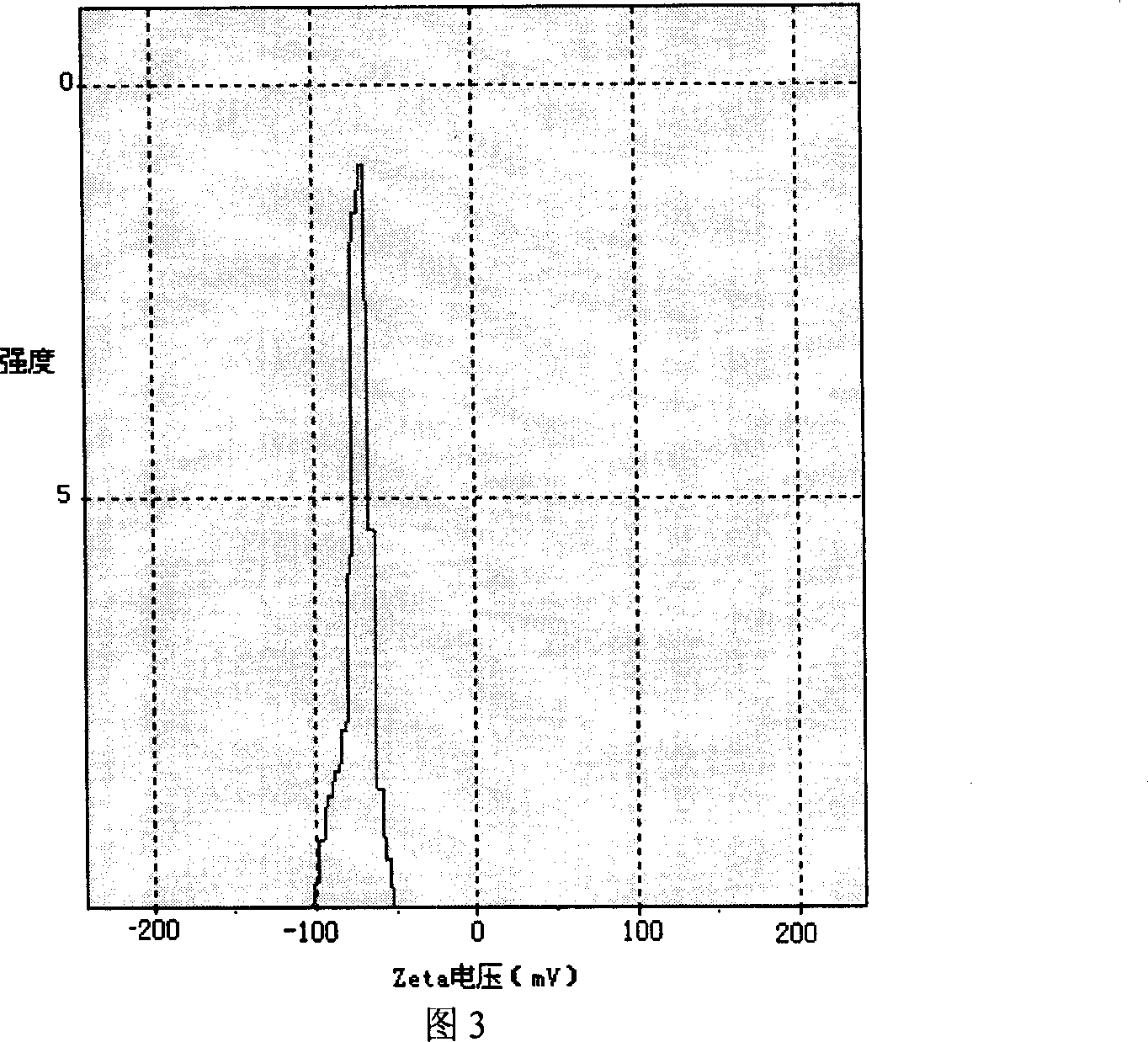
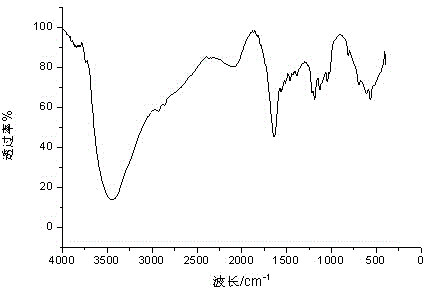
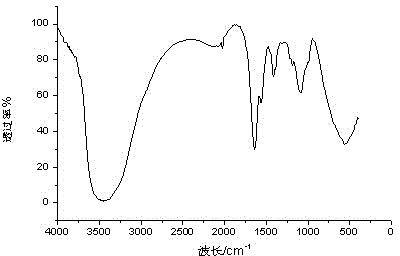
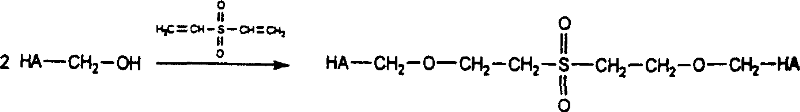Patents
Literature
195results about How to "Antigenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing SIS tissue repair material
The invention provides a method for preparing an SIS tissue restoring material. The method comprises the following steps: carrying out sterilizing treatment, degreasing treatment, cell free treatment and absterging treatment according to an arbitrary sequence at the lower layer of small intestinal mucosa; and finally preparing the material through freezing and drying. The SIS tissue restoring material can be used as an independent and common support vector for a growth factor, a cell, a drug and the like, and becomes a tissue engineering material through in-vitro culture. The material is a natural and nontoxic biological material without antigenicity and immunity. The SIS tissue restoring material can be used for restoring hernia, defect of skin, urinary system tissue defect, mucosa defect and other periphery and internal parenchyma defects.
