Artesunate compound medicine composition with improved mouth feeling and high stability
A technology of artesunate and composition, which is applied in the field of preparations, can solve problems affecting the stability of artesunate, etc., and achieve the effect of fast onset, improved release speed, and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
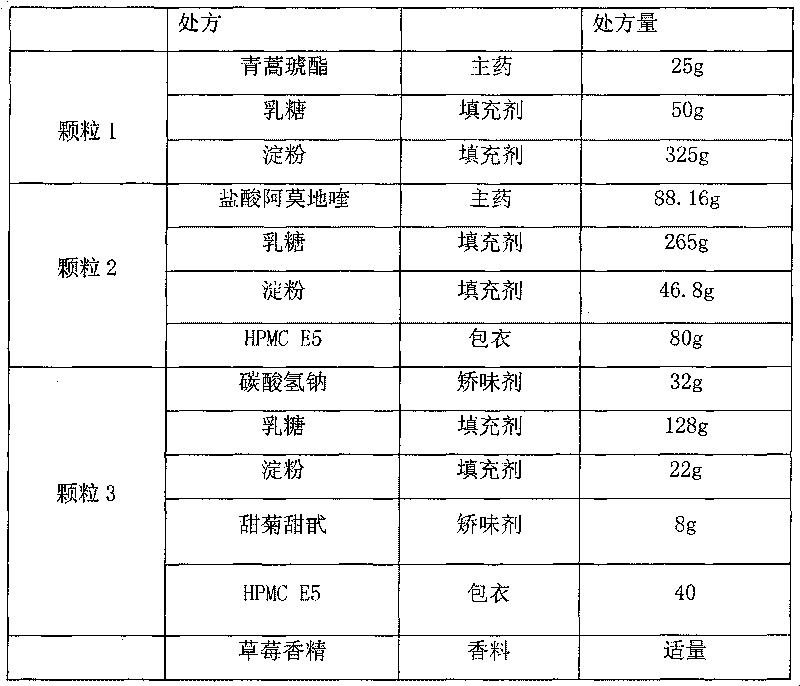
[0040]
[0041] Process:
[0042] Granule 1: mix artesunate, lactose, and starch evenly, granulate 10% starch slurry, dry at 50°C, take 24-60 mesh granules, and set aside.
[0043] Granule 2: Mix amodiaquine hydrochloride, lactose, and starch evenly, granulate with appropriate amount of water, dry at 60°C, take 24-60 mesh granules, coat with 6% HPMC E5 and 50% ethanol to increase the weight by 20%.
[0044] Granule 3: Mix sodium bicarbonate, lactose, and starch evenly, granulate with 50% ethanol, dry at 40°C, get 24-60 mesh granules, coat with 50% ethanol with 6% HPMC E5 to increase the weight by 20%.
[0045] Measure the content of granule 1, granule 2 and granule 3, pack artesunate 25mg, amodiaquine 67.5mg, sodium bicarbonate 32mg per bag.
Embodiment 2
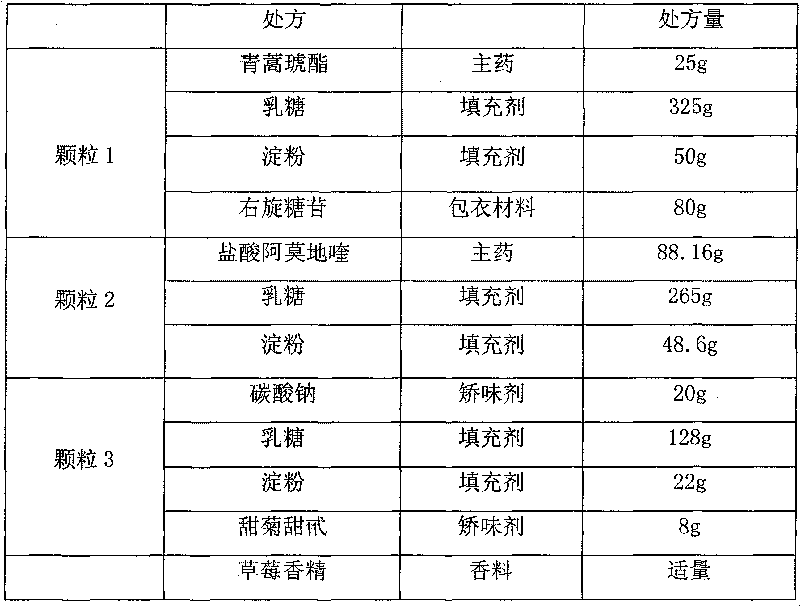
[0047]
[0048] Granule 1: Mix artesunate, lactose, and starch evenly, granulate with 10% starch slurry, dry at 50°C, take 24-60 mesh granules, coat with 20% dextran, and increase the weight of the coating by 20%.
[0049] Granule 2: Mix amodiaquine hydrochloride, lactose, and starch evenly, granulate with appropriate amount of water, dry at 60°C, take 24-60 mesh granules, and set aside.
[0050] Granule 3: Mix sodium bicarbonate, lactose, and starch evenly, granulate with 50% ethanol, dry at 40°C, take 24-60 mesh granules, and set aside.
[0051] Measure the content of granule 1, granule 2 and granule 3, pack artesunate 25mg, amodiaquine 67.5mg, and sodium carbonate granules 20mg per bag.
Embodiment 3
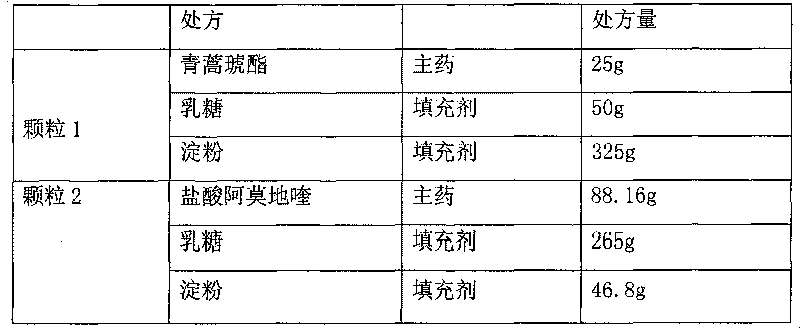
[0053]
[0054]
[0055] Granule 1: Mix artesunate, lactose, and starch evenly, granulate 10% starch slurry, dry at 50°C, and take 24-60 mesh granules
[0056] Granule 2: Mix amodiaquine hydrochloride, lactose, and starch evenly, granulate with appropriate amount of water, dry at 60°C, take 24-60 mesh granules, coat with 20% PVP K29 / 32 aqueous solution, and increase the weight of the coating by 20%.
[0057] Granule 3: Mix sodium bicarbonate, lactose, and starch evenly, granulate with 50% ethanol, dry at 40°C, take 24-60 mesh granules, and coat with 20% PVP K29 / 32 aqueous solution, and the weight gain of the coating is 20%.
[0058] Measure the content of granule 1, granule 2 and granule 3, pack artesunate 25mg, amodiaquine 67.5mg, sodium bicarbonate 32mg per bag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com