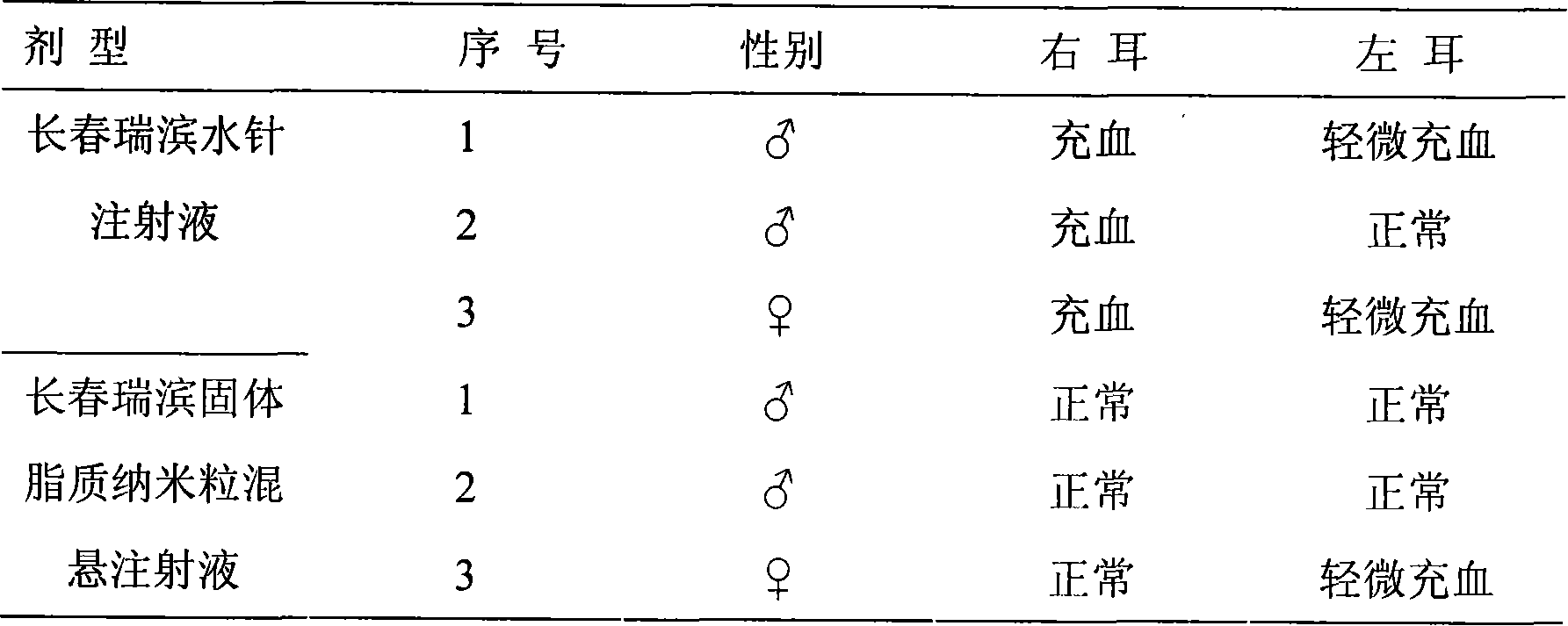
Vinorelbine solid lipid nano granule, freeze drying formulated product and method of preparing the same
A solid lipid nanometer and vinorelbine technology, applied in the field of medicine, can solve the problems of strong blood vessel irritation, high operation temperature, and poor patient compliance, and achieve the goal of avoiding drug degradation, instability, and direct contact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
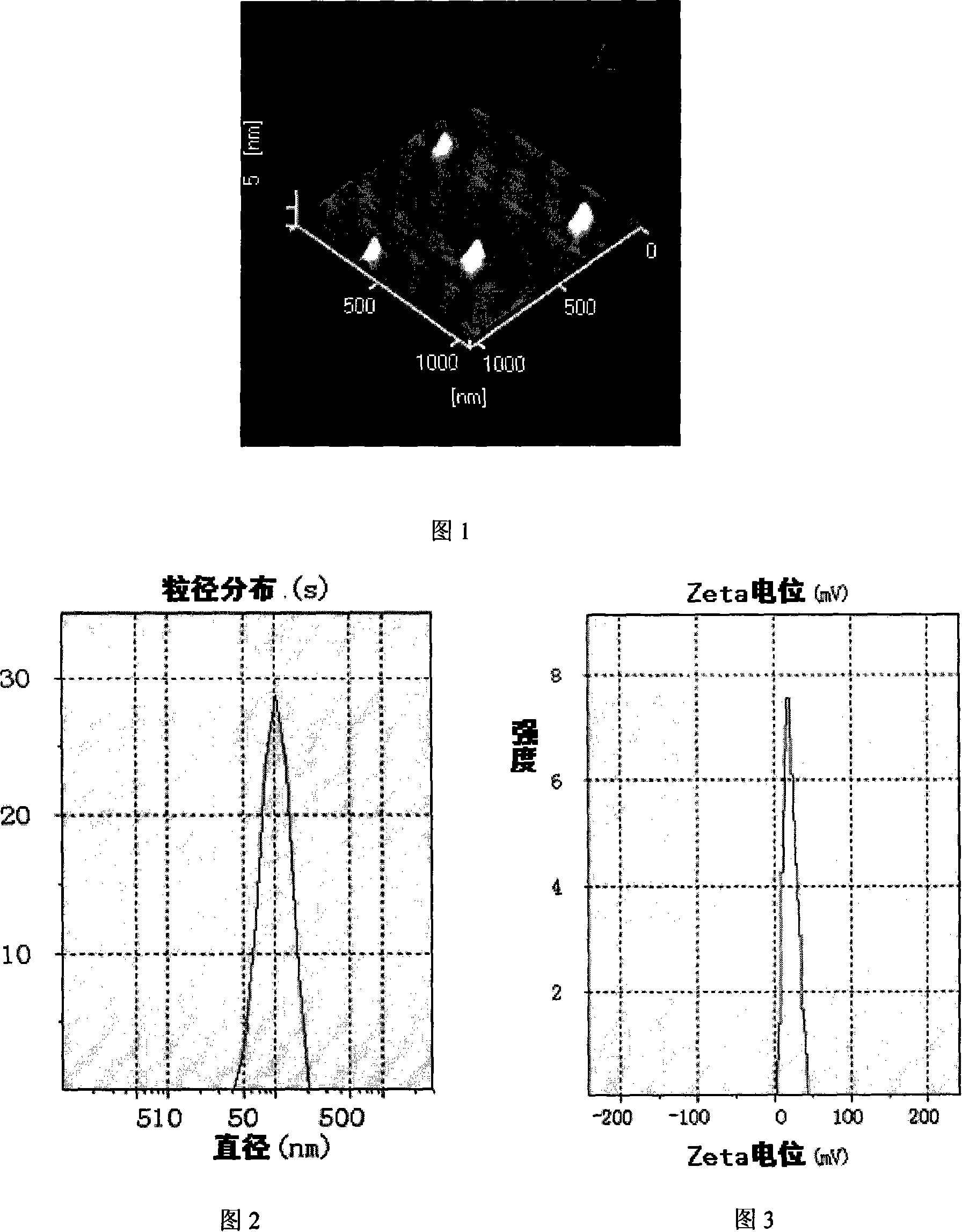
[0064] The vinorelbine solid lipid nanoparticles provided by the present invention are composed of vinorelbine, lipid materials, phospholipids, and oleic acid: vinorelbine 14 mg
[0065] Glyceryl monostearate 200mg
[0066] Lecithin 90mg
[0067] Oleic acid 14mg
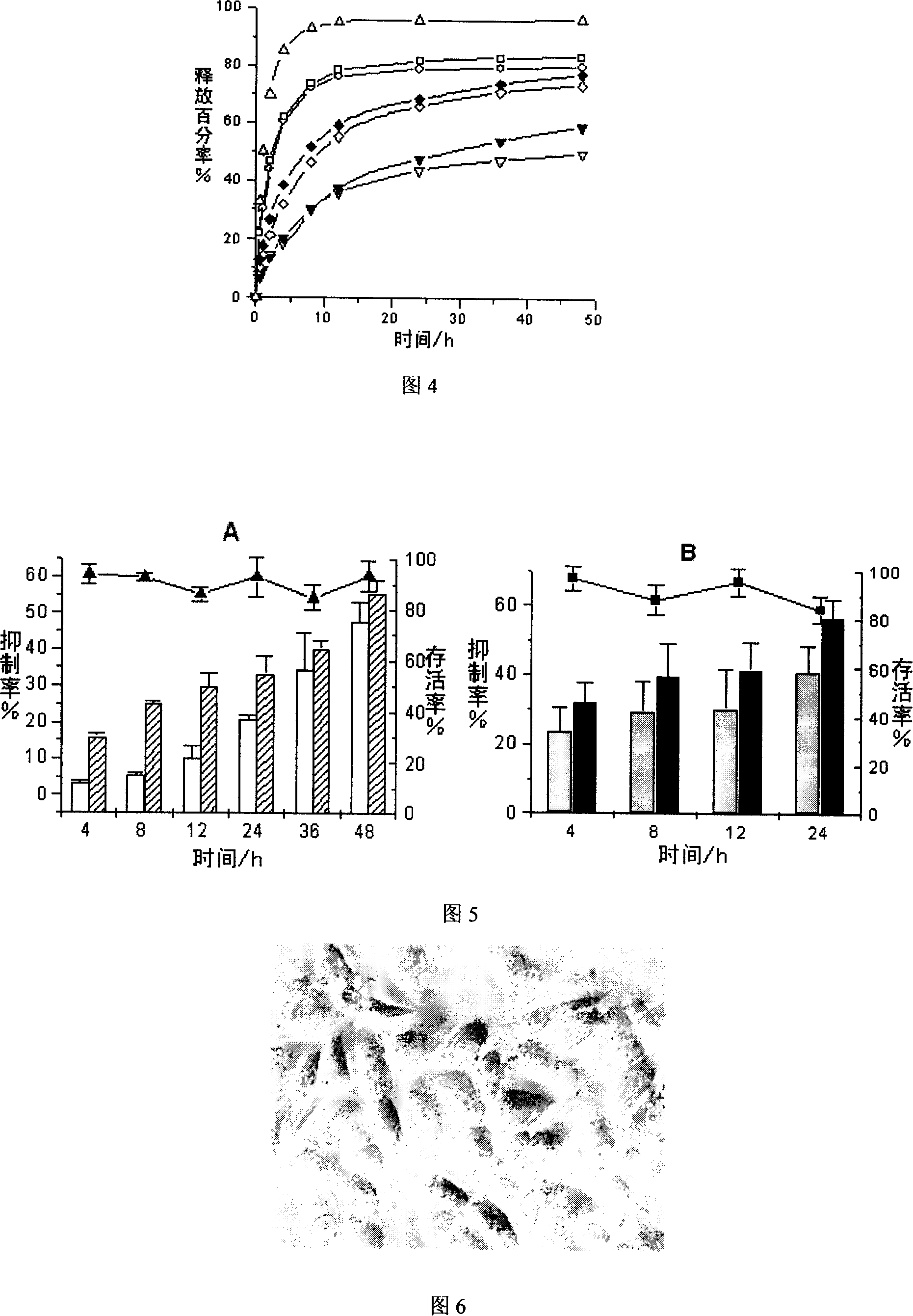
[0068] Preparation method: (1) Melt the prescription amount of lipid material glycerol monostearate under 60℃ water bath conditions; (2) Dissolve the prescription amount of phospholipid, oleic acid, and vinorelbine in ethanol, and then add dropwise Into the molten lipid material; (3) After the ethanol is completely removed under vacuum and reduced pressure, it is quickly placed in liquid nitrogen