Owner:BEIJING DATSING BIO TECH
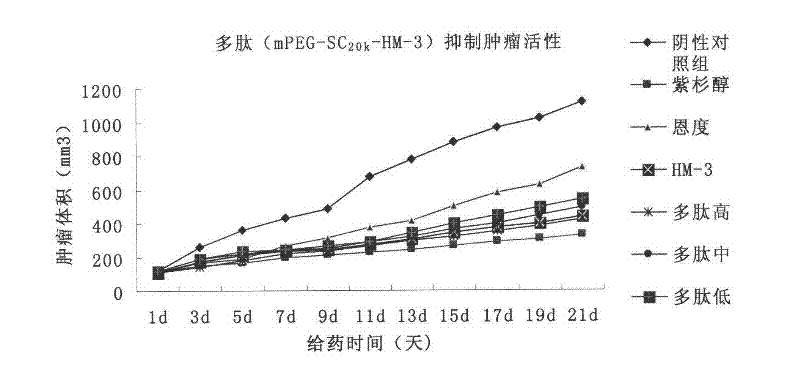
Polyethylene glycol-modified integrin blocking agent HM-3 and application thereof
InactiveCN102417540AExtended half-lifeDoes not affect the activity in vivo and in vitroConnective tissue peptidesPeptide/protein ingredientsTumor angiogenesisPolyethylene glycol
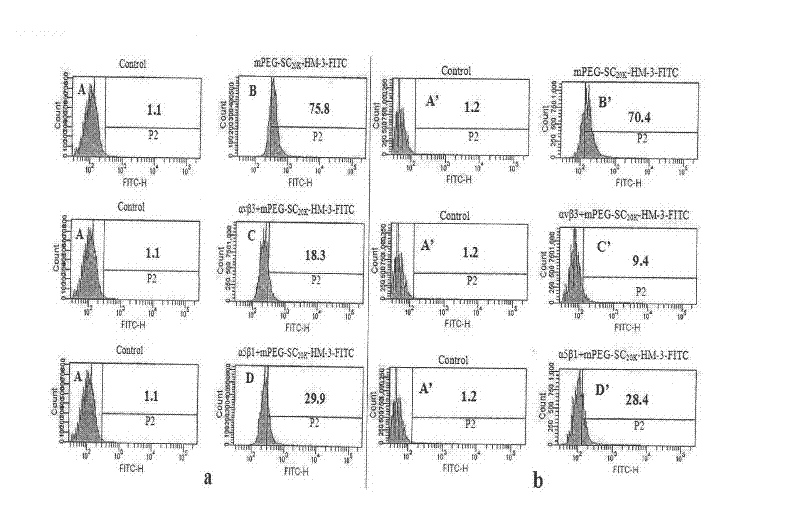
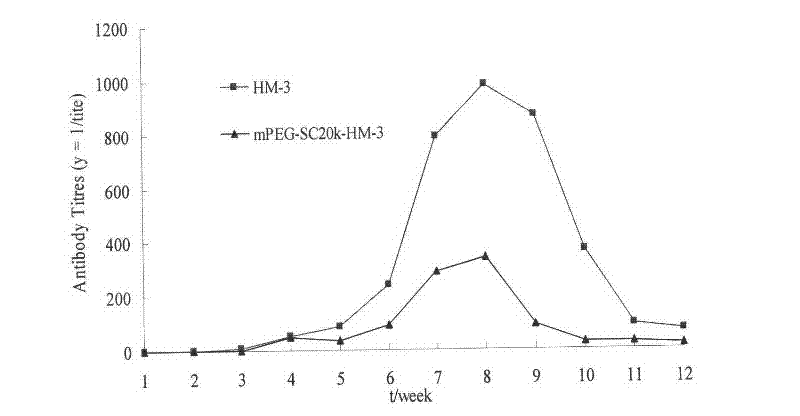
The invention relates to the field of medicaments, in particular to an integrin blocking agent HM-3 which has the function of inhibiting tumor angiogenesis, integrin affinity and a bonding capacity and application thereof. The blocking agent is a polypeptide modified with polyethylene glycol, and the modified integrin blocking agent polypeptide can be applied to treatment of solid tumors. During application of the integrin blocking agent to preparation of a tumor treatment medicament, the sequence and structure of the integrin blocking agent is mPEG-SC20k-Ile-Val-Arg-Arg-Ala-Asp-Arg-Ala-Ala-Val-Pro-Gly-Gly-Gly-Gly-Arg-Gly-Asp. The integrin blocking agent polypeptide designed in the invention is scientific, reasonable, practical and effective, can be used for preparing a treatment medicament for treating human solid tumors, and has remarkable social value and market value; and the treatment spectrum of the integrin blocking agent is expanded greatly, and novel thought and prospect are provided for future development of medicaments.
Owner:CHINA PHARM UNIV
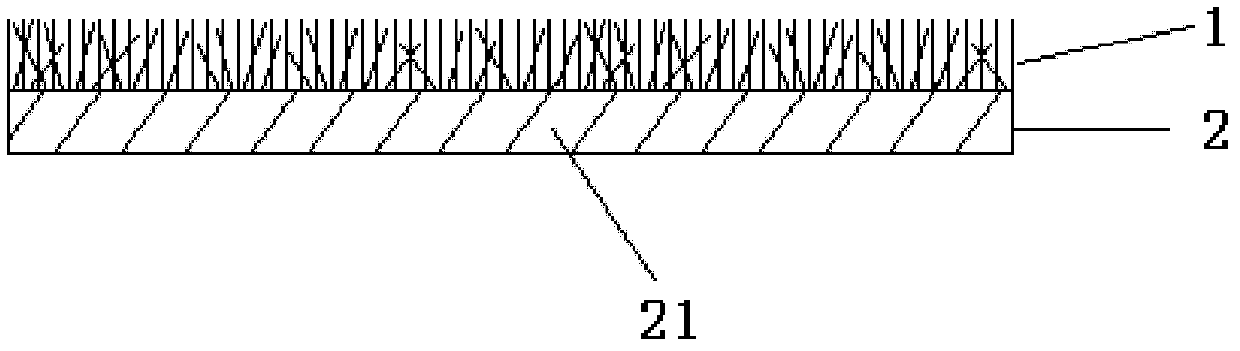
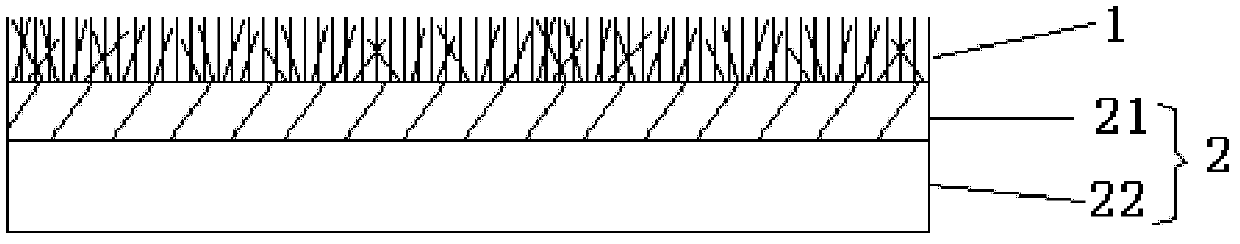
Medical flocking hemostasis material, preparation thereof and application
ActiveCN102600013AAvoid burnsNo toxicityAbsorbent padsMacromolecular non-active ingredientsFiberVein
The invention relates to a medical flocking hemostasis material for stopping bleeding and protecting arteries, veins and wounds of tissues, application of the medical flocking hemostasis material and a method for preparing the medical flocking hemostasis material. The medical flocking hemostasis material comprises a flocking layer and a base layer, the flocking layer is used as a wound contacting layer and made of functional fibers, and the functional fibers are fixed on the upper surface of the base layer in a flocking method. The flocking method comprises a mechanical flocking method and an electrostatic flocking method. The medical flocking hemostasis material can be used for stopping bleeding and protecting the arteries, the veins and the wounds of the tissues, and further can be used as a medicine slow-release carrier. The invention further discloses method for controlling bleeding of the arteries, bleeding of the veins and bleeding of the wounds of the tissues by the aid of the medical flocking hemostasis material. Compared with a hemostasis material in the prior art, the medical flocking hemostasis material has the advantages that a hemostasis performance, comfortableness and the like are greatly improved.
