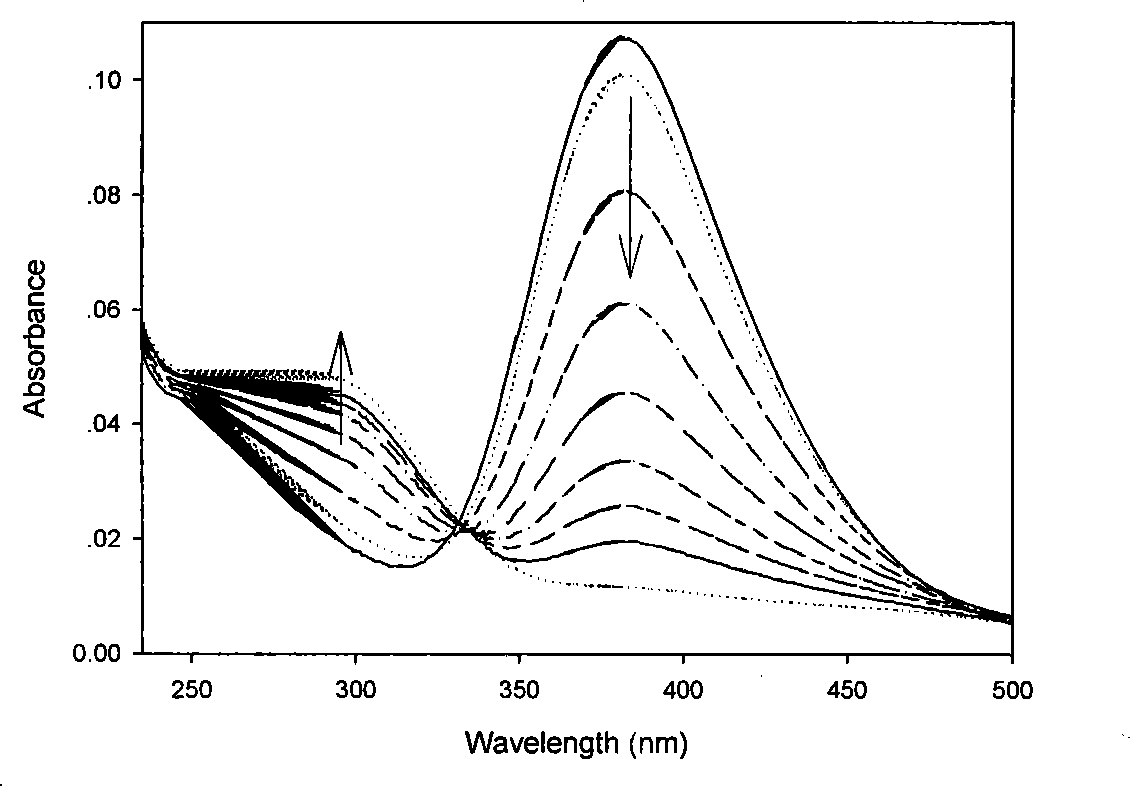
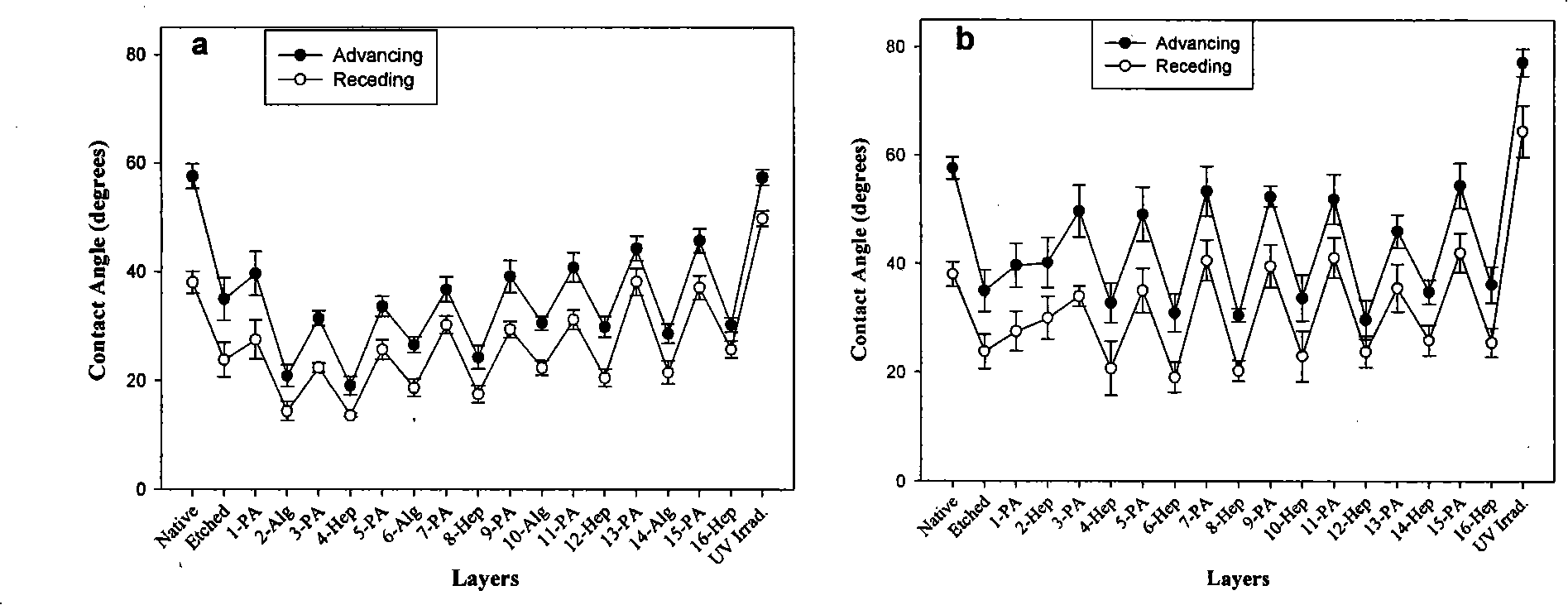
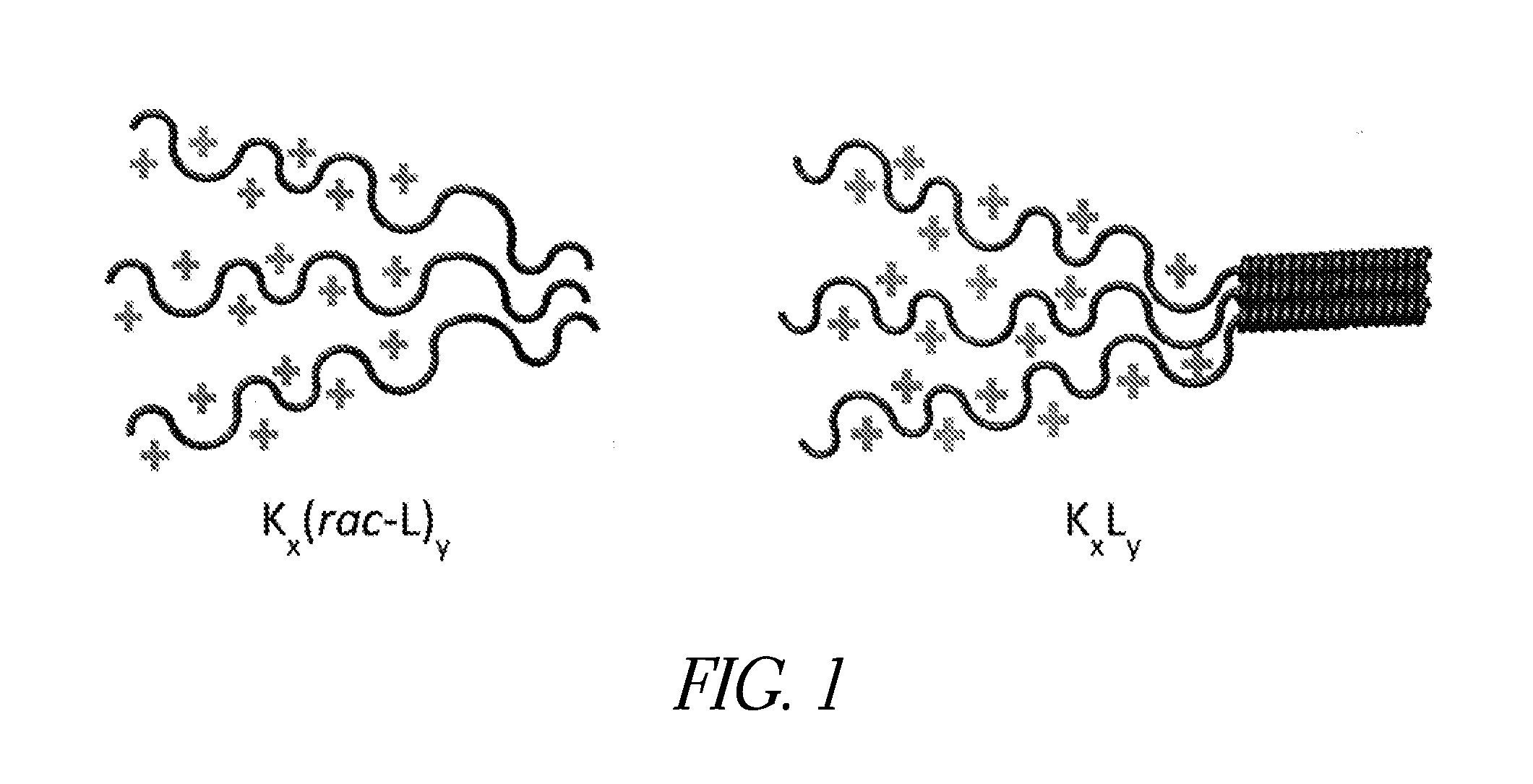
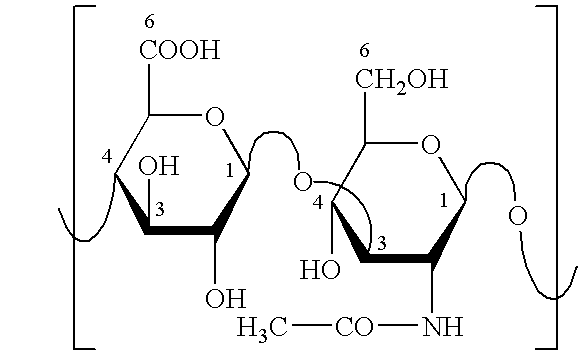
Patents
Literature
292 results about "Tissue Compatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
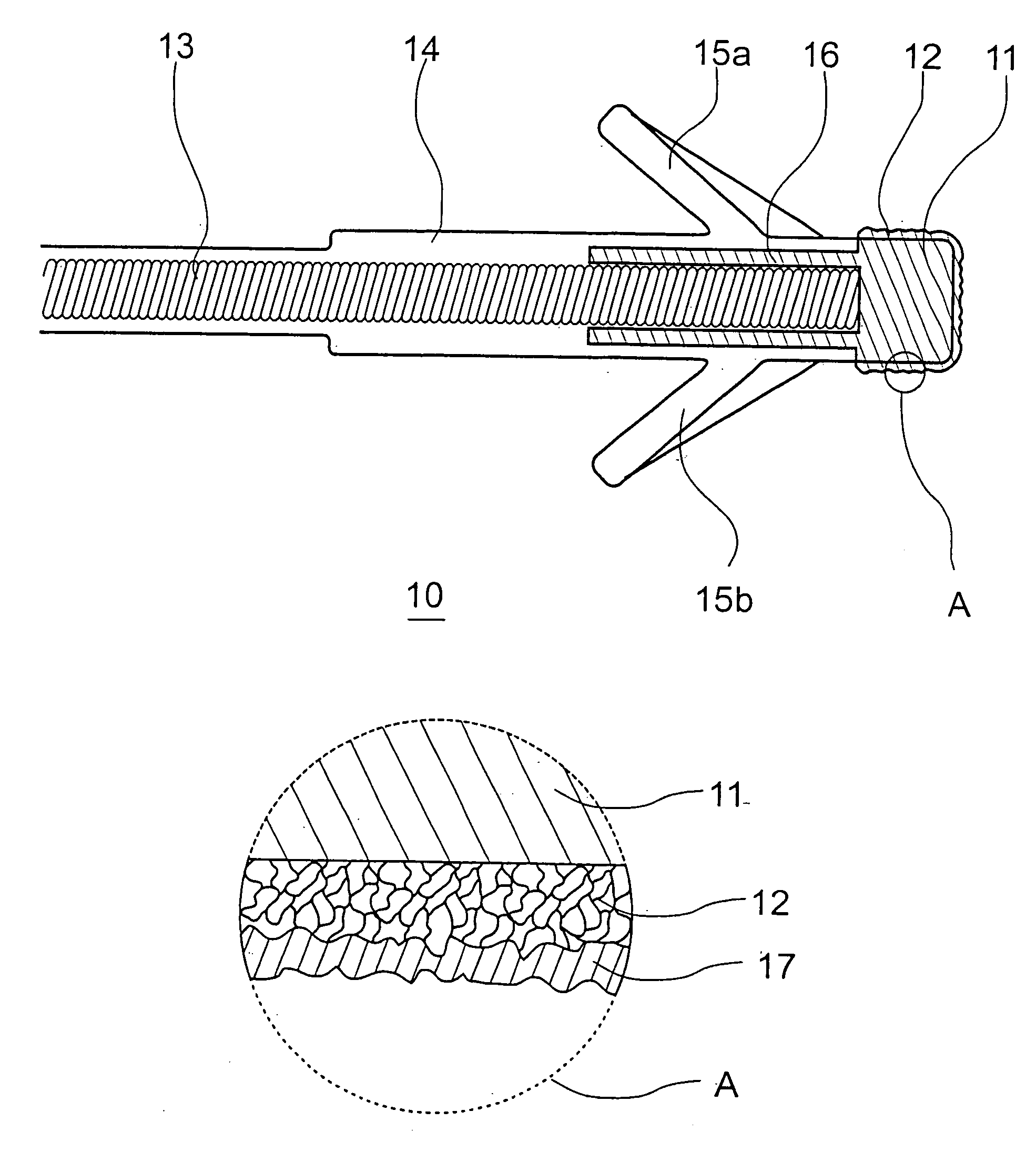
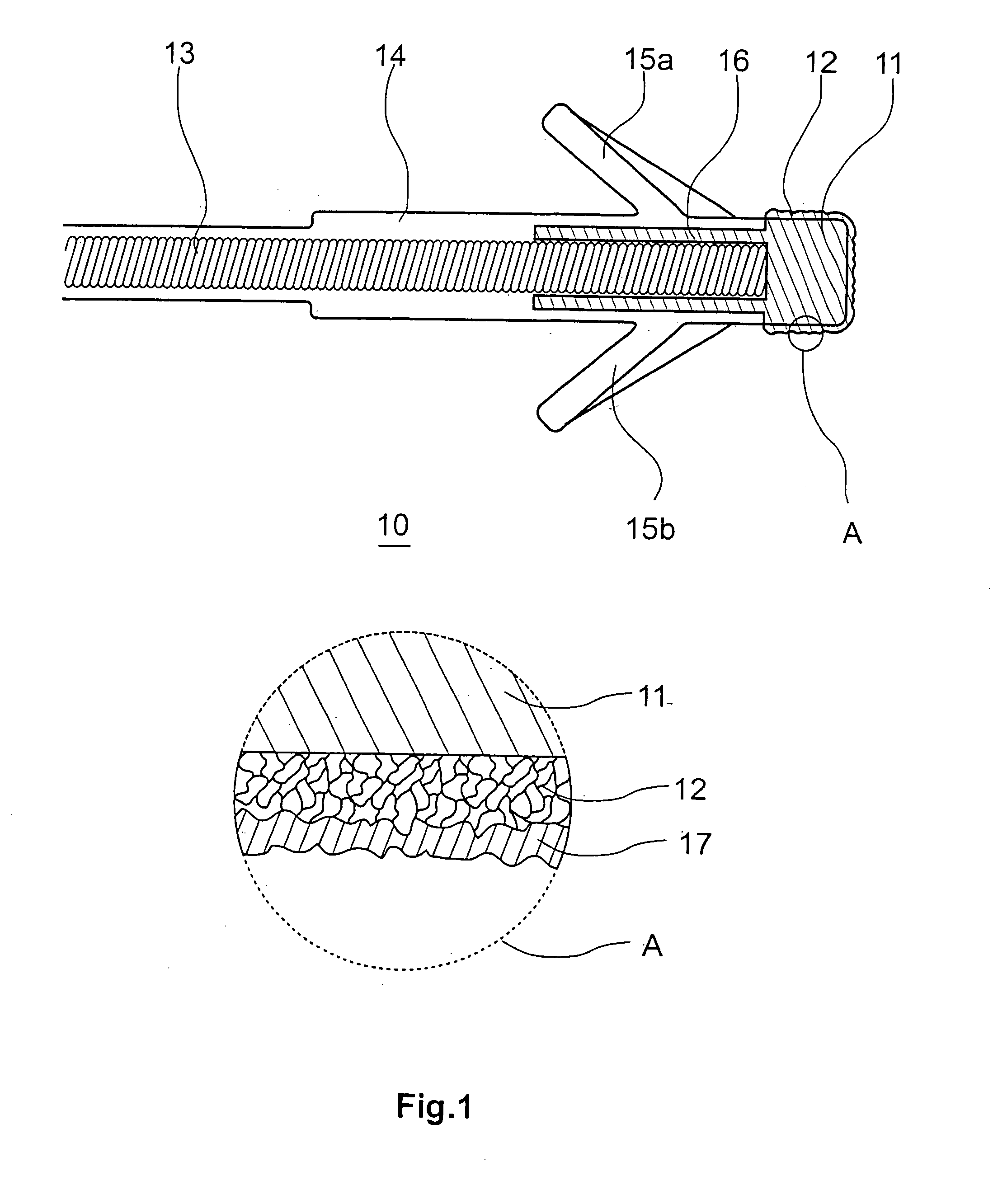
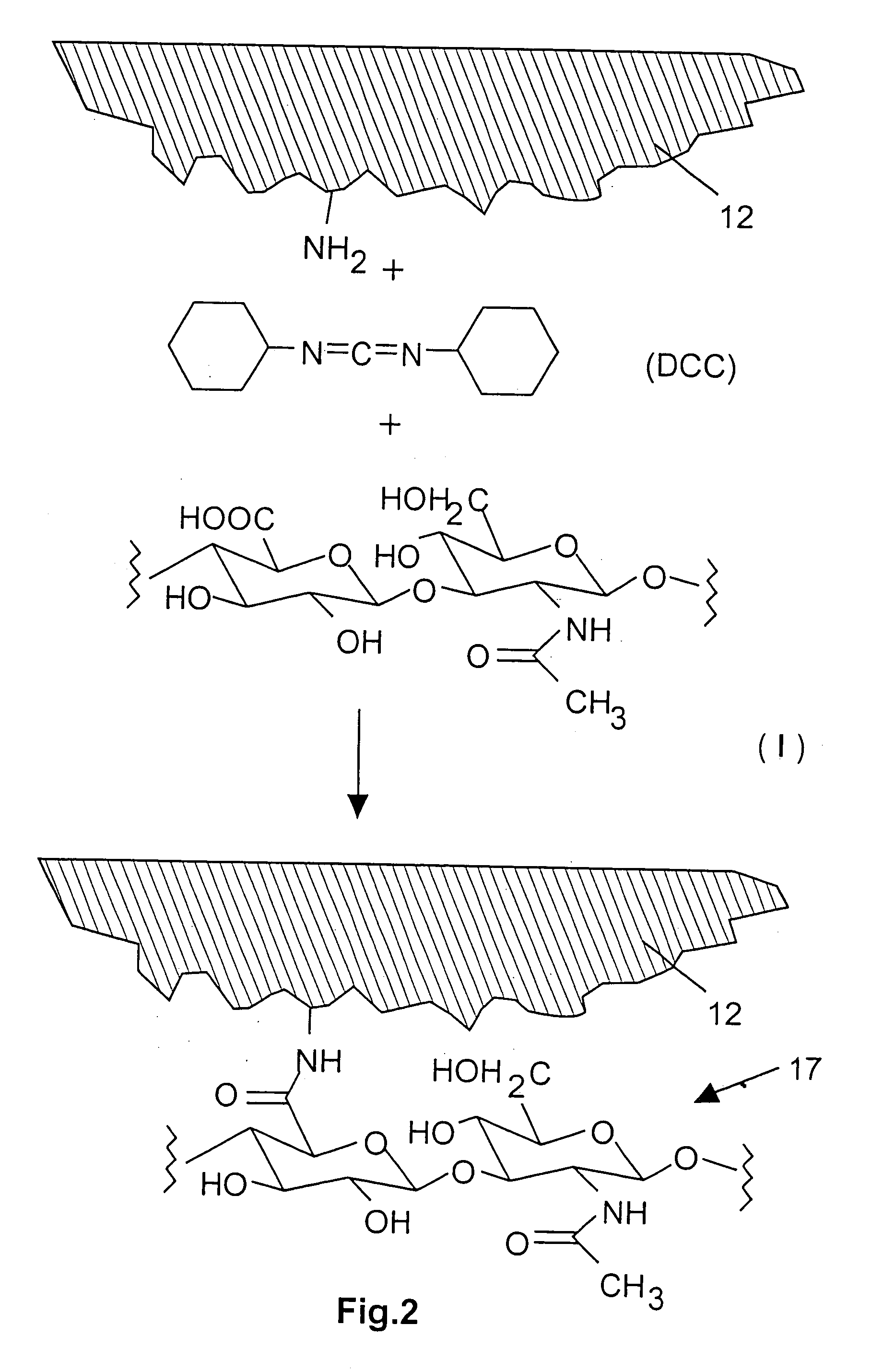
Implantable Stimulation Electrode with a Coating for Increasing Tissue Compatibility
InactiveUS20080234790A1Avoid tissue irritationGood biocompatibilitySurgeryInternal electrodesImplantable Stimulation ElectrodesIrritation
An implantable stimulation electrode for use with an implantable tissue stimulator, especially a pacemaker, a defibrillator, a bone stimulator or a neurostimulator includes a metal base body, optionally one or more intermediate layers disposed on the base body and a coating covering the base body and, optionally, intermediate layers in order to increase tissue compatibility. The coating should prevent tissue irritations after implantation and more particularly increase the stimulus threshold associated therewith, have very high biocompatibility and also has an anti-inflammatory effect. An increase in tissue compatibility is achieved by virtue of the fact that the coating has a polysaccharide layer made of hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Preparation method of injectable human hair keratin soft tissue filling material
