Process method for preparing vinorelbine
A technology of vinorelbine and dehydrated vinblastine, which is applied in the field of chemical pharmaceuticals, can solve the problems of low yield, high cost, and increased production cost, and achieve the effect of reducing production cost and cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
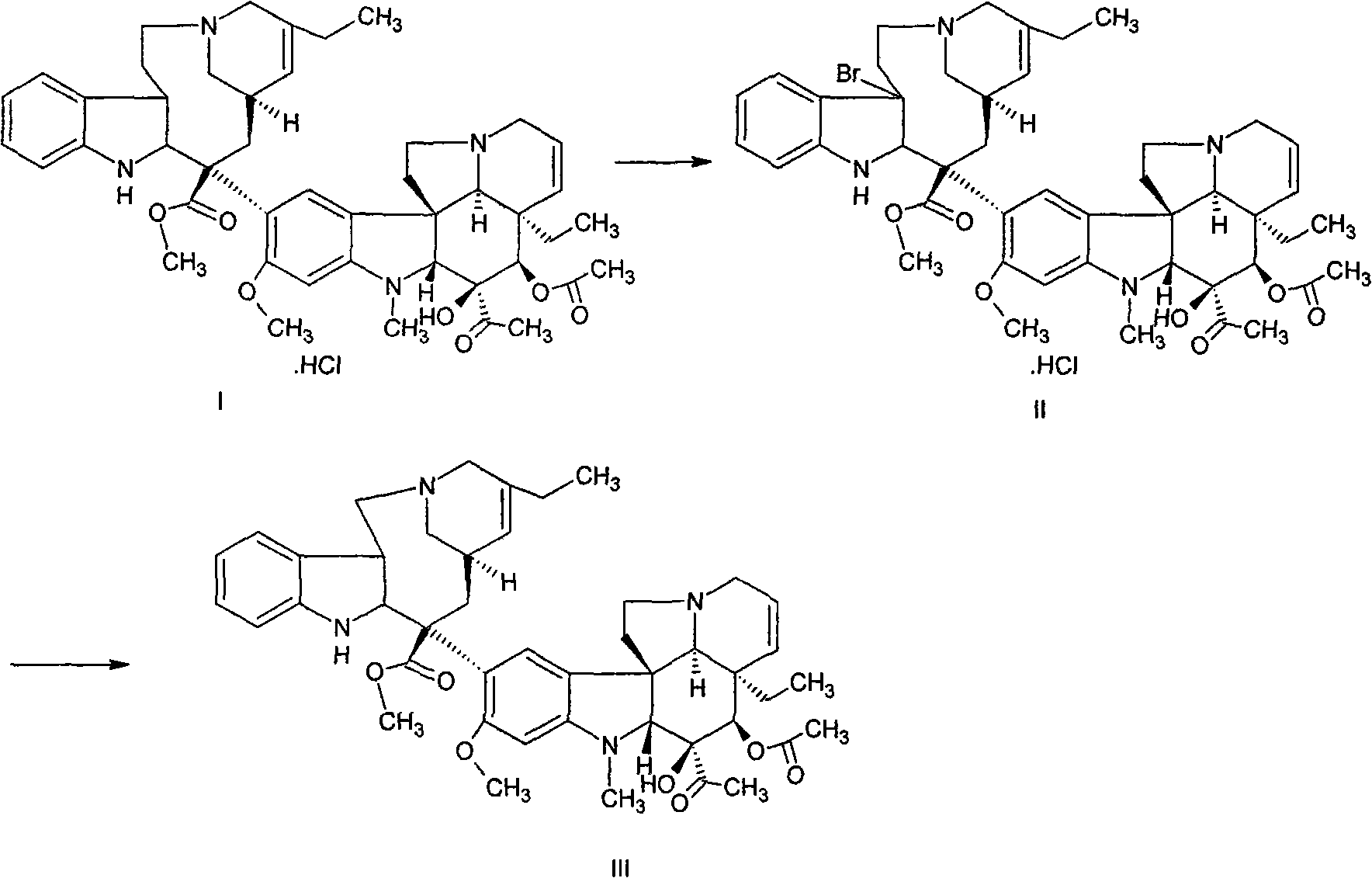
[0013] (1) Preparation of Vinorelbine Crude Product (III) from Dehydrated Vinblastine Hydrochloride (I)
[0014] In a dry round bottom flask, add 20 g of dehydrated vinblastine hydrochloride (prepared in laboratory, HPLC purity 92.5%), under the protection of light and nitrogen, add 1000 ml of dry dichloromethane, stir to dissolve, add 20 ml of pyridine, and use dry ice Cool the acetone bath to below -50°C, add dropwise a mixed solution of 6g of bromosuccinimide, 13ml of trifluoroacetic acid and 1000ml of dry dichloromethane, and keep stirring below -50°C for 2 hours after the dropwise addition. After the reaction, add a mixed solution of 12g silver nitrate, 12g ammonium acetate, 1000ml deionized water and 800ml tetrahydrofuran, stir rapidly, gradually heat up to 20-30°C, maintain this temperature, and stir for 16 hours. After the reaction was completed, 10% sodium carbonate aqueous solution was added dropwise with stirring to make the water phase pH 8-9, and the phases were s...
