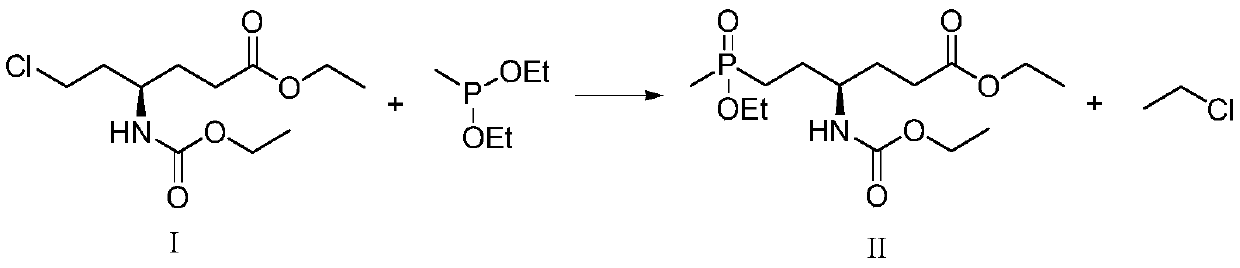
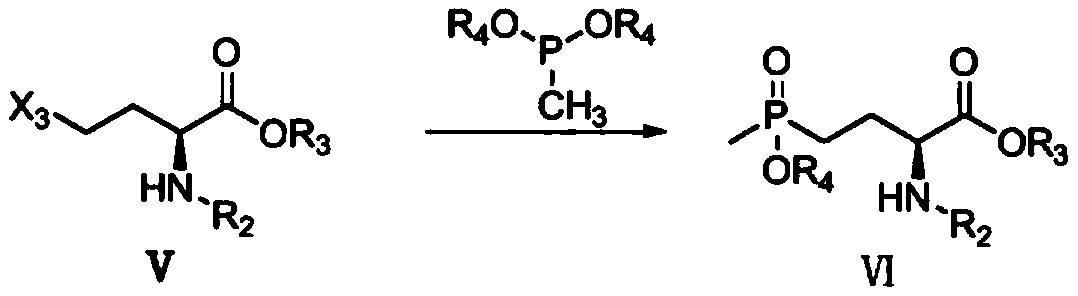
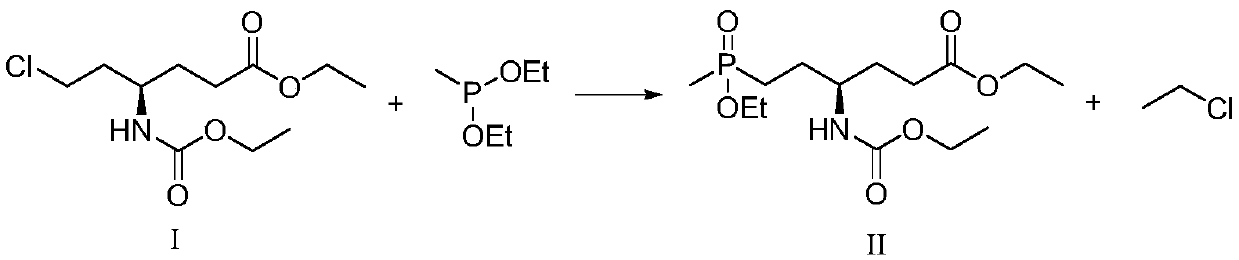
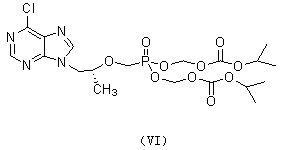
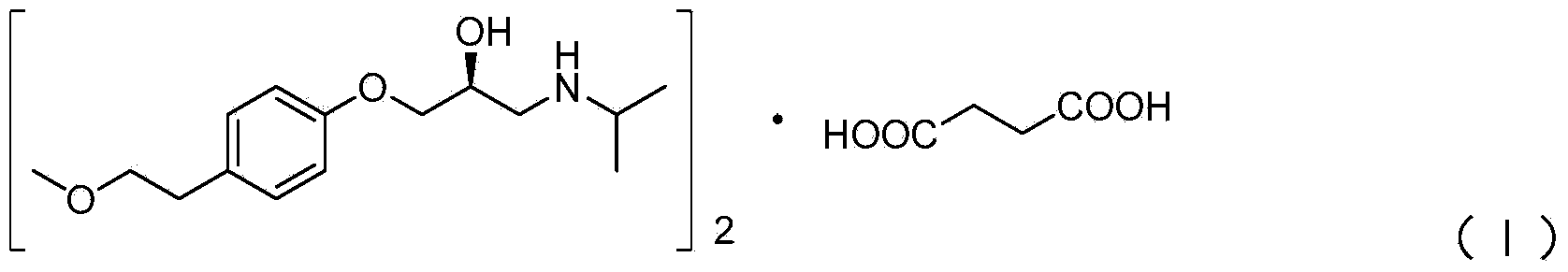Patents
Literature
121results about How to "Avoid racemization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
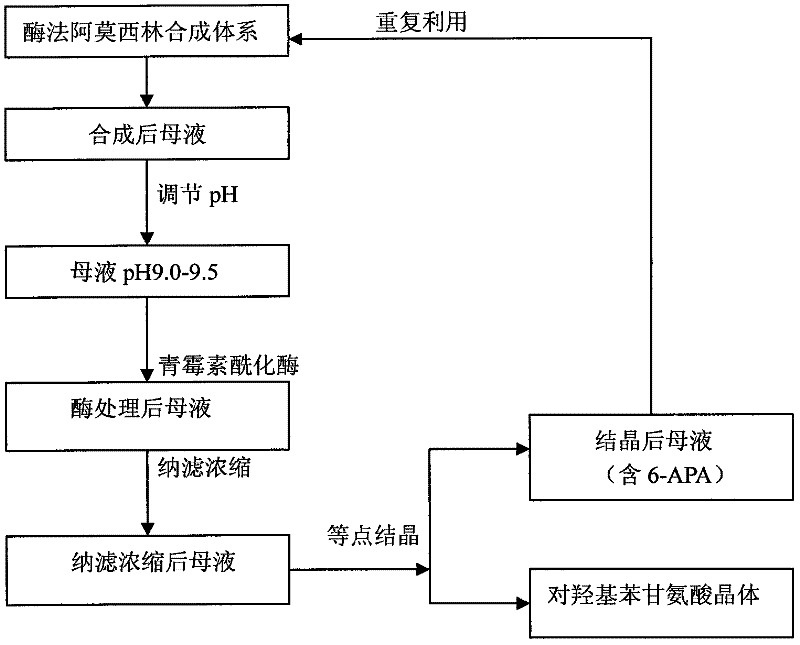
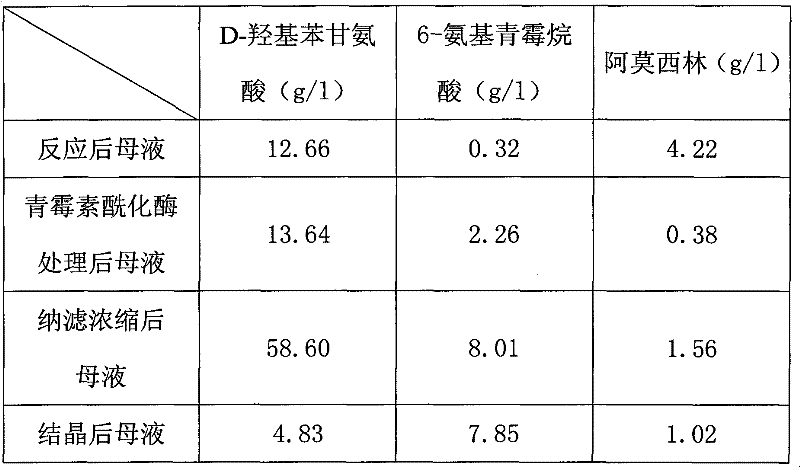
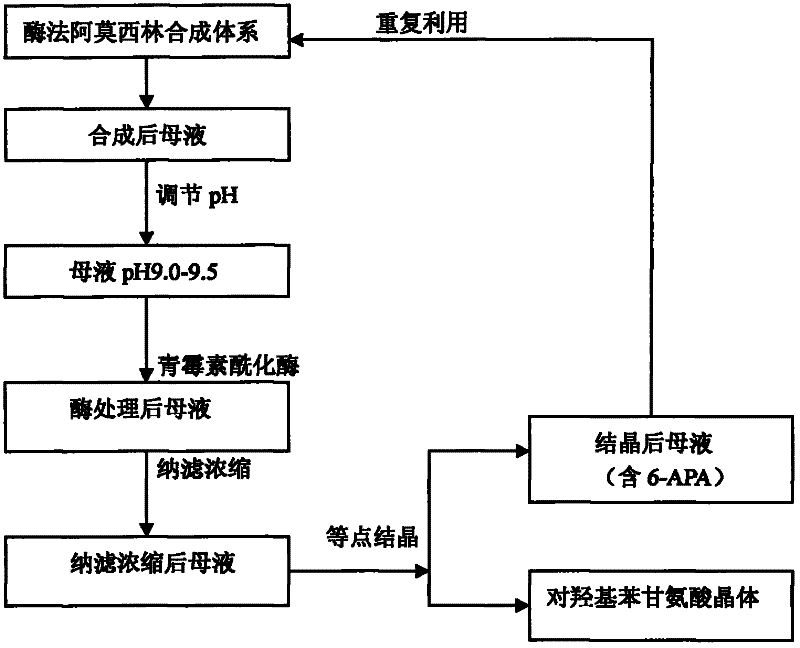
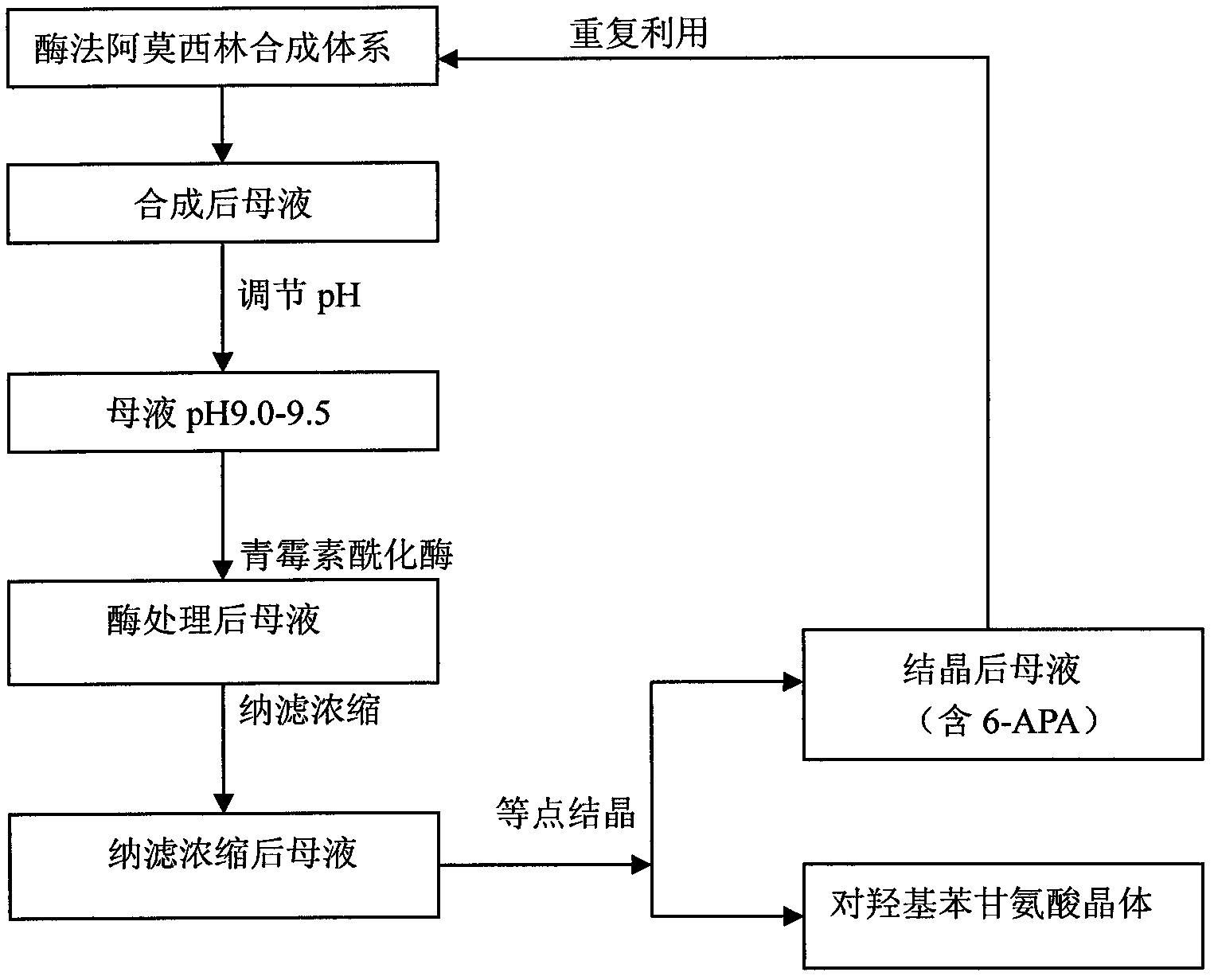
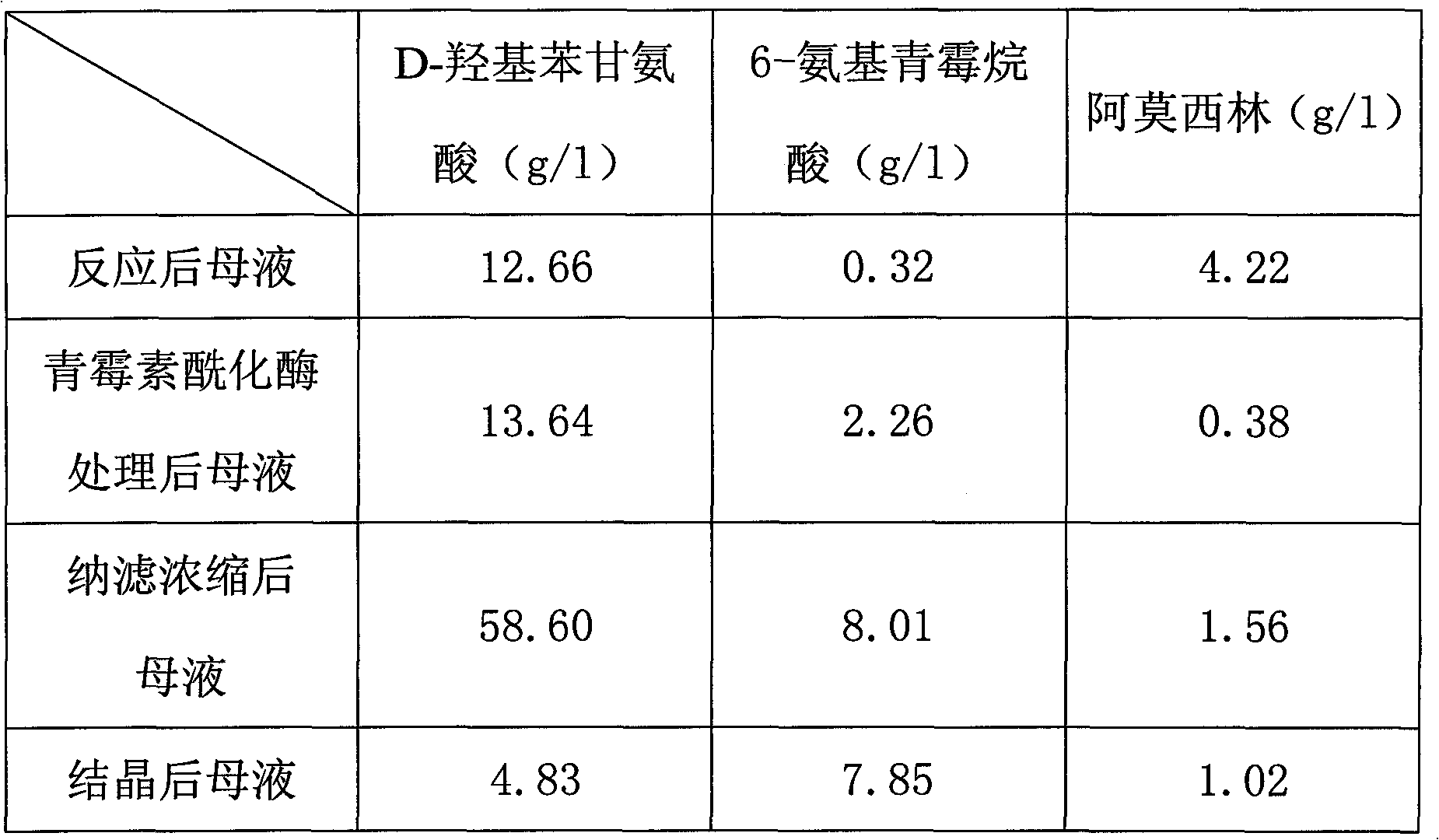
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
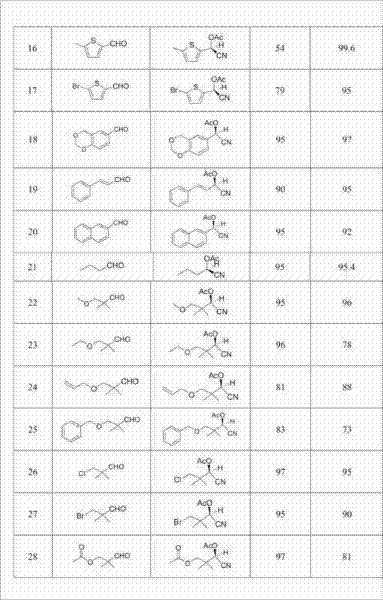
ActiveCN102392060AAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisIsoelectric point
The invention discloses a recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration. The method comprises the following steps: (1) regulating pH of the mother liquor; (2) hydrolyzing amoxicillin in the mother liquor by utilizing penicillin acylase, thus only D-hydroxyphenylglycine and 6-amino-penicillanic acid (6-amino-penicillanic acid) exist in the other liquor; (3) preparing concentrated mother liquor by nanofiltration; and (4) separating and recovering the D-hydroxyphenylglycine and the 6-amino-penicillanic acid (6-amino-penicillanic acid) by isoelectric point crystallization. The method has the advantages that: the recycling rate of an inactive lateral chain can be improved regarding certain amoxicillin synthetase with highhydrolysis activity, so that the enzymatic synthesis of amoxicillin is more economical.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
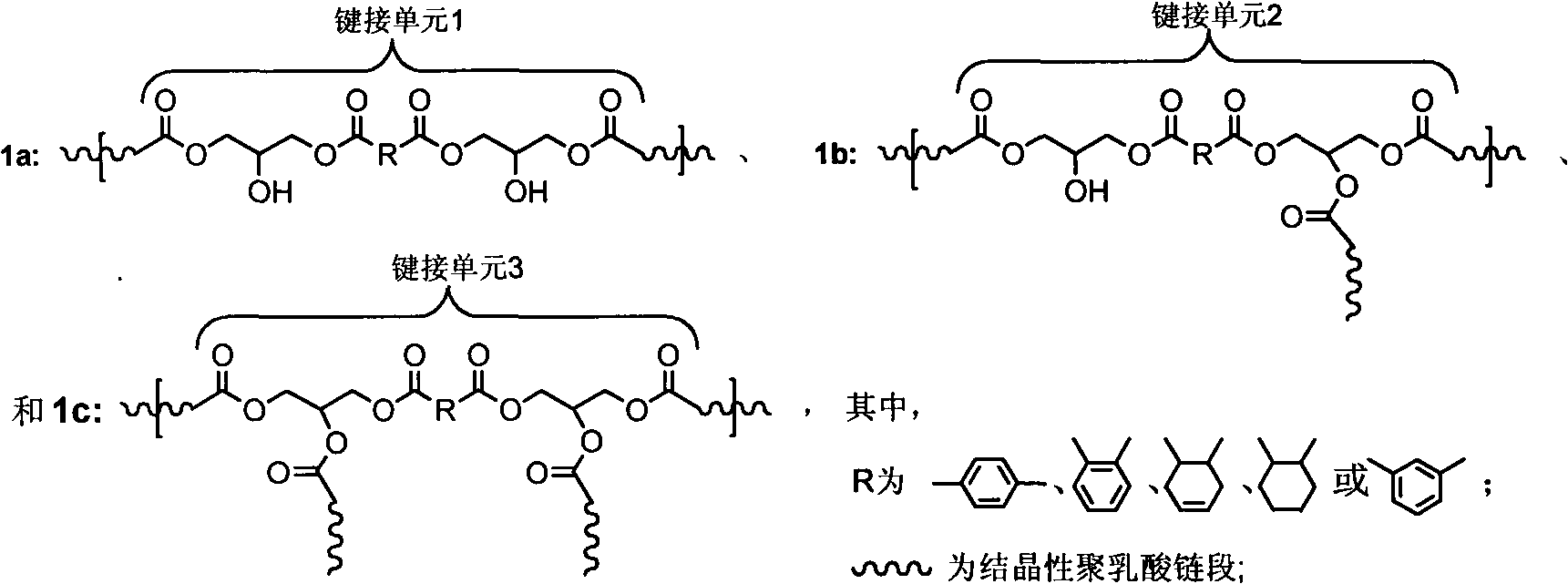
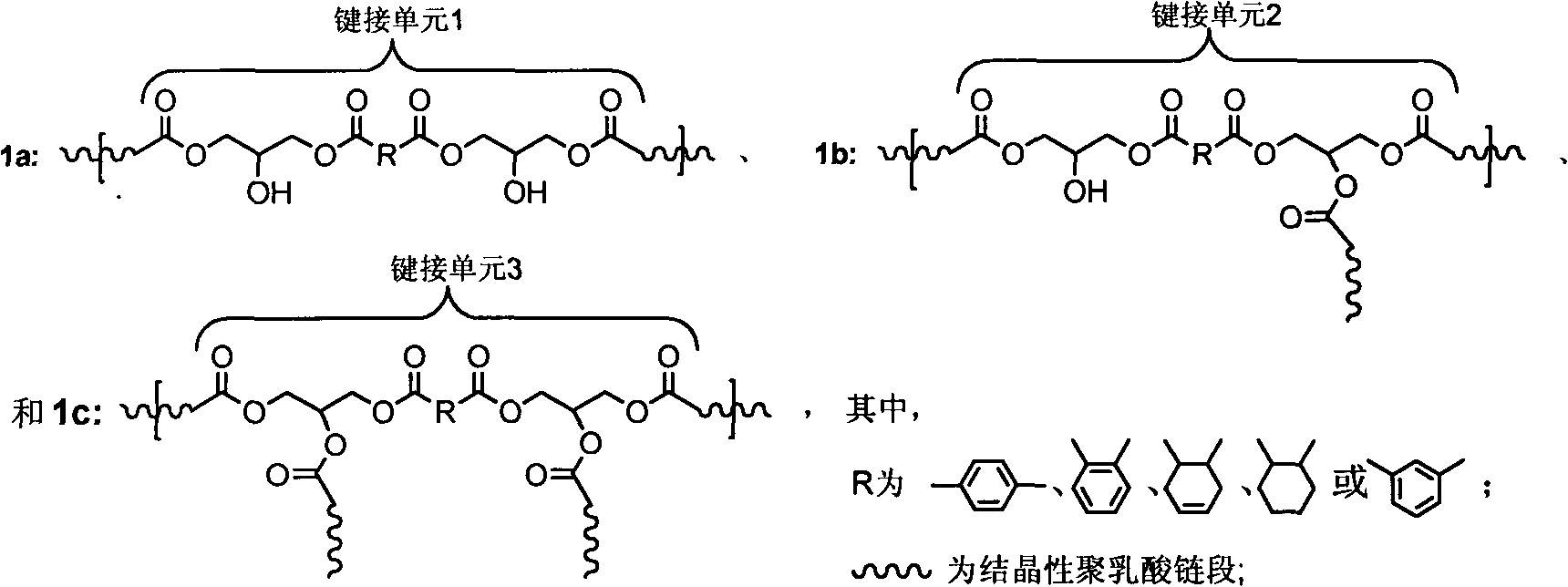
High-molecular weight long-chain branched crystalline polylactic acid material and preparation method thereof
