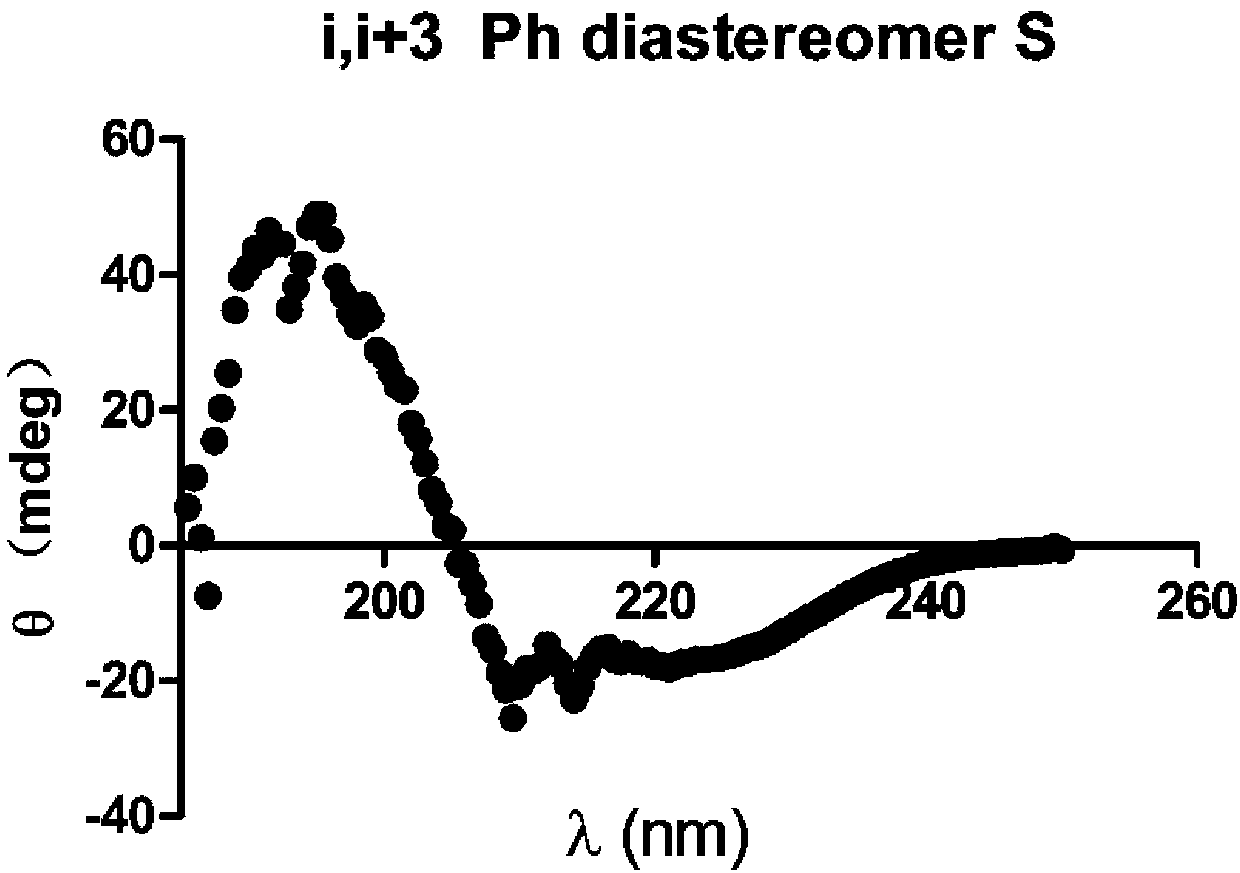
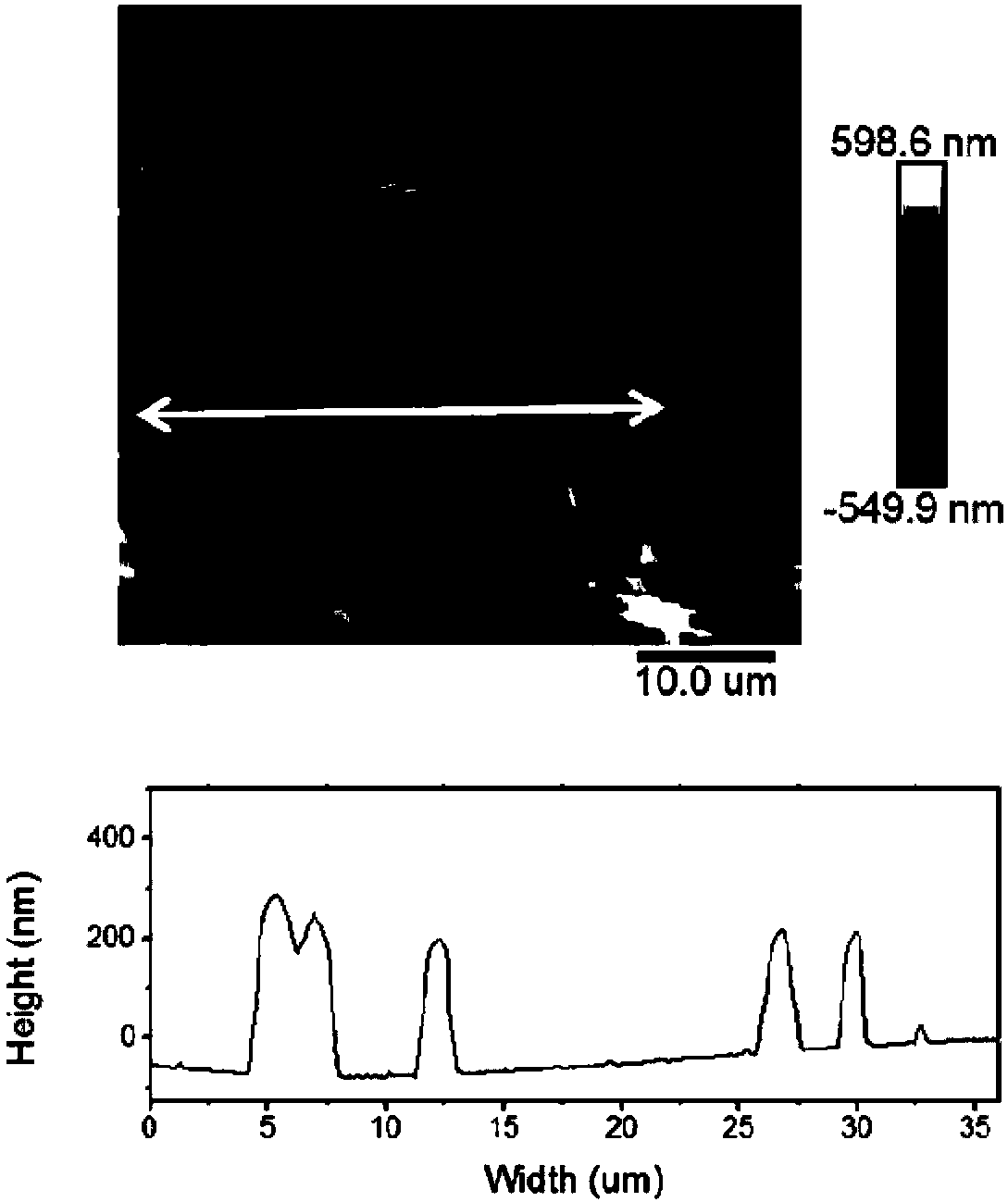
Self-assembled polypeptide nanorod and preparation method thereof
A self-assembly and nanorod technology, applied in the field of bioengineering, can solve problems such as unstable properties of nanorods and difficulties in nanorod methods, and achieve remarkable technological progress, simple and efficient size, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The present invention provides a method for preparing self-assembled polypeptide nanorods, which includes the following steps:
[0039] (I) Synthesis-Fmoc protected non-natural amino acid with chirality at carbon R at position 2 of the side chain; the non-natural amino acid of the present invention can be synthesized by conventional techniques ((a) YNBelokon, VITararov, VI Maleev, TFSavel'eva ,MGRyzhov.Tetrahedron:Asymmetry,1998,9,4249-4252.(b)B.Aillard,NS Robertson,ARBaldwin,S.Robins and AGJamieson,Org.Biomol.Chem.,2014,12,8775-8782 .(c)VASoloshonok,X.Tang,VJHruby and LVMeervelt,Org.Lett.,2001,3,341-343.(d)W.Qiu,VASoloshonok,C.Cai,X.Tang and VJHruby,Tetrahedron , 2000, 56, 2577-2582. (e) X. Tang, VA Soloshonok, VJ Hruby. Tetrahedron: Asymmetry, 2000, 11.2917-2925.), and will not be repeated here.
