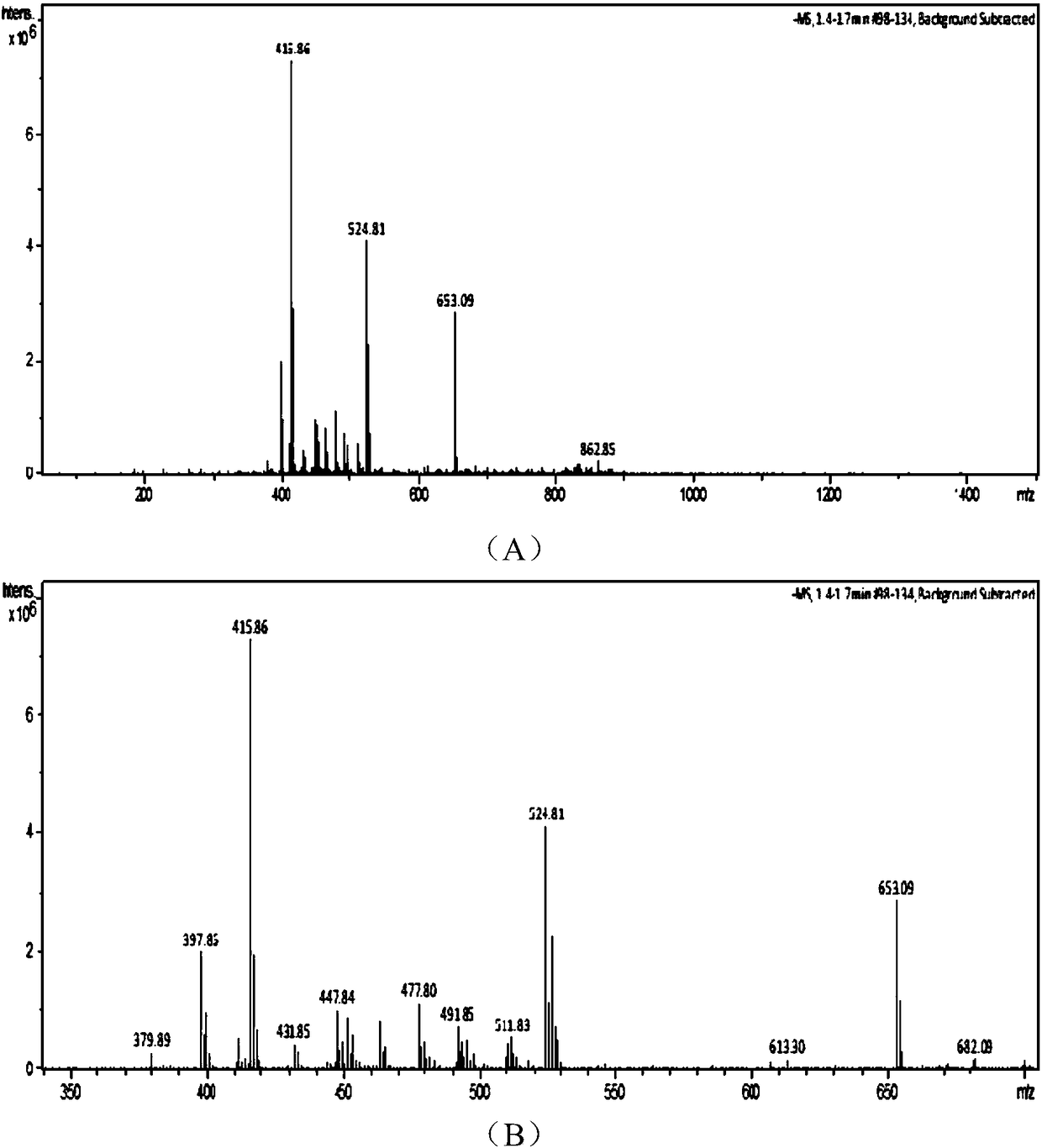
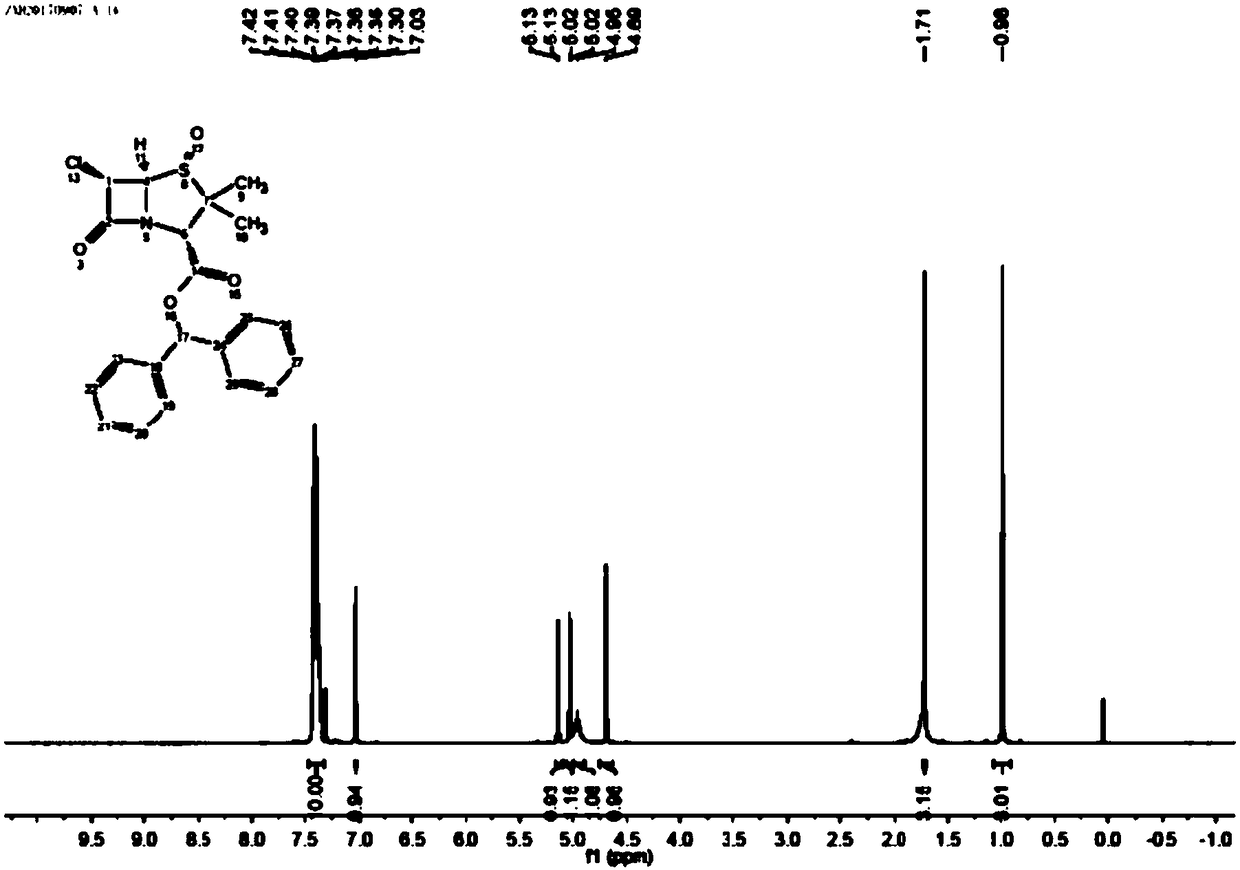
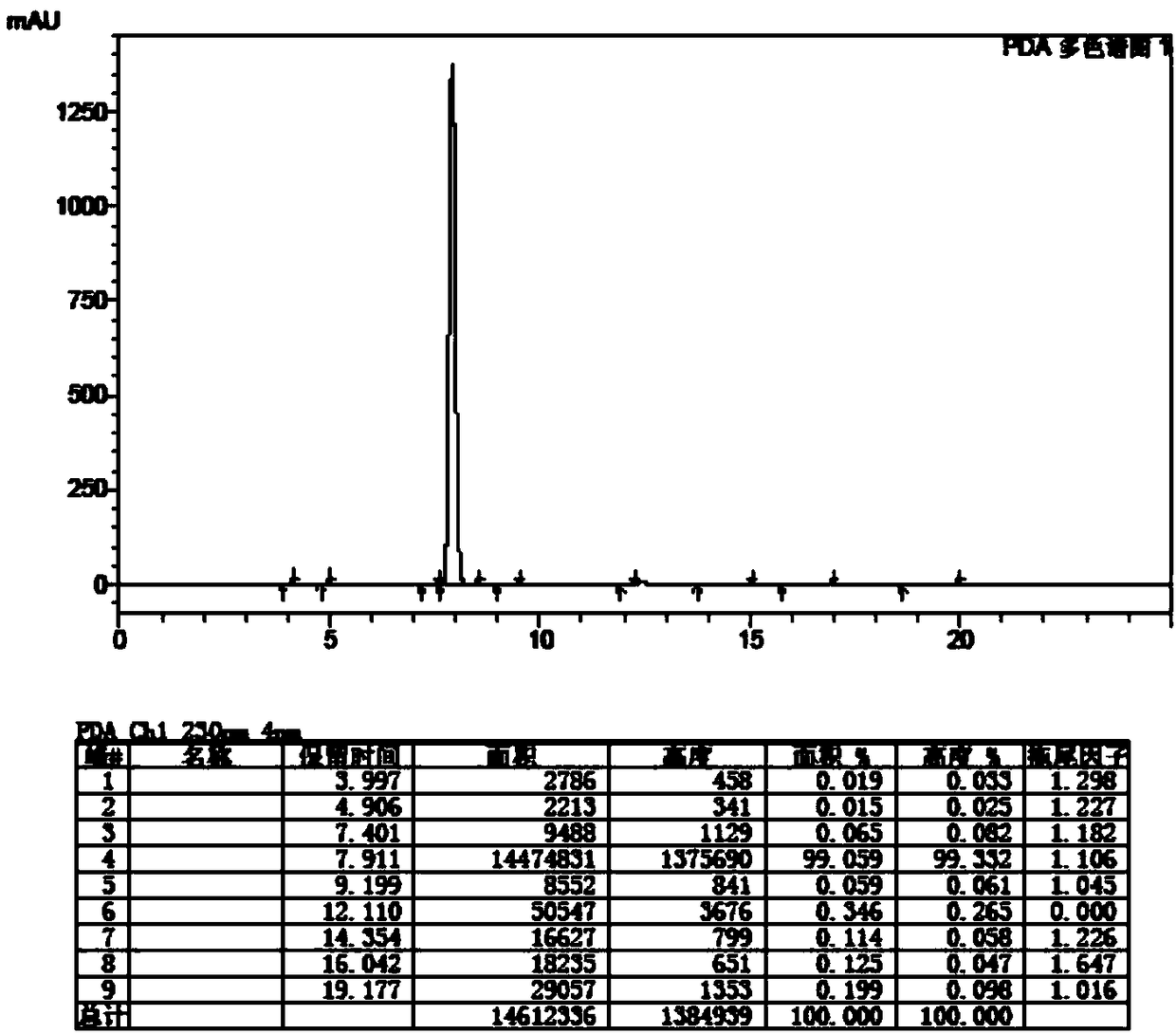
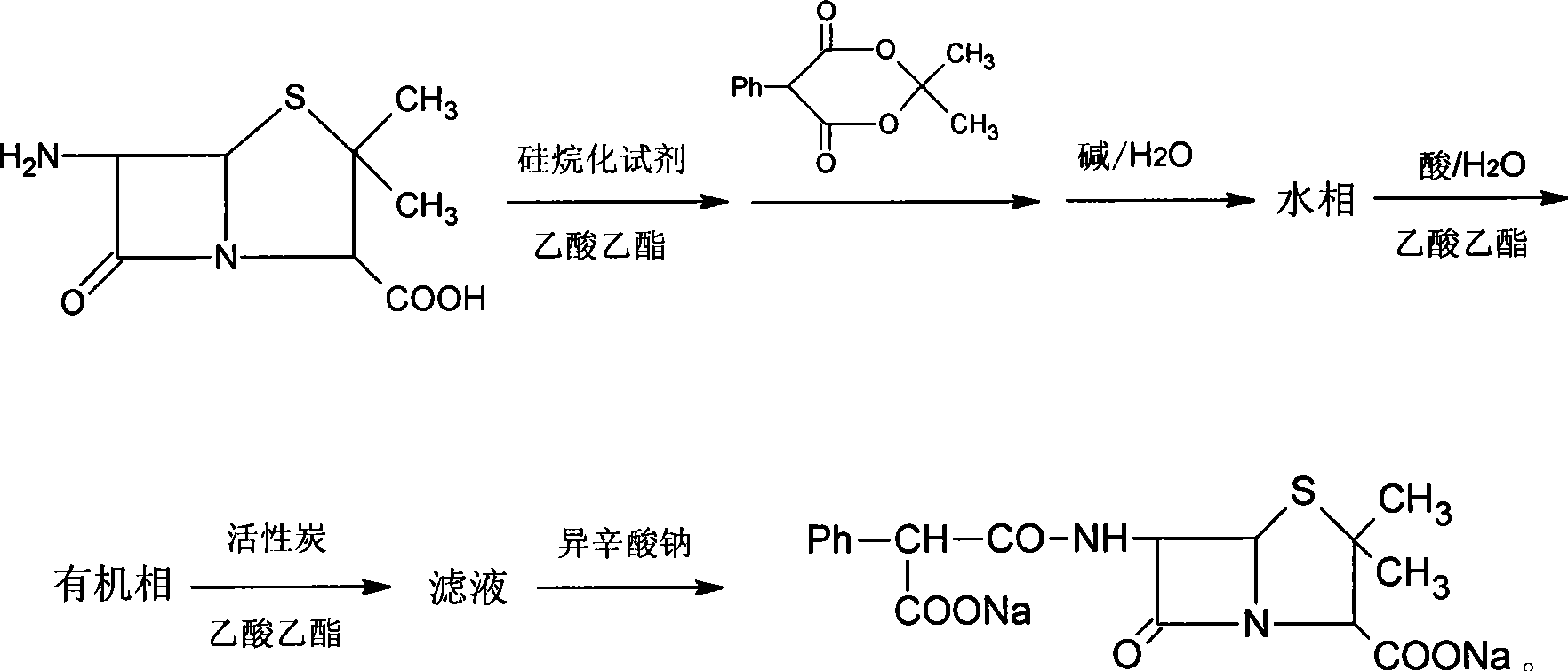
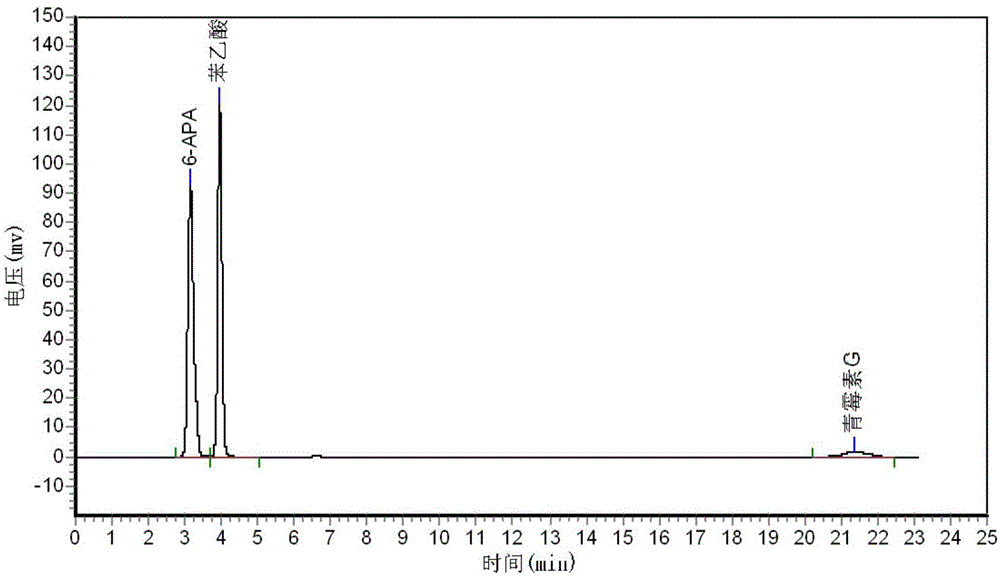
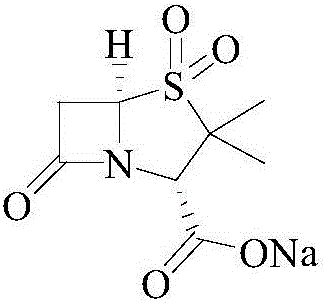
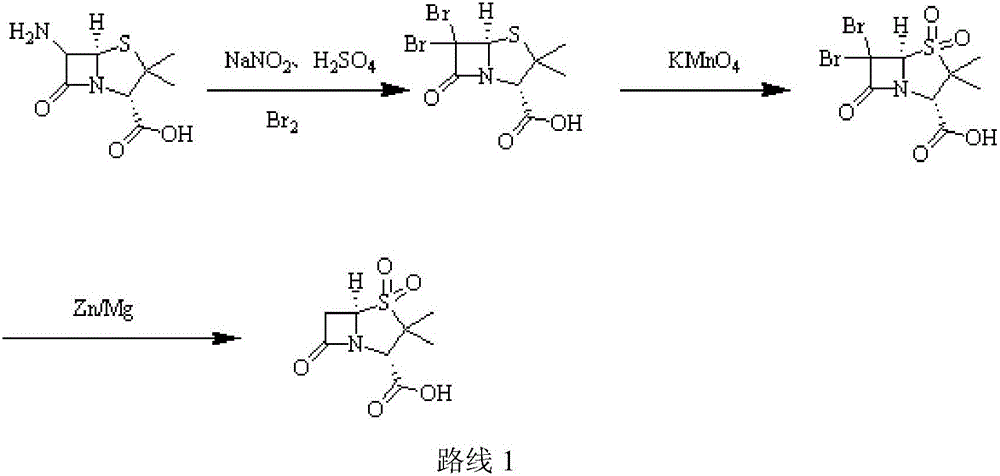
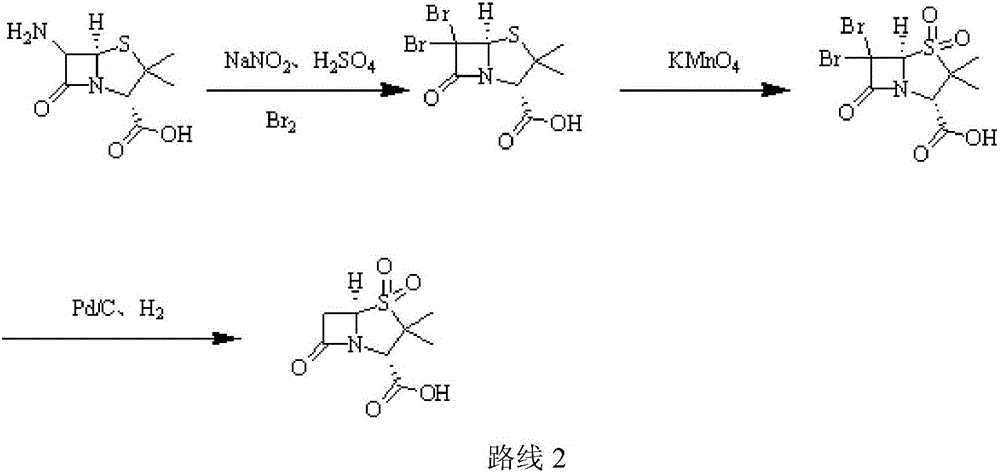
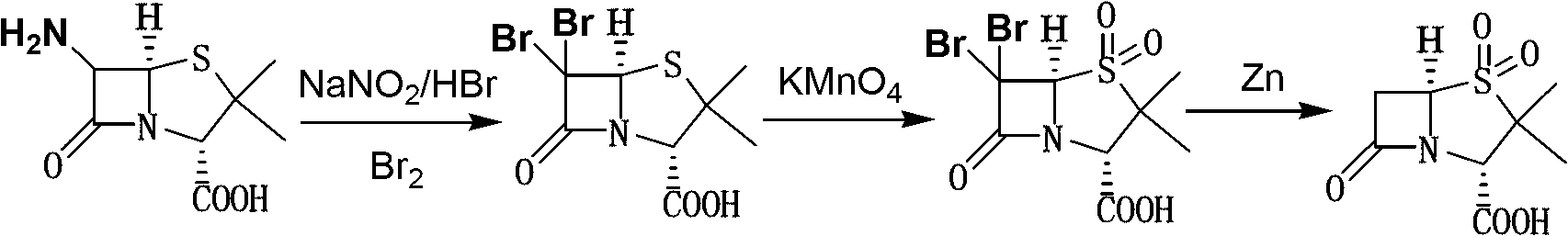Patents
Literature
73 results about "Penicillanic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
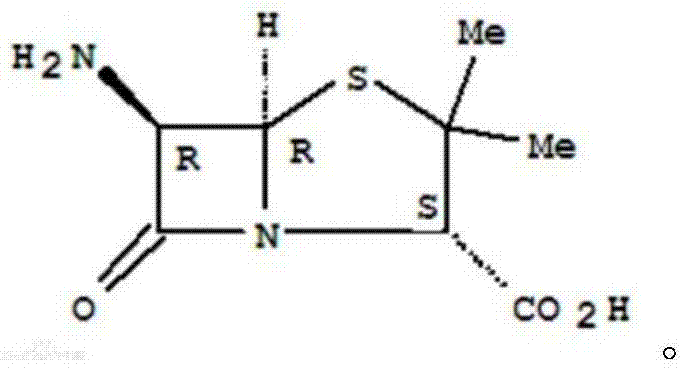
A building block of penicillin, devoid of significant antibacterial activity. (From Merck Index, 11th ed)
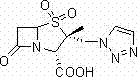
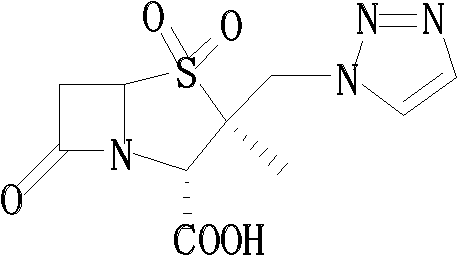
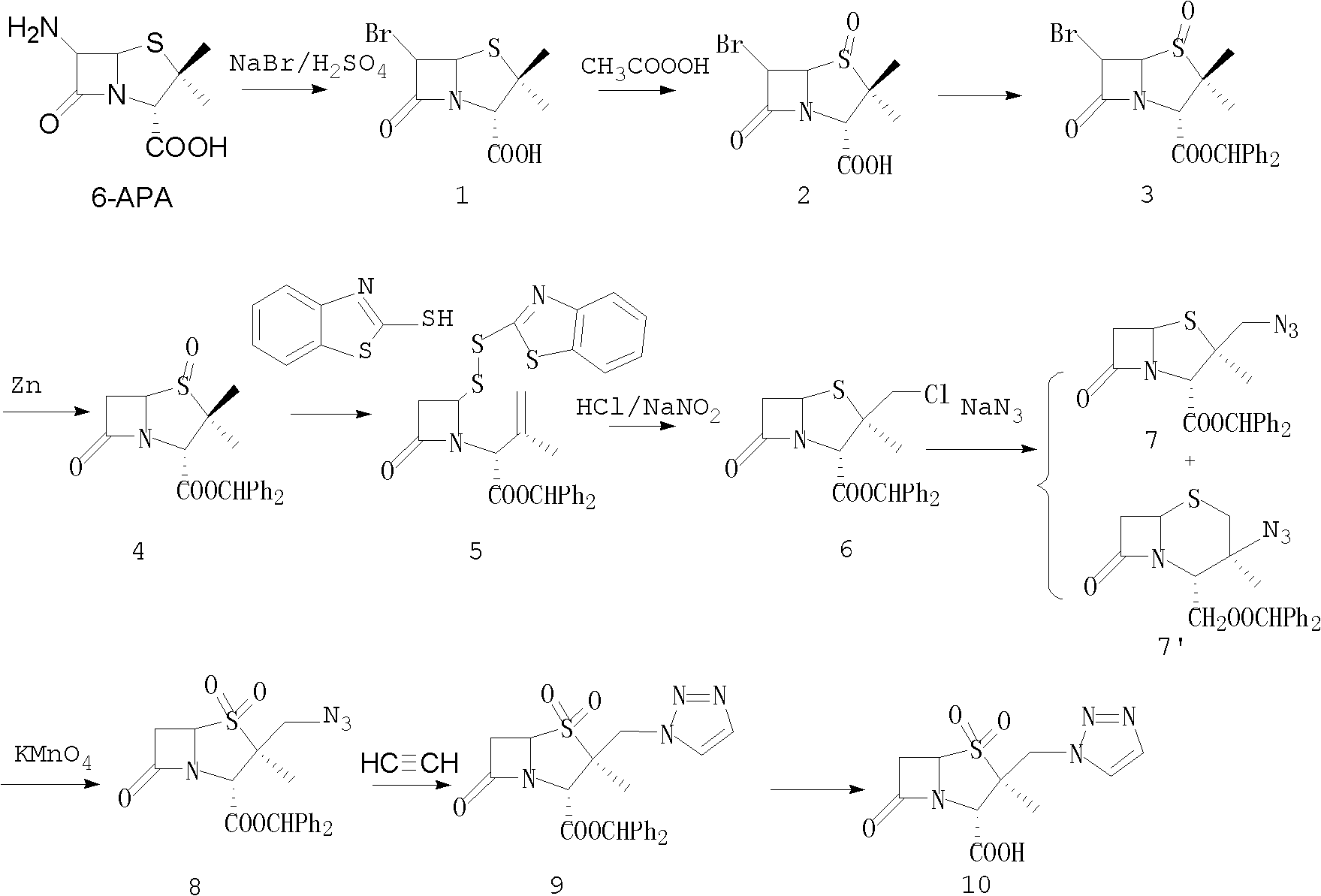
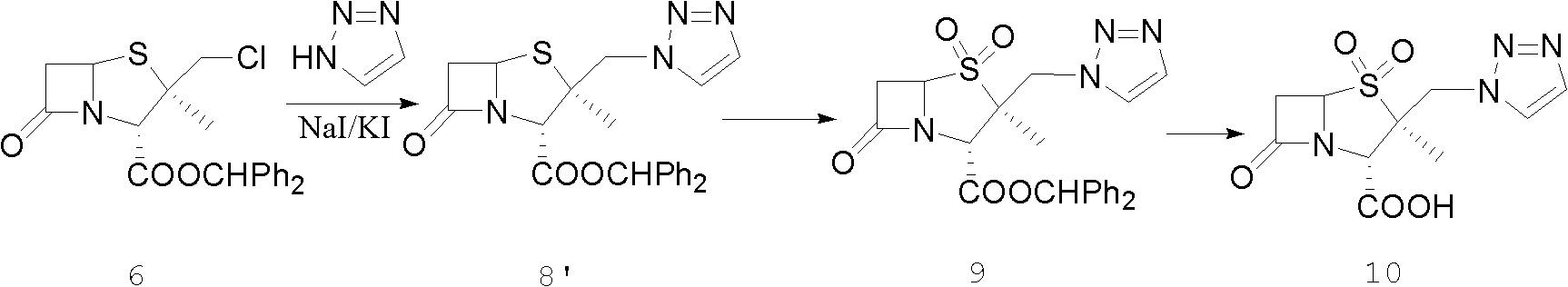
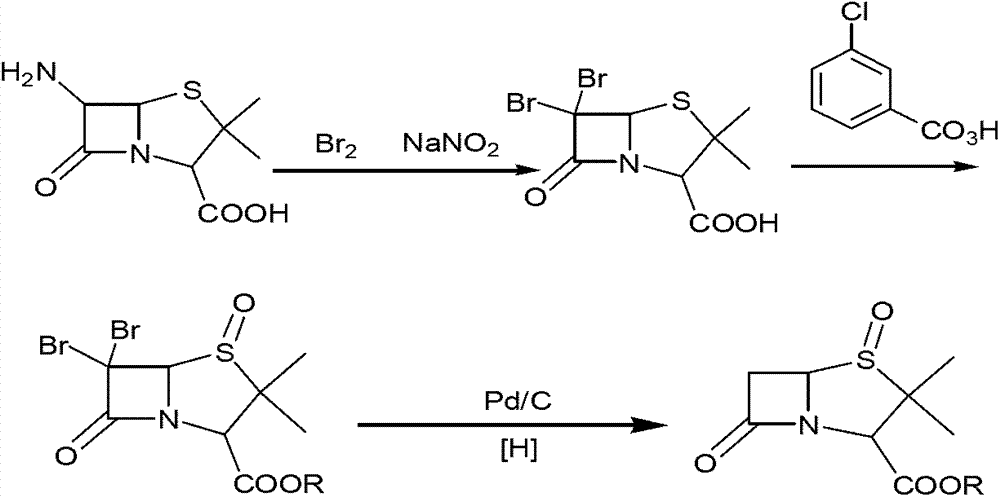
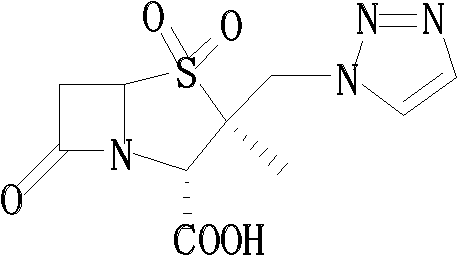
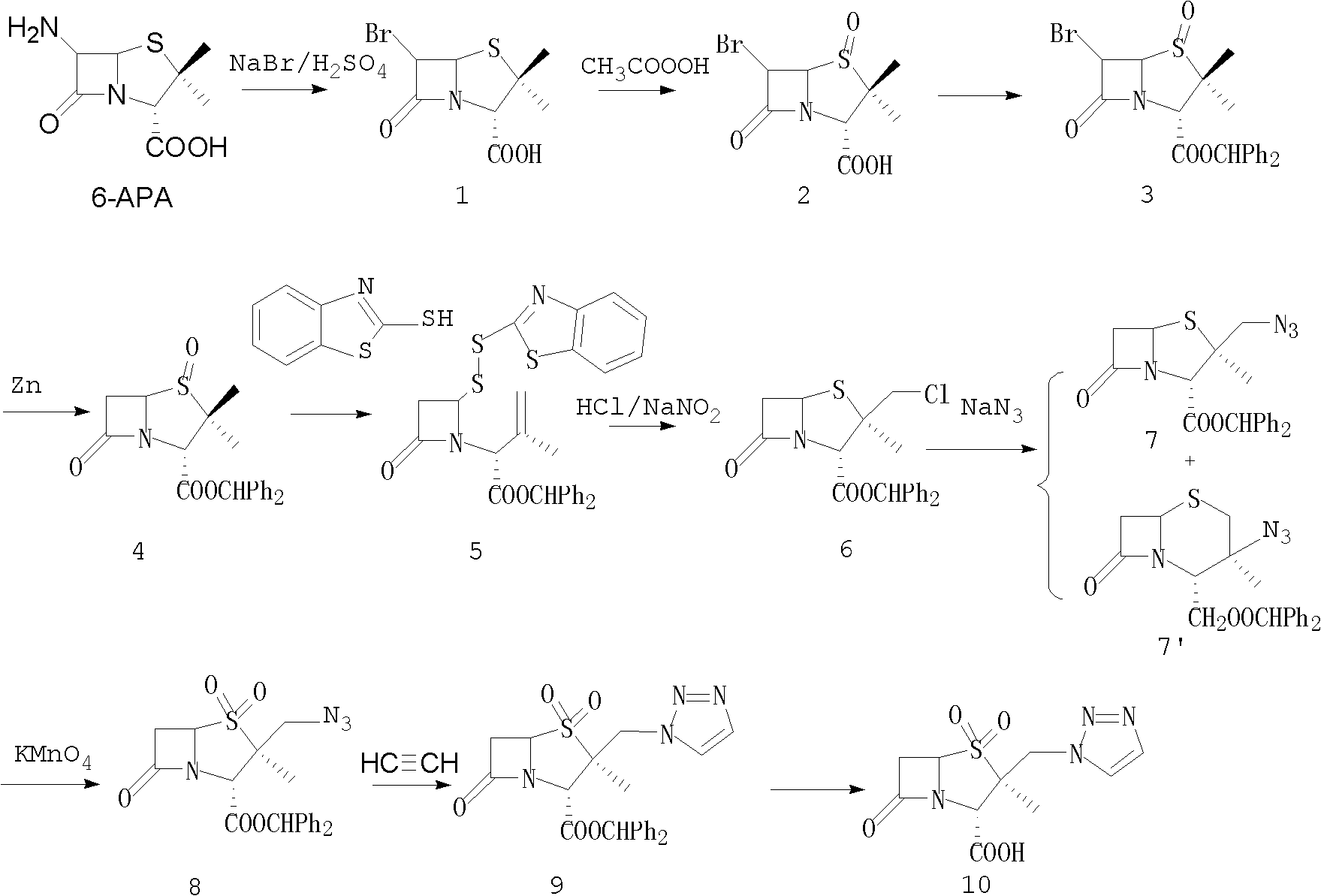
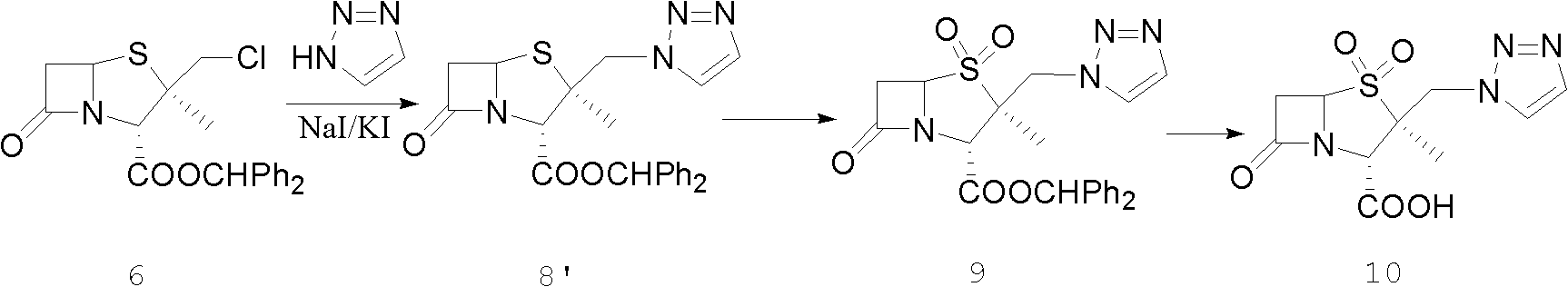
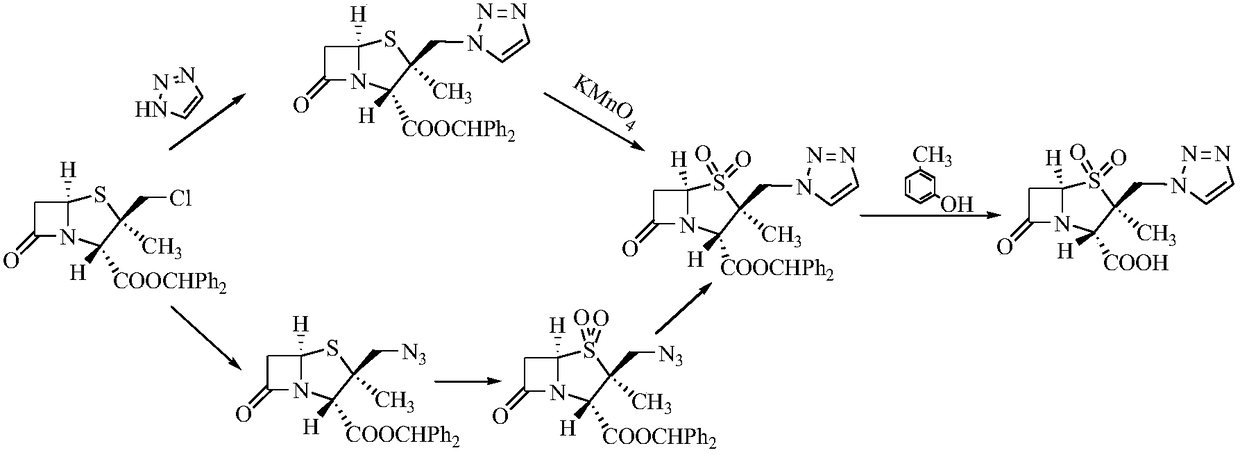
Tazobactam synthesis method
ActiveCN102020663AReduce usageWill not polluteOrganic chemistryChemical recyclingMetacresolSynthesis methods
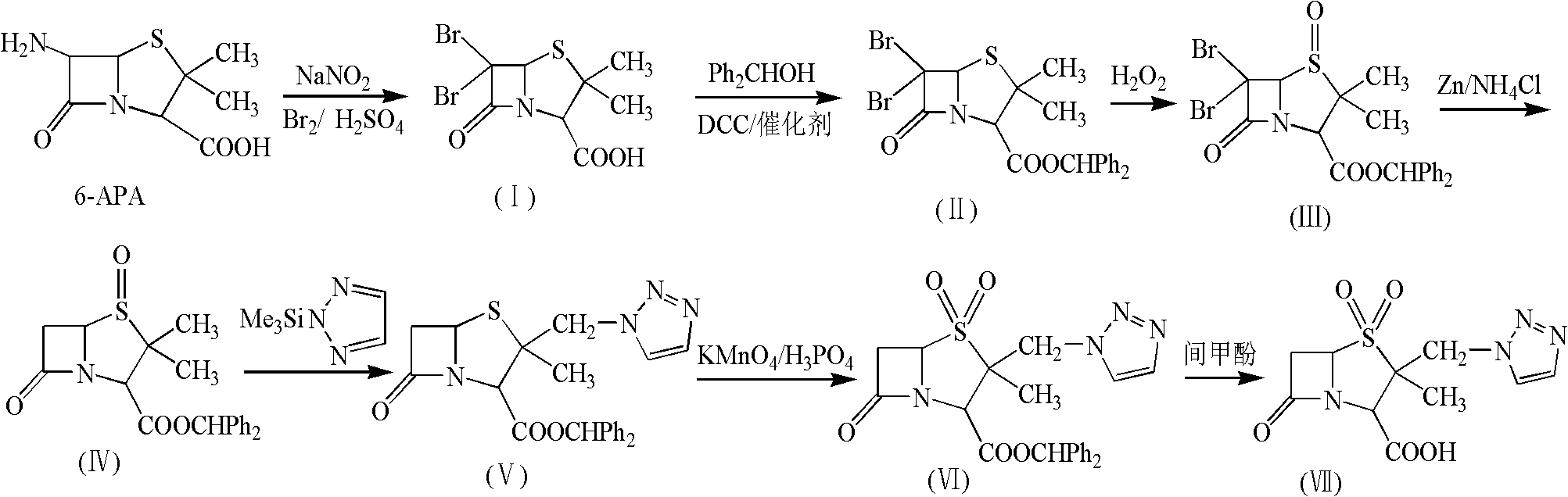
