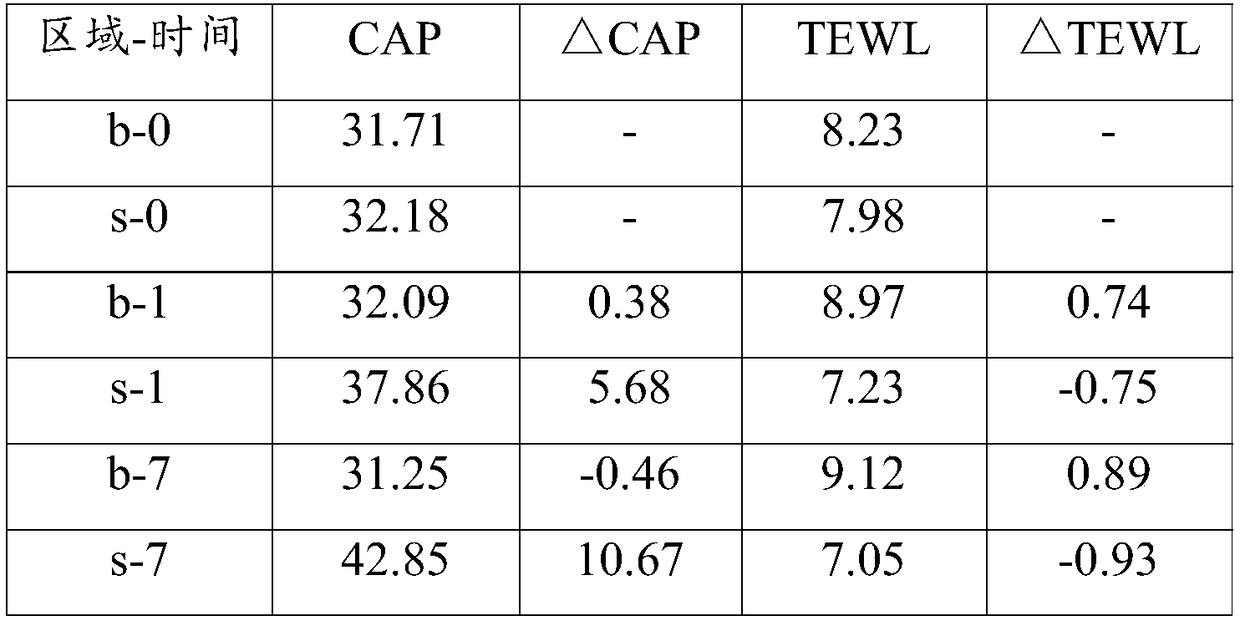
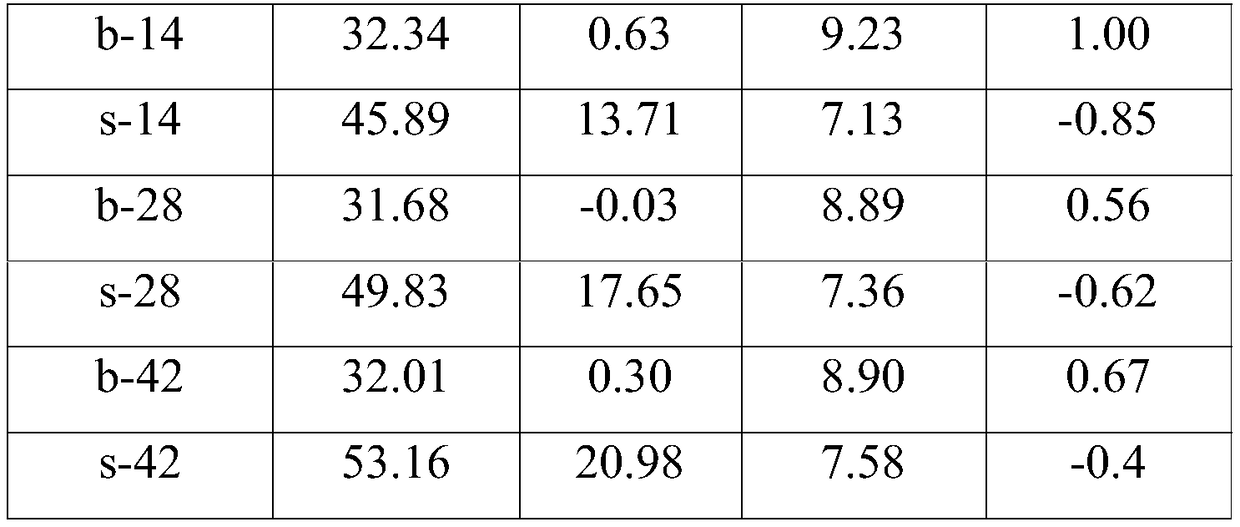
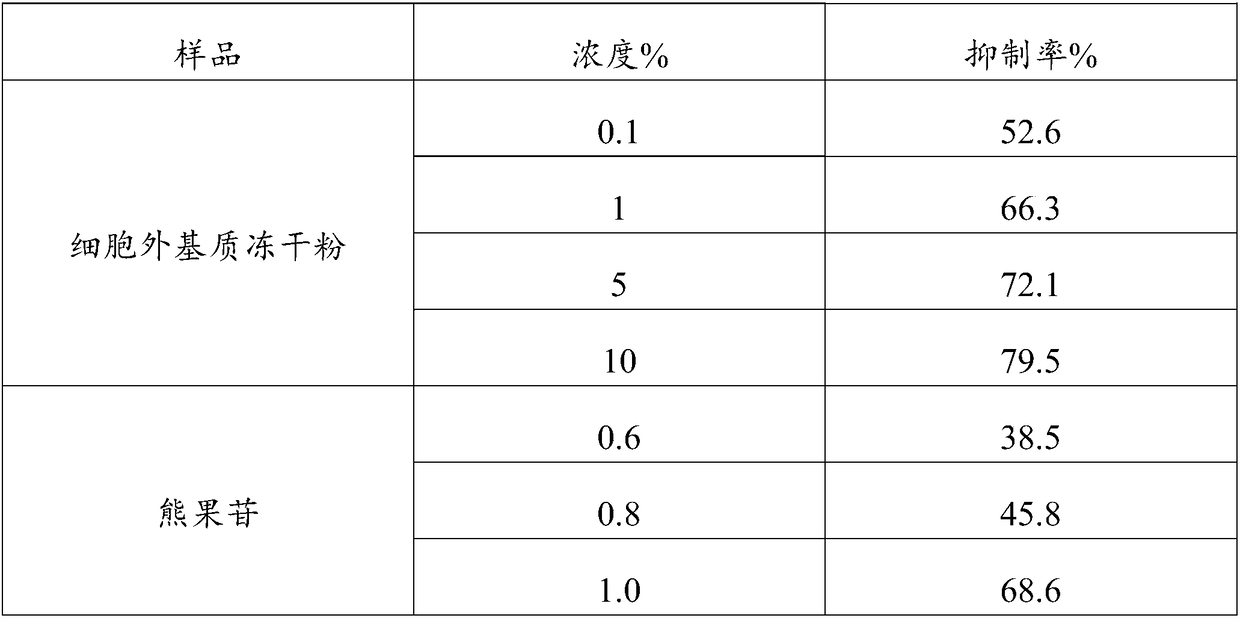
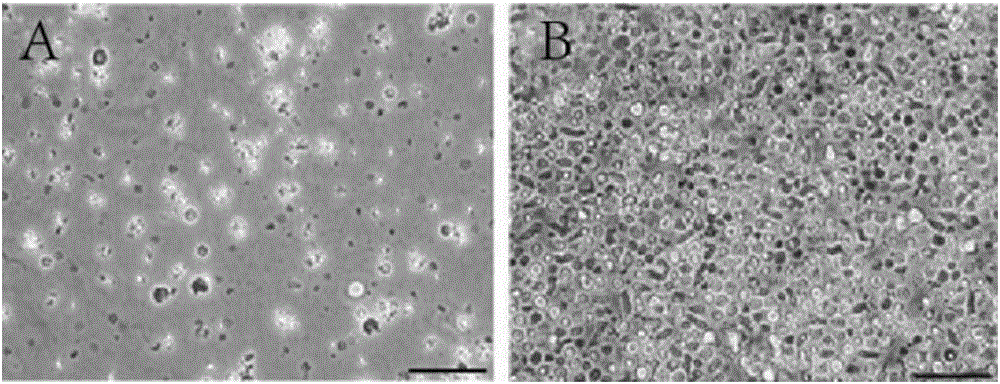
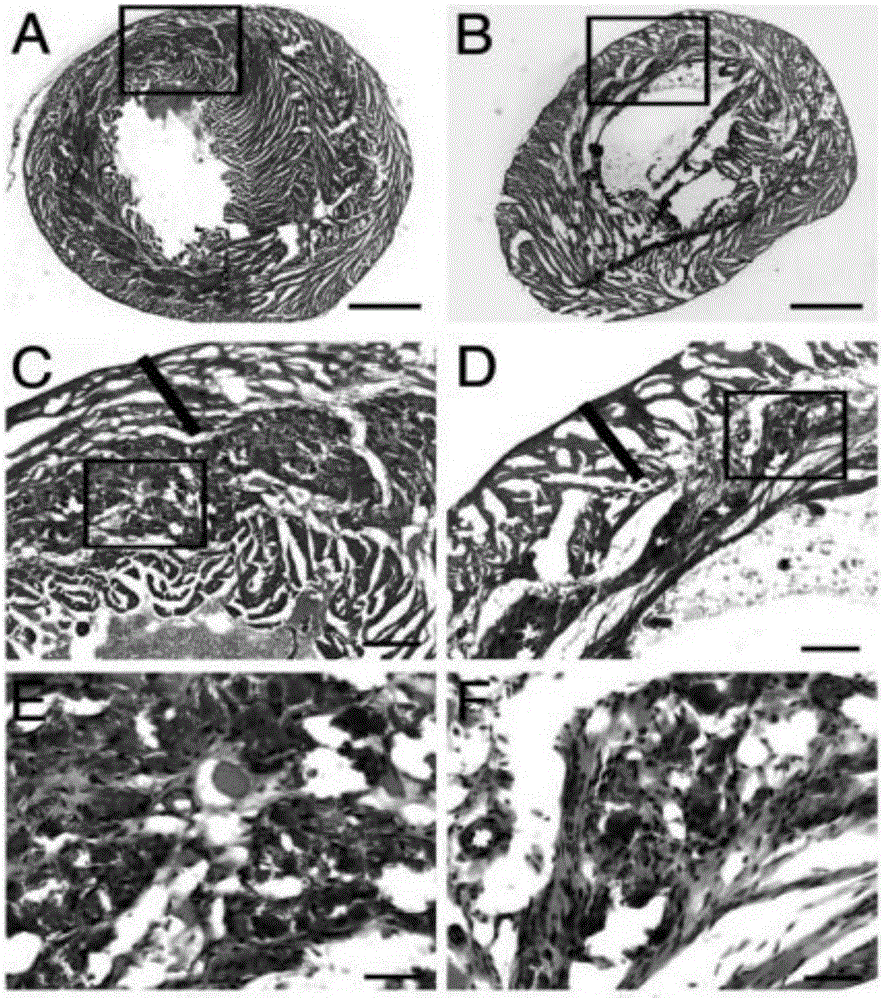
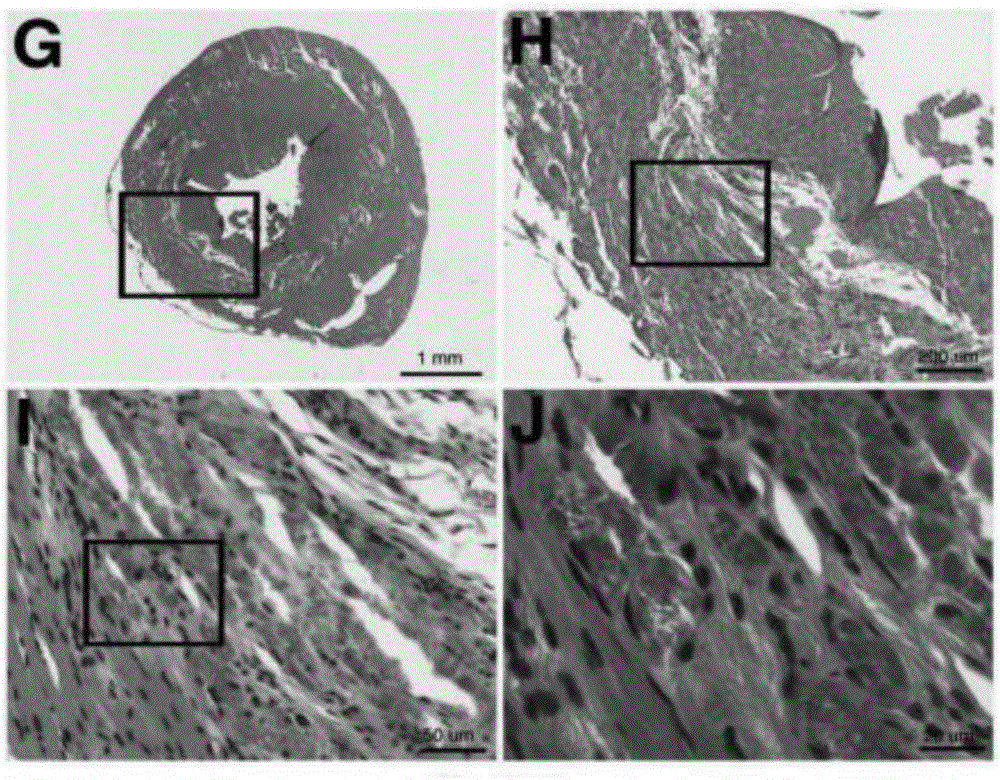
Patents
Literature
77results about How to "Does not cause immune rejection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cardiac muscle tonifying tablet taking acellular biological membrane as carrier as well as preparation method and application of cardiac muscle tonifying tablet
ActiveCN104436305AHelps maintain sterilityImprove decellularization efficiencyProsthesisCross-linkNutrition support
The invention discloses a cardiac muscle tonifying tablet taking an acellular biological membrane as a carrier as well as a preparation method and application of the cardiac muscle tonifying tablet. The cardiac muscle tonifying tablet taking the acellular biological membrane as the carrier is prepared by the following steps: carrying out decellularizing treatment on a natural biological membrane; then, cross-linking nutrient substances on the acellular biological membrane; planting target cells on the acellular biological membrane cross-linked with the nutrient substances; and cultivating a cell sheet which is preliminarily constructed, so that the cardiac muscle tonifying tablet taking the acellular biological membrane as the carrier is prepared. The cardiac muscle tonifying tablet disclosed by the invention is good in proliferation and differentiation activities and mechanical strength, and is sufficient in nutrition support; the cardiac muscle tonifying tablet is not only a breakthrough in tissue engineering but also suitable for the clinical treatment of myocardial infarction. The cardiac muscle tonifying tablet disclosed by the invention is reliable in principle, good in reproducibility, and suitable for standard production.
Owner:JINAN UNIVERSITY
Method for preparing essence of stem cell factor with anti-aging effect
InactiveCN108186548APromote growth and proliferationRelieve agingCosmetic preparationsToilet preparationsGlycerolRepair skin
The invention discloses a method for preparing essence of stem cell factor with an anti-aging effect. The method comprises the steps that step 1, human umbilical cord mesenchyma stem cells are prepared and cultured, stem cell culture supernatant is collected, concentrate is obtained after concentration is conducted, and the concentrate is further frozen and dried to prepare the lyophilized powderof the stem cell factor; step 2, the components of, by volume, 0.1-1% of hyaluronic acid, 1-10% of glycerol, 0.1-1% of collagen protein, 0.1-1% of tea polyphenols and the balance water are mixed to obtain solvent; step 3, the solvent is added into the lyophilized powder of the stem cell factor to be thoroughly dissolved to obtain the essence of the stem cell factor. The prepared essence of the stem cell factor comprises multiple kinds of cell active factor components such as epidermal growth factor, vascular endothelial growth factor, hepatocyte growth factor, nerve growth factor and the like,the essence can nourish skin, promote skin repairing and regenerating, resist wrinkles, remove colored patches and the like, and the essence has better application value in the field of medical beauty.
Owner:上海莱馥生命科学技术有限公司
Vascular stent material of tissue engineering and manufacturing method thereof
ActiveCN101584882AGood biocompatibilityDoes not cause immune rejectionCoatingsBlood vesselsPorositySide effect
