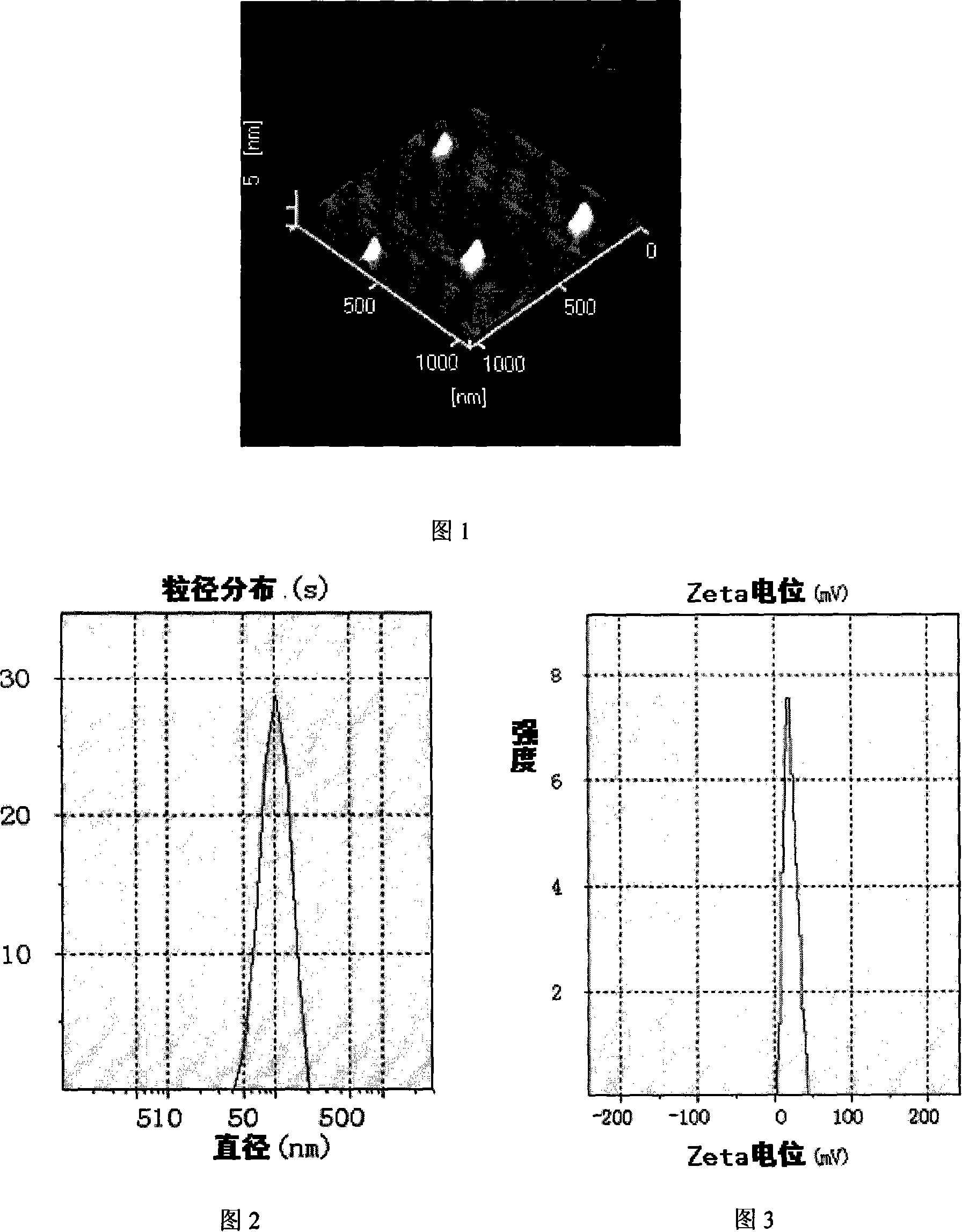
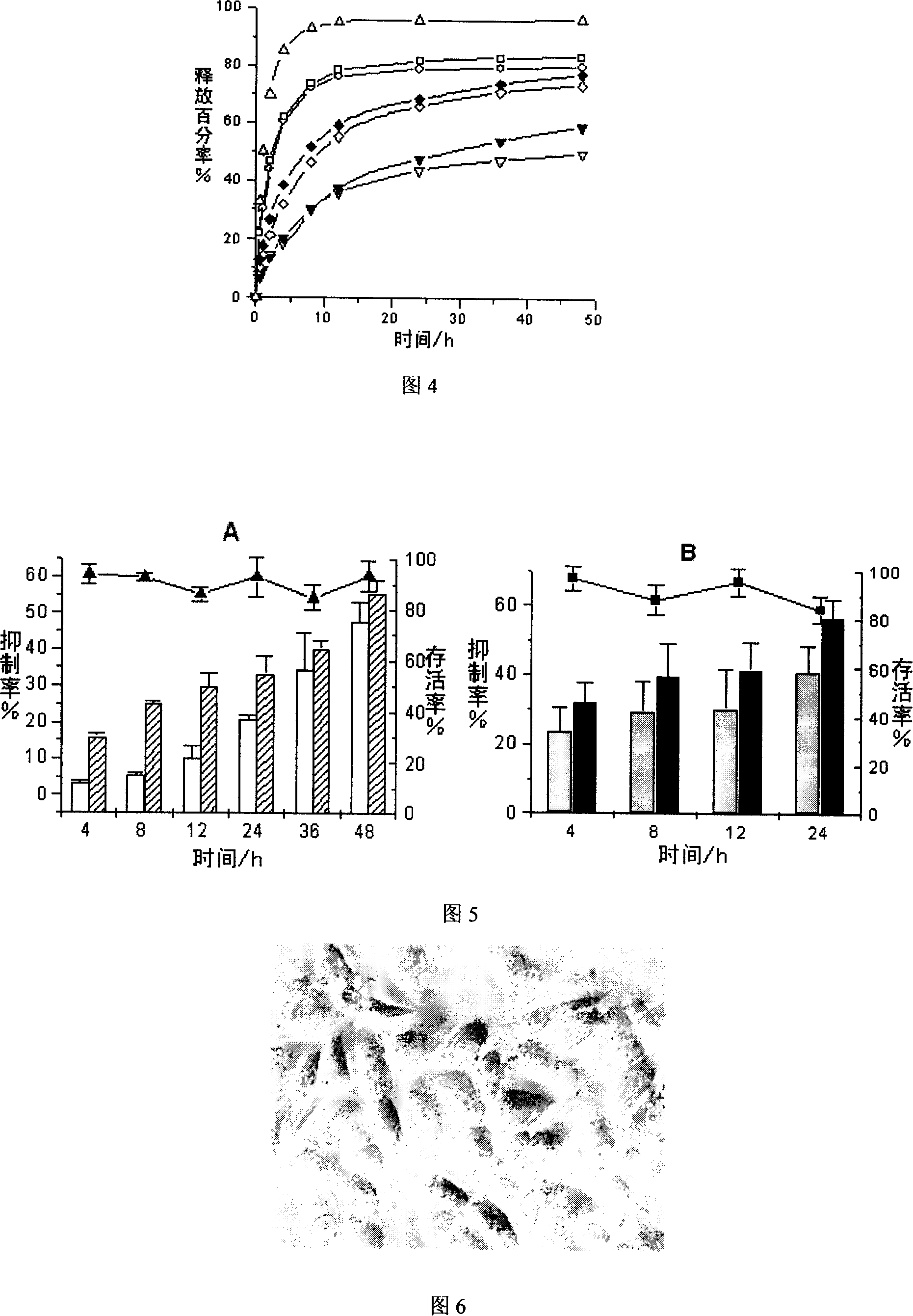
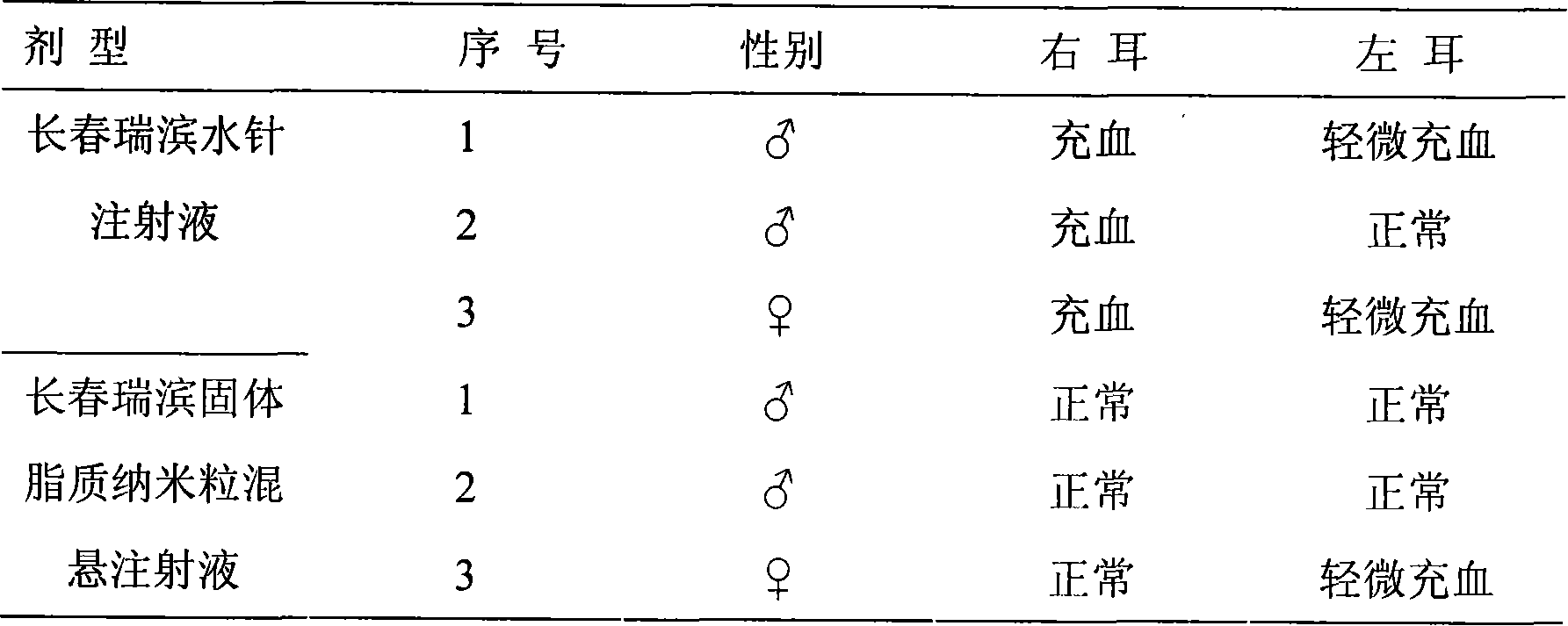
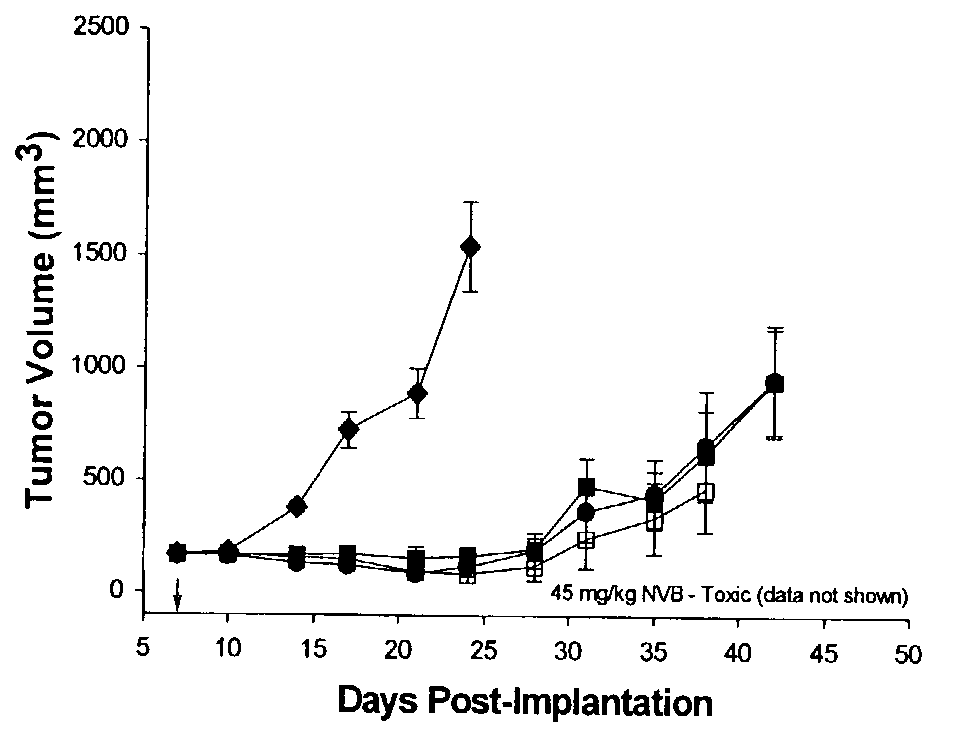
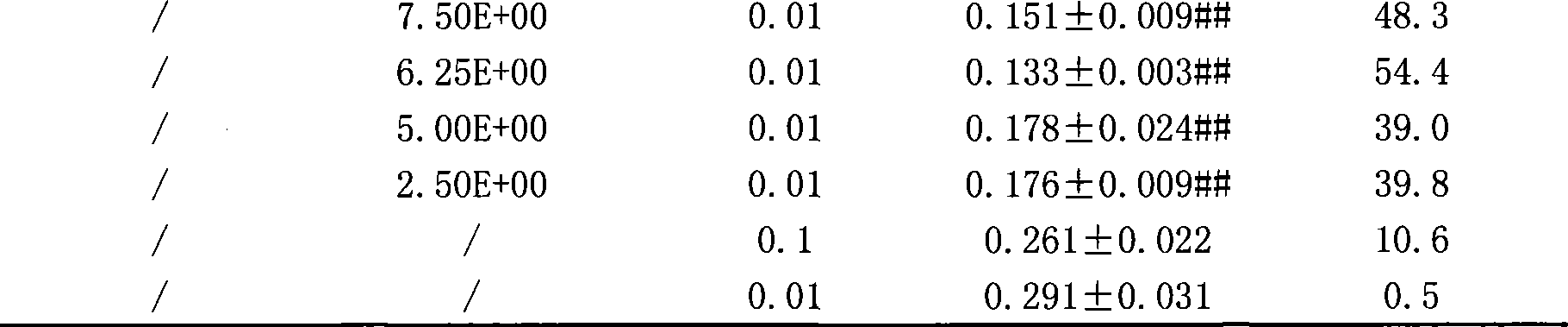Patents
Literature
95 results about "Vinorelbine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vinorelbine is used to treat various types of cancer.