The invention discloses a high-molecular weight long-chain branched crystalline polylactic acid material and a preparation method thereof. The preparation method comprises the following steps of: 1) adding 0.1 to 1 percent of protonic acid catalyst into aqueous solution of lactic acid or mixed solution of the aqueous solution of the lactic acid and silicon dioxide nano particle silica sol containing 0.1 to 10 weight percent of lactic acid, and dehydrating to obtain a product I; 2) adding 0.4 to 2 molar percent of dibasic acid or anhydride into the product I, and reacting to obtain a product II; 3) adding 0.1 to 1 weight percent of lewis acid catalyst into the product II, performing melt polycondensation, and adding 0.1 to 5 weight percent of crystallization accelerator to obtain terminal carboxyl group crystalline polylactic acid prepolymers; and 4) reacting diglycidyl ester and the terminal carboxyl group crystalline polylactic acid prepolymers in a molar ratio of 0.8:1-1.2:1 to obtain the high-molecular weight long-chain branched crystalline polylactic acid material. The preparation method has the advantages of simplicity, short reaction time, high efficiency, low cost and environmental friendliness and capability of contributing to realizing commercialization.
Owner:ZHEJIANG UNIV
Synthetic method for N(2)-L-alanyl-L-glutamine dipeptide
InactiveCN1683391AEasy to separate and purifyThe synthesis process is simpleDipeptidesL-alanyl-l-glutamineDipeptide
The present invention relates to synthesis of dipeptide containing amino acid, and provides a kind of synthesis process of N(2)-L-alanyl-L-glutamine dipeptide with low material cost, simplicity, high yield, no need of separating and purifying intermediate product, easy product separation and purification and environment friendship. The synthesis process includes the reaction of amino acid with protected N-terminal with phosphorus triphenyl oxide and triphosgene in organic solvent to form active ester; the reaction of the active ester with glutamine in water solution of inorganic alkali; acidifying with inorganic acid and eliminating N-terminal protecting radical.