The invention discloses a vascular stent material of tissue engineering and manufacturing method thereof, which comprises selecting acetobacter xylinum as a model strain; using oxygen permeable materials to prepare hollow tubular dies which are arranged in the fermentation liquid and introduced with oxygen for fermentation until the bacterial celluloses completely cover the outer surface of the dies and the required porosity, thickness, uniformity and structural characteristic are reached to obtain a vascular stent raw material of tissue engineering; removing impurities to reduce the impurity rate to below 0.05%, sterilizing and packaging to prepare the vascular stent material of tissue engineering. The invention is simple in process and safe and has no toxic and side effect, and the material has more uniform spatial structure, more suitable porosity, uniform texture of each material in batches by controlling the fermentation process to effectively improve the quality and favourably achieve the scaled production.
Owner:HAINAN YEGUO FOODS
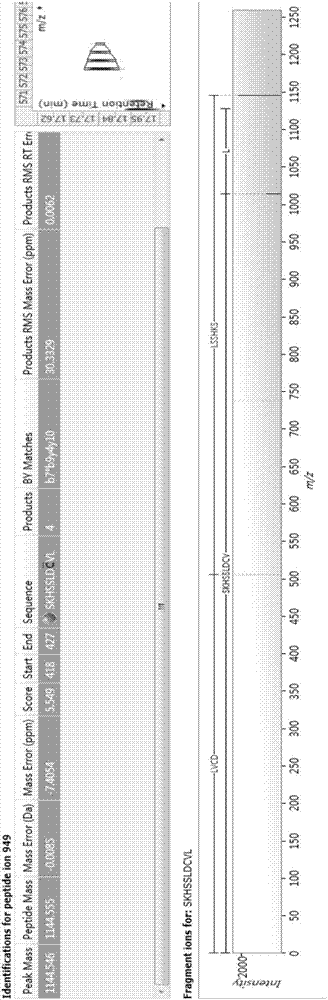
Bioactive polypeptide SKHSSLDCVL, and preparation method and application thereof
ActiveCN107226860AGood antioxidant activityGood for regulating the body's immune systemCosmetic preparationsPeptide/protein ingredientsNeutral red stainPathogen
The invention relates to the field of protein, and in particular to a bioactive polypeptide SKHSSLDCVL, and a preparation method and application thereof. An amino acid sequence of the bioactive polypeptide SKHSSLDCVL is Ser-Lys-His-Ser-Ser-Leu-Asp-Cys-Val-Leu. An in-vitro antioxidant experiment and an in-vitro immunological function promoting experiment verify that the bioactive polypeptide SKHSSLDCVL has better antioxidant biological activity and a better immune modulating function; on one hand, the bioactive polypeptide SKHSSLDCVL has better antioxidant activity and is capable of removing free radicals in a body and increasing the quality of life; on the other hand, the bioactive polypeptide SKHSSLDCVL is capable of enhancing in-vitro proliferation ability of lymphocytes and macrophages, promoting the ability of swallowing neutral red by the macrophages, increasing the ability of defending external pathogen infection by the body and reducing the morbidity of the body, and has a very important significance in developing food, healthcare products and drugs having an immune modulating function and an antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Recombinant human-like collagen and production method thereof
InactiveCN102146135AFast growthIncrease productionFungiConnective tissue peptidesPichia pastorisHuman body
The invention discloses a recombinant human-like collagen. The recombinant human-like collagen has high hydrophilicity and stability. The structure of the recombinant human-like collagen is totally the same with the structure of the corresponding part of a natural collagen gene sequence. When the recombinant human-like collagen is applied to a human body, immunological rejection cannot be caused. The recombinant human-like collagen can be widely applied to the fields of biomedical materials, cosmetics and the like. The invention also discloses a production method of the recombinant human-like collagen, which comprises the following steps of: constructing pichia pastoris gene engineering bacteria; fermenting and cultivating the pichia pastoris gene engineering bacteria; inducing and expressing the recombinant human-like collagen; and purifying the recombinant human-like collagen. The human-like collagen produced by the method has high expression and is convenient to purify. The expression in a fermentation tank can achieve 5.0g / L. In a single batch, about 150g of crude collagens can be produced by a tank with the volume of 50L. Due to high expression, the purity of the crude collagens is over 80 percent and few impurity proteins exist.
