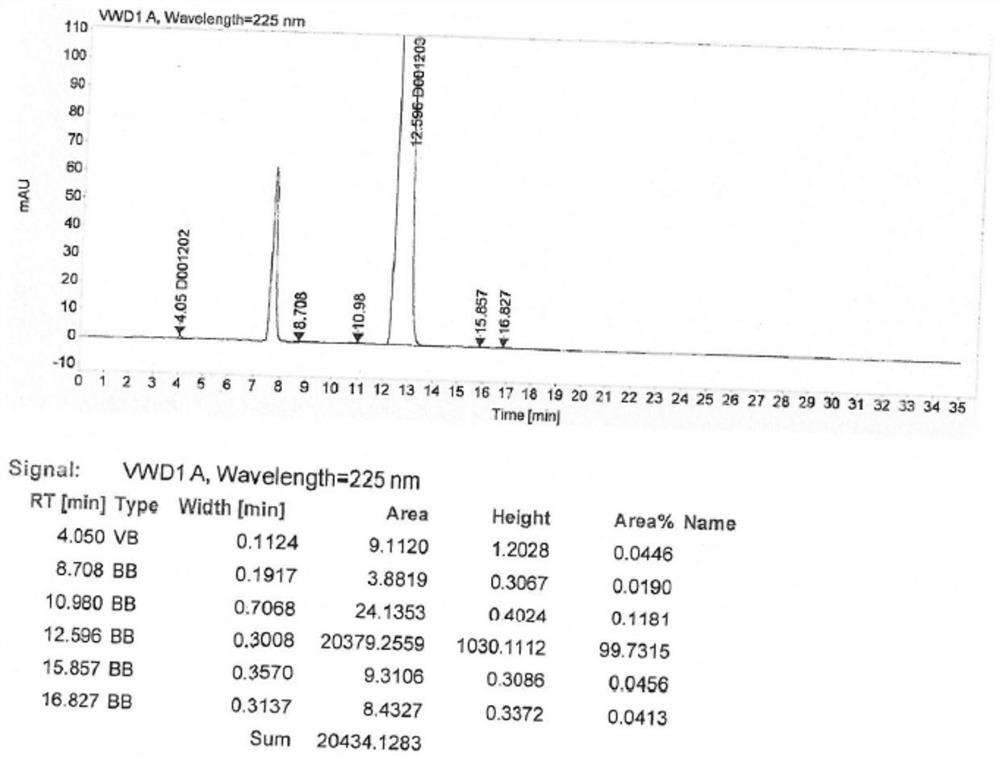
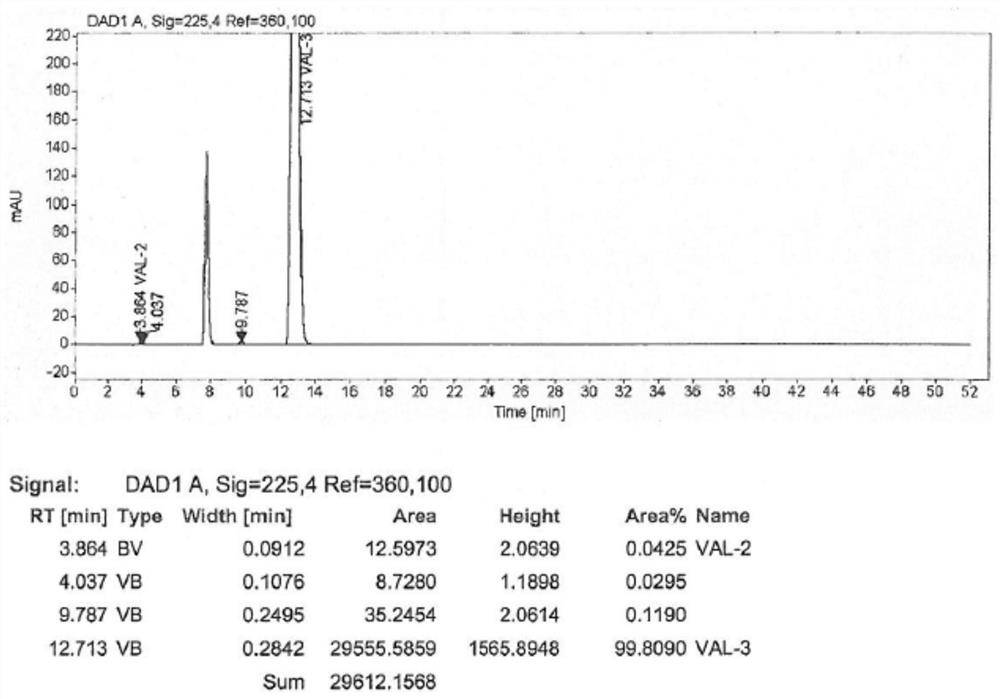
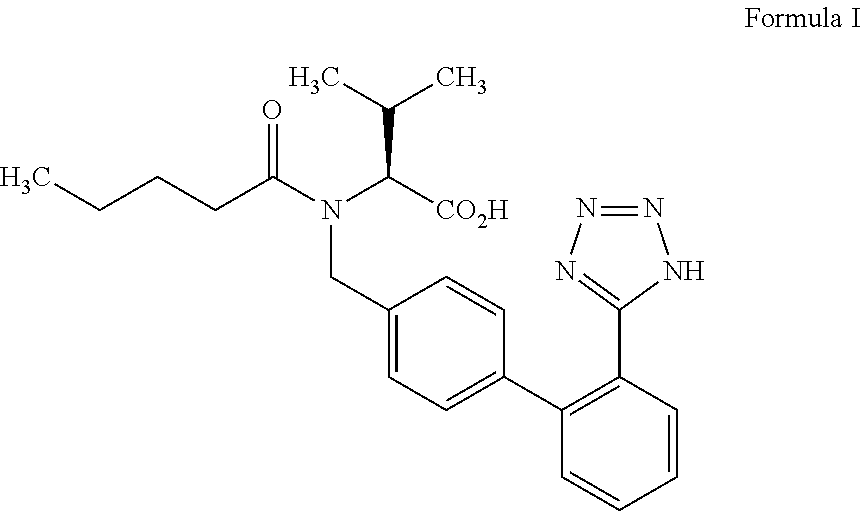
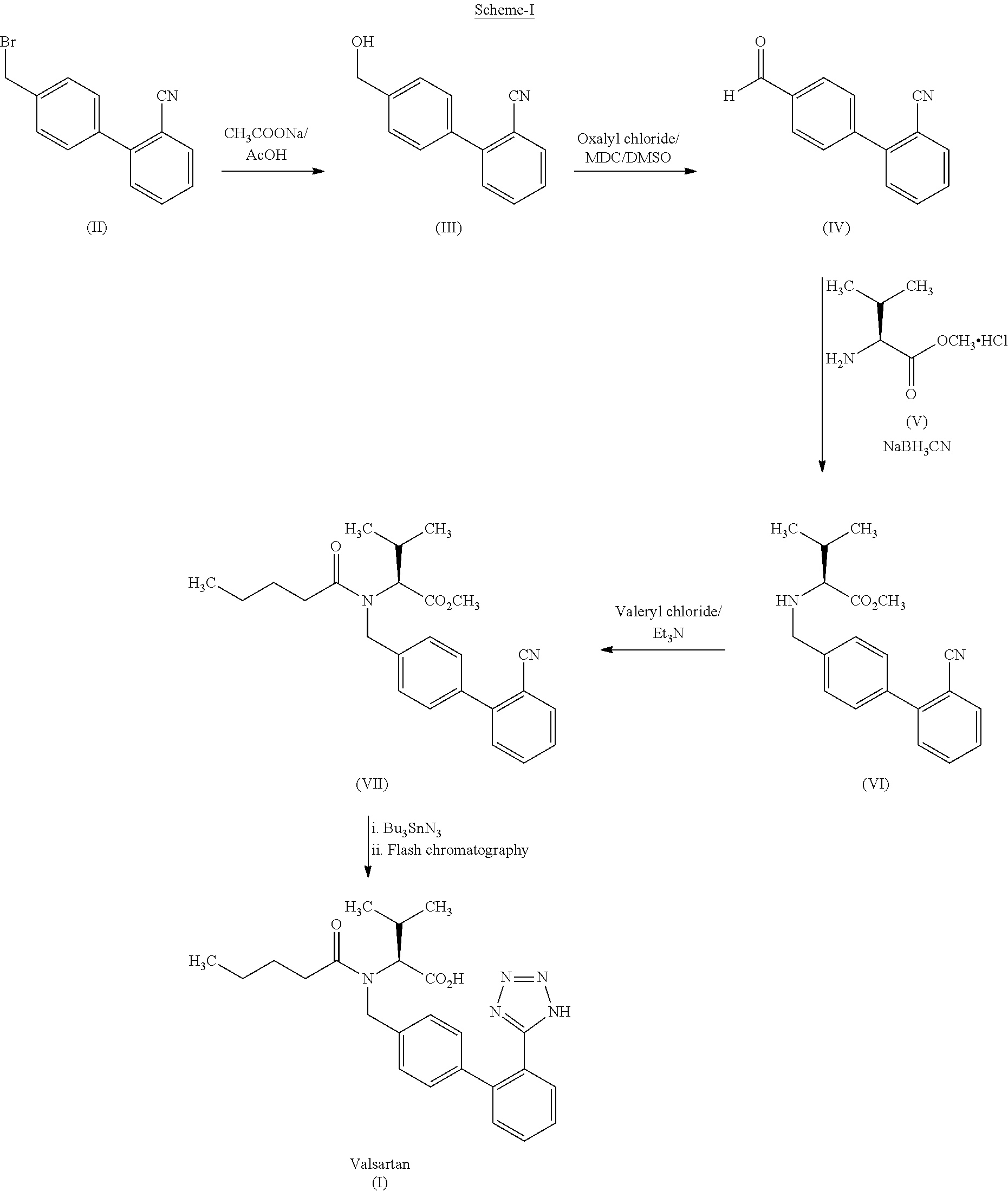
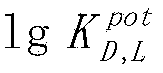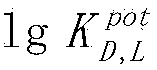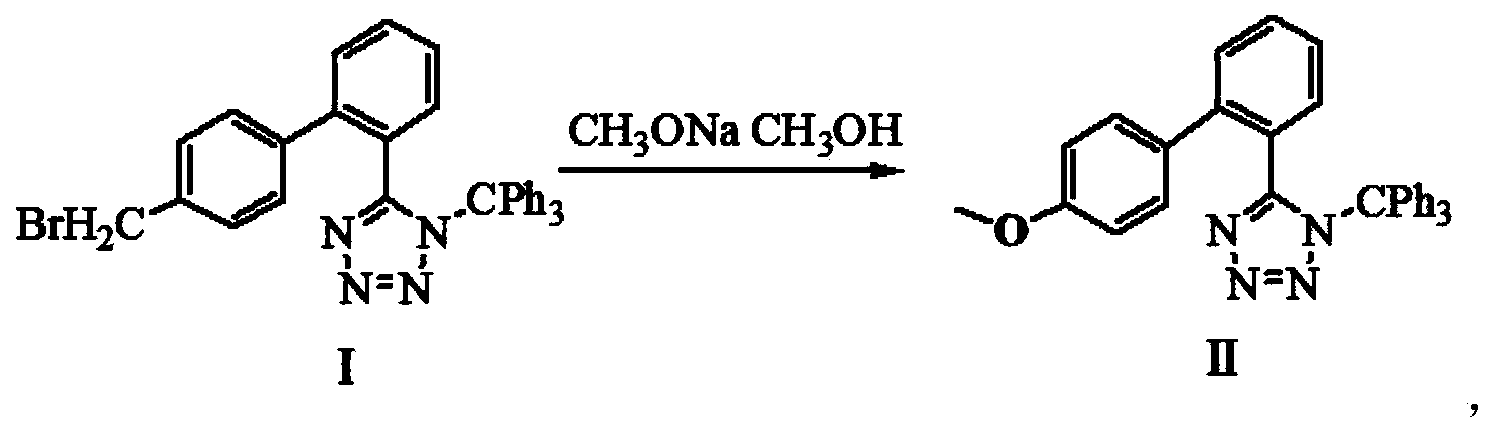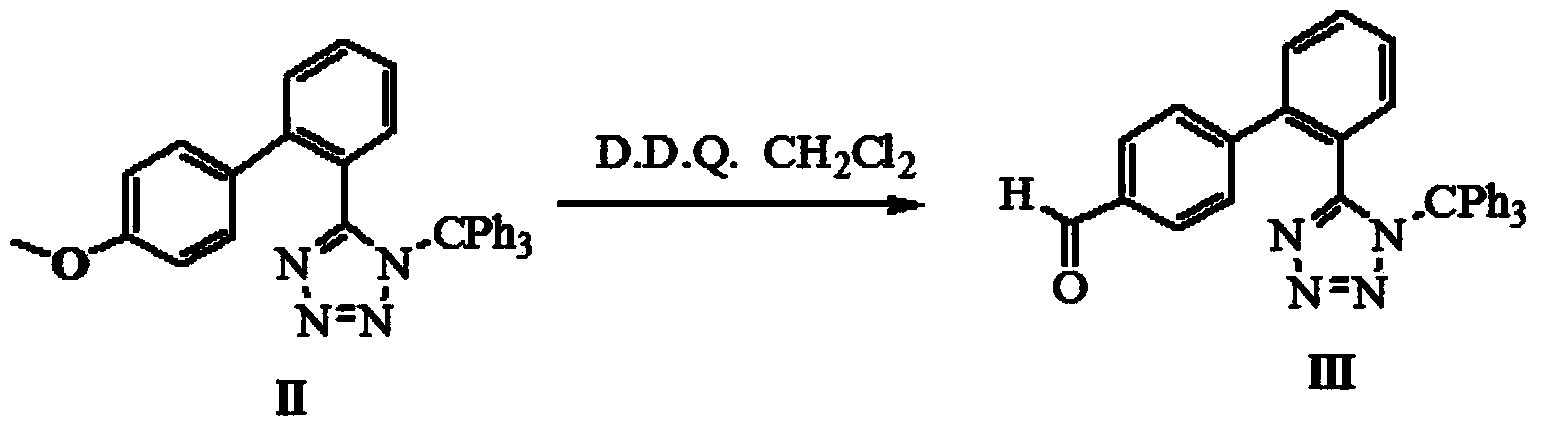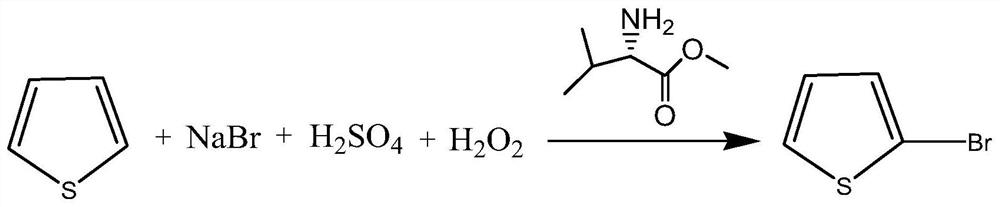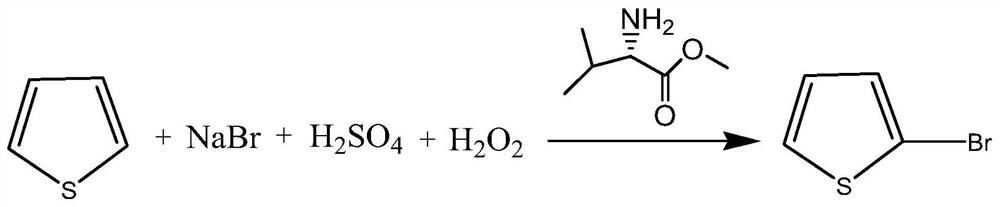Patents
Literature
34 results about "Valine methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
L-Valine methyl ester hydrochloride 99% CAS Number 6306-52-1. Linear Formula (CH 3) 2 CHCH(NH 2)COOCH 3 · HCl . Molecular Weight 167.63 . Beilstein Registry Number 3594960 . EC Number 228-620-9. MDL number MFCD00012497. eCl@ss 32160406 . PubChem Substance ID 24888503. SDS FTNMR (PDF) Similar Products.
