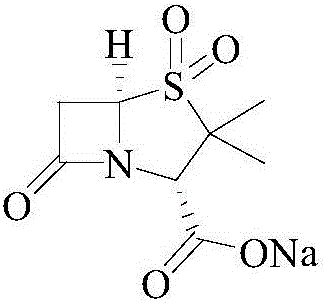
Sulbactam sodium preparation method
The technology of sulbactam sodium and sulbactam acid is applied in the field of β-lactamase inhibitor synthesis, which can solve the problems of high raw material cost, high bromide ion content and high processing cost, and achieves high reaction yield and simple operation. , the effect of reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
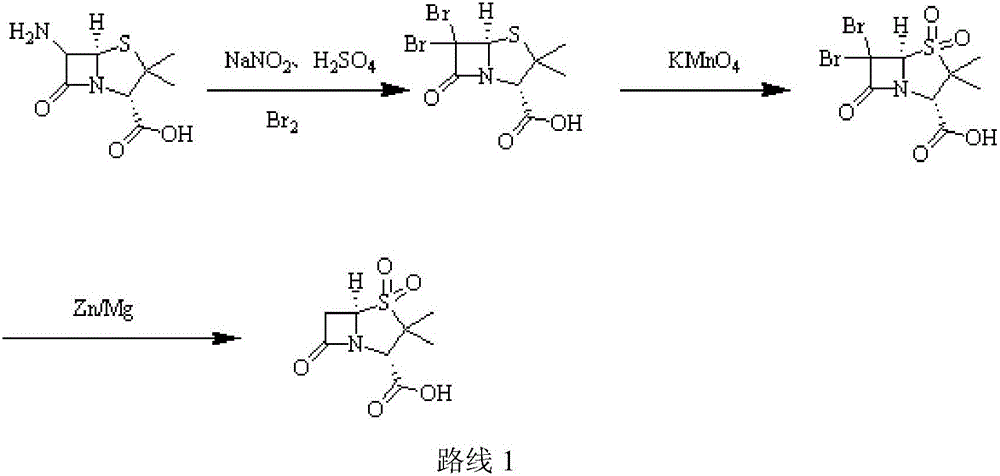
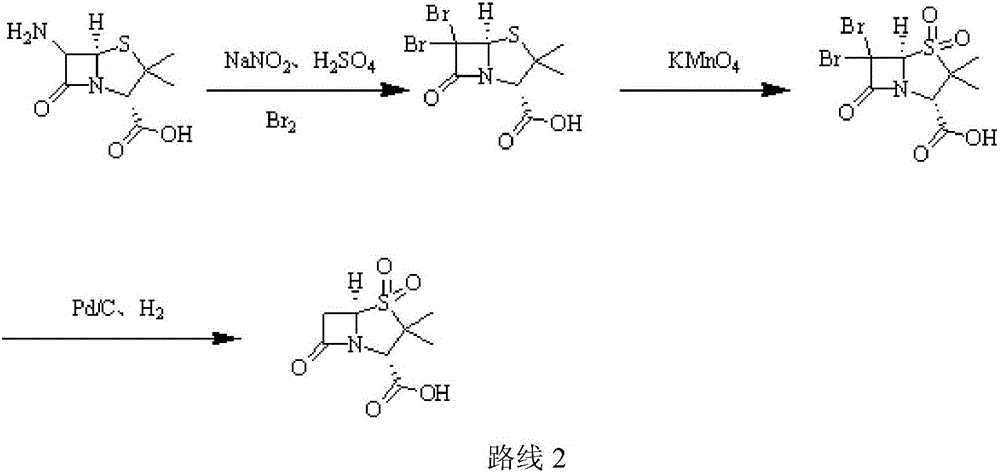
[0031] (1) Add purified water (220ml) to a three-necked flask, add 98% concentrated sulfuric acid (12g, 0.12mol), stir, cool to -5°C, add 6-APA (21.62g, 0.1mol), stir until dissolved; Add 10% sodium nitrite aqueous solution (82.8g, 0.12mol) dropwise, control the temperature at -7±1℃, add dropwise for 2h, keep it for 1h after dripping; add ethyl acetate (220ml), ethanol (50ml), copper Powder (1.9g, 0.03mol), add 50% hypophosphorous acid (15.84g, 0.12mol) dropwise, control the temperature at 5±1℃, add dropwise for 1h, keep warm and stir for 1h; filter after the end of warming, stand still, separate, acetic acid Extract the aqueous phase with ethyl ester and combine the organic phases;
[0032] (2) Cool the organic phase in step (1) to 5°C, add glacial acetic acid (12g, 0.2mol), 3% sulfuric acid aqueous solution (196g, 0.06mol), cool down; maintain the temperature at 7°C, add high manganese in batches Potassium acid (18.96g, 0.12mol), add in 30min, then keep for 2h; after the incub...
Embodiment 2
[0035] (1) Add purified water (320ml) to a three-necked flask, add 98% concentrated sulfuric acid (30g, 0.3mol), stir, and cool to -5°C, add 6-APA (21.62g, 0.1mol), and stir until dissolved; Add 10% sodium nitrite aqueous solution (207g, 0.3mol) dropwise, control the temperature to -2±1℃, add dropwise for 2h, keep the temperature for 4h after dripping; add ethyl acetate (320ml), ethanol (50ml), copper powder (9.53g, 0.15mol), add 50% hypophosphorous acid (66g, 0.5mol) dropwise, control the temperature at 7°C, add dropwise for 1h, keep stirring for 3h; filter after the end of incubation, stand still, separate the liquids, and extract the water with ethyl acetate Phase, combined organic phase;
[0036] (2) Cool the organic phase in step (1) to 5°C, add glacial acetic acid (30g, 0.5mol), 3% sulfuric acid aqueous solution (326.6g, 0.1mol), and cool down; maintain the temperature at 8°C and add high Potassium manganate (47.4g, 0.3mol), add in 30min, then keep for 2h; after the incuba...
Embodiment 3
[0039] (1) Add purified water (390ml) into a three-necked flask, add 38% concentrated hydrochloric acid (7.3g, 0.2mol), stir, cool to -5°C, add 6-APA (32.44g, 0.15mol), stir until dissolved ; Add 10% aqueous sodium nitrite (138g, 0.2mol) dropwise, control the temperature at -5°C, add dropwise for 2h, keep it for 3h after dripping; add dichloromethane (330ml), ethanol (50ml), copper powder ( 9.53g, 0.15mol), add 50% hypophosphorous acid (59.4g, 0.45mol) dropwise, control the temperature at 5°C, add dropwise for 1h, keep warm and stir for 2h; after the end of incubation, filter, stand still, separate liquids, dichloromethane extract water Phase, combined organic phase;
[0040] (2) Cool the organic phase in step (1) to 5°C, add glacial acetic acid (30g, 0.5mol), 3% sulfuric acid aqueous solution (196g, 0.06mol), cool down; maintain the temperature at 8°C, add high manganese in batches Potassium acid (47.41g, 0.3mol), add in 30min, then keep for 2h; after the incubation, add 20% hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com