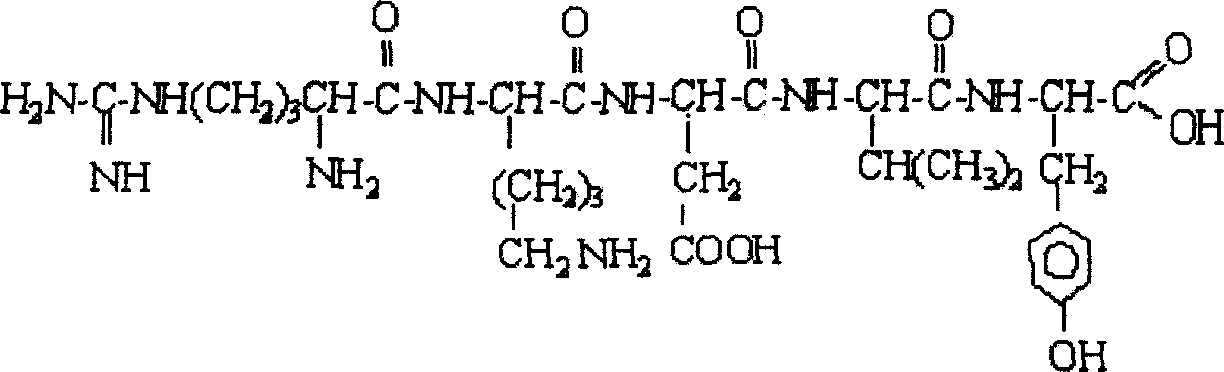
Thymopentapeptide active isomer and application thereof in pharmaceutical preparation
An isomer, thymus technology, applied in antitumor drugs and antioxidant drugs, the active isomer of thymopentin, in the field of preparation of immunomodulatory drugs, can solve problems such as short half-life and unfavorable treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The solid-phase synthesis of embodiment 1 LW501
[0049] Raw materials: Fmoc-Tyr(tBu)-Wang resin, Fmoc-Asp-otBu, Fmoc-Arg(pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Val-OH. The condensing agent for the coupling reaction is benzotriazole-1-oxygen-tris(dimethylamino)phosphorus hexafluorophosphate (BOP), 1-hydroxybenzotriazole (HOBT), N-methylmorpholine (NMM ). The deprotecting agent is 20% piperidine / DMF solution. The cleavage reagent is trifluoroacetic acid.
[0050] Reaction: Fmoc-Tyr(tBu)-Wang resin was swelled in DMF at room temperature for 30 minutes, and washed three times with DMF. Add 20% piperidine / DMF solution and react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-Val-OH, react at room temperature for 3 hours, then wash with DMF three times, add 20% piperidine / DMF solution, react at room temperature for 1 hour to deprotect. Add Fmoc-Asp-otBu, react at room temperature for 3 hours, wash with DMF three times, add 20% piperidine / D...
Embodiment 2
[0054] The solid-phase synthesis of embodiment 2 LW502
[0055] Raw materials: Fmoc-Tyr(tBu)-Wang resin, Fmoc-Glu-otBu, Fmoc-Arg(pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Val-OH. The condensing agent for the coupling reaction is benzotriazole-1-oxygen-tris(dimethylamino)phosphorus hexafluorophosphate (BOP), 1-hydroxybenzotriazole (HOBT), N-methylmorpholine (NMM ). The deprotecting agent is 20% piperidine / DMF solution. The cleavage reagent is trifluoroacetic acid.
[0056] Reaction: Fmoc-Tyr(tBu)-Wang resin was swelled in DMF at room temperature for 30 minutes, and washed three times with DMF. Add 20% piperidine / DMF solution and react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-Val-OH, react at room temperature for 3 hours, then wash with DMF three times, add 20% piperidine / DMF solution, react at room temperature for 1 hour to deprotect. Add Fmoc-Glu-otBu, react at room temperature for 3 hours, wash with DMF three times, add 20% piperidine / D...
Embodiment 3
[0061] The solid-phase synthesis of embodiment 3 LW503, LW504, LW505
[0062] The raw material in Example 1 is replaced with Fmoc-Tyr(tBu)-Wang resin, Fmoc-Val-OHFmoc-Asp-otBu, Fmoc-β-Lys(Boc)-OH, Fmoc-Arg(pbf)-OH, with LW503 can be synthesized by the same method as in Example 1.