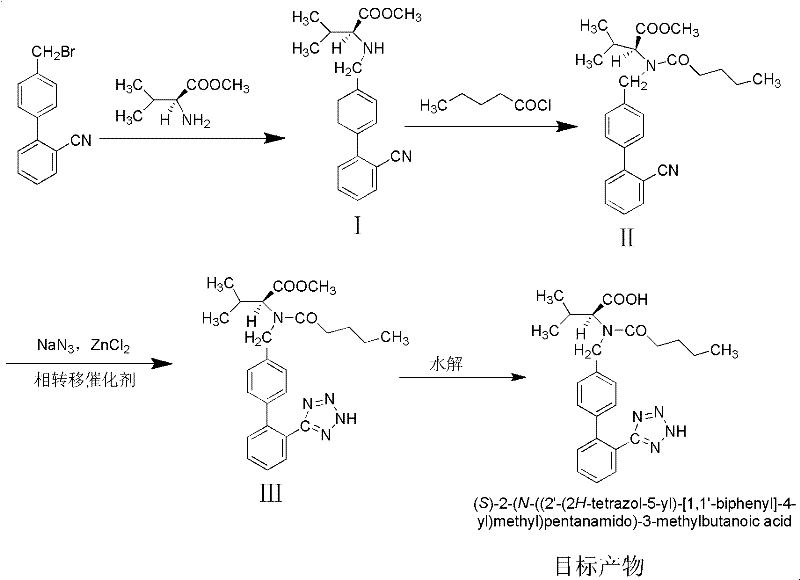
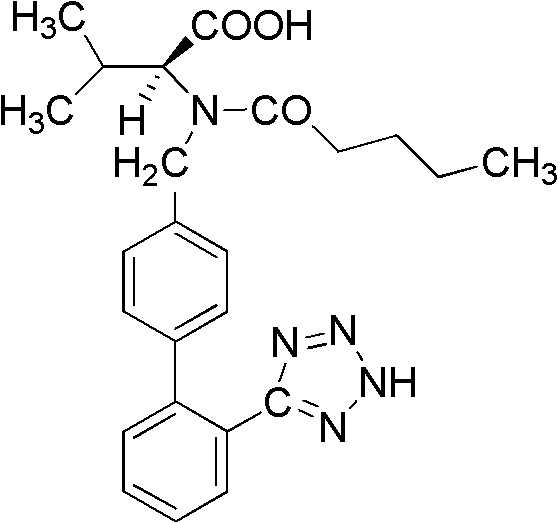
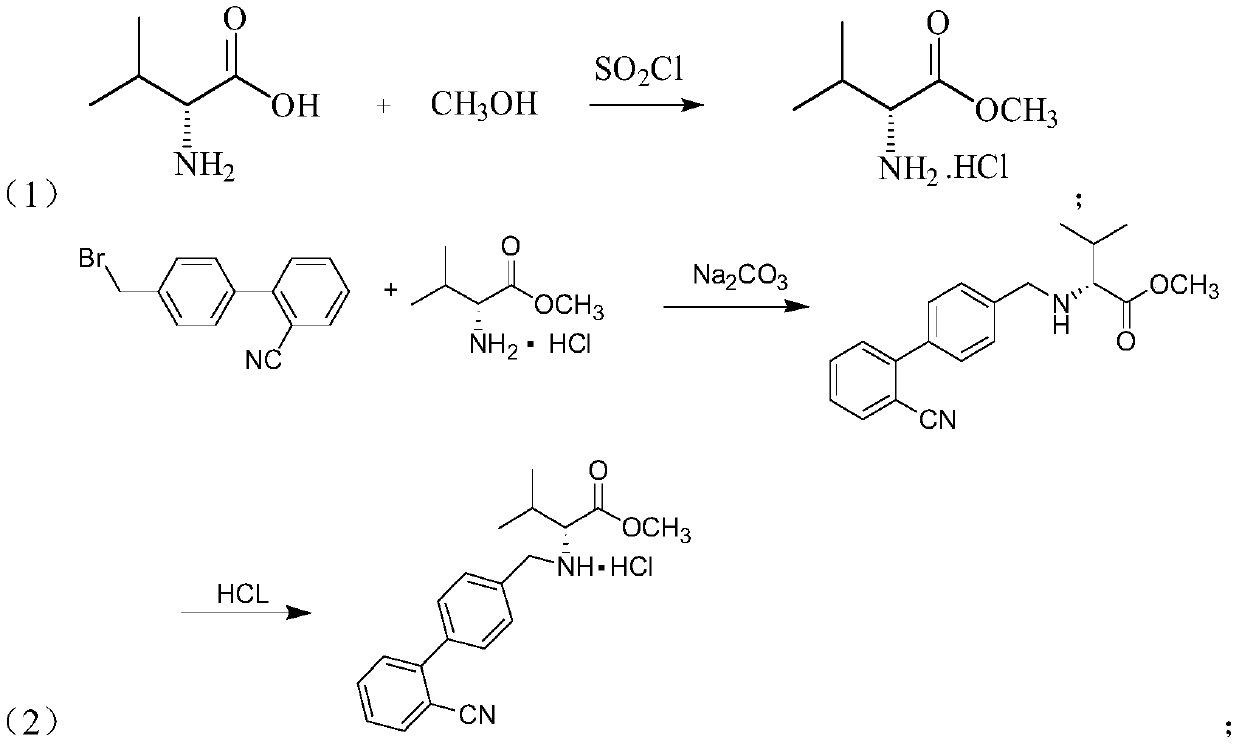
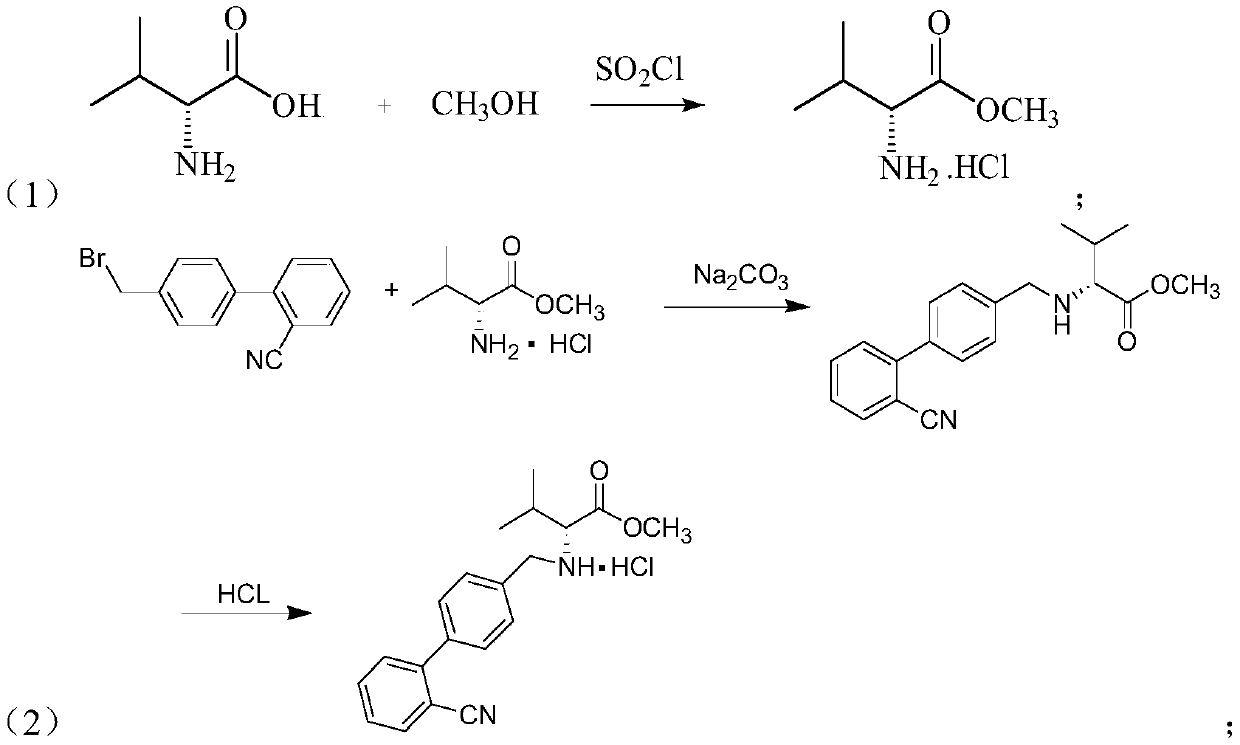
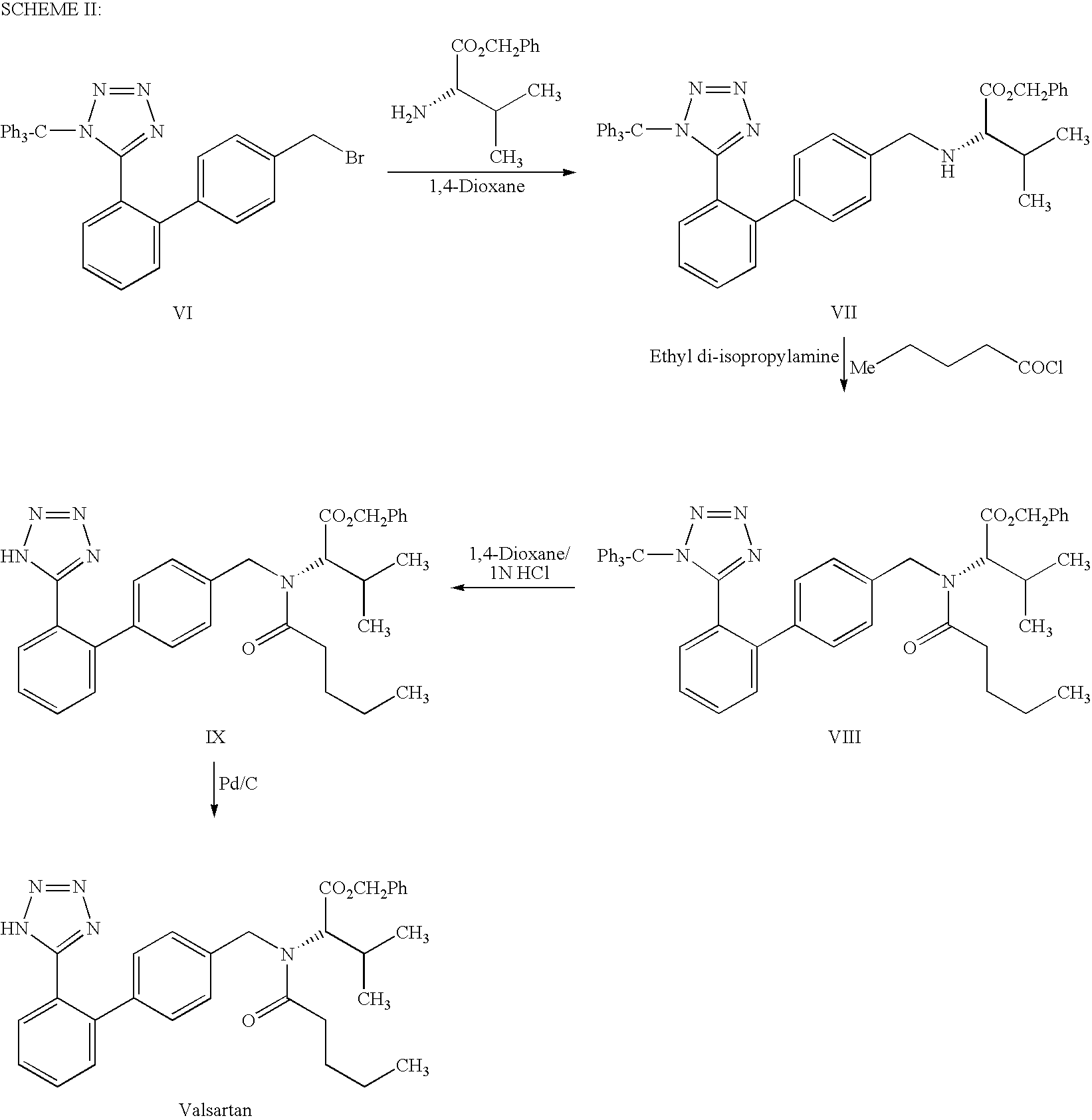
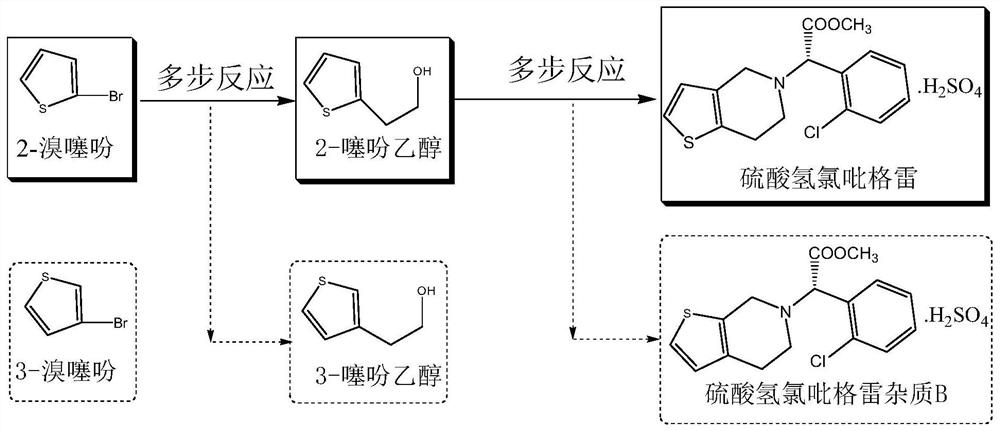
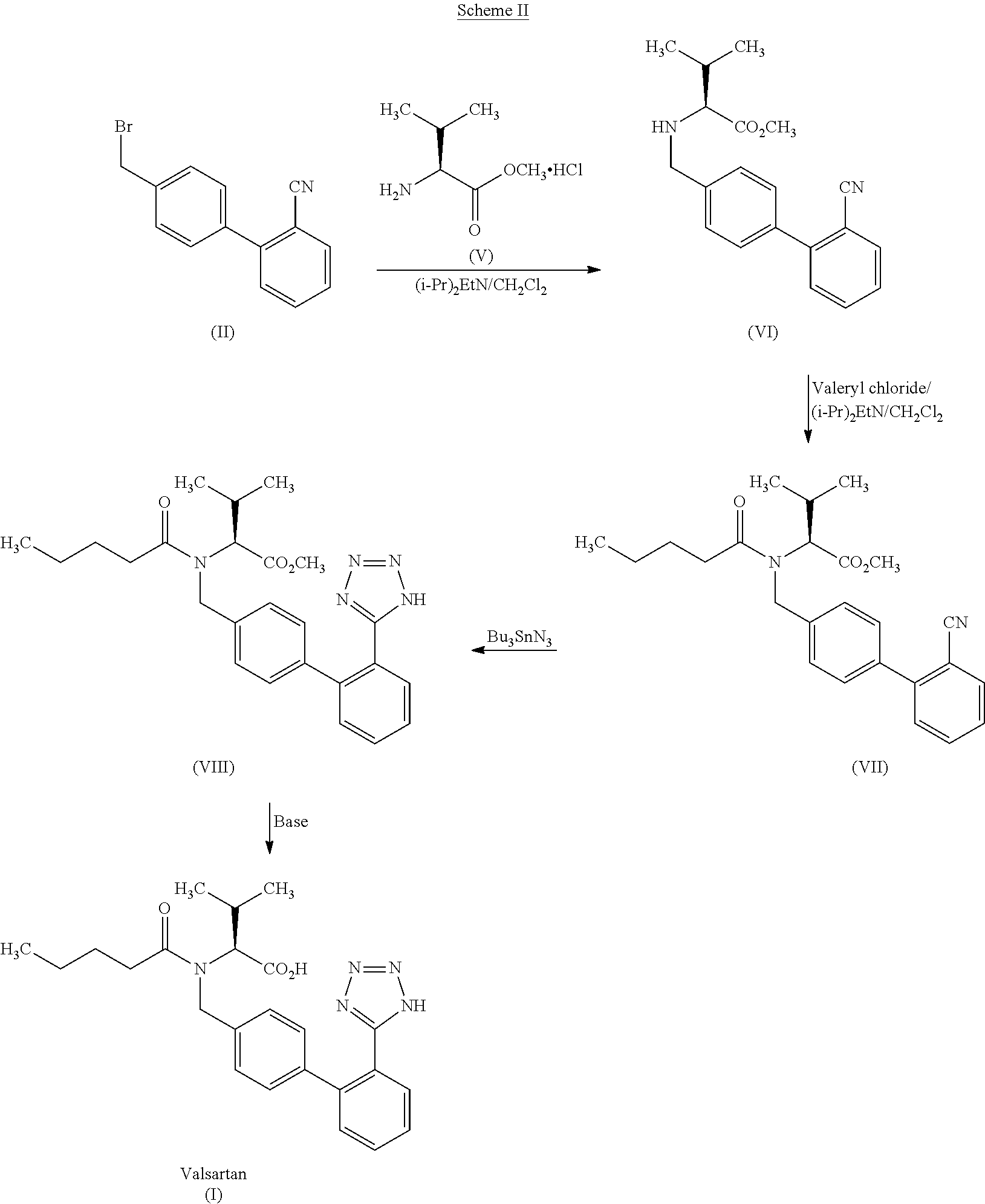
Synthesis method of valsartan
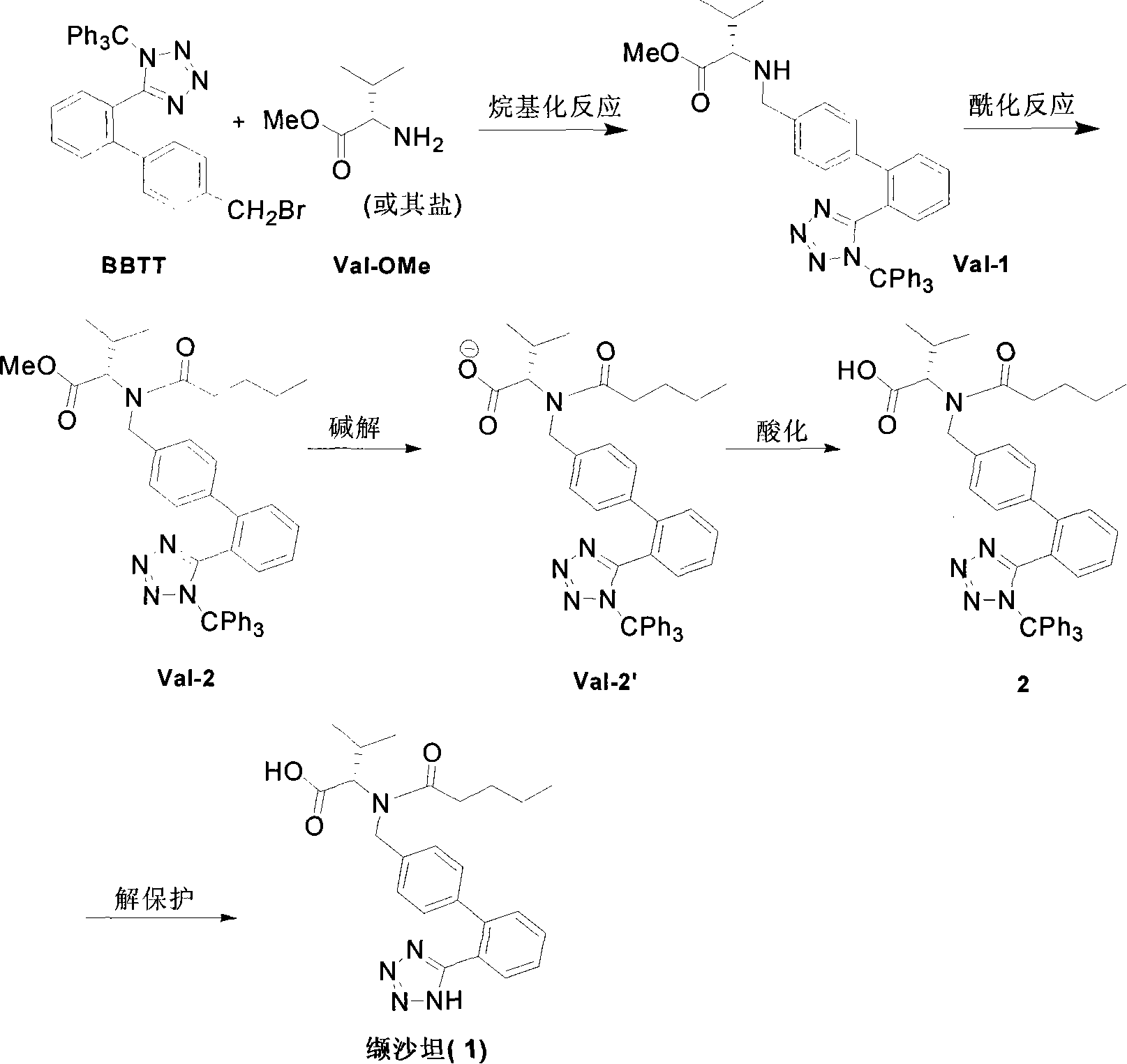
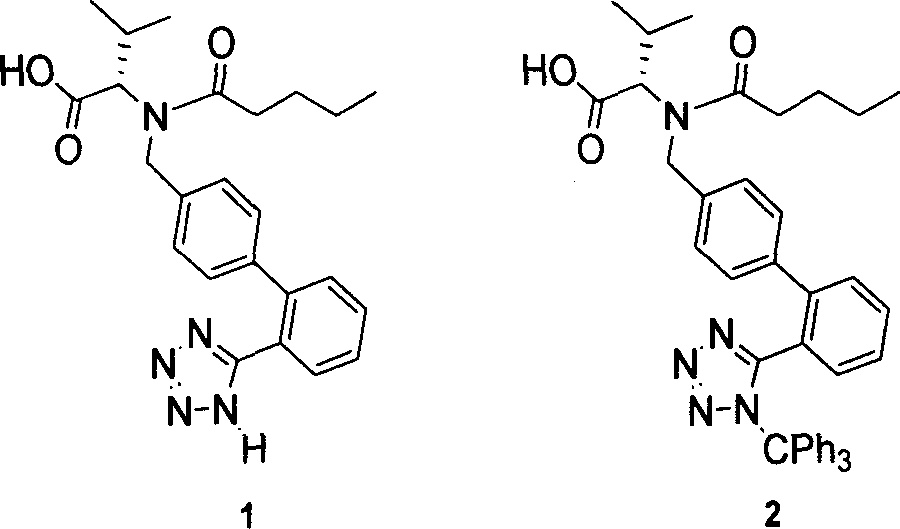
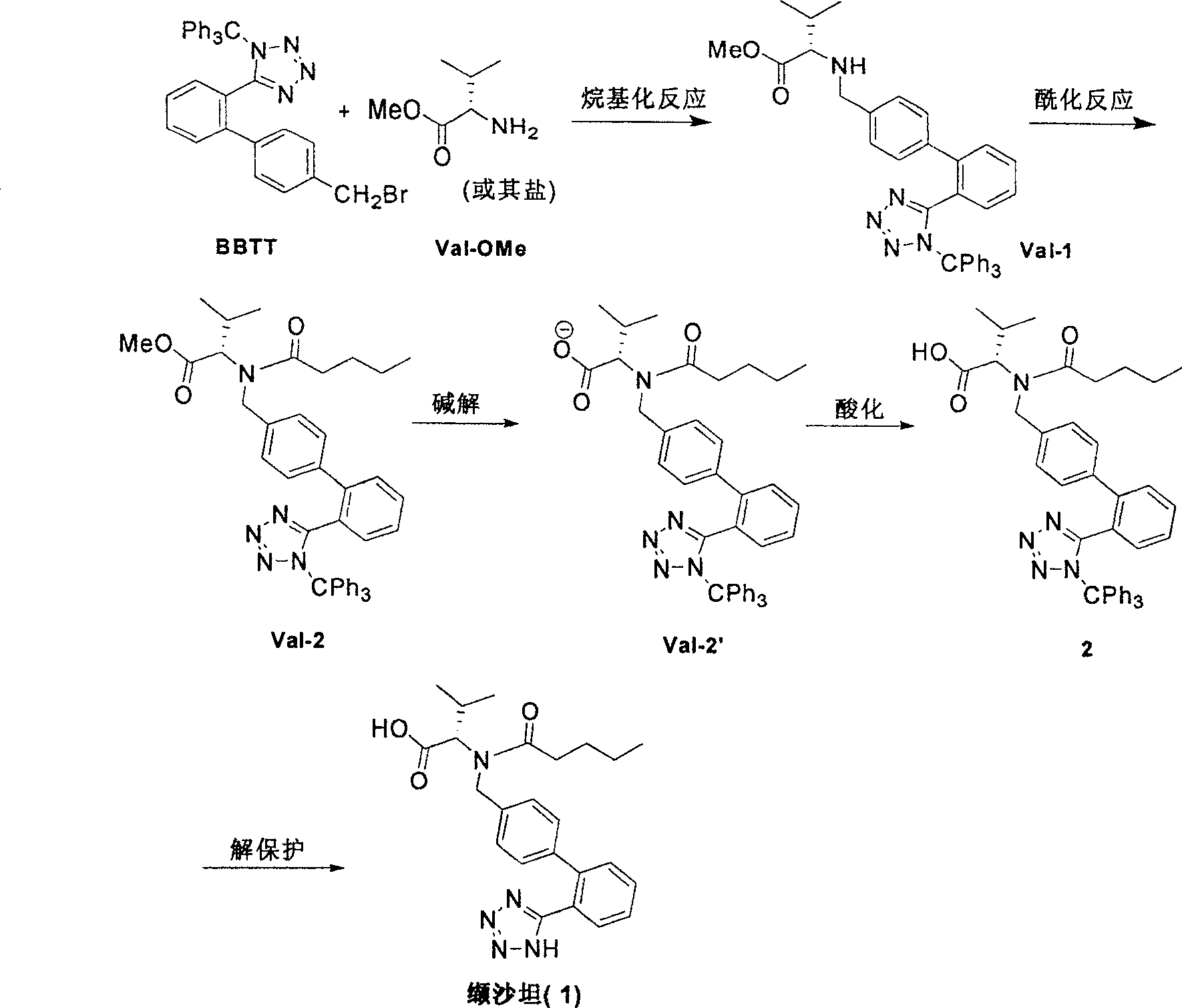
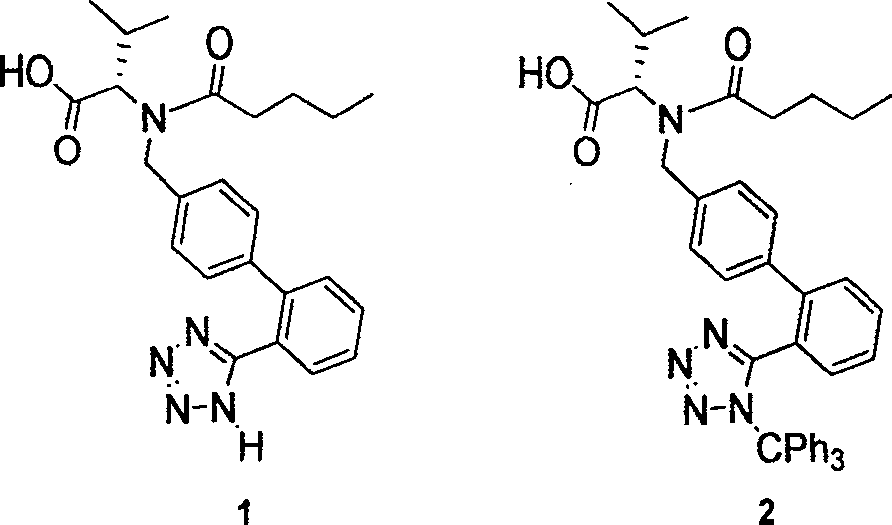
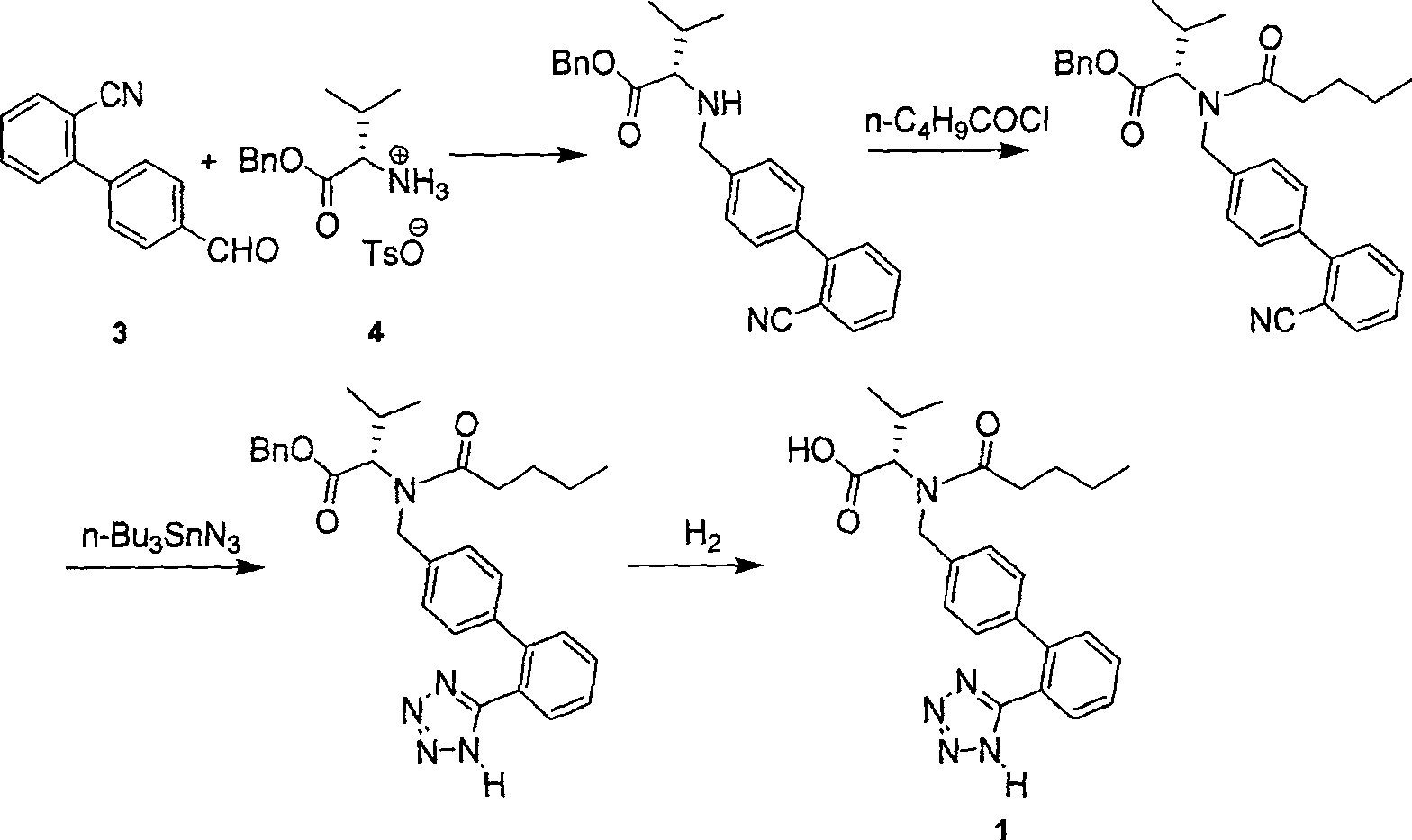
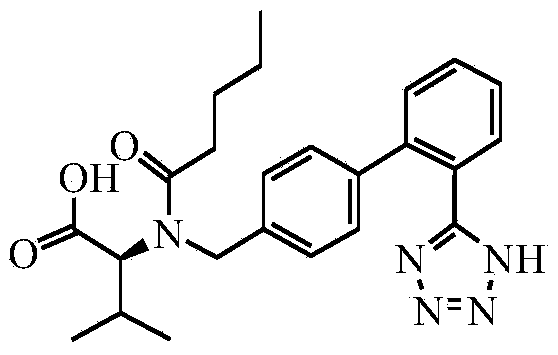
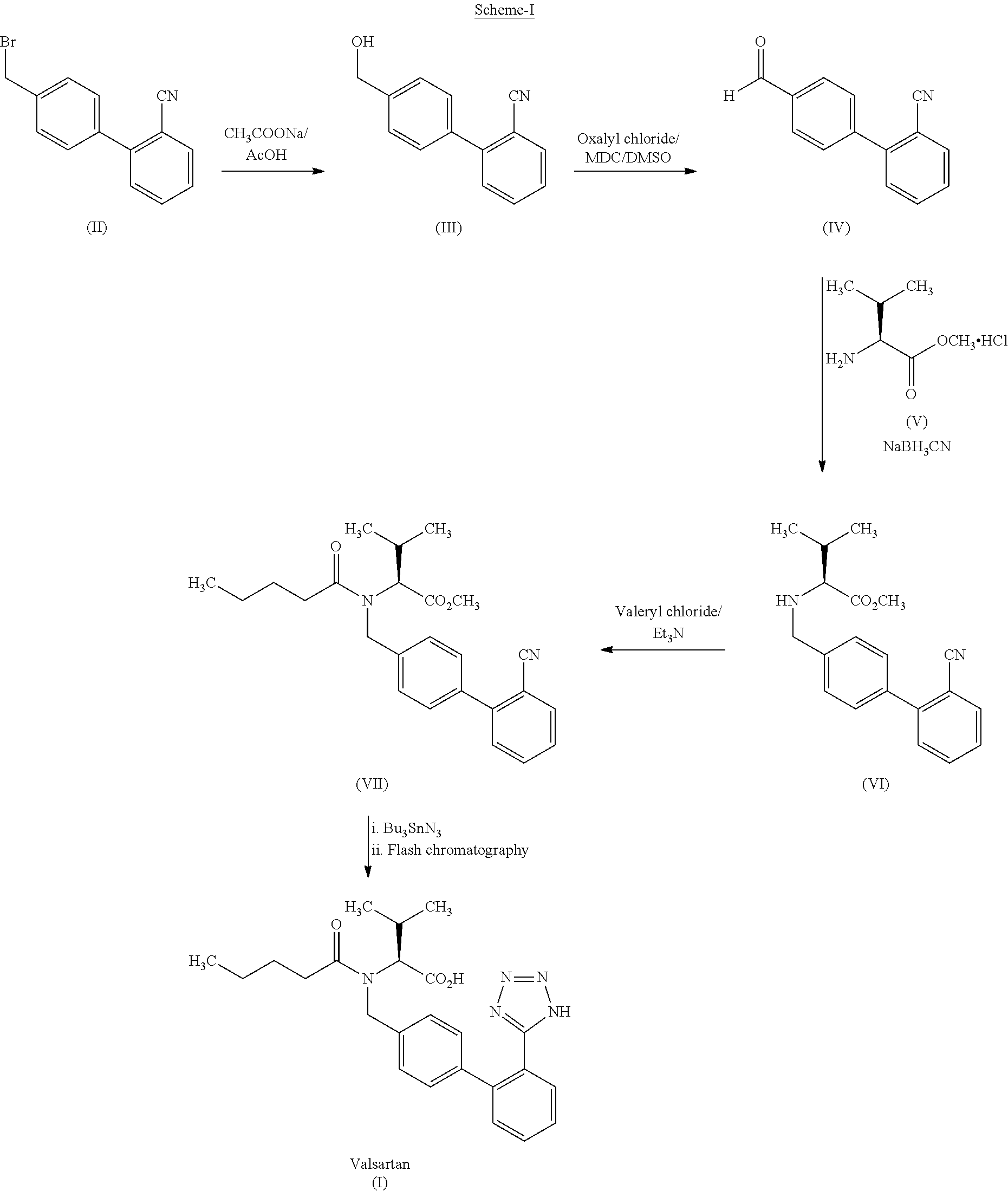
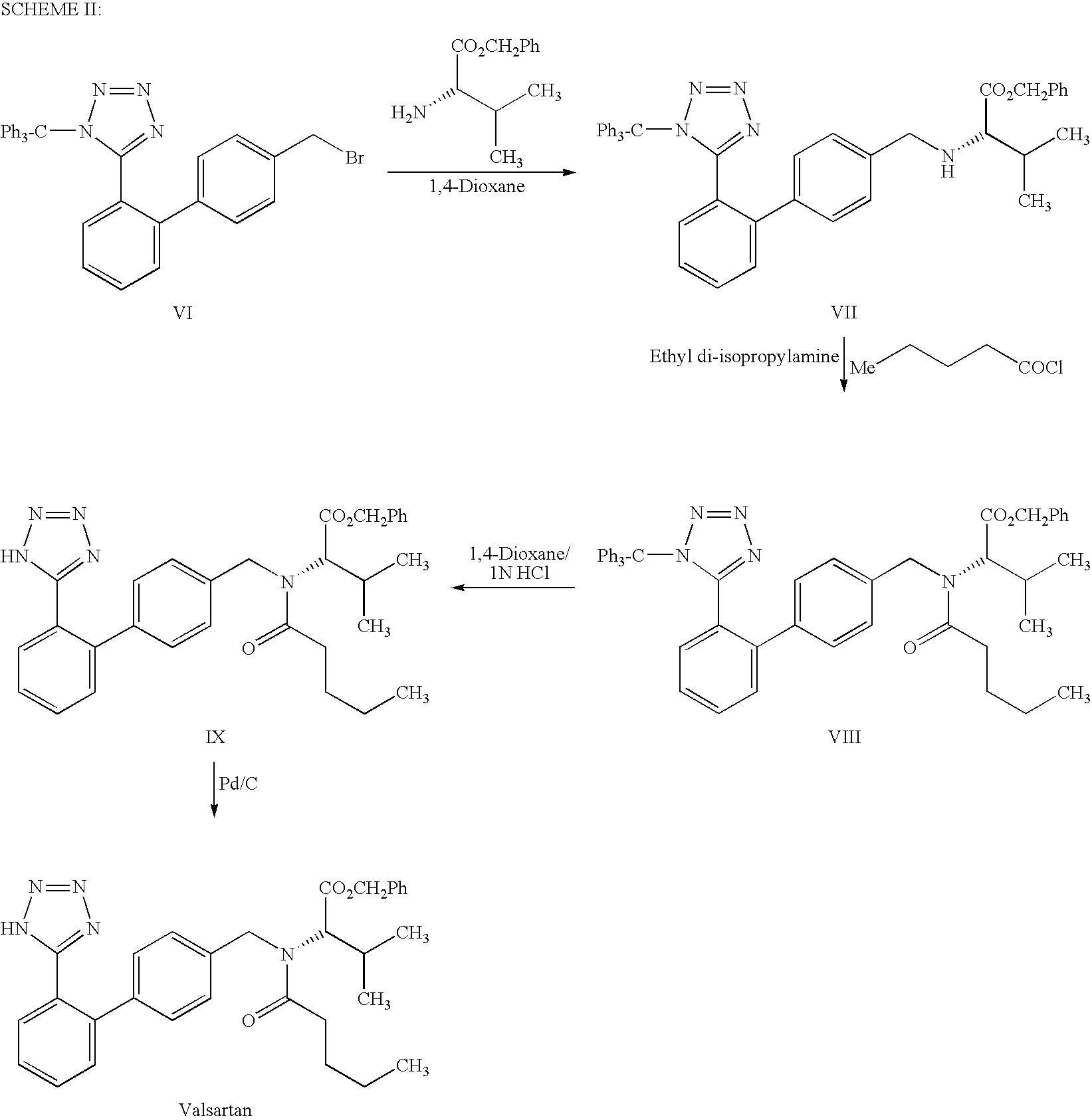
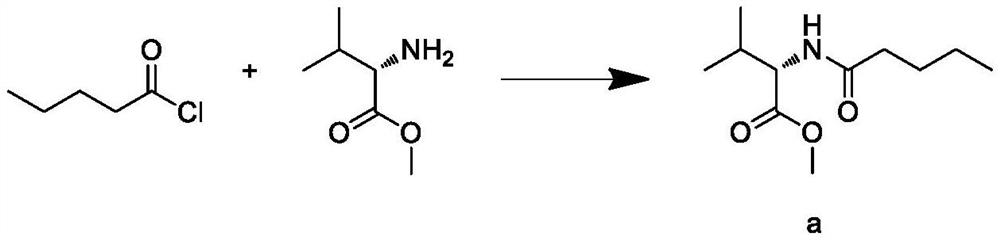
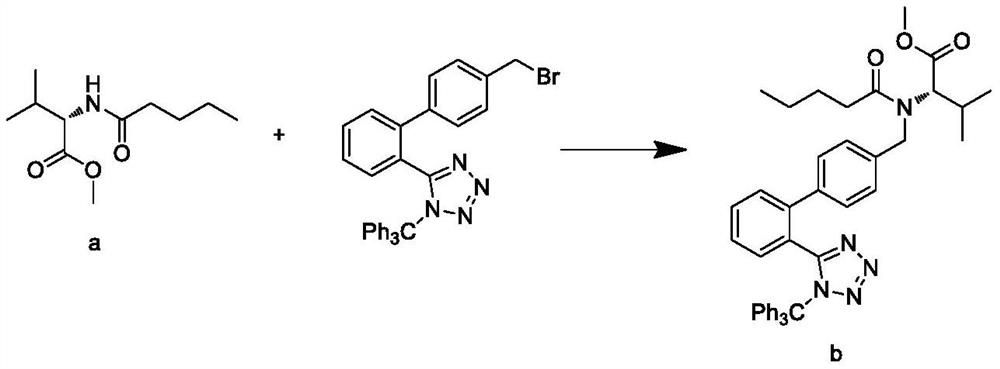
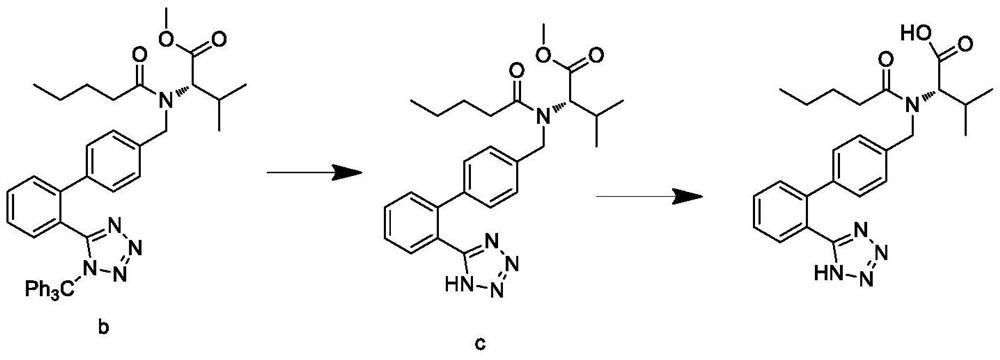
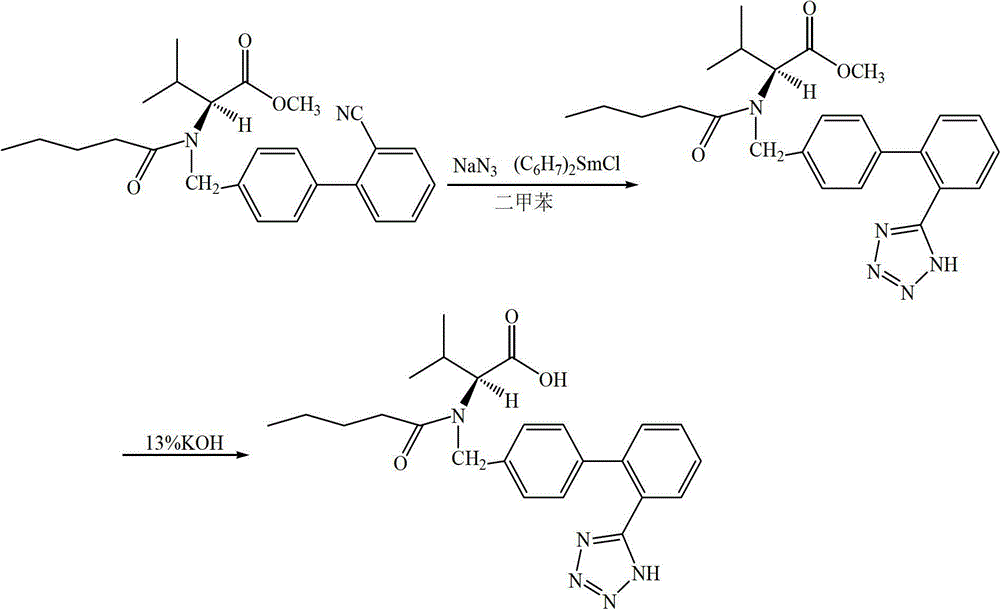
This invention relates to a new synthetic method of valsartan, includes preparation method of intermediate N - ( 1 - oxygen amyl) - N - [ [ 2' - trityl - tetrazolium - 5 - group) - ( 1, 1' - dibenzyl) - 4 - group] - methyl] - L - valine. 2' - ( N - trityl) tetrazolium - 4 - bromoethyl Biphenyl and valine methyl ester ( or other salt) carry out alkylation reaction, after acidylation, by alkaline hydrolysis and acidification to obtain intermediate N - ( 1 - oxygen amyl) - N - [ [ 2' - trityl - tetrazolium - 5 - group) - ( 1, 1' - dibenzyl) - 4 - group] - methyl] - L - valine, then by protection solution to gain Valsartan. This invention belong to pharmaceutical chemistry and organic chemistry region, possess merits of raw material stabilization, yield higher, valsartan optical purity high, industrialization operating easy and so on.
