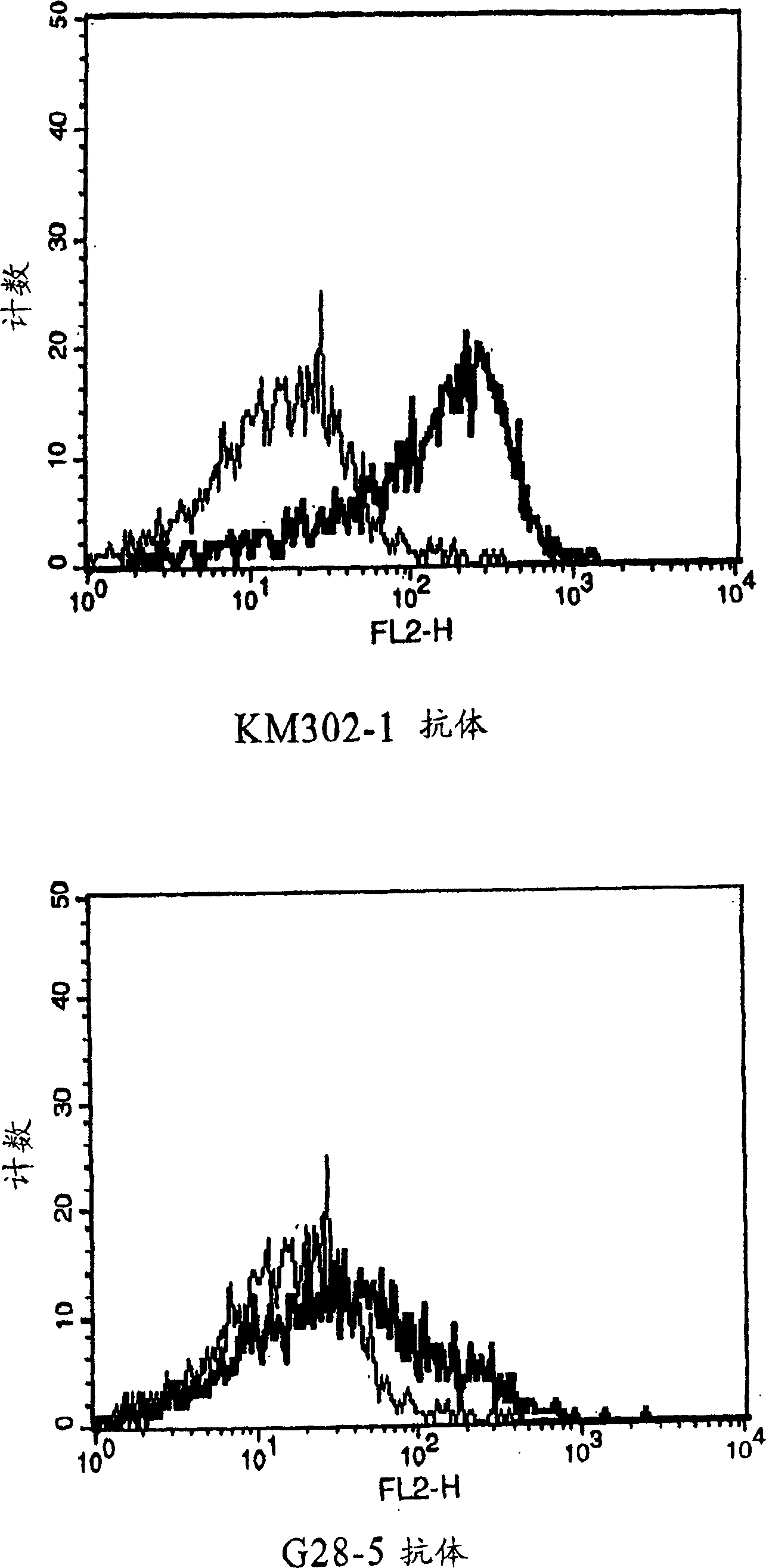
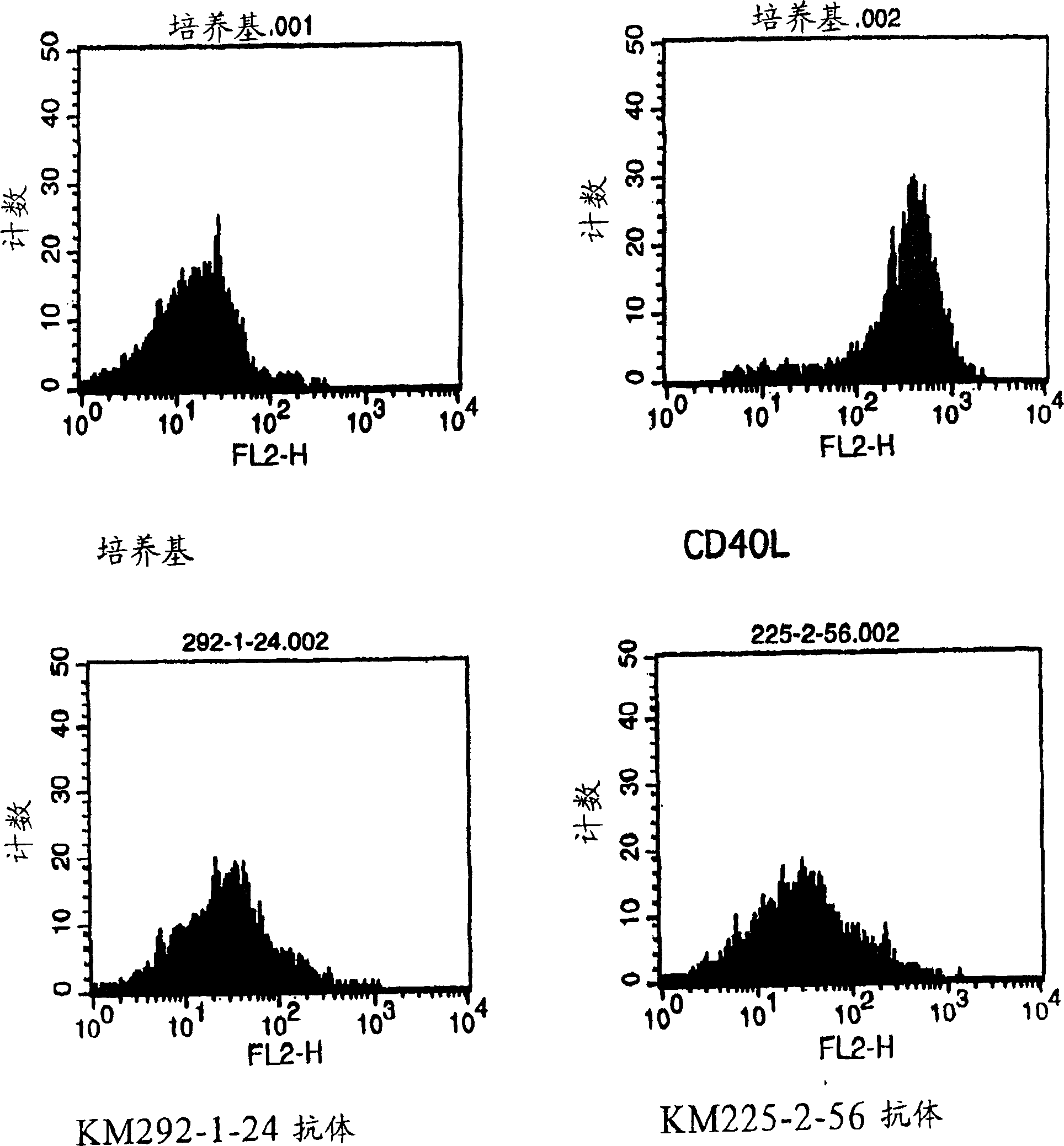
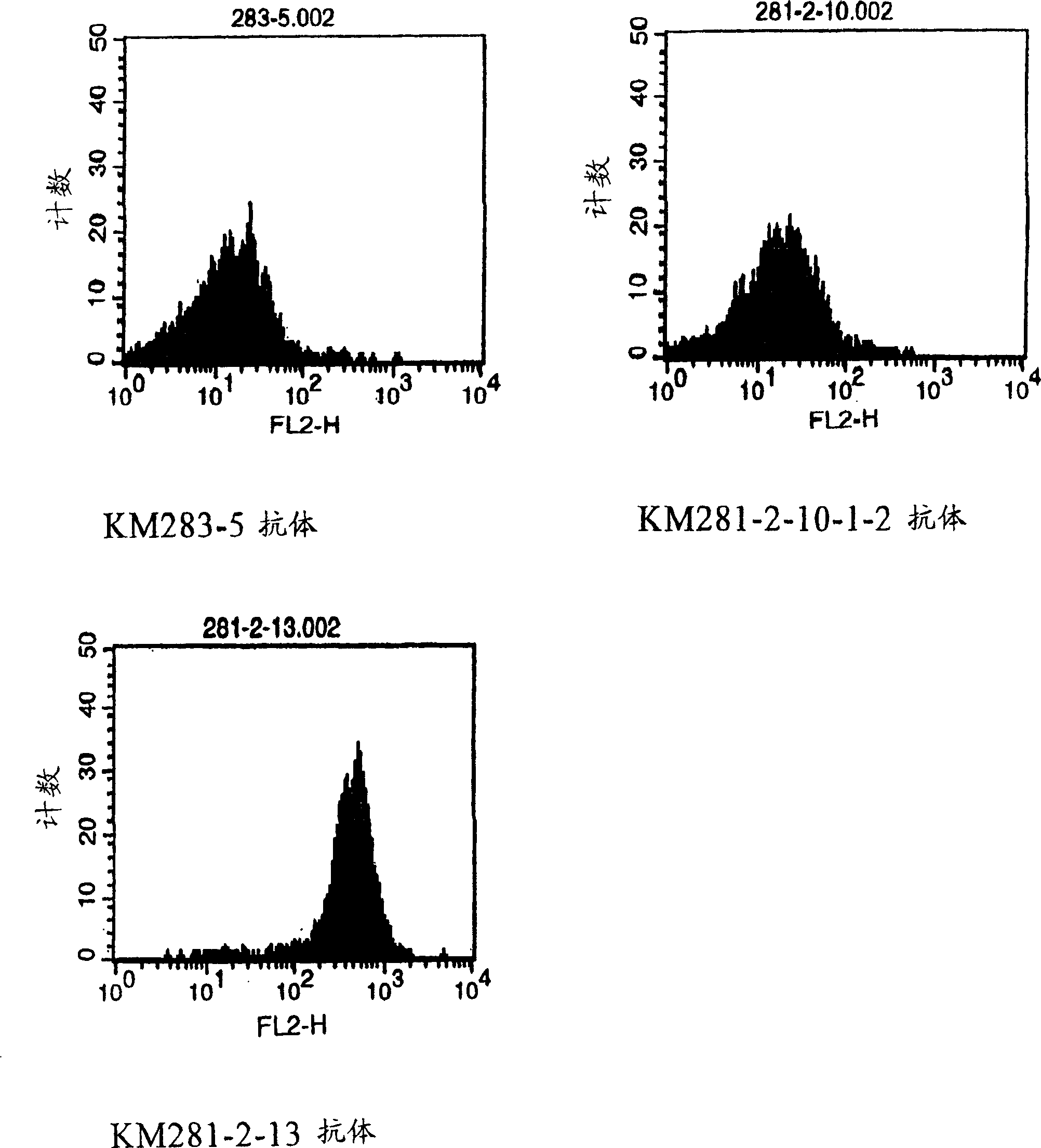
Anti-CD40 monoclonal antibody
An antibody, CD95 technology, applied in the direction of antibodies, antibody mimics/stents, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] The preparation of embodiment 1 antigen
[0120] (1) cell
[0121] EL-4 cells are an established T cell line derived from mice and are readily available (ATCC: TIB-39). Ramos B cells (ATCC No.: CRL-1596) and mouse anti-CD40 antibody-producing hybridomas G28-5 (HB-9110) and 5D12 (HB-11339) were purchased from ATCC.
[0122] (2) Expression and purification of antigen
[0123] Using human CD40 cDNA (Genbank accession number: NM_001250) as a template, and the following primers, the extracellular region was amplified by PCR under the conditions of 20 cycles of 95°C for 5 seconds, 55°C for 30 seconds and 72°C for 30 seconds.
[0124] Primer 1: 5'-CCCAGATCTG TCCATCCAGA ACCACCCACTGCATGCAGAG-3' (SEQ ID NO: 1)
[0125] Primer 2: 5'-ACAAGATCTG GGCTCTACGT ATCTCAGCCGATCCTGGGGA C-3' (SEQ ID NO: 2)
