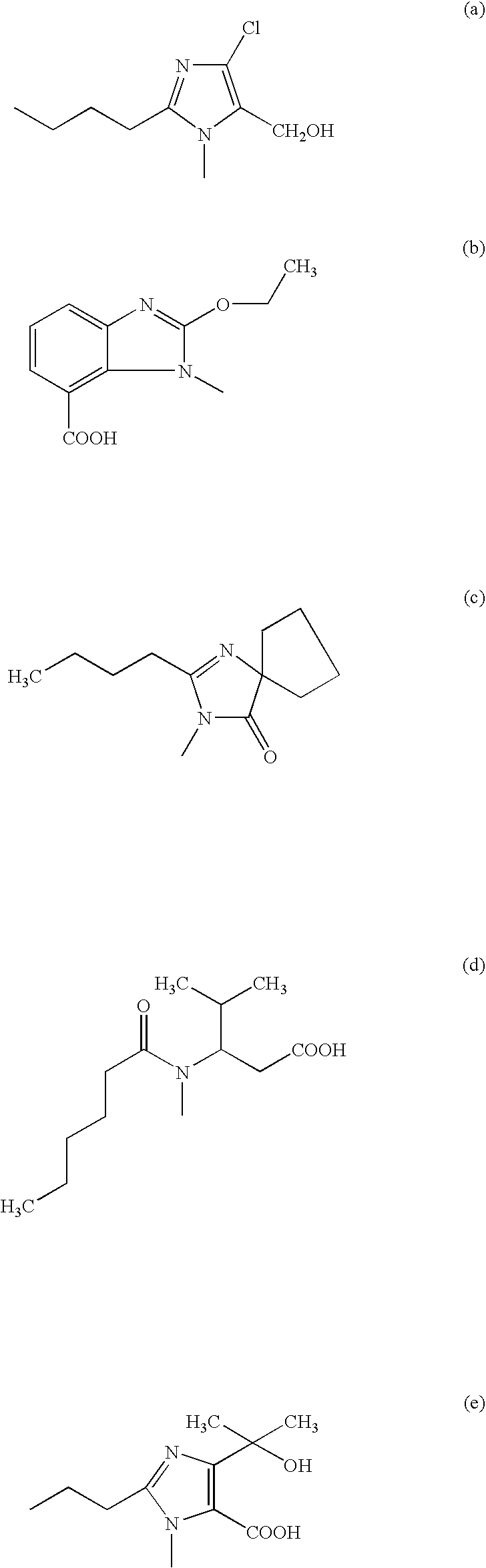
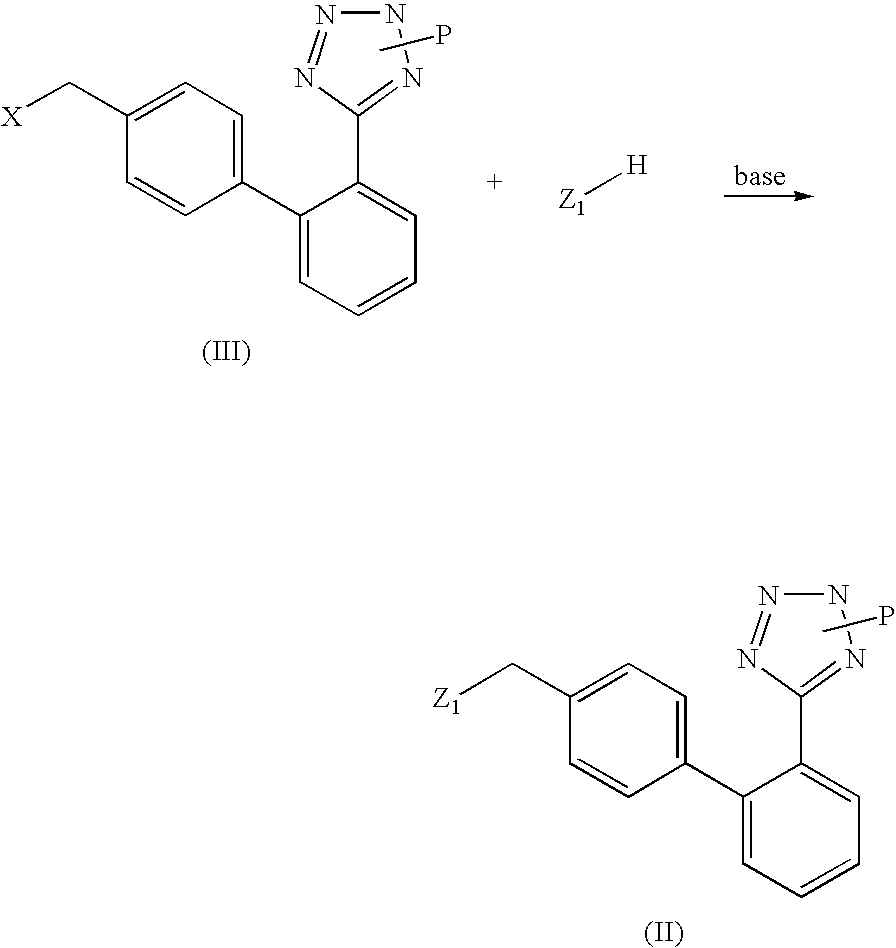
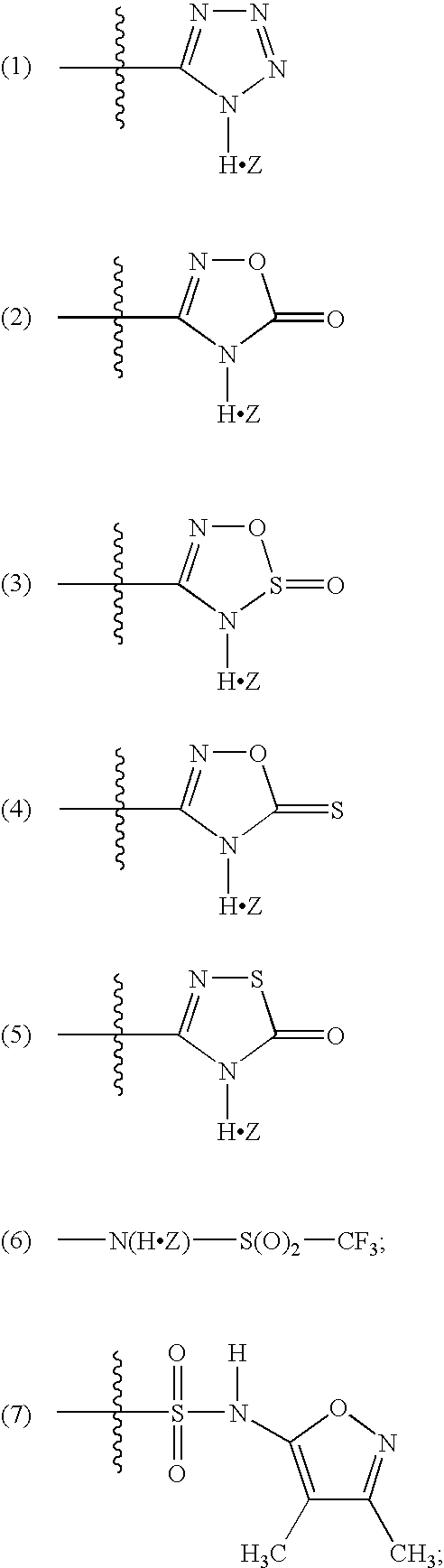
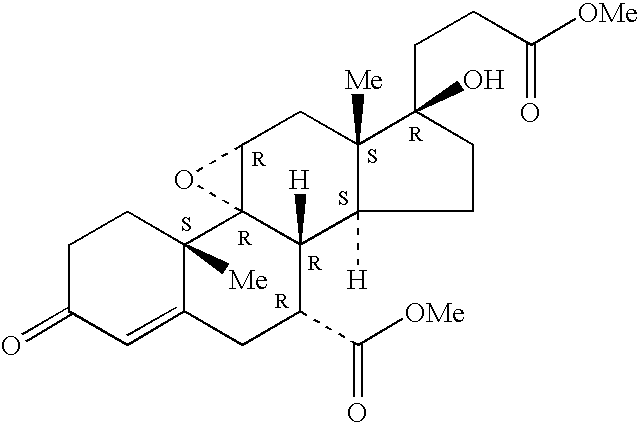
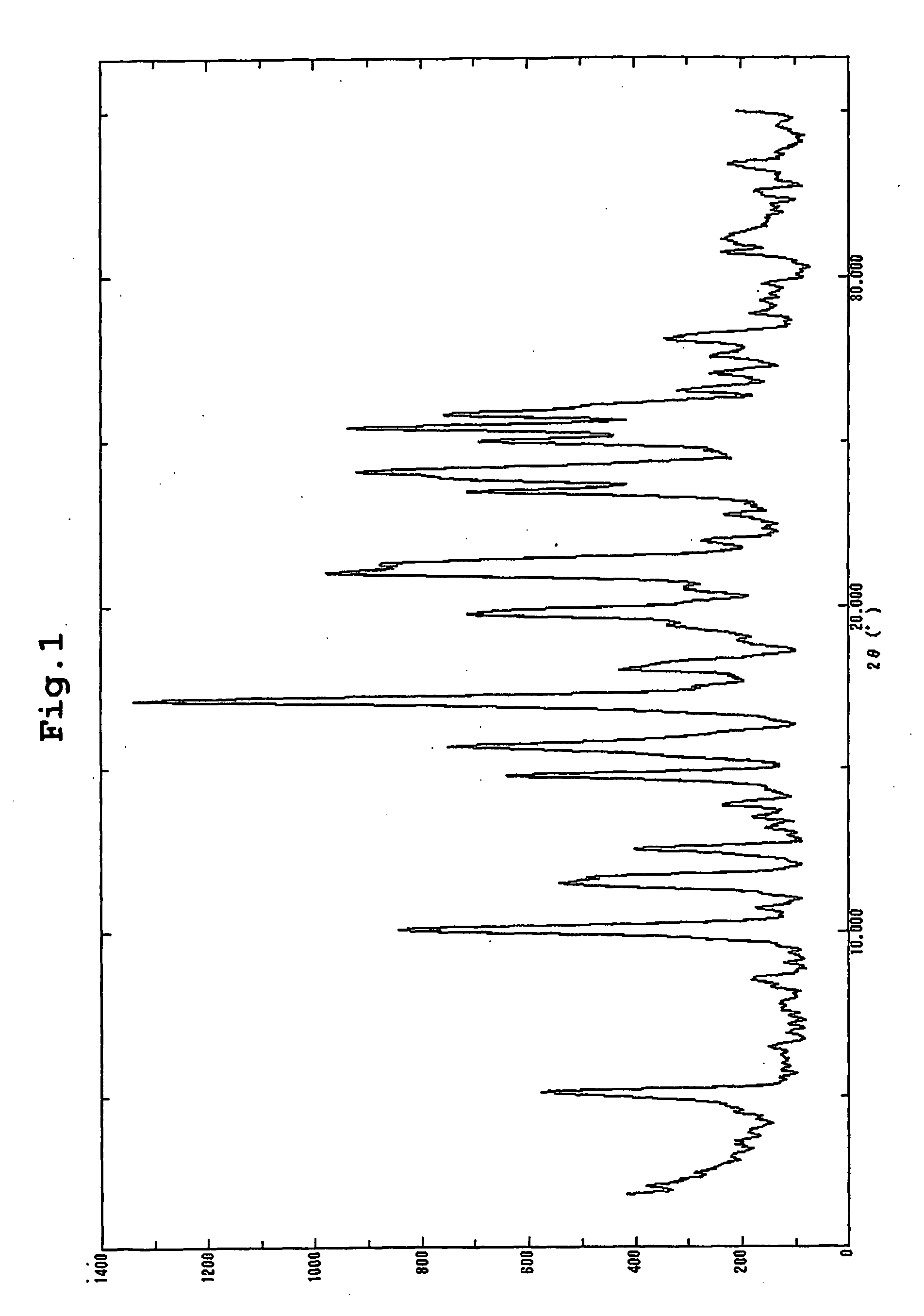
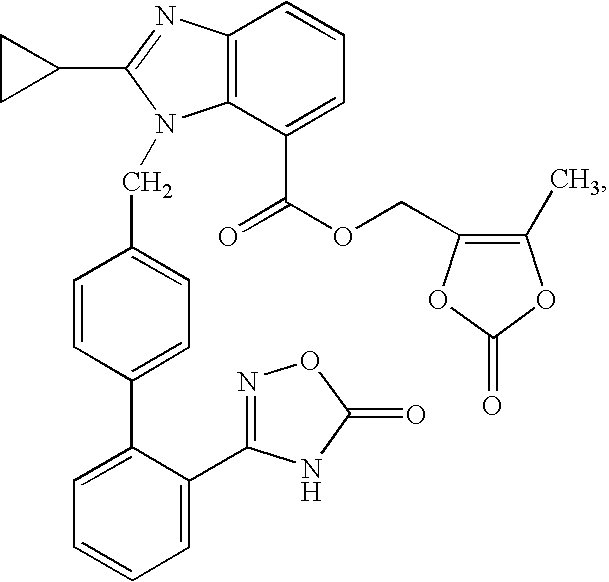
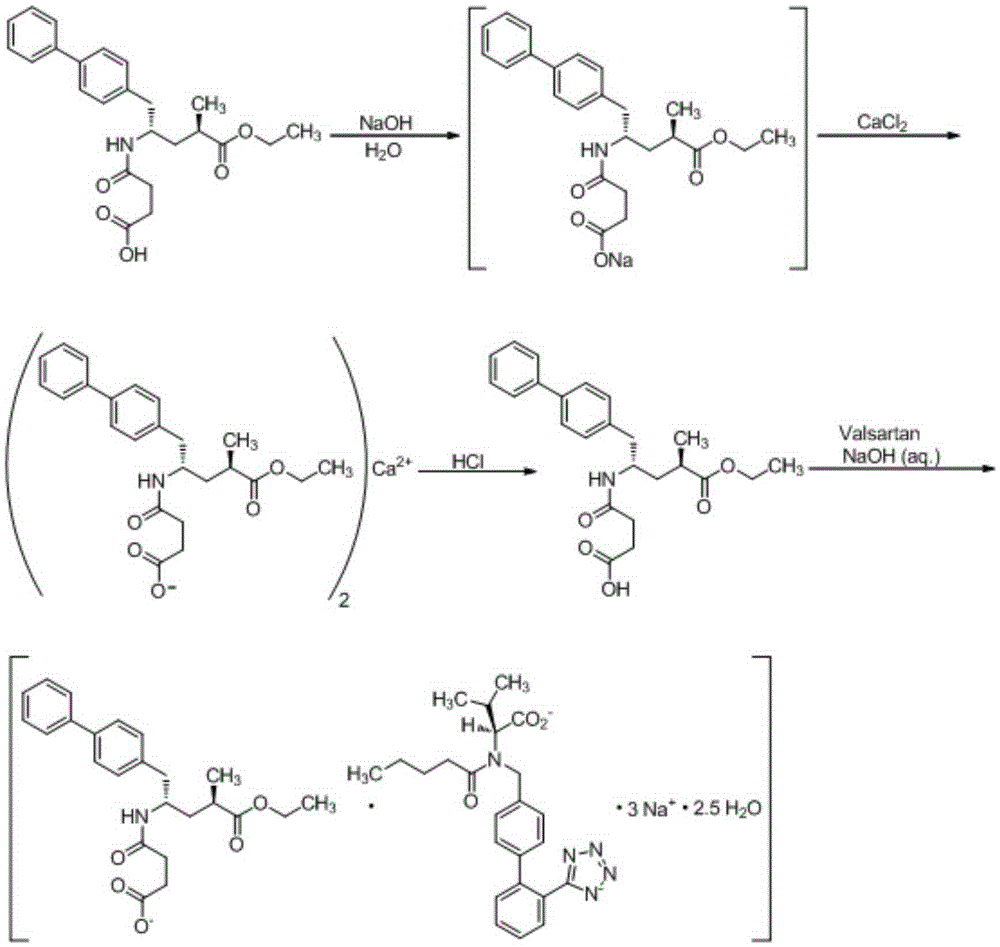
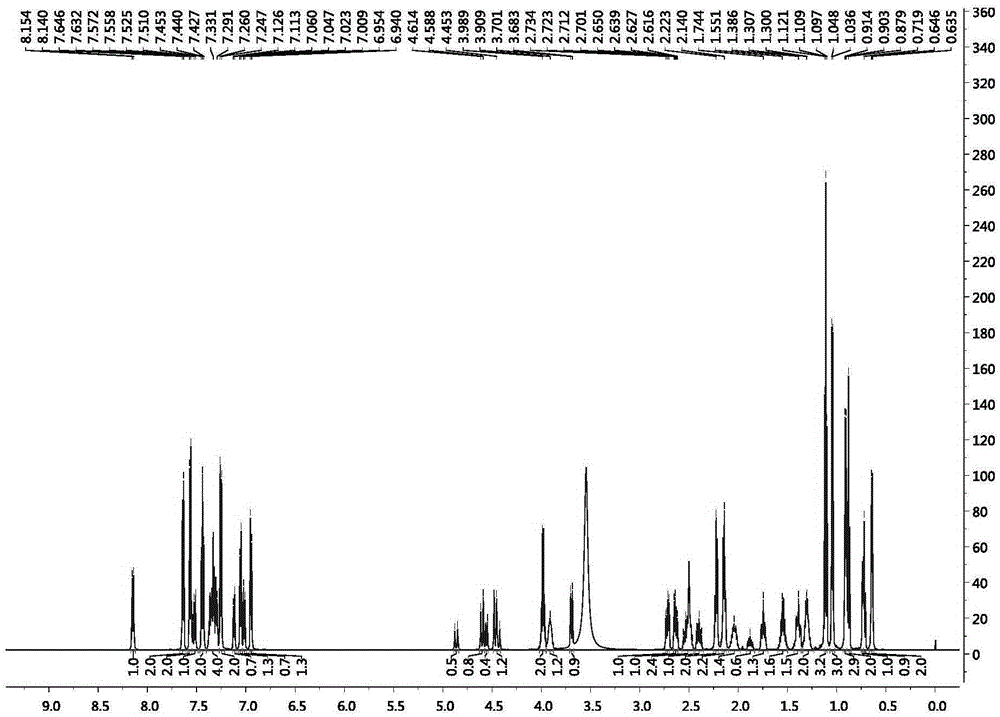
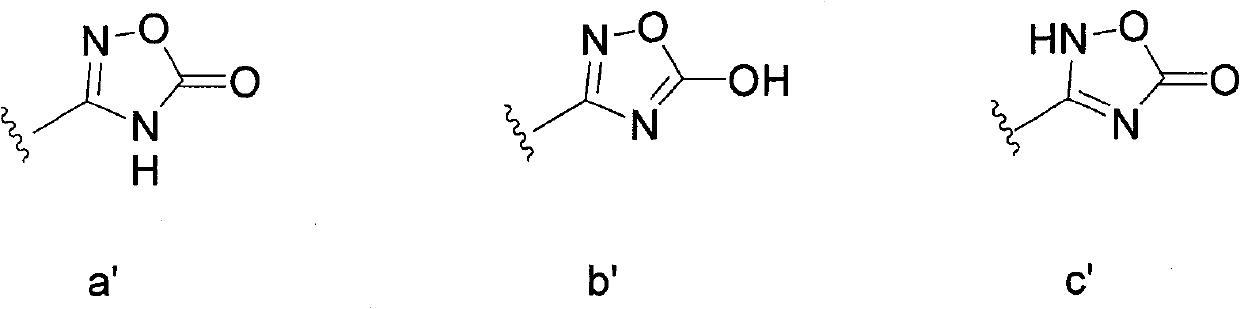
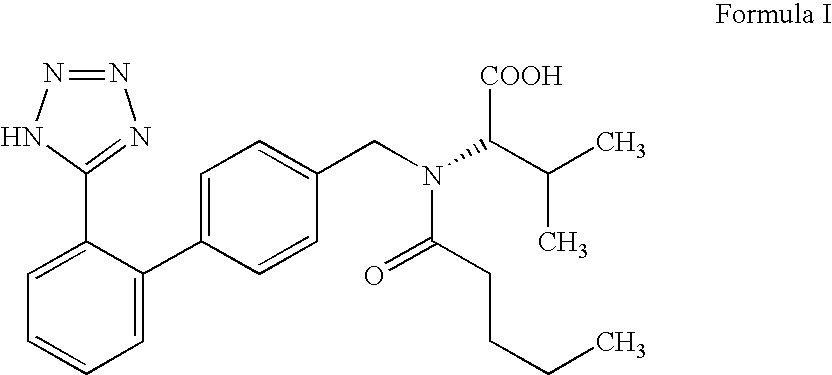
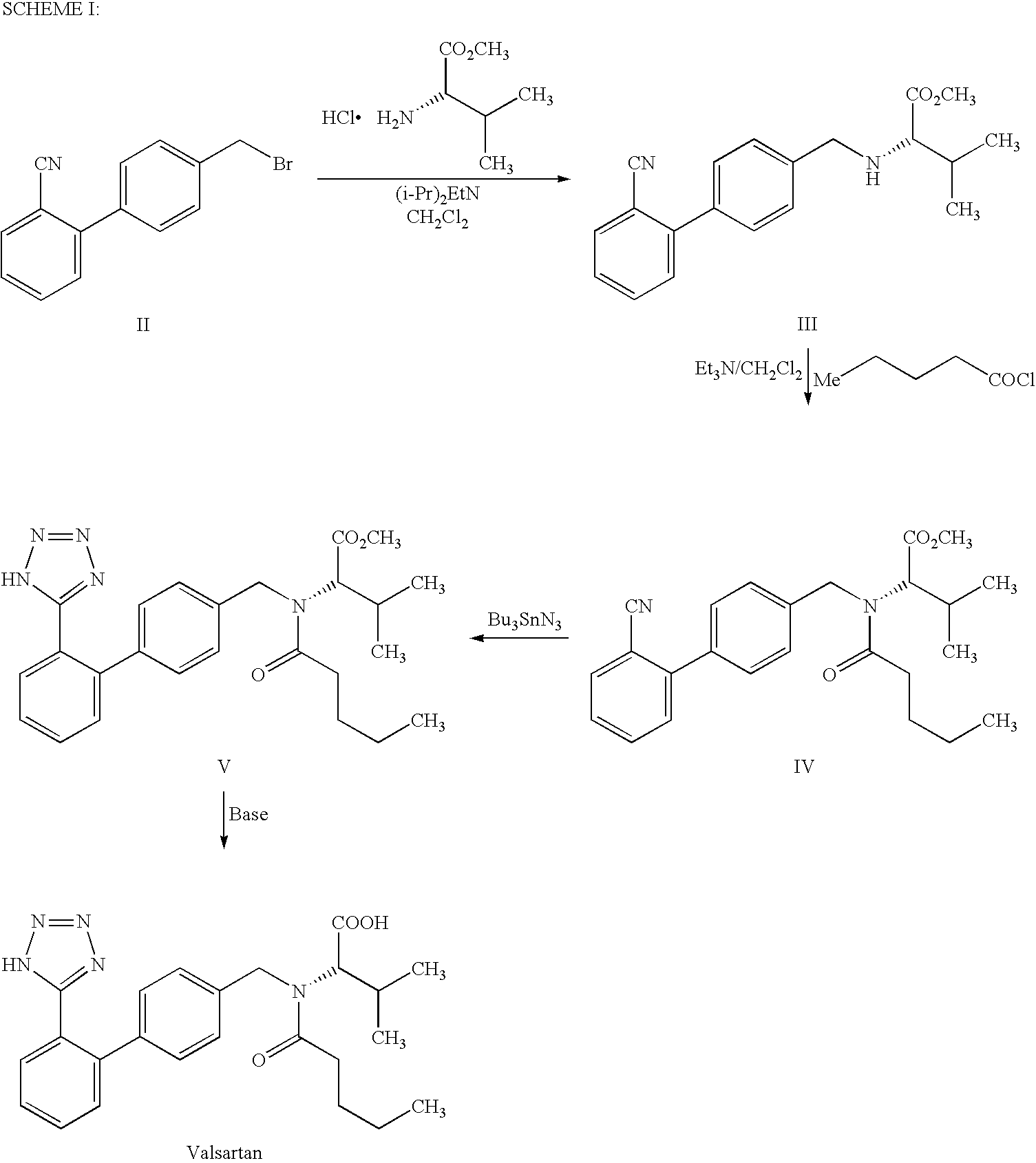
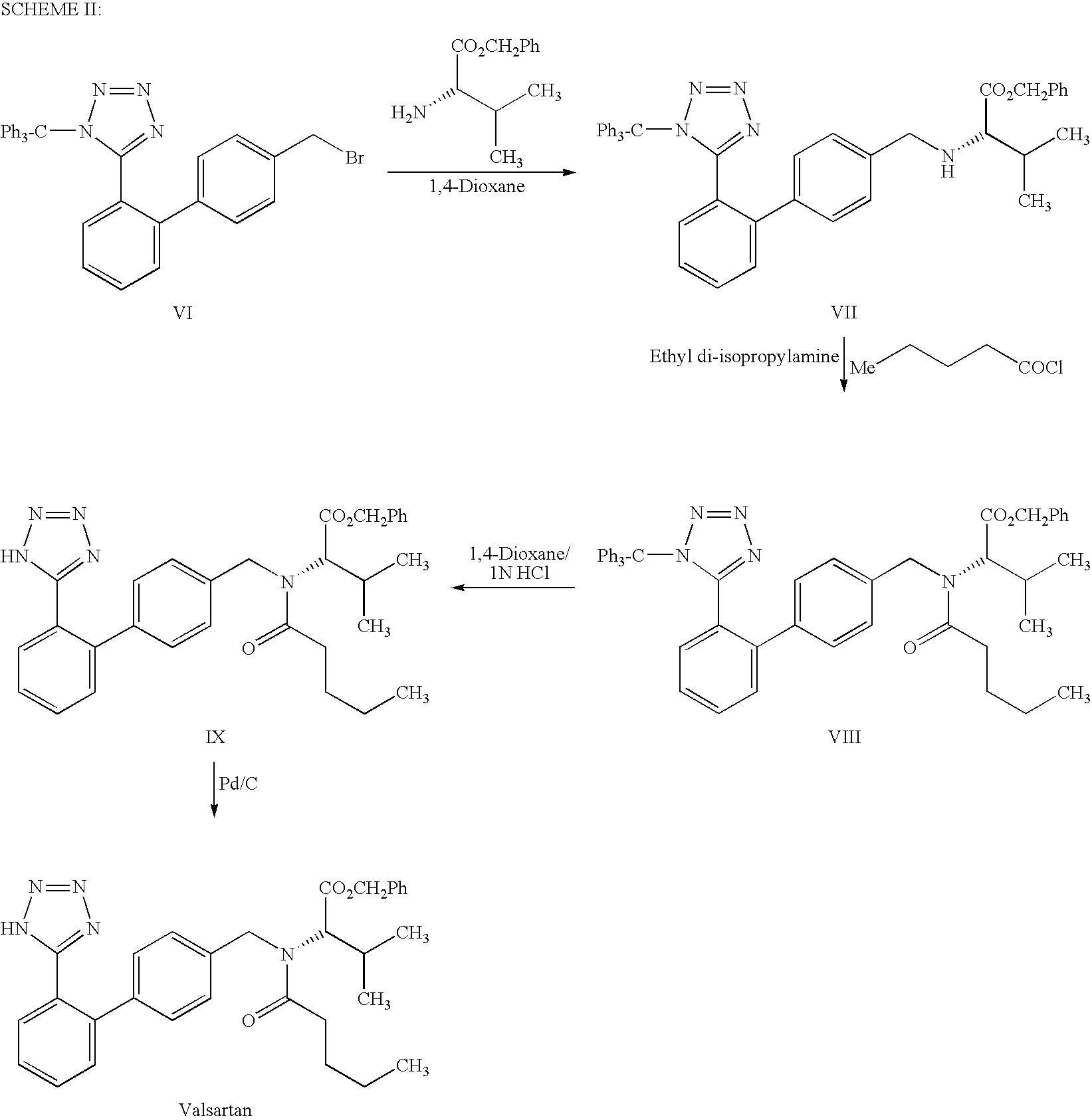
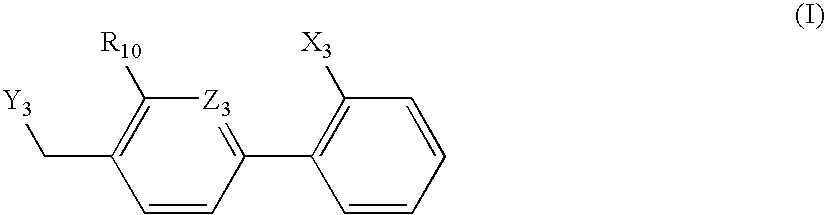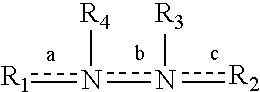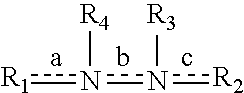Patents
Literature
37 results about "Angiotensin ii antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ANGIOTENSIN II RECEPTOR ANTAGONISTS The angiotensin II receptor antagonists, also known as angiotensin receptor blockers (ARBs), are a family of agents that bind to and inhibit the angiotensin II type 1 receptor (AT1) and thus inhibit the renin-angiotensin system and its cascade of effects in causing arteriolar contraction and sodium retention.
Nitric oxide enhancing angiotensin II antagonist compounds, compositions and methods of use
The invention describes compositions and kits comprising at least one nitric oxide enhancing angiotensin II antagonist compound, or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitric oxide enhancing angiotensin II antagonist compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension (o) treating central nervous system disorders; (p) treating metabolic syndrome; and (q) treating hyperlipidemia. The nitric oxide enhancing angiotensin II antagonist compounds comprise at least one nitric oxide enhancing group linked to the angiotensin II antagonist compound through one or more sites such as carbon, oxygen and / or nitrogen via a bond or moiety that cannot be hydrolyzed.