Owner:ZHEJIANG TIANYU PHARMA
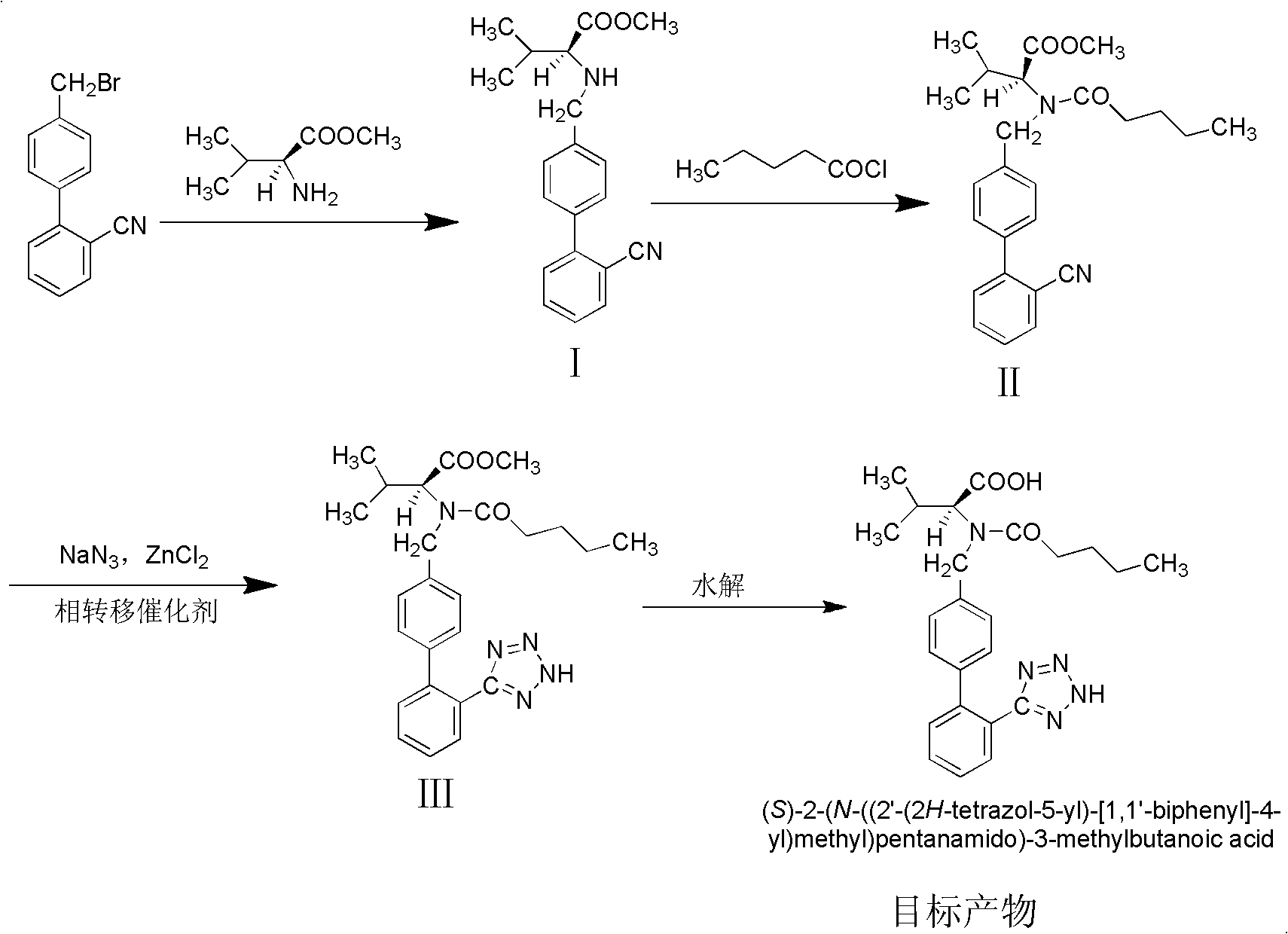
Synthetic method of valsartan
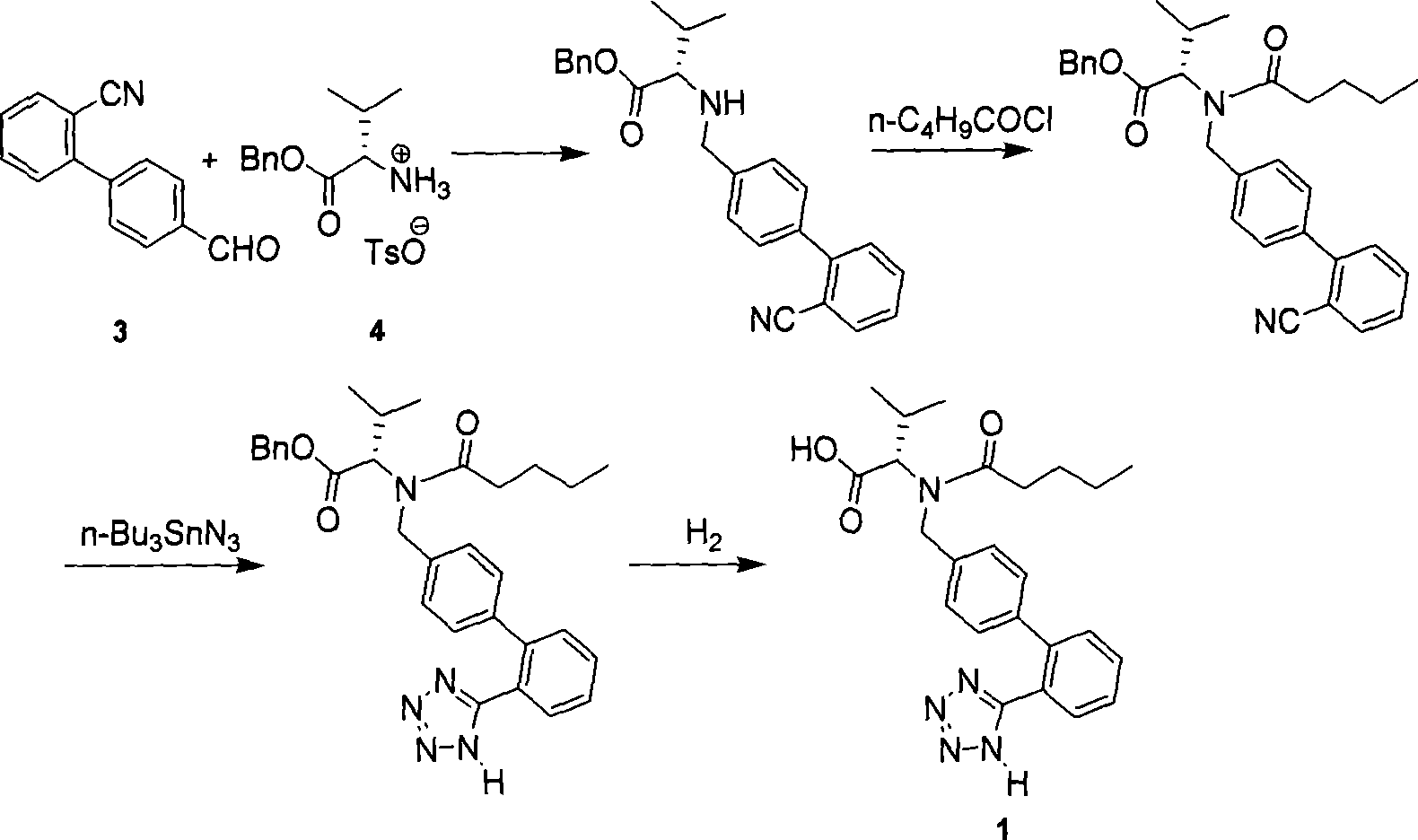
The invention relates to a synthetic method of valsartan, wherein the valsartan is obtained by adopting 4-brooethyl-2'-cyanobiphenyl and L-valine methyl ester hydrochloride as starting materials and synthetizing via steps of coupling, acylating, azidation and hydrolyzing; in the steps of coupling and acrylating, the proper phase transfer catalyst is added to catalyst. In the synthetic method, the phase transfer catalyst is applied to synthetic process of the valsartan, thus, the reactants can contact each other fully; the reaction activity of the reactants is improved; the method has fast reaction speed, thorough reaction and high yield; compared with the method without the phase transfer catalyst, the yield of the target product valsartan can be improved by more than one time; the reaction time is notably shortened so as to further reduce manufacturing cost.
Owner:苏州天绿生物制药有限公司 +1
Method for synthesizing valsartan
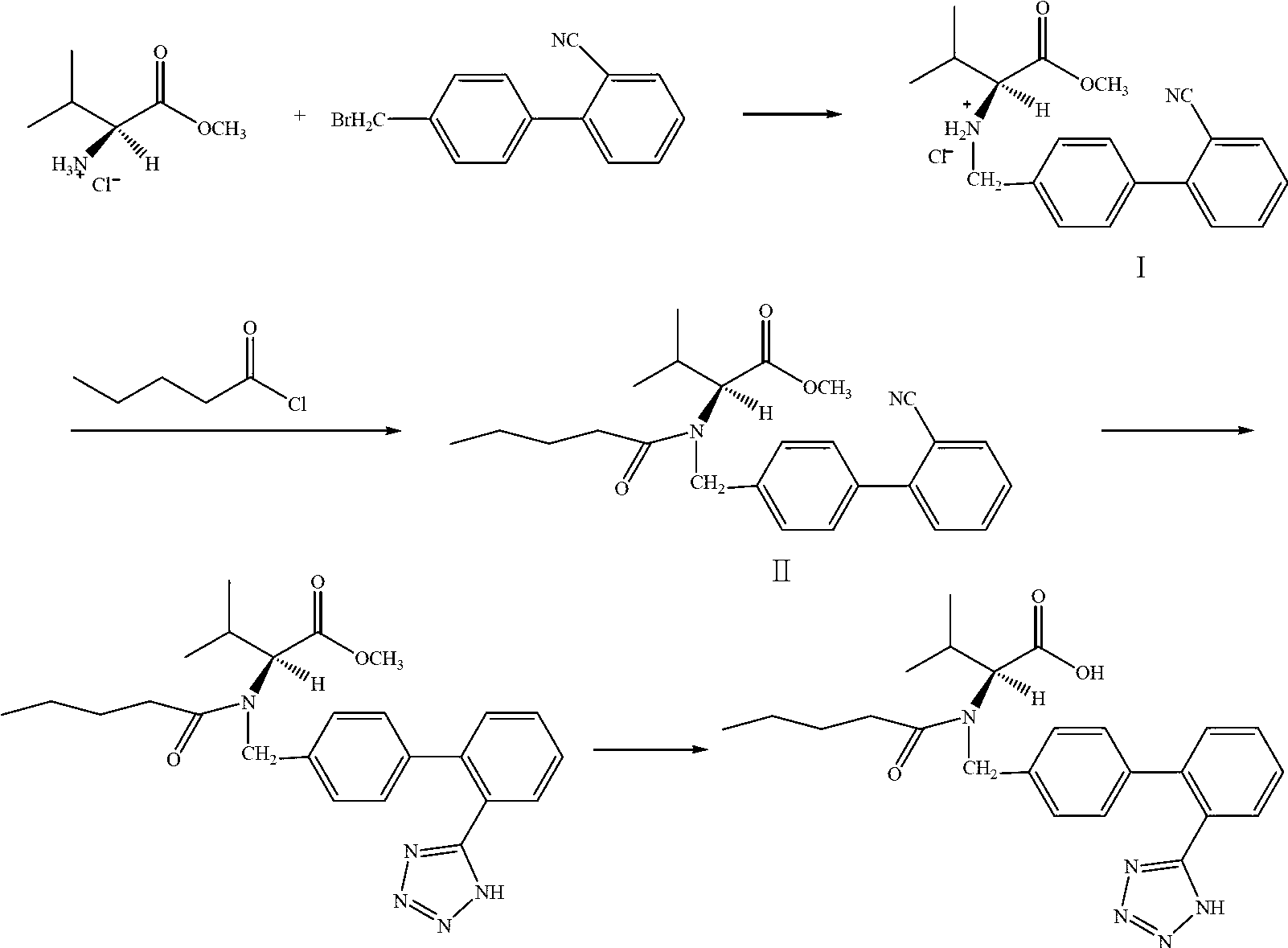
The invention discloses a method for synthesizing valsartan. The method comprises the following steps of: performing coupling reaction of 4'-brooethyl-2-cyanobiphenyl and L-valine methyl ester or L-valine methyl ester hydrochloride to obtain an intermediate I, namely N-(2'-cyanobiphenyl-4-methylene)-L-valine methyl ester or hydrochloride thereof, performing acylation reaction of the intermediate I and valeroyl chloride to obtain an intermediate II, namely N-(2'-cyanobiphenyl-4-methylene)-N-n-valery-L-valine methyl ester, finally putting the intermediate II into a system of zinc chloride and sodium azide and performing azido reaction to obtain an intermediate III, namely valsartan methyl ester, and hydrolyzing the intermediate III under alkaline conditions to obtain a target product. In the method, the yield of the valsartan methyl ester which is taken as an example is over 75 percent; and compared with other prior arts with the total yield of 40 percent, the method has the advantages that raw materials consumed for each ton of product are greatly reduced.
Owner:HEFEI UNIV OF TECH
Improved process for synthesizing valsartan
The invention provides an improved process for synthesizing valsartan. According to the method, N-[(2'-cyano-1, 1'-diphenyl-4-yl) methyl]-L-valine methyl ester hydrochloride is used as a starting raw material and is subjected to improved control of acylation reaction, cyclization reaction, hydrolysis reaction and a crystallization process respectively to obtain high-purity valsartan. The process has the advantages of continuous and simple process operation, high yield and purity and suitability for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Improved method for preparing valsartan
The invention discloses an improved method for preparing valsartan. According to the invention, certain steps required for preparing the valsartan in certain patents in the past are improved. The improved method comprises the following steps of: adding (N-(1-valeryl)-N-[4-[2-(5-cyan)-phenyl]benzyl]-L-valine methyl ester valsartan, sodium azide, triethylamine hydrochloride and a solvent into a reactor, and directly performing diazotization reaction; adding a sodium hydroxide solvent for saponification reaction after the diazotization reaction; and purifying the obtained product to finally obtain the valsartan product with high purity. According to the method, the diazotization reaction time is short, the loss of energy is reduced, the reaction process is environment-friendly, the yield and the purity of the product are high, the industrial production cost is greatly reduced, and the industrial production is easily realized.