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
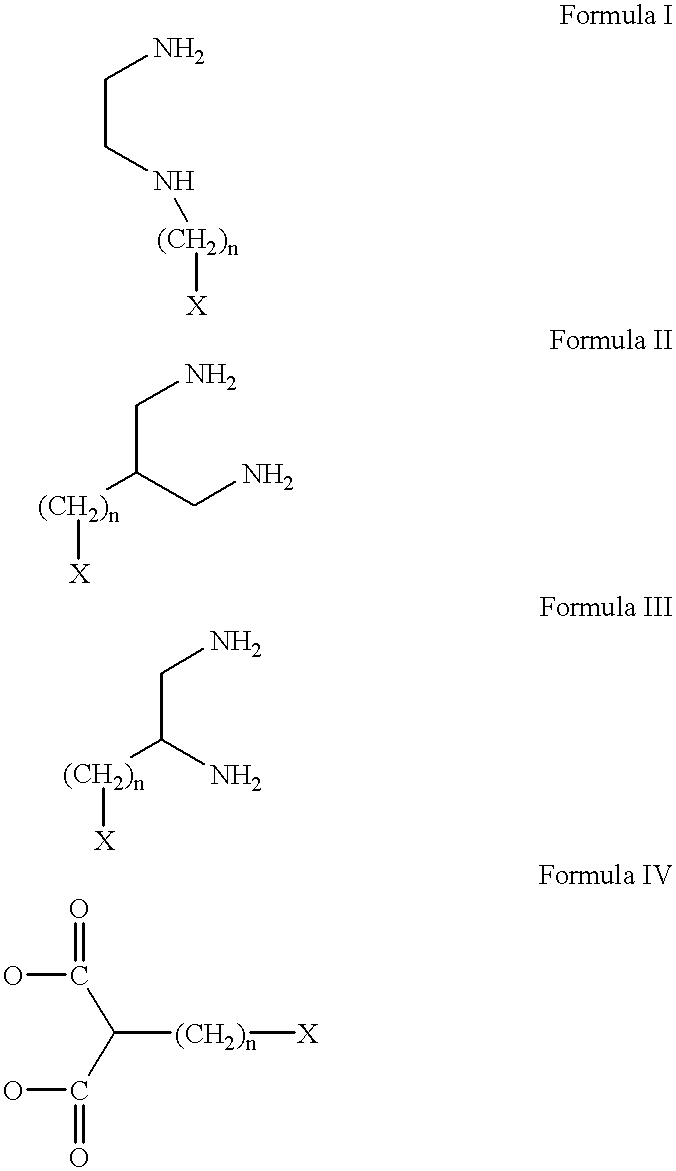
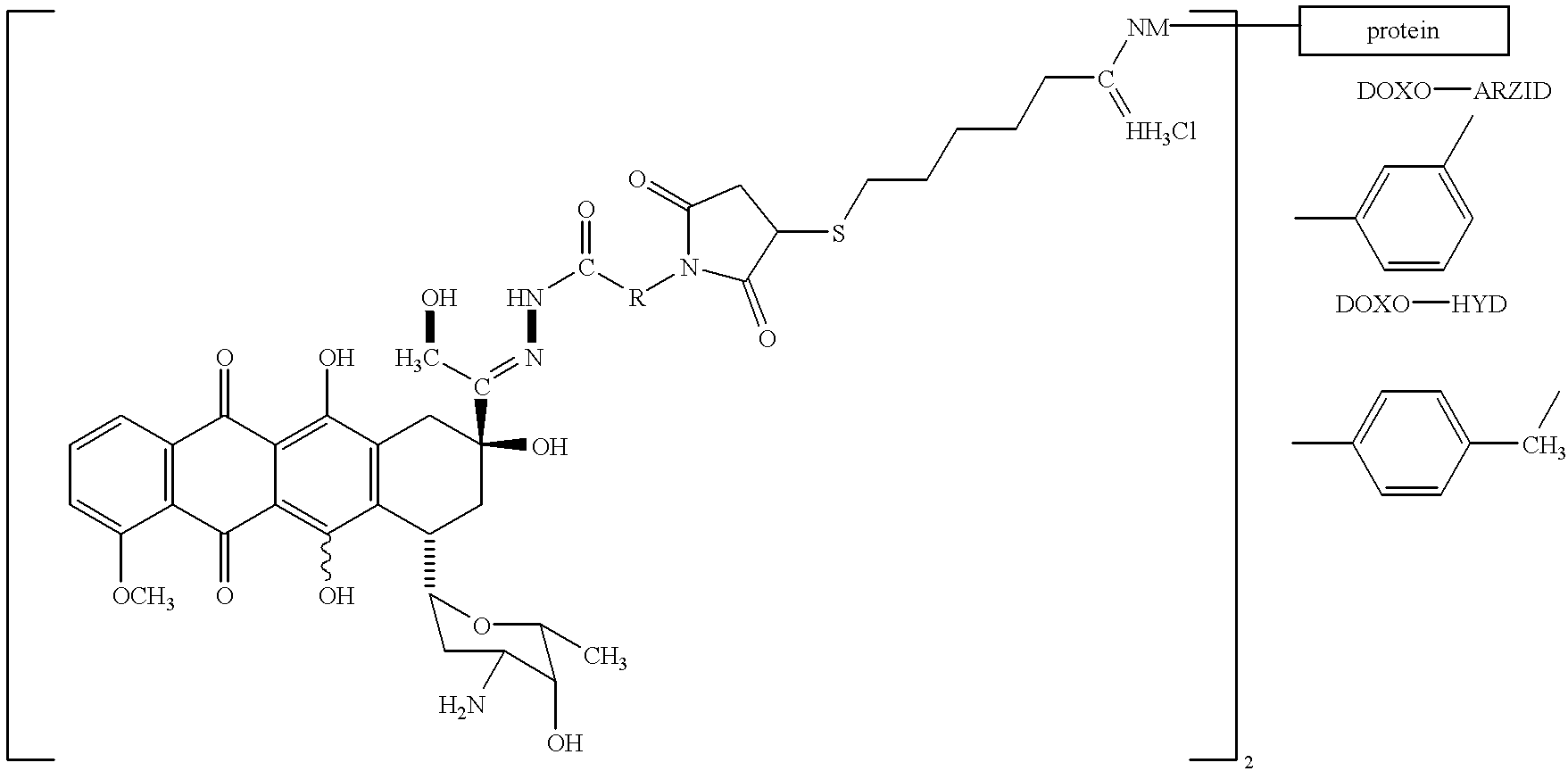
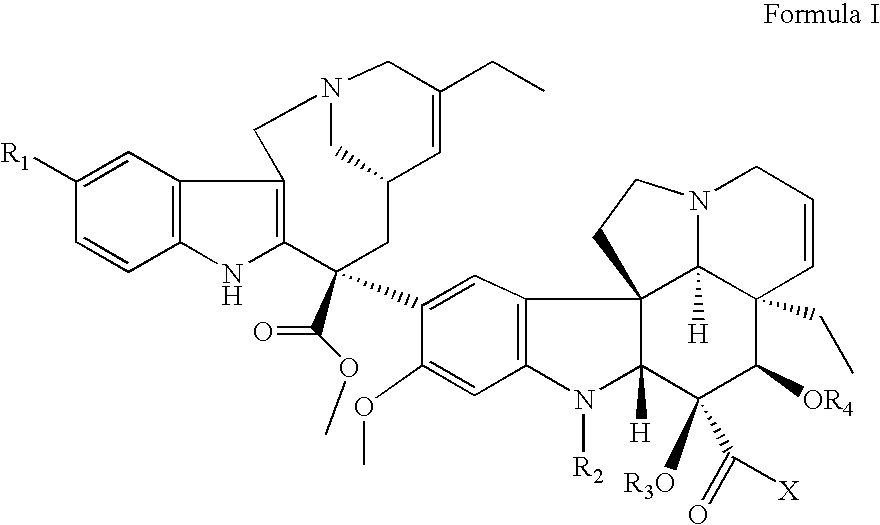
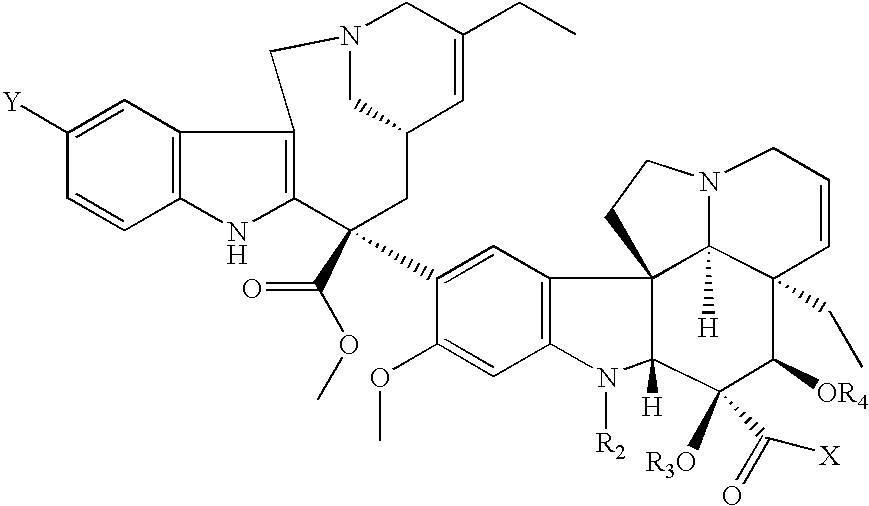
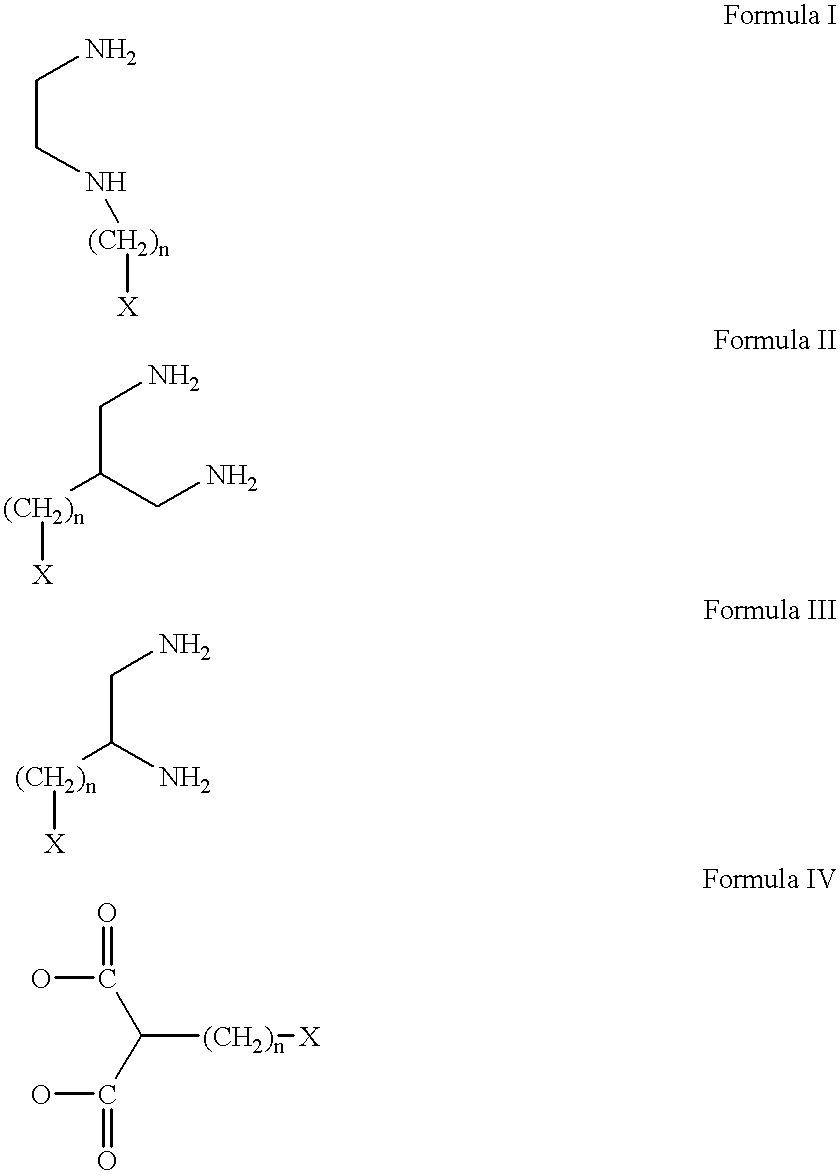
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Method of preparing and using a cold extract from the leaves of nerium oleander
A method of preparing and using a sterile non-toxic pyrogen-free cold extract from the leaves of Nerium oleander as a supplementary medication to cancer chemo-, hormon and / or radiotherapy to restore and / or ameliorate the immune system of the patient and / or to decrease side effects and increase the antitumor effects of radiotherapy and chemotherapeutics, particularly when used in combination with taxol, adriamycin, cisplatin, 5-fluoro-uracil, alimta, cyclophosphamide, mitomycin-C, navelbine, taxotere and topotecan, respectively, and its use in the manufacture of a medicament for the treatment of one or more cancers of bladder, kidney, liver, ovary, pancreas, testicle, uterus, and vagina as well as pleuramesotheliomas and Hodgkin's lymphomas.
Owner:RASHAN JUAY JAMIL
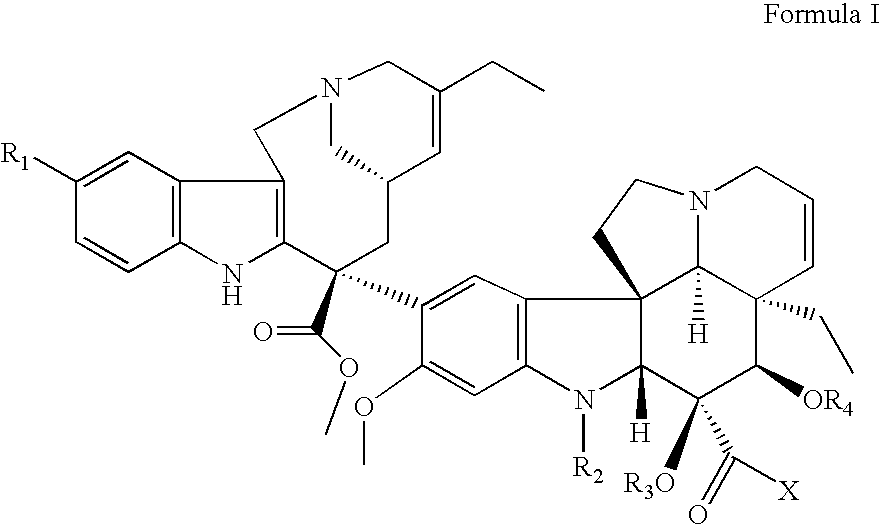
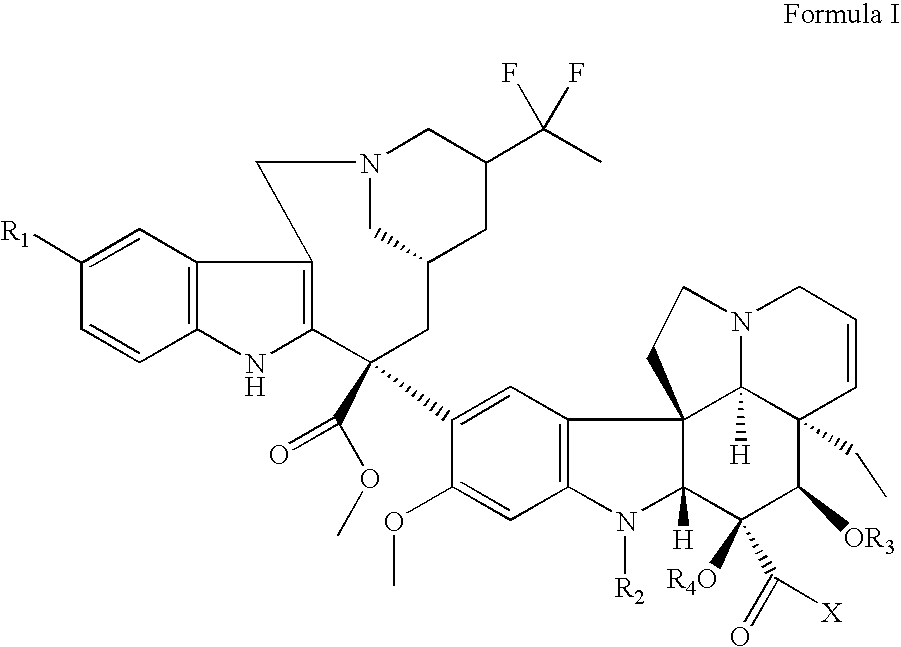
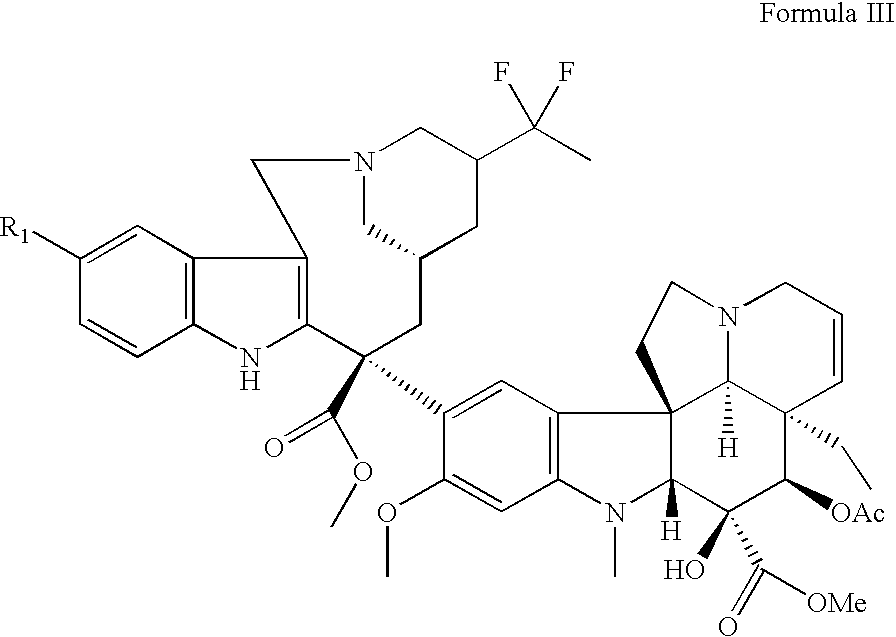
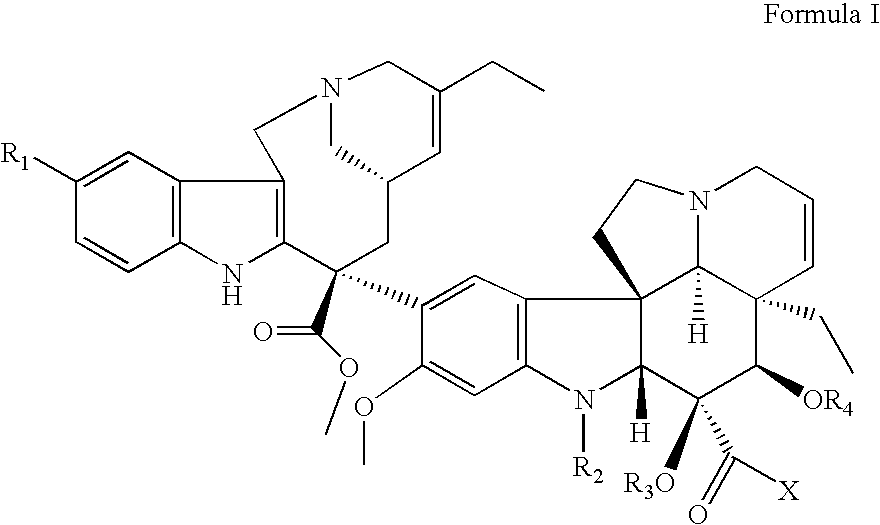
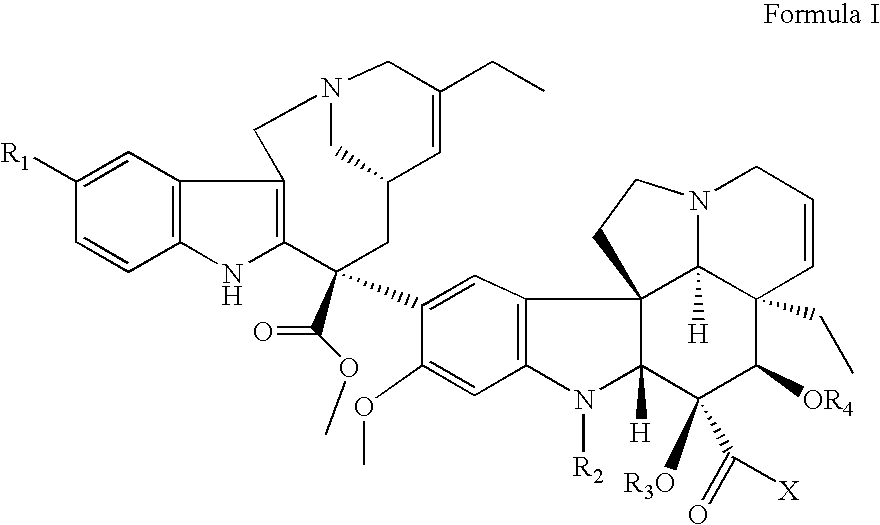
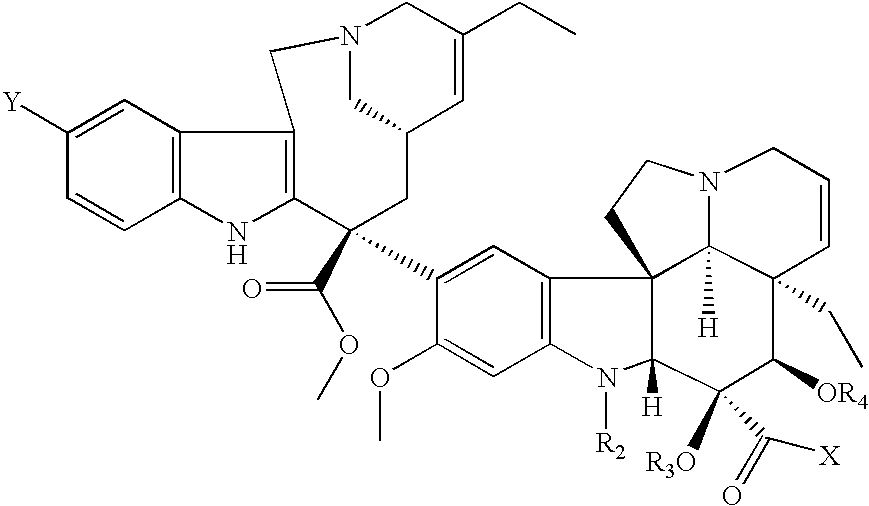
Vinorelbine derivatives
The present invention relates to novel vinorelbine derivatives. Pharmaceutical compositions containing these compounds as well as processes of preparation and processes of use for treatment of various conditions are also disclosed.