to cool; (4) When it is completely frozen, it is taken out, pulverized with a mortar, and dispersed in an appropriate amount of containing 1% F 68 In the aqueous phase, the pH of the aqueous phase is 4; (5) The tissue masher is mashed, 3000rpm, 6000rpm, 15min each to prepare the primary suspension; (6) The prepared primar...
Embodiment 2
[0071] The vinorelbine solid lipid nanoparticles provided by the present invention are composed of vinorelbine, lipid materials, phospholipids, and oleic acid: vinorelbine 42 mg
[0072] Glyceryl monostearate 200mg
[0073] Lecithin 90mg
[0074] Oleic acid 14mg
[0075] Preparation method: (1) Melt the prescription amount of lipid material glycerol monostearate under 60℃ water bath conditions; (2) Dissolve the prescription amount of phospholipid, oleic acid, and vinorelbine in ethanol, and then add dropwise Into the molten lipid material; (3) After the ethanol is completely removed under vacuum and reduced pressure, it is quickly placed in liquid nitrogen to cool; (4) When it is completely frozen, it is taken out, pulverized with a mortar, and dispersed in an appropriate amount of 1% F 68 In the aqueous phase, the pH of the aqueous phase is 3.7; (5) The tissue masher is mashed, 3000rpm, 6000rpm, 15min each to prepare the primary suspension; (6) The prepared primary suspe...
Embodiment 3
[0078] The vinorelbine solid lipid nanoparticles provided by the present invention are composed of vinorelbine, lipid materials, phospholipids, and oleic acid: Vinorelbine 100 mg
[0079] Glyceryl monostearate 150mg
[0080] Lecithin 140mg
[0081] Oleic acid 10mg
[0082] Preparation method: (1) Put the prescription amount of lipid material glyceryl monostearate at 60. Melt in a water bath at ℃; (2) Dissolve the prescribed amount of phospholipids, oleic acid, and vinorelbine in ethanol, and then add them dropwise to the molten lipid material; (3) After the ethanol is completely removed under vacuum and reduced pressure , Quickly place it in liquid nitrogen to cool; (4) Take it out after it is completely frozen, grind it with a mortar, and disperse it in an appropriate amount of 1% F 68 In the aqueous phase, the pH of the aqueous phase is 4; (5) The tissue masher is mashed, 3000rpm, 6000rpm, 15min each to prepare the primary suspension; (6) The prepared primary suspension...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
| Average potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com