Owner:安徽现代天然生物有限公司
Preparation of Valsartan
InactiveCN101475540AAvoid synthetic stepsOperational securityOrganic chemistryBiphenyl derivativesValsartan
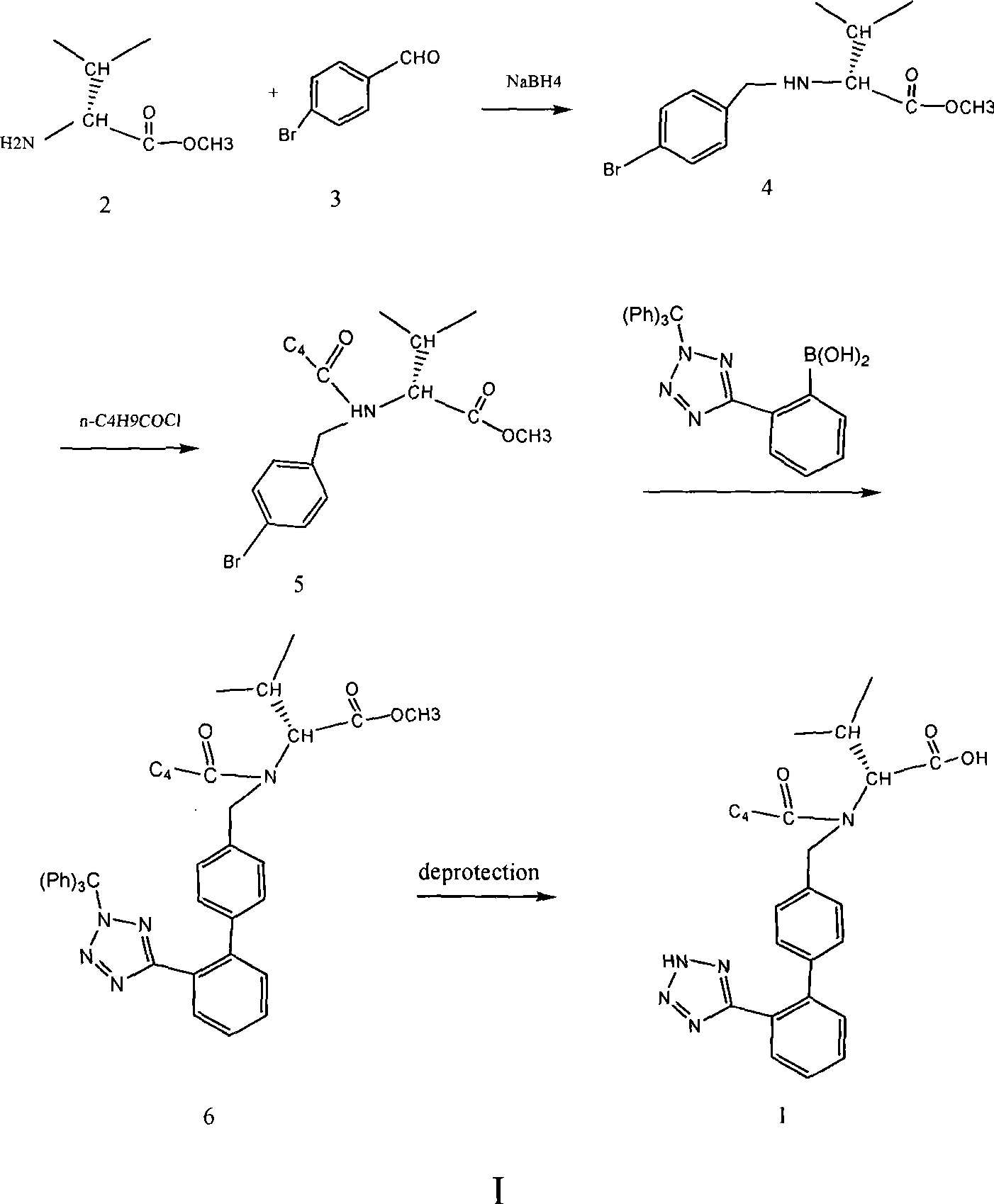
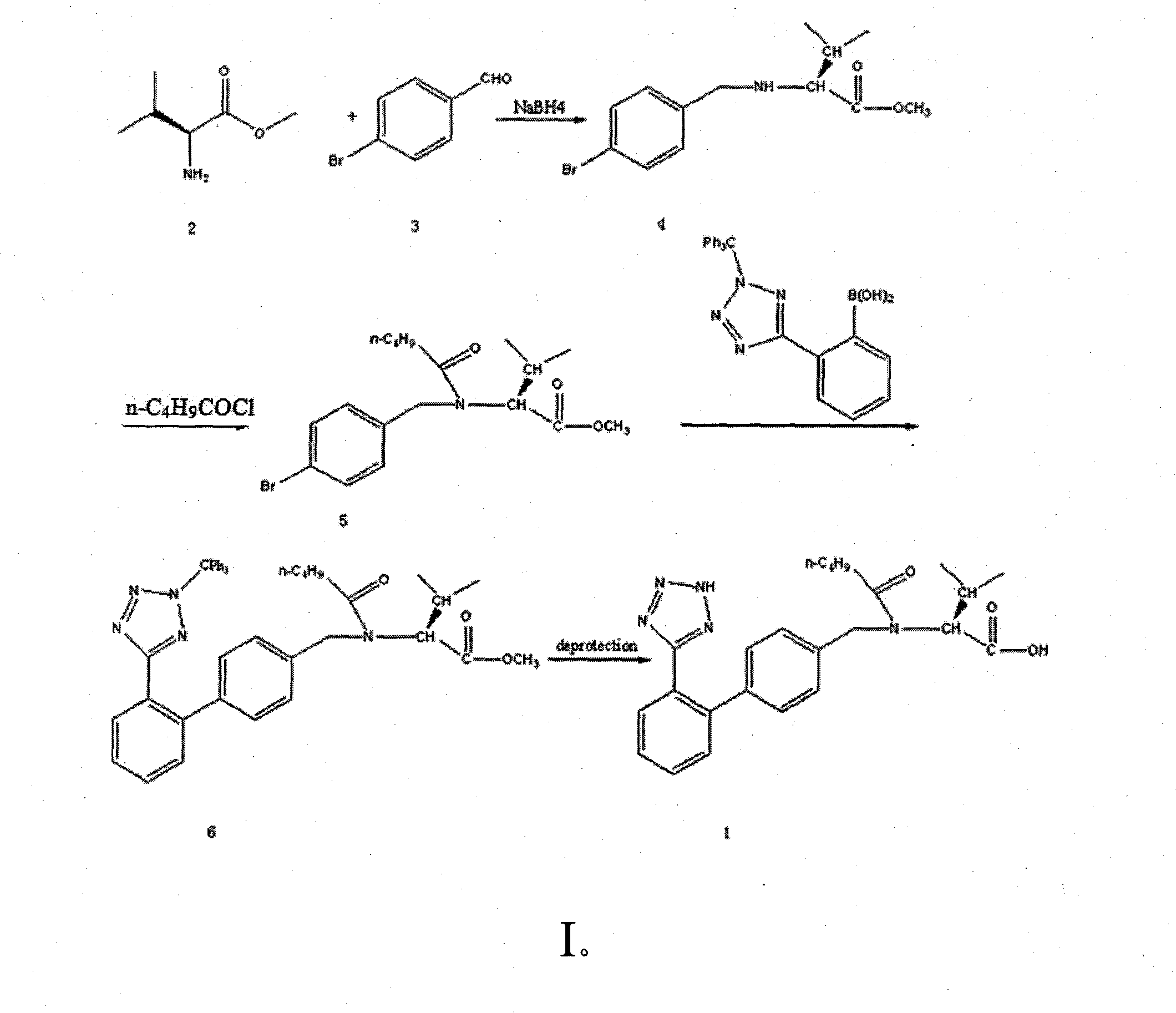
The invention discloses a novel method for preparing valsartan, which comprises the steps: taking L-valine methyl ester or L-valine methyl ester hydrochloride and p-bromobenzaldehyde as raw materials, carrying out reductive amination and acidylation reaction to obtain an intermediate, and performing SUZUKI reaction on the intermediate so as to obtain the valsartan. The method is safely operated, and avoids a step of tetrazole synthesis, and azide and other explosible hazardous compounds are not necessarily used; and the used raw materials can be easily obtained, and most of the raw materials are conventional chemical raw materials. In the method, biphenyl derivatives are not required for preparing the valsartan, and biphenyl products are obtained mainly through the suzuki reaction.
Owner:江苏德峰药业有限公司
Preparation method of LZ969
InactiveCN105001173ASimple processOptimizationOrganic compound preparationCarboxylic acid amides preparationSodium azideSolvent
The invention provides a preparation method of LZ969. According to the preparation method, LZ969 is used for representing a compound of valsartan and 4-(((2S, 4R)-1-(1, 1'-biphenyl-4-yl)-5-ethoxy-4-methyl-5-oxo pentane-2-yl) amino)-4-oxo butyric acid at a molar ratio of 1:1. A preparation method of valsartan comprises following steps: L-valine methyl ester hydrochloride is synthesized; nucleophilic substitution is carried out so as to obtain N-[2'-cyano-(biphenyl-4-yl)-methyl]-L-valine methyl ester hydrochloride; valeryl chloride is added, sodium carbonate is taken as an acid-binding agent, acetone is taken as a solvent, and N-[2'-cyano-(biphenyl-4-yl)-methyl]-N-valeryl is obtained via reaction; and N-[2'-cyano-(biphenyl-4-yl)-methyl]-N-valeryl, sodium azide, and chloro-substituted tributyltin are subjected to heating reaction by taking pyridine as a solvent so as to obtain valsartan. Technical route reaction of the preparation method is mild; operation is simple; and yield is high.
Owner:ZHEJIANG TIANSHUN BIOTECH
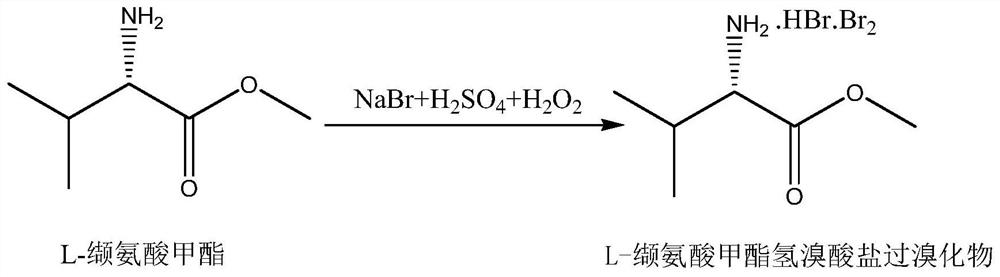
Preparation method of L-valine methyl ester hydrochloride
InactiveCN101898973AHigh purityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationFiltrationChloride
The invention discloses a preparation method of L-valine methyl ester hydrochloride, which comprises the following steps of: firstly, adding absolute methanol in a reactor and controlling the temperature between 8 DEG C below zero and 10 DEG C below zero, dripping thionyl phthalyl chloride by stirring, reacting for 0.8-1.5 hours at the temperature, adding L-valine under the cooling condition, raising the temperature to the ambient temperature and stirring for 2.5-3.5 hours, then heating and refluxing, and reacting for 7-9 hours at the temperature of 60-70 DEG C; and secondly, reducing pressure to distill after the reaction, cooling residues for crystallization, carrying out vacuum filtration, and recrystallizing with absolute methyl-ethyl ether to prepare L-valine methyl ester hydrochloride, wherein the reaction materials have the mole ratio that n (L-valine):n (SOC12):n (absolute methanol)=1.0:1-1.5:20-21. The preparation method of the invention is simple, has fewer steps and small economic investment, and only requires commonly-used devices, and the prepared L-valine methyl ester hydrochloride is high in purity and yield and can satisfy the use requirements.
Owner:张明宝
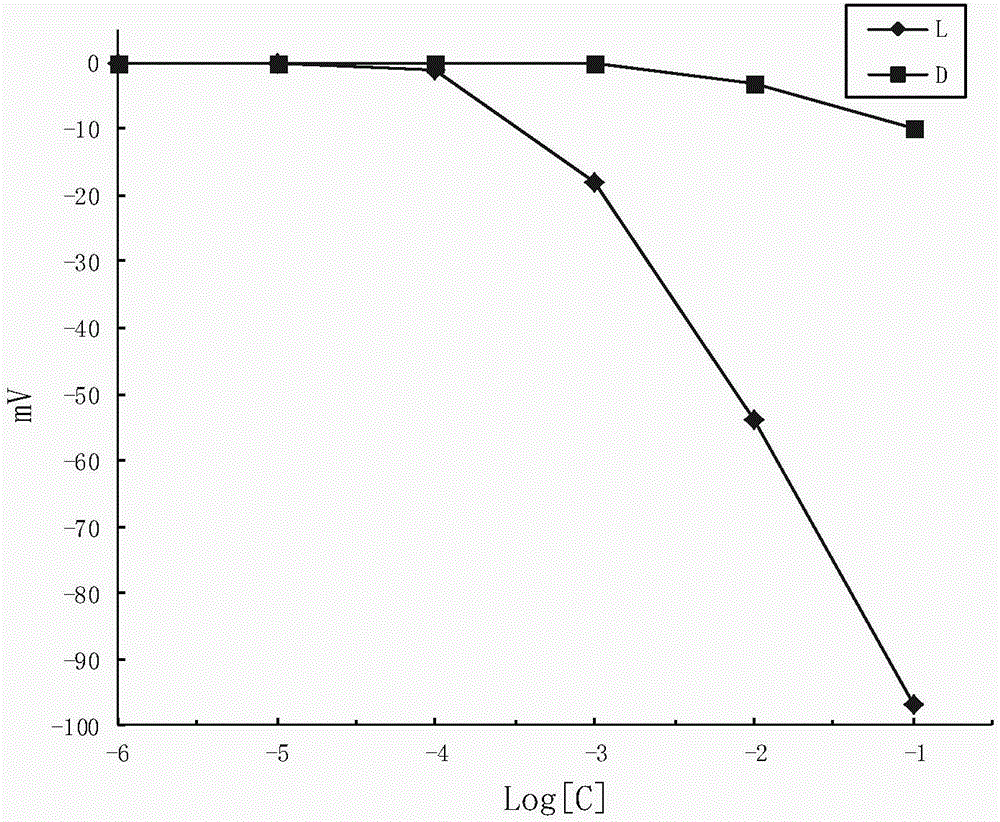
Sodium tetraphenylborate-based enantiomer potential sensor and application of enantiomer potential sensor in detection of valinemethyl ester
InactiveCN103743795AFast analysisLow costMaterial electrochemical variablesPlasticizerSodium tetraphenylborate
The invention discloses a sodium tetraphenylborate-based enantiomer potential sensor and an application of the enantiomer potential sensor in detection of valinemethyl ester. The sodium tetraphenylborate-based enantiomer potential sensor is characterized in that sodium tetraphenylborate, a plasticizer and PVC are dissolved in tetrahydrofuran to prepare a PVC membrane, a step of air drying is carried out, one sheet of the PVC membrane is sliced to paste on one end of a PVC pipe, a filling liquid is injected in the pipe, an Ag / AgCl internal reference electrode is inserted to obtain a PVC membrane electrode, and the membrane electrode forms the enantiomer potential sensor with a calomel electrode. The enantiomer potential sensor is used for detecting a leucinemethyl ester enantiomer. According to the invention, the linearity range is 10<-4>-10<-1> mol<-1>, the slope is -47mV dec<-1>, the LOD(limit of detection) is 3.311*10<-4> mol L<-1>, the electrode enables chiral detection on valinemethyl ester, and the antipodal selective coefficient 1g(img file='DDA0000439081610000011.TIF' wi='160' he='71' / ) is -2.36. The sodium tetraphenylborate-based enantiomer potential sensor and the application of the enantiomer potential sensor in detection of valinemethyl ester have the advantages of rapidity, economy, environmental protection, simple equipment and convenient operation, and can be used for on-site determination.
Owner:YUNNAN NORMAL UNIV
Method for synthesizing valsartan
The invention discloses a method for synthesizing valsartan. The method comprises the following steps of: performing coupling reaction of 4'-brooethyl-2-cyanobiphenyl and L-valine methyl ester or L-valine methyl ester hydrochloride to obtain an intermediate I, namely N-(2'-cyanobiphenyl-4-methylene)-L-valine methyl ester or hydrochloride thereof, performing acylation reaction of the intermediate I and valeroyl chloride to obtain an intermediate II, namely N-(2'-cyanobiphenyl-4-methylene)-N-n-valery-L-valine methyl ester, finally putting the intermediate II into a system of zinc chloride and sodium azide and performing azido reaction to obtain an intermediate III, namely valsartan methyl ester, and hydrolyzing the intermediate III under alkaline conditions to obtain a target product. In the method, the yield of the valsartan methyl ester which is taken as an example is over 75 percent; and compared with other prior arts with the total yield of 40 percent, the method has the advantages that raw materials consumed for each ton of product are greatly reduced.
Owner:HEFEI UNIV OF TECH
Synthesis method of valsartan
Owner:ZHEJIANG TIANYU PHARMA
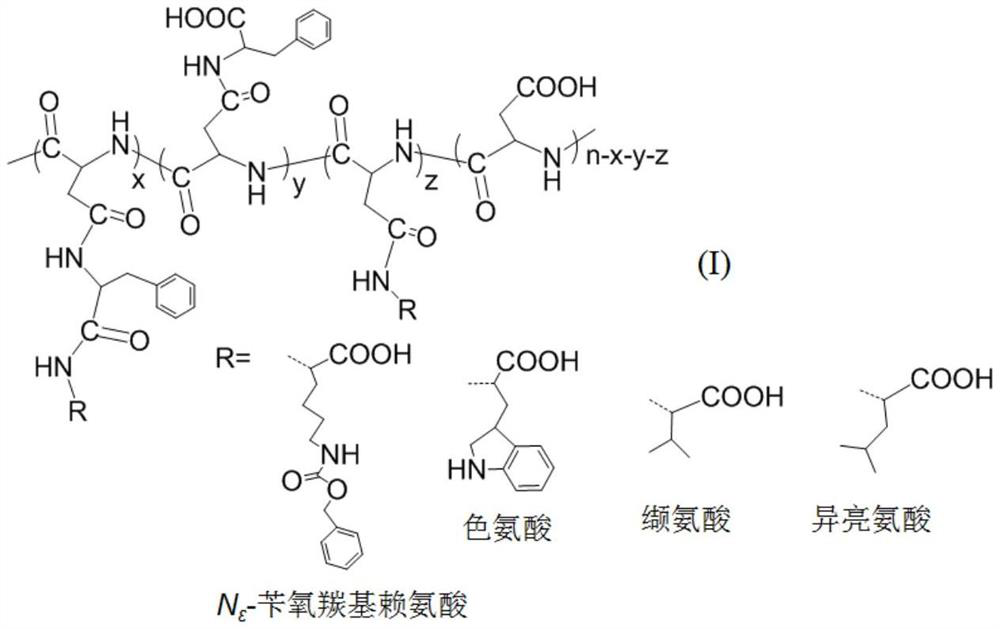
Betulinic acid-amino acid derivative, and preparation method and application thereof
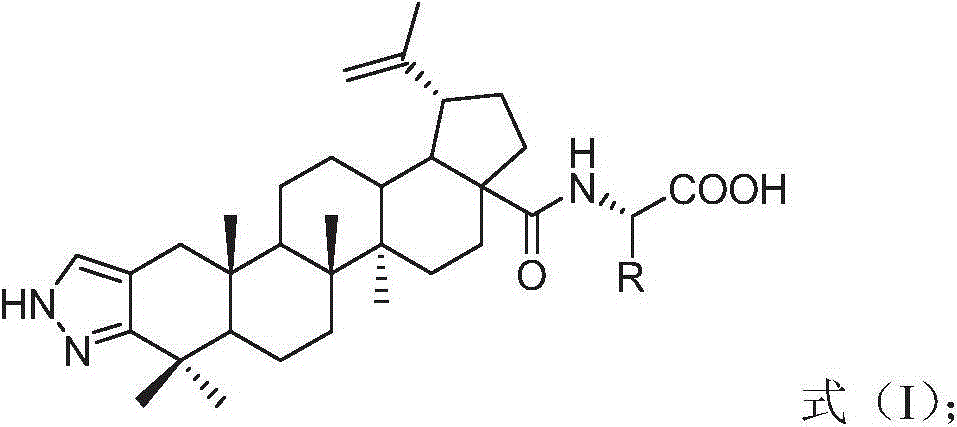
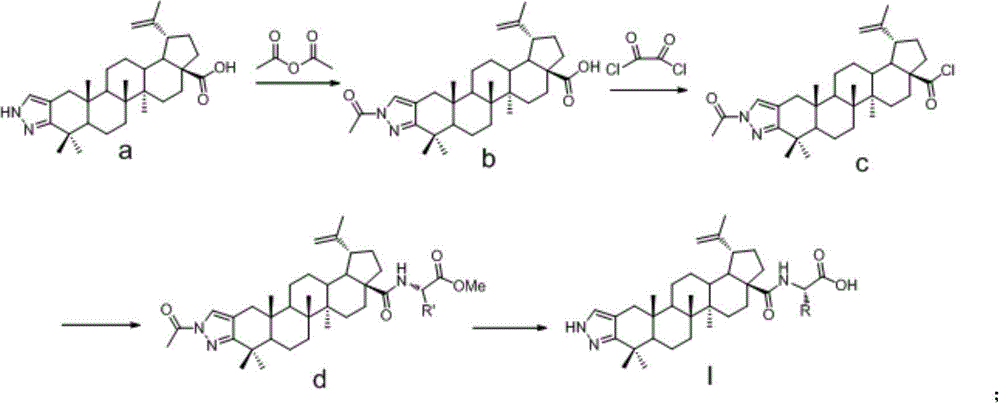
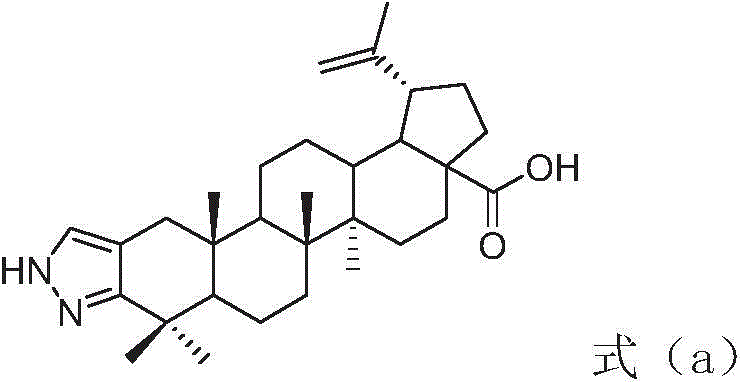
InactiveCN104974215AInhibitory activityHigh activitySkeletal disorderSteroidsAcetic anhydrideN-methyl-L-glutamate
The invention discloses a betulinic acid-amino acid derivative represented by a formula (I) and a preparation method thereof. The preparation method comprises the following steps of carrying out acylation reaction on a compound represented by a formula (a), acetic anhydride and pyridine under the protection of nitrogen so as to obtain a compound represented by a formula (b), reacting the compound of the formula (b) with oxalyl chloride so as to obtain a compound represented by a formula (c), then, respectively reacting the compound of the formula (c) with glycino methyl ester, L-alanine methyl ester, L-valine methyl ester, L-isoleucine methyl ester and L-glutamic acid methyl ester so as to obtain a compound represented by a formula (d), and washing, drying and purifying after reacting the compound of the formula (d) with LiOH so as to obtain the betulinic acid-amino acid derivative represented by the formula (I). The preparation method is high in synthetic efficiency and simple and convenient in process. The invention further discloses application of the betulinic acid-amino acid derivative represented by the formula (I) in preparation of a drug for treating osteoporosis; and the betulinic acid-amino acid derivative can be used as an osteoclast precursor differentiation inhibitor and has the obviously increased osteoclast precursor differentiation activity inhibition effect.