The invention relates to the technical field of the medical reshaping cosmetology, which overcomes the defects of the existing soft tissue filling material by virtue of the advantages of high resistance of the human hair against physical and chemical factors, stable properties, acid and alkali resistance, capability of not being easily absorbed and degraded by tissues, convenient acquisition, repeated material use, easy processing and the like. The middle section of solid-state autologous human hair is used as a raw material and treated with cleaning, bleaching, milling processing, freeze-drying, packaging, and cobalt-60 radiation, so that the human hair is bleached to prepare power homogenate with different thicknesses and liquid human hair keratin granules, thereby providing a human hair keratin soft tissue filling material which is applicable to injection and has the advantages of autologous source, easy obtaining, safety, no toxicity, good tissue compatibility, easiness for a human body to accept and function of stimulating the collagen proliferation. The invention realizes the long-term repair of the skin and soft tissue, and satisfies the requirements of the soft tissue filling material in the field of the reshaping cosmetology.
Owner:泛亚润康(北京)生物科技有限公司
Medical hydrogel dressings and preparation method thereof
ActiveCN101982202AHigh swellingStable in humid environmentAbsorbent padsBandagesCross-linkTissue Compatibility
The invention relates to a medical hydrogel dressings and a preparation method thereof. The hydrogel dressings is prepared by taking 10-30% by mass of starch and 2-15% by mass of water-soluble polymer as raw materials, utilizing a guanidinesalt polycondensate as an anti-bacterial agent, adding a cross-linking agent, and reacting at the temperature of 40-80 DEG C. The operation steps are as follows: adding the starch and the water-soluble polymer in a solvent to prepare into a solution, regulating the pH value of the solution by a NaOH solution, heating for gelatinization, cooling, adding the anti-bacterial agent and the cross-linking agent, stirring to form a mixed solution, pouring the mixed solution into a die, and reacting at the temperature of 40-80 DEG C to obtain the anti-bacterial hydrogel. Compared with the prior art, the hydrogel dressings in the invention has the advantages of good swelling property, high transparency, moderate mechanic strength, excellent bacteria invasion resistance, wide raw material sources, cheap raw materials, good tissue compatibility and favorable medical application prospect, and can effectively prevent wound infection.
