Method for synthesizing amoxicillin and generating byproduct of sodium phenylacetate solution by using semi-direct method
A technology of amoxicillin and sodium phenylacetate, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of phenylacetic acid residue, low 6-APA concentration and low substrate concentration, etc. To achieve the effect of eliminating the generation of waste water, reducing production costs and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
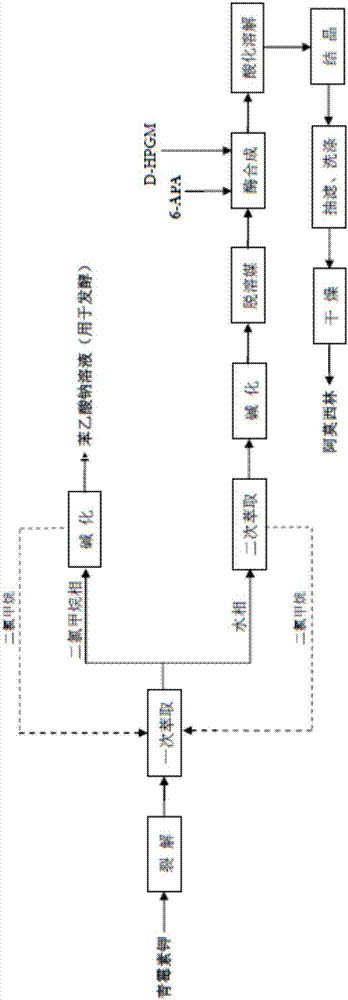
[0037] Embodiment 1 A kind of semi-straight-through method of the present invention synthesizes the method for amoxicillin and by-product sodium phenylacetate solution
[0038] (1) Penicillin Potassium Cleavage
[0039] Weigh 9kg of penicillin potassium, add 91L of purified water, so that the concentration of penicillin potassium is 9%, then add 10.55kg of penicillin G acylase, the enzyme activity is 150u / g; start stirring, add 3mol / L ammonia water to the feed solution , control the pH of the feed solution to be 8.1-8.2, and control the reaction temperature to be 28-30°C; react for 53 minutes, stop the reaction, separate the feed solution from penicillin G acylase to obtain 116L of lysate, and take a sample to determine the concentration of 6-APA to be 44.69 g / L, the concentration of phenylacetic acid is 27.71g / L, the cracking yield is 99.20%, and the lysate is obtained;
[0040] (2) Extraction
[0041] The lysate obtained in step (1) is cooled. When the temperature drops to...
Embodiment 2
[0060] Embodiment 2 A kind of semi-straight-through synthesis method of amoxicillin and by-product sodium phenylacetate solution according to the present invention
[0061] (1) Penicillin Potassium Cleavage
[0062] Weigh 9kg of penicillin potassium, add 66L of purified water, so that the concentration of penicillin potassium is 12%, then add 10.21kg of penicillin G acylase, the enzyme activity is 155u / g; start stirring, add 3mol / L ammonia water to the feed solution , control the pH of the feed solution to be 8.1-8.2, and control the reaction temperature to be 28-30°C; react for 45 minutes, stop the reaction, separate the feed solution from penicillin G acylase to obtain 88L of lysate, and take a sample to determine the concentration of 6-APA to be 58.41 g / L, the concentration of phenylacetic acid is 35.63g / L, and the cleavage yield is 98.36%, and the lysate is obtained;
[0063] (2) Extraction
[0064] The lysate obtained in step (1) was cooled down. When the temperature dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com