Owner:SUZHOU BOCHUANG TONGKANG PHARM TECH CO LTD
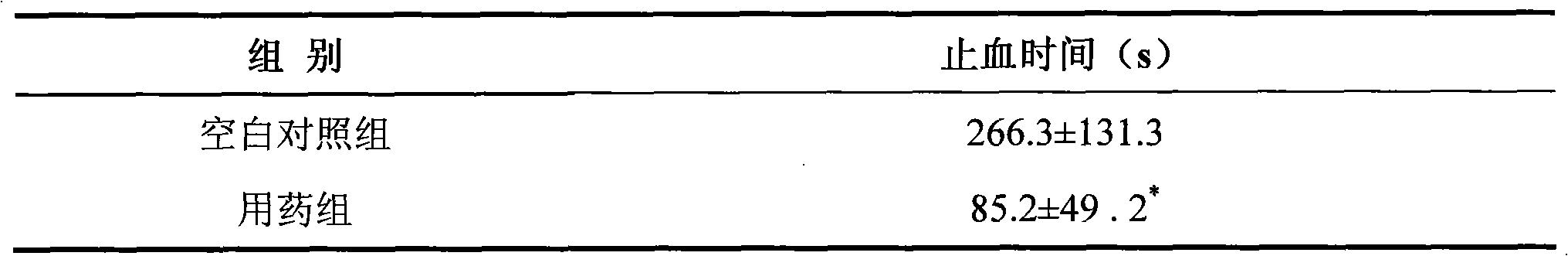
Medical compound micropore polysaccharide and application thereof
ActiveCN101584876AShort hemostatic timeAntigenicAbsorbent padsBandagesSurgical HemostasisWound surface
The invention relates to a medical compound micropore polysaccharide and application in hemostasis on wound surface bleeding areas of various wounds and operation tissues. The medical compound micropore polysaccharide is prepared by adopting a method with the following steps: (1) preparing starch fluid; (2) preparing carboxymethyl chitosan solution; (3) mixing the starch fluid and the carboxymethyl chitosan solution, and adding dispersant and emulsifier into the mixture; (4) emulsifying, crosslinking and co-polymerizing; and (5) refining, drying, packing and sterile treatment. The invention also discloses application of the medical compound micropore polysaccharide in preparing a hemostasis medicament used for the wound surface bleeding areas of wounds and operation tissues. The medical compound micropore polysaccharide of the invention has the following characteristics: 1, the hemostasis time is short, namely the hemostasis is generally finished in 3 to 5 minutes; 2, the medical compound micropore polysaccharide has no antigenicity, has polysaccharide components, and has no protein or immune reaction; 3, the medical compound micropore polysaccharide can be fully degraded and absorbed in vivo; 4, the medical compound micropore polysaccharide is a sterile product and is convenient to use by opening; and 5, the tissue reaction is light. Therefore, the medical compound micropore polysaccharide is a comparatively ideal surgical hemostasis product.
Owner:SAIKE SAISI BIOTECH CO LTD
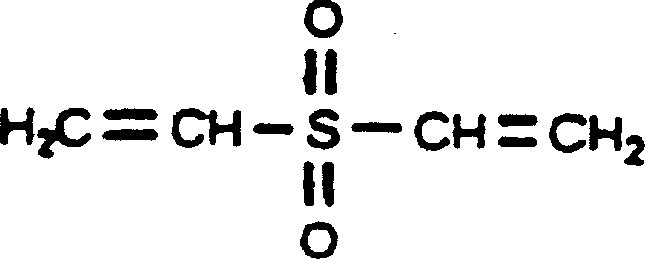
Cross-linked hyaluronic acid derivatives preparation and the preparing technique
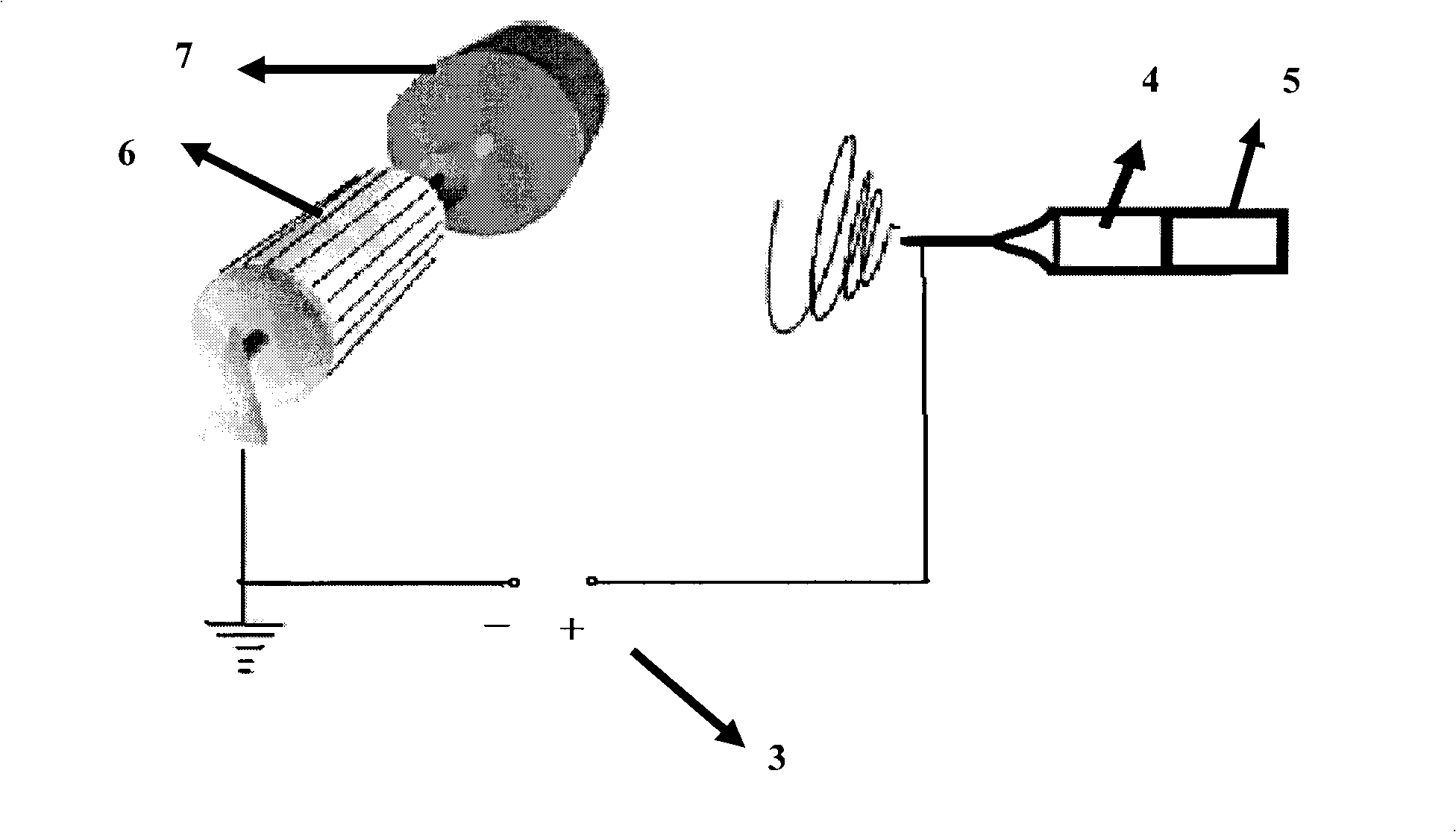
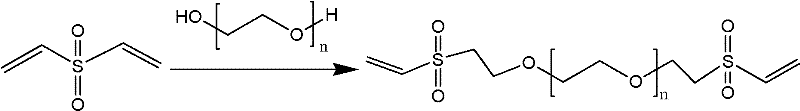
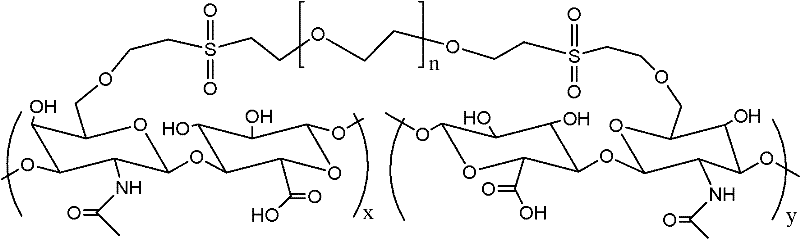
The invention relates to a preparation technique of crosslinked hyaluronic acid gel preparation, characterized in that divinylsulphone reacts with hydroxyl group of hyaluronic acid to form ether linkage. The steps are that the raw material of hyaluronic acid being put into container and dissolving in alkali reagent; stirring to make it thoroughly dissolve; mixing the alkaline hyaluronic acid solution and divinylsulphone in container to form crosslinked hyaluronic acid gel clot; adding substance riched in hydroxyl groups into the reaction solution; extracting the crosslinked hyaluronic acid gel clot and putting it into agitator to stir; and then crosslinked hyaluronic acid gel becoming micro grained. The advantages thereof are that the gel preparation is suitable for injection in vivo, with long residence timen in humans and good filling effect.