Owner:EAST CHINA UNIV OF SCI & TECH
Hydroxylapatite coating magnesium alloy medical inner implantation material and method of preparing the same
The invention relates to a hydroxyapatite coating magnesium alloy medical implantation material and a preparation method thereof. The hydroxyapatite coating magnesium alloy medical implantation material is characterized in that: the surface of a magnesium alloy substrate is attached with a hydroxyapatite coating layer. The preparation method of the hydroxyapatite coating magnesium alloy medical implant material can adopt the bionic solution growing method, the ion beam deposition method, the coating and sintering process, the plasma spray method, the discharge plasma sintering method or the electrophoresis deposition method. The magnesium alloy material with the hydroxyapatite coating structure which is provided by the invention can effectively slow down the degradation rate of the magnesium alloy; at the same time, the coating layer can not only have great tissue compatibility, but can also be conductive to connective tissue attachment and the growth of the bone tissues, improve the bone healing rate and shorten the healing time.
Owner:BEIJING ALLGENS MEDICAL SCI & TECH +1
Chitosan/polylysine in-situ gel and preparation method thereof
InactiveCN102585303APromotes Adhesive GrowthGood tissue compatibilitySurgical adhesivesPharmaceutical non-active ingredientsCell adhesionTissue Compatibility
The invention discloses a chitosan / polylysine in-situ gel and a preparation method thereof. The gel is an in-situ gel prepared from 1-5.5 percent by weight of sulfhydrylization chitosan and 0.1- 3.2 percent by weight of maleimide polylysine. The preparation method comprises the following steps of synthetizing the polylysine containing maleimide double bonds, and preparing a maleimide polylysine solution by taking a PBS (phosphate buffer solution) as a solvent; preparing a sulfhydrylization chitosan solution by taking the PBS as a solvent; and uniformly mixing the two solutions under the condition of physiological pH value to obtain the in-situ gel. The invention has the advantages that the preparation process is simple, and the prepared in-situ gel simulates the components, the structure and the functional characteristics of polysaccharide / polypeptide of a natural extracellular substrate, helps to increase histocompatibility of a material, promotes cell adhesion growth and has wide application prospects in the fields of tissue engineering and drug release.
Owner:TIANJIN UNIV
Cell culturing rack material and its prepn
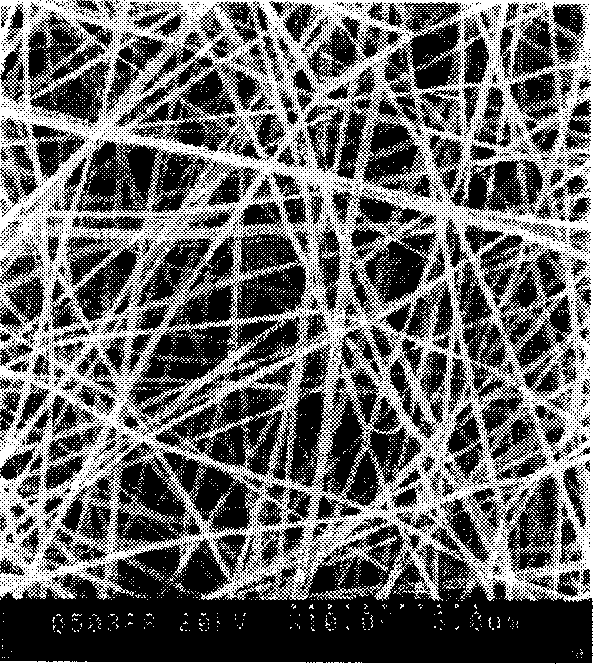
The present invention discloses one kind of cell culturing rack material and its preparation process. The cell culturing rack material is prepared with silk as main material, and through degumming, dissolving, purifying and drying to form fimbrin; dissolving together with collagen or gelatin in the same kind of solvent; high voltage electrostatic spinning to obtain 3D netted nanometer fiber non-woven felt of fimbrin base material, collagen, gelatin, etc. and final organic alcohol treatment. The cell culturing rack material has average fiber diameter smaller than 100 nm, average pore size of 1.0-5.0 microns and porosity of 70-90%. Test shows that the nanometer fiber material has no toxicity, no irritation, no sensibilization, excellent tissue compatibility and perforated pore structure, and may be used as cell culturing rack material for repairing human body tissue.
Owner:SUZHOU UNIV
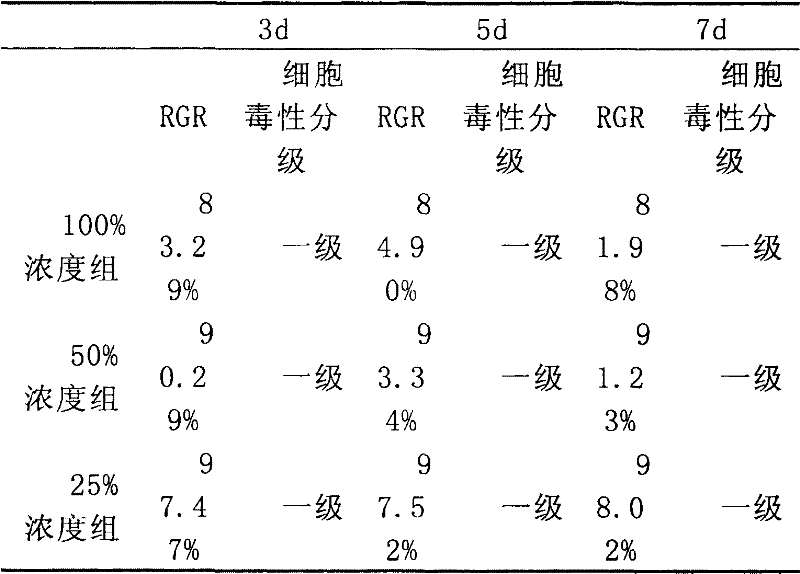
Zn-Sr series zinc alloy as well as preparation method and application of Zn-Sr series zinc alloy
Owner:PEKING UNIV
Preparation method of injectable human hair keratin soft tissue filling material
The invention relates to the technical field of medical plastic surgery. According to the advantages that human hair has high resistance to physical and chemical factors, stable properties, acid and alkali resistance, is not easy to be absorbed and degraded by tissues, is easy to obtain, can be repeatedly obtained, and is easy to process, it overcomes the existing There are deficiencies of soft tissue filler materials. Using the middle section of solid autologous human hair as raw material, after cleaning, bleaching, grinding, freeze-drying, packaging, and cobalt 60 irradiation treatment, the bleached human hair is made into powder homogenate and liquid human hair keratin particles of different thicknesses , to provide a human hair keratin soft tissue filling material that is derived from autologous body, is easy to obtain, is safe and non-toxic, has good tissue compatibility, is easy to be tolerated by the human body, and can stimulate collagen proliferation and is suitable for injection. Realize the long-term repair of skin and soft tissue, and meet the needs of soft tissue filling materials in the field of plastic surgery.