[0126]The amplified cDNA was inserted before the melittin signal sequence and before the human IgGl-derived FC or mouse-derived FC region of the pFastBac vector (Gibco BRL). For prod...
Embodiment 2
[0127] Embodiment 2 is used for the generation of the mouse of immunization
[0128] Mice used for immunization have a genetic background that is homozygous for endogenous Ig heavy chains and kappa light chains, while mice contain a chromosome 14 fragment (SC20) containing the human Ig heavy chain locus, and human Ig kappa Strand transgene (Kco5). These mice were produced by crossing A-line mice with human Ig heavy chain genes and B-line mice with human Ig kappa chain transgenes. Line A mice are homozygous for disrupted endogenous Ig heavy chains and kappa light chains, contain a fragment of chromosome 14 (SC20), and can be passed on to offspring, as described above in the report by Tomizuku et al. (Tomizuka. et al., Proc Natl Acad Sci USA., 2000 Vol. 97: 722). Mice of line A were immunized to obtain the following hybridomas F2-103 and F5-77. In addition, mice of line B (transgenic mice) are homozygous for cleaved endogenous Ig heavy and kappa light chains and contain a hum...
Embodiment 3
[0130] Example 3 Preparation of human monoclonal antibody against human CD40
[0131] The monoclonal antibody in this example was prepared according to the general method described in the introduction of the experimental method of monoclonal antibody (Tamie ANDO et al., KODANSHA, 1991). The human CD40 used as the immunogen here was the human CD40 human FC prepared in Example 1 and the CD40-expressing EL-4-cells. Animals used for immunization herein were human antibody-producing mice producing the human immunoglobulin prepared in Example 2.
[0132] Immunize human antibody producing mice with 2 to 100 μg / CD40:hFc per mouse. Except for the first immunization, the antigen solution was mixed with an equal volume of Freund's incomplete adjuvant (Sigma) and injected subcutaneously into several separate sites. Immunizations are performed about 3 to 4 times every 10 days to 3 weeks. For the first immunization, Freund's incomplete adjuvant (Sigma) was used. Blood was collected from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com