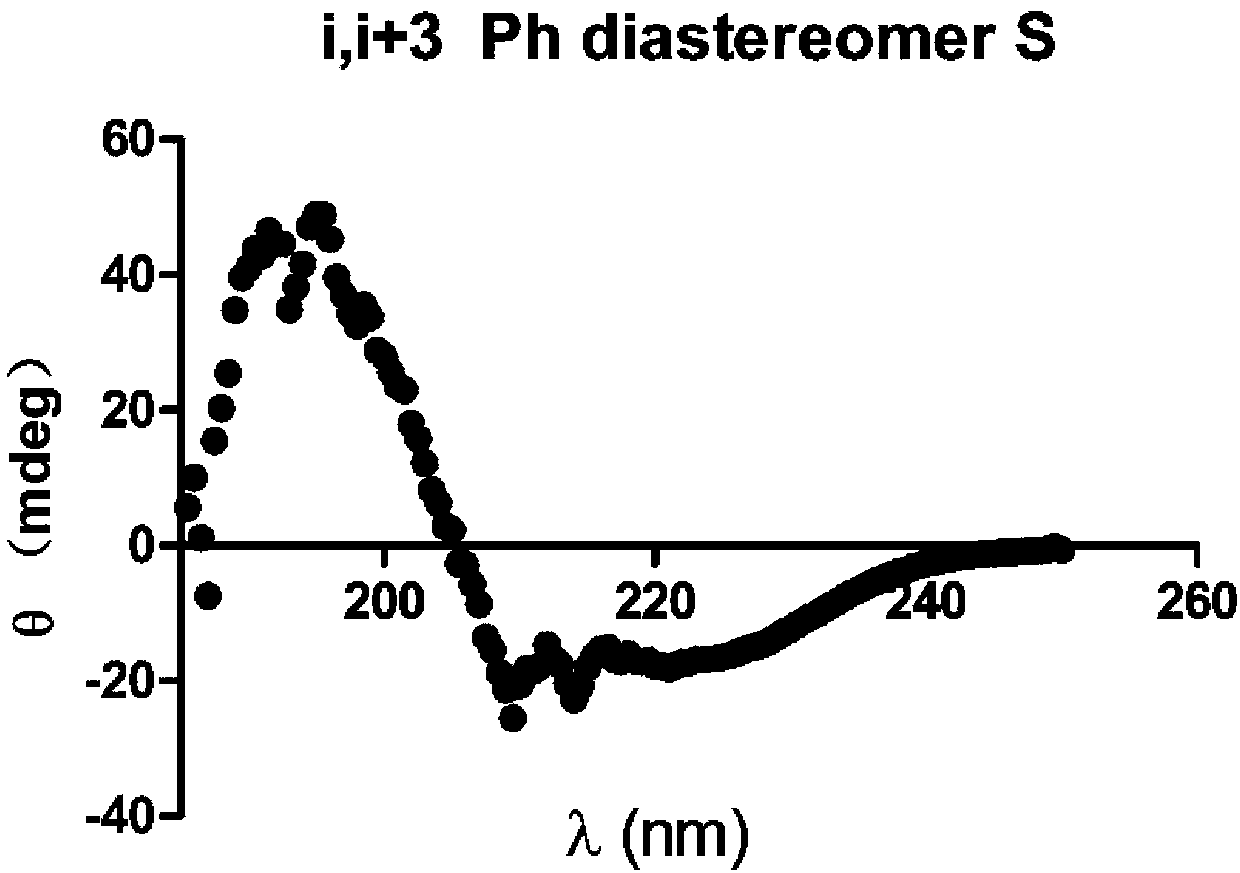
The invention provides a self-assembled polypeptide nanorod. The self-assembled polypeptide nanorod has the structural formula as follows: FORMULA or FORMULA. The invention further provides a preparation method of the self-assembled polypeptide nanorod. The preparation method comprises the following steps: synthesizing non-natural amino acid with side chain aromatic ring substituents and terminal olefins; connecting the carboxyl terminal of the amino acid with the non-natural amino acid, continuing to connecting two amino acids, then connecting cysteine, removing a thiol protecting group of the cysteine from a product, and then performing an intramolecular thiol-ene reaction to obtain a side chain 2-position carbon chiral modified polypeptide compound, wherein the position of carbon chiral side chain coupling amino acid is i / i+3; cutting down polypeptide from resin to obtain a white powdery solid; dispersing the white powdery solid by using ultrapure water and performing ultrasonic treatment to obtain the nanorod.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
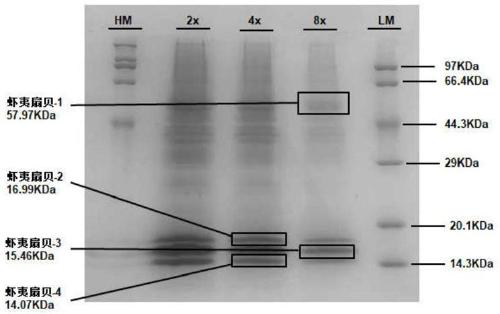
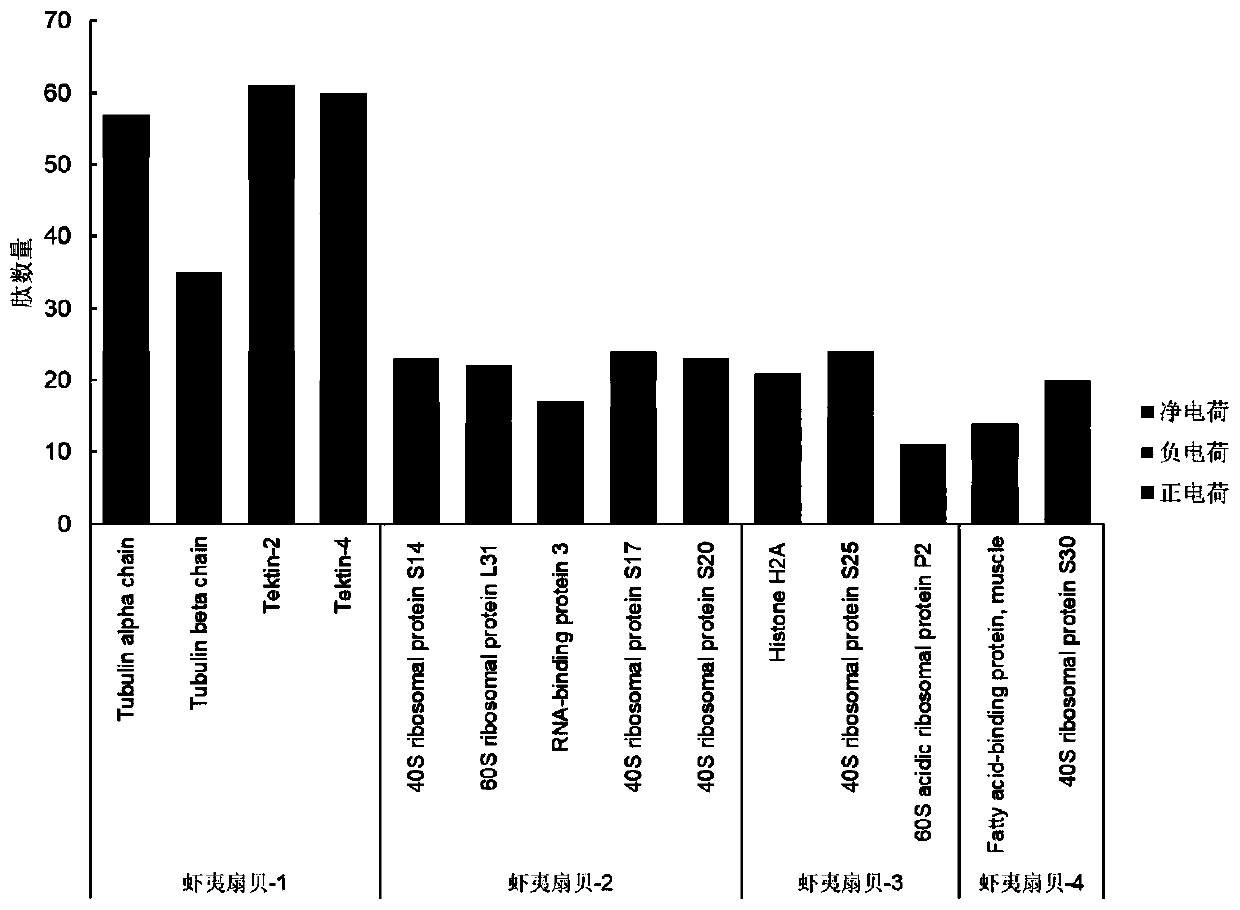