Owner:SHANGHAI QISHENG BIOLOGICAL PREPARATION CO LTD +1
Degradable medical hemostatic non-viscous material and preparation method thereof
InactiveCN101537205AAntigenicExcellent coagulation propertiesAbsorbent padsBandagesAbsorption rateSodium hyaluronate
The invention discloses a degradable medical hemostatic adhesion-resistant material and a preparation method thereof. The invention uses chitosan or derivatives thereof and transparent sodium hyaluronate as main raw materials, adopts methods, such as spray drying, self lamination assembly, and the like to prepare microspheric and gel-type hemostatic adhesion-resistant materials, and medicines and activated substances capable of stimulating hemostasis and antiphlogosis can be compounded and added in the preparation process. The material has high water absorption rate, can rapidly arrest bleeding to form an aquagel protective layer on a wound surface, has favorable tissue adhesiveness, adhesion resistance and bacterinertness, can suit a wound in an irregular shape, truly fully covers the wound surface to fully take the aims of arrest bleeding, resisting adhesion and stimulating wound surface healing, has remarkable postoperative hemostatic adhesion-resistant effects while being used for ear-nose-throat department operations, is convenient to use and brings little pain to patients.
Owner:JINAN UNIVERSITY
Preparation method of gel containing stem cell exosomes for repairing skin wounds
InactiveCN111420117AImprove application securityHigh clinical safetyCulture processSkeletal/connective tissue cellsMesenchymal stem cellEngineering
The invention relates to a preparation method of a gel containing stem cell exosomes for repairing skin wounds. The method comprises the following steps: 1) primary extraction and culture of human umbilical cord mesenchymal stem cells: 1.1) primary extraction of human umbilical cord mesenchymal stem cells, 1.2) subculture, and 1.3) collection of the culture supernatant; 2) extraction of human umbilical cord mesenchymal stem cell exosomes: 2.1) primary centrifugation, 2.2) secondary centrifugation, 2.3) removal of organelles by centrifugation, 2.4) coarse extraction of exosomes, and 2.5) finalextraction of exosomes; 3) preparation of a gel material: 3.1) preparation of chitosan, 3.2) configuration of beta-glycerol phosphate (beta-GP), and 3.3) preparation of the gel material; and 4) gel loading of the exosomes. The gel containing stem cell exosomes can promote repair of skin wounds, shorten the healing time of wounds and reduce scar formation.
Owner:陕西朗泰生物科技有限公司
Method for producing nano-fibre bracket material with levorotation polylactic acid as base material
InactiveCN101401955AFlexible textureGood tissue compatibilityStentsPhysical treatmentPorosityTissue Compatibility
The invention relates to a method for preparing a nanofiber bracket material using levorotatory polylactic acid as matrix. The method comprises the following steps: dissolving the levorotatory polylactic acid as the matrix in a solution of dichloromethane and dimethyl formamide, and stirring and centrifuging the mixture to obtain an electrostatic spinning solution; placing a polylactic acid solution into a 5 milliliter glass syringe, and applying high voltage on the glass syringe; advancing the levorotatory polylactic acid solution in the glass syringe; preparing the mixed solution into a nanofiber material film through a electrostatic spinning technology; and modifying the nanofiber material film to obtain the nanofiber bracket material of which the fiber diameter is between 50 and 500 nanometers and the fiber porosity is more than 90 percent. The method solves the defects that a PLLA porous bracket still has too long degradation time, and degradation products can cause tissue inflammations easily and the like. The method has the advantages of flexible texture, better water permeability and air permeability, excellent tissue compatibility, controllable biodegradability, and no antigenicity.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Construction method for skin tissue engineering rack containing epidermal growth factor
The construction process of skin tissue engineering rack containing epidermal growth factor with fetus calf or ox tendo achillis as basic material includes dewatering, defatting, primary enzyme treatment to reduce the covalent bond among tissue fibers, dialysis, secondary enzyme treatment to remove antigenic determinant, dissolving with acetic acid to obtain no-antigenicity collagen solution and collagen sponge film, soaking in propanetriol to adsorb, drying, mixing water solution of heparin and acetic acid, water solution of epidermal growth factor and propanetriol wetted collagen sponge film, and final freeze drying. The rack has no antigenicity, high strength, high flexibility, convenient operation implantation, no toxicity to wound, and capacity of inducing wound cell growth and promoting wound healing.
Owner:CHINA LEATHER & FOOTWEAR IND RES INST +1
Method for preparing crosslinking hyaluronic acid gel
The invention provides a crosslinking hyaluronic acid gel and its preparation method. The method is characterized in that divinylsulfone is combined with polyethylene glycol for generating a novel cross-linking agent at first, the novel cross-linking agent is reacted with a hyaluronic acid molecule for producing a crosslinking hyaluronic acid gel. The crosslinking sodium hyaluronate gel obtained in the invention has good biological compatibility and longer half life period, and the particle is small and uniform, and is suitable for beautifying shaping, tissue filling, bone articular lubrication or medicine sustained-release preparation and the like.
Owner:XIAN LIBANG PHARMA
Cartilage tissue engineering rack and its application
The present invention provides one kind of double layer cartilage tissue engineering rack comprising one compact layer and one loose layer communicated each other. The rack has excellent biological performance, sufficient cell tissue holding space, certain blocking effect and capacity to allow nutritious matters to pass through freely. It is used in repairing human body's cartilage tissue and constructing extracorporeal cartilage tissue. It is made through preparing solution of different concentrations with acid extracted collagen, freeze drying to obtain collagen sponge, flattening, injecting collagen solution of different concentration, and drying for the second time to obtain the double layer cartilage tissue engineering rack. The rack has the pore size controlled through controlling the concentration of the solution.
Owner:ZHEJIANG UNIV
Bone repair material containing nano hydroxylapatite/collagen particle and preparation thereof
ActiveCN101297980ARetain biocompatibilityGood biocompatibilityProsthesisHuman bodyMinimal invasive surgery
The invention relates to a bone repair material containing nano hydroxyapatite / collagen particles, and a preparation method thereof. The invention provides a bone repair material which comprises: 1 to 2 parts by weight of the nano hydroxyapatite / collagen particles, 1 part by weight of chitosan and 2 to 3 parts by weight of Beta-sodium glycerophosphate (which is supplemented by the weight proportion of the components). The invention also provides a preparation method of the bone repair material; the bone repair material is placed at 37 DEG C or injected into human body, thus forming in-situ gelatin. The bone repair material provided by the invention not only can retain the characteristics of modeled natural bone structure, but also ensures the new performance of syringeability of the material and has temperature sensitivity, thus being applicable to minimal invasive surgery and convenient for clinical application.