[0063] The raw materials in Example 1 were replaced with Fmoc-Tyr(tBu)-Wang resin, Fmoc-Val-OH, Fmoc-Glu-otBu, Fmoc-β-Lys(Boc)-OH, Fmoc-Arg(pbf)-OH , LW504 can be synthesized by the same method as in Example 1.
[0064] The raw materials in Example 1 were replaced with Fmoc-Tyr(tBu)-Wang resin, Fmoc-Val-OH, Fmoc-Glu-otBu, Fmoc-γ-Lys(Boc)-OH, Fmoc-Arg(pbf)-OH , LW505 can be synthesized by the same method as in Example 1.
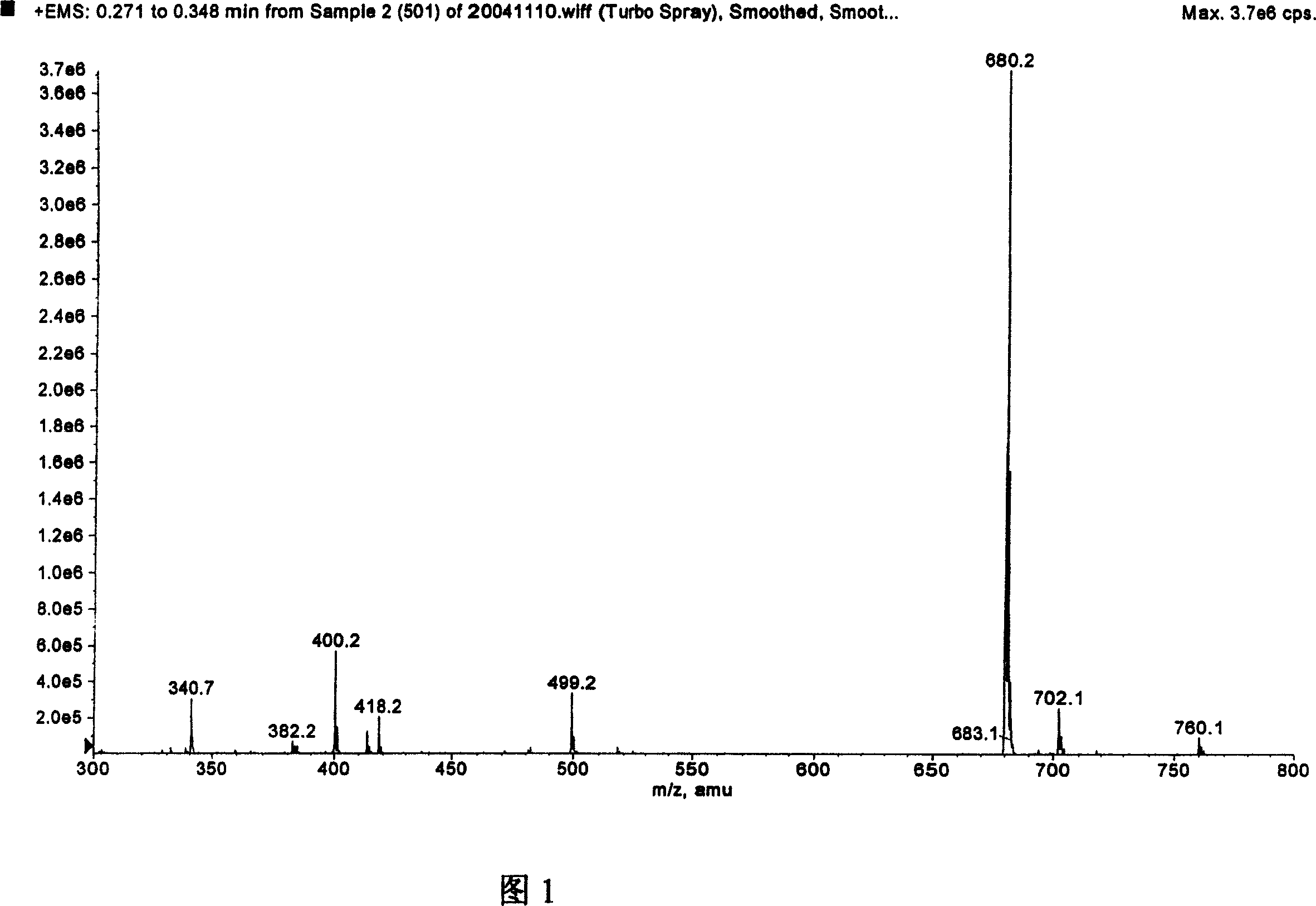
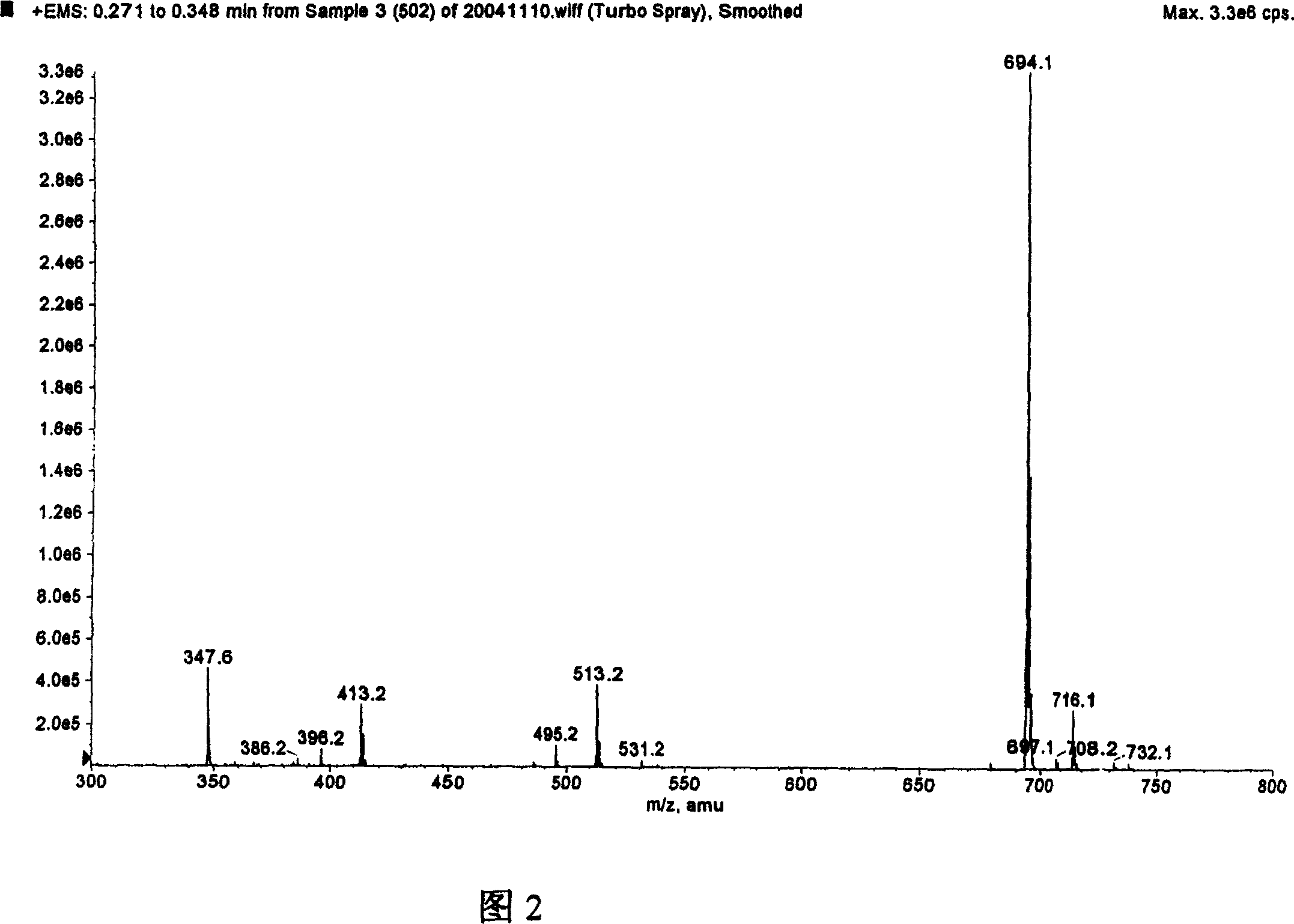
[0065] Its molecular weight was identified by mass spectrometry in full agreement with LW503, LW504, and LW505.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com