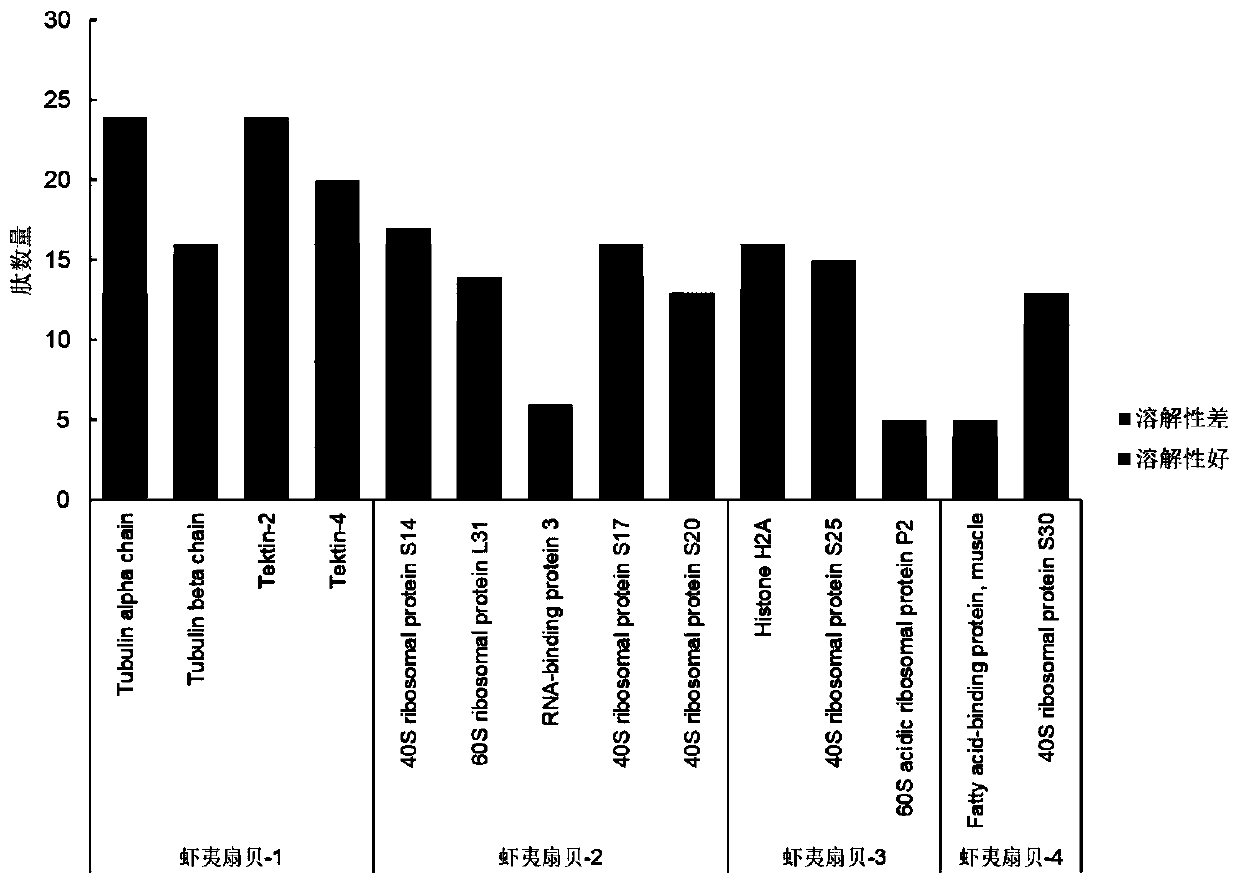
Comb shell oligopeptide, virtual screening method thereof and preparation method of composite gel of comb shell oligopeptide
ActiveCN110156871AReduce utilizationIncrease profitClimate change adaptationPeptidesCarrageenanVirtual screening
The invention discloses comb shell oligopeptide. The comb shelloligopeptide is composed of an amino acid residue A and an amino acid residue K, and the molecular weight is 217.27 Da. The comb shell oligopeptide and lota-carrageenan are mixed at a certain proportion, and composite gel is prepared through the method of heating, swelling and cooling and gelling. Meanwhile, the invention discloses a virtual screeningmethod of the comb shell oligopeptide. The comb shell oligopeptide is obtained through the steps of raw material pretreatment, SDS-PAGE analysis, protein mass spectrum identification,protein sequence screening, in silico analysis, target peptide screening and synthetic peptide preparation. The method is simple in step, convenient to operate and economical, and saves time and labor, the prepared composite gel has the advantages of being high in elasticity performance and high in strength, has high nutritive valueand can serve as a