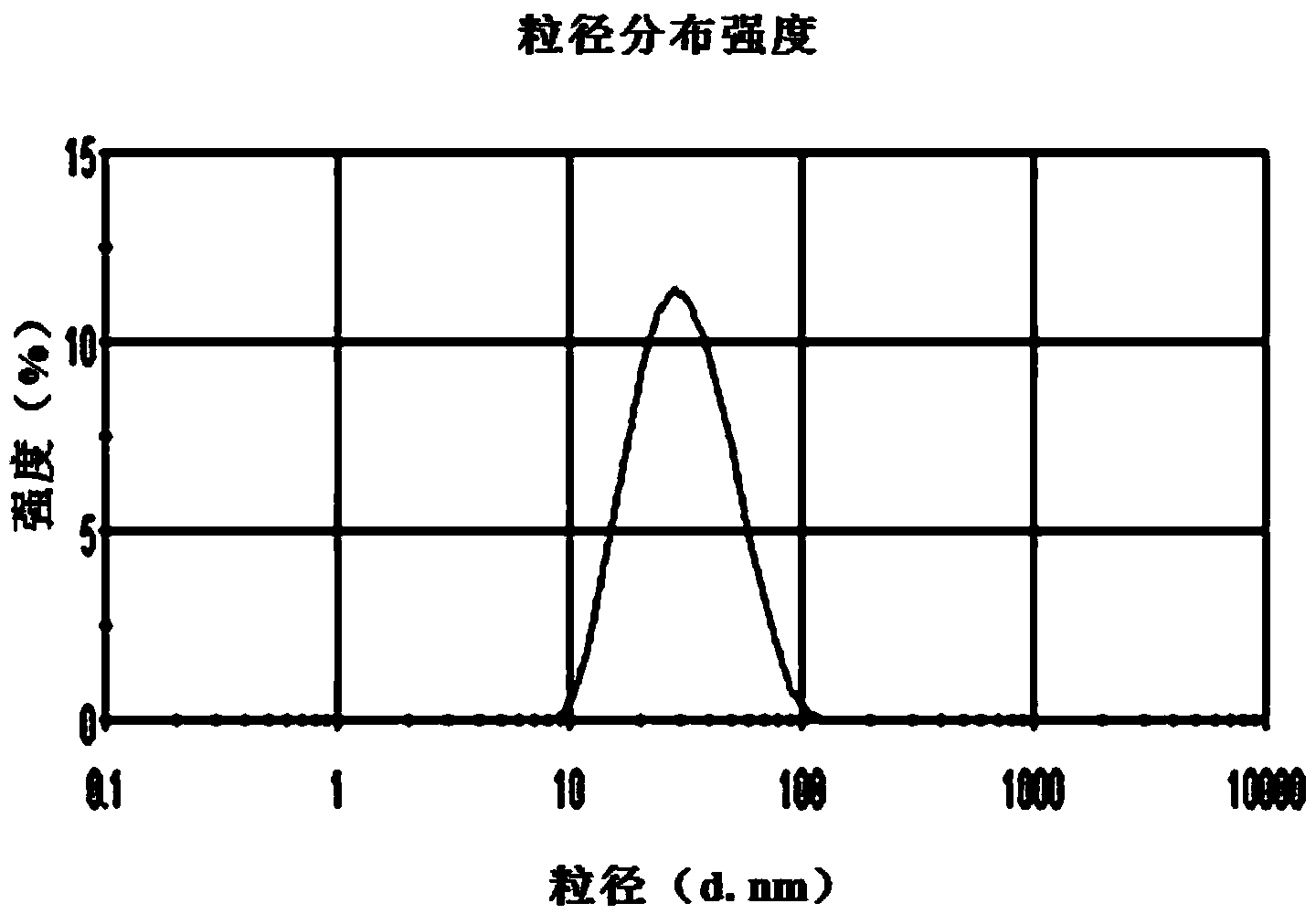
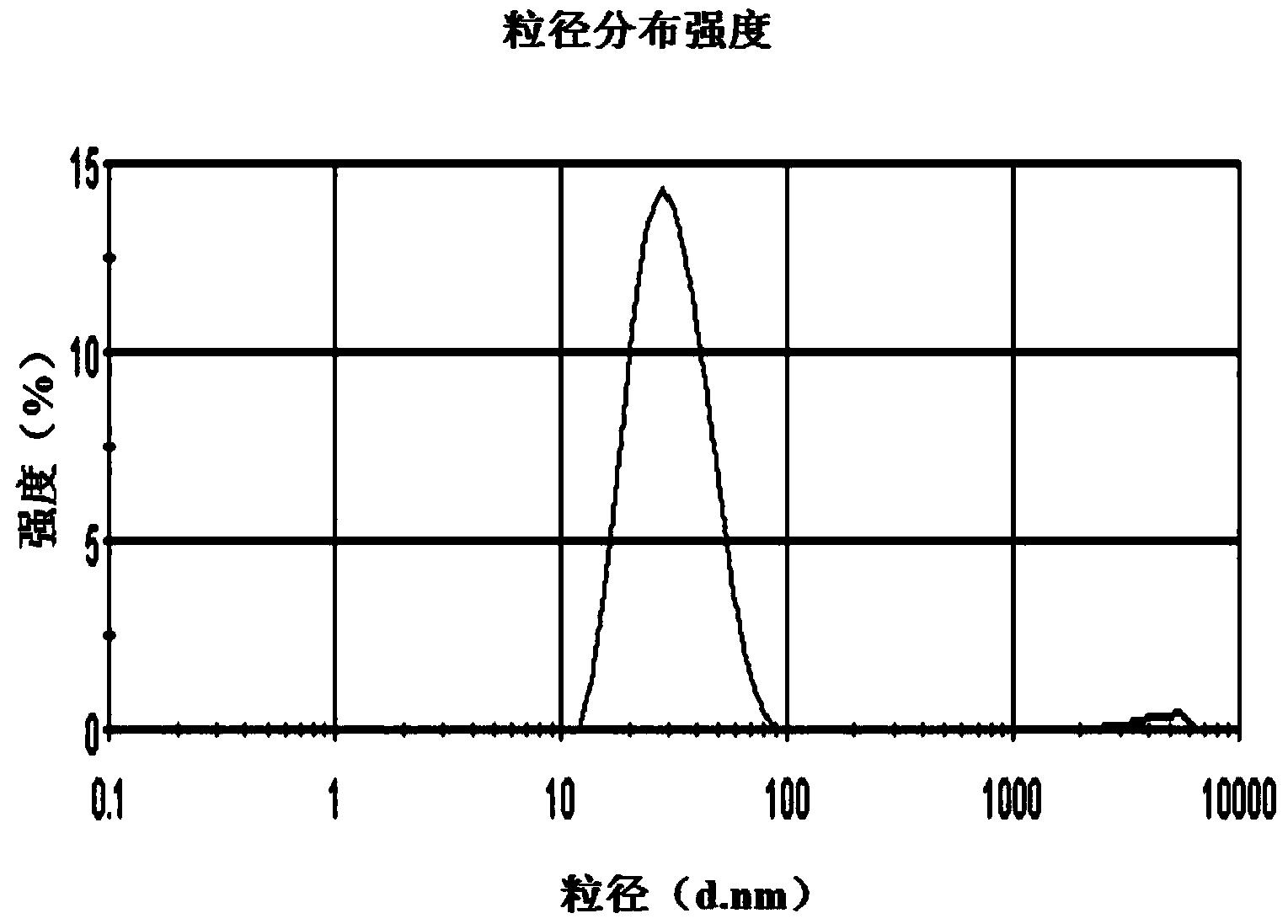
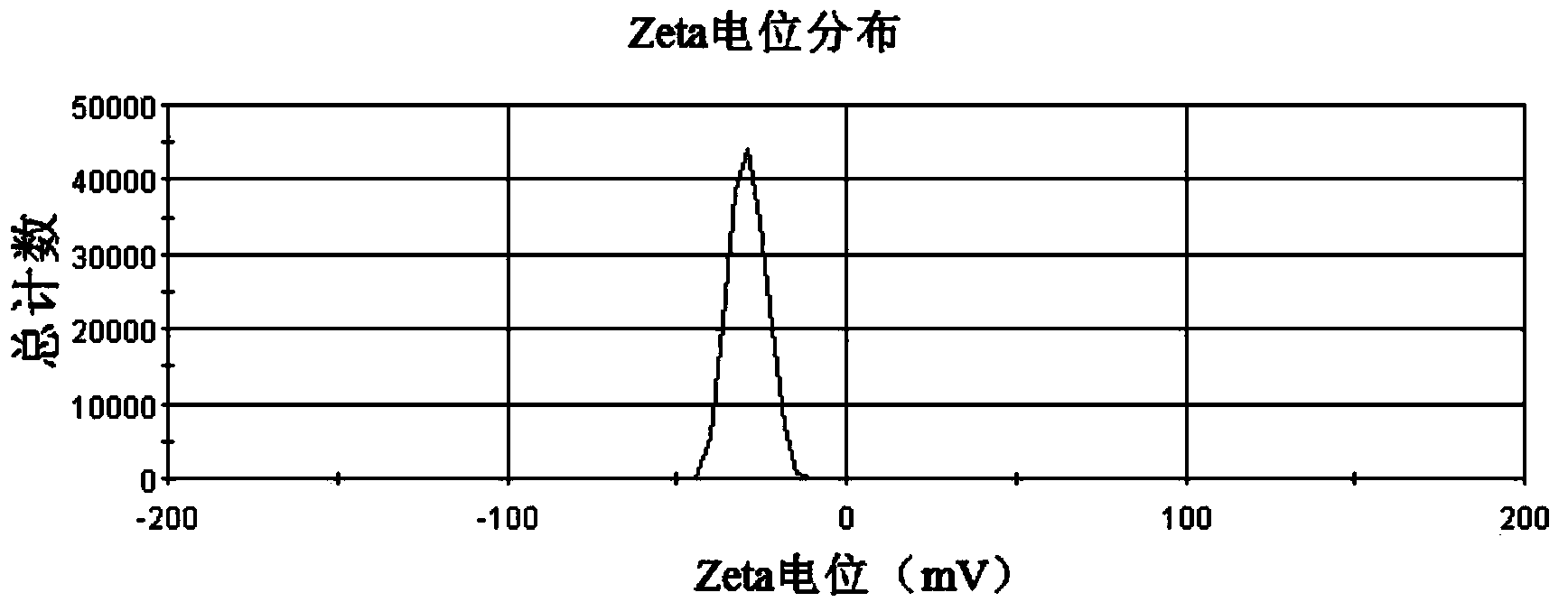
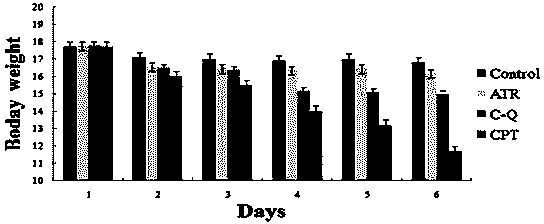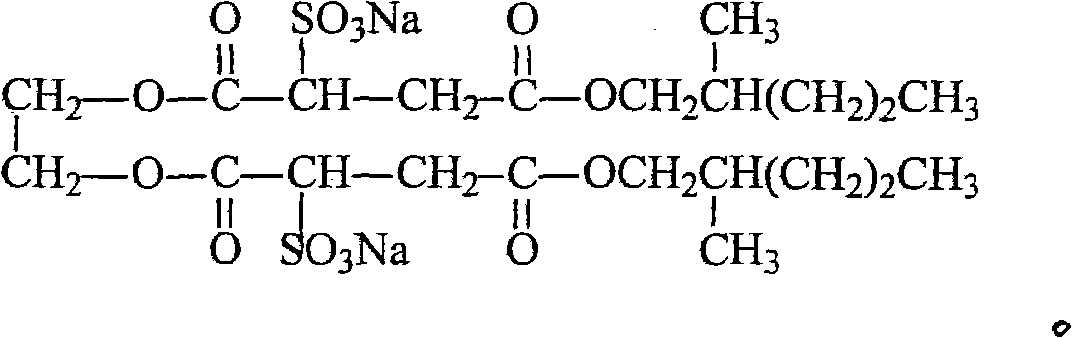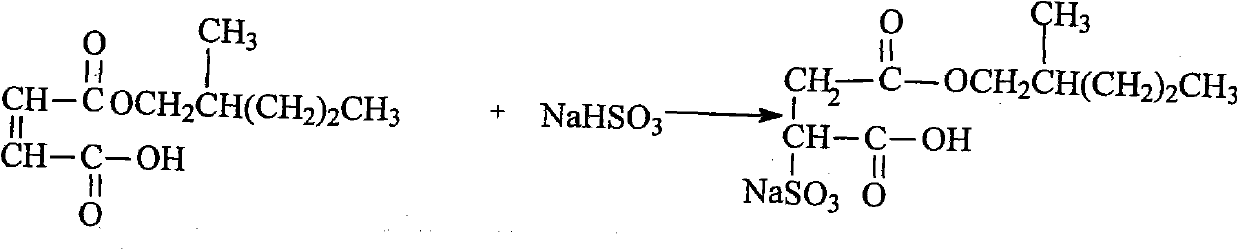Patents
Literature
176 results about "Artesunate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
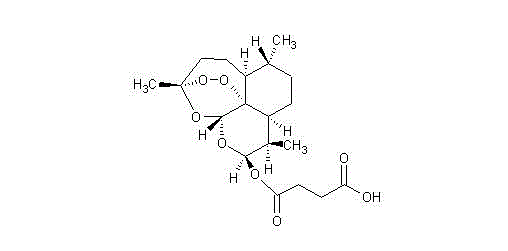
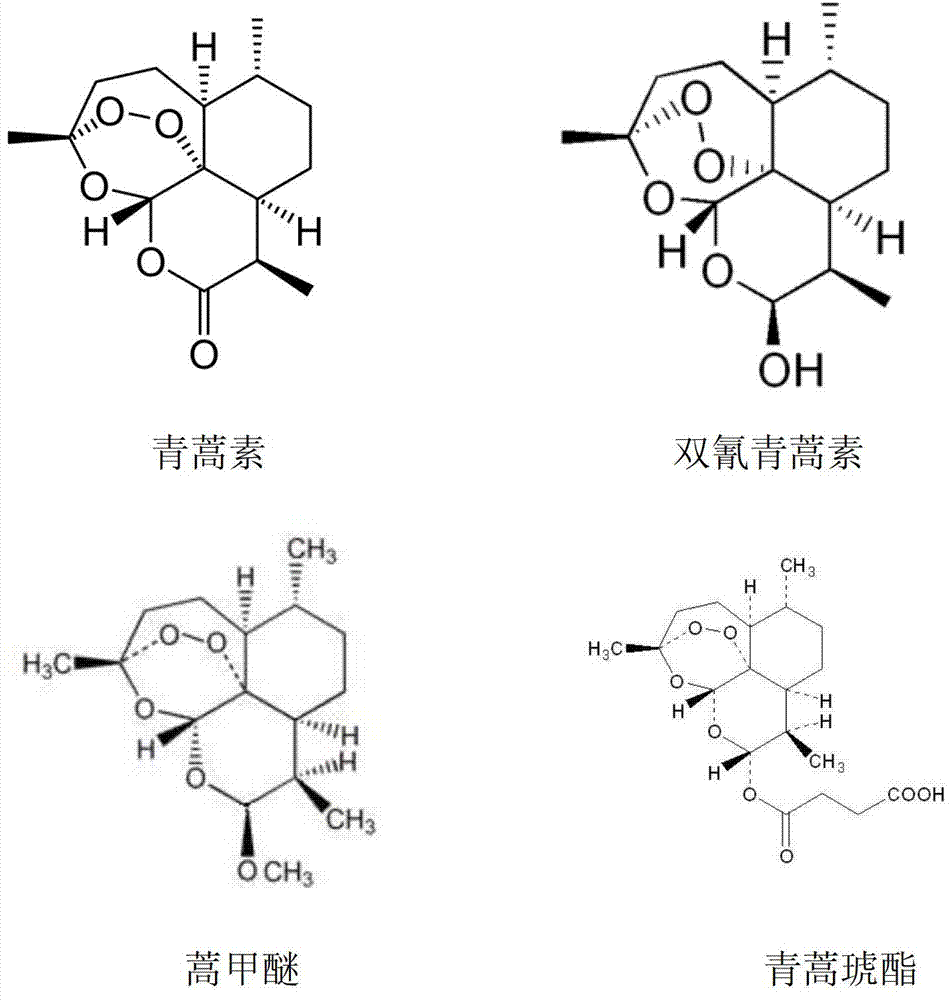
Artesunate (AS) is a medication used to treat malaria, invented by Liu Xu in 1977. The intravenous form is preferred to quinidine for severe malaria. Often it is used as part of combination therapy, such as artesunate plus mefloquine. It is not used for the prevention of malaria. Artesunate can be given by injection into a vein, injection into a muscle, by mouth, and by rectum.