The invention relates to a tazobactam synthesis method which comprises the steps of: with 6-APA(Amino Penicillanic Acid) as raw material, preparing a key intermediate 6,6-dihydro penam sulphoxide acid diphenylcarbinol ester through successive reactions of esterification, oxidation, reduetive debromination and the like without separation; then, reacting with 2-triphenyl silicon-1,2,3-triazole; introducing a triazole ring; and finally obtaining the final product of tazobactam through potassium permanganate oxidation and metacresol deprotection. The tazobactam synthesis method is mainly characterized in that a phase transfer catalyst is introduced in the first step, therefore, the reaction rate and the product purity are improved; since an environment-friendly hydrogen peroxide-cobalt acetate catalytic oxidation system is adopted in the third step, the characteristics of good reaction selectivity, high yield, catalyst recyclability and the like are achieved; a method for synthesizing 2 alpha-methyl-2 beta-(1,2,3- triazole-1- radical) methyl penam-3 alpha-carboxylic acid diphenylcarbinol ester by using 2-triphenyl silicon-1,2,3-triazole is adopted in the fifth step, and the tazobactamsynthesis method is simple and convenient to operate, is safe and reliable, shortens the reaction route and improves the total yield. Compared with the traditional process, the tazobactam synthesis method greatly reduces the production cost and the environment pollution and has greater implementation value and economic benefits.