Owner:陕西九州生物医药科技集团有限公司
Method for preparing goat milk beverage containing ACE (Angiotensin Converting Enzyme) inhibitory peptide
A method for preparing a goat milk beverage containing ACE (Angiotensin Converting Enzyme) inhibitory peptide comprises the following steps of: cooling, centrifuging and fatting reconstituted goat milk, thereby obtaining skimmed milk; preparing casein from the skimmed milk by an isoelectric precipitation method, and obtaining polypeptide powder through enzymolysis; and mixing polypeptide powder with reconstituted goat milk, concentrated fruit juice, polypeptide powder, cane sugar, citric acid, beta-cyclodextrin, purified water and the like to obtain the goat milk beverage containing ACE inhibitory peptide. The goat milk beverage containing ACE inhibitory peptide prepared by the method provided by the invention contains the ACE inhibitory peptide and thereby has obvious effect of reducing blood pressure; if the hyperpietic drinks the goat milk beverage for a long time, the blood pressure of the hyperpietic can be reduced; and the goat milk beverage is mild in properties without side effect on people of normal blood pressure; and the goat milk beverage containing ACE inhibitory peptide has various effects of immunological activity, and prompting digestive absorption and losing weight, and the like.
Owner:SHAANXI UNIV OF SCI & TECH
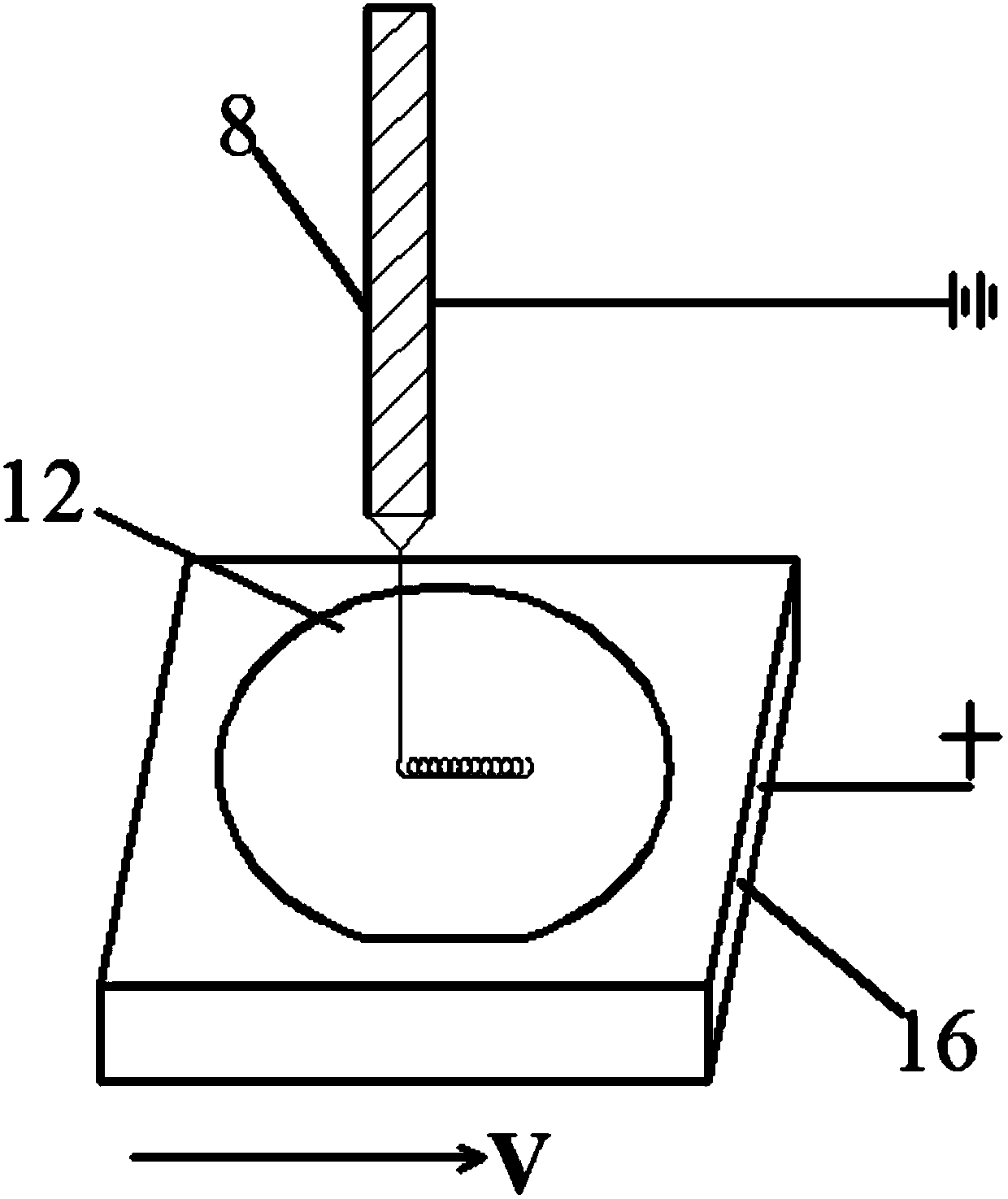
Electrofluid spraying device and method for printing three-dimensional biological scaffold
InactiveCN107718531AImprove adsorption capacityExcellent adsorptionManufacturing platforms/substratesManufacturing driving meansPorosityEngineering
The invention discloses a device and method for printing a three-dimensional biological scaffold. The printing method comprises the following steps that an polycaprolactone (PCL) solution after beingmixed with glacial acetic acid evenly is added into a solution supplying system; the motion speed and path of a motion platform during printing are set through a motion control system, the distance between a spraying needle and a polished silicon crystal substrate is adjusted, and the solution injection rate is set; and a power supply is adjusted, and voltage is exerted between the spraying needleand a loading platform. The three-dimensional biological scaffold which is printed by the method and has various helical structure micro morphologies has higher strength, greater contact area and higher porosity, and thus is more favorable for cell adsorption during cell seeding and differentiation and multiplication during cell culture.
Owner:NAT UNIV OF SINGAPORE SUZHOU RES INST +1
Biological amnion and preparation method thereof
ActiveCN103520780AEasy to prepareWide variety of sourcesLayered productsSurgeryFreeze-dryingDrug biological activity
The invention relates to a biological amnion and a preparation method thereof. The biological amnion has three layers including a slow release layer, an amnion layer and a collagen layer from top to bottom, wherein the slow release layer consists of collagen and biological active factors, the amnion layer consists of an amnion subjected to decellularization treatment, and the collagen layer is formed by freeze-drying and compounding collagen. The biological amnion is prepared through the steps of raw material pretreatment, virus inactivation, decellularization treatm. The biological amnion prepared by the invention has the characteristics of an effect of slowly releasing the active factors, low antigenicity on removing epithelial cells, convenience in product operation, difficulty in curling, good adhesiveness with surrounding tissues, difficulty in sliding and the like. Meanwhile, by using a process for performing the decellularization treatment on the amnion, disclosed by the invention, a natural compact collagen structure in the amnion can be effectively retained, the biological amnion can fully achieve a physical barrier effect after being applied to tenorrhaphy, and an animal experiment proves that the biological amnion can effectively achieve an effect of preventing tissue adhesion.