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
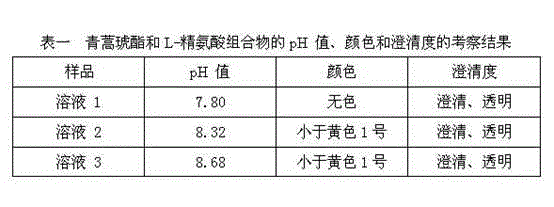
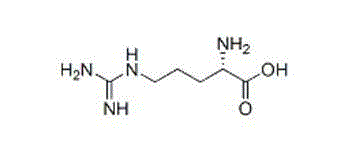
Artesunate and L-arginine composition for injection and preparation method thereof
InactiveCN104414977AImprove stabilityEasy to storeOrganic active ingredientsPowder deliveryFreeze dryArtesunate
The invention discloses an artesunate and L-arginine composition for injection. The artesunate and L-arginine composition comprises the following components in parts by weight: 1,000 parts of artesunate as a raw material and 450-900 parts of L-arginine as an auxiliary material. According to the composition, the artesunate which is poor in solubility can be dissolved; the pH value can be adjusted to be neutral to slightly alkaline for injection; the solution is clear, transparent and colorless; the composition has good solubility and mobility, and can be sub-packaged into a practical powder preparation for injection; and more importantly, the stability of the composition is improved beyond thought. The invention also provides a preparation process of the artesunate and L-arginine composition for injection. The preparation method comprises the following steps: directly mixing the raw materials, namely artesunate and L-arginine; sub-packaging, or dissolving and degerming firstly and then preparing powder for injection by adopting a freeze-drying technology. The artesunate and L-arginine composition for injection disclosed by the invention is simple in preparation process and stable and reliable in quality.
Owner:CHONGQING HUIZHI PHARMA RES INST +1
Application of artemisinin derivative in preparation of medicaments for treating Crohn disease
InactiveCN102048728AFew and mild side effectsAchieving the goal of treating Crohn's diseaseOrganic active ingredientsDigestive systemSide effectHepatic dysfunction
The invention discloses an application of an artemisinin derivative in preparation of medicament for treating Crohn disease, and the artemisinin derivative is selected from artesunate or artemether or styrene monomer 905 (SM905). The artemisinin derivative can be used for achieving the purpose of treating the Crohn disease by inhibiting Th1 / Th17 immune response and inhibiting nuclear factor-kB activation. Compared with the existing medicaments, the artemisinin derivative has less and light side effects, in one year of following visits for the treatment of rheumatoid arthritis, transient reticulocyte decline and hepatic dysfunction only occur in individual cases, and the health can be restored by liver protection treatment without medicament withdrawal. Therefore, the artemisinin derivative can be taken by patients for a long time, the treatment can be maintained, and the purpose of effective treatment can be achieved.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Medicinal composition for resisting non-small cell lung cancer, and application thereof
InactiveCN106668866ASolve the problem of partial drug resistanceInhibition of phosphorylation levelsOrganic active ingredientsAntineoplastic agentsActive componentDihydroartemisinin
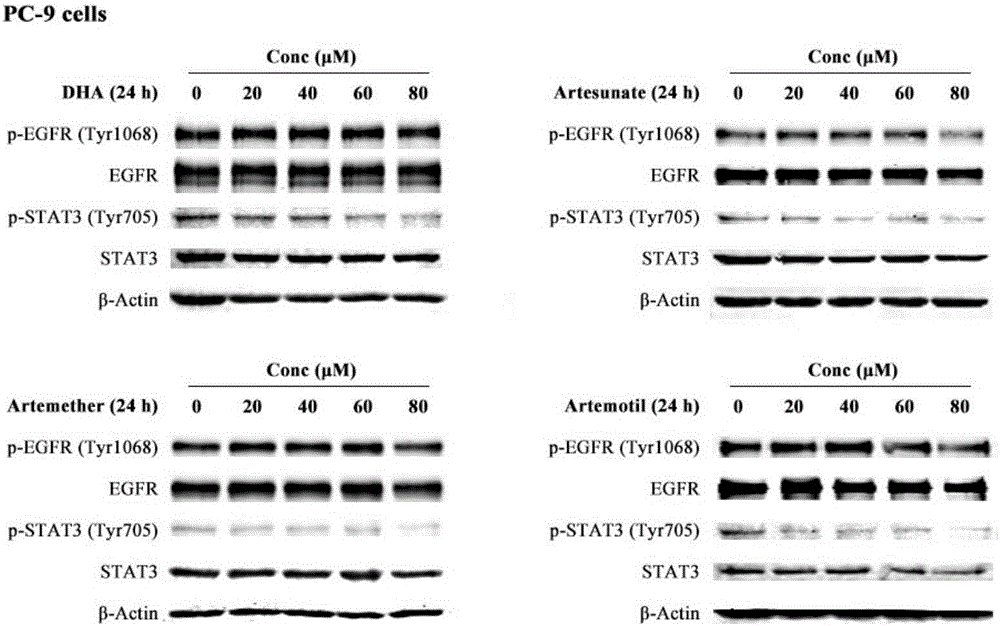
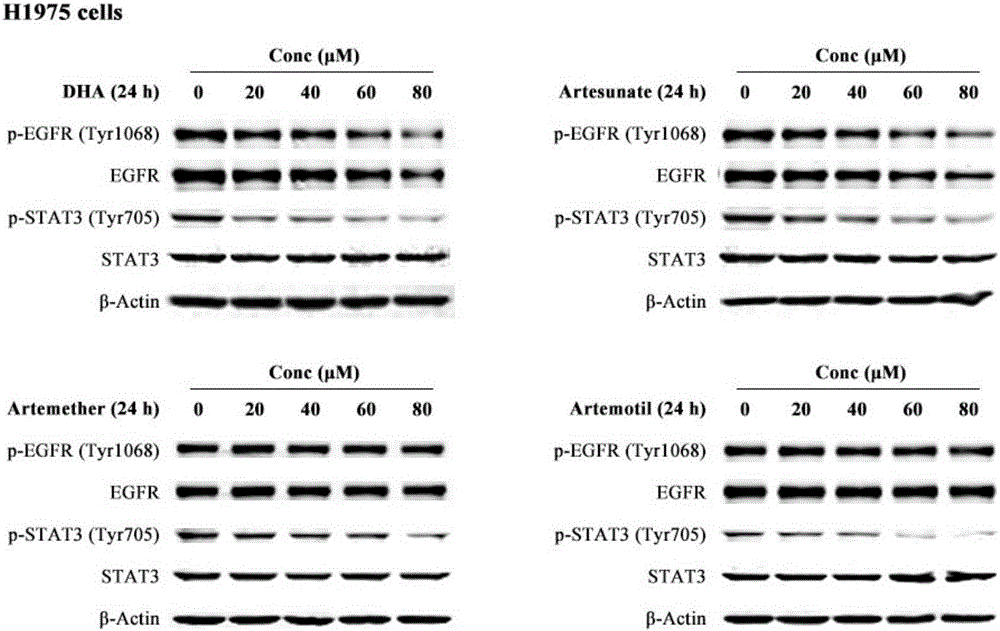
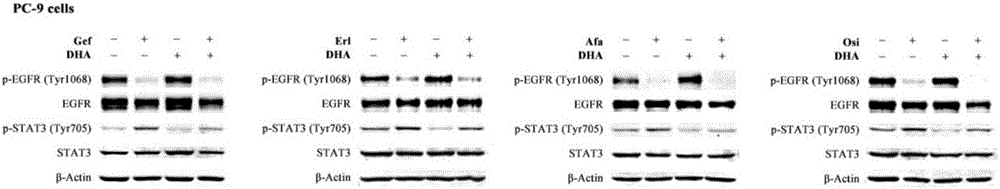
The invention discloses a medicinal composition for resisting non-small cell lung cancer. The active components of the medicinal composition comprise an artemisinin derivative and EGFR-TKI (epidermal growth factor receptor-tyrosine kinase inhibitor), wherein the artemisinin derivative is selected from one of dihydroartemisinin, artesunate, artemether and arteether; and the EGFR-TKI is selected from one of gefitinib, erlotinib, afatinib and osimertinib. The invention also discloses application of the medicinal composition to preparation of medicines for treating and resisting the non-small cell lung cancer. When the medicinal composition provided by the invention is used for treating the non-small cell lung cancer, the medicinal effect which is more excellent than that of the singly used EGFR-TKI can be achieved, the sensitizing effect is achieved, the problem of partial medicine resistance of the non-small cell lung cancer EGFR-TKI is solved, and a scientific basis is provided for the development of new medicines.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Southernwood total flavone, method for preparing its composition and medicine uses thereof
InactiveCN101313927AReduce manufacturing costProcess stabilityOrganic active ingredientsAntipyreticRhizomeMacroporous resin
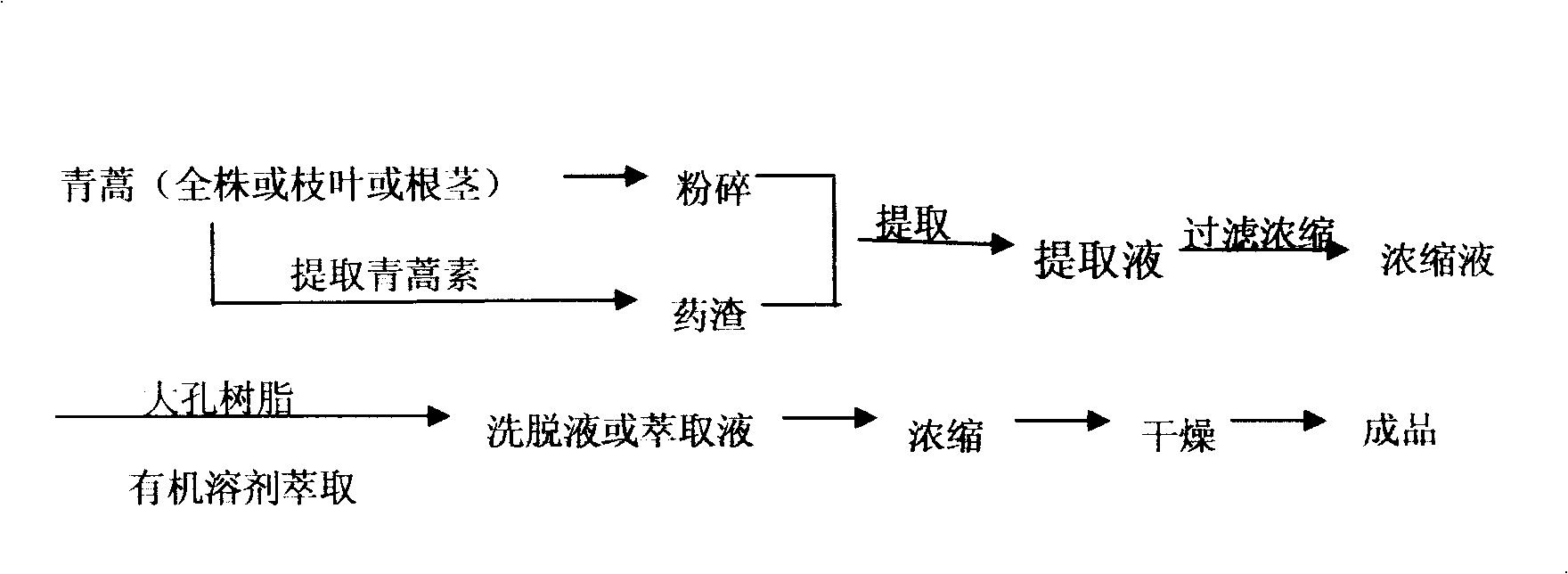
The invention belongs to the Chinese traditional medicine preparation method and the application field, in particular relating to a method for preparing southernwood total flavone and a composition thereof and a medicinal application thereof. The preparation method comprises the following steps that: the whole strain or branches and leaves or rootstalks of southernwood or medical dregs left after artesunate is extracted from the southernwood are used as raw materials, the raw materials are extracted by water or / and alcohols, the obtained extracting solution is subject to filtering and concentration, the obtained concentrates are separated by macroporous resins or extracted and separated by organic solvents, the eluents or extract liquor are concentrated and dried to prepare the southernwood total flavone, and the southernwood total flavone can be used to prepare medicines for treating and preventing tumors, arteriosclerosis, AIDS, flu, herpes, hepatitis and inflammatory reactions caused by pathogenic microbes, or be applied to products related to the diseases. The invention also relates to a medicinal application of a composition in resisting tumors, resisting cardiovascular diseases, resisting inflammation, relieving pain, reducing heat, regulating immunity and resisting bacteria and viruses, wherein the composition comprises 10 to 90 portions of the southernwood total flavone and 90 to 10 portions of artemisia oil.
