Patents
Literature
741 results about "Immune regulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
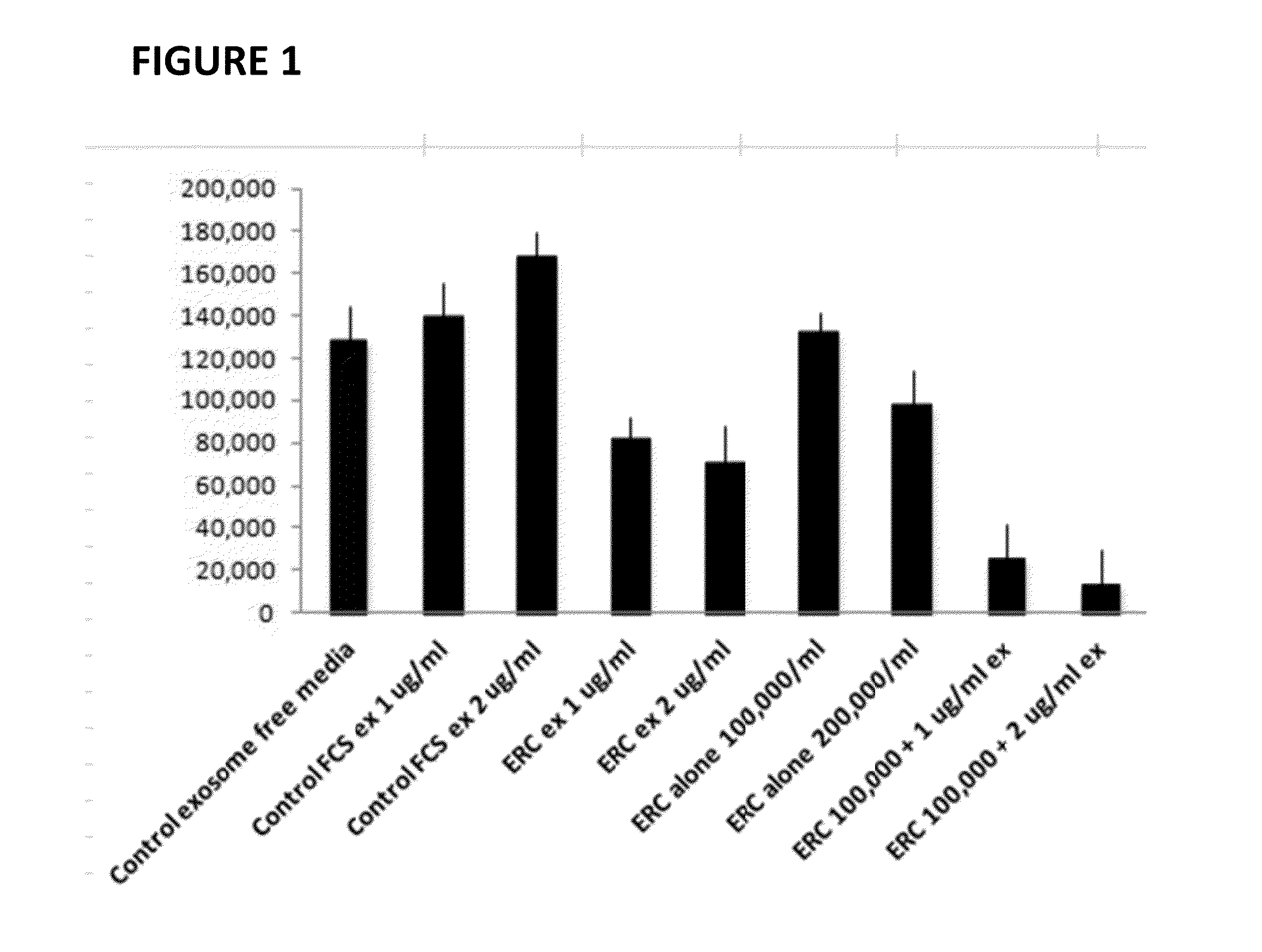
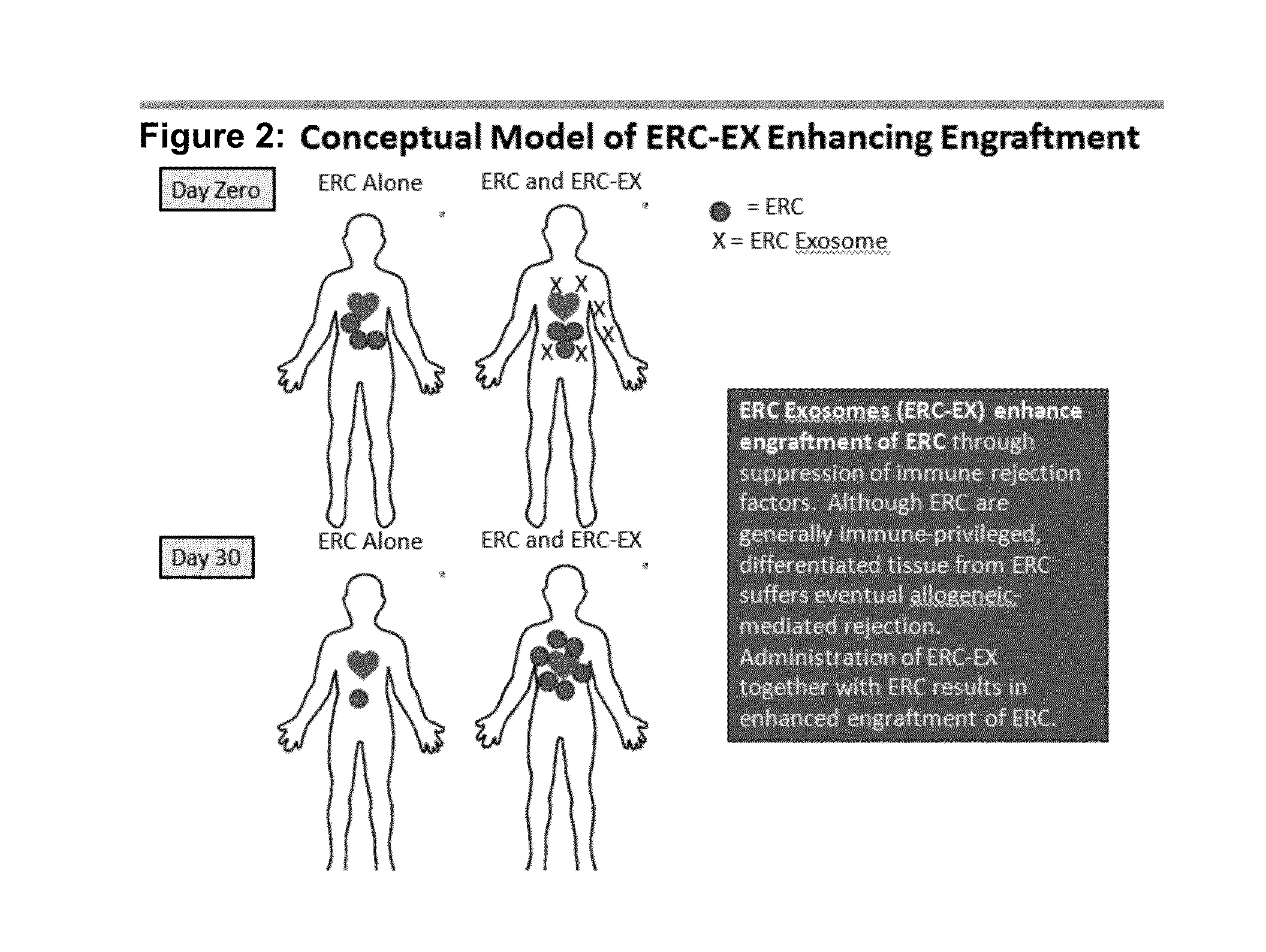
Therapeutic immune modulation by stem cell secreted exosomes
ActiveUS20130195899A1Inhibit inflammationReduce the amount of solutionGenetic material ingredientsSnake antigen ingredientsAutoimmune conditionEndometrium
Disclosed are methods, compositions of matter, and protocols useful for the induction of a therapeutic immune modulatory response through administration of exosomes derived from a stem cell source. In one embodiment, said stem cell source is endometrial regenerative cells. Specifically, in one embodiment stem cell derived exosomes are used as a method of treating an autoimmune condition such as rheumatoid arthritis, multiple sclerosis, or systemic lupus erythromatosis.
Owner:XON CELLS
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS20080159998A1High activityInduce upregulationBiocideGenetic material ingredientsAntigenT cell
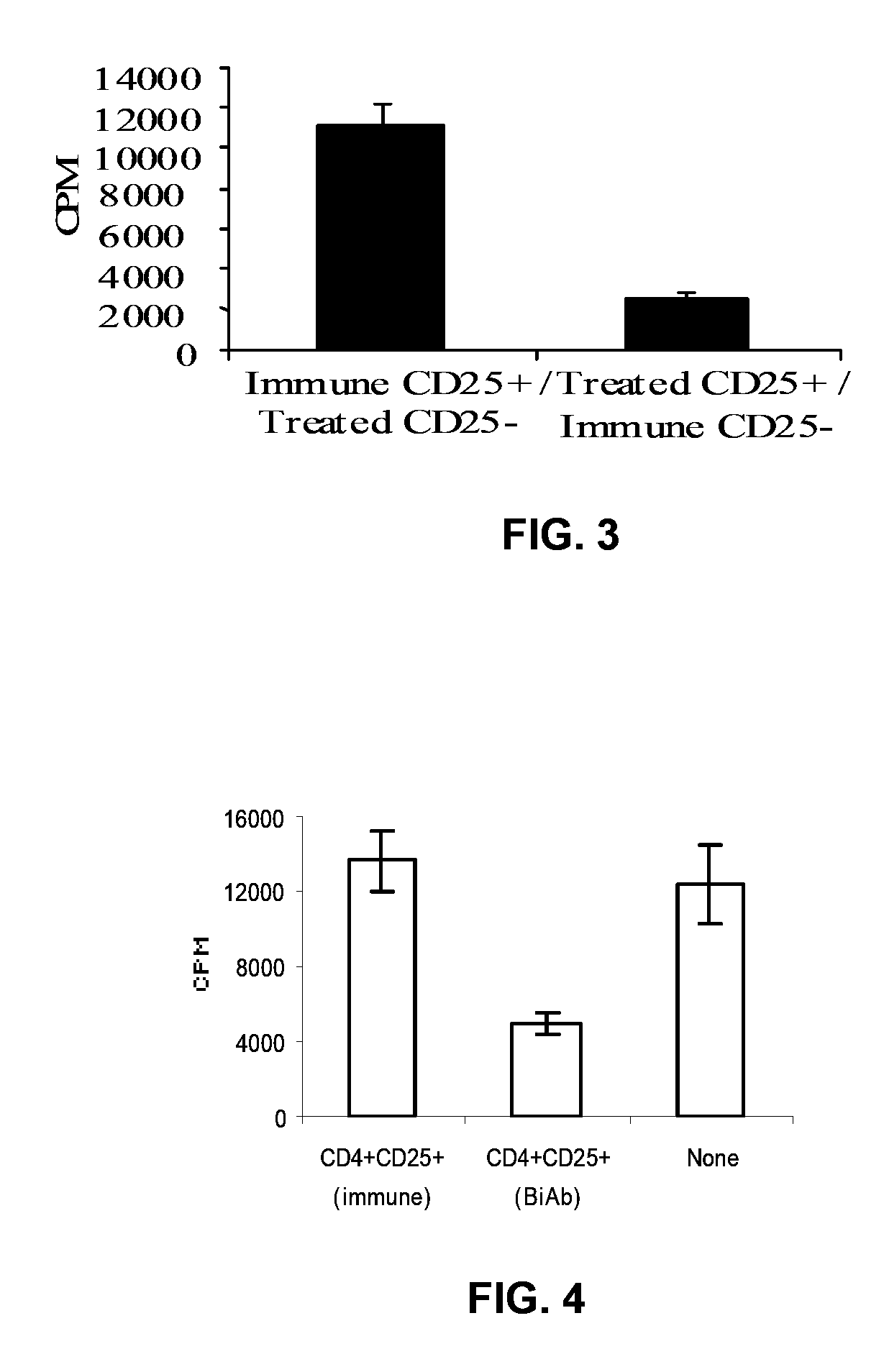
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
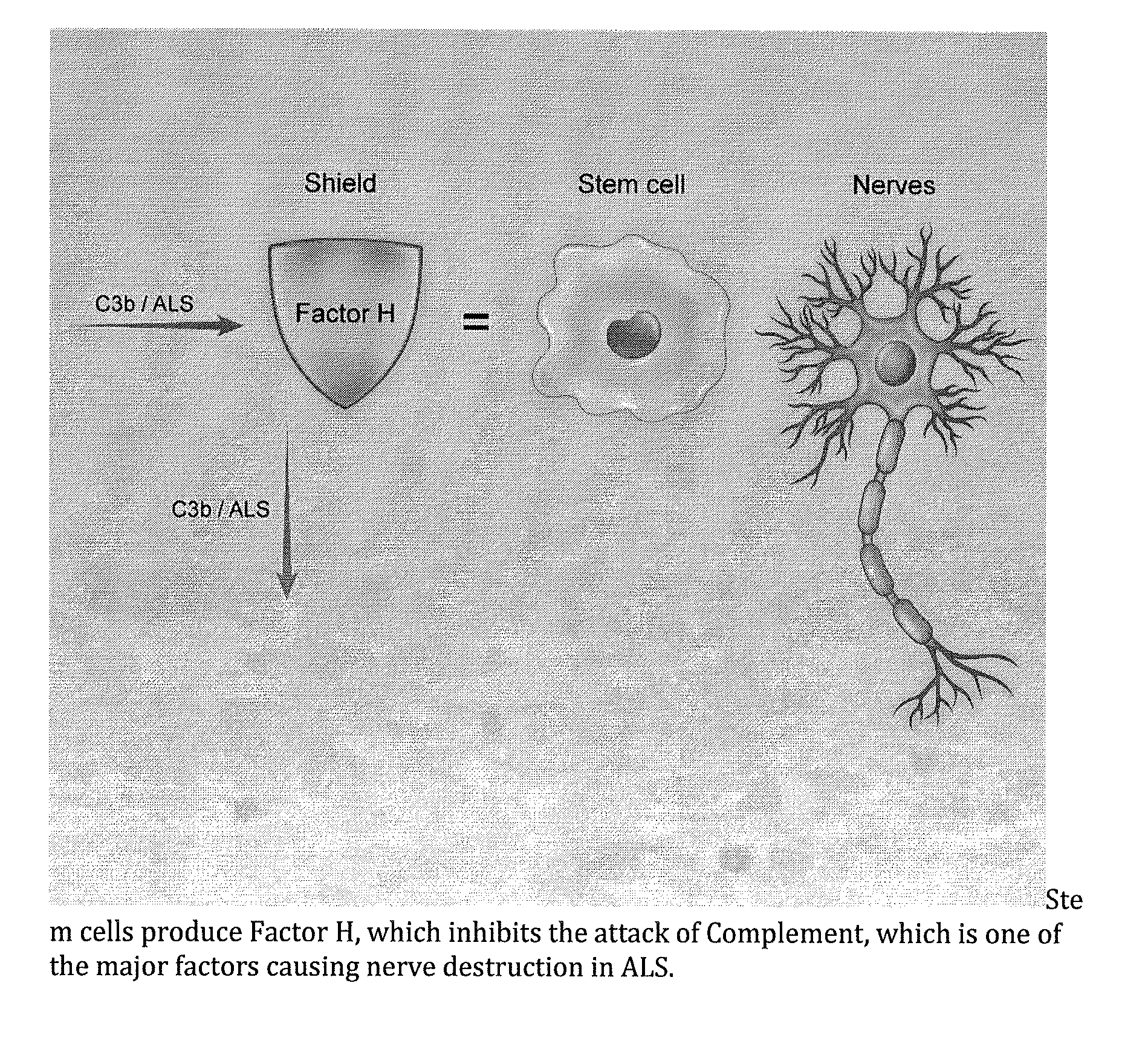
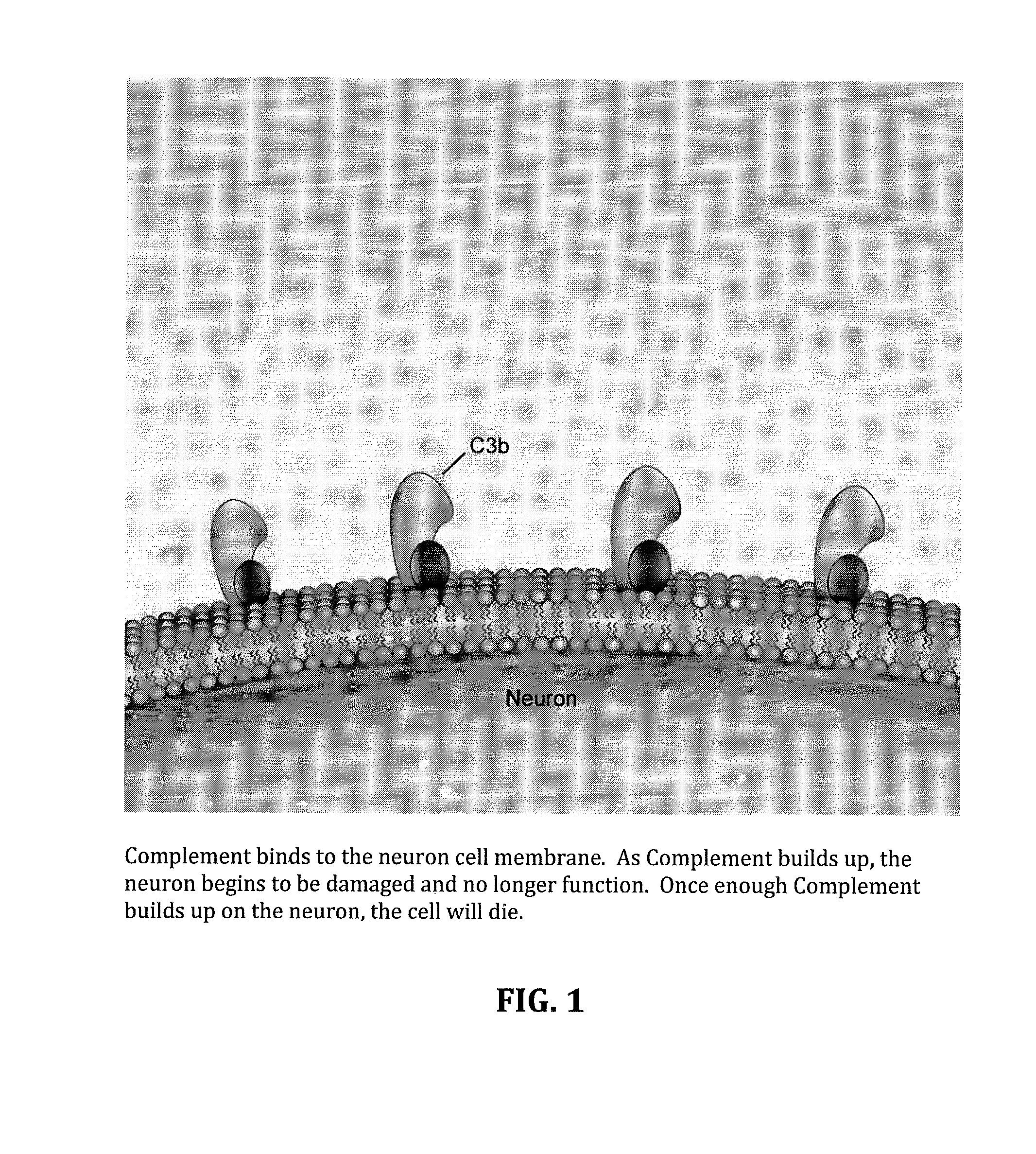
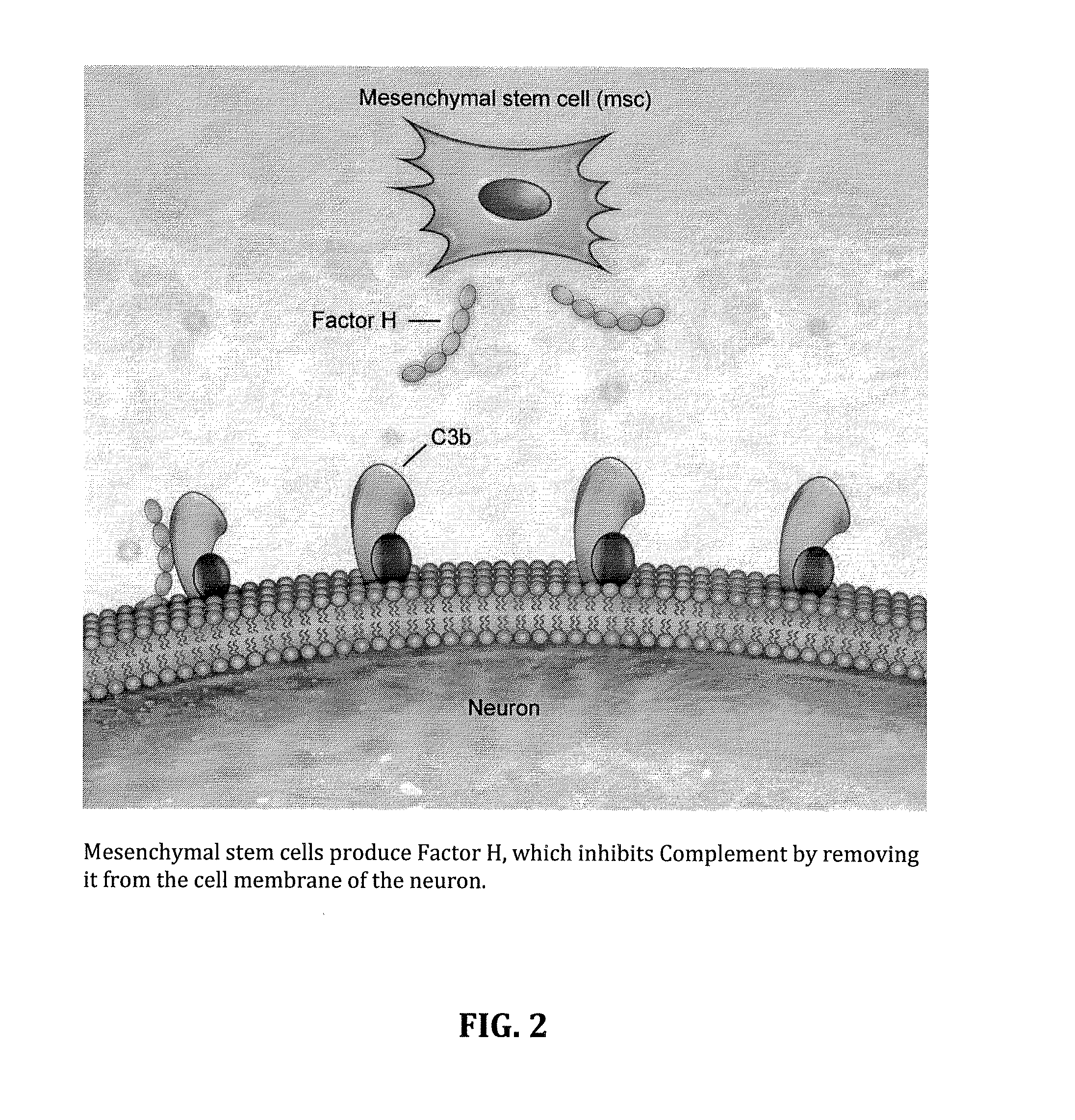
Method for treating ALS via the increased production of factor h
InactiveUS20140234275A1Improve satisfactionInhibition effectBiocideOrganic active ingredientsWhole bodyPrimary motor neuron
Methods and systems for the treatment for ALS incorporating stem cells harvested from the subject to be treated. These stem cells may be genetically altered with the addition of several genes of interest. Then, the patient will receive systemic gene therapy for the muscles and directed specifically at motor neurons. In this multi-pronged treatment approach, the stem cells provide immune regulation and the regeneration of motor neurons. And, the new motor neurons carry the added genes, which are protective against motor neuron death from ALS. The systemic therapy increases the amount of genes, which further reduces the effects of ALS. Additional gene therapy administered in the muscle will be further protective of the axon, while maintaining muscle mass and function.