raw material of the edible colloid industry, the variety of ediblecolloid products is enriched, absorption of the composite gel by the human body is easy, and wide applicationprospects are achieved.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Glucose oxidase mutant
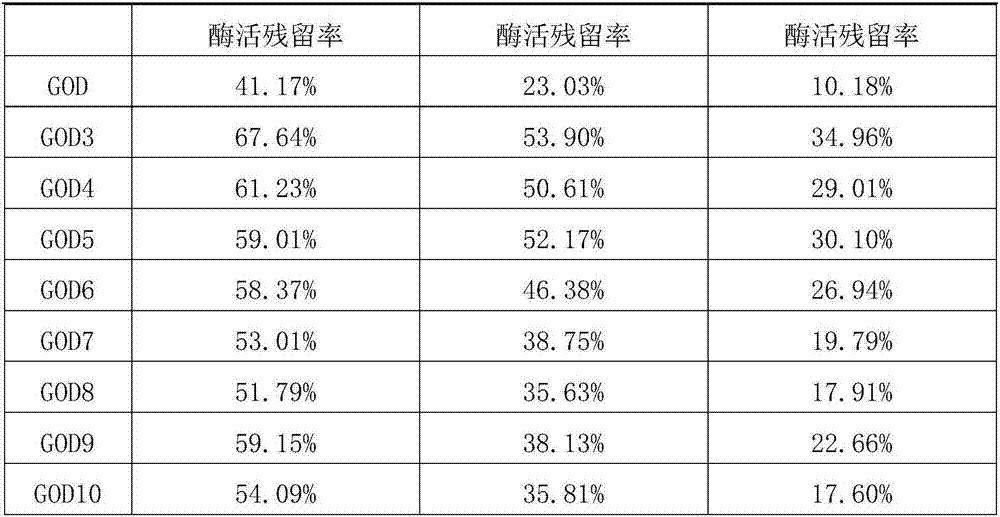
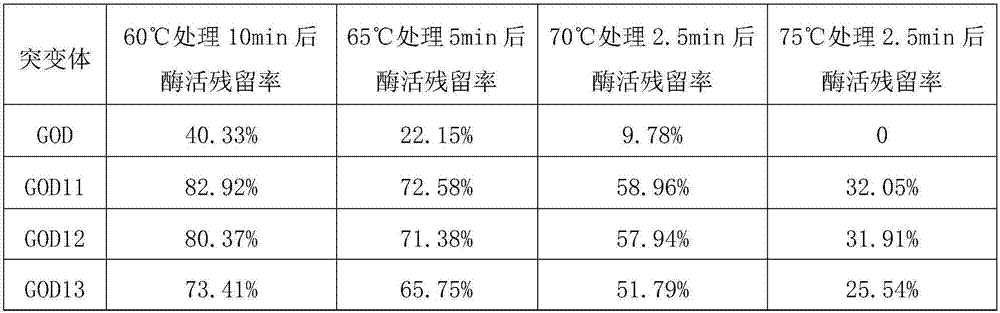
ActiveCN107988177AImprove heat resistanceAccessory food factorsOxidoreductasesEpitopeAfter treatment
The invention aims to provide a glucose oxidase mutant. The glucose oxidase mutant contains (I) a sequence which is at least 70% homologous with the amino acid sequence, shown as SEQ ID NO:1, of glucose oxidase, as well as (II) a sequence containing at least one immune epitope of the glucose oxidase in (I),and the amino acid sequence of the glucose oxidase is obtained by modification, substitution, deletion or addition of one or more amino acids, and substitution refers to substitution for one or two amino acids. The heat resistance of the single-point mutant of the glucose oxidase is generally higher than that of wild glucose oxidase, the heat resistance of a two-point mutant is further improved as compared with the corresponding single-point