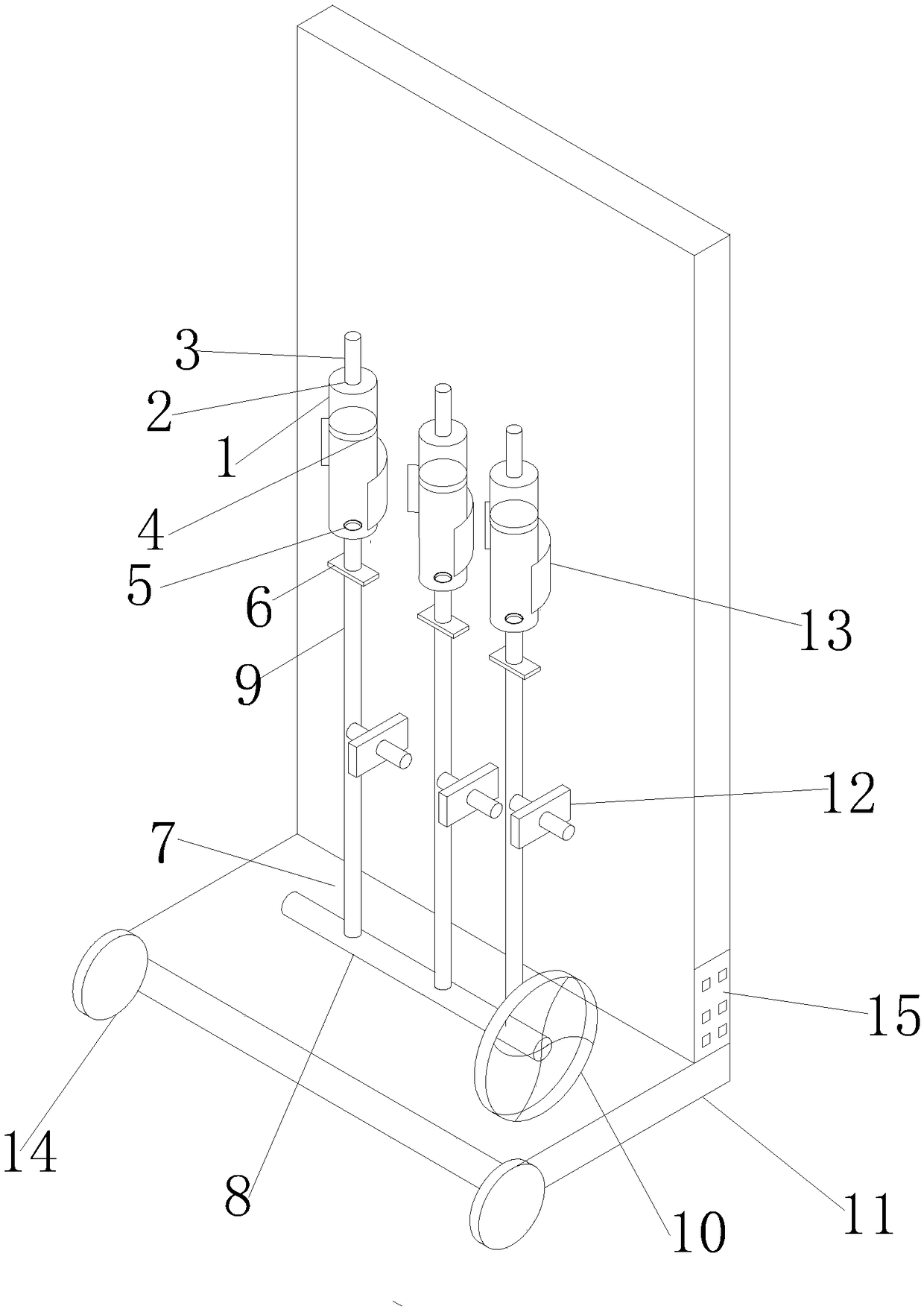
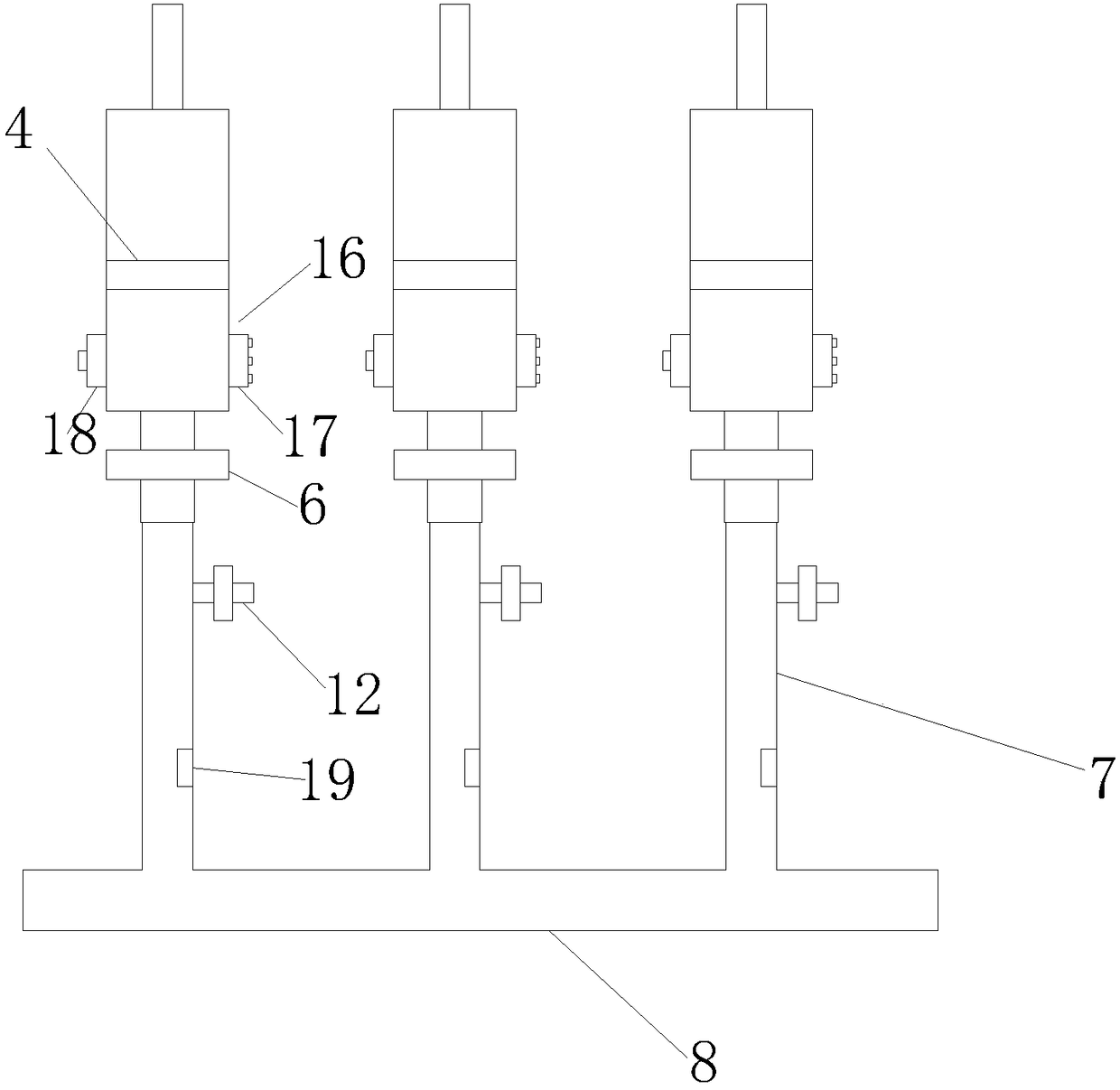
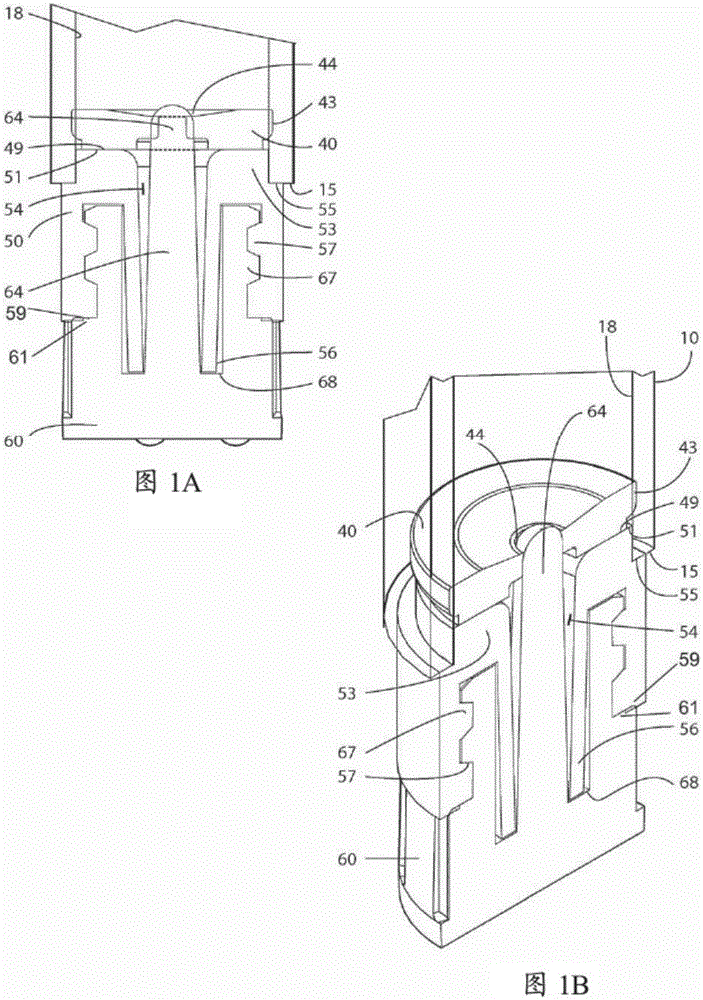
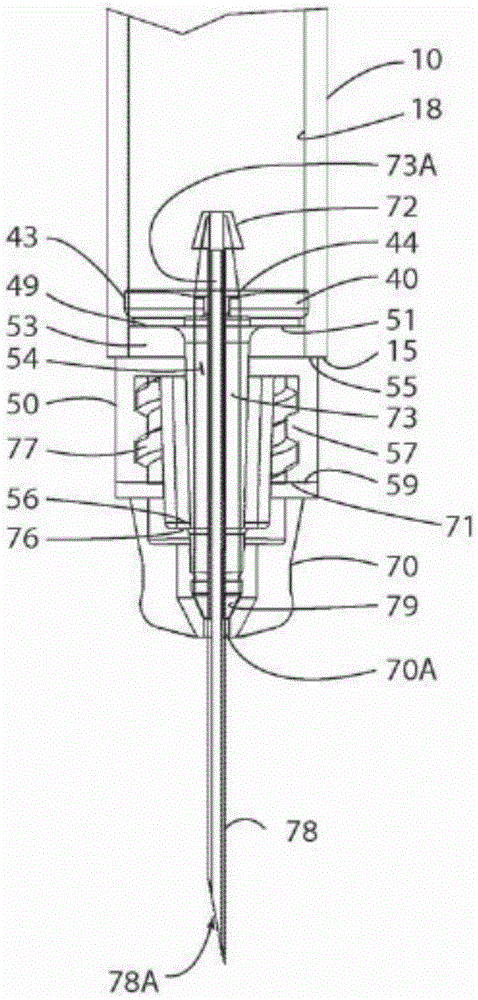
Patents
Literature
93 results about "Glass Syringe" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
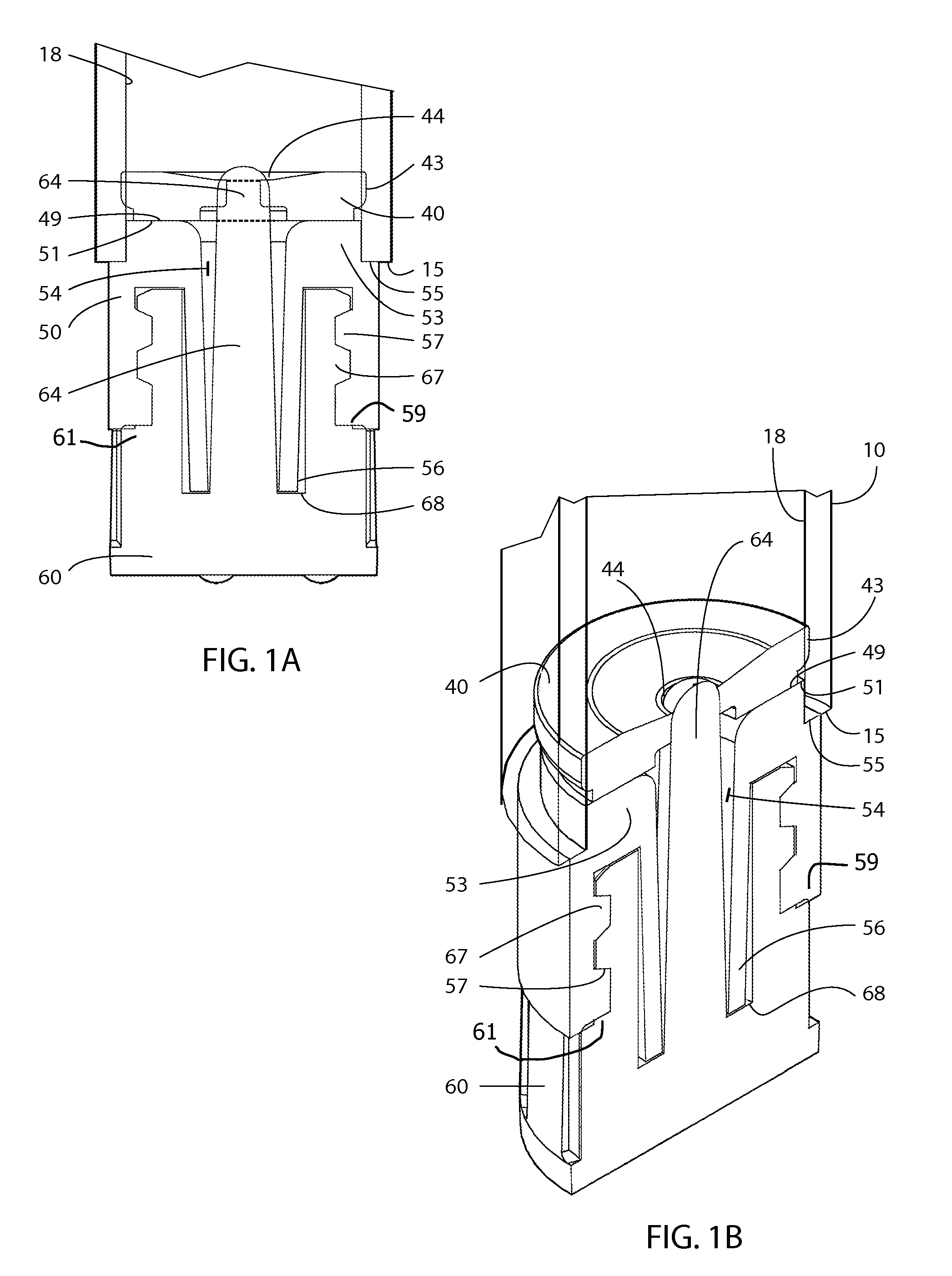
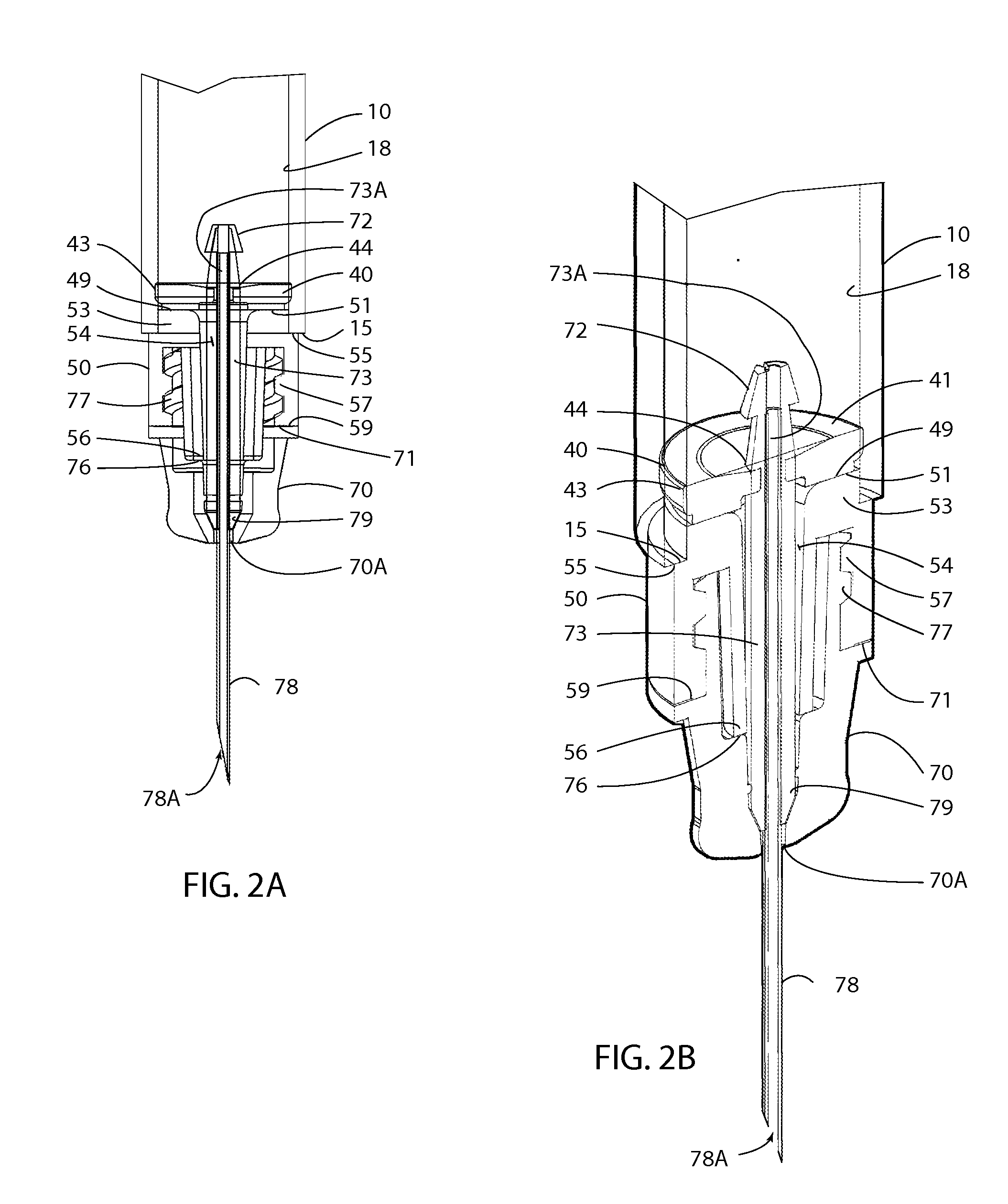
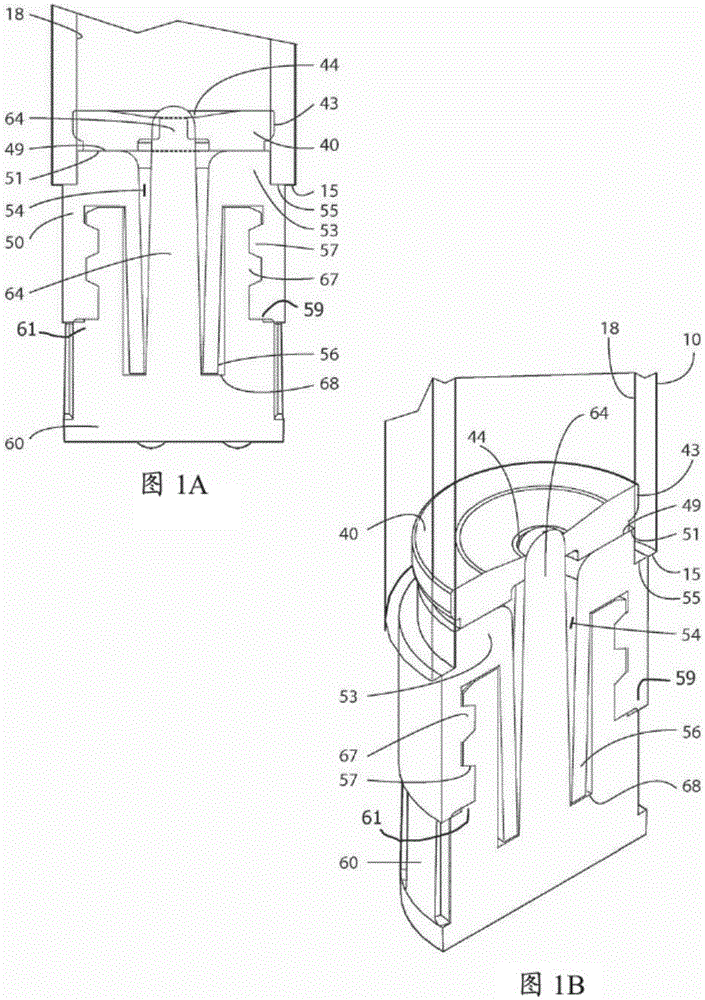
A device for the administration of parenteral drug products that consists of a rigid glass barrel fitted with septum with a plunger at one end and a seal or needle at the other end. The needle assembly may be part of the device or separate.