Owner:BIOVIVA USA
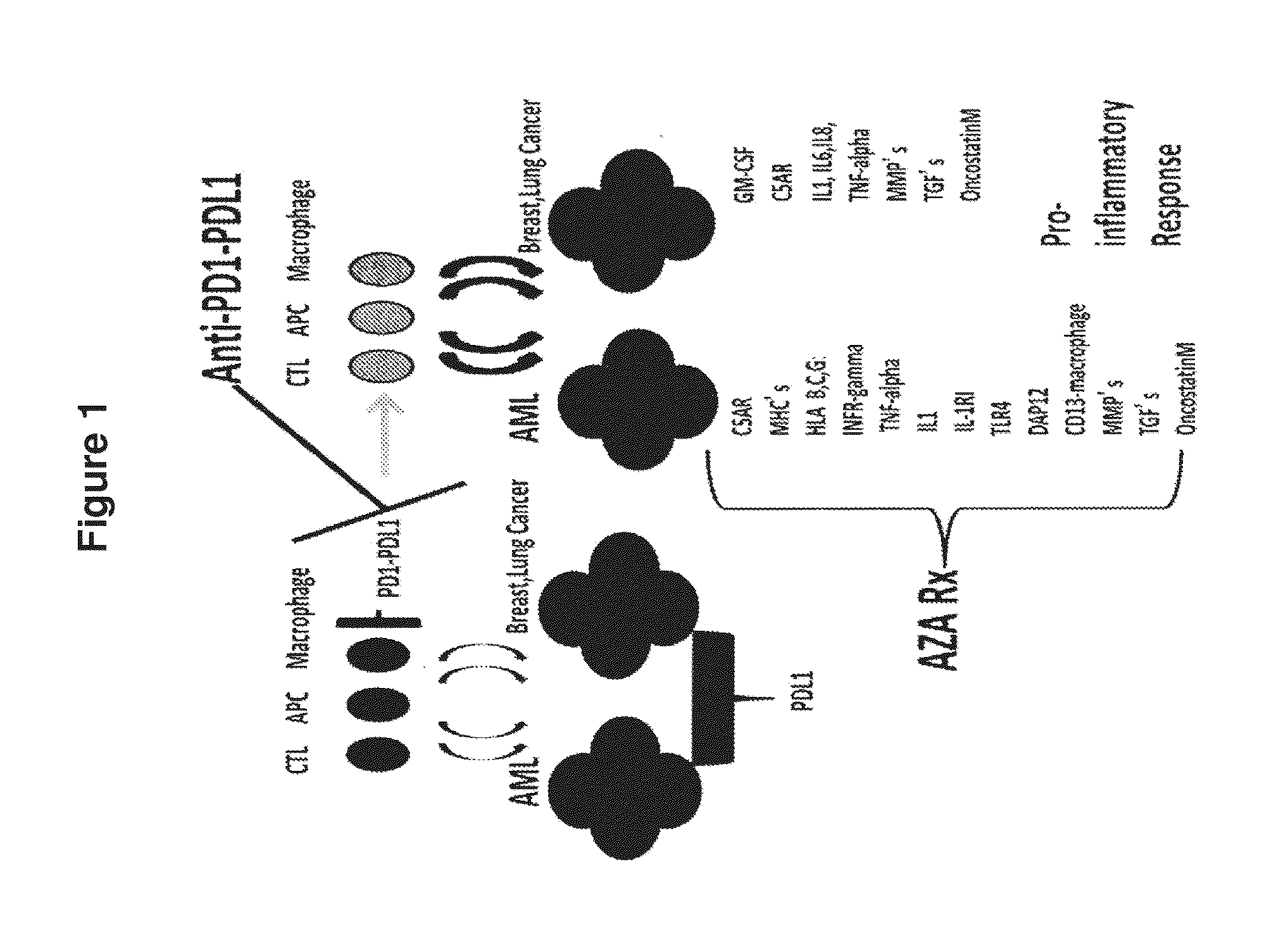
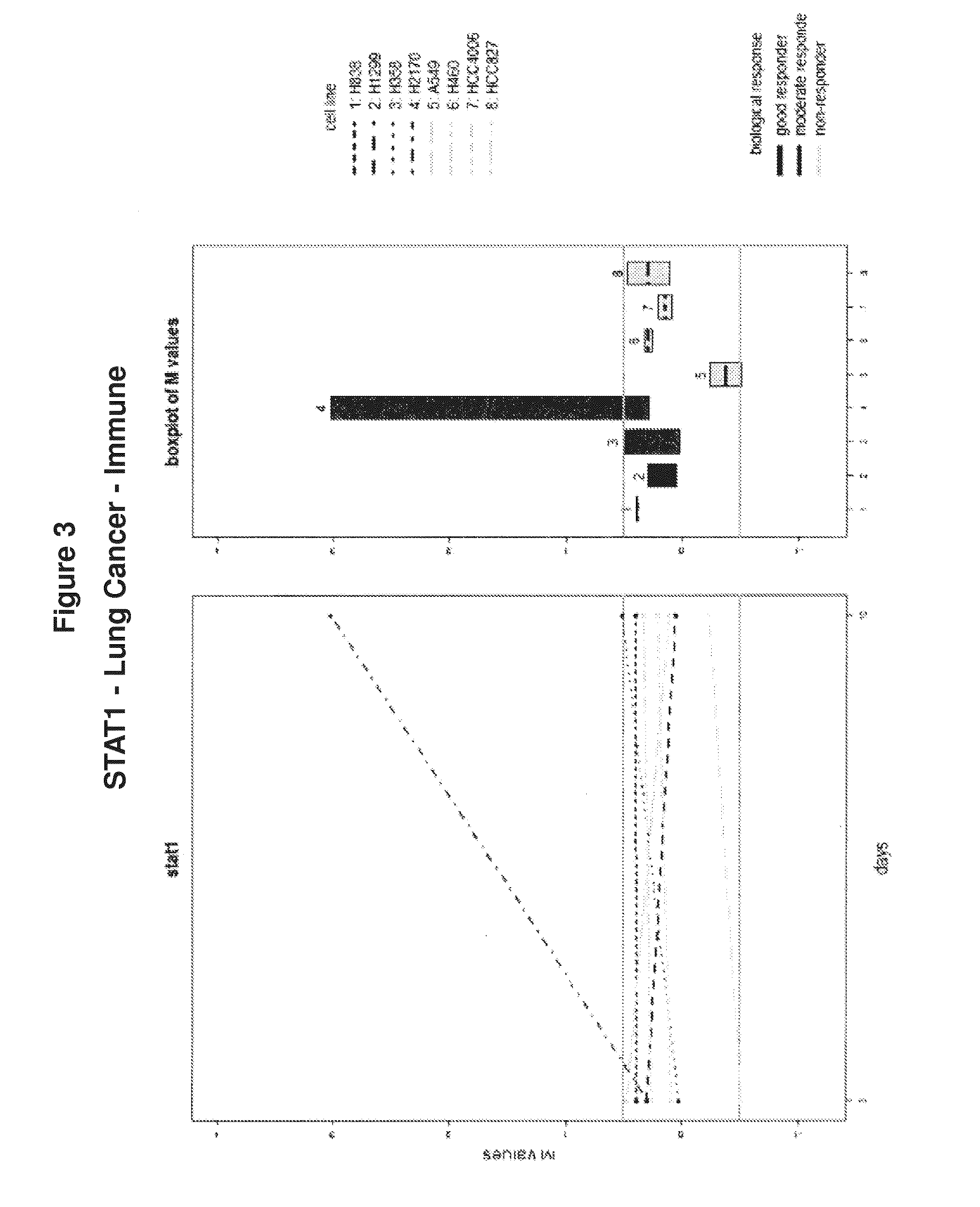
Cancer therapy via a combination of epigenetic modulation and immune modulation
ActiveUS20160193239A1Improve immunityAnti-neoplastic effect of the immune modulating agent in the subject is enhancedOrganic active ingredientsMicrobiological testing/measurementAntiendomysial antibodiesImmune modulator
Cancer therapies that combine epigenetic modulating agent(s) with immune modulating agent(s), which were remarkably identified to provide an improved treatment regimen over single agent therapy, are disclosed. In particular embodiments, the invention provides for improved treatment of NSCLC in patients via administration of exemplary immune modulating agents anti-PD-1 antibody or anti-PD-L1 antibody, which were observed to show enhanced activity in combination with the exemplary epigenetic modulating agent 5-deoxyazacytidine. Further, expression markers of responsive neoplastic cells are also disclosed.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Anti-CD70 antibody and its use for the treatment and prevention of cancer and immune disorders
Disclosed are CD70 binding agents, such as anti-CD70 antibodies and derivatives, that induce a cytotoxic, cytostatic or immunomodulatory without conjugation to a therapeutic agents as well as pharmaceutical compositions and kits comprising the antibody or derivative. Also disclosed are methods for the treatment and prevention of CD70-expressing cancers and immunological disorders comprising administering the CD70 binding agents to a subject.
