Compounds comprising lpa
A compound and selected technology are applied in the direction of medical preparations containing active ingredients, hybrid peptides, chemical instruments and methods, etc., to achieve the effect of preventing myocardial cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
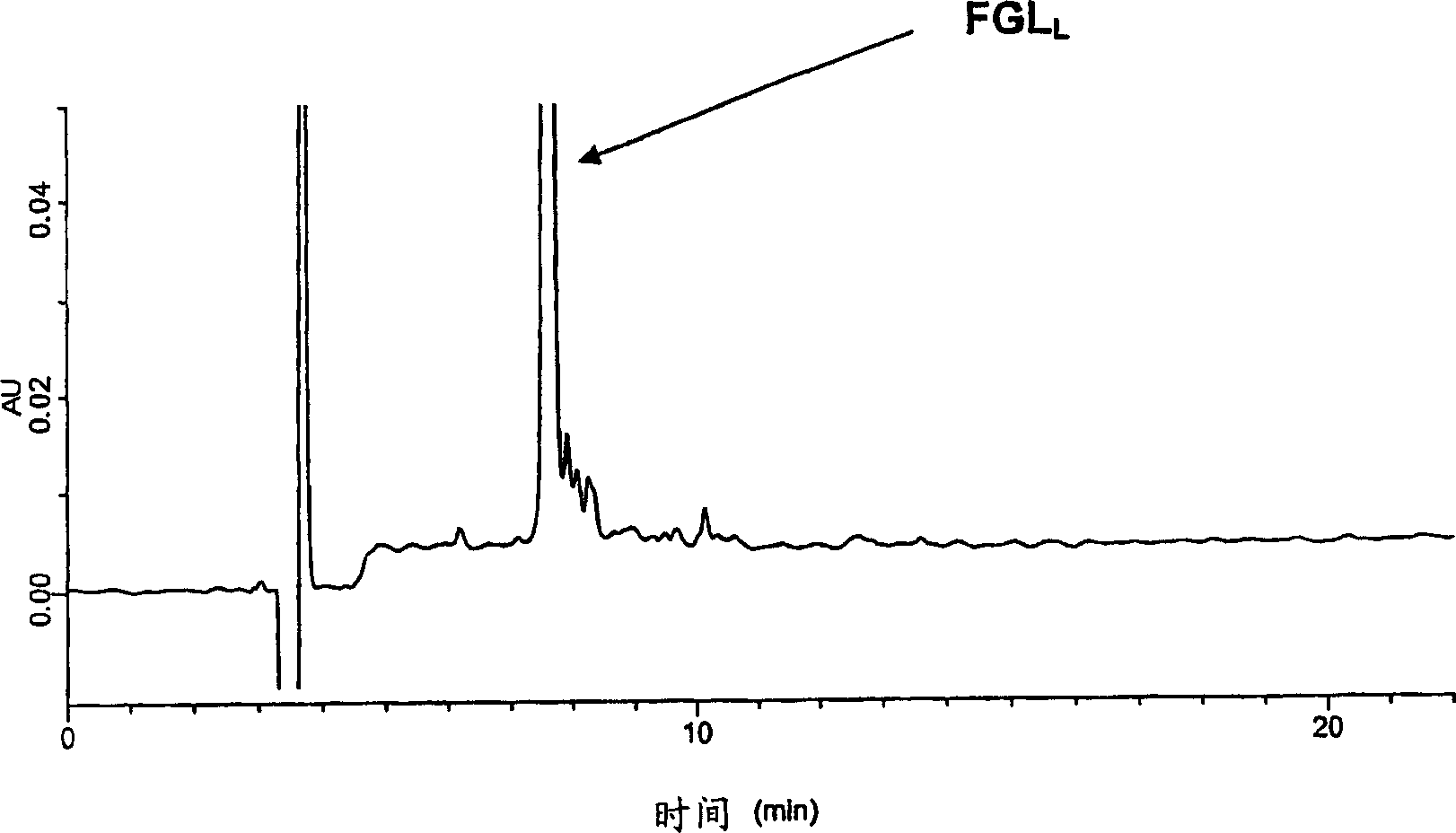
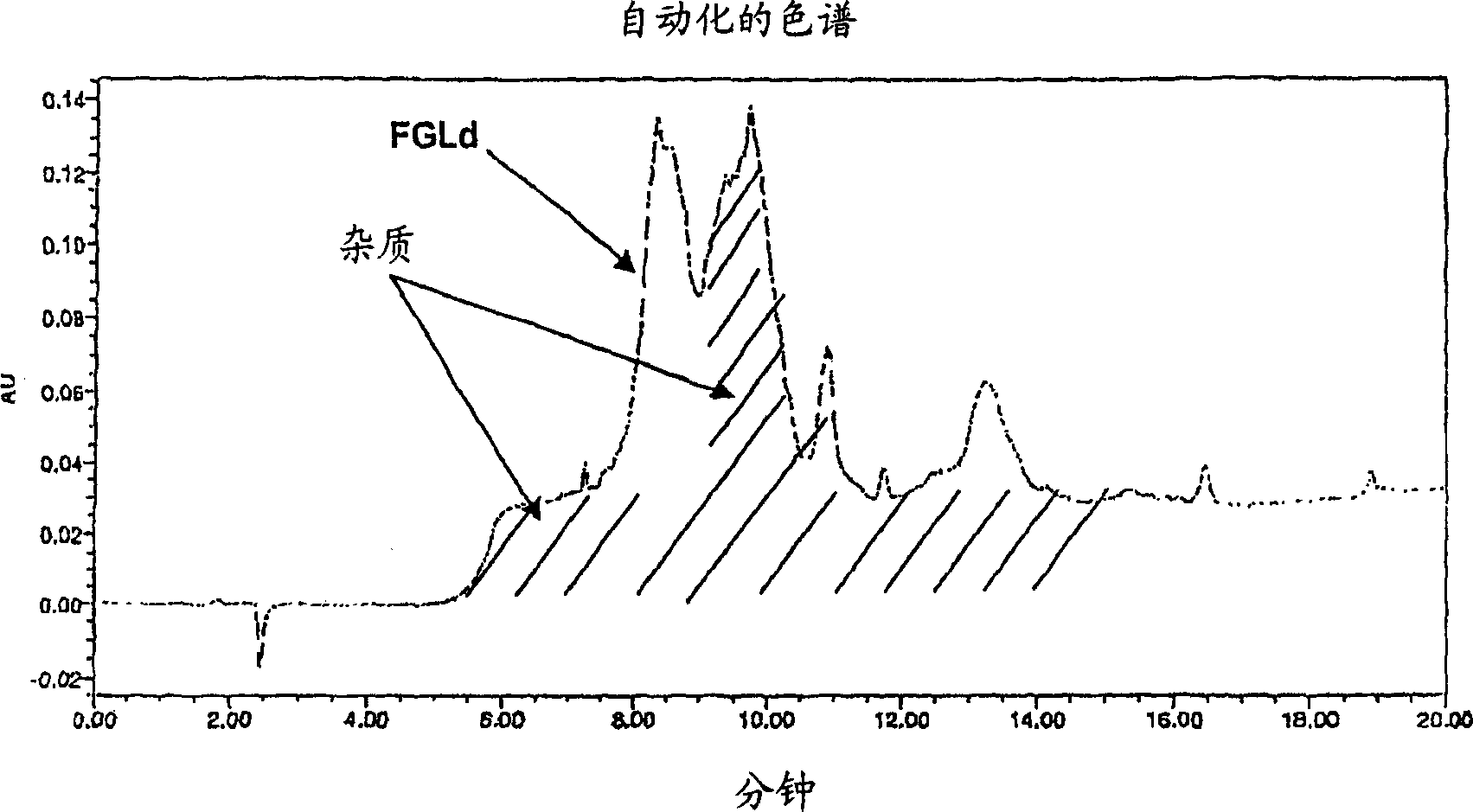
[0670] Example 1. Preparation of different formulations of the FGL peptide of the invention (SEQ ID NO: 1) and other peptides
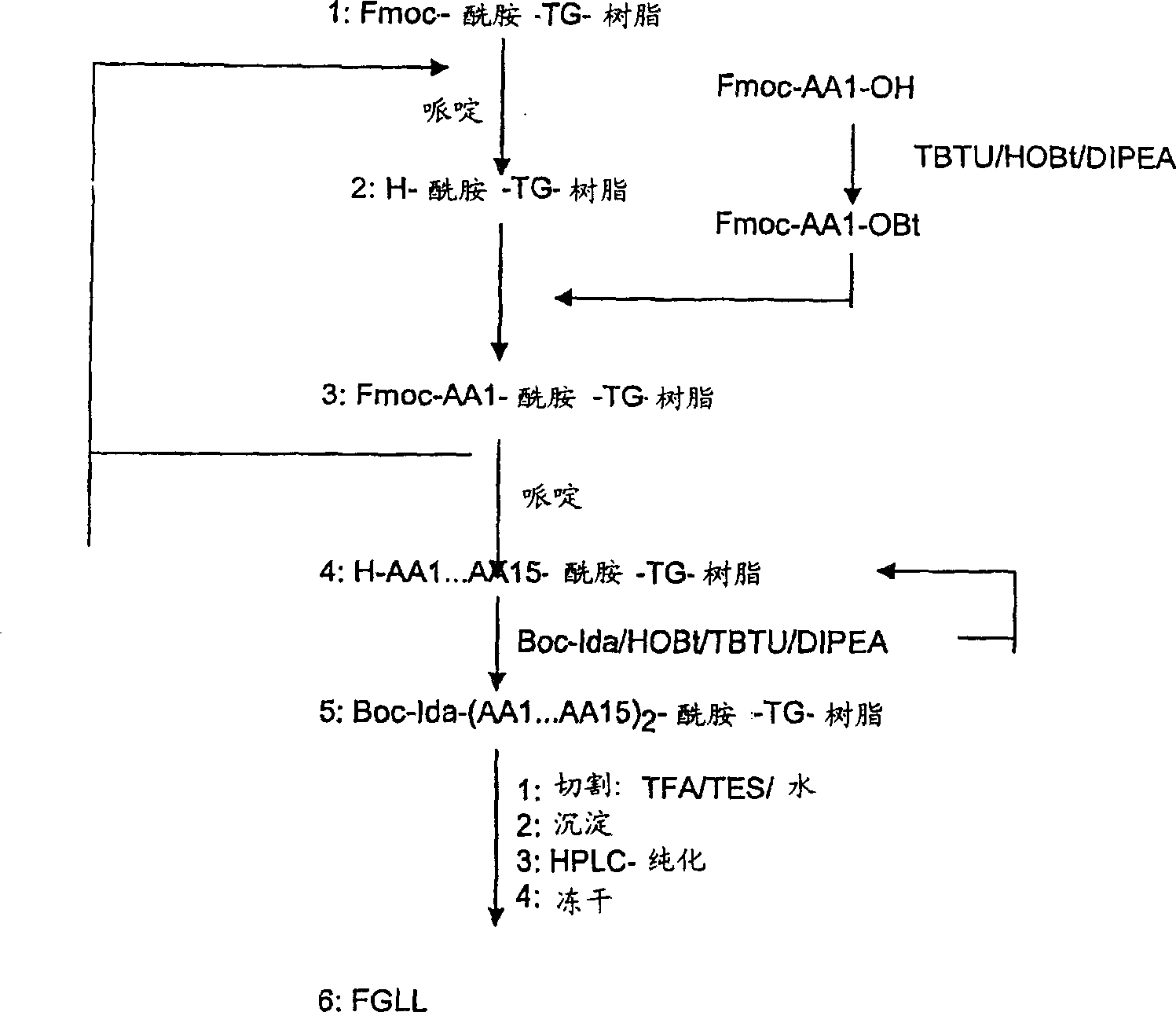
[0671] Solid phase synthesis of individual peptide chains of FGL
[0672] Individual peptide chains of FGL and other peptide sequences of the invention, such as the EFL peptide, were synthesized by standard Fmoc-solid phase methods as described above. Synthesis of peptides used in experiments was performed on TentaGel S RAM resin (90 mg, 0.22 mmol / g). The resin was placed in a polyethylene conduit equipped with a polypropylene filter for filtration. The resin was swollen in DMF and treated with 20% piperidine in DMF to ensure the presence of unprotected amino groups on the resin. The resin was then drained