Owner:SHAANXI RUISHENG BIOTECH
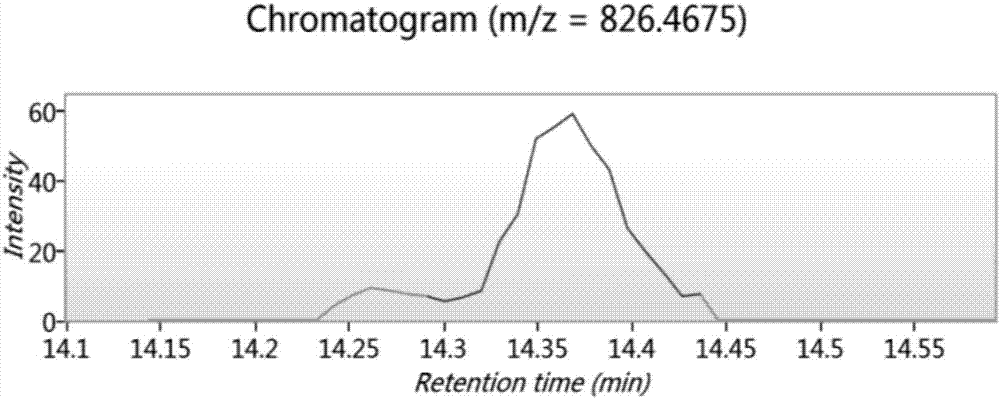
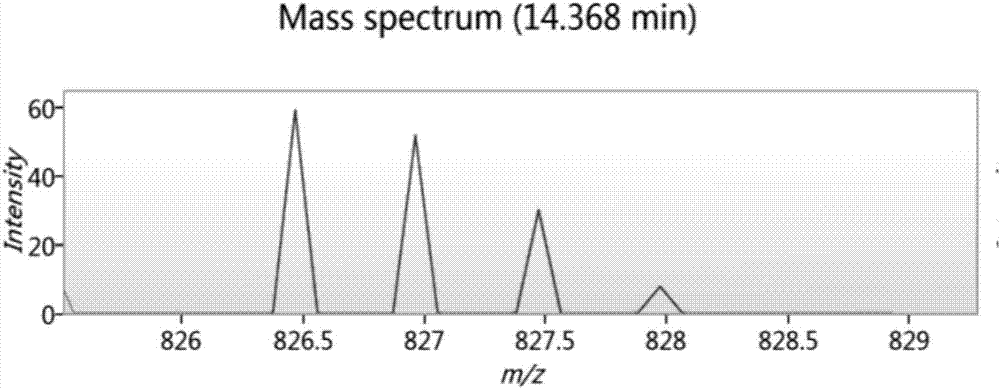
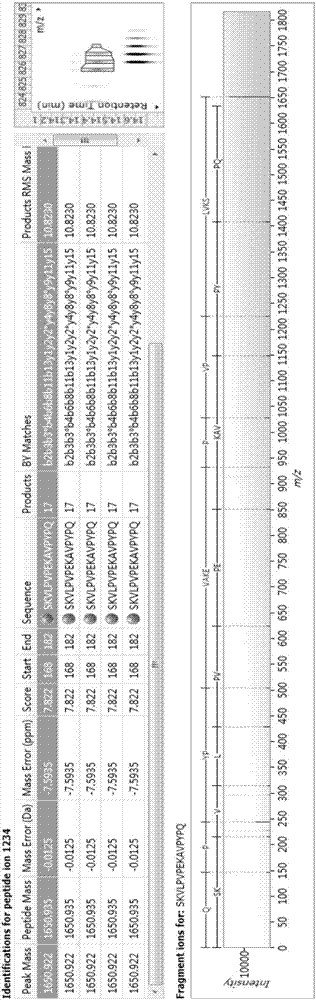
Bioactive peptide SKVLPVPEKAVPYPQ as well as preparation method and application thereof
ActiveCN107176995ARegulate immunityModulate immune activityCosmetic preparationsPeptide/protein ingredientsLife qualityVal-Leu-Pro-Val-Pro
The invention relates to the field of protein, in particular to a bioactive peptide SKVLPVPEKAVPYPQ as well as a preparation method and an application thereof. An amino acid sequence of the bioactive peptide SKVLPVPEKAVPYPQ is Ser-Lys-Val-Leu-Pro-Val-Pro-Glu-Lys-Ala-Val-Pro-Tyr-Pro-Gln. An in-vitro anti-oxidation experiment and an in-vitro immunity function promoting experiment prove that the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation biological activity and immunity regulation function, on one hand, the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation activity, can clear free radicals in an organism and improves life quality; on the other hand, by the aid of the bioactive peptide SKVLPVPEKAVPYPQ, in-vitro proliferation capacity of lymphocytes and macrophages can be enhanced, the phagocytosis capacity of the macrophages for neutral red is improved, the organism's capacity of resisting outside pathogen infection is improved, morbidity of the organism is reduced, and therefore, the bioactive peptide SKVLPVPEKAVPYPQ has quite great significance in development of food, healthcare products and drugs with an immune regulation function and an anti-oxidation function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Recombinant human type III collagen, expression strain and construction method
ActiveCN111363029AHigh purityImprove hydrophilicityFungiConnective tissue peptidesHuman bodyEngineering
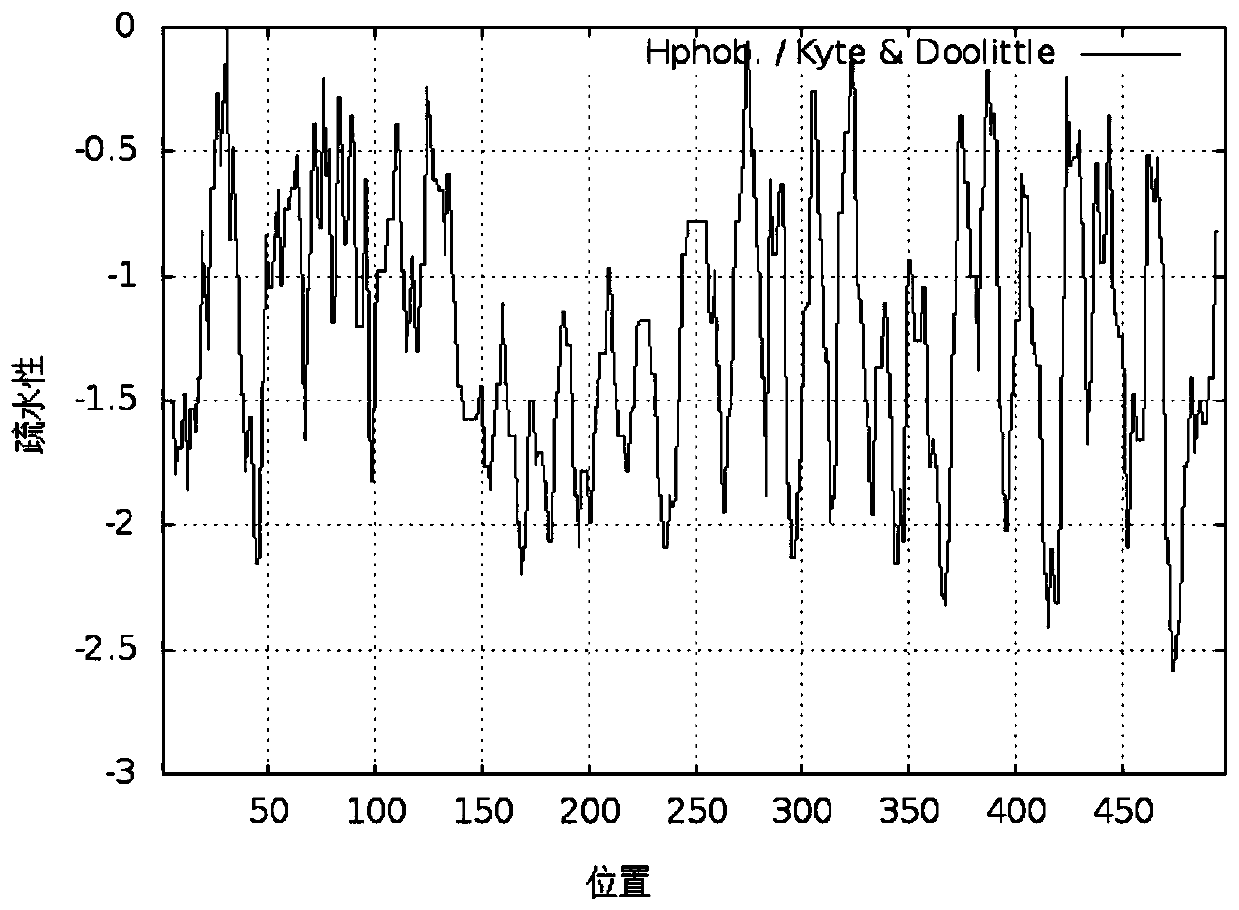
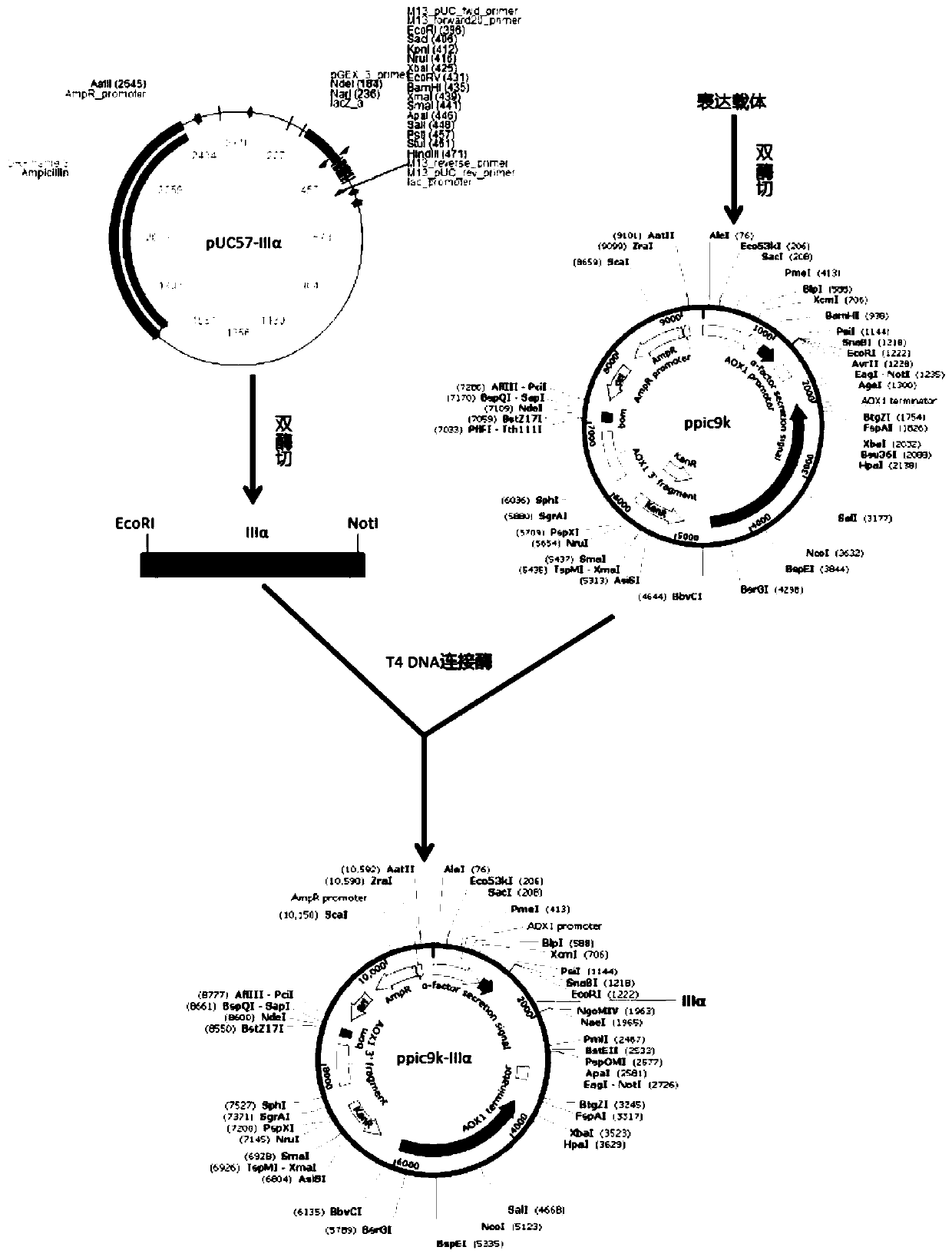
The invention discloses a recombinant human type III collagen protein, an expression strain and a construction method. The recombinant human type III collagen contains 498 amino acids and has a theoretical molecular weight of about 54.5kd. The constructed recombinant human type III collagen expression strain can effectively, stably and largely express the recombinant human type III collagen. The recombinant human collagen has good hydrophilicity and stability, the structure is 100% identical to the corresponding part of a natural collagen gene sequence, immune rejection does not caused when the recombinant human collagen is used in a human body, and the recombinant human collagen can be widely used in fields such as biomedical materials and cosmetics.