Owner:薛永新
Artemisinin-Based Combination Therapy For Treating Parasitic Mediated Disease
The present invention describes a method of treating individuals suffering from microbial infections, including a parasitic disease such as malaria, by using an improved Artemisinin Combination Therapy, known as Tri-ACT. The improved ACT therapy includes administering a combination of three drugs. In one embodiment of the present invention, the method includes administering to an individual a first composition comprising a therapeutically effective amount of an artemether spray sublingually. The individual is then administered a second composition, a therapeutically effective amount of artesunate. A third composition, an effective amount of berberine, or its pharmaceutically acceptable derivatives or salts is administered to the individual.
Owner:KRYPTONITE GRP
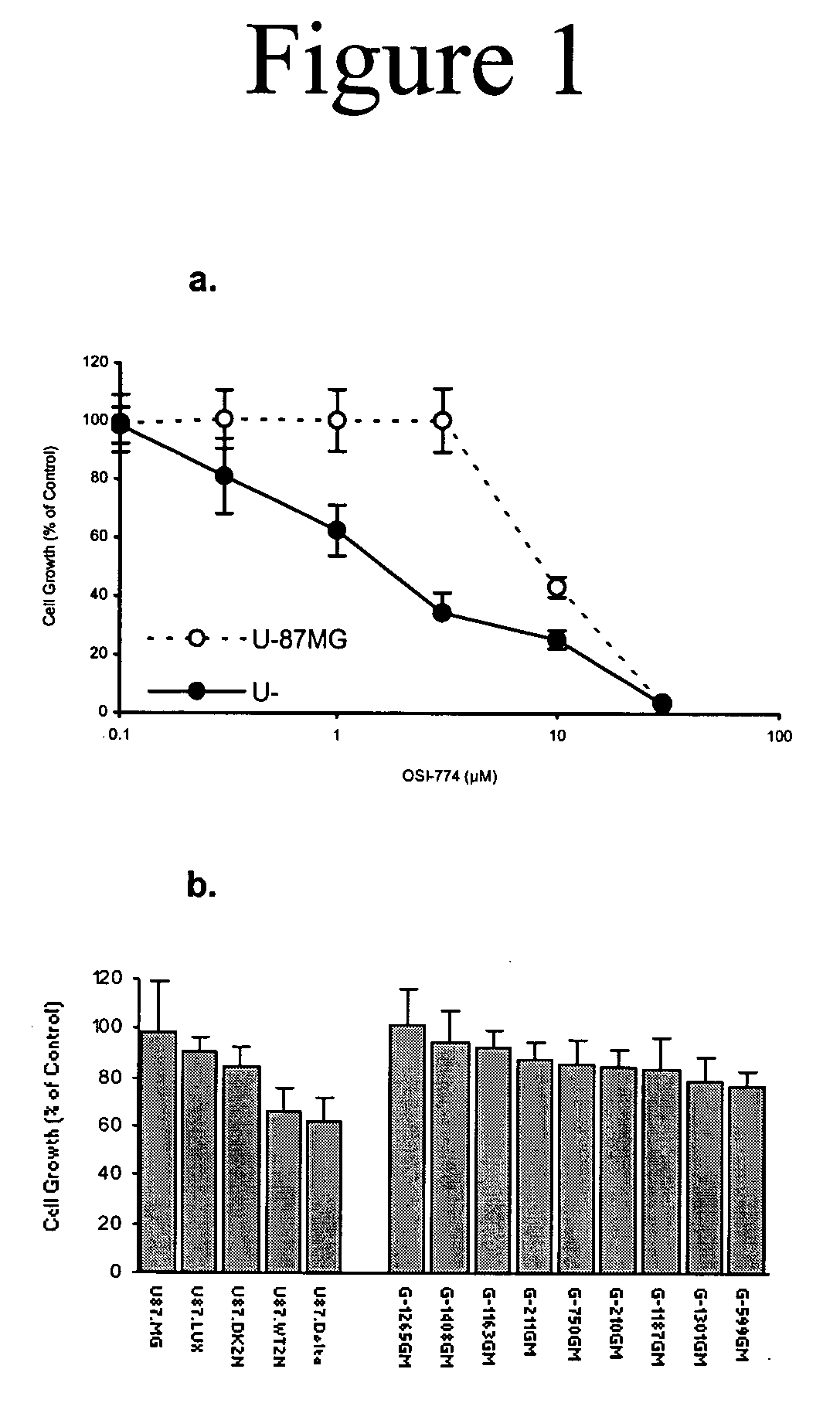
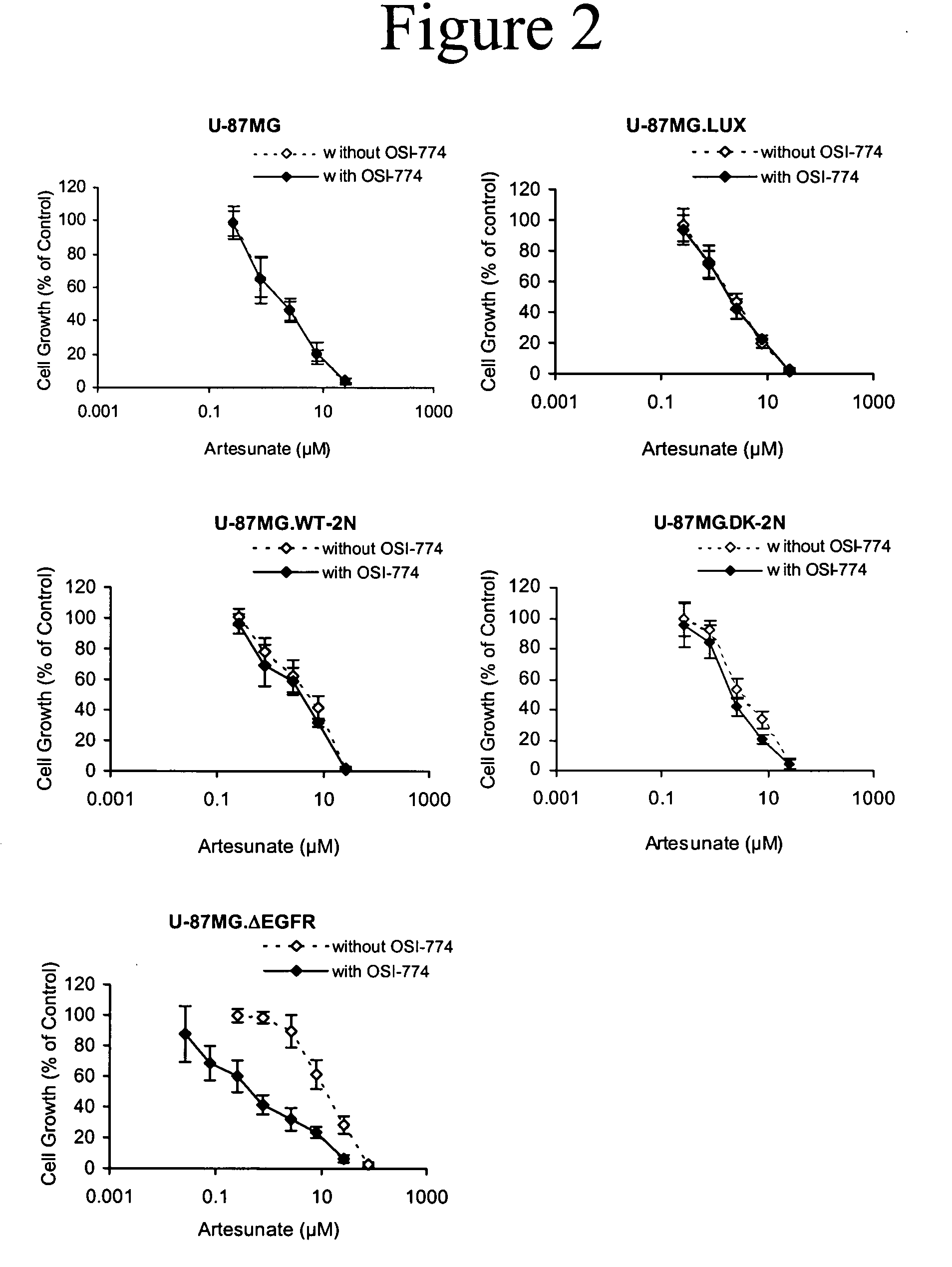
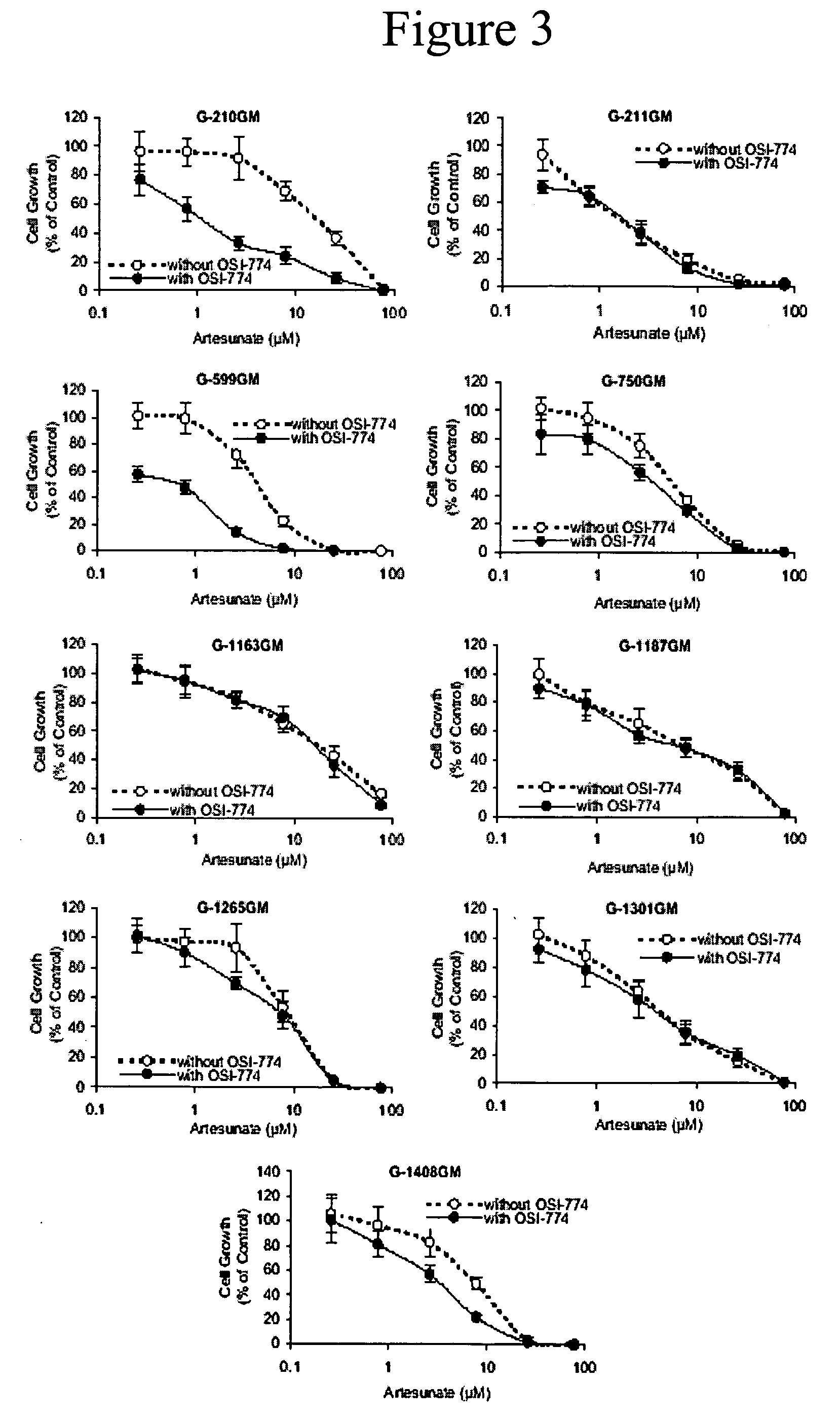
Combined treatment with artesunate and an epidermal growth factor receptor kinase inhibitor
InactiveUS20060084675A1BiocideAnimal repellantsAbnormal tissue growthEpidermal Growth Factor Receptor Kinase
The present invention provides a method for treating tumors or tumor metastases in a patient, comprising administering to the patient simultaneously or sequentially a therapeutically effective amount of an EGFR kinase inhibitor and artesunate combination, with or without additional agents or treatments, such as other anti-cancer drugs or radiation therapy. The invention also encompasses a pharmaceutical composition that is comprised of an EGFR kinase inhibitor and artesunate combination in combination with a pharmaceutically acceptable carrier. A preferred example of an EGFR kinase inhibitor that can be used in practicing this invention is the compound erlotinib HCl (also known as Tarceva™).