Embodiment 2
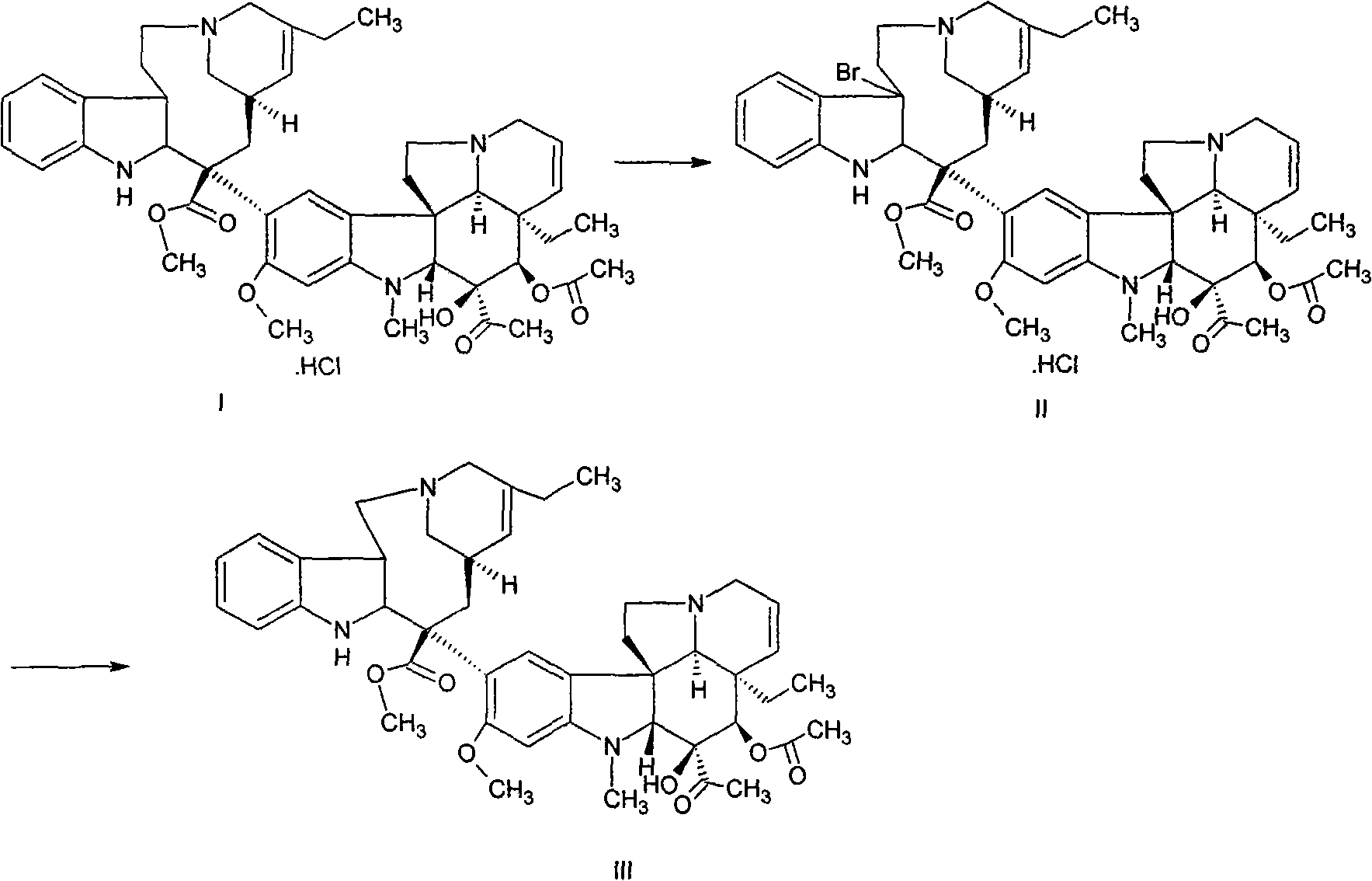
[0018] (1) Preparation of Vinorelbine Crude Product (III) from Dehydrated Vinblastine Hydrochloride (I)
[0019] In a dry round bottom flask, add 20 g of dehydrated vinblastine hydrochloride (prepared in laboratory, HPLC purity 92.5%), under the protection of light and nitrogen, add 1000 ml of dry dichloromethane, stir to dissolve, add 2,6-dichloromethane Take 20ml of picoline, cool down to below -50°C with a dry ice acetone bath, add dropwise a mixed solution of 5.5g of bromosuccinimide, 15ml of trifluoroacetic acid and 1000ml of dry dichloromethane, and keep at -50°C after the dropwise addition The reaction was then stirred for 1.5 hours. After the reaction is over, add a mixed solution of 12g silver nitrate, 12g ammonium acetate, 1000ml deionized water and 800ml tetrahydrofuran, stir rapidly, gradually heat up to 20-30°C, maintain this temperature, and stir for 24 hours. After the reaction was completed, 10% sodium carbonate aqueous solution was added dropwise with stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com