Owner:SEAGEN INC
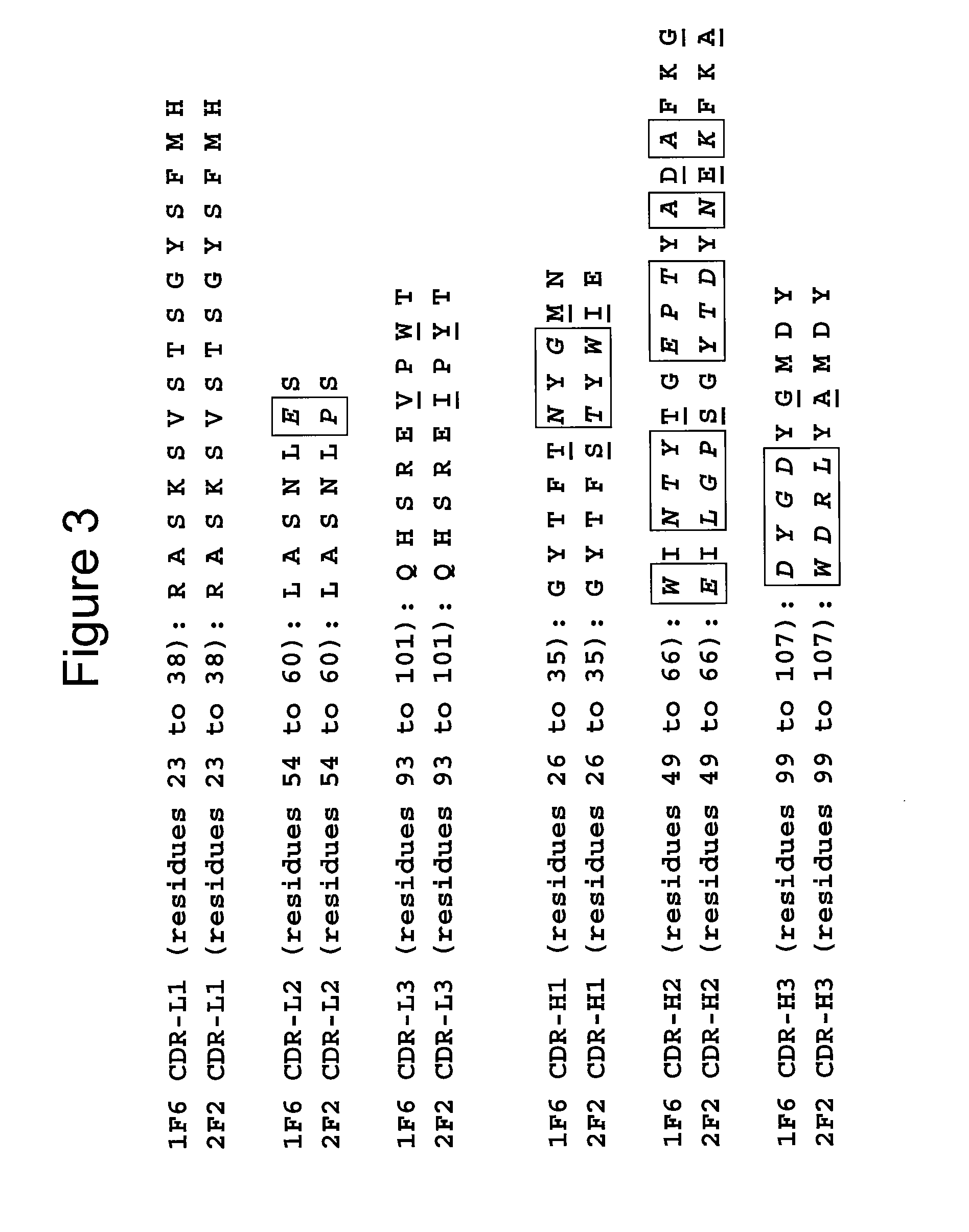
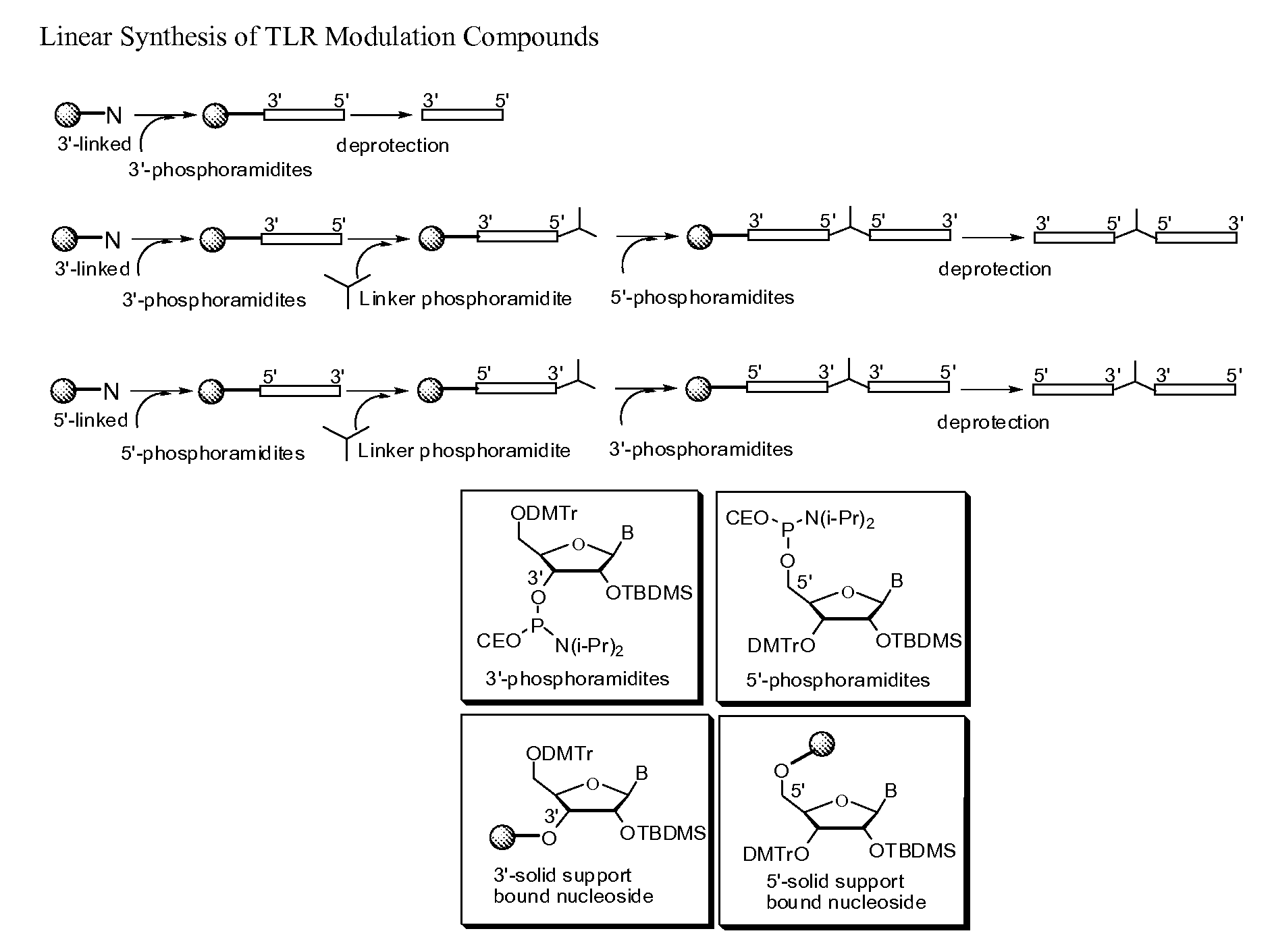
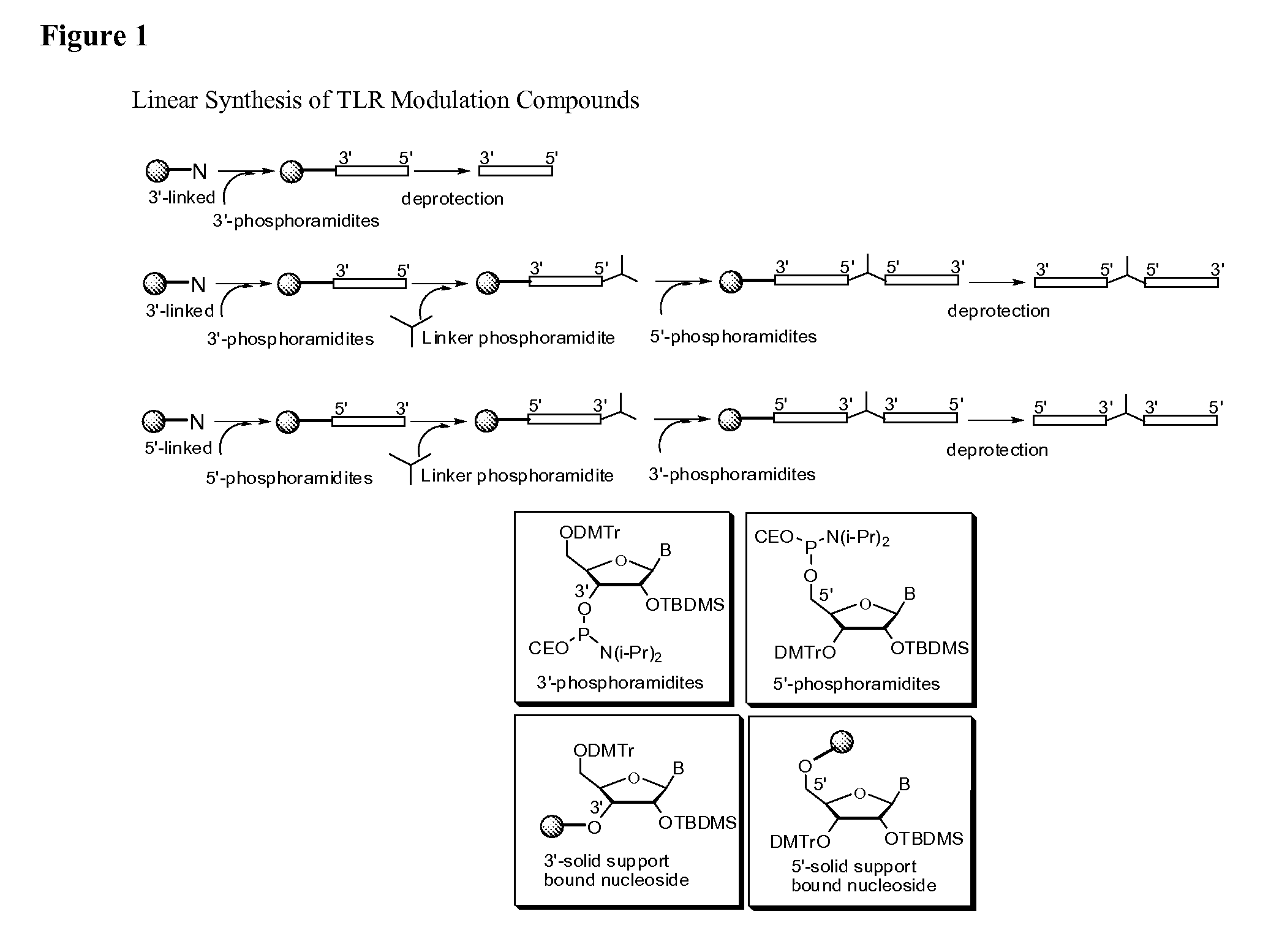
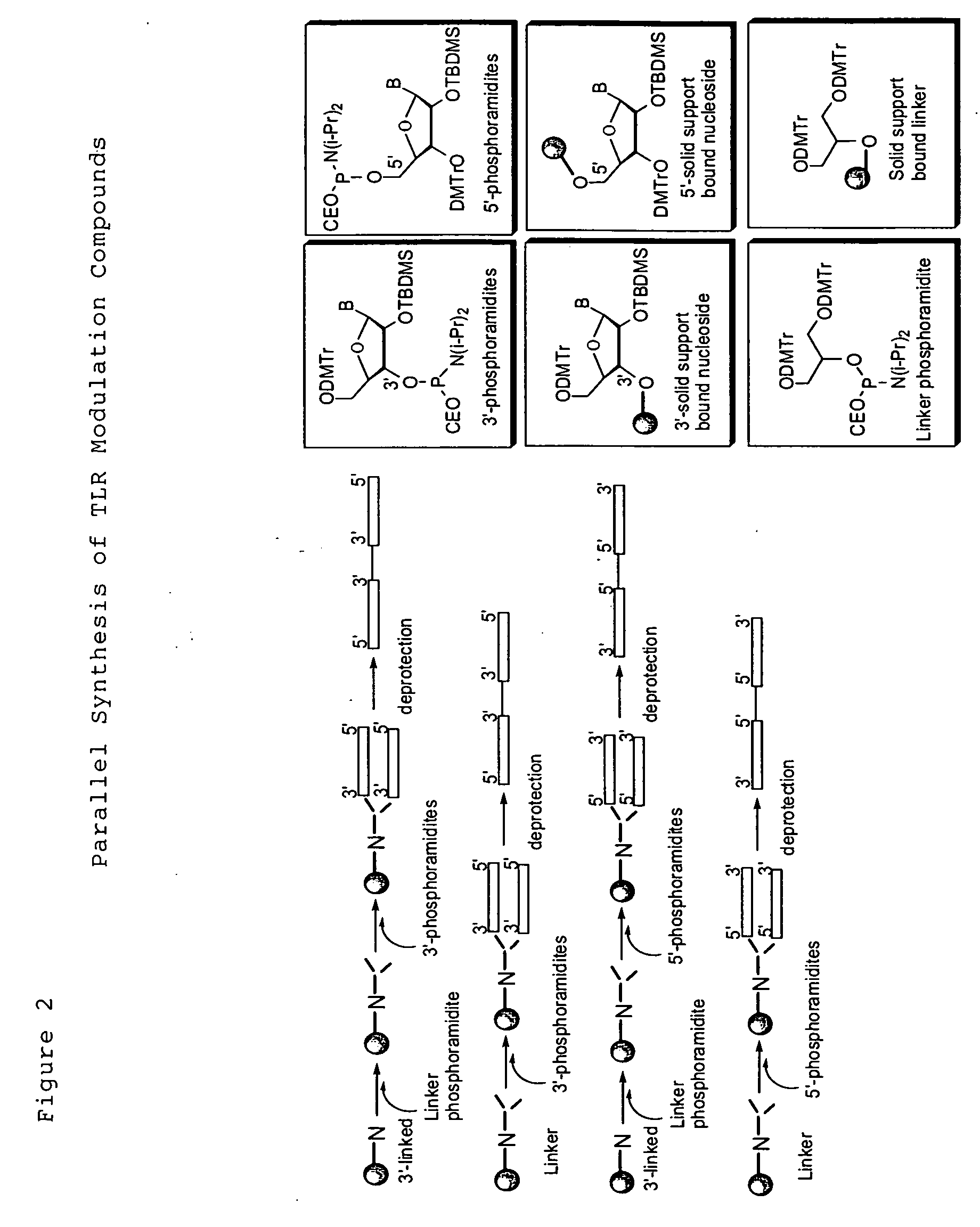
Toll like receptor modulators
ActiveUS20090053148A1Prevent diseaseLower immune responseSenses disorderNervous disorderInflammatory Bowel DiseasesAutoimmune responses
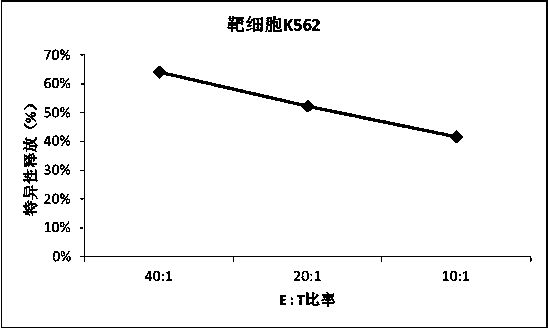
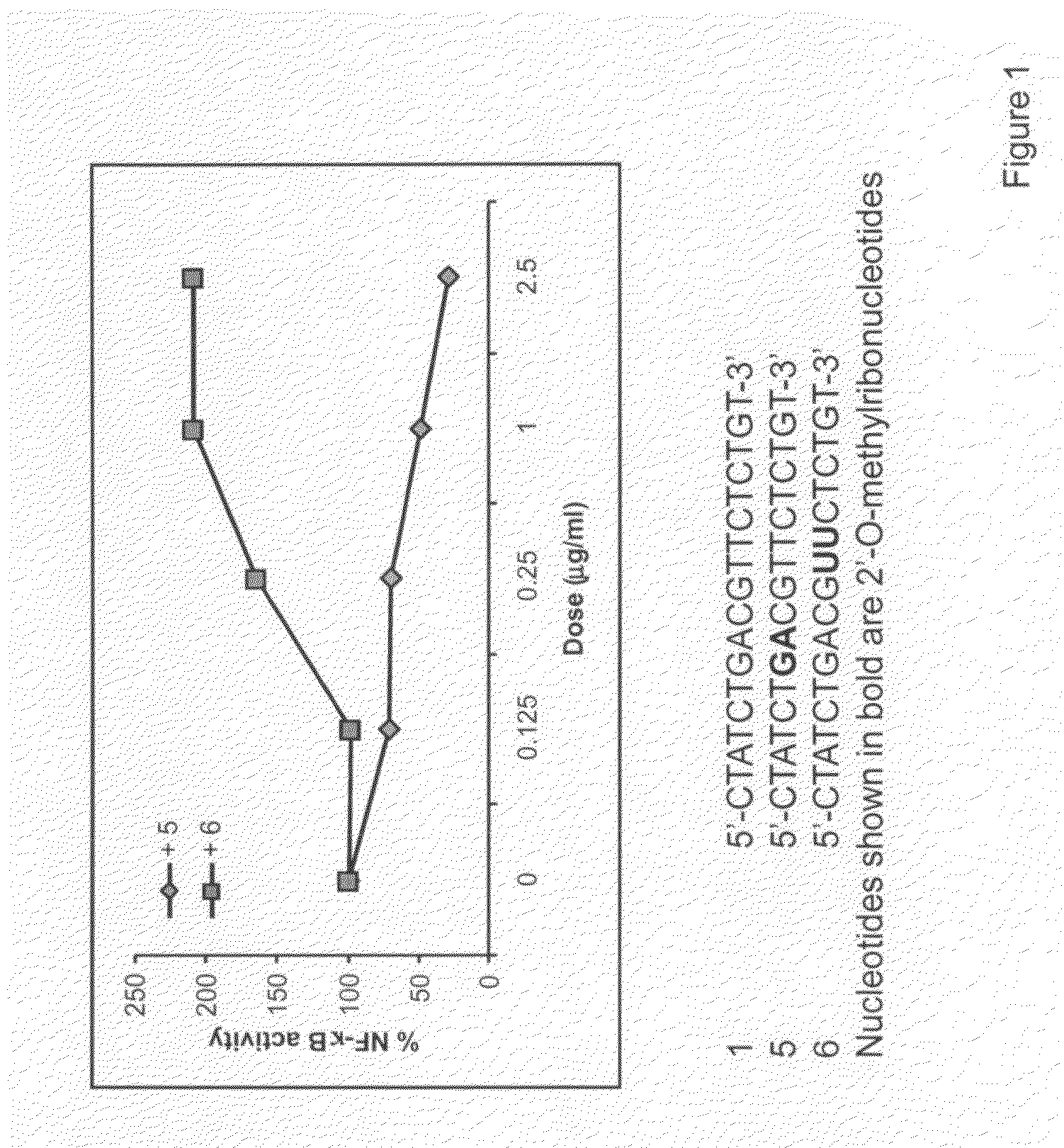
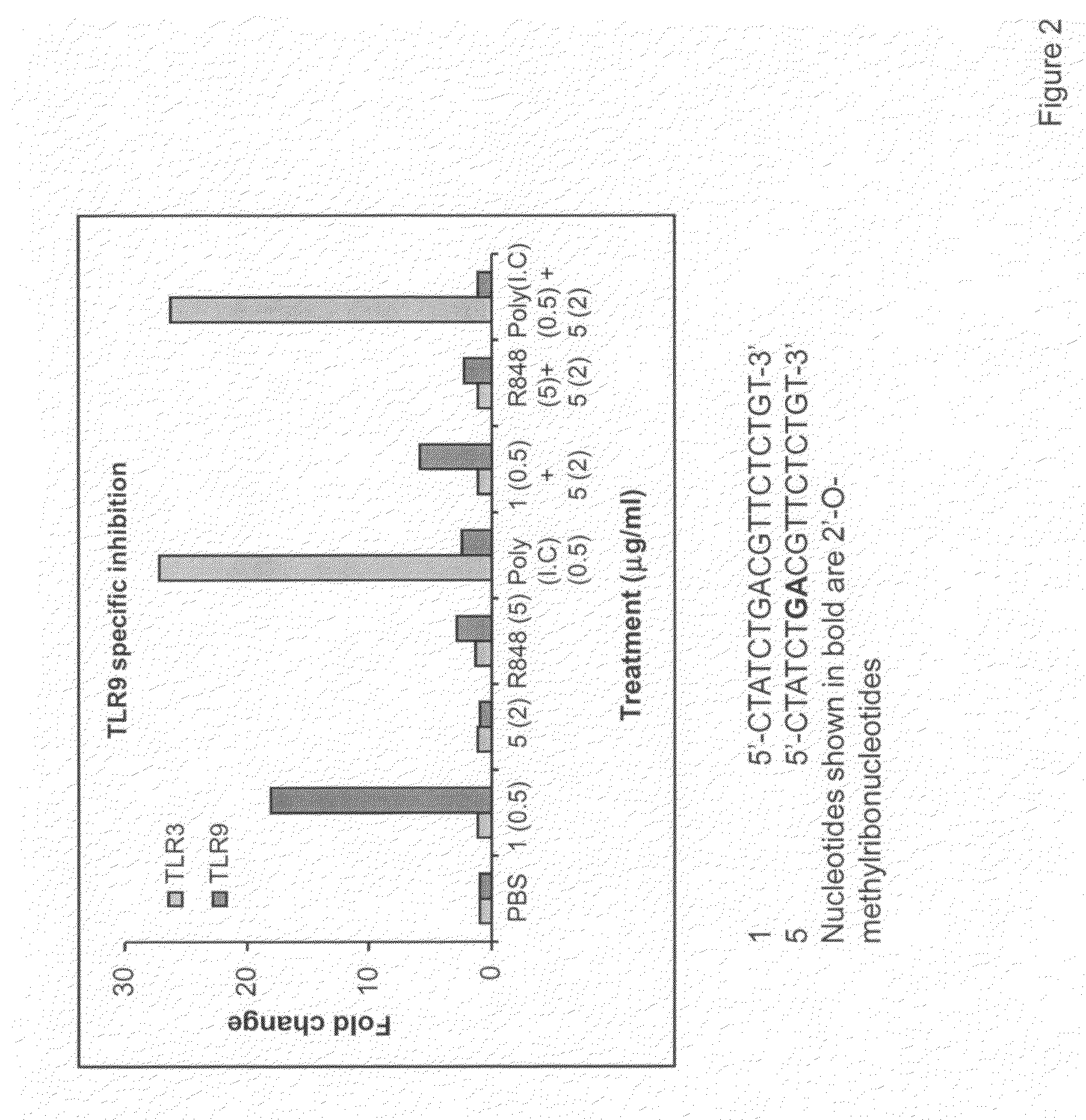
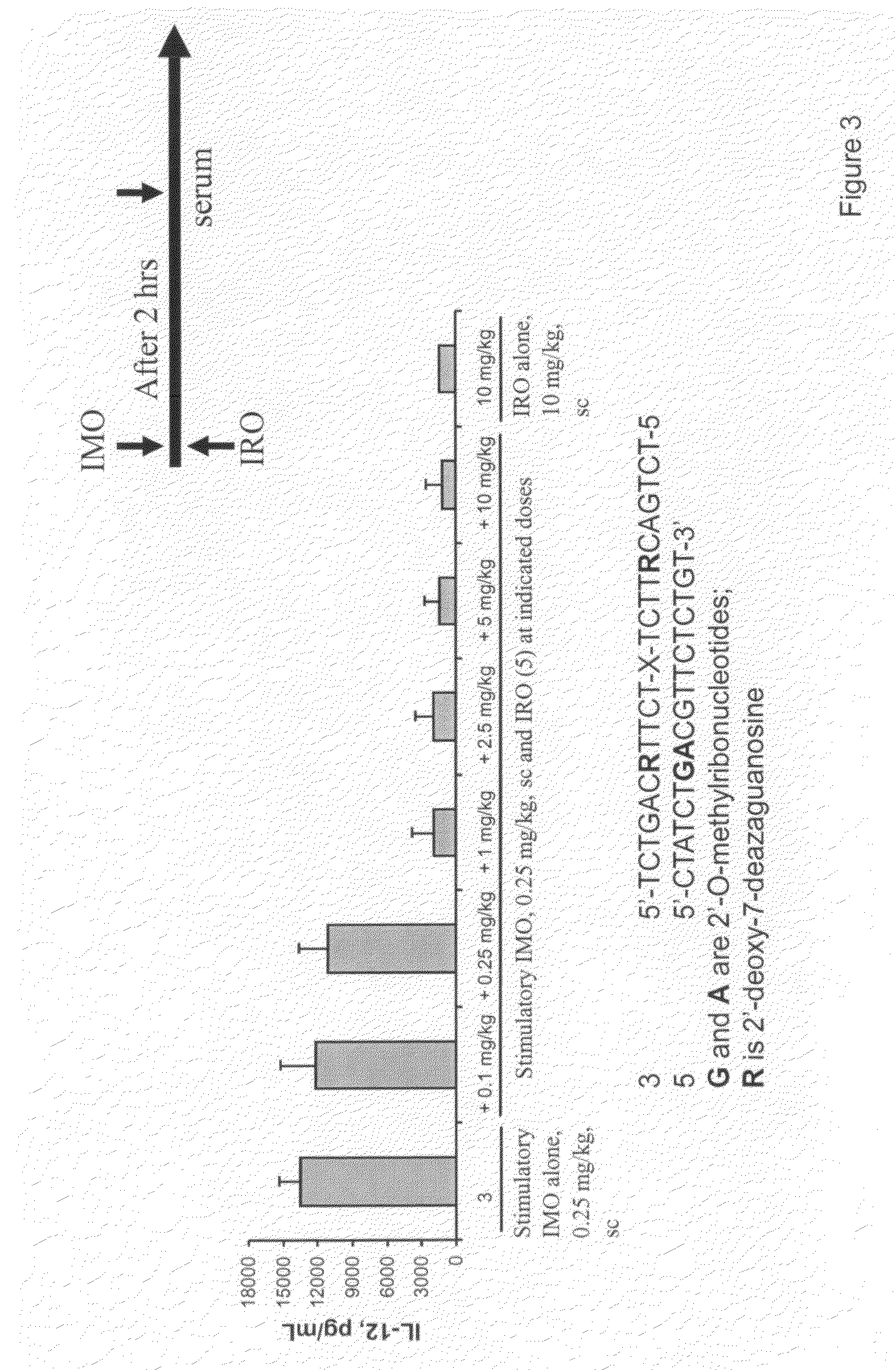
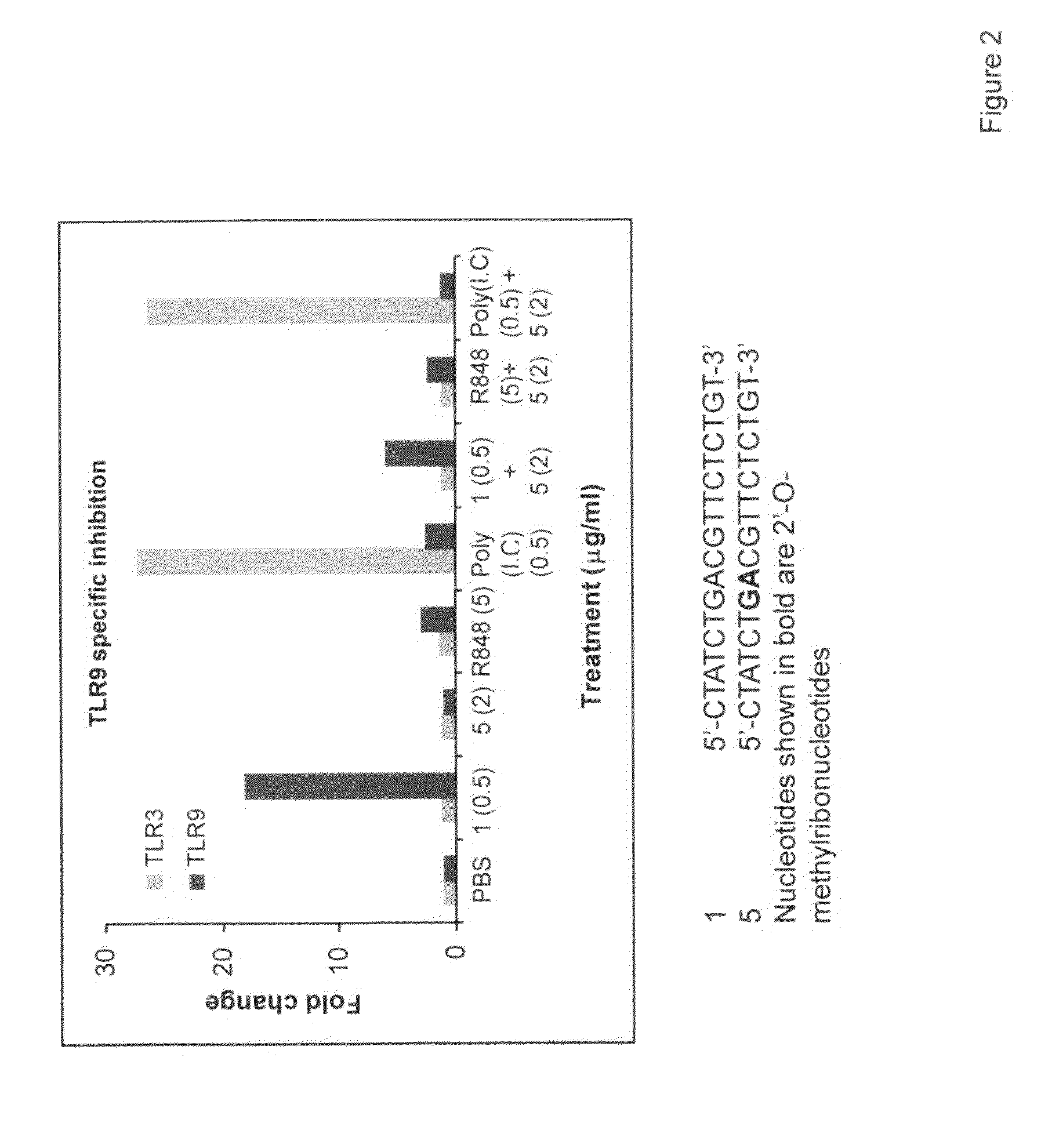
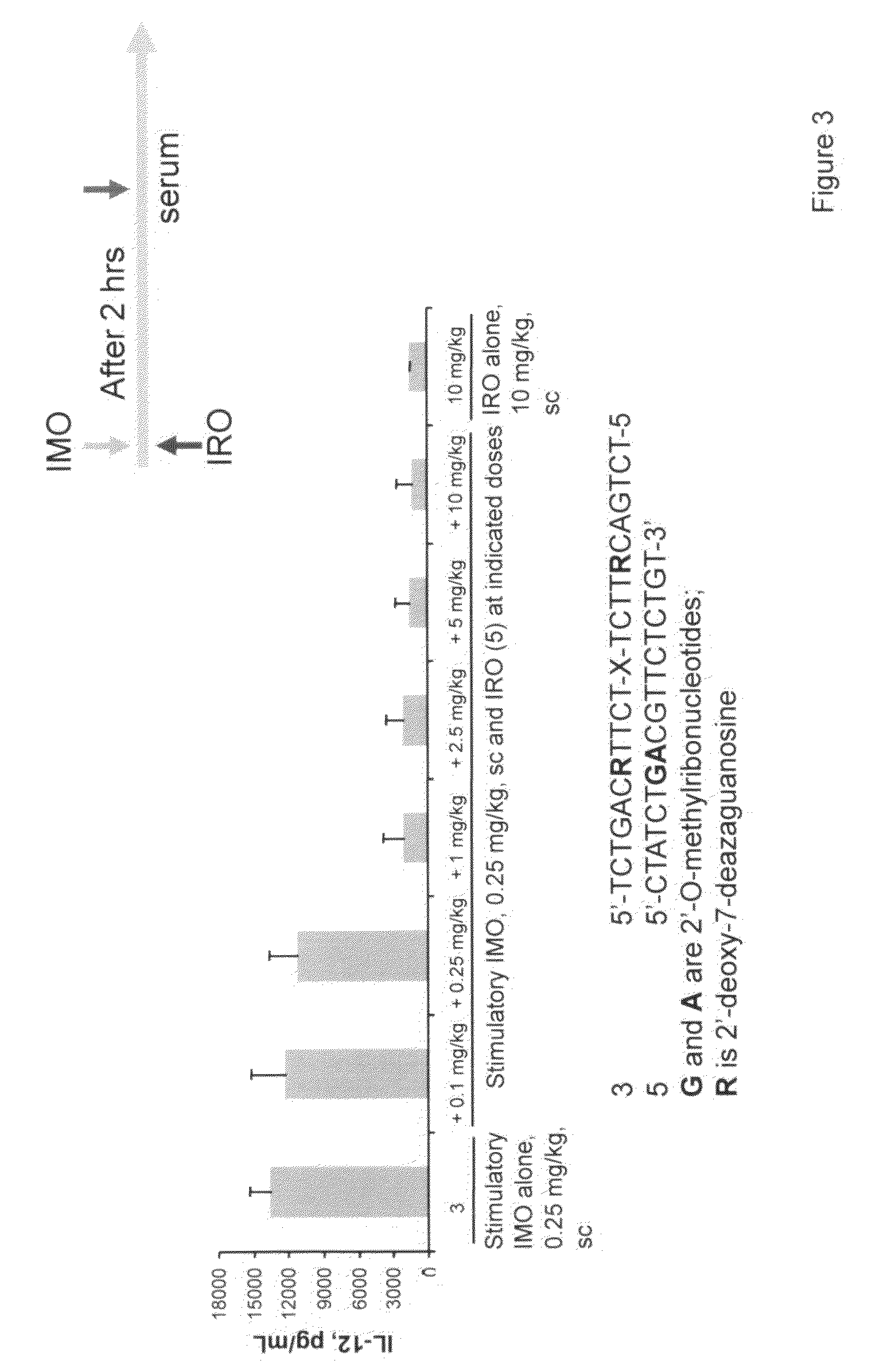
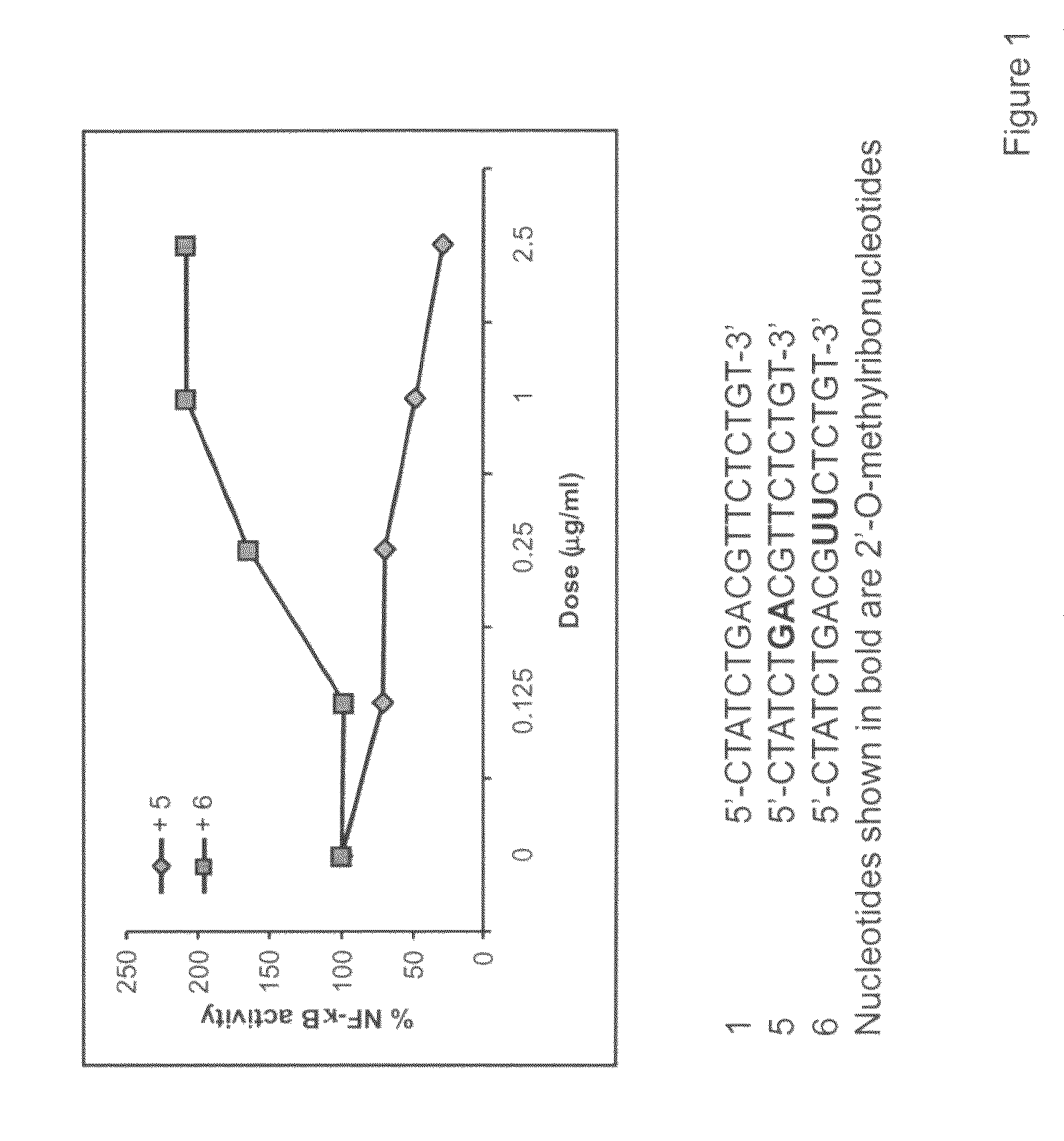
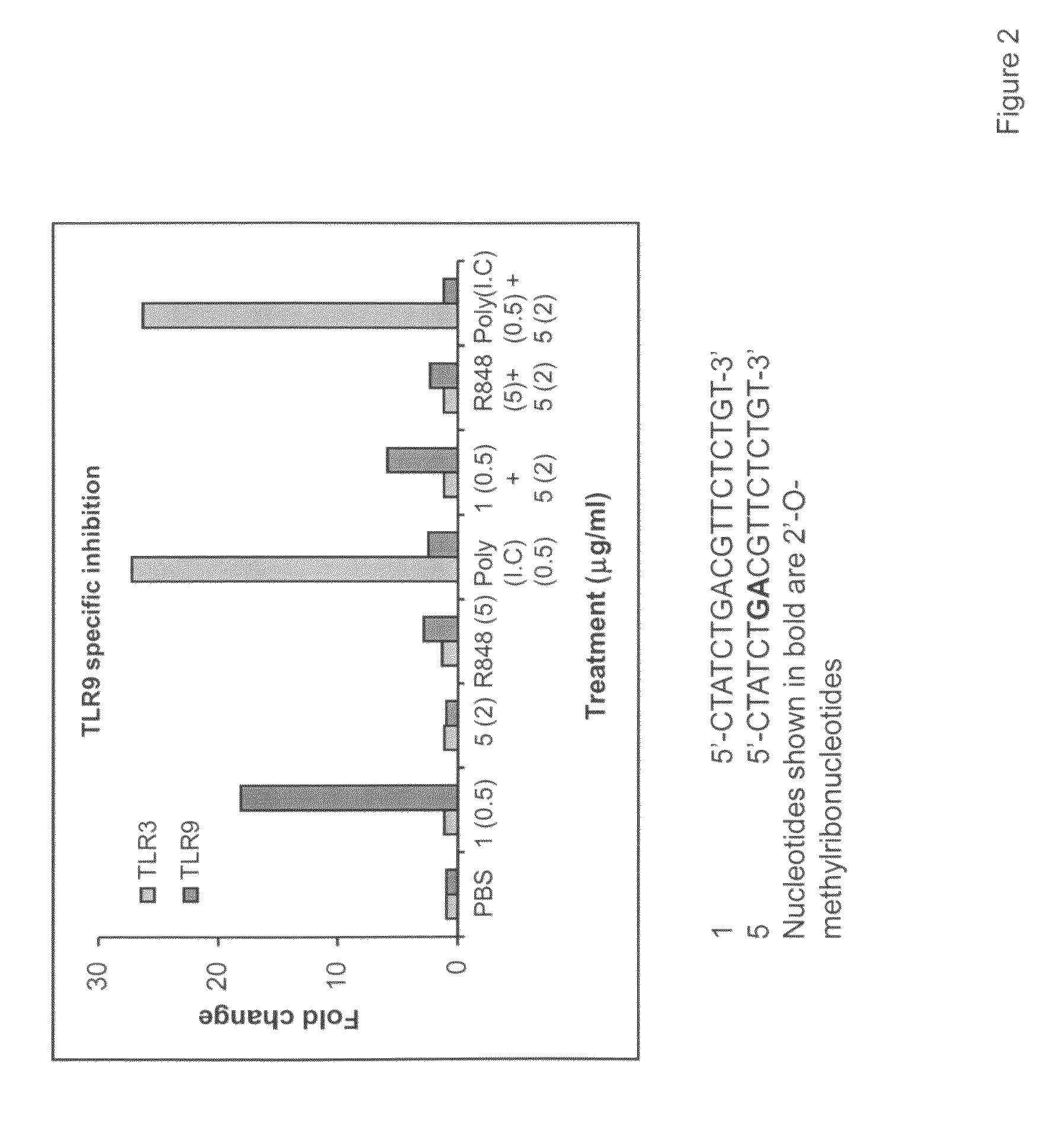
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
Lotus Fuzhuan brick tea and preparation method thereof
The invention relates to lotus Fuzhuan brick tea and a preparation method thereof, in particular to a polymorphic and novel black tea preparation method by optimally combining genuine big leaf tea raw materials in Anhua (China) with medicinal and edible plants. The lotus Fuzhuan brick tea has the characteristics of black tea raw materials in local Anhua, ensures that the original ecological attributes of black tea products, has the obviously functions of nourishing yin to replenish the kidney, generating liquid to moisten dryness, nourishing yin to enrich fluid, regulating immune system, and has the obvious effects of tonifying the spleen to dispel wetness, smoothing qi for digesting and reducing blood lipid.