Owner:泛亚润康(北京)生物科技有限公司
Method for producing nano-fibre bracket material with levorotation polylactic acid as base material
InactiveCN101401955AFlexible textureGood tissue compatibilityStentsPhysical treatmentPorosityTissue Compatibility
The invention relates to a method for preparing a nanofiber bracket material using levorotatory polylactic acid as matrix. The method comprises the following steps: dissolving the levorotatory polylactic acid as the matrix in a solution of dichloromethane and dimethyl formamide, and stirring and centrifuging the mixture to obtain an electrostatic spinning solution; placing a polylactic acid solution into a 5 milliliter glass syringe, and applying high voltage on the glass syringe; advancing the levorotatory polylactic acid solution in the glass syringe; preparing the mixed solution into a nanofiber material film through a electrostatic spinning technology; and modifying the nanofiber material film to obtain the nanofiber bracket material of which the fiber diameter is between 50 and 500 nanometers and the fiber porosity is more than 90 percent. The method solves the defects that a PLLA porous bracket still has too long degradation time, and degradation products can cause tissue inflammations easily and the like. The method has the advantages of flexible texture, better water permeability and air permeability, excellent tissue compatibility, controllable biodegradability, and no antigenicity.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
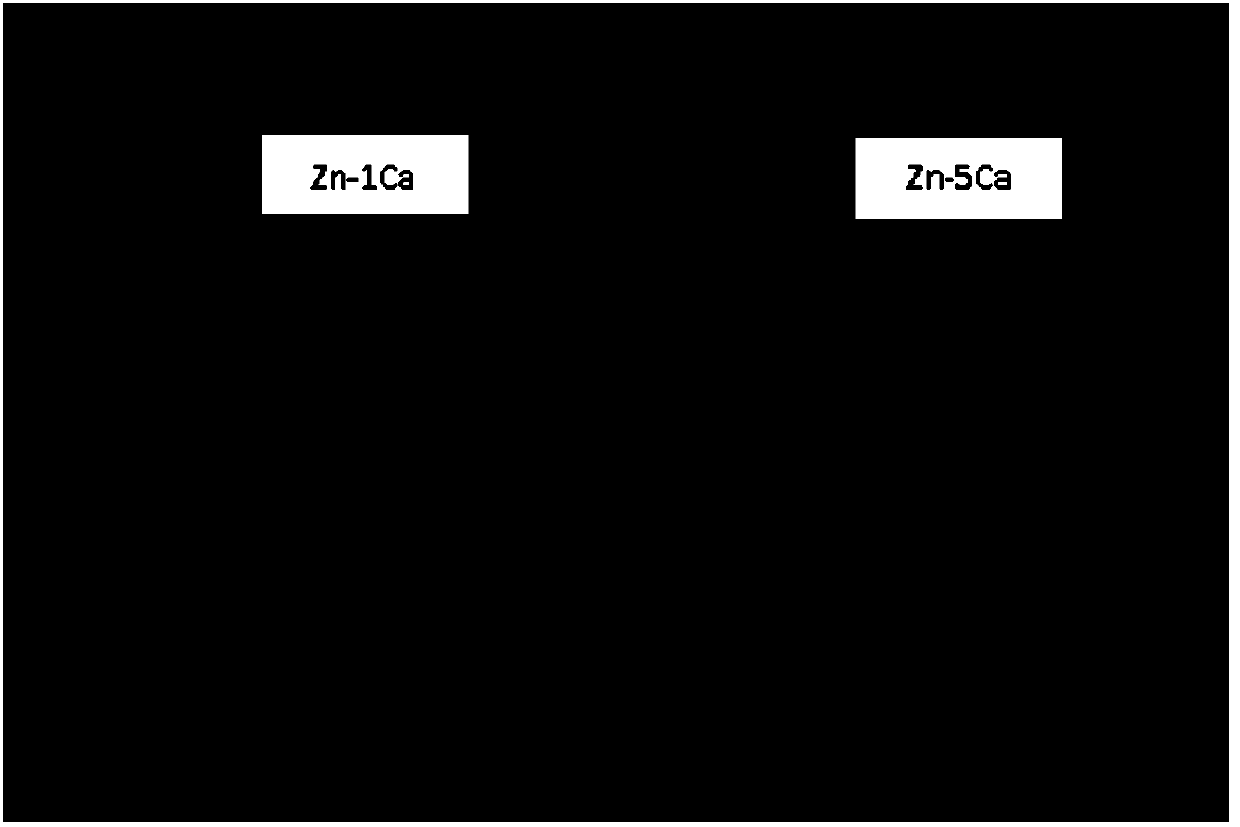
Zn-Ca series zinc alloy as well as preparation method and application of Zn-Ca series zinc alloy
ActiveCN104195369AGood tissue compatibilityMeet strengthProsthesisRare-earth elementTissue Compatibility
The invention discloses a Zn-Ca series zinc alloy as well as a preparation method and application of the Zn-Ca series zinc alloy. The zinc alloy comprises Zn and Ca, wherein the mass percent of Ca in the zinc alloy ranges from 0% to 30%, but is not equal to 0. The zinc alloy also comprises trace elements including Si, P, Li, Ag, Sn and at least one selected from rare earth elements, wherein the mass percent of the trace elements ranges from 0% to 3%, but is not equal to 0. The mechanical property of the Zn-Ca series zinc alloy disclosed by the invention meets the requirements for strength and toughness of a medical implant material, is nontoxic and favorable in tissue compatibility and blood compatibility, can be degraded by using body fluid and can be applied to preparation of medical implants; and the dissolved metal ions can be absorbed and utilized by living bodies to promote bone growth or can be discharged out of bodies through metabolism.
Owner:PEKING UNIV
Method to improve hydroxyapatite implantation and stimulate bone regeneration
InactiveUS20020127261A1Favorable to tissue growth of tissueReduced affinityBone implantJoint implantsApatiteTissue Compatibility
Hydroxyapatite is treated by a combination of nitridation and the application of bone morphogenetic protein to improve the tissue compatibility and affinity of the hydroxyapatite, rendering the hydroxyapatite more useful as a material for biomedical implants.
Owner:RGT UNIV OF CALIFORNIA
Cell-eliminating coanea matrix and its prepn process
InactiveCN101066471AGood scaffoldingMitigation of insufficient contradictionsEye implantsTissue regenerationFiberDisease
The present invention discloses one kind of cell-eliminating cornea matrix, which consists of mainly hundreds of parallel collagen fiber plate layers with voids formed through eliminating cells in between. The cell-eliminating cornea matrix is used in repairing, reconstructing and shaping surficial eye defect, treating surficial eye diseases and constructing tissue engineering cornea. After being transplanted, the cell-eliminating cornea matrix may exist for long term or degrade gradually, and is high transparent. It may allow receptor nerve to grow into and receptor cell to attach on, and has the features of low immunogenicity and high tissue compatibility.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
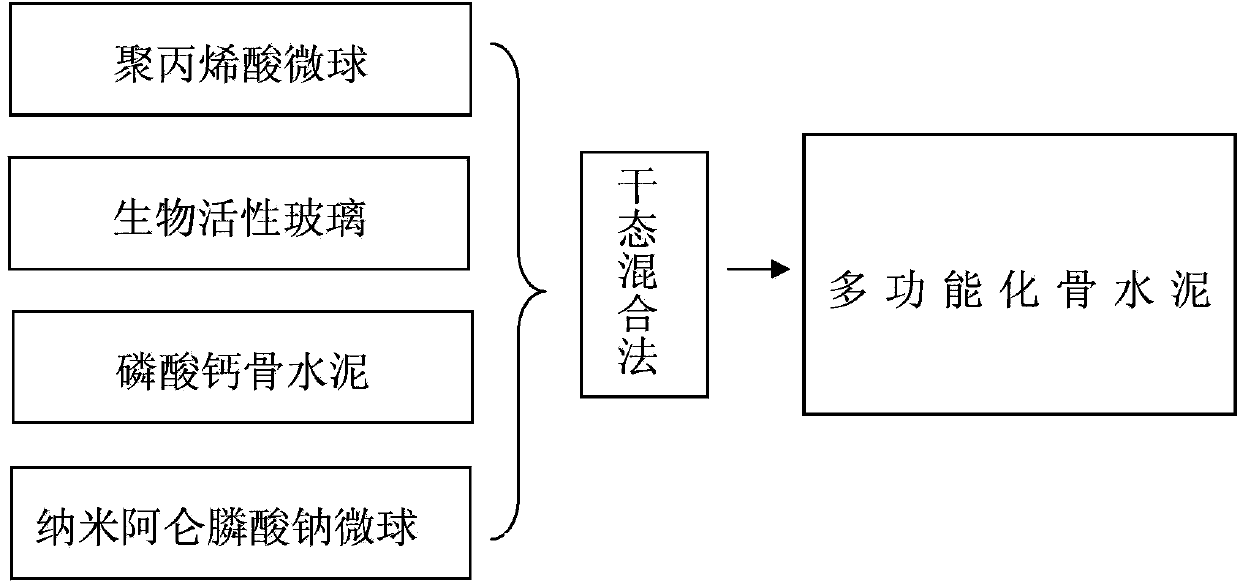
Multifunctional medical biological bone cement
The invention discloses multifunctional medical biological bone cement. The multifunctional medical biological bone cement is prepared from the following raw materials in percentage by mass: 5%-15% of polyacrylic acid microspheres, 20% of bioactive glass, 64.9%-74.9% of calcium phosphate bone cement and 0.1% of nanometer alendronate sodium microspheres, wherein the sum of the mass percentages of the raw materials is 100%. The multifunctional medical biological bone cement has the function of water absorption self-expansion; the using amount of the bone cement can be reduced, and further the leakage of the bone cement is reduced; the multifunctional medical biological bone cement has relatively good tissue compatibility, can be used for inducing the formation of new bones and enhancing the strength of vertebral bodies, is degradable in vivo without in vivo foreign matter residues; and meanwhile, the controlled-release alendronate sodium has the effects of resisting osteoporosis, reducing the absorption of bones and furthering enhancing the vertebral bodies. Therefore, the multifunctional medical biological bone cement has the advantages of little consumption, promotion of bone formation and bone absorption resistance when applied to vertebroplasty.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Bio-derived material with high tissue compatibility and long acting anti-infection as well as preparation method and application thereof