Owner:XIAMEN UNIV
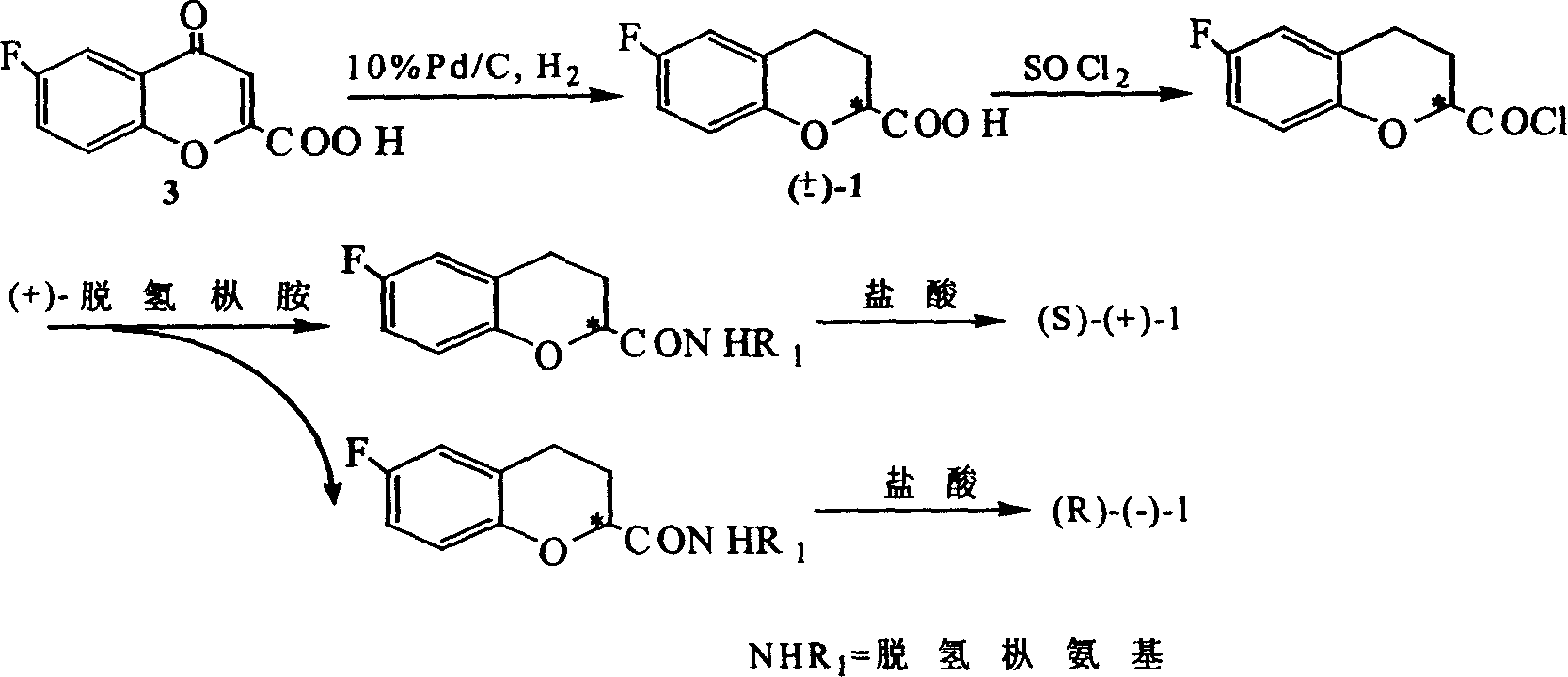
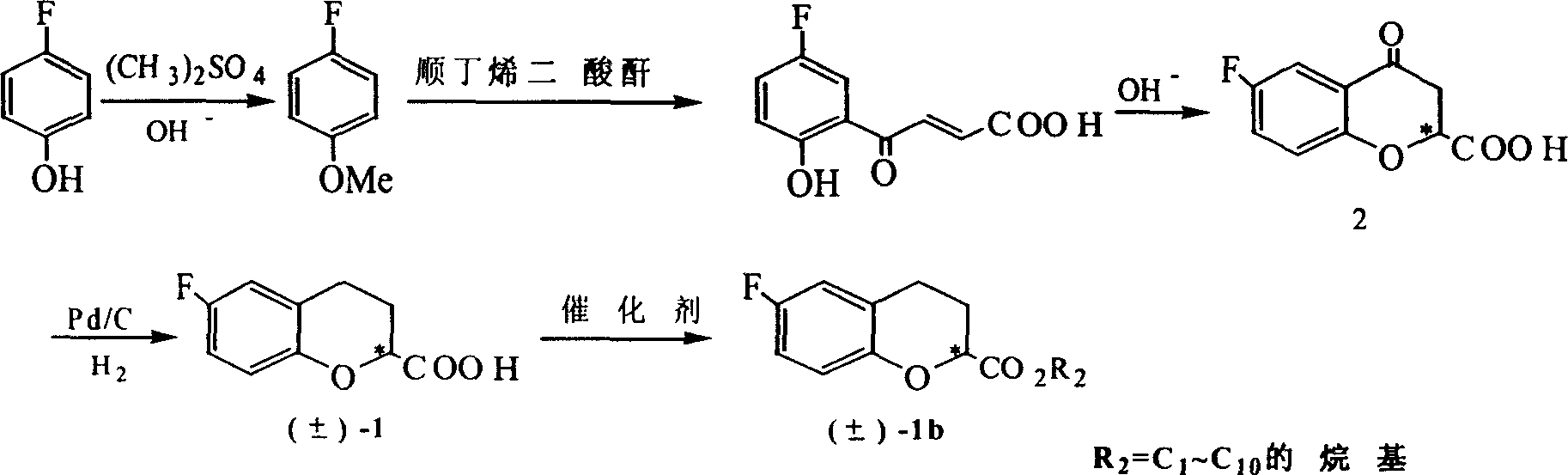
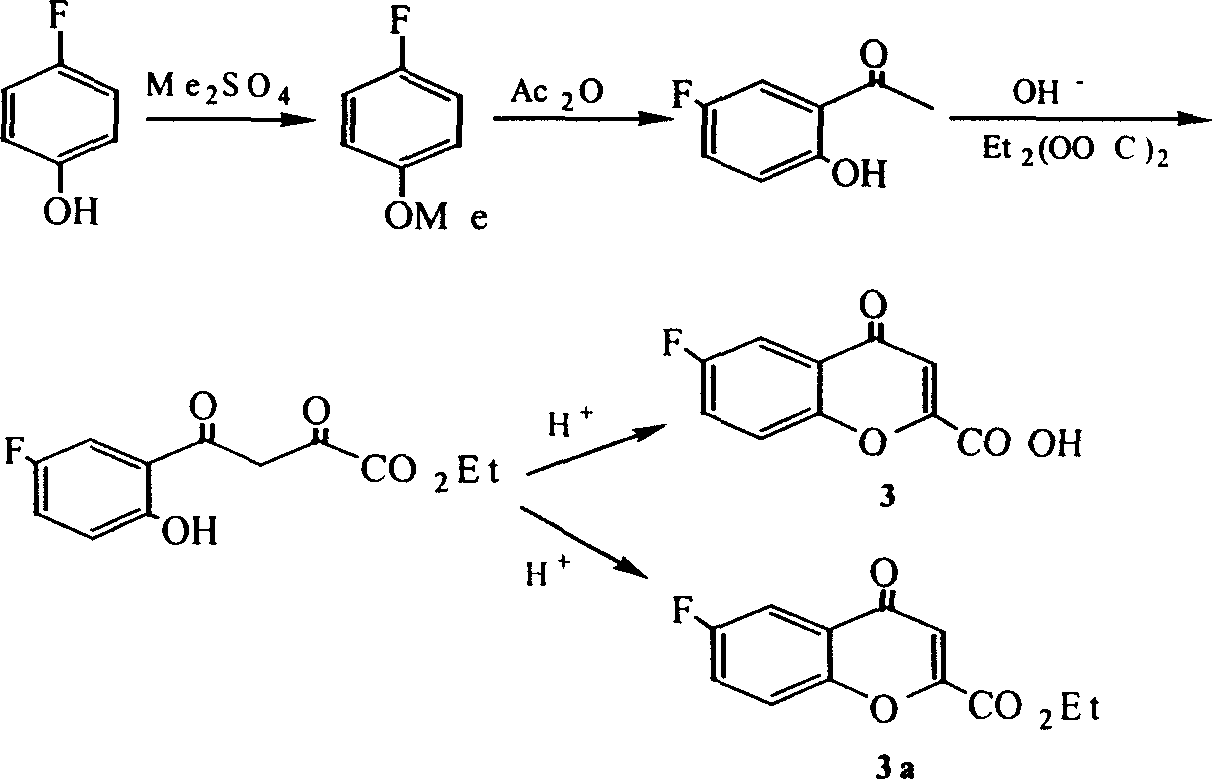
Method for synthesizing optical enantiomer 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylic acid and 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylate
InactiveCN1629154AThe synthesis method is simpleThe synthesis method is reasonableOrganic chemistryBenzopyranAlcohol
The invention provides the method for synthesizing destination substance of (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid through two paths, the (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid and C1-C10 alcohols are subject to esterification reaction so as to synthesize the corresponding racemic ester. Then chemical dismantling is conducted to (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid, in order to obtain prepare optical purity of (R)-(-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid and (S)-(+)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
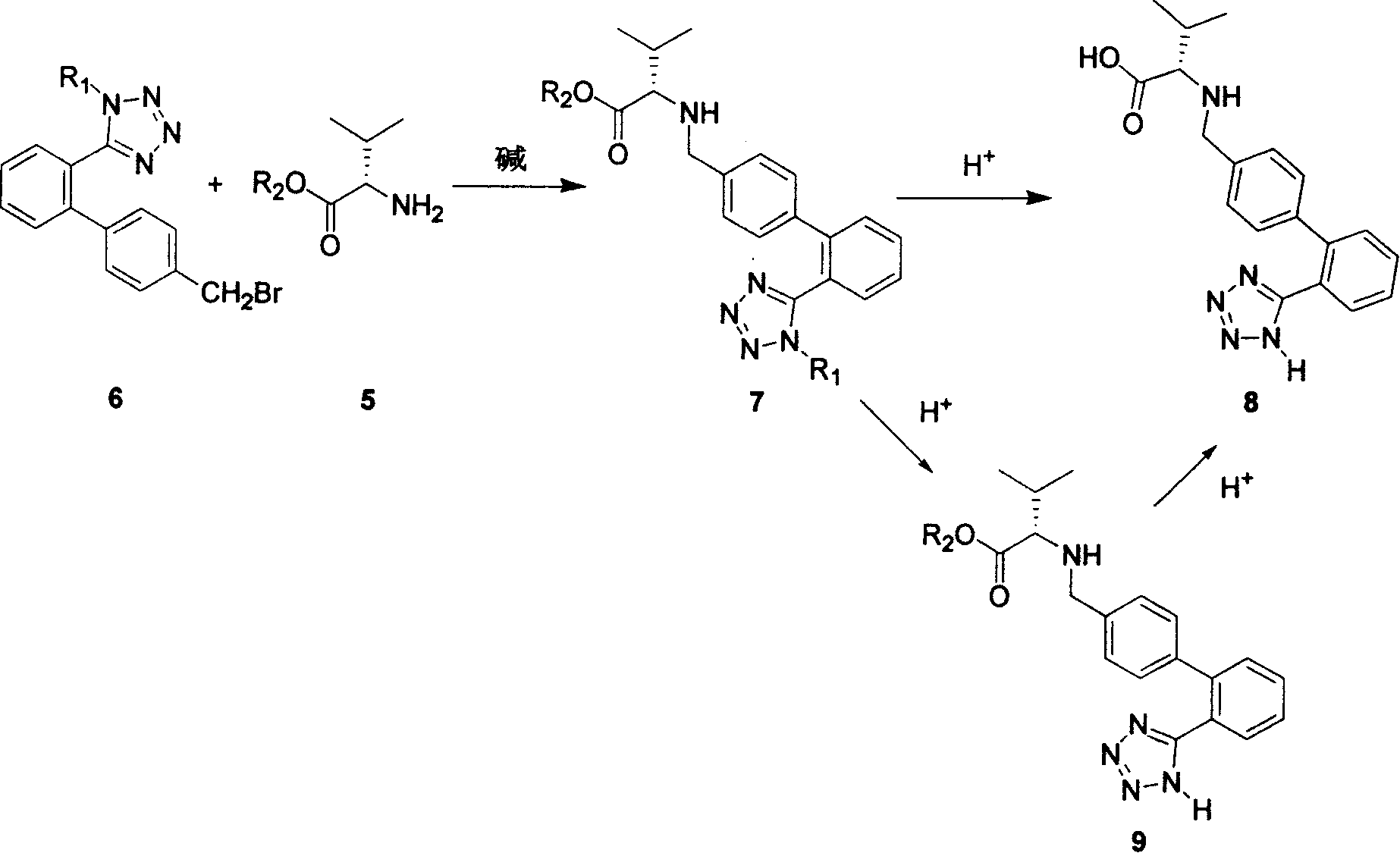
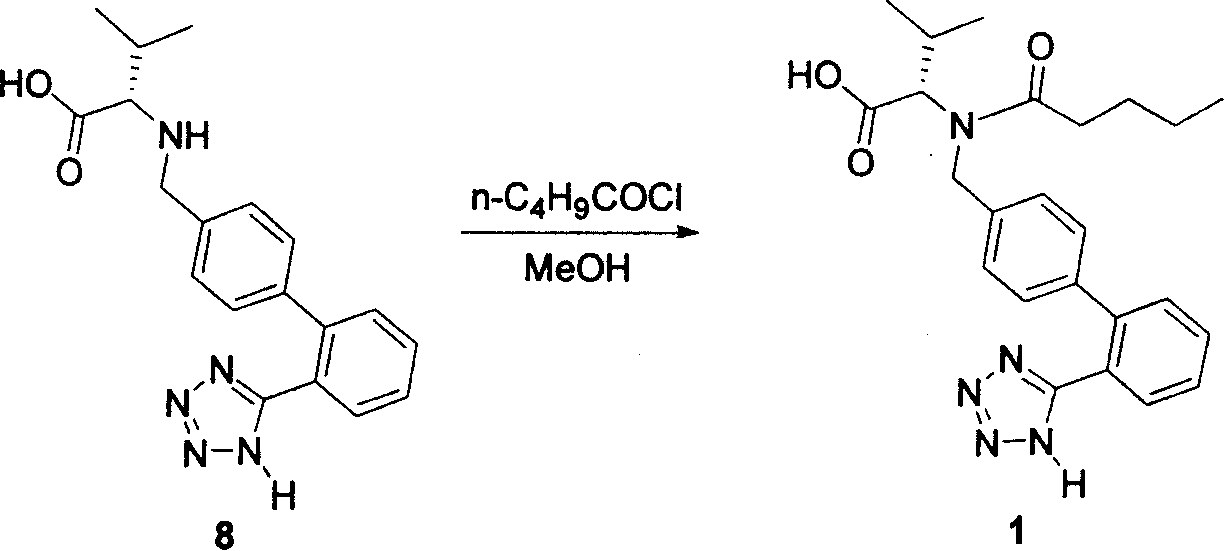
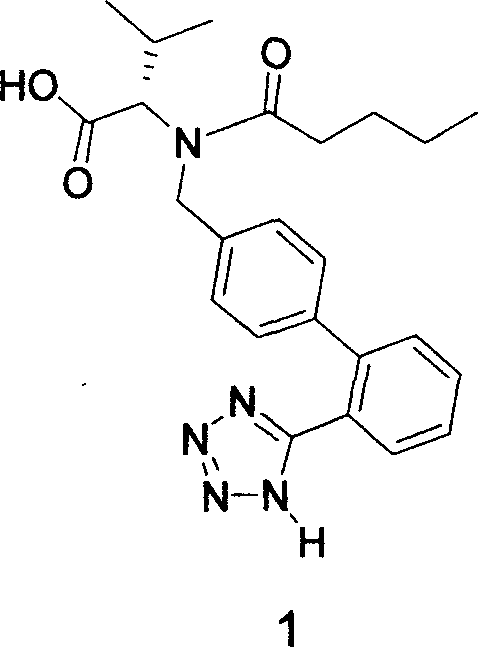
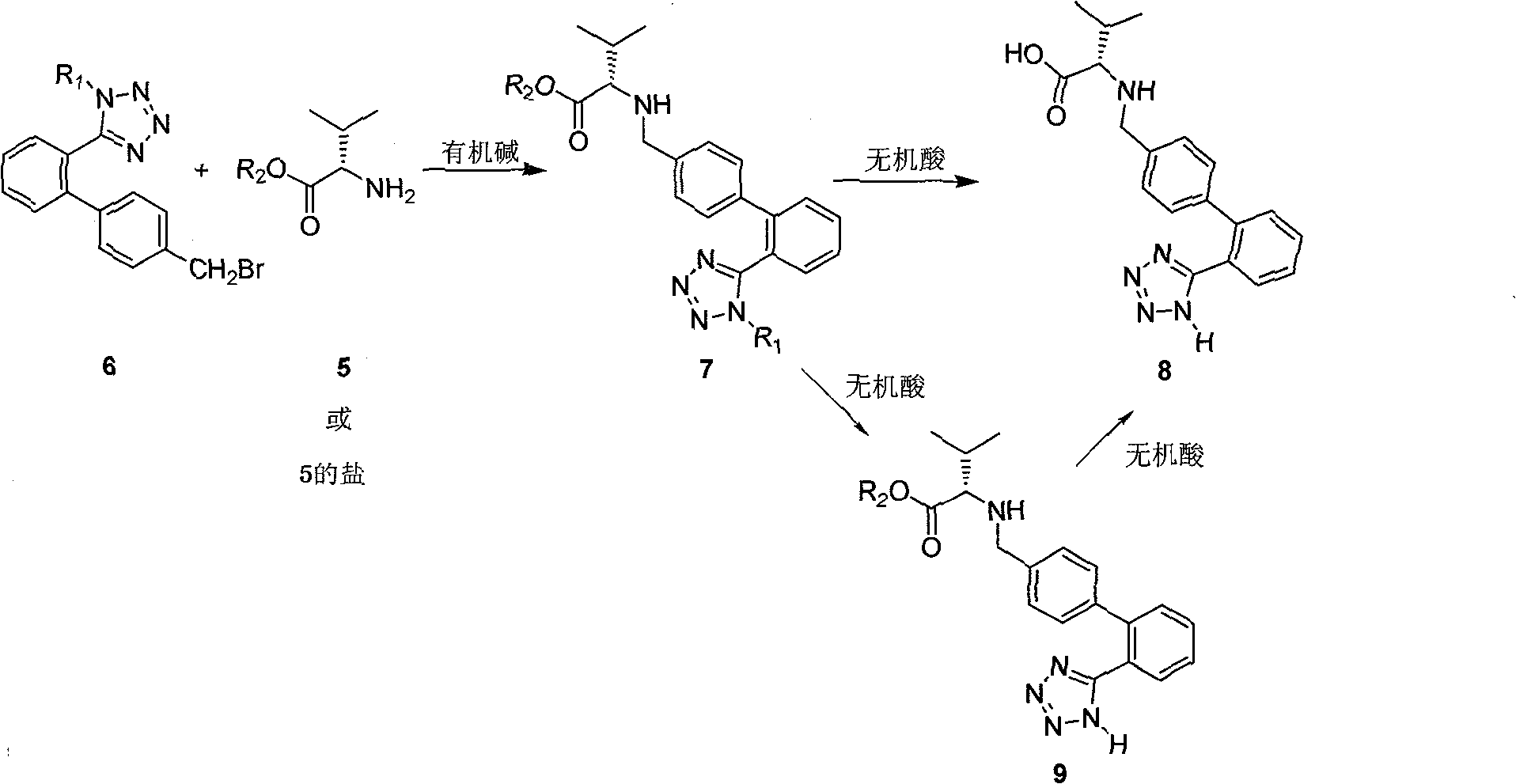
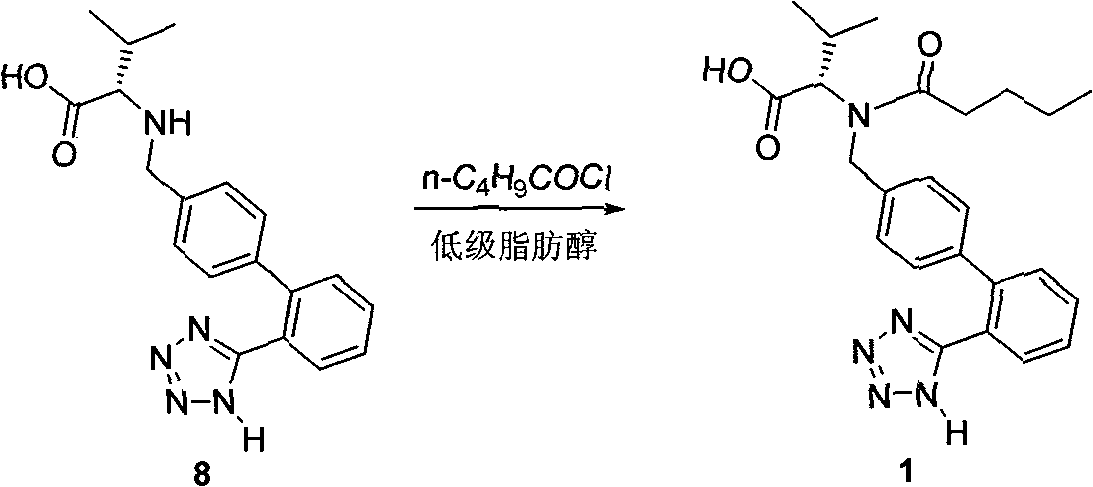
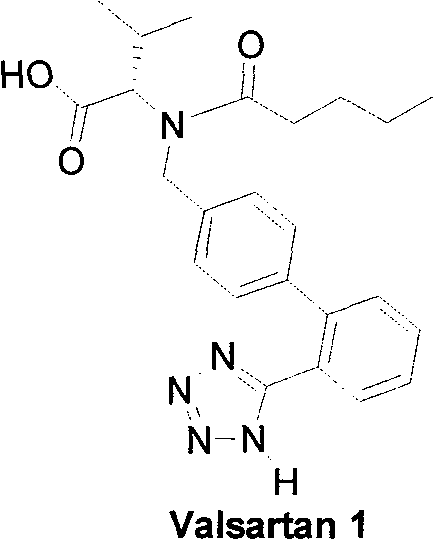
Method for synthesizing Valsartan with high optical purity
The invention relates to a synthesis of high optical purify N-[[2'-(1H- tetrazoline-5-group)- (1,1'- diphenyl)-4-group]-methyl-]-L-valine, and using it as raw material in the synthesis of high optical valsartan. Using the 2'- tetrazoline group-4-methyl bromide biphenyl protected by tetrazoline and esterification protected carboxyl of L- valine(its salt) as raw material, by nucleophilic substitution, doing deprotective reaction in acidic condition, getting high optical purify N-[[2'-(1H-tetrazoline-5-group)-(1,1'-diphenyl)-4-group]-methyl-]-L-valine; in the existence of lower aliphatic alcohol, condensing with n-valeryl chloride to synthesize the valsartan in temperature range -20deg C-40deg C, controlling pH value between 1.5-5.0.
Owner:LINHAI TIANYU PHARMA
Preparation method of Quizalofop-p-ethyl with high optical content