Owner:EFFERTH THOMAS +1
Use of artemisinin and its derivatives in cancer therapy
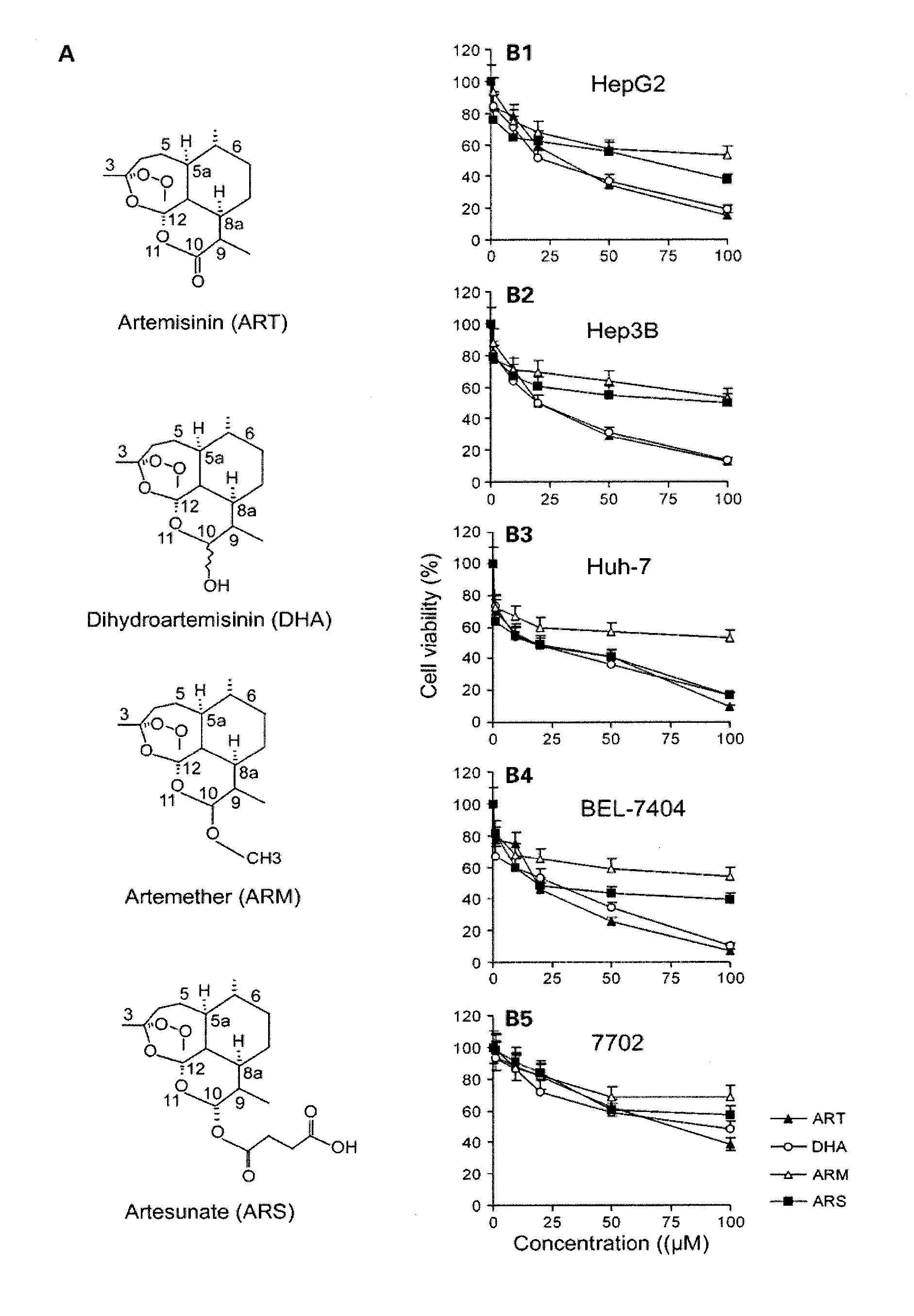
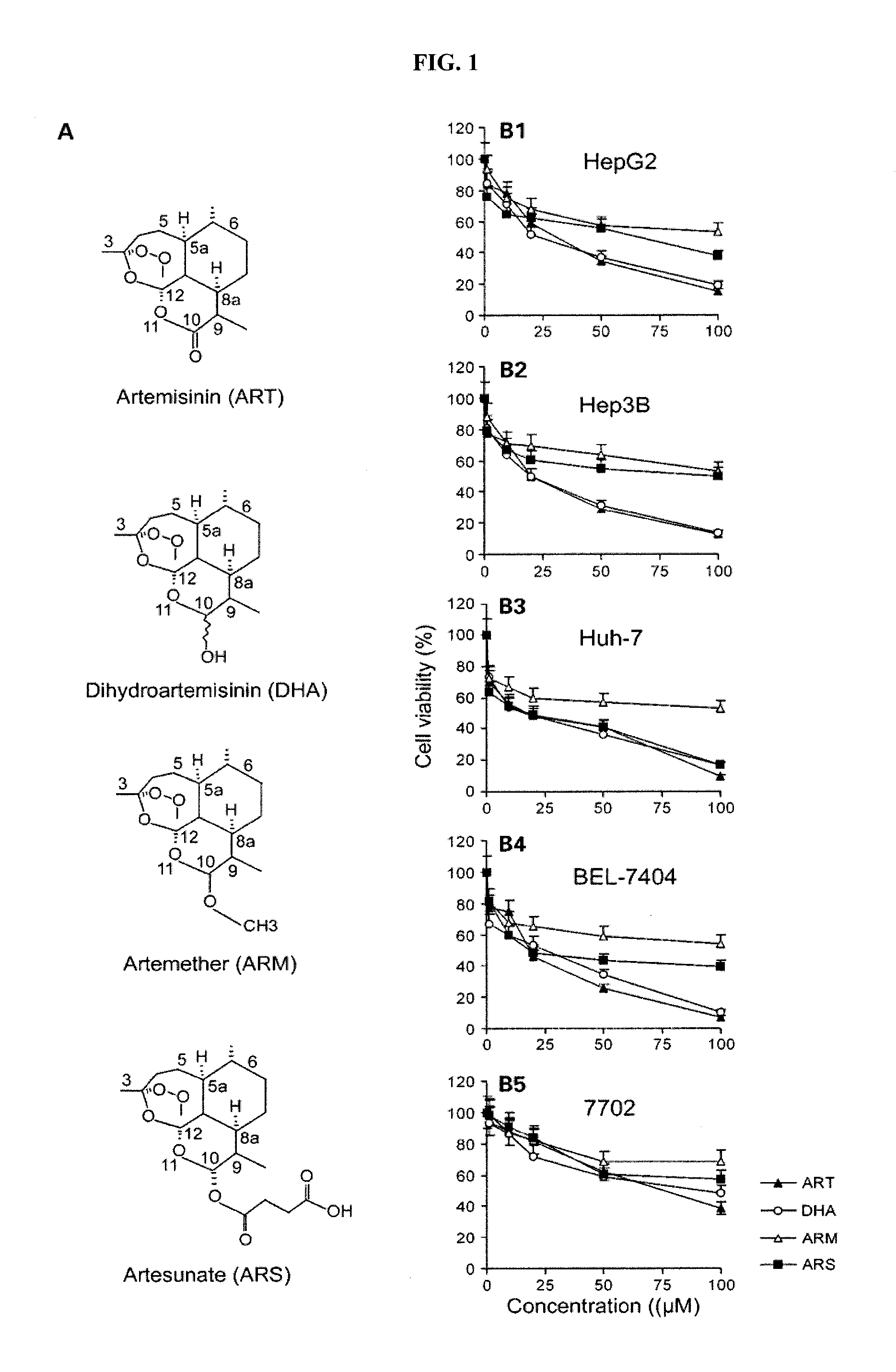
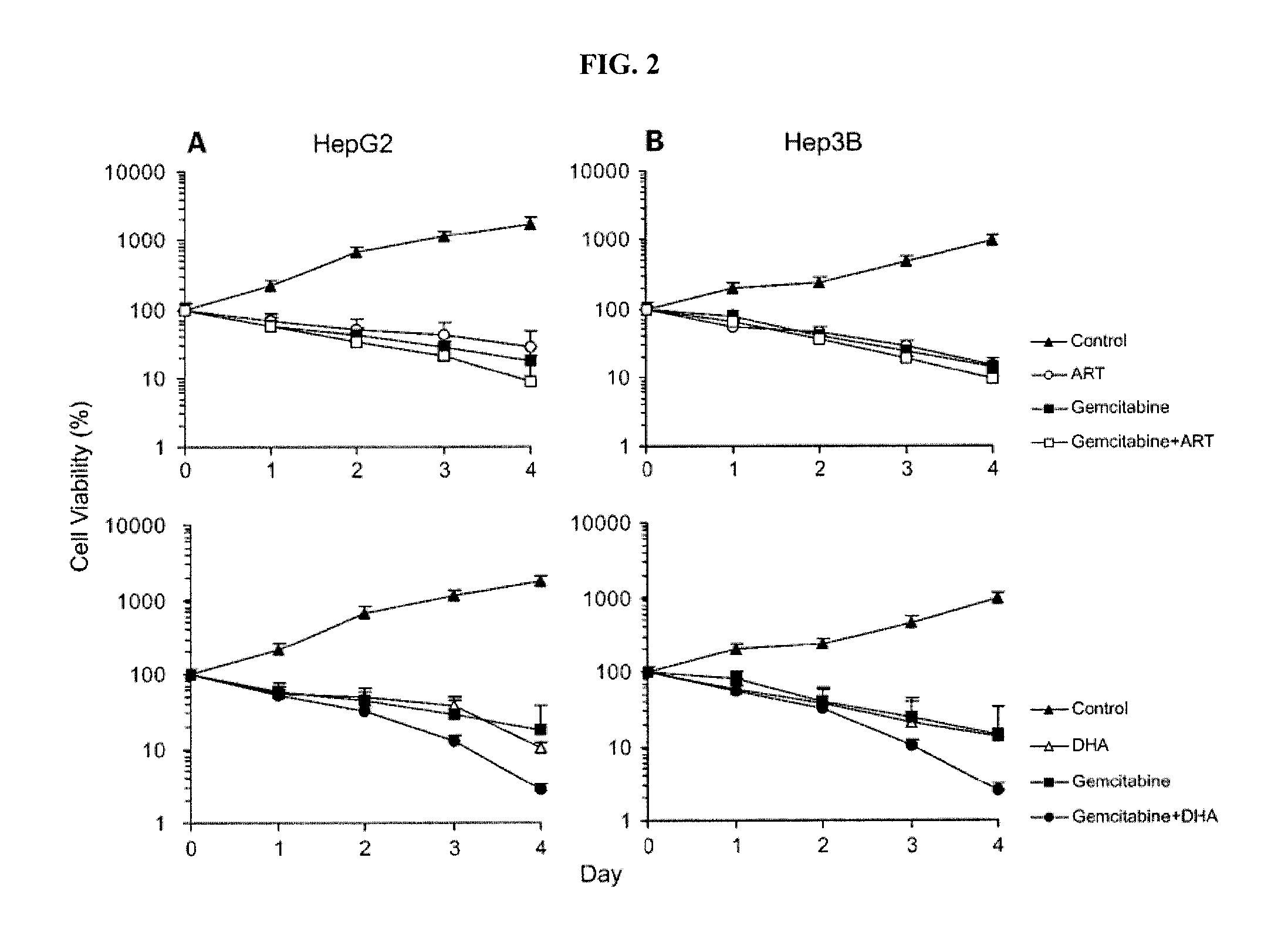
A method for treating cancer in a mammal includes administering to the mammal in need thereof a therapeutically effective amount of artemisinin (ART) or its derivative, such as dihydroartemisinin (DHA), artemether (ARM), or artesunate (ARS) alone or in combination with a chemotherapeutic agent, such as gemcitabine and carboplatin. A method for inhibiting tumor cell proliferation includes contacting a tumor cell with ART or its derivative, such as DHA, ARM, and ARS, in an amount effective to inhibit tumor cell proliferation or in combination with a chemotherapeutic agent, such as gemcitabine and carboplatin.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
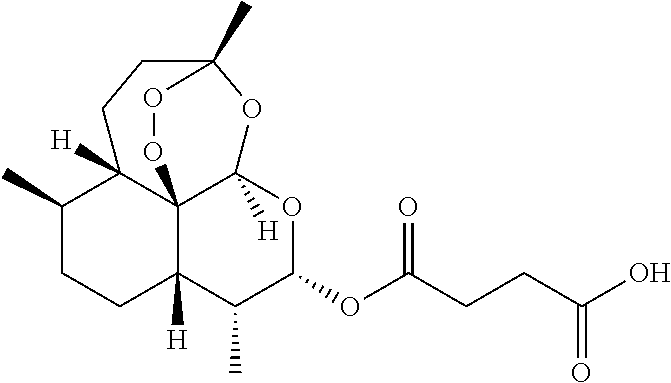
Camptothecin and artesunate conjugate, preparation method and application thereof
ActiveCN104163823AHas antitumor activityReduce harmOrganic active ingredientsOrganic chemistryOrganic solventMedicinal chemistry
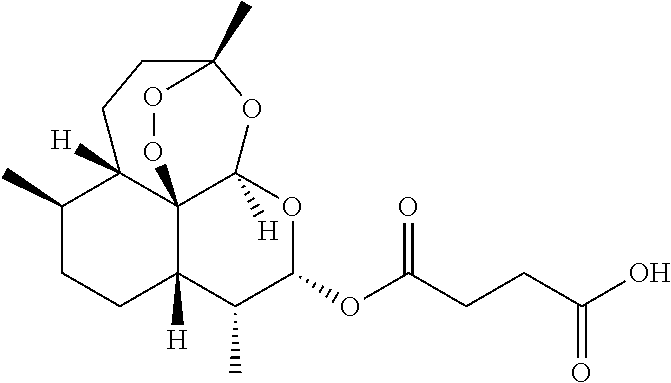
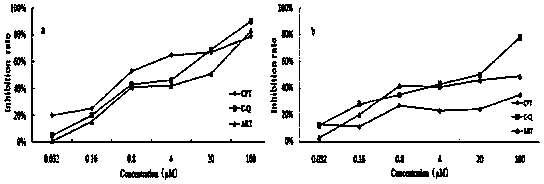
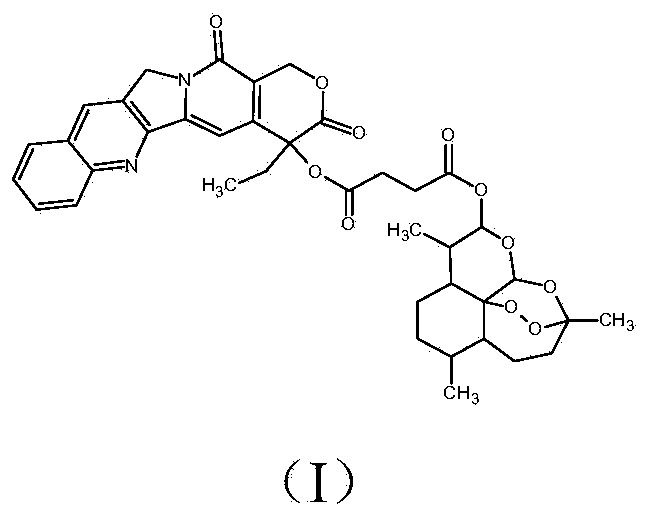
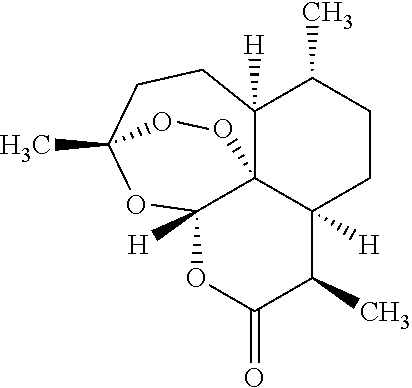
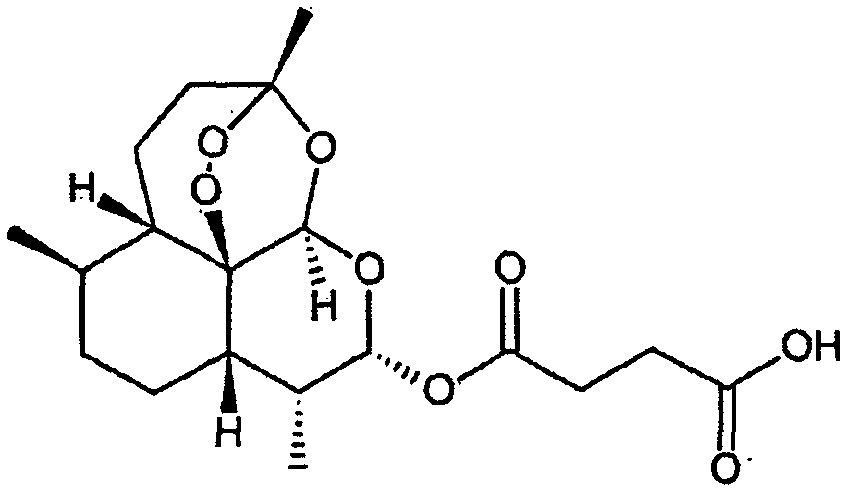
The present invention discloses a camptothecin and artesunate conjugate represented by a formula (I). The preparation method comprises the steps of dissolving camptothecin represented by a formula (II) and artesunate represented by a formula (III) in an organic solvent, carrying out a stirring reaction at a temperature of 15-50 DEG C under the effects of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 4-dimethylaminopyridine, performing TLC tracking monitoring until the reaction is over, and post-treating the reaction solution to obtain the camptothecin and artesunate conjugate represented by the formula (I). The camptothecin and artesunate conjugate of the present invention can be used for preparing various anti-tumor drugs. The formulas (I), (II) and (III) are shown as follows.