mutant, residual enzyme activity after treatment at 60 DEG C for 10 min reaches 71.19%-82.92%, residual enzyme activity after treatment at 65 DEG C for 5 min reaches 63.03%-72.58%, residual enzyme activity after treatment at 70 DEG C for 2.5 minreaches 49.91%-58.96%, and residual enzyme activity after treatment at 75 DEG C for 2.5 min reaches 24.78%-32.05%.
Owner:WEIFANG KANGDIEN BIOTECH +2
Mutant enzyme and application thereof
ActiveUS8883434B2Increase probabilitySimple structureSugar derivativesBacteriaGlucose dehydrogenase activityMicroorganism
An object is to provide a novel enzyme that exhibits glucose dehydrogenase activity. Furthermore, another object is to provide a novel method pertaining to enzyme modification. Provided is a mutated enzyme containing an amino acid sequence wherein one or at least two amino acids selected from the group consisting of (1) to (13) are substituted with another amino acid in the amino acid sequence of a microorganism-derived glucose oxidase: (1) the amino acid corresponding to the amino acid at position 115 of the amino acid sequence of SEQ ID NO: 1; (2) the amino acid corresponding to the amino acid at position 131 of the amino acid sequence of SEQ ID NO: 1; (3) the amino acid corresponding to the amino acid at position 132 of the amino acid sequence of SEQ ID NO: 1; (4) the amino acid corresponding to the amino acid at position 193 of the amino acid sequence of SEQ ID NO: 1; (5) the amino acid corresponding to the amino acid at position 353 of the amino acid sequence of SEQ ID NO: 1; (6) the amino acid corresponding to the amino acid at position 436 of the amino acid sequence of SEQ ID NO: 1; (7) the amino acid corresponding to the amino acid at position 446 of the amino acid sequence of SEQ ID NO: 1; (8) the amino acid corresponding to the amino acid at position 472 of the amino acid sequence of SEQ ID NO: 1; (9) the amino acid corresponding to the amino acid at position 511 of the amino acid sequence of SEQ ID NO: 1; (10) the amino acid corresponding to the amino acid at position 535 of the amino acid sequence of SEQ ID NO: 1; (11) the amino acid corresponding to the amino acid at position 537 of the amino acid sequence of SEQ ID NO: 1; (12) the amino acid corresponding to the amino acid at position 582 of the amino acid sequence of SEQ ID NO: 1; and (13) the amino acid corresponding to the amino acid at position 583 of the amino acid sequence of SEQ ID NO: 1.