ActiveCN101531640AHigh optical purityQuick responseBiocideOrganic chemistryQuinoxalineOrganic solvent
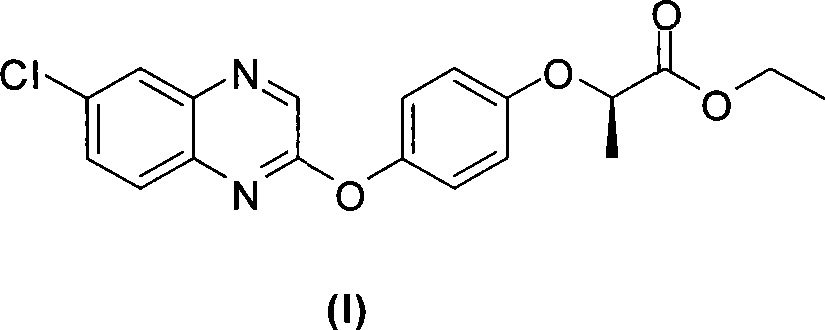
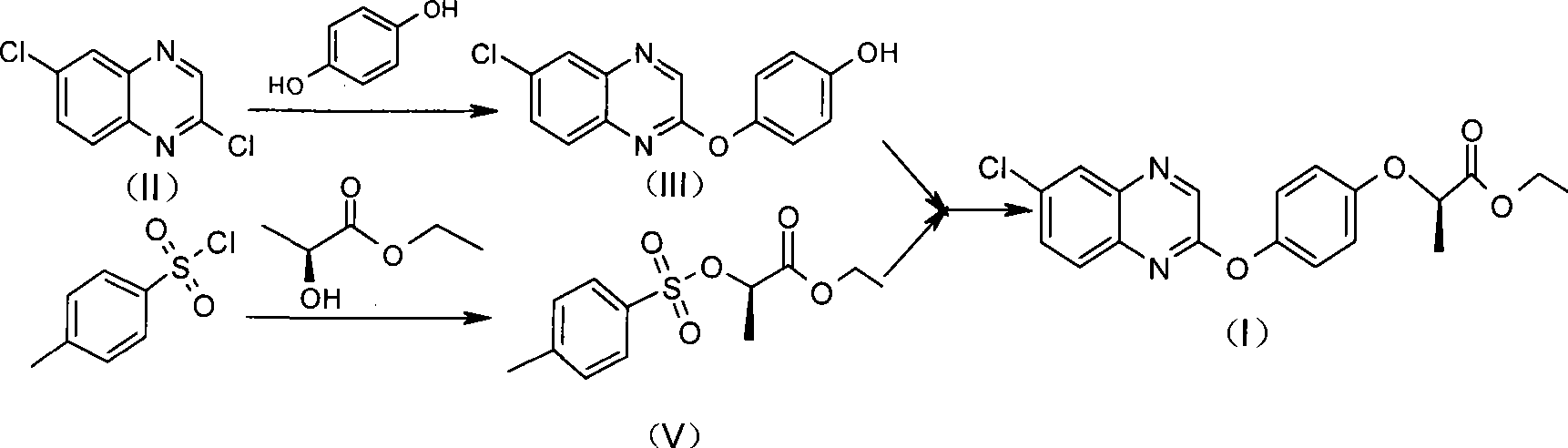
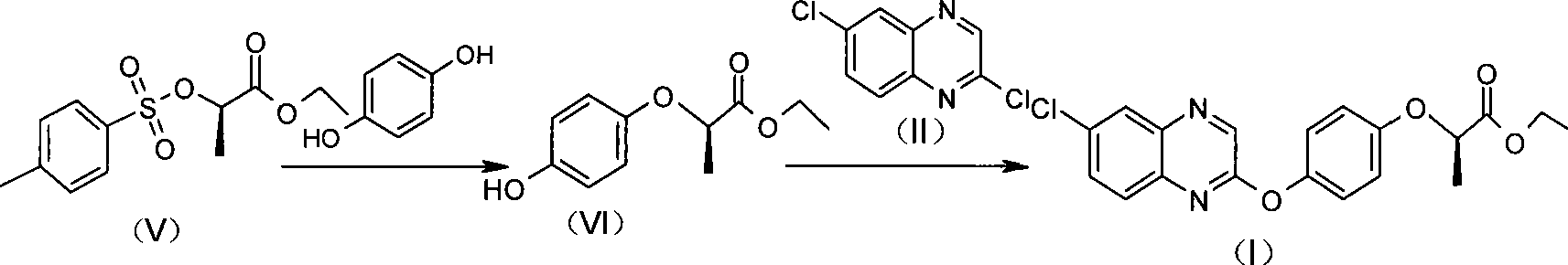
The invention relates to a preparation method of Quizalofop-p-ethyl with high optical content. In the method, 6-chorine-(4-hydroxyl group phenoxyl) quinoxaline and S(-)p-toluenesulfonyl ethyl lactate are reacted in non-polar organic solvent for 4-8h under the temperature of 60-150 DEG C with metal carbonate of 100-800mesh as the catalyst. The optical antimer proportion of the Quizalofop-p-ethyl prepared by the method R:S is more than 98.5:1.5, which is suitable for industrial mass production, is easy for operation and low in cost.
Owner:NUTRICHEM LAB CO LTD
Dapoxetine hydrochloride synthetic method
InactiveCN104496830AAvoid it happening againAvoid racemizationOrganic compound preparationAmino-hyroxy compound preparationAlkyl transferOrganic solvent
The invention provides a dapoxetine hydrochloride synthetic method. The method includes the steps of (1) subjecting a compound (9) and (-)-beta-chlorodiisopinocampheylborane to reduction reaction in an organic solvent at the temperature of 10 DEG C-80 DEG C to obtain a compound (12); (2) subjecting a compound (8) and alkali to reaction in an organic solvent for 2 hours-14 hours at the temperature of 0 DEG C-10 DEG C, adding the compound (12) obtained in step (1), and performing alkylation reaction for 15 hours-40 hours at the temperature of 0 DEG C-10 DEG C to obtain a compound (13); (3) subjecting the compound (13) obtained in step (2) and thionyl chloride to reaction in an organic solvent at the temperature of -10 DEG C-0 DEG C to obtain a compound (14); (4) subjecting the compound (14) obtained in step (3) and dimethylamine to reaction in an organic solvent at the temperature of 20 DEG C-30 DEG C to obtain dapoxetine free amine and to reaction with an ethanol solution of hydrogen chloride to obtain hydrochloric acid dapoxetine. The dapoxetine hydrochloride synthetic method is high in yield and product optical purity. The reaction scheme is shown in the description.