Owner:YIYUAN XINQUAN CHEM
Penam iodide, preparation and use thereof
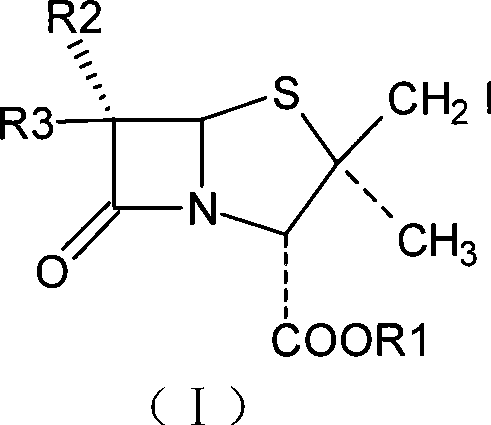
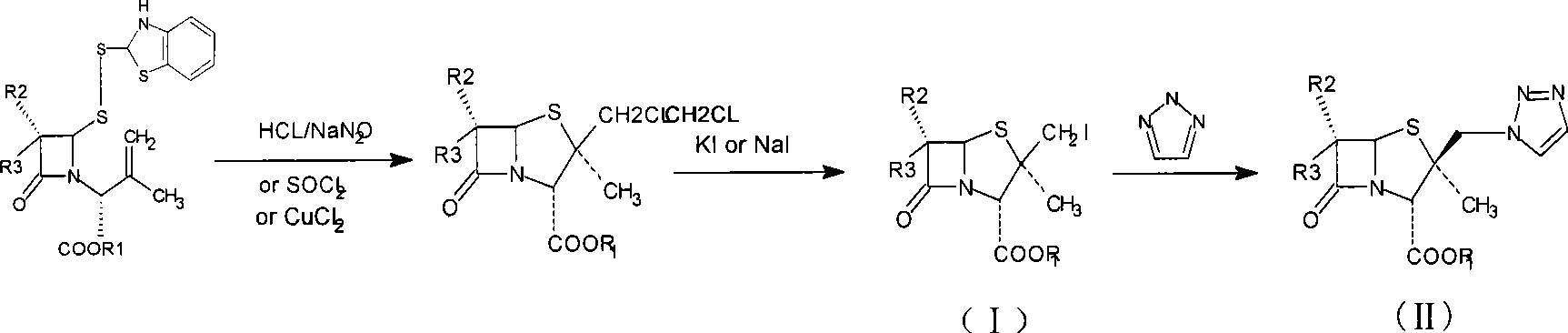
ActiveCN101434610AReduce pollutionLow production costOrganic chemistryBulk chemical productionIodideStructural formula
The invention relates to an intermediate iodide in the synthesis of Tazobactam, and a preparation method and the application thereof in preparing Tazobactam, and discloses the structural formula (I) of 2Beta-iodomethyl penicillanic acid ester. The invention has unique technique, stable product quality, mild technique condition and high yield, is easy to be controlled, requires relatively low production cost, reduces environmental pollution, needs no special equipment and is suitable for industrialized mass production.
Owner:QILU PHARMA HAINAN +1
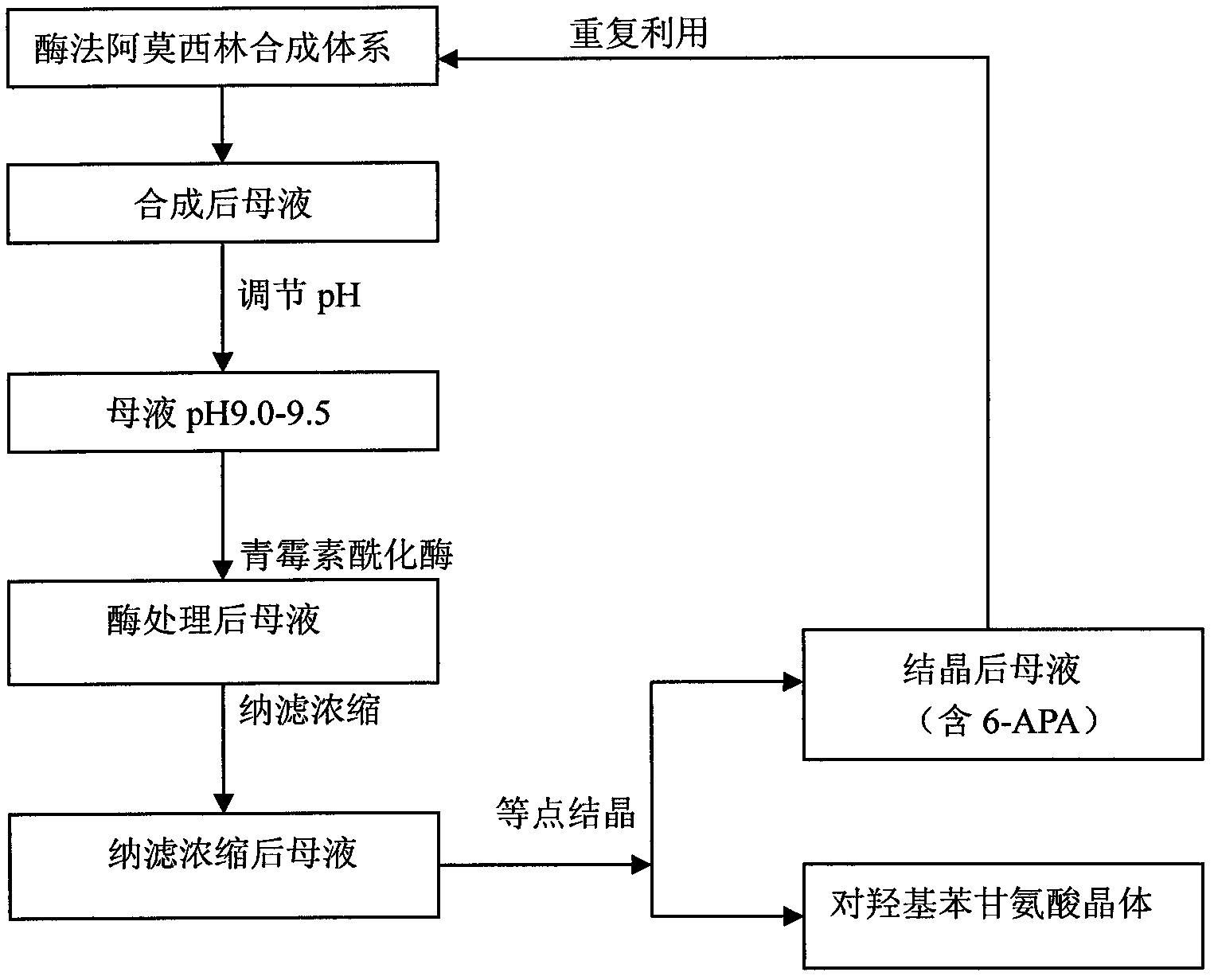
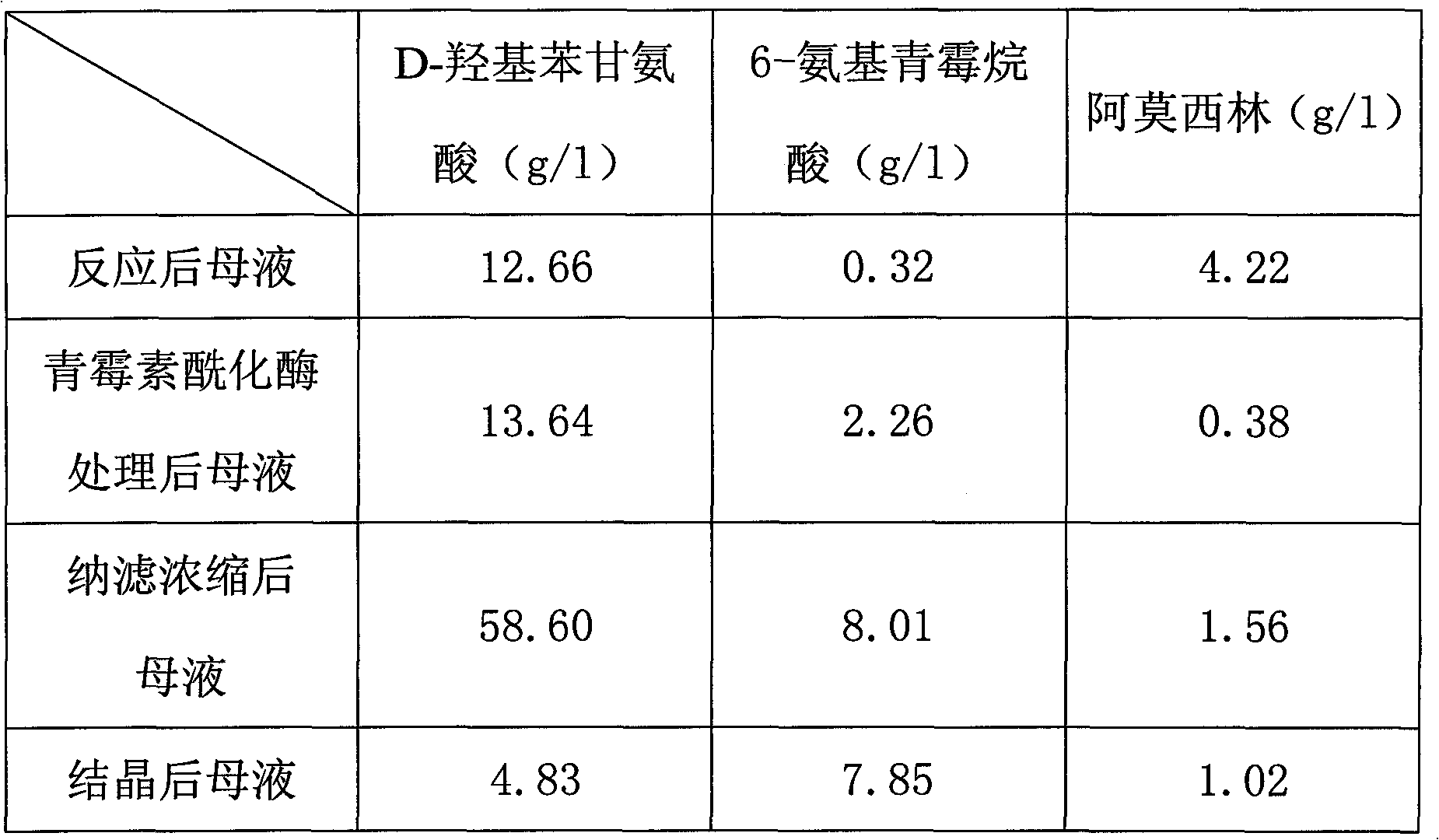
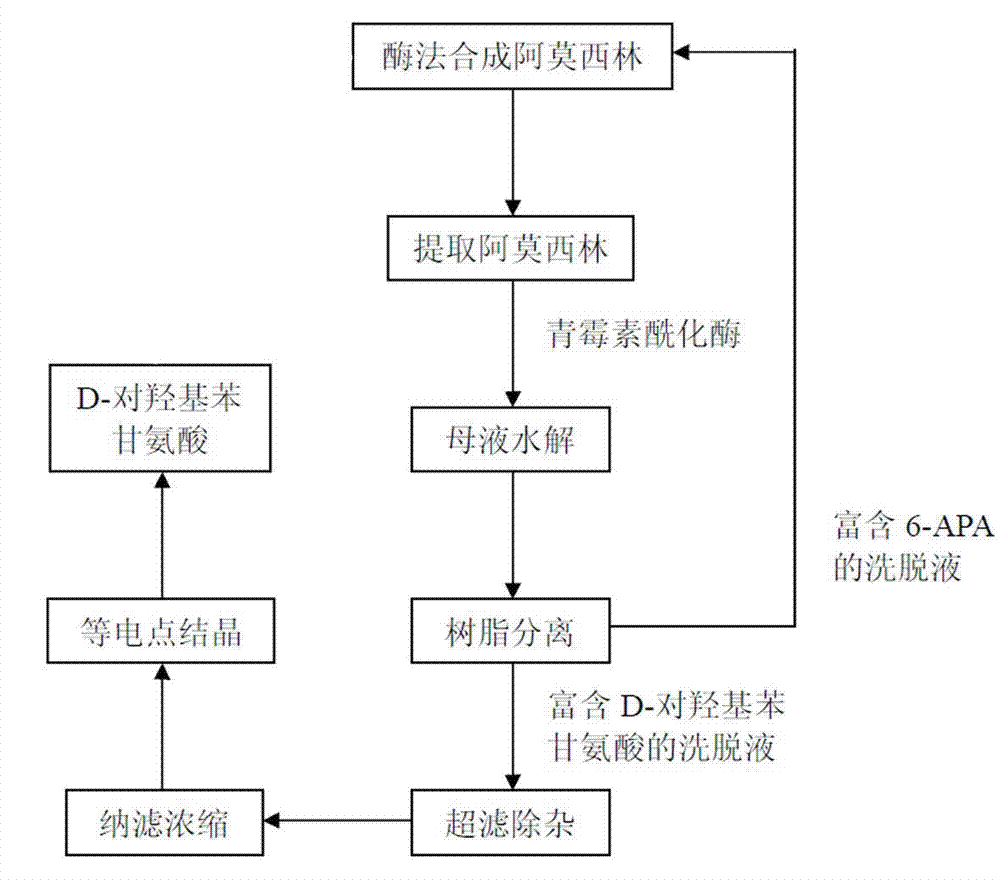
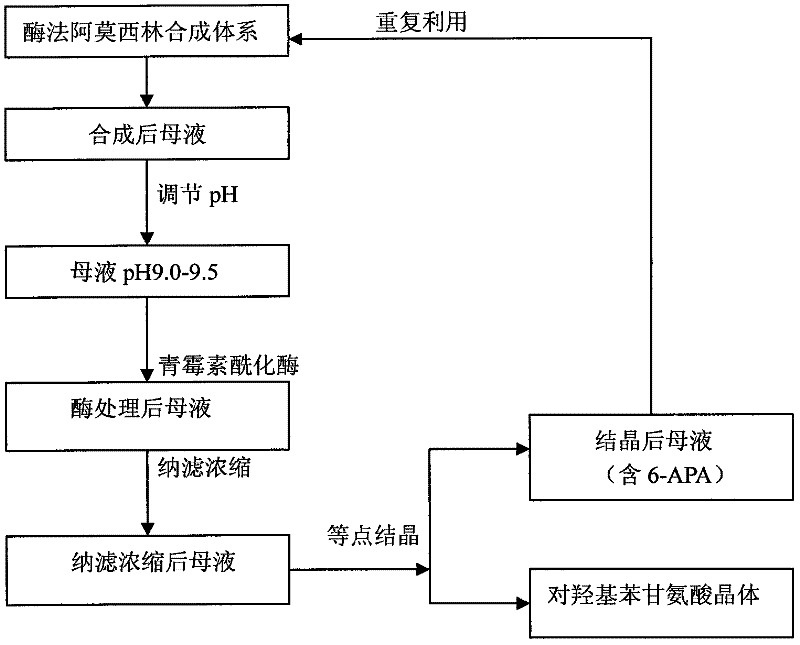
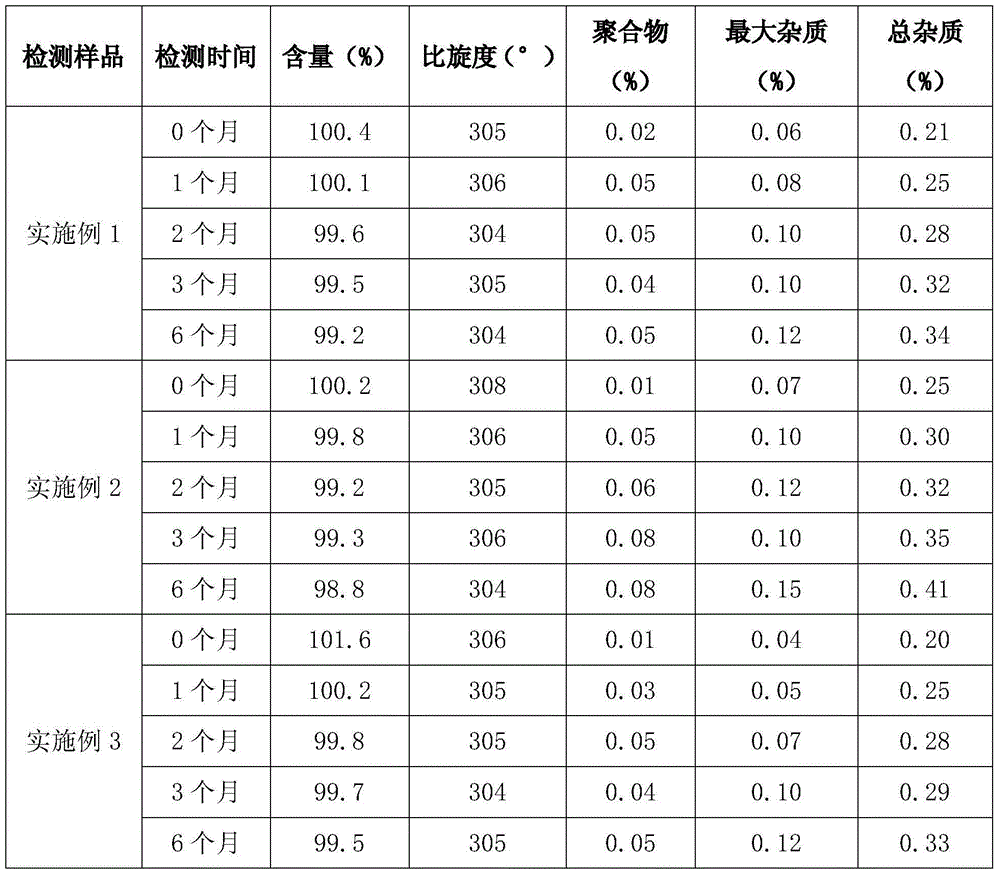
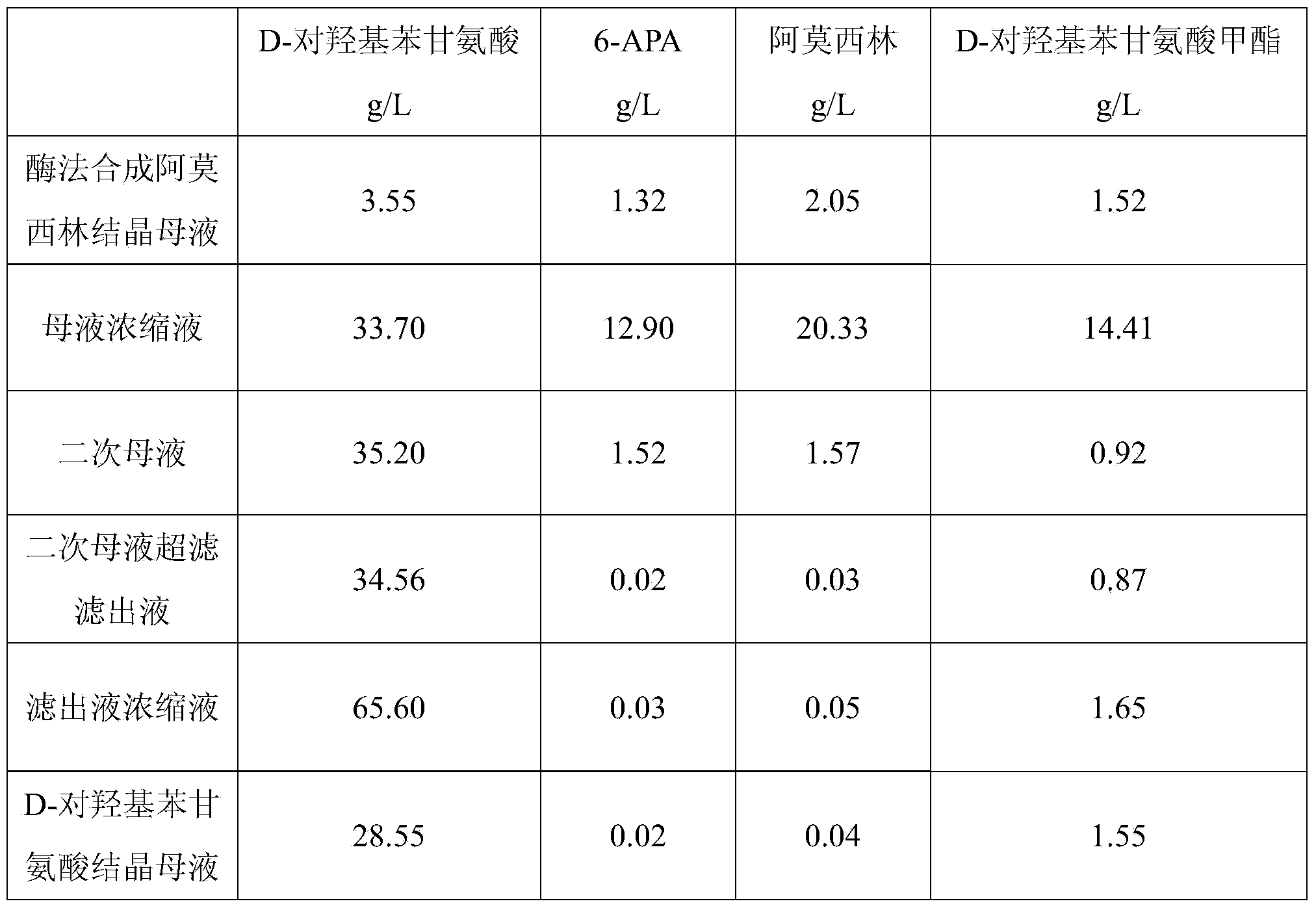
Method for recycling active ingredients in amoxicillin mother liquor synthesized by enzymatic method
ActiveCN102816803AAvoid adverse reactionsRelieve pressureOrganic compound preparationChemical industryBULK ACTIVE INGREDIENTP-hydroxyphenylglycine
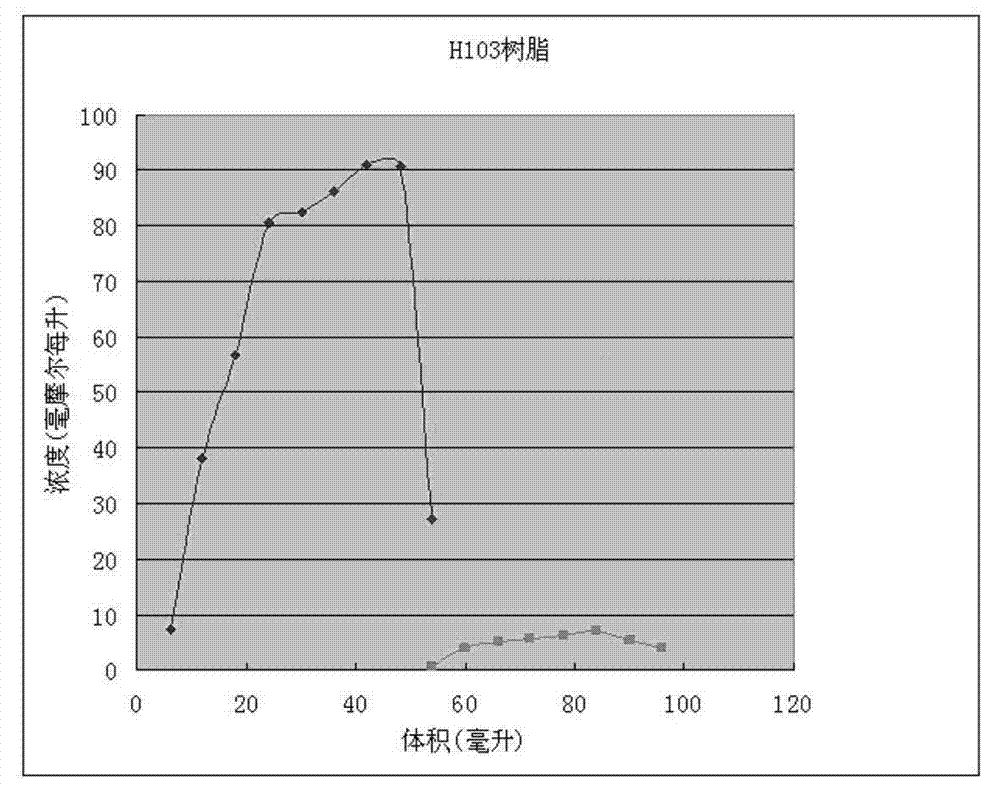
The invention discloses a method for recycling active ingredients in the amoxicillin mother liquor synthesized by an enzymatic method. The method includes synthesizing the amoxicillin mother liquor by the enzymatic method; separating the amoxicillin mother liquor through a macro-porous resin column, eluting the separated amoxicillin mother liquor by deionized water, and collecting an eluant rich in D-4-Hydroxyphenylglycine and an eluant rich in 6-amino penicillanic acid (APA) respectively; filtering the eluant rich in D-4-Hydroxyphenylglycine with Daltonian ultrafiltration membranes with a cutoff molecular weight of 150 to 200; performing nanofiltration concentration on the Daltonian ultrafiltration membranes for the filtered liquor with the cutoff molecular weight of 150 to 200, and standing, crystallizing and filtering the concentrated liquor to obtain solids, and drying the solids to obtain the D-4-Hydroxyphenylglycine. According to the method, the technological design is reasonable, the operation is convenient, the recycling effect is good, and energy is saved and the environment is protected.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
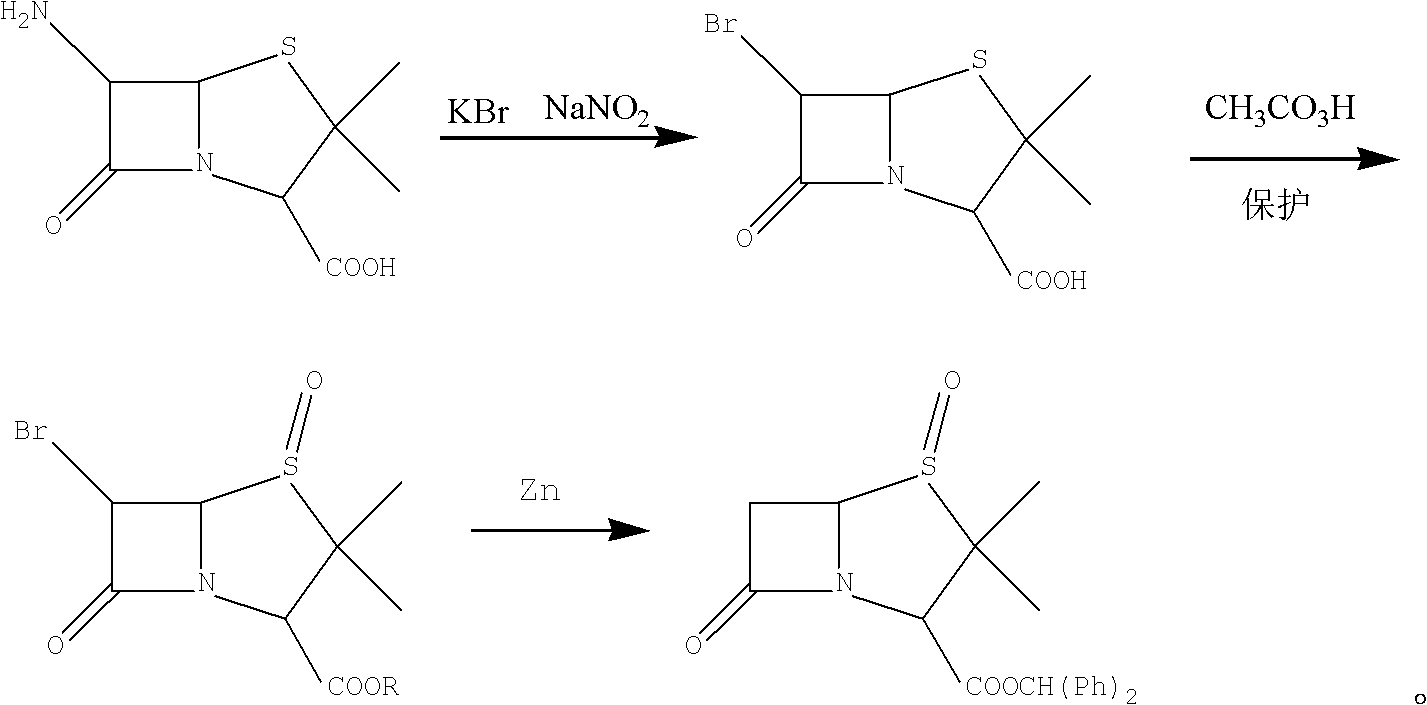
Preparation method of benzhydryl s-oxopenicillanate
ActiveCN103044447AThe synthesis steps are simpleShort reaction cycleOrganic chemistryHydrogenation reactionBromine
The invention provides a preparation method of benzhydryl s-oxopenicillanate, which comprises the following steps: reacting 6-aminopenicillanic acid to obtain a penicillanic acid (iii) solution; adding catalyst molybdenum acetopyruvate, and dropwisely adding oxydol while controlling the temperature; after the reaction finishes, centrifuging to obtain oxopenicillanic acid; and reacting to obtain the benzhydryl s-oxopenicillanate. The method provided by the invention has the following advantages: 1. simplified