Owner:贺志弘 +2
Cell preparation for treating osteoarthritis and preparation method thereof
InactiveCN104958320AGood reliefEasily damagedSkeletal disorderMammal material medical ingredientsJoint cavityMesenchymal stem cell
The invention provides a cell preparation for treating osteoarthritis and a preparation method thereof. The cell preparation mainly comprises mesenchymal stem cells, differentiation-induced stem cells, platelet-rich plasma and dispersion media thereof. Compared with the prior art, the cell preparation for treating osteoarthritis has the advantages that the mesenchymal stem cells are mixed with the differentiation-induced stem cells and compounded with the platelet-rich plasma and can be dispersed in the joint cavity, so that the immune regulation function of the mesenchymal stem cells can be exerted, the microenvironment of the whole joint cavity can be improved, and inflammation remission and injury repair are facilitated; tissues can be repaired after the differentiated mesenchymal stem cells are implanted into the body, so that the repair process is shortened.
Owner:广东佰鸿干细胞再生医学有限公司
Immune modulating compounds from fungi
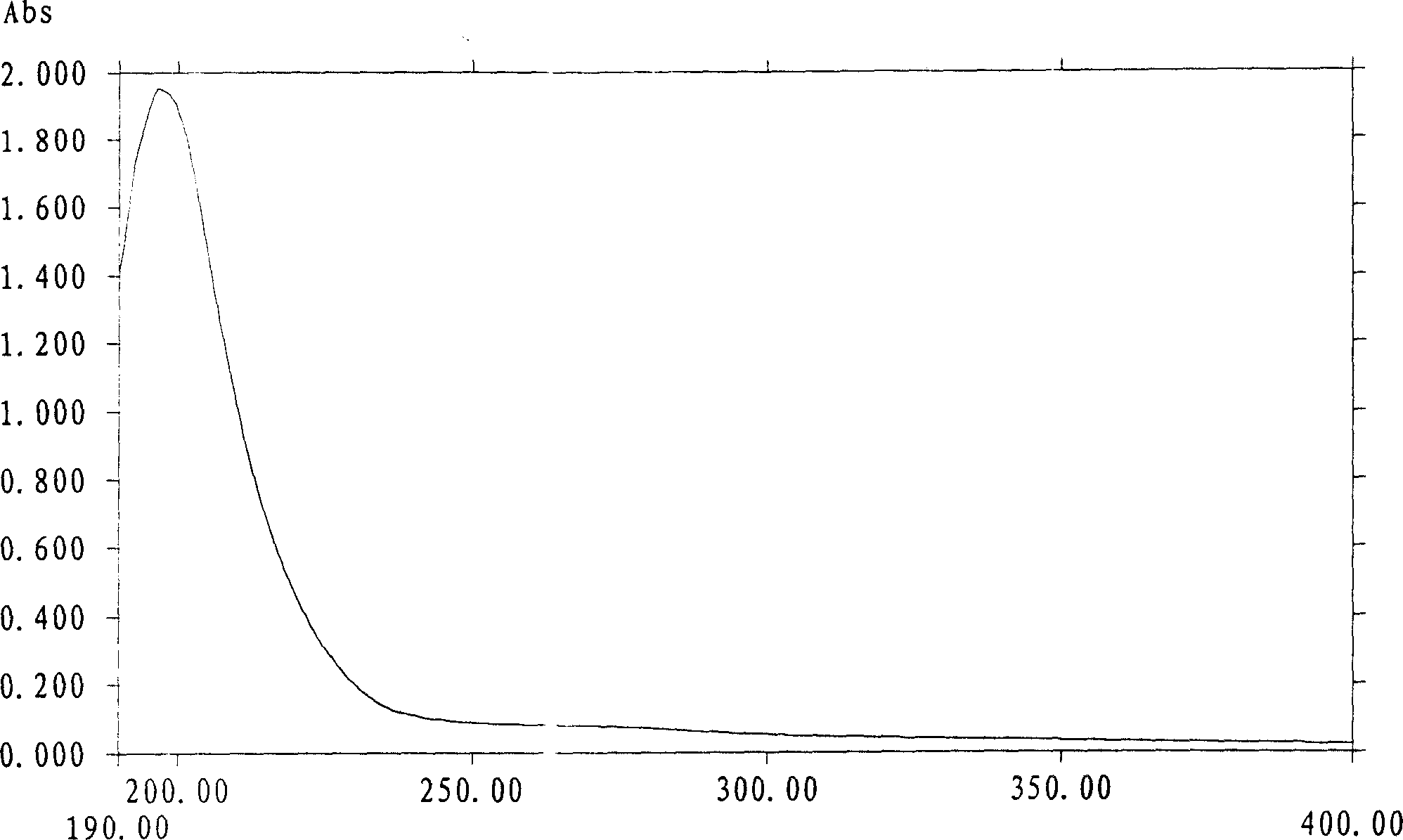
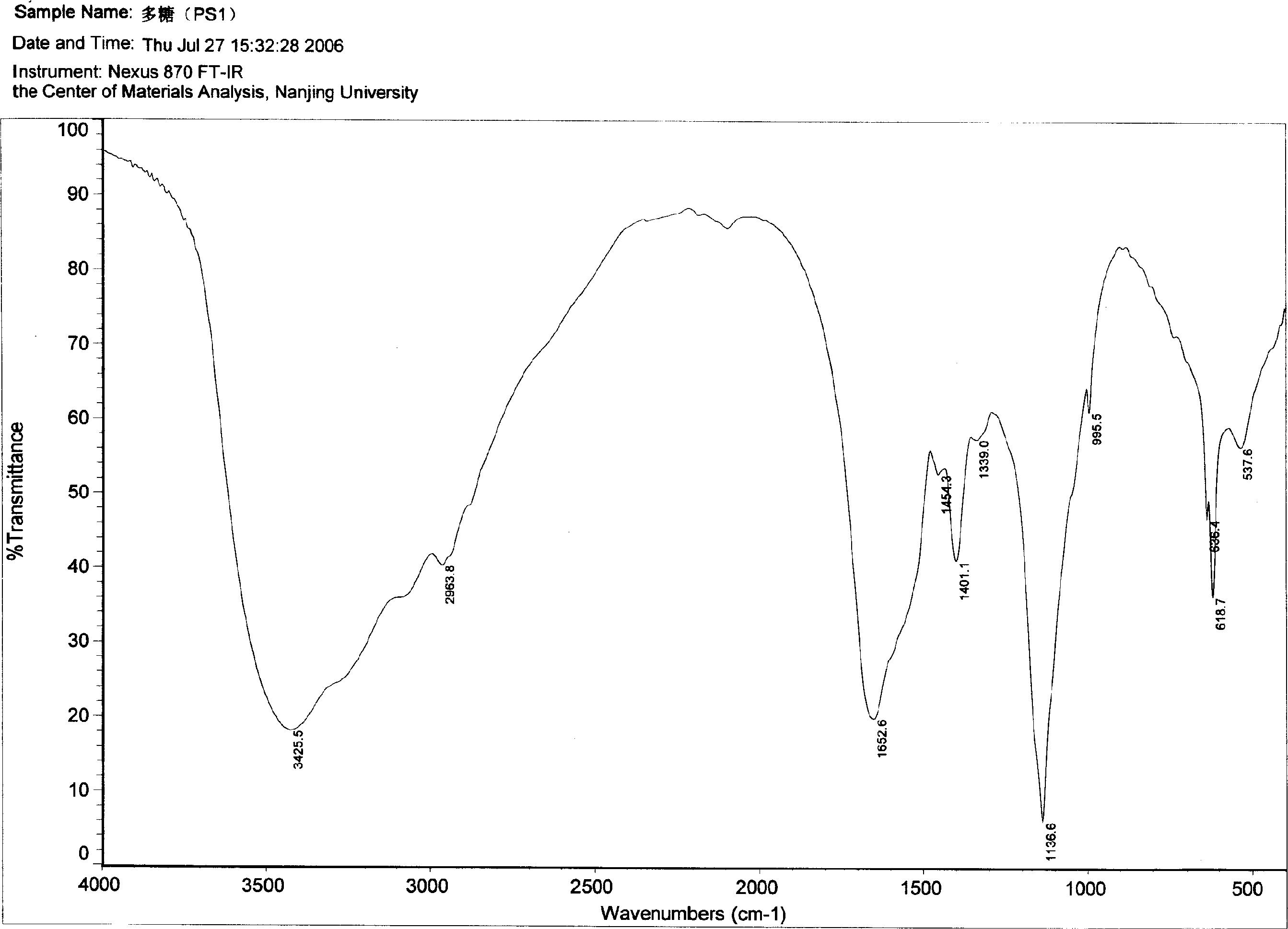
The present invention relates to compositions comprising polypeptides and polysaccharides. The compositions are in general immune modulating. The invention also discloses methods of producing these compositions using filamentous fungi cultivated in liquid medium. The compositions are useful for example in the treatment of immune compromised conditions.
Owner:GLYCANOVA
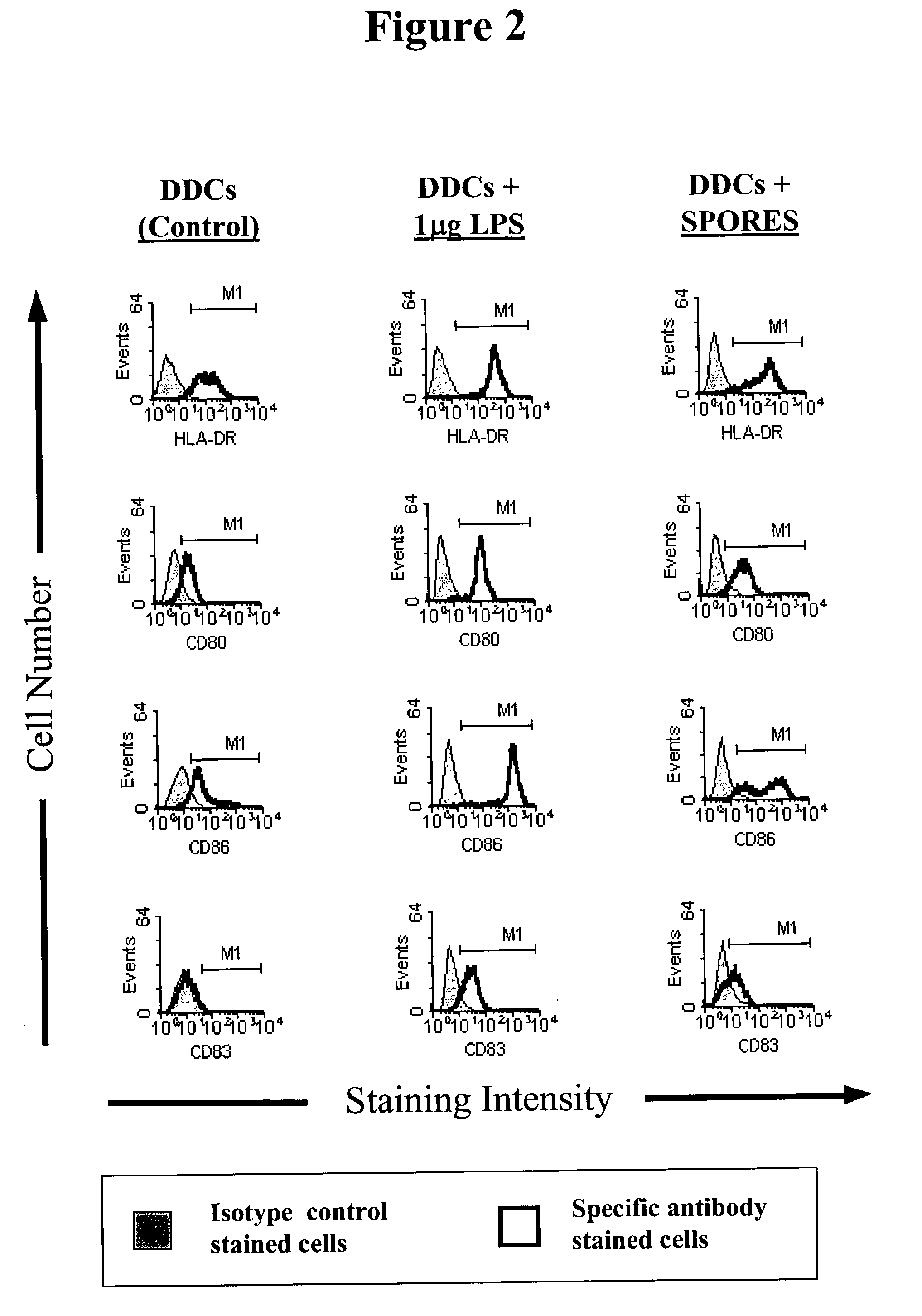
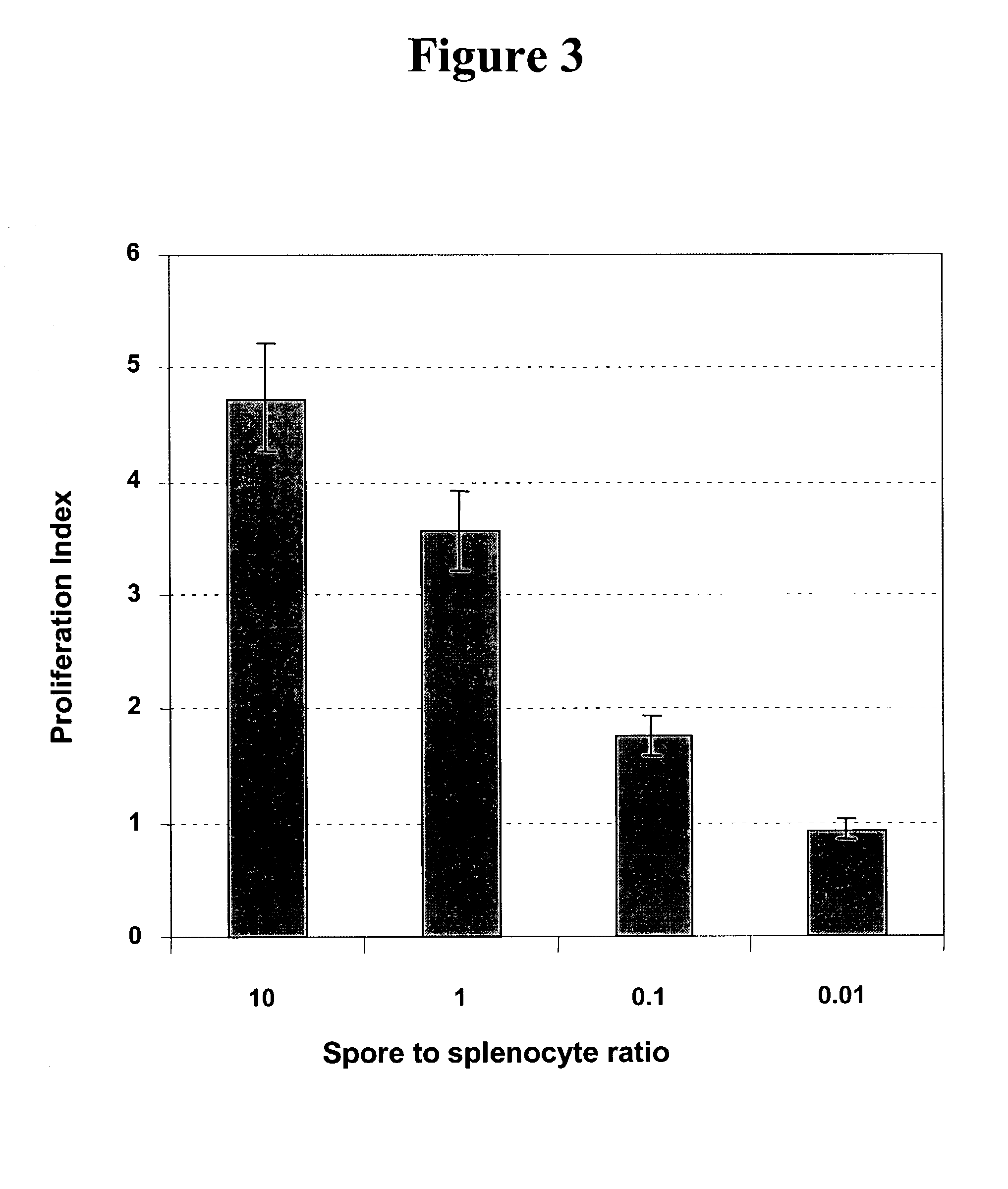
Composition and corresponding method of using spores of Bacillus subtilis to stimulate and/or enhance immune response in mammals
Disclosed is a composition for administration to a human or animal subject, the composition containing spores of Bacillus subtilis in an amount effective to stimulate immune responsiveness in the subject. The spores have an adjuvant, immunomodulatory, immune potentiation, or dendritic cell maturation effect, or a combination thereof Also disclosed is a method of boosting an immune response in an animal subject by administering B. subtilis spores to an animal or human subject. Further disclosed is a vaccine containing B. subtilis spores, in the presence or absence of an antigen.