Owner:JIANGSU JLAND BIOTECH CO LTD
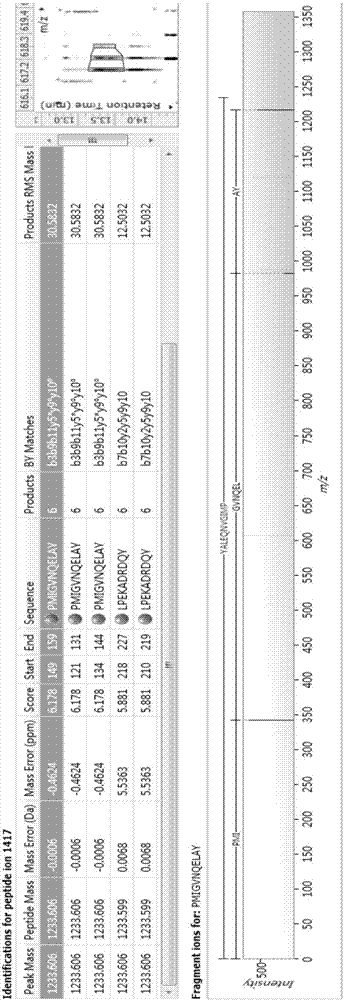
Bioactive polypeptide PMIGVNQELAY, and preparation method and application thereof
ActiveCN107236031AGood antioxidant activityGood for regulating the body's immune systemCosmetic preparationsPeptide/protein ingredientsDrugChemistry
The invention relates to the field of protein, in particular to a bioactive polypeptide PMIGVNQELAY, and a preparation method and application thereof. The amino acid sequence of the bioactive polypeptide PMIGVNQELAY is Pro-Met-Ile-Gly-Val-Asn-Gln-Glu-Leu-Ala-Tyr. In vitro antioxidant experiments and in vitro immune function promoting experiments prove that the polypeptide PMIGVNQELAY has better antioxidant biological activity and immune modulating functions; on one hand, good antioxidant activity is realized; free radicals in the body can be cleared away; the life quality is improved; on the other hand, the bioactive polypeptide PMIGVNQELAY can enhance the in vitro multiplication capacity of lymphocytes and macrophages; the capability of the macrophages for devouring toluylene red is promoted; the capability of the body for resisting the external pathogen infection is improved; the body morbidity is reduced; very important significance is realized for developing food, health care products and medicines with the immune modulating function and the antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Indirect ELISA reagent kit for detecting antibodies against rabbit hemorrhagic disease virus
InactiveCN101533016AGood reactogenicityGuaranteed high sensitivityColor/spectral properties measurementsHemorrhagic diseasesAntigenicity
The invention relates to an ELISA reagent kit for detecting antibodies against rabbit hemorrhagic disease virus (RHDV), and belongs to the field of biotechnology. The reagent kit adopts recombined VP60 capsid protein expressed by a baculovirus expression system as envelope antigen, detects the antibodies against rabbit hemorrhagic disease virus in serum of a rabbit according to an indirect ELISA principle, and is used for rabbit hemorrhagic disease vaccine serological detection in a rabbit group. The core substance envelope antigen of the reagent kit is the recombined VP60 capsid protein expressed by the baculovirus expression system, which has good antigenicity like natural RHDV but no potential poison spreading danger like the RHDV. The reagent kit used to detect serum samples has the advantages of low background reaction and obvious contrast of OD values of negative and positive serum development. The reagent kit has low production cost, strong specificity and high sensitivity, can detect samples in a large quantity, and has important application value in aspects such as rabbit hemorrhagic disease immunity monitoring and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Preparation method of composite soft-tissue patch
ActiveCN101773687APromote ingrowthPromote proliferationProsthesisMicrosphereBiocompatibility Testing
The invention discloses a preparation method of a composite soft-tissue patch. Sustained-release microspheres containing human cell culture secretion are mixed with gel solution, then the mixture is compounded with an acellular dermal matrix under vacuum, and after drying and sterilization, the composite soft-tissue patch is obtained. The prepared composite patch can improve the biocompatibility and the bioactivity of the dermal matrix effectively. The experiment shows that compared with the single acellular dermal matrix, the patch can promote cell proliferation and collagen formation effectively and has better biocompatibility and better tissue remolding. The patch can be widely applied to closure of various wound surfaces, repair of various soft-tissue defects and reinforcement of weakplaces and can be prepared into micro particles to be used for filling various fine and weak or dented places by injection, thus having wider application prospect in clinic.
Owner:SHAANXI RUISHENG BIOTECH
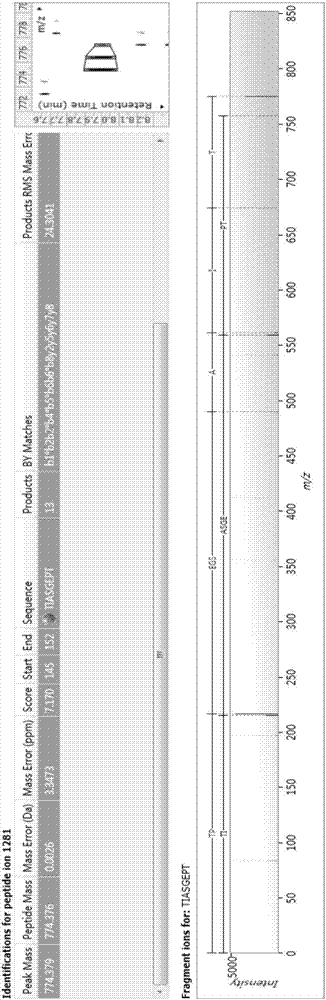
Bioactive polypeptide TIASGEPT, and preparation method and application thereof
ActiveCN107226857AReduce harmRegulate immunityCosmetic preparationsPeptide/protein ingredientsNitric oxideDrug biological activity
The invention relates to the field of protein, and in particular to a bioactive polypeptide TIASGEPT, and a preparation method and application thereof. An amino acid sequence of the bioactive polypeptide TIASGEPT is Thr-Ile-Ala-Ser-Gly-Glu-Pro-Thr. An in-vitro antioxidant experiment and an in-vitro immunological function promoting experiment verify that the bioactive polypeptide TIASGEPT has better antioxidant biological activity and a better immune modulating function; on one hand, the bioactive polypeptide TIASGEPT has better antioxidant activity and is capable of removing free radicals in a body and increasing the quality of life; on the other hand, the bioactive polypeptide TIASGEPT is capable of enhancing in-vitro proliferation ability of lymphocytes and macrophages, promoting the increment of nitric oxide induction amount of the macrophages, increasing the ability of defending external pathogen infection by the body and reducing the morbidity of the body, and has a very important significance in developing food, healthcare products and drugs, which have an immune modulating function and an antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