[0040] The structural formula is as follows:
[0041] Where X is
[0042]
[0043]
[0044] or Any one of the groups.
[0045] (Ii) Use the method of solid-phase synthesis of th...
Embodiment 2
[0056] Stapler cyclic peptide Ac-cyclo(1,4)-CAAS 5 (2-phenyl)-NH 2 Preparation method of peptide nanorods formed by self-assembly,
[0057] Unnatural amino acid S protected by Fmoc in R configuration 5 The structural formula of (2-phenyl) is:
[0058] First, NH is synthesized by Fmoc solid-phase peptide synthesis 2 -CAAS 5 (2-phenyl)-MBHA resin, the specific route is as follows:
[0059]
[0060]
[0061]
[0062]
[0063]
[0064] The specific operation is:
[0065] 1. Connect the first amino acid: Weigh 1.0g MBHA resin into a 100ml peptide tube, add 20ml N-methylpyrrolidone (NMP) and swell with nitrogen for 30 minutes; filter out the solvent and add 25% morpholine NMP solution by volume , Blow nitrogen for 30min, wash; ligation reaction: add Fmoc-S5(2-phenyl)-OH(0.4M in NMP) solution, HCUT(0.38M in NMP), DIEA according to 5.0ml / 5.0ml / 0.71ml, mix and add Nitrogen was bubbled through the resin for 120 minutes, and the reaction liquid was filtered off. Washing: drain the solvent in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com