Itraconazole liposome and preparation process thereof
A technology of itraconazole lipid and itraconazole, applied in the field of liposome and its preparation, can solve the problems of limitation, drug accumulation, strong side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
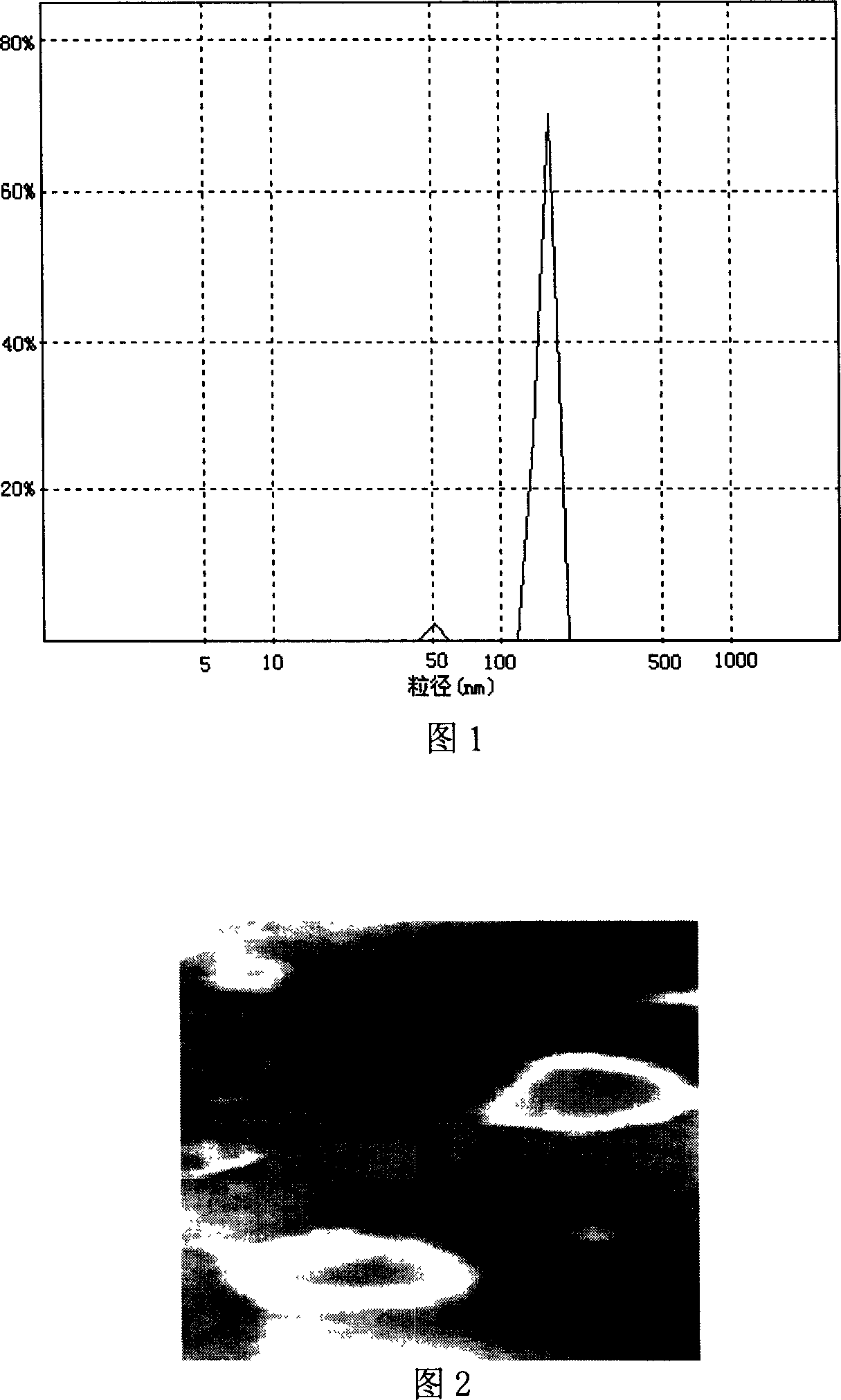
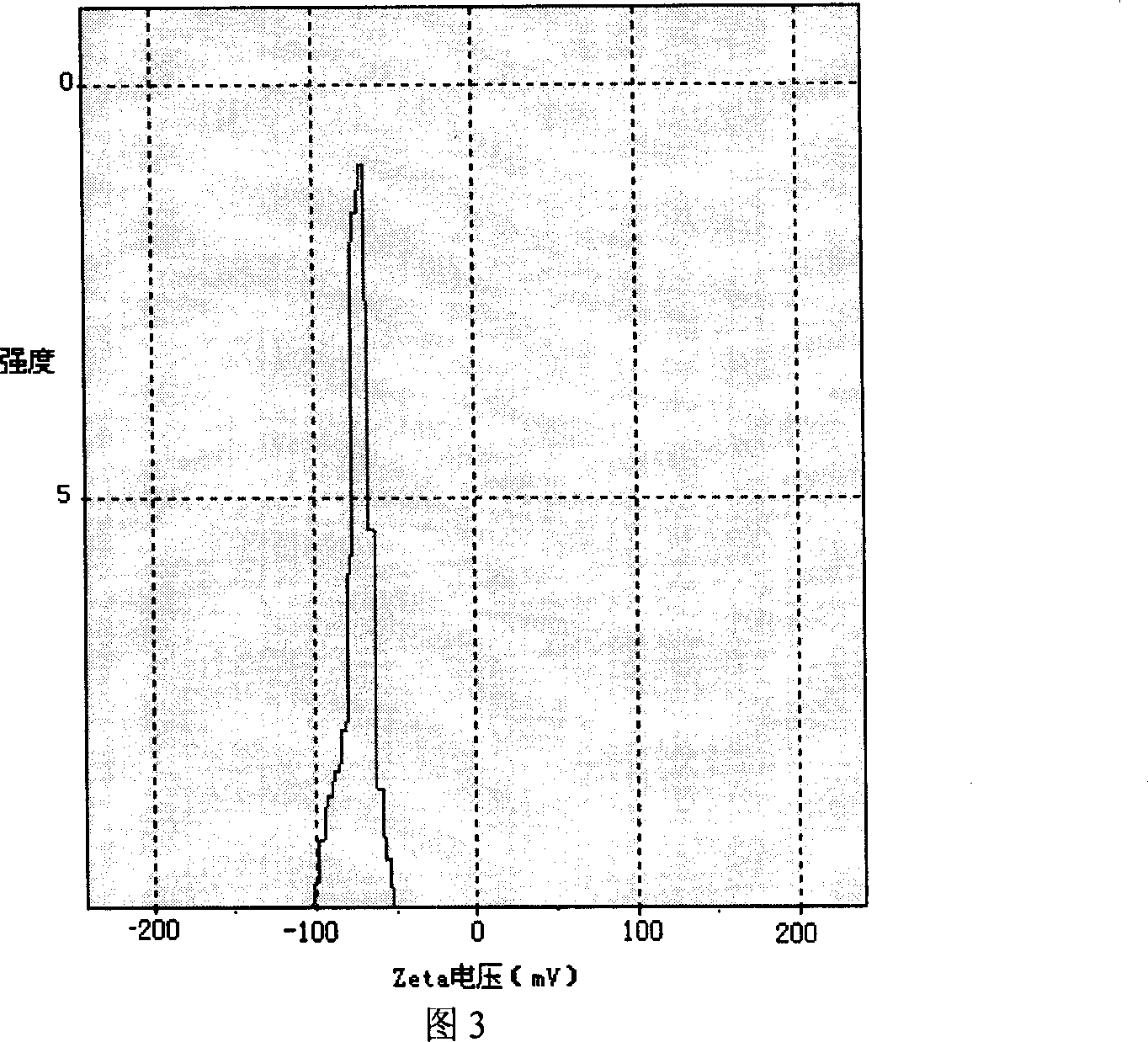
Image
Examples
specific Embodiment approach 1
[0008] Embodiment 1: The itraconazole liposome in this embodiment is composed of 18-22 mg itraconazole, 505-509 mg lecithin, 90-95 mg cholesterol, 73-77 mg sodium deoxycholate, 505-509 mg in the following proportions Mannitol, 505~509mg lactose and 18~22mL phosphate buffer with pH value of 5.5~6.5 are prepared.
[0009] In this embodiment, the drug prepared by itraconazole liposome can be used between meals or after meals, which can increase the absorption rate of the drug, but cannot be combined with terfenadine, astemizole or cisapride.
specific Embodiment approach 2
[0010] Embodiment 2: The difference between this embodiment and Embodiment 1 is: the itraconazole liposome is composed of 20 mg itraconazole, 507.7 mg lecithin, 92.3 mg cholesterol, 75 mg sodium deoxycholate in the following proportions , 507.7 mg of mannitol, 507.7 mg of lactose and 20 mL of phosphate buffer with a pH value of 5.5 to 6.5.
specific Embodiment approach 3
[0011] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the pH value of the phosphate buffer is 6.0. Others are the same as the first or second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com