Owner:ZHEJIANG UNIV OF TECH
Artemisinin-Based Combination Therapy For Treating Viral Mediated Disease
The present invention describes a method of treating individuals suffering from microbial infections, including a viral infection such as Dengue Fever, using an improved Artemisinin Combination Therapy (ACT), known as Tri-ACT. The improved ACT therapy includes administering a combination of three drugs. In one embodiment of the present invention, the method includes administering to an individual a first composition comprising a therapeutically effective amount of an artemether spray sublingually. The individual is then administered a second composition, a therapeutically effective amount of artesunate. A third composition, an effective amount of berberine, or its pharmaceutically acceptable derivatives or salts is then administered to the individual.
Owner:KRYPTONITE GRP
Method for rapidly breeding transgene abrotanum using micro adventive bud technique
The invention relates to a method for rapidly propagating transgenic Artemisia annua by microadventitious bud technology in the field of biotechnology. In the present invention, after the transgenic Artemisia annua explants are sterilized, they are placed on the medium for inducing adventitious buds, cultivated under light, and a large number of adventitious buds grow around the explants; the explants with a large number of adventitious buds are transferred to Micro-adventitious buds are elongated in the medium for elongation of adventitious buds; the elongated adventitious buds are cut and transferred to the rooting medium of adventitious buds to produce adventitious roots; the complete plants that grow robustly are transplanted into a medium containing transplanting A large number of normal-growing transgenic Artemisia annua plants were obtained through domestication. The present invention screens out a medium suitable for induction and rooting of transgenic Artemisia annua explants, the induction rate of adventitious buds of transgenic Artemisia annua explants exceeds 90%, the rooting rate of adventitious buds is 100%, and the survival rate of transplanted regenerated plants is 100%. , The reproduction coefficient reaches 80-100.
Owner:SUZHOU TANGJI BIOTECH CO LTD
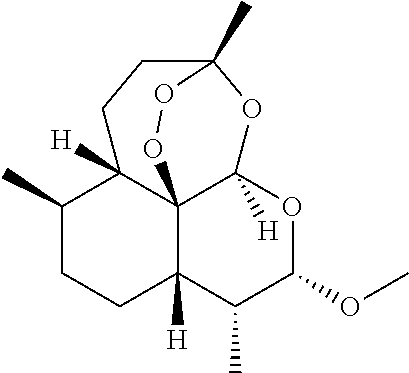
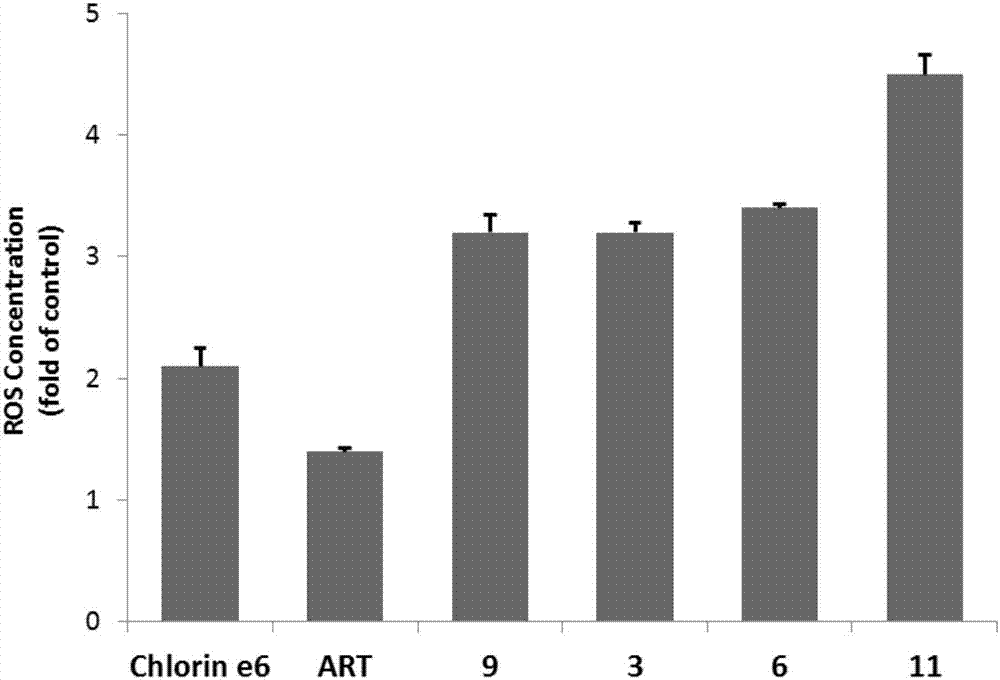
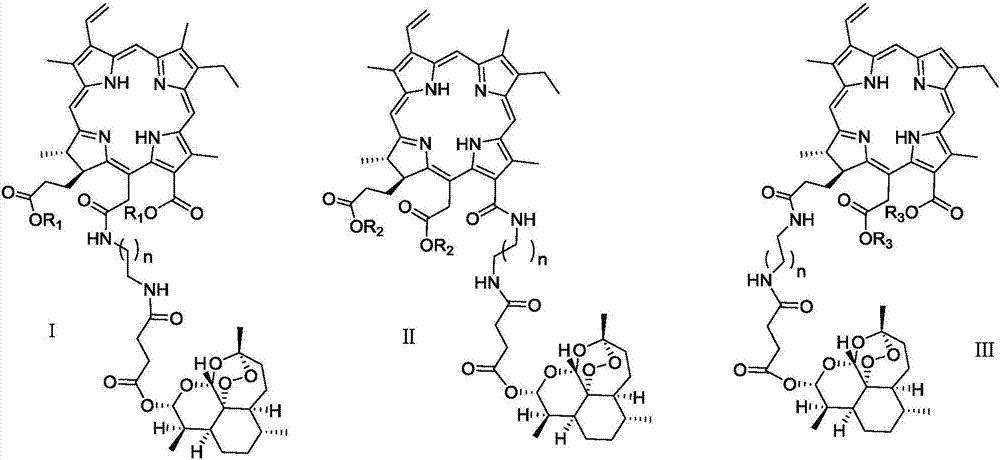
Chlorin and artesunate compound with photosensitive and sound-sensitive activity as well as preparation method and application of compound
ActiveCN107417706AHigh activityHigh photoactivityOrganic chemistryAntineoplastic agentsPositive controlSonodynamic therapy
The invention relates to a chlorin and artesunate compound with photosensitive and sound-sensitive activity as well as a preparation method and an application of the compound and belongs to the technical field of chemical medicines. The chlorin and artesunate compound with photosensitive and sound-sensitive activity has different degrees of inhibition effects on human hepatoma cells Hep G2 in in-vitro anti-tumor activity evaluation. The photoactivity and ultrasonic activity are both higher than that of chlorin e6 and artesunate which are used as positive controls. The compound can be used for preparing a photosensitizer and a sound-sensitive agent in photodynamic therapy and sonodynamic therapy methods for tumor therapy.
Owner:DALIAN UNIV OF TECH
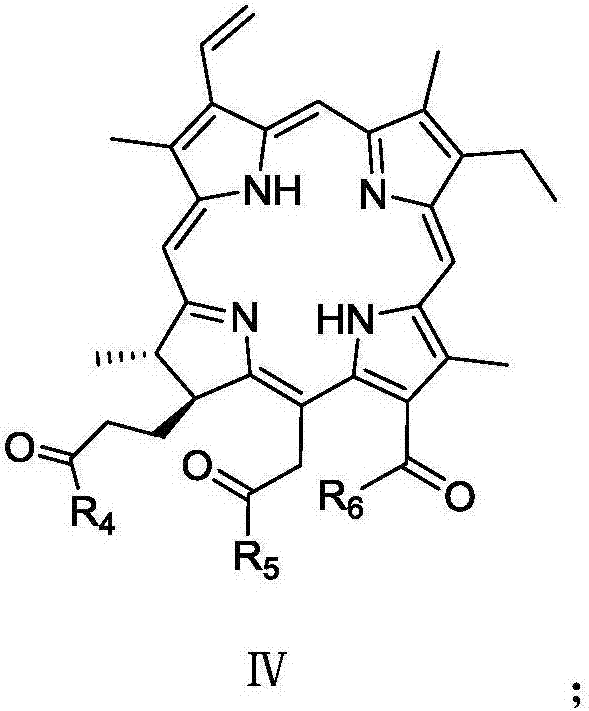
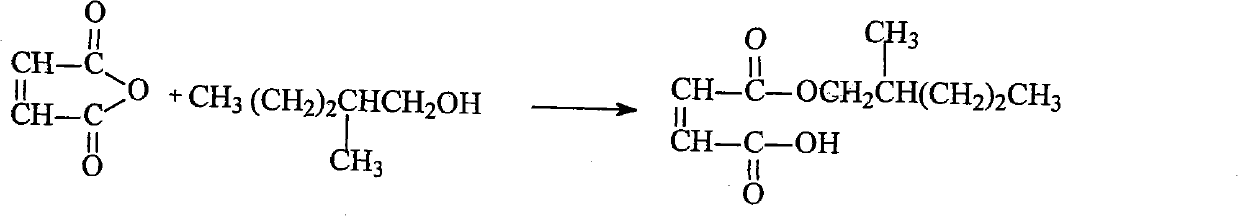
Ethylene glycol bi-sulpho succinic acid bi(2-methyl amyl) artesunate and production method thereof
InactiveCN102001975AGood moisturizationGood permeabilityTransportation and packagingSulfonic acids salts preparation1-PentanolSulfite salt
The invention discloses an ethylene glycol bi-sulpho succinic acid bi(2-methyl amyl) artesunate and a production method thereof. The product is prepared by reacting maleic anhydride with 2-methyl-1-amyl alcohol to form a monoesterfication product, reacting the monoesterfication output with sodium sulfite to form a sulphonated product, and then reacting the sulphonated product with ethylene glycol. The product has good performance and is simple to synthesize.
Owner:NANTONG UNIVERSITY
Method for enhancing arteannuin content in southernwood using gene hmgr and fps co-transformation
InactiveCN101182545AIncrease contentStable new drug sourcesComponent separationMicrobiological testing/measurementArtemisia annuaDNA
The invention relates to a method for co-transforming hmgr and fps in the field of biotechnology to increase the content of artemisinin in artemisia annua. The present invention clones hmgr and fps genes from Artemisia annua, constructs a plant expression vector containing said DNA molecules, uses Agrobacterium tumefaciens to mediate, simultaneously introduces hmgr and fps genes into Artemisia annua and regenerates plants, and detects exogenous objects by PCR The integration of genes hmgr and fps was determined, and the content of artemisinin in Artemisia annua was determined by high performance liquid chromatography-evaporative light scattering detector, and the transgenic plants of Artemisia annua with increased artemisinin content were screened. The content of artemisinin in the transgenic Artemisia annua obtained by the present invention is significantly increased, up to 2.32 times that of the non-transformed control plants.