Owner:ALBANY MOLECULAR RESEARCH INC
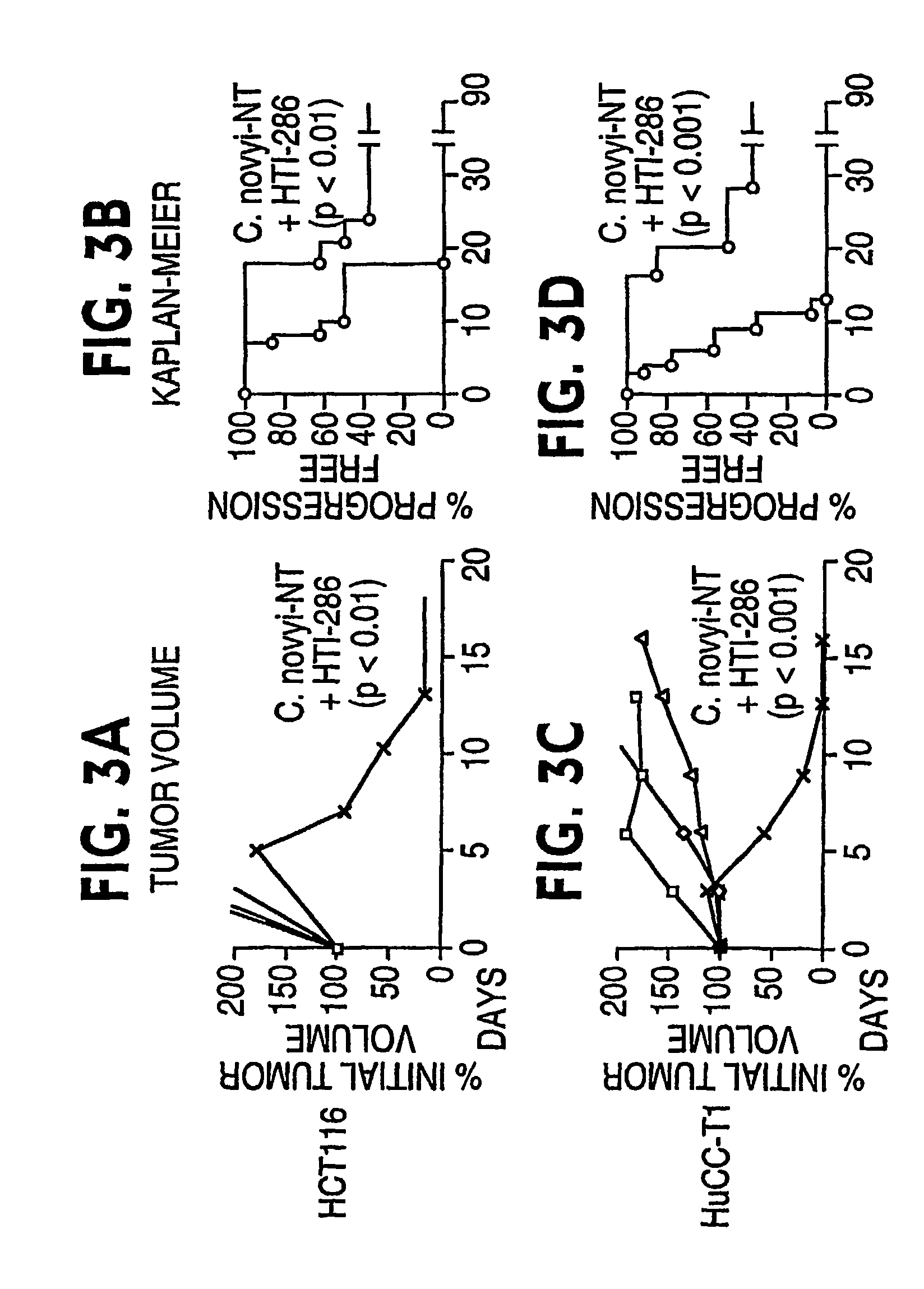
Combination bacteriolytic therapy for the treatment of tumors
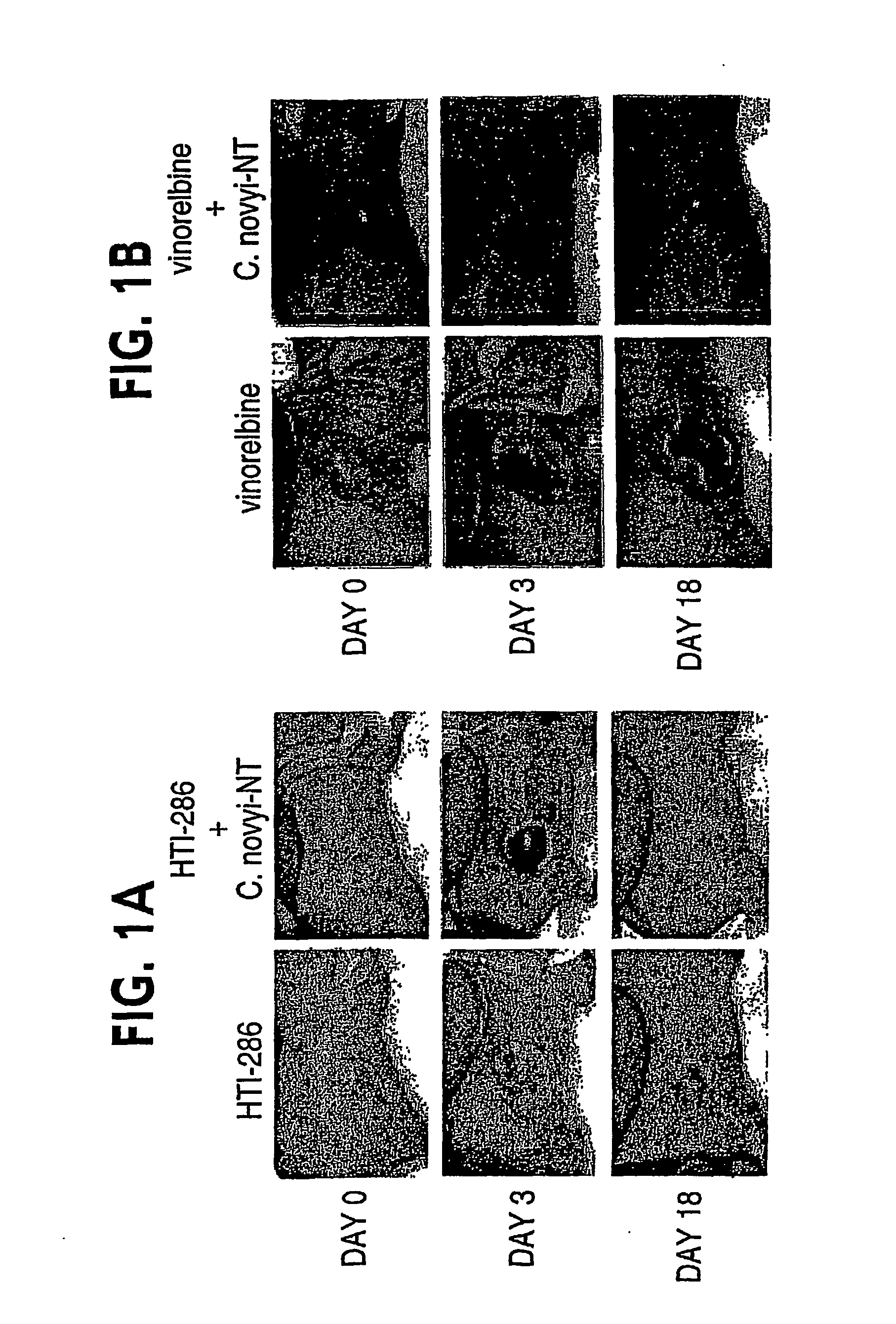
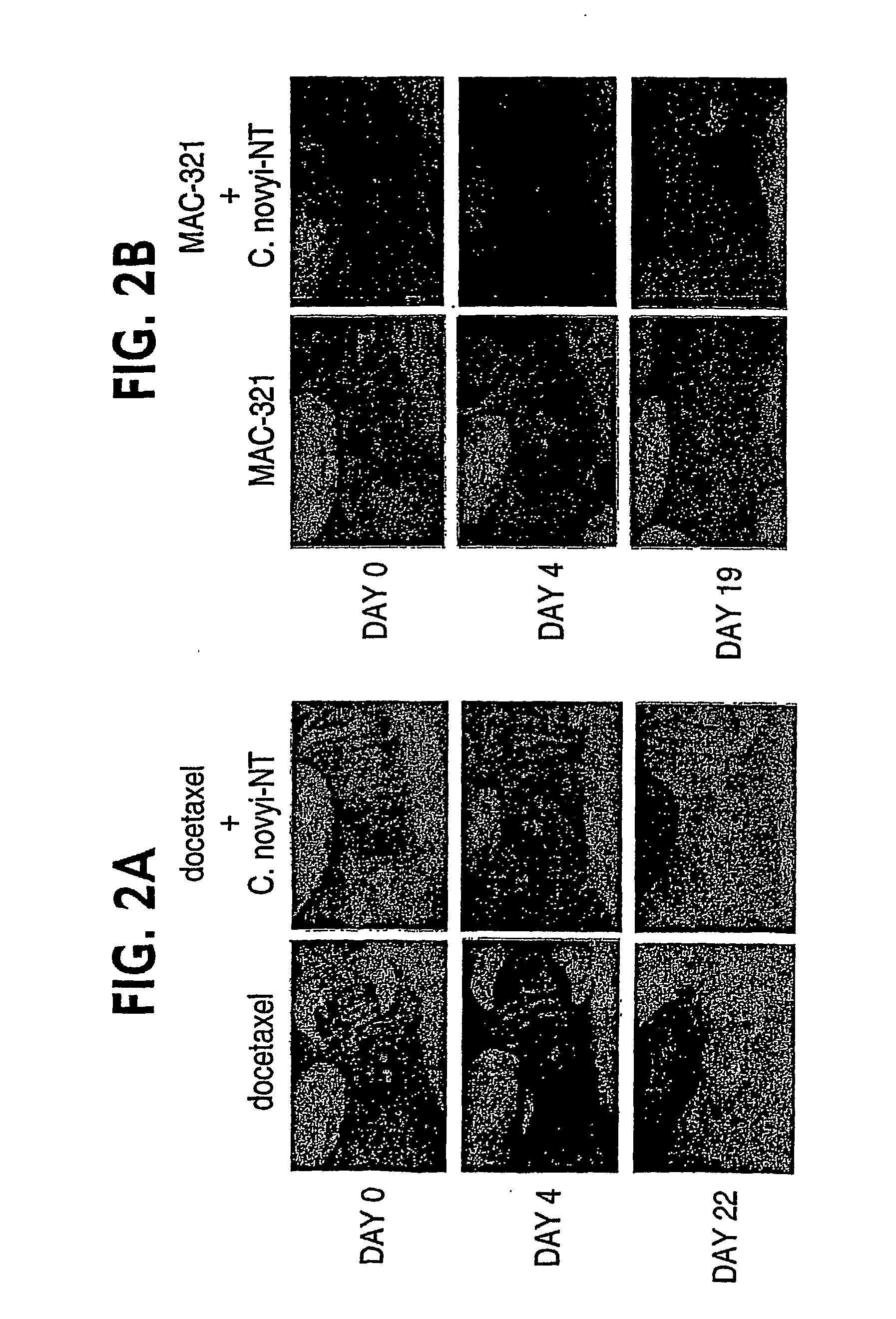
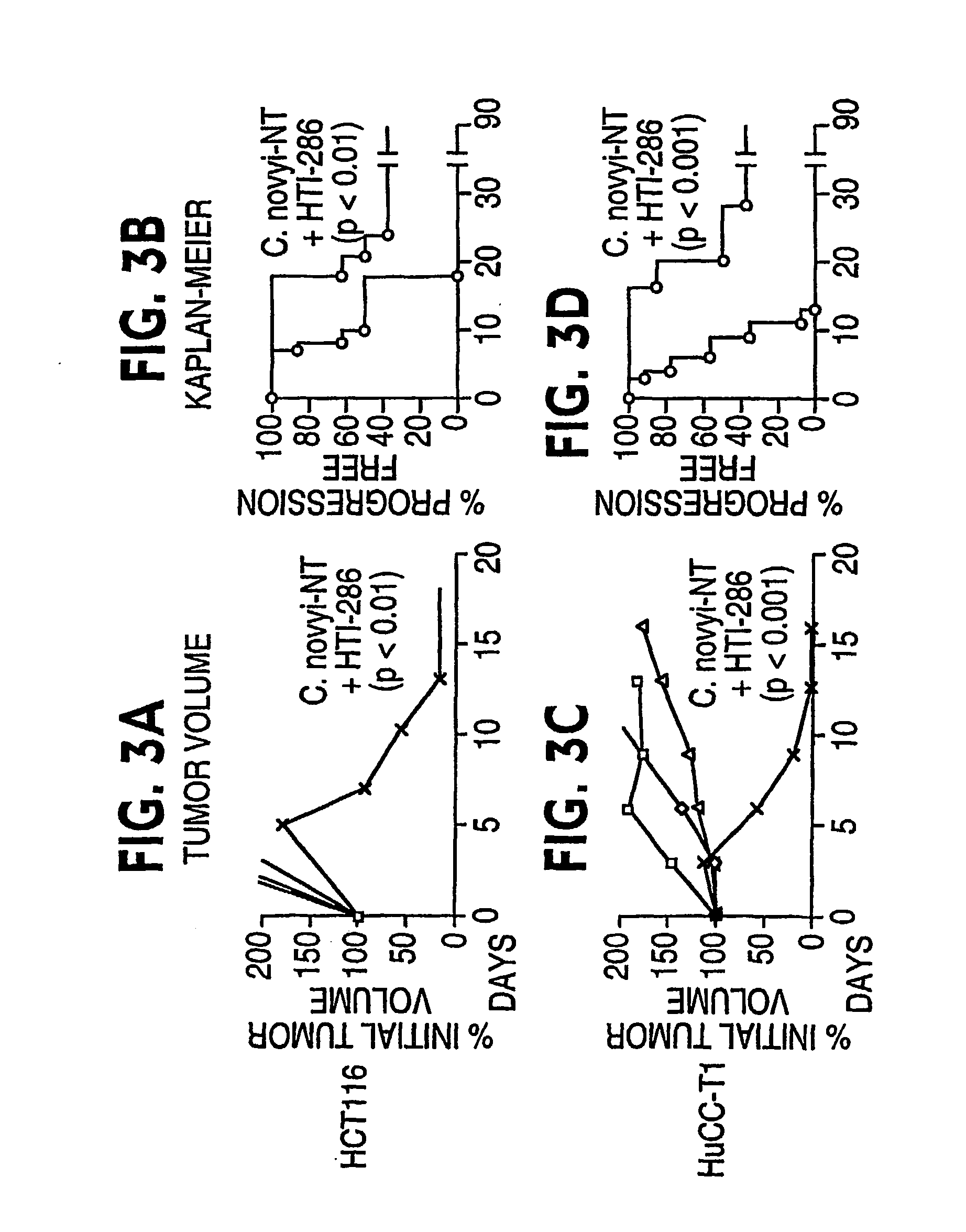
Current approaches for treating cancer are limited, in part, by the inability of drugs to affect the poorly vascularized regions of tumors. We have found that spores of anaerobic bacteria in combination with agents which interact with microtubules can cause the destruction of both the vascular and avascular compartments of tumors. Two classes of microtubule inhibitors were found to exert markedly different effects. Some agents that inhibited microtubule synthesis, such as vinorelbine, caused rapid, massive hemorrhagic necrosis when used in combination with spores. In contrast, agents that stabilized microtubules, such as the taxane docetaxel, resulted in slow tumor regressions that killed most neoplastic cells. Remaining cells in the poorly perfused regions of tumors could be eradicated by sponzlated bacteria. Mechanistic studies showed that the microtubule destabilizers, but not the microtubule stabilizers, radically reduced blood flow to tumors, thereby enlarging the hypoxic niche in which spores could germinate. A single intravenous injection of spores plus selected microtubule-interacting agents was able to cause regressions of several tumors in the absence of excessive toxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vinorelbine liposome micro ball injection and its prepn
InactiveCN1771954ALess irritatingLow toxicityOrganic active ingredientsGranular deliveryMedicineIrritation
The present invention relates to one kind of vinorelbine liposome micro ball injection and its preparation process. The present invention has vinorelbine medicine in 90-98 % coated inside the oil phase and oil-water interface of liposome micro ball, reduces the clinical toxicity and irritation of vinorelbine greatly and raises its antitumor effect. The preparation of the present invention as one kind of antitumor injection has low irritation, low toxicity and high curative effect.
