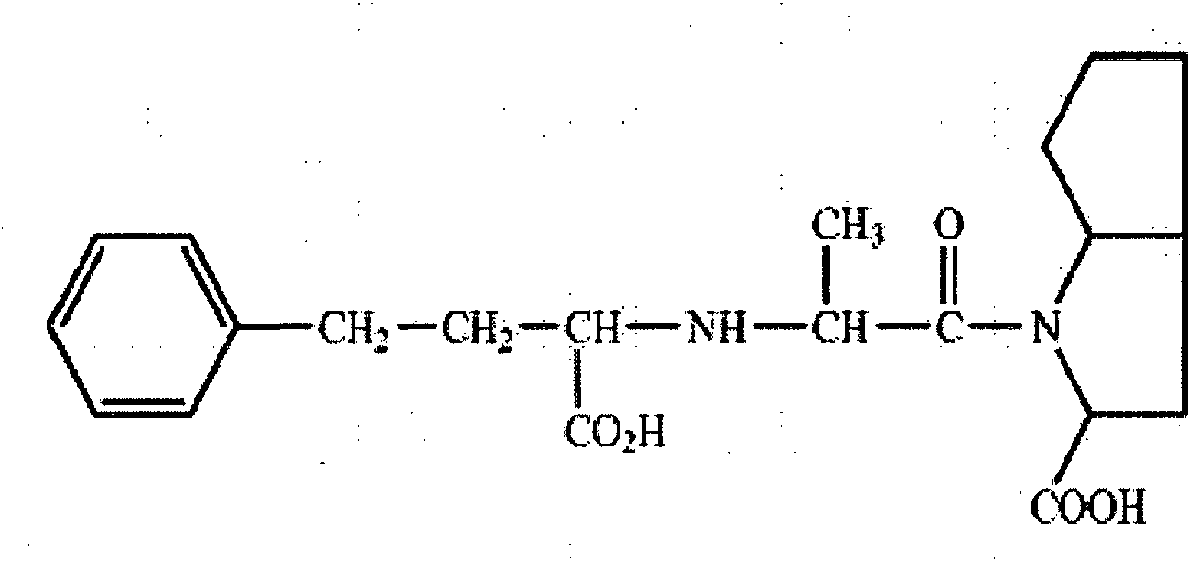Patents
Literature
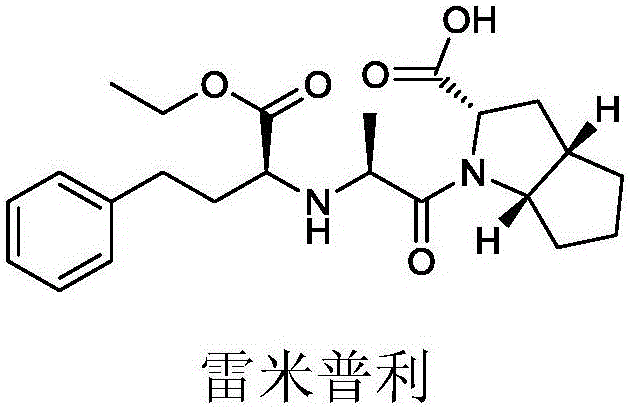
62 results about "Ramipril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
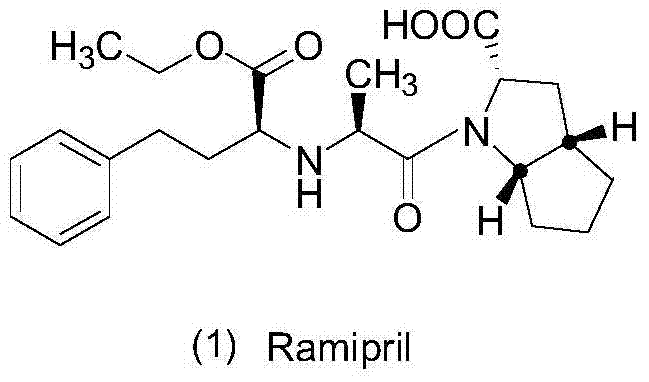
Ramipril is used to treat high blood pressure (hypertension).
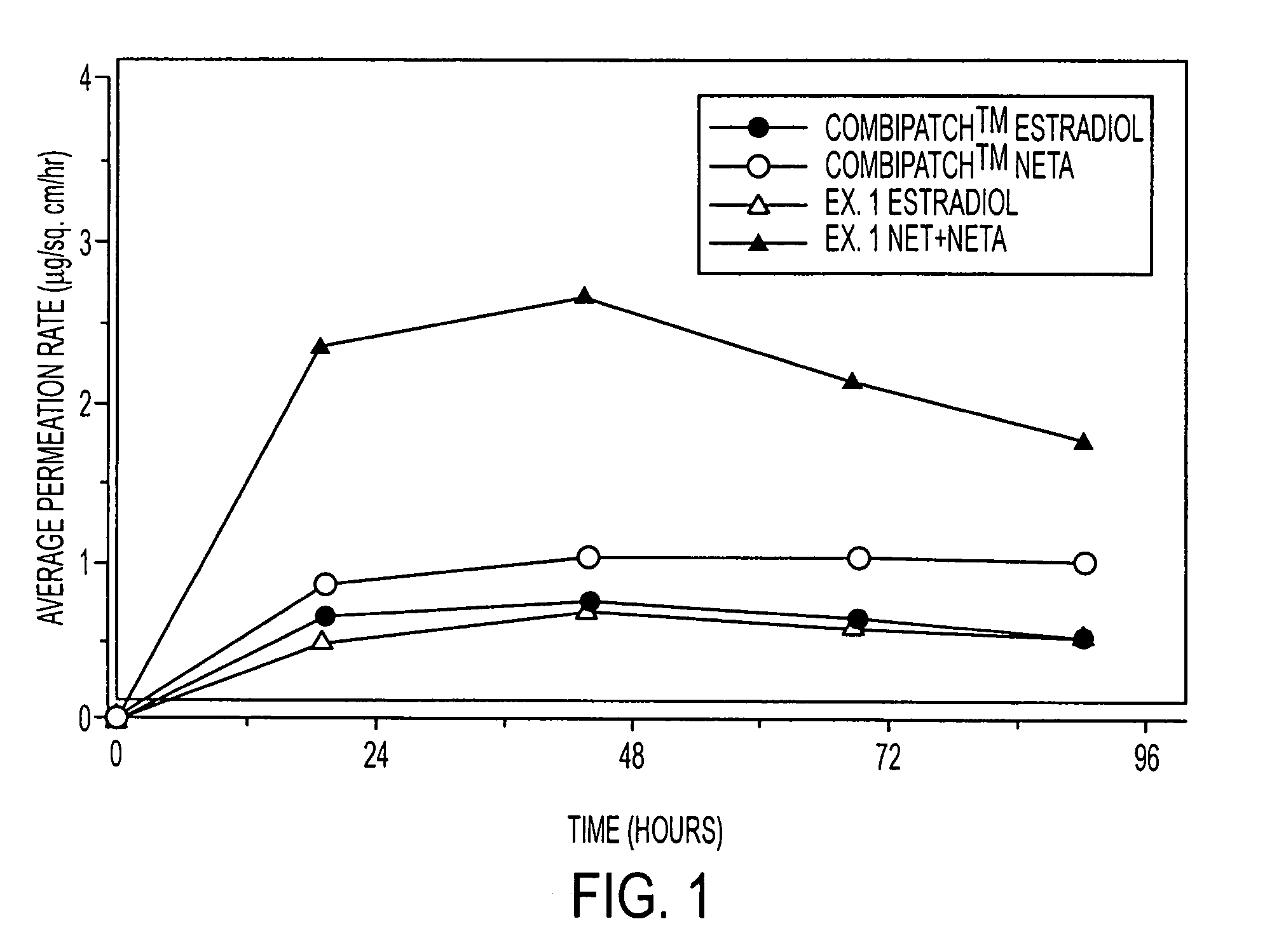
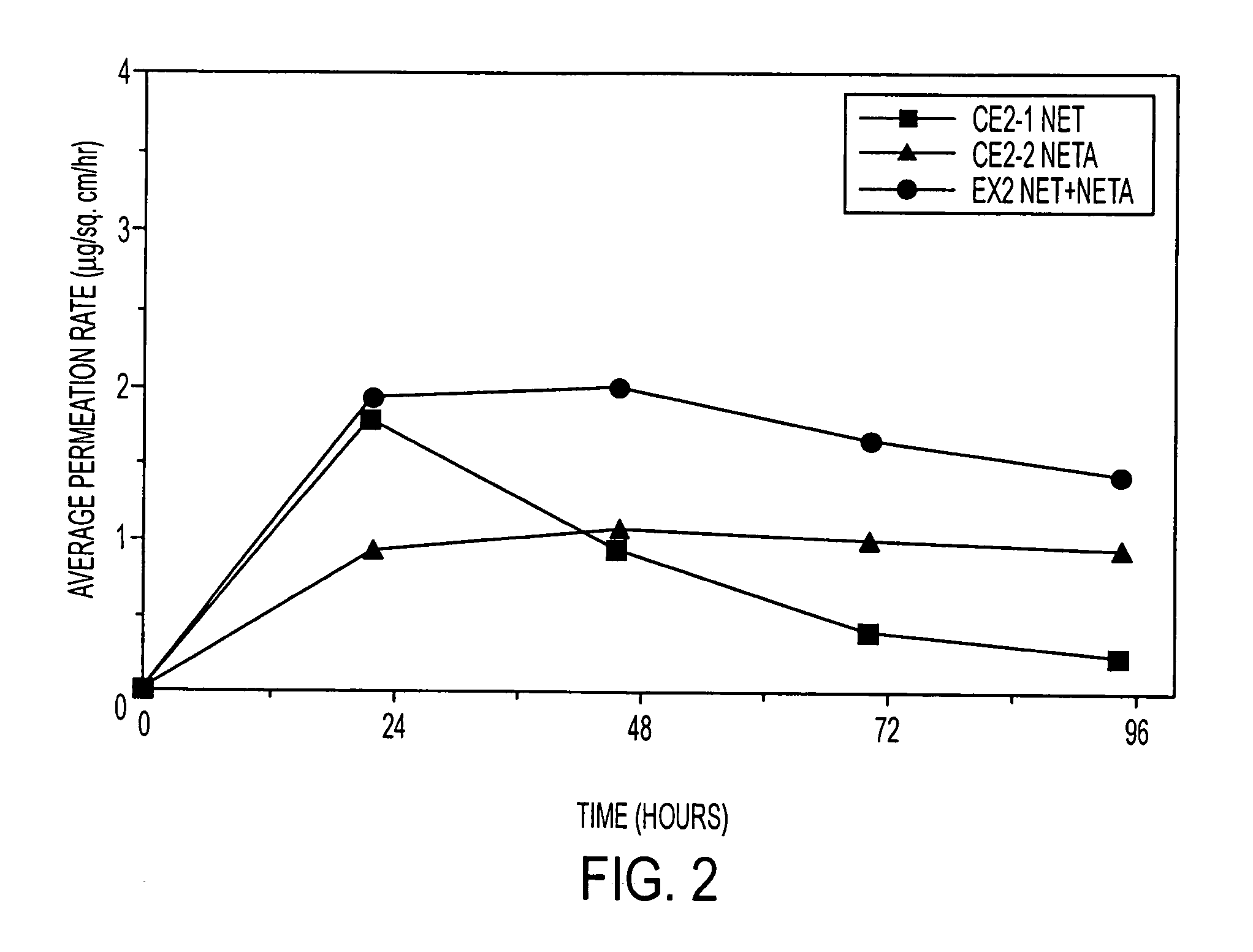
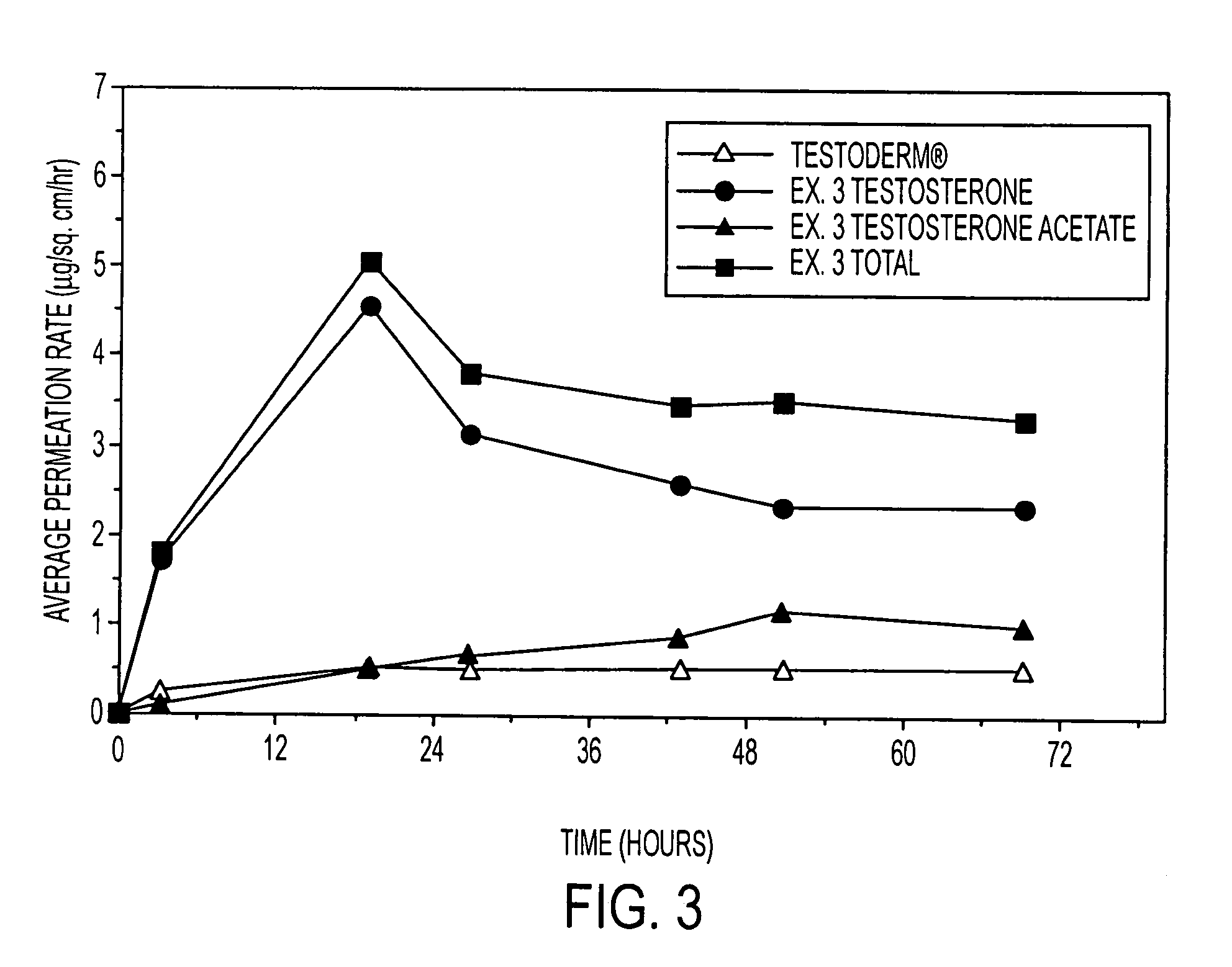
Enhanced drug delivery in transdermal systems
InactiveUS7456159B2Convenient amountGood synergyOrganic active ingredientsBiocideActive agentEthyl ester
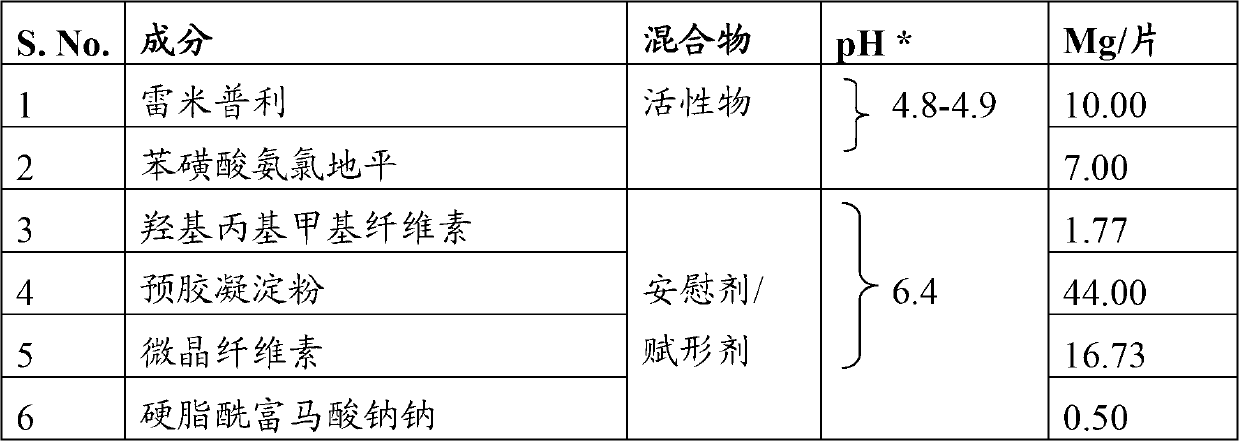
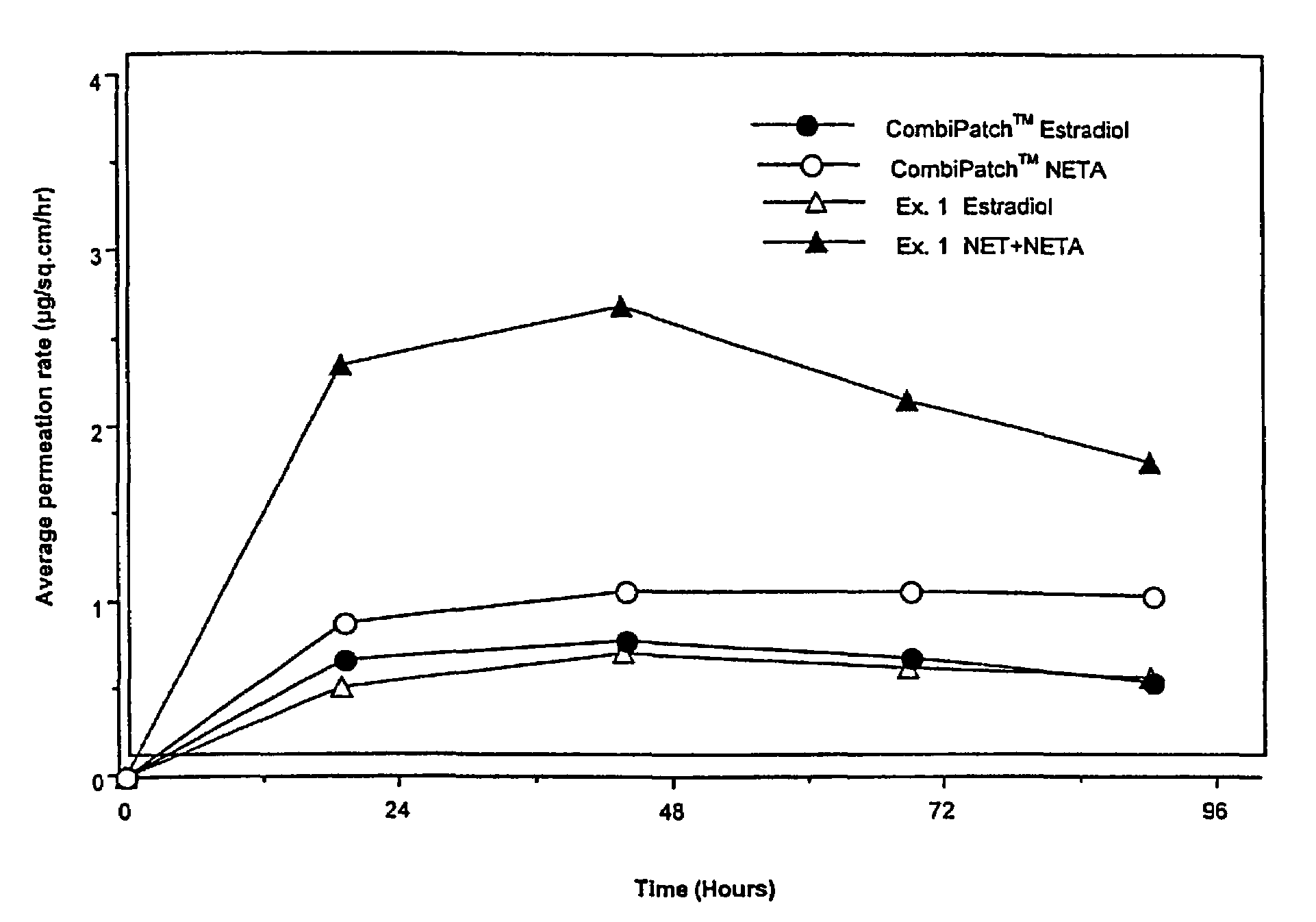
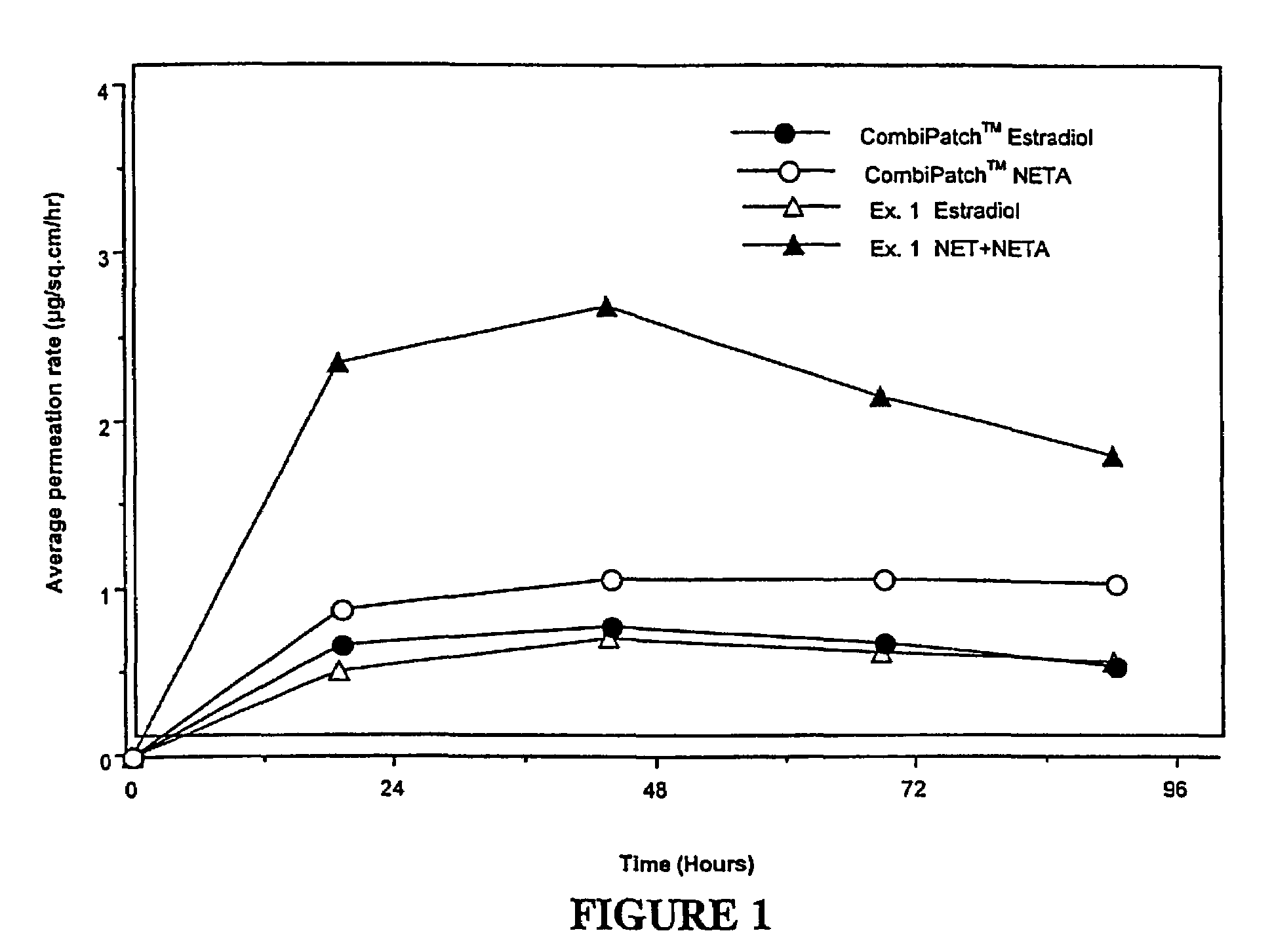
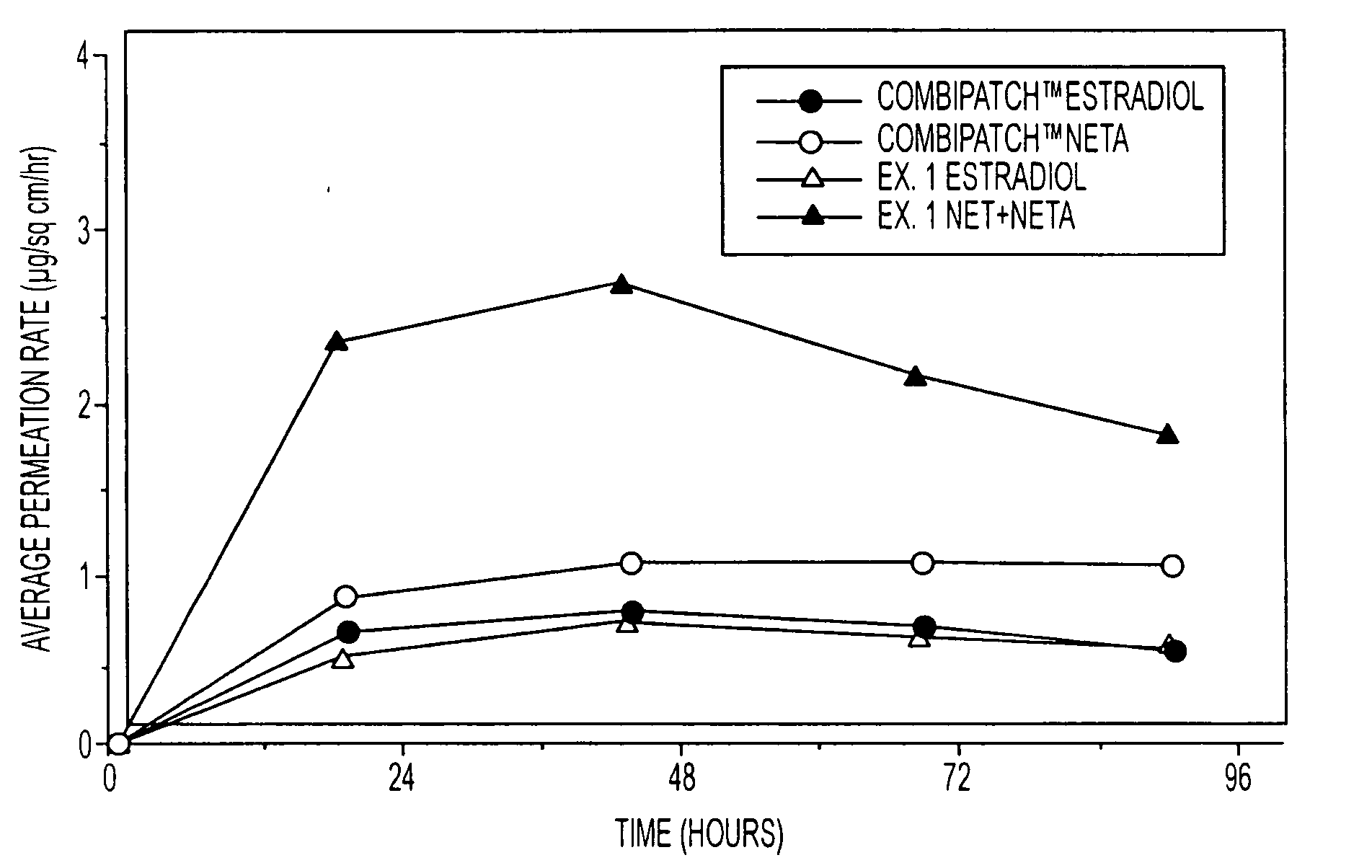
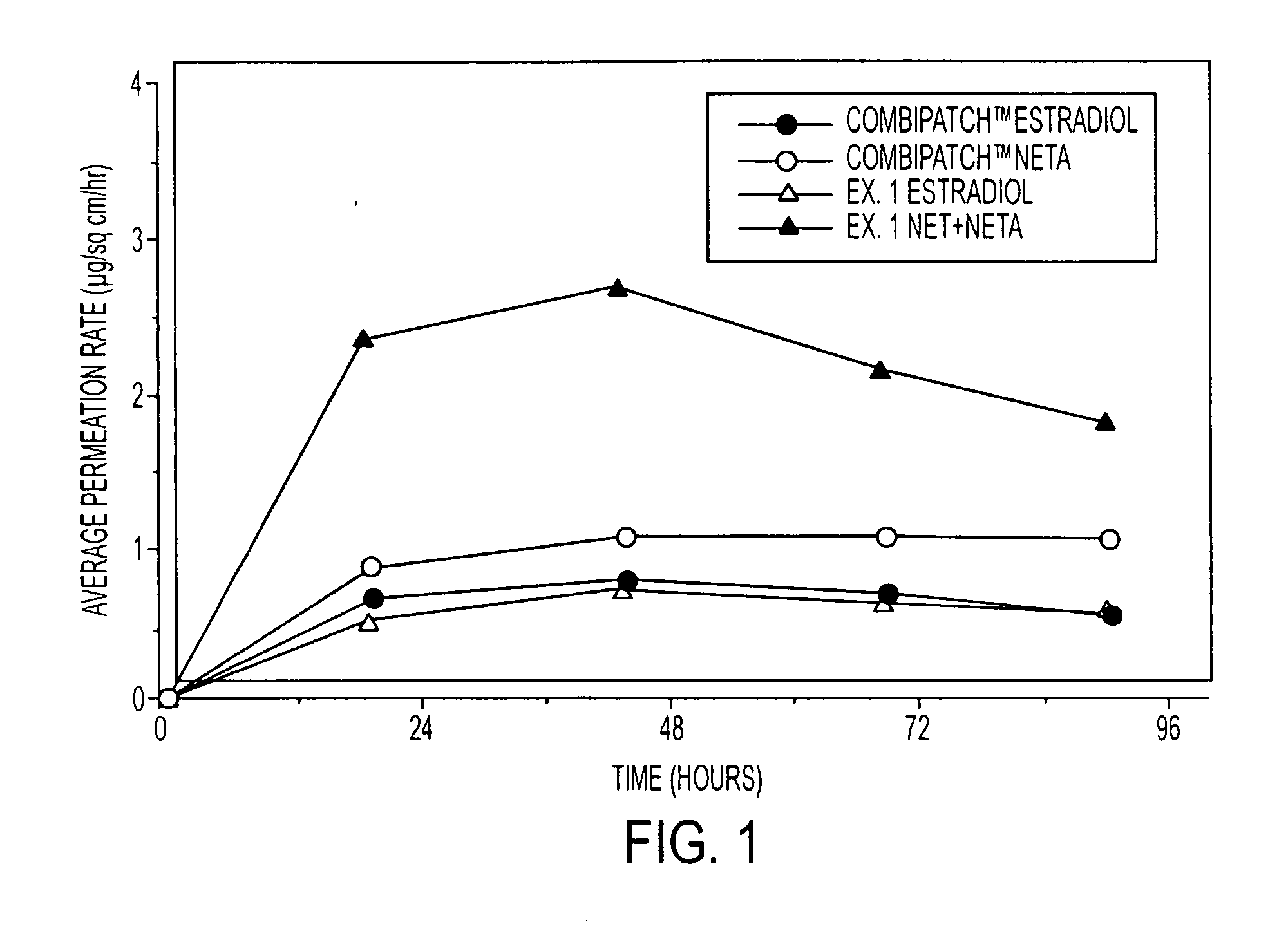
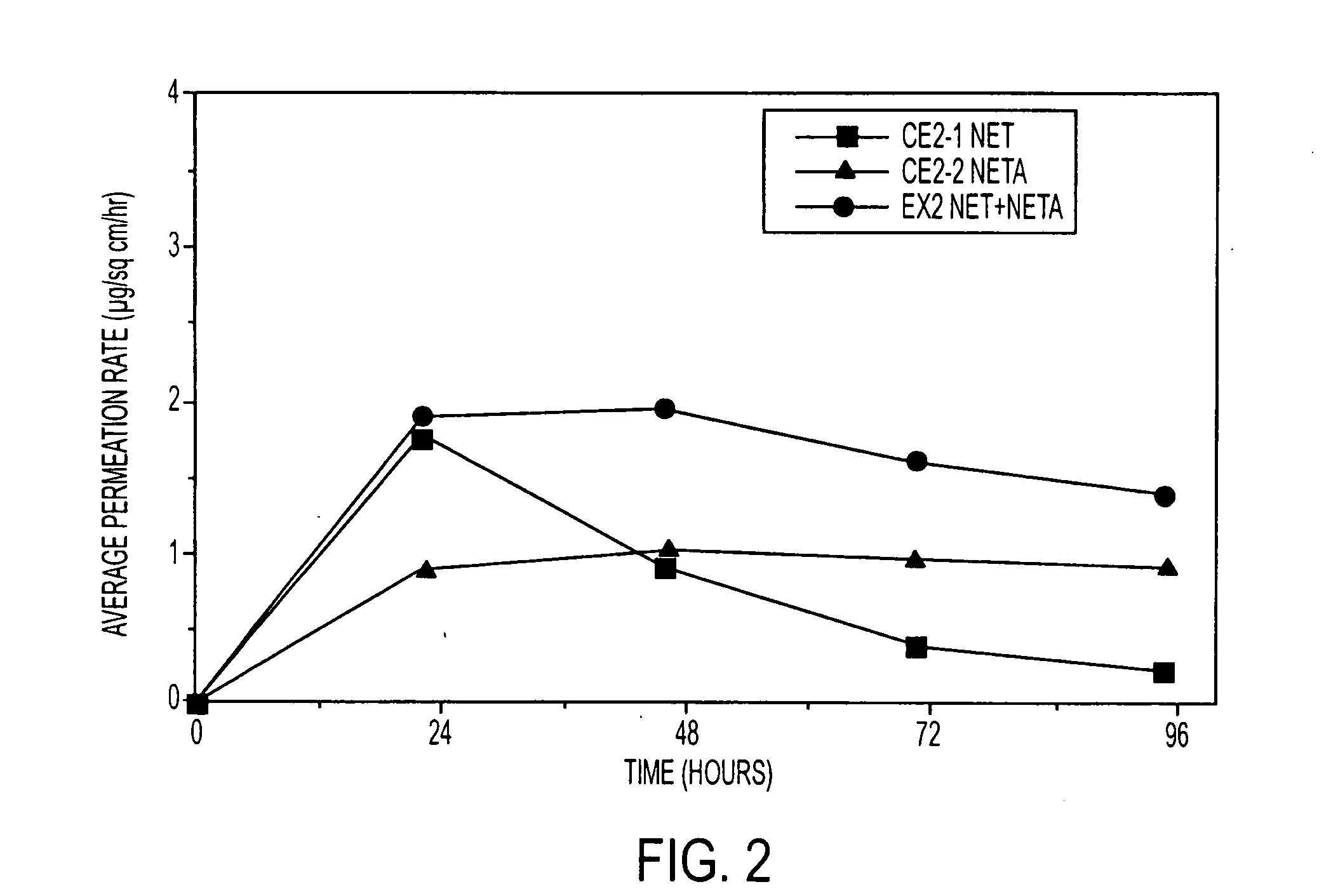
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid: corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
Stable pharmaceutical compositions containing an ACE inhibitor