InactiveCN101810883AAchieving Self-Assembly PreparationImprove proliferative abilityProsthesisCell freeTissue Compatibility
The invention relates to a bio-derived material with high tissue compatibility and long acting anti-infection. The bio-derived material comprises the components of a cell-free matrix and absorbable anti-infective nanometer particles, wherein the amount of the absorbable anti-infective nanometer particles implanted into the cell-free matrix have the range of 100-1000 micrograms / cm<3> according to the content of the inorganic antibacterial agent. A preparation method of the bio-derived material comprises the following steps of: (1) preparing absorbable inorganic antibacterial agent nanometer particles; (2) carrying out cell-free modifying treatment on the bio-derived material; and (3) constructing a nanometer-modified bio-derived material by using a self-assembly technology. The bio-derivedmaterial has the advantages of directly contacting an abdominal cavity, long acting anti-infection, high tissue compatibility and strong ability to promote cell proliferation and can be used for contaminated wounds or infected wounds and the like; after implanted in vivo, the inorganic antibacterial nanometer particles burst a medicament within a short period (not more than 2 h) to quickly realize effective concentration so that the duration time of the effective blood concentration reaches 2 weeks to 3 months or even longer; and the invention has favorable application potential in the rehabilitation treatment of various tissue organs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Biomembrane material strip belt for high-myopia posterior scleral reinforcement surgery and manufacture method thereof
The invention relates to an ophthalmic medical appliance and a manufacture method of the ophthalmic medical appliance, in particularly to a biomembrane material strip belt for a high-myopia posterior scleral reinforcement surgery. Base materials of the biomembrane material strip belt have a wide resource, the biomembrane material strip belt is blue after the treatment, the manufactured blue biomembrane material strip belt has good visuality and cannot easily get lost in the implantation process; the hardness and the elasticity are proper, the adaptability is good, the implantation is easy, and the biomembrane material strip belt is easily pasted, sewed and fixed with a receptor sclera; after the implantation into the human body, the toxicity is low, the mechanical strength is high, the tissue compatibility is good, the degradation resistance capability is high, and the calcification is not easy to occur; and the whole body of the biomembrane material strip belt is in a fusiform shape, the thickness is 0.15 to 0.45mm, the side part is an inclined surface, the middle section is widest, the middle section is gradually narrowed in arc-shaped transition respectively towards the left end and the right end, the length is 30 to 60mm, the width of the middle section is 10 to 16mm, the width of the left and the right end is 2 to 3mm, through the special shape design of the strip belt, the venae vorticosae can be prevented from being pressed, the reinforcement range is enlarged, the inclined surface of the side part is in 30 to 45 degrees, the marking at the positive surface and the opposite surface is realized, and the twisting turning is not easy to cause during the implantation.
Owner:BEIJING XINKANGCHEN MEDICAL SCI & TECH DEV
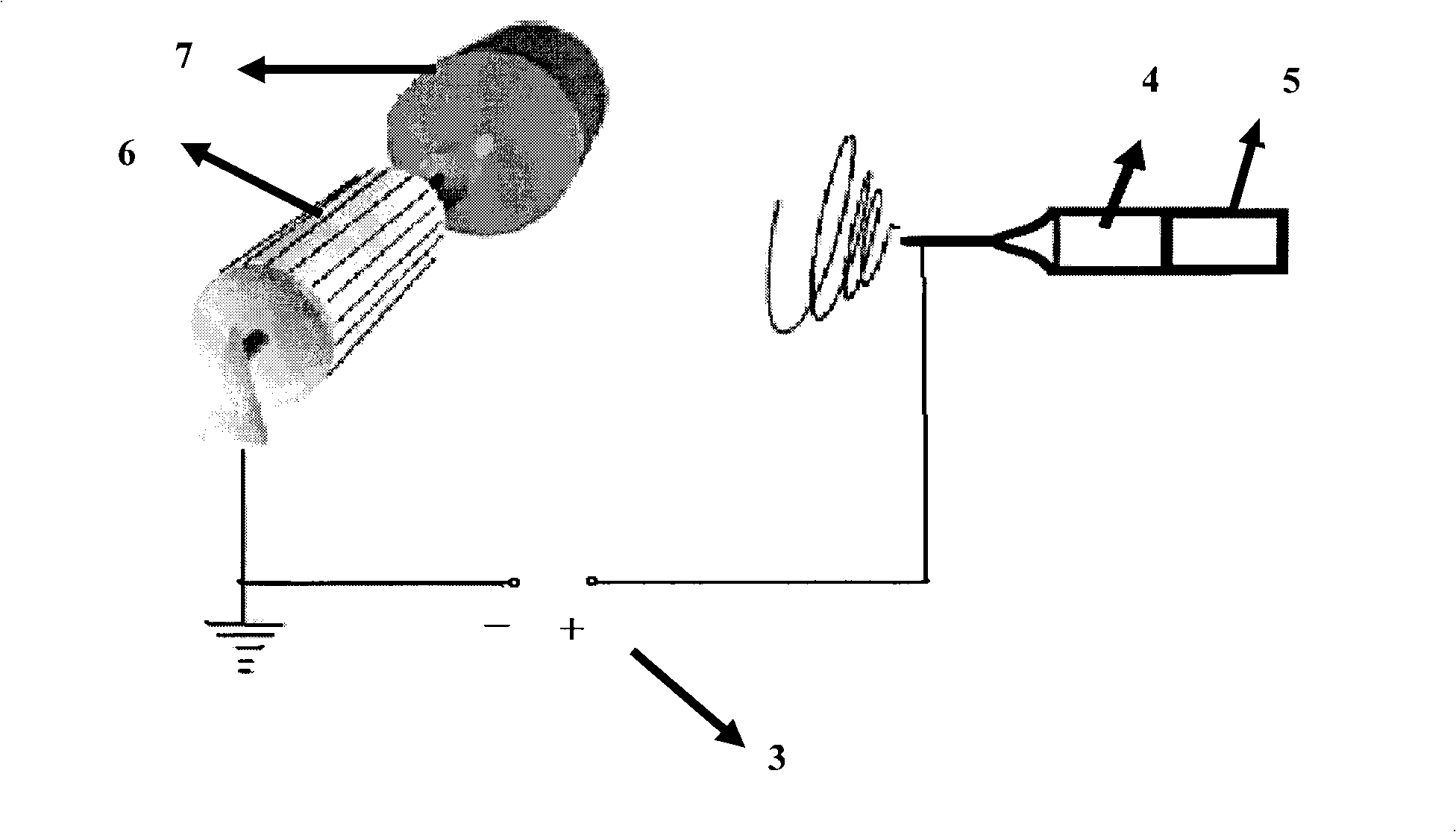
Method for preparing anticoagulant vascular stent
InactiveCN101927037AImprove bindingNot easy to fall offPharmaceutical containersMedical packagingMedicineDistilled water
The invention discloses a method for preparing an anticoagulant vascular stent, which comprises the following steps of: A, depositing a plasma-polymerized allyl amine functional film on the surface of the vascular stent by using a pulse plasma polymerization method; B, preparing heparin sodium mixed solution; and C, precipitating heparin sodium, namely soaking the vascular stent on which the plasma-polymerized allyl amine functional film is deposited prepared in the step A into the heparin sodium mixed solution prepared in the step B, reacting at the temperature of between 4 and 20 DEG C for 12 to 48 hours, and after the reaction, fully rinsing by using distilled water, and drying to obtain the anticoagulant vascular stent. The vascular stent prepared by the method has the advantages of high binding force between an anticoagulant film layer on the surface of the vascular stent and the vascular stent, and excellent tissue compatibility and blood compatibility.