Owner:沈阳东星医药科技有限公司
Stabilized oil-in-water emulsion of vinca alkaloids for vein and production thereof
InactiveCN1679576ALow toxicityLess irritatingOrganic active ingredientsEmulsion deliveryAdditive ingredientVinorelbine
A stable oil-in-water emulsion of vinorelbine for intravenous injection is prepared from vinorelbine, the oil for injection, emulsifier, and the water for injection. Its advantages are high tageting function, low poison, and high safety.
Owner:JIANGSU QINGJIANG PHARMA
Vinorelbine emulsion and its preparing method
The invention relates to a vinorelbine emulsion which comprises (by weight ratio) vinorelbine 0.001-10%, emulsifying agent 0.1-20%, triglyceride compounds 0.1-30%, osmoregulation agent 0.1-10%, stabilizing agent 0.05-5%, anti-oxidant 0.01-5%, and balancing water for injection. The invention has the advantages of increased medicament stability and improved curative effect.
Owner:李晓祥
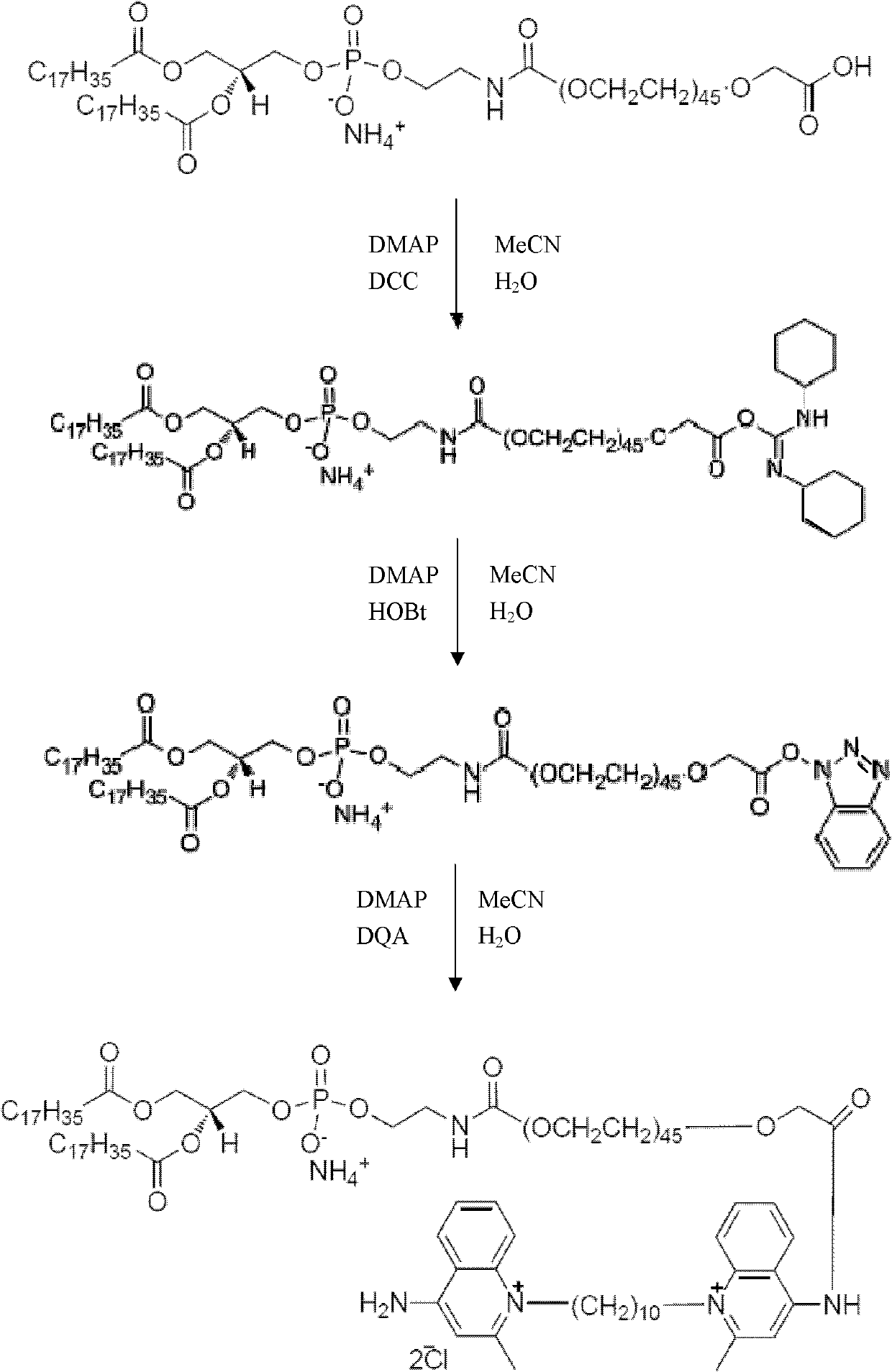
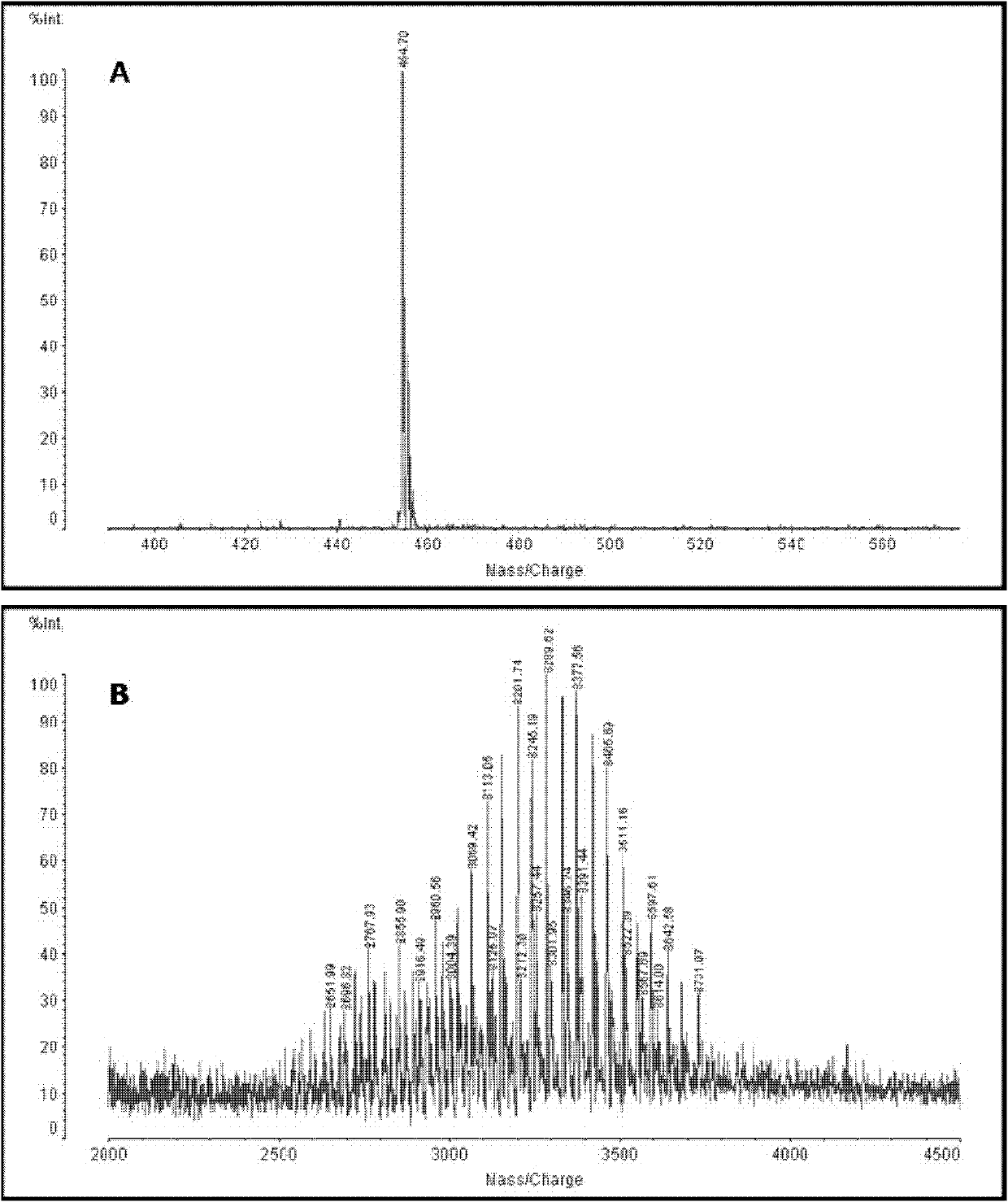
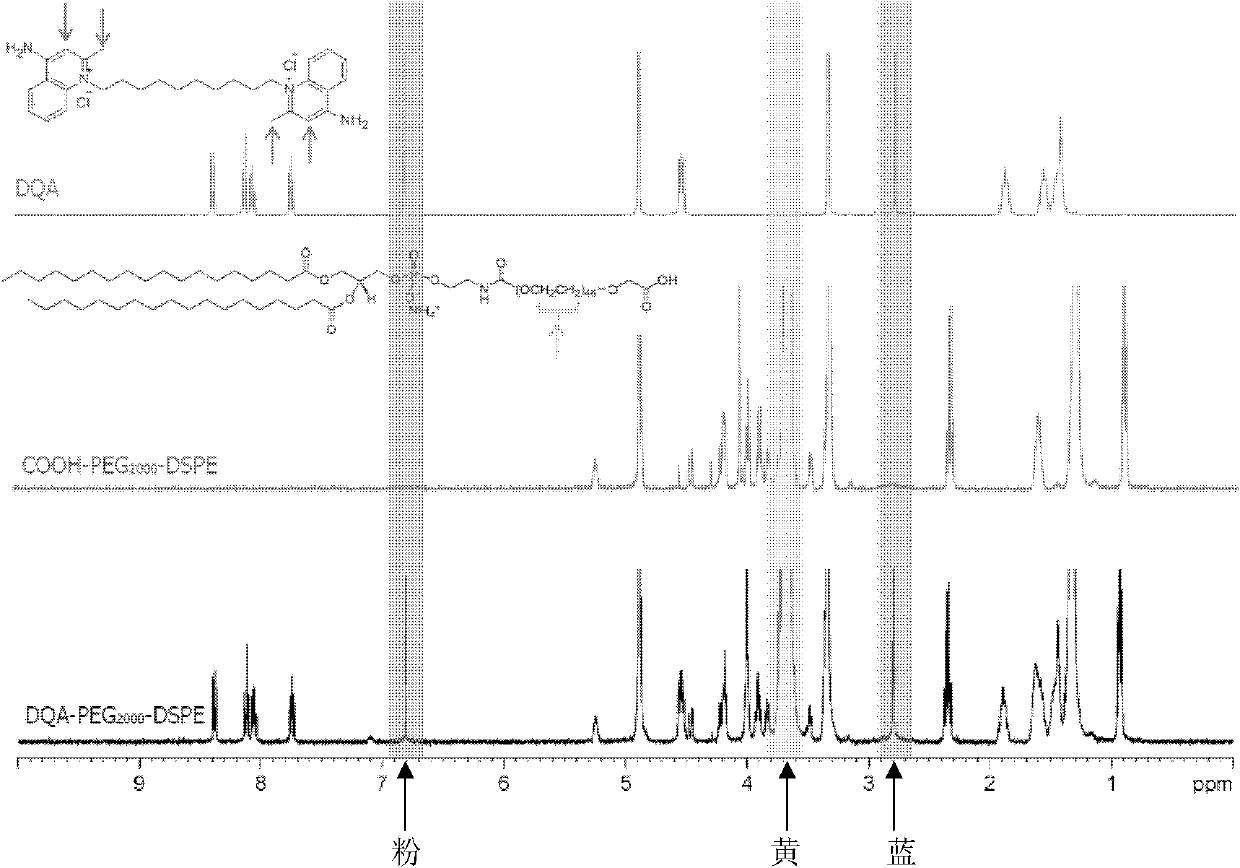
Dequalinium chloride-polyethylene glycol-distearoyl phosphatidyl ethanolamine conjugated compound and resveratrol liposome modified thereby
InactiveCN102757555AAvoid drug resistanceIncrease intakeHydroxy compound active ingredientsRespiratory disorderYolkCholesterol
The invention discloses a DQA-PEG 2000-DSPE (Dequalinium Chloride-Polyethylene Glycol-Distearoyl Phosphatidyl Ethanolamine) conjugated compound and resveratrol liposome modified thereby. The structural formula of the compound is shown by a formula I. In terms of composition, the resveratrol liposome comprises resveratrol and a fat material, wherein the mass ratio of the resveratrol to the fat material is (1:20)-(1:40); and the fat material consists of egg yolk lecithin, cholesterol and the compound shown by the formula I in the molar ratio of (63-67):(18-22):(2-4.35) in sequence. Pharmacodynamic tests prove that mitochondrial targeted resveratrol liposome has an extremely strong cell toxic effect in in-vitro cell experiments of human lung adenocarcinoma A549 cells and drug-resistant A549 / cDDP cells thereof, a tumor sphere model and an in-vivo transplantation tumor model and can penetrate through the core of the tumor sphere. The anti-tumor effect of vinorelbine liposome on the drug-resistant A549 / cDDP cells can be obviously improved by combining the resveratrol liposome with the vinorelbine liposome for use (formula I).