synthesis steps: the processes of bromination and reduction debromination are not adopted; 2. short reaction period: only three reaction steps are adopted, thereby greatly shortening the production period; 3. favorable reaction selectivity and high yield: the total yield of the three steps for preparing the benzhydryl s-oxopenicillanate is higher than 72%; 4. mild reaction conditions: the high-risk hydrogenation reaction is not needed; and 5. the separation of the product and the intermediates is simple to operate, the two intermediates in the benzhydryl s-oxopenicillanate synthesis route can be directly used for synthesizing the required compound without refinement, and the end product can be directly filtered and separated.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Tazobactam synthesis method
ActiveCN102643292ASteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
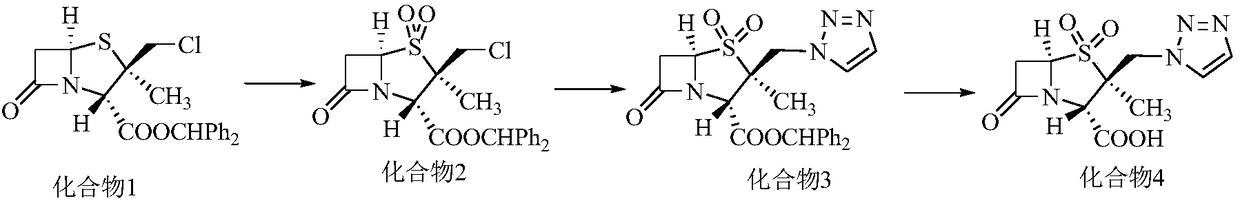
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
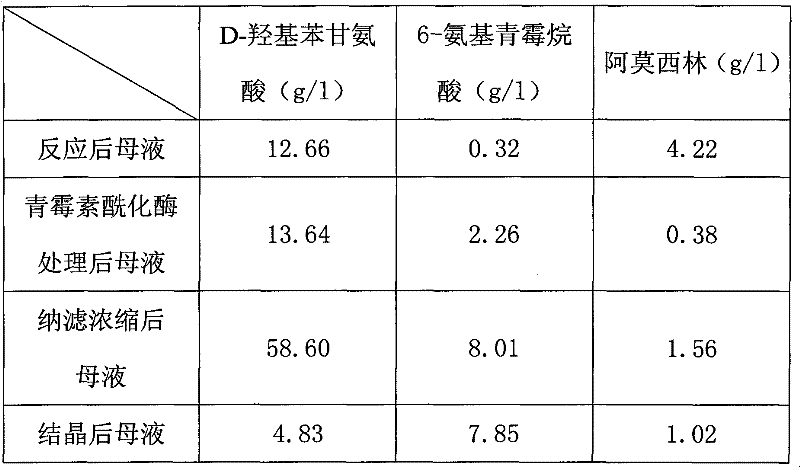
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
ActiveCN102392060AAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisIsoelectric point
The invention discloses a recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration. The method comprises the following steps: (1) regulating pH of the mother liquor; (2) hydrolyzing amoxicillin in the mother liquor by utilizing penicillin acylase, thus only D-hydroxyphenylglycine and 6-amino-penicillanic acid (6-amino-penicillanic acid) exist in the other liquor; (3) preparing concentrated mother liquor by nanofiltration; and (4) separating and recovering the D-hydroxyphenylglycine and the 6-amino-penicillanic acid (6-amino-penicillanic acid) by isoelectric point crystallization. The method has the advantages that: the recycling rate of an inactive lateral chain can be improved regarding certain amoxicillin synthetase with highhydrolysis activity, so that the enzymatic synthesis of amoxicillin is more economical.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
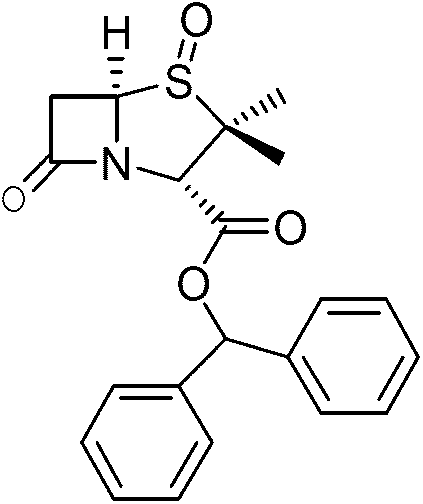
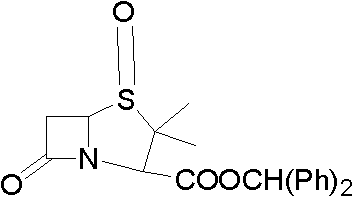
Method for preparing penicillanic acid sulfoxide diphenyl methyl ester
ActiveCN101935324AQuality improvementIncrease profitOrganic chemistryHigh pressureMeta-Chloroperoxybenzoic acid
The invention provides a method for preparing penicillanic acid sulfoxide diphenyl methyl ester. Penicillanic acid sulfoxide is prepared by the following four-step reaction. Hydrobromic acid is used as a brominating agent for substituting potassium bromide and bromine, and hydrogen peroxide is used as an oxidant for substituting peracetic acid and chloroperoxybenzoic acid, so the preparation method is safe and environment-friendly; in addition, the hydrogen peroxide is reacted with a compound (iii) under the catalysis action of acetyl molybdenum pyruvate so that the reaction mole yield can be obviously improved and reaches 90 percent; zinc powder which substitutes palladium and carbon is used as a reducing agent, so high-pressure equipment is not needed in the reaction; and the preparation method simplifies the process operation step, is easy for industrialized production, and reduces the production cost.
