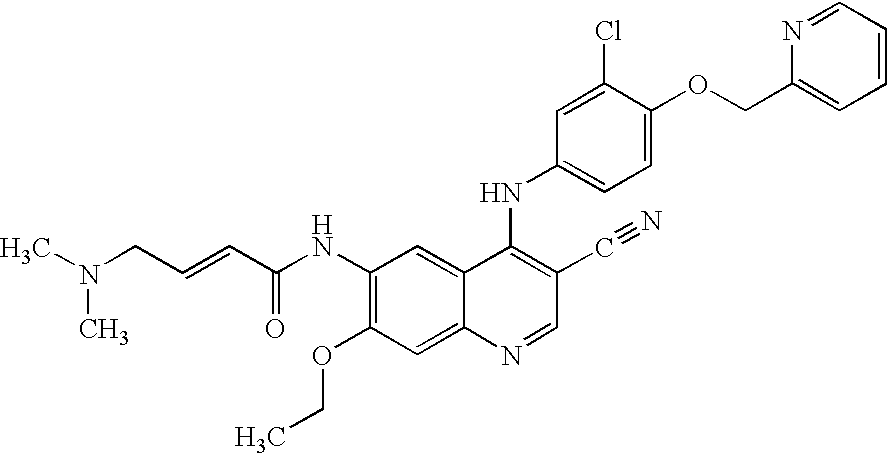
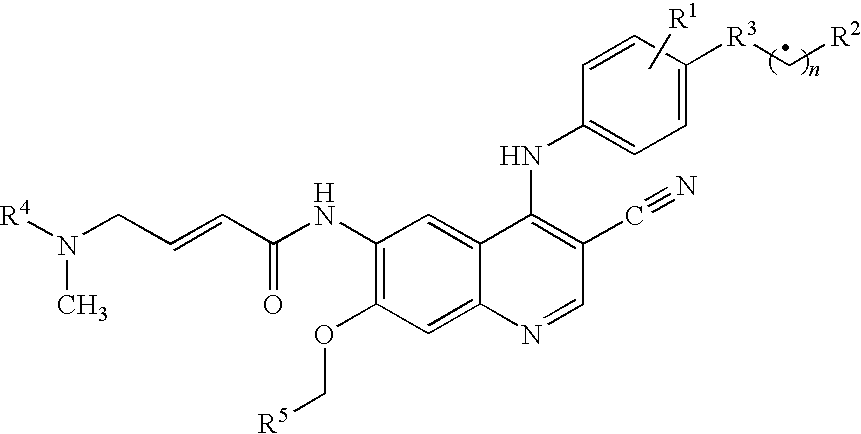
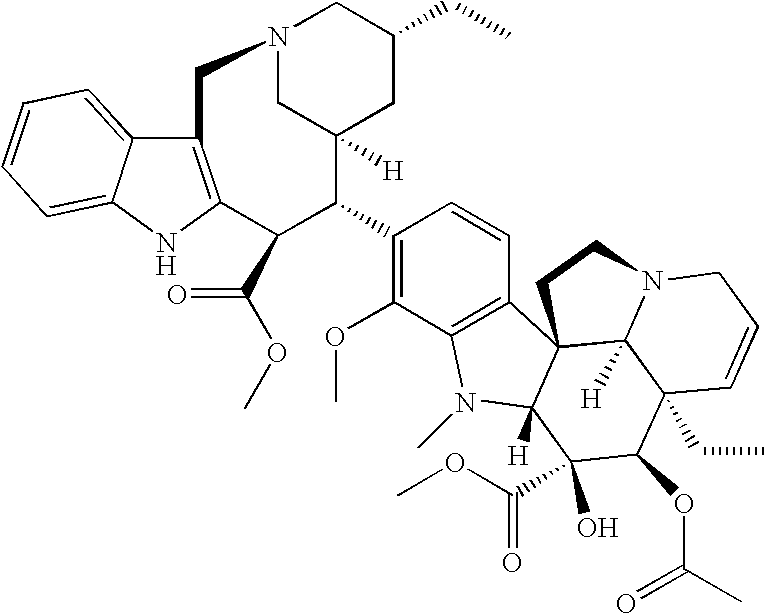
Antineoplastic Combinations Containing HKI-272 and Vinorelbine
a technology of hki-272 and vinorelbine, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of cancer cells which are not recognized, the risk of toxicity increases, and the association of trastuzumab-based therapy with potential cardiac toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Combination Regimen of HKI-272 and Vinorelbine in Lung Cancer Cell Proliferation Assays
[0108]A standard cell proliferation assay was utilized to independently analyze the response of lung cell lines NC1-H1666, NC1-H1650, and NC1-H1975 to various dilutions of HKI-272 and vinorelbine in combination. Briefly, fetal bovine serum (FBS) RPM1-1640 (Media) was added to each well of 96 well plates containing one of the cell lines. Each column of wells contained a different dilution of HKI-272 and solutions of vinorelbine were added to each well at a variety of dilutions with respect to the HKI-272 dilutions (the highest final concentration of HKI-272 was 1 μM for the H1650 and H1666 cell lines; the highest final concentration of HKI-272 was 9 μM for the H1975 cell line; and the highest final concentration of vinorelbine was 0.1 μM for all of the cell lines). Following incubation of the cell plates at 37° C., 5% CO2 for 72 hours, cell proliferation was assessed.
[0109]Cell proliferation was re...
example 2
Combination Regimen of HKI-272 and Vinorelbine in Treatment of Non-Metastatic Breast Cancers
[0110]Patients having diagnosed non-metastatic breast cancers are treated using a regimen of HKI-272 and vinorelbine. Patients are administered HKI-272 at either dose level 1 or 2. Dosing of HKI-272 begins at cycle 1, day 1 with daily oral administration of HKI-272 at the dosages in Table 3. HKI-272 is taken orally on the remaining days of the each cycle. On those days that HKI-272 and vinorelbine are administered on the same day, i.e., days 1 and 8 of the cycle, HKI-272 is administered prior to the vinorelbine infusion.
TABLE 3Dose LevelHKI-272 Dose (mg)Vinorelbine Dose (mg / m2)1160252240
[0111]Vinorelbine is administered on days 1 and 8 of each 21-day cycle, provided that the combination of HKI-272 and vinorelbine is well tolerated and there is no evidence of disease progression. Vinorelbine is administered intravenously using preferentially a central venous route, through a free-flowing IV li...
example 3
Combination Regimen of HKI-272 and Vonorelbine in Treatment of Metastatic Breast Cancers
[0114]Patients having diagnosed metastatic breast cancers are treated using a regimen of HKI-272 and vinorelbine.
[0115]Vinorelbine is administered on day 1 and day 8 of the cycle using the dosages described in Example 2, Table 3 or 5. The vinorelbine is administered over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to vinorelbine infusion.
[0116]Dosing of HKI-272 begins at cycle 1, day 2 with daily oral administration of HKI-272 at the dosages provided in Example 2, Table 3 or 4. HKI-272 is taken orally on the remaining days of the each cycle. On those days that HKI-272 and vinorelbine are administered on the same day, i.e., day 8 of the cycle, HKI-272 is administered prior to the vinorelbine infusion. If the patient has any serious side-effects durin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com