Owner:SHANGHAI JIAO TONG UNIV
Artesunate nanoemulsion drug composition and preparation method thereof
InactiveCN101623255AHigh thermodynamic stabilityGood storage stabilityElcosanoid active ingredientsPharmaceutical non-active ingredientsSide effectAcute toxicity testing
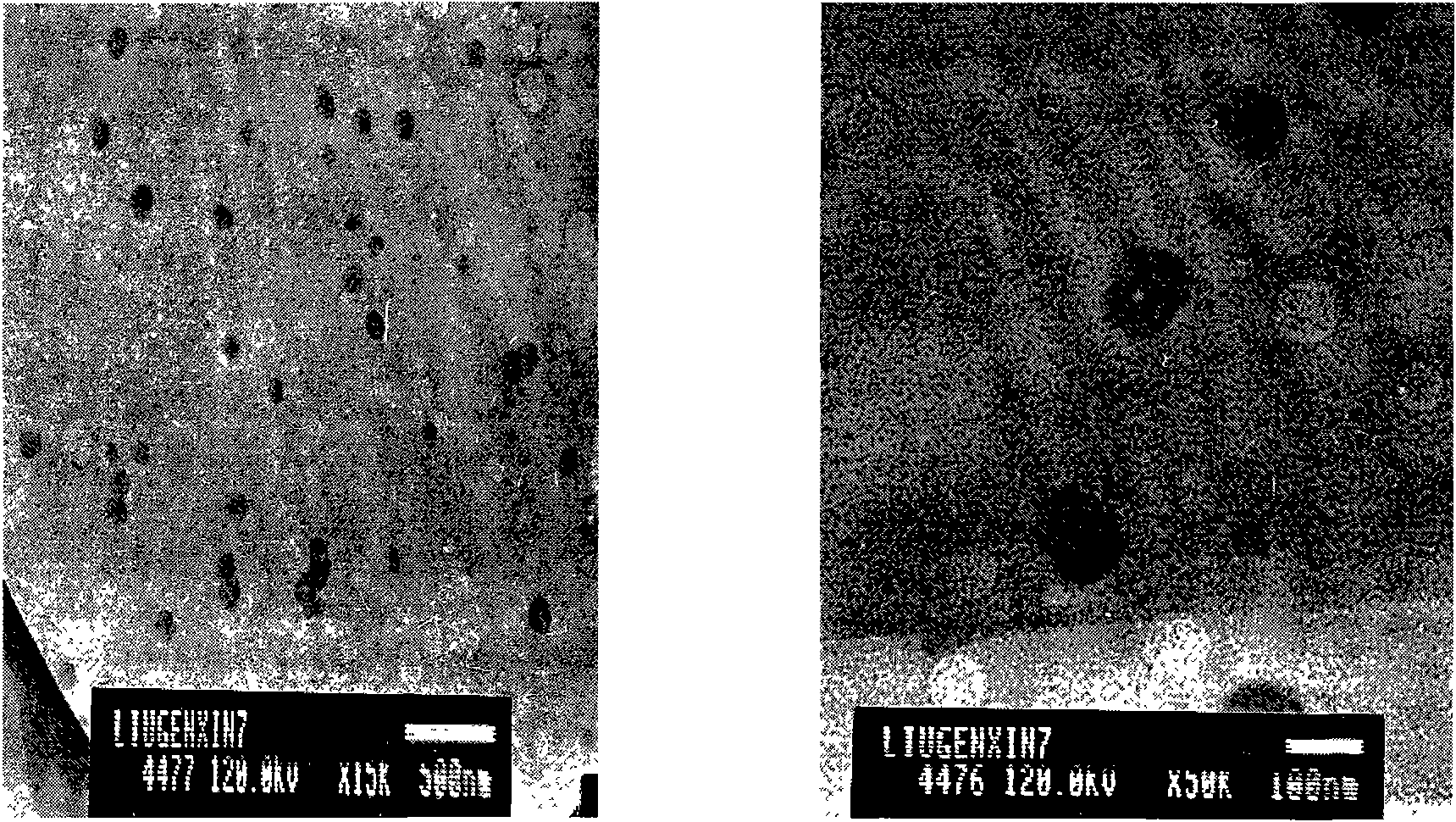
The invention discloses an Artesunate nanoemulsion drug that is an O / W (oil-in-water type) nanoemulsion system consisting of ethyl oleate, Tween-80, normal butanol, ultrapure water and Artesunate. The nanoemulsion drug effectively overcomes the first-pass effect of traditional tablets in liver, causes no sense of pain in injection and greatly improves the insecticidal and helminthic effect of original Artesunate. Meanwhile, the nanoemulsion system has high drug-loading rate, is stable when stored, has certain slow release function compared with merchant Artesunate injection, and further is convenient for administration, thus greatly improving the bioavailability. Besides, shown by acute toxicity tests and clinical drug effect tests on mice, the nanoemulsion has no obvious toxic or side effect and is a safe, reliable and high-efficiency nano level antiparasitic drug.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Preparation method and application of transferrin modified hollow mesoporous copper sulfide/artesunate nanoparticles
ActiveCN105126113ASmall toxicityThe synthesis process is simpleOrganic active ingredientsEnergy modified materialsSide effectTherapeutic effect
The invention relates to a preparation method and an application of transferrin modified hollow mesoporous copper sulfide / artesunate nanoparticles and effectively and simultaneously achieves targeting, photothermal therapy, photodynamic therapy, photoacoustic tomography, drug therapy and DMR (diffusion molecular retention) effect to realize integration of diagnosis and treatment. The method includes: synthesizing hollow mesoporous copper sulfide nanoparticles, loading artesunate in copper sulfide in a hollow mesoporous structure, and modifying with transferrin through electrostatic adherence to obtain the transferrin modified hollow mesoporous copper sulfide / artesunate nanoparticles. The synthesis process is simple, anti-cancer drugs are sent to cancerous parts by means of peri-cancerous injection, and accordingly treatment effects can be further improved, and toxic and side effects on normal tissues and cells are reduced. A function of infrared photothermal therapy is realized while photodynamic therapy and photoacoustic tomography can be carried out, and accordingly integration of photothermal therapy, photodynamic therapy, chemotherapy, tumor diagnosis and comprehensive therapy is realized. The transferrin modified hollow mesoporous copper sulfide / artesunate nanoparticles realize a great innovation of drugs for tumor treatment.
Owner:ZHENGZHOU UNIV
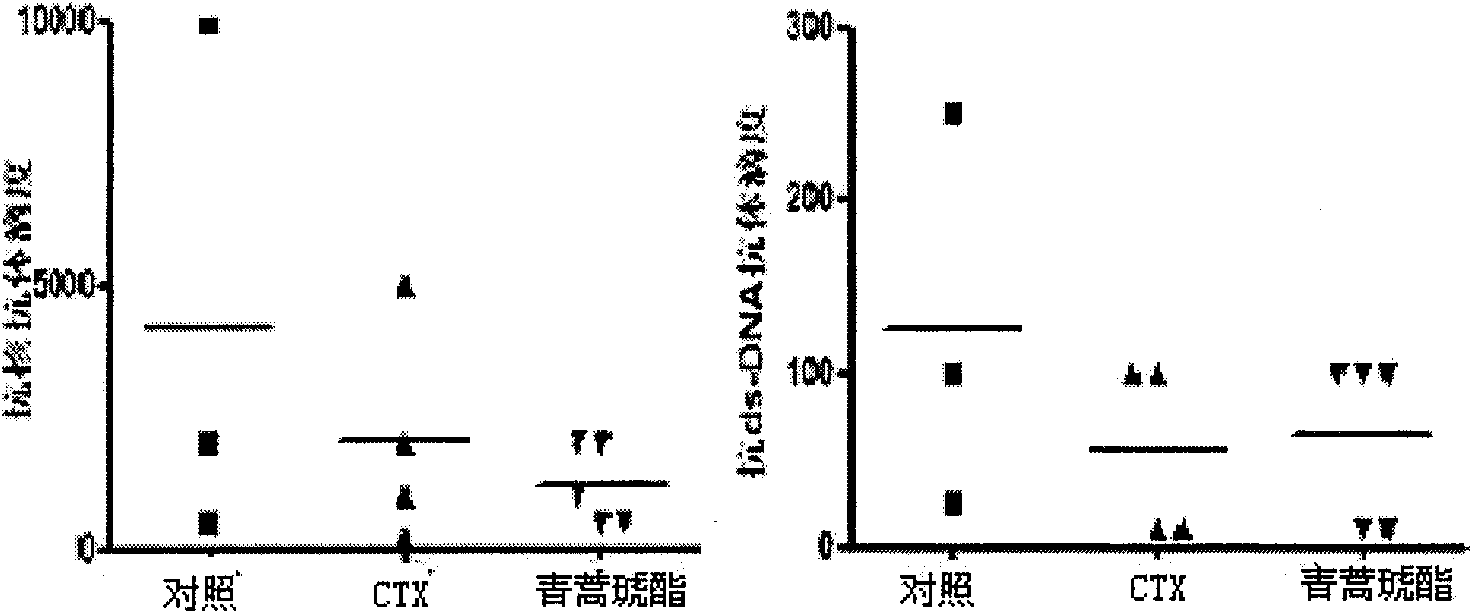
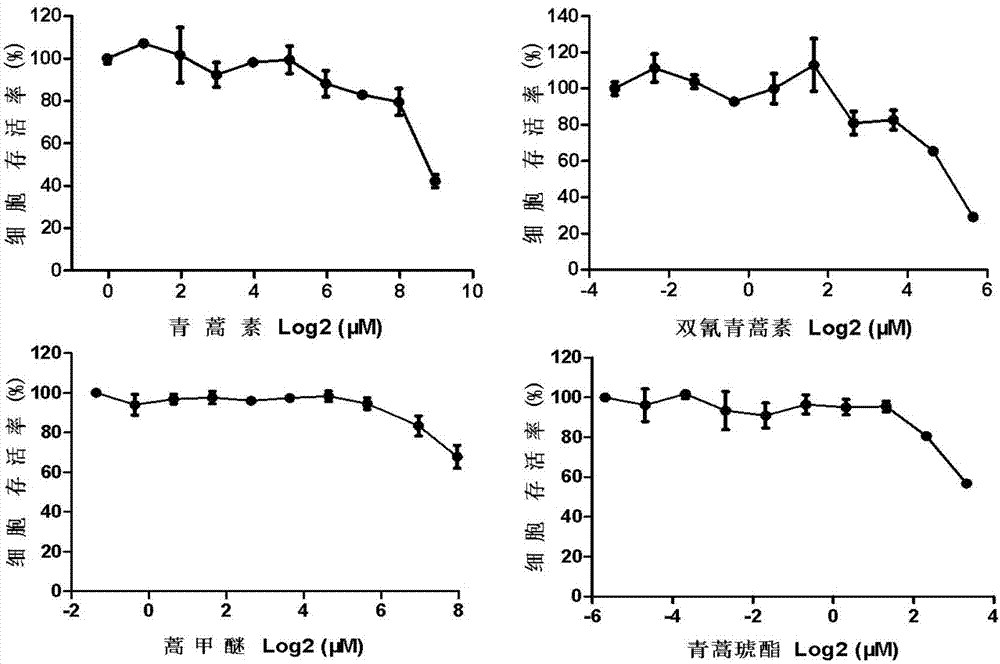
Application of artesunate as drug for treating systemic lupus erythematosus
InactiveCN101632657AMature production processQuality improvementOrganic active ingredientsSkeletal disorderLupus pernioSide effect
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
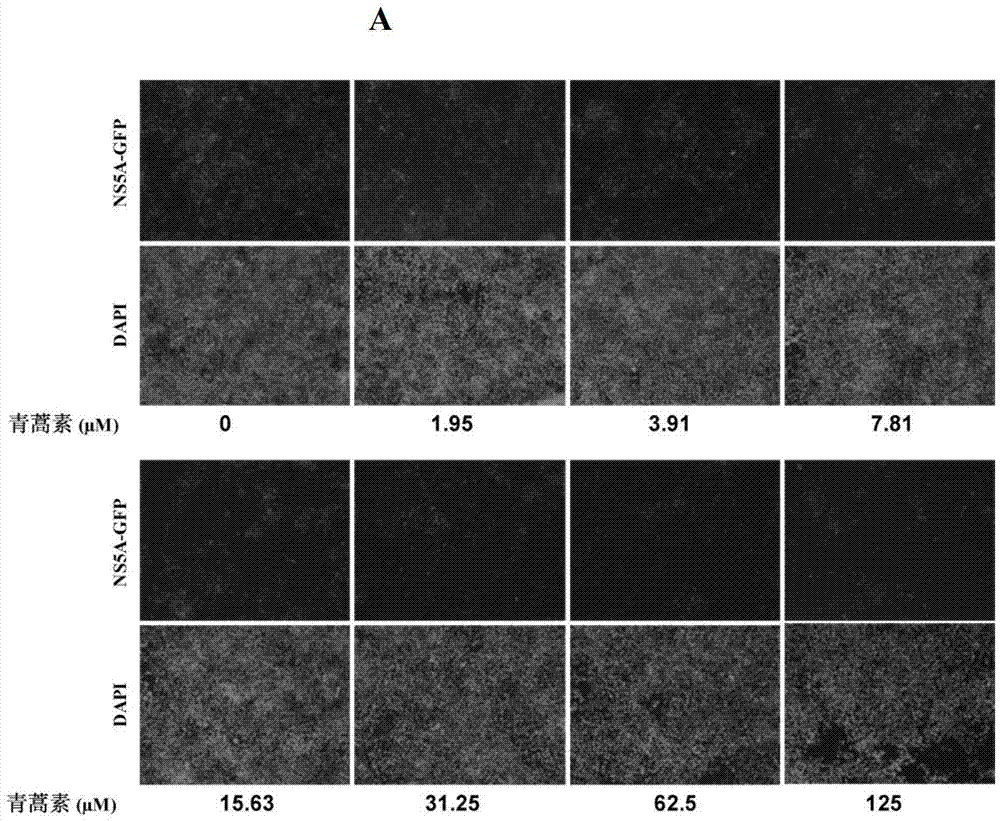
Application of artemisinin and derivatives thereof in preparation of medicaments for treating hepatitis C viruses
InactiveCN102755316ASmall side effectsOrganic active ingredientsDigestive systemAnti virusSide effect
The invention discloses an application of artemisinin and derivatives thereof in preparation of medicaments for treating hepatitis C viruses (HCV). The medicaments which almost do not have toxic concentration are used for anti-virus test; and by detecting the influence of the artemisinin and the derivatives thereof such as dicyanogen artemisinin, artemether and artesunate on HCV replication, the artemisinin and the derivatives thereof serving as small molecular compounds have remarkable viral activity resistance and are dose-dependent. The artemisinin and the derivatives thereof which have already been used for clinical antimalarials have low toxic or side effect on a human body. The artemisinin and the derivatives thereof which almost do not have toxicity can inhibit about 90 percent of HCV replication, and are efficient and low-toxic anti-HCV compounds. The compounds have a broad prospect for developing anti-HCV medicaments.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Nano-Artesunate capsule and preparation process thereof
InactiveCN101642448AOvercome insoluble in water, fast metabolismOvercoming utilizationOrganic active ingredientsAntiparasitic agentsWater bathsSide effect
The invention relates to a nano-Artesunate capsule and a preparation process thereof. A capsule heart is Artesunate, a capsule film is gelatin, a capsule shape is in a ball shape or an ellipsoid shape, and the diameter of the capsule is 30 nm. The invention adopts the following preparation process: swelling the gelatin by distilled water to form colloid, dissolving powdered Artesunate in ethanol at the room temperature, dissolving glacial acetic acid in the distilled water, taking gelatin solution, slowly dropping the Artesunate ethanol solution under the stirring in a water bath with the constant temperature of 40 DEG C, adjusting pH value with glacial acetic acid solution, slowly dropping formaldehyde under the condition of ice bath, solidifying to form the capsule, and freezing and drying to obtain powder of the nano-Artesunate capsule. The capsule prepared by the invention can be taken orally as well as injected by injection, the dosage is small, and no toxic or side effect occursby taking orally; and the injection cannot cause thrombus, and the like in blood vessels, can support sustained release of medicine delivery, and the injection has high efficiency and long effective period. The grain size of the capsule is uniform, the covering state is good, and the covering rate is 50%; the addition of emulsifier and coagulant aid is not needed during preparation, the process issimple, and the operation is convenient.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Application of artesunate for preparing medicine to treat fibrosis of liver
InactiveCN1903191AHas anti-hepatic fibrosis effectPrevent liver cancerOrganic active ingredientsDigestive systemHepatic stellate cell proliferationLiver cancer
An application of artesunate in preparing the medicines for preventing and treating the hepatic fibrosis and preventing heptocirrhosis and liver cancer is disclosed.