Owner:江西祥太生命科学有限公司
Process for producing 6-amino-penicillanic acid and phenylacetic acid
PCT No. PCT / ES97 / 00066 Sec. 371 Date Feb. 25, 1998 Sec. 102(e) Date Feb. 25, 1998 PCT Filed Mar. 14, 1997 PCT Pub. No. WO97 / 35029 PCT Pub. Date Sep. 25, 1997Alternative process for obtaining 6-aminopenicillanic acid. The process comprises replacing the stages of extraction with organic solvents and isolation and separation of the intermediate penicillin salt as a solid by a process of ultrafiltration of the culture broth in at least 2 successive stages. The first stage has a cut-off for molecular weights of 20,000 Dalton and the second, 2000 Dalton. Subsequent to the enzyme conversion stage the products from that stage are subjected to a series of anionic exchange chromatography steps.
Owner:ANTIBIOTICOS SA
Preparation method for 6-amino penicillanic acid
The invention discloses a preparation method for 6-amino penicillanic acid, which comprises the following steps: a, performing ultrafiltration membrane separation and nanofiltration membrane concentration on a penicillin fermentation liquor to obtain a concentrated filter liquor; b, placing the concentrated filter liquor into a reaction tank, adding an immobilized penicillin acylase 4MU / m<3> concentrated filter liquor and performing conversion reaction to obtain a 6-amino penicillanic acid conversion solution; c, performing actived carbon decoloration and filtering on the conversion solution to obtain a 6-amino penicillanic acid filter liquor; and d, adding seed grain into the 6-amino penicillanic acid filter liquor obtained through the procedures in the step c, growing the grain, crystallizing, filtering, washing and drying. The preparation method has the advantages of simple process flow, easiness for operation, safety, environmental protection, and capabilities of effectively improving the yield of 6-APA, reducing the production cost and improving the labor productivity.
Owner:NORTH CHINA PHARMA COMPANY
Technology for preparing amoxicillin by straight-through method
The invention discloses a technology for preparing amoxicillin by a straight-through method. The technology takes a penicillin degreasing fluid as a starting raw material; 6-APA (6-amino penicillanic acid) crystallization, suction filtration, washing and drying steps are saved; the amoxicillin can be prepared without obtaining solid 6-APA via separation; and the prepared amoxicillin meets a requirement of a medicinal standard. The technology saves an operation procedure, reduces the labor intensity of production personnel, lowers the production cost, reduces environmental protection pressure, avoids contact between the production personnel and 6-APA dry powder, and reduces anaphylaxis.
Owner:石药集团中诺药业(石家庄)有限公司
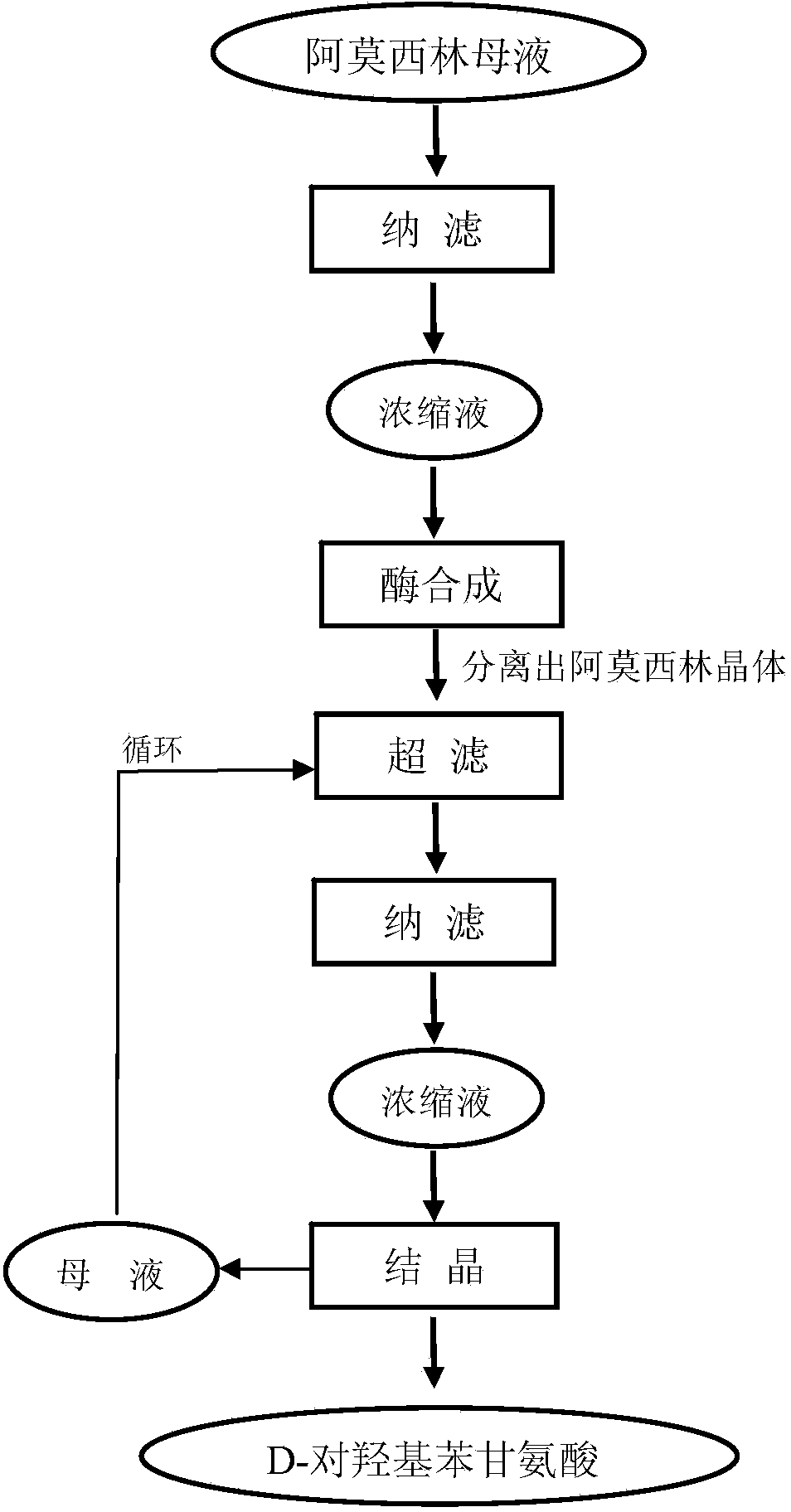
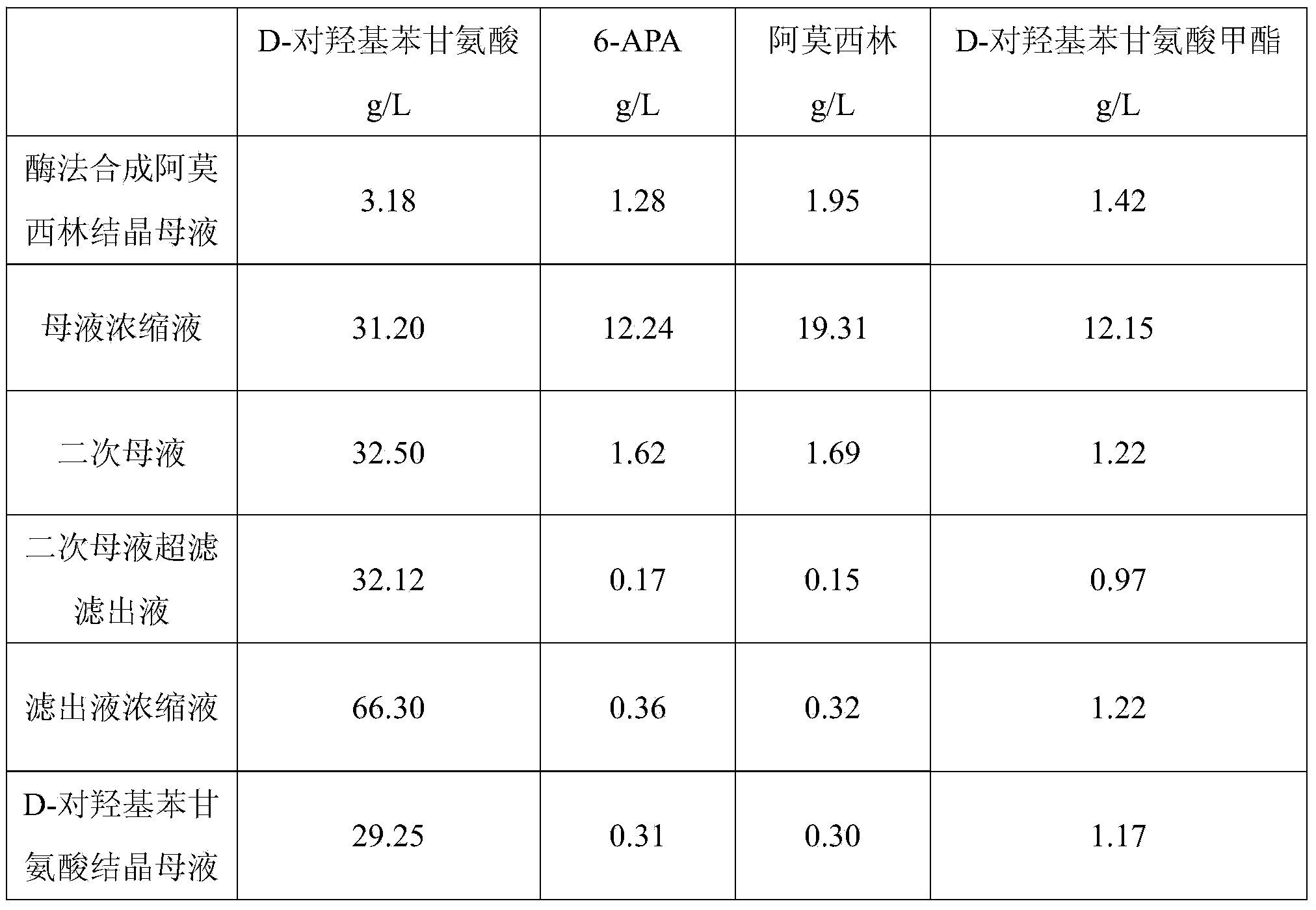
Method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by enzyme process
ActiveCN104357528AImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationChemistry
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
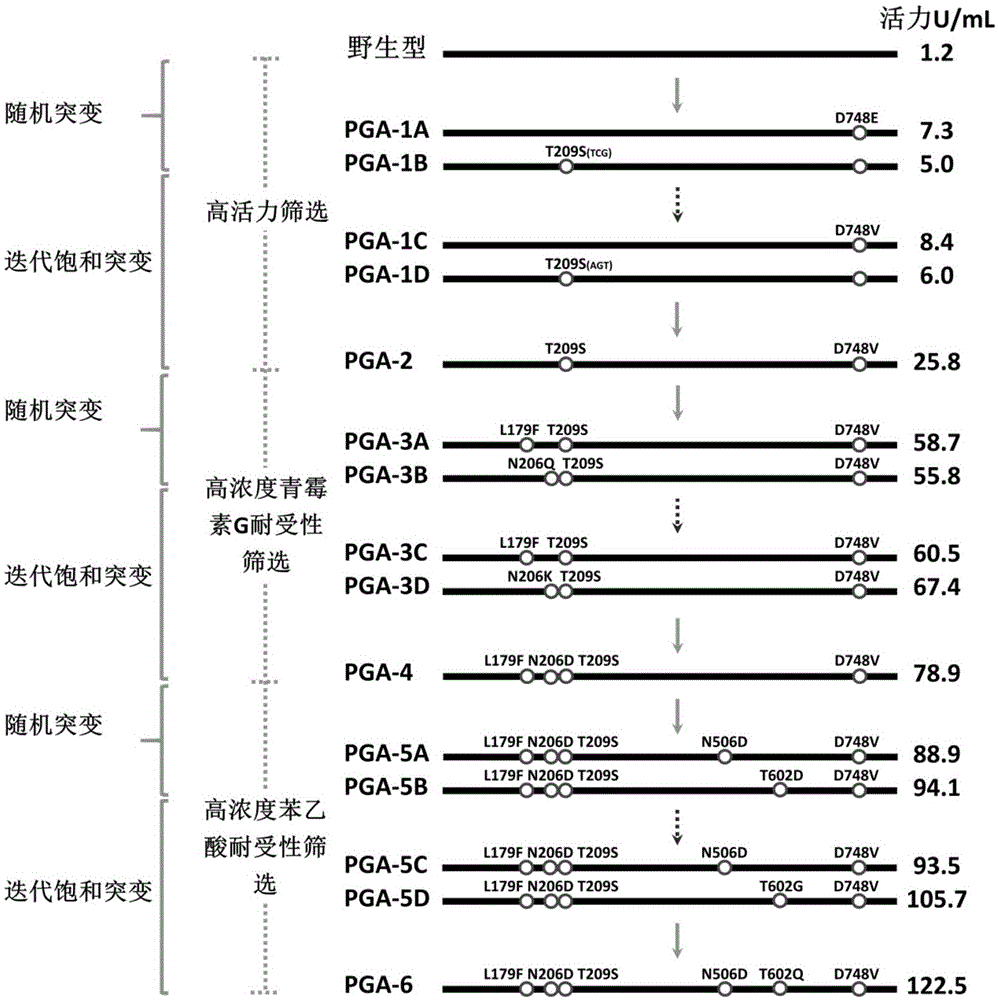
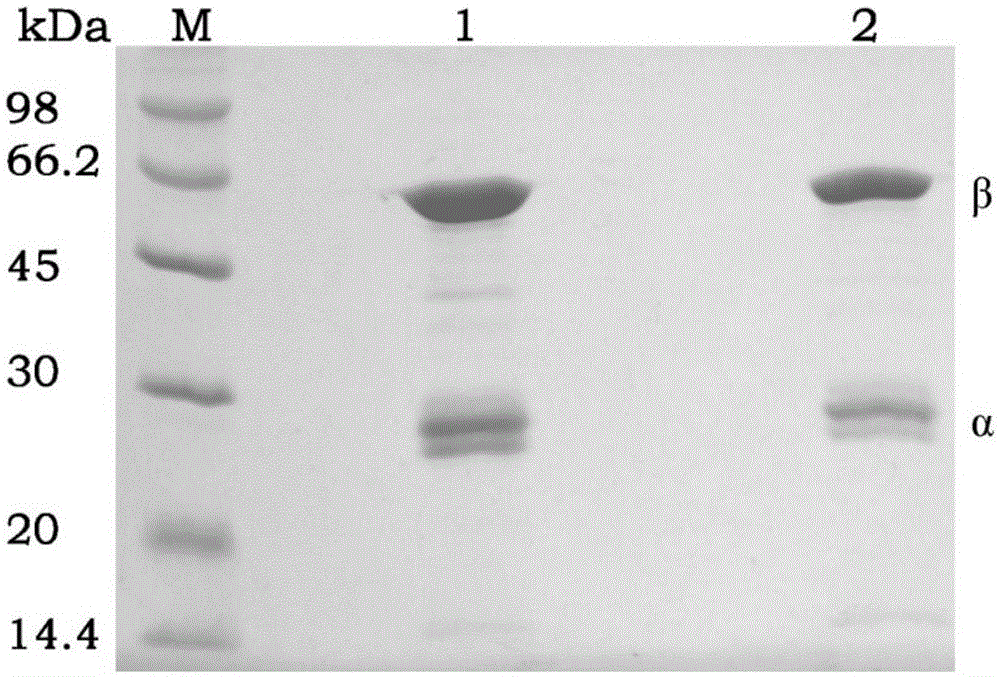
Mutant of penicillin G acylase (PGA) and preparation method and application of mutant
ActiveCN105087533AStrong concentration toleranceIncreased concentration toleranceHydrolasesFermentationPhenyl acetic acidEscherichia coli
The invention provides a mutant of penicillin G acylase (PGA) and a preparation method and application of the mutant. The non-rationally and semi-rationally designed enzyme engineering reconstruction technology is adopted for mutation of penicillin G acylase obtained from Escherichia coli ATCC 11105, so that a PGA mutant with higher reactivity, higher reaction rate, better conversion rate, stronger in penicillihe concentration tolerance, less substrate residue, and higher in phenyl acetic acid concentration tolerance; meanwhile, the mutant is subjected to recombinant expression, bacteria strain construction, fermenting cultivation, immobilization and application to prepare 6-amino-penicillanic acid (6-APA). The activity of the PGA-6 mutant prepared by the invention is increased by 102 times, the substrate penicillihe concentration tolerance is increased to 30%, and the phenyl acetic acid concentration tolerance is increased to 20 mmol / L; meanwhile, the immobilized PGA-6 mutant is used to decompose penicillihe with a concentration of 25% under the condition of pH 8.0 and 25 DEG C so as to prepare 6-APA, and the reaction time is shortened to 55 minutes, the substrate conversion rate is 98% or above, and after being used for 600 batches and above, the activity is not lost obviously, therefore, good operation stability is achieved.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
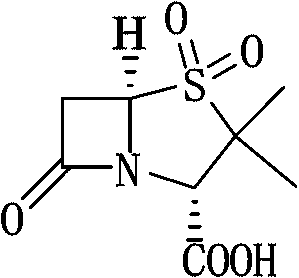
Sulbactam sodium preparation method
InactiveCN106699774AReduce pressure on environmental protectionEasy to operateOrganic chemistryStrong acidsReaction step
The invention relates to a sulbactam sodium preparation method belongs to the technical field of synthesis of beta-lactamase inhibitors. The sulbactam sodium preparation method comprises the steps that 6-amino penicillanic acid (6-APA) is used as a raw material, reacts in strong acid and a sodium nitrite water solution, then reacts under the effects of copper powder and hypophosphorous acid, and then a target product sulbactam sodium is prepared through oxidation and substitution reaction. The reaction process is simple in operation, the method includes few reaction steps, a by-product is reduced, the final reaction yield is high, the treatment difficulty and cost after wastewater production are reduced, the pressure of environmental protection is reduced for enterprises, the product production cost is reduced, and the sulbactam sodium preparation method is suitable for industrial production.