Owner:PEKING UNIV
Antineoplastic Combinations Containing HKI-272 and Vinorelbine
A combination of HKI-272 compound and a vinorelbine compound in the treatment of a neoplasm is provided. Regimens, kits, and methods for treatment of neoplasm, including breast cancer including metastatic breast cancer, and lung cancer, using this combination, optionally in combination with other anti-neoplastic agents, or immune modulators are also described.
Owner:WYETH LLC
Vinorelbine long circulation liposome preparation and preparation method thereof
InactiveCN101933904AImprove stabilityEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsVinorelbineLiposome
The invention relates to a Vinorelbine long circulation liposome preparation and a preparation method thereof, the preparation comprises three sub-packaging units, i.e. an empty liposome, sodium phosphate buffer solution and heavy tartaric acid Vinorelbine; and before being used, the three sub-packaging units are mixed together and heated appropriately. The preparation method has the advantages of simple process and easy operation and is suitable for industrial production, and the Vinorelbine long circulation liposome which is prepared by adopting the method has high encapsulation rate and good stability.
Owner:QILU PHARMA CO LTD
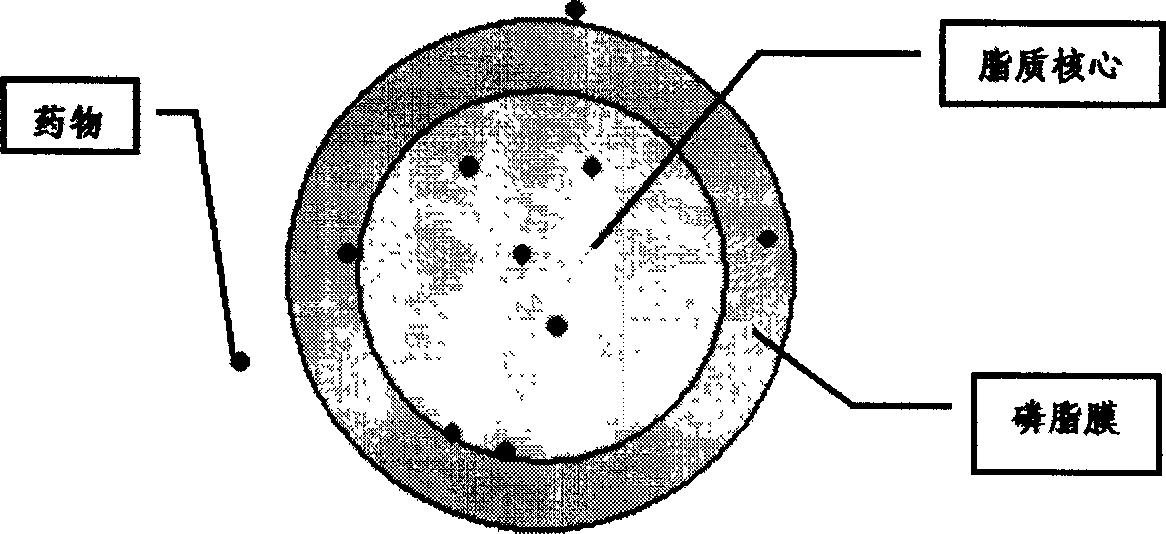
Vinorelbine solid lipid nano granule, freeze drying formulated product and method of preparing the same
InactiveCN101129375AAvoid degradationPrevent proliferationOrganic active ingredientsPowder deliveryWater bathsLipid formation
Disclosed is kind of Vinorelbine solid lipid nanoparticles which comprise (by weight percent) vinorelbine 1-25%, grease material 30-90%, phospholipids 5-50% oleinic acid 0-20%. The preparing process consists of melting liposome material through water-bath, dissolving phosphatides, oleic acid and Vnorelbine with ethanol, dropping into the liposome material, removing ethanol under decompression condition, cooling down and freezing, placing into pH 2-5 aqueous phase, triturating and homogenizing the turbid liquor to obtain the freeze-dried preparation with improved anticancer reactivity of Vinorelbine.
Owner:ZHEJIANG UNIV
Compositions and methods for treating cancer
This invention provides compositions and methods for treating neoplasias in a mammal. In particular, the invention provides liposome-encapsulated vinca alkaloids, e.g., vinorelbine, and methods of treating a mammal using such compositions.
Owner:UNIV OF TEXAS MD ANDERSON CANCER CENT THE +1
Vinorelbine derivatives
The present invention relates to novel vinorelbine derivatives. Pharmaceutical compositions containing these compounds as well as processes of preparation and processes of use for treatment of various conditions are also disclosed.
Owner:ALBANY MOLECULAR RESEARCH INC
Vinorelbine derivatives
InactiveUS20050176748A1Inhibit cell proliferationBiocideOrganic chemistryVinorelbineMedicinal chemistry
The present invention relates to novel vinorelbine derivatives. Pharmaceutical compositions containing these compounds as well as processes of preparation and processes of use for treatment of various conditions are also disclosed.
Owner:ALBANY MOLECULAR RESEARCH INC
Preparation of vinorelbine lipoplast, for injection
InactiveCN100998562AAvoid local phlebitisReduce myelosuppressionOrganic active ingredientsPharmaceutical non-active ingredientsDrugs solutionOrganic solvent
A process for preparing the vinorelbine liposome for injection, especially the vinorelbine liposome tartrate, includes such steps as dissolving multiple kinds of lipoid in organic solvent, mixing it with buffer liquid in HPLC gradient pump while pressing, ultrafiltering to remove organic solvent and obtain simple liposome, regulating pH=7-9 by special alkali solution, mixing with medicine solution, and encapsulating to obtain medicine.
Owner:XIAN LIBANG PHARMA TECH
Lung cancer-targeted peptides and applications thereof
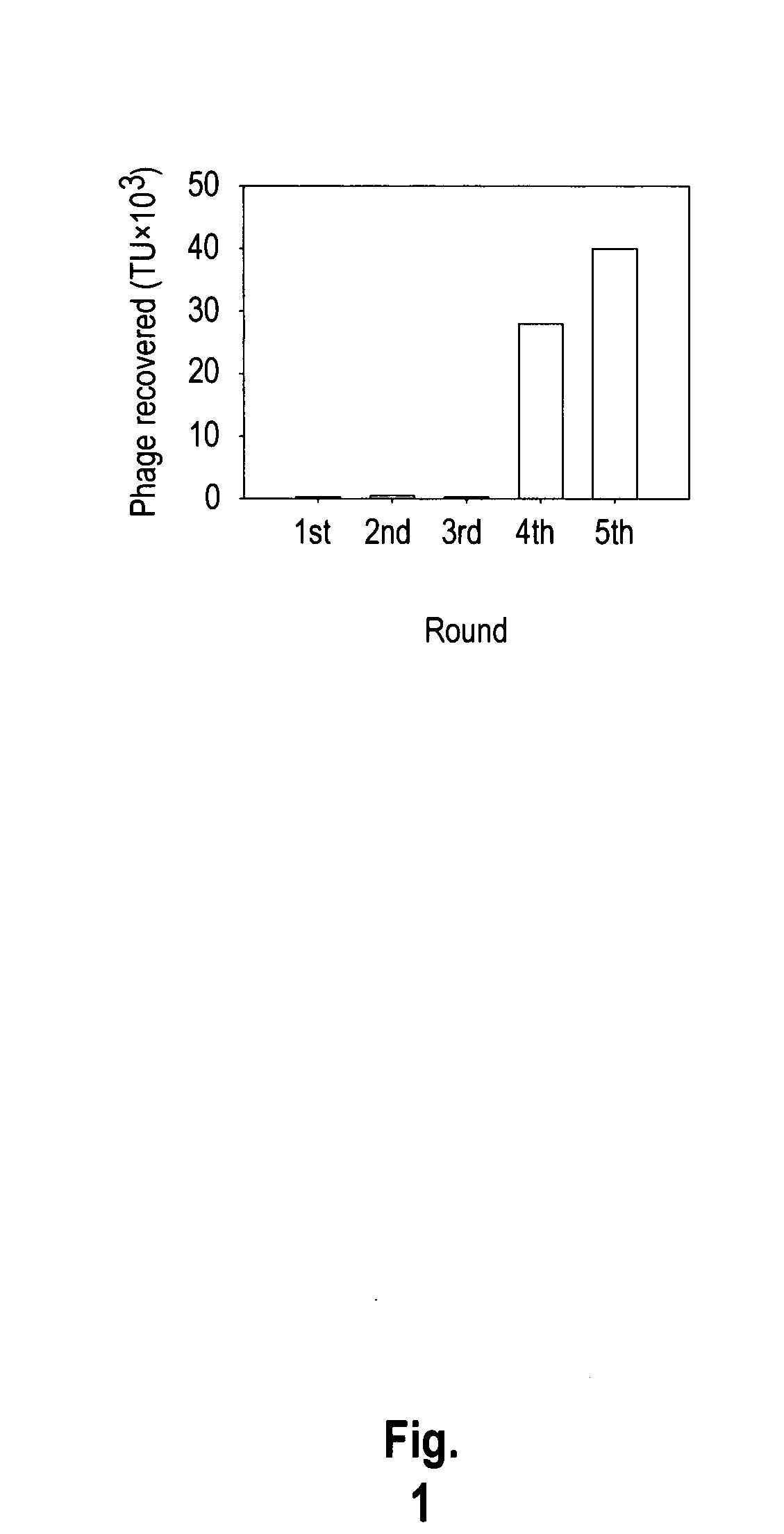
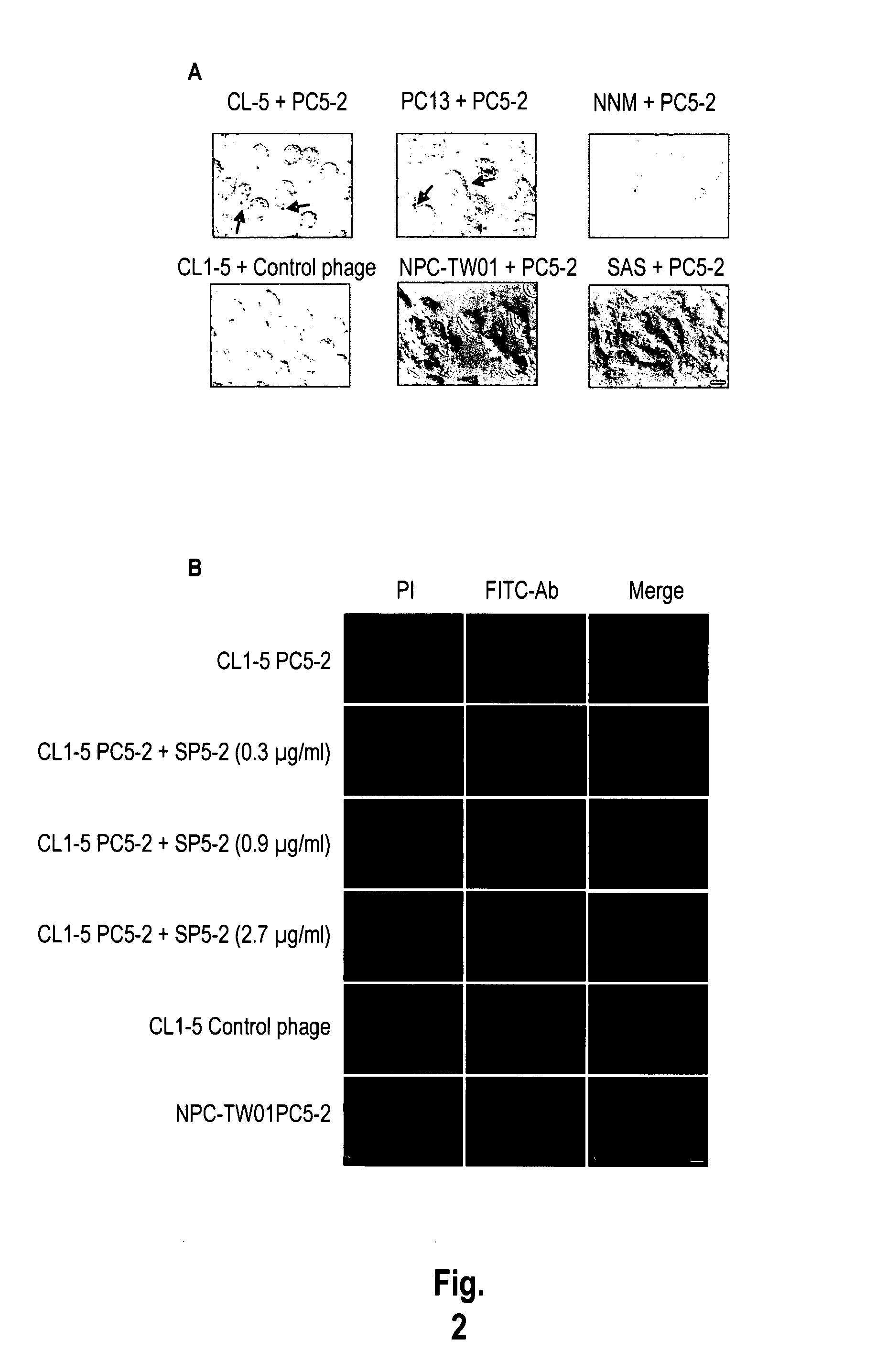
The invention provides nucleic acids, peptides, and antibodies for use in applications including diagnosis and therapy. The peptides target lung cancer and were identified by phage display. Targeting phage PC5-2 and synthetic peptide SP5-2 were both able to recognize human pulmonary tumor specimens from lung cancer patients. In SCID mice bearing NSCLC xenografts, the targeting phage was able to target tumor masses specifically. When the peptide was coupled to liposomes containing the anti-cancer drugs vinorelbine or doxorubicin, the efficacy of these drugs against human lung cancer xenografts was improved, the survival rate increased, and the drug toxicity was reduced.