Owner:BEIJING ALLGENS MEDICAL SCI & TECH
Artificially recombinant H9N2 hypotype influenza virus and its uses
The invention relates to an artificial recombination N9 hypotype influenza virus A / Harbin / Re-2 / 2003 (H9N2), artificial recombination H9 hypotype influenza virus A / Harbin / Re-2 / 2003(H9N2) characterized in that, it includes a HA of H9N2 hypotype fowl influenza virus A / chicken / Guangxi / 10 / 99(H9N2) and six internal genes of influenza virus A / PR / 8 / 34(H1N1), PB2, PB1, PA, NP, M and NS. its storage number is CCTCC-V200311.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Artificial skin containing human bone marrow mesenchymal stem cell and its construction method
InactiveCN1511593AHigh strengthGood flexibility and enzyme resistanceTissue cultureProsthesisGrowth cellGrowth factor
The present invention relates to construction process of artificial skin containing human bone marrow mesenchymal stem cell. Skin tissue engineering rack is first constructed with fetus calf or ox tendo achillis as basic material, collagen sponge film through twice enzyme treatments and the mixture of heparin and epidermal growth factor; and human bone marrow mesenchymal stem cell obtained via purifying human bone marrow blood is implanted on two sides of the rack to constitute the artificial skin with bone marrow mesenchymal stem cell on the two sides of and in the internal of the rack. The artificial skin has no antigenicity, high strength, high flexibility, no toxicity to wound, and capacity of inducing wound cell growth and promoting wound healing.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA +1
Cross-linked sodium hyaluronate and preparation method and application thereof
ActiveCN106397846AMild reaction conditionsLess side effectsCosmetic preparationsAerosol deliverySodium hyaluronateChemistry
The invention relates to cross-linked sodium hyaluronate and a preparation method and application thereof. The cross-linked sodium hyaluronate is prepared from raw materials, including sodium hyaluronate, an inorganic salt and a cross-linking agent. The preparation method comprises the steps: dissolving a certain amount of sodium hyaluronate in an alkaline solution with the pH value of 7.5 to 9.0, then, adding a certain amount of inorganic salt into the solution, and carrying out stirring until the inorganic salt is completely dissolved; heating the solution to the temperature of 20 DEG C to 60 DEG C, then, slowly dripping a certain volume of cross-linking agent solution into the solution, carrying out stirring, and carrying out a reaction for a period of time while carrying out heat preservation; adjusting the pH to 7.1 to 7.5 with acid, carrying out balancing for 1 to 2 hours, then, carrying out precipitation with ethanol or an ethanol solution, so as to obtain lumpy solids, i.e., the cross-linked sodium hyaluronate. By the method provided by the invention, the cross-linking efficiency of the cross-linked sodium hyaluronate can be increased, the reaction yield can be increased, side reactions can be reduced, and the residual of the cross-linking agent can be reduced; the prepared cross-linked sodium hyaluronate is safe and non-toxic, is good in stability, long in in-vivo retention time and good in filling effect and is applicable to in-vivo injection.
Owner:BEIJING DATSING BIO TECH
Medical hemostatic polysaccharide starch microsphere and preparation method thereof
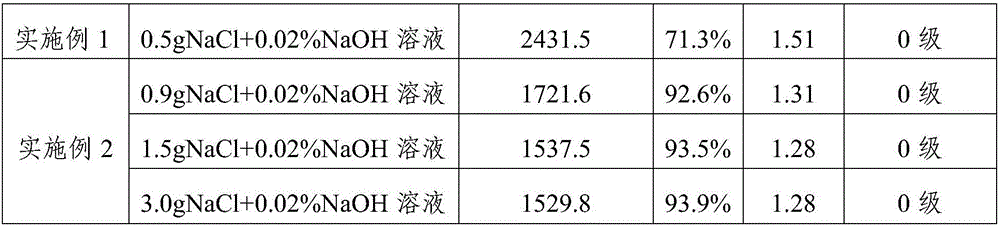
ActiveCN104311870AAbsorb quickly and completelyDisorganized reactionOrganic active ingredientsPotato starchStarch Microspheres
The invention relates to a preparation method and a refining method of medical hemostatic polysaccharide starch. With pretreated potato starch as a raw material and epichlorohydrin as a crosslinking agent, a microsphere adopting a three-dimensional network structure is synthesized by an emulsifying and crosslinking technology. The medical hemostatic polysaccharide starch is high in biocompatibility; the medical hemostatic polysaccharide starch is full with wrinkles on the surface, so that the particle surface area is increased, the water absorbing rate is significantly improved and the hemostatic time is greatly reduced; the medical hemostatic polysaccharide starch is especially applicable to large-area blood seepage, deep bleeding, and bleeding at a part difficult to reach by operative procedures.
Owner:SHIJIAZHUANG YISHENGTANG MEDICAL SUPPLIES
Carbazochrome Sodium Sulfonate infusion and its preparation method
InactiveCN1557302AGood effectEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismDiseaseUpper gastrointestinal
The present invention belongs to the field of medicine technology. The Carbazochrome liquid contains Carbazochrome 0.002-0.5 wt%, antioxidant 0-10 wt%, osmotic pressure regulator 0.8-75 wt%, and injection water 20-99.1 wt%. The preparation process of the Carbazochrome liquid includes dissolving osmotic pressure regulator with partial injection water in a compounding tank; adding injection level active carbon through heating and stirring for adsorption for 30 min; filtering and decarbonizing with titanium rod and adding rest injection water to obtain the solution I; dissolving Carbazochrome and antioxidant in solution I, adding injection level active carbon for adsorption for 30 min, filtering and decarbonizing with titanium rod, regulating pH value, fine filtering, detecting, packing in bottle, disinfecting and other steps. The medicine is used in intravenous transfusion for treating various hemorrhage diseases.
