Biologically active polypeptide WNIPMGLIVNQ and preparation method and application thereof
A technology of biologically active polypeptides and derivatives, applied in the field of proteins, can solve problems such as human risks, and achieve the effects of reducing injury, enhancing proliferation ability, and improving ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
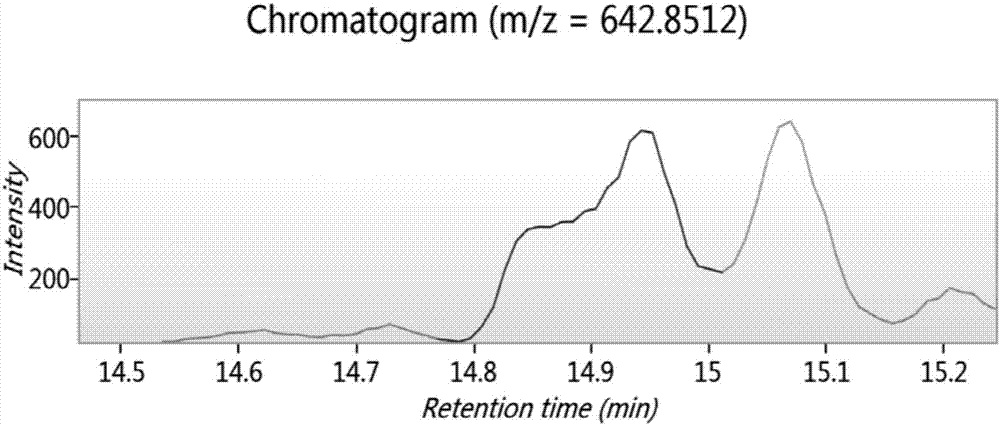
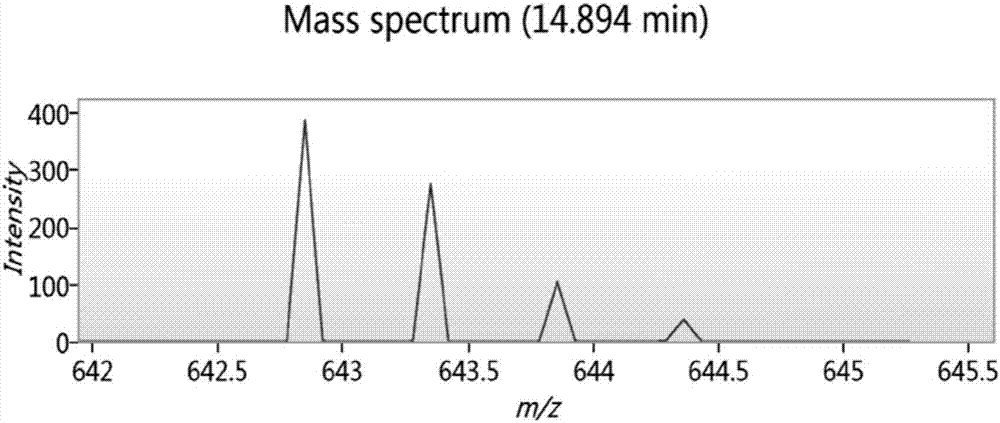
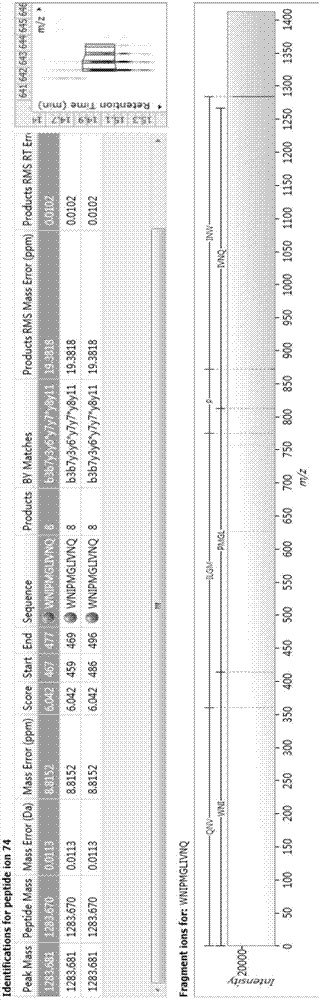
Image
Examples
Embodiment 1
[0033] The artificial synthesis of embodiment 1 active peptide WNIPMGLIVNQ
[0034] 1. Synthesis of Bioactive Peptides
[0035] 1. Weigh 3g of RINK resin (degree of substitution: 0.3mmol / g) into a 150ml reactor and soak in 50ml of dichloromethane (DCM).
[0036] After 2.2 hours, the resin was washed with nitrogen-dimethylformamide (DMF) 3 times the volume of the resin, and then drained. This was repeated four times, and the resin was drained for use.
[0037] 3. Add a certain amount of 20% piperidine (piperidine / DMF=1:4, v:v) into the reactor, and shake it on a decolorizing shaker for 20 minutes to remove the Fmoc protecting group on the resin. After deprotection, wash four times with 3 times the resin volume of DMF, and then drain.
[0038] 4. Take a small amount of resin and use ninhydrin (Nine Wells Ninhydrin) method to test it (Two drops each of Test A and Test B, react at 100°C for 1 min), if the resin is colored, it means that the deprotection is successful.
[0039] ...
Embodiment 2
[0082] Antioxidant activity experiment of embodiment 2 bioactive peptides
[0083]The antioxidant activity of the biologically active polypeptide WNIPMGLIVNQ obtained in Example 1 was tested by free radical scavenging method (DPPH·method) and total antioxidant capacity method (Ferric Reducing AbilityPower FRAP method).
[0084] 1. Determination of in vitro antioxidant activity of bioactive peptide WNIPMGLIVNQ by [DPPH·] method
[0085] 1) Experimental reagents and instruments
[0086] Reagent: 1,1-diphenyl-2-trinitrophenylhydrazine (1,1-Diphenyl-2-picrylhydrazyl[DPPH·]), produced by Wako Company of Japan; Methanol, provided by Shanghai Sinopharm Company; obtained in Example 1 Milk-derived biologically active polypeptide WNIPMGLIVNQ.
[0087] Main instruments: Sunrise microplate reader, product of Tecan Company of Austria; 96-well cell culture plate, manufactured by Millipore Company of the United States; analytical balance, product of Meitelei-tolido Company.
[0088] 2) Ex...
Embodiment 3
[0123] Example 3 Bioactive Peptide Promoting Body Immunity Activity Experiment
[0124] 1. MTT method to determine the in vitro lymphocyte proliferation ability of biologically active polypeptide WNIPMGLIVNQ
[0125] 1) Experimental materials and instruments:
[0126] Reagents and materials: experimental animals balb / c mice (male 6-8 weeks old, Animal Experiment Center, School of Agriculture and Biology, Shanghai Jiaotong University); milk-derived bioactive polypeptide WNIPMGLIVNQ obtained in Example 1; mouse lymphocyte extract (purchased from Suo Laibao); RPMI1640 medium (purchased from GIBCO); 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT, purchased from from Amresco Company); Concanavalin (ConA, purchased from Sigma Company); bovine serum albumin (BSA, purchased from Genebase Company); pepsin (purchased from Sigma Company); pancreatin (Corolase PP, purchased from AB Company ).
[0127] Instruments: LRH-250F biochemical incubator, Shanghai Heng Technolog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion source temperature | aaaaa | aaaaa |
| Collision energy | aaaaa | aaaaa |
| Quality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com