InactiveUS6869963B2Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalInstability
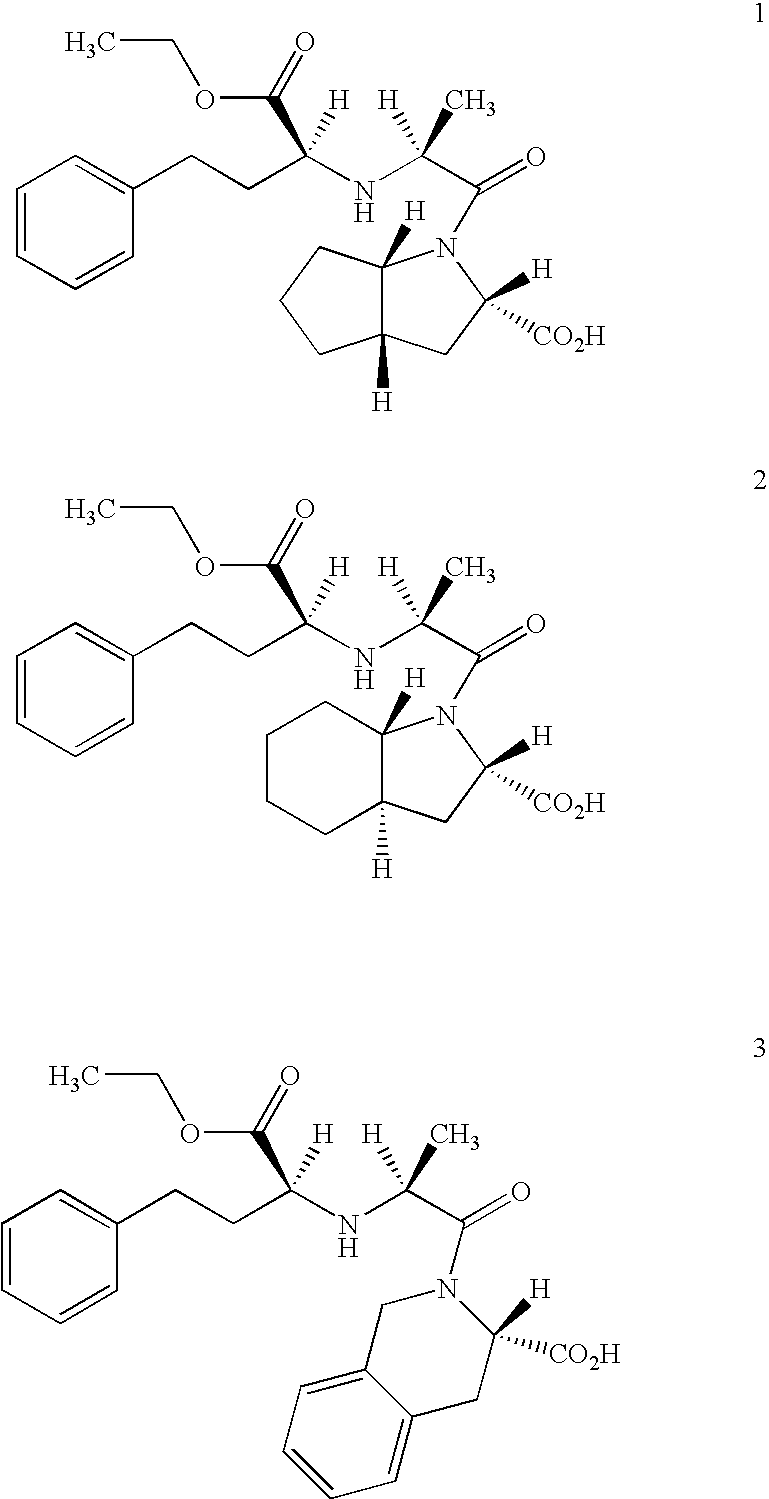
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Preparation of ramipril and stable pharmaceutical compositions
InactiveUS20070232680A1Minimizes hydrolysisReduce impurityBiocideOrganic chemistryMedicinal chemistryRamipril
Owner:DR REDDYS LAB LTD +1
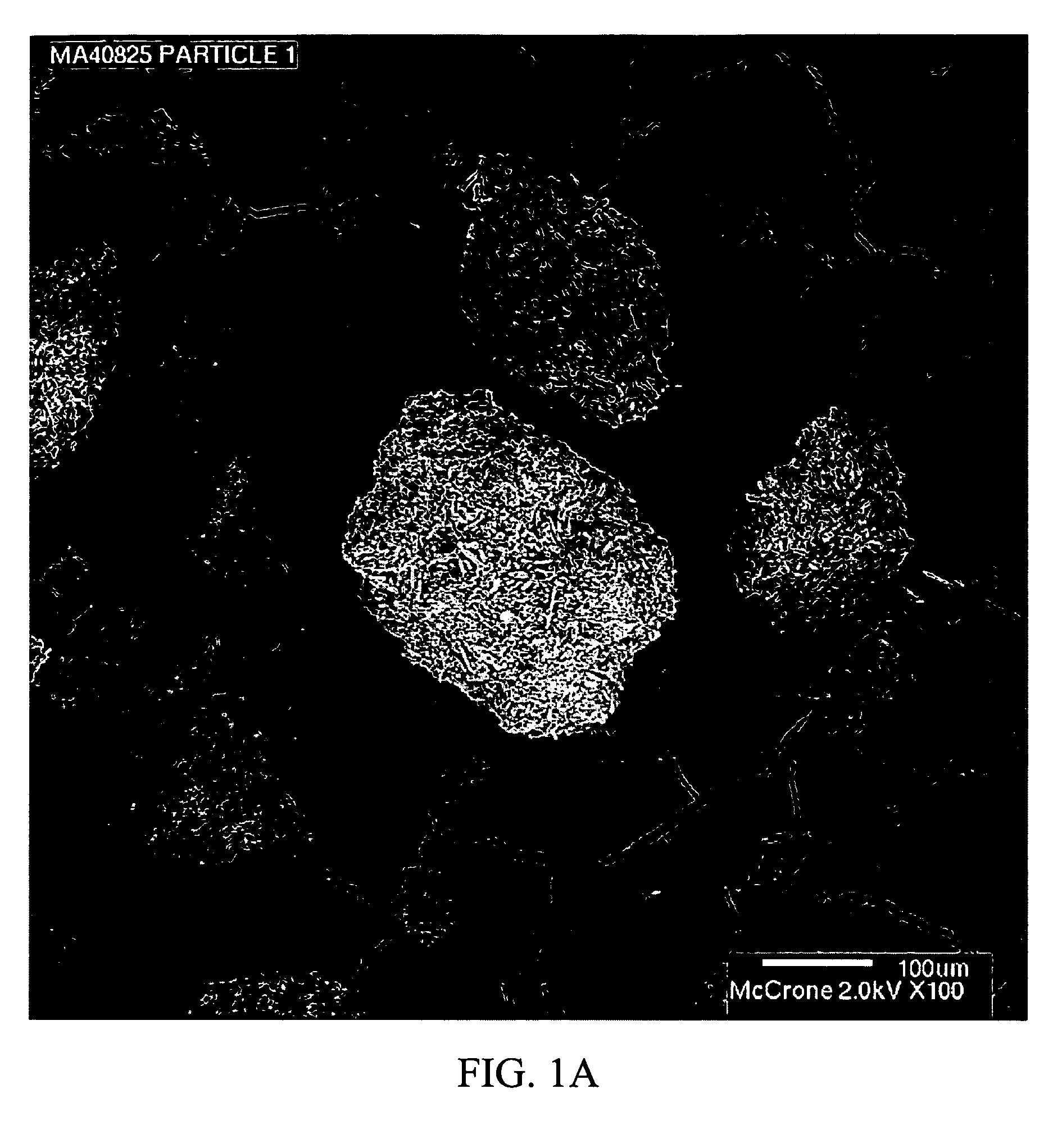
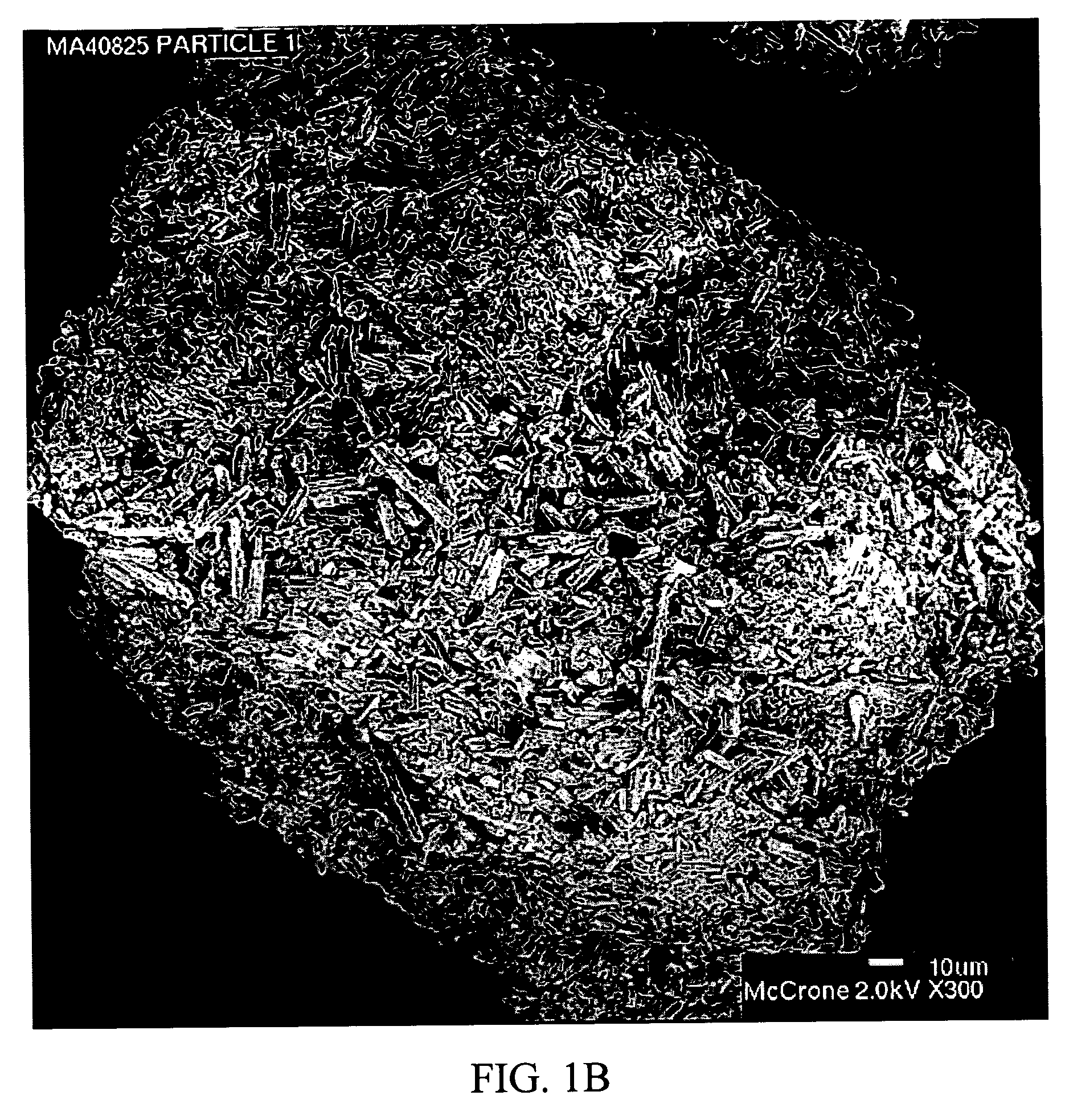
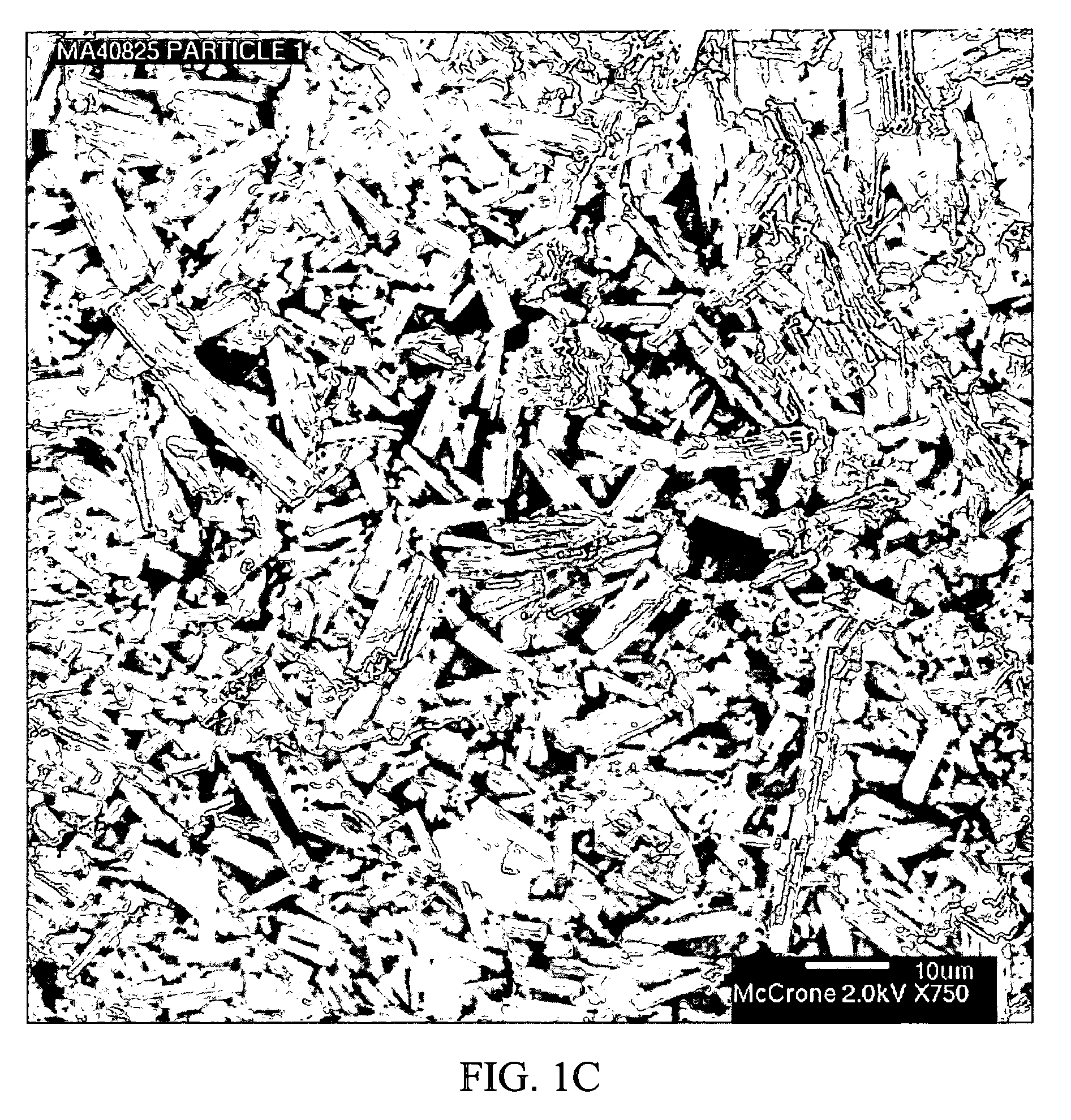
Stabilized individually coated ramipril particles, compositions and methods
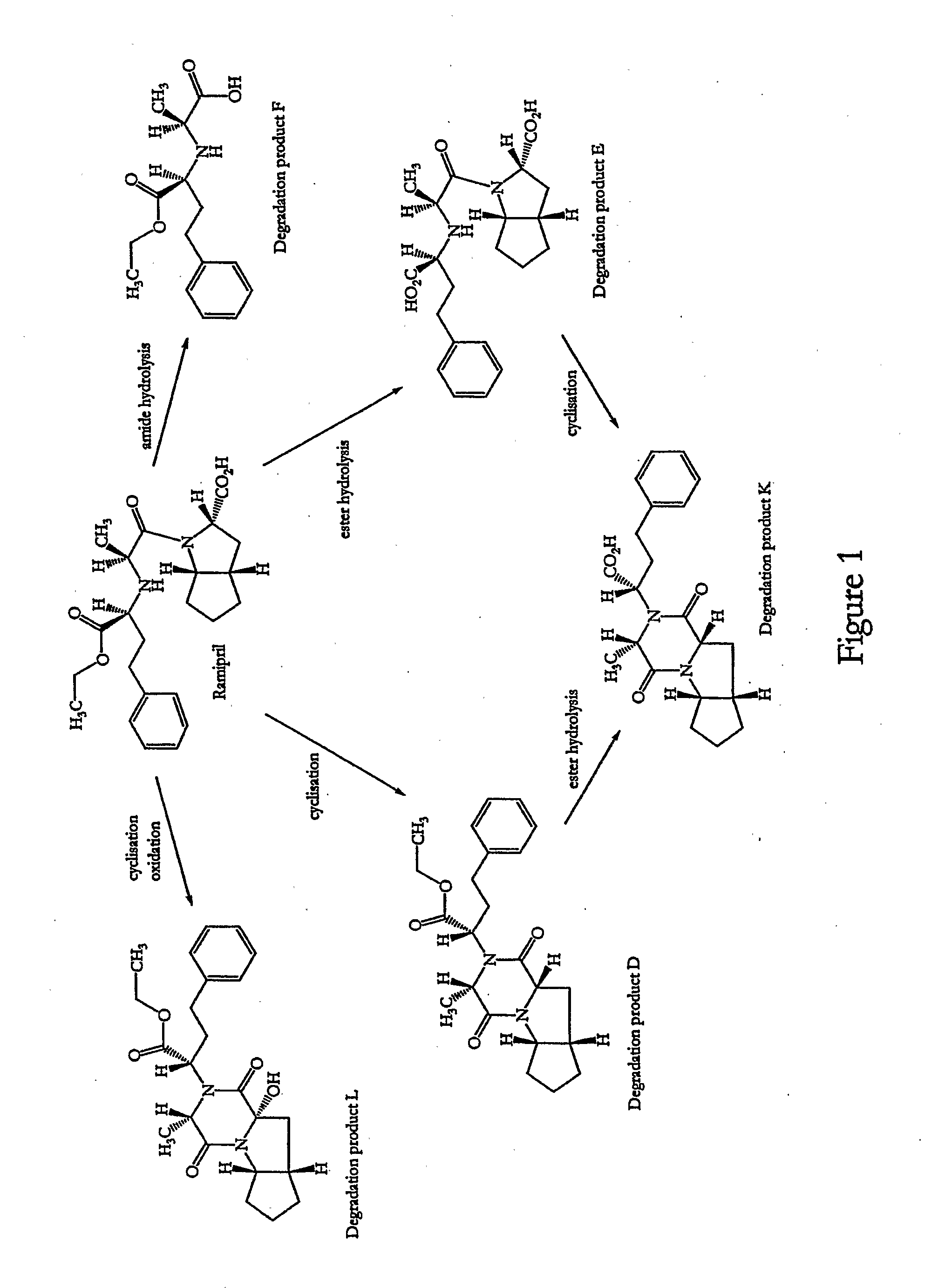
InactiveUS20060159742A1Improve stabilityMaintain potencyBiocidePowder deliveryDecompositionOral therapy
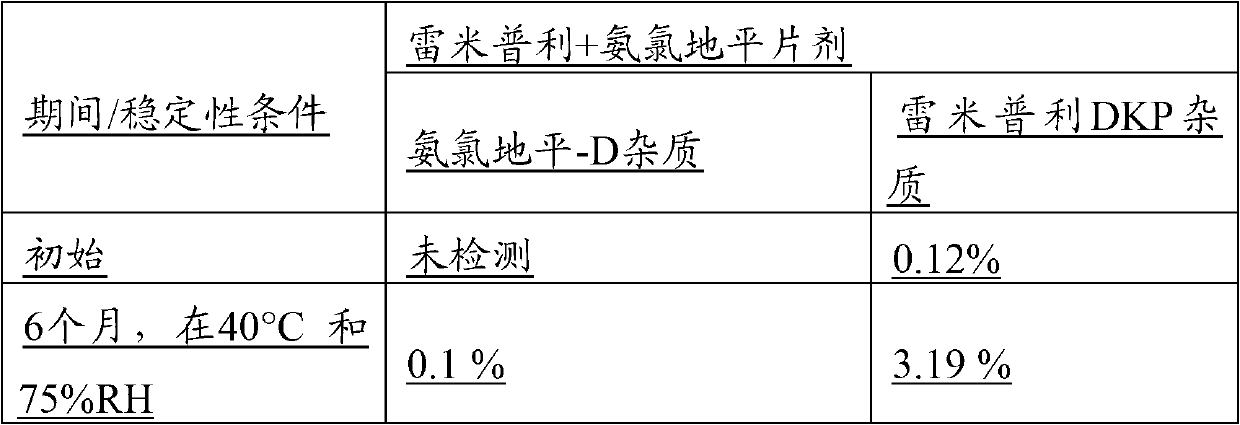
The present invention relates to novel ramipril crystalline particles with improved stability and bioavailability. More particularly, the present invention is directed to individually