Owner:肖广常 +1
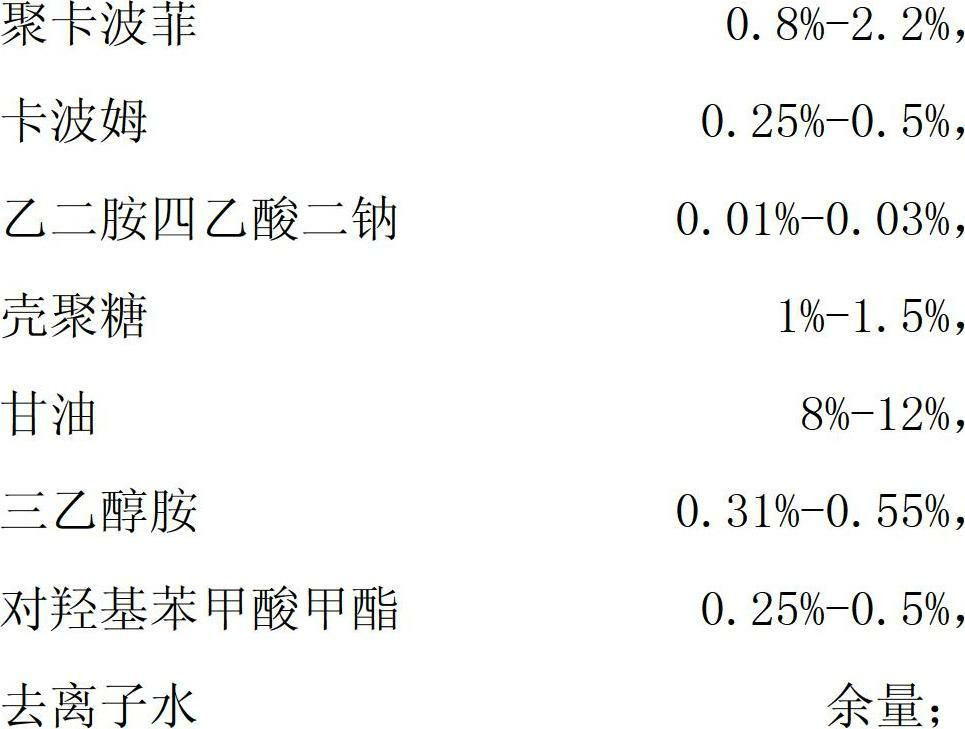
Vagina pH buffer antibacterial gel and preparation method thereof
InactiveCN102688182AImprove self-cleaning abilityReduce buildAntibacterial agentsAerosol deliveryAntigenDisease
The invention provides vagina pH buffer antibacterial gel, a preparation method thereof, and a using method thereof. The gel mainly comprises the components of polycarbophil, carbomer, ethylenediamine tetraacetic acid disodium, chitosan, glycerol, triethanolamine, methyl parahydroxybenzoats and deionized water. By using the weak acidity of polycarbophil and the antibacterial performance of chitosan, the gel can eliminate the source of recurrence of vagina inflammatory diseases and recovers the self-cleaning ability of the vagina. The gel is free from antigen performance, allergy and irritation, and convenient to use, and tissue reaction is avoided.
Owner:天津枫盛阳医疗器械技术股份有限公司
Method for preparing biological scaffold by 3D printing
InactiveCN105903078AGood biocompatibilityNo toxicityAdditive manufacturing apparatusProsthesisBiologic scaffoldNormal bone
The invention provides a method for preparing a biological scaffold by 3D printing. The method comprises the steps of grinding allogeneic bones or heterogeneous bones at a low temperature in a freezing grinder to obtain a printing material, modeling 3D printing with the aid of a computer, and then performing post treatment in a genipin solution. The prepared biological scaffold has good biological compatibility, and the bone graft material does not have toxicity, rejection, mutagenicity or antigenicity in vivo, and does not disturb bone and tissue regeneration; the prepared biological scaffold can be gradually degraded and absorbed and replaced by autologous bone tissues, can bear the pressure close to 20MPa with normal bone cortex, and has good initial mechanical properties; and the elastic modulus is gradually reduced, stress shielding is avoided, fracture, collapse and loosening of implants in the long-term healing process are avoided, bone fusion can be accelerated, and the implants can be finally completely converted into autologous bone tissues.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
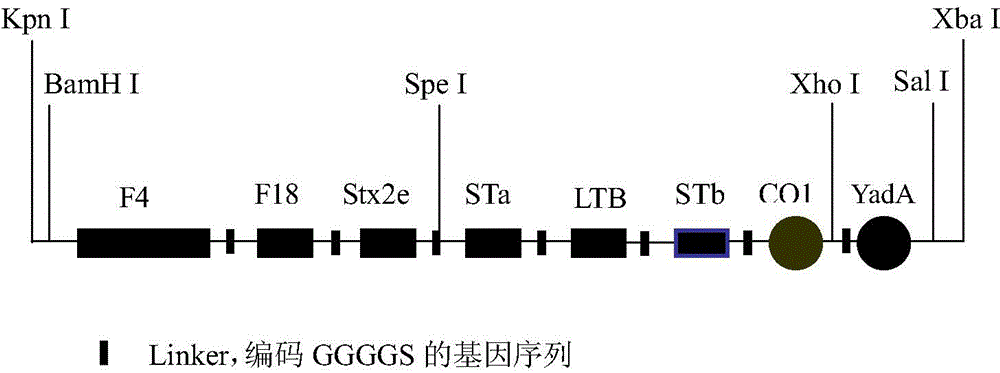
Optimized enterotoxigenic escherichia coli-producing polyvalent antigen gene sequence and application thereof in preventing weaned piglet diarrhea
InactiveCN104593397AFull protective responseHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInclusion bodies
The invention discloses an optimized enterotoxigenic escherichia coli (ETEC)-producing polyvalent antigen gene sequence and the application thereof in preventing weaned piglet diarrhea, and belongs to the technical field of biology. The polyvalent antigen gene sequence is synthesized by a large fragment according to the preference of the lactococcus lactis codon, and the synthesized polyvalent antigen gene sequence contains six antigen genes, namely common ETEC dominant serotype F4<+> primary structure protein FaeG causing the weaned piglet diarrhea, the receptor binding domain RBD of F18<+>, and toxins Stx2e and STa mutant, LTB subunit and STb, and molecular peptides CO1 and YadA31 genes targeting the M cells and the intestinal cells, respectively; the genes are connected by GGGGS. The genes are applicable to constructing a lactic acid bacterium living-vector vaccine and remarkably improving the secretory expression quantity of the target protein, and have excellent immunogenicity and protection effect; the genes also are suitable for high-efficiency expression in the escherichia coli; experiments prove that the inclusion body of the genes has excellent immunocompetence and can be taken as the vaccine for preventing the weaned piglets from F4<+> and F18<+> ETEC infection.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Multifunctional female pelvic cavity biological sticking patch
ActiveCN101427947AExcellent tissue compatibilityImprove stabilityLigamentsMusclesRectovaginal fistulaDIAPHRAGM DEFECT
The invention discloses a structure and a preparation method of a multifunctional woman pelvic biological repair plate. The biological repair plate takes natural biomembranes as raw material, is processed through a series of biochemical technology, increases the stability greatly, and is made through remachining shaping and activity-modifying. The stability of the material is high, the biocompatibility is good, the ingrowth for autologous nerve structures of a patient can be induced, and the supporting and fixing functions of the autologous nerve structures can be served. The morphological structure of the multifunctional woman pelvic biological repair plate comprises a head, double wings and a bosom body, and likes a tailless mosquito hawk, as shown in an attached drawing. The structure is designed according to years of clinical experience of an inventer, the adaptability is wide, the practicability is strong, and the multifunctional woman pelvic biological repair plate can be used in multiple situations such as posterior vaginal wall prolapse, musculus levator ani laceration, uterosacral ligament slack, rectovaginal diaphragm defect, rectovaginal fistula repair, vaginal contraction, etc.