Owner:淄博鑫泉医药技术服务有限公司
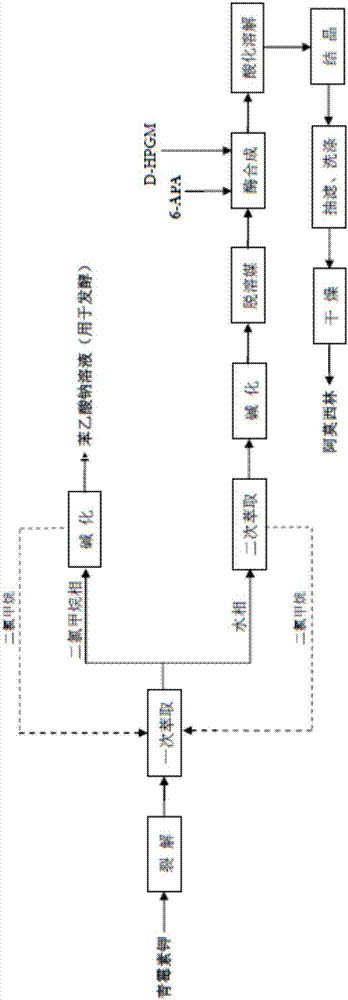
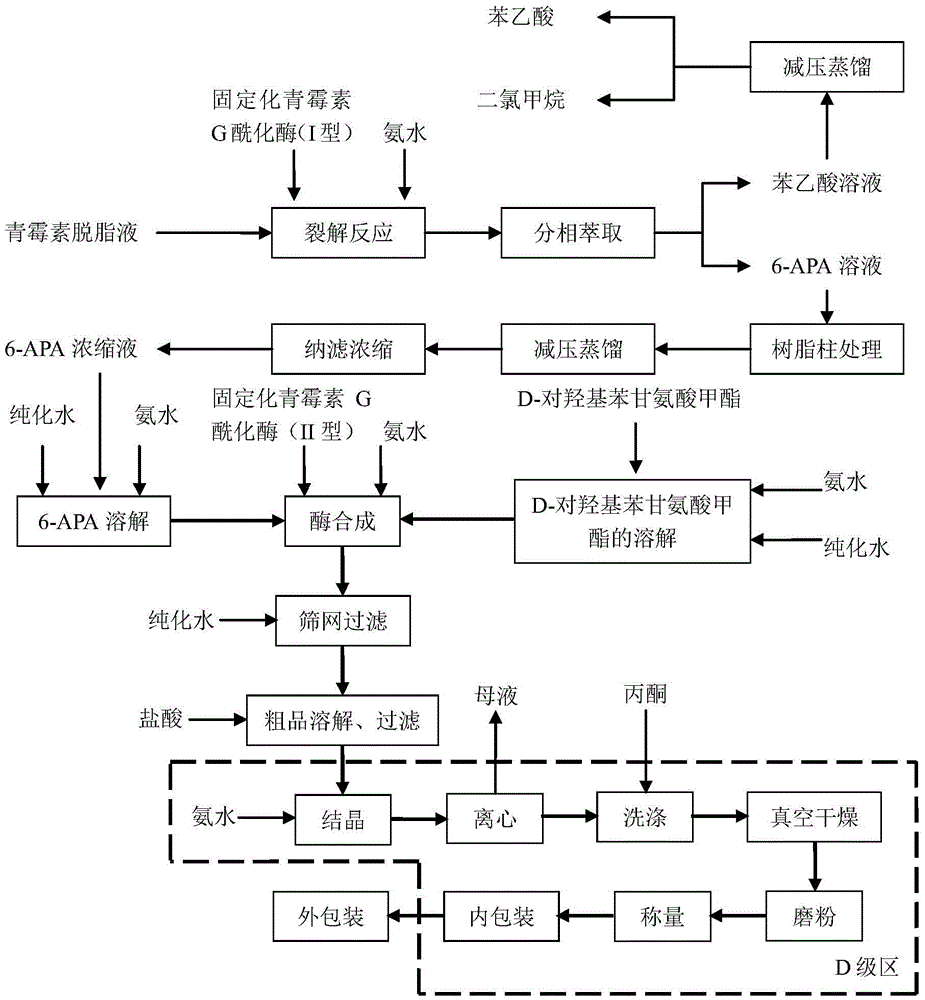
Process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid)
The invention belongs to the technical field of medicine preparation and relates to a process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid). The process includes: taking penicillin degreasing solution as an initial material, sequentially performing cracking reaction, extraction, phase splitting, resin column adsorptive purification, distillation and concentration to obtain a 6-APA solution with the concentration being 80-100g / L, and synthesizing with p-hydroxyphenylglycine methyl ester under a catalytic action of type-II penicillin G acylase to obtain the amoxicillin. Compared with a traditional method, the process has the advantages that subsequent steps of 6-APA crystallization, centrifuging, drying and the like are avoided, investment of fixed assets is reduced, energy loss, equipment loss and cost are reduced, profits are increased, and physical injuries of staffs are reduced. Compared with existing direct amoxicillin preparation methods, the process has the advantages that by adoption of dichloromethane as an extracting agent, total mole yield of amoxicillin is higher relatively, the extracting agent is easy for distillation separation, the content of residual solvents in products is greatly reduced, medication safety is improved, and the process is worthy of popularization in production.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
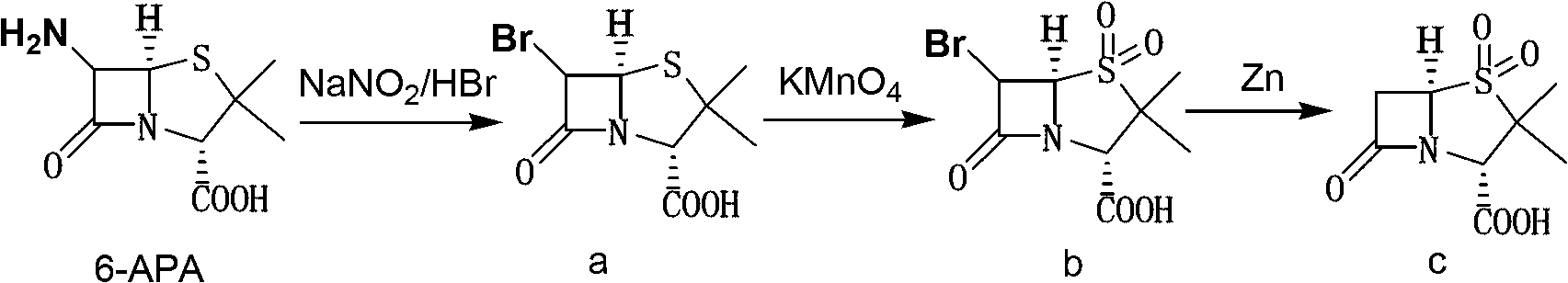
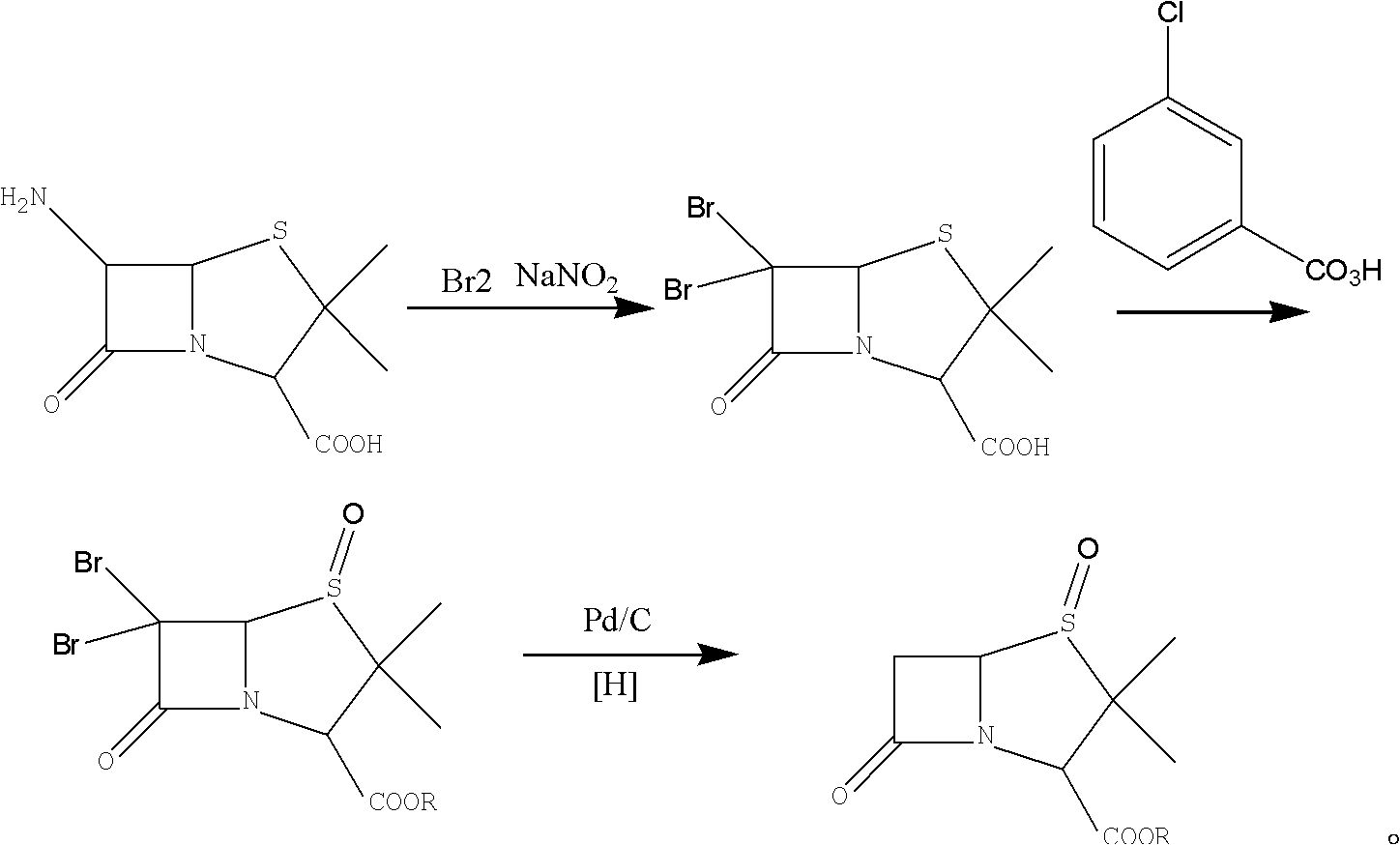
Sulbactam preparation method
The invention discloses a sulbactam preparation method. In the method, 6-amino penicillanic acid (6-APA) serving as a raw material undergoes a dibromination reaction with bromine serving as a bromating agent in the presence of sulfuric acid or hydrobromic acid; the product of the dibromination reaction is oxidized by potassium permanganate; the product of the oxidization is subjected to debromination by using zinc powder or magnesium powder as a reducing agent; and thus, the sulbactam is prepared. In a manganese dioxide removing process in an oxidization phase, 50-percent sulfuric acid and 20 to 28-percent hydrogen peroxide are added alternately; in a pH value adjusting process in a reduction phase, the pH value is adjusted by adding sodium bicarbonate; and in a layer-based extraction process in the reduction phase, an organic layer is washed by 4 to 10 percent solution of potassium permanganate first till red color is stable and then washed by saturated aqueous solution of sodium chloride. Thus, byproducts are reduced, the yield of main products is increased, the product is very stable and avoids turning yellow, and a good basis is provided by down-stream products.
Owner:JIANGSU HUAXU PHARMA
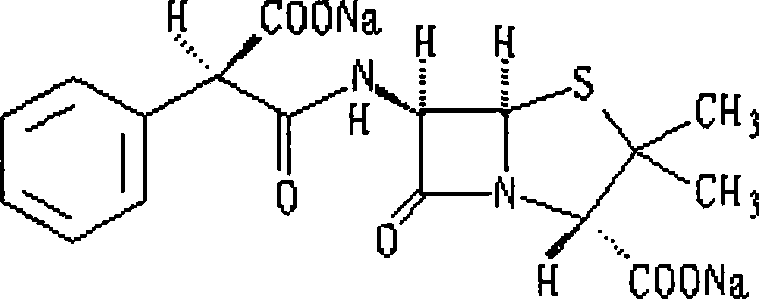
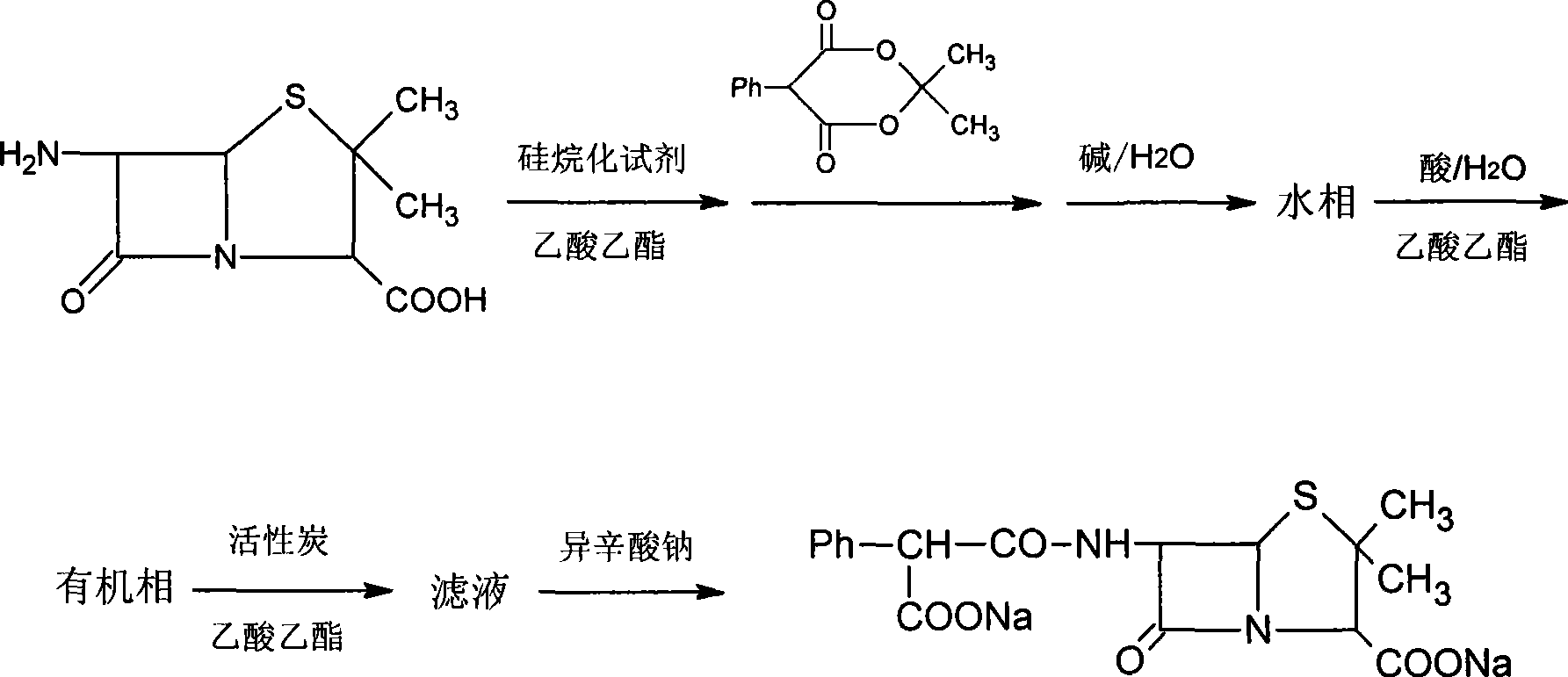
Process for the preparation of the sodium salt of 6[D-(-)alpha-4-(ethyl-2,3-dioxo-1-piperazinocarbonylamino) phenylacetamido]penicillanic acid
The present invention refers to the preparation process of the sodium salt of 6[D-(-)alpha-4-(ethyl-2,3-dioxo-1-piperazinocarbonylamino) phenylacetamido]penicillanic acid, comprising the reaction of the acid with a reagent selected from the group consisting of sodium hydroxide, sodium carboxylates and sodium alcoholates, followed by a separation step of the so obtained sodium salt by precipitation.