Owner:ACAD SINIC +1
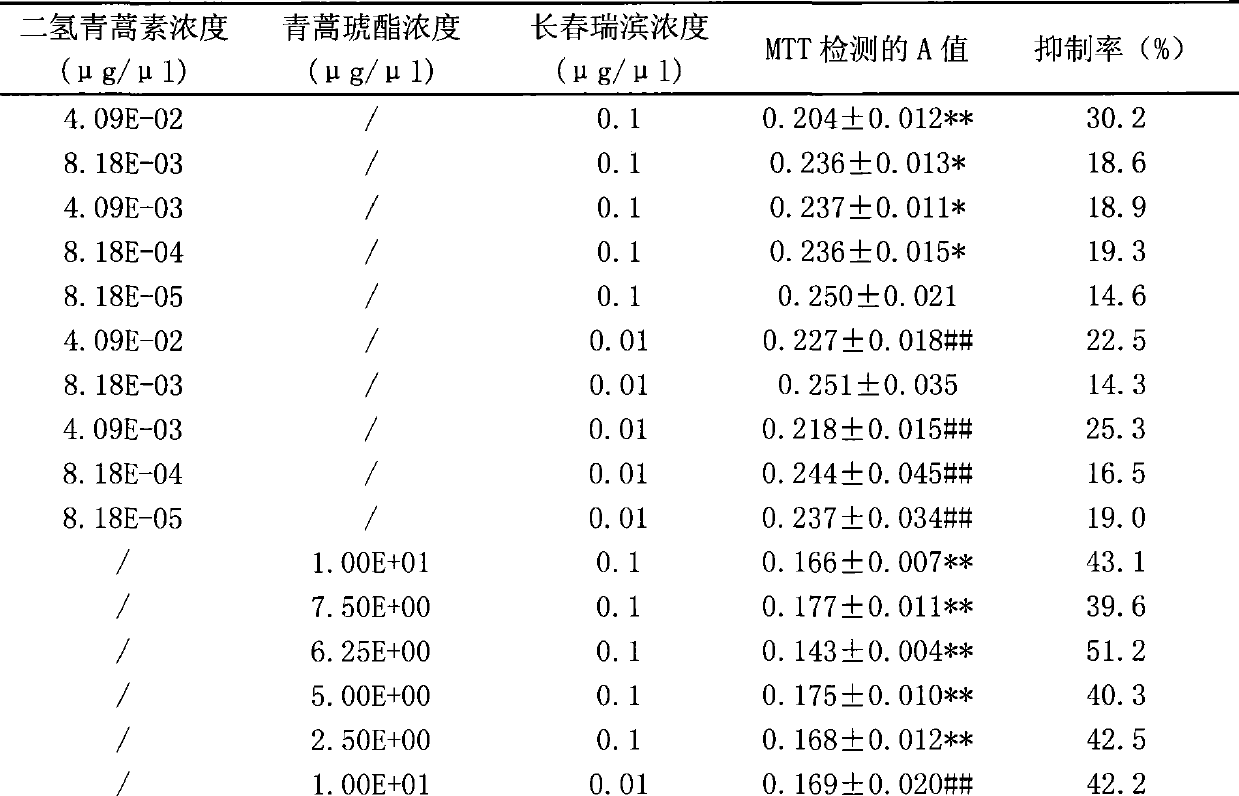
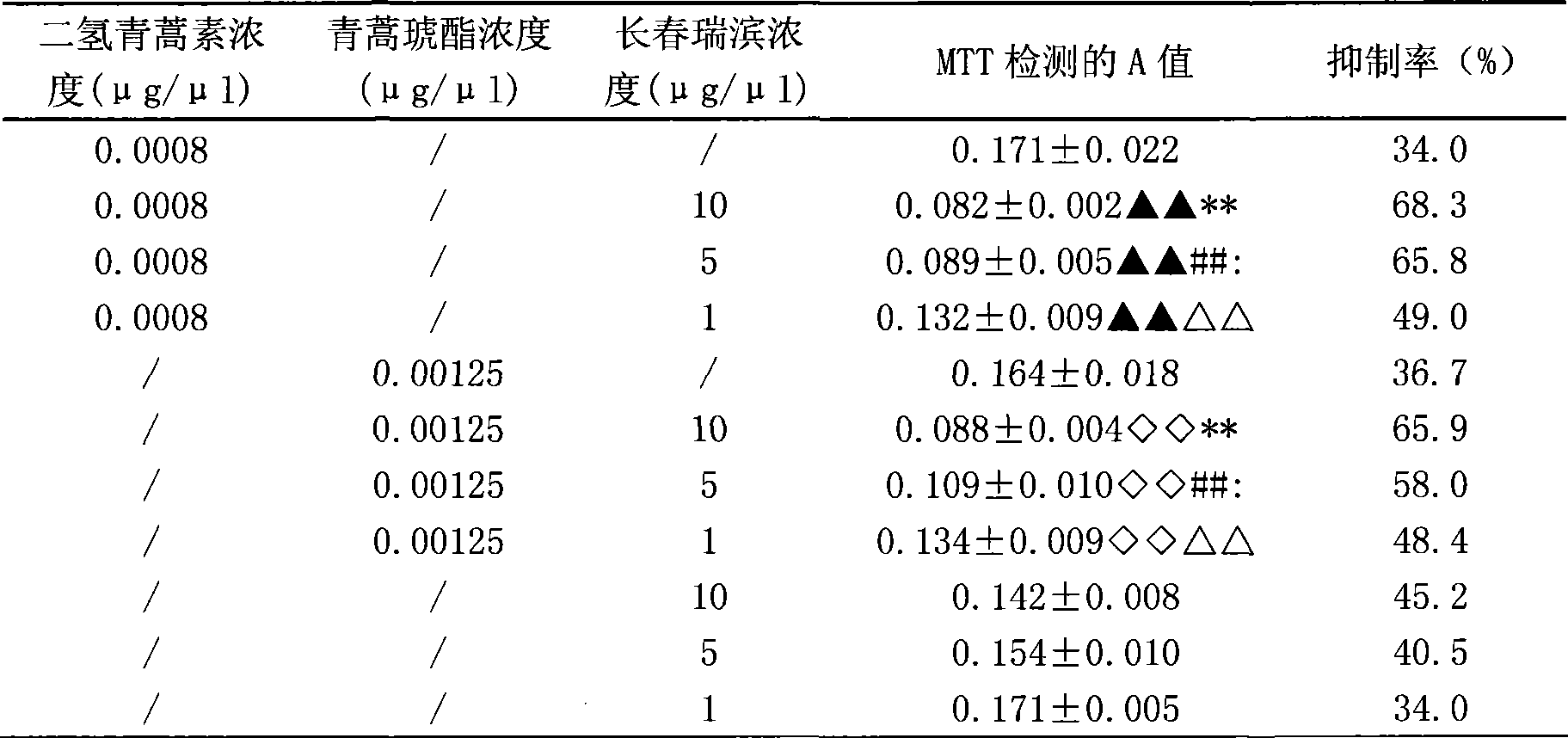
Composition of artemisinin derivative and vinorelbine, and use thereof
InactiveCN101380325AReduce dosageSynergisticOrganic active ingredientsAntineoplastic agentsAdjuvantOral medication
The invention relates to a composite of an artemisinin derivative and vinorelbine in the technical field of pharmaceuticals and the application thereof; wherein, the ratio of the artemisinin derivative (including dihydroartemisinin or artesunate) and the vinorelbine is 1:500 to 500:1. The composite can be applied to preparing antineoplastics and made into the dosage forms of injection administration and oral administration by adding pharmaceutically acceptable adjuvant carriers. The two effective components (the artemisinin derivative and the vinorelbine) of the composite not only have obvious synergistic effect and but also can be cooperatively applied so as to reduce the amount of the vinorelbine and lower the production cost. By adding the pharmaceutically acceptable adjuvant carriers to prepare the dosage forms of injection administration and oral administration, the composite can be applied to the anti-tumor field.
Owner:SHANGHAI JIAO TONG UNIV
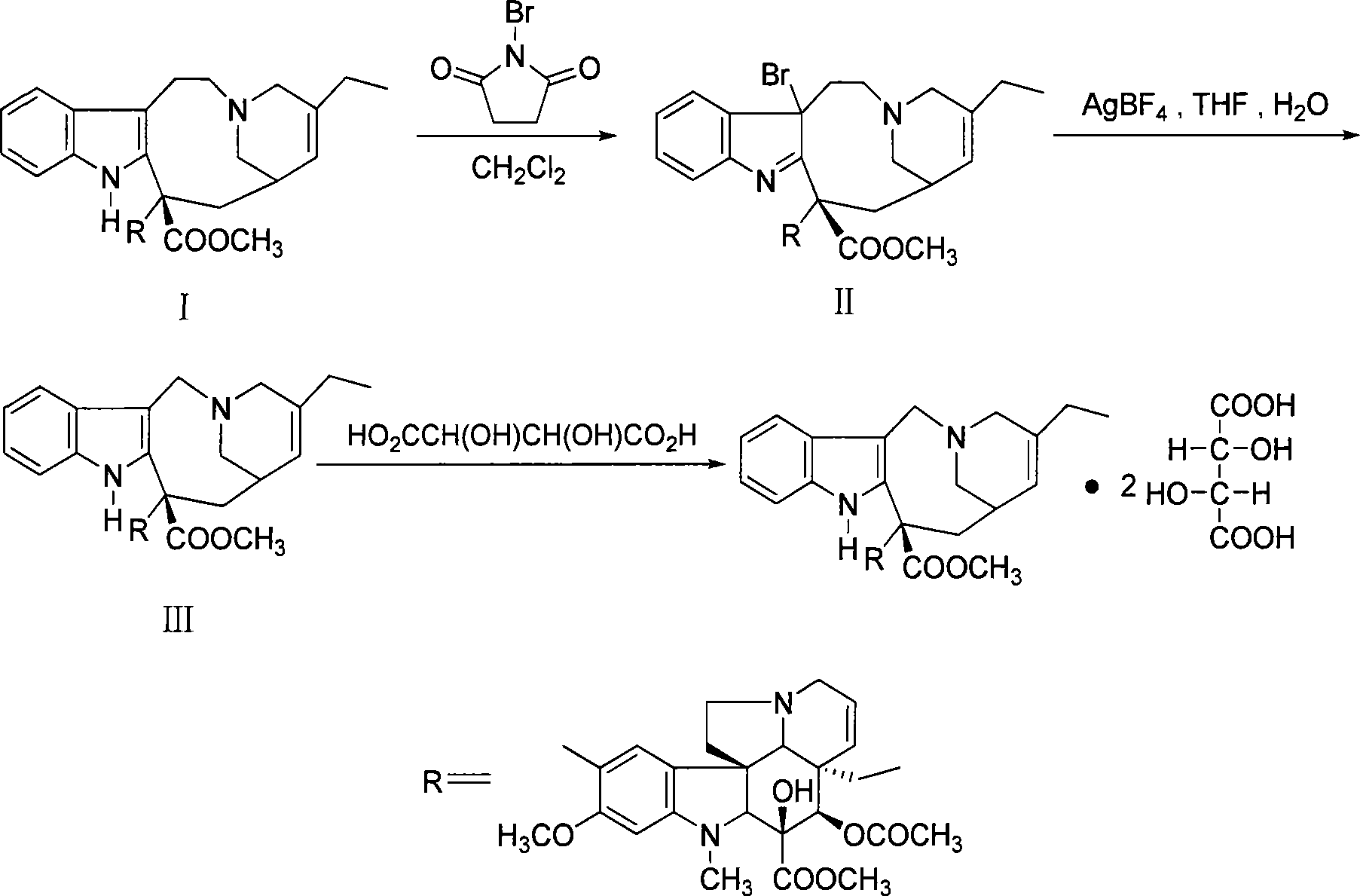
Preparation method for vinorelbine
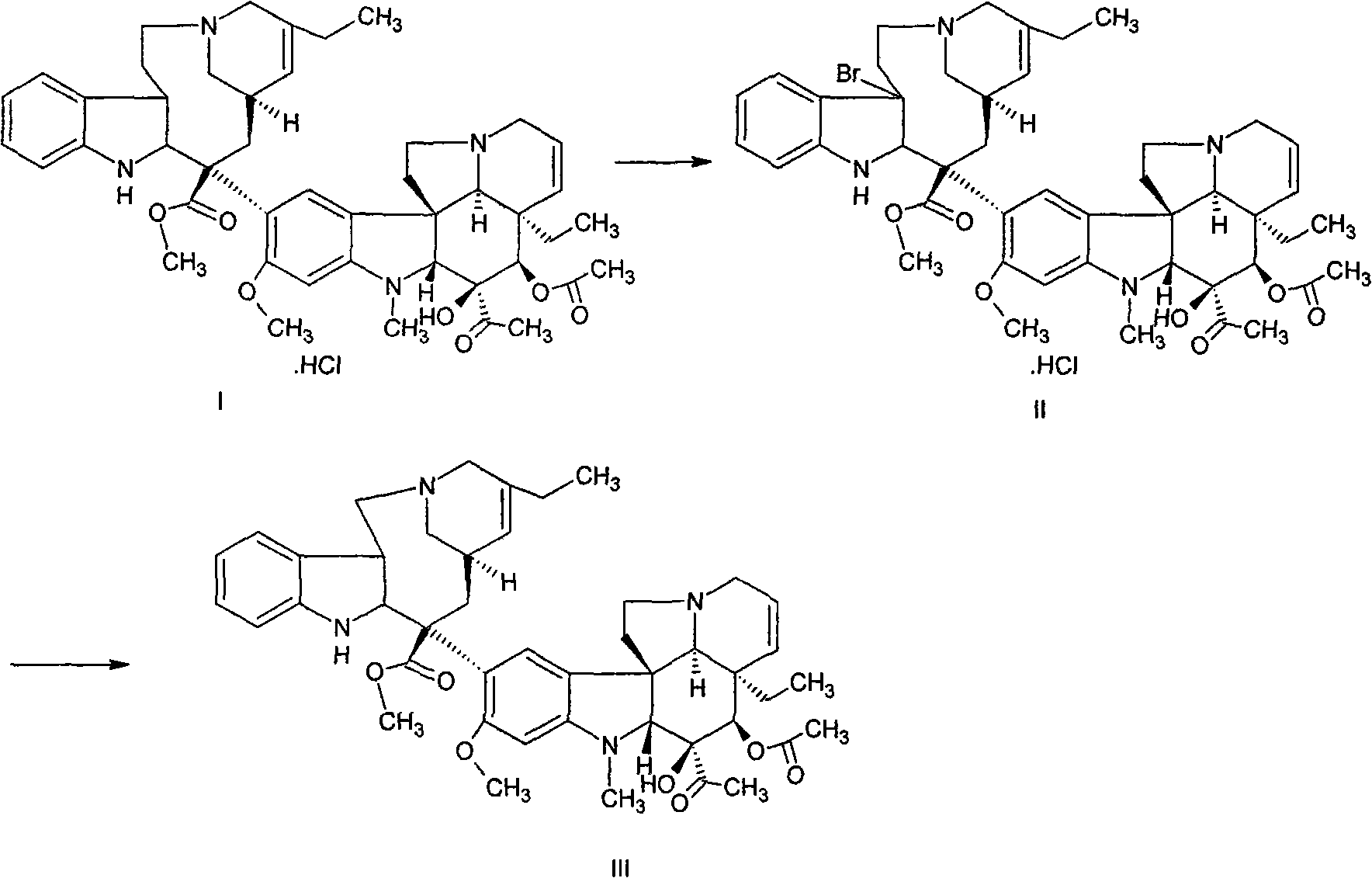
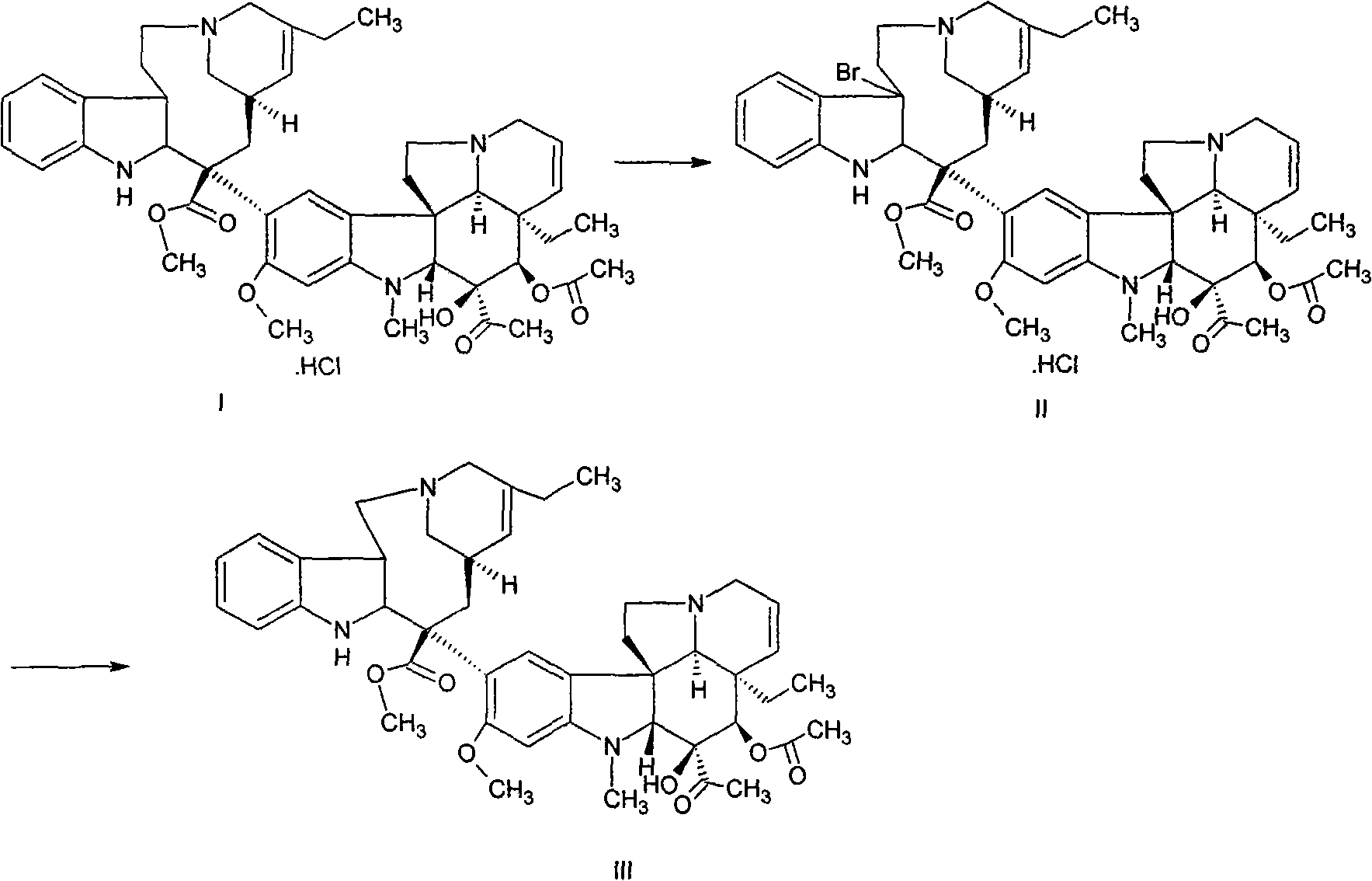
ActiveCN101508697ASimple preparation processExpensive to fixOrganic chemistrySolid sorbent liquid separationSilver tetrafluoroborateChromatographic separation
The invention discloses a preparation method of vinorelbine. The method comprises the following steps: taking anhydrovinblastine as a raw material; performing a bromination reaction to prepare bromide; and then performing separation by alumina column chromatography. The bromide is condensed and cyclized to vinorelbine during a column chromatographic separation process without using silver tetrafluoroborate and tetrahydrofuran, and product purity can be up to 65% (HPLC). The method helps avoid using the expensive silver tetrafluoroborate reagent, shorten process flow, lower raw material cost and reduce environmental pollution; meanwhile, the method also helps solve the disadvantages of unstable product quality, yield and the like in a silver tetrafluoroborate process and has good popularization and application prospect.