Owner:ARROMAX PHARMATECH
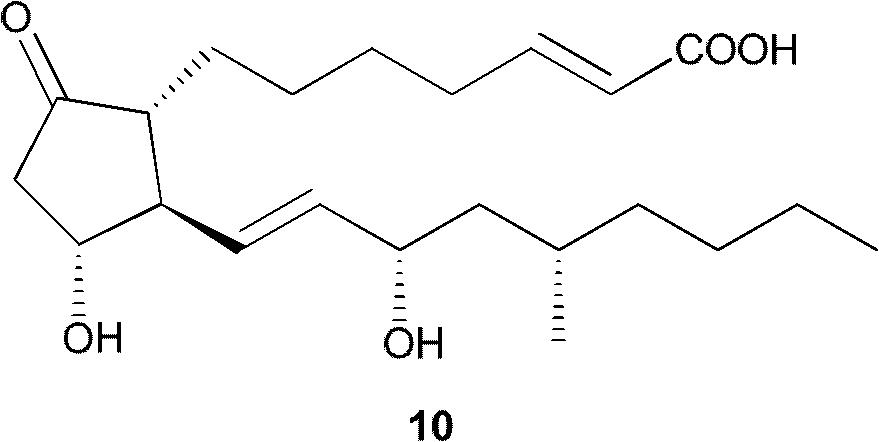
Method for preparing key intermediate of limaprost
InactiveCN102875586AReserved configurationMild reaction conditionsGroup 4/14 element organic compoundsStructural formulaLimaprost-alfadex
The invention discloses a method for preparing the key intermediate of limaprost, particularly provides a chiral compound of formula I and a preparation method thereof. The method comprises the following steps: (1) reacting a compound of formula 3 with a compound of formula 4 to obtain a compound of formula 5; (2) reacting a compound of formula 6 with the compound of formula 5 to obtain a compound of formula 7; (3) mixing the compound of formula 7 with a compound of formula 8 to obtain the compound of formula 1; (4) reacting the compound of formula 1 with a reducing agent to obtain a raw product, separating and purifying the raw product by column chromatography to obtain a compound of formula 2; (5) reacting the compound of formula 2 with a protector reduction agent to obtain a compound of formula 9, wherein each structural formula and substituent are defined as the specification. According to the invention, the method has mild reaction conditions, and the high-purity chiral compound can be conveniently and efficiently prepared.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Azeotropic distillation of cyclic esters of hydroxy organic acids
InactiveUS6984293B2Increase formation rateAvoid racemizationOrganic compound preparationVacuum distillation separationOrganic acidOrganic Ester
Cyclic esters of hydroxy organic acids can be produced and recovered via azeotropic distillation. In certain embodiments cyclic esters, such as glycolide and lactide, can be produced from a fermentation broth or other feed stream that contains a hydroxy organic acid, an ammonium salt of a hydroxy organic acid, an amide of a hydroxy organic acid, or an ester of a hydroxy organic acid using azeotropic distillation. The hydroxy organic acid of the feed stream or the hydroxy organic acid derived from the feed stream by decomposition is reacted to produce the cyclic ester. In other embodiments a crude composition of a cyclic ester of an organic ester can be purified using azeotropic distillation.
Owner:TATE & LYLE INGREDIENTS AMERICAS INC
Synthesis method of enantiomer-enriched indoline-2-formic acid
ActiveCN104672124ASimple reaction conditionsImprove conversion rateOrganic chemistryEnantiomerSynthesis methods
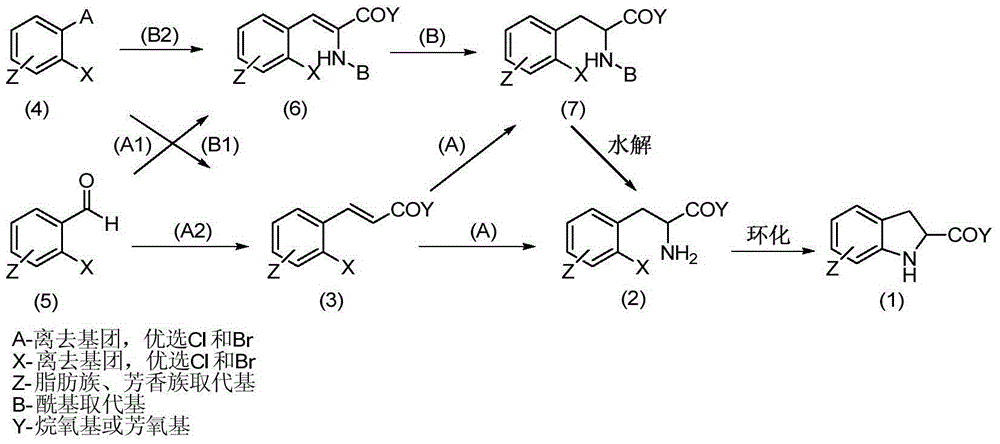
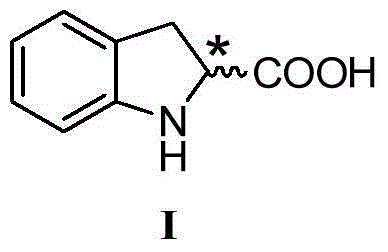
The invention discloses a synthesis method of enantiomer-enriched indoline-2-formic acid shown in a formula (I). The synthesis method of the enantiomer-enriched indoline-2-formic acid comprises the following steps: by adopting low-cost and available ortho-position halogen substituted benzaldehyde and N-benzoyl substituted glycine as starting materials, carrying out Erlenmeyer-Plochl cyclization, alkaline hydrolysis and asymmetric catalytic hydrogen for constructing a chiral center, and then carrying out acid catalysis, deprotection and cyclization sequentially or cyclization, acid catalysis and deprotection sequentially, so that the enantiomer-enriched indoline-2-formic acid is obtained. The synthesis method of the enantiomer-enriched indoline-2-formic acid has the advantages that raw materials used in the whole process route are low-cost and easily available, harmful substances or multiple danger special processes are not used, reaction conditions are mild, technological operation is simple, production is safe and stable, the product yield is high, the purity is high, less three wastes are produced, and the energy consumption is low, so that the synthesis method of the enantiomer-enriched indoline-2-formic acid is a process route especially applicable to industrial production. The formula (1) is described in the specification.
Owner:ZHEJIANG CHANGMING PHARMA
Method for anaerobically degrading feather keratin with microbial strain 18D-TA
ActiveCN102326668AReduce energy consumptionLow costBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention relates to a method for anaerobically degrading feather keratin with a microbial strain 18D-TA, belonging to the technical field of microorganisms. The method comprises the following specific steps of: A, treating feathers: cleaning feathers with water, putting into a drying oven of which the temperature is 45-55 DEG C, and drying for 2-3 days for later use; and B, inoculating the strain: performing high-temperature moist-heat sterilization on a culture medium and the feathers treated in the step A, inoculating the strain 18D-TA in the amount of 2-10 percent, and anaerobically standing and culturing at the temperature of 45-60 DEG C for 8-10 hours till feather down falls off, wherein the feathers are completely separated from feather stems 18-24 hours later, and the feather stems are degraded in different degrees. Compared with other aerobic bacteria, the strain used in the method has the advantages: more products can be accumulated, so that ventilation equipment is not required to be provided, and the energy consumption is lowered greatly; and meanwhile, the efficiency is high, the fermenting period is short, racemization of most amino acids is avoided, and the absorption applicability of a feather hydrolysis product is enhanced.
Owner:BIOGAS SCI RES INST MIN OF AGRI
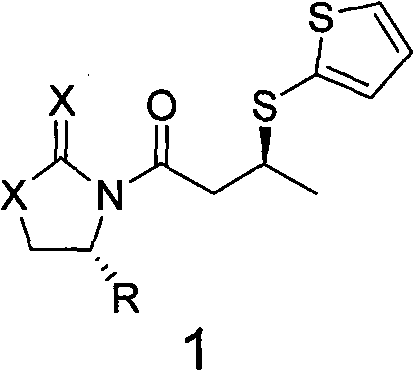
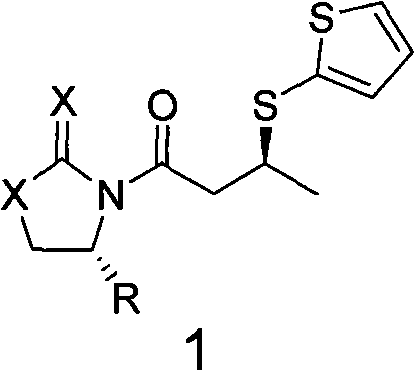
Method for synthesizing chiral dorzolamide hydrochloride
InactiveCN101735209AHigh optical purityHigh yieldAsymmetric synthesesDORZOLAMIDE HYDROCHLORIDEStructural formula
The invention discloses a compound represented by a general formula 1 and a preparation method thereof. In the structural formula, X=Y=O, or X=S, Y=O, or X=Y=S; and R represents phenyl, benzyl or isopropyl. The invention also discloses a method for synthesizing a compound represented by a general formula 3, and a method for synthesizing chiral dorzolamide hydrochloride, which are formed by carrying out one step reaction and multi-step reaction on the compound represented by the general formula 1. The compound synthesizes the chiral dorzolamide hydrochloride used for treating glaucoma by adopting a classical chiral prosthetic group induction method, and has the advantages of good yield, no racemization and high optical purity.
Owner:FUJIAN SOUTH PHARMA CO LTD
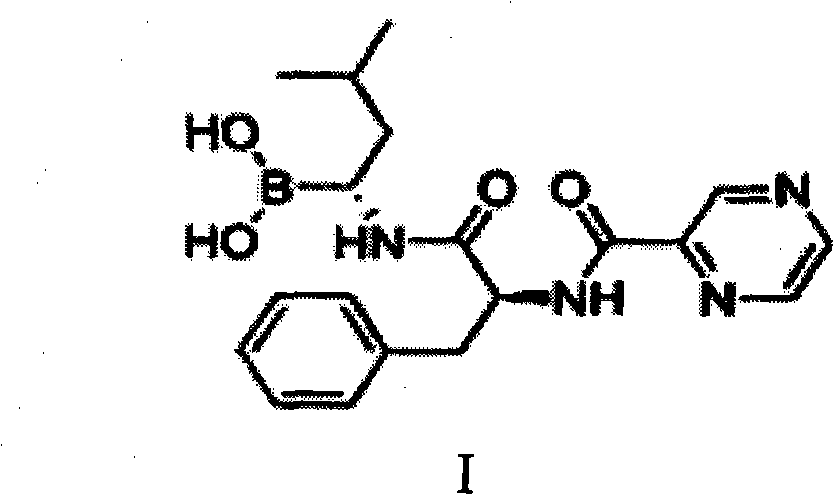
Improved method of bortezomib process
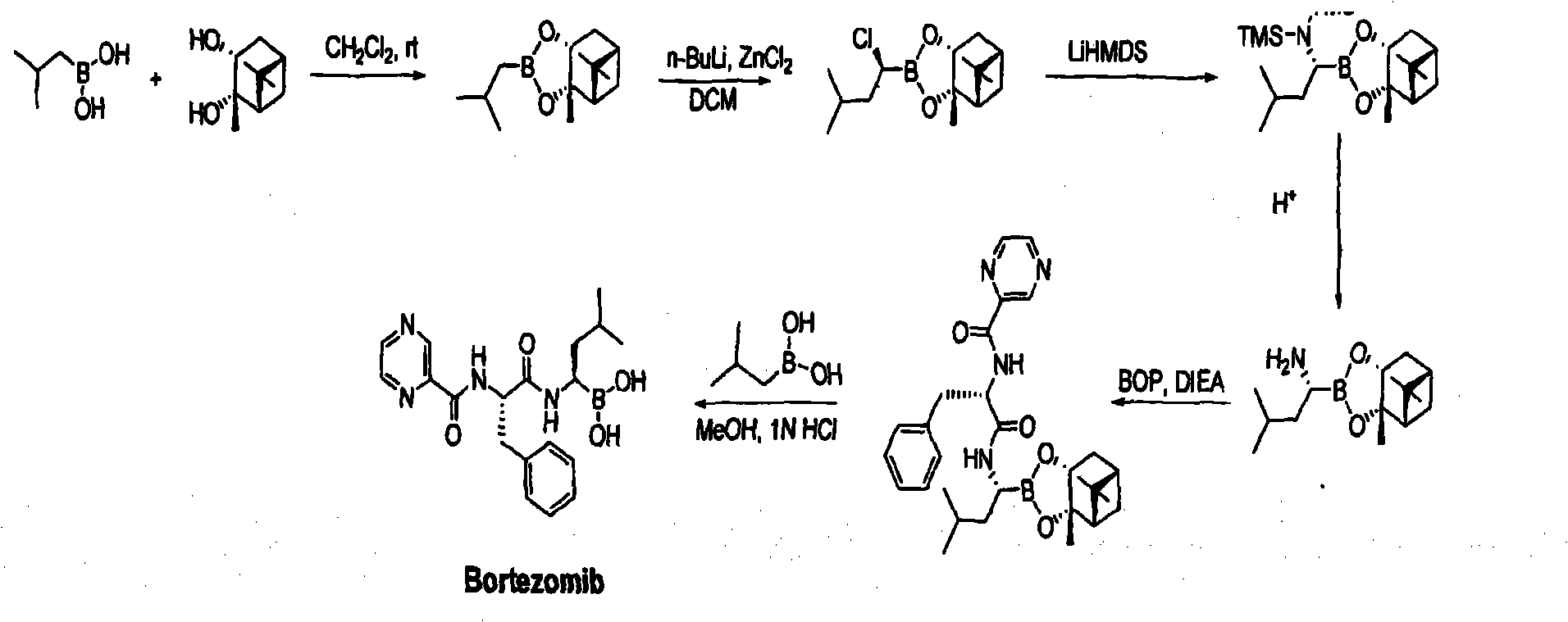
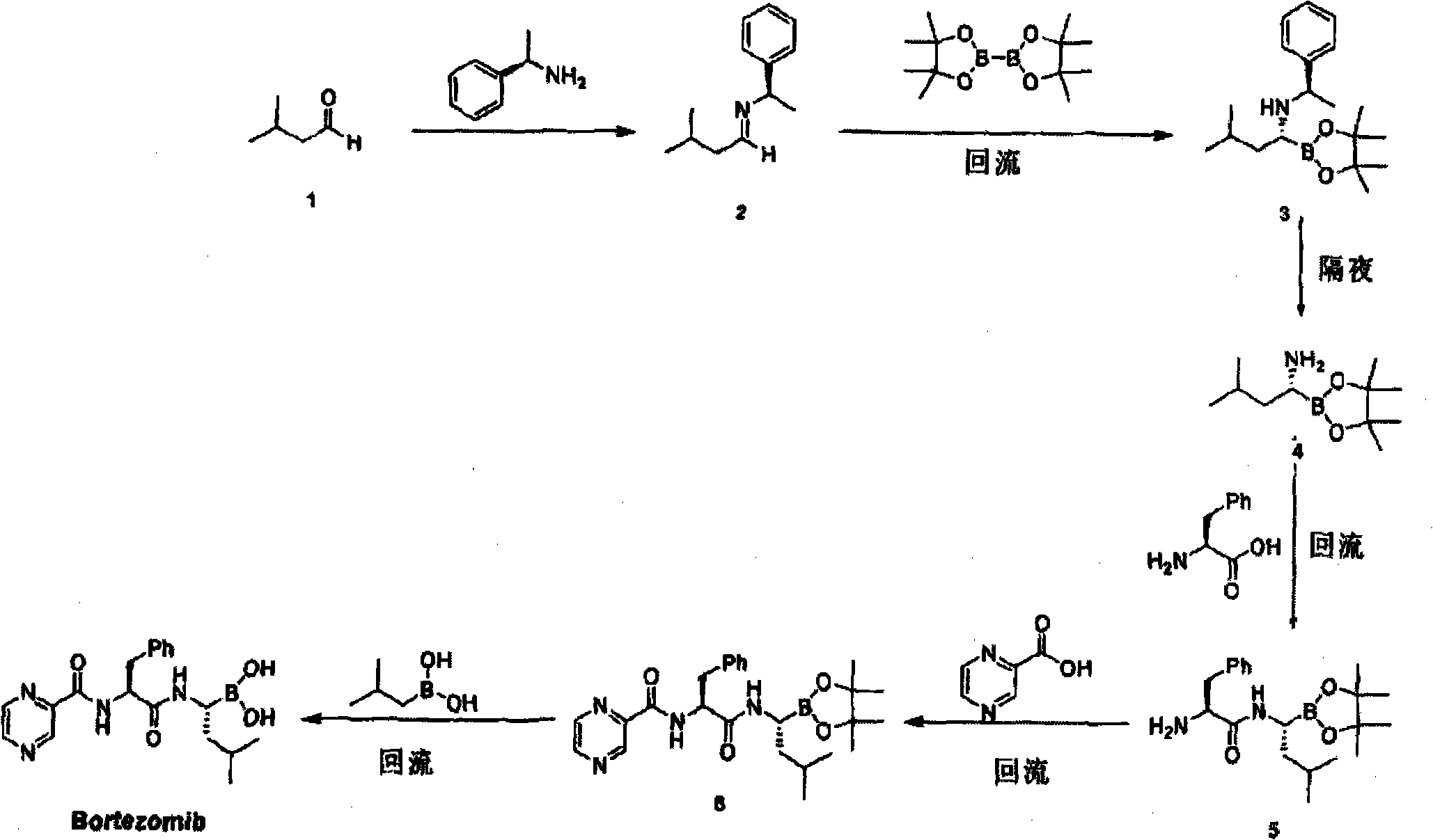
ActiveCN103539832AInhibition of product racemizationSuppresses formation of condensation by-productsPeptidesChemistryBortezomib
The invention belongs to the field of pharmaceutical synthesis, and specifically relates to an improved method of a medical compound bortezomib process. Bortezomib is prepared by the following steps of condensation, deprotection, coupling and the like by taking N-trifluoroacetyl-L-phenylalanine, alpha R)-(1S, 2S, 3S, 5S)-pinane diol-1-amino-3-methyl butane-1-borate trifluoroacetate. Side reactions are effectively avoided through optimization of raw materials and proportioning, control of reaction conditions and an appropriate treatment process, so that the product yield and the purity are improved to a greater extent. The total recovery of four steps is over 50% and the liquid detection purity reaches 99.83%.