coated, single ramipril crystalline particles for pharmaceutical and biopharmaceutical applications in oral therapies that are stabilized against decomposition into degradation products, namely, ramipril-DKP and ramipril-diacid, during formulation and storage conditions. The present invention also relates to stabilized ramipril pharmaceutical compositions, novel anhydrous pharmaceutical grade ramipril powders, methods for improving ramipril bioavailability, and methods of manufacture and stabilization of ramipril formulations. The novel, anhydrous pharmaceutical grade ramipril powders and ramipril compositions and dosage forms formed therewith are useful in the treatment of cardiovascular disorders and have the advantage that they provide greater stability against decomposition into ramipril-DKPs and ramipril-diacids under formulation and storage conditions. In addition, they maintain consistent label ramipril potency over extended shelf-life and provide reduced in vivo variability in the bioavailability of ramipril among subjects when administered orally.
Owner:KING PHARMA RES & DEV
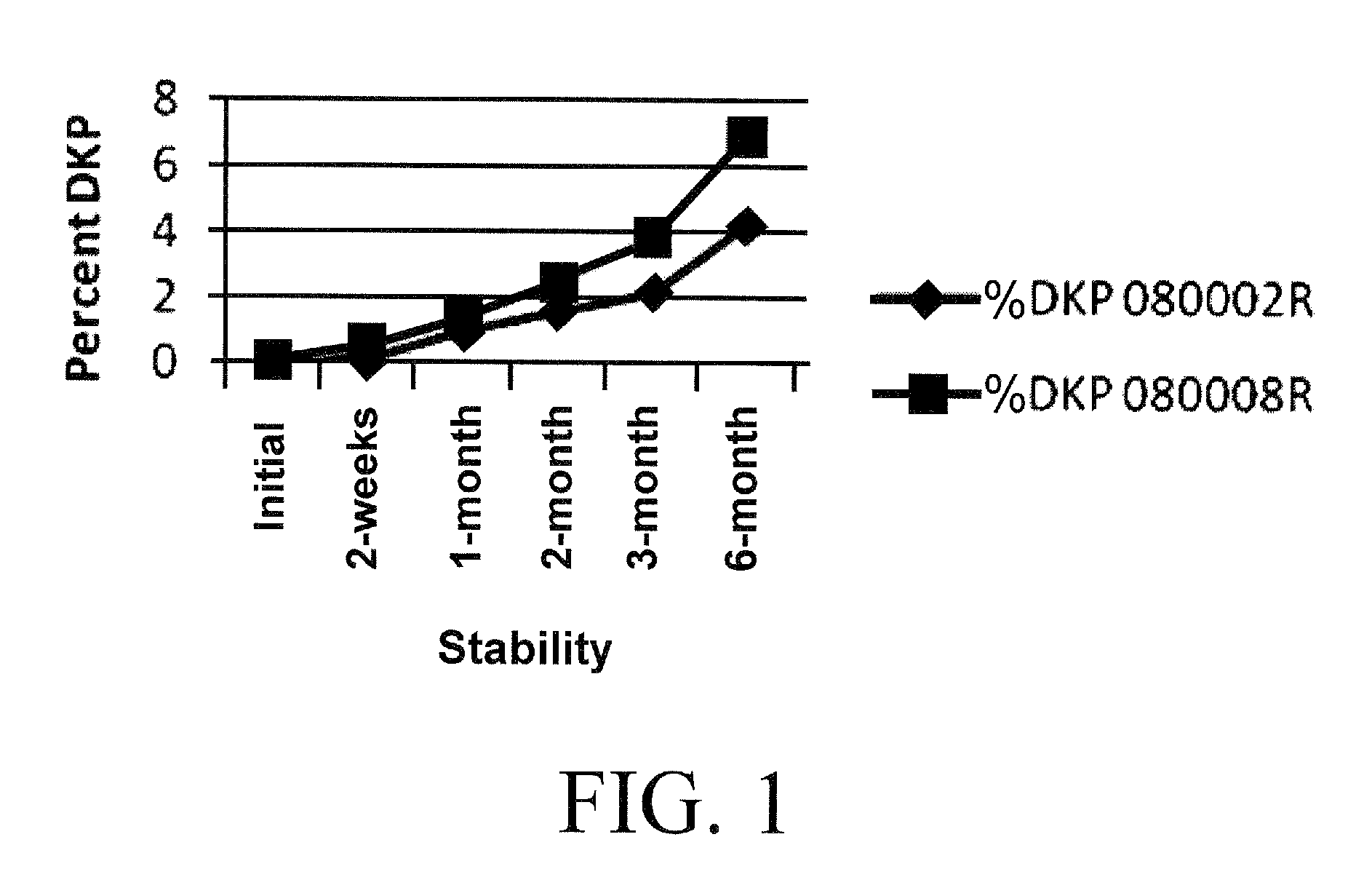
Stabilized Coating for Pharmaceutical Formulations
InactiveUS20100062062A1Improve stabilityEfficiently coat and stabilizeBiocidePharmaceutical non-active ingredientsPolyvinyl alcoholDiketopiperazines
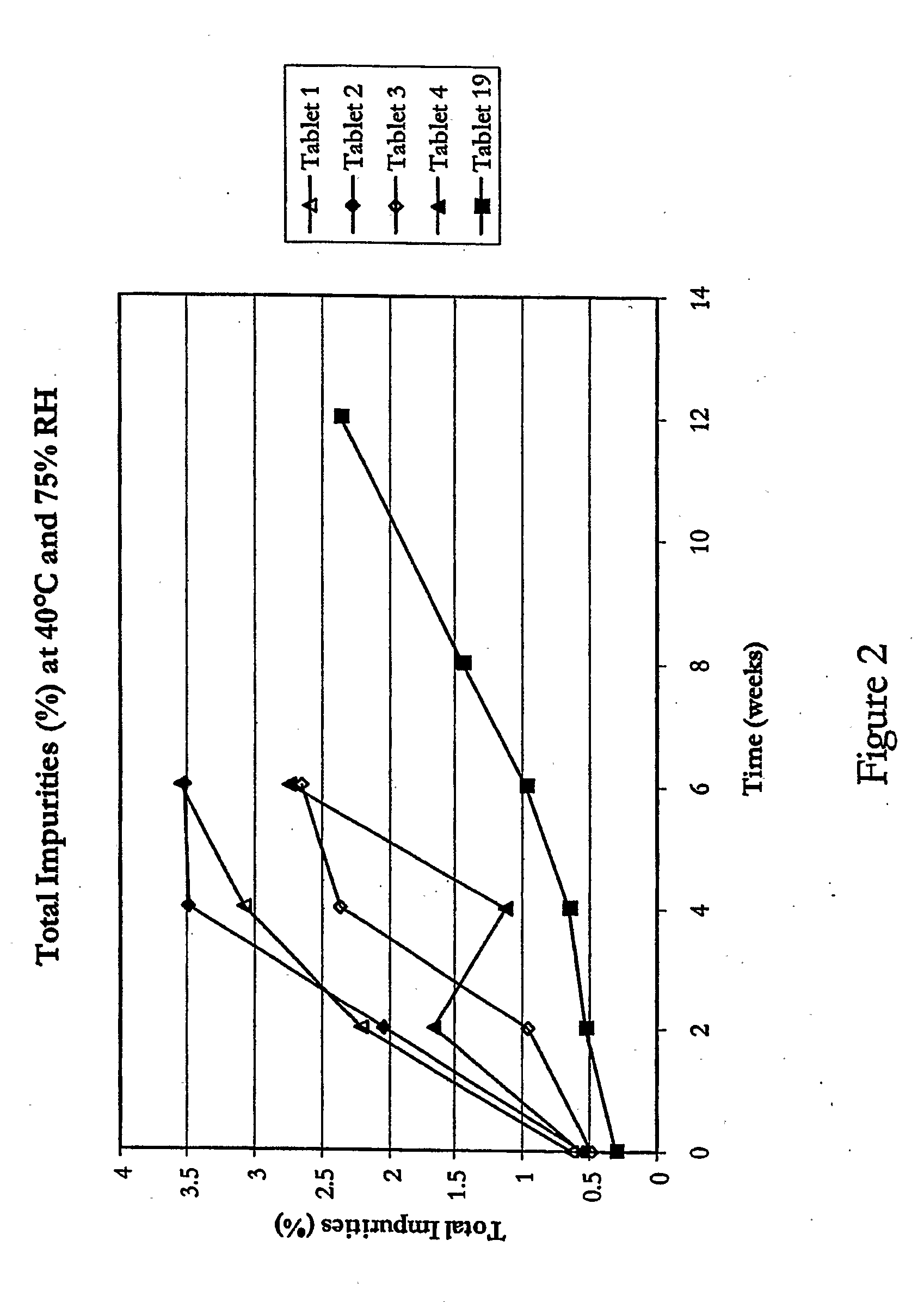
A process is described for preparing stabilized tablet formulations for temperature and moisture sensitive active drugs. Water soluble polyvinyl alcohol is processed with drugs such as angiotensin converting enzyme (ACE) inhibitors and compressed into solid form once excess water is removed. Low dose polyvinyl alcohol ramipril tablets prepared by this process are stable under conditions of high humidity and heat for periods of at least up to six months with less than 8% hydrolysis of the prodrug to the active metabolite diketopiperazine (DKP).