Owner:EAST CHINA NORMAL UNIV
Preparation method of valsartan intermediate
PendingCN111393327AMild reaction conditionsShort reaction timeOrganic compound preparationOrganic chemistry methodsValsartanOrganosolv
The invention belongs to the technical field of preparation of medical intermediates, and particularly relates to a preparation method of a valsartan intermediate. The method comprises the following steps: using L-valine and thionyl chloride as raw materials, in the presence of an organic solvent, carrying out esterification reaction to obtain L-valine methyl ester hydrochloride, then reacting theL-valine methyl ester hydrochloride, with 4-Bromomethyl-2-cyanobiphenyl under an alkaline condition, carrying out organic solvent extracting, crystallization, centrifugation and drying to obtain a crude product of N-[(2 '-cyano-1, 1'-biphenyl-4-yl) methyl]-L-valine methyl ester hydrochloride, and refining to obtain the product with main content of 99.5% or above and individual impurity content of0.1% or below. The method is mild in reaction condition, short in reaction time, simple and convenient to operate, simple in aftertreatment, high in yield, few in obtained product impurity, small inenvironmental pollution, low in cost and easy for industrial production.
Owner:河南豫辰药业股份有限公司
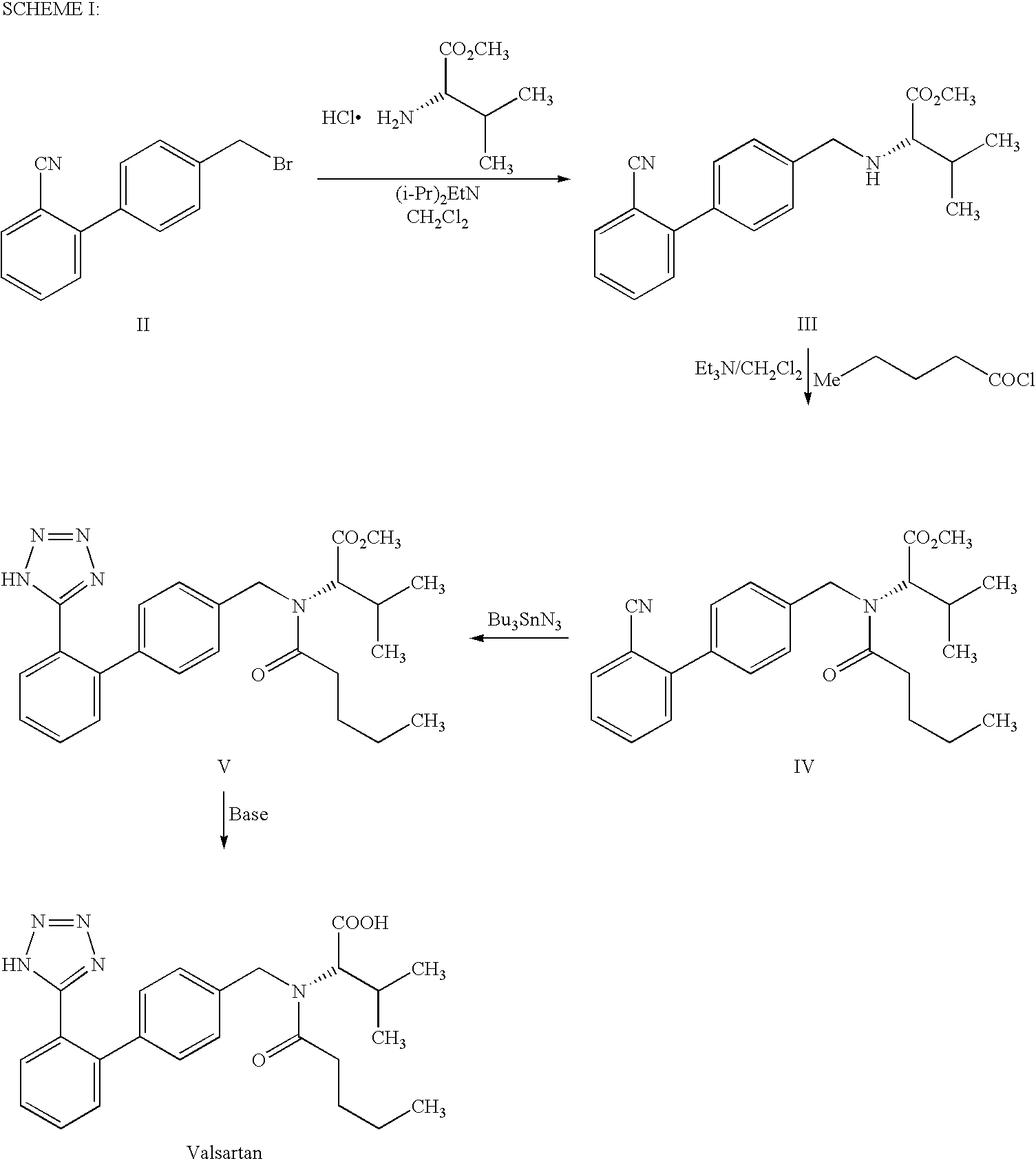
Process for the preparation of angiotensin II antagonist
InactiveUS7880015B2Simple and cost-effective processHigh purityOrganic chemistryOXALIC ACID DIHYDRATEOxalate
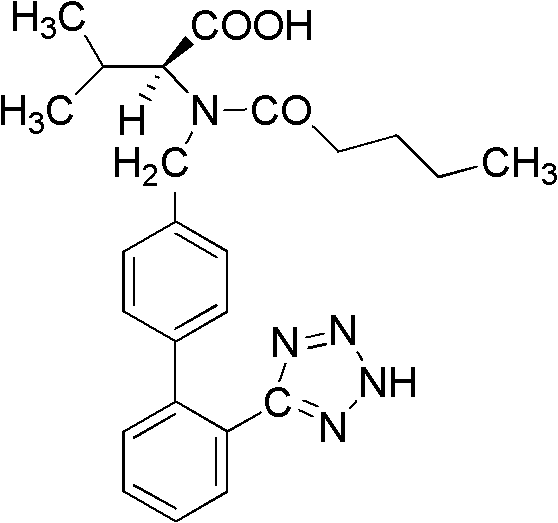
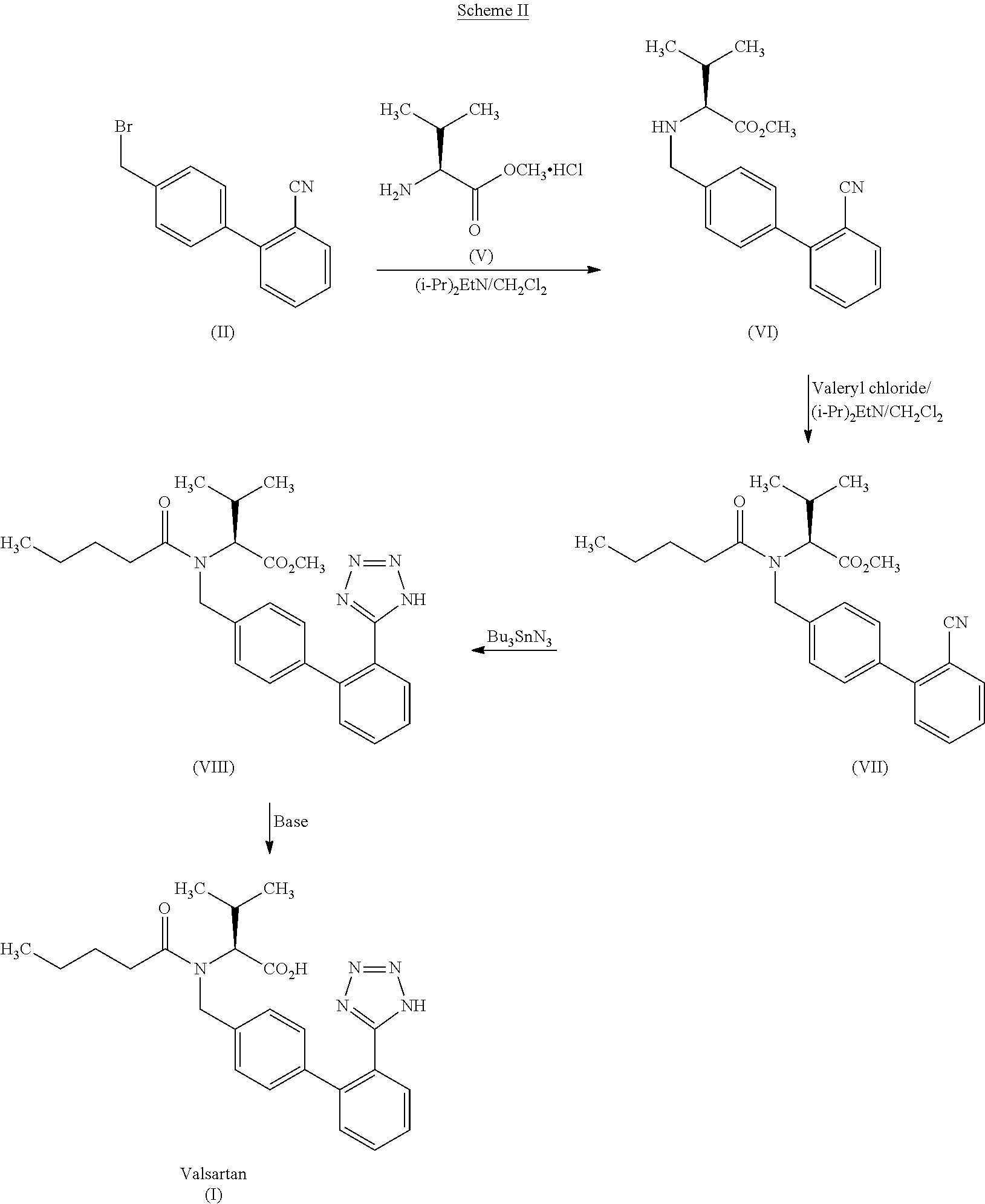
The present invention provides a method for the preparation of N-(1-oxopentyl)-N-[[2′-(1H-tetra-zol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine (Valsartan) which comprises; treating N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)biphenyl-4-yl]methyl]-L-valine methyl ester (X) with oxalic acid or its hydrates in a solvent to produce N-[[2′-(1-triph-enylmethyl-tetrazol-5-yl)biphenyl-4-yl]methy]-L-valine methyl ester oxalate (Xa) and treating the compound (Xa) with a base in a solvent followed by reacting with valeryl chloride in presence of base in a solvent to produce N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)[1,1′biphenyl]-4-yl]methyl]-N-valeryl-L-valine methyl ester (XI), de-protecting the compound (XI) using anhydrous acidic conditions to produce N-(1-oxopentyl)-N-[[2′-(1-H-tetrazol-5-yl)[1,1′biphenyl]-4-yl]methyl-L-valine methyl ester (V) followed by treating with base in a solvent to produce Valsartan.
Owner:AUROBINDO PHARMA LTD
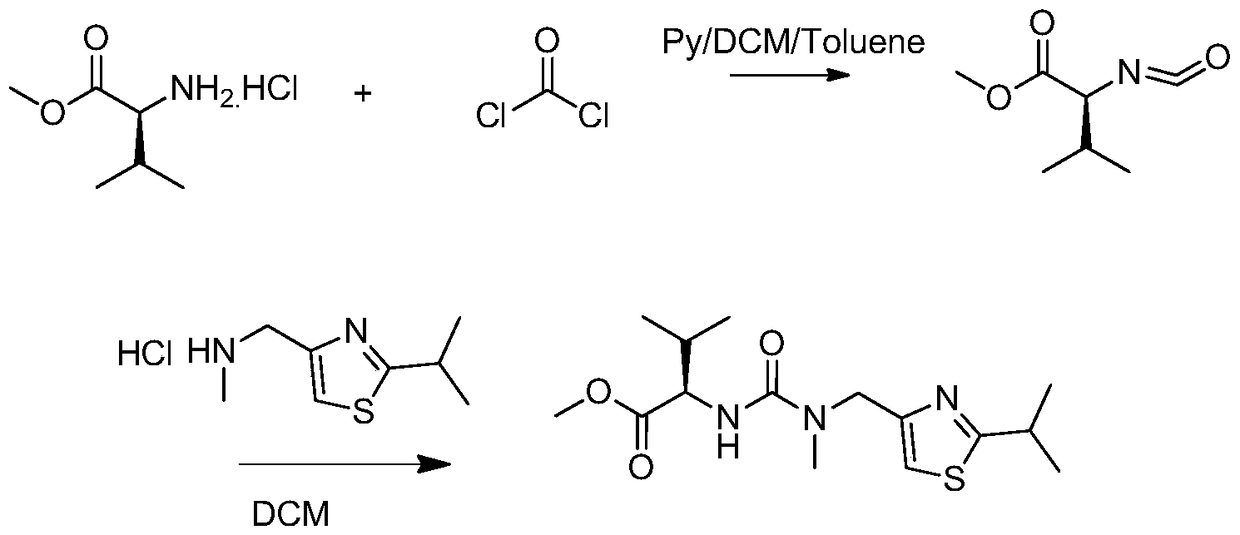
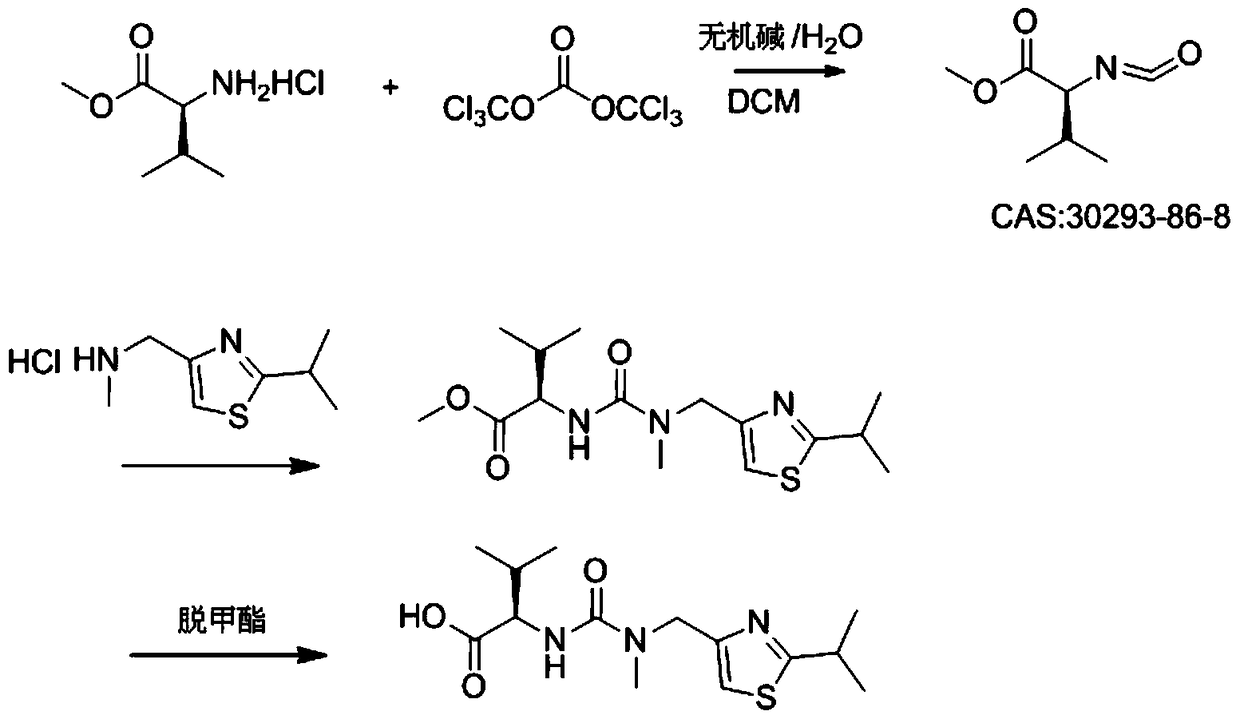
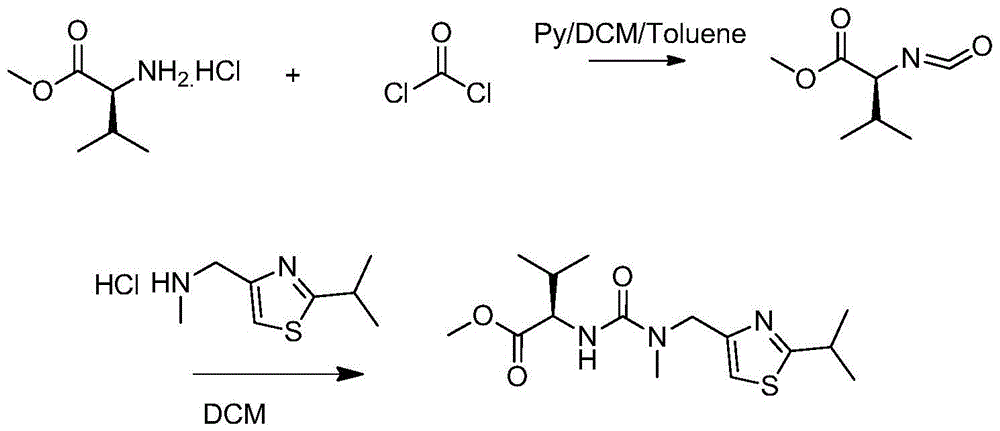
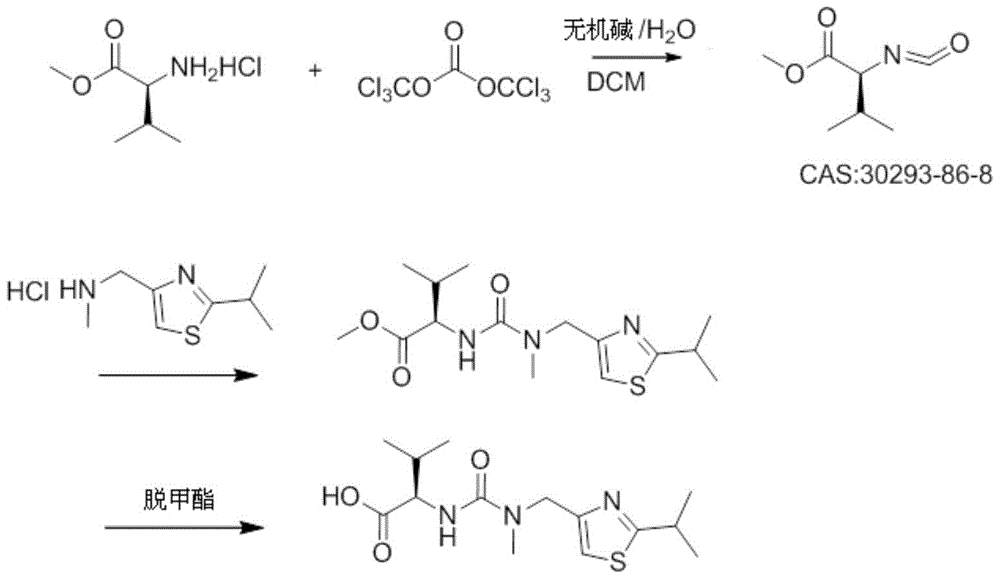
A kind of heterogeneous synthesis method and application of ritonavir intermediate
ActiveCN105753806BReduced Moisture RequirementsReduce manufacturing costOrganic chemistryThiazoleIsopropyl
The invention discloses a heterogeneous synthetic method and application of a Ritonavir intermediate. The heterogeneous synthetic method comprises the following steps: dissolving L-valine methyl ester hydrochloride and triphosgene in dichloromethane by utilizing a heterogeneous method; adding inorganic alkali liquor dropwise at a low temperature to generate isocyanate compounds; adding 2-isopropyl- 4-(methyl amino methyl) thiazole into a pot without separating to obtain N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)formyl)methyl-L-valine. According to the heterogeneous synthetic method, one-pot synthesis of the Ritonavir intermediate MTV methyl ester is realized; all products can be purified through pulping and crystallizing; the purity of the products is higher than 98 percent. The pollution in a production process is low; the heterogeneous synthetic method has low harm to an operator, and is beneficial to environment, easy to operate, and particularly suitable for large-scale industrial production.