Owner:SHANDONG NEWTIME PHARMA
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
ActiveCN102392060BAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisNanofiltration
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Preparation method of succinic acid S-metoprolol
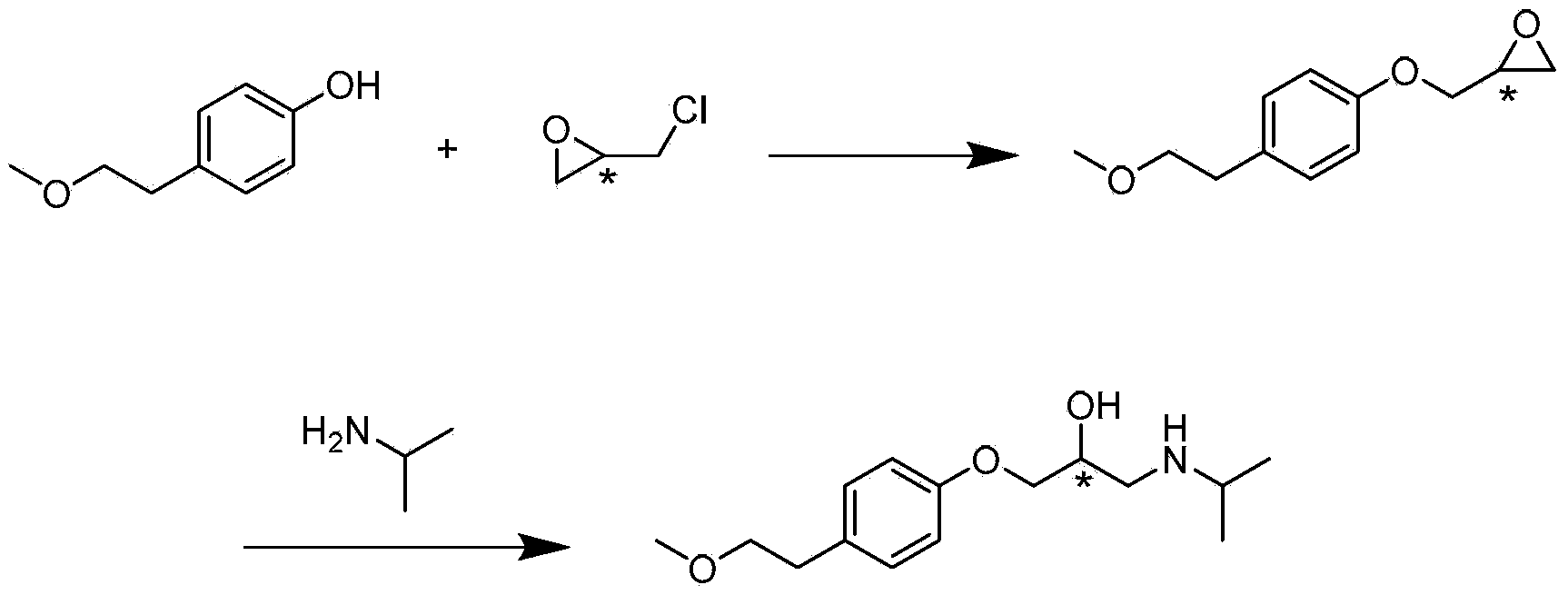
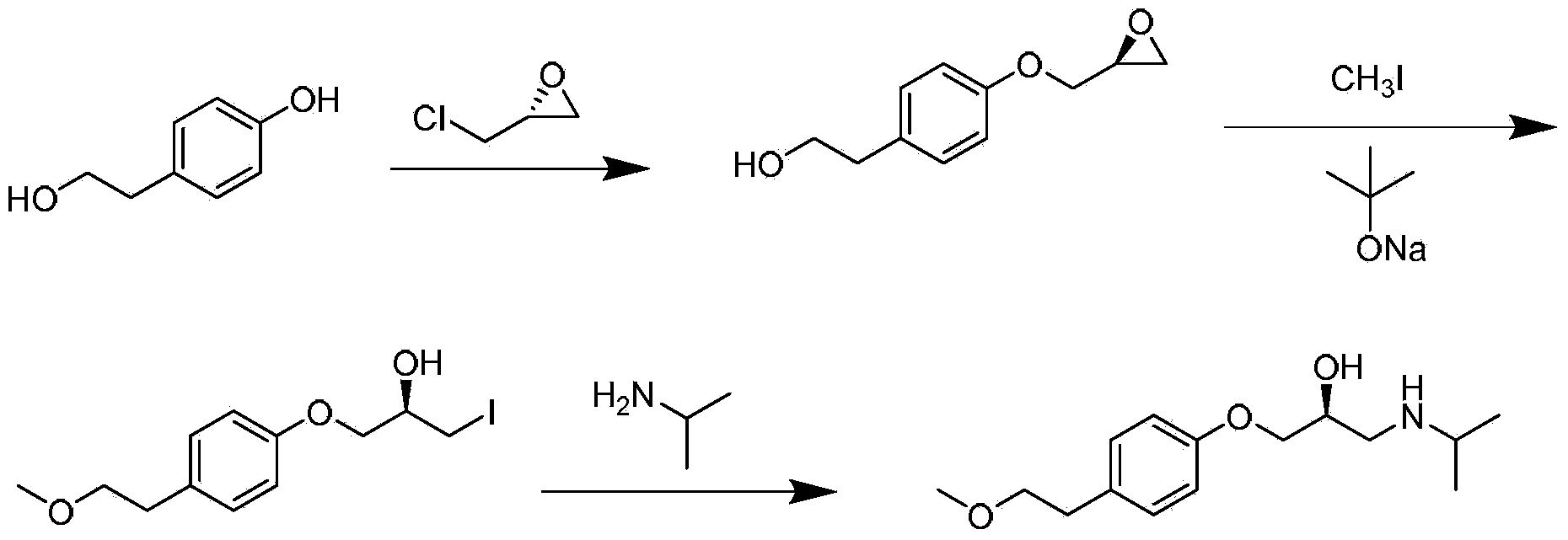
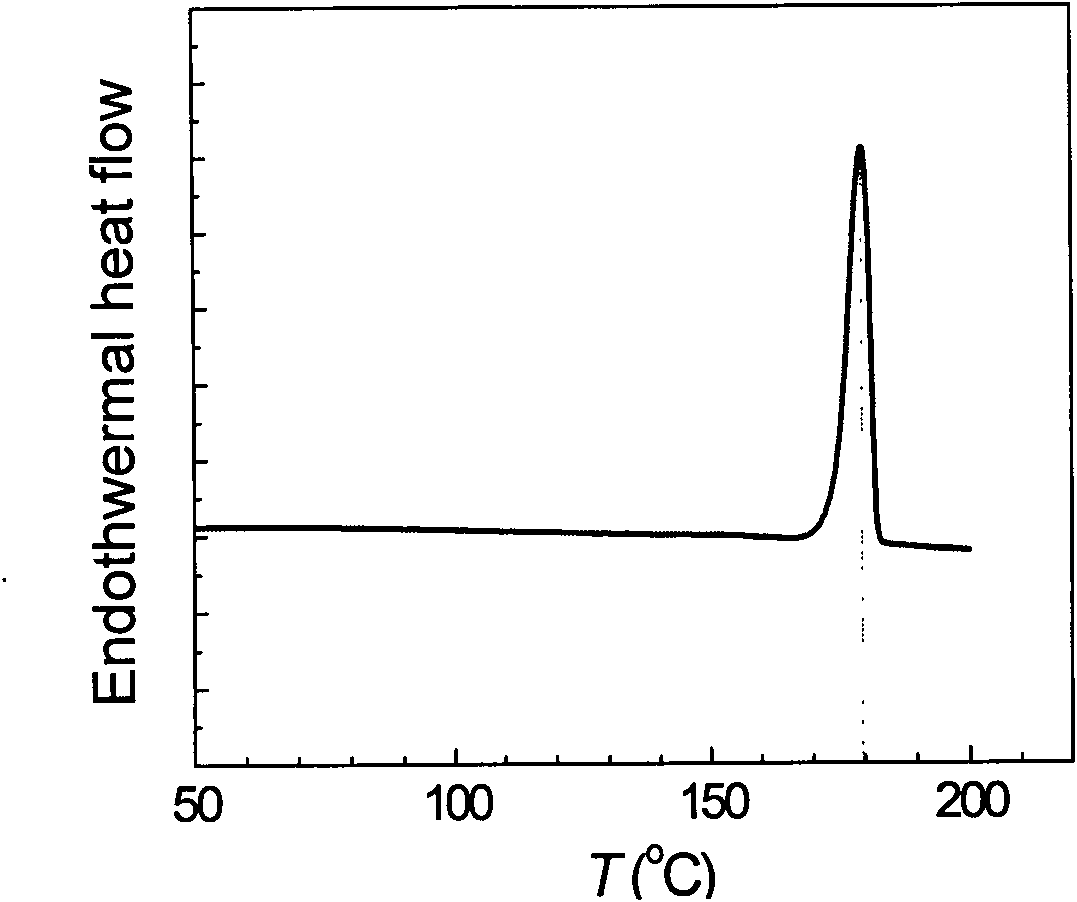
ActiveCN103980134AAvoid product racemizationLow equipment requirementsOrganic compound preparationCarboxylic acid salt preparationMetoprololMedicinal chemistry
The invention discloses a preparation method of succinic acid S-metoprolol. The preparation method of the succinic acid S-metoprolol comprises that methoxyl ethyl phenol is taken as an initial raw material, (R)-epoxy chloropropane is taken as a reaction agent, and etherification reaction and amination reaction are carried out, so that an S-metoprolol crude product is obtained; then the S-metoprolol crude product and succinic acid form salt, dissociation is carried out by virtue of alkali for obtaining oily metoprolol, dibenzoyl-D-tartaric acid is used for splitting, so that an S-metoprolol pure product is obtained, and the obtained S-metoprolol pure product and succinic acid form salt, so that the succinic acid S-metoprolol is obtained. The preparation method of the succinic acid S-metoprolol has the advantages that S-metoprolol is salified and then purified, then splitting is carried out, and purity and ee value of the succinic acid S-metoprolol product are increased, and quality requirement in the field of medicines can be met.