Owner:方步武
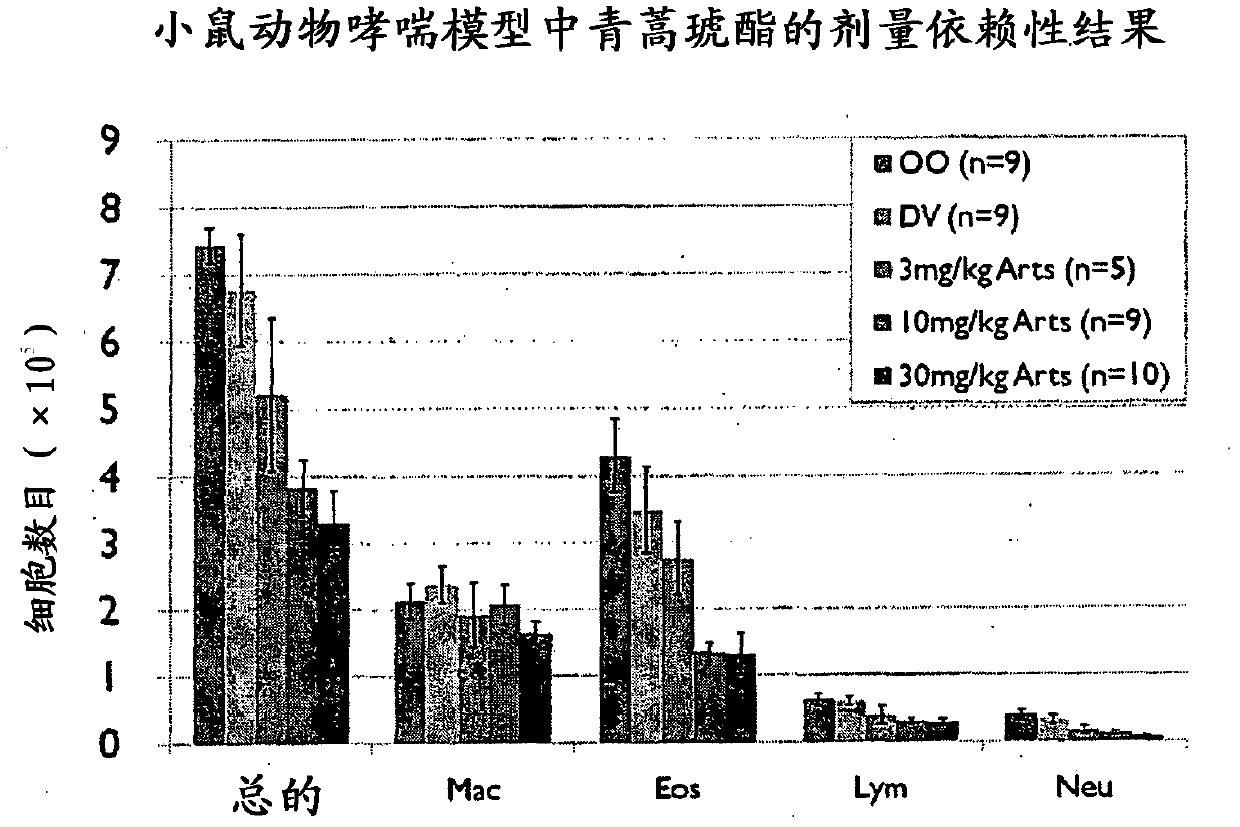
Use of artemisinin derivatives for treatment of asthma and chronic obstructive pulmonary disease (copd)
InactiveCN102481282AOrganic active ingredientsOrganic chemistryObstructive Pulmonary DiseasesMalaria
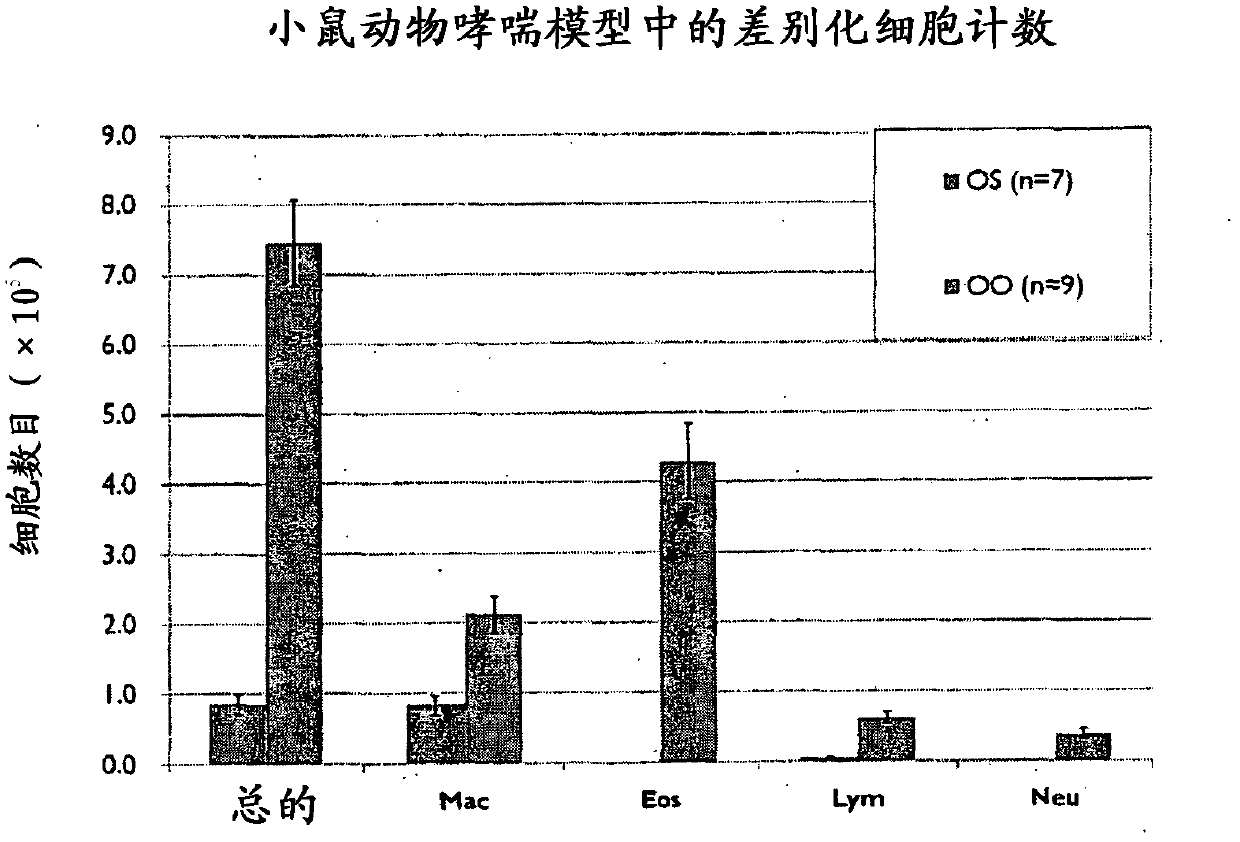
Artesunate is a derivative of artemisinin isolated from a Chinese herb Artemisia annua L. It is used clinically for the treatment of malaria. We investigated potential anti-inflammatory actions of artemisinin derivatives. artemisinin derivatives significantly inhibited OVA-induced signs, symptoms and parameter of airway disorders Taken together, our results clearly demonstrate anti-inflammatory effects of artemisinin derivatives. Artemisinin derivatives can be used to complement or to replace oral steroids during asthma exacerbation treatment. Further artemisinin derivatives can be used as an anti-inflammatory agent for controlling airway disorders.
Owner:NAT UNIV OF SINGAPORE
Application of artesunate in preparing medicine for treating and preventing central nerve injury
InactiveCN104523679AReduce infarctionImprove neurological deficitsOrganic active ingredientsNervous disorderSide effectCerebral infarction
The invention provides an application of artesunate in preparing medicine for treating and preventing central nerve injury. The artesunate has the facilitating function on the multiplication of neural stem cells cultured in a vitro, meanwhile infarct lesions of a big mouse with cerebral infarction is effectively reduced, and the neurologic impairment caused by cerebral apoplexy is improved. The artesunate is safe and free of obvious toxic and side effects.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Enhanced artemisinin-based combination therapy for treating parasitic mediated disease
PendingUS20140256761A1Facilitates early administrationIncidence of recurrenceBiocideAnimal repellantsBerberineCombined Modality Therapy
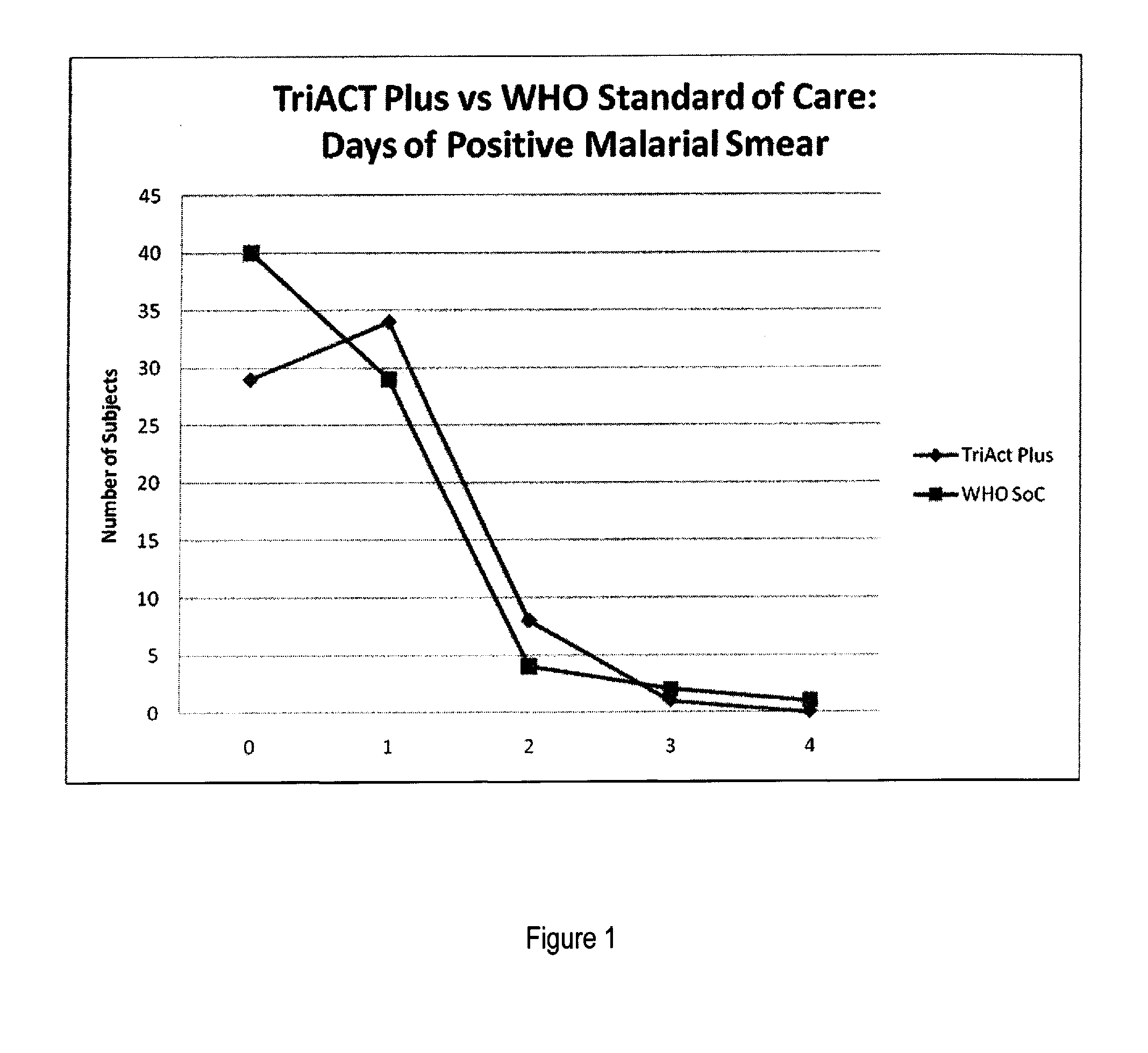
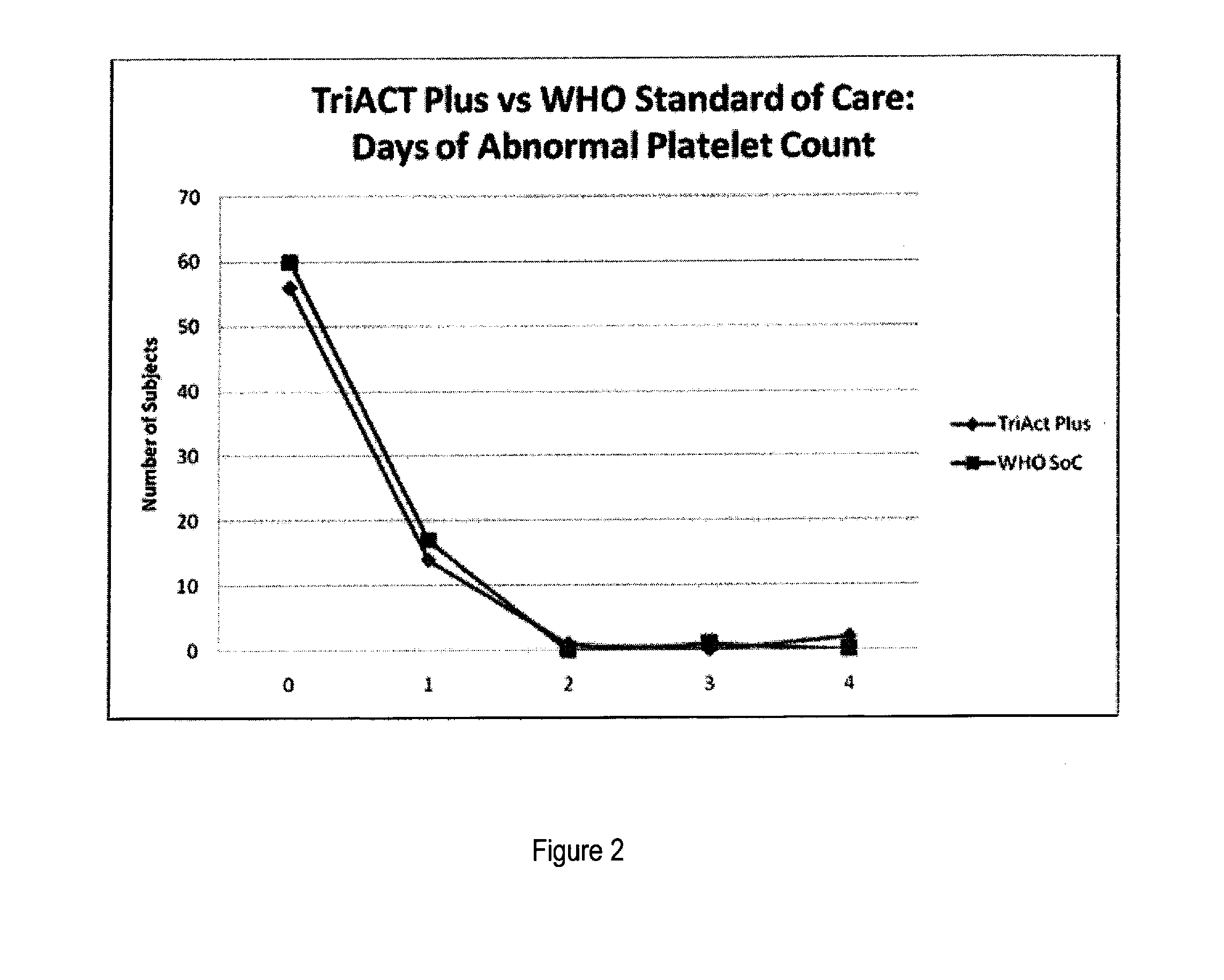
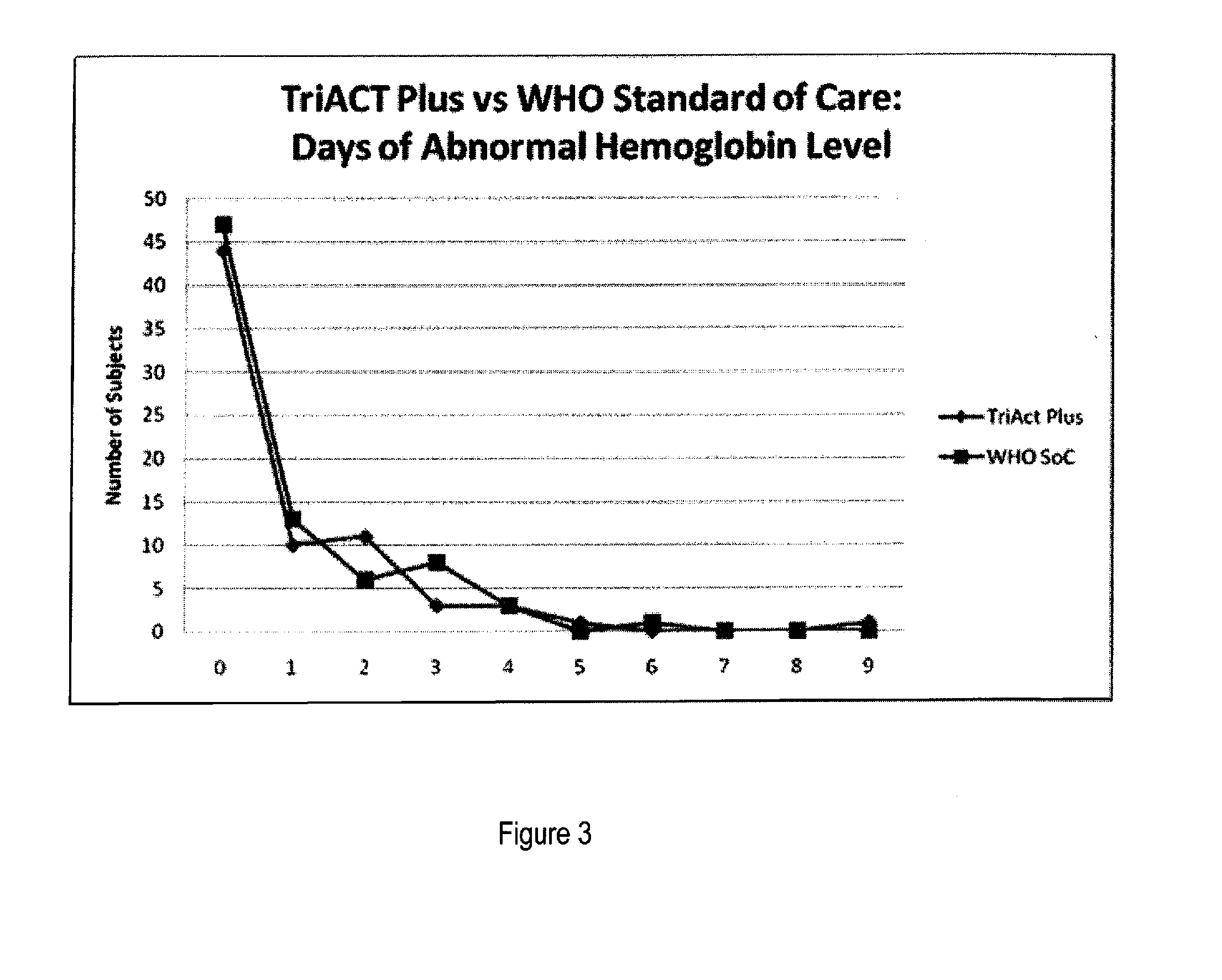
The present invention describes a method of treating individuals suffering from microbial infections, including a parasitic disease such as malaria, by using an improved Artemisinin Combination Therapy (ACT), known as ActRx Tri-ACT Plus. The improved ACT therapy includes administering a combination of four drugs. In one embodiment of the present invention, the method includes administering to an individual a first composition comprising a therapeutically effective amount of an artemether spray sublingually. The individual is then administered a second composition, a therapeutically effective amount of artesunate. A third composition, an effective amount of berberine, or its pharmaceutically acceptable derivatives or salts is administered to the individual. A fourth composition, an effective amount of primaquine, or its pharmaceutically acceptable derivatives or salts is administered to the individual.