Owner:AMANO ENZYME INC
Short peptide for inhibiting human hepatitis B virus infection and its application
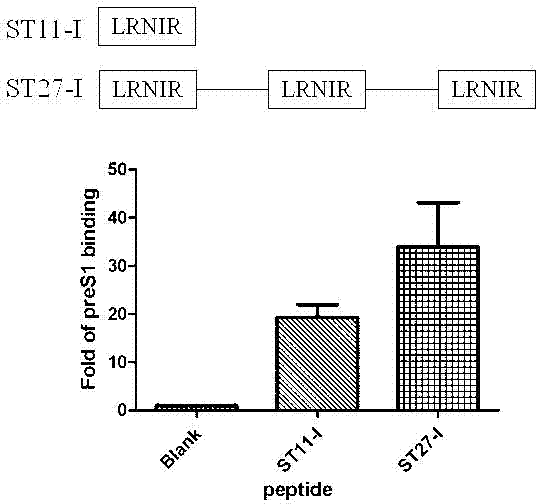
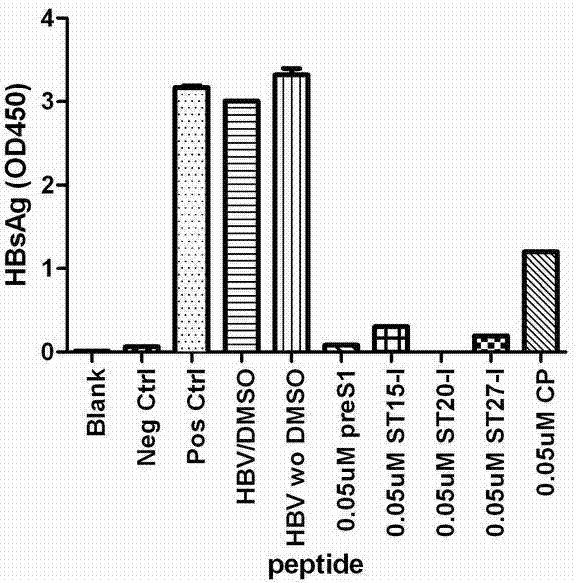
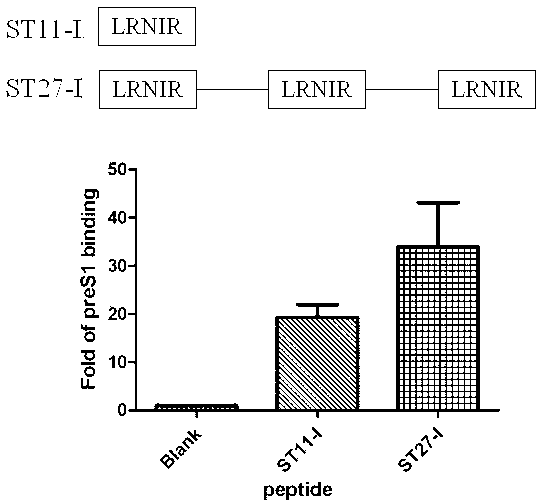
ActiveCN102775470AHigh suppression efficiencyStrong specificityBacteriaDigestive systemArginineBiomedicine
The invention belongs to the biomedicine field, and relates to a short peptide for inhibiting human hepatitis B virus infection. The short peptide comprises anyone of the following amino acid sequences: 1, N-leucine-arginine-asparagine-isoleucine-arginine-C; 2, an amino acid sequence obtained through deleting one or two amino acids from the above amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 3, an amino acid sequence obtained through substituting one or two amino acids in the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 4, adding one or more amino acids to the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; and 5, an amino acid sequence obtained through repeated splicing of the amino acid sequences 1-4 and capable of inhibiting the human hepatitis B virus infection. The invention also provides a construct and a cell of an amino acid sequence comprising the short peptide or of a nucleic acid coding sequence of the short peptide, and an application of the short peptide.
Owner:FUDAN UNIV
Pro-drug peptide with improved pharmaceutical properties
The present disclosure relates to a pro-drug peptide, or a salt thereof, having improvement for at least one biological property relative to a parent peptide or peptidomimetic, wherein the biological property is selected from the group consisting of therapeutic index, stability, solubility, toxicity, adsorption, and pre-systemic metabolism. The pro-drug peptide comprising the following structure: Z-pep, wherein: pep is the parent peptide or peptidomimetic; Z is a sequence of n amino acids, Z is cleaved in vivo releasing pep; n≥2 amino acids. The present disclosure also relates to methods of making and using the pro-drug peptide of the present disclosure. For example, the present disclosure describes a pro-drug peptide that may be used to prevent, treat, or ameliorate at least one symptom of hypoglycemia or a hypoglycemia-related disease or disorder.