Owner:AETHOS PHARMA
Stable pharmaceutical compositions containing an ace inhibitor
InactiveUS20050009806A1Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalDepressant
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Stable pharmaceutical formulations
A stable oral pharmaceutical formulation comprising ramipril or its pharmaceutically acceptable salt and a stabilizing amount of an ammoniomethacrylate copolymer in a pharmaceutically acceptable carrier medium is described.
Owner:SUN PHARMA INDS
Stable Pharmaceutical Composition Comprising an Ace Inhibitor
InactiveUS20080038342A1Minimize degradationStable pharmaceutical compositionBiocidePill deliveryStress inducedCoronary heart disease
The present invention relates to a stable pharmaceutical composition comprising an ACE inhibitor or a pharmaceutically acceptable salt or derivative thereof. In particular, the invention relates to a pharmaceutical composition, which comprises an ACE inhibitor, or a pharmaceutically acceptable salt or a derivative thereof, and a C16-C28 glyceride. ACE inhibitors useful in the present invention are susceptible to heat and / or mechanical stress-induced degradation. Preferred ACE inhibitors are ramipril, trandolapril, quinapril and pharmaceutically acceptable salts and derivatives thereof. The composition of the present invention may be for use as a medicament for the treatment or prevention of a cardiovascular disease, a coronary heart disease, a cerebrovascular disease, a peripheral vascular disease, arrhythmia, hypertension, cardiac failure, cardiovascular death, myocardial infraction, stroke or angina. The present invention further relates to a method of preparing the pharmaceutical composition of the present invention. The present invention also relates to a method of providing a stable pharmaceutical composition comprising an ACE inhibitor, or a pharmaceutically acceptable salt or derivative thereof, by incorporating a C16-C28 glyceride into the composition. The present invention further relates to a use of C16-C28 glyceride to provide a stable pharmaceutical composition comprising an ACE inhibitor or a pharmaceutically acceptable salt or derivative thereof.
Owner:NICHE GENERICS
A method for preparing ramipril
ActiveCN103282350AAvoid wastingIncrease productionCarboxylic acid amides optical isomer preparationPropanoic acidSerine endopeptidase
Enantio-specific synthesis of optically pure (2S)-acetylamino-3-(2-oxo-cyclopentyl)- propionic acid (I) comprising converting enantiomeric mixture of (1 -4C alkyl)-2- acetylamino-3-(2-oxocyclopentyl) propionoate (II) (+ and -) under the influence of an Alkaline serine endopeptidase is disclosed. The invention further describes use of optically pure (2S)-acetylamino-3-(2-oxocyclopentyl)-propionic acid (I) formed by the process of present invention, in the preparation of Ramipril.
Owner:AARTI HEALTHCARE LTD
Ramipril formulation
InactiveUS20070053975A1Fast absorptionGreat C max valueBiocideNervous disorderFOOD EFFECTPharmacology
A Ramipril formulation rapidly disintegrates after ingestion and exhibits substantially no food effect.
Owner:SELAMINE
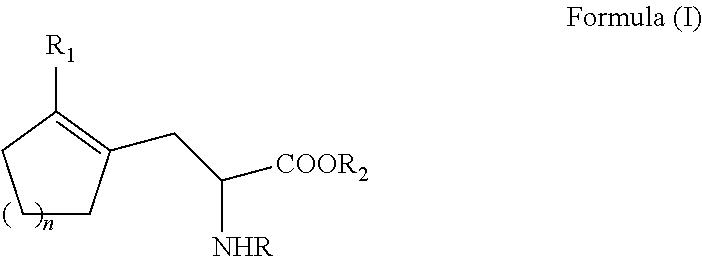
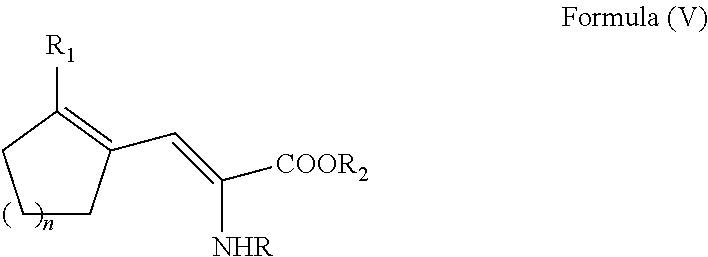
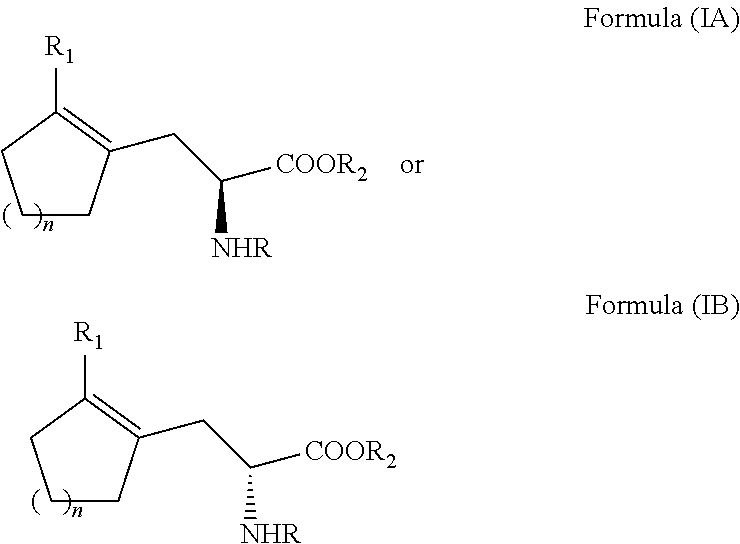
Enantioselective process for cycloalkenyl ?-substituted alanines
ActiveUS20110257408A1Easy to convertHigh yieldOrganic compound preparationCarboxylic acid amides optical isomer preparationCycloalkeneAsymmetric hydrogenation
A process for preparing an enantiomerically enriched cycloalkene-substituted alanine compound having the structure:by asymmetrically hydrogenating a dehydro amino acid compound having the structure:in a suitable reaction media in the presence of a catalyst having a transition metal moiety complexed to a chiral phosphine ligand to prepare enantiomerically enriched cycloalkene substituted alanine compounds having the structure of Formula (IA) or (IB), which are key intermediates for the ACE inhibitors ramipril and perindolpril:
Owner:CHIRAL QUEST
Enhanced drug delivery in transdermal systems
InactiveUS20080167280A1Convenient amountGood synergyBiocidePeptide/protein ingredientsActive agentEthyl ester
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid:corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
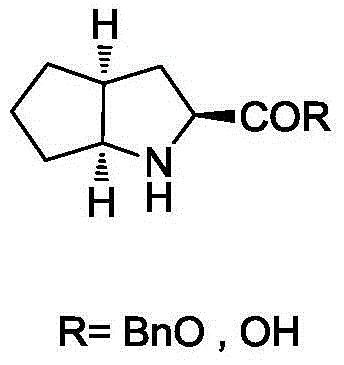
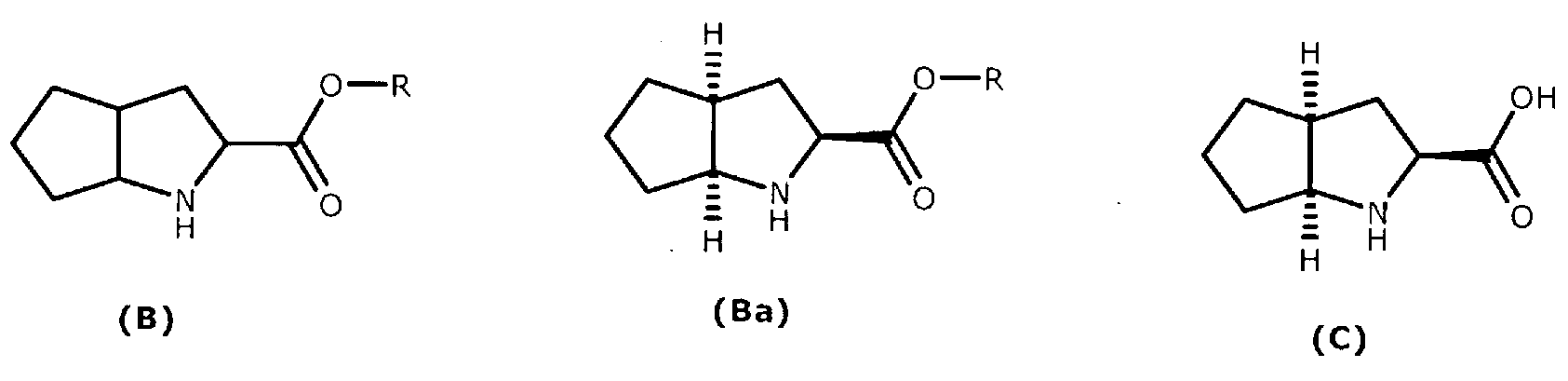
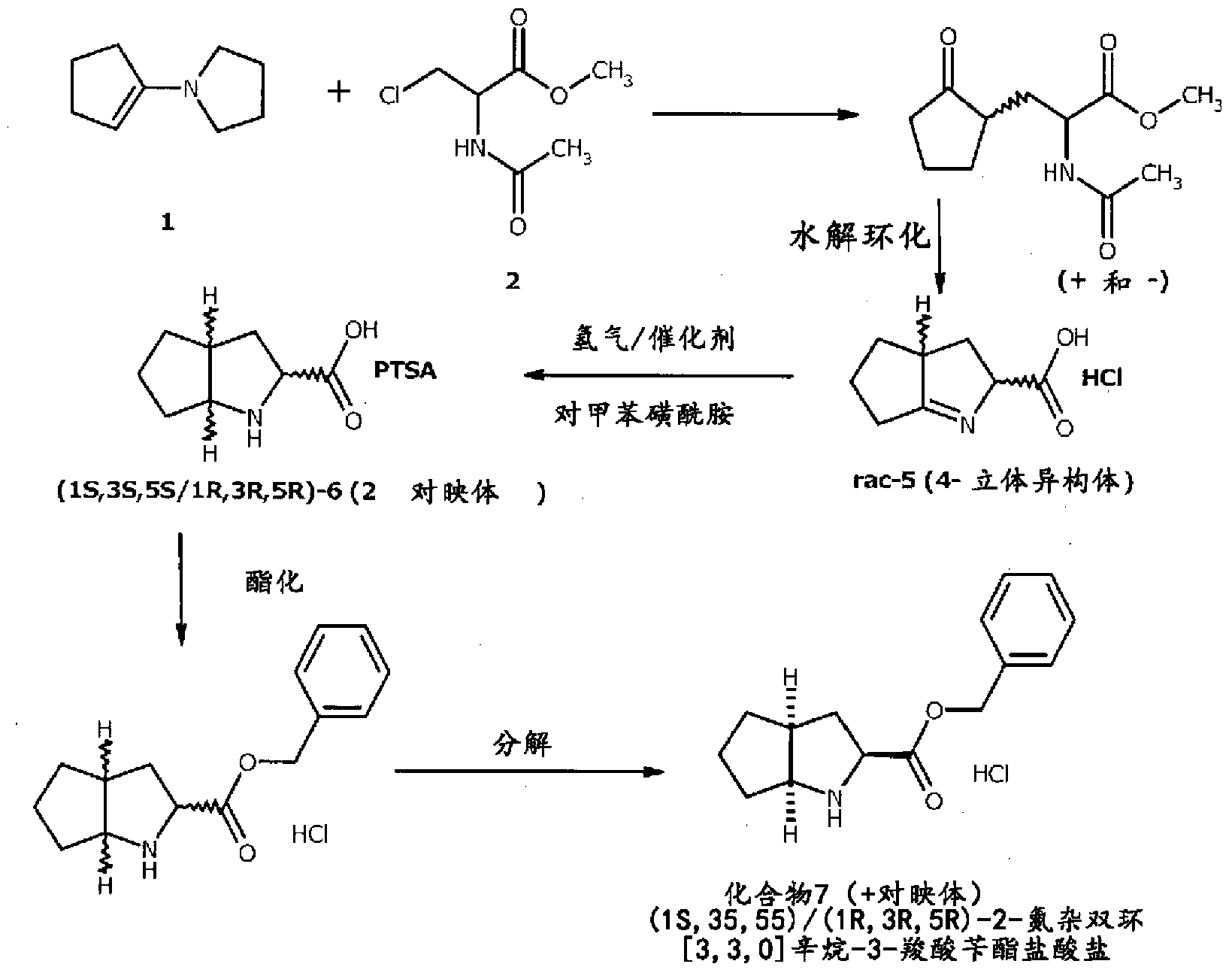
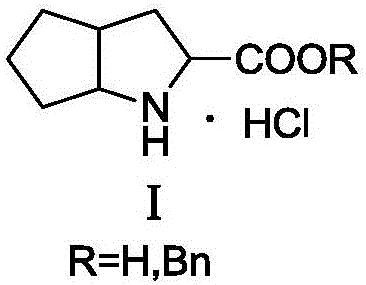
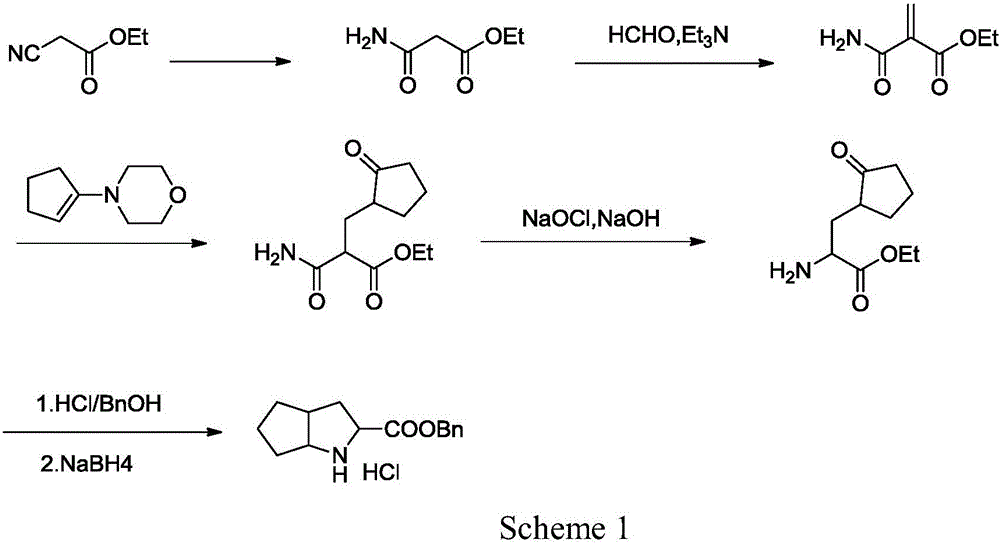
Synthesizing method using serine to prepare Ramipril key intermediate
InactiveCN105777611ASimple preparation processSimple and fast operationOrganic chemistrySynthesis methodsCarboxylic acid
The invention relates to a synthesizing method using serine to prepare a Ramipril key intermediate. The Ramipril key intermediate is 2-azabicyclo[3.3.0] octane-3-carboxylic acid hydrochloride or benzyl ester hydrochloride. The synthesizing method includes: using the serine as the initial raw material, and sequentially performing esterification, acyl chloride acylation, deacidification, Michael addition, hydrolysis and hydrogenation reduction to obtain the Ramipril key intermediate. The synthesizing method has the advantages that the key intermediate is synthesized by a five-step method, and the synthesizing method is cheap in raw material, environmentally friendly, simple in preparation process, simple to operate, mild in reaction condition, short in reaction cycle, convenient in post-treatment, low in equipment requirement, capable of avoiding heavy metal pollution and the use of expensive catalysts, few in three wastes, high in product yield and purity and suitable for industrial production.