Owner:CHENGDU SOUTHWEST JIAOTONG UNIV SCI & TECH GARDEN MANAGEMENT
Method for preparing small-diameter artificial blood vessels on basis of nanotechnologies
InactiveCN105079874AGood tissue compatibilityHigh porosityFilament/thread formingProsthesisFiberPorosity
The invention belongs to the field of medicine and high-polymer materials, and discloses a method for preparing small-diameter artificial blood vessels on the basis of nanotechnologies. The method includes steps of dissolving, by weight, 8% of fibroin and 5% of polycaprolactone in hexafluoroisopropanol to obtain spinning liquor; manufacturing the nano-fiber blood vessels with the wall thicknesses of 130-170 micrometers on cylindrical rod-shaped receiving screens with the diameters of 1-1.2mm by means of electrospinning by the aid of electrospinning technologies. Compared with the prior art, the method has the advantages that requirements of tissue engineering on high tissue compatibility, high porosity, plasticity and degradability can be met owing to the electrospinning technologies, vascular stents can be modified by cell activity factors (mechanical growth factors) and medicine (heparin) with anticoagulant activity, and accordingly shortcomings of technological complexity and poor tissue compatibility of existing cell-modified vascular stents can be overcome by the aid of the method.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Preparation method of hyperstable endovascular stent anticoagulant coatings
InactiveCN101156970AImprove anticoagulant performanceGood tissue compatibilityPharmaceutical containersMedical packagingCross-linkChemical reaction
The invention takes diazo-resin or multivalence metal ion solution as cross-linking agent. Through the electrostatic attraction LBL self-assembly technology, overstable endovascular stent graft anti-coagulation coating is prepared to lead the anti-coagulation coating to have good blood compatibility and tissue compatibility. The invention is realized in the following steps: a, the surface of an endovascular stent graft is cleaned, firstly, the surface of the endovascular stent graft is ultrasonically cleaned with multiple organic solvents for 1 to 120 minutes, and then the surface thereof is rinsed to be clean with water; b, oxidation treatment: H2O2 is boiled for 1 to 120 minutes, and then is rinsed with fresh water, KOH solution is etched for 1 to 120 minutes and then flushed to be clean with a large amount of fresh water; c, the preparation of the anti-coagulation coating: the diazo-resin with positive electricity and a polymer with anti-coagulation reactivity and / or the other polyanion solution are alternately deposited on the surface of the material through the electrostatic attraction function, ultraviolet light or visible light is adopted to irradiate surface coating of the material, through the photochemical reaction, ionic linkage between the inner layers of the coating is converted into covalent linkage, so as to attain the overstable anti-coagulation coating; or multivalence metal positive ion and the polymer or polyanion with anti-coagulation reactivity are alternately deposited on the surface of the stent graft material through the electrostatic attraction function, so as to attain the stable anti-coagulation coating.
Owner:HARBIN INST OF TECH
Gelatin-chitosan/montmorillonite drug carried microspheres and preparation method thereof
InactiveCN101703480AGood tissue compatibilityImprove performancePeptide/protein ingredientsInorganic non-active ingredientsMicrosphereTissue Compatibility
The invention discloses a gelatin-chitosan / montmorillonite drug carried microsphere and a preparation method thereof. The drug carried microsphere is prepared by the following steps: dissolving the hydrophilic drug in the gelatin water solution, adding montmorillonite suspended water solution, carrying out intercalation reaction to obtain solution A; dissolving the chitosan in the acetic acid water solution, stirring and dissolving to obtain solution B; stirring and emulsifying liquid paraffin wax and span-80 to obtain C; adding solution B into solution A, carrying out intercalation reaction, adding C, stirring, standing, dropwise adding glutaric dialdehyde water solution, crosslinking and curing, adding isopropyl alcohol and stirring, removing supernate after standing and delaminating, vacuum filtrating to remove liquid; and washing with anhydrous ether and isopropyl alcohol in sequence, and drying to obtain the microsphere. The drug carried microsphere in the invention has good tissue compatibility, low toxicity or no toxicity, and is a drug carried material with excellent performance. The method in the invention can reduce the dosage of chemical crosslinking agent, lower the toxity of the microsphere, dramatically lower the rate of drug release, and further improve slowly controlled release ability of the drugs.
Owner:TIANJIN UNIV
Compositions and uses of antimicrobial materials with tissue-compatible properties
Compositions comprising a mixture of an antimicrobial cationic polypeptide and a second pharmaceutically-acceptable polymer are disclosed, as well as methods and uses thereof for the treatment and prevention of infections that occur when our natural barriers of defense are broken.
Owner:AMICROBE
Acellular dermal matrix guided tissue regeneration membrane material as well as preparation method and application thereof
ActiveCN108355171AAvoid carryingAvoid risk of transmissionTissue regenerationProsthesisFiberTissue repair
The invention provides an acellular dermal matrix guided tissue regeneration membrane material as well as a preparation method and application thereof, and relates to the technical field of medical biological materials. The preparation method provided by the invention comprises the following steps: pretreating, removing an epidermal layer, preparing a loose surface, stabilizing, performing the accellular treatment, washing, and sterilizing. A membrane material prepared by the method provided by the invention adopts fish skin as a raw material and has a three-dimensional space framework structure, and comprises a compact surface formed by a biological substrate membrane and a collagen loose surface formed by a dermal scaffold. By adopting the membrane material provided by the invention, therisk of the existing tissue restoration material in carrying and propagating the human-animal viruses can be avoided, the cell components can be completely removed, the immunogenicity is low, the clearance of the collagen fibers is increased, a corium layer structure is reserved, the tissue compatibility is good, the mechanical shielding effect is good, and the generation of new bones can be facilitated; and when used for restoring and treating the bone deficiency of an oral implantation operation, the material is digestible, and the second operation and the injury of the membrane material topatients can be effectively avoided.
Owner:QINGDAO MARINE BIOPHARMACEUTICAL RES INST
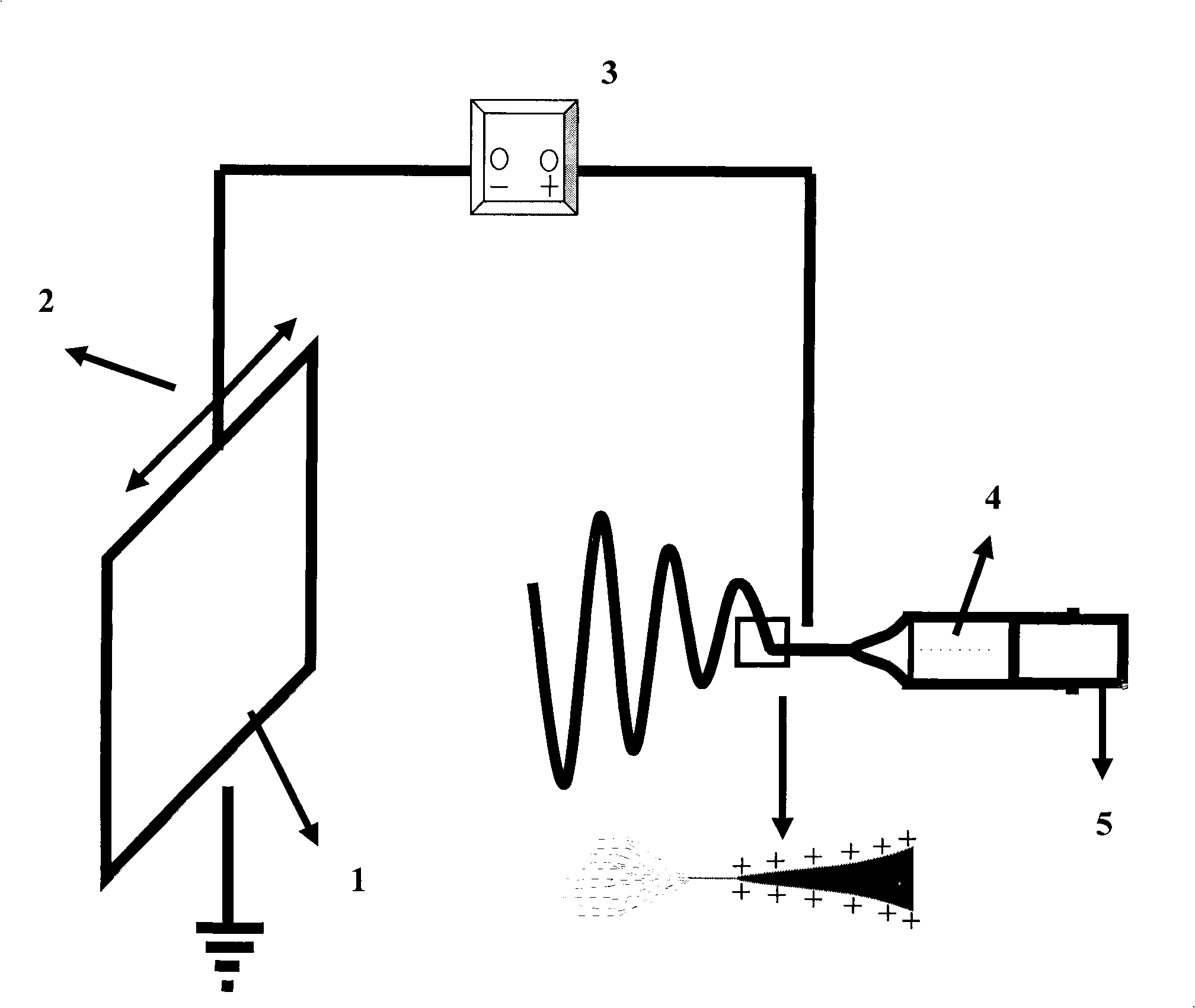
Coating system for implants for increasing tissue compatibility
InactiveUS20060165962A1Good biocompatibilityEasy to processDecorative surface effectsDuplicating/marking methodsIrritationCoating system
The invention relates to a coating system for implants comprising a metal base body, which is optionally covered with one or several intermediate layers. Said coating system comprises a coating which is disposed thereon in order to increase tissue compatibility. The coating prevents tissue irritations after implantation has an extremely high biocompatibility and has an anti-inflammatory effect. This is achieved by virtue of the fact that the coating comprises a polysaccharide layer made of a) chitosane and b) hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Chitosan biofilm polypropylene mesh and preparation method thereof