Owner:ANHUI NEW STAR PHARMA DEV
Melt/solid-phase polycondensation preparation method of polylactic acid material with high molecular weight and high crystallinity
The invention discloses a melt / solid-phase polycondensation preparation method of a polylactic acid material with high molecular weight and high crystallinity. The method comprises the following steps: adding 0.1-1% of protonic acid catalyst into the aqueous solution of lactic acid or the mixed solution of the aqueous solution of lactic acid and the silica nano particle silica sol containing 0.1-10% of lactic acid by weight, and dehydrating; performing an oligomerization reaction; adding 0.1-1% of lewis acid catalyst for a melt polycondensation reaction; adding 0-5% of crystallization accelerator, mixing uniformly and granulating, and crystallizing in an inert gas flow; and finally performing a solid-phase polycondensation reaction in the inert gas flow to obtain polylactic acid with high molecular weight and high crystallinity. In the invention, as two catalysts with different properties are added at different steps, the dehydration time is shortened, racemization is suppressed and the molecular weight and crystallinity of an oligomer are improved; moreover, the crystallization accelerator is introduced in situ, and thus the crystallinity and melting point of the polylactic acid are improved at low cost; and finally the heat resistance is improved. The invention is favorable for realizing industrialization.
Owner:ZHEJIANG UNIV
Cyclic antimicrobial peptides
InactiveUS20140303071A1Easily synthesizeSalt insensitiveAntibacterial agentsBiocidePolymicrobial InfectionsAntimicrobial peptides
Owner:NOVABIOTICS LTD
Esomeprazole sodium sterile lyophilized powder for injection and preparation process of lyophilized powder
InactiveCN103768028AGuaranteed efficacy and safetyLow costOrganic active ingredientsPowder deliveryChemistryActive component
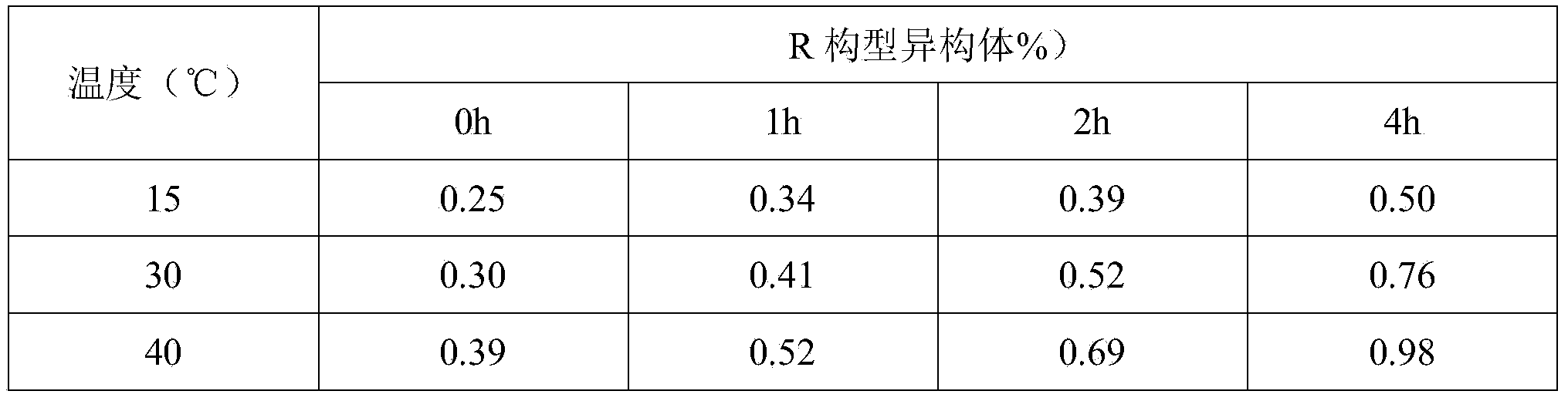
The invention relates to sterile lyophilized powder for inhibiting esomeprazole sodium racemization and a preparation process of the lyophilized powder. The active component esomeprazole sodium is included in a molecular 'hole' structure of esomeprazole sodium by adopting beta-cyclodextrin and derivatives thereof, so that racemization of esomeprazole sodium can be stopped to guarantee the curative effect and safety of a product. The R configurational isomer of the product is less than 0.5 percent when placed for 24 months; the preparation process is simple and practical, and is suitable for industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
Synthetic method of L-glufosinate intermediate
PendingCN109912649AReduce dosageImprove securityGroup 5/15 element organic compoundsOrganic synthesisReaction rate
The invention provides a synthetic method of L-glufosinate intermediate shown as a formula II and belongs to the technical field of organic synthesis. The synthetic method comprises the step: under aprotective gas atmosphere, reacting a compound in a formula I with diethyl methyl phosphonate in the presence of a catalyst to obtain an intermediate shown as the formula II; the catalyst is MgBr2+PEG-400, LiBr+PEG-400, KCl+PEG-400, MgBr2+TBAB, MgCl2,MgBr2, TMSBr+TBAB, LaCl3+TBAB or CaCl2. The synthetic method provided by the invention has the benefits that as the catalyst is added, the dosage ofthe diethyl methyl phosphonate is reduced, and the process safety and the reaction rate are improved; meanwhile, the problem of reaction racemization can be solved, so that the ee value of a product is improved, the post-processing operation is simplified, and the process cost is reduced, therefore, the industrial application is more easy to perform.
Owner:LIER CHEM CO LTD +1
Synthetic method for N(2)-L-alanyl-L-glutamine dipeptide
InactiveCN1302008CEasy to separate and purifyThe synthesis process is simpleDipeptidesL-alanyl-l-glutamineDipeptide
The present invention relates to synthesis of dipeptide containing amino acid, and provides a kind of synthesis process of N(2)-L-alanyl-L-glutamine dipeptide with low material cost, simplicity, high yield, no need of separating and purifying intermediate product, easy product separation and purification and environment friendship. The synthesis process includes the reaction of amino acid with protected N-terminal with phosphorus triphenyl oxide and triphosgene in organic solvent to form active ester; the reaction of the active ester with glutamine in water solution of inorganic alkali; acidifying with inorganic acid and eliminating N-terminal protecting radical.
Owner:XIAMEN UNIV
New process for synthesizing tenofovir disoproxil fumarate
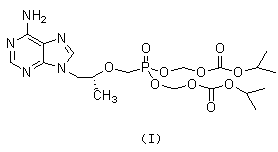
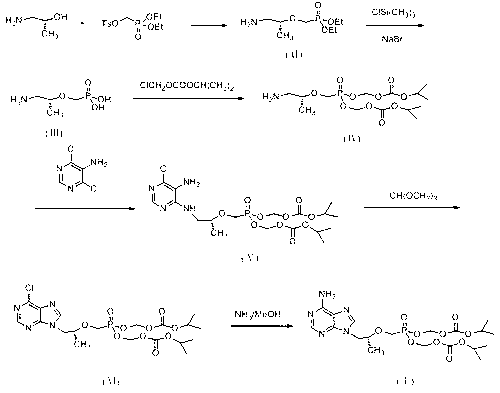
ActiveCN103304601AFew reaction stepsEffective controlGroup 5/15 element organic compounds1-Amino-2-propanolPhosphoric acid
The invention discloses a new process for synthesizing tenofovir disoproxil fumarate. The new process comprises the following steps of: reacting (R)-(-)-1-amino-2-propanol serving as a raw material with p-benzenesulfonyloxymethyl phosphoric acid diethylester, and then performing hydrolysis and condensation to obtain (R)-2-O-[bis(isopropoxycarbonyloxomethyl)phosphon-methyl]-1-amino-2-propanol (IV); and condensing the compound shown in the formula (IV) with 5-amino-4,6-dichloropyrimidine, and then performing cyclization and aminolysis, thereby obtaining the tenofovir disoproxil fumarate. The new process for synthesizing tenofovir disoproxil fumarate provided by the invention is low in production cost, safe in process, good in product quality and suitable for industrial production.
Owner:APELOA PHARM CO LTD +1
Production technology of 3-hydroxytetrahydrofuran with high optical purity
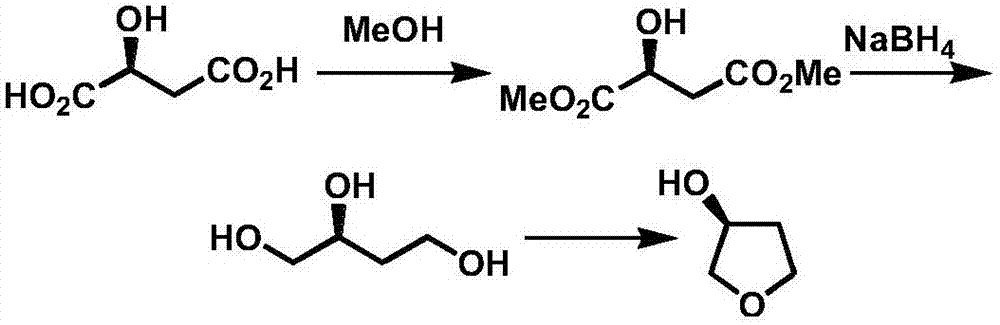
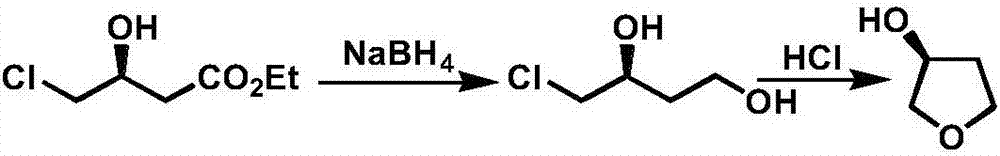
InactiveCN107098872AAvoid racemizationInhibit side effectsOrganic chemistry3-HydroxytetrahydrofuranSolvent
The invention discloses a production technology of3-hydroxytetrahydrofuran with high optical purity. The production technology comprises the following steps: (1) taking chloroacetoacetic acid ethyl ester as a starting raw material, adding appropriate amount of solvents, chiral catalysts and reducing agents, and reacting at an appropriate temperature to obtain chiral ethyl 4-chloro-3-hydroxybutyrate; (2) taking the chiral ethyl 4-chloro-3-hydroxybutyrate obtained in step (1) as a raw material, adding the appropriate amount of solvents and metal borohydride reducing agents, and reacting at the appropriate temperature to obtain chiral 4-chloro-3-hydroxy-1-butanol; (3) taking the chiral 4-chloro-3-hydroxy-1-butanol obtained in step (2) as the raw material, adding appropriate amount of catalysts and solvents, and reacting at the appropriate temperature to obtain chiral 3-hydroxytetrahydrofuran. According to the production technology of the 3-hydroxytetrahydrofuran with the high optical purity, the chiral 3-hydroxytetrahydrofuran can be produced through a three-step reaction, the shortcomings of complicated production operation and high production cost are solved, and products with high optical purity can be produced.
Owner:SUZHOU HUADAO BIOLOGICAL PHARMA
L-pantoyl lactone dehydrogenase derived from Cnuibacter physcomitrellae
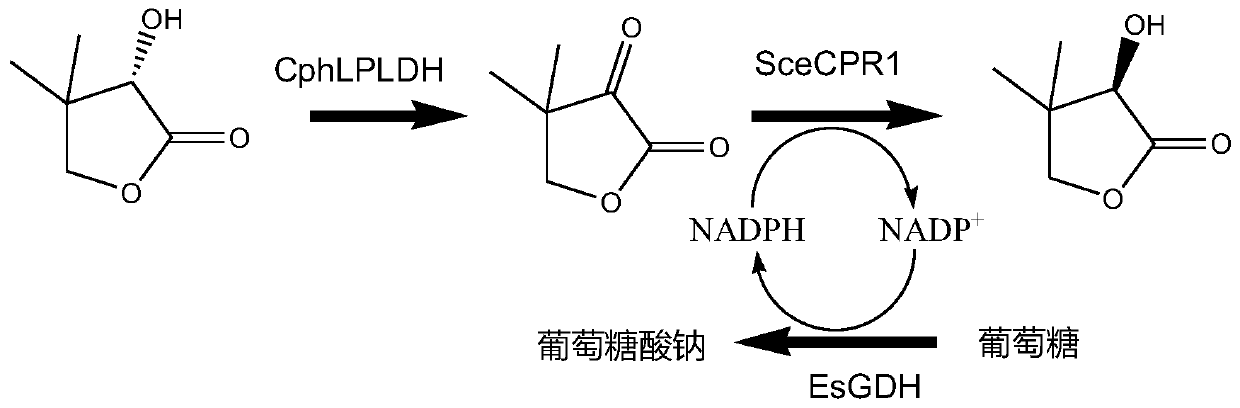
ActiveCN110396507AIncrease enzyme activityAvoid racemizationBacteriaMicroorganism based processesPantoyl lactoneSpontaneous hydrolysis
The invention relates to the technical field of genetic engineering, particularly relates to L-pantoyl lactone dehydrogenase and application thereof, and discloses L-pantoyl lactone dehydrogenase derived from Cnuibacter physcomitrellae for the first time. The L-pantoyl lactone dehydrogenase has excellent enzyme activity on L-pantoyl lactone and can be applied to catalytic synthesis of D-pantoyl lactone. A system for chiral inversion synthesis of D-pantoyl lactone with L-pantoyl lactone by multi-enzyme cascade catalysis is constructed, and a genetically engineered bacterial cell co-expressing L-pantoyl lactone dehydrogenase, D-ketopantolactone reductase and glucose dehydrogenase is used as a catalyst, accumulation and spontaneous hydrolysis of the intermediate product ketopantolactone is avoided, and the steps of separating the intermediate product, racemizing the L-pantoyl lactone, lactonizing D-pantoic acid under an acidic condition are omitted, so that the reacting process is simplified, use of acid and alkali is reduced, and the reaction efficiency is improved.