Disposable self-shielding unit dose syringe guard
InactiveUS20050054987A1Prevent movementMaximize bearing surface engagement therebetweenInfusion syringesInfusion needlesDetentEngineering
Owner:SAFETY SYRINGES
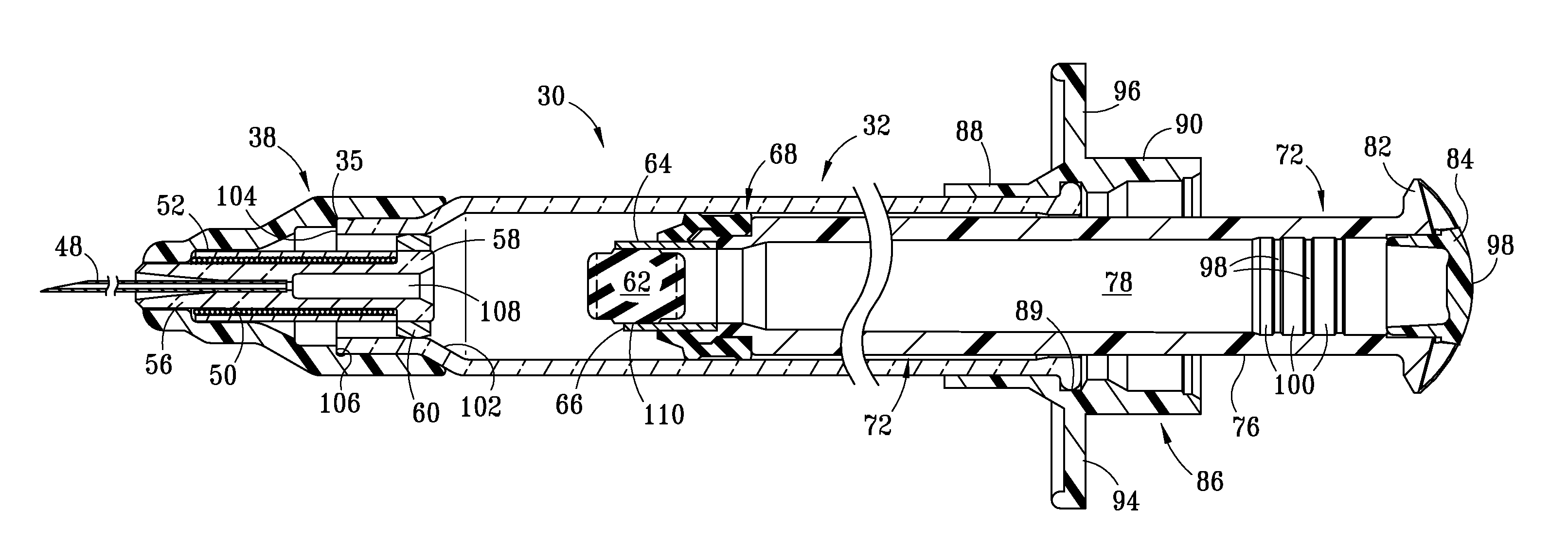
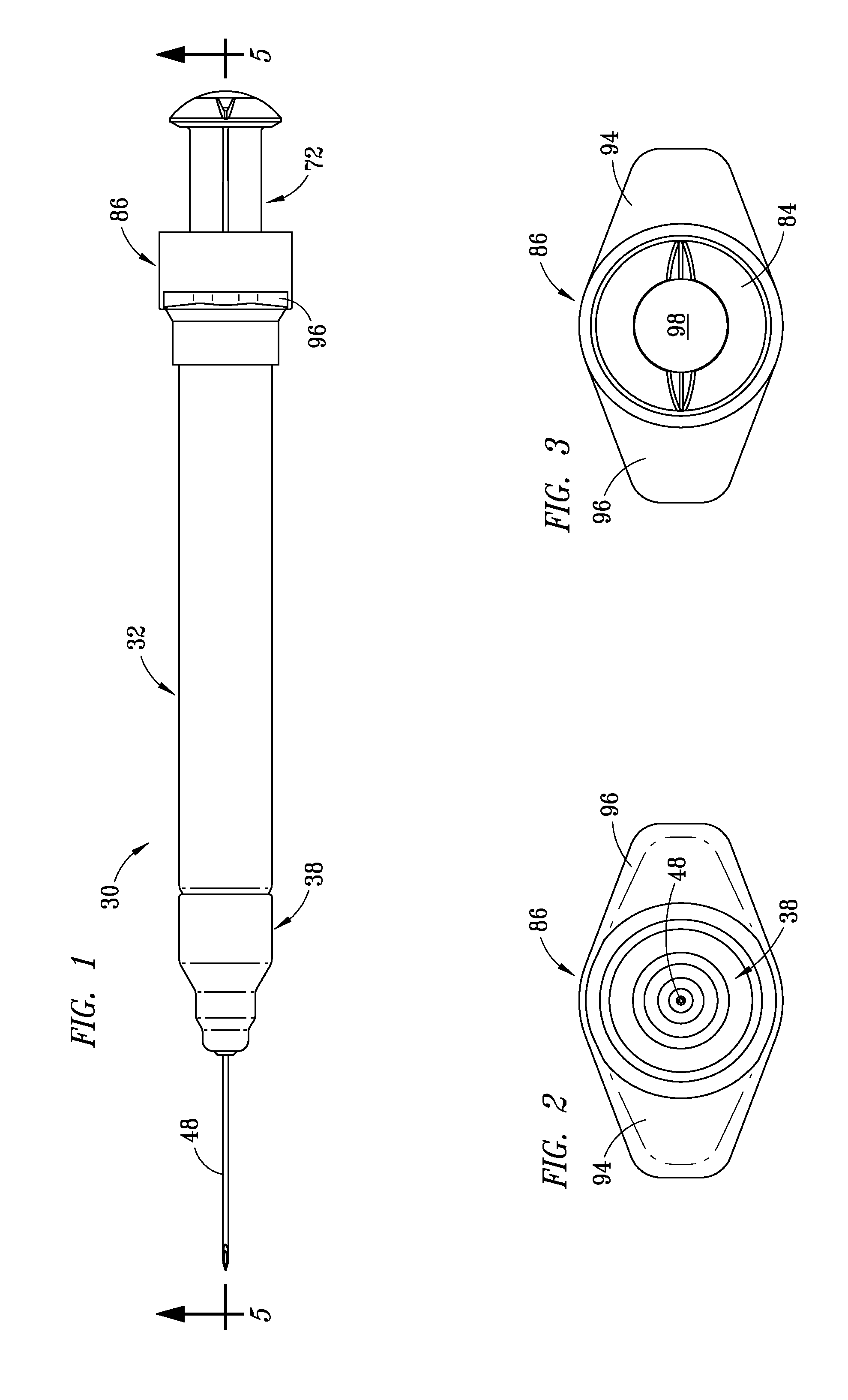
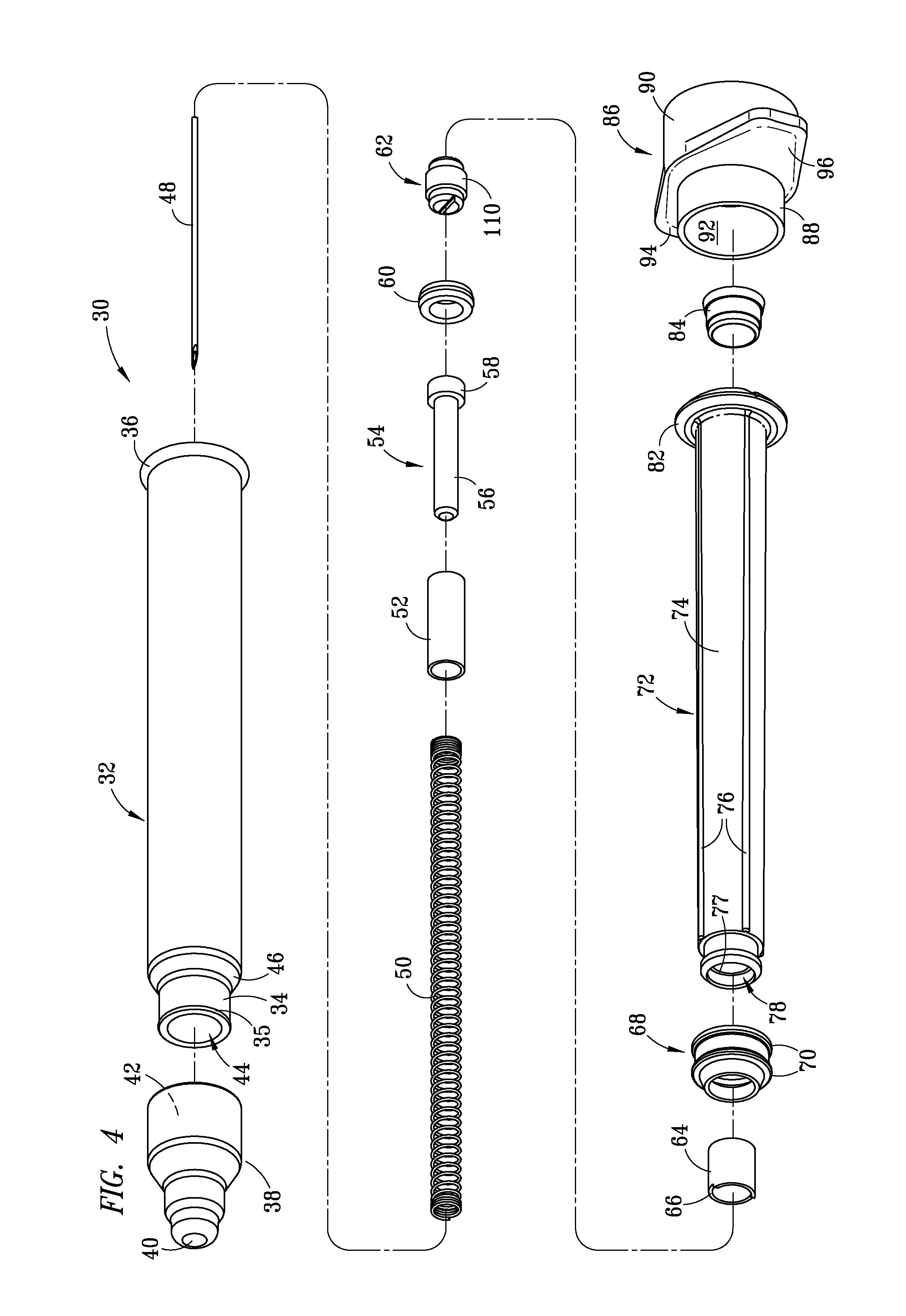
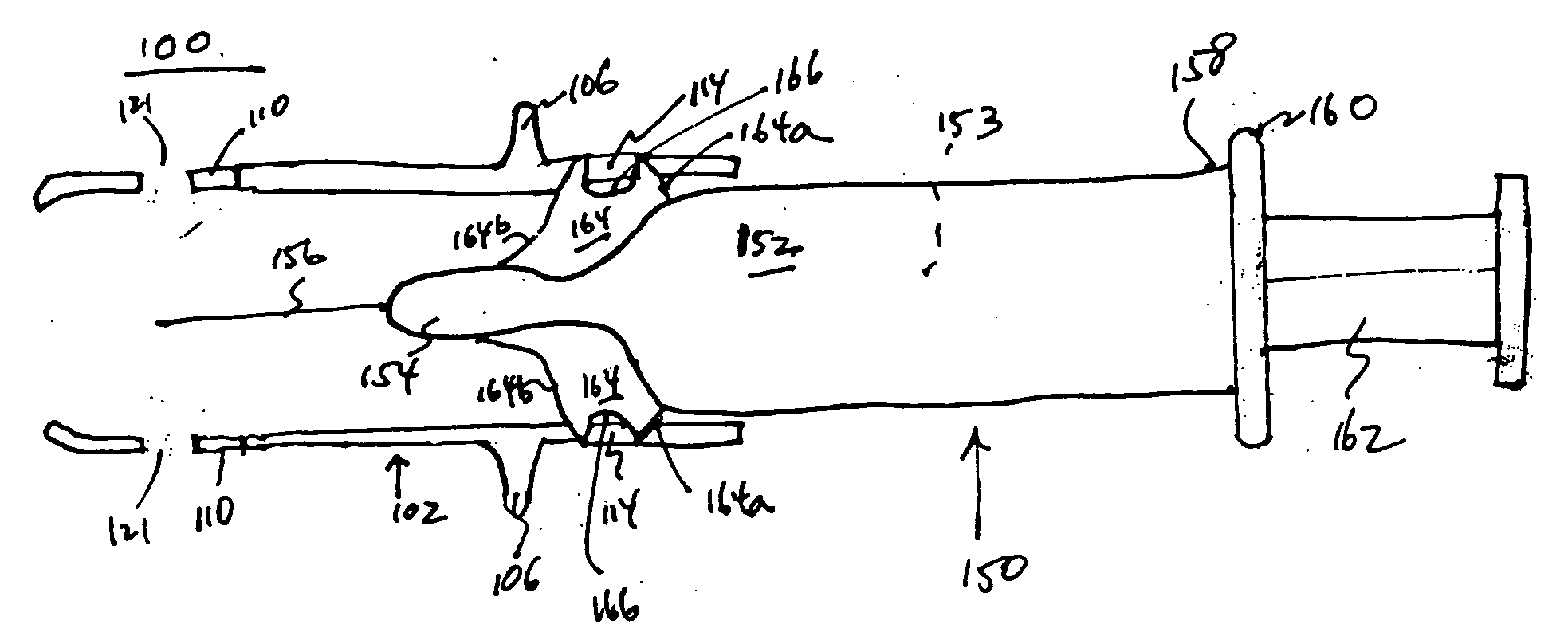
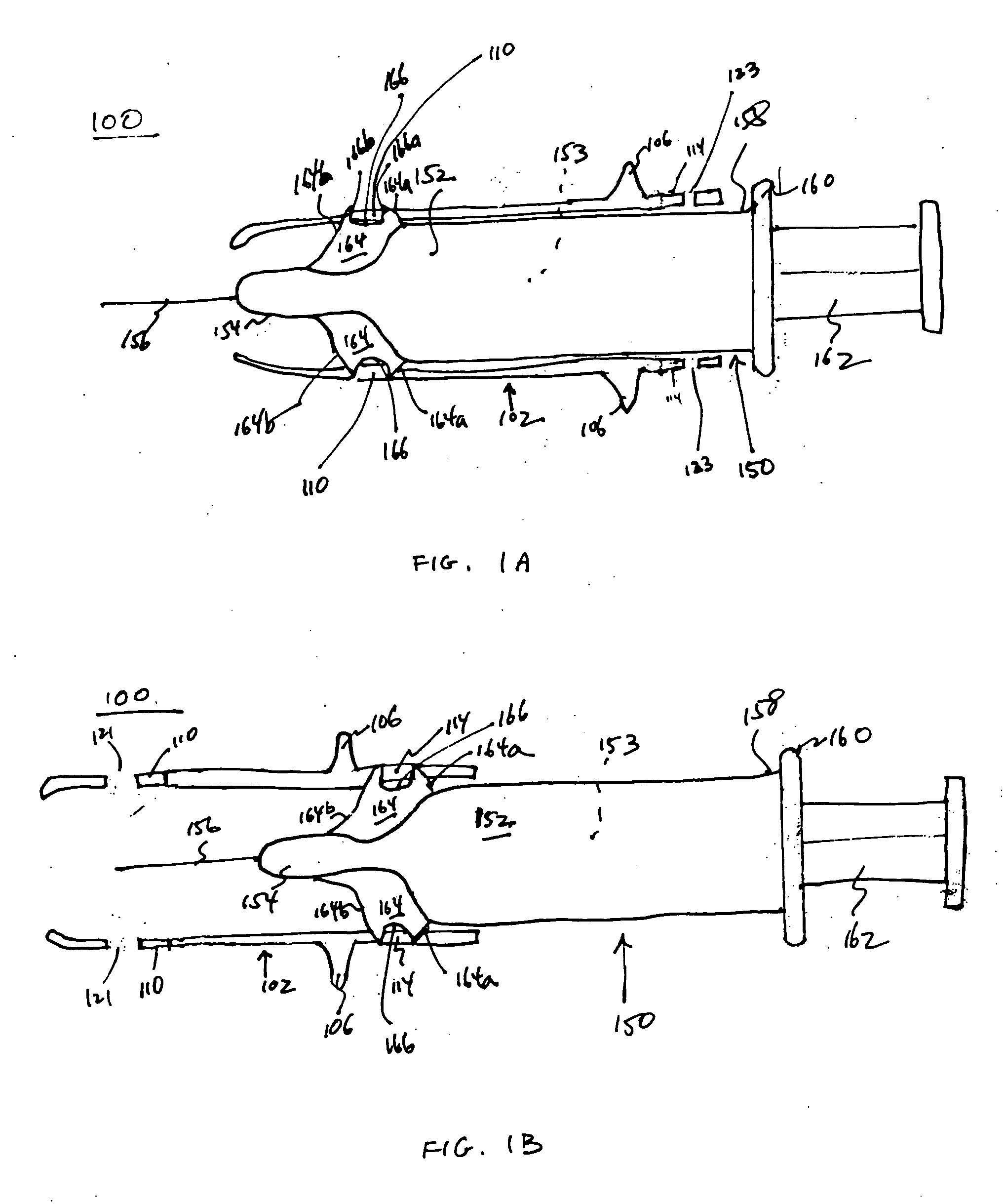
Injection Device
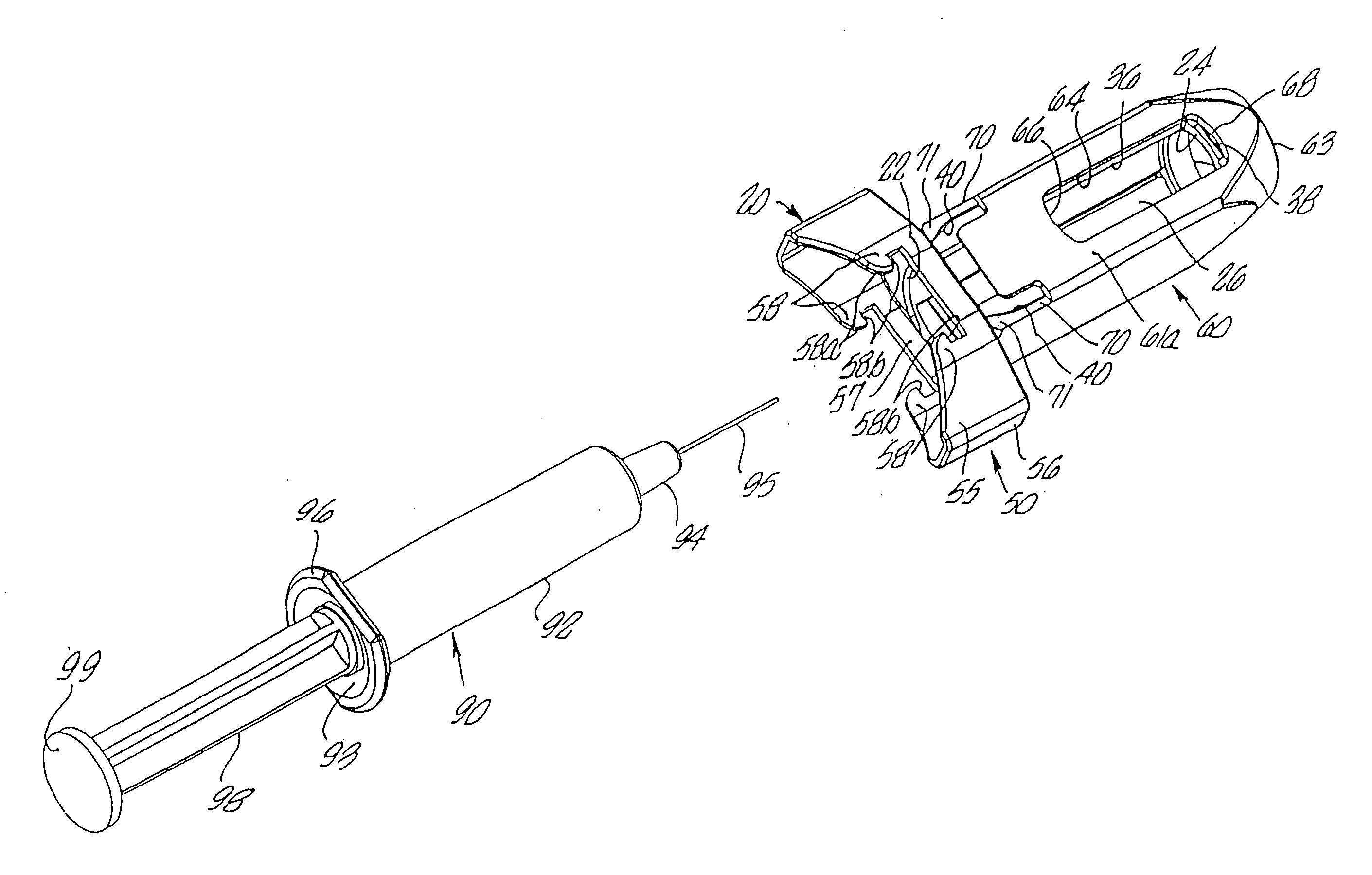
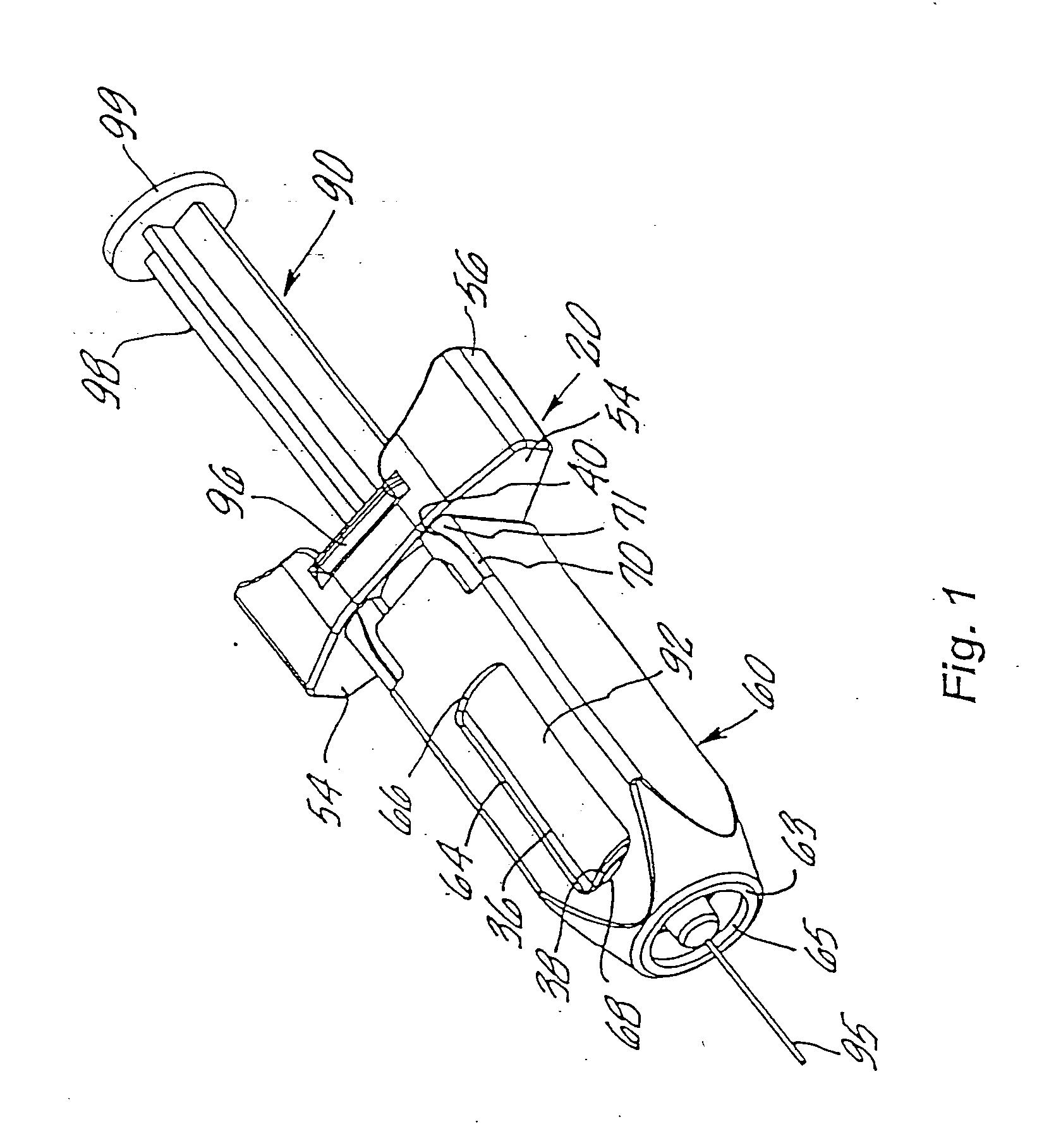
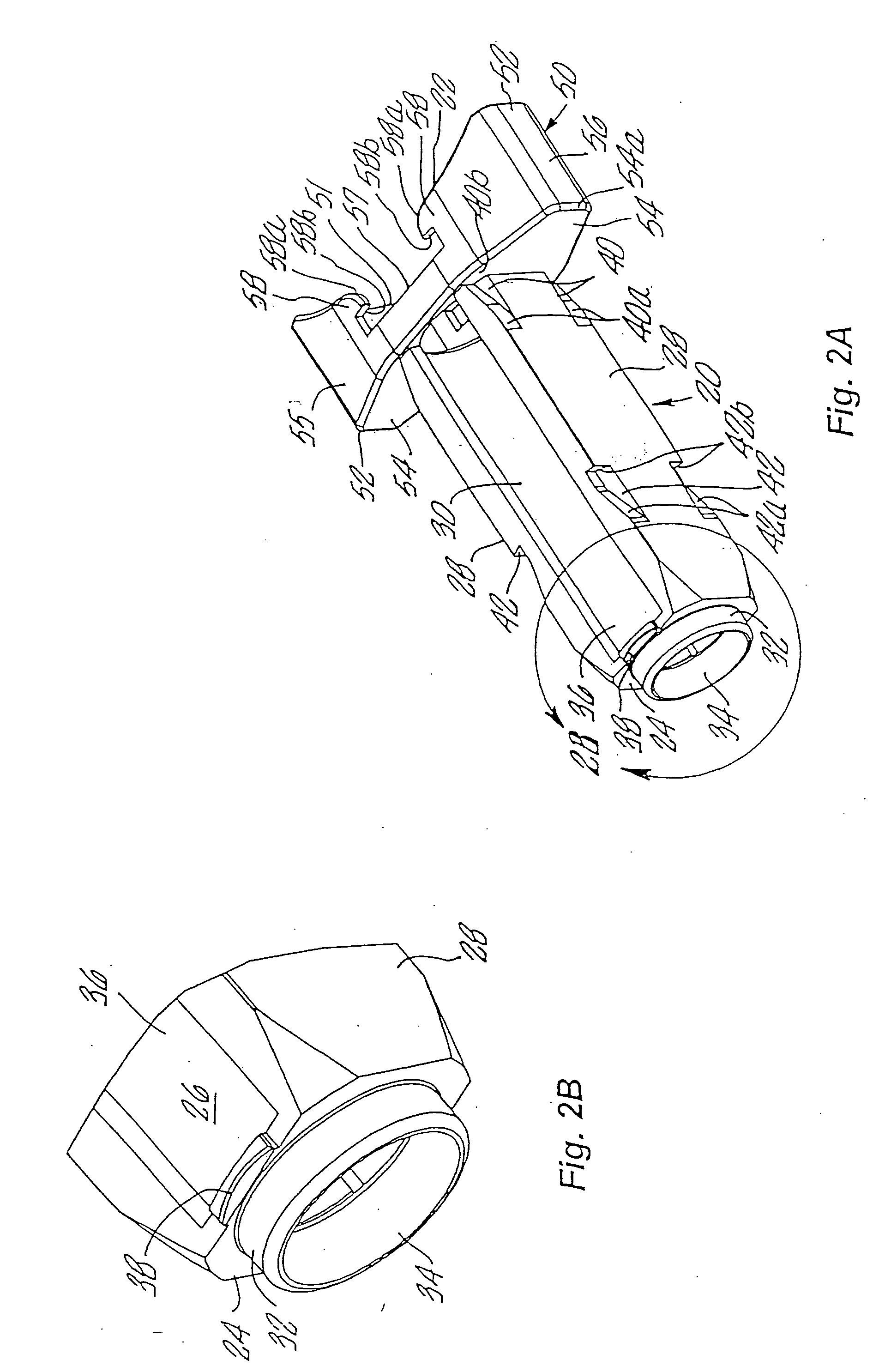
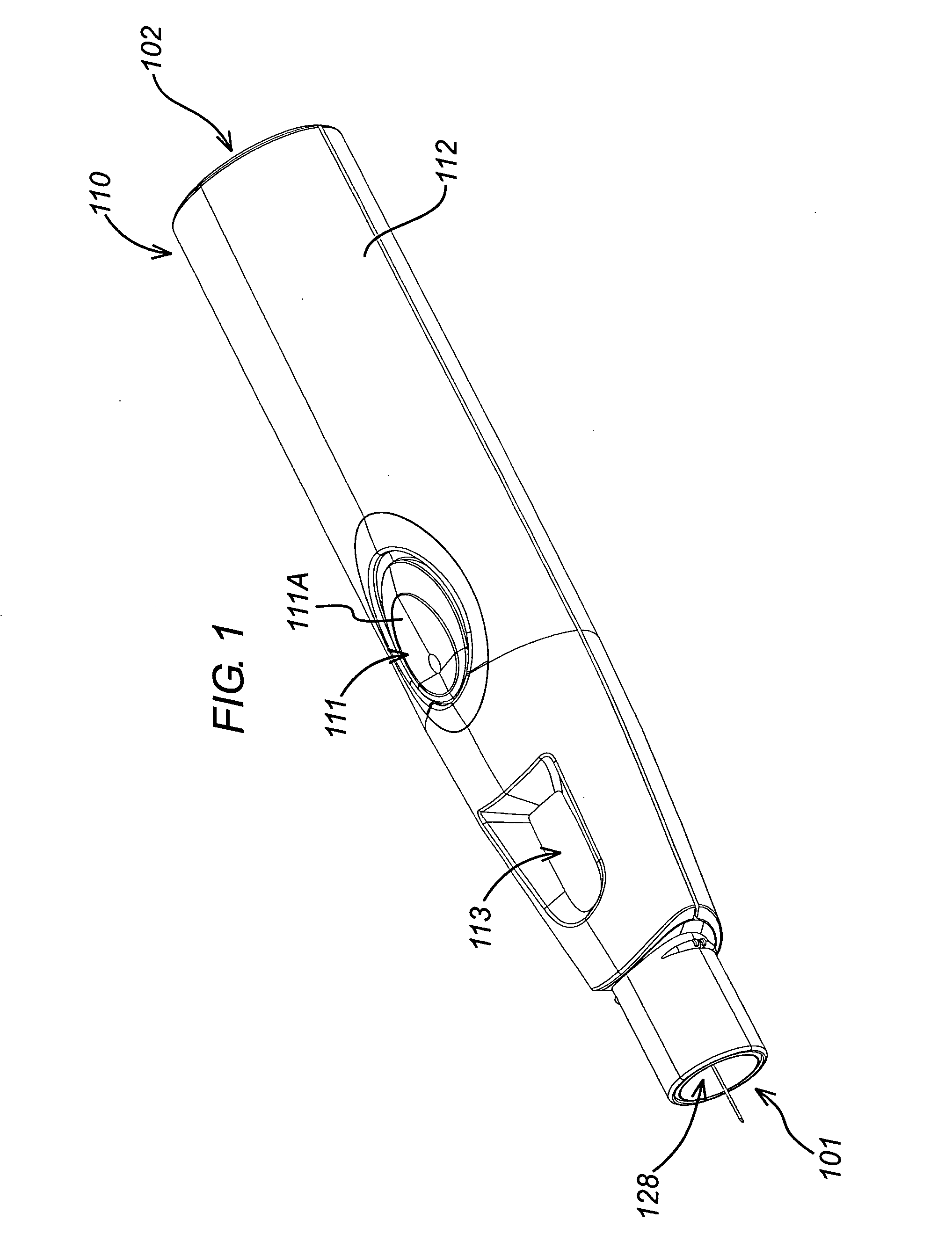
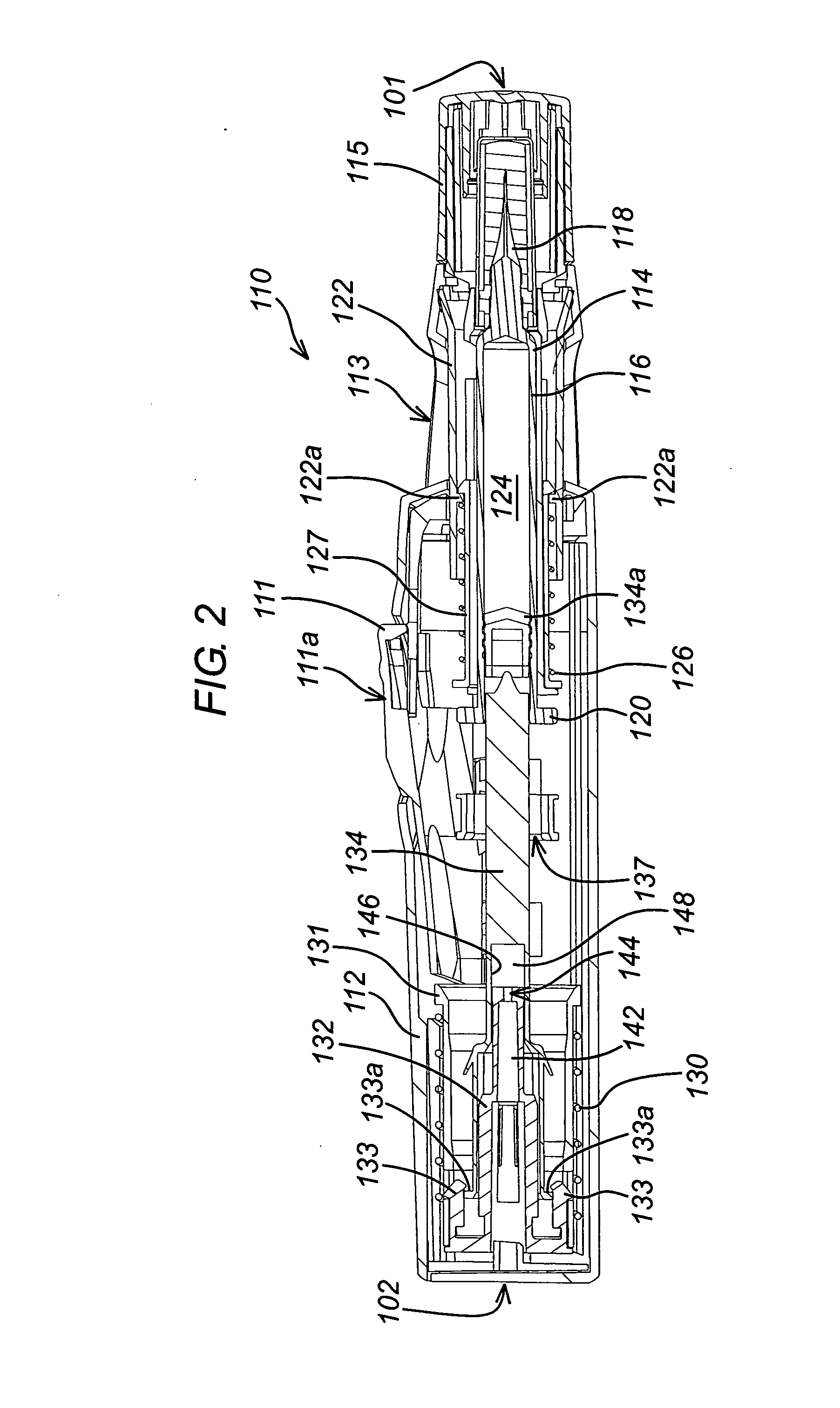
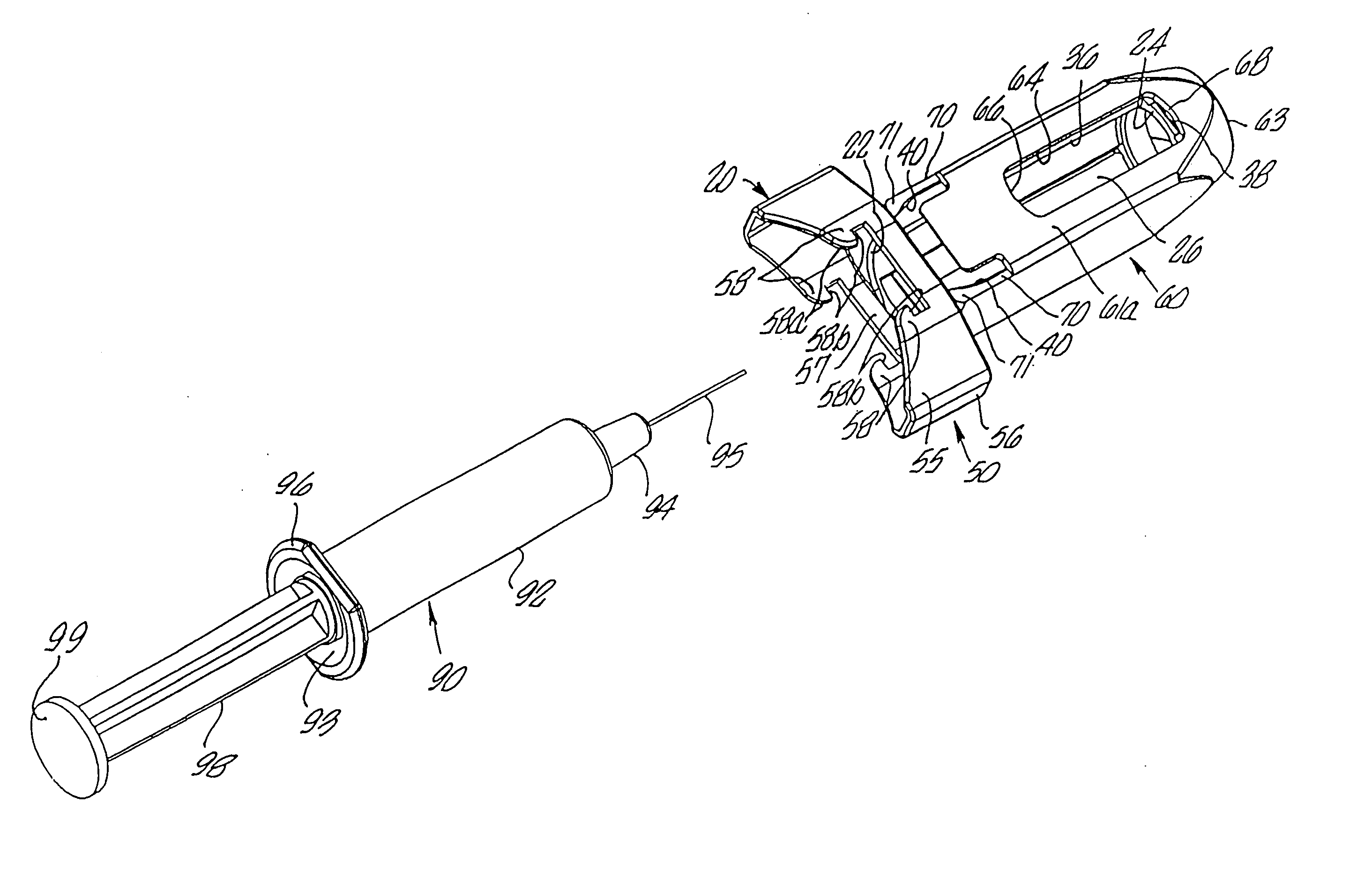
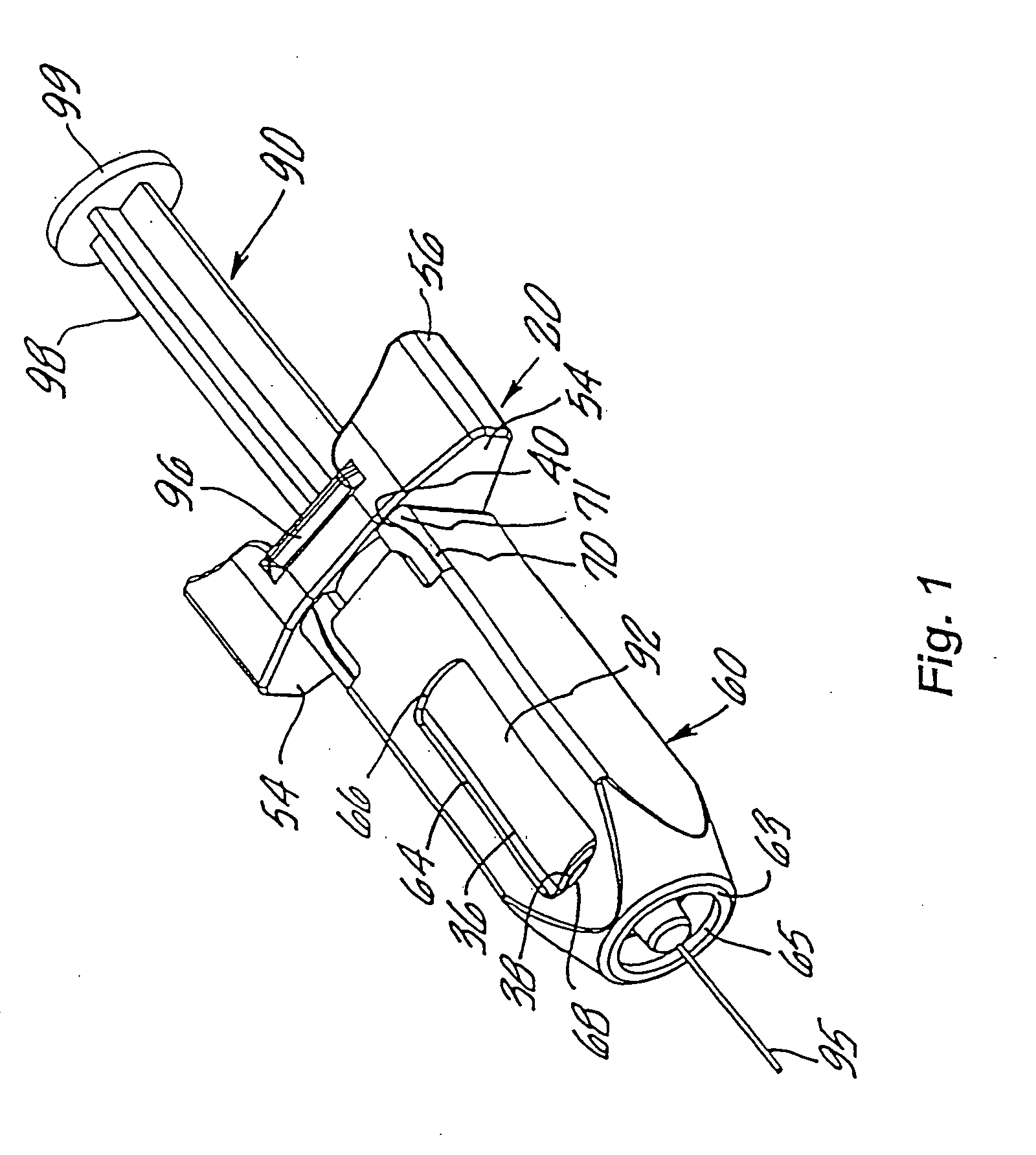
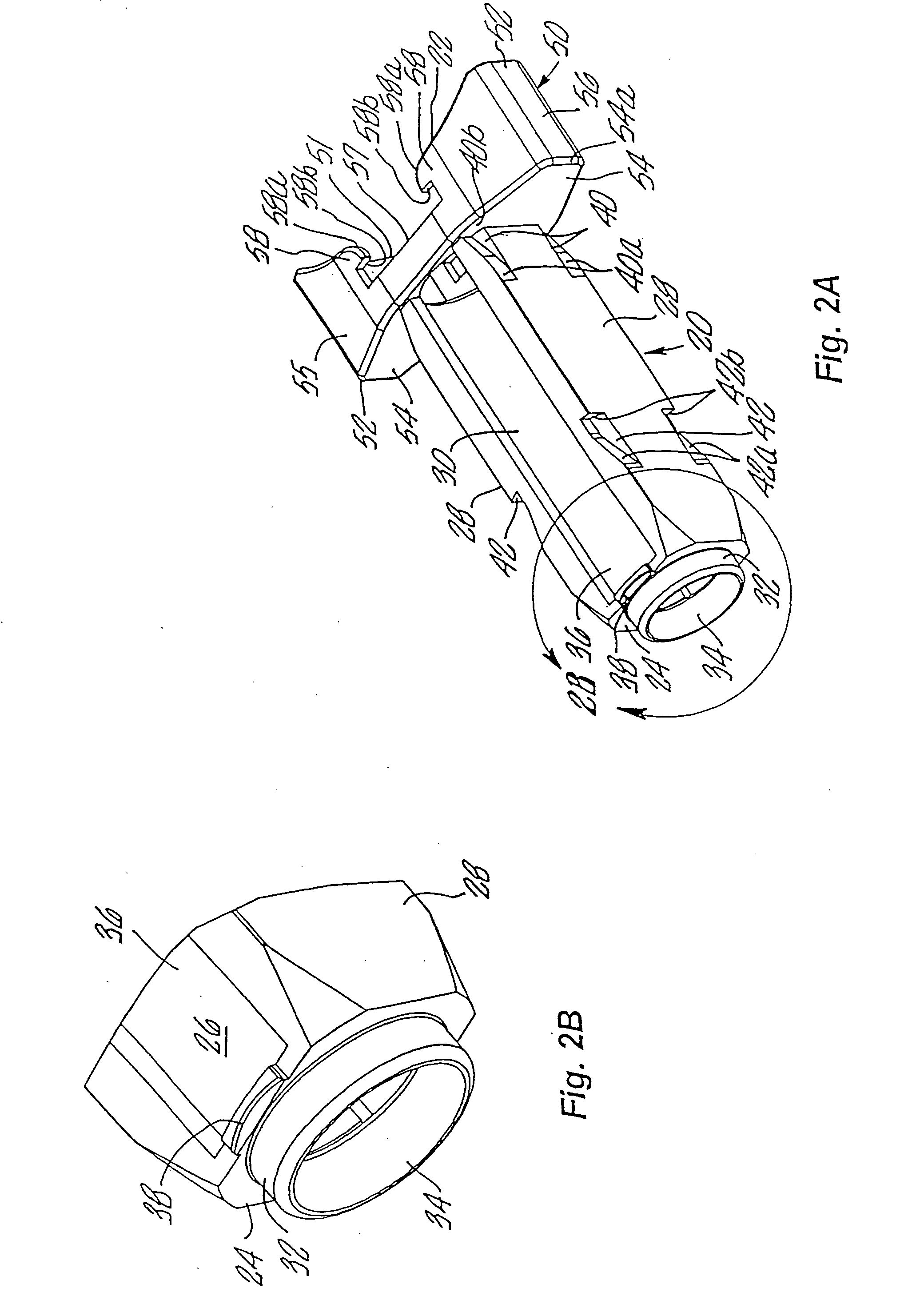
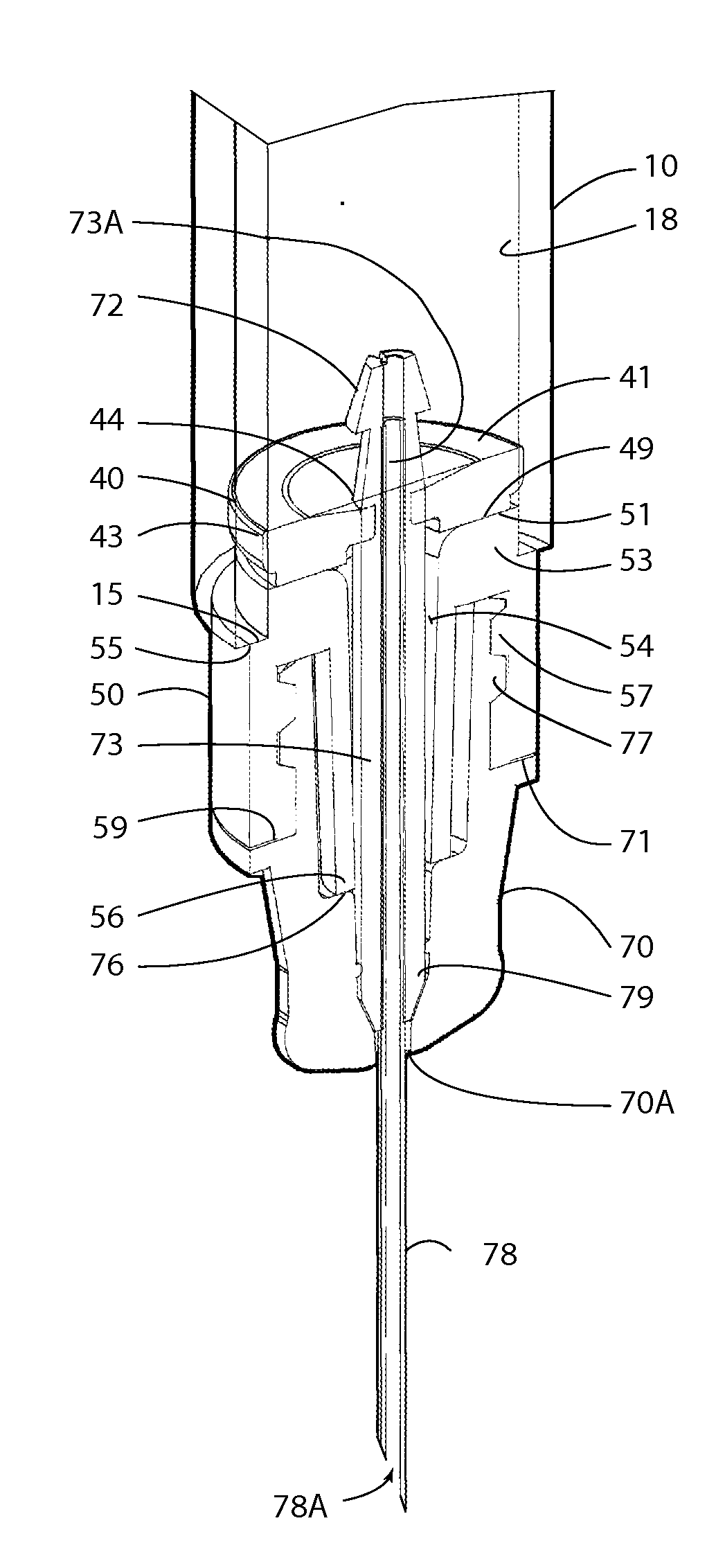
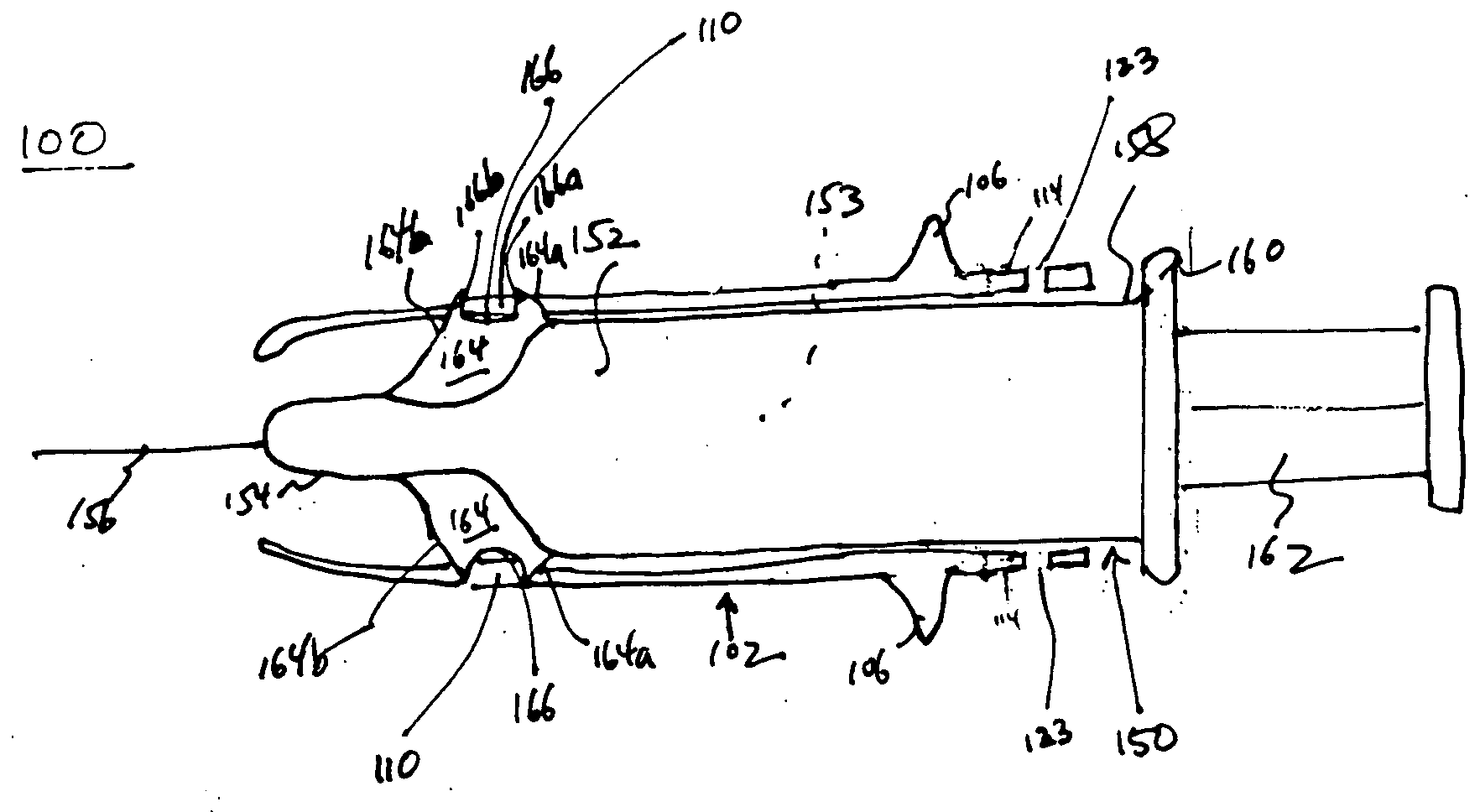
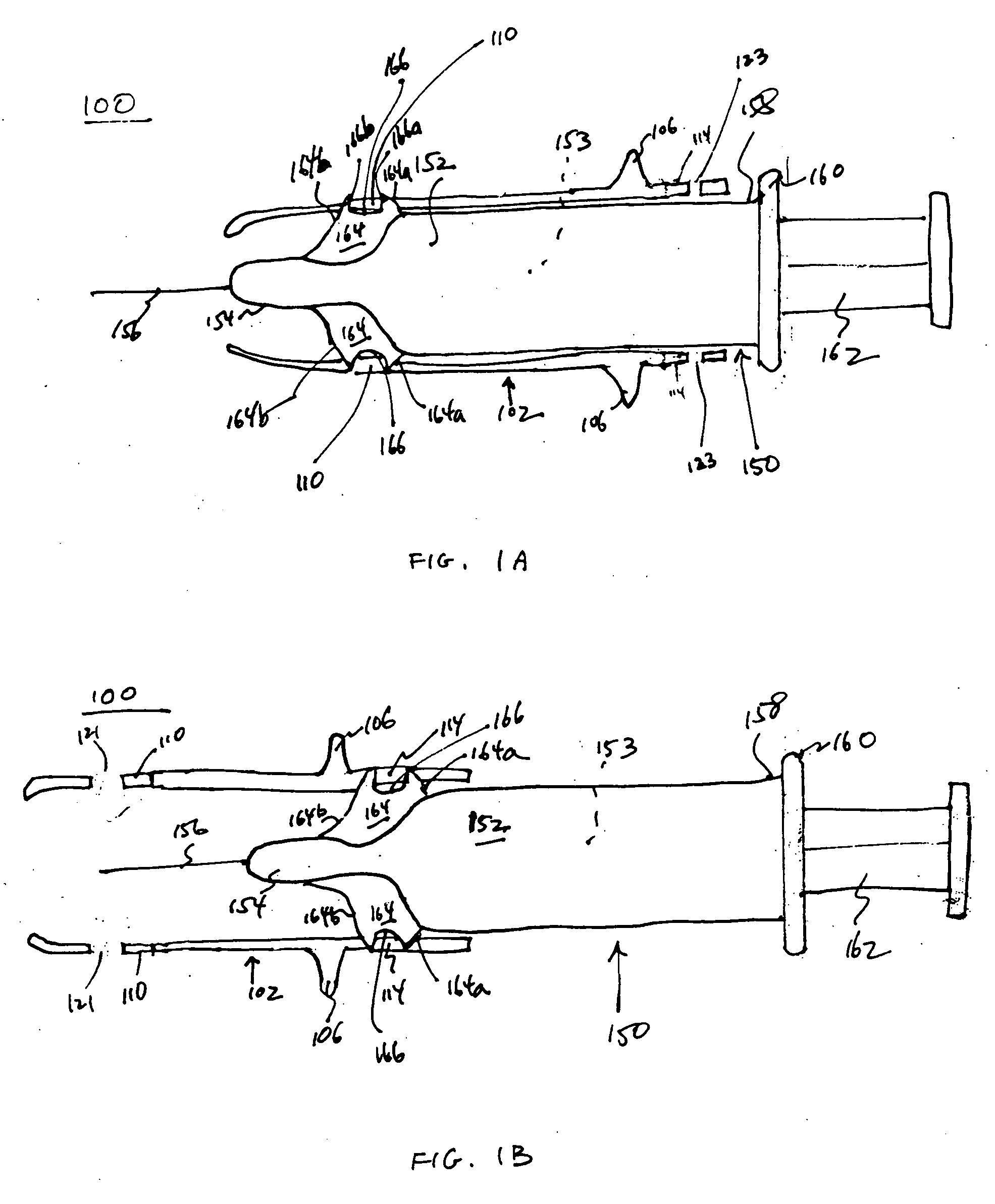