Owner:NICOX SA
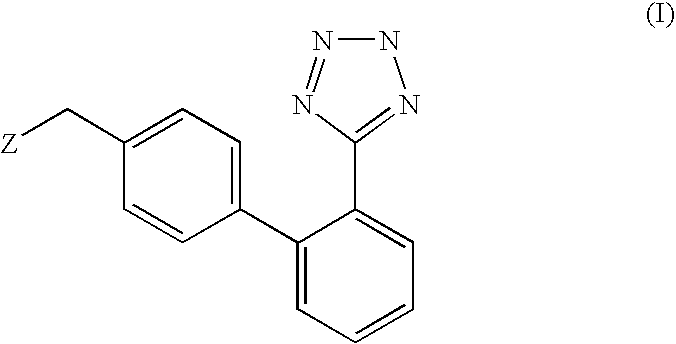
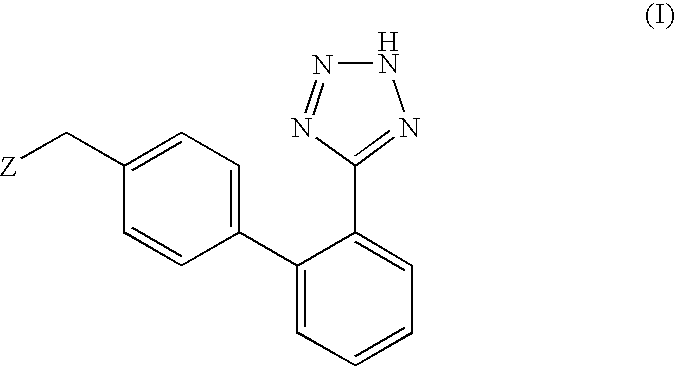
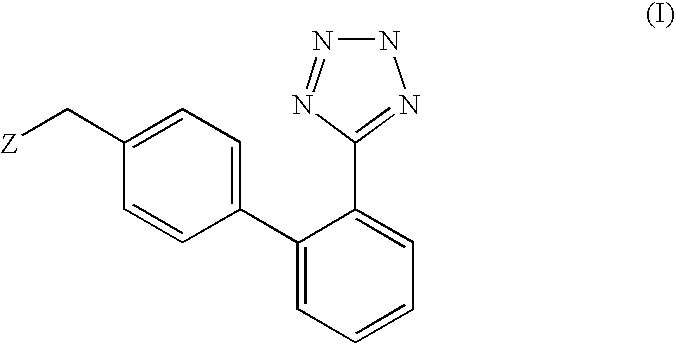
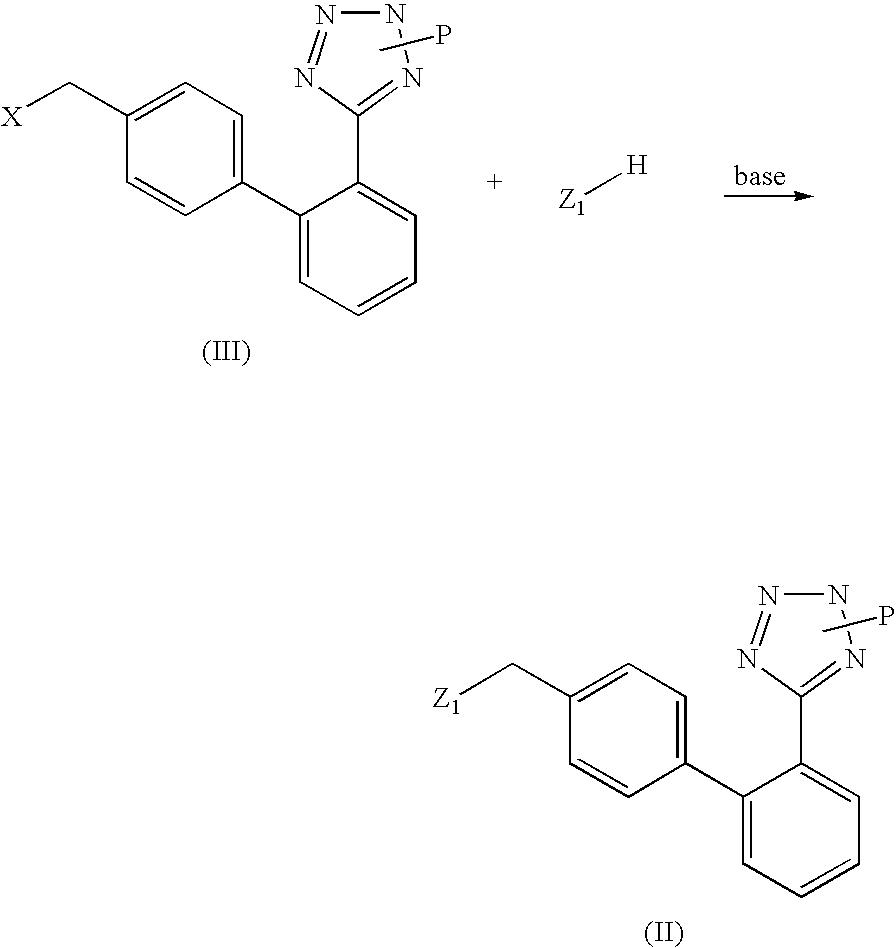
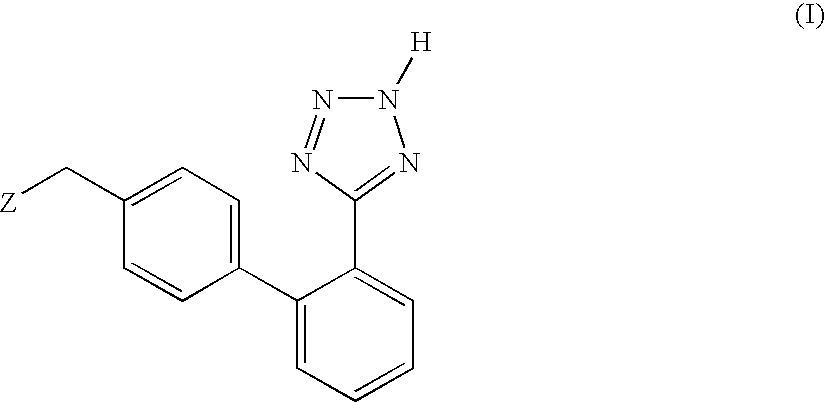
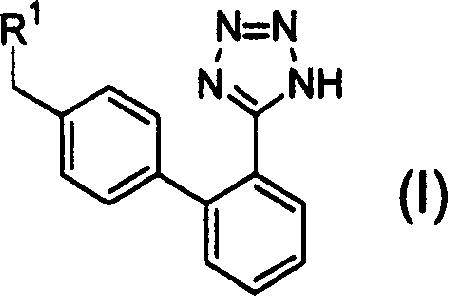
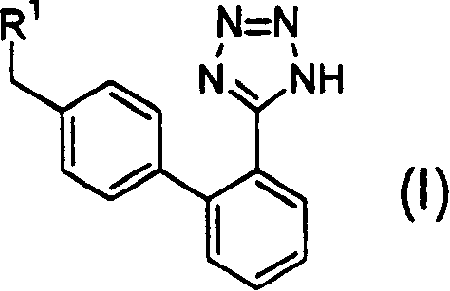
Phenyltetrazole compounds
Novel phenyltetrazole compounds useful as intermediates in the preparation of angiotensin II antagonists and the processes for the conversion thereof to biologically active molecules.
Owner:DIPHARMA SPA
Organic nitric oxide enhancing salts of angiotensin ii antagonists, compositions and methods of use
The invention describes compositions and kits comprising at least one organic nitric oxide enhancing salt of an angiotensin π antagonist, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The organic nitric oxide enhancing compounds that form salts with the