and washed with DMF until no yellow color was detected upon addition of Dhbt-OH to the drained DMF. The removal of the Fmoc-groups in turn allows the incorporation of amino acids one at a time. Fmoc-amino acids were preactivated by TBTU / HOBt in ...
Embodiment 2
[0716] Example 2. Effects of different formulations of FGL on the survival of neurons in vitro
[0717] The biological activity of different formulations of FGL peptides was compared in a survival assay well known in the art and described in detail below.
[0718] dopaminergic neuron (DN)
[0719] Dopaminergic neurons were prepared from embryonic day 15 Wistar rat embryos (Charles River, Sulzfeld, Germany or Mollegaard, Denmark). Pregnant rats were sacrificed, uteri were removed and stored in Hank's Balanced Salt Solution (HBSS; Gibco, BRL) on ice. Ventral parts of the midbrain were dissected from embryonic brains, homogenized on ice in Gey's Balanced Salt Solution (GBSS; Gibco, BRL) supplemented with 5 g glucose / l (Sigma-Aldrich), followed by trypsinization . Dissociated cells were washed in the presence of DNAsel and soybean trypsin inhibitor (Sigma-Aldrich).
[0720] For survival assays, isolate neurons at 150,000 cells / cm as described above. 2 The density was seeded i...
Embodiment 3
[0754] Example 3. In vitro stimulation of axonal outgrowth by different formulations of FGL
[0755] Primary cultures of DN, HN and CGN were prepared as described above.
[0756] dopaminergic neuron (DN)
[0757] DN at 100,000 cells / cm 2 Inoculated at a density of 24-well cell culture plates (previously coated with 12.5 μg / ml poly-D-lysine; Sigma-Aldrich) containing 50% (v / v) Optimem1 (Gibco, BRL), 25% ( v / v) In a medium with horse serum (Gibco, BRL), 25% (v / v) HBSS and 5 g glucose / l. After switching the medium to supplemented with 2% (v / v) B27 Neurobasal supplement, 0.5% (v / v) glutamine, 100 U / ml penicillin and 100 μg / ml streptomycin (all from Gibco, BRL) , before placing the cells in Neurobasal medium without or with various concentrations of peptides to attach for 1-2 hours. After 72 hours of culture, neurons were fixed with 4% (v / v) formaldehyde and treated with a primary mouse monoclonal antibody against tyrosine hydroxylase and a biotinylated goat anti-mouse secondar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com