The invention relates to a chitosan biofilm polypropylene mesh and a preparation method thereof. According to the invention, a polypropylene mesh is processed by using waterless ethanol and deionized water; the polypropylene mesh is modified by using 1.0 to 5.0% of a chitosan film forming solution through a process of biofilm forming, such that a chitosan biofilm polypropylene mesh is obtained. The method of the invention is simple and operable. No toxic or harmful reagent is included. No adverse effect is brought to subsequent applications of the medical mesh. According to the invention, the polypropylene mesh has a high porosity, peripheral tissue can well contact and grow on the polypropylene mesh, such that the anti-stretching property of a repaired part is enhanced; also, chitosan has a good biocompatibility, such that complications such as polypropylene mesh erosion, exposure and tissue adhesion at an implantation position are effectively reduced. The chitosan biofilm polypropylene mesh is especially suitable for clinical women pelvic reconstructive surgery. With the chitosan biofilm polypropylene mesh, histocompatibility of the polypropylene materials in pelvic reconstructive operations is improved, and pelvic reconstruction postoperative complications are reduced.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Loaded drug sustained release composite tissue repairing material and preparation method thereof
The invention relates to a loaded drug sustained release composite tissue repairing material and a preparation method thereof. The repairing material comprises a material having a three dimensional structure, porosity, and permeability, a drug or a drug loading medium. The repairing material can sustained release loaded drugs. Moreover, the release curve is controllable. The tissue compatibility and host-material immune reaction type of the repairing material are not changed. The repairing material is biocompatible, nontoxic, and harmless. The biological functions and special functions of the repairing material are enhanced, and the clinical application prospect is good.
Owner:EXCELLENCE MEDICAL TECH SUZHOU CO LTD +2
Carboxymethyl chitin membrane for postoperative adhesion prevention and its preparation method
InactiveCN1569262AHigh mechanical strengthGood biocompatibilityPharmaceutical containersMedical packagingChitin formationTissue Compatibility
A post operation adhesion proof carboxymethyl chitin membrane characterizing in containing carboxymethyl chitin and cross linking agent or chitosan is provided. The weight ratio of carboxymethyl chitin with cross linking agent is 100:0.1í½0.5, while the weight ratio of carboxymethyl chitin with chitosan is 100:10í½1000. The preparing process: carboxy methylating chitin to carboxymethyl chitin, combining with cross linking agent or chitosan to prepare carboxy methylating chitin water soluble collagen solution or acid solution, preparing to carboxy methylating chitin dry membrane, flushing and drying. The adhesion proof membrane provided by the invention has favorable tissue compatibility, favorable adhesion with body wound surface, rapid degradation rate, in addition, the degradation product can be absorbed and utilized by body without adverse reaction.
Owner:OCEAN UNIV OF CHINA
A kind of silk support and its preparation and application
ActiveCN102293688ASmooth structureImprove mechanical propertiesCoatingsProsthesisPolymer scienceTissue Compatibility
The invention discloses a silk stent a preparation method and application to repairing and constructing of organism tissues. The silk stent is mainly composed of a base layer membrane and a water-soluble natural polymer material layer membrane, wherein the base layer membrane is a netting structure woven from silk, and the size of meshes is between 0.25mm<2> and 25mm<2>; and the water-soluble natural polymer material is silk fibroin, hyaluronic acid, collagen or chitosan. The invention has the advantages that: (1) the silk stent has a flat structure, excellent biological and mechanical performances and excellent tissue compatibility; (2) the meshes of the silk stent are uniform in size, and can be regulated; (3) the materials are easily available, and the preparation method is simple and convenient; (4) the natural polymer material adopted in the silk stent has good biological compatibility, and the operation is easy; and (5) the silk stent is suitable for repairing or reinforcing tissues inside an organism, and constructing tissues outside the organism.
Owner:ZHEJIANG XINGYUE BIOTECH
Composite extracellular matrix ingredient biological material
ActiveCN105920669ALight adhesionImprove adhesionTissue regenerationProsthesisCell-Extracellular MatrixAdditive ingredient
Owner:SHANGHAI EXCELLENCE MEDICAL TECH CO LTD
Nano silver-porcine acellular dermal matrix (PADM) biological dressing and preparation method thereof
InactiveCN102018991ALow antigenicityReduce the number of dressing changesAbsorbent padsBandagesBiological dressingTissue Compatibility
The invention discloses a nano silver-porcine acellular dermal matrix (PADM) biological dressing and a preparation method thereof. The biological dressing comprises PADM and nano-silver particles assembled on the PADM, wherein, the content of the nano-silver particles is 435.66-447.66 mu g / g, and the particle size of the nano-silver particles is 55-75nm. The preparation method of the biological dressing comprises the following steps: (1) reducing silver nitrate with trisodium citrate to prepare nano silver solution; (2) carrying out gradient dehydration with alcohol and ultrasonic treatment on the PADM; and (3) placing the treated PADM obtained from the step (2) in the nano silver solution for self-assembly, thus finally obtaining the nano silver-PADM biological dressing. The biological dressing obtained in the invention has the advantages of strong antibacterial property, high biological safety and good tissue compatibility; and simultaneously, the process is simple, and the use is convenient.
Owner:NANTONG UNIVERSITY +1
Ocular surface biomembrane and preparation method thereof
The invention provides a preparation method of an ocular surface biofilm, which uses animal pericardium, intestinal membrane and adipose omentum as raw materials through degreasing, decellularization, multi-directional antigen removal, epoxy fixation, curved surface molding, surface Modification, cobalt-60γ-ray irradiation sterilization and other process steps. The ocular surface biofilm obtained by the method of the present invention has a curved surface similar to that of the eyeball, can be close to the eyeball, has no antigenicity, good histocompatibility, can participate in the healing process of ocular surface wounds, and is beneficial to wound healing; Good air permeability and bacterial filtration can play a good role in the protection and treatment of the ocular surface; it has good strength, can be pasted and sewed, and is easy to use. In addition, through the surface modification of drug sustained release, it can also be made into a therapeutic ocular surface biofilm that can release anti-infective drugs slowly.
Owner:SUMMIT GD BIOTECH
Method for preparing biodegradable filling materials, product prepared by aid of method and application of product
ActiveCN106492284AEasy to fillEasy to operatePowder deliveryAerosol deliveryGel preparationMass ratio
The invention relates to a method for preparing biodegradable filling materials, a product prepared by the aid of the method and application of the product. The method mainly includes steps of (1), mixing matrix materials and dispersion-phase materials according to a mass ratio of 10:0-6:4 with one another and dissolving the matrix materials and the dispersion-phase materials in organic solvents to obtain high-polymer organic solvents; (2), treating the high-polymer organic solvents by the aid of a mechanical process or an emulsification process to obtain a micro-sphere. The matrix materials comprise polydioxanone; the dispersion-phase materials comprise a type of or a plurality of types of poly-L-lactic acid, polycaprolactone, polylactic acid and polyglycolic acid. The method, the product and the application have the advantages that the method is easy to implement, low in cost and good in repeatability, the product comprises tiny and stable particles, and the micro-sphere prepared by the aid of the method has similar and controllable particle sizes, is good in filling performance and tissue compatibility and is safe and degradable; the product can be degraded slowly, the degradation time can be regulated and controlled according to selection of the types and the adding proportions of the dispersion-phase materials; the micro-sphere can be used for preparing freeze-dried powder preparations and gel preparations, and the product is convenient to transport, store and clinically use.
Owner:重庆瑞凡德生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com