angiotensin II antagonists are organic nitrates, organic nitrites, nitrosothiols, thionitrites, thionitrates, NONOates, heterocyclic nitric oxide donors and / or nitroxides. The heterocyclic nitric oxide donors are furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines.
Owner:NICOX SA
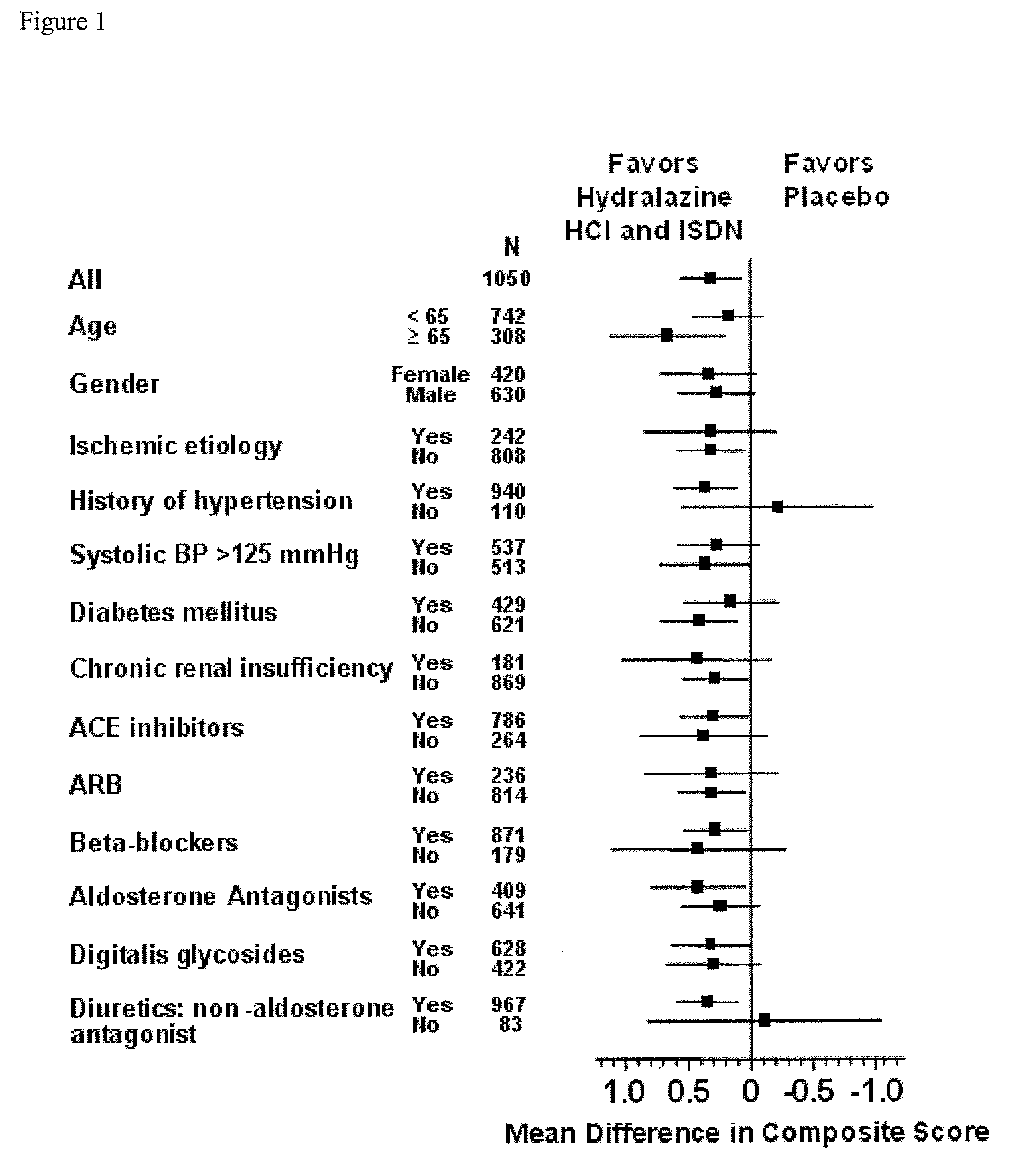
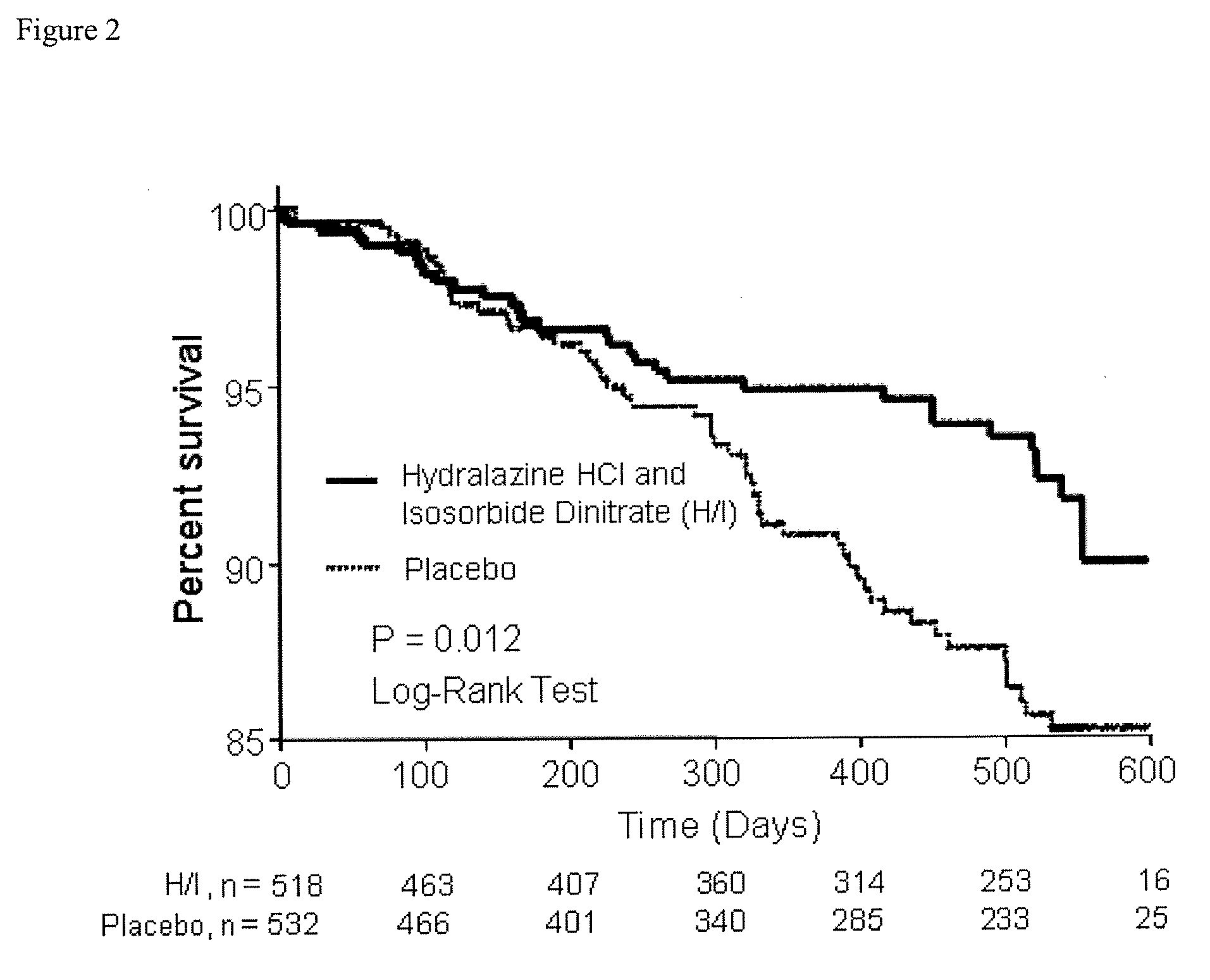
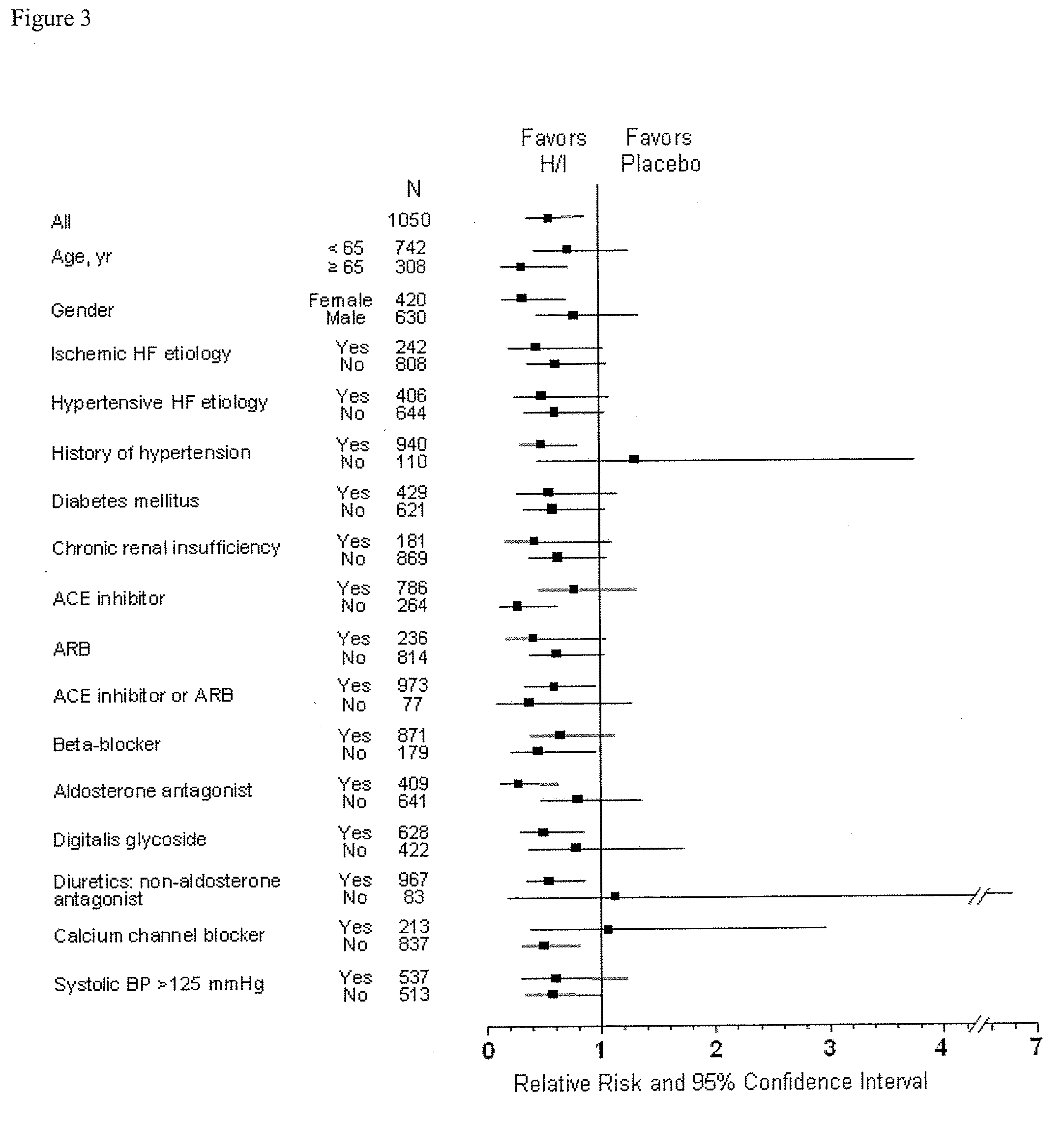
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
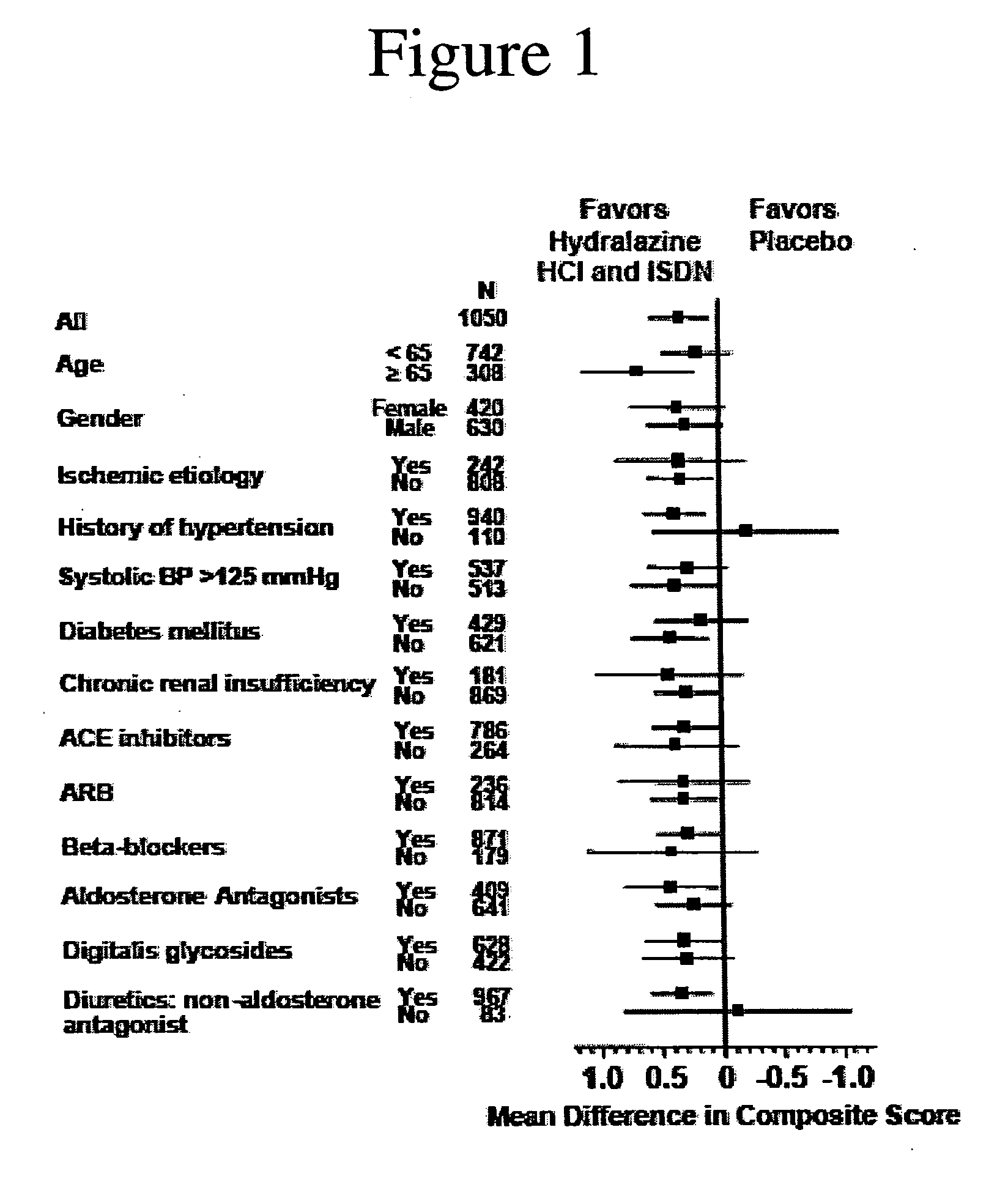
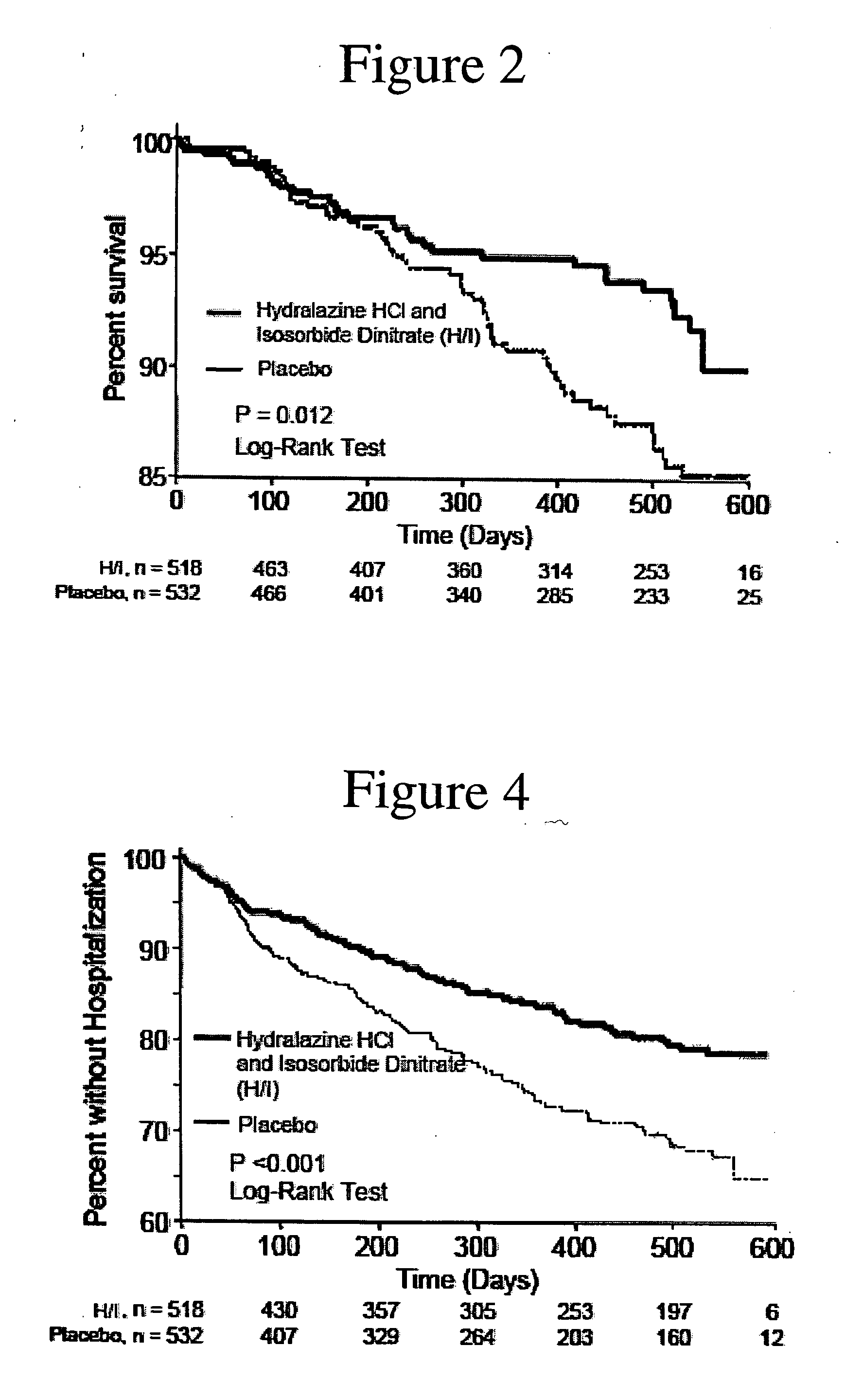
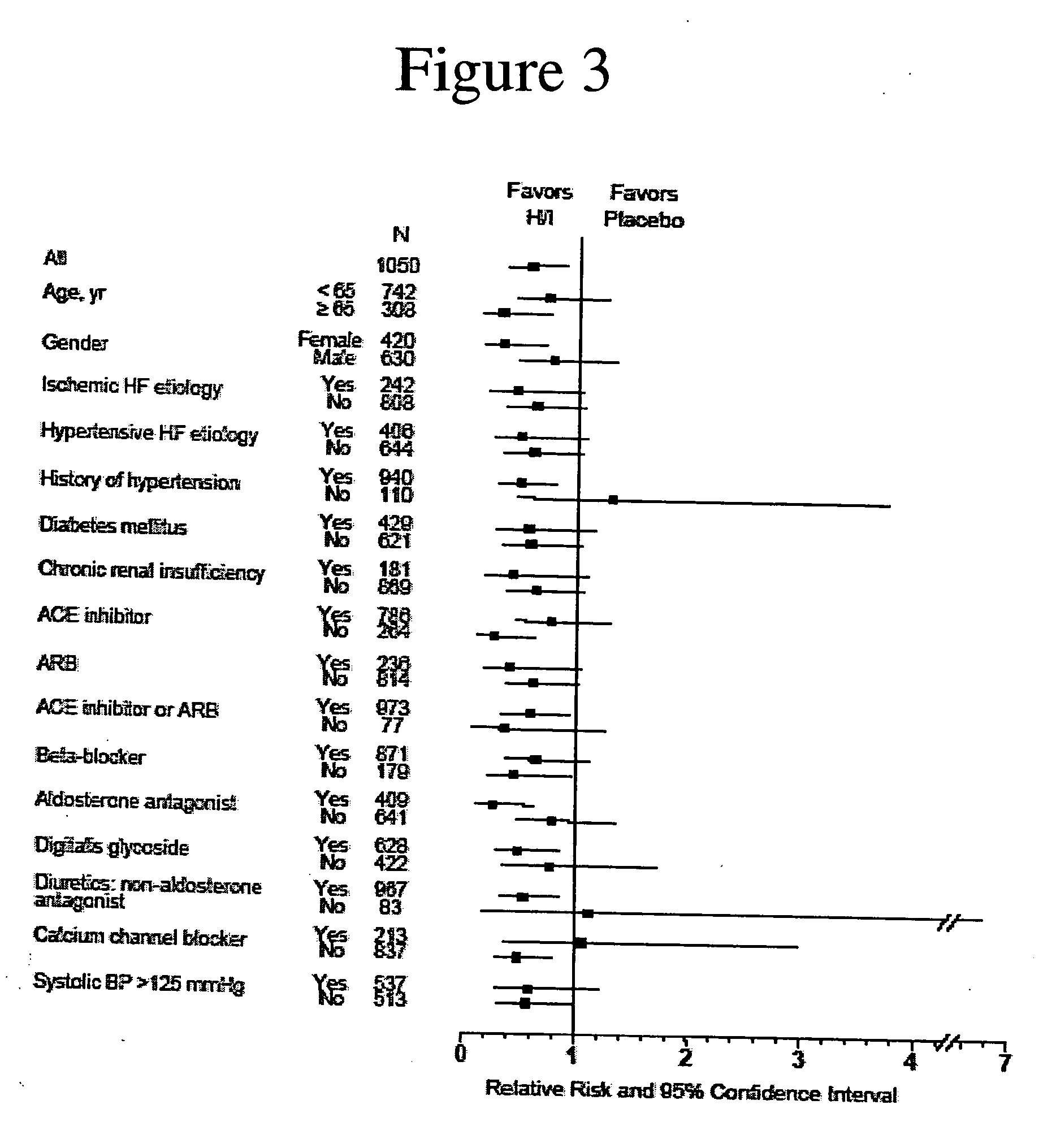
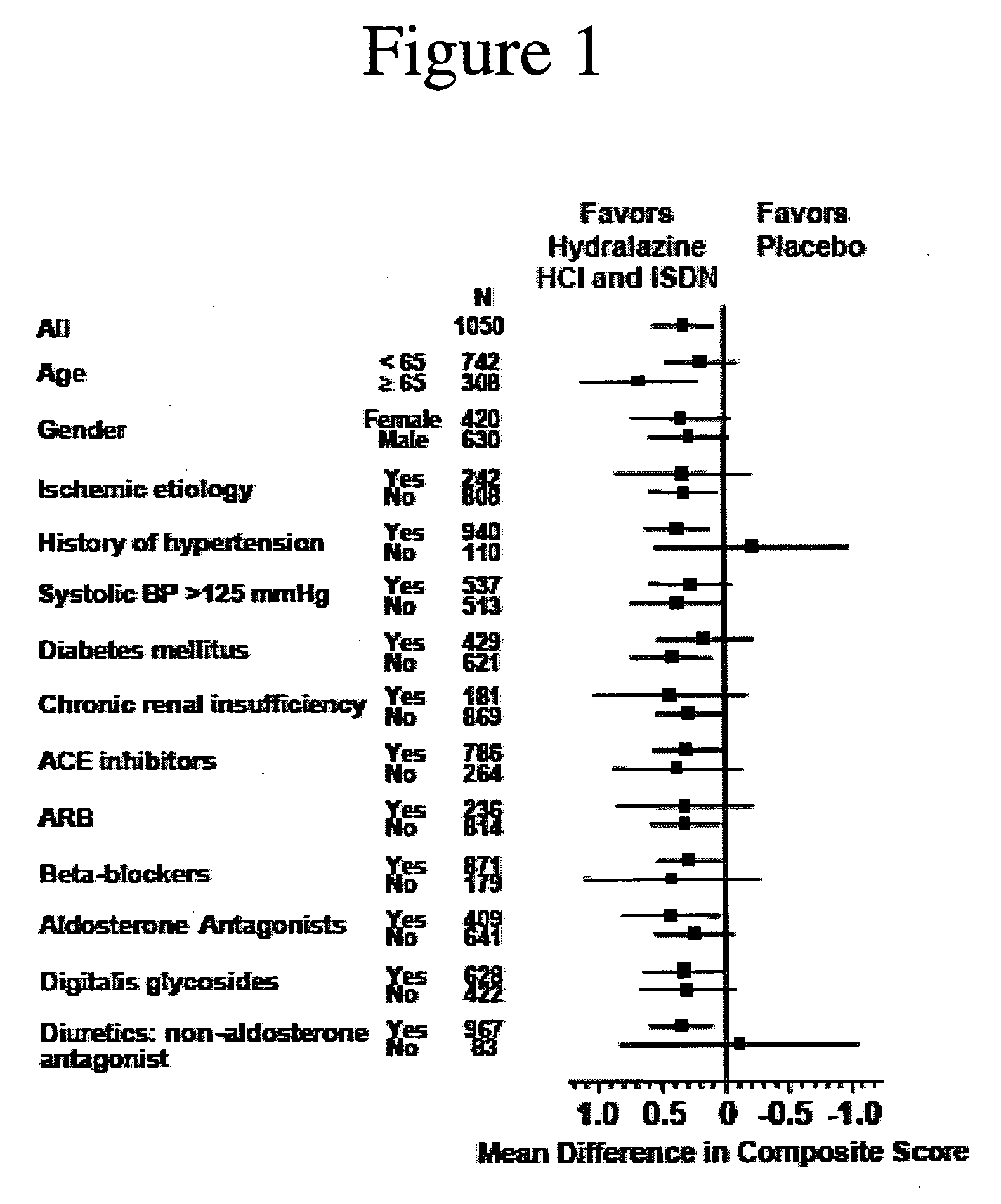
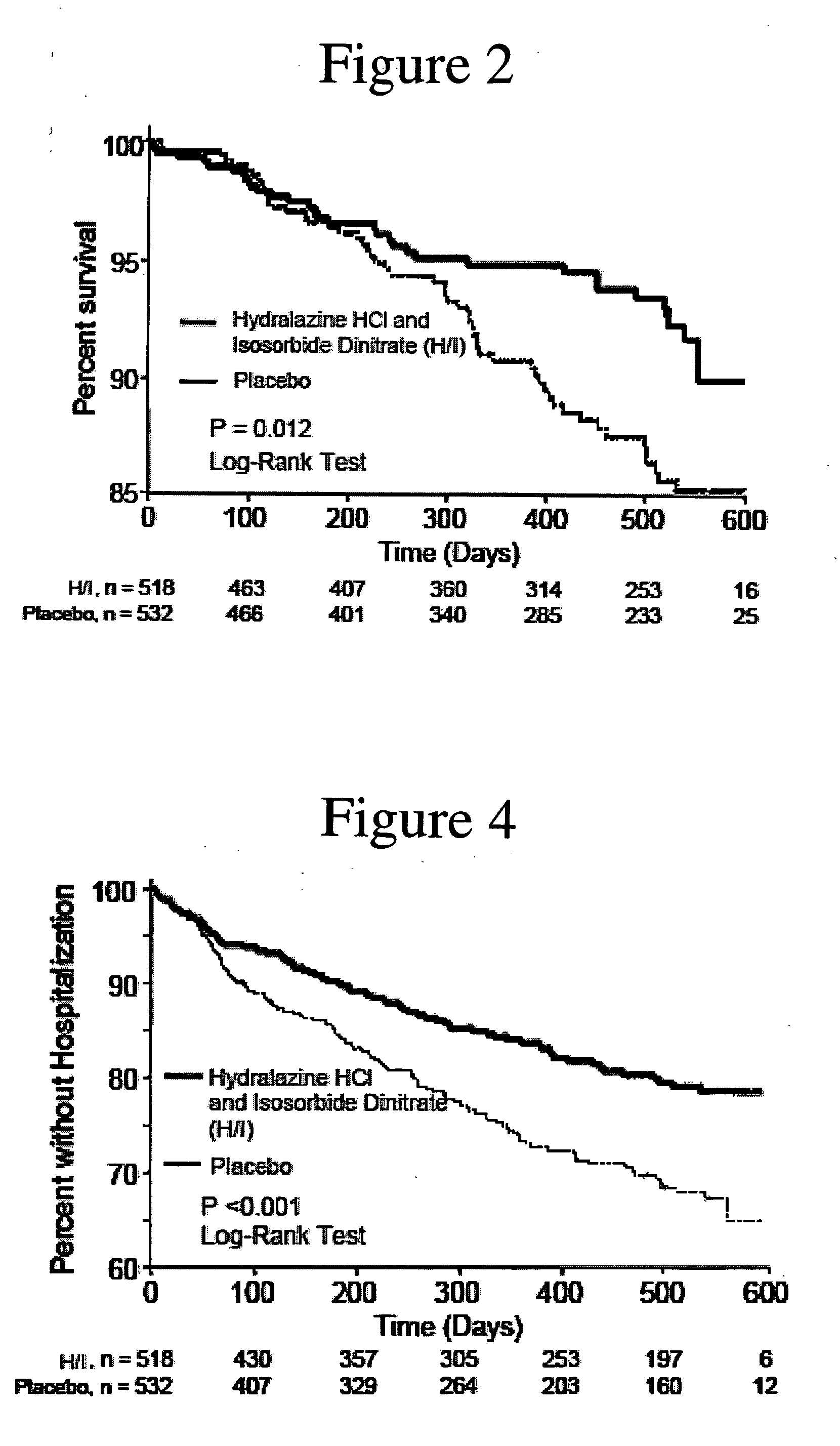
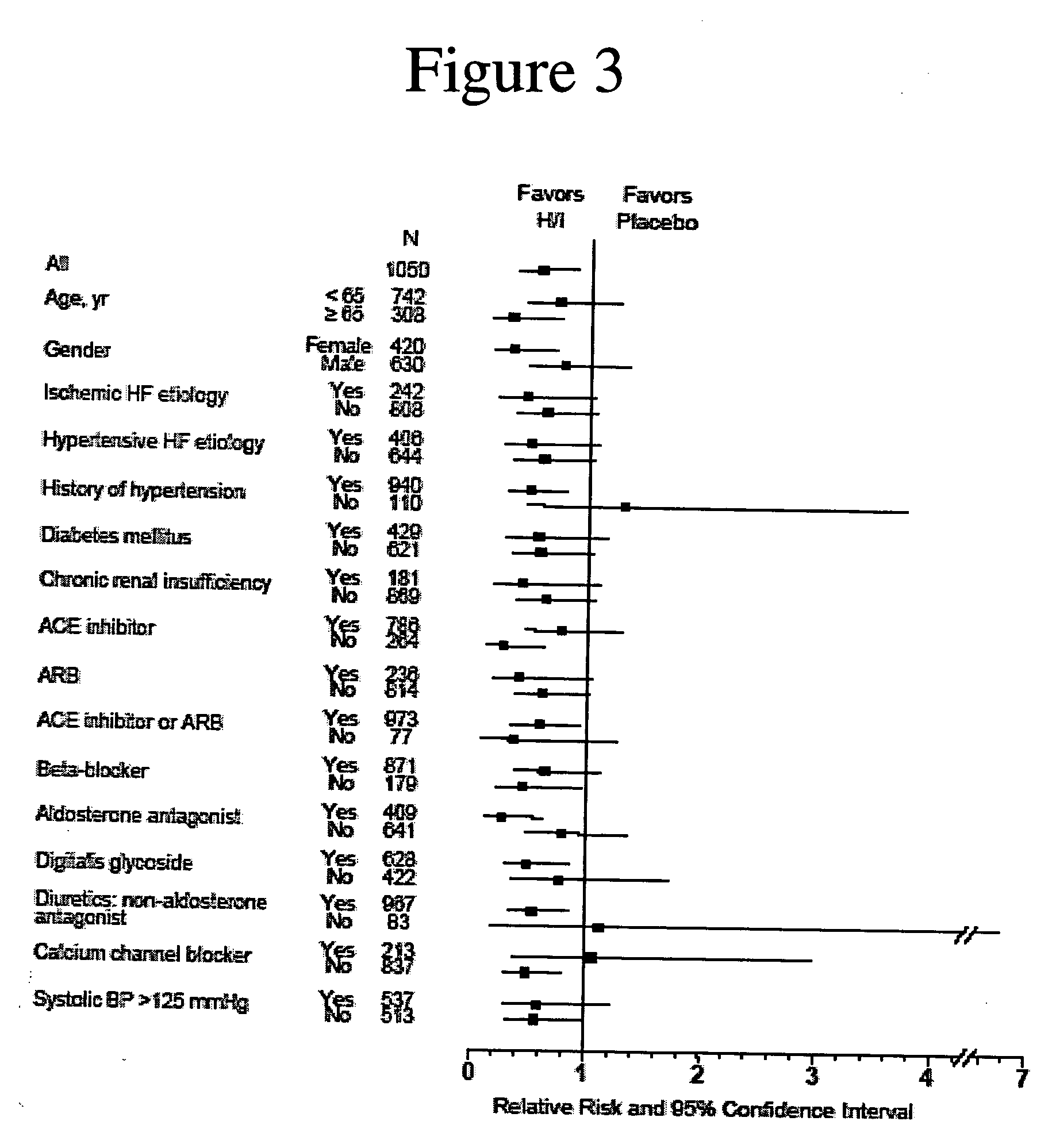
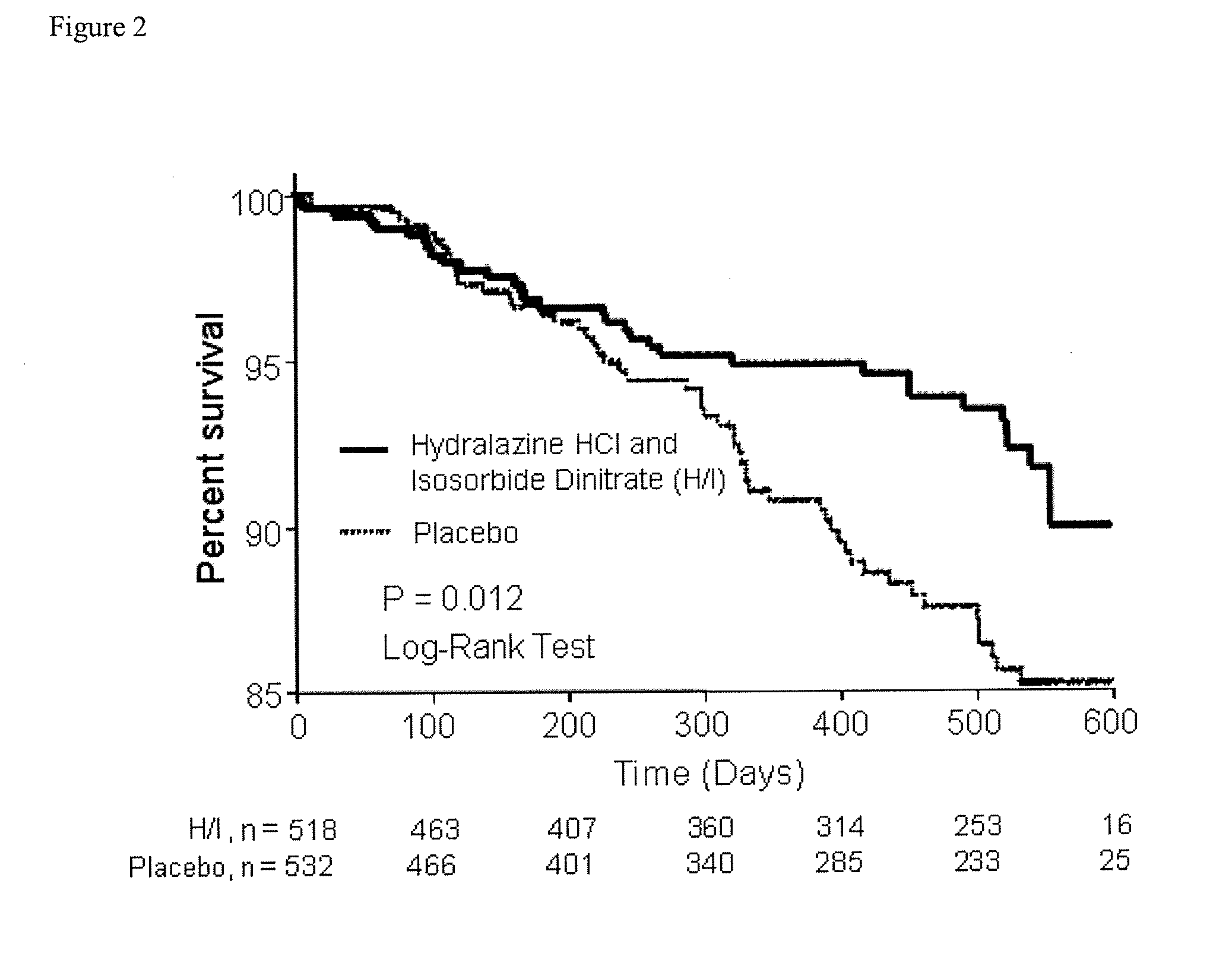
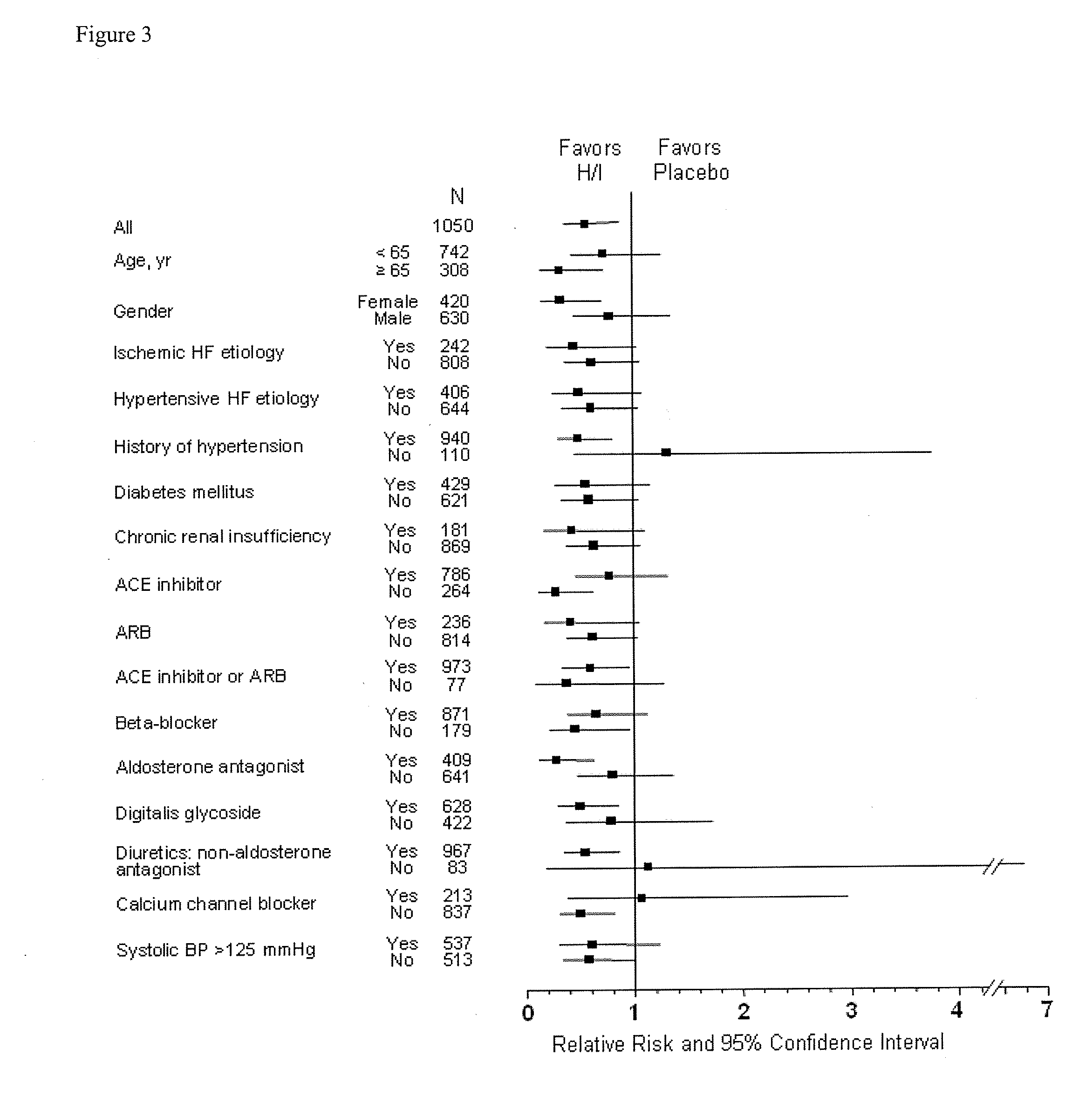
