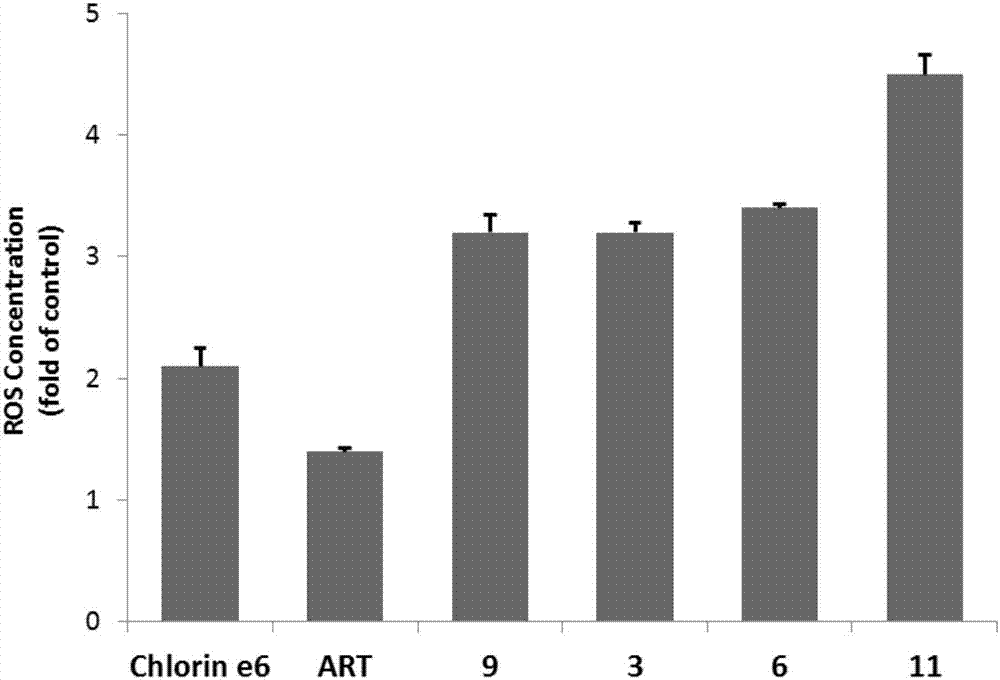
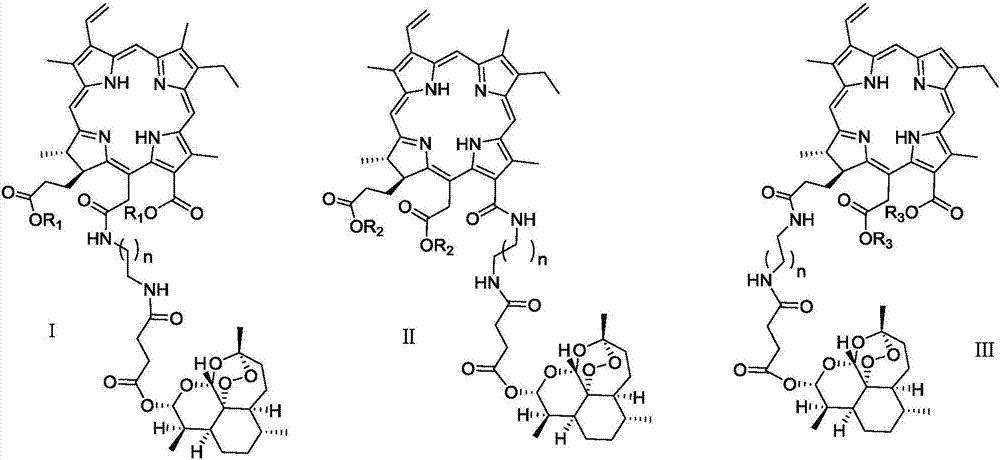
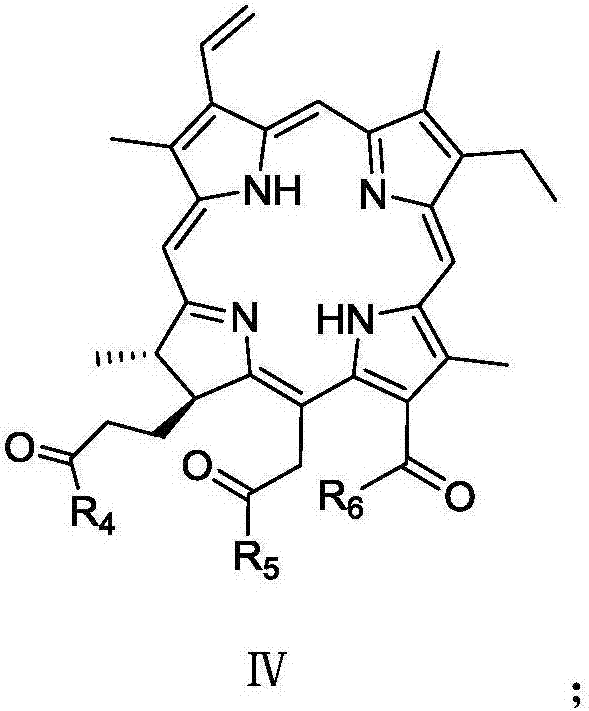
Chlorin and artesunate compound with photosensitive and sound-sensitive activity as well as preparation method and application of compound
A technology of chlorin artemisia annua and its conjugates, which is applied in the field of chemical medicine and can solve the problems of low production of reactive oxygen species, low selectivity of tumor cells, and large doses required
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of intermediate artesunate NHS ester
[0037] Dissolve Artesunate in CH 2 Cl 2 (5ml). At 0°C, add EDCI (129.0mg), react the mixed solution at room temperature under nitrogen protection for 10min, add N-Hydroxysuccinimide (NHS, 78.2mg) mixed solution and react at room temperature for 10h, after the reaction, add Ionized water (20mL) with CH 2 Cl 2 Extraction (50mL×3), the organic phase was dried with anhydrous sodium sulfate, concentrated under low pressure, the concentrate was subjected to silica gel column chromatography, and the elution condition was (petroleum ether / ethyl acetate=1:1) to obtain the intermediate Artesunate NHS (248.1 mg, 99%). 1 H-NMR (CDCl 3 ,400MHz,ppm)δ:5.71(d,J=8.0Hz,1H),5.35(s,1H),2.81~2.97(m,2H),2.71~2.79(m,6H),2.41~2.49(m, 1H), 2.27(td, J=12.0Hz, 4.0Hz, 1H), 1.95(dt, J=12.0Hz, 4.0Hz, 1H), 1.77~1.85(m, 1H), 1.61~1.73(m, 2H) ,1.54(dt,J=12.0Hz,4.0Hz,1H),1.33~1.45(m,1H),1.33(s,3H),1.24~1.33(m,2H),1.20(dd,1H,J=8.0 Hz, 4.0Hz), 0.90~...
Embodiment 2
[0045] Synthesis of Compound 4