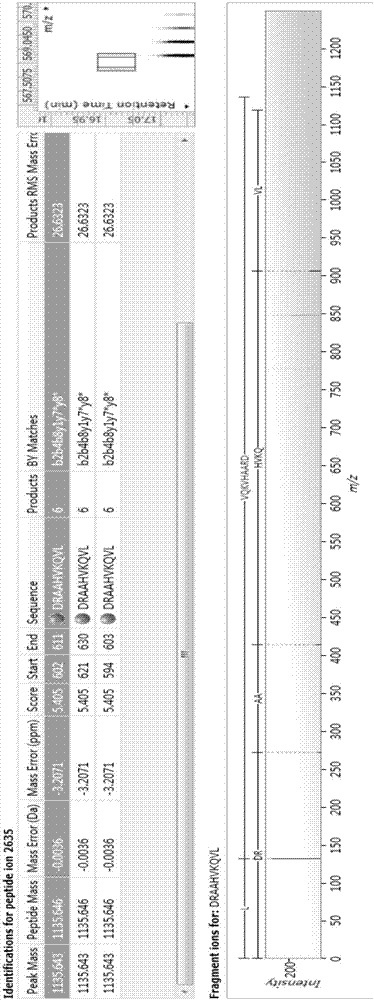
Bioactive peptide DRAAHVKQVL as well as preparation method and application thereof
ActiveCN107177000AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsLife qualityCytokine
The invention relates to the field of protein, in particular to a bioactive peptide DRAAHVKQVL as well as a preparation method and an application thereof. An amino acid sequence of the bioactive peptide DRAAHVKQVL is Asp-Arg-Ala-Ala-His-Vla-Lys-Gln-Val-Leu. An in-vitro anti-oxidation experiment and an in-vitro immunity function promoting experiment prove that the bioactive peptide DRAAHVKQVL has better anti-oxidation biological activity and immunity regulation function, on one hand, the bioactive peptide DRAAHVKQVL has better anti-oxidation activity, can clear free radicals in an organism and improves life quality; on the other hand, by the aid of the bioactive peptide DRAAHVKQVL, in-vitro proliferation capacity of lymphocytes and macrophages can be enhanced, the macrophages are promoted to secrete cytokines, the organism's capacity of resisting outside pathogen infection is improved, morbidity of the organism is reduced, and therefore, the bioactive peptide DRAAHVKQVL has quite great significance in development of food, healthcare products and drugs with an immune regulation function and an anti-oxidation function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
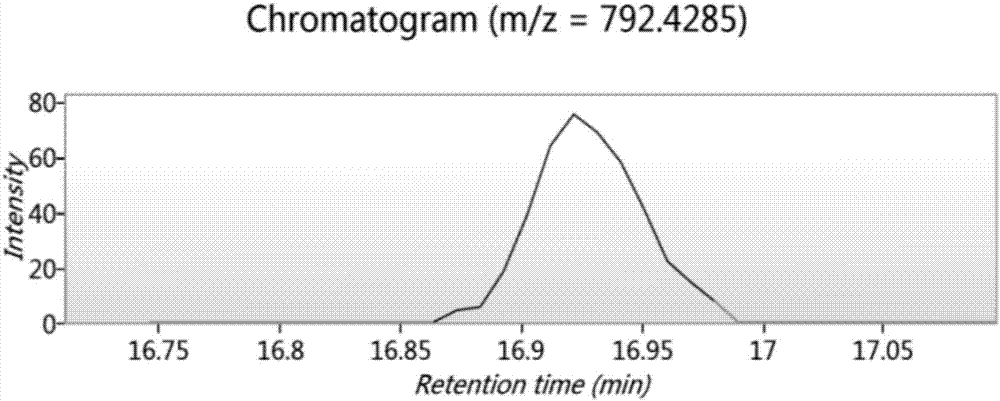
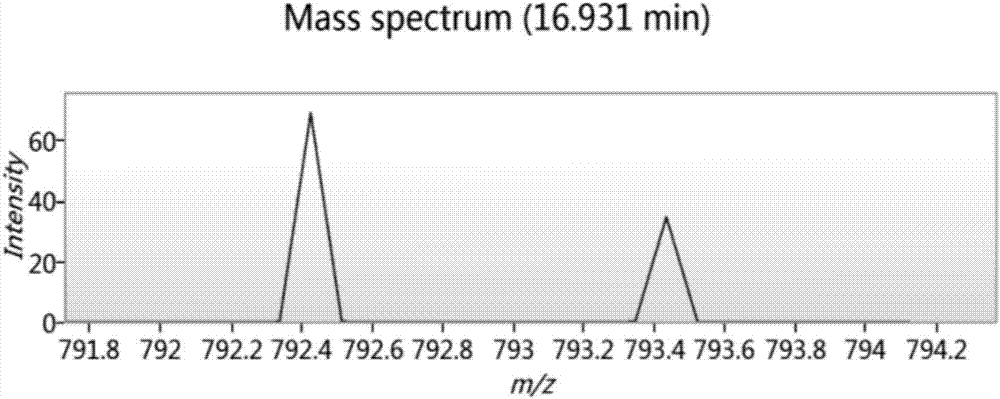
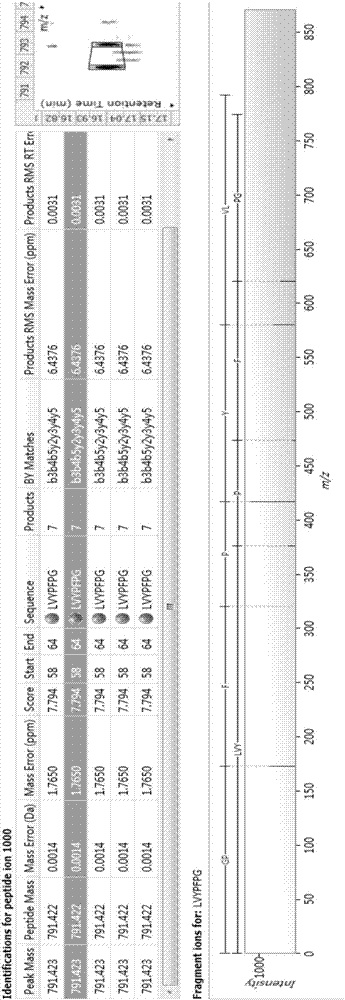
Bioactive polypeptide LVYPFPG as well as preparation method and application thereof
InactiveCN107200780AGood antioxidant activityGood for regulating the body's immune systemCosmetic preparationsPeptide/protein ingredientsPathogenNeutral red stain
The invention relates to the field of proteins, in particular to bioactive polypeptide LVYPFPG as well as a preparation method and application thereof. An amino acid sequence of the bioactive polypeptide LVYPFPG is Leu-Val-Tyr-Pro-Phe-Pro-Gly. In-vitro antioxidant experiments and in-vitro immune function promotion experiments prove that the polypeptide LVYPFPG has better antioxidant biological activity and an immune regulation function; on the one hand, the polypeptide LVYPFPG has the better antioxidant activity, thus being capable of removing free radicals in a human body and further improving the quality of life; on the other hand, the bioactive polypeptide LVYPFPG can enhance the in vitro proliferative capacity of lymphocytes and macrophages and promote the neutral red phagocytosis of the macrophages, thus improving the external pathogen infection resistance of the organism and reducing the morbidity of the organism; therefore, the bioactive polypeptide LVYPFPG has a very important significance for developing food, health care products and medicines which have the immune regulation function and the antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
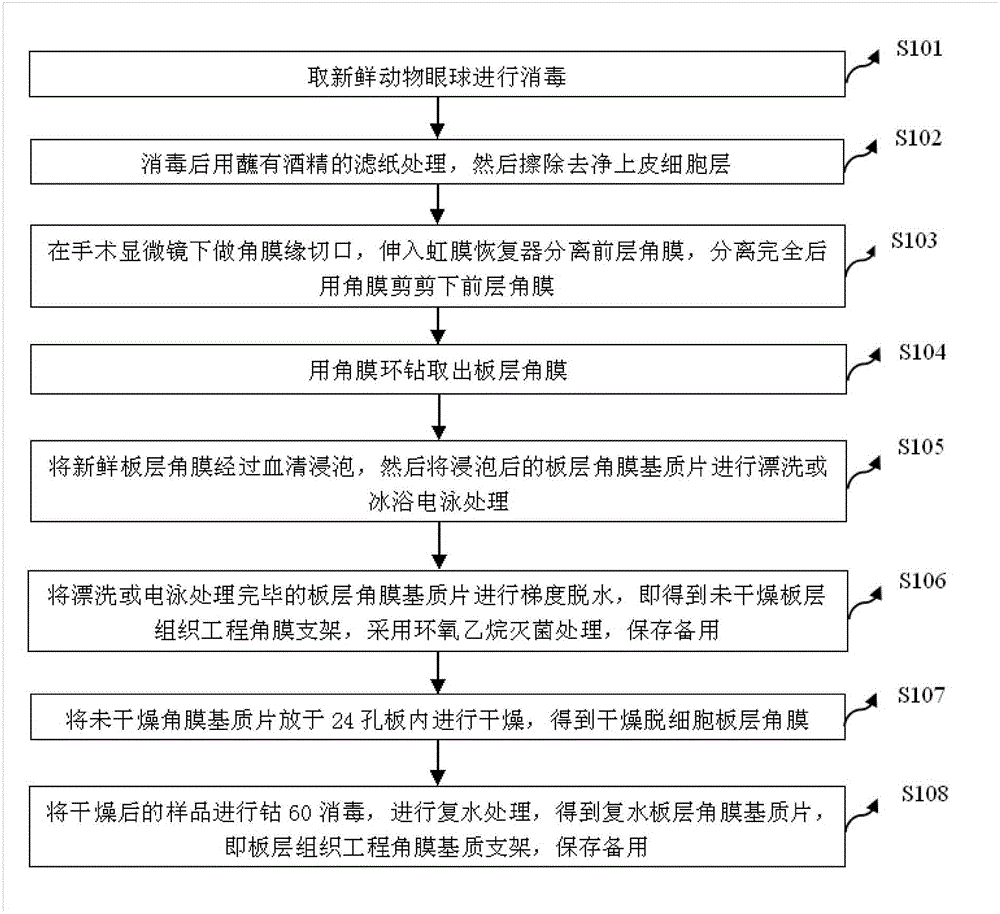
Method for preparing decellularized lamellar cornea matrix sheet
InactiveCN104645415AThe production process is simple and reliableShorten the timeProsthesisAnterior corneaEthylene Oxide Sterilization
The invention discloses a method for preparing a decellularized lamellar cornea matrix sheet. The method comprises the steps of disinfecting a fresh animal eyeball; treating with filter paper dipped with alcohol after disinfecting, and then erasing an epithelial cell layer; incising the corneal limbus under an operation microscope, stretching into an iris restorer to separate the anterior cornea, and then shearing the anterior cornea with corneal scissors; drilling the lamellar cornea with a corneal annulus; soaking the fresh lamellar cornea into serum, and then rinsing or performing ice-bath electrophoresis treatment; performing gradient dehydration after the treatment to obtain a non-dried lamellar tissue engineering corneal frame, sterilizing with ethylene oxide, and preserving for later use; drying the non-dried cornea matrix sheet in a 24-pore plate to obtain dried decellularized lamellar cornea; and sterilizing the dried sample with cobalt 60, and performing rehydration treatment to obtain a rehydrated lamellar cornea matrix sheet. The obtained cornea matrix sheet is high in transparency, low in structural destroy, good in biocompatibility, close to fresh cornea in performance and thorough in decellularization.