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Cardiovascular Compounds Comprising Nitric Oxide Enhancing Groups, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one nitric oxide enhancing group, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; Q) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The cardiovascular compounds are angiotensin II antagonists, aldosterone antagonists, endothelin antagonists, hydralazine compounds, neutral endopeptidase inhibitors and renin inhibitors. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors.
Owner:NICOX SA
Method to treat cardiofibrosis with a combination therapy of an angiotensin II antagonist and epoxymexrenone
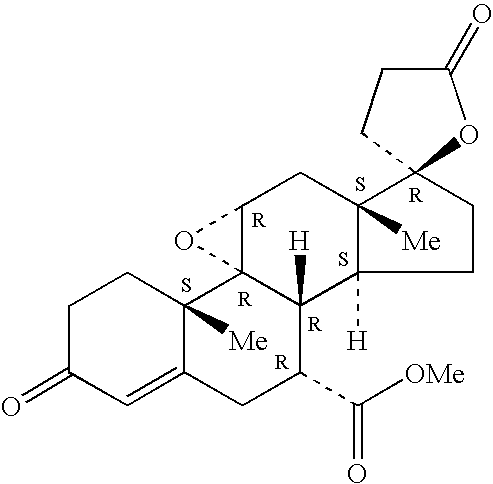
A therapeutic method is described for treating cardiofibrosis or cardiac hypertrophy using a combination therapy comprising a therapeutically-effective amount of an angiotensin II receptor antagonist and a therapeutically-effective amount of expoxymexrenone.