[0046] Pheophytin a (100.0mg) was dissolved in DMF (1ml). Add EDCI (38.0mg) and N-Boc-ethylenediamine (30.0mg) in turn. The mixed solution was reacted at room temperature for 10 hours. After the reaction, 10ml of water was added to dissolve it, extracted with dichloromethane (50mL×3), the organic phase was dried with anhydrous sodium sulfate, concentrated under low pressure, and the concentrate was subjected to silica gel column chromatography. The elution condition was for (CH 2 Cl 2 / MeOH=50:1), the intermediate product 17-(N-Boc-ethylenediamide)pheophytina was obtained. At 0°C, the intermediate product was dissolved in Acetone / 5%KOH (2ml, 50:50, v / v). Mixed solution React at room temperature for 10 h, add 5% citric acid solution after the reaction, adjust the pH to 5, and filter with suction to obtain compound 4 (53.6 mg, 43%). 1 H-NMR (DMSO-d 6 ,400MHz,ppm)δ:9.77(s,1H,10-H),9.69(s,1H,5-H),9.12(s,1H,20-H),8.34(dd,1H,J=16.0, 8.0Hz,3 1 -...
Embodiment 3
[0052] Synthesis of compound 7
[0053] Methylated chlorophyllin a (102.0 mg) dissolved in CHCl 3 (2ml), after stirring for 10 minutes, 0.2ml ethylenediamine was added, and the mixed solution was reacted at room temperature for 10h. After the reaction was completed, the solvent was concentrated and removed under low pressure, and the concentrate was subjected to silica gel column chromatography, and the elution condition was (CH 2 Cl 2 / MeOH=3:1), to obtain compound 7 (92.2mg, 82%). 1 H-NMR (CDCl 3 ,400MHz,ppm)δ:9.56(s,1H,10-H),9.50(s,1H,5-H),8.78(s,1H,20-H),7.91(dd,1H,J=20.0, 12.0Hz,3 1 -H),7.05(t,1H,J=4Hz,N-H),6.20(d,1H,J=20.0Hz,3 2a -H),5.99(d,1H,J=12.0Hz,3 2b -H),5.47(d,1H,J=20.0Hz,15 1a -H),5.18(d,1H,J=20.0Hz,15 1b -H),4.42~4.46(m,1H),4.33(dd,1H,J=8.0,4.0Hz),3.64(s,3H,12 1 -CH 3 ),3.60~3.64(m,2H),3.57(s,3H,2 1 -CH 3 ),3.39(s,3H),3.37(s,3H),3.24~3.32(m,2H,-CH 2 -),3.15(s,3H,7 1 -CH 3 ),2.76(t,2H,J=8.0Hz,-CH 2 -),2.48~2.54(m,1H,172a -H),2.11~2.20(m,2H,17 2b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com