Owner:GUANGZHOU HANFANG PHARMA
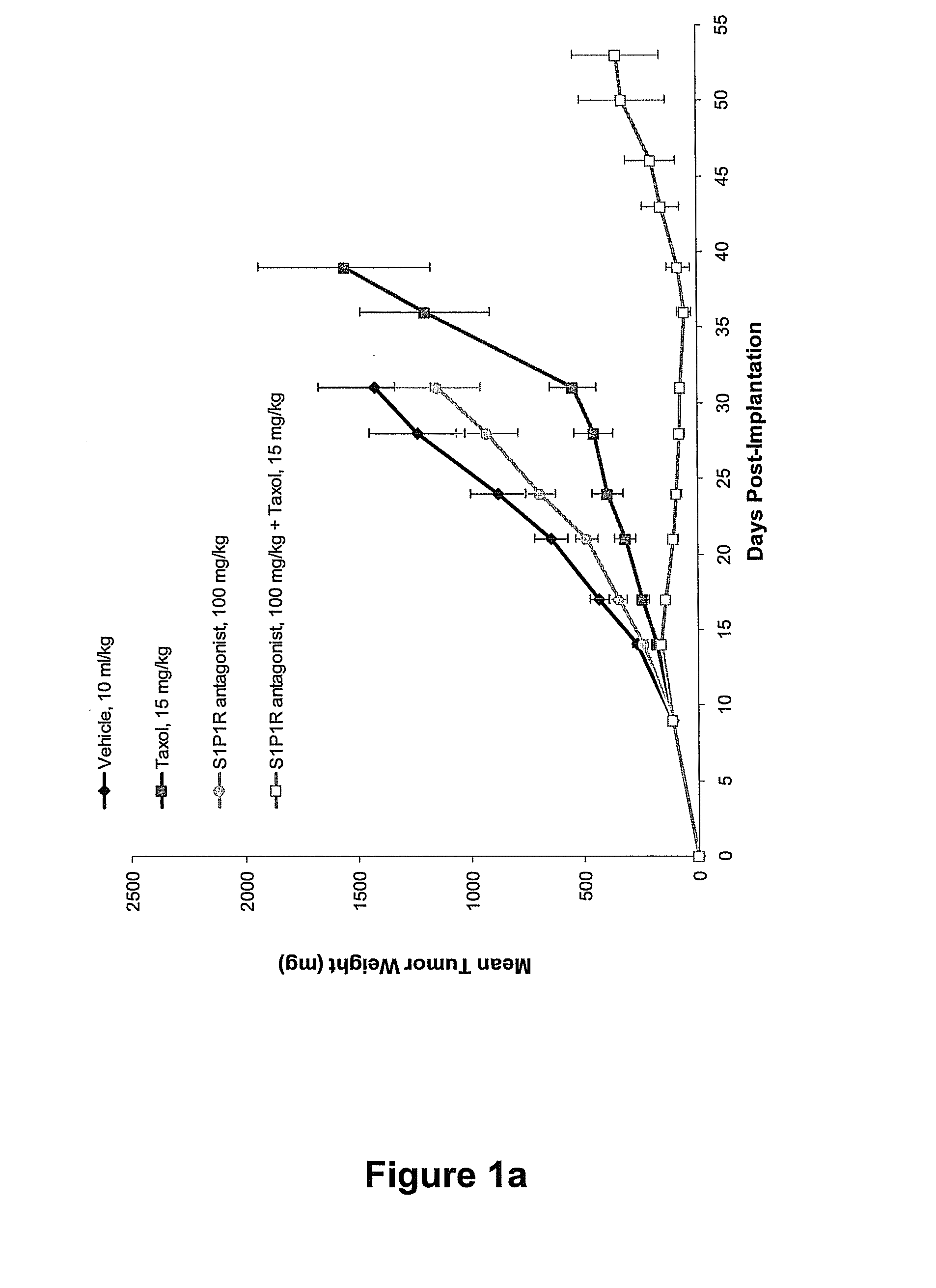
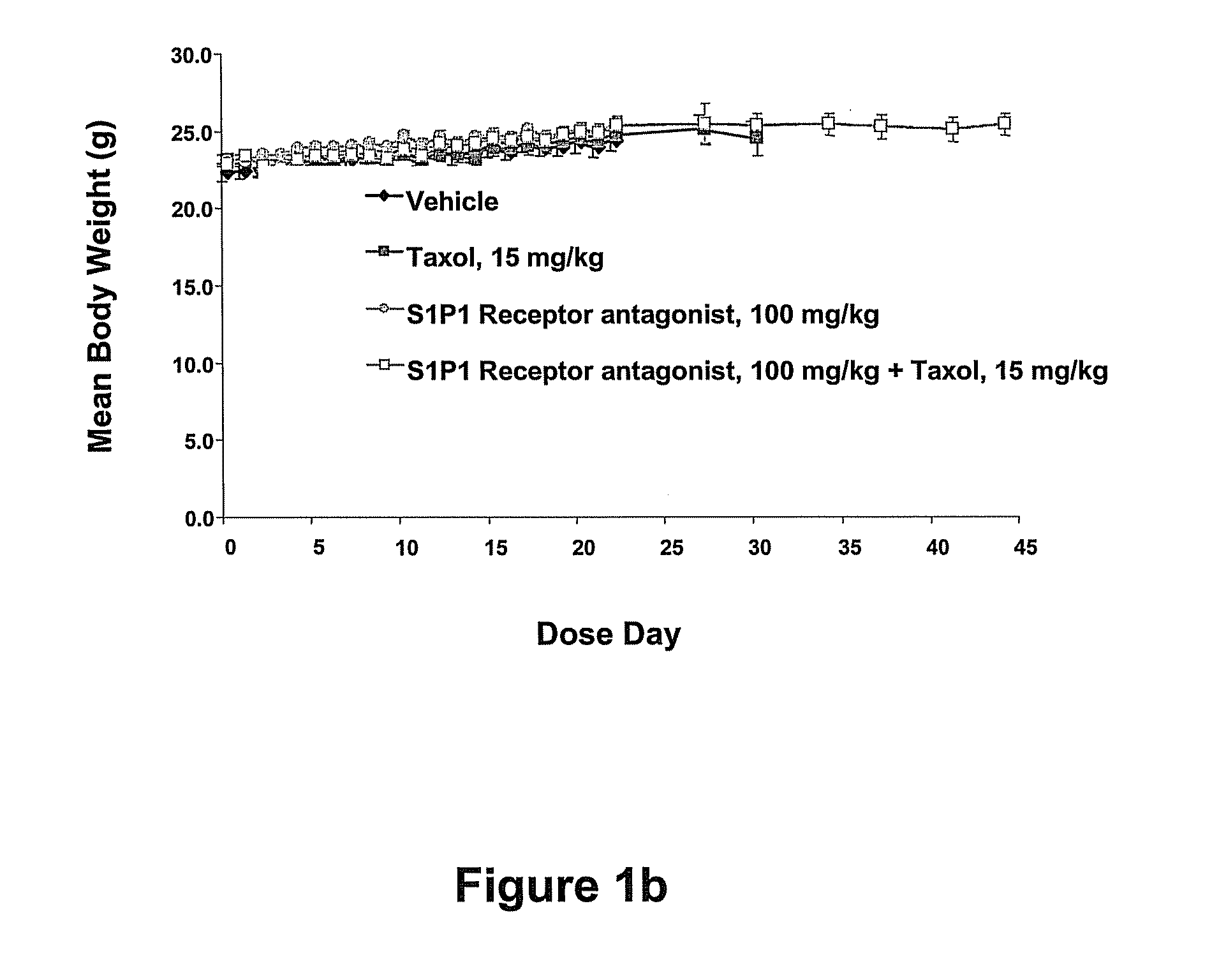
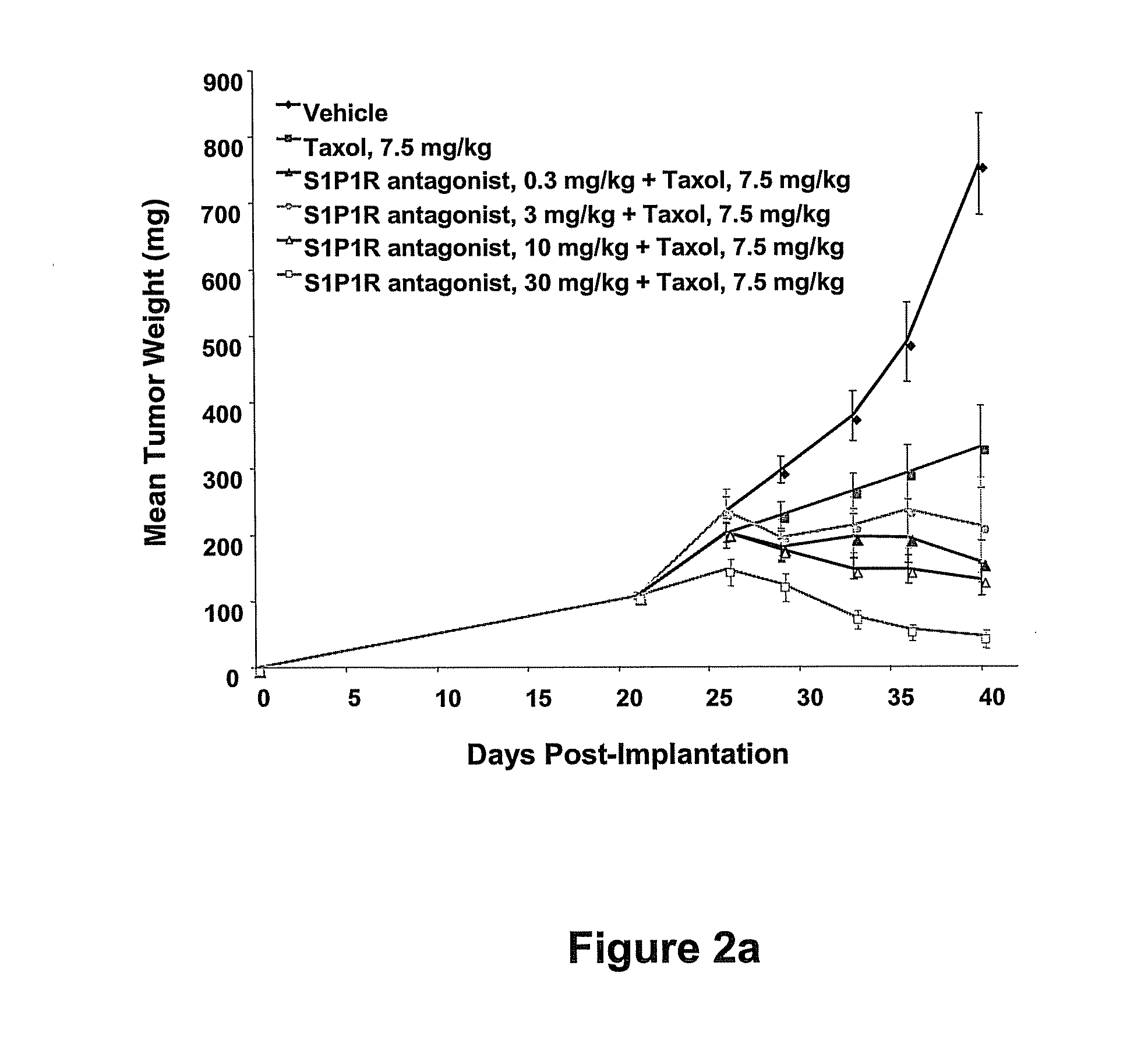
Synergistic Effects Between Sphingosine-1-Phosphate Receptor Antagonists and Antimicrotubule Agents
This invention is based on the discovery that the administration of a sphingosine-1-phosphate receptor antagonist (S1P) and at least one chemotherapeutic agent selected from the the group of antimicrotubule agents provides an unexpectedly superior treatment for cancer. Antimicrobial agents such as the taxane compounds are known in the art, for example, paclitaxel (available as TAXOL® from Bristol-Myers Squibb, Princeton, N.J.), docetaxel (available as TAXOTERE® from Sanofi-aventis, Bridgewater, N.J.) and the like and other compounds that act as antimicrotubule agents, such as Vincristine (ONCOVIN®, VINCASAR PFS®, VCR), Vinblastin (VELBAN®, VELSAR®) and Vinorelbine, and similar compounds. The present invention also provides methods of modulating the growth of selected cell populations, such as cancer cells, by administering a therapeutically effective amount of at least one sphingosine-1-phosphate 1 (S1P1R) receptor antagonists, and at least one antimicrotubule agent.
Owner:EXELIXIS INC
Vinorelbine compositions and methods of use
The present invention is for novel compositions and methods for treating cancer, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include liposome entrapped vinorelbine in which the liposome can contain any of a variety of neutral or charged liposome-forming compounds and cardiolipin. The liposomes of the present invention can be either multilamellar vesicles or unilamellar vesicles, as desired.
Owner:NEOPHARMA INC
Slow-release injection contg. platinum compounds and cellulotoxic medicines
InactiveCN1868453AEasy to operateGood repeatabilitySolution deliveryPharmaceutical non-active ingredientsMicrosphereActive component
A slow-release anticancer injection is composed of the slow-release microballs containing active component and slow-releasing auxiliary and the special solvent containing the suspending aid. Said active component is the combination of Pt compound chosen from dicycloplatinum, etc and the cellulotoxic medicine chosen from doxorubicin, vinorelbine, etc. Said slow-releasing auxiliary is chosen from polylactic acid or its copolymer, monoethyl polyethanediol or its copolymer, EVAc, etc.
Owner:SHANDONG LANJIN PHARMA +1
Combination bacteriolytic therapy for the treatment of tumors
Current approaches for treating cancer are limited, in part, by the inability of drugs to affect the poorly vascularized regions of tumors. We have found that spores of anaerobic bacteria in combination with agents which interact with microtubules can cause the destruction of both the vascular and avascular compartments of tumors. Two classes of microtubule inhibitors were found to exert markedly different effects. Some agents that inhibited microtubule synthesis, such as vinorelbine, caused rapid, massive hemorrhagic necrosis when used in combination with spores. In contrast, agents that stabilized microtubules, such as the taxane docetaxel, resulted in slow tumor regressions that killed most neoplastic cells. Remaining cells in the poorly perfused regions of tumors could be eradicated by sponzlated bacteria. Mechanistic studies showed that the microtubule destabilizers, but not the microtubule stabilizers, radically reduced blood flow to tumors, thereby enlarging the hypoxic niche in which spores could germinate. A single intravenous injection of spores plus selected microtubule-interacting agents was able to cause regressions of several tumors in the absence of excessive toxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vinorelbine Bitartrate lipsome freeze-drying powder injection and its preparation method
InactiveCN1839800AExtend cycle timeSmall toxicityOrganic active ingredientsPowder deliveryFreeze-dryingAdditive ingredient
The invention relates to an antineoplastic vinorelbine bitartrate liposome, its freeze-dried powder injection and preparing process, wherein the preparation comprises liposome of vinorelbine bitartrate and pharmaceutically acceptable carrying agent, the liposome of vinorelbine bitartrate contains the following constituents: vinorelbine bitartrate, phosphatide, cholesterin and vitamin E, their weight ratio being 1 : 1-100 : 1-15 : 0.01-0.05.