Owner:ZHEJIANG UNIV OF TECH +1
Enhanced drug delivery in transdermal systems
ActiveUS20080167365A1Convenient amountGood synergyBiocidePeptide/protein ingredientsActive agentEthyl ester
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid: corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
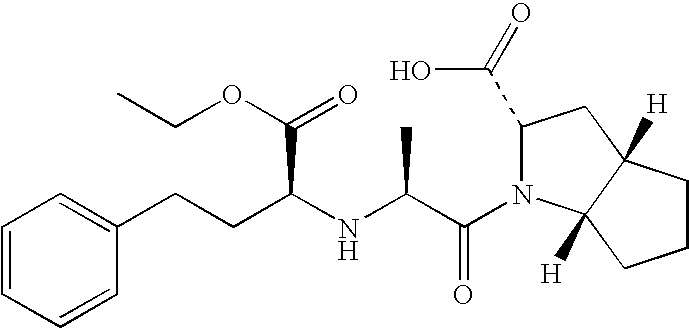
Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride
ActiveCN1611494AReduce dosageReduce manufacturing costOrganic chemistryChemical synthesisOrganic solvent
The invention relates to a kind of chemosynthesis method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which is the medicine midbody for synthesizing Pulitzer series medicine such as Enalapril, Ramipril, Lisinopril and so on. The invention uses couple (trichloromethyl) ester carbonate and N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine as row material, and get the product by reacting with catalyst action in organic solvent. This chemosynthesis method is a manufacturing method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which has acquirable raw material, low production cost, and no three wastes normally.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of N-carboxyalkyl dipeptide type angiotensin converting enzyme inhibitor
InactiveCN1429835ASimple and efficient processSuitable for industrialized mass productionDipeptidesCardiovascular disorderDipeptideDepressant
A process for preparing N-carboxyalkyl dipeptide type angiotensin converting enzyme (ACE) depressant (such as enalapril maleate, ramipril, etc) includes such steps as preparing N-carboxylic acid anhydride from bis (trichloromethyl) carbonate, and coupling with relative alpha-amino acid.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
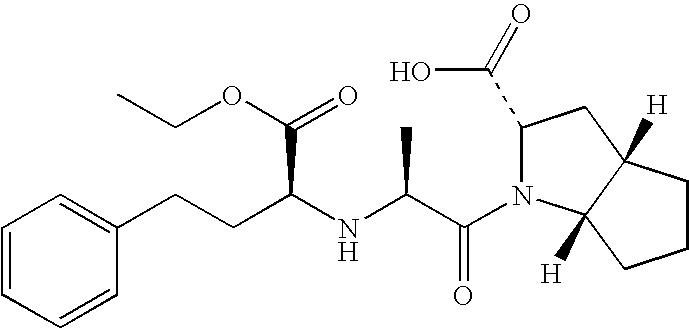
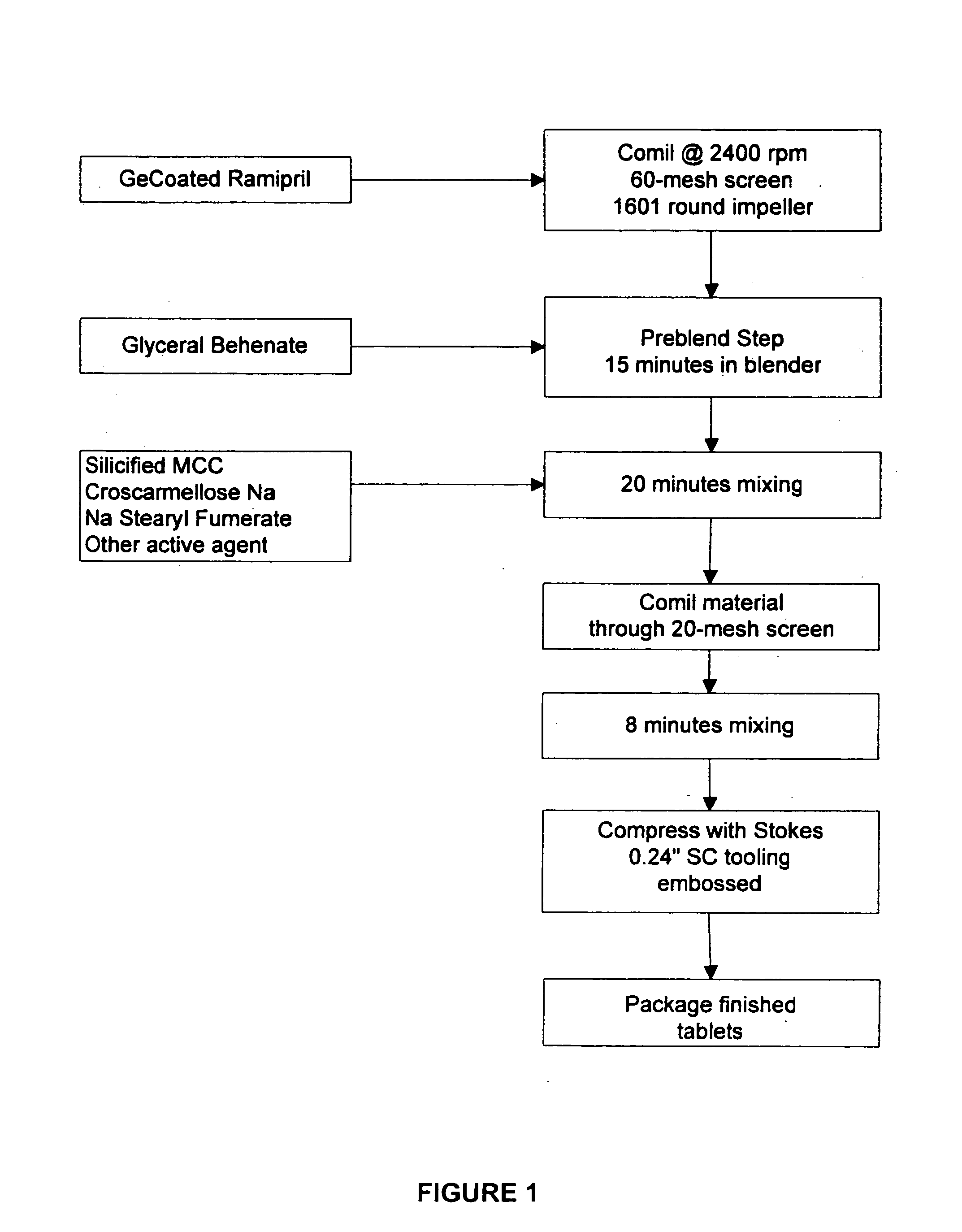
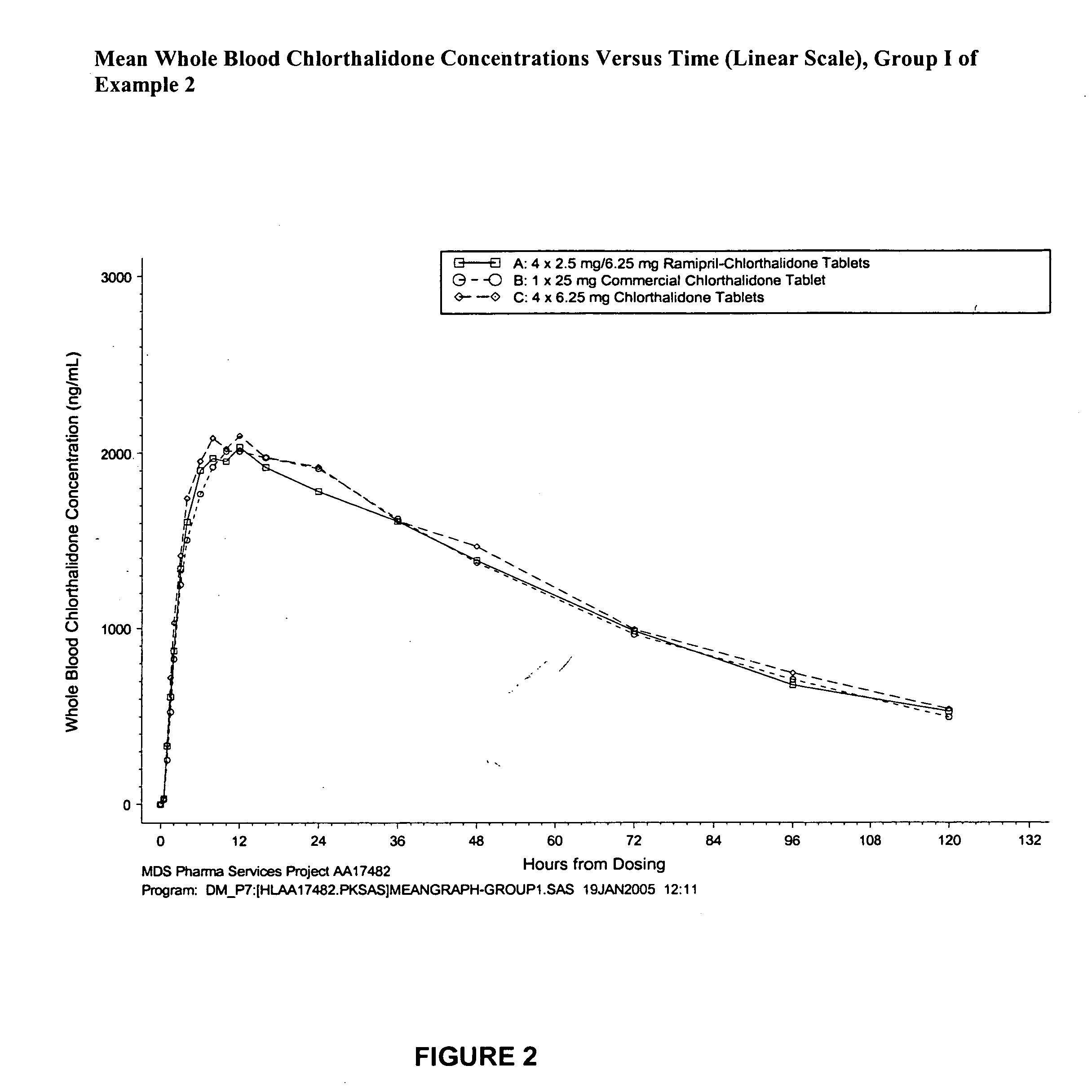
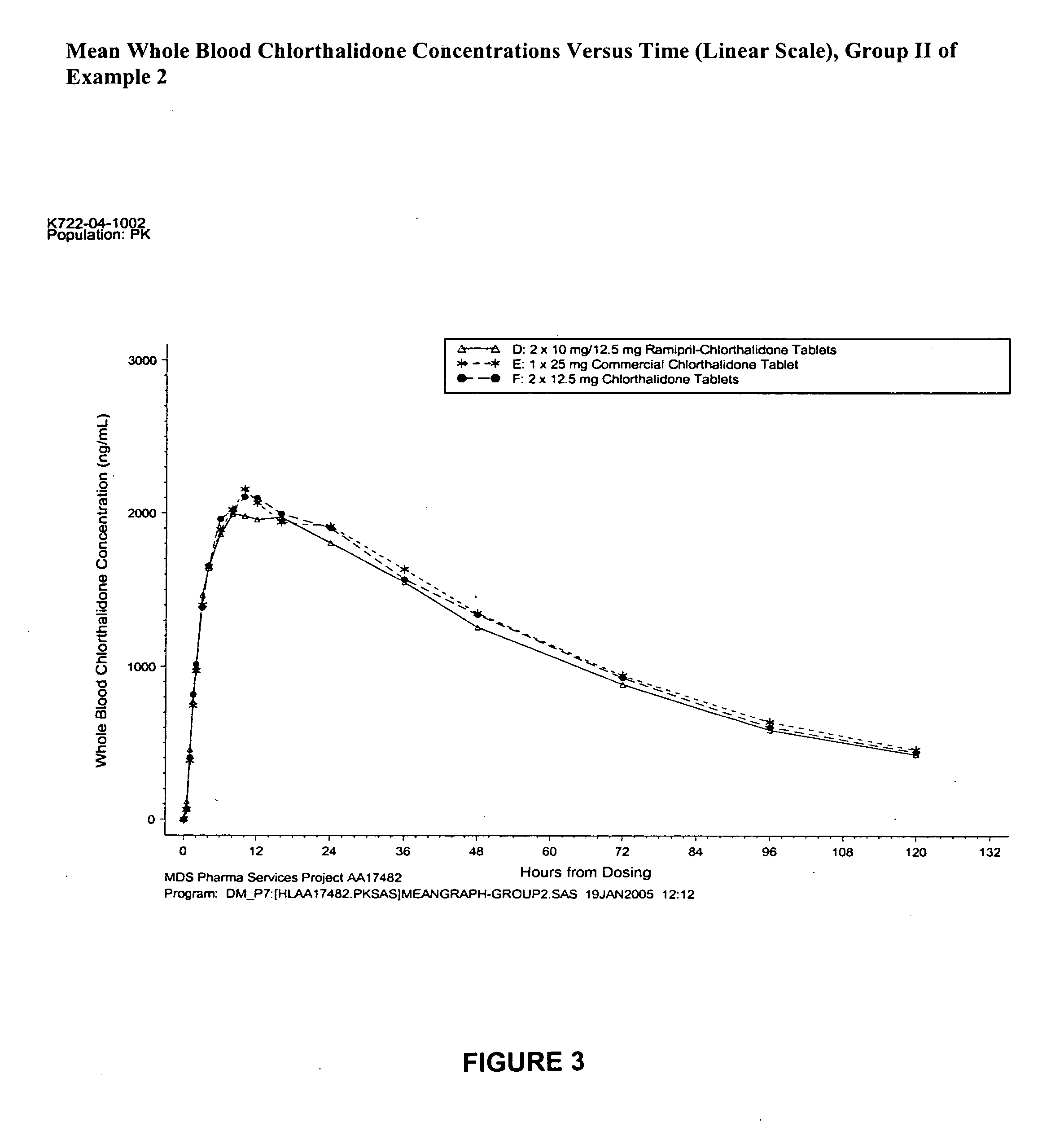
Compositions of stabilized ramipril in combination with another active agent
A pharmaceutical composition comprising ramipril, another active agent, and a blending agent, wherein in the ramipril is coated by the blending agent, and wherein the blending agent is glyceryl behenate, glyceryl stearate, stearyl alcohol, macrogol stearate ether, palmitosearate, ethylene glycol, polyethylene glycol, stearic acid, cetyl alcohol, lauryl alcohol, amylopectin, poloxymer or combinations thereof.