Owner:IST BIOCHIM ITALANO GIOVANNI LORENZINI
Preparation method of tazobactam
The invention discloses a preparation method of tazobactam. The preparation method comprises the following steps: performing double oxidization on 2beta-chloromethyl penicillanic acid diphenyl methylester by adopting a solution prepared from potassium permanganate, glacial acetic acid and concentrated sulfuric acid; then loading triazole by taking crown ether as a phase transfer catalyst and taking potassium iodide as a catalyst; then performing deprotection to obtain tazobactam. Compared with the prior art, the preparation method disclosed by the invention has the advantages that although sulfur atoms are oxidized into sulfone to lower chlorine atom activity, the use of the crown ether as the phase transfer catalyst and the potassium iodide as the catalyst compensates for the inactivation well. By adopting the method, the stability of the 2beta-chloromethyl penicillanic acid diphenyl methyl ester is improved, the reaction time is shortened, the reaction yield is improved, the operation risk is lowered, and the industrial production is facilitated.
Owner:山东安信制药有限公司 +1
Method for recovering 6-amino penicillanic acid by employing membrane
InactiveCN101041663AEliminate degradationNo generationSemi-permeable membranesOrganic chemistryOrganic solventFilter system
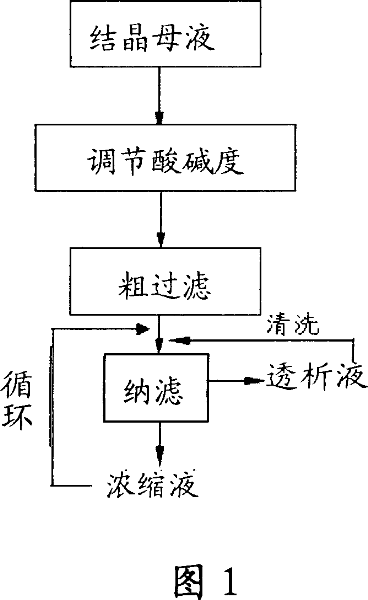
The invention discloses a recycling method of 6-amino penicillanic acid through film, which comprises the following steps: adjusting pH value of crystal mother liquid with 6-amino penicillanic acid to 5.0-8.5; proceeding rough filter; adding mother liquid into nano-filtering system; separating through nano-filtering film; obtaining the condensate and dislysate with condensing times at 5-20; adopting the organic solvent tolerant film with stop molecular weight at 5-800 at 1-10 deg.c under 15-40bar; washing the nano-filtering system.
Owner:BEIJING DAKING EASTERN TECH
Method for preparing (3R,4R)-3-[(1R)tert-butyl dimethyl silica ethyl]-4-acetoxy-2-aza ring butanone
InactiveCN101220050AHigh yieldLow reaction temperatureGroup 4/14 element organic compoundsBromine4-acetoxy-2-azetidinone
The invention discloses a method for preparation of (3R, 4R) -3-[(1R)-(tert-butyl dimethylsilyloxy) ethyl]-4-acetoxy-2-azetidinone. The method contains procedures such as diazotization, bromination, esterification, Griganard reaction, reduction, hydroxyl protection, ring opening, oxidation, etc., wherein through reaction time adjustment during the process of esterification, reaction temperature decrease in ring opening and application of pyridine as the solvent in synthesis of (3S, 5R, 6S)-6-bromine-6-[(1R)-hydroxyethyl] penicillanic acid methyl ester of the Griganard reaction, the reaction of procedure 3) can be carried out ranging from minus 25 DEG C to minus 5 DEG C with equivalent total yield, thereby greatly reducing the equipment investment and energy consumption.
Owner:HEBEI UNIV OF TECH
Method for synthesizing general artificial antigen of beta-lactam drugs
InactiveCN101307092AThe synthesis steps are simpleThe synthetic step worksSerum albuminPeptide preparation methodsBovine serum albuminCarrier protein
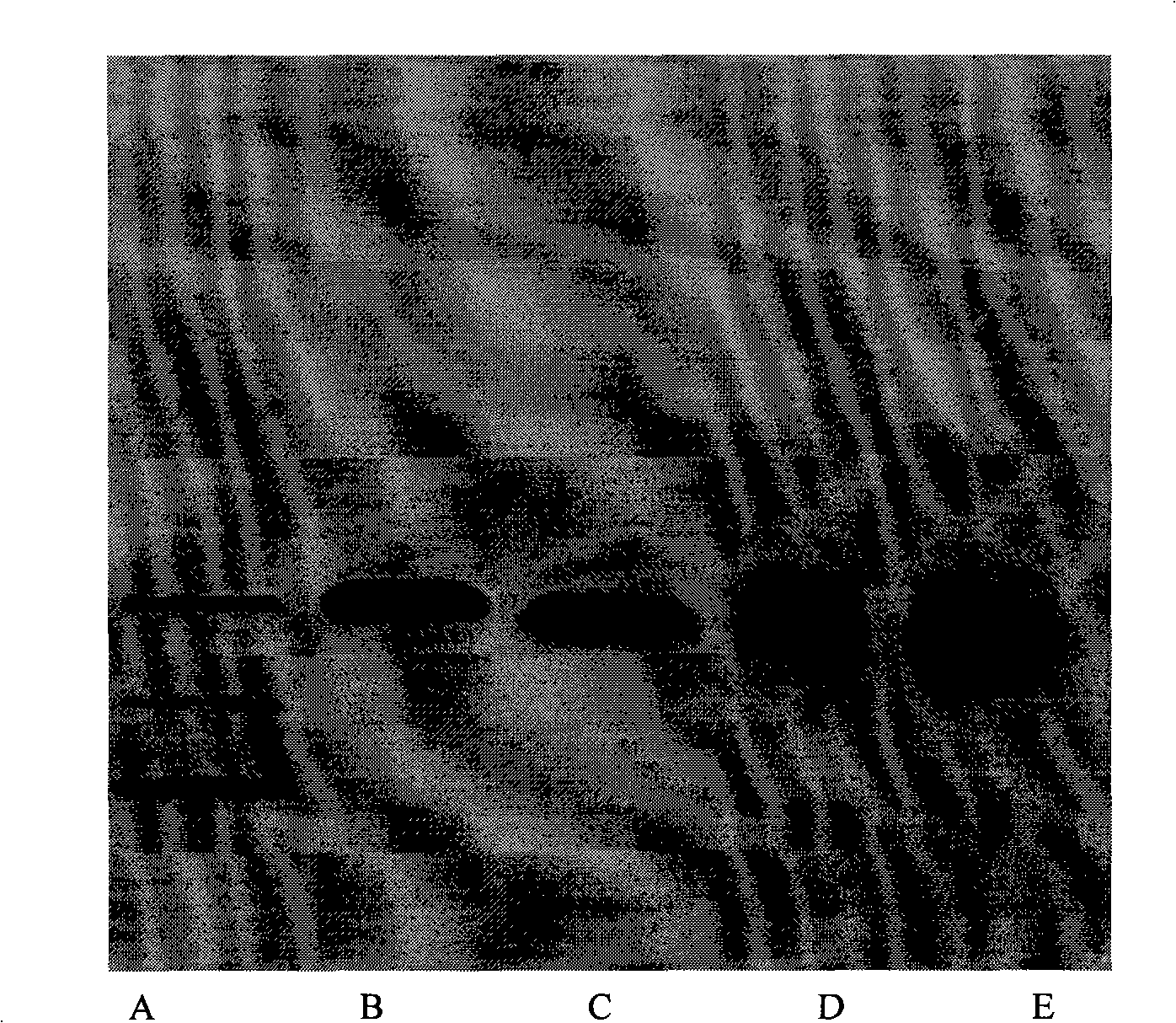
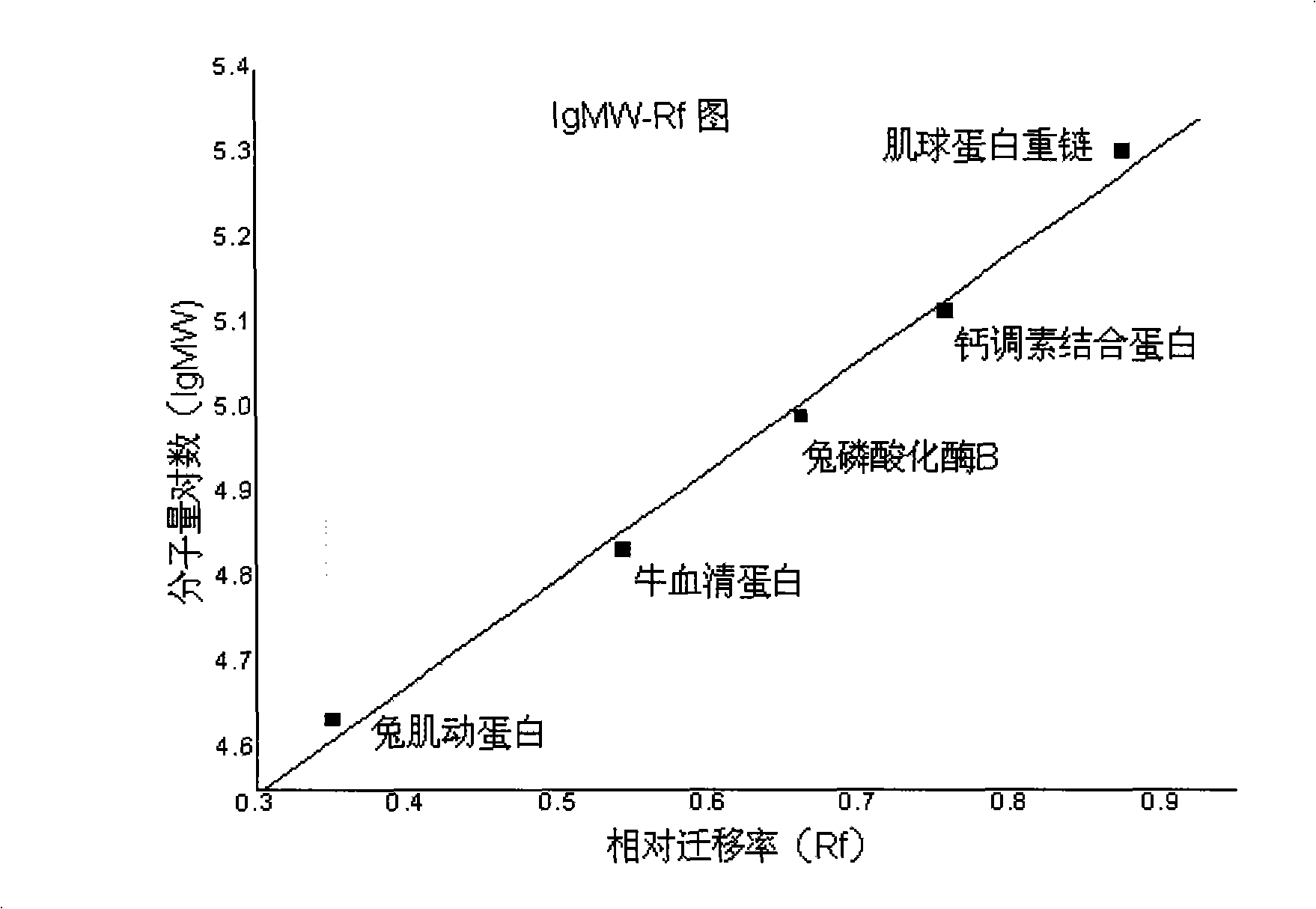
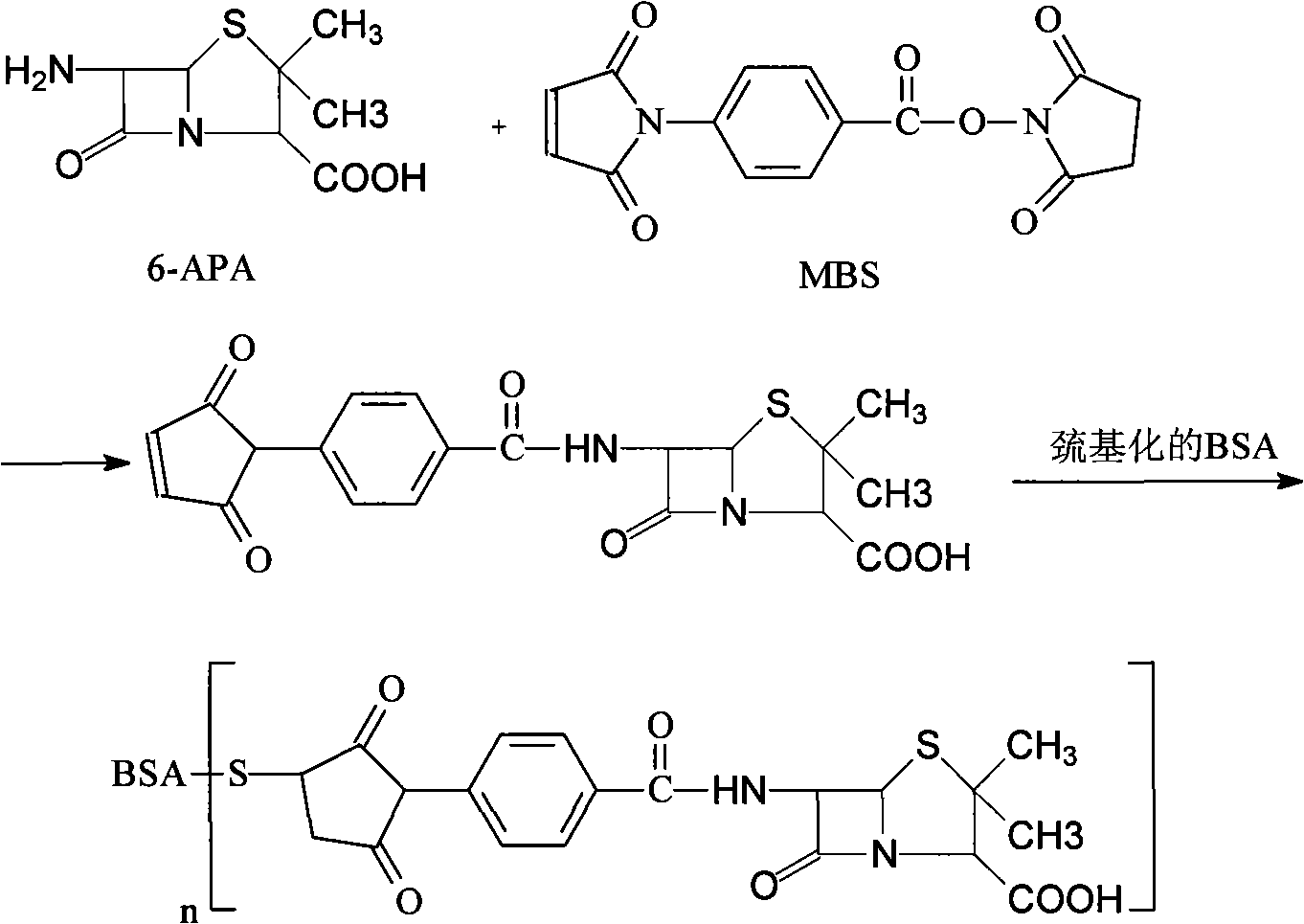
A synthetic method for the general artificial antigen of a beta-lactam medicine belongs to the biochemical technical field. The invention adopts 6-amino penicillanic acid as hapten, couples the hapten with the carrier protein of bovine serum albumin BSA through an N-(m-maleimide group benzene methanoyl) succinimides MBS method, and determines the coupling ratio of the coupled matter through a gel electrophoresis method. The method successfully synthesizes the 6-APA general artificial antigen with a simple and effective synthesis process. The method which can completely be used for immune analysis provides necessary artificial antigen for future study and meets the requirements of domestic study on the artificial antigen.