Owner:GD SEARLE & CO
Process for the preparation of angiotensin ii antagonistic compounds
A process for the preparation of angiotensin II antagonists and novel intermediates useful for the synthesis thereof.
Owner:DIPHARMA SPA
Benzimidazole derivative and use as angiotensin ii antagonist
InactiveCN101151260AEasy to solveGood treatment effectOrganic active ingredientsOrganic chemistryBenzimidazole derivativeCarboxylic acid
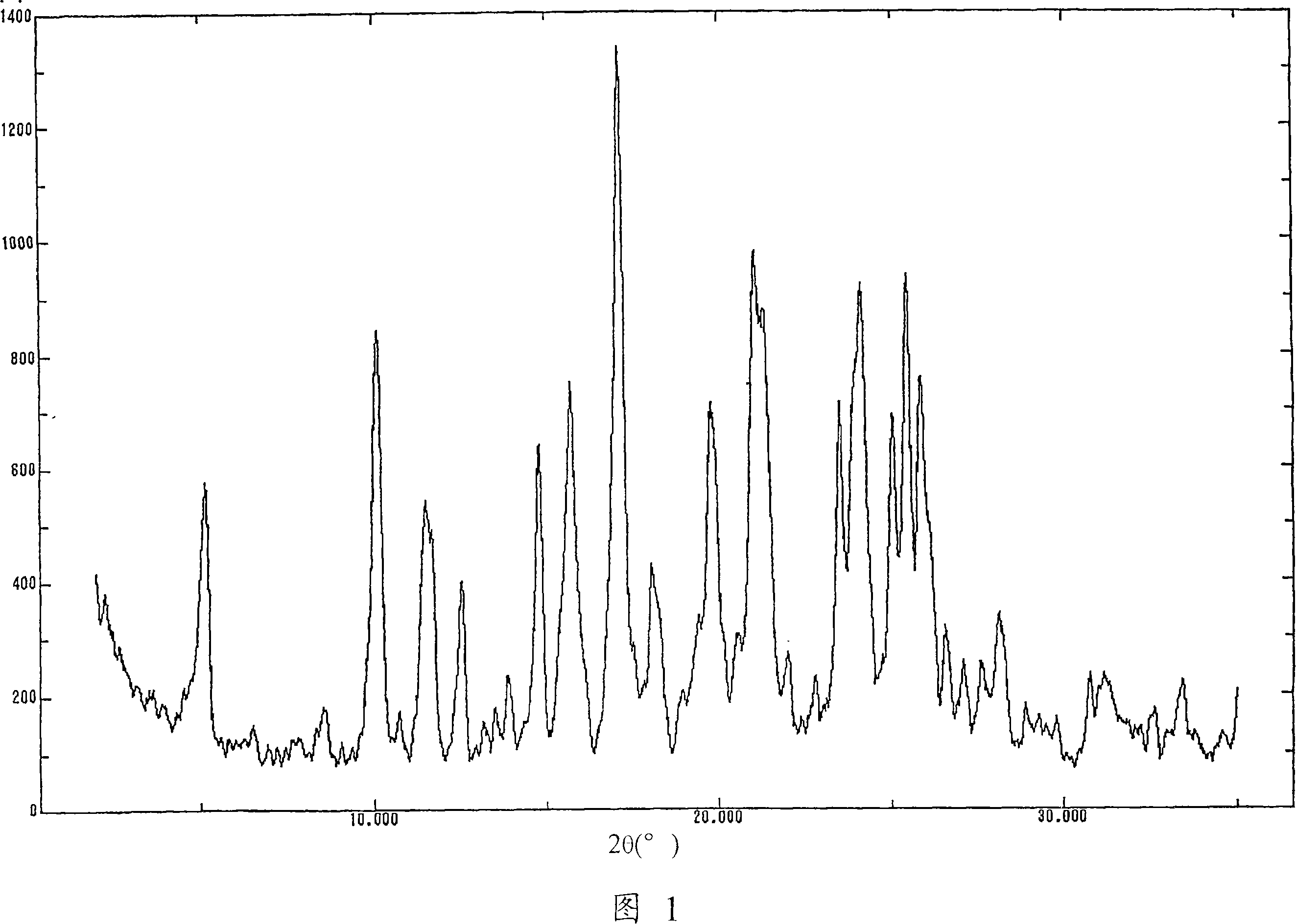
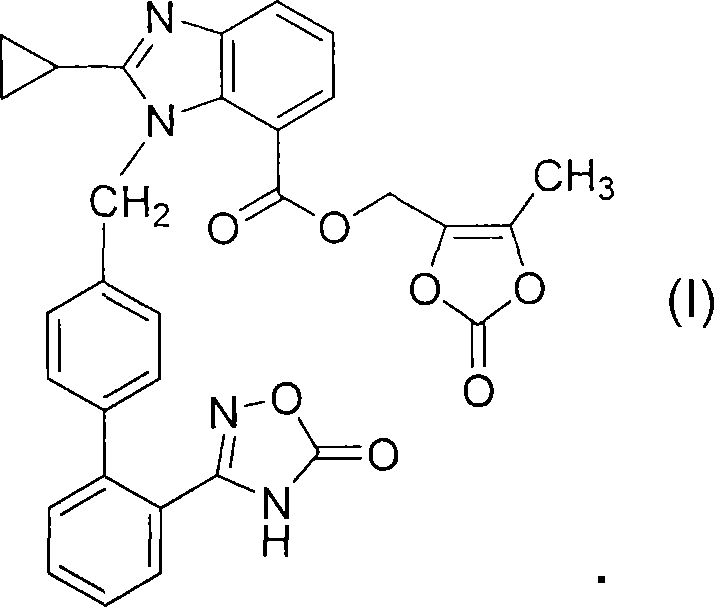
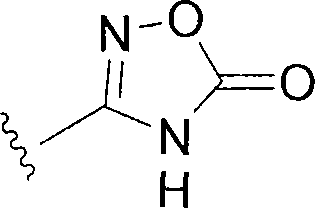
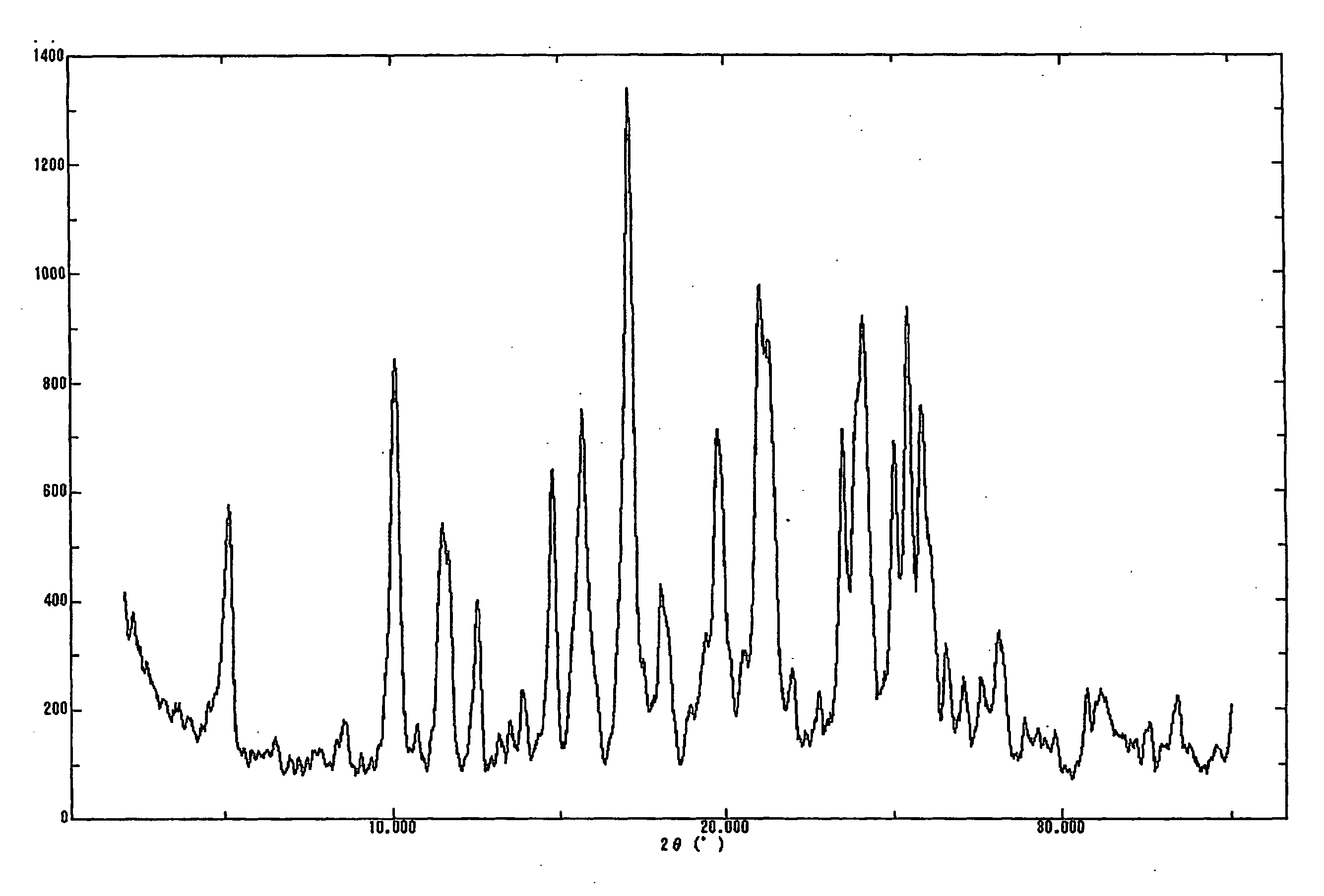
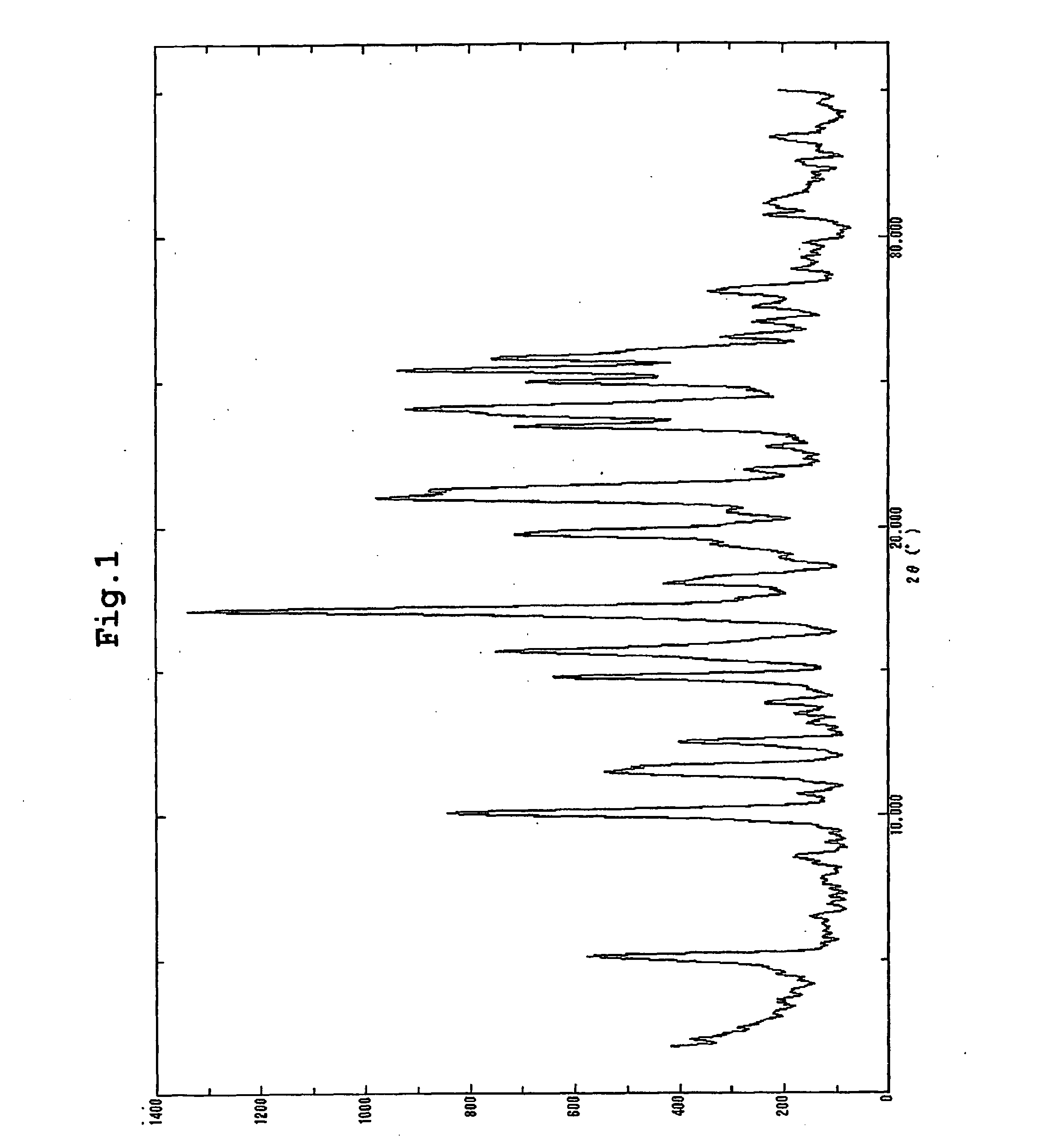
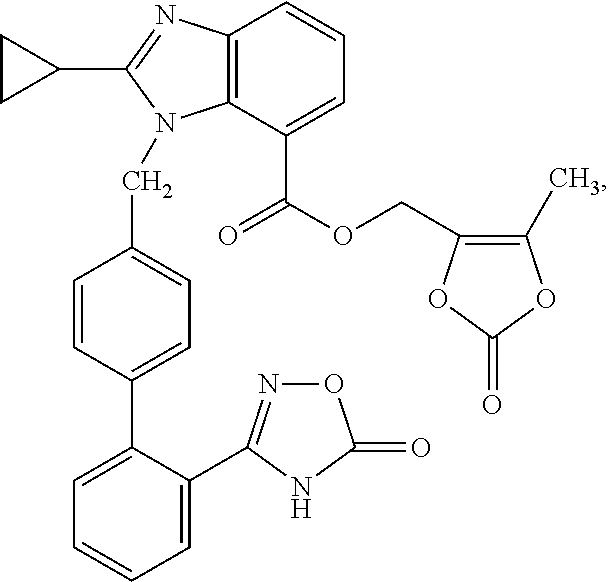
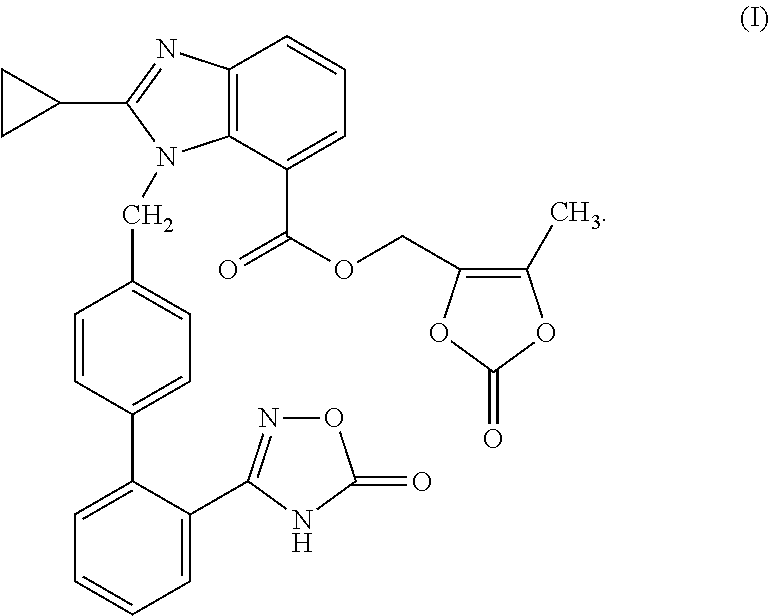
The present invention provides (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-cyclopropyl-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate represented by the formula: which has superior properties as a pharmaceutical agent, a salt thereof, a crystal thereof, a pharmaceutical agent containing the compound, and the like.