Owner:KING'S COLLEGE LONDON
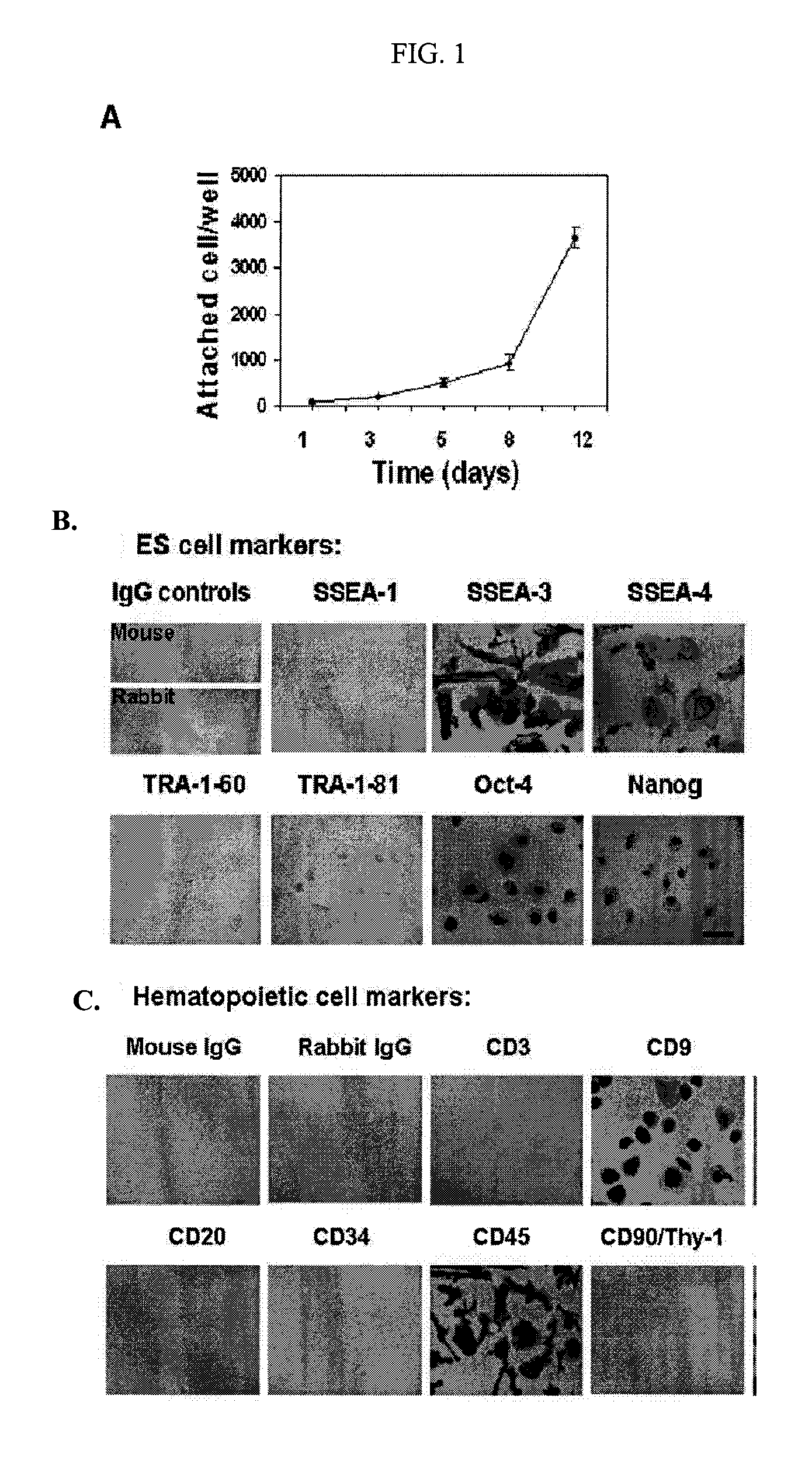
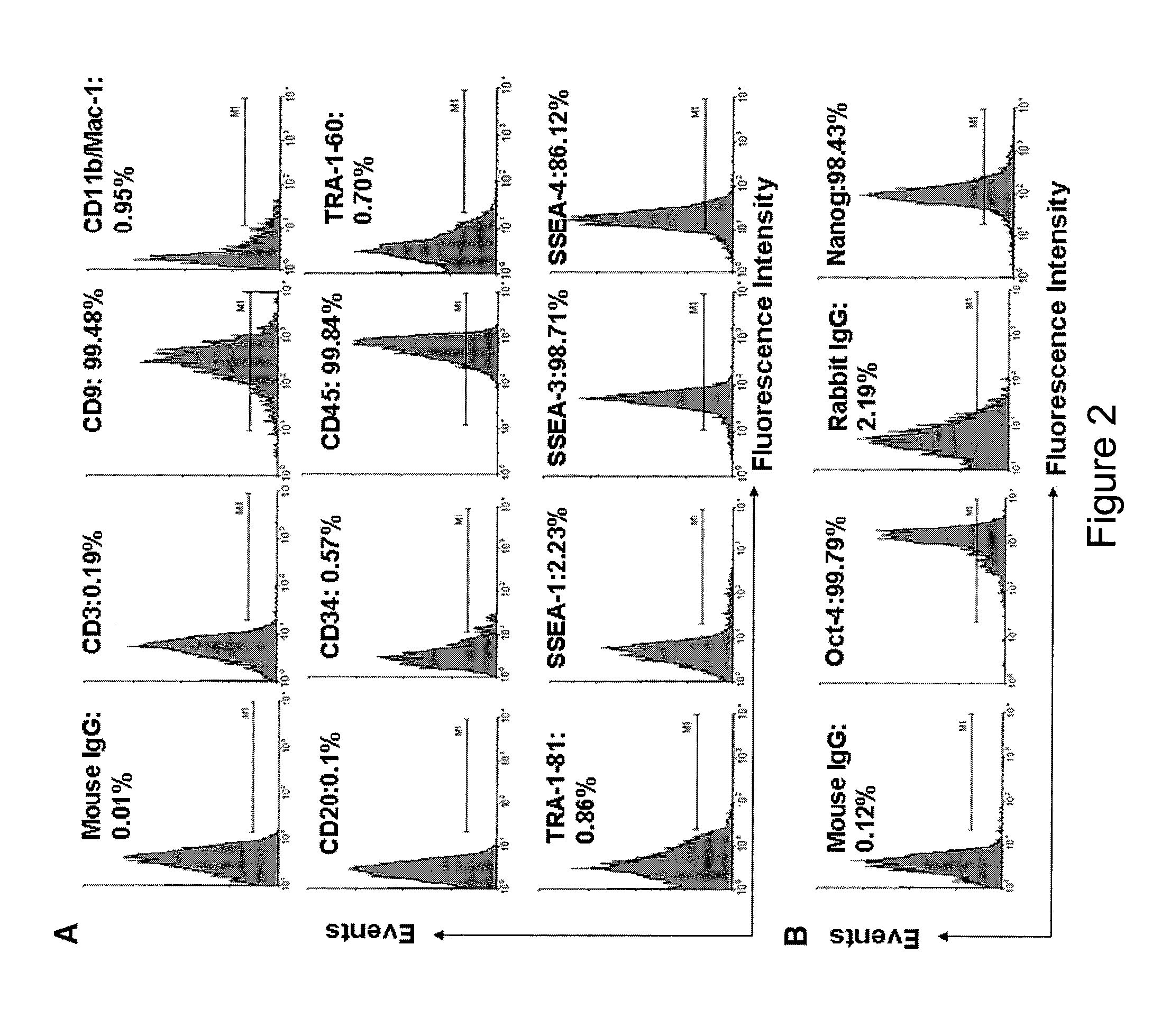
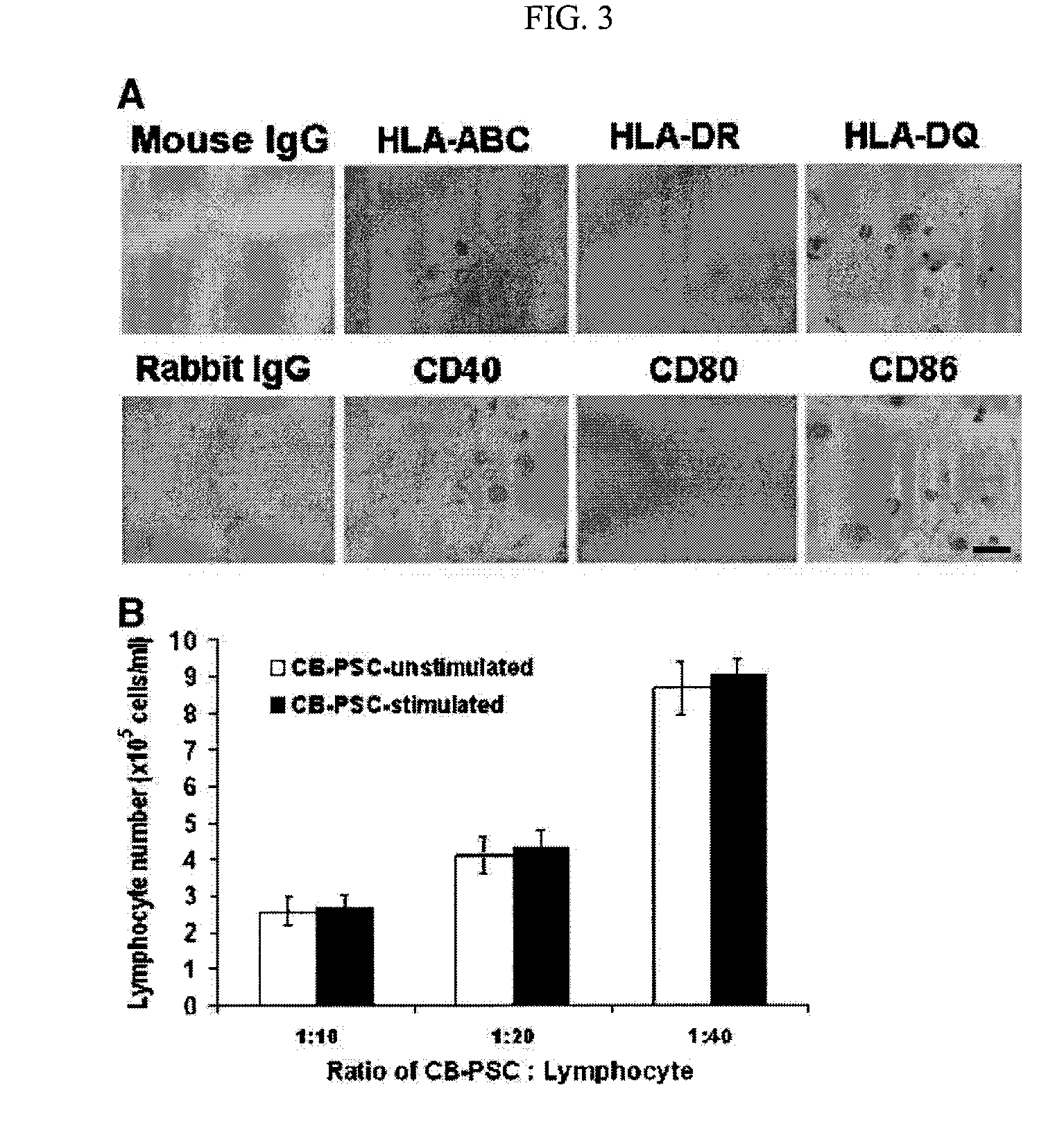
Isolated Embryonic-Like Stem Cells Derived From Human Umbilical Cord Blood
ActiveUS20090175832A1Low immunogenicityAbility to proliferateBiocideMetabolism disorderHematopoietic cellAutoimmune condition
The present invention is related generally to embryonic-like stem cells isolated from human umbilical cord blood, designated herein as cord blood-stem cells (CB-SC's), which display the characteristics of embryonic stem cells and hematopoietic cells. These cells have the capability of proliferation and are able to differentiate to multiple types of cells. In addition, the CB-SC display low immunogenicity and immune regulation. These cells are, therefore, suitable for use in stem cell-based therapies for the treatment of diseases such as Parkinson's disease, diabetes, spinal cord damage, multiple sclerosis, cardiovascular disease, stroke and birth defects, and for preventing, treating and / or reducing an autoimmune disease in a mammalian subject.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
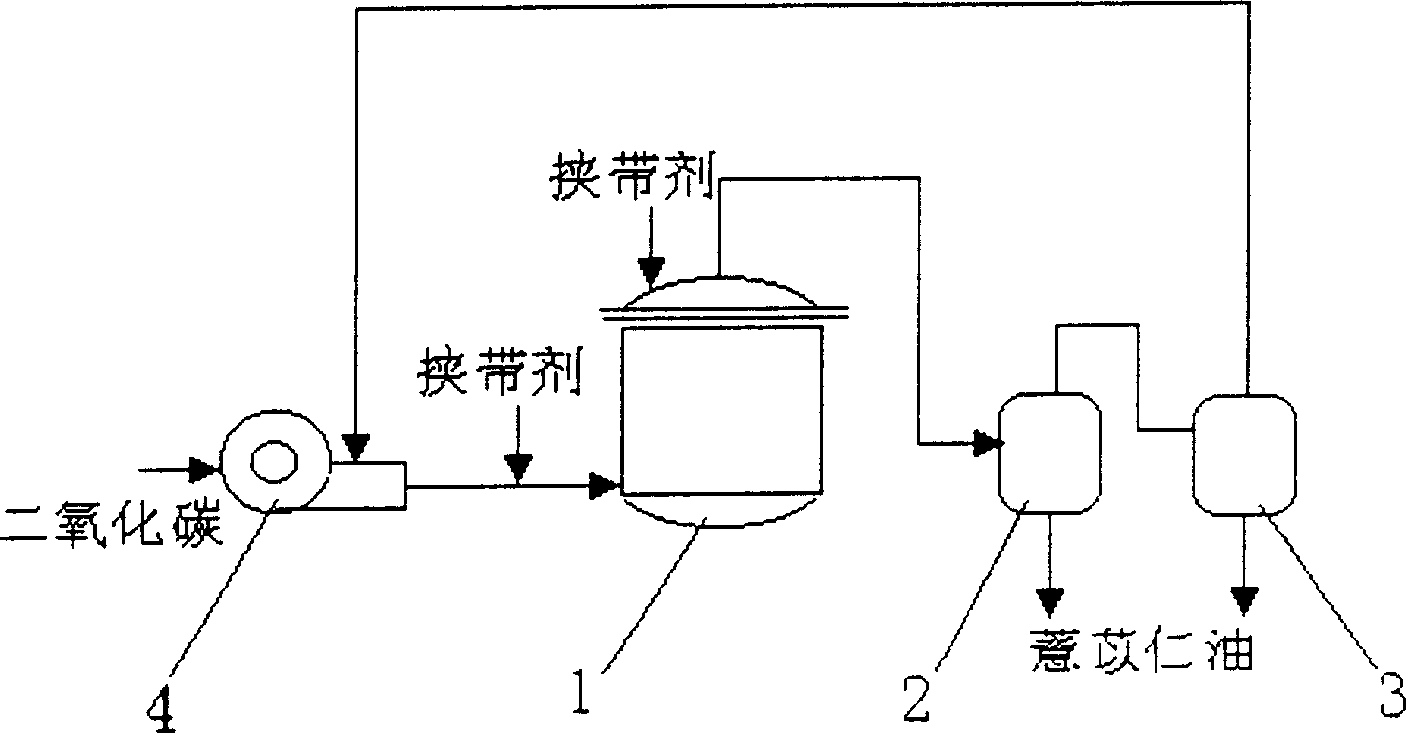
Process for extracting coix seed oil by using supercritical CO2 extraction method
InactiveCN1502680AImprove solubilityHigh yieldFatty-oils/fats productionFunctional foodExtraction methods
The present invention discloses a method for extracting coix seed oil by using supercritical CO2 extraction process, and is characterized by that it makes the CO2 being in supercritical state by matched with entraining agent to implement extraction of coix seed, and its yield of coix seed oil can be up to above 8.18%. Said coix seed oil contains lots of unsaturated fatty acid and protein, can be used for preparing the medicine for curing cancer, making immune regulation, reducing blood fat, relieving inflammation and relieving cough, at the same time can be used for developing various function health-care foods.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
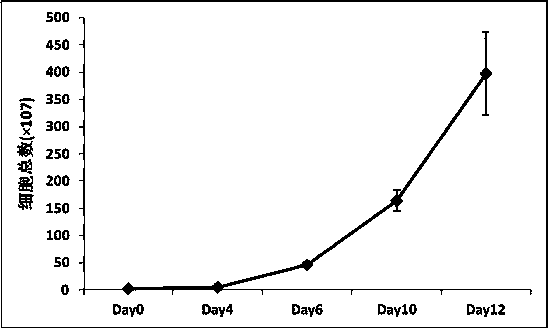
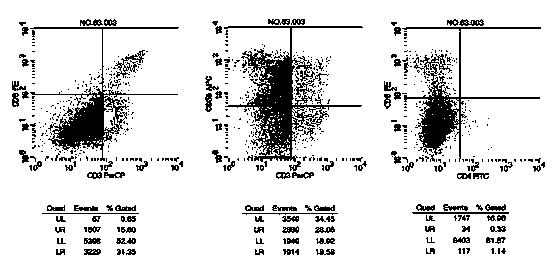
Culture medium efficiently amplifying autologous NK cells and cultural method
The invention relates to a culture medium efficiently amplifying autologous NK cells and a culture method. The culture medium internally contains interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNFalpha) and CD3-antibody, can efficiently amplify and activate NK cells so as to obtain a large quantity of highly active immune cells with majority of NK cells to be transferred back to human bodies, and has remarkable curative effect in anti-tumor, anti-virus infection and immune adjustment related diseases. The culture medium efficiently amplifying autologous NK cells comprises the culture medium for cultivating cells and added factors added into the culture medium for cultivating cells, is used for cultivating and amplifying a large quantity of autologous activated NK cells, and is characterized in that the added factor comprises interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNF alpha) and CD3-antibody.