Owner:JIANGNAN UNIV
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
ActiveCN102392060BAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisNanofiltration
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Method for preparing sulbactam
The invention discloses a method for preparing sulbactam and belongs to the technical field of pharmaceuticals. The method is characterized in that a 'one-pot' method is adopted, starting from 6-aminopenicillanic acid (6-APA), under the action of sodium nitrite and hydrobromic acid, a reaction solution which contains product 6 alpha-penicillanic acid is obtained through bromination reaction, and then, solid sulbactam is obtained through further oxidation and reduction reaction. The solid sulbactam is directly prepared during the reaction preparation process without separation, thus the method is simple to operate and easy for industrial production; the method adopts single-bromination reaction to substitute double-bromination reaction; moreover, bromine which has greater pollution is not used as a raw material, but hydrobromic acid is taken as a raw material, and the method is simple to operate and is low in cost; and the sulbactam solid which is prepared through the method is high in yield and good in quality.
Owner:QILU TIANHE PHARMA
Method of preparing semisynthetic antibiotic 6-amino penicillanic acid by ion liquid extraction penicillin and enzymic catalytic reaction coupling
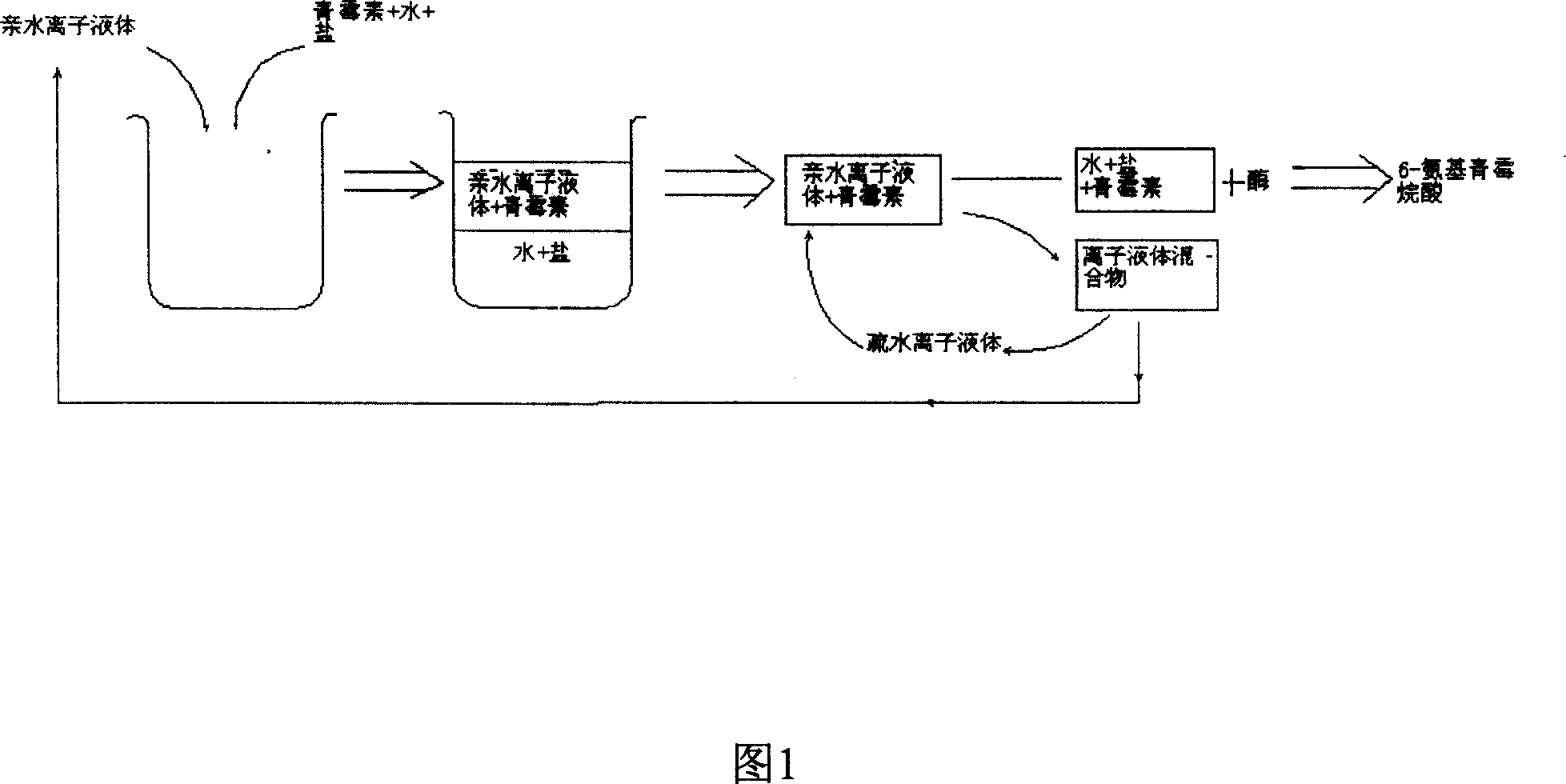
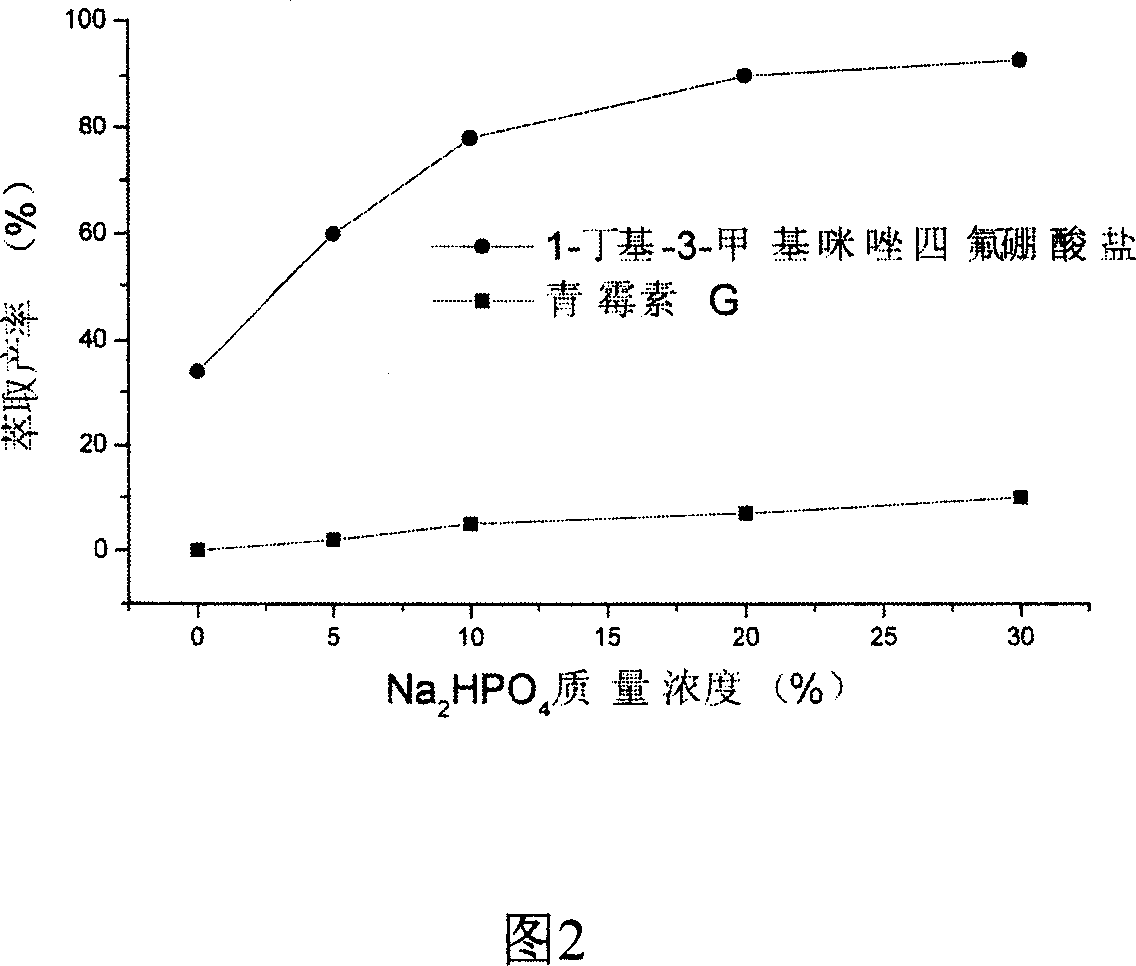
InactiveCN1948316AAchieve separationReduced hydrophilic ionic liquid contentOrganic chemistryFermentationOrganic solventHydrophile
The present invention relates to a technological process for preparing semi-synthetic antibiotic 6-aminopenicillanic acid by utilizing integrative process composed of penicillin fermentation liquor extraction and enzymatic catalysis reaction. It is characterized by that it utilizes the aqueous two-phase formed by hydrophilic ionic liquor to make penicillin be extracted into the upper phase containing ionic liquor, then utilizes hydrophobic ionic liquor to make secondary extraction so as to make the hydrophilic ionic liquor be extracted into hydrophobic phase, and the penicillin aqueous solution of raffinate phase can be directly undergone the process of enzymatic catalysis reaction, the enzyme activity can be up to above 80%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
The preparation method of latamoxef sodium intermediate
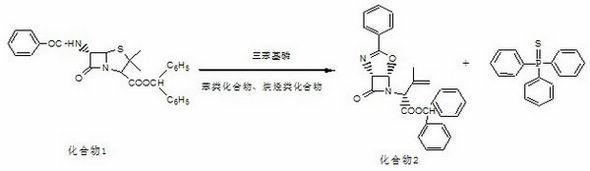
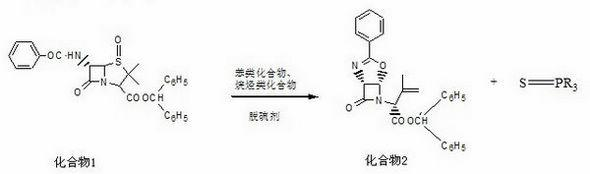
InactiveCN102286004ASimple production equipmentEase of industrial productionOrganic chemistryAlkaneLatamoxef
The invention discloses a preparation method of Latamoxef sodium intermediate, which comprises adding 6-beta-benzamide-4-oxo-penicillanic acid diphenylmethyl ester and triphenylphosphine into benzene and alkanes In the solvent, the reaction is carried out under reflux; after concentration under reduced pressure, acetonitrile and alcohol mixed solvent are added to the concentrate, crystal growth, filtration, concentration, cooling and crystallization, and filtration are carried out to obtain the present invention. Since triphenylphosphine was chosen to replace the highly toxic and harmful tributylphosphine desulfurizer, the uncontrollable dehydration device was eliminated, the production equipment was simplified, and the mixed system of the generated by-product triphenylthiophosphine in acetonitrile and alcohol solvents It can be crystallized in solid form, which reduces the subsequent impurity removal process and is beneficial to ensure the improvement of product quality and yield. The product yield can be increased from 40% to 58% of the original process, and the cost of materials can be reduced by about 600 yuan / Kg, which provides technical conditions for the large-scale production of oxycephem parent core, with significant economic benefits and strong market competitiveness .
Owner:河北九派制药股份有限公司
Preparation method of 6-chloropenicillin sulfoxide diphenylmethyl ester and application thereof
ActiveCN108264519AReduce degradation rateImprove the overall synthesis yieldOrganic chemistryWater insolubleCarboxylic acid
The invention provides a preparation method of 6-chloropenicillin sulfoxide diphenylmethyl ester and application thereof. The preparation method comprises the following steps that in mixed liquid of hydrochloric acid and alcohol, 6-APA and sodium nitrite are subjected to chlorination to obtain 6-chloropenicillanic acid (AT-0); the AT-0 is extracted with water-insoluble solvent, and then is oxidized with hydrogen peroxide to obtain 6-3-3-dimethyl-7-oxo-4-fluorenyl-1-azabicyclo[3.2.0] n-heptane-2-carboxylic acid (AT-1); AT-1 is reacted with ATMD to obtain chloropenicillin Diphenyl sulfoxide (AT-2) in the water-insoluble solvent. The invention also provides a method for preparing pentamidine sulfoxide diphenylmethyl ester using AT-2 wet products. The molar yield of the method is much higher than the prior art due to the fact that bromide of penicillanic acid and the pentamidine sulfoxide diphenylmethyl ester is less stable compared with chloride of the penicillanic acid and the pentamidine sulfoxide diphenylmethyl ester.
Owner:潍坊奥通药业有限公司
Method for synthesizing carbenicillin sodium
InactiveCN101469002AReaction raw materials are cheap and easy to obtainMild reaction conditionsOrganic chemistryFiltrationSynthesis methods
The invention relates to a synthesis method for carbenicillindisodium. The carbenicillindisodium is prepared sequentially through the following steps: adding 6-amino penicillanic acid and a silanized agent into ethyl acetate, and after stirring, adding 2,2-dimethyl-5-phenyl-1,3-dioxane-4,6-diketone till the reaction is completed; adding water and alkaline into the reaction mixture, and regulating the pH value to between 5.0 and 8.5 percent; after carrying out the phase separation, collecting the water phase, adding ethyl acetate, regulating the pH value to between 1.0 and 4.0 through acid, carrying out the phase separation again, and collecting the organic phase; and adding ethyl acetate and active carbon into the organic phase for stirring and filtration, adding sodium iso-octoate solution into the filtrate, filtrating out the precipitate, cleaning the precipitate through acetone, and carrying out the vacuum drying of the precipitate to obtain the carbenicillindisodium. The synthesis method has easily obtained and cheap raw materials, moderate reaction conditions and high yield, and is suitable for the industrialized production. The solvents used are only water and ethyl acetate, thereby reducing the harm of the solvents on operating personnel, and bringing about the convenient reclamation and treatment of the solvents.
Owner:上海新先锋药业有限公司 +1
Method for synthesizing amoxicillin and generating byproduct of sodium phenylacetate solution by using semi-direct method
InactiveCN107988306AHigh yieldImprove product qualityOrganic compound preparationCarboxylic acid salt preparationPhenylacetic acidBenzylpenicillin potassium
The invention relates to a method for synthesizing amoxicillin and generating a byproduct of a sodium phenylacetate solution by using a semi-direct method and belongs to the technical field of medicine preparation. The method comprises the following steps: splitting benzylpenicillin potassium into 6-APA (Amino Penicillanic Acid) and phenylacetic acid under the action of penicillin acylase, extracting a splitting solution with dichloromethane to separate 6-APA from the phenylacetic acid, putting ammonia water into an extraction water phase, adjusting the pH value of a material liquid to be neutral, removing the dichloromethane, putting the solid 6-APA into the obtained 6-APA dissolving liquid, and synthesizing the amoxicillin together with D-methyl p-hydroxyphenylglycinate under the actionof amoxicillin synthetase. A sodium phenylacetate solution can be prepared by alkalizing the dichloromethane obtained by extracting the splitting solution, the sodium phenylacetate solution can be directly applied to fermentation production of penicillin, the step of recycling the phenylacetic acid is avoided, and the production cost is reduced.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Method for continuously crystallizing 6-amino-penicillanic acid
ActiveCN104725402AReduce stepsImprove work efficiencyOrganic chemistryAutomatic controlEnergy source
The invention discloses a method for continuously crystallizing 6-amino-penicillanic acid and relates to the technical field of medical intermediate synthesis. The method is used for overcoming the defect of great quality difference between different batches of products obtained by use of an intermittent split-phase crystallization method in the prior art by virtue of a continuous crystallization; and as a result, the uniformity of the products is greatly improved, and meanwhile, the automatic control is applied, the production efficiency is improved and the energy source is saved.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
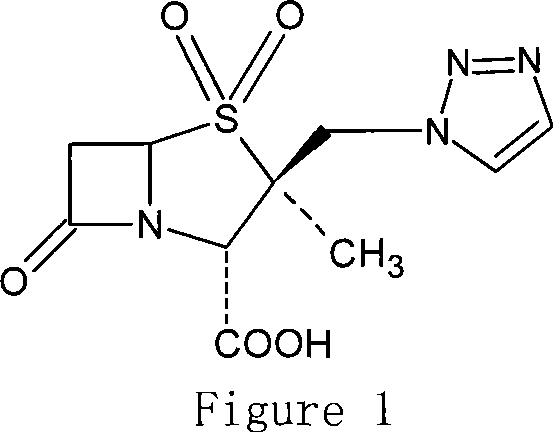
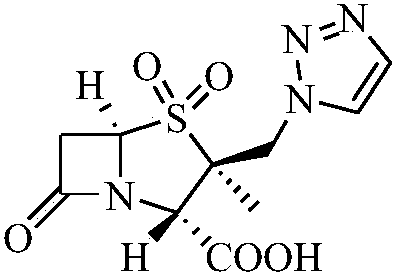
Synthetic method for tazobactam
The invention discloses a synthetic method for tazobactam. The method comprises the following steps: adding propiolic acid, 2 beta-azidomethyl penicillanic acid-1 beta-oxide, sodium ascorbate and a catalyst containing cuprous ions or copper ions to a solvent in sequence; stirring and reacting these materials for 0.5-72 h at 20-180 DEG C; and after the reaction is finished, extracting a reactant and carrying out column chromatography on the reactant so as to obtain the tazobactam. Through the method, the synthetic method for the tazobactam, disclosed by the invention, has the advantages as follows: the tazobactam is a novel sulbactam type beta-lactamase inhibitor and can be used for treating a plurality of bacterial infections; and compared with a method with acetylene as raw material, the method has the advantages as follows: through using the propiolic acid as the raw material, the safety in the reaction process is enhanced and an electricity absorbing carboxyls on a molecule is beneficial for carrying out cycloaddition reaction and preferably compatible with a reaction substrate; the reaction condition is mild; the reaction can be conducted at normal temperature; the operation process is convenient and simple; the yield of obtained products is high; and industrial production can be carried out on a large scale.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Tazobactam synthesis method
ActiveCN102643292BSteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing (3R,4R)-3-[(1R)tert-butyl dimethyl silica ethyl]-4-acetoxy-2-aza ring butanone Method for preparing (3R,4R)-3-[(1R)tert-butyl dimethyl silica ethyl]-4-acetoxy-2-aza ring butanone](https://images-eureka.patsnap.com/patent_img/39a8c06e-36db-4484-84c9-d69ce5ba9d3a/s2008100522519d00011.PNG)