Owner:TAKEDA PHARMA CO LTD
Benzimidazole Derivative and Use as Angiotensin II Antagonist
InactiveUS20090054502A1Superior prophylacticGood treatment effectBiocideSenses disorderBenzimidazole derivativeAngiotensin ii antagonist
The present invention provides (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-cyclopropyl-1-{[2′-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate represented by the formula:which has superior properties as a pharmaceutical agent, a salt thereof, a crystal thereof, a pharmaceutical agent containing the compound, and the like.
Owner:TAKEDA PHARMA CO LTD
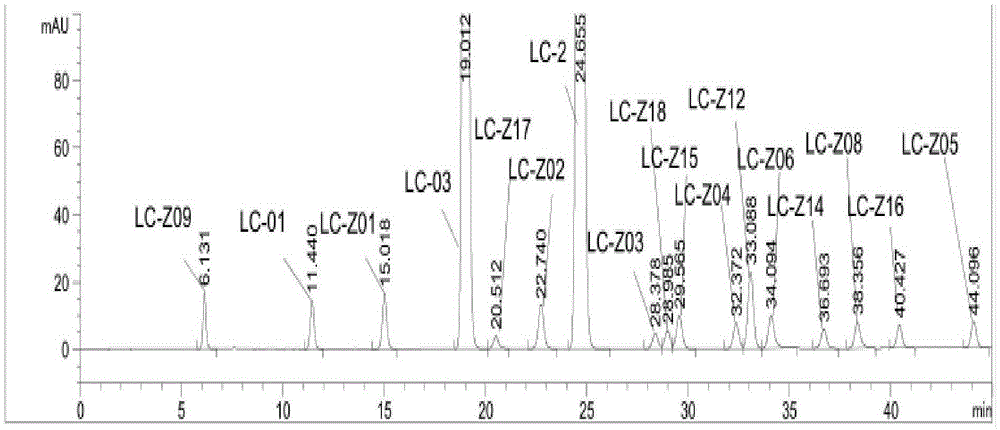
Separation method of impurities in angiotensin receptor antagonist and NEP inhibitor compound
ActiveCN105929031AEfficient separationSimple processComponent separationHplc methodAngiotensin ii antagonist
The invention provides a separation method of impurities in an angiotensin receptor antagonist and NEP inhibitor compound. The method is as below: separating impurities in pharmaceutical composition containing angiotensin receptor antagonist and NEP inhibitor by HPLC method. A mobile phase in the high performance liquid chromatography includes an aqueous phase and an organic phase; the aqueous phase comprises a phosphate buffer solution, trifluoroacetic acid solution or formic acid solution; the organic phase contains an organic mixture or acetonitrile; the organic mixture includes acetonitrile and methanol; and the aqueous phase and organic phase are in the volume ratio of (45%-65%):(55%-35%). The invention adopts the high performance liquid chromatography for separation of impurities; the mobile phase used in the high performance liquid chromatography includes aqueous phase with specific components and the organic phase, and the mobile phase can effectively separate impurities in the angiotensin receptor antagonist and NEP inhibitor compound.
Owner:LIANGJIANG MEDICINE CO LTD
Phenyltetrazole compounds
Novel phenyltetrazole compounds useful as intermediates in the preparation of angiotensin II antagonists and the processes for the conversion thereof to biologically active molecules.
Owner:DIPHARMA SPA
Benzimidazole derivative and use as angiotensin ii antagonist
InactiveUS20120172401A1Maintain good propertiesStrong actionBiocideSenses disorderBenzimidazole derivativeCarboxylic salt
Owner:TAKEDA PHARMA CO LTD
Use of dipyridamole in combination with acetylsalicylic acid and an angiotensin ll antagonist for stroke prevention
InactiveUS20070004687A1InhibitionReduce riskBiocideSalicyclic acid active ingredientsDipyridamoleSalicylic acid
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical formulations and use thereof in prevention of stroke, diabetes and/or congestive heart failure
InactiveCN1384756AReduce in quantityNervous disorderOrganic chemistryAngiotensin ii antagonistPharmaceutical formulation
The present invention relates to the production of renin-angiotensin system (RAS) inhibitors or pharmaceutically acceptable derivatives thereof, in particular ramipril or ramipril, for the prevention of stroke, diabetes and / or congestive heart failure. (CHF) in medicines. The present invention also relates to a method for preventing and / or treating stroke, diabetes and / or CHF, the method comprising administering a therapeutically effective amount of a RAS inhibitor or a pharmaceutically acceptable derivative thereof to a patient in need of said prevention and / or treatment, Specifically ramipril or ramipril.
Owner:SANOFI AVENTIS DEUT GMBH
Dipyridamole, acetylsalicylic acid, and angiotensin II antagonist pharmaceutical compositions
InactiveUS20060241089A1Inhibition effectStroke preventionBiocideSalicyclic acid active ingredientsDipyridamoleRisk stroke
A pharmaceutical composition comprising a therapeutically effective amount of: (a) dipyridamole or a pharmaceutically acceptable salt thereof; (b) acetylsalicylic acid; and (c) an angiotensin II antagonist, kits containing these three compounds, and methods for preventing stroke or reducing the risk of stroke or secondary stroke in a patient in need thereof by administering an effective amount of these compounds to the patient.
Owner:BOEHRINGER INGELHEIM INT GMBH
Nitrosated And Nitrosylated Cardiovascular Compounds, Compositions And Methods Of Use
InactiveUS20070238740A1Improve propertiesBiocideSenses disorderRenovascular diseaseAngiotensin ii antagonist
The invention describes novel nitrosated and / or nitrosylated cardiovascular compounds or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitrosated and / or nitrosylated cardiovascular compound, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel compositions and kits comprising at least one cardiovascular compound of the invention, that is optionally nitrosated and / or nitrosylated, and, optionally, at least one nitric oxide donor compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; and (k) treating nephropathy. The nitrosated and / or nitrosylated cardiovascular compounds are preferably nitrosated and / or nitrosylated aldosterone antagonists, nitrosated and / or nitrosylated angiotensin II antagonists, nitrosated and / or nitrosylated calcium channel blockers, nitrosated and / or nitrosylated endothelin antagonists, nitrosated and / or nitrosylated hydralazine compounds, nitrosated and / or nitrosylated neutral endopeptidase inhibitors and nitrosated and / or nitrosylated renin inhibitors.
Owner:NICOX SA
2'-halobiphenyl-4-yl intermediates in the synthesis of angiotensin ii antagonists
A process for obtaining 2′-halo-4-methylbiphenyls is described, which comprises reacting 4 halotoluene with a 1,2-dihalobenzene in the presence of elemental metal such as magnesium, lithium or zinc, wherein 0 to 0.9 molar, particularly 0 to 0.2 molar excess of 4-halotoluene in regard to 1,2-dihalobenzene is used, and arised organometal intermediates are quenched by elemental mental halogen. In addition, the coupling of arised 2′-halo-4-methylbiphenyls with 2-(1-propyl)-4-methyl-6-(1′-methylbenzimidazole-2-il)benzimidazole to afford 3′-(2′-halo-biphenyl-4-ylmethyl)-1,7′-dimethyl-2′-propyl-1H,3′H-[2,5′]bibenzoimidazolyl, which can be further converted to organometallic compound and said organometallic compound is further reacted with formic acid derivative, such as N,N-dimethylformamide, alkylformiate or carbon dioxide to obtain telmisartan, is also described. Further described is use of in line analytics for monitoring the aforementioned reactions, process for preparing a pharmaceutical composition and / or dosage for, or use in preparing a medicament.
Owner:LEK PHARMA D D
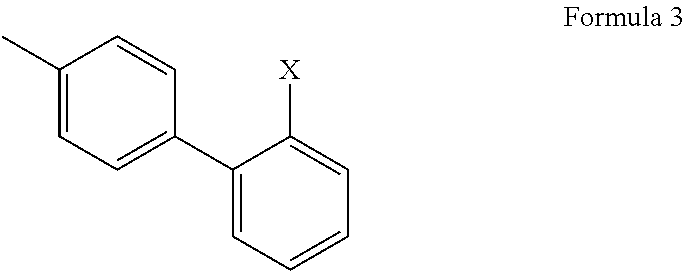
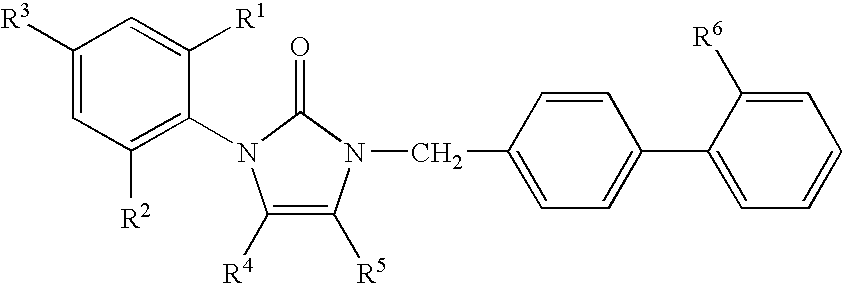
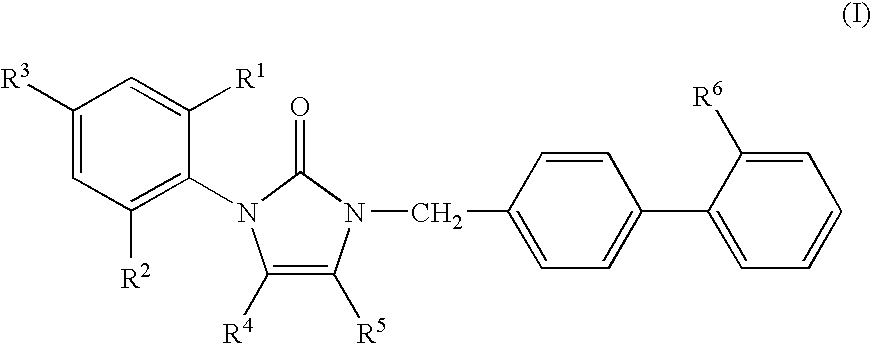
1-phenyl imidazol-2-one biphenylmethyl compounds for treatment of circulatory disorders
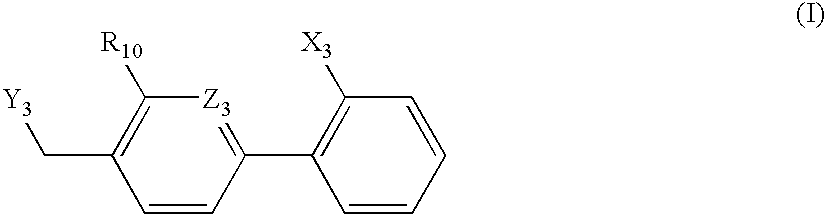
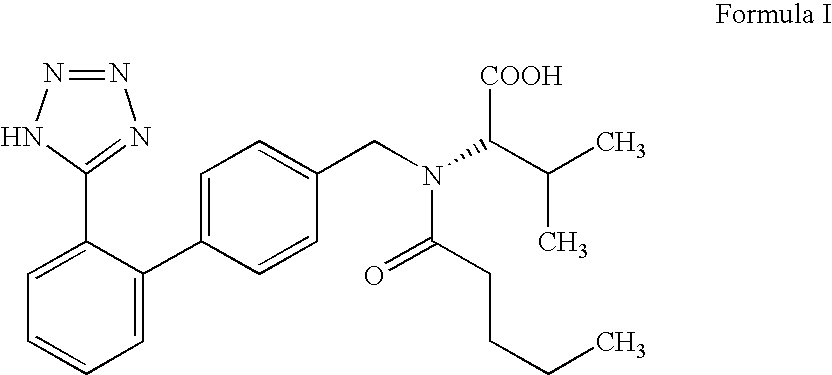
A class of 1-phenyl imidazol-2-one biphenylmethyl compounds is described for use in treatment of circulatory disorders. Compounds of particular interest are angiotensin II antagonists of the formula wherein each of R1, R2 and R3 is independently selected from hydrido, alkyl, alkoxy, cyano, halo, hydroxy, carboxyl, alkoxycarbonyl, formyl and acetyl; alkylcarbonyl and haloalkylcarbonyl; with the proviso that at least one of R1, R2 and R3 must be a substituent other than hydrido, and with the further proviso that when each of R1 and R3 is hydrido, then R2 cannot be chloro; wherein R4 is hydrido; wherein R5 is alkyl; and wherein R6 is tetrazolyl; or a stereoisomer or a tautomer thereof or a pharmaceutically-acceptable salt thereof. These compounds are particularly useful in treatment or control of hypertension and congestive heart failure.
Owner:GD SEARLE & CO
Pharmaceutical combination of angiotensin II antagonists and angiotensin I converting enzyme inhibitors
InactiveUS20060154976A1Good effectMaximum preventionAntibacterial agentsBiocideDepressantRisk stroke
A method of treatment of indications which can be positively influenced by inhibition of AT1 mediated effects with maintenance of AT2 receptor mediated effects of angiotensin II and by ACE inhibition, thus also increasing bradykinin mediated effects, e.g., to reduce the incidence of stroke, acute myocardial infarction or cardiovascular death, or of indications associated with the increase of AT1 receptors in the subepithelial area or increase of AT2 receptors in the epithelia, comprising coadministration of effective amounts of an angiotensin II antagonist and an ACE inhibitor, pharmaceutical compositions containing an angiotensin II antagonist together with an ACE inhibitor and the use of an angiotensin II antagonist and an ACE inhibitor for the manufacture of corresponding pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARM KG
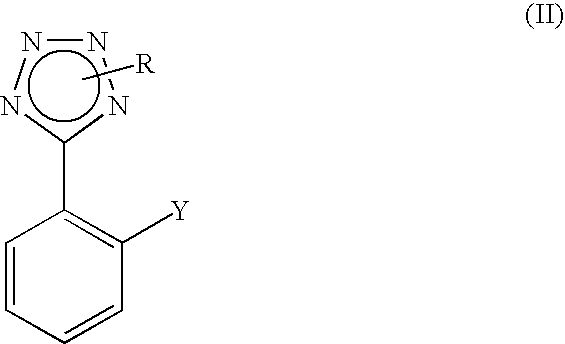
Process for the preparation of phenyltetrazole derivatives
A process for the preparation of phenyltetrazole derivatives of formula (II) wherein R and Y are as defined in the disclosure, by direct ortho-metallation of (tetrazol-5-γl)benzene. The compounds of formula (II) are useful intermediates for the preparation of angiotensin II antagonists.
Owner:DIPHARMA SPA
Synergistic therapeutic compositions and methods
InactiveCN1879884APeptide/protein ingredientsHeterocyclic compound active ingredientsAngiotensin I Converting Enzyme InhibitorsTherapeutic effect
This invention relates to compositions and methods for achieving a therapeutic effect such as lowering blood pressure and treating congestive heart failure in a mammal. More specifically, this invention relates to synergistic compositions comprising amounts of at least two therapeutic agents selected from the group consisting of a renin inhibitor, an angiotensin I converting enzyme inhibitor and an angiotensin II antagonist, which inhibitors and antagonists are present in amounts sufficient to cause synergistic therapeutic effects such as lowering blood pressure and treating congestive heart failure in a mammal. Further, this invention relates to methods for achieving synergistic therapeutic effects such as lowering blood pressure and treating congestive heart failure in a mammal which methods comprise administering to said mammal, either sequentially in any order or simultaneously, amounts of at least two therapeutic agents selected from the group consisting of a renin inhibitor, an angiotensin I converting enzyme inhibitor and an agiotensin II antagonist, in amounts sufficient to achieve a synergistic therapeutic effect.