Owner:UREKA
T-CELL RECEPTORS WHICH RECOGNISE FRAMESHIFT MUTANTS OF TGFbetaRII
The present invention relates to TCR molecules which recognise neopeptides produced as a result of the cancer-associated '-1A' frameshift mutation in human TGFbetaRII. The TCR molecules are capable ofbinding a peptide of SEQ ID NO: 1 when said peptide is presented by a Class I MHC, and comprise an alpha-chain domain and a beta-chain domain, each chain domain comprising three CDR sequences, wherein a) CDRs 1, 2 and 3 of the alpha-chain domain have the sequences of SEQ ID NOs: 2, 3 and 4 respectively; and b) CDRs 1, 2 and 3 of the beta-chain domain have the sequences of SEQ ID NOs: 5, 6 and 7 respectively, and wherein one or more of said CDR sequences may optionally be modified by substitution, addition or deletion of 1 or 2 amino acids. Nucleic acid molecules encoding such TCRs are provided, as are soluble TCR molecules with these CDR sequences. The nucleic acid molecules of the invention can be used to modify immune effector cells to express a TCR as defined herein, and such modifiedimmune effector cells are useful in therapy for cancer, as are soluble TCRs as defined above.
Owner:UNIV OSLO HF
Polymeric biosurfactants
InactiveCN101600441AAntibacterial agentsPeptide/protein ingredientsCritical micelle concentrationAcyl group
The present invention is directed to biosurfactants that can self-assemble or auto- aggregate into polymeric micellar structures and their use in topically-applied dermatologic products. The invention relates in particular to polymeric acylated biosurfactants (PABs) conforming to the formula Acyl-AA-Term where Acyl is an 8- to 22-membered carbon chain, branched or unbranched, saturated or unsaturated, AA is a consecutive sequence of four to nine amino acid residues, where at least one, preferably at least two of the amino acid residues is charged, and Term is an acid C-terminus or an amide C-terminus. PABs of the present invention have low critical micelle concentrations (predominantly less than about 100 ppm) in an aqueous environment of Minimal Essential Media (MEM) and can lower the surface tension in the aqueous MEM environment to less about 50 dynes / cm2. They also have the ability to increase metabolic soluble proteins.
Owner:THERAPEUTIC PEPTIDES
Method for producing blattella germanica allergen Bla g 7 protein in bacilliform virus-insect expression system
InactiveCN101492692BSolve the complicated purification processMicroorganism based processesFermentationBacteroidesTotal rna
The invention discloses a method for producing German cockroach allergen Bla g 7 protein in a rhabdovirus-insect expression system, comprising the following steps: total RNA is extracted from the tissue of the German cockroach, the total RNA is reversely transcripted into cDNA, a coding gene of German cockroach allergen Bla g 7 is obtained by the method of PCR; the coding gene is cloned to a rhabdovirus carrier, insect cells are transformed for expression; the method of affinity chromatography is adopted for purification, thus obtaining the German cockroach allergen Bla g 7 protein. The protein is different from Bla g 7 protein of foreign sources in terms of 2 amino acid locuses. In the invention, the rhabdovirus-insect expression system is adopted to produce German cockroach allergen Blag 7 protein, thus avoiding influence on Bla g 7 allergen structures in different expression systems (such as bacterial and microzyme); in addition, the invention provides the method of affinity chromatography, thus solving the problem that the Bla g 7 allergen has complex purification process.