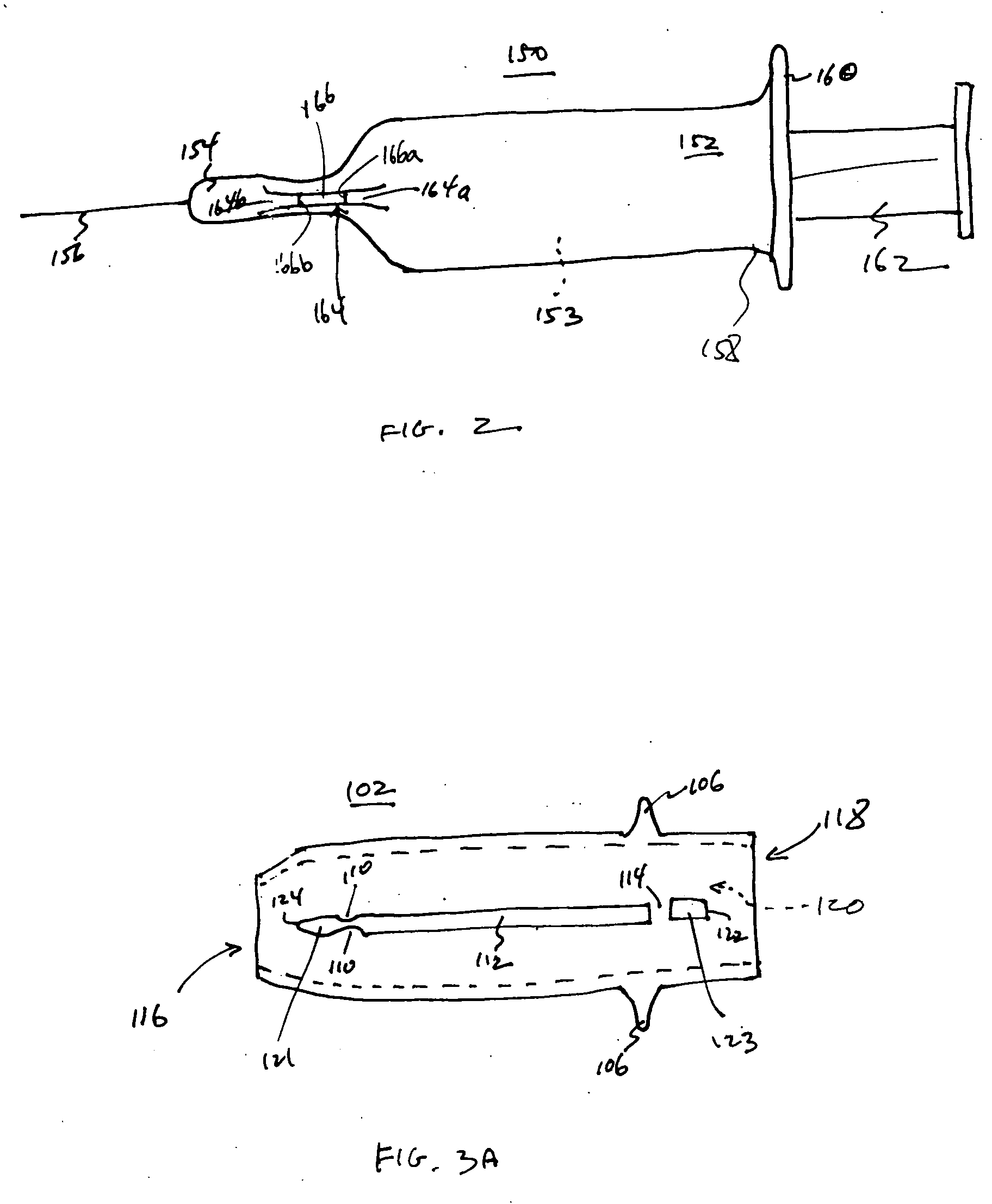
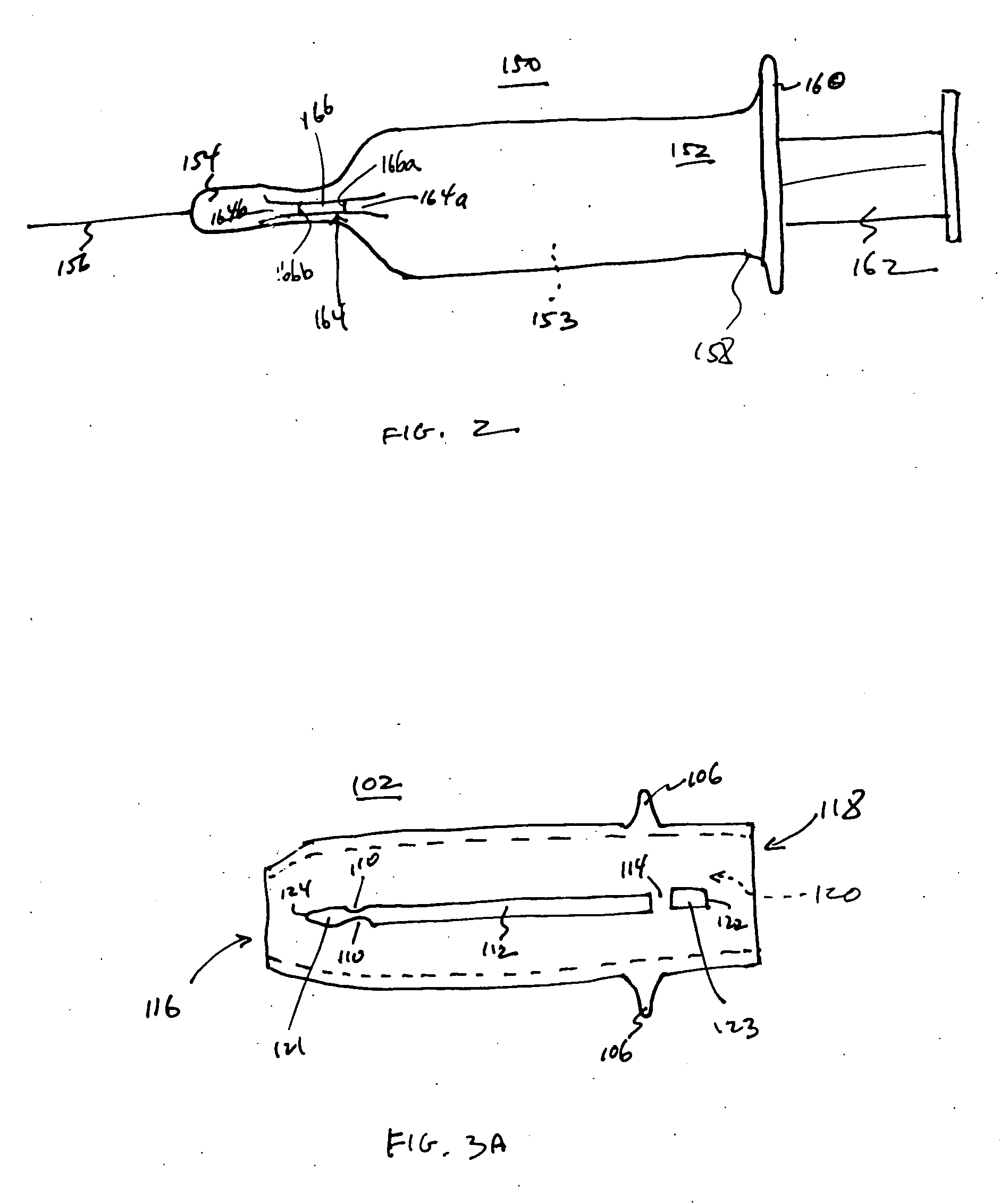
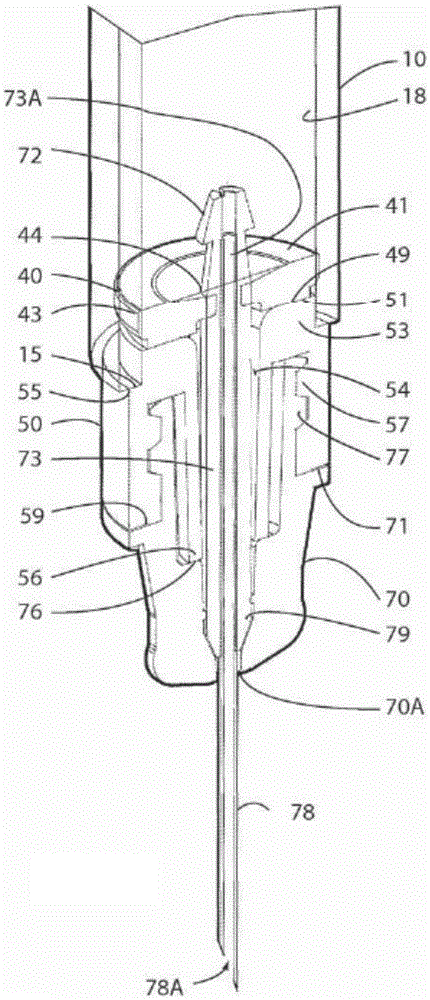
An injection device is described. A syringe 114 is received within a housing, the syringe having a bore terminating at a forward end in a hypodermic needle 118 and at a rearward end in a flared opening 210 in which a bung 134a having a bore 206 surrounded by a skirt 208 is inserted. A drive element 134 has a forward end consisting of a substantially flat annular region 200 that bears upon the skirt 208 of the bung 134a and surrounds a conical middle region 202 that is received in the bore 206 of the bung 134a. An actuator advances the drive element 134 so as to advance the bung 134a and discharge the contents of the syringe through the needle 118. The opening 210 in the rear of the glass syringe is flared by being provided with a radius. The combination of the radius at the opening 210 and the projecting conical middle region 202 of the drive element allows misalignments of the two to be managed during automated assembly. This is because the conical middle region 202 either pass straight into the opening 210 of the syringe, or contact the radius, which guides them towards the centre of the syringe bore. The radius and the substantially flat annular region 200 and the central conical portion 202 of the drive element 134 are so shaped and dimensioned that axial misalignment between the syringe 114 and the drive element 134 during assembly of the injection device are corrected by, firstly, the conical middle region 202 of the drive element 134 riding up the radius to a point at which, secondly, the substantially flat annular region 200 also makes contact with and rides up the radius, to align the drive element 134 in the bore of the syringe 114.