Owner:上海冠昊医疗器械有限公司
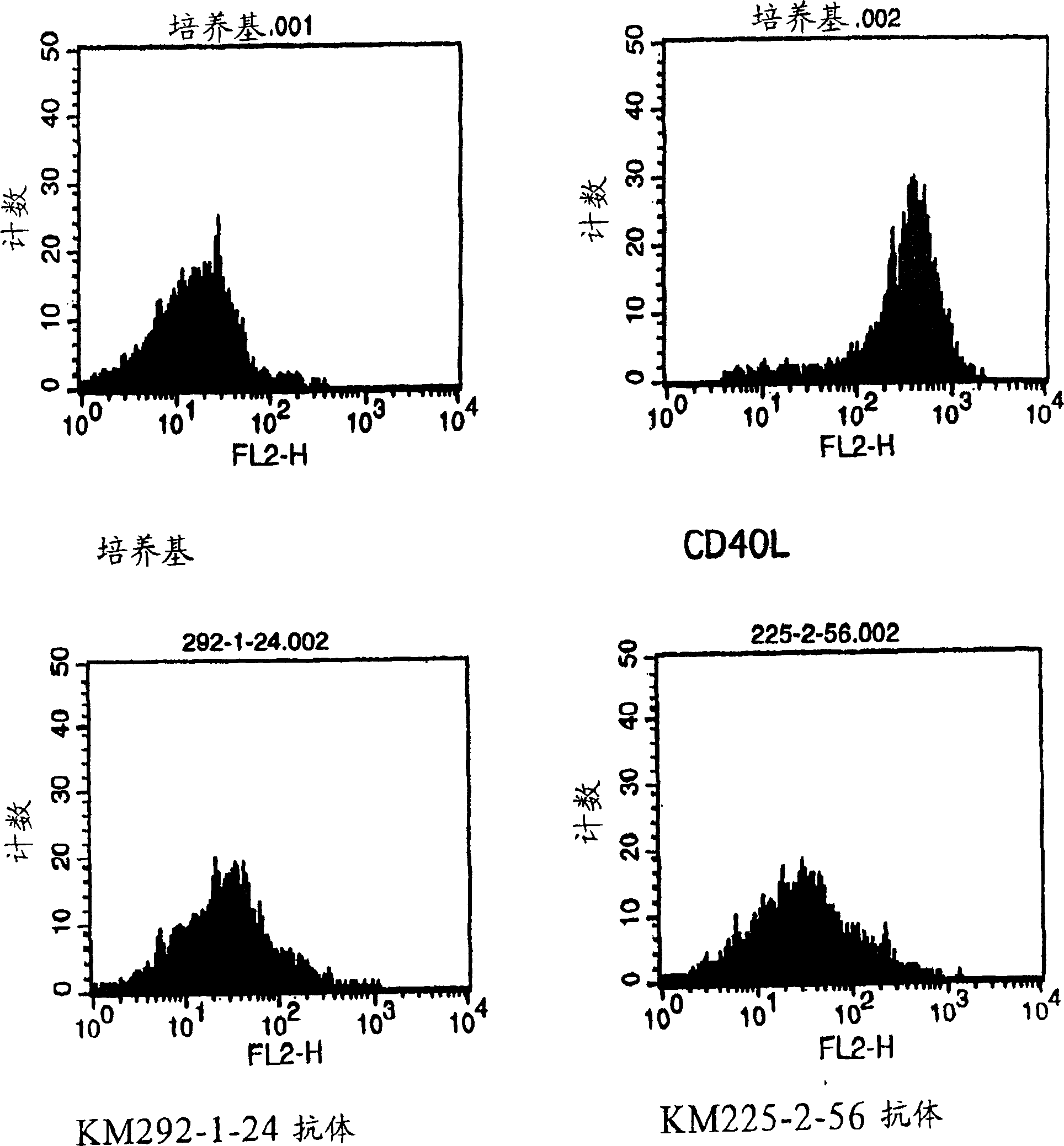
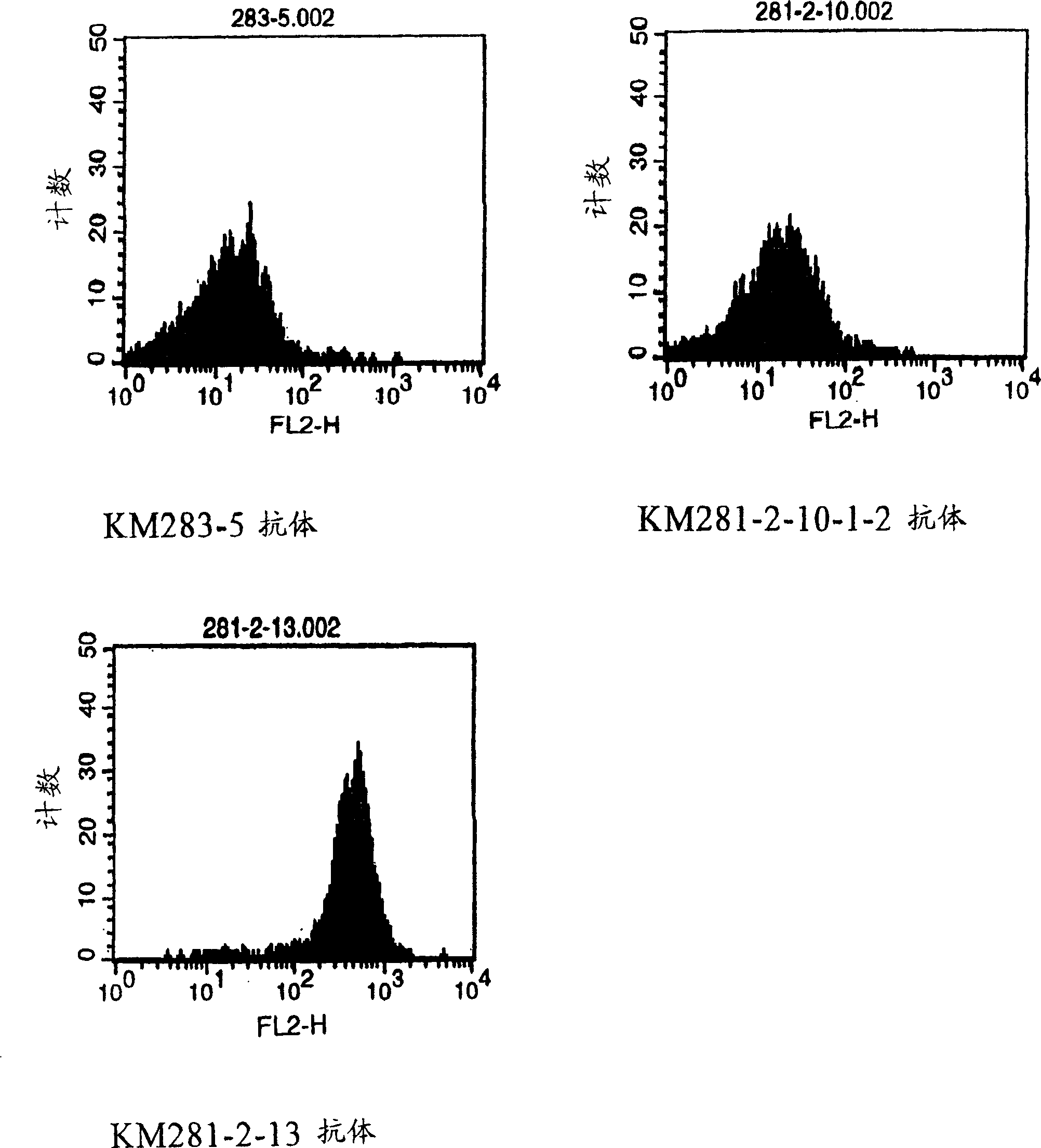
Anti-CD40 monoclonal antibody
InactiveCN1522264AAntigenicInhibition of ligand actionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAnti cd40Antiendomysial antibodies
Owner:KYOWA HAKKO KIRIN CO LTD
Bovine lactoferricin and preparation method thereof
InactiveCN102993296AHeat resistantHas a broad-spectrum antibacterial effectBacteriaTransferrinsFusion Protein ExpressionPepsin
The invention relates to bovine lactoferricin and a preparation method thereof. Bovine lactoferricin (Lfcin B) is 25 amino acid residues hydrolyzed from a bovine lactoferrin (BLF) N-terminal (17-41) by pepsin, and has a molecular weight of approximately 3.1kd. The preparation method comprises the steps of: (2) recombinant expression vector construction; (2) fusion protein expression induction; and (3) protein separation and purification. Bovine lactoferricin has the advantages that: Lfcin B has the highest activity among all the Lfcins, and an antibacterial effect 400 times that of bovine lactoferrin, such that Lfcin B can play an important role in gastrointestinal tracts.