Owner:南昌大学第一附属医院 +1
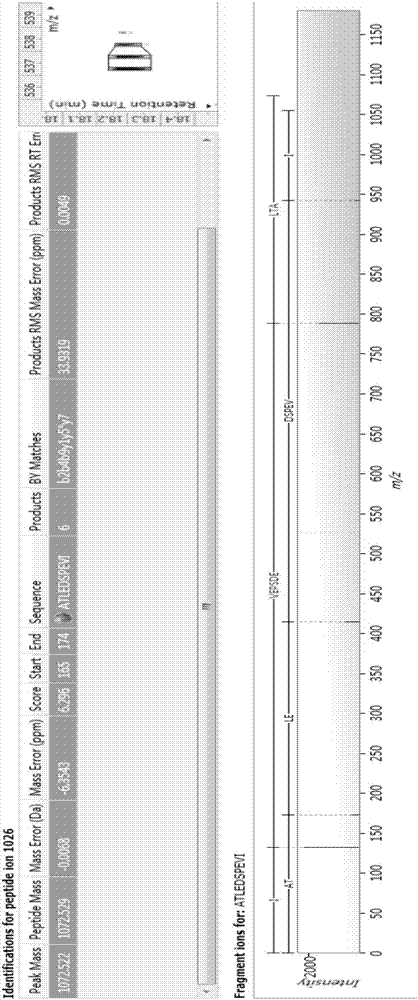
Bioactive polypeptide ATLEDSPEVI as well as preparation method and application thereof
ActiveCN107141346AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsQuality of lifeLymphocyte
The invention relates to the field of proteins and particularly relates to bioactive polypeptide ATLEDSPEVI as well as a preparation method and application thereof. The amino acid sequence of the bioactive polypeptide ATLEDSPEVI is Ala-Thr-Leu-Glu-Asp-Ser-Pro-Glu-Val-Ile. In-vitro anti-oxidation experiments and in-vitro immune function promotion experiments verify that the polypeptide ATLEDSPEVI has better antioxidant biological activity and immune modulating function, so that on one hand, the polypeptide ATLEDSPEVI has better antioxidant activity, so that free radicals in a body can be removed, and the quality of life is improved; on the other hand, the polypeptide ATLEDSPEVI can enhance the in-vitro multiplication capacity of lymphocytes and macrophages, the macrophages are promoted to secrete cytokines, the capability of the body in defense for external pathogen infection is enhanced, and the incidence rate of the body is reduced. Therefore, the polypeptide ATLEDSPEVI has great significance in development of food, health-care products and drugs with immune modulating function and antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Bioactive polypeptide QEPVL, and preparation and application thereof
ActiveCN102964427AImproves antioxidant activityBoosts immune activityPeptide/protein ingredientsMicroorganism based processesLymphocyteDrug biological activity
The invention relates to the field of a protein and particularly relates to a milk-derived bioactive polypeptide QEPVL with in-vitro antioxidant activity and body immunity promoting activity, wherein an amino acid sequence of the bioactive polypeptide QEPVL is Gln-Glu-Pro-Val-Leu. Through an in-vitro antioxidant test and an in-vitro immunity promoting test, the polypeptide QEPVL is proved to have relatively good antioxidant biological activity and immunity improving function; on one hand, free radicals in the body can be removed to reduce the harm on the human body caused by the free radicals; on the other hand, according to the bioactive polypeptide QEPVL, the immunity of the body can be further enhanced and the multiplication capacity of lymphocytes in vitro can be promoted, thereby improving the ability of the body to resist infections of external pathogens and reducing the incidence rate of the body without causing immunological rejections. The bioactive polypeptide QEPVL has great significance for the development of milk products, healthcare products and medicines with the antioxidant function and the immunity enhancement.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Tissue engineered esophagus
InactiveCN1410034AAntigenicDoes not cause immune rejectionTubular organ implantsCapillary networkVascular endothelium
A histo-enigineered esophagus is disclosed, and it contains a dual-layer structure of epithelial layer and dermis layer constructed by scaffold material and seed cells. The vascular endothelial cellsin the dermis layer directly take part in the creation of capillary network. Two revascularization methods are used for quickly communicating with the blood supply channel of receptor.
Owner:谭强
Hyaluronic acid-cationic drug ion pair particle
ActiveCN105125522AVarious particle sizesSimple preparation processPharmaceutical non-active ingredientsAntineoplastic agentsIon pairingRadiochemistry
The invention provides a hyaluronic acid-cationic drug ion pair particle as well as a preparation method and application thereof. A positively charged cationic drug serves as a hydrophobic core and negatively charged hyaluronic acid functions as a hydrophilic shell, so that the ion pair particle has double targeting properties, namely active targeting and passive targeting.
Owner:SICHUAN UNIV
Method for preparing ACE (Angiotensin Converting Enzyme) inhibitory peptides through compound enzymatic hydrolysis of feta protein
InactiveCN103215331APromote generationStrong inhibitory activityFermentationNeutral proteaseSide effect
The invention provides a method for preparing ACE (Angiotensin Converting Enzyme) inhibitory peptides through compound enzymatic hydrolysis of feta protein. The method comprises the following steps of: centrifuging and degreasing recovered goat milk taken as a raw material, preparing casein by an isoelectric precipitation method, carrying out compound hydrolysis on the casein through neutral protease and trypsase under the conditions of specific temperature and pH, optimizing the hydrolysis condition, treating the hydrolytic process with ultrasound waves to obtain small-molecule ACE inhibitory peptides and determining the activity of the small-molecule ACE inhibitory peptides. The inhibitory proportion of an ACE inhibitory peptide product prepared by the method can reach above 80%; the ACE inhibitory peptide product has small molecular mass, can be absorbed by a human body in the form of complete peptide fragments and cannot cause the immunological rejection of the human body; and the ACE inhibitory peptide product has an obvious antihypertensive effect on hypertensive, has no effect on people with normal blood pressure and has the characteristics of little dosage, no toxic or side effect, mild property and strong physiological function.
Owner:SHAANXI UNIV OF SCI & TECH
Bioactive polypeptide EINTVQVTST and preparation method and application thereof
ActiveCN107188949AGood antioxidant activityGood for regulating the body's immune systemCosmetic preparationsPeptide/protein ingredientsDrugPathogen
The invention relates to the field of proteins, in particular to a bioactive polypeptide EINTVQVTST and a preparation method and application thereof. An amino acid sequence of the bioactive polypeptide EINTVQVTST is Glu-Ile-Asn-Thr-Val-Gln-Val-Thr-Ser-Thr. By virtue of in-vitro antioxidant experiment and in-vitro immunity promoting experiment, the polypeptide EINTVQVTST is proved to have good antioxidant bioactivity and immunity regulation function, has good antioxidant activity on one hand, and can eliminate free radicals in the body and improve the quality of life; and on the other hand, the bioactive polypeptide EINTVQVTST can improve the in-vitro proliferation capacity of lymphocyte and macrophage, promotes the macrophage to secrete cell factors, improves the capacity of the body for resisting external pathogenic infection, decreases the disease rate of the body, and has important significance for developing food, healthcare products and drugs with an immunity regulation function and an antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Polyvinyl alcohol hydrogel, method for preparing same and application of polyvinyl alcohol hydrogel
The invention relates to polyvinyl alcohol hydrogel, a method for preparing the same and application of the polyvinyl alcohol hydrogel, and belongs to the field of polymeric hydrogel. The method for preparing the polyvinyl alcohol hydrogel includes heating and dissolving polyvinyl alcohol and organic solvents at the temperature of 110-145 DEG C to obtain polyvinyl alcohol solution; allowing the obtained polyvinyl alcohol solution to stand still to naturally cool the polyvinyl alcohol solution until the temperature of the polyvinyl alcohol solution reaches the room temperature so as to obtain polyvinyl alcohol with gel structures; removing the organic solvents from the polyvinyl alcohol with the gel structures to obtain the polyvinyl alcohol hydrogel. The organic solvents are blended solvents with dimethyl sulfoxide and N-N, dimethylformamide, and a volume ratio of the dimethyl sulfoxide to the N-N, dimethylformamide is 0.25-1; the mass concentration of the obtained polyvinyl alcohol solution is 10-30%. The polyvinyl alcohol hydrogel, the method and the application have the advantages that an appropriate proportion of solvents are applied when the polyvinyl alcohol solution is prepared, and accordingly the polyvinyl alcohol hydrogel can be prepared from the obtained polyvinyl alcohol solution in standing and natural cooling modes.