Owner:JIANGSU PROVINCE HOSPITAL
Short peptide for inhibiting human hepatitis B virus infection and its application
ActiveCN102775470BHigh suppression efficiencyStrong specificityBacteriaDigestive systemArginineTert-leucine
The invention belongs to the biomedicine field, and relates to a short peptide for inhibiting human hepatitis B virus infection. The short peptide comprises anyone of the following amino acid sequences: 1, N-leucine-arginine-asparagine-isoleucine-arginine-C; 2, an amino acid sequence obtained through deleting one or two amino acids from the above amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 3, an amino acid sequence obtained through substituting one or two amino acids in the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; 4, adding one or more amino acids to the amino acid sequence 1 and capable of inhibiting the human hepatitis B virus infection; and 5, an amino acid sequence obtained through repeated splicing of the amino acid sequences 1-4 and capable of inhibiting the human hepatitis B virus infection. The invention also provides a construct and a cell of an amino acid sequence comprising the short peptide or of a nucleic acid coding sequence of the short peptide, and an application of the short peptide.
Owner:FUDAN UNIV
Configuration of cell facter IL-24 eucargon expression carrier and application
InactiveCN1322135CGrowth Prevention and TreatmentPrevent relapseGenetic material ingredientsFermentationMelanomaSmall intestine cancer
A method for configuring the eucaryotic expression carrier of cell factor IL-24 includes designing the ascending and descending primers, PCR amplifying begining from the second amino acid in the code at human IL-24 N terminal by using TRAPIL-24 plasmid as template, cutting off target band, purifying it with reagent kit, configuring and determining recombinant plasmid pEGFP-CI-IL-24, respectively enzyme-severing the PCR product and carrier pEGFP by Bgl II and Sal I, purifying them by reagent kit, linking fragment, culturing, cloning, determining and sequencing. The recombinant cell factor Il-24 can be used to treat liver cancer, small intestine cancer and melanoma.
Owner:WUHAN UNIV
Carboxyl-terminal specific anti-human amyloid protein monoclonal antibody gene and its encoded polypeptide and application
Owner:BEIJING JIAOTONG UNIV
Thymopentapeptide active isomer and application thereof in pharmaceutical preparation
InactiveCN1927879BImprove stabilityExtended half-lifePeptide/protein ingredientsPill deliveryHalf-lifeCurative effect
The present invention is active thymic peptide 5 (TP-5) isomer and its pharmaceutical application, and belongs to the fields of organic chemistry and chemical medicine. Structurally, the active TP-5 isomer has TP-5 amino sequence of Arg-Lys-Asp-Val-Tyr with one or two amino acids substituted by corresponding beta-amino acids or gamma-amino acids. It is prepares through a conventional Fmoc solid peptide synthesizing process. Phamacodynamical experiment shows that the active TP-5 isomer possesses obvious immunological enhancement effect, antitumor effect and antioxidant effect. Extracorporeal activity and stability measurement shows that the active TP-5 isomer has activity not lower than that of TP-5, raised stability, longer half life and thus enhanced curative effect. The present invention provides theoretic base for the research on the activity mechanism of thymic peptide and the development of new medicine.
Owner:JILIN UNIV
Method for producing blattella germanica allergen Bla g 7 protein in bacilliform virus-insect expression system
InactiveCN101492692ASolve the complicated purification processAvoid problems with allergen structure influencesMicroorganism based processesGenetic engineeringBacteroidesTotal rna
The invention discloses a method for producing German cockroach allergen Bla g 7 protein in a rhabdovirus-insect expression system, comprising the following steps: total RNA is extracted from the tissue of the German cockroach, the total RNA is reversely transcripted into cDNA, a coding gene of German cockroach allergen Bla g 7 is obtained by the method of PCR; the coding gene is cloned to a rhabdovirus carrier, insect cells are transformed for expression; the method of affinity chromatography is adopted for purification, thus obtaining the German cockroach allergen Bla g 7 protein. The protein is different from Bla g 7 protein of foreign sources in terms of 2 amino acid locuses. In the invention, the rhabdovirus-insect expression system is adopted to produce German cockroach allergen Bla g 7 protein, thus avoiding influence on Bla g 7 allergen structures in different expression systems (such as bacterial and microzyme); in addition, the invention provides the method of affinity chromatography, thus solving the problem that the Bla g 7 allergen has complex purification process.
Owner:JIANGSU PROVINCE HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com