Owner:GUANGZHOU GLAM BIOTECH
Tissue engineered esophagus
InactiveCN1410034AAntigenicDoes not cause immune rejectionTubular organ implantsCapillary networkVascular endothelium
A histo-enigineered esophagus is disclosed, and it contains a dual-layer structure of epithelial layer and dermis layer constructed by scaffold material and seed cells. The vascular endothelial cellsin the dermis layer directly take part in the creation of capillary network. Two revascularization methods are used for quickly communicating with the blood supply channel of receptor.
Owner:谭强
O-type foot and mouth disease virus antigen epitope molecular mimic peptide and application thereof
InactiveCN101597327AAntigenicNon-toxicAntiviralsPeptidesVirulent characteristicsMolecular Immunology
The invention relates to the molecular immunology field. Although the existing foot-and-mouth disease weak poison vaccines and inactivated vaccines and other routine vaccines have good immunogenicity, but the vaccines have some unsafe factors such as reversion of virulence, incomplete virus blanching, the escape of live-virus from a preparation factory and the like so that people are motivated to find a more safe and effective FMD vaccine. In the molecular mimic peptide invention, phage display techniques are used to sieve effective FMDV epitopes to provide an O-type foot and mouth disease virus antigen epitope molecular mimic peptide and the invention also provides an application thereof in preparing immunogen and swine foot-and-mouth disease epitope peptide vaccine. The FMDV epitope peptide vaccine using the molecular mimic peptide of the invention has the antigenicity of target molecule without toxicity, is very safe and has low mass production cost and good economic effect and social effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Recombinant novel coronavirus COVID-19S-RBD protein and preparation method and application thereof
ActiveCN112079907AEasy to useGood effectSsRNA viruses positive-senseVirus peptidesInclusion bodiesAntigenicity
The invention discloses a recombinant novel coronavirus COVID-19S-RBD protein and a preparation method and application thereof. The preparation method comprises the steps that an artificially synthesized S-RBD fusion protein gene is constructed into an escherichia coli expression system, and then the soluble purified recombinant COVID-19S-RBD protein with high expression quantity and antigenicityis obtained through a special inclusion body renaturation technology. The preparation method of the recombinant protein is easy and convenient to operate, low in production cost and high in efficiency, and the prepared recombinant protein can be used for preparing a reagent strip for detecting new coronavirus; the using effect of the reagent strip is verified by the preparation method, the detection result is accurate, the efficiency is high, the result is popular and easy to understand, and the preparation method is particularly suitable for rapid screening of the new coronavirus of a large number of people.
Owner:SHANGHAI JIEMEN BIO TECH
Itraconazole liposome and preparation process thereof
InactiveCN1969830AHighly toxicStrong side effectsOrganic active ingredientsAntimycoticsMANNITOL/SORBITOLPhosphate
The invention discloses an eosin liposome and making method, which comprises the following parts: 18-22mg eosin, 505-509mg lecithin, 90-95mg cholesterol, 73-77mg sodium deoxycholate, 505-509mg mannitol, 505-509mg lactose and 18-22mL phosphate buffer with pH value at 5.5-6.5. the making method comprises the following steps: (1) adding eosin, sodium deoxycholate, lecithin and cholesterol into chloroform; (2) decompressing; evaporating chloroform; (3) adding lactose and mannitol into phosphate buffer; (4) emulsifying phosphate buffer without fat film; (5) freezing; drying to obtain the product.
Owner:HARBIN MEDICAL UNIVERSITY
Preparation and application of cell-eliminating pig corium substrate without cytotoxicity
InactiveCN1587390AIncrease success rateGood repeatabilitySkin implantsTissue cultureChemistryTissue engineering
The preparation process of cell-eliminating pig corium substrate includes sustained vibration of the corium substrate in 0.5 mol / L NaOH solution compounded with thrice distilled water, rinsing with thrice distilled water and balanced phosphate salt solution separately, freeze drying, packing and sterilizing. The cell-eliminating pig corium substrate is used as the in vitro corium culturing rack in composite skin preparing tissue engineering process, and the preparation process includes coating the corium substrate with acetic acid dissolved type-I ox collagen, regulating pH to 7.0-7.4, soaking subsequently in balanced phosphate salt solution and no serum culture medium, inoculating passage epidermal cell, and no serum culture inside a CO2 hatcher. The tissue engineering composite skin is used as human skin substitute.
Owner:中国人民解放军三〇四医院
Paper additive and preparation method thereof
InactiveCN104805734AHas antibacterial propertiesAntigenicNon-fibrous pulp additionPaper/cardboardBiochemical engineeringPolyhexamethylene guanidine
The invention discloses a paper additive and a preparation method. During preparation, the paper additive comprises raw materials in parts by weight as follows: 0.6-6 parts of an antibacterial agent, namely, polyhexamethylene guanidine hydrochloride, 1-4 parts of carboxymethyl chitosan, 0.4-2 parts of a condensing agent, 0.1-0.5 parts of an activating agent and 0.05-0.2 parts of a cross-linking agent. The preparation method comprises steps as follows: the carboxymethyl chitosan is dissolved in water, then the pH value is adjusted to range from 5 to 5.5 with hydrochloric acid, the condensing agent and the activating agent are added to the mixture for activation, the antibacterial agent, namely, the polyhexamethylene guanidine hydrochloride, is added for a reaction, an obtained reaction liquid is subjected to suction-filtration, washing, drying and crushing and then evenly mixed with the cross-linking agent, namely, N,N-dimethyl bisacrylamide, an obtained mixture is dissolved in deionized water, and the paper additive is obtained. The paper additive has the advantages that the paper additive is broad in spectrum, high in efficiency, low in toxicity, free of drug resistance, high in antibacterial endurance and the like in the antibacterial aspect and has the advantages that the paper additive is capable of significantly enhancing the paper strength, non-toxic and harmless to human bodies and the like in the enhancing aspect.
Owner:SHANGHAI INST OF TECH
Dressing with synergistic effect with skin as well as preparation method and application thereof
InactiveCN104027836AFast self-healingSpot-free self-healingAbsorbent padsBandagesEthylene diamineCollagenan
The invention provides dressing with a synergistic effect with skin. The dressing is characterized by comprising the following raw materials in percentage by mass: 0.03-0.5% of hyaluronic acid, 0.09-0.5% of collagen, 0.01-0.05% of epidermi growth factor, 0.1-0.5% of sodium hydroxide, 1.5-3.0% of EDTA (Ethylene Diamine Tetraacetic Acid) disodium and the balance of water. Due to adoption of the dressing, a better micro environment can be provided, the nutrient components can be replenished, and the rapid and non-spot self-healing of skin is promoted.
Owner:南京仁天生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com