Owner:KING PHARMA RES & DEV
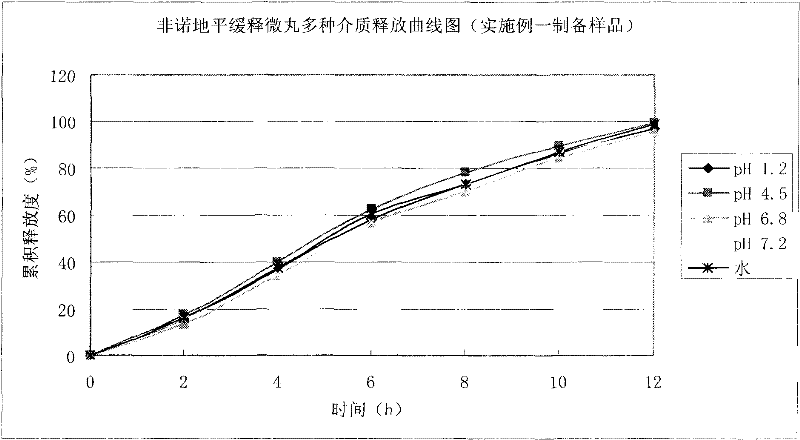
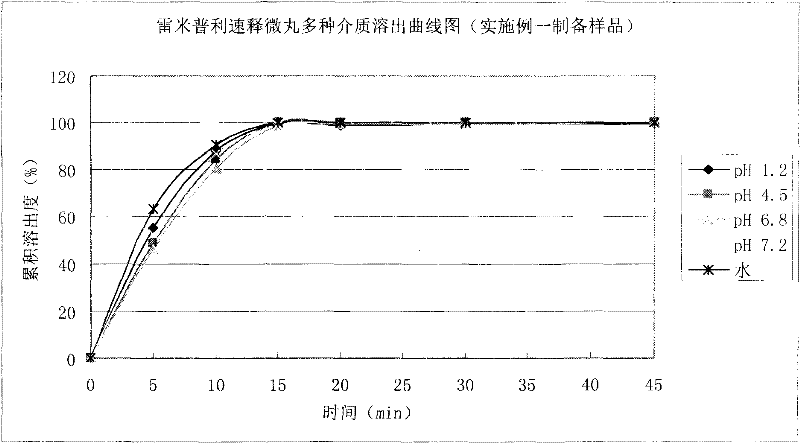
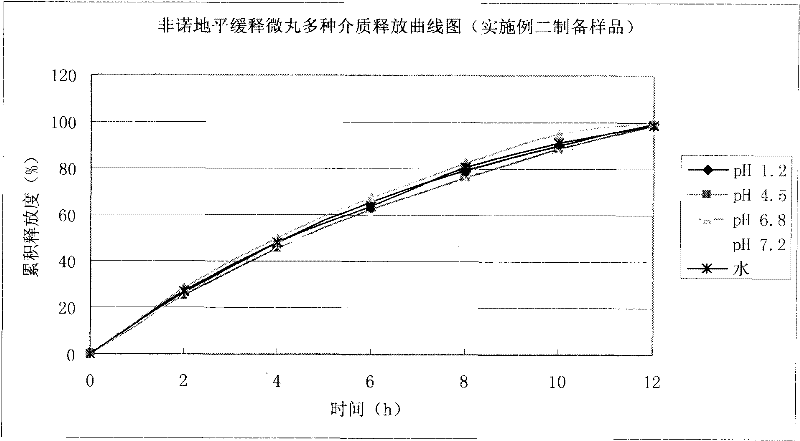
Felodipine ramipril compound sustained-release preparation and preparation method thereof
InactiveCN102600451AUniform absorption rateSmall differences in individual bioavailabilityOrganic active ingredientsDipeptide ingredientsSustained release pelletsSide effect
The invention discloses a compound sustained-release preparation containing felodipine and ramipril and a preparation method of the compound sustained-release preparation, wherein the compound sustained-release preparation is prepared by mixing felodipine sustained-release pellets and ramipril immediate-release pellets according to a specific dose proportion. The felodipine sustained-release pellets can sustainably release for 12 hours, and can be taken once a day, so as to keep the antihypertensive effect for a long time, the ramipril immediate-release pellets can be quickly absorbed so as to achieve the antihypertensive effect and treat the plasma concentration, due to the combination of the two kinds of pellets, the antihypertensive effect can be greatly enhanced, the toxic and side effect of the medicine can be reduced, the administration frequency can be reduced, sustainably stable antihypertensive effect can be achieved in a human body, the patient compliance can be enhanced, the compound sustained-release preparation consists of hundreds and thousands of pellets in uniform particle sizes, so that the damage of individual pellets will not cause burst release of the whole preparation, and compared with the sustained release tablets, the compound sustained-release preparation disclosed by the invention has the advantages of higher safety, lower irritation to gastrointestinal tract, more stable plasma concentration and capability of effectively improving the clinical effect.
Owner:广州科的信医药技术有限公司
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
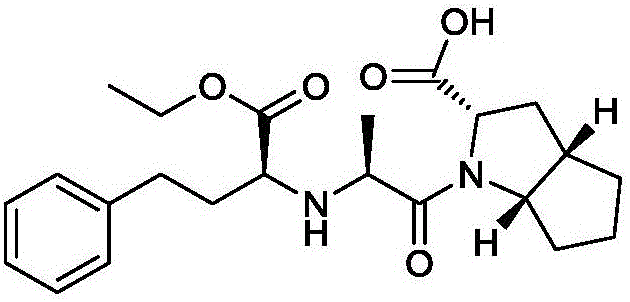
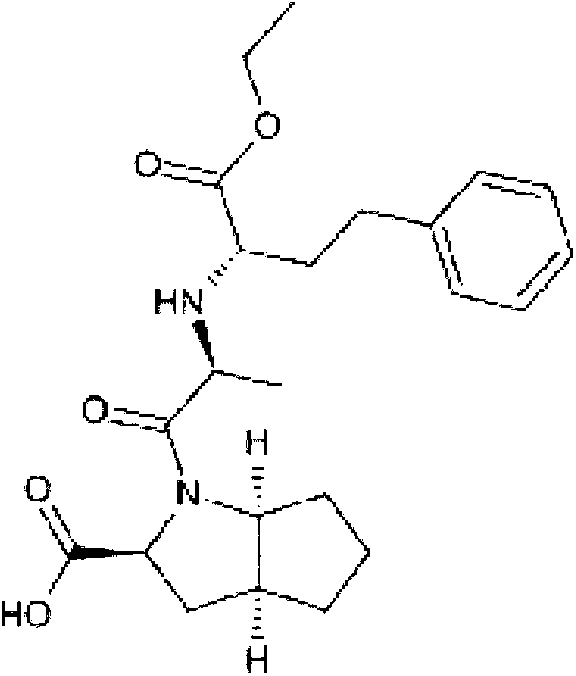
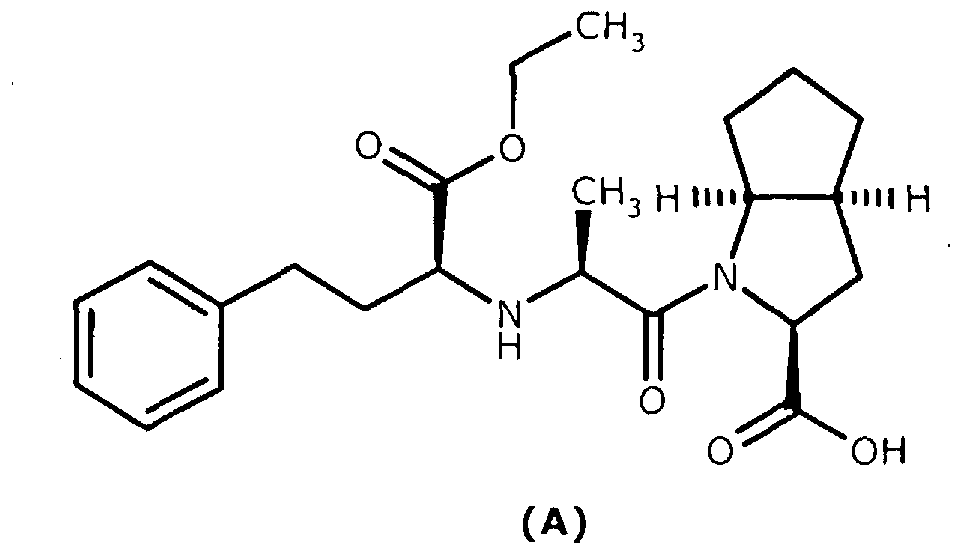
Preparation method of ramipril
The invention relates to a large-scale industrialized preparation method of ramipril. The preparation method includes following steps; (1) performing a reaction directly from a compound (s,s,s)-2-azobicyclo[3,3,0]octane-3-carboxylic acid derivative and N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-L-alanine under action of a condensation reagent; and (2) performing catalytic hydrogenation deprotection and a purifying process to obtain the ramipril. In the preparation method, by means of optimization of technology conditions, generation of impurities can be reduced and a DCU residual problem can be solved. The preparation method is more than 60% in total yield and can reach a HPLC detected purity of 99.95%.
Owner:SHANDONG NEWTIME PHARMA
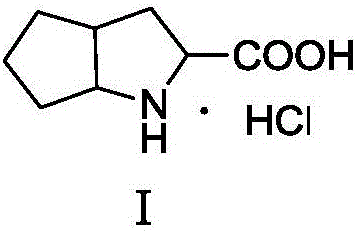
Synthesis method of ramipril key intermediate
ActiveCN106748966ASimple and fast operationMild reaction conditionsOrganic chemistrySynthesis methodsCarboxylic acid
The invention discloses a synthesis method of a ramipril key intermediate. The ramipril key intermediate is 2-azabicyalo [3.3.0] octane-3-carboxylic acid hydrochloride. The 2-azabicyalo [3.3.0] octane-3-carboxylic acid hydrochloride is obtained through sequential dehydration cyclization, formaldehyde condensation, hydrolysis, removal, Michael addition, cyclization and palladium-carbon catalytic hydrogenation reduction of N-benzoyl-glycine as a raw material. According to the synthesis method of the ramipril key intermediate, the required raw material and reagent are cheap and available, the yield is relatively high, the operation is simple, the cost is low and the method is suitable for industrial production.
Owner:ZHEJIANG UNIV OF TECH
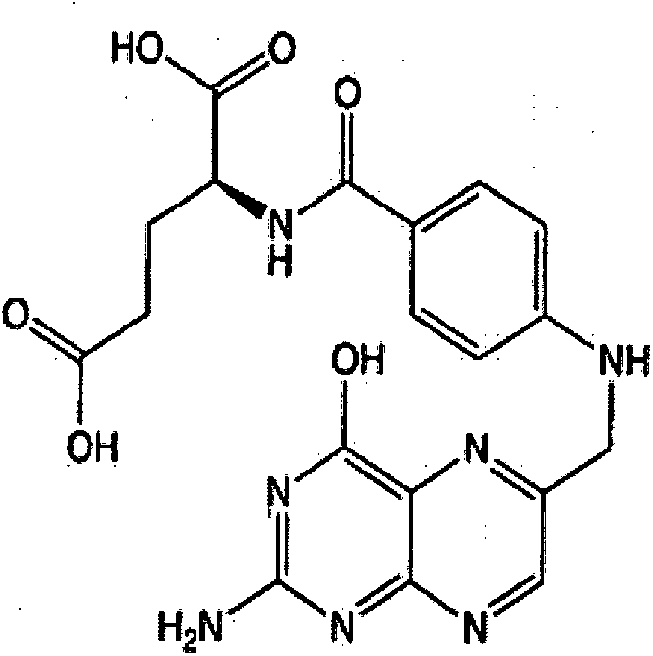
Folic acid - ramipril combination: cellprotective, neuroprotective and retinoprotective ophtalmologic compositions
The invention relates to a cellprotective, neuroprotective and retinoprotective composition. In an embodiment of the invention, said composition comprises (i) Ramipril or Ramiprilate and (ii) folic acid. The composition of the invention can be used, in particular, for the prevention of loss of vision, or even for improving visual acuity and visual field in normal subjects, as well as for treating ophthalmologic pathologies, in particular: glaucoma, diabetic retinopathy, age related macular degeneration, hereditary dystrophy of the retina, uveitis, ammetropia (myopia, presbyopia). This combination of active principles could also be used in general conditions for treating general pathologies (cancer...).