Owner:CILAG GMBH INT
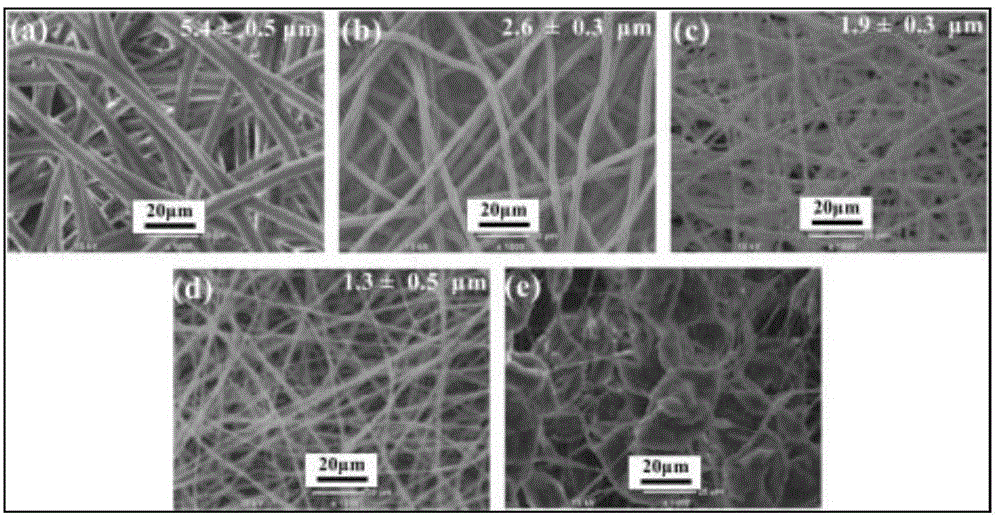
Method for producing nano-fibre bracket material with levorotation polylactic acid as base material
InactiveCN101401955AFlexible textureGood tissue compatibilityStentsPhysical treatmentPorosityTissue Compatibility
The invention relates to a method for preparing a nanofiber bracket material using levorotatory polylactic acid as matrix. The method comprises the following steps: dissolving the levorotatory polylactic acid as the matrix in a solution of dichloromethane and dimethyl formamide, and stirring and centrifuging the mixture to obtain an electrostatic spinning solution; placing a polylactic acid solution into a 5 milliliter glass syringe, and applying high voltage on the glass syringe; advancing the levorotatory polylactic acid solution in the glass syringe; preparing the mixed solution into a nanofiber material film through a electrostatic spinning technology; and modifying the nanofiber material film to obtain the nanofiber bracket material of which the fiber diameter is between 50 and 500 nanometers and the fiber porosity is more than 90 percent. The method solves the defects that a PLLA porous bracket still has too long degradation time, and degradation products can cause tissue inflammations easily and the like. The method has the advantages of flexible texture, better water permeability and air permeability, excellent tissue compatibility, controllable biodegradability, and no antigenicity.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Disposable self-shielding unit dose syringe guard
InactiveUS20050033242A1Prevent accidental releaseEasy to mass produceInfusion syringesInfusion needlesDetentPrefilled Syringe
An improved guard for a medical cartridge, such as a unit dose pre-filled glass syringe, comprising a body for receiving the cartridge, and a shield slidably attached to the body which are pre-assembled and ready to receive a cartridge therein. The body has a mechanism on a proximal end thereof which holds the cartridge therein. The body and shield have cooperating detents and detent pockets which allow the shield to be directed distally, from an unguarded position in which the needle on the cartridge is uncovered for delivery of medication, to a guarded position in which the needle is permanently covered for disposal. The body may also include a substantially rectangular-shaped finger grip on its proximal end for receiving a similarly shaped proximal flange on the cartridge, whereby the cartridge is received in a predetermined orientation. The body may also include one or more ribs within the cavity for accommodating a cartridge with a large needle cap, such as a 0.5 mL capacity pre-filled syringe. In addition, the guard may include a finger grip plug lockably attachable to the proximal end of the body, and a plunger insertable through the finger grip plug to engage a piston in a cartridge not having its own plunger. The plunger may include a one-way locking member to prevent removal of the plunger from the finger grip plug after assembly.
Owner:SAFETY SYRINGES
Luer connection adapters for syringes
ActiveUS20150045744A1Easy to installEasy to adaptInfusion syringesMedical devicesBiomedical engineeringGlass Syringe
A connector mountable to a syringe barrel has a proximal barrel-engaging portion, a distal luer fitment portion, and a fluid aperture therethrough. The barrel-engaging portion of the connector includes an axial ledge configured to abut the axial distal edge of a glass syringe barrel. The connector facilitates mounting a luer assembly to the barrel. The luer assembly may be a tip cap, a luer needle assembly, or a luer needle-less assembly, having a complementary luer fitment for connection to the luer fitment portion of the connector. The connector and syringe may further include an immobile, compressible needle seal. The needle seal is adjacent to or engageable with the barrel-engaging portion of the connector.
Owner:HIKMA PHARMA LLC
Container
InactiveUS20070158234A1Improve shock absorptionDispensing apparatusOther accessoriesElectrical and Electronics engineeringPlastic bag
A package has a rectangular parallelepiped container including vertical front and rear panel sections connected by a pair of vertical side panel sections, a top wall connected to the upper edge of the rear panel section and a bottom wall connected to the at least one of the lower edges of the vertical panels and a top cushion panel disposed beneath the top wall and rotatably connected to at least one upper edge of the side panel sections. A holder is disposed, in the container, for holding a plurality of glass syringes each of which is sealingly contained in a plastic bag.
Owner:BAYER SCHERING PHARMA AG
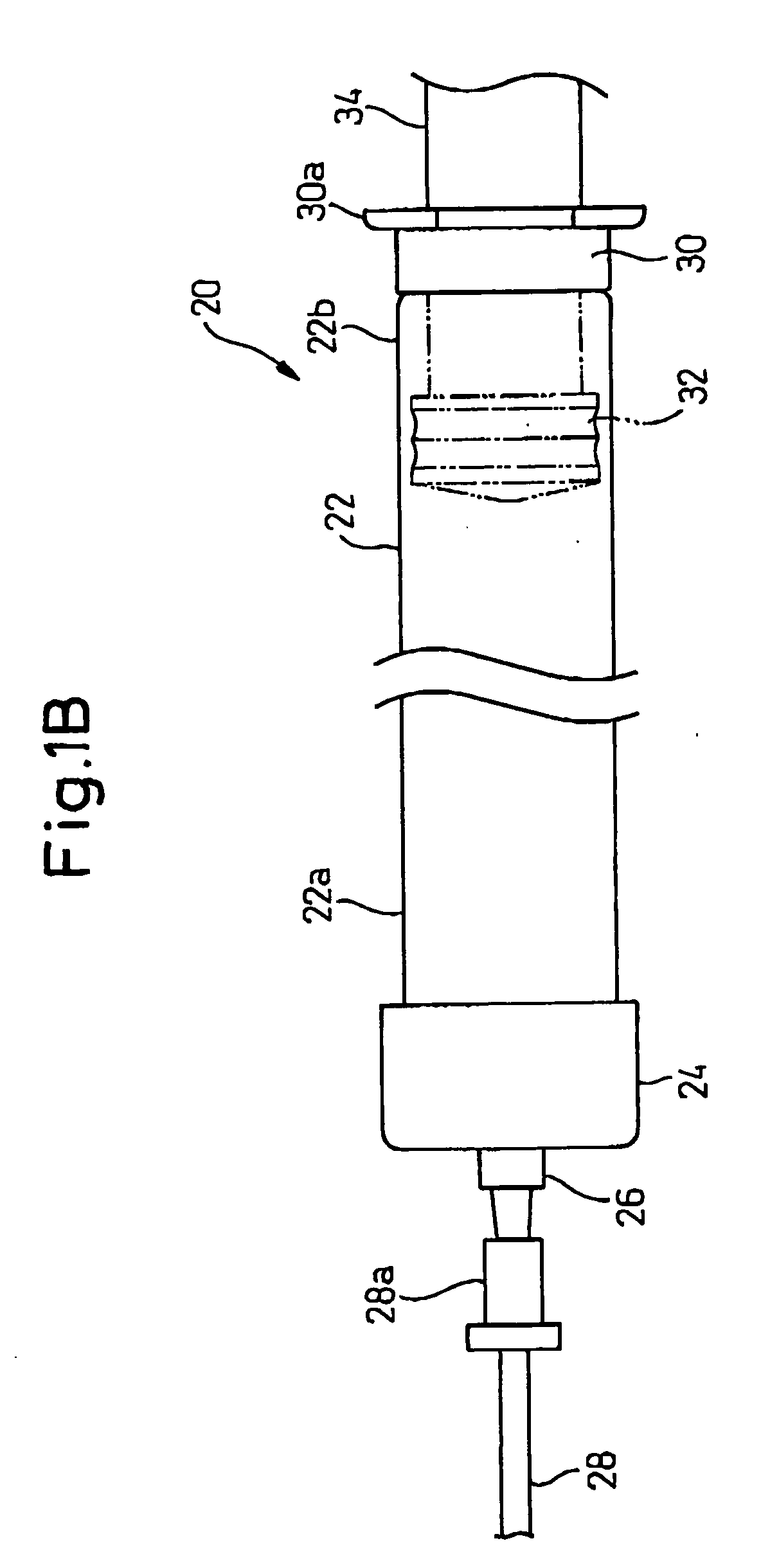
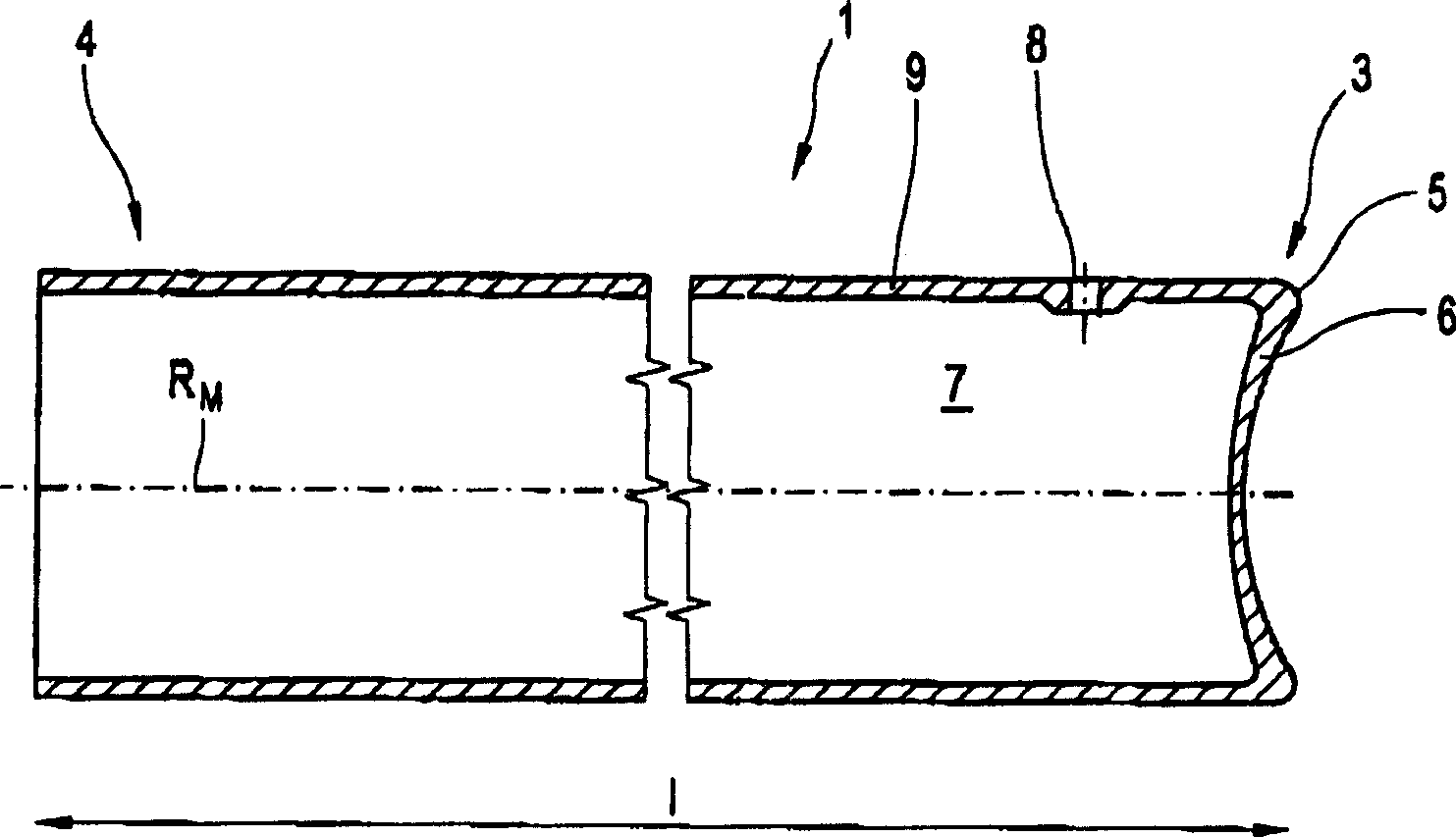
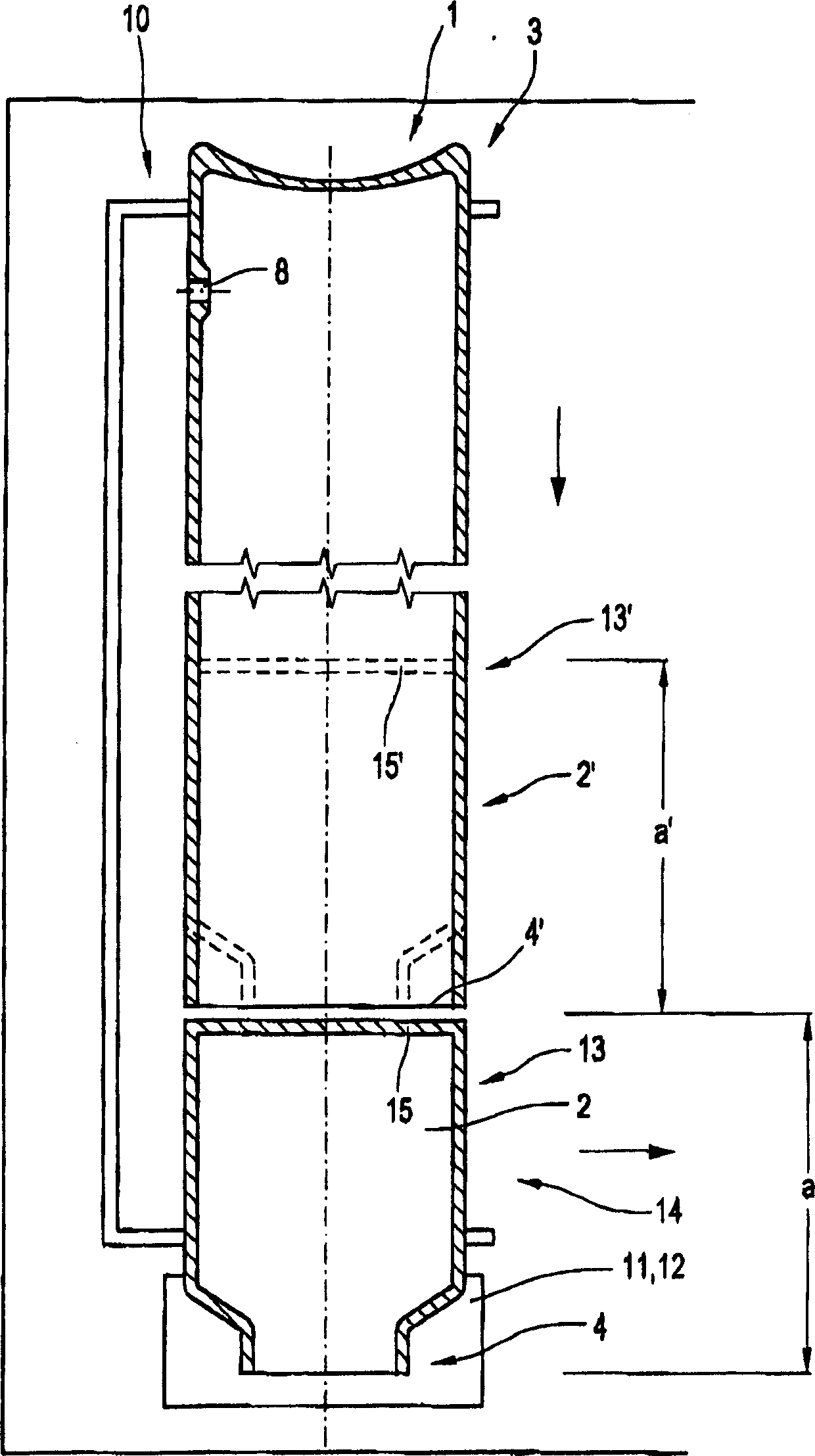
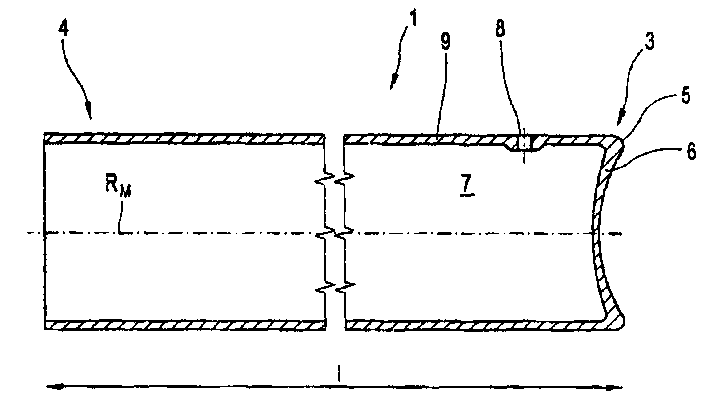
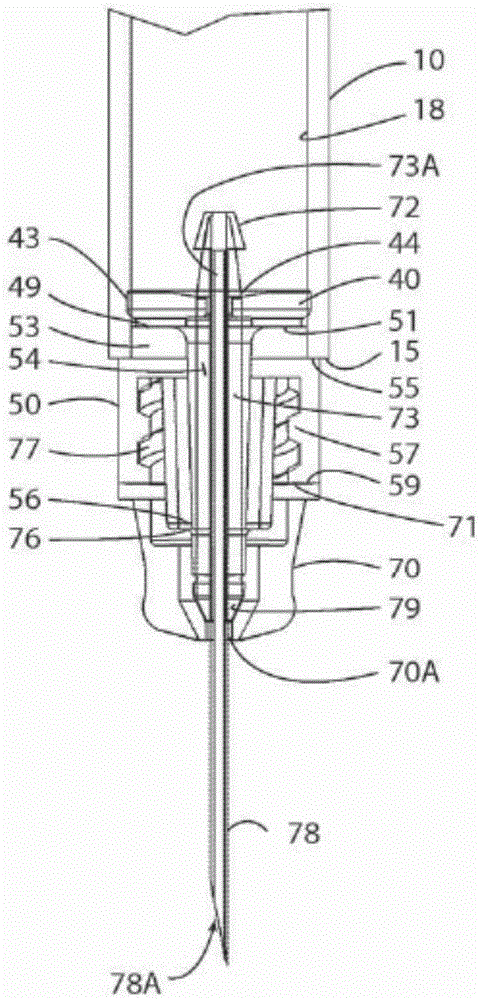
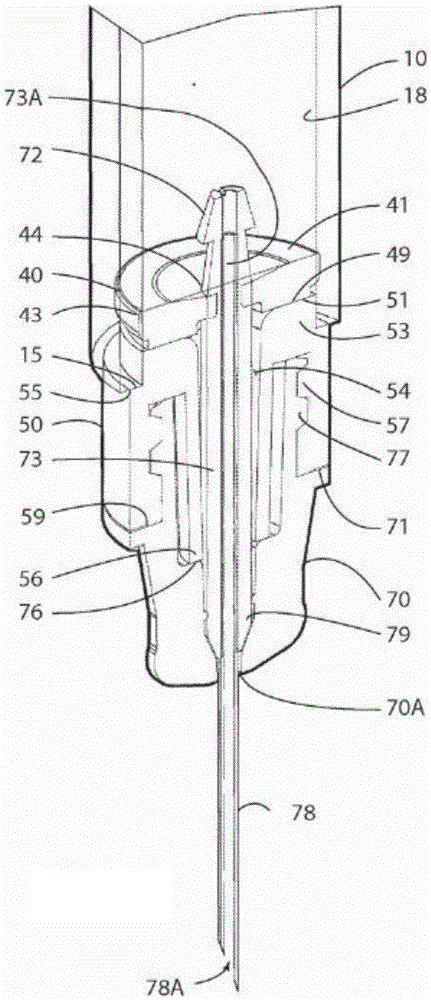
Glass Syringe with Retractable Needle
A syringe, particularly preferred for use as a prefilled syringe, that has a retractable needle and is characterized by a liquid containment chamber of variable volume, the liquid containment chamber being further defined by surfaces made of glass or an elastomeric material, and not of plastic, and the syringe having no glass part directly contacting another glass part.