Owner:PFIZER INC
Process for the preparation of angiotensin II antagonist
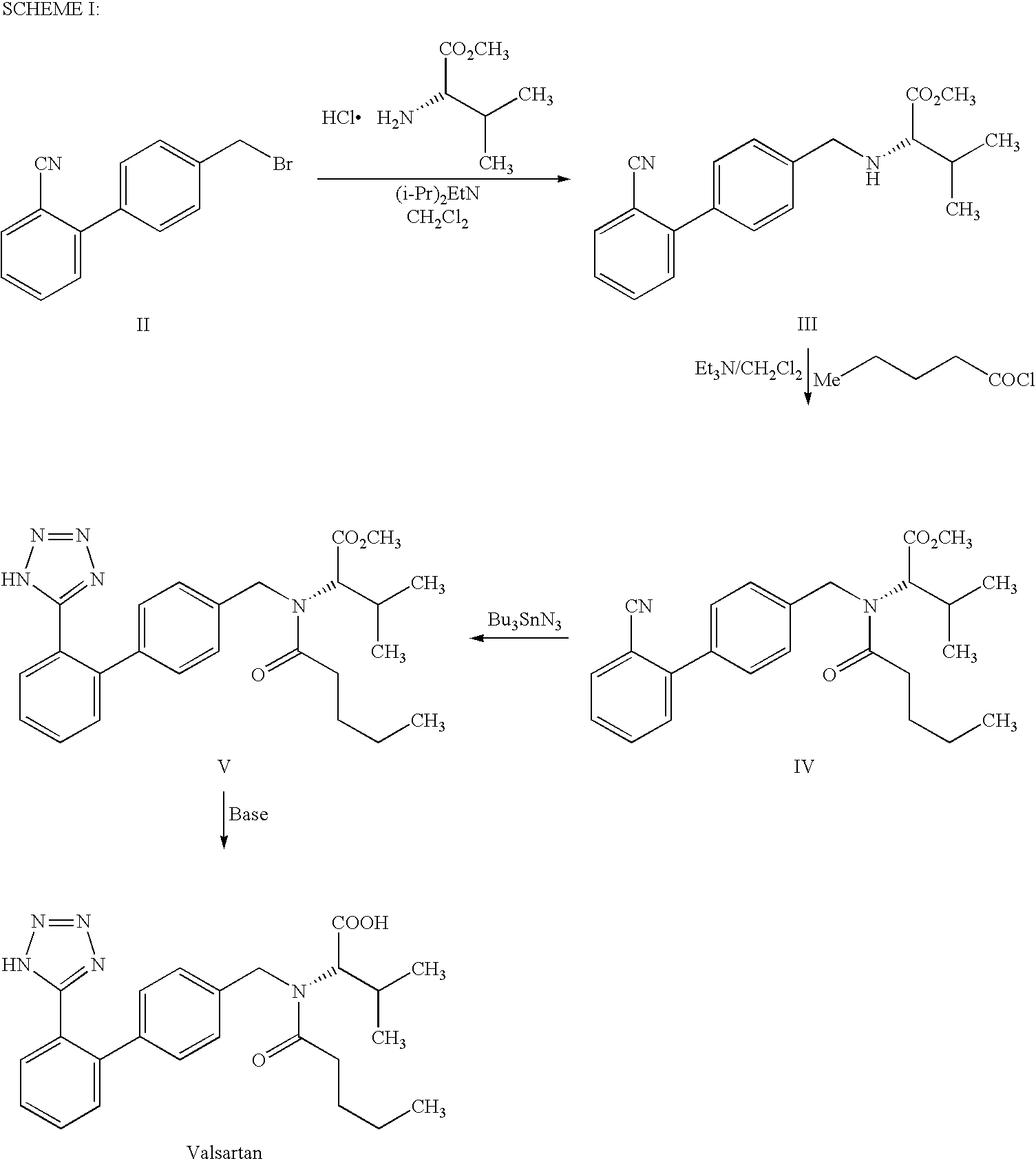
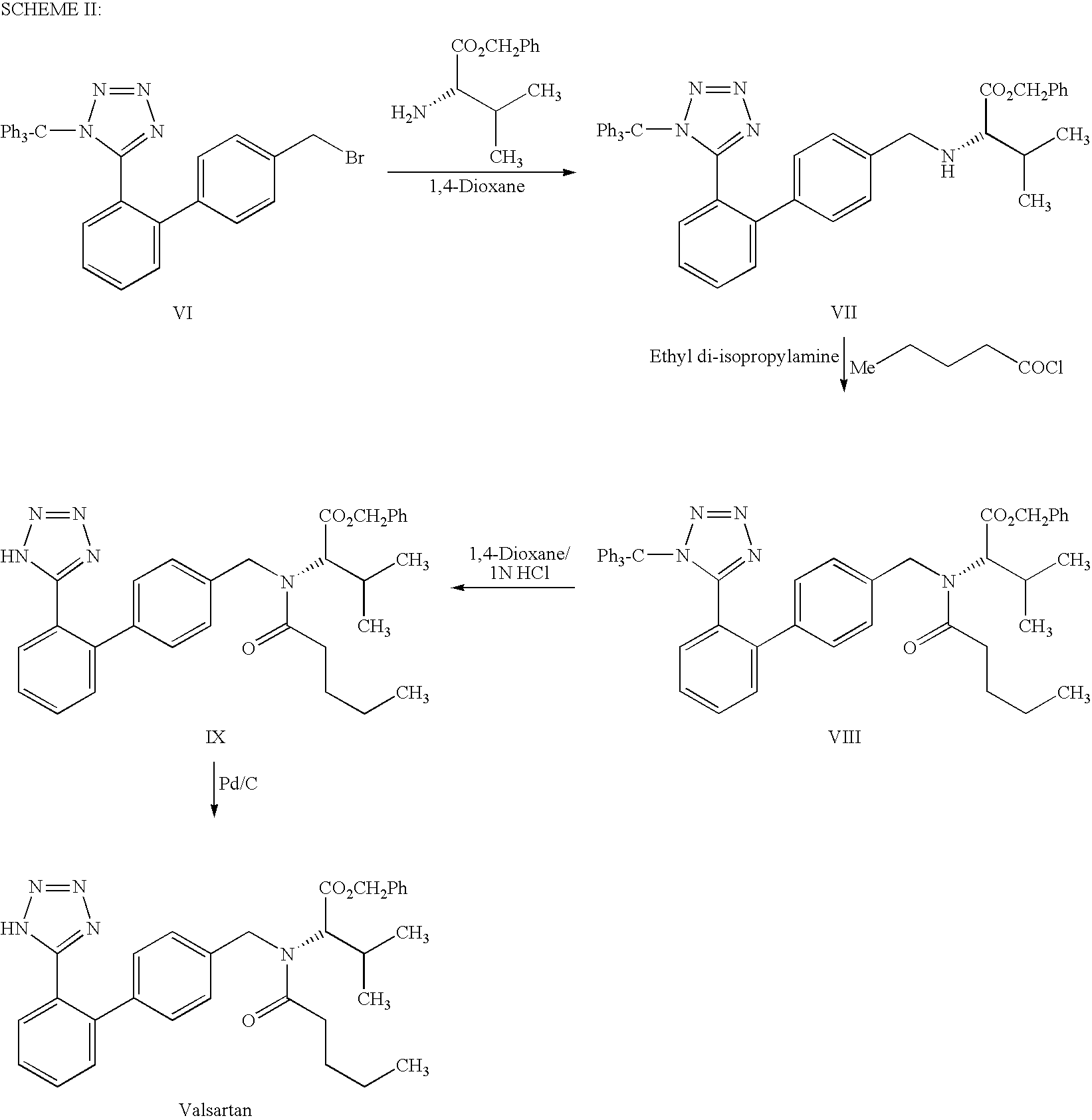
InactiveUS7880015B2Simple and cost-effective processHigh purityOrganic chemistryOXALIC ACID DIHYDRATEOxalate
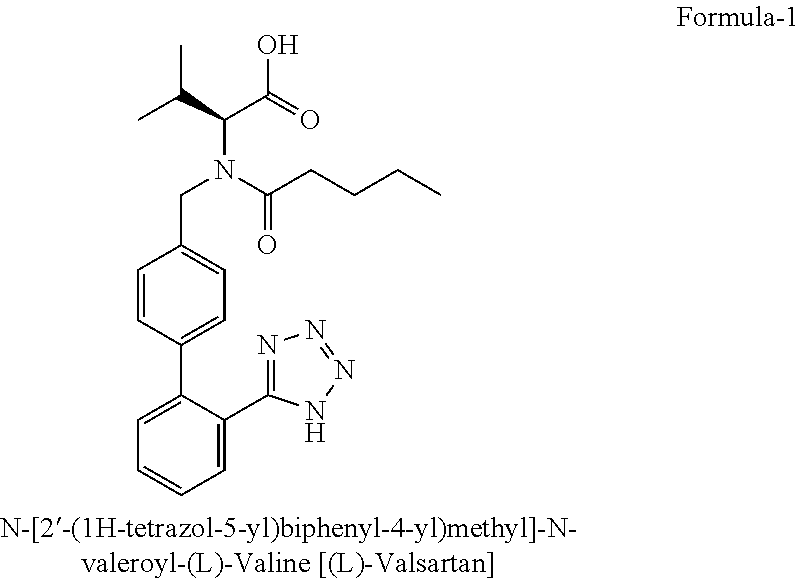
The present invention provides a method for the preparation of N-(1-oxopentyl)-N-[[2′-(1H-tetra-zol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine (Valsartan) which comprises; treating N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)biphenyl-4-yl]methyl]-L-valine methyl ester (X) with oxalic acid or its hydrates in a solvent to produce N-[[2′-(1-triph-enylmethyl-tetrazol-5-yl)biphenyl-4-yl]methy]-L-valine methyl ester oxalate (Xa) and treating the compound (Xa) with a base in a solvent followed by reacting with valeryl chloride in presence of base in a solvent to produce N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)[1,1′biphenyl]-4-yl]methyl]-N-valeryl-L-valine methyl ester (XI), de-protecting the compound (XI) using anhydrous acidic conditions to produce N-(1-oxopentyl)-N-[[2′-(1-H-tetrazol-5-yl)[1,1′biphenyl]-4-yl]methyl-L-valine methyl ester (V) followed by treating with base in a solvent to produce Valsartan.
Owner:AUROBINDO PHARMA LTD
Methods for reducing hospitalizations related to heart failure
InactiveUS20090118293A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Benzimidazole derivative and use as angiotensin ii antagonist
InactiveCN101151260BEasy to solveGood treatment effectOrganic active ingredientsOrganic chemistryBenzimidazole derivativeMethyl benzene
The present invention provides (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-cyclopropyl-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate represented by the formula: which has superior properties as a pharmaceutical agent, a salt thereof, a crystal thereof, a pharmaceutical agent containing the compound, and the like.
Owner:TAKEDA PHARMA CO LTD
Process For the Preparation of Angiotensin II Antagonist
InactiveUS20090281326A1Simple and cost-effective processHigh purityOrganic chemistryOxalateValsartan
The present invention provides a method for the preparation of N-(1-oxopentyl)-N-[[2′-(1H-tetra-zol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine (Valsartan) which comprises; treating N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)biphenyl-4-yl]methyl]-L-valine methyl ester (X) with oxalic acid or its hydrates in a solvent to produce N-[[2′-(1-triph-enylmethyl-tetrazol-5-yl)biphenyl-4-yl]methy]-L-valine methyl ester oxalate (Xa) and treating the compound (Xa) with a base in a solvent followed by reacting with valeryl chloride in presence of base in a solvent to produce N-[[2′-(1-triphenylmethyl-tetra-=zol-5-yl)[1,1′biphenyl]-4-yl]methyl]-N-valeryl-L-valine methyl ester (XI), de-protecting the compound (XI) using anhydrous acidic conditions to produce N-(1-oxopentyl)-N-[[2′-(1-H-tetrazol-5-yl)[1,1′biphenyl]-4-yl]methyl-L-valine methyl ester (V) followed by <treating with base in a solvent to produce Valsartan.
Owner:AUROBINDO PHARMA LTD
Combined agents for treatment of glaucoma
InactiveCN1615880ASenses disorderElcosanoid active ingredientsBULK ACTIVE INGREDIENTAngiotensin ii antagonist
The present invention provides a preventive or therapeutic agent for glaucoma, which contains an angiotensin II antagonist and dorzolamide or a pharmacologically acceptable salt thereof as active ingredients, and uses these active ingredients simultaneously, separately or sequentially, wherein the angiotensin II antagonist Factor II antagonists are compounds with the following general formula (I) or their pharmacologically acceptable salts, esters or other derivatives, wherein R1 is as defined in the claims.
Owner:SANKYO CO LTD
Compositions and methods related to heart failure
InactiveUS20090118294A1Reduce in quantityShorten the construction periodBiocideNervous disorderAdrenergic antagonistPyridazine
The invention provides methods for reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); reducing the number of hospital admissions for heart failure; and reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); by administering to a patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), or diuretic compounds.
Owner:NITROMED
Process for the preparation of angiotensin ii antagonists and intermediates thereof
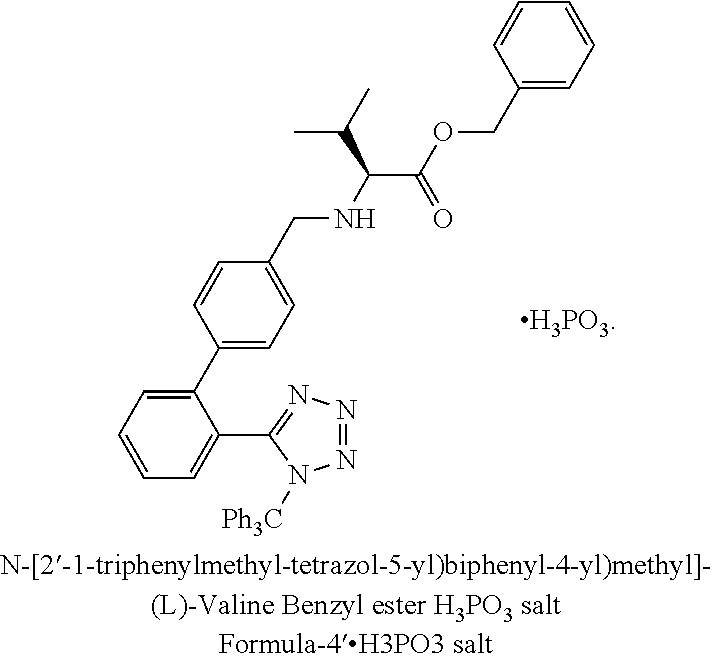
InactiveUS20140316142A1Easy to handleHigh purityOrganic chemistryPhosphite saltAngiotensin ii antagonist
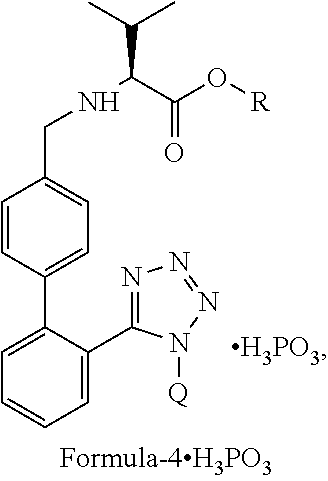
The present invention relates to an improved process for the preparation of angiotensin receptor antagonists and intermediates thereof. Particularly the present invention relates to an improved process for the preparation of N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine of Formula 1via a phosphite salt of Formula-4.H3PO3preferably a phosphite salt of Formula-4′.H3PO3
Owner:ALKEM LAB LTD
Pharmaceutical Combination of Angiotensin II Antagonists and Angiotensin I Converting Enzyme Inhibitors
InactiveUS20080146639A1Good effectMaximum preventionAntibacterial agentsBiocideRisk strokeAngiotensin ii antagonist
A method of treatment of indications which can be positively influenced by inhibition of AT1 mediated effects with maintenance of AT2 receptor mediated effects of angiotensin II and by ACE inhibition, thus also increasing bradykinin mediated effects, e.g., to reduce the incidence of stroke, acute myocardial infarction or cardiovascular death, or of indications associated with the increase of AT1 receptors in the subepithelial area or increase of AT2 receptors in the epithelia, comprising coadministration of effective amounts of an angiotensin II antagonist and an ACE inhibitor, pharmaceutical compositions containing an angiotensin II antagonist together with an ACE inhibitor and the use of an angiotensin II antagonist and an ACE inhibitor for the manufacture of corresponding pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARMA KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com