Owner:拉乌夫·雷基克
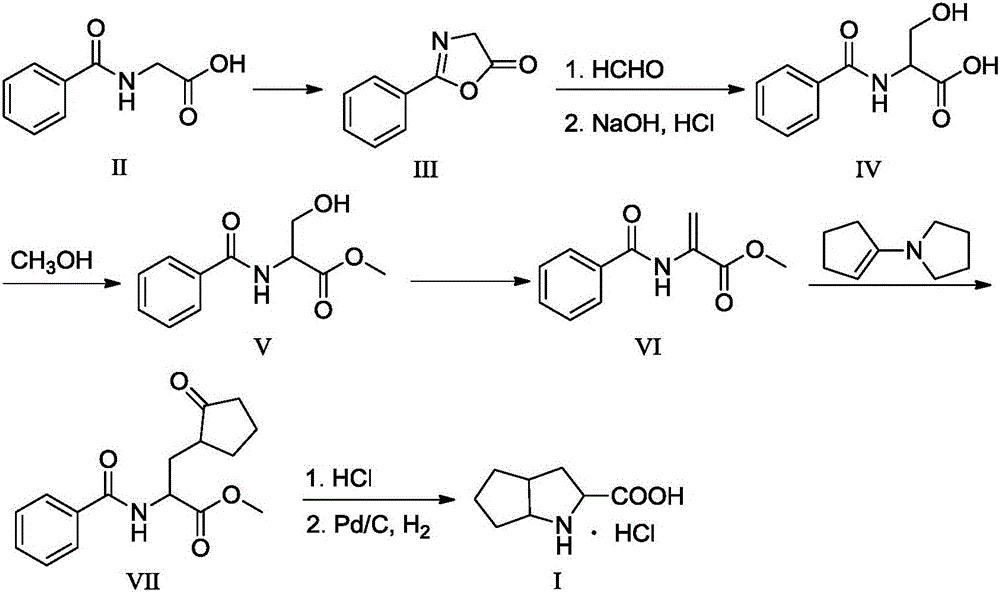
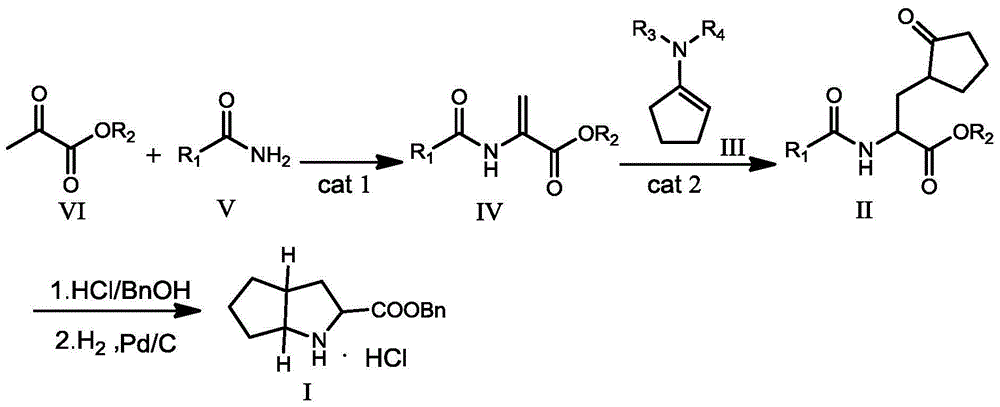
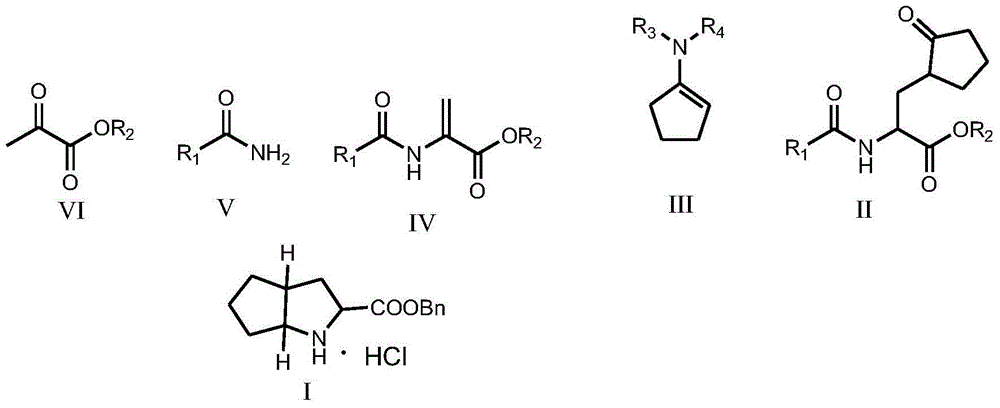
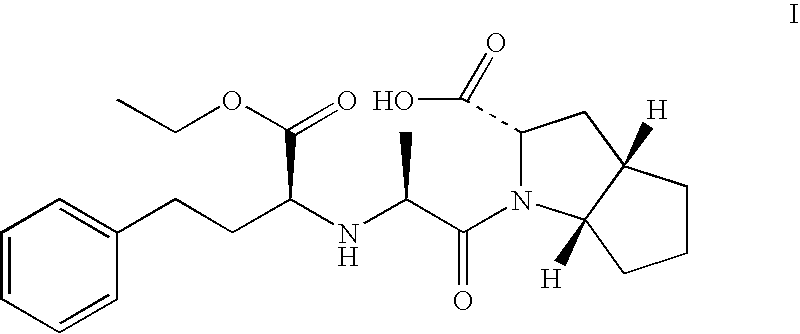
Ramipril intermediate synthesis method
ActiveCN104817486ASimple processFew synthetic stepsOrganic chemistrySynthesis methodsBENZYL ALCOHOL/WATER
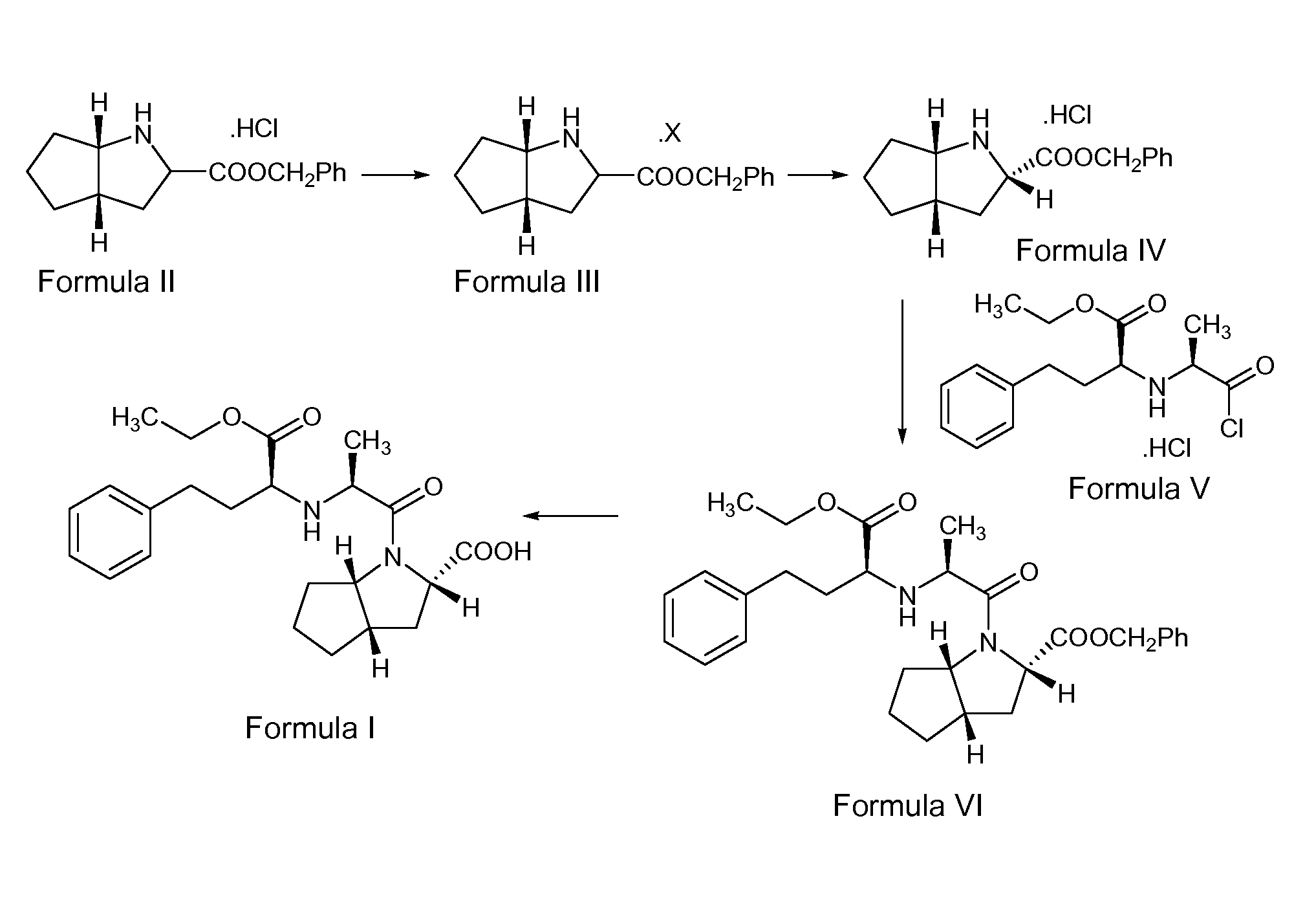
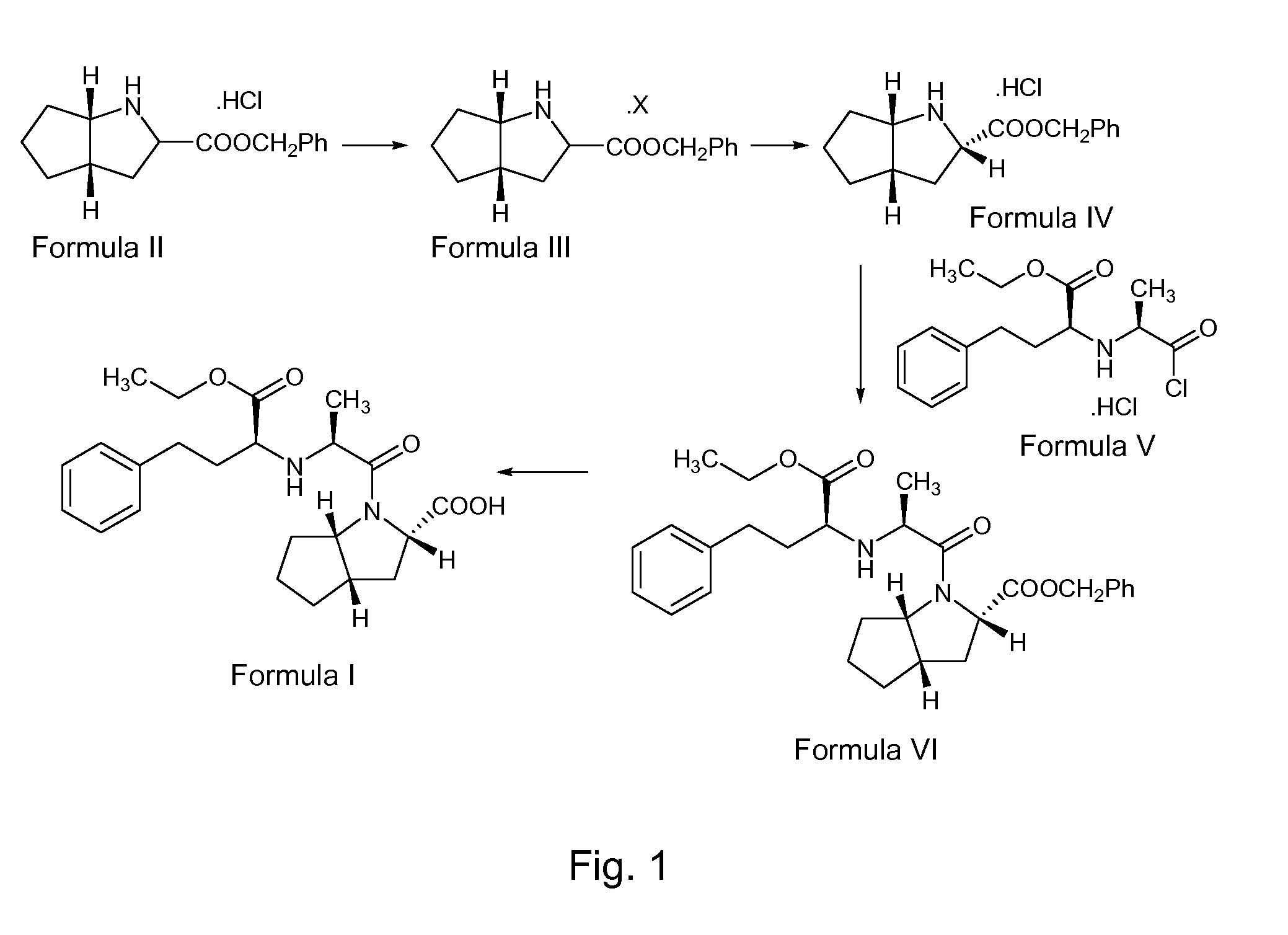
The invention discloses a ramipril key intermediate synthesis method. The ramipril key intermediate is shown in the formula I. The method comprises that a compound shown in the formula VI and an amide compound shown in the formula V undergo a condensation reaction to produce a compound shown in the formula IV, the compound shown in the formula IV and an enamine compound shown in the formula III undergo an addition reaction to produce a compound shown in the formula II, the compound shown in the formula II and benzyl alcohol undergo a hydrolytic cyclization reaction and an esterification reaction in the acid solution, and the product is reduced by a reducer to form the ramipril key intermediate shown in the formula I. The ramipril key intermediate synthesis method has the advantages of simple processes, mild reaction conditions, no heavy metal pollution, use of a chlorinated reagent, simple post-treatment, safe and reliable operation and less three wastes.
Owner:ZHEJIANG UNIV OF TECH +1
Ramipril-amlodipine salt
Owner:SELAMINE
Solid pharmaceutical formulations of ramipril and amlodipine besylate, and their preparation
InactiveCN102781430AOrganic active ingredientsPharmaceutical product form changePharmaceutical drugAmlodipine besilate
The present invention is directed to solid stable pharmaceutical fixed dose compositions comprising ramipril, amlodipine besilate and pharmaceutically acceptable excipients, and to their preparation.
Owner:SANOFI AVENTIS DEUT GMBH
Method to treat pulmonary hypoplasia in the newborn
A method of treating pulmonary hypoplasia in infants has been developed, wherein epidermal growth factor (EGF) is administered to the pulmonary system of an infant in need of treatment thereof. The EGF is administered as an aerosol or dry powder directly to the pulmonary tree, or into the amniotic fluid before birth if a situation such as oligohydramnios is recognized pre-term. The method can also be used to treat persistent pulmonary hypertension of the newborn. A hydrophobic angiotensin I-converting enzyme (ACE) inhibitor such as ramipril can also be used for the oral treatment of persistent pulmonary hypertension of the newborn.
Owner:GENOMED
Pharmaceutical formulations and use thereof in prevention of stroke, diabetes and/or congestive heart failure
InactiveCN1384756AReduce in quantityNervous disorderOrganic chemistryAngiotensin ii antagonistPharmaceutical formulation
The present invention relates to the production of renin-angiotensin system (RAS) inhibitors or pharmaceutically acceptable derivatives thereof, in particular ramipril or ramipril, for the prevention of stroke, diabetes and / or congestive heart failure. (CHF) in medicines. The present invention also relates to a method for preventing and / or treating stroke, diabetes and / or CHF, the method comprising administering a therapeutically effective amount of a RAS inhibitor or a pharmaceutically acceptable derivative thereof to a patient in need of said prevention and / or treatment, Specifically ramipril or ramipril.
Owner:SANOFI AVENTIS DEUT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C1.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C2.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C3.PNG)