Owner:厦门蔚嘉制药有限公司
Preparation of Valsartan
InactiveCN101475540BAvoid synthetic stepsOperational securityOrganic chemistryBiphenyl derivativesValsartan
The invention discloses a novel method for preparing valsartan, which comprises the steps: taking L-valine methyl ester or L-valine methyl ester hydrochloride and p-bromobenzaldehyde as raw materials, carrying out reductive amination and acidylation reaction to obtain an intermediate, and performing SUZUKI reaction on the intermediate so as to obtain the valsartan. The method is safely operated, and avoids a step of tetrazole synthesis, and azide and other explosible hazardous compounds are not necessarily used; and the used raw materials can be easily obtained, and most of the raw materials are conventional chemical raw materials. In the method, biphenyl derivatives are not required for preparing the valsartan, and biphenyl products are obtained mainly through the suzuki reaction.
Owner:江苏德峰药业有限公司
Method for preparing valsartan
InactiveCN104292174AAvoid racemizationGuaranteed optical purityOrganic chemistrySodium methoxideValsartan
The invention provides a method for preparing valsartan. The method comprises the following steps: firstly, reacting N-(triphenylmethyl)-5-(4'-formyl-biphenyl-2-yl) tetrazolium with sodium methoxide, secondly, adding dichlorodicyanobenzoquinone (DDQ) and oxidizing, thirdly, reacting with L-valine methyl ester, fourthly, alkylating with valeryl chloride, fifthly, removing triphenylmethyl group and sixthly removing ester group and acidifying to obtain the target product valsartan. According to the method, a novel method for preparing valsartan is provided by virtue of the Williamson ether synthesis step and DDQ oxidation step, since potassium dihydrogen phosphate is adopted as a buffering reagent in the triphenylmethyl group removal process and a 1.3mol / L sodium hydroxide solution is used in the hydrolysis process, the problem of racemization of the product generated caused by too strong alkaline is avoided and the method is of important significance in maintaining the optical purity of the valsartan product.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of 2-bromothiophene
ActiveCN111763194AHigh boiling pointReduce generationOrganic chemistryCombinatorial chemistryChemical products
The invention discloses a preparation method of 2-bromothiophene, relates to a method for improving thiophene 2-site substitution selectivity and reducing the generation amount of 3-bromothiophene, and belongs to the technical field of preparation of fine chemical products such as medicines. The method is characterized in that thiophene is converted into 2-bromothiophene in the presence of L-valine methyl ester. The preparation method comprises the steps of adding sodium bromide, L-valine methyl ester and water, adding sulfuric acid, adding thiophene, and adding hydrogen peroxide. The reactionformula is shown in the attached drawing. The method has the advantages of being high in thiophene 2-sit substitution selectivity, small in 3-bromothiophene generation amount, environmentally friendly and low in cost.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
Heterogeneous synthetic method and application of Ritonavir intermediate
ActiveCN105753806AReduced Moisture RequirementsReduce manufacturing costOrganic chemistryThiazoleMethyl group
The invention discloses a heterogeneous synthetic method and application of a Ritonavir intermediate. The heterogeneous synthetic method comprises the following steps: dissolving L-valine methyl ester hydrochloride and triphosgene in dichloromethane by utilizing a heterogeneous method; adding inorganic alkali liquor dropwise at a low temperature to generate isocyanate compounds; adding 2-isopropyl- 4-(methyl amino methyl) thiazole into a pot without separating to obtain N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)formyl)methyl-L-valine. According to the heterogeneous synthetic method, one-pot synthesis of the Ritonavir intermediate MTV methyl ester is realized; all products can be purified through pulping and crystallizing; the purity of the products is higher than 98 percent. The pollution in a production process is low; the heterogeneous synthetic method has low harm to an operator, and is beneficial to environment, easy to operate, and particularly suitable for large-scale industrial production.
Owner:厦门蔚嘉制药有限公司
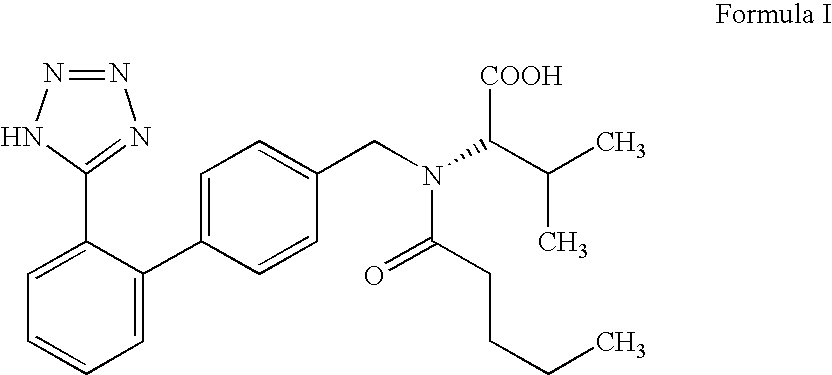
Valsartan impurity and preparation method thereof
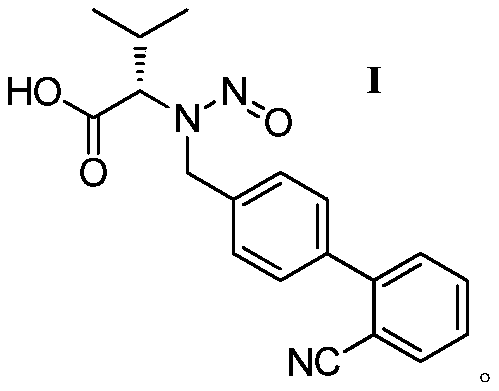
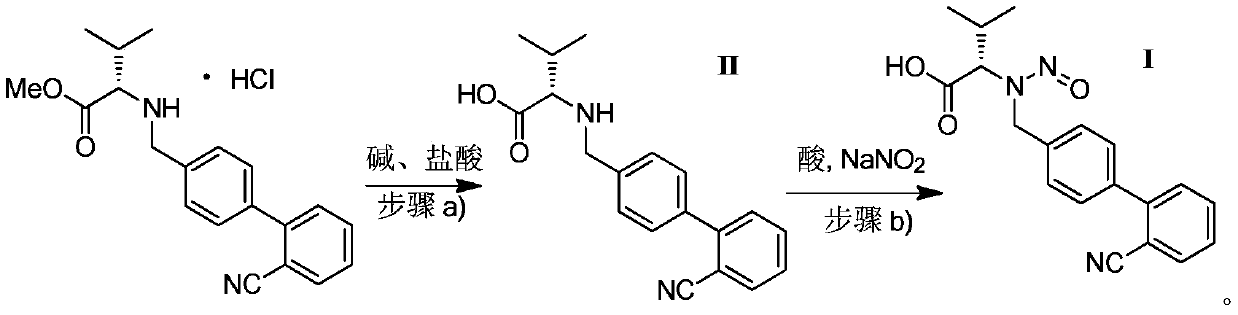
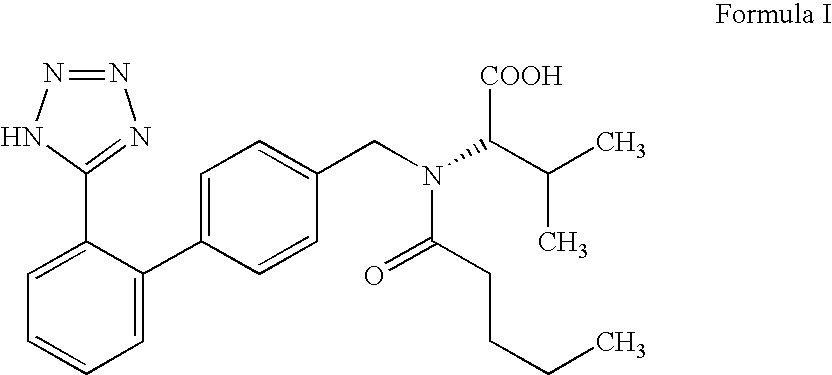
The invention discloses a valsartan impurity and a preparation method of the valsartan impurity. The valsartan impurity has a structure shown as formula I as shown in the specification. The preparation method of the valsartan impurity comprises the following steps: a) allowing a compound, N-((2'-cyan-[1,1'-biphenyl]-4) methyl)-L-valine methyl ester hydrochloride as a starting material and alkali to give an esterification reaction in a solvent and then performing acidification to prepare a compound shown as formula II as shown in the specification, and b) allowing the compound shown as the formula II and sodium nitrite in an organic solvent to be subjected to nitrosation under an acidic condition to form the valsartan impurity shown as the formula I. The preparation method is mild in reaction condition, simple in technology, short in reaction time and good in yield; the high-purity valsartan impurity shown as the formula I is obtained; a reliable impurity control is provided for qualitycontrol research of a valsartan product; and the valsartan impurity has very important significance.
Owner:ZHEJIANG HUAHAI ZHICHENG PHARMA CO LTD +2
Process for the preparation of valsartan
InactiveUS20130144067A1Simple and cost-effective processHigh purityOrganic chemistryValsartanChloride
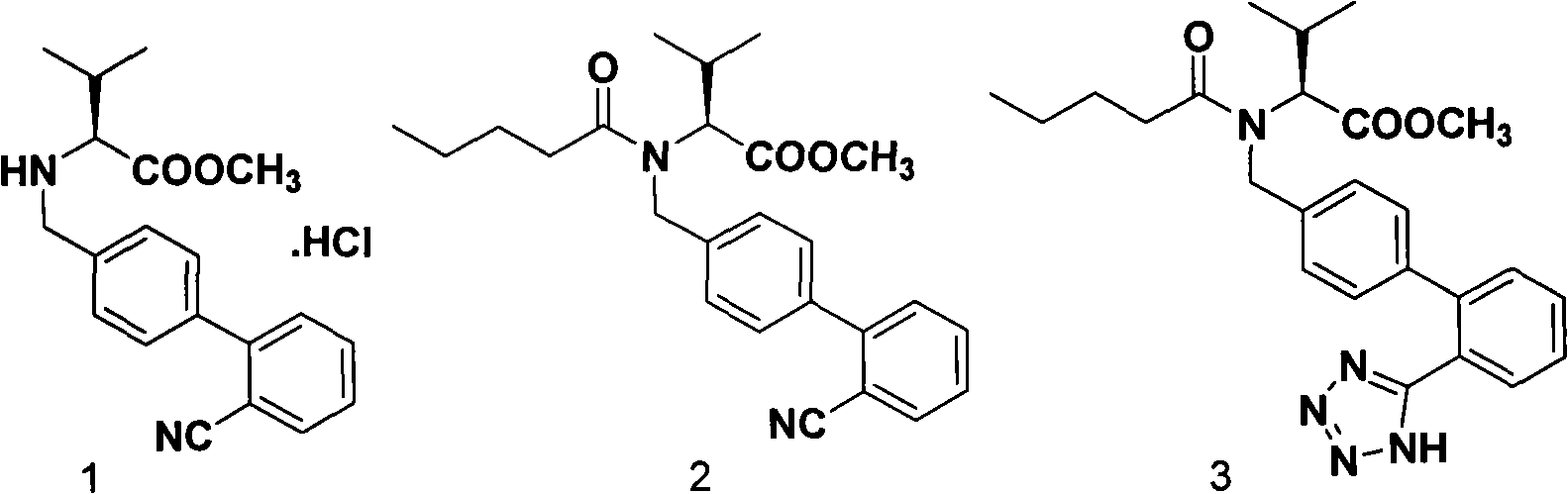
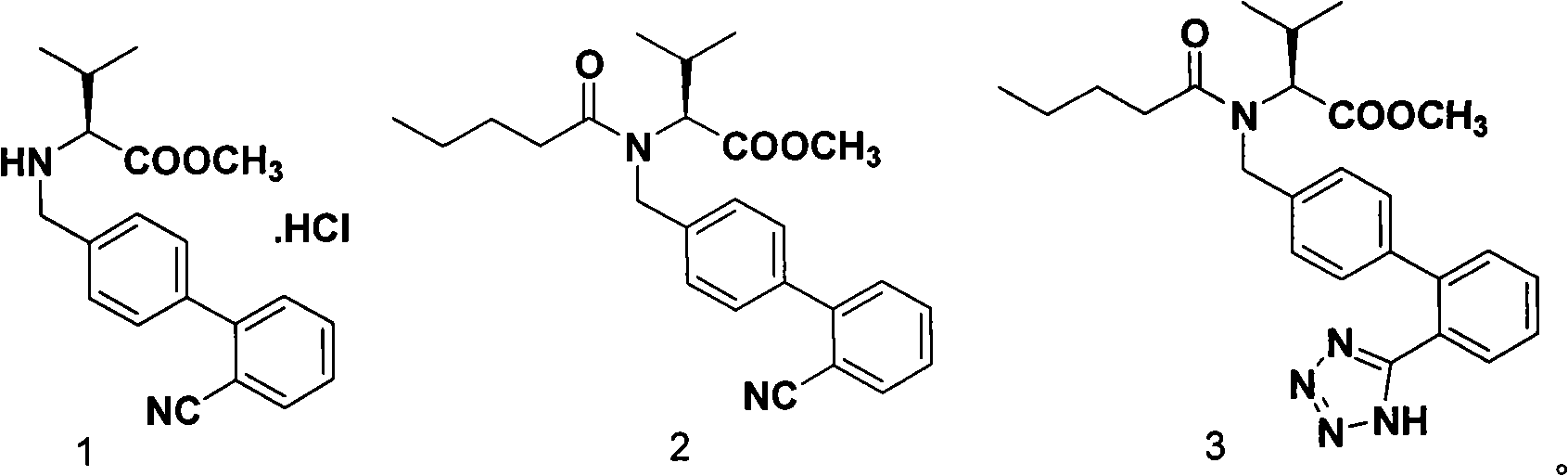
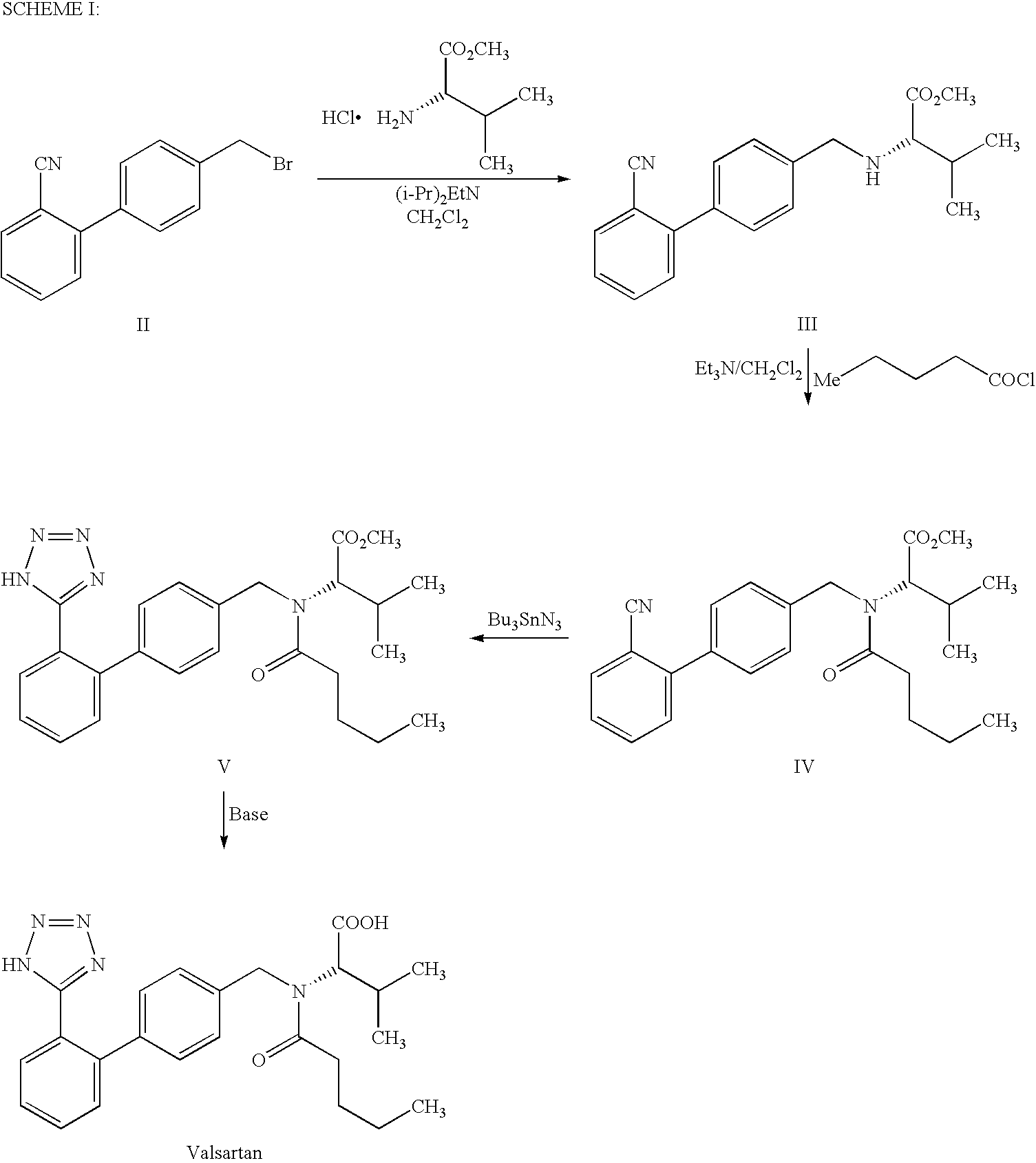
The present invention relates to a process for the preparation of pure Valsartan (I) substantially free from impurities of formulae (Ia), (Ib), and (Ic), which comprises: (i) condensing 2-(4′-bromomethylphenyl)benzonitrile of formula (II) with L-valine methyl ester hydrochloride of formula (V) in the presence of a base in a solvent to produce N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester of formula (VI); (ii) treating the compound VI of step (i) with acid followed by treating with base to produce pure compound VI substantially free from dimeric impurity of formula (Via); (iii) reacting the pure compound of formula (VI) with n-valeryl chloride in the presence of a base to produce pure N-valeryl-N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester (VII) substantially free from alkene impurity of formula (Vila); (iv) reacting the compound of formula (VII) with trialkyltin chloride and a metal azide in a solvent at a reflux temperature to produce N-(1-oxopentyl)-N-[[2′-(2-tributyltinte-trazol-5-yl)-(1,1′-biphenyl)-4-yl]methyl]-(L)-valine methyl ester of formula (VHIb) free from thermal degradation impurity (Villa); (v) hydrolyzing the compound of formula (VHIb) in the presence of alkaline conditions to produce Valsartan (I).
Owner:AUROBINDO PHARMA LTD
Process For the Preparation of Angiotensin II Antagonist
InactiveUS20090281326A1Simple and cost-effective processHigh purityOrganic chemistryOxalateValsartan
The present invention provides a method for the preparation of N-(1-oxopentyl)-N-[[2′-(1H-tetra-zol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine (Valsartan) which comprises; treating N-[[2′-(1-triphenylmethyl-tetra-zol-5-yl)biphenyl-4-yl]methyl]-L-valine methyl ester (X) with oxalic acid or its hydrates in a solvent to produce N-[[2′-(1-triph-enylmethyl-tetrazol-5-yl)biphenyl-4-yl]methy]-L-valine methyl ester oxalate (Xa) and treating the compound (Xa) with a base in a solvent followed by reacting with valeryl chloride in presence of base in a solvent to produce N-[[2′-(1-triphenylmethyl-tetra-=zol-5-yl)[1,1′biphenyl]-4-yl]methyl]-N-valeryl-L-valine methyl ester (XI), de-protecting the compound (XI) using anhydrous acidic conditions to produce N-(1-oxopentyl)-N-[[2′-(1-H-tetrazol-5-yl)[1,1′biphenyl]-4-yl]methyl-L-valine methyl ester (V) followed by <treating with base in a solvent to produce Valsartan.