Owner:康思葆(北京)生物技术有限公司
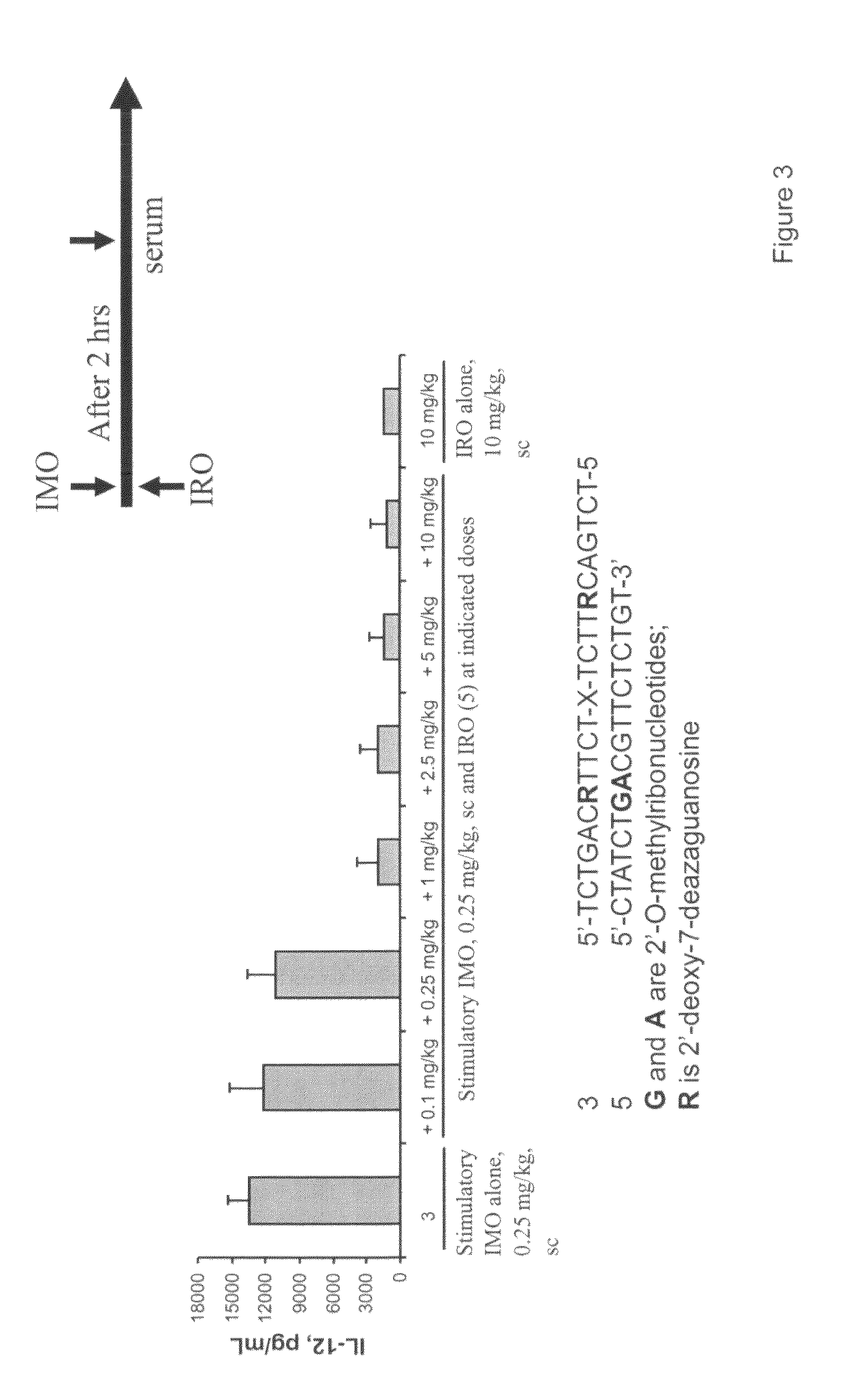
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Giant salamander functional food with immune regulation action and preparation method thereof
InactiveCN101720933AMaintain nutritional activityLong shelf lifeFood preparationDiseaseEnzymatic hydrolysis
The invention discloses a giant salamander functional food with an immune regulation action and a preparation method thereof. The giant salamander functional food is prepared by obtaining giant salamander enzymatic hydrolysis powder by carrying out treatment of cell disruption, enzymatic hydrolysis and spray drying on giant salamander meat and then mixing the giant salamander enzymatic hydrolysis powder with extractive powder of medical and edible dual purpose plants including medlar, jujube and hawthorn and auxiliary materials according to a certain proportion. The preparation method keeps the nutrition activity of the giant salamander meat to the maximum extent, enhances the nutrition utilization ratio of a human body and also has the advantages of simple preparation process, convenient operation, safety, reliability, low production cost, and the like. The giant salamander meat functional food has the effects of eliminating oxygen free radicals, delaying aging and enhancing the immunity, is combined with the nourishing function of other medical and edible dual purpose plants so as to enhance the resistant ability of the human body to various diseases through long-term eating, strengthen the adaptability of the human body to the environment and be beneficial to the recovery of a patient after a surgery or with a weak constitution and is an ideal functional food.
Owner:张家界金鲵生物工程股份有限公司
Anti-CD70 Antibody And Its Use For The Treatment And Prevention Of Cancer And Immune Disorders
ActiveUS20090232806A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer preventionCD70
Owner:SEAGEN INC
Immune modulatory activity of human ribonucleases
InactiveUS20040009503A1Microbiological testing/measurementBiological material analysisPhases of clinical researchWhite blood cell
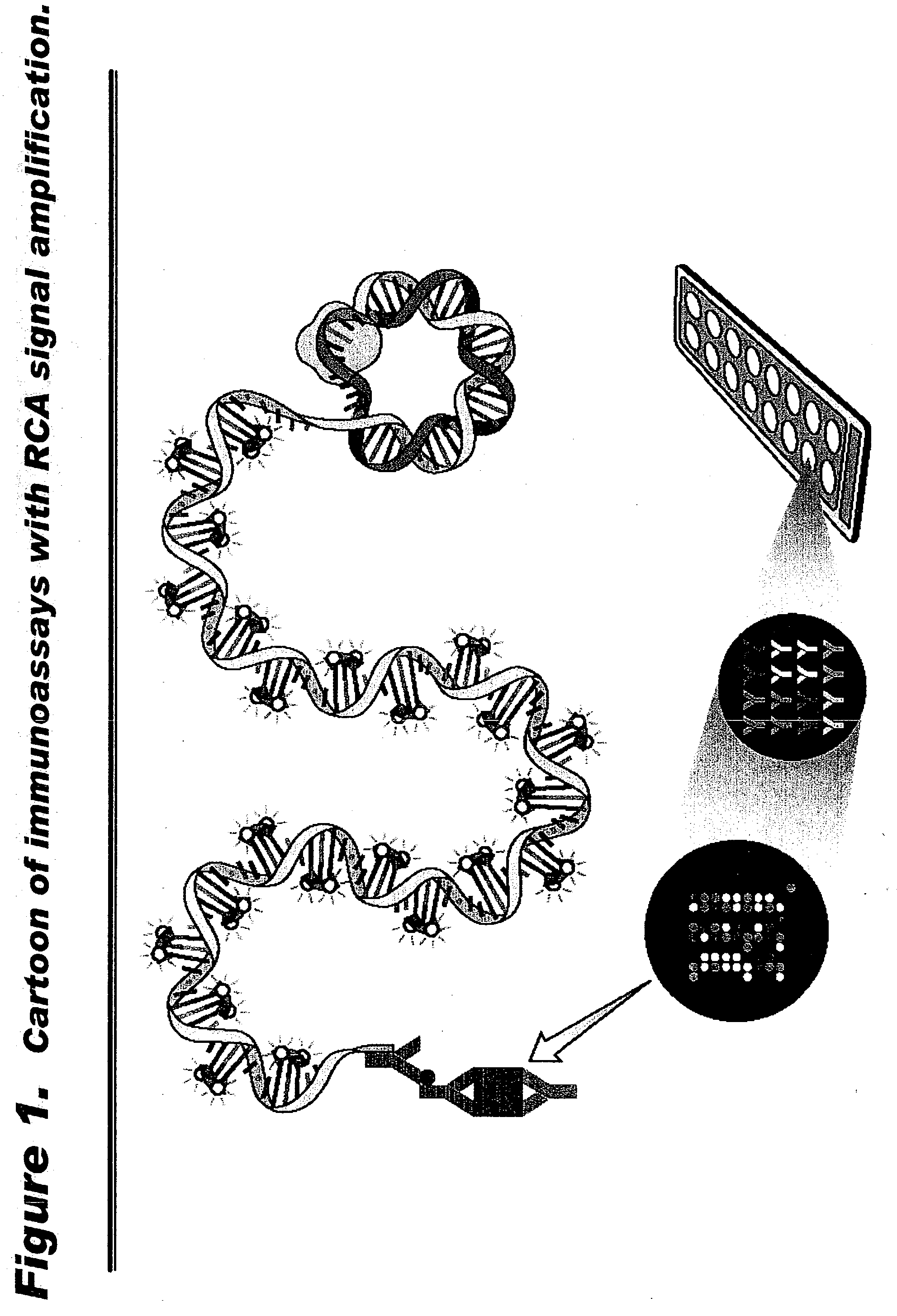
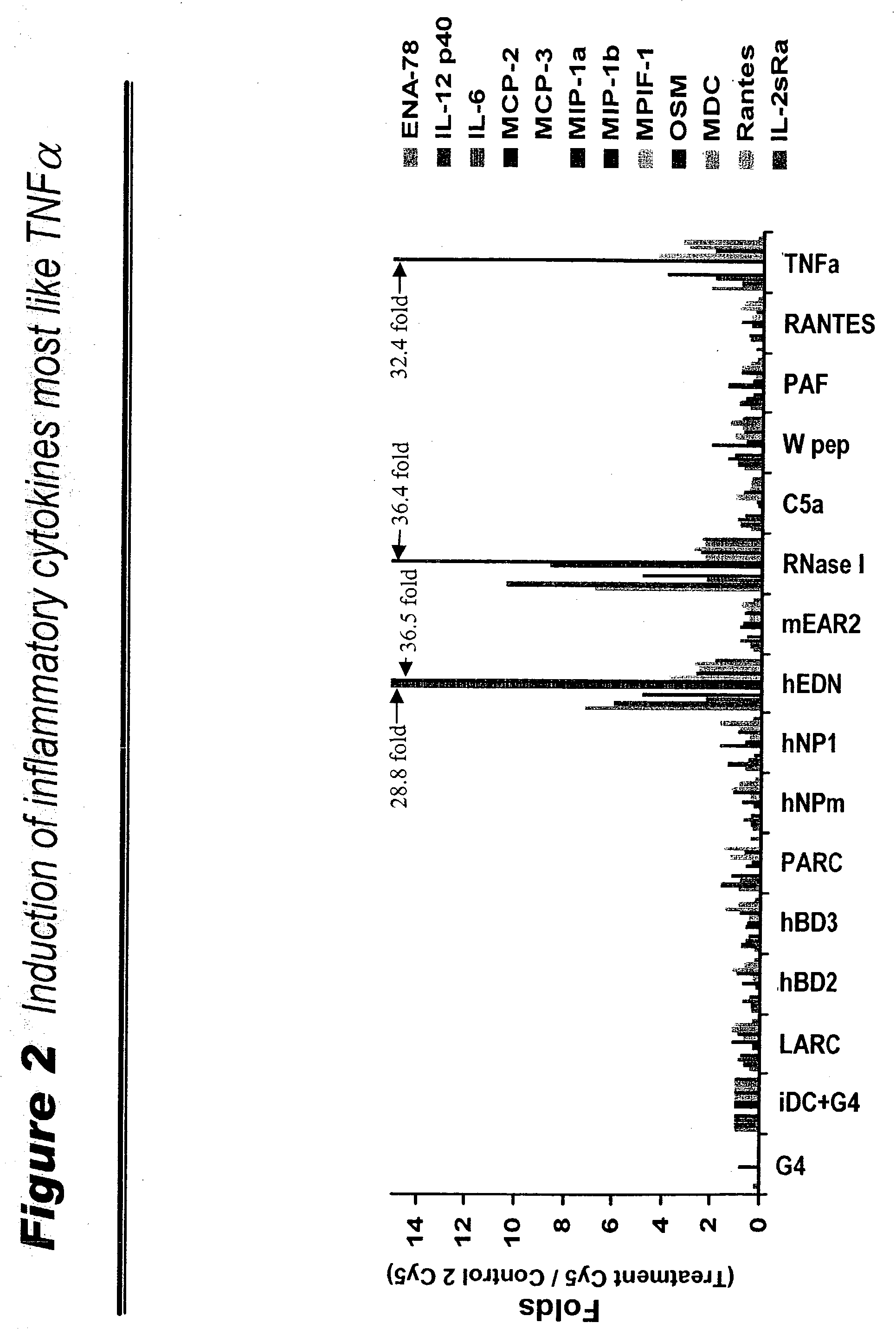
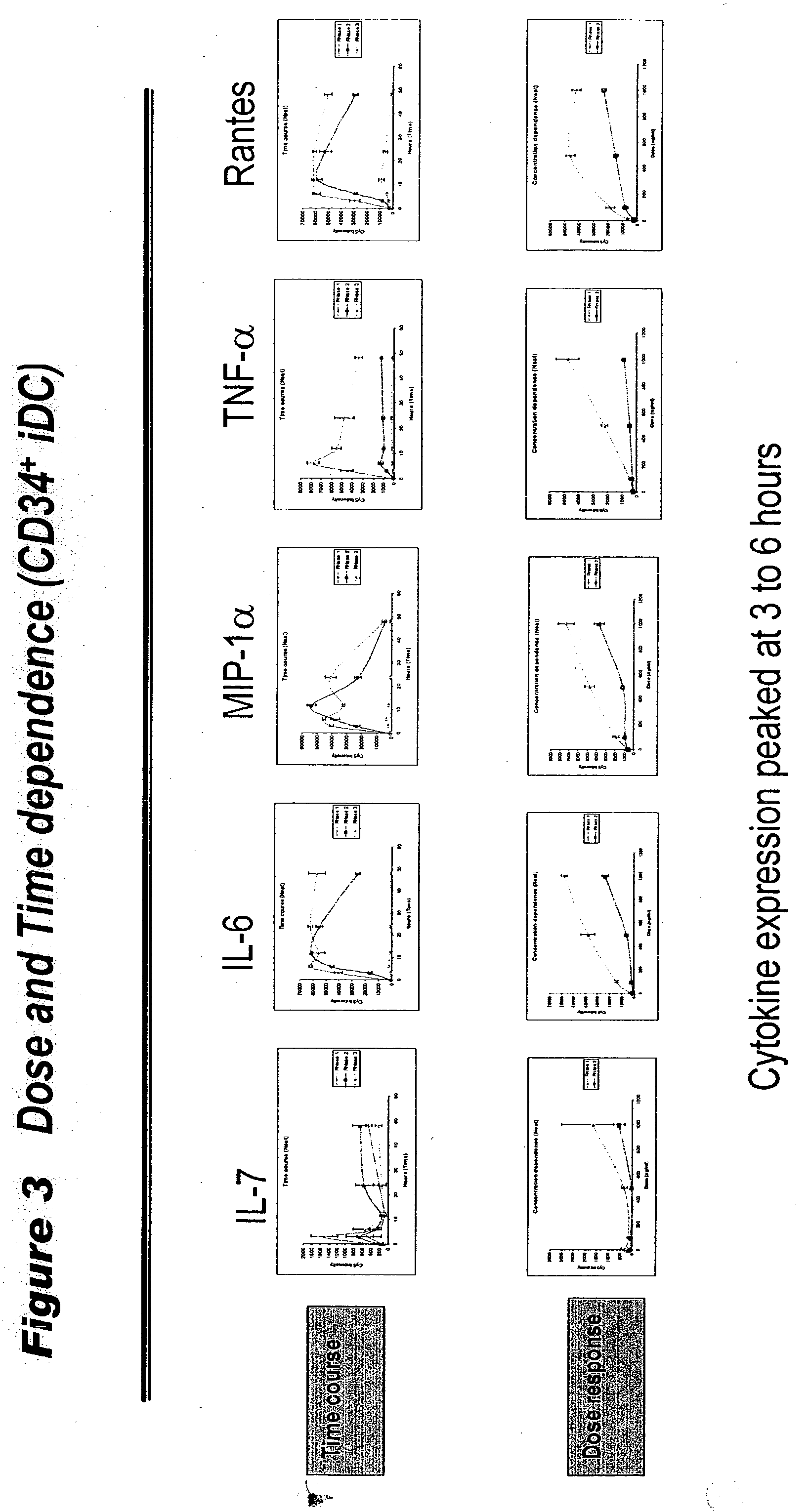
Human extracellular ribonucleases (RNases) are widely distributed in various organs and body fluids and together with other members of the mammalian RNase A superfamily. In addition to their RNase activity, several RNases have been shown to have special biological actions, i.e., antitumor, antiviral and angiogenic properties. However, the molecular mechanisms of such activities are unclear. Using protein microarrays amplified rolling circle amplification (RCA), we investigated the effects of EDN (Rnase 2), ECP (Rnase 3) and RNase 1 on leukocytes cytokine production. We measured the levels of 78 different cytokines and growth factors in culture supernatants to determine the cytokine profiles of cells treated with different combinations of RNases and RNase inhibitors. Members of human ribonuclease family (such as Rnase 1, hEDN (Rnase 2) and Rnase 3) induced expression of certain sets of cytokines in human leukocytes, including ENA-78, EOT2, BLC, GDNF, 1309, IFN-alpha, IFN-gamma, IL-10, IL-12P40, IL-12p70, IL-13, IL-16, IL-18, IL-1beta, IL-1ra, IL-2Sra, IL-3, IL-6, IL-6sR, IL-7, IL-8, IP-10, MCP-1, MCP-2, MCP-3, MCSF, MIG, MDC, MIP-1alpha, MIP-1beta, MPIF-1, NAP-2, RANTES, sCD23, OSM, TARC, TNF-alpha, TNF-R1 and uPAR. Thus members of the Rnase superfamily are therapeutic targets for treatment of inflammatory diseases and clinical conditions. Inhibition or augmentation of Rnase expression is used to modulate the immune system and is beneficial for host defense against various diseases and is exploited as an adjuvant. The expression of Rnases is a diagnostic marker for inflammation related conditions and is used to determine various disease stages. In addition, expression of cytokines, chemokines, growth factors is used to monitor efficacy of Rnase-base therapies.
Owner:PATHWAY DIAGNOSTICS CORP INC
Methods of administering microencapsulated materials for immune modulated diseases
InactiveUS7105158B1Reduce doseInhibit cytokine synthesisOrganic active ingredientsAntibody ingredientsDiseaseMicrosphere
Compositions useful in treating immune modulated disease comprising an anticytokine antibody or immune active drug capable of modifying cytokine activity or modulating the immune system microencapsulated with a biodegradable nonantigenic material, such as albumin or PLGA. When the composition is introduced into a subject, it is phagocytosed by the target organ, the target organ digests the microsphere, releasing the drug or an active form or fragment thereof intracellularly. The drug then modifies the target organ function, thereby modulating it's activity. A method is disclosed for preparation of the microencapsulated composition.