Owner:KRYPTONITE GRP
Artemisia apiacea toothpaste and preparation method thereof
InactiveCN106880554AAvoid bleedingEffective antibacterialCosmetic preparationsToilet preparationsAdhesiveToothpaste
The invention relates to the technical field of daily chemical products, and in particular relates to artemisia apiacea toothpaste and a preparation method thereof. The artemisia apiacea toothpaste is prepared from the following raw materials in percentage by weight: 18 to 25 percent of friction agent, 0.3 to 0.6 percent of adhesive, 54 to 64 percent of humectant, 2 to 7 percent of foaming agent, 0.05 to 0.2 percent of sweetening agent, 0.1 to 0.4 percent of preservative, 0.3 to 0.6 percent of sodium pyrophosphate, 1 to 2 percent of essence, 0.1 to 0.3 percent of artemisinin or artesunate and the balance of artemisia apiacea grass extract aqueous solution. By matching the fresh artemisia apiacea grass extract aqueous solution with the artemisinin or the artesunate, the artemisia apiacea toothpaste disclosed by the invention has the functions of killing bacteria, resisting bacteria and inhibiting bacteria and can be used for preventing gingival bleeding; in addition, by adding the sodium pyrophosphate, the artemisia apiacea toothpaste has good effects of preventing and treating dental calculus and dental plaque.
Owner:SICHUAN LAMBERT BIOLOGICAL TECH CO LTD
Artesunate purification process
ActiveCN103374016ASimplify separabilitySimple purification processOrganic chemistryAgainst vector-borne diseasesSolventEnergy consumption
The invention relates to an artesunate purification process, and aims to provide an artesunate purification process with simple process, high yield and short crystallization time, and a preparation method. The artesunate purification process comprises six steps including esterification, extraction, concentration, crystallization, purification and drying. The process provided by the invention simplifies the purification and separation of artesunate, has advantages of short reaction time, especially greatly shortened crystallization time of only 4-6 min, product yield above 93%, product purity higher than 99%, recyclable solvents, low energy consumption and environmental protection, so as to greatly reduce costs of artesunate anti-malaria drugs and contribute to the public welfare of the global against malaria resistance.
Owner:重庆恒星生物技术有限责任公司
Novel application of artesunate
PendingCN106727586AAddressing drug resistanceImprove lung cancer prognosisOrganic active ingredientsAntineoplastic agentsErlotinibAdenocarcinoma
The invention provides novel application of artesunate. The artesunate is used for reversing erlotinib drug resistance of lung adenocarcinoma, or the artesunate is used together with erlotinib. The problem that clinical application of a conventional molecular targeted drug is limited as drug resistance can be resulted ultimately can be solved. The invention belongs to the field of lung cancer treatment.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Preparation of artesunate fat emulsion for injection and application of artesunate fat emulsion in treatment of malaria
InactiveCN102895186AImprove lymphocyte activityContributes to resistanceOrganic active ingredientsAntiparasitic agentsSide effectBiocompatibility Testing
The invention discloses an artesunate fat emulsion injection preparation which contains 0.01 to 30 wt% of artesunate, 10.0 to 30.0 wt% of oil for injection, 0.6 to 30.0 wt% of an emulsifier, 0 to 10 wt% of a solubilizer, 0 to 5 wt% of a co-emulsifier, 2.25 to 7 wt% of an isotonic agent and 0.002 to 0.075 wt% of an anti-oxidant, with the balance being injection water. The invention also discloses a preparation method and pharmacodynamic and safety evaluation for the artesunate fat emulsion injection preparation. The artesunate fat emulsion provided by the invention has the characteristics of good biocompatibility, high physical stability, convenient preparation, good security, high drug loading capacity and the like, has a particle size of less than 200 mu m and is suitable for injection, e.g, intravenous injection and intramuscular injection. According to the invention, the characteristic that a soybean oil fat emulsion inhibits growth of plasmodium falciparum is utilized to enhance anti-malarial effects of drugs, to improve bioavailability of the drugs and to reduce toxic and side effects of the drugs; nutrients needed by the body of a patient with malaria can be provided, the activity of lymphocytes of the patient is improved, which assists the patient in resisting malaria and recovering.
Owner:福州欣瑞普医药科技有限公司
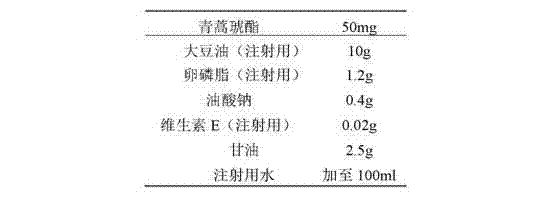
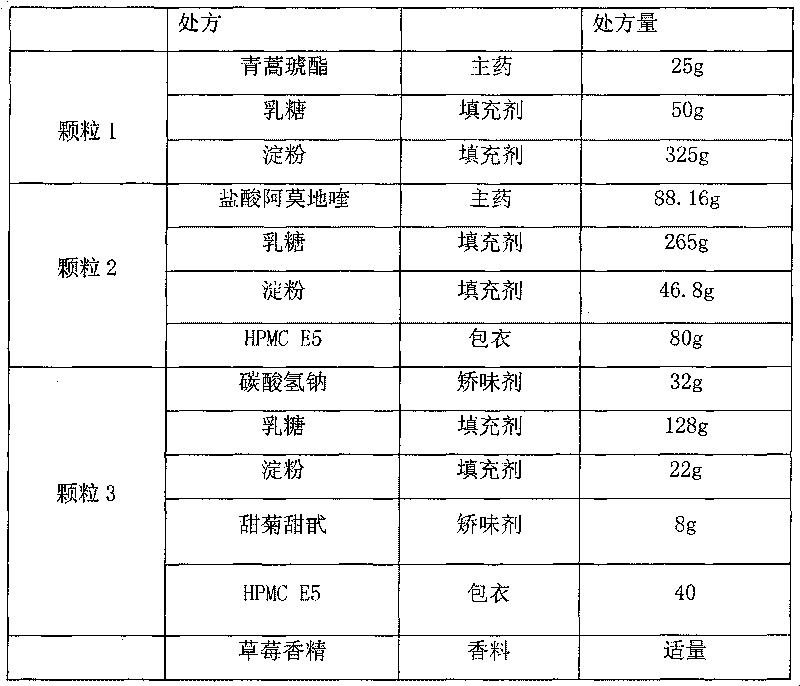
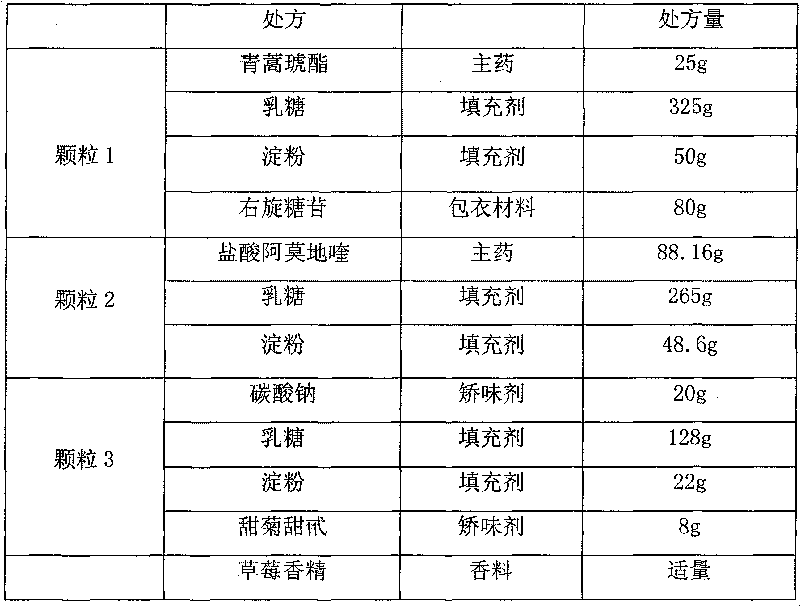
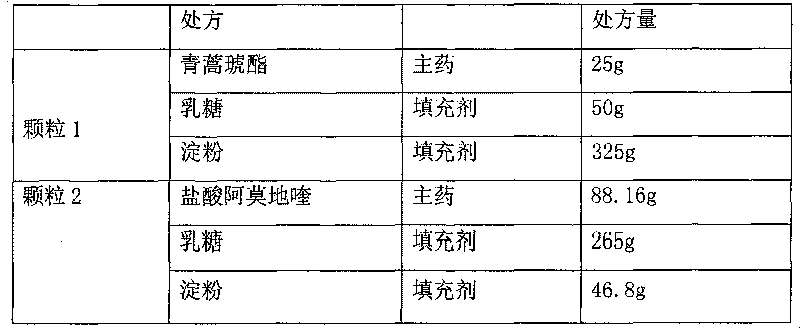
Artesunate compound medicine composition with improved mouth feeling and high stability
InactiveCN101756982AOrganic active ingredientsInorganic non-active ingredientsTraditional medicineMacromolecule
The invention belongs to the field of preparations, in particular to an artesunate compound medicine composition with improved mouth feeling and high stability. The invention is characterized in that 1) the artesunate compound medicine composition comprises three kinds of granules: artesunate granules, alkaline matter granules and camoquin granules; and 2) the lagging cover is realized through polymer materials, and the artesunate granules are separated from the alkaline matter granules and the camoquin granules. The medicine composition improves the release speed of the artesunate, inhibits the bitterness of the camoquin, and simultaneously maintains the stability of the artesunate.
Owner:CHONGQING PHARMA RES INST
Compound antimalarial composition
InactiveCN104224787ASmall particle sizeIncrease the speed of transdermalOrganic active ingredientsAerosol deliveryOrganic solventMedicine
The invention relates to a compound antimalarial composition. Particularly, the invention relates to a compound antimalarial composition containing artesunate and febrifugine and in particular relates to a compound antimalarial ethosome composition containing artesunate ethosomes and febrifugine ethosomes, a compound antimalarial ethosome gel paste as well as a preparation method and an application of the compound antimalarial ethosome gel paste. The compound antimalarial ethosome gel paste is prepared from the following components including a gel paste matrix, the artesunate ethosomes, the febrifugine ethosomes, a backing and an antisticking layer. According to the compound antimalarial ethosome gel paste disclosed by the invention, by utilizing the carrier permeation enhancing property of the ethosomes, the percutaneous permeability of an antimalarial medicament and the antimalarial effect of the medicament are improved; in addition, with the adoption of the compound antimalarial ethosome gel paste, the preparation technology is simple, high-temperature heating is not needed in the preparation process, and a harmful organic solvent is avoided.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com