Owner:AUROBINDO PHARMA LTD
Synthesis process of valsartan
ActiveCN113717118AEfficient synthesis processHigh purityOrganic chemistrySolid sorbent liquid separationValsartanTetrazole
The invention relates to a synthesis process of valsartan, belonging to the technical field of medicine synthesis. The synthesis process comprises the following steps: mixing DMF, L-valine methyl ester hydrochloride and N,N-diisopropylethylamine, carrying out magnetic stirring at 5 DEG C, dropwise adding n-valeryl chloride, and conducting stirring at a room temperature for a reaction for 1.5 hours after dropwise adding to obtain an intermediate product a; producing an intermediate product b from the intermediate product a and N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazole under the action of a hydrogen pulling agent and a condensing agent; and subjecting the intermediate product b to protection group removal and ester group hydrolysis to obtain a crude valsartan product, and finally, carrying out chiral separation to obtain the valsartan target product with a high S-enantiomer content. According to the invention, an existing raw material is used as a substrate, so generation of impurities is reduced, a synthesis route is shortened, product purity is improved, and production efficiency is improved; and moreover, reaction conditions are mild, and the process is an efficient valsartan synthesis process.
Owner:安徽美诺华药物化学有限公司
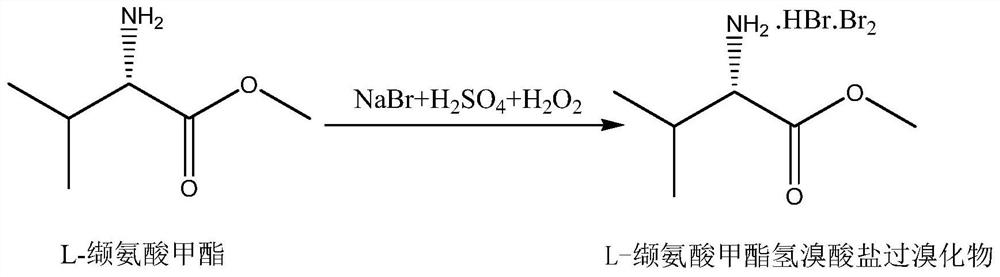
Sodium bromide application experiment method
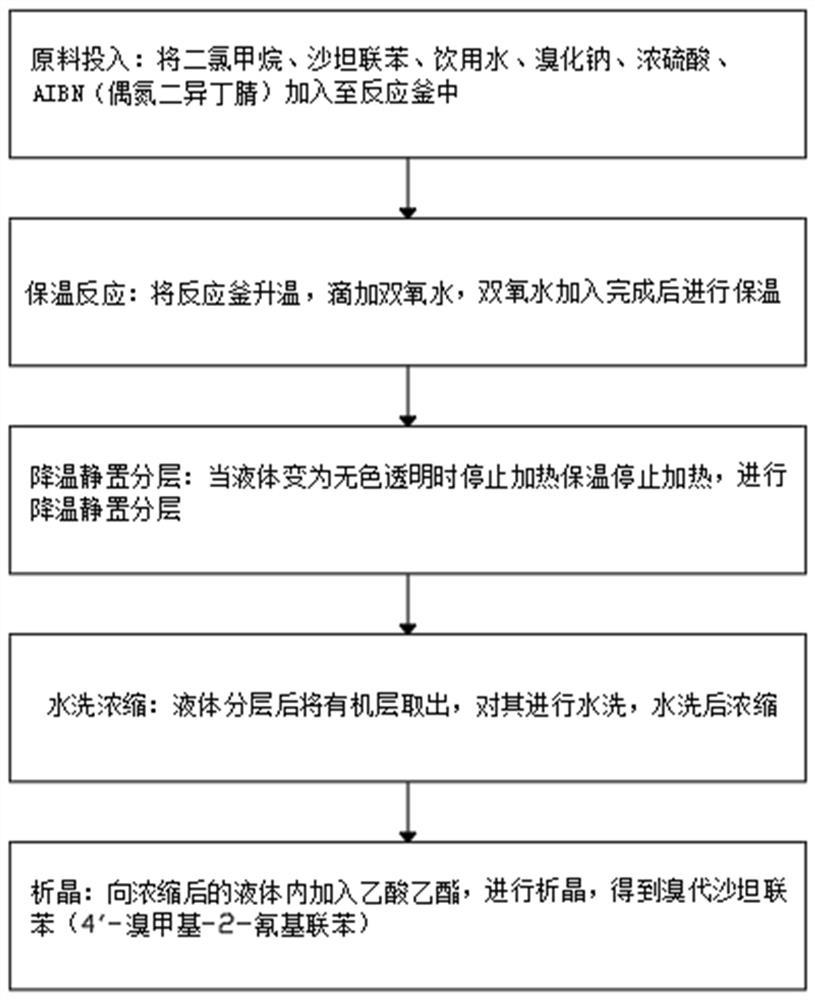
PendingCN112851549ARealize inner loopLow costOrganic compound preparationCarboxylic acid nitrile purification/separationValsartanEthyl acetate
The invention belongs to the technical field of valsartan intermediate synthetic product treatment, and particularly relates to a sodium bromide application experimental method. The method comprises the following steps: adding raw materials into a reaction kettle according to the weight ratio of sartan biphenyl, AIBN (azodiisobutyronitrile), sodium bromide, concentrated sulfuric acid, dichloromethane, drinking water and hydrogen peroxide, heating the reaction kettle, and dropwise adding hydrogen peroxide; after hydrogen peroxide is added, carrying out the heat preservation; when liquid becomes colorless and transparent, stopping heating, and carrying out the heat preservation; carrying out cooling, standing and layering; after liquid layering, taking out an organic layer and washing the organic layer; performing concentration after water washing, adding ethyl acetate into concentrated liquid, carrying out the crystallization, obtaining bromo-sartan biphenyl (4'-bromomethyl-2-cyanobiphenyl), and achieving the recycling of the bromine element, wherein the bromo-sartan biphenyl (4'-bromomethyl-2-cyanobiphenyl) obtained after recycling can be continuously used for valsartan intermediate N-[(2'-cyanobiphenyl-4-yl) methyl]-L-valine methyl ester hydrochloride, and therefore internal circulation of bromine is achieved, the cost is reduced, and environment-friendly emission is reduced.
Owner:安徽云帆药业有限公司
Synthetic method for sartan drug intermediate and application of intermediate
Owner:苏州天绿生物制药有限公司 +1
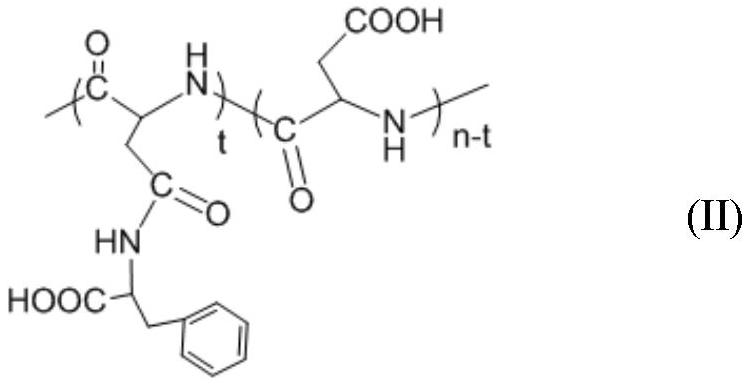
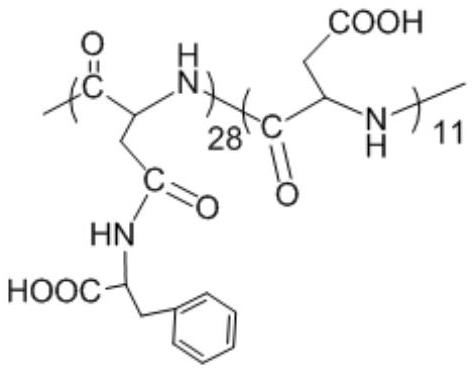
Ph-responsive polyaspartic acid grafted with hydrophobic amino acid and preparation method thereof
ActiveCN109369913BSimple and fast operationLow costPharmaceutical non-active ingredientsEthyl groupPhenylalanine
The invention relates to a pH-responsive polyaspartic acid grafted with hydrophobic amino acids and a preparation method thereof, using polyaspartic acid and L-phenylalanine methyl ester hydrochloride as raw materials, through 1-(3- Dimethylaminopropyl)-3-ethyl carbodiimide coupling reaction and alkali washing, obtain polyaspartic acid-grafting-phenylalanine; use polyaspartic acid-grafting-phenylalanine Amino acid and hydrophobic amino acid hydrochloride are used as raw materials, and the product is obtained through coupling reaction and alkali washing. Hydrophobic amino acid hydrochloride including N ε ‑Benzyloxycarbonyllysine benzyl ester hydrochloride, tryptophan methyl ester hydrochloride, valine methyl ester hydrochloride and isoleucine methyl ester hydrochloride; The amino acid contains a pH-responsive anionic carboxyl group and has good biocompatibility. When the polymer concentration is less than 0.1 mg / mL, the survival rate of smooth muscle cells is above 80%. The operation is simple and the cost is low. It is suitable for the field of biomedical drug carrier materials.
Owner:TIANJIN UNIV
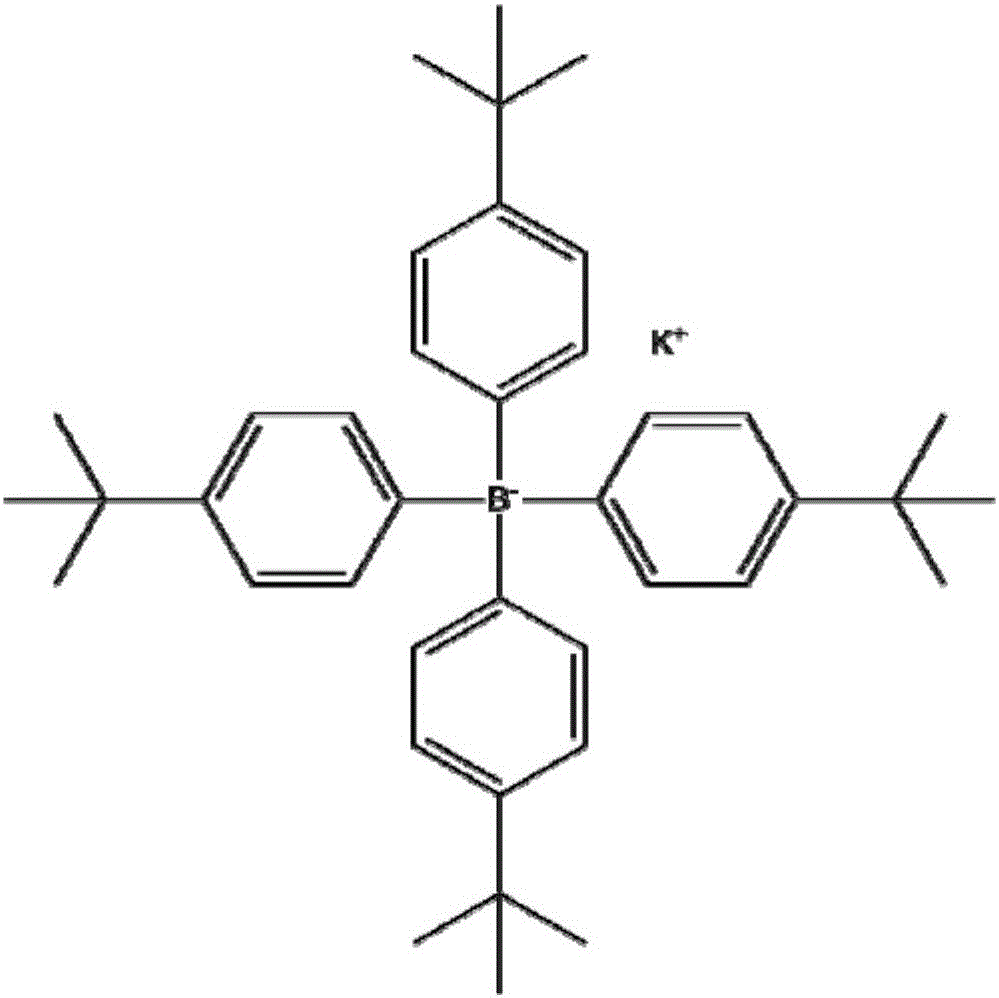
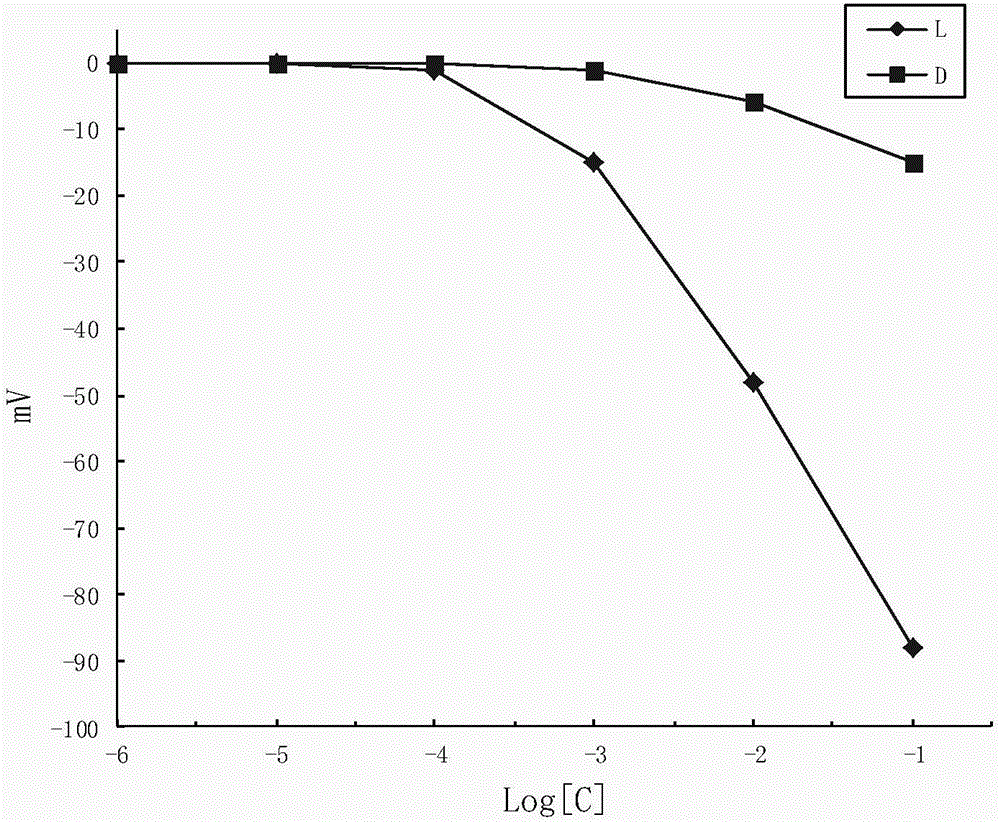
Potassium tetrakis(4-tert-butylphenyl)borate-based enantiopotential sensor and its application in the detection of valine methyl ester
InactiveCN103743784BFast analysisLow costMaterial analysis by electric/magnetic meansEnantiomerPotassium
The invention discloses an enantiomer potential sensor based on potassium tetrakis(4-t-butylphenyl)borate and application of the enantiomer potential sensor to methyl valinate detection. The enantiomer potential sensor is made by taking potassium tetrakis(4-t-butylphenyl)borate, a plasticizer and PVC, dissolving in tetrahydrofuran so as to prepare a PVC membrane, drying in air, cutting and getting a sheet and adhering to one end of a PVC pipe perfused with an internal perfusion fluid, inserting Ag / AgCl internal reference electrode to obtain a PVC membrane electrode, and combining with a calomel electrode for forming the enantiomer potential sensor. The enantiomer potential sensor is applied to detect enantiomers of methyl valinate. The linearity range is 10<-4>-10<-1> mol*L<-1>, the gradient is -39.5 mVdec<-1>, the detection limit is 3.548*10<-4> mold*L<-1>, the electrodes can help to perform chiral detection on methyl valinate, and the enantioselectivity coefficient is -2.20, wherein the calculation expression of the enantioselectivity coefficient is shown in the specification. The enantiomer potential sensor provided by the invention has the advantages of being rapid, economic, environment-friendly, simple in equipment, convenient to operate and applicable to on-site detection.
Owner:YUNNAN NORMAL UNIV
A kind of preparation method of 2-bromothiophene
ActiveCN111763194BHigh boiling pointReduce generationOrganic chemistryChemical productsSodium bromide
A preparation method of 2-bromothiophene relates to a method for improving the 2-position substitution selectivity of thiophene and reducing the production amount of 3-bromothiophene, and belongs to the technical field of preparation of fine chemical products such as medicine. It is characterized in that thiophene is converted into 2-bromothiophene in the presence of L-valine methyl ester. It comprises the following steps: adding sodium bromide and L-valine methyl ester and water, adding sulfuric acid, adding thiophene, and adding hydrogen peroxide. The reaction formula is shown in the accompanying drawing. The invention has the advantages of high thiophene 2-position substitution selectivity, less 3-bromothiophene generation, environmental friendliness and low cost.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
A kind of synthetic method of valsartan intermediate
ActiveCN110078640BReduce dosageReduce pollutionOrganic compound preparationCarboxylic acid nitrile purification/separationValsartanOrganic solvent
The present invention relates to a kind of synthetic method of valsartan intermediate, comprises the following steps: (1) first dissolving following formula II compound in organic solvent, adding acid-binding agent, sodium chloride or potassium chloride and water, stirring evenly , after adding n-valeryl chloride dropwise, carry out insulation reaction; (2) add water to quench after completion of the reaction, carry out alkali washing, pickling, and alkali washing successively, and finally obtain the following formula I compound of the target product by distillation under reduced pressure, i.e. N-[( 2'-cyanobiphenyl-4-yl)methyl]-N-(1-oxopentyl)-L-valine methyl ester. In the present invention, by adding an appropriate amount of sodium chloride or potassium chloride to the reaction system, the occurrence of the hydrolysis reaction of n-valeryl chloride is suppressed by using the same ion effect, thereby reducing the amount of n-valeryl chloride, which is beneficial to improving the purity and yield of the product , reduces production cost, reduces environmental pollution, and is more suitable for industrial production.
Owner:ZHEJIANG MENOVO PHARMA
Process for the preparation of valsartan
InactiveUS8981109B2Simple and cost-effective processHigh purityOrganic chemistryValsartanPhenyl group
The present invention relates to a process for the preparation of pure Valsartan (I) substantially free from impurities of formulae (Ia), (Ib), and (Ic), which comprises: (i) condensing 2-(4′-bromomethylphenyl)benzonitrile of formula (II) with L-valine methyl ester hydrochloride of formula (V) in the presence of a base in a solvent to produce N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester of formula (VI); (ii) treating the compound VI of step (i) with acid followed by treating with base to produce pure compound VI substantially free from dimeric impurity of formula (Via); (iii) reacting the pure compound of formula (VI) with n-valeryl chloride in the presence of a base to produce pure N-valeryl-N-[(2′-cyanobiphenyl-4-yl)methyl]-(L)-valine methyl ester (VII) substantially free from alkene impurity of formula (Vila); (iv) reacting the compound of formula (VII) with trialkyltin chloride and a metal azide in a solvent at a reflux temperature to produce N-(1-oxopentyl)-N-[[2′-(2-tributyltintetrazol-5-yl)-(1,1′-biphenyl)-4-yl]methyl]-(L)-valine methyl ester of formula (VHIb) free from thermal degradation impurity (Villa); (v) hydrolyzing the compound of formula (VHIb) in the presence of alkaline conditions to produce Valsartan (I).
Owner:AUROBINDO PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com