Owner:OF MERCER UNIV THE
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
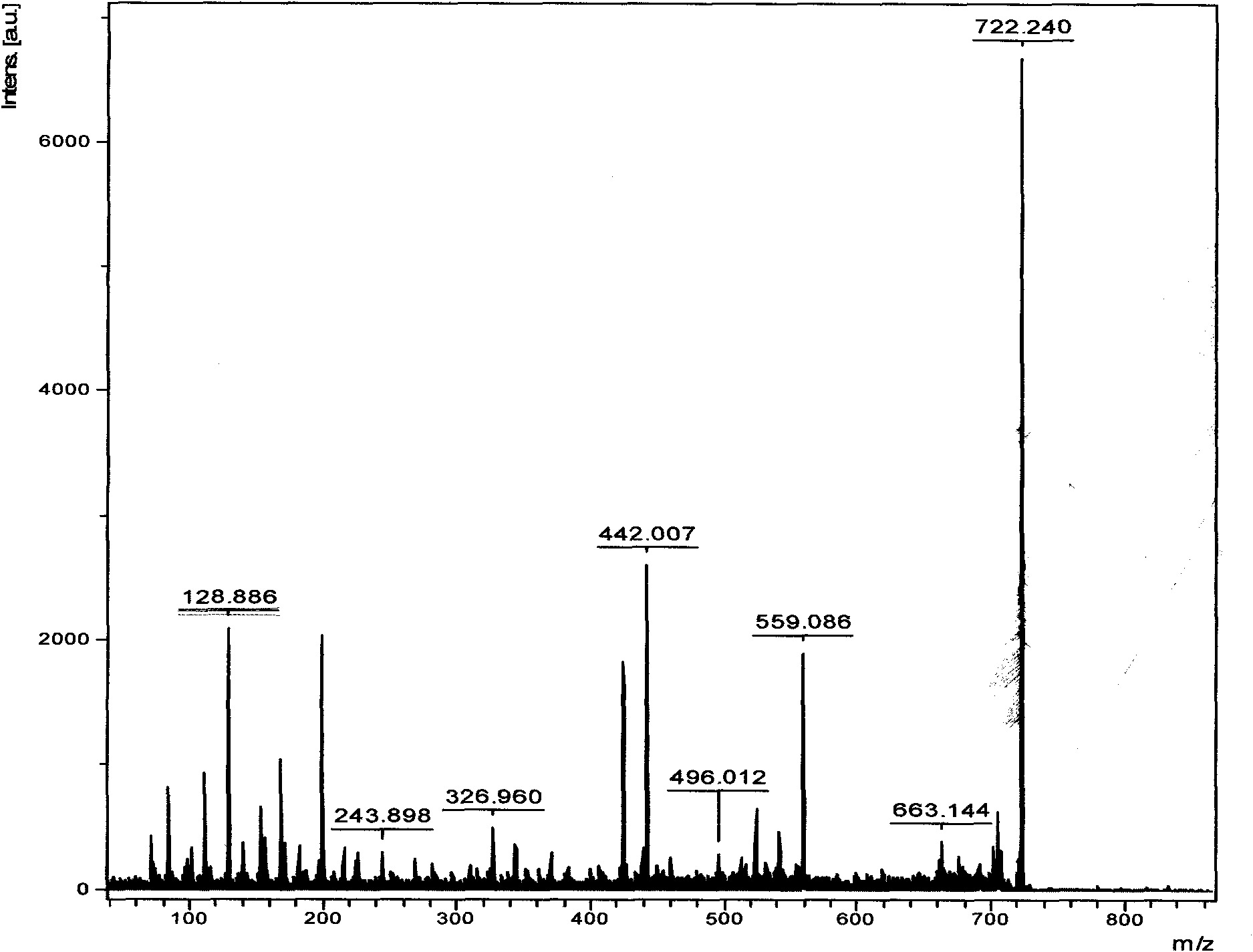
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Haw vinegar healthy beverage and preparing process thereof
The invention provides a hawthorn vinegar healthy beverage, which is characterized by comprising the following raw materials in weight portion: 40 to 80 portions of hawthorn, 40 to 80 portions of honey, 40 to 80 portions of xylitol, 50 to 100 portions of white vinegar, 0.25 to 0.5 portion of aspartame, and 650 to 850 portions of water, and the hawthorn vinegar is prepared by a certain method. The hawthorn vinegar has disease prevention efficacy of table vinegar, good flavor of juice, palateful sweet and sour and sweet taste, and is suitable to be directly drunk. The hawthorn vinegar is rich in various amino acids, organic acid, vitamin, mineral and other nutritious ingredients, and has efficacies of blood-fat reduction, vessel relaxing, oxidation resistance, immunity regulation, harmful bacteria elimination, balance maintenance for blood acid alkali, weight reduction, digestion help and sobering up and the like.
Owner:昆明振华制药厂有限公司
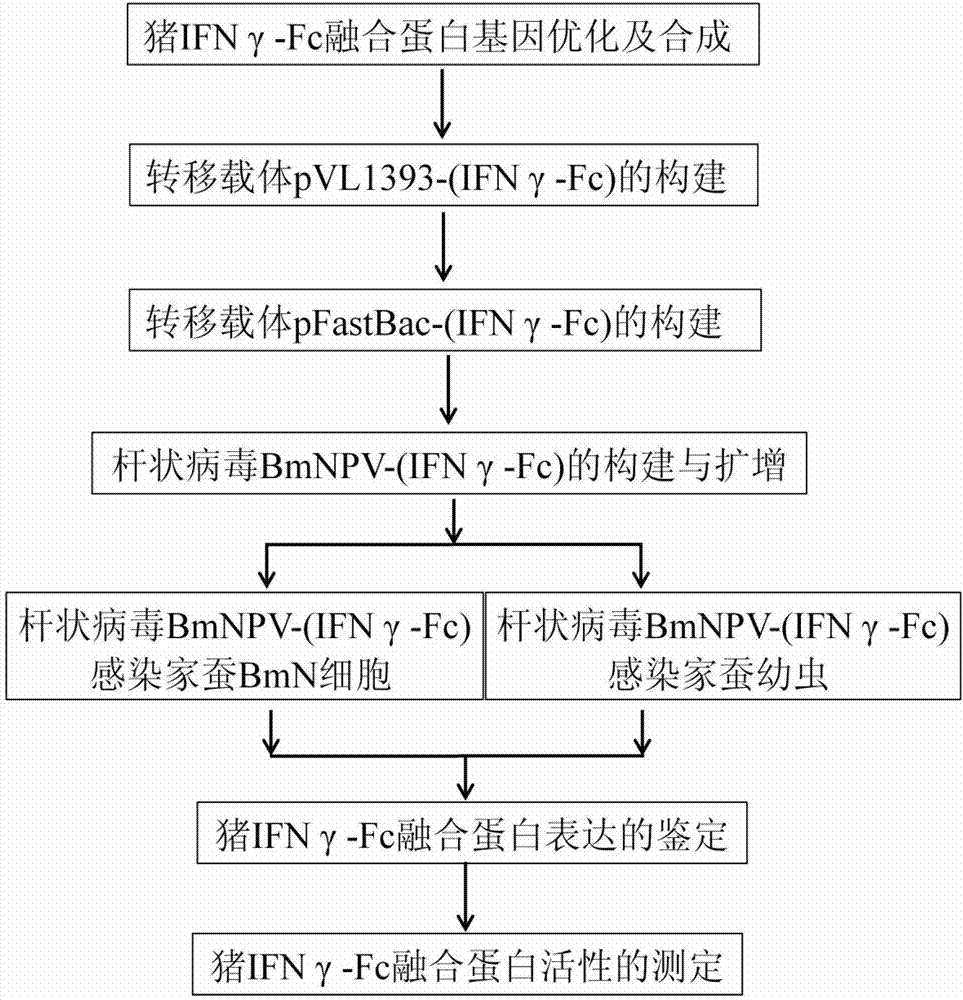
Pig IFN (interferon) gamma-Fc fusion protein as well as coding gene and expression method of pig IFN (interferon) gamma-Fc fusion protein
ActiveCN103087200AReduce manufacturing costPrecise structurePeptide/protein ingredientsAnimal feeding stuffBiotechnologyProtein target
The invention provides a pig IFN (interferon) gamma-Fc fusion protein optimized according to a silkworm reaction system and a coding gene of the pig IFNgamma-Fc fusion protein and a method of expressing pig IFNgamma-Fc fusion protein by using a silkworm bioreactor, and belongs to the field of a biological genetic engineering. The pig interferon gamma serving as a non-special broad spectrum antiviral biological agent has a wide medicinal prospect in a veterinary drug field; but like most of genetic engineering veterinary drugs, the pig interferon gamma also has the problems such as insufficient output, expensive price, irregular quality, short acting time and the like. A target protein with a high expression efficiency and strong activity is obtained by expressing the pig IFNgamma-Fc fusion protein optimized by using a silkworm expression system. In addition, the acting time of the pig interferon gamma is prolonged by addition of an Fc segment, so that the overall immune regulation effect is enhanced, and purification at a later stage is facilitated, thus a pig IFNgamma-Fc fusion protein preparation produced by using a genetic engineering method is possible, and a condition is also created for developing a novel feed containing the fusion protein.
Owner:江苏晶红生物医药科技股份有限公司
Immune regulation based on the targeting of early activation molecules
InactiveUS20050002929A1Increase concentrationIncrease proliferative activitySenses disorderNervous disorderDiseaseAgonist
Disclosed are methods of treating subjects having disorders or conditions characterized by an unwanted immune response including administering an effective amount of an early activation molecule agonist, antagonist or depletor, to the subject. Human monoclonal antibodies specific to the early activation molecules, and methods of use, are also disclosed.
Owner:GOMEZ PILAR LAUZURICA +2
Uses of bispecific antibody coated dendritic cells pulsed with antigens and gm-CSF in immune regulation
GM-CSF administered before immunization exerted a sustained suppressive effect against the induction of myasthenia gravis (MG). This suppression was associated with lowered serum autoantibody levels, reduced T cell proliferative responses to AChR, and an expansion in the population of FoxP3+ regulatory T cells. Manipulating DCs to expand regulatory T cells is useful for the control of autoimmune diseases such as myasthenia gravis MG.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Preparation method of human adipose-derived MSCs (mesenchymal stem cells) and application of human adipose-derived mesenchymal stem cell in preparation of medicine for treating diseases
InactiveCN104357383ABroad basic researchSolid basic researchAntipyreticMetabolism disorderAutoimmune conditionSide effect
The invention discloses a preparation method of human adipose-derived MSCs (mesenchymal stem cells) and an application of the human adipose-derived MSCs in preparation of medicine for treating diseases. The preparation method of the human adipose-derived MSCs comprises steps as follows: adipose tissue collection, adipose tissue transportation, adipose tissue connection, adipose tissue microblock preparation, SVF (stromal vascular fraction) extraction, primary culture, subculturing, cell cryopreservation, cell bank establishment and cell recovery. The human adipose-derived MSCs have a powerful immunity regulation function and can be applied to preparation of medicine for treating autoimmune diseases, and compared with conventional medicine, the human adipose-derived MSCs have the advantages as follows: 1, the treatment is convenient, the curative effect is lasting, the variety and the number of medicine for treatment and toxic and side effects caused by medicine treatment can be reduced, even the medicine such as immunosuppressant and the like for treatment is out of service, the death rate and the disability rate are reduced, and life quality is improved; 2, the safety is good, and toxic and side effects are avoided; 3, the source of adipose tissue is wide, the collection is convenient, fundamental research of the human adipose-derived MSCs is solid, the preparation technology is mature, the popularization degree is high, and the technology is controllable; and 4, the preparation cost of the human adipose-derived MSCs is lower than that of other biological preparation and can be accepted by most patients.
Owner:张炳强
Preparation for amylovorin of bacillus and application thereof
InactiveCN1908182AIncrease productionHigh yieldOrganic active ingredientsBacteria material medical ingredientsLymphocyteWhite blood cell number
the invention discloses a preparing method of extracellular polysaccharide of Bacillus subtilis and application in the tumour facet, which is characterized by the following: inhibiting tumour from growing; accelerating the growth of macrophage, leucocyte and lymph cell; fitting for health or immune adjusting drug.
Owner:NANJING UNIV
Drug-carrying liposome modified by antimicrobial peptide and preparation method and application thereof
ActiveCN107441501AGood treatment effectHas an immunomodulatory effectAntibacterial agentsOrganic active ingredientsAntibiotic resistanceAntimicrobial peptides
The invention belongs to the technical field of biological medicines, and particularly relates to drug-carrying liposome modified by antimicrobial peptide and a preparation method and application thereof. The liposome modified by the antimicrobial peptide is provided, the antimicrobial peptide is modified by a hydrophobic segment, the hydrophobic modified antimicrobial peptide is prepared by coupling the hydrophobic segment (or named as nitrogen tail end coupling a hydrophobic compound) at the nitrogen tail end, the amino acid sequence of the antimicrobial peptide is VQWRIRVAVIRK-NH2 beginning from the nitrogen tail end. The drug-carrying liposome is further provided, the drug-carrying liposome is obtained by loading antibiotics on the liposome. According to the liposome which are loaded with antibiotics, the antimicrobial peptide and the antibiotics are loaded into the liposome simultaneously, and good antibacterial activity in vivo, the immune regulation effect and good safety are achieved; and the liposome which are loaded with antibiotics is an ideal candidate drug for treating MRSA, and relieving of antibiotic resistance is facilitated, and new thoughts and strategies are provided for treating bacterial infection.
Owner:SICHUAN UNIV
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
Owner:IDERA PHARMA INC
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
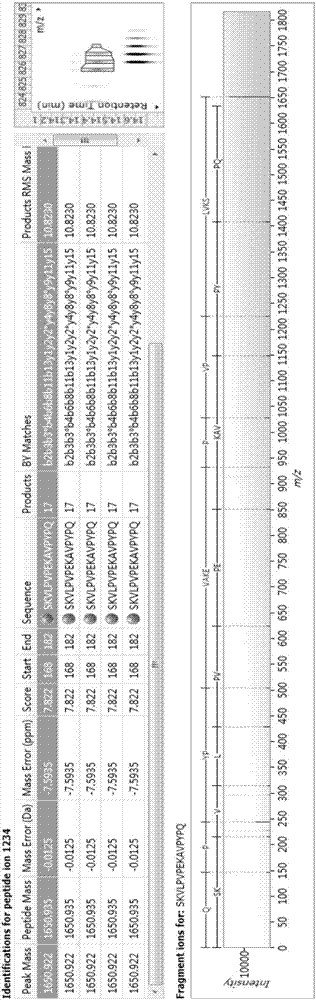
Bioactive peptide SKVLPVPEKAVPYPQ as well as preparation method and application thereof
ActiveCN107176995ARegulate immunityModulate immune activityCosmetic preparationsPeptide/protein ingredientsLife qualityVal-Leu-Pro-Val-Pro
The invention relates to the field of protein, in particular to a bioactive peptide SKVLPVPEKAVPYPQ as well as a preparation method and an application thereof. An amino acid sequence of the bioactive peptide SKVLPVPEKAVPYPQ is Ser-Lys-Val-Leu-Pro-Val-Pro-Glu-Lys-Ala-Val-Pro-Tyr-Pro-Gln. An in-vitro anti-oxidation experiment and an in-vitro immunity function promoting experiment prove that the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation biological activity and immunity regulation function, on one hand, the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation activity, can clear free radicals in an organism and improves life quality; on the other hand, by the aid of the bioactive peptide SKVLPVPEKAVPYPQ, in-vitro proliferation capacity of lymphocytes and macrophages can be enhanced, the phagocytosis capacity of the macrophages for neutral red is improved, the organism's capacity of resisting outside pathogen infection is improved, morbidity of the organism is reduced, and therefore, the bioactive peptide SKVLPVPEKAVPYPQ has quite great significance in development of food, healthcare products and drugs with an immune regulation function and an anti-oxidation function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
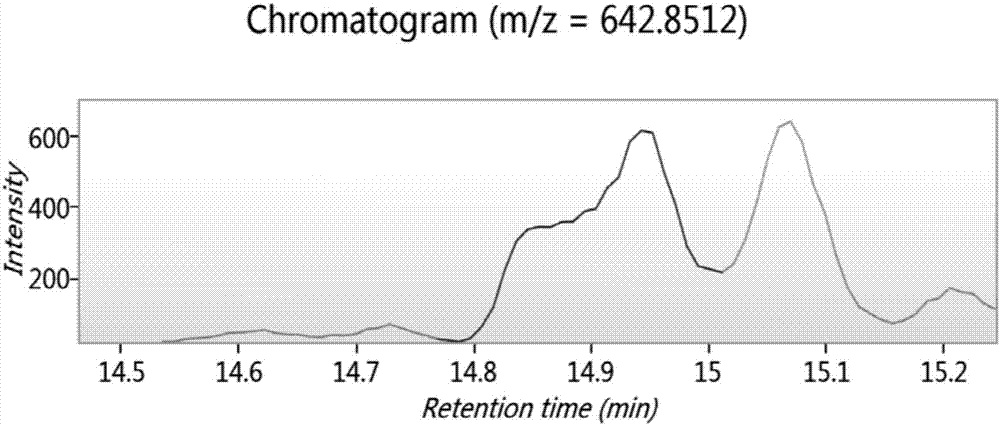
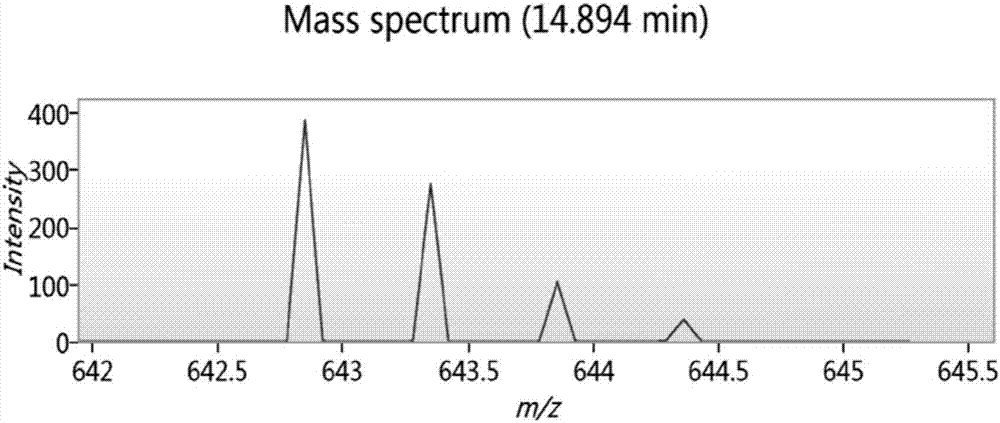
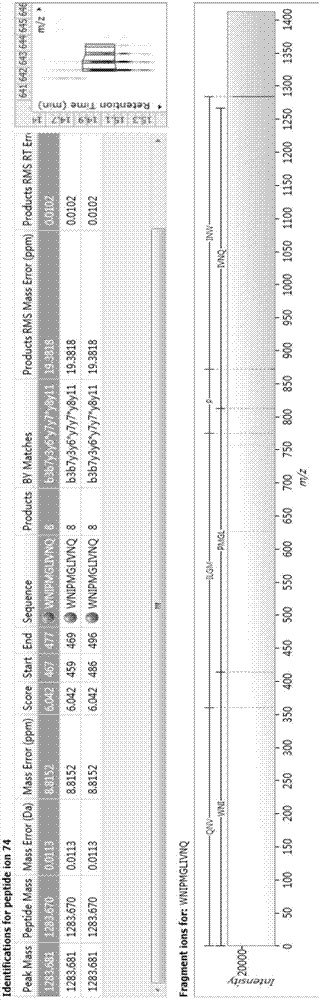
Biologically active polypeptide WNIPMGLIVNQ and preparation method and application thereof
ActiveCN107163136AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsDiseaseNitric oxide
The present invention relates to the field of protein, and in particular relates to biologically active polypeptide WNIPMGLIVNQ and a preparation method and the application thereof, and the amino acid sequence of the biologically active polypeptide WNIPMGLIVNQ is Trp-Asn-Ile-Pro-Met-Gly-Leu-Ile-Val-Asn-Gln. In vitro antioxidant experiments and in vitro immune function promotion experiments verify the biologically active polypeptide WNIPMGLIVNQ has good antioxidant biological activity and immunity regulating function, on the one hand, the biologically active polypeptide WNIPMGLIVNQ has good antioxidant activity to remove free radicals in the body and improve the quality of life; on the other hand, the biologically active polypeptide WNIPMGLIVNQ can enhance the in-vitro proliferation ability of lymphocytes and macrophages to promote the increase of macrophage induced nitric oxide, the biologically active polypeptide WNIPMGLIVNQ can improve the external pathogen infection resistance of the body and reduce the disease incidence of the body, and has very important significance for development of food, health care products and drugs with immune function regulating and antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com