Owner:HANGZHOU XINFU TECH CO LTD +1
Method for synthesizing Valsartan with high optical purity
The invention relates to a synthesis of high optical purify N-[[2'-(1H- tetrazoline-5-group)- (1,1'- diphenyl)-4-group]-methyl-]-L-valine, and using it as raw material in the synthesis of high optical valsartan. Using the 2'- tetrazoline group-4-methyl bromide biphenyl protected by tetrazoline and esterification protected carboxyl of L- valine(its salt) as raw material, by nucleophilic substitution, doing deprotective reaction in acidic condition, getting high optical purify N-[[2'-(1H-tetrazoline-5-group)-(1,1'-diphenyl)-4-group]-methyl-]-L-valine; in the existence of lower aliphatic alcohol, condensing with n-valeryl chloride to synthesize the valsartan in temperature range -20deg C-40deg C, controlling pH value between 1.5-5.0.
Owner:LINHAI TIANYU PHARMA
Method for enzymatically synthesizing chiral acetic cyanhydrin ester by directly using cyanogen salt as cyanogens source
InactiveCN102417917ASimple methodHigh industrial application valueFermentationAcetic anhydrideAddition reaction
The invention discloses a method for enzymatically synthesizing chiral acetic cyanhydrin ester by directly using cyanogen salt as a cyanogens source. The method includes the following steps: enzyme, the cyanogen salt, water, organic solvent and acetic acid are sequentially added into a reaction vessel and uniformly mixed, aldehyde compound is dripped, reaction takes place under the condition of room temperature, and the reaction time is 6 to 100 hours; after the reaction is finished, separated organic phase is dipped into mixture containing acetic anhydride and pyridine to react under the room temperature for 3 to 24 hours; and after saturated sodium bicarbonate and water washing, drying, concentration and column chromatography purification, the corresponding target compound is obtained. The method directly uses the cyanogen salt as the cyanogens source and the cheap, easy-to-obtain acetic acid as a proton donor, and effectively inhibits the spontaneous chemical addition reaction of HCN and aldehyde ketone by controlling the proportion between the water and the organic phase, so that enzymatic reaction under hydroxynitrile lyase can be efficiently carried out. The method is simple and safe, can obtain high yield of high-optical purity chiral acetic cyanhydrin ester, and has high industrial application value.
Owner:EAST CHINA NORMAL UNIVERSITY
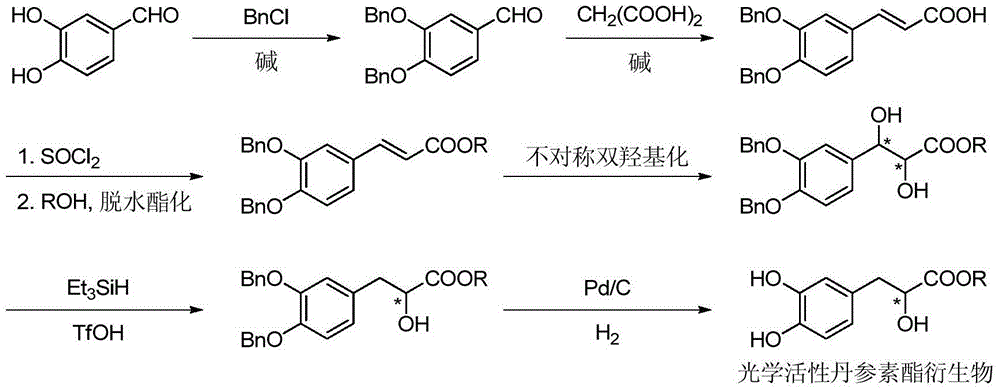
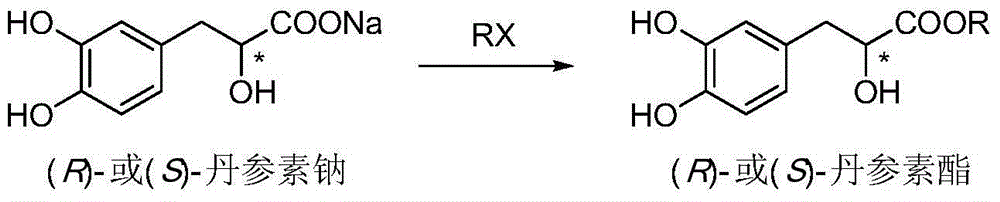
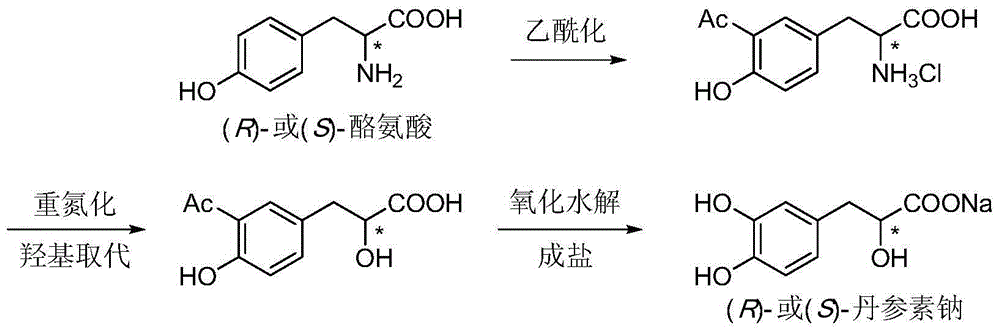
Asymmetric synthesis method for tanshinol ester derivative
InactiveCN105085264AAvoid racemizationAddressing Reaction Selectivity IssuesOrganic compound preparationCarboxylic acid esters preparationEnantioselective synthesisCarbonate ester
The invention discloses an asymmetric synthesis method for a tanshinol ester derivative. The method comprises: carrying out direct reaction on (R)- or (S)-sodium tanshinol (the formula is shown in the description) and halogenated hydrocarbon, sulphonate, sulphate or carbonic ester to obtain an (R)- or (S)-tanshinol ester derivative (the formula is shown in the description) with high optical activity, wherein R is C1-C7 alkyl or bornyl. The method disclosed by the invention is free of complex separation steps in the whole course, is simple in preparation process, does not pass through chromatographic column, is low in production cost, and is quite suitable for industrialized batch production.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing L-pyroglutamic acid
The invention discloses a method for preparing L-pyroglutamic acid, which comprises the following steps: placing L-glutamic acid in an ethanol system; adding a proper amount of a dehydration catalyst; heating to 88 to 100 DEG C to perform the dehydration reaction; after the reaction, carrying out filtering, concentration and crystallization; carrying out suction filtration on crystals to obtain the L-pyroglutamic acid competitive product. The method disclosed by the invention belongs to a closed process, is simple to operate and adopts a proper proportion; the L-pyroglutamic acid generated through dehydration is soluble to ethanol, the L-glutamic acid which is not dehydrated is insoluble to ethanol, the L-glutamic acid which is not dehydrated is directly removed by filtering, crystallization is carried out to obtain the high-purity L-pyroglutamic acid, and the obtained L-pyroglutamic acid has purity of over 99.5 percent and yield of over 85 percent; due to addition of the dehydration catalyst concentrated sulfuric acid, the dehydration reaction is accelerated; moreover, the reaction is performed at a lower reaction temperature, so that the L-pyroglutamic acid is prevented from generating racemization; the concentrated sulfuric acid is in a mother liquor obtained by suction filtration and can be returned together with the L-glutamic acid for next batching; the method is low in cost and is suitable for industrial production.
Owner:宜兴市前成生物有限公司
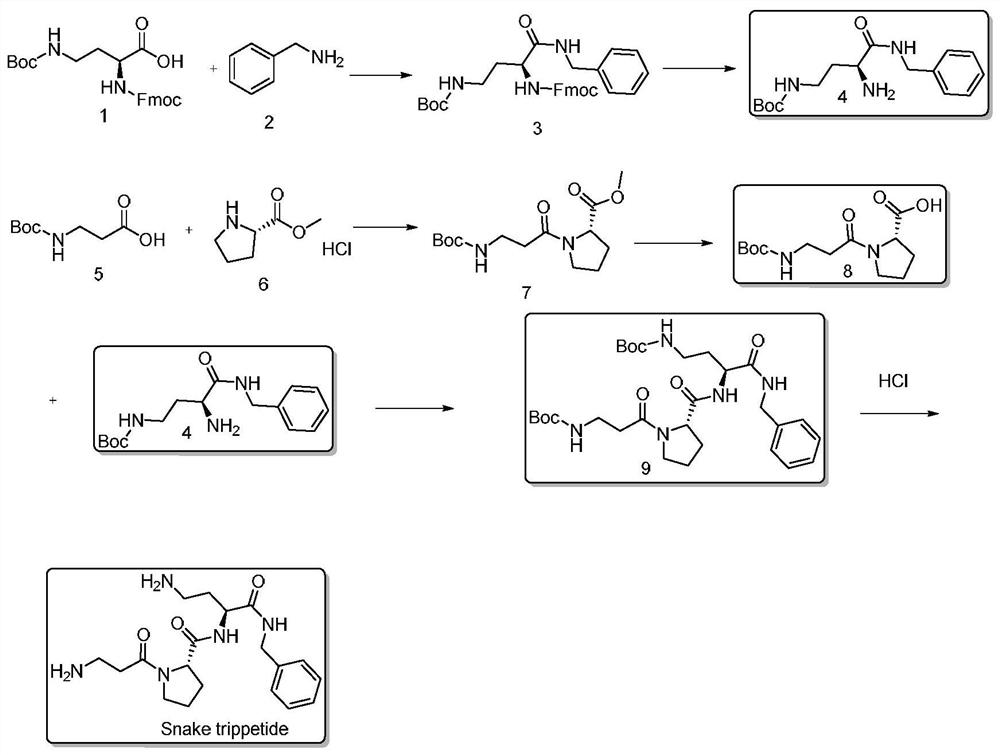
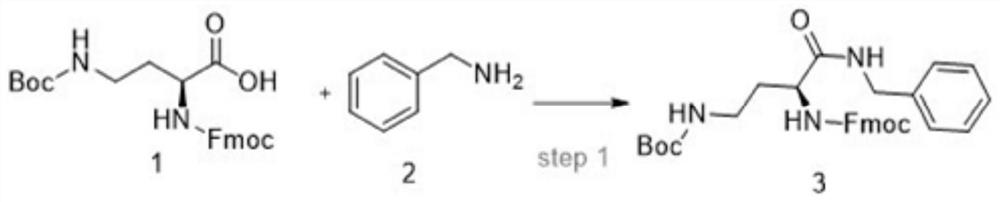
Liquid-phase synthesis method of snake venom-like tripeptide
InactiveCN112430253ADoes not involve racemization issuesAvoid racemizationPeptide preparation methodsBulk chemical productionFluid phaseHATU
The invention discloses a liquid-phase synthesis method of snake venom-like tripeptide. The method comprises the following steps: using raw materials Fmoc-Da (Boc)-OH, benzylamine, Boc-beta-Ala-OH andH-Pro-OMe. HCl / H-Pro-OBzl. HCl as raw materials and DIC / HOBt, DIC / HOAt, EDC / HOBT, HATU, HBTU and the like as reagents, synthesizing polypeptide H-beta-Ala-Pro-Dab(Boc)-NHBzl, then cutting off a protective group Boc under an acidic condition and purifying to obtain the product. The product H-beta-Ala-Pro-Dab(Boc)-NHBzl is directly obtained by using a solid-phase synthesis method, the stability problem of CTC resin is solved in the product, the racemization problem of Dab is avoided by adopting a method of connecting Fmoc-Db (Boc)-OH and benzylamine, and the synthesis method is short in processroute, very short in production period, suitable for large-scale production, good in method stability and suitable for large-scale production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
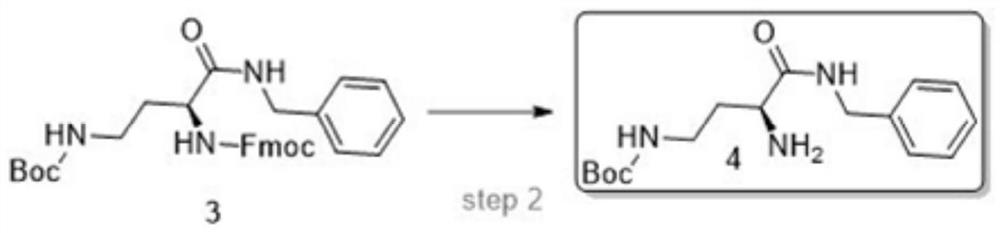
Synthesis method of semaglutide
InactiveCN112028986AHigh purityAvoid efficiencyPeptide preparation methodsBulk chemical productionAmino acid side chainDipeptide
The invention discloses a synthesis method of semaglutide. The synthesis method comprises the following steps: (1) taking Fmoc-Gly-Wang resin as a solid-phase carrier and removing an Fmoc protection group; gradually coupling amino acids from a C end to an N end in sequence; coupling to 20 amino acid R-Lys (Fmoc)-OH at the N end of a main chain; (2) removing a side chain Fmoc protection groupof the 20 amino acid at the N end of the main chain and coupling side chain amino acids in sequence; (3) removing a 20 amino acid R protection group at the N end of the main chain and coupling residual amino acids of the main chain in sequence according to a peptide sequence to obtain semaglutide full-protection peptide resin, wherein dipeptide fragments are selected as 18 -19 amino acids at the N end and 1 -2 <nd> amino acids at the N end; and (4) cracking and precipitating the semaglutide full-protection peptide resin through a cracking solution to obtain semaglutide crudepeptide. The method disclosed by the invention is simple and convenient; the prepared semaglutide has high purity and industrial production is facilitated.
Owner:哈尔滨吉象隆生物技术有限公司
Synthesis process of rivastigmine hydrogen tartrate
ActiveCN104072391AAvoid racemizationControllable optical purityCarbamic acid derivatives preparationOrganic compound preparationReaction temperatureRivastigmine
The invention aims to solve the technical problem that during industrial production, the yield of rivastigmine hydrogen tartrate is low, and provides a synthesis process of rivastigmine hydrogen tartrate. A racemized raw material is adopted to firstly synthesize racemized rivastigmine, and then direct separation is performed to obtain an optically pure S-shaped isomer, so that racemization in the reaction process is effectively avoided, the optical purity is controllable, and further the product quality is controllable. Through simplified process flow, the optimal reaction solvent, reaction time and reaction temperature can be discovered via exploration, and the method for preparing rivastigmine hydrogen tartrate, which is high in yield, low in cost and simple to operate, has easily accessible raw materials and is suitable for being realized in industrial production, is found.
Owner:SICHUAN XINSIDUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com