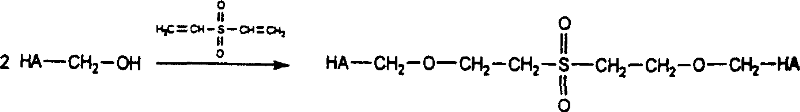Cross-linked hyaluronic acid derivatives preparation and the preparing technique
A technology of cross-linking hyaluronic acid and hyaluronic acid, which is applied in medical science, surgery, etc., can solve the problem of short residence time and achieve the effect of increasing viscoelasticity and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
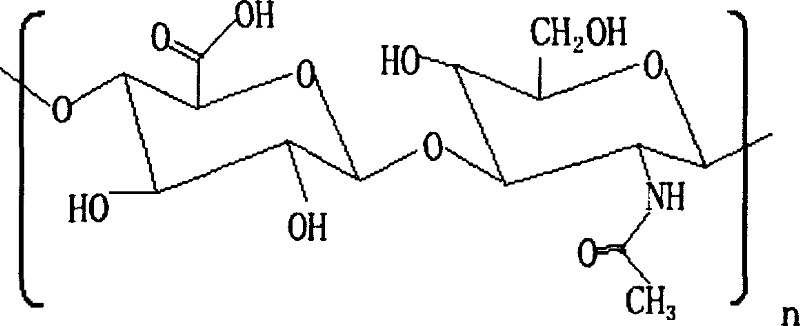
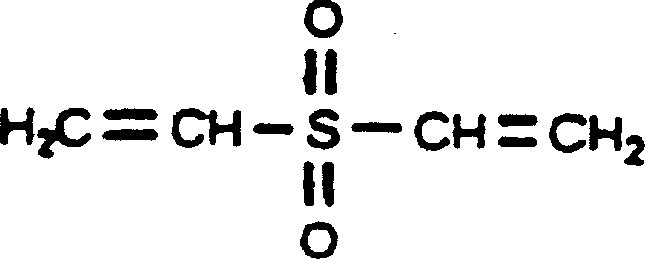
[0026] Dissolve 3 g of hyaluronic acid dry powder (molecular weight: 1,500,000 Daltons) in 200 ml of 1M NaOH solution, stir to dissolve completely. Add 2 g of divinyl sulfone and stir well. Place in a thermostat at 25°C for 24 hours to form cross-linked hyaluronic acid. Glucose solution was added to the cross-linked hyaluronic acid, and the reaction concentration of the glucose solution was 4%, and placed in a 25° C. incubator for 24 hours. The pH value of the cross-linked hyaluronic acid gel was adjusted to neutral with HCl solution. Take out the cross-linked hyaluronic acid gel block and put it into an electric mixer, stir, and the cross-linked hyaluronic acid block gel becomes a particulate gel. Sterilize by autoclaving at 121°C for 30 minutes.
Embodiment 2
[0028] Dissolve 5g of hyaluronic acid dry powder (molecular weight: 1,500,000 Daltons) in 200ml of 1M KOH solution, stir to dissolve completely. Add 5 g of divinyl sulfone and stir well. Place in a thermostat at 25°C for 24-48 hours to form cross-linked hyaluronic acid. Glucose solution was added to the cross-linked hyaluronic acid curd, and the reaction concentration of the glucose solution was 8%, and placed in a 25°C thermostat for 36 hours. The pH value of the cross-linked hyaluronic acid gel was adjusted to neutral with HCl solution. Take out the cross-linked hyaluronic acid gel block and put it into an electric mixer, stir, and the cross-linked hyaluronic acid block gel becomes a particulate gel. Sterilize by autoclaving at 121°C for 15 minutes.
Embodiment 3
[0030] Dissolve 4.5g of hyaluronic acid dry powder (molecular weight: 1,500,000 Daltons) in 200ml of 2M Na 2 CO 3 solution, stir to dissolve completely. Add 3.5 g of divinyl sulfone and stir well. Place in a thermostat at 25°C for 36 hours to form cross-linked hyaluronic acid. Glucose solution was added to the cross-linked hyaluronic acid curd, and the reaction concentration of the glucose solution was 6%, and placed in a 25° C. incubator for 32 hours. The pH value of the cross-linked hyaluronic acid gel was adjusted to neutral with HCl solution. Take out the cross-linked hyaluronic acid gel block and put it into an electric mixer, stir, and the cross-linked hyaluronic acid block gel becomes a particulate gel. Sterilize by autoclaving at 121°C for 20 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com