Owner:ZHEJIANG UNIV +1
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
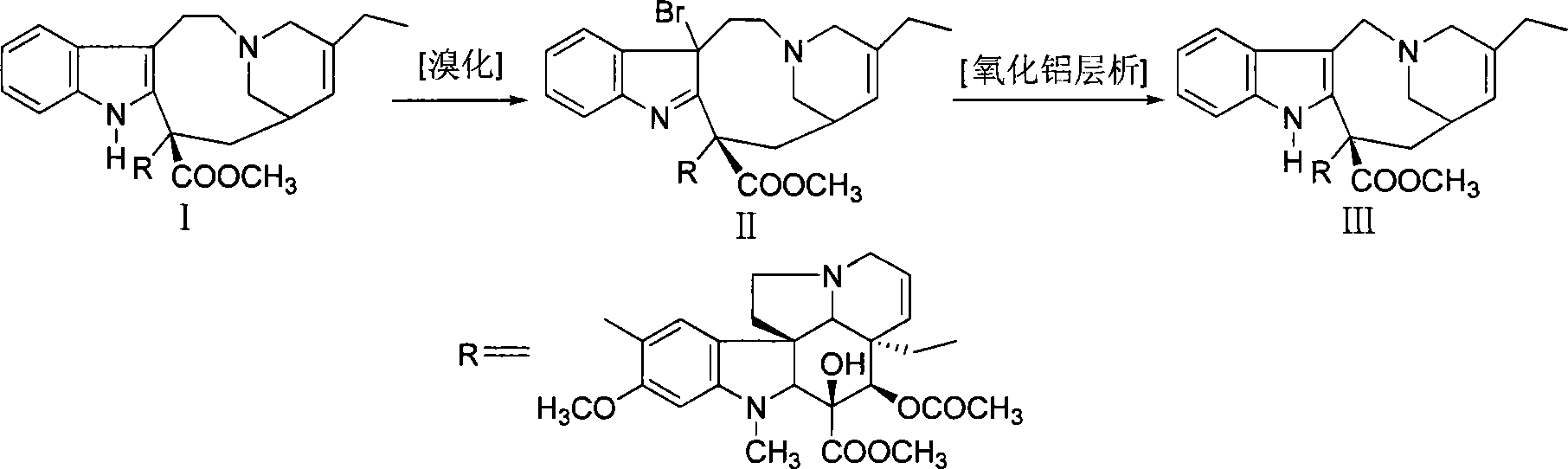
Process method for preparing vinorelbine
InactiveCN101781322ALow priceDoes not affect yieldOrganic chemistryChromatographic separationVinorelbine
The invention discloses a process method for preparing vinorelbine, which comprises the following steps: taking dehydration vinblastine or salt thereof as a starting material, obtaining rough vinorelbine after bromoforming and a ring contraction reaction, and obtaining vinorelbine through column chromatography. The method adopts silver nitrate to replace silver boron tetrafluoride to be served as a reduction cyclization reagent, and thereby not influencing the yield of the reaction and the purity of crude products, and further reducing the production cost.
Owner:深圳万乐药业有限公司
Method for purifying Vinorelbine Bitartrate
A process for purifying vinorelbine includes preparing silica gel column, negative-pressure chromatography by filling liquid from its bottom, segmental cutting, eluting, filtering, low-temp vacuum concentrating, dripping it in water to obtain crystals, centrifugal depositing, and drying the deposit.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Dual sustained-release anticancer injection
InactiveCN101301264APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideMicrosphere
A double sustained release anticancer gel sustained release injection consists of anticancer medicine and amphiphilic block copolymer hydrogel, wherein the anticancer medicine comprises vincristine, vinorelbine, vinblastine, daunomycin, mitoxantrone, mitozolomide and temozolomide, etc., and exists in sustained release preparation injection in the forms of sustained release microsphere, microsphere or micropowder, i.e. the anticancer medicine in anticancer useful quantity is partly or completely wrapped inside the sustained release microsphere. Sustained release gel has temperature-sensitive gelling characteristics and is in the state of fluxible liquid in an environment with the temperature lower than body temperature; moreover, the sustained release gel can be automatically converted into non-flowing water-insoluble gel capable of biodegradation and absorption inside the body of a warm blood so as to slowly release medicine inside part of a tumor; the sustained release microsphere is propitious to release medicine smoothly and slowly, and double sustained release is propitious to control tumor cells entering a dormancy stage; moreover, the medicine which exists in the sustained release gel in the form of micropowder is propitious to release the medicine relatively faster and to control cells in faster proliferation. The double sustained release anticancer gel sustained release injection can used together with radiotherapeutic particle, etc.
Owner:济南基福医药科技有限公司
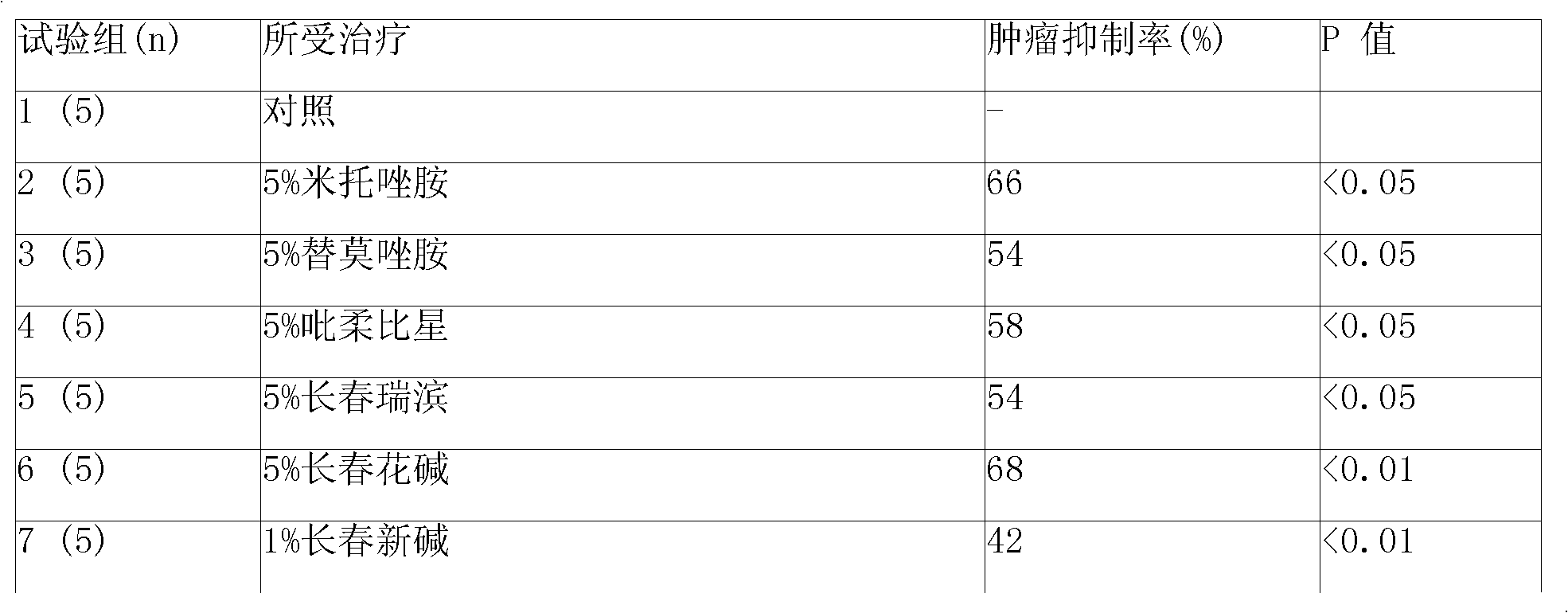
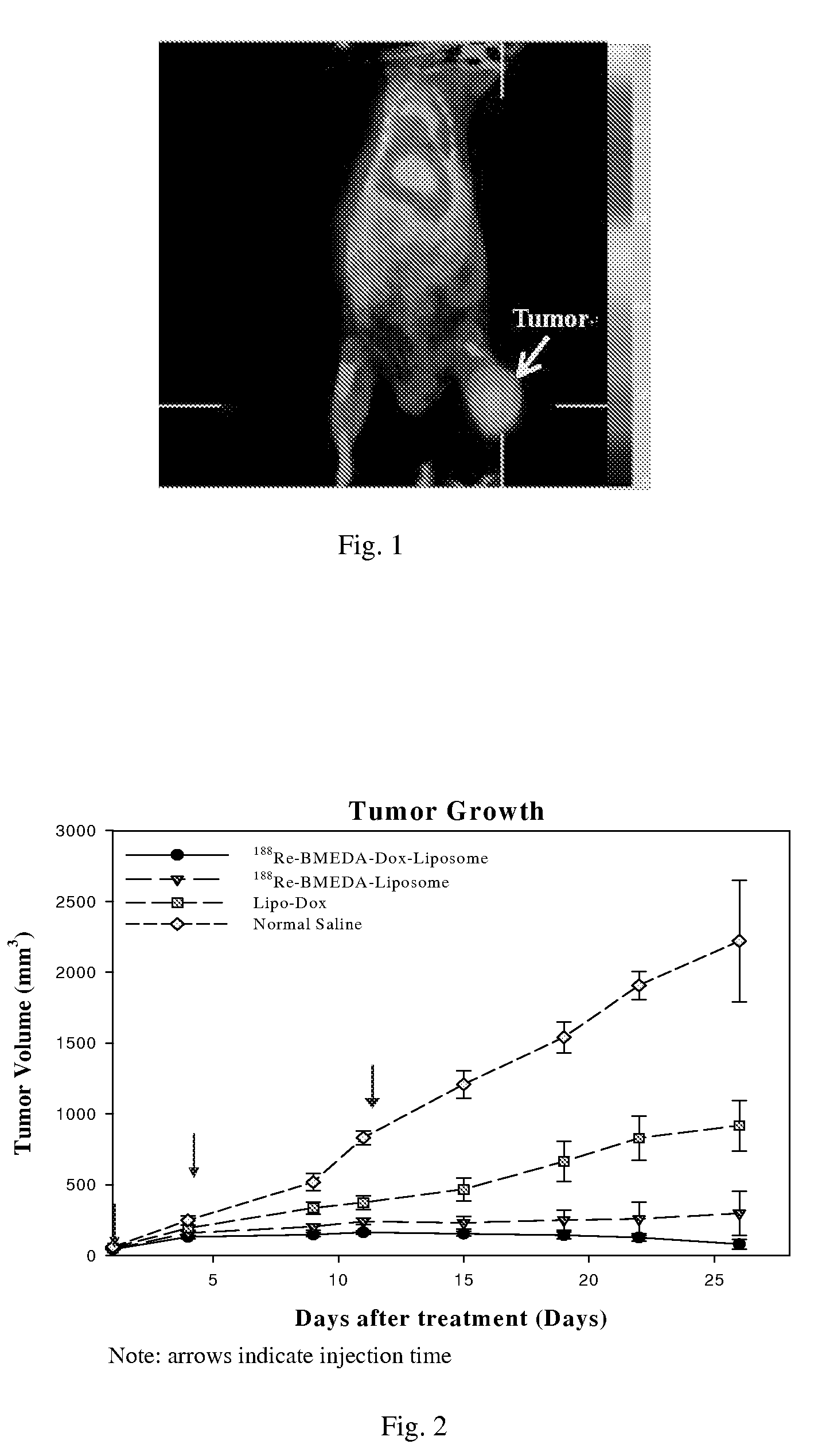
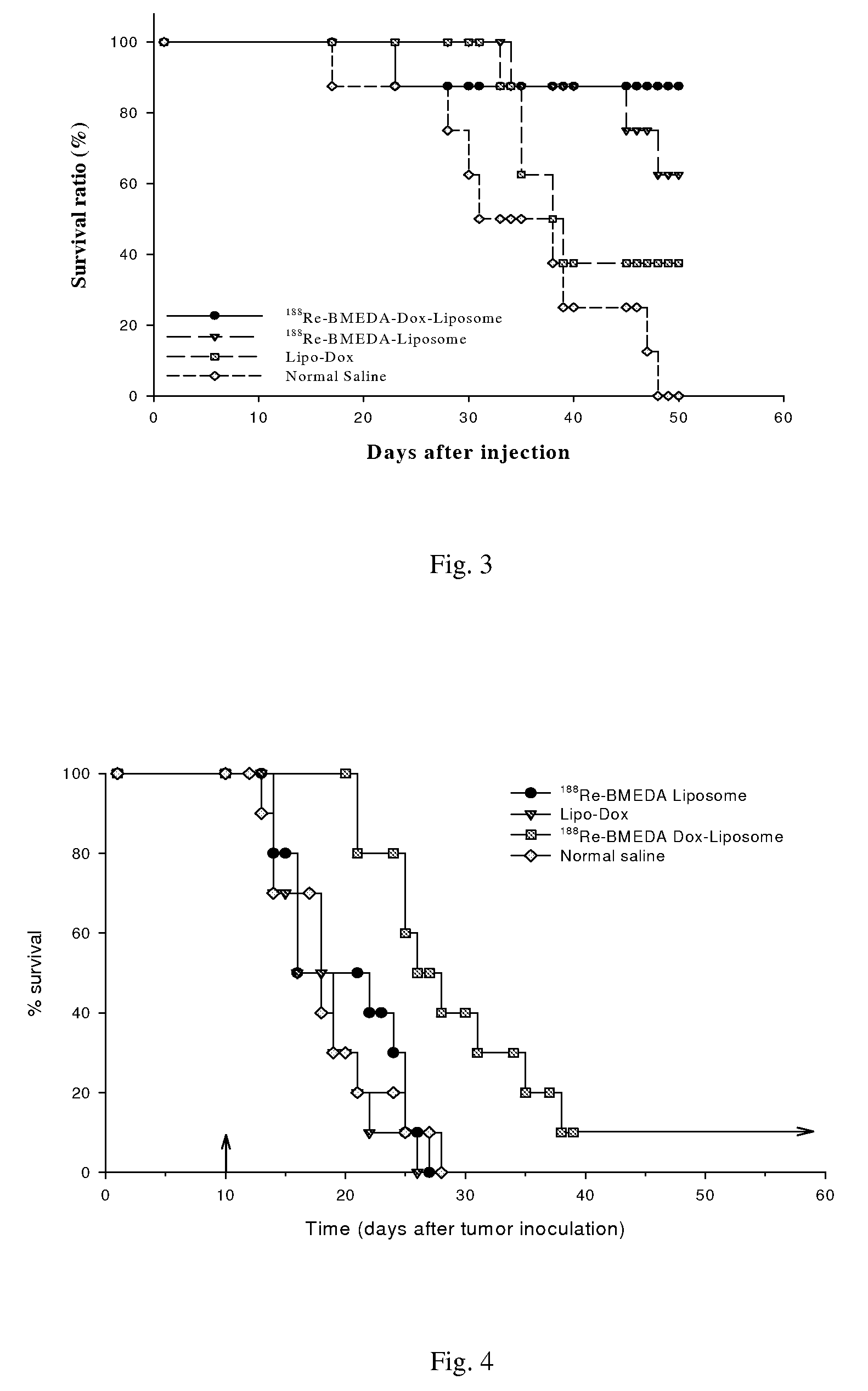
Kit for preparation of nano-targeted liposome drug in combined radionuclide therapy and chemotherapy
InactiveUS20080226546A1Simple and convenient and effectiveSimple and convenient and effective and easyOrganic active ingredientsRadioactive preparation carriersCholesterolDspe peg
This invention is to manufacture a kit for preparation of nano-targeted liposome drugs in combined chemotherapy and radionuclide therapy. It is a kit consisting of three components: (1) A 10 ml vial A which contains BMEDA, gluconate acetate, SnCl2. (2) A 10 ml vial B which contains DSPC, cholesterol, DSPE-PEG, and Doxorubicin(DXR) (or Daunorubicin, Vinolbine). (3) A 10 ml vial C which contains 188ReO4− (or 186ReO4−) solution. The procedure of using the kit is as follows: (1) Remove the contents of the 188ReO4− (or 186ReO4−) solution from vial C. (2) Inject the 188ReO4− (or 186ReO4−) solution into the vial A, and the mixtures react in appropriate temperature. (3) Remove the contents of the 188Re-BMEDA (or 186Re-BMEDA) solution from vial A. (4) Inject the 188Re-BMEDA (or 186Re-BMEDA) solution into the vial B, and the mixtures react in appropriate temperature. The reconstituted solution in the vial B is applied to combine bimodality radiochemotherapy for treatment of tumor and ascites.
Owner:INST NUCLEAR ENERGY RES ROCAEC +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com