Owner:RETRACTABLE TECH INC
Syringe with integral safety system
An injection device for delivery of a therapeutic agent is provided that includes a glass syringe with an integrally molded lug extending radially from the syringe and a guard slidably attached to the syringe. One set of detents on the guard and the lug of the syringe retain the guard in the retracted position. Another set of detents on the guard and the lug of the syringe retain the guard in the extended position. Another injection device for delivery of a therapeutic agent is provided that includes a glass syringe with an integrally molded disk that extends radially from the syringe and a guard slidably coupled to the syringe. One set of detents on the guard and the integrally molded disk of the syringe retain the guard in a retracted position. Another set of detents on the guard and the integrally molded disk retain the guard in an extended position.
Owner:SAFETY SYRINGES
Electrospinning ultrafine conductive polymeric fibers
InactiveUS7264762B2Electroconductive/antistatic filament manufactureElectric discharge heatingFiberOrganic solvent
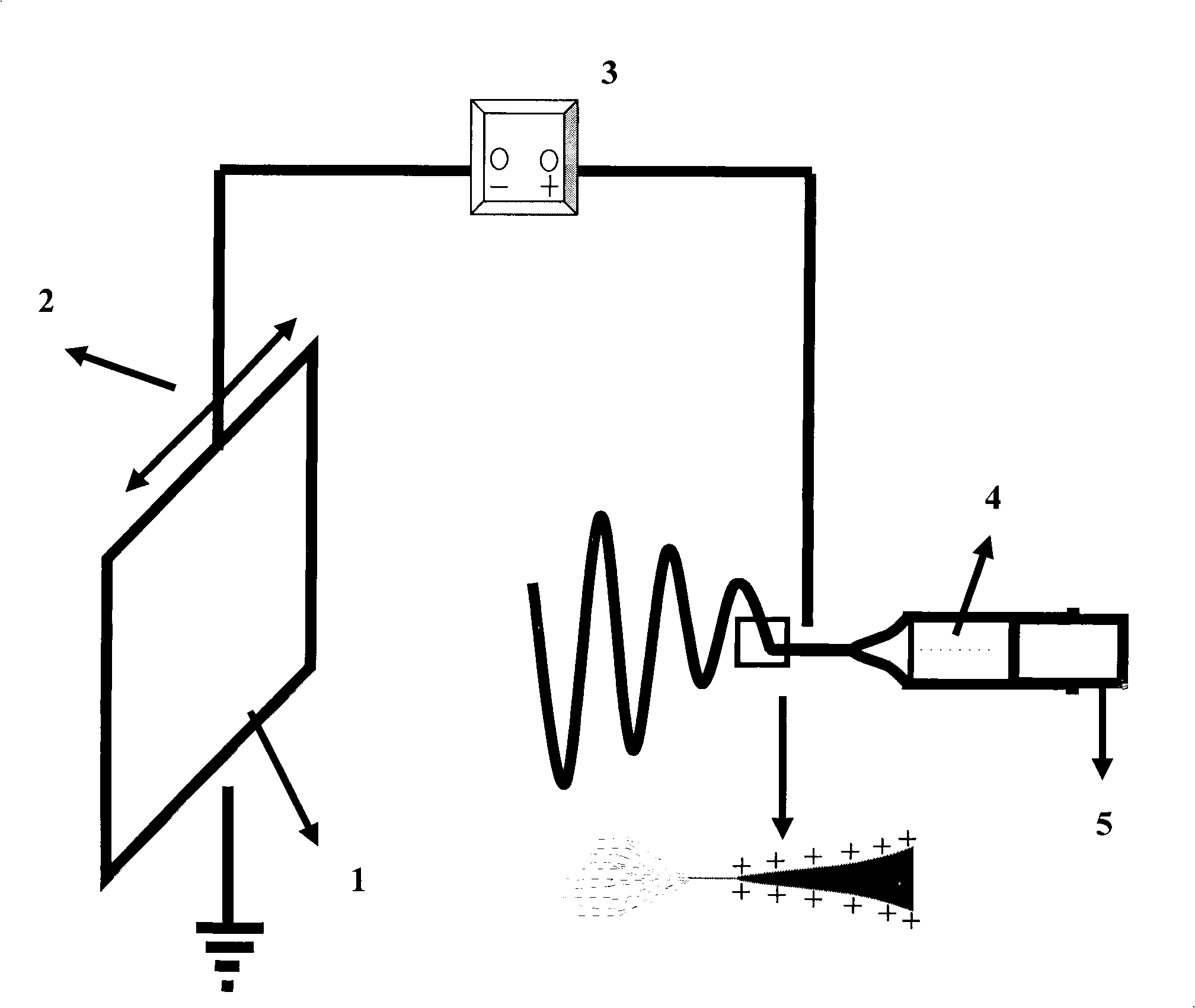
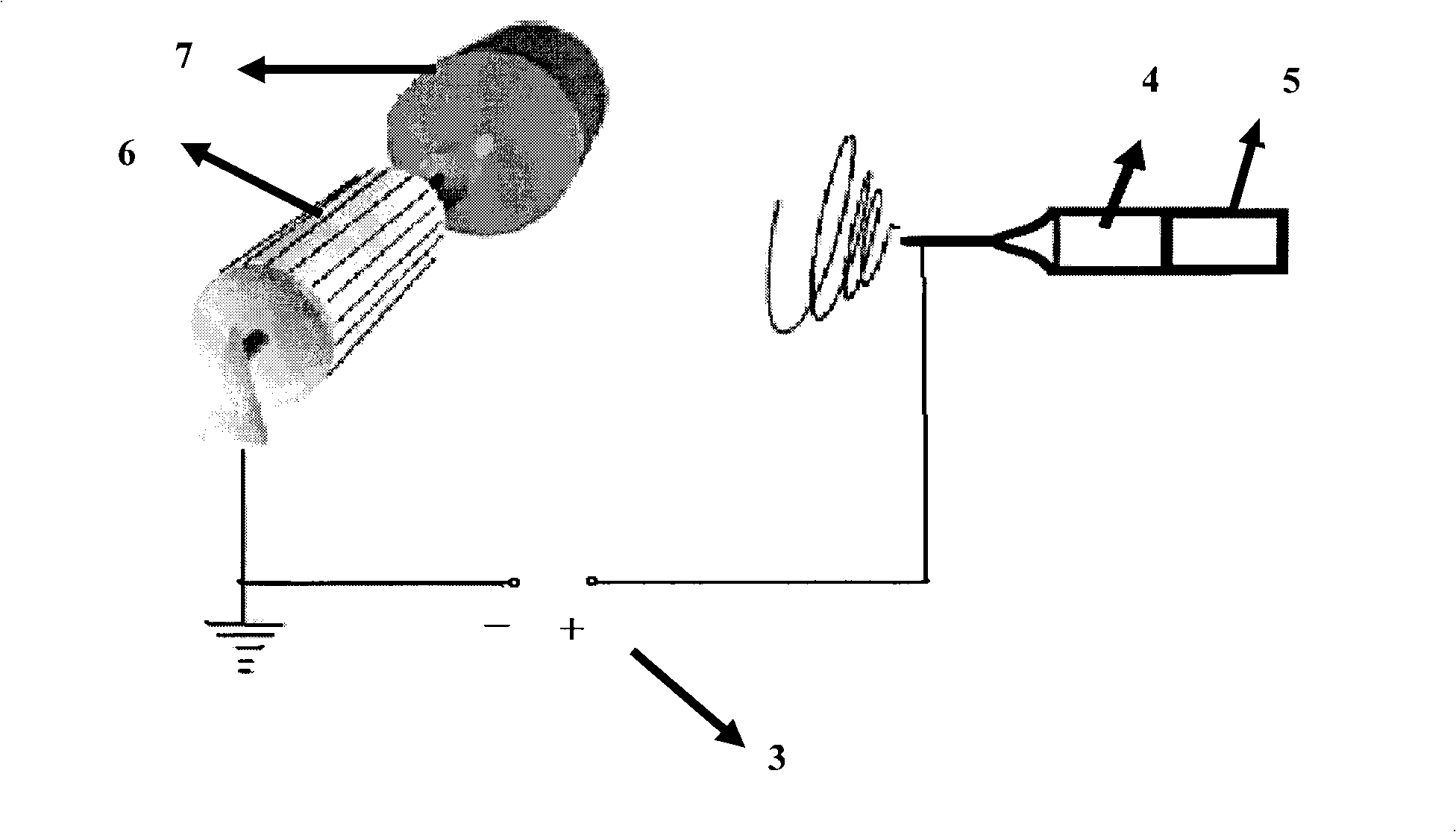
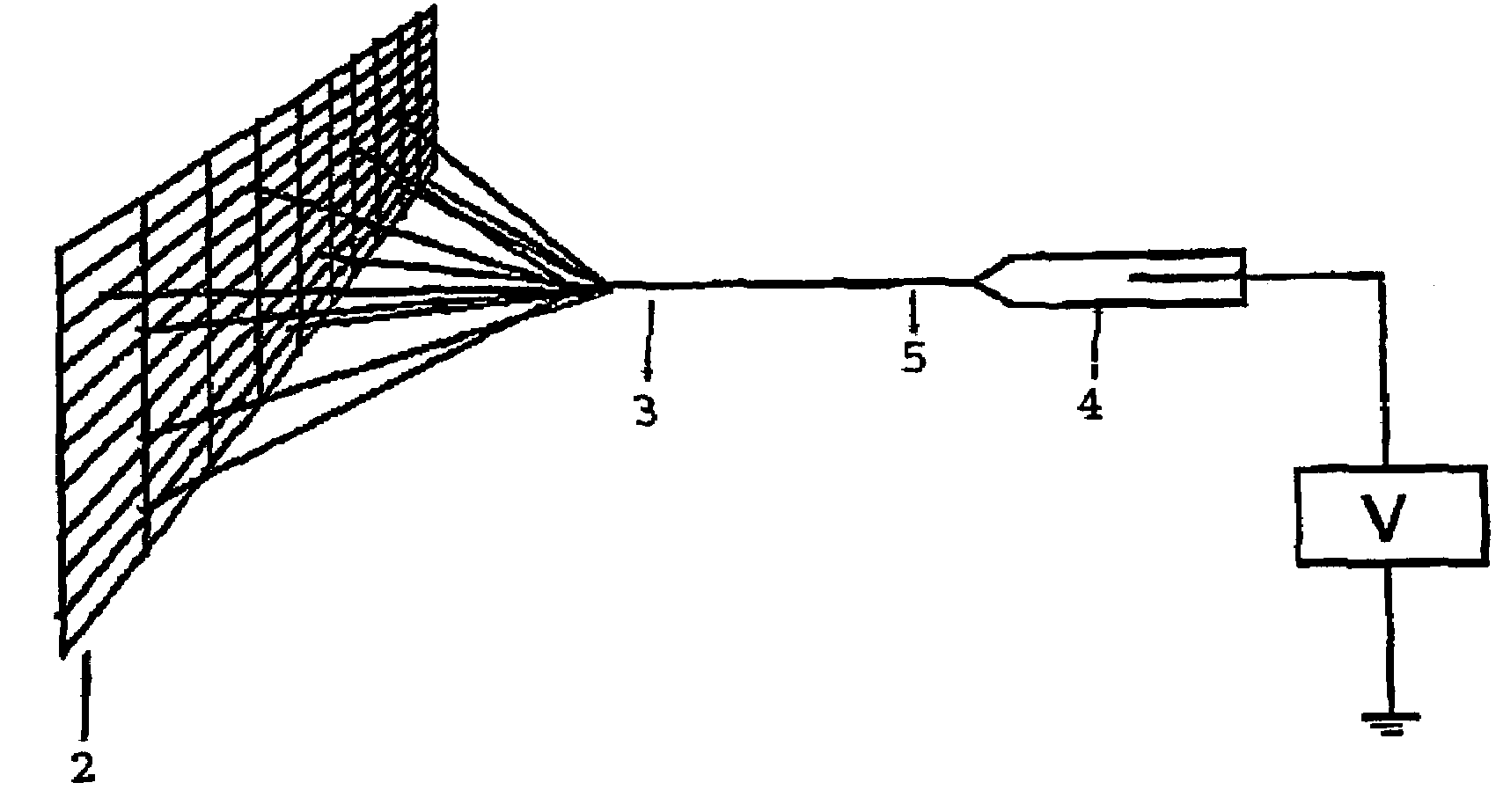
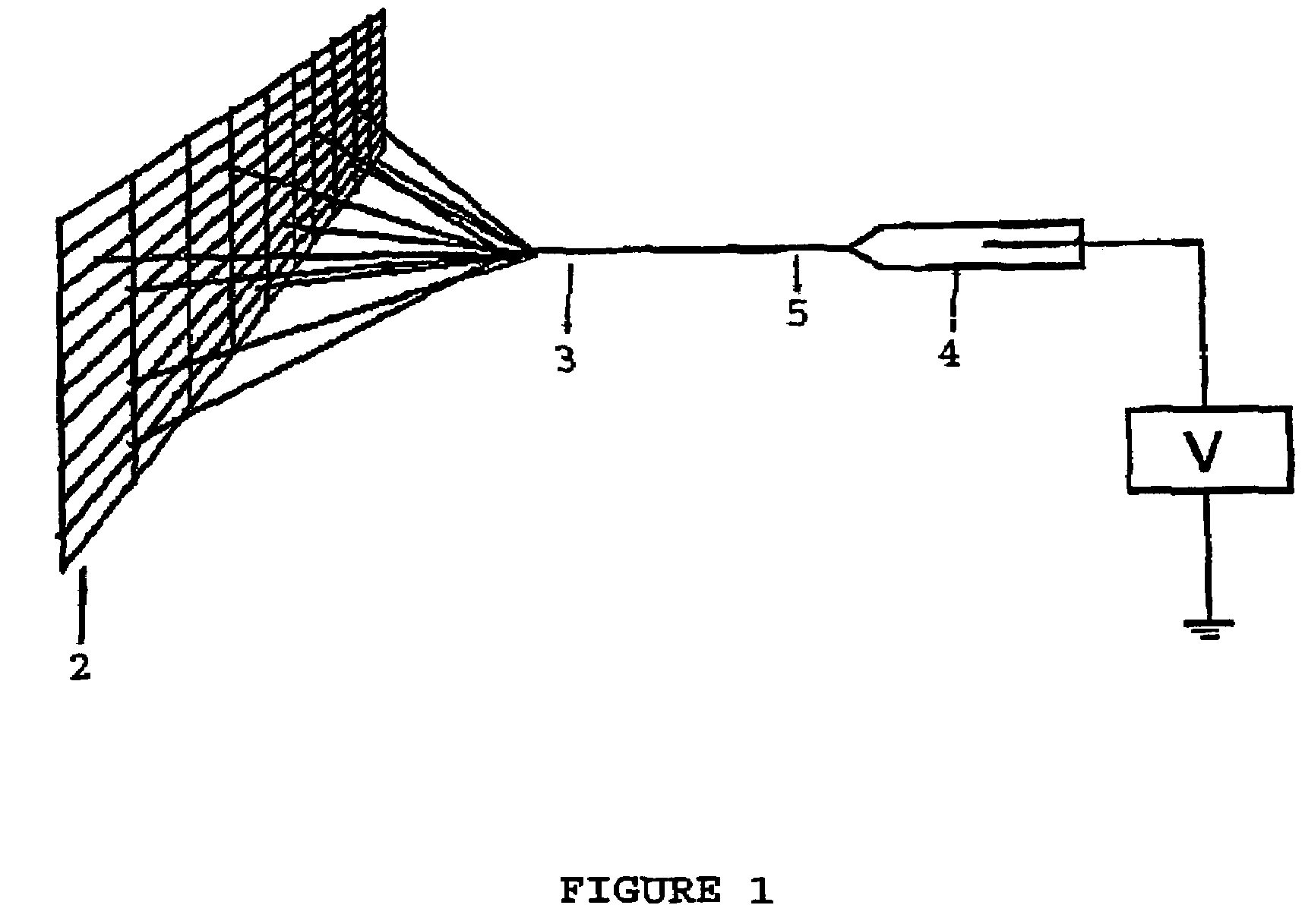
A process of making conductive polymeric fibers by electrospinning fibers from a blend of polymers dissolved in an organic solvent includes generating a high voltage electric field between oppositely charged polymer fluid in a glass syringe (4) with a capillary tip (5) and a metallic collection screen (2) and causing a polymer jet (3) to flow to the screen (2) as solvent evaporates and collecting fibers on the screen (2).
Owner:DREXEL UNIV +1
Botulinum toxin prefilled glass syringe
ActiveUS10549042B2Extended shelf lifeConvenient and safe and easy to useAntibacterial agentsCosmetic preparationsMedicineC. botulinum
Owner:MERZ PHARMA GMBH & CO KGAA
Tube preform and method for producing glass containers from a tube preform
InactiveCN1467166ASignificant economic advantagesReliable control of pressure conditionsGlass reforming apparatusWork in processEngineering
A tube blank is disclosed for producing glass receptacles, in particular glass tube vials, glass ampules, or glass syringes, particularly those suitable for pharmaceutical applications, having a tube wall, including two end regions-a first end region and the second end region-the first end region being sealed to form a floor and a ventilation hole being introduced into the tube wall in the region of the end region, distinguished in that the tube end characterizing the second end region has an opening.