Owner:ZHENGZHOU UNIV
Application of extracellular matrix freeze-drying powder
InactiveCN108815094APromote growthInduced growthCosmetic preparationsToilet preparationsCell-Extracellular MatrixFreeze-drying
The invention discloses application of an extracellular matrix freeze-drying powder. The extracellular matrix freeze-drying powder is prepared from fresh mammal tissues by rinsing, disinfecting, degreasing, rinsing, cell removal, cleaning, pulping, extracting and freeze-drying. The extracellular matrix freeze-drying powder has the advantages that the multiple active matters of polytype collagen, proteoglycan, growth factors and the like in the animal tissues are reserved; the multiple biological activities are realized, the biocompatibility is good, and the tissue regeneration is promoted; theanti-oxidizing, repair and aging-resisting effects are realized, and the proliferation, transfer and differentiation of the cells are induced; the extracellular matrix freeze-drying powder can be used as the structure support, and the filling function is realized.
Owner:广州昕生医学材料有限公司
Esophageal tissue constructed by tissue engineering
The invention belongs to the field of biomedical engineering and specifically relates to an esophageal tissue constructed by tissue engineering. A tissue-engineered esophagus containing an epithelial layer and a dermal layer is consisted of scaffold materials and seed cells according to the basic principles and methods of tissue engineering. Vascular endothelial cells in the dermal layer directly participate in the establishment of a capillary network, and two revascularization methods are used to quickly communicate the capillary network in the dermal layer with the blood supply channel of a receptor, thus providing blood to the epithelium of the tissue-engineered esophagus. The esophageal tissue of the invention changes a former therapeutic mode of repairing damage by damage, and can be used for simplifying esophagectomy and one-stage reconstruction of digestive tracts, thus reducing medical trauma and benefiting the majority of patients with esophageal diseases.
Owner:DALIAN CHUANGDA TECH TRADE MARKET
Extraction method and purpose of renewable particles of umbilical cord blood
ActiveCN106377547ADoes not cause immune rejectionSolve puzzlesDigestive systemMammal material medical ingredientsDiseaseLow speed
The invention provides an extraction method of renewable particles of umbilical cord blood. The extraction method comprises the following steps of taking the umbilical cord blood, carrying out low-speed centrifugation, adding a buffer solution in lower layer precipitates A to carry out washing, carrying out low-speed centrifugation, adding erythrocyte lysate in lower layer precipitates B to carry out cracking, carrying out middle-speed centrifugation, adding the buffer solution in lower layer precipitates C, violently shaking, and carrying out low-speed centrifugation; respectively taking an upper layer solution A, an upper layer solution B, and an upper layer solution D, and carrying out high-speed centrifugation; collecting all the precipitates, and cultivating in an MEM (minimum essential medium); and collecting the medium after being cultivated for 10-30 days, and carrying out 3000-7000g centrifugation so as to enrich the renewable particles of the umbilical cord blood. The renewable particles of the umbilical cord blood can be used for treating ischemic myocardial injury as one of major diseases.
Owner:孔五一
Folate-receptor-mediated pH-sensitive Decoy-ODN (Decoy-oligodeoxynucleotide) nanoparticle preparation and preparation method thereof
InactiveCN102240267AAvoid degradationEasy to retouchPowder deliveryGenetic material ingredientsFolic acidFolate receptor
The invention relates to a folate-receptor-mediated pH-sensitive Decoy-ODN (Decoy-oligodeoxynucleotide) nanoparticle preparation and a preparation method thereof. A nanoparticle suspension is prepared from 5-200 mu g / ml of the Decoy-ODN used as a generic medicament, 0.01-6.0 mg / ml of folate-conjugated trimethyl chitosan used as a medicament carrier and 10-500 mu g / ml of pH-sensitive material by a complex coacervation method, wherein the folate conjugation rate of the folate-conjugated trimethyl chitosan is 5-30%, and the ratio of the total volume of the Decoy-ODN solution and the pH-sensitive material solution to the volume of the folate-conjugated trimethyl chitosan solution is 1:(1-4). The folate-receptor-mediated pH-sensitive Decoy-ODN nanoparticle preparation provided by the invention has the advantages of good shaping property, uniform particle size distribution, high DNA (deoxyribonucleic acid) binding rate, better stability, low cytotoxicity and high in-vitro transfection efficiency.
Owner:SHANDONG UNIV
Preparation method of lymphoma-targeted blood platelet targeting drug loading system carrying and loading anti-tumor drug and connecting CD22 monoclonal antibody
InactiveCN107137717AGood biocompatibilityIncrease contentOrganic active ingredientsPharmaceutical non-active ingredientsSide effectLife quality
The invention discloses a preparation method of a lymphoma-targeted blood platelet targeting drug loading system which carries and loads a CD22 monoclonal antibody and an anti-tumor drug. The preparation method comprises the following steps: firstly deeply centrifuging and washing blood platelet-enriched blood plasma obtained through whole blood anticoagulation and centrifugation, obtaining washed blood platelets, treating a maleimide group with the surface modified with a pointing sulfhydryl by adopting an MBS reagent, and then reacting with an anti-CD22 monoclonal antibody subjected to sulfhydrylation by virtue of a Traut's reagent, so that the blood platelets which connect the CD22 monoclonal antibody and target lymphoma cells are obtained; and then carrying out mixed incubation on the blood platelets and the anti-tumor drug, centrifuging, and dispersing, so that the target drug loading system is obtained finally. The drug loading system has the advantages of wide source, high drug loading ratio, good biocompatibility, active and accurate targeting, long cycling time and the like, the anti-tumor effect of the drug can be enhanced, and the toxicity on normal tissues is also reduced, so that toxic and side effects of a chemotherapy drug are alleviated, and the drug loading system is significant for improving the life quality and medical quality of a patient with lymphoma.
Owner:NANJING DRUM TOWER HOSPITAL
Human umbilical cord mesenchymal stem cell serum-free medium
PendingCN109402051AControl infection riskDoes not cause immune rejectionCulture processSkeletal/connective tissue cellsSerum free mediaVitamin C
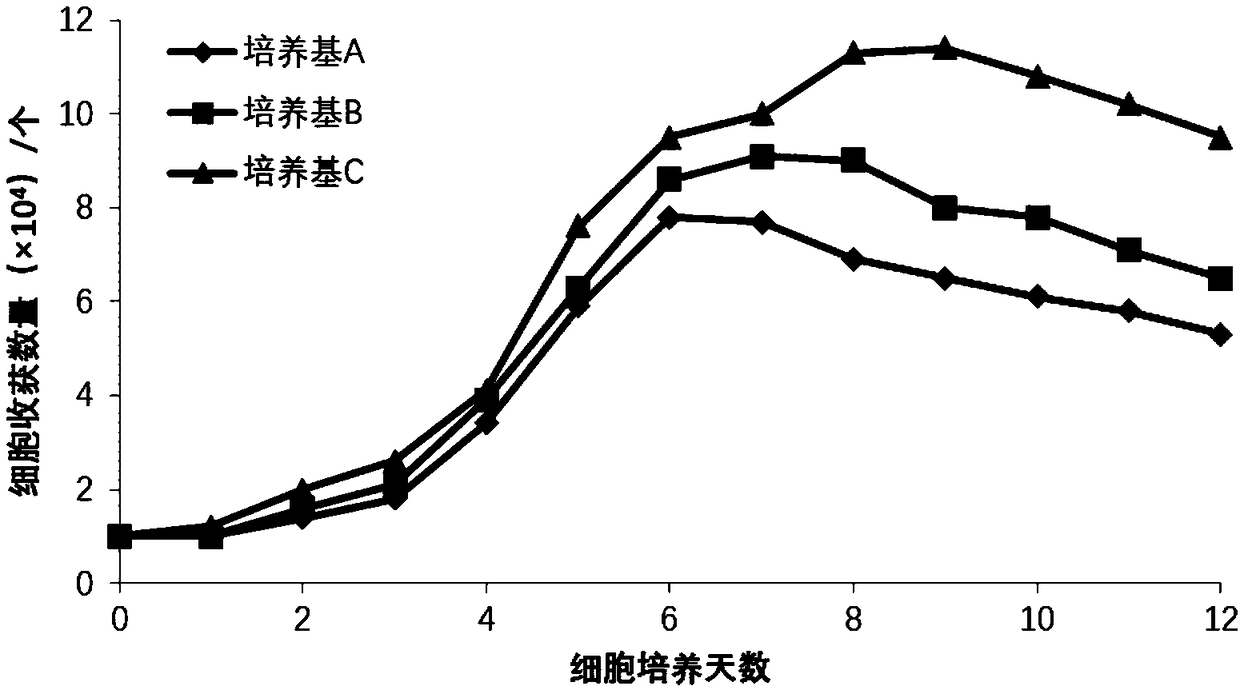
The invention provides a human umbilical cord mesenchymal stem cell serum-free medium characterized by comprising an alpha-MEM basal medium and further comprising the following components by final concentration: 1-20 mM of glutamine, 1-20 mM of HEPEs, 10-100 muM of putrescine, 0.1-10 muM of transferrin, 10-400 muM of vitamin C, 1-10 muM of recombinant insulin, 1-20 nM of progesterone, 10-200 nM ofcortisol, 1-20 mg / mL of human serum albumin, 1-10 ng / mL of basic fibroblast growth factor, beta 11-10 ng / mL of transforming growth factor and 1-50 mg / mL of spirulina fast-soluble proteoglycan. The human umbilical cord mesenchymal stem cell serum-free medium can significantly improve the proliferation rate and the adherence performance of human umbilical cord mesenchymal stem cells, is beneficialto the proliferation of the human umbilical cord mesenchymal stem cells and the maintenance of stem cell characteristics, and has the potential of adipogenic osteogenic induced differentiation. The medium is simple, clear and stable, does not contain any serum components, overcomes the risk of heterologous protein contamination and pathogenic microorganisms, and has high safety.
Owner:青岛麦迪赛斯生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com