Owner:舱壁玻璃公司
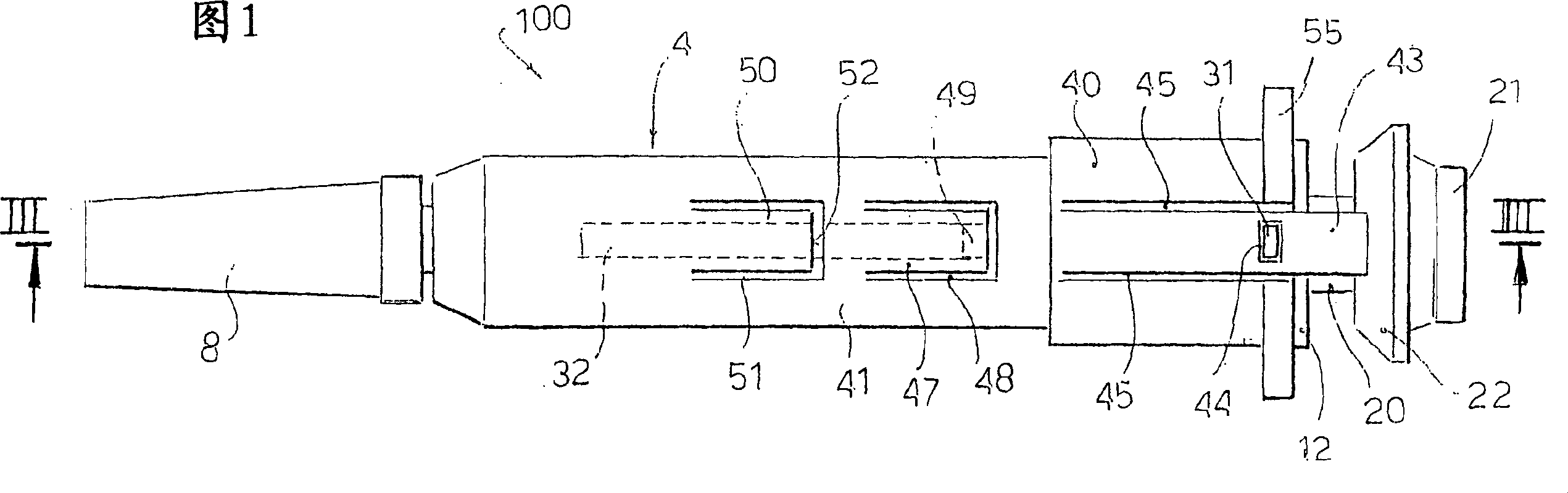
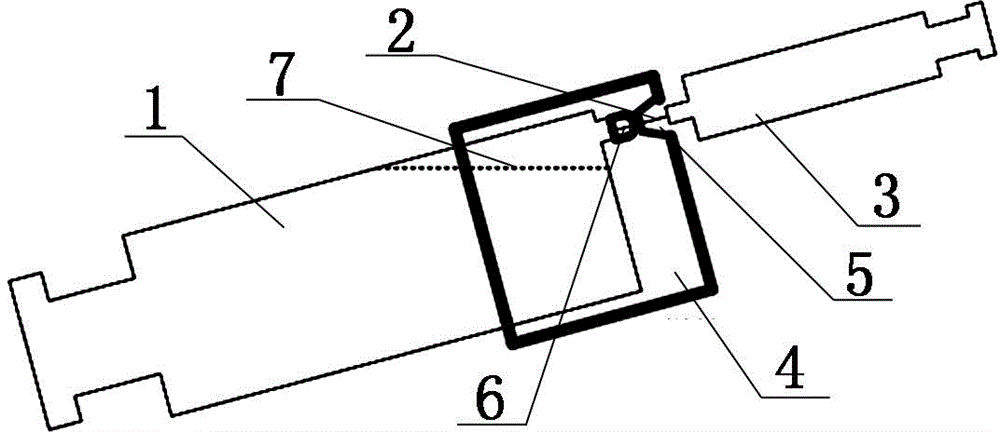
Glass safety syringe and relative safety kit for glass syringe
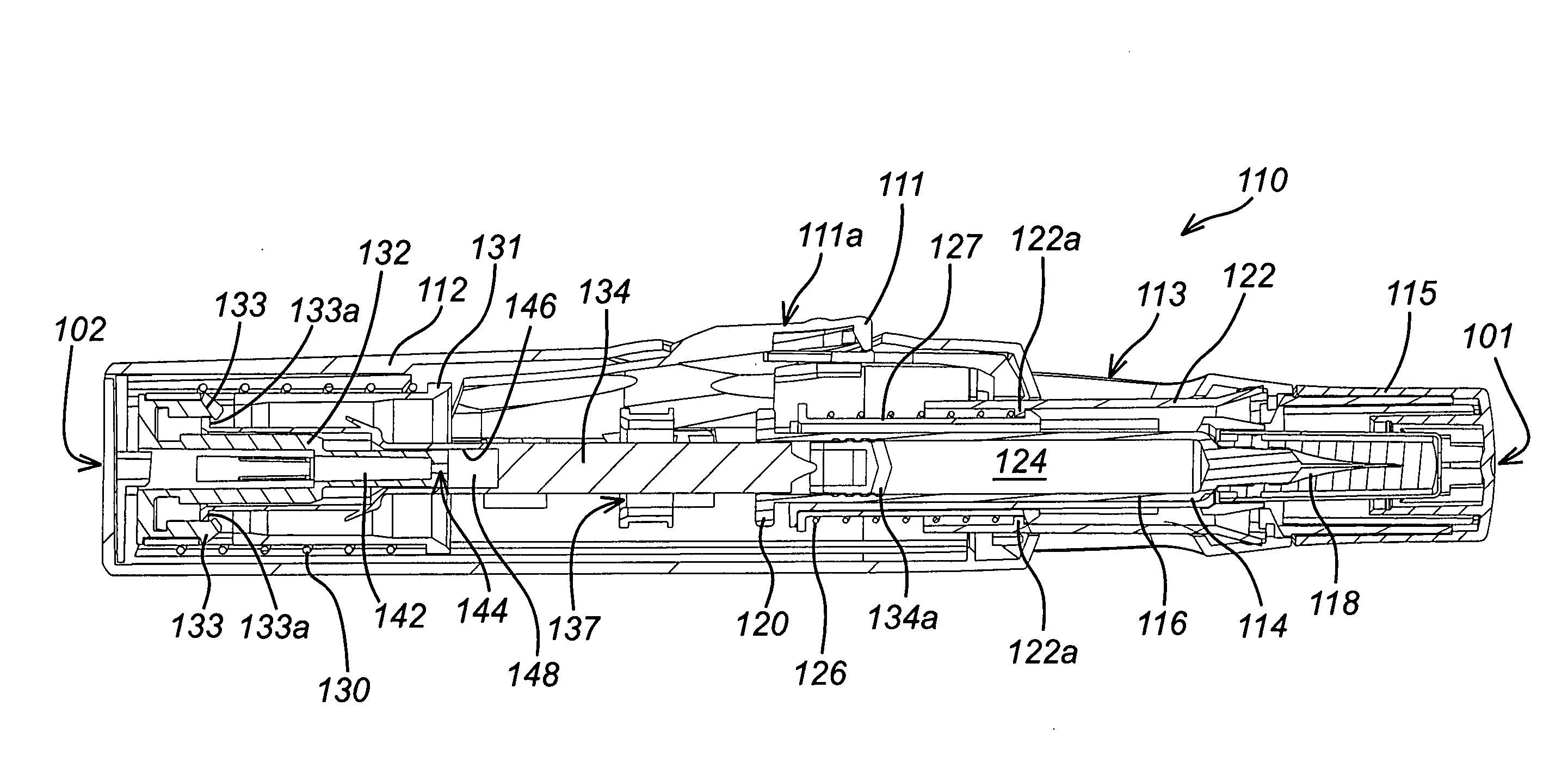
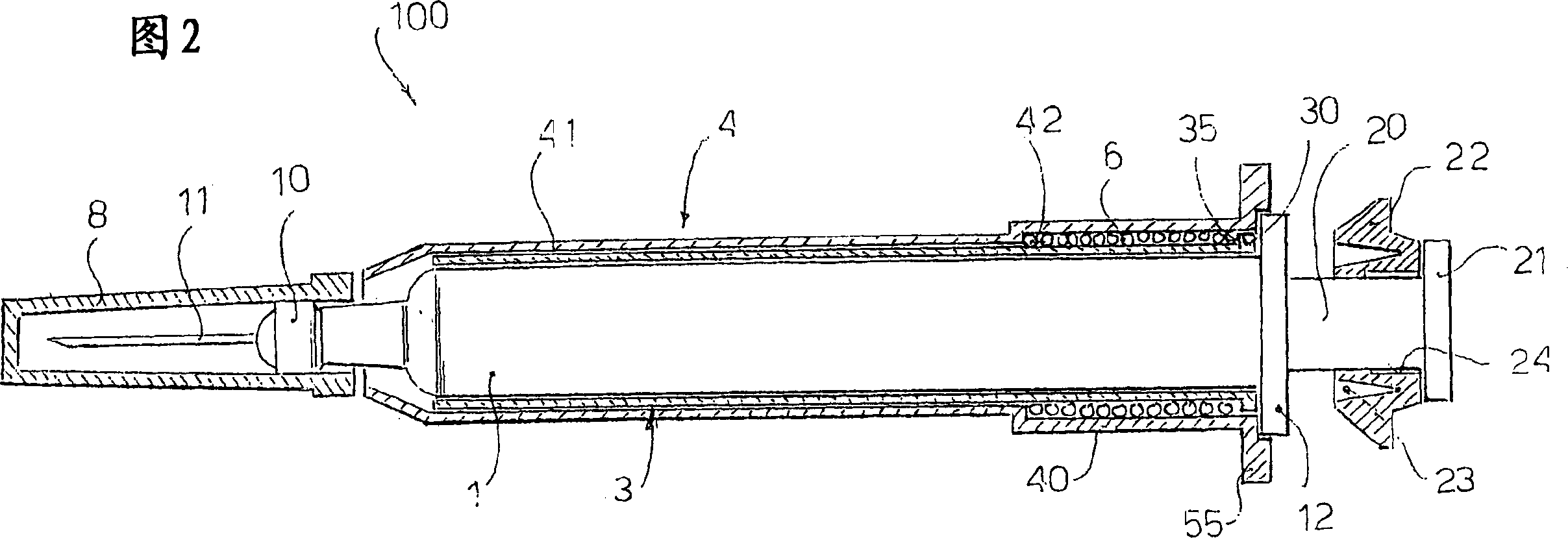
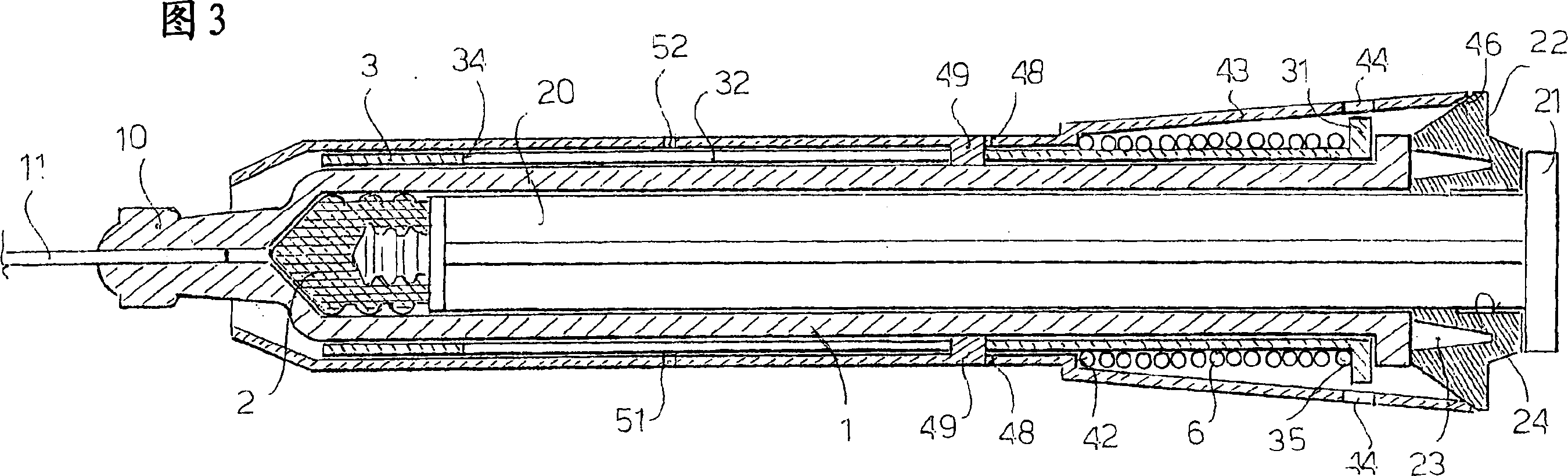
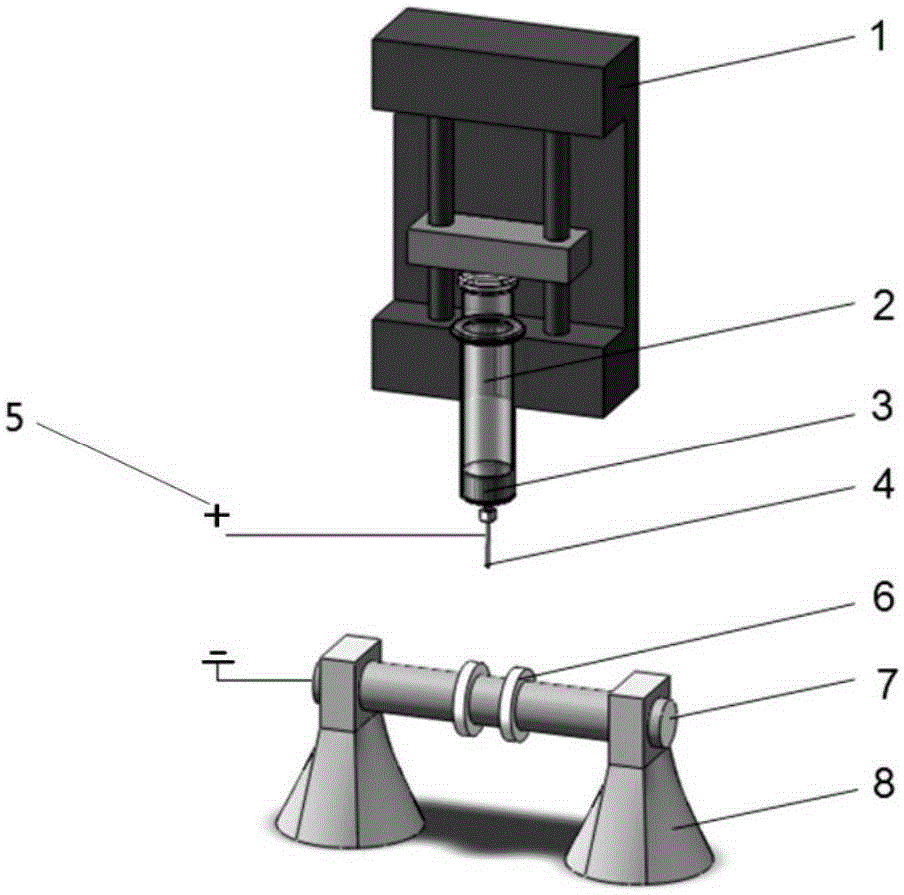
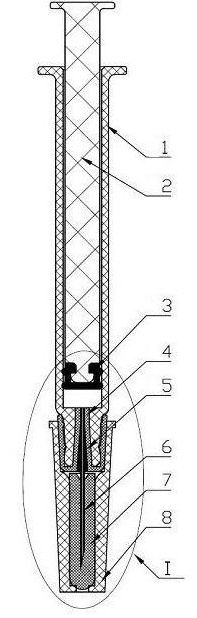
A safety syringe (100) comprises a glass syringe body (1), a plunger (2) slidable inside the syringe body (1) and provided at the rear with a manually operable stem (20), an injection needle (11) mounted at the front end (10) of the syringe body (1), a sheath (3) mounted integrally on the syringe body (1), a sleeve (4) mounted slidably on the shcath to pass from a retracted use position to an advanced safety position, in which the needle (11) is protected by the sleeve (4), locking means mutually provided in the sheath and in the sleeve to lock the sleeve (4) in position when it is in the retracted use position and in the forward safety position, and operating means (22) integrally mounted with the stem (20) of the piston to cooperate with locking means (43) so as to allow movement of the sleeve (4) from the retracted use position to the advanced safety position. <IMAGE>
Owner:塞尔焦・雷斯特利 +2
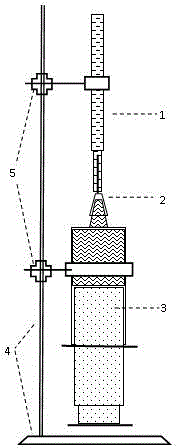
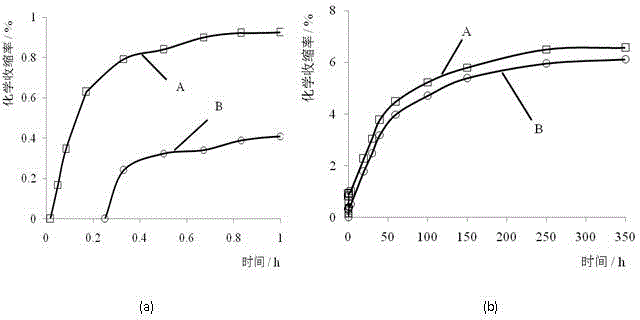
Cement chemical shrinkage test device and method
InactiveCN104569361ASimple and fast operationShort operating timeMaterial testing goodsCement pasteSyringe needle
The invention provides a cement chemical shrinkage test device and method. The cement chemical shrinkage test device consists of a glass graded tube, a syringe needle, a glass syringe and a support. By use of favorable leak tightness and disassembly and assembly convenience of the syringe and the needle, the quick test operation can be realized, and chemical shrinkage information of the relatively early cement hydration age can be obtained; meanwhile, the needle is utilizes for controlling the water amount so as to perform the chemical shrinkage test on cement pastes with different water-cement ratios; and the test device is easy to prepare, convenient to operate and reliable in test results.
Owner:TONGJI UNIV
Electrostatic spinning device for preparing axial orderly arranged tubes by means of magnetic field inducement
InactiveCN105200540ABending Instability SuppressionGood orientationFilament/thread formingFiberHigh pressure
The invention provides an electrostatic spinning device for preparing axial orderly arranged tubes by means of magnetic field inducement. The device can be used for preparing three-dimensional orderly arranged tubular tissue engineering scaffold materials. The device comprises a micro-injection pump, a reservoir, a conductive capillary tube, a direct-current high-voltage power supply, a receiving device and annular ferrite magnets, wherein the reservoir is a glass injector and fixed to the micro-injection pump, the micro-injection pump can accurately control spinning solution extrusion rate, the conductive capillary tube is a stainless steel injection needle with the top end ground flat, the receiving device is a roller connected with a motor, the positive pole of the direct-current high-voltage power supply is connected with the stainless steel injection needle, and the roller serving as the receiving device is grounded through a wire so that a high-voltage electric field can be generated between the head of the stainless steel injection needle and the receiving device; the roller is sleeved with a pair of annular ferrite magnets which rotate together with the roller, and the distance between the two magnets is 4-10 cm so that the receiving device can be in a stable magnetic field. Compared with the single roller method for preparing a tubular support, the device has the advantages that the orderliness of prepared fibers is improved greatly, and the application range of electrostatic spinning is widened.
Owner:BEIHANG UNIV
Container
InactiveUS7806262B2Improve shock absorptionDispensing apparatusIntravenous devicesGlass SyringePlastic bag
A package has a rectangular parallelepiped container including vertical front and rear panel sections connected by a pair of vertical side panel sections, a top wall connected to the upper edge of the rear panel section and a bottom wall connected to the at least one of the lower edges of the vertical panels and a top cushion panel disposed beneath the top wall and rotatably connected to at least one upper edge of the side panel sections. A holder is disposed, in the container, for holding a plurality of glass syringes each of which is sealingly contained in a plastic bag.
Owner:BAYER SCHERING PHARMA AG
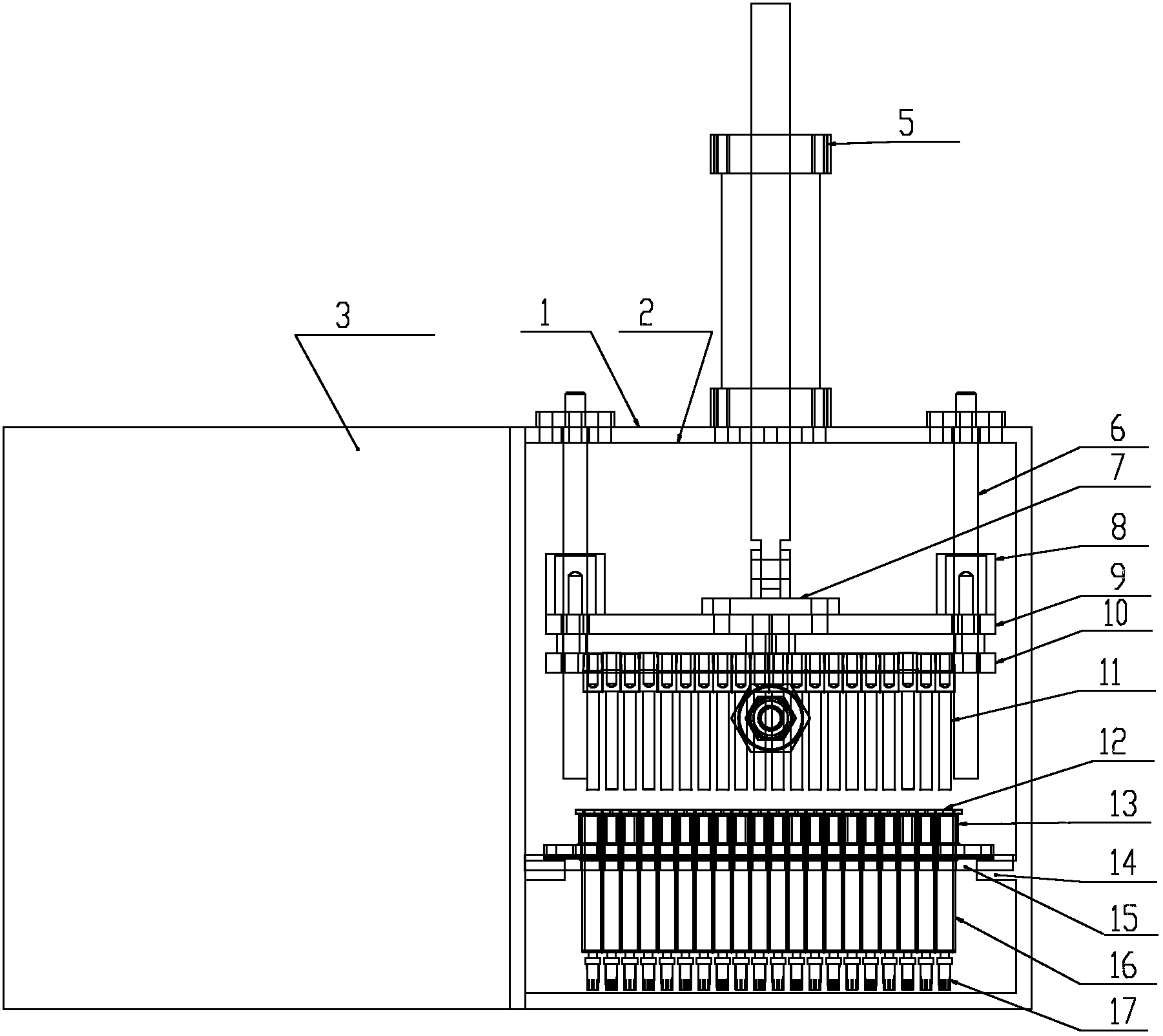
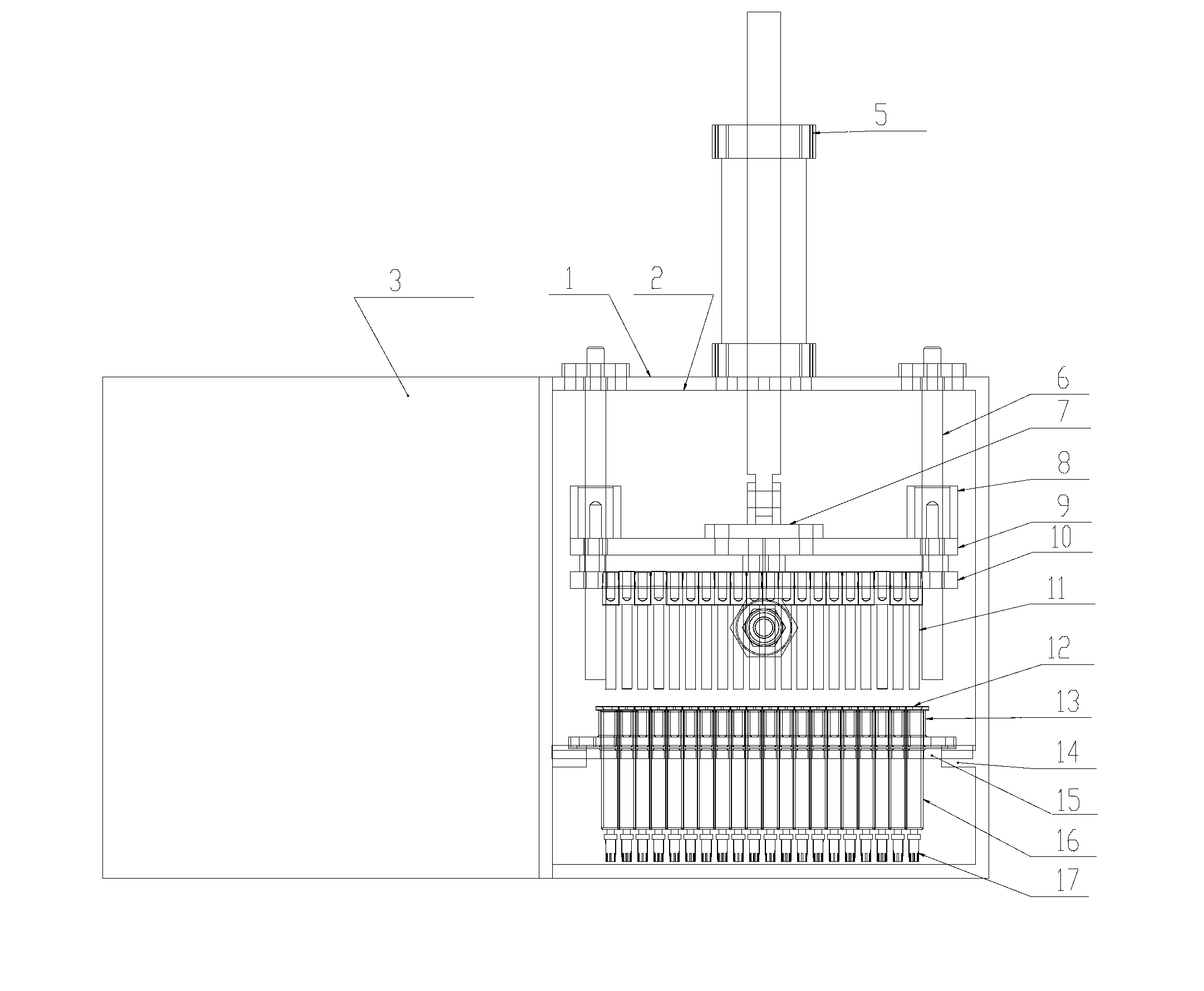
Pre-encapsulating glass syringe vacuum plugging machine
InactiveCN102838062AImprove stabilityImprove processing qualityThreadless stopper insertionEngineeringMedicine use
The invention discloses a pre-encapsulating glass syringe vacuum plugging machine which comprises a sealing box body, wherein the box body is provided with a door which can be opened, and a contact part of the door and a side plate of the box body is provided with a sealing piece; the side wall of the middle lower part in the box body is provided with a positioning tool plate, and a plugging mechanism is arranged above the positioning tool plate; the side surface of the box body is provided with a connector which is communicated with the interior of the box body; and the plugging mechanism and a vacuum generator are both connected with a controller. According to the pre-encapsulating glass syringe vacuum plugging machine, air in the lower part of liquid medicine in a glass syringe can all be exhausted to avoid the overflowing of the liquid medicine, and the lower edge of a rubber plug connected with a push rod in the glass syringe is just contacted with the upper surface of the liquid medicine, so that when the glass syringe is used by working personnel, after a packing is opened and a syringe needle is loaded, the syringe can be directly used by pushing the push rod without worrying about the problem that the liquid medicine contains bubbles; and the pre-encapsulating glass syringe vacuum plugging machine has the advantages of time saving, working efficiency improvement and improvement of the liquid medicine use safety and is suitable for long-distance transport and long-time storage.
Owner:山东淄博民康药业包装有限公司
Pre-encapsulating glass syringe vacuum plugging method
InactiveCN102838063AImprove stabilityImprove processing qualityThreadless stopper insertionLiquid productEngineering
The invention discloses a pre-encapsulating glass syringe vacuum plugging method which comprises the following steps of: (1) loading a liquid product tool plate into a box; (2) tool positioning: clamping a part of edge of a glass syringe tool plate in a stage of positioning clamp trough of a positioning tool plate; (3) rubber plug charging tool plate positioning: placing a rubber plug tool plate filled with rubber plugs on the upper part of the glass syringe tool plate, and clamping a part of edge of the rubber plug tool plate in the other stage of positioning clamp trough of the positioning tool plate; (4) closing a door of the box body; (5) pumping with vacuum to eliminate liquid medicine bubbles; (6) releasing pressure and charging plugs; and (7) encapsulating. According to the pre-encapsulating glass syringe vacuum plugging method, air in the lower part of the liquid medicine in a glass syringe can all be exhausted to avoid the overflowing of the liquid medicine, and the lower edge of the rubber plug connected with a push rod in the a syringe is just contacted with the upper surface of the liquid medicine, so that when the glass syringe is used, after a packing is opened and a syringe needle is loaded, the syringe can be directly used by pushing the push rod, and the pre-encapsulating glass syringe vacuum plugging method has the advantages of time saving, working efficiency improvement, use safety improvement of the liquid medicine and is suitable for long-distance transport and long-time storage.
Owner:山东淄博民康药业包装有限公司
High-elasticity PHA porous fiber material and preparing method thereof
InactiveCN104452107AControllable diameterAdjustable elongation at breakFilament/thread formingNon-woven fabricsElectrospinningSolvent
The invention relates to a high-elasticity PHA porous fiber material and a preparing method of the high-elasticity PHA porous fiber material. The method is characterized by comprising the steps that (1), PHA (P3HB4HB, 10-18 mol%4HB) is added into a mixed solvent of chloroform and acetone under the condition of stirring, and a mixed solution is obtained through stirring; (2), the temperature of the mixed solution is risen to 50 DEG C-80 DEG C under the condition of stirring, and through sufficiently stirring the mixed solution for 5-8 minutes, a P3HB4HB electrostatic spinning solution evenly dissolved is obtained, wherein the concentration of polymer is 6 wt%-12 wt%; (3), the P3HB4HB spinning solution is transferred into a glass syringe, then the glass syringe where the P3HB4HB spinning solution is contained is fixed to an electrostatic spinning device for electrostatic spinning, collected fibrous membranes are dried for 6-12 hours under a vacuum condition at the temperature of 40 DEG C-60 DEG C, and the high-elasticity P3HB4HB porous fiber membrane is obtained through preparation, wherein the elongation at break can reach above 500%. The preparing process is simple, controllable and efficient, biological degradability and biological compatibility are good, the diameter and elongation mechanical property of fibers are adjustable within a certain range, and the high-elasticity PHA porous fiber material has the good elasticity and the high elongation at break.
Owner:JIANGNAN UNIV
Pre-filled medicinal liquid injector
The invention relates to an improved pre-filled medicinal liquid injector and belongs to a clinical medical appliance. The injector comprises a needle, a syringe and a push rod, wherein the syringe isan integrated glass syringe; the needle is fixedly inserted into a cone arranged on the front end of the glass syringe; the needle hole of the needle is communicated with the inner cavity of the glass syringe; the needle is provided with a needle sealing cap; the medical rubber piston is arranged in the inner cavity of the glass syringe; and the piston is in thread connection with the push rod; the turn-up of the bottom end of the glass syringe is provided with double side cuts. The injector is reasonable in structure and reliable in installation, can be directly used to contain medicinal liquid, is convenient in use, improves the utilization rate of the medicinal liquid, reduces medicinal liquid waste, reduces usage cost, facilitates the destruction and management after dispensable use,avoids repeated use and reduces pollution. The injector reduces intermediate processes between the delivery and the use by injection into human body of medicines, ensures the safety and reliability ofthe use of injection medicines of people and creates obvious economic and social benefits.
Owner:山东淄博民康药业包装有限公司
Experiment platform for carbon deposition and coking of fuel
InactiveCN108181425ACorrespondence is clearHigh reliability of explanationChemical analysis using combustionTime conditionEngineering
The invention relates to an experiment platform for carbon deposition and coking of fuel in the technical field of fuel power devices. The experiment platform comprises an injection pump, a glass injector, a long needle, a heating disc, experiment sheets, a thermocouple, an electric heating wire, temperature controllers, a weight sensor, a mass display, a sealed exhaust hood and other components.The flat plate temperature condition required for experiments is obtained in the electric heating manner, fuel is slowly dropped on a flat plate, experimental procedures and experimental conditions such as temperature conditions, time conditions, environment atmosphere and the like are flexibly set according to research backgrounds, and flat plate carbon deposition of the fuel under specific conditions is obtained. The temperature condition can be controlled in a larger range, and the carbon deposition problem of all types of fuel can be researched. The experiment platform is reasonable in design, simple in structure and applicable to optimization design of the experiment platform for carbon deposition and coking of the fuel.
Owner:SHANGHAI JIAO TONG UNIV
Pipettor close performance detection device
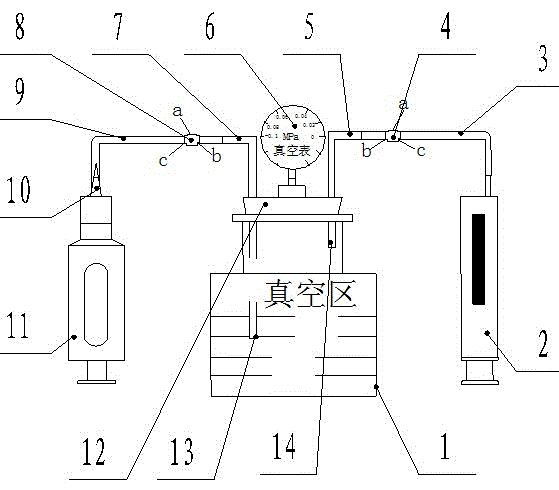
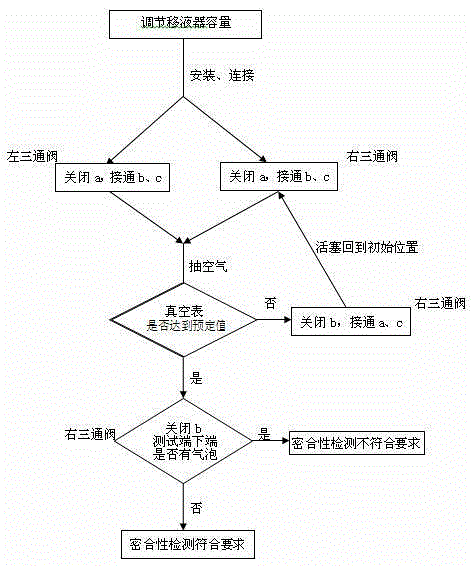
InactiveCN104990673AImprove pass rateTroublesomeDetection of fluid at leakage pointVacuum pressurePipette
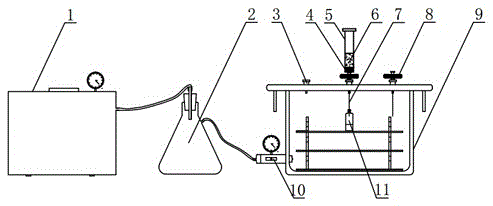
The invention relates to a pipettor close performance detection device. The detection device is formed by a transparent reagent bottle, a glass syringe, a right latex tube, a right three-way valve, a right glass tube, a vacuum pressure gauge, a left glass tube, a left three-way valve, a left latex tube, a liquid-absorbing nozzle and a soft rubber bottle plug. A pipettor close performance detection method of the pipettor close performance detection device is characterized by adjusting a pipettor to be detected to nominal capacity; installing the liquid-absorbing nozzle to the pipettor to be detected and screwing the liquid-absorbing nozzle tightly; carrying out connection according to the installation connection step; adjusting rotary knobs of the left and right three-way valves and extracting air with the glass syringe and repeating the operation until a pointer of the vacuum pressure gauge points to -0.04 MPa; and carrying out close performance detection of the pipettor to be detected through a closed loop formed by the pipettor to be detected, the left three-way valve, the transparent reagent bottle, the vacuum pressure gauge and the b end of the right three-way valve in sequence. The detection device solves the problems that it is troublesome and not easy to carry out the detection and efficiency is not high when carrying out close performance detection on the pipettor.
Owner:ZUNYI INST OF PROD QUALITY INSPECTION & TESTING
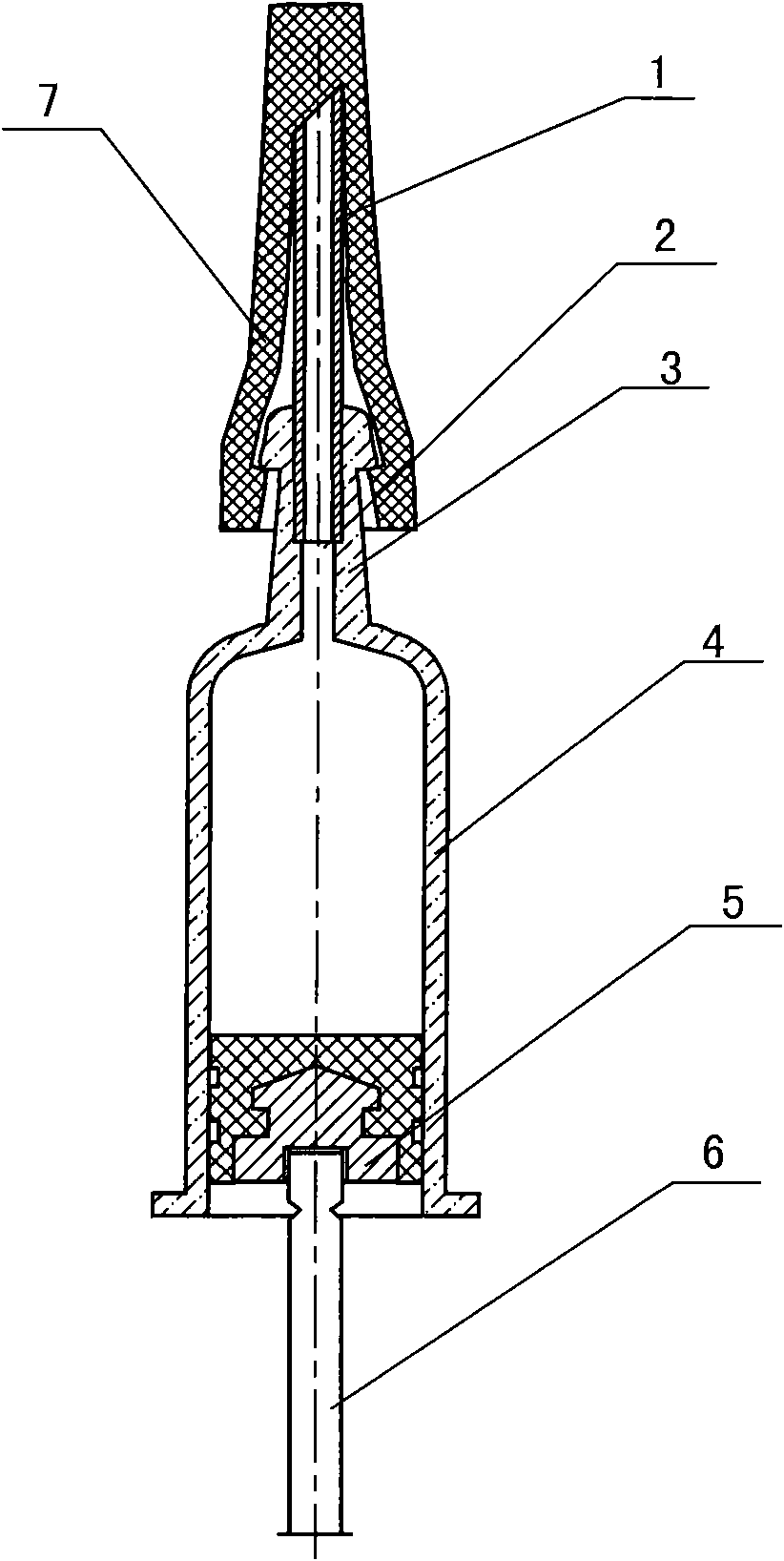
Pre-filled glass syringe
The invention relates to a pre-filled glass syringe. The glass syringe comprises a glass outer bushing, a core bar, a piston, a needle and a protective cap, wherein a needle seat is arranged on the front end of the glass outer bushing; the needle is fixedly arranged in a needle hole at a center pole of the needle seat by utilizing an adhesive; the protective cap is composed of a silica gel cap and a glass sheath, the glass sheath is sheathed on the needle seat, and the silica gel cap is arranged in a cavity of the glass sheath; and the needle is directly inserted into a needle protective body of the silica gel cap via a guide hole on the silica gel cap. The pre-filled glass syringe has the advantages of reasonable structure and low manufacture cost, is convenient in operation and use, is convenient and reliable in machining, and can be utilized as a liquid medicine reservoir as well as a syringe, thus being an ideal disposable pre-filled syringe.
Owner:SHANDONG WEIGAO GROUP MEDICAL POLYMER
Syringe with integral safety system
Owner:SAFETY SYRINGES
Method for preparing microencapsulated drink of Nanguo pear liver-protecting anti-alcohol agent
The invention relates to a method for preparing a microencapsulated drink of a Nanguo pear liver-protecting anti-alcohol agent, which is particularly used for Nanguo pear fruit and provides a theoretical basis for quantification of ethanol and acetaldehyde dehydrogenase in Nanguo pear liver-protecting anti-alcohol drink production. The process of the method comprises: raw material selection, washing, block cutting, water-bath soakage, ice-bath grinding, ice-bath homogenization, one-hour 0 to 4 DEG C extraction, 15-minute 400-rpm centrifugation, freezing concentration, the preparation of enteric microencapsulates of mixed concentrates of the ethanol, the acetaldehyde dehydrogenase and the like and the preparation of the drink by blending according to required package. The preparation of enteric microencapsulates of the mixed concentrates of the ethanol, the acetaldehyde dehydrogenase and the like is implemented by the following steps: mixing a wet enzyme paste mixture and 10 percent gelatin and 5 percent xanthan gum in a volume ratio of 1:5.5; fully and uniformly mixing the materials; uniformly mixing the mixed materials with a certain amount of 2-percent sodium alga acid; injecting the mixed solution into cooled solution of CaCl2 by using a glass syringe with a No.4 syringe needle to perform emulsification for 10 minutes with a stirring speed or 400rpm to obtain colloid-calcium alginate double-layer gel beads; subjecting the colloid-calcium alginate double-layer gel beads to a film forming reaction with solution of chitosan for 30 minutes; removing unreacted chitosan by washing to obtain wet Nanguo pear-dehydrogenase combined microencapsulate gel beads; and placing the microencapsulated dehydrogenase microencapsulate gel beads in a regulation tank for regulating concentration according to an anti-alcohol ratio, adding a proper amount of sugar and other seasoning matters, homogenizing the mixture, and automatically filling and sealing the resulting product.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Desorption device for saxitoxin in macroporous resin and method of desorption device
ActiveCN105651575AAvoid residuePrevent dumpingPreparing sample for investigationDesorptionFiltration
The invention relates to a desorption device for saxitoxin in macroporous resin and a method of the desorption device. The desorption device comprises a vacuum pump and a solid-phase extraction instrument which are connected to the two ends of a transition bottle. A small column joint of the solid phase extraction instrument is connected with a microporus filter. A glass syringe is installed on the microporus filter. A filtering material layer is arranged at the bottom of the glass syringe. By means of the filtering material layer, macroporous resin can be effectively prevented from blocking the syringe. The method mainly includes the steps of flushing, moisture filtering, stirring, desorption, desorption solution collection and the like, filtering is completed at the same time during desorption, operation is easy, convenient and rapid, the time consumption is low, the steps of filtration and concentration can be omitted, and the use amount of a desorption solution can be effectively decreased.
Owner:ZHEJIANG UNIV
Luer connection adapters for syringes
A connector mountable to a syringe barrel has a proximal barrel-engaging portion, a distal luer fitment portion, and a fluid aperture therethrough. The barrel-engaging portion of the connector includes an axial ledge configured to abut the axial distal edge of a glass syringe barrel. The connector facilitates mounting a luer assembly to the barrel. The luer assembly may be a tip cap, a luer needle assembly, or a luer needle-less assembly, having a complementary luer fitment for connection to the luer fitment portion of the connector. The connector and syringe may further include an immobile, compressible needle seal. The needle seal is adjacent to or engageable with the barrel-engaging portion of the connector.
Owner:UNITRACT SYRINGE
Glass syringe seal sampling auxiliary joint device used for insulating oil gas chromatography
InactiveCN104913952APrevent slippingEliminate interpolation phenomenonComponent separationWithdrawing sample devicesGas liquid chromatographicPhysical chemistry
Provided is a glass syringe seal sampling auxiliary joint device used for insulating oil gas chromatography. The glass syringe seal sampling comprises a glass syringe A containing a sampling detection gas inside and a glass syringe B equipped with a sampling syringe needle. A sampling auxiliary joint which can be inserted from the head of the glass syringe and inserted into the glass syringe A is arranged on the glass syringe. A funnel-shaped syringe needle guider is arranged at the side end part of the sampling auxiliary joint. The funnel-shaped syringe needle guider and a rubber sealing cap of the head of the glass syringe A can achieve butt joint and clamping after plugging positioning. The sampling auxiliary joint comprises a joint support contacting the glass syringe A and achieving a fixed function. Windows are formed in two sides of the joint support and the windows do not affect observation view during sampling. The structure is simple, usage is convenient and reliable, the work efficiency of chromatography of dissolved gases in oil can be raised, and the problem of smooth transferring of gas between syringes is solved.
Owner:STATE GRID CORP OF CHINA +1
Pneumatic syringe
InactiveCN108392697AAllows for multiple injectionsImprove work efficiencyInfusion syringesMedical devicesMulti injectionEngineering
The invention provides a pneumatic syringe. The pneumatic syringe comprises a plurality of glass syringe bodies and is characterized in that the end face of one end of each syringe body is provided with a liquid outlet, the other end face of each syringe body is provided with an air inlet, a control switch set is arranged on the end face of each syringe body, the air inlets are connected with a fan through air inlet tubes, each liquid outlet is fixedly connected with a liquid outlet tube, a needle can sleeve the outer wall of each liquid outlet tube, one end of the fan is arranged on a frame,the bottom of the frame is provided with rotary wheels, the end face of one end of the frame is provided with a plurality of clamp rings, the end face of the frame is provided with a control panel, and the control panel is electrically connected with the control switch set and the fan. The pneumatic syringe has the advantages that air suction and air discharge can be achieved by the fan so as to allow the syringe to complete suction and injection, repeated injection can be performed by a user conveniently by the multiple syringe bodies, work efficiency is increased, and problems in the prior art are solved effectively.
Owner:合肥钰芹信息科技有限公司
Luer connection adapters for retractable needle syringes
A connector mountable to a syringe barrel has a proximal barrel-engaging portion, a distal luer fitment portion, and a fluid aperture therethrough. The barrel-engaging portion of the connector includes an axial ledge configured to abut the axial distal edge of a glass syringe barrel. The connector facilitates mounting a luer assembly to the barrel. The luer assembly may be a tip cap having a complementary luer fitment for connection to the luer fitment portion of the connector. The luer assembly may be a luer needle assembly having a complementary luer fitment for connection to the luer fitment portion of the connector. The connector and syringe may further include an immobile, compressible needle seal. The needle seal is adjacent to or engageable with the barrel-engaging portion of the connector. The syringe may be configured with a plunger capable of engaging a retractable needle.
Owner:UNITRACT SYRINGE
Preparation method and application of PHBV/rosiglitazone (RSG) sustained-release membrane
InactiveCN108743566ALittle side effectsDetermining the duration of sustained and effective anti-fibrosis actionOrganic active ingredientsSenses disorderPostoperative scarsGlaucoma
The invention discloses a preparation method and application of a PHBV / rosiglitazone (RSG) sustained-release membrane and belongs to the technical field of medicines. The preparation method comprisesthe following steps: mixing PHBV powder, chloroform and dimethylformamide and preparing a PHBV-TCM / DMF spinning solution with a mass percentage of 5 percent for later use; dissolving rosiglitazone ina dimethyl sulfoxide solution and preparing RSG / DMSO solutions with different concentration gradients; then adding in the spinning solution, preparing spinning solutions with different final concentrations and transferring into a glass syringe for electrostatic spinning to form the rosiglitazone / PHBV sustained-release membrane; the rosiglitazone / PHBV sustained-release membrane is used in the anti-glaucoma filtration operation, is safe, effective and lasting in anti-glaucoma postoperative scar formation and improves the long-term the glaucoma operation success rate, thereby having a very important clinical significance.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com